SUMMARY
Acute symptomatic seizures caused by either diffuse or focal perinatal hypoxic–ischemic insults and intracranial hemorrhage in term newborns make up the large majority of all neonatal seizures. Acute seizures are one of the most common neurological disorders in term newborns who require admission to the neonatal intensive care unit. Despite elucidation of seizure pathogenesis in this population using animal models, treatment is limited by a lack of good evidence-based guidelines because of a paucity of rigorously conducted clinical trials or prospective studies in human newborns. A result of this knowledge gap is that management, particularly drug choice, is guided by clinical experience rather than by data informing drug efficacy and safety. This review summarizes the common etiologies and pathogenesis of acute symptomatic seizures, and the current data informing their treatment, including potential novel drugs, together with a suggested treatment algorithm.
Keywords: Neonatal seizures, EEG, Hypoxic–ischemic encephalopathy, Stroke, Intracranial hemorrhage
1. Introduction
Acute symptomatic neonatal seizures represent the majority of neonatal seizures, and are one of the most common neurological disorders in term newborns admitted to the neonatal intensive care unit (NICU). The three most common etiologies of neonatal seizures are all acute neurologic disorders, namely the ischemic/hemorrhagic brain disorders of hypoxic–ischemic encephalopathy (HIE; 38%), ischemic stroke (18%), and intracranial hemorrhage (12%) [1]. Other etiologies of acute symptomatic neonatal seizures include transient metabolic derangements (4%) and central nervous system (CNS) infections (4%). The proportion of seizures caused by these etiologies has not changed much in the last decade [2], although acute meningitis and/or encephalitis are much less common in developed countries now compared with earlier decades. Currently, acute CNS infections are a relatively uncommon cause of neonatal seizures, on par with many other congenital causes, such as brain malformations (4%), inborn metabolic disorders (3%), and other genetic causes of benign (3%) or severe neonatal onset epilepsies (6%). Additionally, the proportion of neonatal seizures caused by ischemic/hemorrhagic brain injury is probably higher than the numbers cited above, given that these data were derived from a study of tertiary/quaternary NICUs where there is likely a higher proportion of newborns with congenital etiologies of seizures or epilepsy, such as brain malformations, inborn errors of metabolism and genetic epilepsies. This review will focus on the etiologies, pathogenesis and management of acute symptomatic seizures in term newborns, related to acute acquired rather than congenital disorders.
2. Diagnosis of acute symptomatic seizures
Acute symptomatic seizures in newborns can have a subtle, clonic, myoclonic, or less commonly tonic semiology, depending which brain region(s) is/are involved in the seizure activity, or the seizures may be entirely subclinical. Even when clinically evident, brief seizures without change in vital signs may not be detected by a caregiver or medical provider unless the newborn is under direct observation at the time of the seizure [3]. Additionally, many paroxysmal behaviors in newborns are not seizures, so clinical assessment of seizures may result in both over- and underdiagnosis [3]. Thus the diagnosis of seizures in newborns requires electroencephalography (EEG), ideally continuous video-EEG with a conventional 10–20 montage modified for neonates [4]. A recent large cohort study showed that 62% of all newborns with seizures had at least one subclinical seizure and 16% of newborns had only subclinical seizures [1]. Subclinical seizures are also more likely to occur in the setting of severe encephalopathy and administration of antiseizure or sedative medications, and clearly if paralytic medications are administered. Neonatal seizures are often of short duration (<1–2 min), and are highly focal with very little spread to other brain regions, thus are often not detected by one- or two-channel amplitude-integrated (a) EEG monitoring [5]. Full montage video-EEG is needed to detect very brief and/or focal seizures [5], and video is needed to distinguish frequent EEG artifacts from movement or routine care that may be mistaken for seizure. Finally, continuous video-EEG monitoring (cvEEG) is required to determine the effect of antiseizure medications, since some medications increase the chance of subclinical seizures or less clinically evident seizures (e.g., seizures may be briefer or more focal with treatment) [6,7], and will lead to the false conclusion that the medication has been effective.
Several historical and clinical characteristics of seizures help point towards a specific etiology. Timing of seizure onset provides the first clue regarding seizure etiology, as seizures caused by HIE often present in the first 12–24 h after birth, whereas seizures resulting from perinatal stroke, hemorrhage or infection often have later onset [8], with some infections presenting with seizures weeks after birth. Seizure type and location are also suggestive of the underlying etiology. Focal clonic seizures that are unilateral often point to a focal ischemic stroke or hemorrhage, whereas multiple different semiologies and/or locations of seizures point to diffuse processes such as HIE, metabolic disorder or CNS infection, or multifocal stroke or hemorrhage. Interestingly, a large cohort study showed that seizure burden was not appreciably different among the three ischemic/hemorrhagic etiologies of acute symptomatic seizures [1].
3. Hypoxic–ischemic encephalopathy
The most common and important cause of acute symptomatic neonatal seizures is hypoxic–ischemic encephalopathy (HIE) [1]. Its importance relates not only to HIE being the most common etiology, but to the emergent detection and treatment of HIE with therapeutic hypothermia to reduce mortality and neurologic morbidity, and the increasing evidence that the acute seizures occurring in ~25–50% exacerbate the perinatal brain injury. The evidence that seizures exacerbate ischemic brain injury is more readily derived from animal models [9], but there is also supportive evidence from human studies [10–12]. Another common feature of HIE is the frequent occurrence of subclinical seizures, in addition to the usual clinical seizure semiologies that can occur in newborns (subtle, clonic, myoclonic, tonic). While there remains some debate about the utility of treating electrographic-only seizures, current evidence points to a harmful effect and a potential benefit of treatment of subclinical seizures on long-term neurologic outcome [11]. This notion that improved detection and treatment of electrographic as well as electroclinical seizures may improve outcome underlies the current emphasis to use continuous video-EEG monitoring (cvEEG) with a full neonatal EEG montage to both detect seizures in newborns with HIE (or encephalopathy of unclear etiology), and to improve treatment of acute symptomatic seizures. Thus the current American Clinical Neurophysiology Society guideline supports a minimum of 24 h of cvEEG for all newborns with encephalopathy and/or suspected seizures for adequate detection of seizures [4] (discussed by Katsarou et al. in this issue). The duration of cvEEG monitoring for suspected HIE varies among institutions, as, although seizures often have onset within 6–12 h of birth, there are instances of seizure onset occurring as late as 36 h, or rarely, new onset or recurrence of seizures during rewarming after therapeutic hypothermia [1].
4. Stroke
Perinatal stroke is the second most common cause of symptomatic seizures in newborns. Although focal ischemic stroke has similarities with the diffuse ischemia of HIE, there are a few important differences. Seizures caused by focal stroke often present at an older age than they do for HIE, up to 24–48 h after birth or older. Since the middle cerebral artery territory is most commonly affected [13], newborns often present with focal clonic seizures, presumably emanating from the injured motor cortex. Seizures may have focal sharp waves/spike–polyspikes with frequency of 1–2 Hz and phase reversal over the central region. Perinatal stroke may be hemorrhagic, whether venous or arterial in origin, but hemorrhage is more common with venous infarction [14]. Hemorrhagic transformation of perinatal arterial stroke is uncommon, occurring in <15% of all neonatal arterial stroke in the largest report, which includes neonates with congenital heart disease or other underlying conditions [13].
5. Intracranial hemorrhage
Almost all types of intracranial hemorrhage (ICH) can be associated with seizures, depending on location and size [2]. Often, a newborn will have more than one type or location of ICH, with seizures resulting from one or more of the different hemorrhages or locations of ICH. Epidural hemorrhage is rare in newborns, and associated seizures may reflect other injury, whether from other ICH or ischemic brain injury [15]. Parturitional subdural hemorrhage (SDH) in the posterior fossa, typically posterior to the cerebellum and/or lining the tentorium, is probably the most common type of ICH, does not cause seizures, and has been documented to occur in asymptomatic newborns [16]. In contrast, seizures related to SDH in the anterior and middle cranial fossae may be related to associated subarachnoid hemorrhage (SAH), although large supratentorial SDH with cerebral compression can result in seizures. Subpial hemorrhage, a subtype of SAH that often causes seizures, is most often related to trauma with contusion or from venous compression or obstruction and is most often found in the temporal lobe [17]. Unsurprisingly, cerebral parenchymal hemorrhage involving cortical or subcortical gray matter commonly causes seizures. The cause of parenchymal hemorrhage may not be identified, even with magnetic resonance angiography or venography, and may sometimes be related to rupture of underlying vascular malformations. Thalamic hemorrhage is often a venous hemorrhagic infarction with associated intraventricular hemorrhage, which may be the consequence of thrombosis in the internal cerebral veins or more diffuse sinus venous thrombosis [14,18]. Isolated intraventricular hemorrhage (IVH) is rarely associated with seizures unless the IVH is large, but IVH with seizures is most often associated with parenchymal brain injury, whether ischemic or hemorrhagic, and may occur in the setting of perinatal asphyxia and HIE [1,19]. Since cranial ultrasound (CUS) can detect most types of ICH, and almost always detects large ICH that requires surgical intervention, obtaining an early CUS in newborns with seizures is a useful step in diagnostic testing, when magnetic resonance imaging cannot be obtained quickly.
6. Metabolic disorders and derangements
Transient disturbances in electrolytes may result in acute seizures in newborns, as can occur in older children and adults [1]. For example, hyponatremia and hypocalcemia can result in acute seizures in the first days after birth. In these cases, treatment is directed at determining the cause and correcting the underlying etiology of the electrolyte or metabolic derangement, as conventional antiseizure medications are often ineffective and usually unnecessary. Transient metabolic derangements may result from congenital or acquired renal or hepatic disorders, or multisystem genetic disorders, such as hypocalcemia associated with 22q11 mutations or Beckwith–Wiedemann syndrome, so an etiology of any metabolic abnormality should be investigated. Importantly, metabolic derangements often occur with perinatal asphyxia (e.g. hyponatremia from SIADH), so the possibility of HIE needs to be considered when there are signs of multi-organ dysfunction that could be related to systemic hypoxia–ischemia. Hypoglycemia may occur with systemic asphyxia, but isolated hypoglycemia is more often transient or related to maternal diabetes, or, less commonly, to congenital hyperinsulinism. Additionally, there may be iatrogenic causes of, or contributions to, electrolyte disturbances in the setting of hypoxia–ischemia and associated renal dysfunction with anuria/oliguria and intravenous fluid administration leading to hyponatremia.
7. Central nervous system infection
Acute seizures may result from infection of the brain/meninges with bacterial, viral or other microbial pathogens, and the infection may start in the antenatal, perinatal or postnatal periods. Although acute infections of the brain represent only about 5% of the cases of neonatal seizures [1], they are important to recognize and diagnose accurately, as specific antimicrobial treatment of the underlying infection is critical to minimize brain injury and other CNS or systemic complications. Seizures from CNS infections may present at any point during the neonatal period, occurring as late as several weeks of age, for example in the case of late group B streptococcal or herpes simplex virus infection. There has been increasing recognition of postnatally acquired parechovirus infection, which results in predominantly diffuse white matter injury, although gray matter may also be affected with accompanying acute seizures. There are many different types of microbes that can cause in-utero or perinatal CNS infections; a detailed description of these infections can be found elsewhere [20]. For the purposes of this review, it is important to note that acute seizures resulting from infection may persist longer than with ischemic or hemorrhagic brain injury, possibly related to ongoing inflammation that persists until the infection is treated adequately in the brain with antimicrobial agents that cross the blood– brain barrier. Thus there may be need for more prolonged cvEEG monitoring and treatment with ASDs to ensure complete resolution of symptomatic seizures from CNS infection.
8. Pathogenesis
The pathophysiology of acute symptomatic seizures in newborns relates in part to the energy deficit associated with hypoxia–ischemia, combined with the unique properties of immature neurons with regard to expression and action of neurotransmitter receptors and ion transport [21]. Immature neurons have high expression of N-methyl-D-aspartate and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors for excitatory neurotransmitter glutamate, and relatively low expression of the inhibitory GABA receptors, resulting in a tendency to excitation over inhibition. Additionally, GABAA receptors in immature neurons are excitatory, in part because high expression of the sodium/potassium/chloride (NKCC1) co-transporter around term age results in high intracellular chloride (Cl−) [22]. Thus when activated GABAA receptors open Cl− channels, intracellular Cl− flows down its concentration gradient, out of the neuron, and causes excitation rather than inhibition [22]. Since NKCC1 is upregulated by hypoxia and can be blocked by the diuretic medication bumetanide, subsequent studies showed that bumetanide combined with phenobarbital was effective in treating acute seizures in hypoxic and other neonatal rodent models [23,24]. Interestingly, since mature neurons in the thalami and brainstem have low expression of NKCC1 in newborns, the differential effect of GABAA receptor activation in cortical versus subcortical neurons provides an explanation for uncoupling of cortical seizure activity from motor manifestations after administration of GABAergic drugs [6]. Recent data show that Cl− homeostasis is likely set by local impermeant anions rather than just ion co-transporters [25], thus further work is needed to elucidate the complex interplay of ion transport and neurotransmitter action that is unique to the pathogenesis of neonatal seizures. The reader is referred to other sources for more detailed discussion of the cellular and molecular aspects of seizure pathophysiology and consequences in newborns [21,25,26]. (see Katsarou et al in this issue).
9. Treatment overview
Unlike neonatal genetic/metabolic epilepsies, where treatment may be guided by knowledge of gene mutations affecting specific ion channels, enzymatic reactions, co-factors or neurotransmitters, the treatment of acute symptomatic seizures is guided by limited data from small trials and observational studies, and an incomplete understanding of the pathophysiology of acute seizures. As mentioned previously, the treatment of symptomatic seizures is directed not only at resolving the acute seizures, but potentially reducing the severity of acute brain injury and ideally decreasing the incidence/severity of later epilepsy and/or neurologic disability [9,10,12,26]. While there is supportive but not incontrovertible evidence that treatment of electrographic-only seizures will improve outcome in human newborns [11], most experts in the field currently aim to control both electroclinical and electrographic-only (subclinical) seizures. Unfortunately, the treatment of symptomatic seizures is limited by the lack of adequate evidence-based data because of a paucity of randomized trials or other well-designed prospective studies of the efficacy and safety of antiseizure drugs (ASDs).
There has been one randomized, double-blind trial comparing phenobarbital to phenytoin in a crossover design [27], and all other ASD efficacy and safety data are derived from small open-label trials and observational studies, such that the evidence for efficacy or safety for any ASD is weak. Since acute symptomatic seizures typically resolve within hours to days, it is also difficult to determine whether the ASD response reported in many studies is a true drug effect or the natural resolution of seizures. For example, the randomized trial cited above showed that both ASDs were more effective in newborns whose seizures were decreasing (81%) rather than increasing (30%) in frequency, and a large retrospective study showed much greater efficacy of midazolam or lidocaine when used as third-line rather than second-line therapy [27,28]. These data suggest that the reported drug efficacy may be a result, at least in part, of the natural resolution of acute seizures, and underscore the importance of testing ASDs early in the course of neonatal seizures to determine their true efficacy. It is also unknown what magnitude of seizure burden might be harmful (e.g., exacerbate brain injury), or conversely, what magnitude of reduction in seizure burden might improve long-term outcome. The field is in dire need of well-designed, controlled trials that test the efficacy and safety of ASDs soon after onset of seizures, in newborns whose seizure burden is significant and not easily controlled with a single ASD. Until better evidence is available from such trials and studies, current treatment will continue to be based on clinical experience and the limited available data.
Current treatment practices and a suggested algorithm are described below, with a summary of the mechanism, potential efficacy and adverse effects of the common ASDs used, as well as a brief discussion of less commonly used ASDs and potential ASDs being tested in trials. More detailed discussion of the pharmacokinetic and other properties of ASDs used to treat neonatal seizures can be obtained in focused reviews of these medications [29,30].
10. Hypothermia
Whereas hypothermia was not developed to treat seizures specifically, the introduction of therapeutic hypothermia for the treatment of neonatal HIE led to the observation that seizure burden appeared to be reduced in newborns treated with hypothermia [31]. Given the multiple mechanisms of action of hypothermia in reducing energy consumption, neurotransmitter release and excitotoxicity (mechanisms that play a role in seizure pathogenesis), it is rational to think that hypothermia might also mitigate seizures. Although hypothermia is not indicated for the treatment of acute seizures as it is for HIE, there are reports showing that hypothermia appears to reduce seizures related to focal stroke and recurrent seizures that occurred during rewarming after hypothermia. Certainly hyperthermia should be avoided in the setting of neonatal HIE, and given the known effect of fever in exacerbating ischemic brain injury and lowering the seizure threshold. Avoidance of fever is also recommended for any newborn with acute symptomatic seizures, whether related to ischemic, hemorrhagic or infectious etiologies. In addition to hypothermia, other agents are being tested in ongoing trials to determine whether these agents provide an additional neuroprotective effect that further improves neurologic outcome in neonatal HIE (e.g., NCT02811263), and ideally these agents should be evaluated with regard to any reduction of neonatal seizure burden.
11. Phenobarbital
Phenobarbital (PB) remains the mainstay of treatment for neonatal seizures [1,32,33], not only because of its long history of use, but its easy intravenous or enteral administration with good absorption, long half-life with predictable first-order pharmacokinetics, and some data suggesting efficacy. Phenobarbital acts on the GABAA receptors to decrease neuronal excitability, but, as noted in Section 8, there may be paradoxical excitation with GABAergic activation because of high intracellular Cl− concentration and associated reversal of Cl− currents. Nevertheless, a likely mix of mature and immature neurons in the cortex may be the reason that PB has some efficacy in controlling seizures. The best efficacy data are from the one randomized, double-blind trial showing that an initial loading dose of PB ~25 g/L resulted in seizure cessation in nearly half of newborns, although more frequently in newborns whose seizures were subsiding or whose seizure burden was relatively low [27]. The major adverse effects of PB are related to CNS depression and secondary effects on respiration, such that high PB doses or levels may require increased respiratory support. Data showing that high doses of PB (>40 mg/kg) induced excess neuronal apoptosis in the developing rat brain, compared with controls [34], led to concerns regarding potential long-term adverse effects of PB and other ASDs used in newborns. Many clinicians avoid high doses of PB (e.g., >40 mg/kg) because of excessive sedation at high levels, long half-life with slow elimination (particularly with hepatic dysfunction), and the potential for adverse neurodevelopmental effects. In the absence of convincing data of superior efficacy or safety of other antiseizure drugs, however, PB continues to be widely used as first-line, or less commonly as second-line, treatment for neonatal seizures.
12. Phenytoin/fosphenytoin
Phenytoin (PHT) has also been used for decades to treat neonatal seizures, but, unlike PB, PHT acts via a voltage-dependent blockade of membrane sodium channels responsible for the action potential. The randomized trial that compared PB with PHT showed that PHT had similar efficacy to PB, as a first loading dose of PHT was also effective at controlling seizures in nearly half of newborns [27]. This trial also showed that adding either PB or PHT as a second ASD resulted in successful seizure treatment in nearly 60% of newborns. Unlike PB, PHT has less predictable pharmacokinetics, a short half-life, and poor enteral absorption, requiring more frequent dosing and more frequent blood level measurements to ensure that the loading and maintenance doses achieve levels within the therapeutic range. Because of second-order pharmacokinetics, further increases in the PHT maintenance dose will at a certain point result in much larger increases in serum levels than at lower doses, which may increase the chance of adverse effects. The development of the pro-drug fosphenytoin reduced the incidence of adverse effects such as hypotension and irritation at the injection site, but the pharmacokinetic properties are the same as for PHT. Similar to the case for PB, PHT was also shown to induce apoptosis in developing rodent neurons, at supratherapeutic doses of >20 mg/kg [34]. Thus, although PHT may provide additional seizure reduction when added to PB, PB is usually preferred over PHT for maintenance therapy, because the favourable properties of PB allow stable levels to be easily maintained with once-daily dosing.
13. Levetiracetam
Levetiracetam (LEV) has been used increasingly in recent years to treat neonatal seizures, despite a lack of clinical trial data or rigorous studies to demonstrate its efficacy or safety [1,33]. The mechanism of action of LEV is unclear, as it appears to reduce presynaptic neurotransmitter release by binding to a synaptic vesicle glycoprotein (SV2A), with greatest effect in rapidly discharging neurons [35]. Its popularity is likely related to the availability of both intravenous and oral suspension formulations and its relatively safe track record in older infants and children with epilepsy, as well as published pharmacokinetic data in newborns [36]. While awaiting data from an ongoing trial of LEV compared with PB (NCT01720667), available data from retrospective observational reports show that LEV was possibly effective in 35% of 23 newborns from the one study that used prolonged cvEEG monitoring to confirm both initial seizures and drug response, with LEV administered mostly as second- or third-line therapy [37]. Even then, precise data are limited regarding the efficacy and response time in terms of seizure reduction, as that study reported a >50% reduction within 24 h [37]. Other published data regarding LEV are much more limited, as those studies did not have cvEEG to demonstrate either that the clinically suspected seizures prior to LEV administration were EEG-proven seizures, or that LEV resulted in EEG-proven reduction in seizures [38,39]. Additionally, LEV was usually administered as second- or third-line therapy, when acute seizures were likely subsiding [38,39]. There are mixed results from rodent studies regarding any neuroprotective effect, so it is unclear whether there is any additive benefit to using LEV for acute symptomatic seizures in newborns [40,41]. Currently, whereas LEV appears to be safe and has predictable pharmacokinetics, its use in newborns is limited by the uncertainty regarding its efficacy and optimal dosing – data which may become available with completion of ongoing or future clinical trials.
14. Benzodiazepines, such as midazolam, lorazepam
The benzodiazepines midazolam (MDZ) and lorazepam (LZP) have both been used for many years for the treatment of neonatal seizures, either as an intravenous infusion (typically MDZ) or with intermittent dosing (LZP > MDZ). These two GABAergic drugs are commonly used to treat acute seizures in older children and adults in emergency rooms, ICUs and throughout the hospital, so it is not surprising that they would be used in newborns, despite a paucity of data supporting their utility. Although either drug could be used as an initial ASD for suspected clinical seizures prior to start of cvEEG with few untoward effects, administration of frequent doses or infusions results in sedation and respiratory depression, as for the GABAergic drug PB. An early retrospective study of midazolam infusion in just 13 newborns reported excellent efficacy, although it was given as third-line therapy, so may have been administered when acute seizures were resolving naturally [42]. Since then, a large retrospective study of lidocaine reported limited efficacy of MDZ as second-line therapy, with MDZ showing efficacy in only ~13% of newborns [28]. Although MDZ and LZP have not been studied specifically with regard to the potential for apoptosis, the benzodiazepines clonazepam and diazepam were shown to cause apoptosis at threshold doses of 0.5 mg/kg and 10 mg/kg, respectively [34].
15. Lidocaine
Lidocaine is used widely in Europe but is rarely used in North America, which underscores the influence of physician experience and institutional culture in the treatment of neonatal seizures [32,33]. Lidocaine, like phenytoin, blocks voltage-gated sodium channels, but has a risk of cardiac arrhythmias and cannot be used in conjunction with phenytoin/fosphenytoin [43]. It is administered as an intravenous infusion, and efficacy data are available only from retrospective studies, the largest of which was also limited by the use of aEEG instead of cvEEG for seizure detection. This study showed that ~20% of newborns had cessation of aEEG seizures for >4 h after lidocaine administration without need for other ASDs, suggesting modest efficacy [28].
16. Bumetanide
As described in the section on pathogenesis, bumetanide was proposed as a potential ASD specifically for symptomatic seizures in term newborns because high expression of NKCC1 in neurons peaks around term age, and several rodent models suggesting its efficacy for hypoxic and other neonatal seizures [22,23]. An open-label trial of bumetanide in 14 human newborns with HIE was terminated early, although there has been debate both about the reported poor efficacy and safety concerns [44–47]. The small number of subjects and lack of a comparison control group prevented a definitive interpretation of either the efficacy or safety or bumetanide. A larger randomized, controlled, double-blind trial of bumetanide that recently completed enrollment will yield more detailed data regarding the use of bumetanide to treat neonatal seizures (NCT00830531), particularly since a comparison control group was used to determine drug response and safety.
17. Topiramate, perampanel
Blockade of excitatory glutamatergic AMPA receptors is a potentially important mechanism for reducing symptomatic neonatal seizures as well as later epilepsy, as AMPA receptors have been demonstrated to be potentiated by hypoxia, and AMPA receptor antagonists decrease this effect and the accompanying epileptogenesis in rodent models [48]. Through their antagonistic effect at AMPA receptors and other mechanisms, topiramate (TOP) and perampanel are potentially attractive ASDs for symptomatic seizures in newborns, for possible antiseizure, antiepileptogenic and neuroprotective properties [49,50]. The lack of an intravenous formulation of TOP has limited its use in newborns with HIE or other acute symptomatic etiologies, as enteral medications are often avoided in critically ill newborns, but an intravenous formulation tested in phase I trials in adults may eventually become available for neonatal testing. A pilot randomized trial of enteral TOP in 44 newborns with HIE provided some preliminary PK and safety data, and suggested a reduced incidence of later epilepsy, but did not employ cvEEG to determine if there was any beneficial effect on acute neonatal seizures (NCT01241019) [51]. Despite the lack of efficacy and safety data, clinicians have reported using topiramate to treat neonatal seizures [33]. There are no published reports yet of perampanel use in human newborns.
18. Valproate, carbamazepine, and newer ASDs oxcarbazepine, lacosamide
These four ASDs all act at least in part by blocking voltage-sensitive sodium channels to achieve their anticonvulsant effect in the brain, similar to phenytoin. Valproate and carbamazepine are older ASDs that have been reported to treat neonatal seizures successfully in small case-series, but the use of intravenous or enteral valproate is limited by the risk of potentially life-threatening adverse effects (hepatic and bone marrow failure), and carbamazepine and the newer related compound oxcarbazepine both lack an intravenous formulation. Lacosamide is a newer ASD that is available intravenously, with scattered reports of efficacy and safety in case series of infants, but no published data in newborns.
19. Suggested management
A suggested algorithm for treatment of acute symptomatic seizures in newborns is shown in Fig. 1, reflecting an approach to management based on the available, admittedly limited, published data. Note that the first steps involve management of airway, breathing and circulation as for any neurologic emergency, and testing to rule out and treat any metabolic derangements or treatable infections. Continuous video-EEG is the reference standard to ensure accurate diagnosis of seizures and to assess the response to ASDs administered. Although there are no clear data to guide the severity of seizure burden that requires ASD treatment, expert consensus opinion is that 30 s/h of EEG-proven seizure duration should be required for randomization and treatment in a drug trial (International Neonatal Consortium document in preparation; https://c-path.org/programs/inc/). Some physicians report administering an ASD for a single 10 s seizure, whereas others would require a seizure burden of ≥2 min/h. Since seizures are refractory to the initial loading dose of an ASD (usually PB) in ~50–64% of all newborns [1,27], a second or third ASD may be needed to achieve good seizure control. The high rate of persistent seizures following a first- or second-line ASD means that continued monitoring of cvEEG is needed after administration of each ASD, with prompt addition of the next ASD if >30–120 s/h of seizure activity recurs after the peak ASD level has been reached, depending on expected pharmacokinetics of each ASD. Note also that whereas it may be useful to obtain ASD levels to guide management, doses of ASDs should be provided promptly when seizures persist without waiting for results of ASD levels, as there are data suggesting that earlier/more rapid treatment reduces overall seizure burden [11,52]. Whereas some clinicians use LEV as second-line therapy, the algorithm below suggests PHT as second-line therapy because of data supporting higher efficacy of PHT versus LEV; however, data from ongoing clinical trials cited throughout this review may provide support for different treatment algorithms or dosing regimens in the near future. Lidocaine has not been included as a third-line drug here because of low efficacy from retrospective studies and reported adverse effects, although this drug may still be used in some centers in place of midazolam, given relatively few data regarding efficacy or safety of midazolam.
Fig. 1.
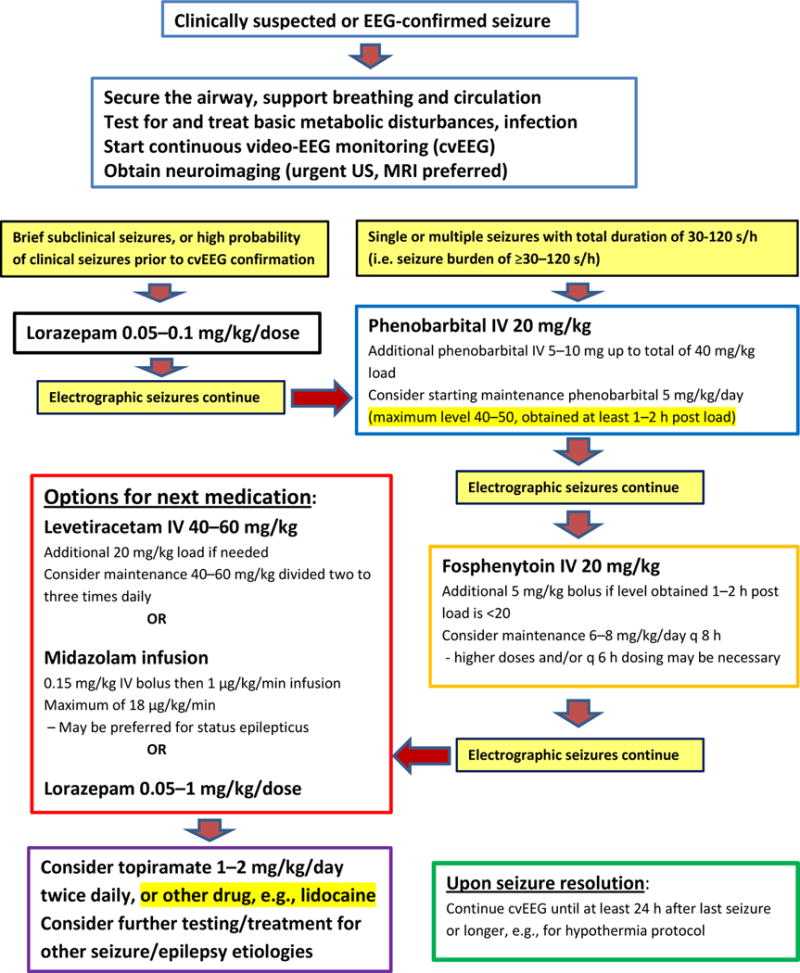
Neonatal seizure treatment algorithm. EEG, electroencephalography; US, ultrasound; MRI, magnetic resonanc imaging; IV, intravenous.
20. Duration of ASD treatment
The duration of ASD treatment for acute symptomatic seizures is another area where practice is highly variable, as there are few data regarding the need for, or potential adverse effects of, ASD use after cessation of acute neonatal seizures. A recent large cohort study showed that the decision to continue ASDs was most strongly associated with study site and seizure etiology, with an ASD being continued at NICU discharge in 72% of newborns with HIE, stroke or ICH, most commonly PB (63%) or LEV (24%) [53]. A multicenter randomized trial to test the neurodevelopmental outcome and post-neonatal seizure incidence after neonatal seizures was terminated early due to low enrollment (NCT01089504), but a comparative efficacy study to test these questions is currently underway (NCT02789176). Further discussion of neurodevelopmental outcome after neonatal seizures is included in a separate review in the same issue.
Practice points
The large majority of neonatal seizures are acute symptomatic seizures caused by ischemic/hemorrhagic brain disorders.
Basic science and human data suggest that acute symptomatic seizures in newborns contribute to later epilepsy and worse neurodevelopmental outcome.
Treatment is often guided by experience and preference, as there are few randomized, controlled trials or large prospective studies to inform drug choice, dosing and treatment duration.
Research directions
Further basic science research is needed to elucidate pathophysiology of acute neonatal seizures caused by hypoxia–ischemia and to identify potential therapeutic targets.
More work is needed to determine the severity of seizure burden that contributes to worse neurologic outcome, and hence would benefit from treatment to improve outcome.
Development of accurate and reliable automated seizure detection algorithms for general use would be invaluable for management, given the labor-intensive nature of EEG review and paucity of expert EEG readers at most centers.
Randomized, controlled trials of antiseizure drugs are critically needed to determine both the efficacy and safety of currently used and emerging new drugs.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1*.Glass HC, Shellhaas RA, Wusthoff CJ, et al. Contemporary profile of seizures in neonates: a prospective cohort study. J Pediatr. 2016;174:98–103 e101. doi: 10.1016/j.jpeds.2016.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tekgul H, Gauvreau K, Soul J, et al. The current etiologic profile and neurodevelopmental outcome of seizures in term newborn infants. Pediatrics. 2006;117:1270–80. doi: 10.1542/peds.2005-1178. [DOI] [PubMed] [Google Scholar]
- 3.Murray DM, Boylan GB, Ali I, Ryan CA, Murphy BP, Connolly S. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Archs Dis Childh Fetal Neonatal Ed. 2008;93:F187–91. doi: 10.1136/adc.2005.086314. [DOI] [PubMed] [Google Scholar]
- 4*.Shellhaas RA, Chang T, Tsuchida T, et al. The American Clinical Neurophysiology Society’s Guideline on continuous electroencephalography monitoring in neonates. J Clin Neurophysiol. 2011;28:611–17. doi: 10.1097/WNP.0b013e31823e96d7. [DOI] [PubMed] [Google Scholar]
- 5.Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics. 2007;120:770–7. doi: 10.1542/peds.2007-0514. [DOI] [PubMed] [Google Scholar]
- 6.Glykys J, Dzhala VI, Kuchibhotla KV, et al. Differences in cortical versus subcortical GABAergic signaling: a candidate mechanism of electroclinical uncoupling of neonatal seizures. Neuron. 2009;63:657–72. doi: 10.1016/j.neuron.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathieson SR, Livingstone V, Low E, Pressler R, Rennie JM, Boylan GB. Phenobarbital reduces EEG amplitude and propagation of neonatal seizures but does not alter performance of automated seizure detection. Clin Neurophysiol. 2016;127:3343–50. doi: 10.1016/j.clinph.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rafay MF, Cortez MA, de Veber GA, et al. Predictive value of clinical and EEG features in the diagnosis of stroke and hypoxic ischemic encephalopathy in neonates with seizures. Stroke. 2009;40:2402–7. doi: 10.1161/STROKEAHA.109.547281. [DOI] [PubMed] [Google Scholar]
- 9.Wirrell EC, Armstrong EA, Osman LD, Yager JY. Prolonged seizures exacerbate perinatal hypoxic–ischemic brain damage. Pediatr Res. 2001;50:445–54. doi: 10.1203/00006450-200110000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Shah DK, Wusthoff CJ, Clarke P, et al. Electrographic seizures are associated with brain injury in newborns undergoing therapeutic hypothermia. Archs Dis Childh Fetal Neonatal Ed. 2014;99:F219–24. doi: 10.1136/archdischild-2013-305206. [DOI] [PubMed] [Google Scholar]
- 11.Srinivasakumar P, Zempel J, Trivedi S, et al. Treating EEG seizures in hypoxic ischemic encephalopathy: a randomized controlled trial. Pediatrics. 2015;136:e1302–9. doi: 10.1542/peds.2014-3777. [DOI] [PubMed] [Google Scholar]
- 12.Kharoshankaya L, Stevenson NJ, Livingstone V, et al. Seizure burden and neurodevelopmental outcome in neonates with hypoxic–ischemic encephalopathy. Dev Med Child Neurol. 2016;58:1242–8. doi: 10.1111/dmcn.13215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kirton A, Armstrong-Wells J, Chang T, et al. Symptomatic neonatal arterial ischemic stroke: the International Pediatric Stroke Study. Pediatrics. 2011;128:e1402–10. doi: 10.1542/peds.2011-1148. [DOI] [PubMed] [Google Scholar]
- 14.Bruno CJ, Beslow LA, Witmer CM, et al. Haemorrhagic stroke in term and late preterm neonates. Archs Dis Childh Fetal Neonatal Ed. 2014;99:F48–53. doi: 10.1136/archdischild-2013-304068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heyman R, Heckly A, Magagi J, Pladys P, Hamlat A. Intracranial epidural hematoma in newborn infants: clinical study of 15 cases. Neurosurgery. 2005;57:924–9. doi: 10.1227/01.neu.0000180026.73246.bf. discussion 924–9. [DOI] [PubMed] [Google Scholar]
- 16.Sirgiovanni I, Avignone S, Groppo M, et al. Intracranial haemorrhage: an incidental finding at magnetic resonance imaging in a cohort of late preterm and term infants. Pediatr Radiol. 2014;44:289–96. doi: 10.1007/s00247-013-2826-7. [DOI] [PubMed] [Google Scholar]
- 17.Huang AH, Robertson RL. Spontaneous superficial parenchymal and leptomeningeal hemorrhage in term neonates. Am J Neuroradiol. 2004;25:469–75. [PMC free article] [PubMed] [Google Scholar]
- 18.Wu YW, Hamrick SE, Miller SP, et al. Intraventricular hemorrhage in term neonates caused by sinovenous thrombosis. Ann Neurol. 2003;54:123–6. doi: 10.1002/ana.10619. [DOI] [PubMed] [Google Scholar]
- 19.Al Yazidi G, Boudes E, Tan X, Saint-Martin C, Shevell M, Wintermark P. Intraventricular hemorrhage in asphyxiated newborns treated with hypothermia: a look into incidence, timing and risk factors. BMC Pediatrics. 2015;15:106. doi: 10.1186/s12887-015-0415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Vries LS, Volpe JJ, Unit IX. intracranial infections Volpe’s neurology of the newborn. 6th. Philadelphia: Elsevier; 2018. pp. 971–1089. [Google Scholar]
- 21*.Silverstein FS, Jensen FE. Neonatal seizures. Ann Neurol. 2007;62:112–20. doi: 10.1002/ana.21167. [DOI] [PubMed] [Google Scholar]
- 22*.Dzhala VI, Talos DM, Sdrulla DA, et al. NKCC1 transporter facilitates seizures in the developing brain. Nat Med. 2005;11:1205–13. doi: 10.1038/nm1301. [DOI] [PubMed] [Google Scholar]
- 23.Dzhala VI, Brumback AC, Staley KJ. Bumetanide enhances phenobarbital efficacy in a neonatal seizure model. Ann Neurol. 2008;63:222–35. doi: 10.1002/ana.21229. [DOI] [PubMed] [Google Scholar]
- 24.Rheims S, Minlebaev M, Ivanov A, et al. Excitatory GABA in rodent developing neocortex in vitro. J Neurophysiol. 2008;100:609–19. doi: 10.1152/jn.90402.2008. [DOI] [PubMed] [Google Scholar]
- 25*.Glykys J, Dzhala V, Egawa K, et al. Local impermeant anions establish the neuronal chloride concentration. Science. 2014;343:670–5. doi: 10.1126/science.1245423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26*.Holmes GL. The long-term effects of neonatal seizures. Clin Perinatol. 2009;36:901–14. vii–viii. doi: 10.1016/j.clp.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 27*.Painter MJ, Scher MS, Stein AD, et al. Phenobarbital compared with phenytoin for the treatment of neonatal seizures. N Engl J Med. 1999;341:485–9. doi: 10.1056/NEJM199908123410704. [DOI] [PubMed] [Google Scholar]
- 28.Weeke LC, Toet MC, van Rooij LG, et al. Lidocaine response rate in aEEG-confirmed neonatal seizures: retrospective study of 413 full-term and preterm infants. Epilepsia. 2016;57:233–42. doi: 10.1111/epi.13286. [DOI] [PubMed] [Google Scholar]
- 29.Donovan MD, Griffin BT, Kharoshankaya L, Cryan JF, Boylan GB. Pharmacotherapy for neonatal seizures: current knowledge and future perspectives. Drugs. 2016;76:647–61. doi: 10.1007/s40265-016-0554-7. [DOI] [PubMed] [Google Scholar]
- 30*.El-Dib M, Soul JS. The use of phenobarbital and other anti-seizure drugs in newborns. Semin Fetal Neonatal Med. 2017;22:321–7. doi: 10.1016/j.siny.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 31.Orbach SA, Bonifacio SL, Kuzniewicz MW, Glass HC. Lower incidence of seizure among neonates treated with therapeutic hypothermia. J Child Neurol. 2014;29:1502–7. doi: 10.1177/0883073813507978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32*.Vento M, de Vries LS, Alberola A, et al. Approach to seizures in the neonatal period: a European perspective. Acta Paediatr. 2010;99:497–501. doi: 10.1111/j.1651-2227.2009.01659.x. [DOI] [PubMed] [Google Scholar]
- 33.Silverstein FS, Ferriero DM. Off-label use of antiepileptic drugs for the treatment of neonatal seizures. Pediatr Neurol. 2008;39:77–9. doi: 10.1016/j.pediatrneurol.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 34*.Bittigau P, Sifringer M, Genz K, et al. Antiepileptic drugs and apoptotic neurodegeneration in the developing brain. Proc Natl Acad Sci USA. 2002;99:15089–94. doi: 10.1073/pnas.222550499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meehan AL, Yang X, McAdams BD, Yuan L, Rothman SM. A new mechanism for antiepileptic drug action: vesicular entry may mediate the effects of levetiracetam. J Neurophysiol. 2011;106:1227–39. doi: 10.1152/jn.00279.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sharpe CM, Capparelli EV, Mower A, Farrell MJ, Soldin SJ, Haas RH. A seven-day study of the pharmacokinetics of intravenous levetiracetam in neonates: marked changes in pharmacokinetics occur during the first week of life. Pediatr Res. 2012;72:43–9. doi: 10.1038/pr.2012.51. [DOI] [PubMed] [Google Scholar]
- 37.Abend NS, Gutierrez-Colina AM, Monk HM, Dlugos DJ, Clancy RR. Levetiracetam for treatment of neonatal seizures. J Child Neurol. 2011;26:465–70. doi: 10.1177/0883073810384263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramantani G, Ikonomidou C, Walter B, Rating D, Dinger J. Levetiracetam: safety and efficacy in neonatal seizures. Eur J Paediatr Neurol. 2011;15:1–7. doi: 10.1016/j.ejpn.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 39.Khan O, Chang E, Cipriani C, Wright C, Crisp E, Kirmani B. Use of intravenous levetiracetam for management of acute seizures in neonates. Pediatr Neurol. 2011;44:265–9. doi: 10.1016/j.pediatrneurol.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 40.Griesmaier E, Stock K, Medek K, et al. Levetiracetam increases neonatal hypoxic–ischemic brain injury under normothermic, but not hypothermic conditions. Brain Res. 2014;1556:10–18. doi: 10.1016/j.brainres.2014.01.034. [DOI] [PubMed] [Google Scholar]
- 41.Komur M, Okuyaz C, Celik Y, et al. Neuroprotective effect of levetiracetam on hypoxic ischemic brain injury in neonatal rats. Childs Nerv Syst. 2014;30:1001–9. doi: 10.1007/s00381-014-2375-x. [DOI] [PubMed] [Google Scholar]
- 42.Castro Conde JR, Hernandez Borges AA, Domenech Martinez E, Gonzalez Campo C, Perera Soler R. Midazolam in neonatal seizures with no response to phenobarbital. Neurology. 2005;64:876–9. doi: 10.1212/01.WNL.0000152891.58694.71. [DOI] [PubMed] [Google Scholar]
- 43.Weeke LC, Schalkwijk S, Toet MC, van Rooij LG, de Vries LS, van den Broek MP. Lidocaine-associated cardiac events in newborns with seizures: incidence, symptoms and contributing factors. Neonatology. 2015;108:130–6. doi: 10.1159/000430767. [DOI] [PubMed] [Google Scholar]
- 44.Pressler RM, Boylan GB, Marlow N, et al. Bumetanide for the treatment of seizures in newborn babies with hypoxic ischaemic encephalopathy (NEMO): an open-label, dose finding, and feasibility phase 1/2 trial. Lancet Neurol. 2015;14:469–77. doi: 10.1016/S1474-4422(14)70303-5. [DOI] [PubMed] [Google Scholar]
- 45.Glass HC. Bumetanide for treatment of seizures in neonates. Lancet Neurol. 2015;14:456–7. doi: 10.1016/S1474-4422(15)70024-4. [DOI] [PubMed] [Google Scholar]
- 46.Thoresen M, Sabir H. Epilepsy: neonatal seizures still lack safe and effective treatment. Nat Rev Neurol. 2015;11:311–12. doi: 10.1038/nrneurol.2015.74. [DOI] [PubMed] [Google Scholar]
- 47.Pressler RM, Boylan GB, Marlow N, et al. Bumetanide for neonatal seizures – back from the cotside. Nat Rev Neurol. 2015;11:724. doi: 10.1038/nrneurol.2015.116. [DOI] [PubMed] [Google Scholar]
- 48.Rakhade SN, Zhou C, Aujla PK, Fishman R, Sucher NJ, Jensen FE. Early alterations of AMPA receptors mediate synaptic potentiation induced by neonatal seizures. J Neurosci. 2008;28:7979–90. doi: 10.1523/JNEUROSCI.1734-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao Q, Hu Y, Holmes GL. Effect of topiramate on cognitive function and activity level following neonatal seizures. Epilepsy Behav. 2005;6:529–36. doi: 10.1016/j.yebeh.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 50.Sfaello I, Baud O, Arzimanoglou A, Gressens P. Topiramate prevents excitotoxic damage in the newborn rodent brain. Neurobiol Dis. 2005;20:837–48. doi: 10.1016/j.nbd.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 51.Filippi L, Fiorini P, Catarzi S, et al. Safety and efficacy of topiramate in neonates with hypoxic ischemic encephalopathy treated with hypothermia (NeoNATI): a feasibility study. J Matern Fetal Neonatal Med. 2017:1–8. doi: 10.1080/14767058.2017.1304536. [DOI] [PubMed] [Google Scholar]
- 52.van Rooij LG, Toet MC, van Huffelen AC, et al. Effect of treatment of subclinical neonatal seizures detected with aEEG: randomized, controlled trial. Pediatrics. 2010;125:e358–66. doi: 10.1542/peds.2009-0136. [DOI] [PubMed] [Google Scholar]
- 53.Shellhaas RA, Chang T, Wusthoff CJ, et al. Treatment duration after acute symptomatic seizures in neonates: a multicenter cohort study. J Pediatr. 2017;181:298–301 e291. doi: 10.1016/j.jpeds.2016.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]


