Abstract
GABAA receptors play a dominant role in mediating inhibition in the mature mammalian brain, and defects of GABAergic neurotransmission contribute to the pathogenesis of a variety of neurological and psychiatric disorders. Two types of GABAergic inhibition have been described: αβγ receptors mediate phasic inhibition in response to transient high-concentrations of synaptic GABA release, and αβδ receptors produce tonic inhibitory currents activated by low-concentration extrasynaptic GABA. Both αβδ and αβγ receptors are important targets for general anesthetics, which induce apparently different changes both in GABA-dependent receptor activation and in desensitization in currents mediated by αβγ vs. αβδ receptors. Many of these differences are explained by correcting for the high agonist efficacy of GABA at most αβγ receptors vs. much lower efficacy at αβδ receptors. The stoichiometry and subunit arrangement of recombinant αβγ receptors are well established as β-α-γ-β-α, while those of αβδ receptors remain controversial. Importantly, some potent general anesthetics selectively bind in transmembrane inter-subunit pockets of αβγ receptors: etomidate acts at β+/α− interfaces, and the barbiturate R-5-allyl-1-methyl-5-(m-trifluoromethyl-diazirynylphenyl) barbituric acid (R-mTFD-MPAB) acts at α+/β− and γ+/β− interfaces. Thus, these drugs are useful as structural probes in αβδ receptors formed from free subunits or concatenated subunit assemblies designed to constrain subunit arrangement. Although a definite conclusion cannot be drawn, studies using etomidate and R-mTFD-MPAB support the idea that recombinant α1β3δ receptors may share stoichiometry and subunit arrangement with α1β3γ2 receptors.
Keywords: αβδ, αβγ, GABAA Receptors, Allosteric Modulation, Concatemers, General Anesthetics
Graphical abstract

1. Introduction
γ-Aminobutyric acid type A (GABAA) receptors are major mediators of inhibition in the CNS [1, 2]. The GABAA receptor is a chloride ion channel, a member of the pentameric Cys-loop superfamily of ligand-gated ion channels (pLGICs), which also include nicotinic acetylcholine, glycine, 5-HT3 and zinc-activated receptors [3]. Multiple GABAA receptor subunit subtypes and splice variants have been identified: α1-α6, β1-β3, γ1-γ3, δ, ε, π, θ and ρ1–3 [4, 5]. Each subunit is composed of a N-terminal extracellular hydrophilic domain, four transmembrane α-helices (M1-M4), three inter-helix loops, and a C-terminal extracellular domain [6, 7]. GABAA receptors mostly consist of two α subunits, two β subunits and one additional subunit from either a γ or δ [4]. The composition, stoichiometry and subunit arrangement of receptors containing ε, π and θ are unknown. The predominant GABAA receptor isoforms in the brain are αβγ and αβδ [8]. αβγ receptors, with α1β2γ2 as the most common isoform, are widely distributed in the brain [8, 9] and at the cellular level are mostly localized in synapses [10, 11], although α5βγ receptors [12–14] and a small fraction of other αβγ receptors are also extrasynaptic [10, 11]. In contrast, αβδ receptors only constitute a small proportion of GABAA receptors in the brain [8, 15] and at the cellular level are located outside of synapses [10, 16]. The δ subunits co-assemble with α6 subunits in the cerebellum [17–19] and with α4 subunits in the hippocampus, striatum, thalamus and cortex [19–23]. Co-assembly of δ and α1 subunits is observed in the hippocampus [24]. Although α1 and δ subunit mRNAs are found in the thalamus [25], co-immunoprecipitation of α1 and δ subunit protein is undetectable in this brain structure [21]. The most common isoform of δ subunit-containing GABAA receptors in the CNS is the α4βδ receptor [8].
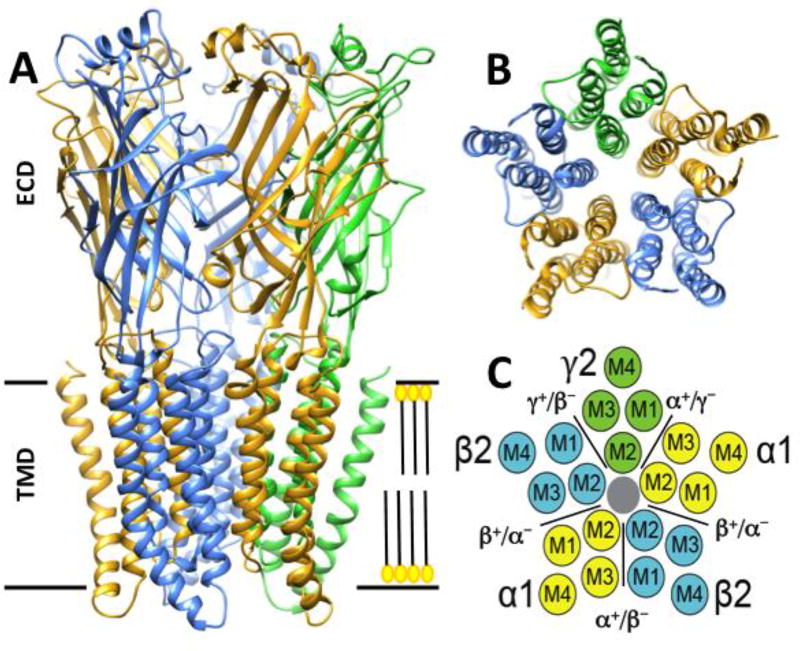
Structural models of heteropentameric GABAA receptors (Figure 1) are based on high-resolution structural data from snail Lymnaea stagnalis acetylcholine binding protein [26], bacterial and nematode homomeric pLGICs, including Gloeobacter violaceus ion channel (GLIC) [27, 28], Erwinia chrysanthemi ion channel (ELIC) [29, 30] and Caenorhabditis elegans glutamate-gated chloride channel α (GluCl) [31, 32], a human GABAA homomeric β3 receptor [33] and a zebrafish α1 glycine receptor [34]. The interfacial surfaces of each subunit are denoted as minus (−) and plus (+) viewed from above and scanning anticlockwise. There are two GABA binding sites on typical synaptic αβγ GABAA receptors, each located in extracellular domains between α and β subunits (β+/α− interfaces) [35]. GABA binding induces a series of conformational changes that propagates to transmembrane domains, leading to opening of the channel gate [36].
Figure 1. Structural model of α1β2γ2 synaptic GABAA receptors.
Panel A depicts a side view of α1β2γ2 receptor homology model, showing ribbon representation of extracellular domains (ECDs) and transmembrane domains (TMDs). Subunits are color-coded: α = gold, β = blue, γ = green. Intracellular domains (between M3 and M4) have been shortened -- their actual size is variable and their structures are not well established. Panel B shows the transmembrane domain of the homology model viewed from the extra-synaptic space (with the ECD removed). Panel C is a schematic representation of panel B, with transmembrane domains of each subunit and intra-subunit clefts (black) labeled. In this and the following figures, we observed the “Guidelines for preparing color figures for everyone including the colorblind” [179].
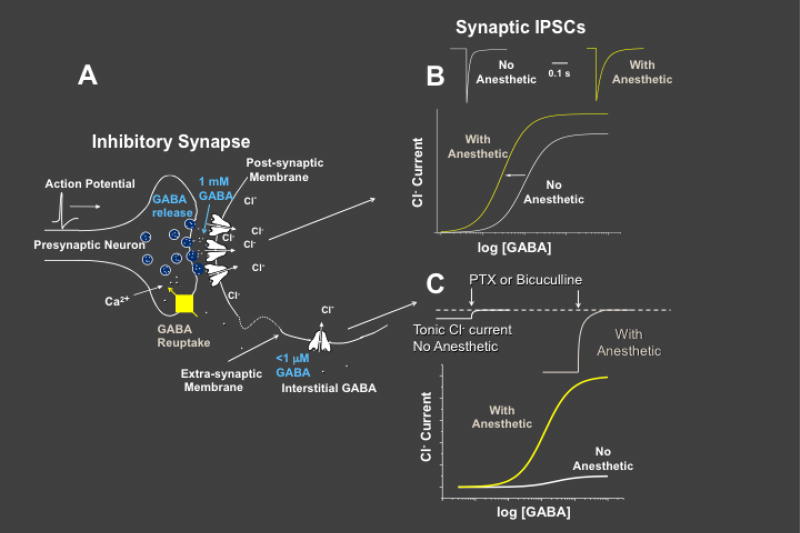
GABAA receptor-mediated inhibition is both phasic and tonic (Figure 2A) [11, 37]. Phasic inhibition is evoked by transient activation of synaptic GABAA receptors in response to brief high concentrations of GABA. Voltage-clamp recordings of neuronal inhibitory postsynaptic currents (IPSCs) measure the amplitude and kinetic properties of phasic currents. In contrast, tonic inhibition is mediated by extrasynaptic GABAA receptors, which are continuously activated by slowly varying low concentrations of ambient GABA [11, 37–40]. These steady-state tonic currents are measured in voltage-clamped neurons as the holding current that is blocked by GABAA receptor antagonists such as bicuculline or picrotoxin. Tonic inhibition plays a critical role in modulating neuronal excitability in many brain areas. For example, in thalamus and hippocampus, tonic currents account for ~ 75–90% of total GABAA receptor-mediated inhibitory currents [37, 41, 42].
Figure 2. Anesthetic effects on synaptic vs. extrasynaptic neuronal GABAergic currents.
Panel A depicts a GABAergic synapse with a presynaptic neuron on the left and a postsynaptic neuron on the right. In response to action potentials triggering calcium influx and increased intracellular calcium in presynaptic boutons, GABA release into the synapse transiently reaches millimolar concentrations that activate most of the nearby postsynaptic GABAA receptors. Transporter-mediated reuptake into neurons rapidly reduces synaptic GABA, resulting in postsynaptic receptor deactivation. Together, these events generate inhibitory postsynaptic currents (IPSCs; panel B, top left). In the presence of anesthetic drugs, receptor deactivation and IPSC decay is slowed (panel B, top right), increasing GABA-dependent chloride flux, and reducing GABA EC50 (panel B, bottom). GABA leakage from synapses activates extrasynaptic GABAA receptors (panel A, lower right), generating tonic currents (panel C, top left). In the presence of anesthetics, tonic currents increase (panel C, top right). Because GABA is a weak agonist at extrasynaptic receptors containing δ-subunit, anesthetics induce smaller “leftward shifts” and larger “upward shifts” in concentration-response experiments of these receptors (panel C, bottom).
Evaluating the properties of individual GABAA receptor isoforms is carried out by heterologous expression of specific subunits in cells such as human embryonic kidney (HEK) cells and Xenopus oocytes. To mimic fast IPSCs and study fast components of receptor mediated currents, small cells are selected or macropatches are pulled from cell membranes, and a rapid solution-switching device (exchange times range from hundreds of µsec to several msec) is used to apply agonists and/or drugs [43, 44]. This “artificial synapse” approach assesses rates of fast current phases such as desensitization (current reduction during agonist application) and deactivation (current return to baseline after terminating agonist application) [7]. Pseudo steady-state GABAA receptor responses are investigated using relatively slow solution exchange devices (solution exchange time > 10 msec) in HEK cells and larger cells [45, 46]. In classical GABA concentration-response analysis, GABA EC50 (the GABA concentration that elicits 50% of maximal response) and Hill slope, a coefficient that may give information on the number of GABA interacting sites [47], are measured.
GABAA receptors are allosterically modulated by a myriad of compounds. Widely used positive GABAA receptor modulators include benzodiazepines, general anesthetics, and some anticonvulsants [6]. Inhibitors such as bicuculline, picrotoxin, gabazine and other convulsants, are mostly used as research tools. This review focuses on potent general anesthetics.
A variety of approaches have been used to locate and study general anesthetic sites on heteromeric GABAA receptors, including photolabeling, mutagenesis, subunit chimeras and concatenated subunit assemblies. Receptor labeling with photoreactive anesthetic analogs has identified several binding sites on α1β2/3γ2 GABAA receptors [35]. Mutant studies have helped define the functional roles of amino acid residues on GABAA receptors [48]. Chimeric subunits constructed with complementary portions of subunit isoforms associated with different pharmacological sensitivity to anesthetics can identify subunit domains of interest [49]. Concatenated subunit assemblies are formed by tethering two or three subunits together using amino acid linkers to constrain subunit arrangement [50].
2. Structure and function of synaptic αβγ GABAA receptors
The stoichiometry and subunit arrangement of recombinant αβγ receptors have been well established as β-α-γ-β-α (counterclockwise viewed from the extracellular space) (Figure 3A) [51–53]. Mutation of the conserved M2-9´ leucine to serine (L9´S) on α1, β2 or γ2 subunits induces a large reduction in GABA EC50. Co-expression of receptor messenger RNAs encoding mixtures of wild type and L9´S mutant α1, β2, or γ2 subunits produced mixed populations of receptors and multi-phasic GABA concentration-responses [51]. From the number of phases in such concentration-response curves, it was inferred that α1β2γ2 receptors contain two α1, two β2 and one γ2 subunits [51]. Baumann et al examined the subunit arrangement of α1β2γ2 receptors by co-expressing concatenated subunit assemblies that constrain different subunit arrangements [54]. Electrophysiological studies demonstrated that the subunit arrangement of β2-α1-γ2-β2-α1 exhibits pharmacological properties similar to α1β2γ2 receptors assembled from free subunits [54]. Biochemical and fluorescence energy transfer experiments [52, 53] support this stoichiometry and subunit arrangement of α1β2/3γ2 receptors.
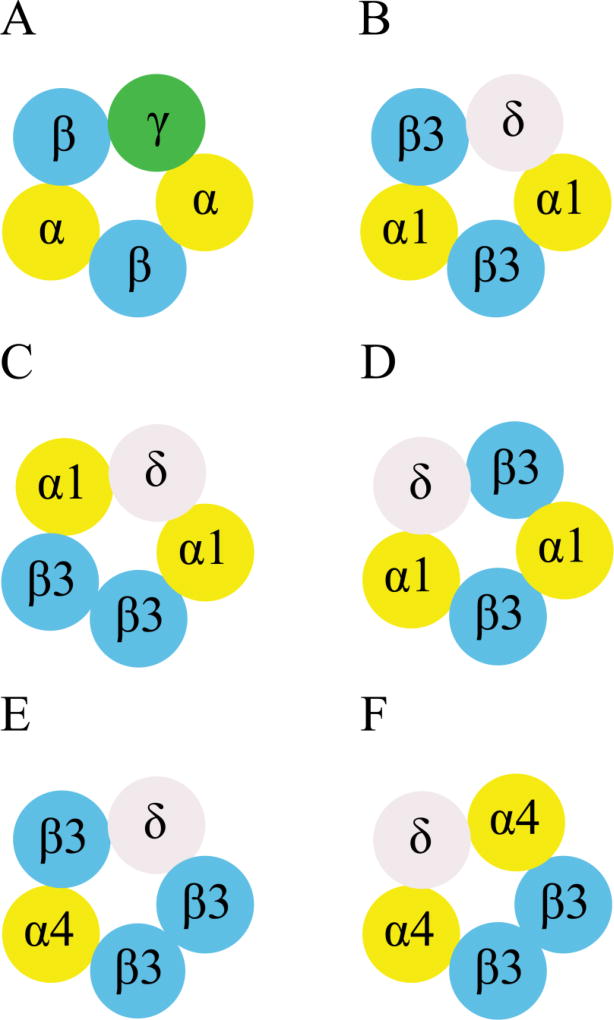
Figure 3. Cartoons to depict the subunit arrangement of recombinant αβγ and αβδ receptors.
A, The stoichiometry and subunit arrangement of recombinant αβγ receptors are well established as β-α-γ-β-α [51–53], which is also illustrated in Figure 1B, C. B, Our data in oocytes using MWC model analysis support the idea that the subunit arrangement of α1β3δ receptors is β3-α1-δ-β3-α1 [46], which was also proposed by Kaur et al [76]. C, D, The other two subunit arrangements proposed by Kaur et al [76] are β3-α1-δ-α1-β3 and α1-β3-α1-β3-δ. E, F, Photolabeling studies indicate that the possible subunit arrangement of α4β3δ receptors stably expressed in HEK293 cells is either β3-β3-δ-β3-α4 or β3-β3-α4-δ-α4. (all cartoons counterclockwise viewed from the extracellular space).
Most recombinant αβγ receptors exhibit very infrequent spontaneous gating [55–57]. It is estimated, for instance, that only about 1 in 20,000 channels is spontaneously open in α1β2γ2 receptors [57]. Maximally GABA-activated (at 1–10 mM) peak currents in oocyte-expressed α1β2/3γ2 receptors are enhanced about 10–20% with addition of positive allosteric modulators [38, 58–60], indicating that GABA alone activates about 85% of the receptors. Both receptor desensitization and deactivation apparently contribute to shaping IPSC time course [61]. Receptor desensitization in α1β2/3γ2, α4β3γ2, α5β3γ2 or α6β3γ2 receptors displays multiple exponential components during prolonged GABA exposure [49, 62–67]. Receptor desensitization reduces the speed of deactivation (desensitization-deactivation coupling) [43] in α1β2/3γ2, α4β3γ2, α5β3γ2 or α6β3γ2 receptors [65, 67–69].
Co-expression of α1, β2 and γ2 subunits in oocytes or HEK cells could form a mixture of α1β2 and α1β2γ2 receptors. In oocytes, increasing the ratio of γ2 subunit mRNA/cDNA relative to α1 and β2 subunits (up to 10:1:1) facilitated the formation of more homogenous α1β2γ2 receptors [70, 71]. In contrast, in HEK cells, reduced ratio of γ2 subunit cDNA to α1 and β2 subunits (0.3:1:1) led to homogenous α1β2γ2 receptors, while higher γ2:α1/β2 ratios may result in incorporation of more than one γ2 subunit per receptor [72]. It is currently unknown how receptor assembly differs in various cell types. However, co-expression of concatenated subunit assemblies appears to constrain subunit stoichiometry and arrangement in αβγ receptors [54, 72–74].
3. Structure and function of extrasynaptic αβδ GABAA receptors
The stoichiometry and subunit arrangement of αβδ GABAA receptors is uncertain. Several approaches that were used to investigate αβγ subunit stoichiometry and arrangement were unsuccessful in αβδ receptors. For instance, δL9´T mutations produce small EC50 shifts (< 4-fold; our unpublished observations) and GABA concentration-responses using mixtures of mutant and wild type subunits do not support strong inferences about stoichiometry of expressed αβδ receptors [75]. Comparison of concatenated subunit assemblies to free α1/4β2/3δ receptors gives no “best match” result based on GABA EC50 and neurosteroid enhancement of maximal GABA-induced currents [76, 77]. Both dimer (β-α) and trimer (β-α-γ or β-α-δ) can form functional receptors alone. In αβγ receptors, the current amplitude resulting from β-α-γ and β-α co-expression is much bigger than that of receptors formed from either dimer or trimer alone. Thus, it is evident that pentameric β-α-γ/β-α receptor currents dominate when both dimer and trimer are co-expressed [74]. However, in αβδ receptors, the current amplitude resulting from co-expression of β-α-δ and β-α is similar to that from receptors formed from either dimer or trimer alone. Thus, it is uncertain if pentameric β-α-δ/β-α receptors are the predominant form when both dimer and trimer are co-expressed. Moreover, α4β2δ receptors display very different neurosteroid activation efficacy relative to GABA in oocytes and HEK cells, although results in the two cell types agree better when β2-δ concatemers are co-expressed with free α4 subunits. This implies that free subunits assemble differently in the two cell types [45].
Spontaneous activity is not observed in recombinant α1β2δ receptors, but has been reported in α4β3δ and α6β2δ receptors [78, 79]. Maximally activating GABA concentrations evoke peak currents from recombinant αβδ receptors that are enhanced over 20-fold by positive allosteric modulators [59, 64, 80, 81]. These observations led us to estimate that saturating GABA concentrations activate only 3–5% of α1β3δ receptors [46]. α4/6β2/3δ receptors are very sensitive to GABA, with GABA EC50 less than 1 µM [64, 80, 82]. Neuronal tonic currents appear to be non-desensitizing even with addition of exogenous GABA [83], and α1β2/3δ receptors activated by maximal GABA exhibit very little or no desensitization [58, 59, 84]. Although α4β2/3δ and α6β3δ receptors desensitize in response to > 1 mM GABA [64, 67, 80, 85], the rate is slower than in α4β3γ2 and α6β3γ2 receptors [65, 85]. Deactivation of αβδ receptors is faster than that of αβγ receptors, consistent with low GABA efficacy.
In some recombinant expression systems, δ subunits may not efficiently incorporate into ternary αβδ receptors [46, 86–88]. This likely explains why one study found identical general anesthetic modulation in both α4β3δ and α4β3 receptors [89]. The subunit stoichiometry of surface αβδ receptors apparently depends on the ratios of α:β:δ mRNAs or cDNAs used for heterologous expression [72, 88, 90, 91]. By varying the subunit ratios and the concentrations of mRNAs injected into oocytes, we observed that increasing the δ subunit ratio (α:β:δ ratio = 1:1:3) and reducing total injected mRNA (10-fold from the 5 ng/oocyte typically used for α1βγ2 receptors) maximized current enhancement by allosteric modulators, suggesting optimal incorporation of the δ subunit into α1β3δ receptors [46]. It is unknown if this approach can be used to monitor the incorporation of the δ subunit into α4βδ and α6βδ receptors. In expression studies of α1β2δ in HEK cells, 10-fold less δ subunit cDNA is required to eliminate the functional signature of α1β2 receptors [72]. Thus, consistent incorporation of δ subunit into heterologously expressed receptors requires close attention to experimental conditions.
4. Functional effects of anesthetics on synaptic αβγ GABAA receptors
Many potent general anesthetics allosterically enhance the activation of GABAA receptors [7, 92–95]. In studies of phasic inhibition associated with αβγ receptors, etomidate [96, 97], propofol [98, 99], the barbiturate pentobarbital [100, 101], and the neurosteroids tetrahydrodeoxycorticosterone (THDOC) [102] and alphaxalone [103] all prolong IPSC decay. Potent general anesthetics also enhance GABA-evoked activation of oocyte-expressed α1β2/3γ2 receptors (Figure 2B), producing large “leftward shifts” (decreased EC50) and a small “upward shift” (increased maximal GABA current response) in concentration-responses [46, 60, 69, 104, 105]. This pattern of anesthetic-induced changes indicates high GABA intrinsic efficacy in αβγ receptors, consistent with previous single channel studies [59, 106, 107]. At high concentrations, general anesthetics also directly activate αβγ receptors (GABA-independent activation or allosteric agonism) [7]. Both positive modulation of orthosteric agonist responses and direct receptor activation by the anesthetic etomidate display similar pharmacological characteristics [57, 108, 109]. In α1β3γ2 receptors, the direct effect of pentobarbital appears bi-phasic, with maximal currents due to direct agonism at ~1 mM and inhibition at higher concentrations via a distinct inhibitory site [59].
In rapid kinetic studies of α1β3γ2 receptors, pentobarbital, propofol and THDOC do not alter or slightly reduce peak currents evoked by maximal GABA [58, 59, 64]. Pentobarbital and propofol decrease the extent of desensitization of α1β3γ2 and/or α6β3γ2 receptors [49, 59, 64], while THDOC and etomidate do not alter desensitization of α1β2/3γ2 receptors [58, 66]. General anesthetics prolong the deactivation of α1β2/3γ2 and/or α6β3γ2 receptors [58, 59, 64, 66]. Thus, desensitization may contribute to the differences in apparent maximal intrinsic efficacy of GABA in αβγ receptors studied in Xenopus oocytes vs. HEK293 cells.
5. Functional effects of anesthetics on extrasynaptic αβδ GABAA receptors
Extrasynaptic αβδ receptors are also important targets for general anesthetics [7], which enhance tonic currents in multiple brain regions [41, 42, 83, 98, 110–117]. General anesthetics alter GABA-dependent responses in oocyte-expressed αβδ receptors (Figure 2C) by inducing a small “leftward shift” and a large “upward shift”, contrasting with the pattern in αβγ receptors [46, 81]. The large increase in maximal GABA currents indicates low GABA intrinsic efficacy in αβδ receptors. Consistent with this, single channel recordings in the presence of high GABA show smaller mean channel open times in αβδ than in αβγ receptors [58, 59, 106, 118, 119]. Application of general anesthetics with maximal GABA increases the mean channel open time by inducing long-lived open states in α1β3δ receptors in single channel recordings [58, 59, 68, 119]. General anesthetics at high concentration also directly activate αβδ receptors. Pentobarbital alone directly activates α1β3δ receptors, with maximal responses at ~3 mM [59].
Artificial synapse experiments also show that general anesthetics substantially enhance peak currents of α1β3δ or α4β3δ receptors expressed in HEK cells [44, 58, 59, 64, 85, 89, 120]. Propofol and THDOC appear to evoke a greater augmentation of maximal GABA currents in α1β3δ than in α4/6β3δ receptors [58, 64, 76, 85, 89]. Different anesthetics evoke variable changes in αβδ receptor desensitization. Etomidate reduces the extent of desensitization for α1β3δ receptors [44]. This effect contrasts with studies showing apparent acceleration of α1β3δ receptor desensitization by both pentobarbital and THDOC [49, 58, 59]. Propofol does not alter desensitization of either α1β3δ or α6β3δ receptors [64]. Etomidate, pentobarbital and THDOC prolong deactivation of α1β3δ receptor currents [44, 49, 58, 59]. Propofol prolongs the deactivation of α6β3δ receptors but not α1β3δ receptors [64].
Endogenous neurosteroids are mainly synthesized in glial cells [121, 122], and their concentrations in the brain (10–300 nM) are dynamically regulated [123, 124]. The endogenous neurosteroid THDOC at physiological concentrations selectively enhances tonic currents mediated by αβδ receptors [83]. Both expression and function of αβδ receptors change with fluctuating neurosteroid levels in rodent brain [125–130]. Sensitivity of tonic GABAergic currents to neurosteroid modulation varies among brain regions. In hippocampus, 10 nM THDOC reduces neuronal excitability by augmenting tonic αβδ receptor currents [83]. In thalamocortical neurons, although 100 nM THDOC enhances tonic currents, 10 nM THDOC does not [42].
Several factors likely contribute to region-specific modulation of tonic inhibition by neurosteroids: First, the distribution of GABAA receptors in the brain is heterogeneous [8, 9]. The major αβδ receptor subtype expressed in thalamus is α4βδ, while α1βδ subtype is undetectable [20, 21]. In contrast, the hippocampus expresses both α4βδ and α1βδ receptors [22, 24]. α4βδ and α1βδ receptors exhibit differential sensitivity both to GABA [59, 80, 82] and to neurosteroids (see above). Second, the different effects of neurosteroids on tonic inhibition in the hippocampus and thalamus may be partly due to phosphorylation of αβδ receptors. The function and modulatory effect of α4β3δ receptors are regulated by both PKA and PKC [79, 131–133].
6. Shift analysis using an allosteric co-agonist model
A Monod-Wyman-Changeux (MWC) allosteric model was introduced by Chang & Weiss to describe the activation of wild type and gating mutant α1β2γ2 receptors [134]. In this model, estimated open probability (Popen) in a population of receptors is calculated by adding normalized spontaneous receptor activity to activated currents and correcting for the intrinsic efficacy of GABA. GABA efficacy is estimated by measuring how much maximal GABA currents are enhanced by strong positive modulators such as anesthetics or neurosteroids. MWC models allow and account for spontaneous channel activity, and were shown to account for effects on both GABA EC50 and spontaneous activation associated with L9’S mutations [134].
The effects of general anesthetics on αβγ receptor function are similar to those of L9’S gating mutations [134]. The combined effects of GABA and anesthetics, including etomidate, propofol, and pentobarbital, can be quantitatively accounted for using MWC co-agonist models (Figure 4) [60, 104, 135, 136]. Because MWC models explicitly account for agonist intrinsic efficacy, MWC shift analysis also unifies leftward-shift, increased maximal response, and direct anesthetic activation to quantify anesthetic modulation in different GABAA receptor subtypes [46, 136] or when a partial orthosteric agonist is combined with an anesthetic [135]. We modeled the allosteric modulation of etomidate on α1β3δ receptors using an MWC model that accounted for all of above effects when normalized responses were corrected for the low intrinsic efficacy of GABA in these receptors [46].
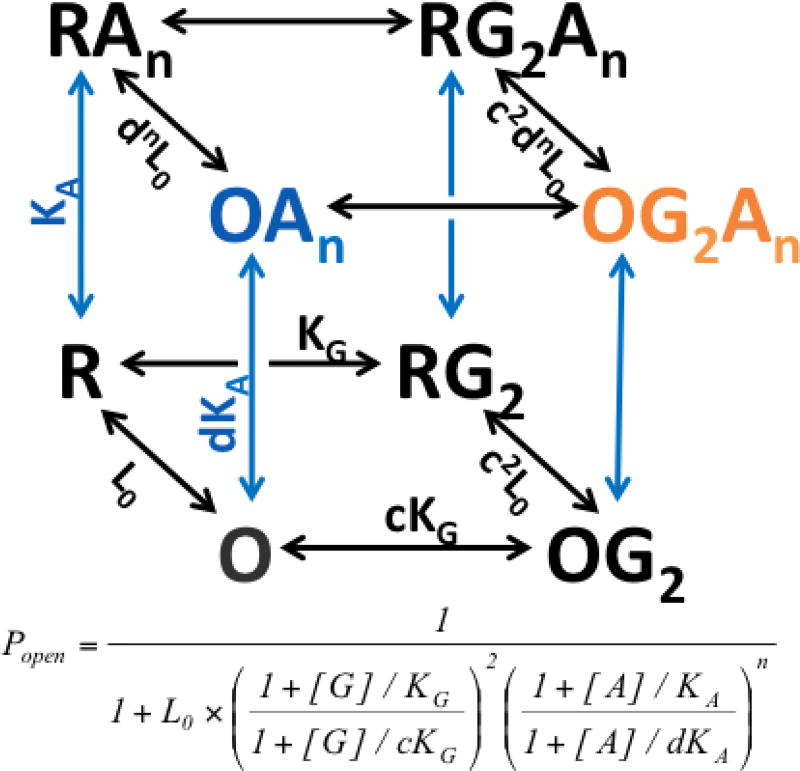
Figure 4. Monod-Wyman-Changeux allosteric co-agonism model in GABAA receptors.
This equilibrium scheme depicts a receptor activation model with two canonical states (R = closed, O = open) that can be activated by the orthosteric agonist GABA (G) and allosteric agonists such as anesthetics (A). The number of GABA binding sites is 2, based on structural and functional studies of synaptic GABAA receptors, and the number of anesthetic sites is a variable, n. GABA binding transitions and the GABA-activated state OG2 are shown in black and anesthetic binding transitions and the anesthetic-activated state OAn are shown in blue. The co-activated OG2An is shown in orange. An equation for the equilibrium open probability (in a population of receptors) of this model is shown below the scheme.
The basal equilibrium between unliganded R and O states (R:O) is L0 and basal open probability = (1+L0)−1; thus low L0 values indicate a higher probability of spontaneous receptor activation. The dissociation constant for GABA interactions with closed receptors is KG, and the dissociation constant for anesthetic interactions with closed receptors is KA. Agonist efficacy in the model depends on the relative affinity of ligands for open vs. closed receptors. For GABA, the efficacy parameter is c and for anesthetics, it is d; c and d values less than 1.0 indicate agonist activity and lower values indicate greater relative affinity for open states and thus greater agonist efficacy. The influence of agonists on closed:open equilibria is shown in black. When GABA concentrations are high enough to occupy all receptors, the RG2:OG2 equilibrium is c2L0 and Popen = (1+c2L0)−1. Similarly, anesthetic-bound receptors display an RAn:OAn equilibrium of dnL0 and Popen = (1+dnL0)−1. When both GABA and anesthetics are bound, the RG2An:OG2An equilibrium is c2dnL0 and Popen = (1+c2dnL0)−1. Modified from [104].
Our MWC models are based on data collected from oocyte recordings and do not include desensitized states. As the solution exchange times are slow in oocyte recordings, fast components of desensitization are obscured. However, MWC models that include desensitized states are consistent with the kinetics of patch-clamp recordings obtained with rapid solution exchange [137].
7. Structural identification of selective anesthetic sites on synaptic αβγ GABAA receptors
Multiple approaches have been used to locate general anesthetic sites on GABAA receptors, revealing evidence of remarkable site selectivity for some drugs. Both photolabeling and chimera strategies are unbiased approaches that can locate potential sites, while approaches based on point mutations can provide hypothesis-based complementary evidence. Each of these approaches has multiple limitations and weaknesses. Here, we briefly review these approaches and how they have contributed to identifying GABAA receptor sites for four potent anesthetics.
Chimeric constructs and point mutations have long been used to evaluate structure-function relationships of general anesthetics and other GABAA receptor modulators [138–141]. However, these studies have limited value in identifying drug contacts. Chimeric approaches have proven useful in cases where two homologous subunits display widely different drug sensitivities. For example, differential etomidate sensitivity in GABAA receptors containing β1 vs. β2 led to identification of the M2-15´ residue (β1S265 vs. β2N265) as key residues. However, care must be taken in evaluating efficacy and potency metrics--a set of β3/ρ1 chimeras that were constructed to locate pentobarbital sites showed varying effects on pentobarbital agonism vs. maximal GABA effects, but variance and single channel analyses indicated that GABA efficacy was altered without affecting pentobarbital efficacy [142]. In the case of point mutations, we tested whether the etomidate-insensitive phenotype of α1M236W and β2M286W mutations was generalizable to other anesthetic-photolabeled residues, and found that Trp mutations do not reliably identify drug contact vs. non-contact residues [143].
Photolabeling has provided unbiased regional identification of anesthetic binding sites on GABAA receptors [3, 94, 144]. However, many of these studies used GABAA receptors with only one or two subunit isotypes (e.g. β3 or α1β3) instead of the most common α1β2/3γ2 construct. These models limit data analysis and comparisons with functional studies [35, 145]. More generally, photoreactive groups, if appended to parent drug molecular structures in key binding regions, may disrupt pharmacological activity. Thus, photolabeling with many anesthetic derivatives likely fails to identify important contact residues.
Substituted cysteine accessibility modification-protection (SCAMP) was first used by Bali and Akabas to test propofol interactions in GABAA receptors [146] and applied more extensively by us to identify contact sites for multiple anesthetics [35]. SCAMP involves mutation of a putative anesthetic contact residue to cysteine, introducing a free sulfhydryl. Formation of covalent bond formation after exposure to a sulfhydryl-reactive chemical agent is detected with electrophysiology. If the cysteine-substitution is in an anesthetic binding pocket, drugs occupying the site should reduce the rate of covalent bond formation through steric competition [35]. Our recent analysis shows that SCAMP results agree with photolabeling results, indicating that SCAMP is a reliable technique to identify new contact residues [143]. In SCAMP experiments, it is also important to demonstrate drug-specific effects on cysteine modification. If all anesthetics similarly affect cysteine modification rate, this may indicate an allosteric rather than a steric interaction [35].
Etomidate is a potent stereoselective imidazole ester anesthetic. R-(+)-etomidate positively modulates and directly activates α1β2γ2 receptors about 20-fold more potently than S-(−)-etomidate, similar to the relative anesthetic potency of etomidate enantiomers in animals [147]. Heterologous αβγ receptors with β2 or β3, but not β1 are modulated by etomidate [109]. This observation led to chimera and point mutant studies that identified an asparagine residue (N265) in the M2 helices of β2 or β3 subunits as a major determinant of etomidate sensitivity [108, 148]. Quantitative MWC co-agonist modeling of etomidate effects in α1β2γ2 receptors indicated the presence of two equivalent sites [135]. MWC analysis of etomidate effects in α1β2N265Sγ2 and α1β2N265Mγ2 receptors suggested effects on drug binding to open-channel states more than closed states [69]. Photoreactive etomidate derivatives incorporate at α1M236 in α-M1 and βM286 in β-M3, which are located in structural models at transmembrane β+/α− interfaces [149, 150]. Tryptophan mutations at either α1M236 or β2M286 induce spontaneously active GABA-sensitive receptors that are weakly modulated by etomidate, suggesting that the Trp side chains both mimic etomidate binding and occlude the sites [151]. Concatemeric subunit β2-α1 and β2-α1-γ2 assemblies with α1M236W mutations produce equivalent functional changes in expressed receptors, supporting equivalence of the two etomidate sites [74]. Photolabeling also indicated etomidate binding at β3+/β3− interfaces in α1β3 receptors [152], accounting for etomidate activation of β3 homomeric receptors [153]. SCAMP has mapped additional etomidate contact residues in α1β2/3γ2 receptors [143, 154], all of which map to transmembrane β+/α− interfaces.
In summary, there is abundant evidence that etomidate binds selectively in the two β+/α− interfaces of α1β2/3γ GABAA receptors.
R-5-allyl-1-methyl-5-(m-trifluoromethyl-diazirynylphenyl) barbituric acid (R-mTFD-MPAB) was developed as a barbiturate photolabel that was found to be both potent and stereoselective in α1β2/3γ2 receptors and in animals [155]. Photolabeling reveals that it binds specifically at transmembrane subunit interfaces between γ2-M3 and β3-M1 (γ+/β−) and between α1-M3 and β3-M1 (α+/β−) helices in α1β3γ2 receptors [156]. SCAMP studies have confirmed some of these contact residues in α1β3γ2 receptors and identified additional contacts [143]. The convulsant barbiturate photolabel S-1-methyl-5-propyl-5-(m-trifluoromethyl-diazirynylphenyl) barbituric acid (S-mTFD-MPPB) inhibits the function of α1β3γ2 receptors [157, 158], and also incorporates at γ+/β− interfaces on α1β3γ2 receptors [159]. This suggests that the γ+/β− interface can mediate allosteric channel gating shifts in opposing directions, perhaps depending on the specific orientation of hypnotic vs. convulsant barbiturates within the site.
In summary, m-substituted MPAB and MPPB derivatives selectively bind in α+/β− and γ+/β− interfaces of α1β2/3γ2 receptors. Studies are underway to establish if mutations in these sites display asymmetrical functional effects.
Propofol contacts were tentatively identified in mutant studies at β2M286 in β2-M3 [160]. MWC shift analysis indicates that propofol possesses more than two allosteric sites on α1β2γ2 receptors [104]. In line with this prediction, photolabeling studies demonstrated that propofol binds at β+/α− and α+/β− interfaces in α1β3 receptors, which are homologues of the inter-subunit sites for both etomidate and R-mTFD-MPAB, respectively [35, 161]. Mutant studies support this conclusion [141]. Thus, propofol shares binding sites with both etomidate and barbiturates. Photolabeling in β3 homomeric and α1β3 receptors also suggested propofol contact with β3H267 [162], and β3H267 may be close to the sites for R-mTFD-MPAB [163]. However, studies using mutational analysis and SCAMP techniques do not support the idea that β3H267 contacts propofol [163, 164].
Neurosteroids bind in sites that are distinct from those where etomidate, propofol, and barbiturates bind based on functional and photolabeling results. Neurosteroids enhance GABAA receptor activation by etomidate and barbiturates, synergize with etomidate in anesthetizing animals [165], and enhance photolabeling by etomidate analogs [166]. Photolabeling in β3 homomeric receptors identified F301 in β3-M3 as a possible neurosteroid binding site [167]. Recent published data supports the hypothesis that neurosteroid enhancing sites are located in β+/α− interfaces near the inner end of β-M3 and α-M1 helices. Crystal structures of homomeric chimeric channels that include α subunit transmembrane domains reveal THDOC interactions with αQ242 [168, 169], a site where mutations significantly alter neurosteroid sensitivity [170, 171]. Possible neurosteroid contact residues in α1β2γ2 receptors include S241 and Q242 in α1-M1 and N407 and Y410 in α1-M4 [170–173]. SCAMP experiments comparing contacts for propofol, etomidate and alphaxalone on β3-M3 in α1β3γ2 receptors show that alphaxalone protects cysteine substitutions at β3 residues V290, F293, L297 and F301, and some of these mutations also reduce neurosteroid sensitivity [174]. In addition, SCAMP studies confirm that neurosteroids do not interact with established etomidate and R-mTFD-MPAB contacts [105]. Neurosteroid sites are thus adjacent and intracellular to the sites where etomidate binds in β+/α− interfaces, and the effects of mutations in these sites suggest that they account for the majority of neurosteroid effects on channel gating.
8. General anesthetics as structural probes in αβδ GABAA receptors
Chimera studies have aimed at identifying the structures underlying the differential patterns of general anesthetic modulation in αβγ vs. αβδ receptors. For example, general anesthetics strongly enhance the peak currents of α1β3δ receptors but only slightly potentiate α1β3γ2 currents in the presence of saturating GABA [49, 58]. Feng and Macdonald [49] compared pentobarbital effects in receptors containing γ2/δ or δ/γ2 subunit chimeras vs. wild type α1β3γ2 and α1β3δ receptors. The relative efficacy of pentobarbital vs. GABA depended on δ sequences between the N-terminus and the middle of M1. However, GABA efficacy in the chimeric receptors was not assessed, so it remains uncertain whether the chimeras affected GABA efficacy, pentobarbital efficacy, or both. It is also possible that the chimeras affected subunit assembly.
The formalism of MWC allosteric models requires assessment of GABA efficacy and thus establishes a stronger framework for functional analysis of structural changes. Taking advantage of the unique binding sites of etomidate on αβγ GABAA receptors, we inferred if putative αβδ receptors possess the same β+/α− interfaces by quantifying the allosteric modulation of these two receptor isoforms by etomidate using MWC allosteric model. Our hypothesis was that if the stoichiometry and subunit arrangement of α1β3γ2 and α1β3δ receptors are different, the number of etomidate binding sites on both receptor isoforms would be different, which would result in divergent allosteric effects of etomidate [46]. We observed that etomidate at 3.2 µM produced a 10-fold leftward shift and 24% upward shift of GABA concentration-responses in α1β3γ2 receptors. However, the same concentration of etomidate evoked a 3-fold leftward shift and a 17-fold upward shift of GABA concentration-responses in α1β3δ receptors [46]. We quantitatively compared etomidate (3.2 µM) modulation of GABA-dependent activation in α1β3γ2 vs. α1β3δ receptors using MWC model analysis. Even though etomidate effects on GABA-dependent responses are very different in α1β3γ2 vs. α1β3δ receptors, after correcting data for the different GABA efficacies in these two receptors, our analysis indicated comparable allosteric shifts [46]. Comparable allosteric modulation of α4β2γ2 and α4β2δ receptors by alfaxalone was also observed using the similar approach [175]. Moreover, because etomidate selectively acts via two β+/α− interfacial sites in α1β3γ2 receptors [35], this result may indicate that α1β3δ receptors also contain two β+/α− interfaces. If this is the case, our data tend to support previous studies that δ simply replaces γ in the established synaptic subunit arrangement [72, 75, 88, 176]. These data also agree with a previous observation that pentobarbital evokes similar modulation in both α1β3δ and α1β3γ2 currents activated with low GABA concentrations [59]. Indeed, synaptic αβγ receptors may contribute significantly to modulation of tonic currents by general anesthetics [177].
Using site-selective anesthetics is also advantageous in studying GABAA receptors formed from subunit concatemers. THDOC was used as a probe drug to seek information about receptor subunit arrangement of free α1/6β3δ receptors vs. various concatenated α1/6β3δ receptors [50, 76]. It was proposed that free α1β3δ receptors may display at least three subunit arrangements: β3-α1-δ-β3-α1, β3-α1-δ-α1-β3 and α1-β3-α1-β3-δ (Figure 3B, C, D) [76]. We further examined the allosteric modulation of concatenated β3-α1-δ/β3-α1 receptors (formed by injection of β3-α1-δ trimer and β3-α1 dimer mRNAs in oocytes) by etomidate at 3.2 µM and found that etomidate produced a 3-fold leftward shift and a 14-fold upward shift of GABA concentration-responses [46]. We quantitatively compared etomidate modulation of free α1β3δ receptors and concatenated β3-α1-δ/β3-α1 receptors, and found close agreement, further supporting the β3-α1-δ-β3-α1 subunit arrangement for oocyte-expressed α1β3δ receptors (Figure 3B) [46]. However, it should be noted that our data do not determine the definite stoichiometry and subunit arrangement of α1β3δ receptors. For example, it is possible that etomidate may bind to other intersubunit sites in α1β3δ receptors, such as δ+/α− or β+/β– interface, which may exert similar allosteric effect to those β+/α− sites. R-mTFD-MPAB acts in αβγ receptors via both α+/β− and γ+/β− sites, but its effects in αβδ receptors remain unexplored. We measured allosteric modulation by R-mTFD-MPAB in free and concatenated β3-α1/β3-α1-δ receptors and found them to be similar (unpublished observations). Further mutant studies to compare the contributions of α+/β− sites vs. δ+/β− sites and other possible interfacial sites are needed to strengthen our conclusion.
Photolabeling with site-selective anesthetics provides additional insight into αβδ receptor subunit arrangements. Chiara et al [178] identified azi-etomidate and R-mTFD-MPAB incorporation sites in human α4β3δ receptors that were expressed in HEK cells and purified in detergent. Neither photolabel incorporated into the δ subunit. Azi-etomidate incorporated in β3M286 (M3) and α4M269 (M1), consistent with β+/α− sites. Both photolabels incorporated at β3M227 (M1), suggesting anesthetic sites in β3+/β3 interfaces. Assuming homogeneous assembly, possible subunit assemblies are β3-β3-δ-β3-α4 or β3-β3-α4-δ-α4 (Figure 3E, F) [178]. If α1β3δ receptors share similar stoichiometry and subunit arrangement with α1β3γ2 receptors, these data suggest that the stoichiometry and subunit arrangement of α4β3δ receptors may be different from those of α1β3δ receptors.
In summary, anesthetics, particularly etomidate, neurosteroids and R-mTFD-MPAB that display strong specificity for certain subunit interfacial sites, are informative structural probes for heteromeric GABAA receptors. Some of these drugs apparently can also act via β+/β− interfaces, requiring chimera studies to test inferences. Propofol acts via four sites per αβγ receptor, and is thus of no value as a probe of αβδ receptor subunit arrangements. Interpretation of anesthetic functional effects must include independent assessment of GABA efficacy, and formal MWC model analyses enforce this, while also enabling quantitative comparisons of drug effects in different receptor isoforms.
9. Conclusions
The structure of neuronal αβδ receptors remains uncertain, and the structure of expressed recombinant αβδ receptors may vary with cell type and other variables. Because certain general anesthetics act at well-defined αβγ subunit interfaces, they and subunit modifications that affect anesthetic sensitivity in αβγ receptors represent useful tools for probing the subunit arrangement of αβδ receptors. As we learn more about anesthetic binding sites, the value of these tools as structural probes will also grow.
Acknowledgments
Funding
This work was supported by P01GM058448 and R01GM089745 to SAF and funds from Department of Anesthesia, Critical Care and Pain Medicine at Massachusetts General Hospital to HJF and SAF.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors have no conflict of interest to report
Chemical compounds
Alphaxalone Etomidate
Pentobarbital
Propofol
R-5-allyl-1-methyl-5-(m-trifluoromethyl-diazirynylphenyl) barbituric acid (R-mTFD-MPAB)
S-1-methyl-5-propyl-5-(m-trifluoromethyl-diazirynylphenyl) barbituric acid (S-mTFD-MPPB)
Tetrahydrodeoxycorticosterone (THDOC)
References
- 1.Roberts E. Gaba: The road to neurotransmitter status. In: Olsen RW, Venter CJ, editors. Benzodiazepine/gaba receptors and chloride channels: Structure and functional properties. New York: Wiley; 1986. pp. 1–39. [Google Scholar]
- 2.Beleboni RO, Carolino RO, Pizzo AB, Castellan-Baldan L, Coutinho-Netto J, dos Santos WF, Coimbra NC. Pharmacological and biochemical aspects of gabaergic neurotransmission: Pathological and neuropsychobiological relationships. Cellular and molecular neurobiology. 2004;24:707–728. doi: 10.1007/s10571-004-6913-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Forman SA, Chiara DC, Miller KW. Anesthetics target interfacial transmembrane sites in nicotinic acetylcholine receptors. Neuropharmacology. 2015;96:169–177. doi: 10.1016/j.neuropharm.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olsen RW, Sieghart W. International union of pharmacology. Lxx. Subtypes of gamma-aminobutyric acid(a) receptors: Classification on the basis of subunit composition, pharmacology, and function. Update. Pharmacol Rev. 2008;60:243–260. doi: 10.1124/pr.108.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chua HC, Chebib M. Gabaa receptors and the diversity in their structure and pharmacology. Advances in pharmacology (San Diego, Calif. 2017;79:1–34. doi: 10.1016/bs.apha.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 6.Olsen RW, Sieghart W. Gaba a receptors: Subtypes provide diversity of function and pharmacology. Neuropharmacology. 2009;56:141–148. doi: 10.1016/j.neuropharm.2008.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng HJ. Allosteric modulation of alphabetadelta gabaa receptors. Pharmaceuticals. 2010;3:3461–3477. [Google Scholar]
- 8.McKernan RM, Whiting PJ. Which gabaa-receptor subtypes really occur in the brain? Trends Neurosci. 1996;19:139–143. doi: 10.1016/s0166-2236(96)80023-3. [DOI] [PubMed] [Google Scholar]
- 9.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. Gaba(a) receptors: Immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 10.Nusser Z, Sieghart W, Somogyi P. Segregation of different gabaa receptors to synaptic and extrasynaptic membranes of cerebellar granule cells. J Neurosci. 1998;18:1693–1703. doi: 10.1523/JNEUROSCI.18-05-01693.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farrant M, Nusser Z. Variations on an inhibitory theme: Phasic and tonic activation of gaba(a) receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- 12.Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 gaba(a) receptors. Proc Natl Acad Sci U S A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serwanski DR, Miralles CP, Christie SB, Mehta AK, Li X, De Blas AL. Synaptic and nonsynaptic localization of gabaa receptors containing the alpha5 subunit in the rat brain. The Journal of comparative neurology. 2006;499:458–470. doi: 10.1002/cne.21115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin LJ, Bonin RP, Orser BA. The physiological properties and therapeutic potential of alpha5-gabaa receptors. Biochem Soc Trans. 2009;37:1334–1337. doi: 10.1042/BST0371334. [DOI] [PubMed] [Google Scholar]
- 15.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 gabaa receptor subunit mrnas in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing gaba(a) receptors and their activation by gaba spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones A, Korpi ER, McKernan RM, Pelz R, Nusser Z, Makela R, Mellor JR, Pollard S, Bahn S, Stephenson FA, Randall AD, Sieghart W, Somogyi P, Smith AJ, Wisden W. Ligand-gated ion channel subunit partnerships: Gabaa receptor alpha6 subunit gene inactivation inhibits delta subunit expression. J Neurosci. 1997;17:1350–1362. doi: 10.1523/JNEUROSCI.17-04-01350.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Poltl A, Hauer B, Fuchs K, Tretter V, Sieghart W. Subunit composition and quantitative importance of gaba(a) receptor subtypes in the cerebellum of mouse and rat. J Neurochem. 2003;87:1444–1455. doi: 10.1046/j.1471-4159.2003.02135.x. [DOI] [PubMed] [Google Scholar]
- 19.Hortnagl H, Tasan RO, Wieselthaler A, Kirchmair E, Sieghart W, Sperk G. Patterns of mrna and protein expression for 12 gabaa receptor subunits in the mouse brain. Neuroscience. 2013;236:345–372. doi: 10.1016/j.neuroscience.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sur C, Farrar SJ, Kerby J, Whiting PJ, Atack JR, McKernan RM. Preferential coassembly of alpha4 and delta subunits of the gamma-aminobutyric acida receptor in rat thalamus. Mol Pharmacol. 1999;56:110–115. doi: 10.1124/mol.56.1.110. [DOI] [PubMed] [Google Scholar]
- 21.Jia F, Pignataro L, Schofield CM, Yue M, Harrison NL, Goldstein PA. An extrasynaptic gabaa receptor mediates tonic inhibition in thalamic vb neurons. J Neurophysiol. 2005;94:4491–4501. doi: 10.1152/jn.00421.2005. [DOI] [PubMed] [Google Scholar]
- 22.Chandra D, Jia F, Liang J, Peng Z, Suryanarayanan A, Werner DF, Spigelman I, Houser CR, Olsen RW, Harrison NL, Homanics GE. Gabaa receptor alpha 4 subunits mediate extrasynaptic inhibition in thalamus and dentate gyrus and the action of gaboxadol. Proc Natl Acad Sci U S A. 2006;103:15230–15235. doi: 10.1073/pnas.0604304103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drasbek KR, Hoestgaard-Jensen K, Jensen K. Modulation of extrasynaptic thip conductances by gabaa-receptor modulators in mouse neocortex. J Neurophysiol. 2007;97:2293–2300. doi: 10.1152/jn.00651.2006. [DOI] [PubMed] [Google Scholar]
- 24.Glykys J, Peng Z, Chandra D, Homanics GE, Houser CR, Mody I. A new naturally occurring gaba(a) receptor subunit partnership with high sensitivity to ethanol. Nat Neurosci. 2007;10:40–48. doi: 10.1038/nn1813. [DOI] [PubMed] [Google Scholar]
- 25.Browne SH, Kang J, Akk G, Chiang LW, Schulman H, Huguenard JR, Prince DA. Kinetic and pharmacological properties of gaba(a) receptors in single thalamic neurons and gaba(a) subunit expression. J Neurophysiol. 2001;86:2312–2322. doi: 10.1152/jn.2001.86.5.2312. [DOI] [PubMed] [Google Scholar]
- 26.Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, Smit AB, Sixma TK. Crystal structure of an ach-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature. 2001;411:269–276. doi: 10.1038/35077011. [DOI] [PubMed] [Google Scholar]
- 27.Bocquet N, Prado de Carvalho L, Cartaud J, Neyton J, Le Poupon C, Taly A, Grutter T, Changeux JP, Corringer PJ. A prokaryotic proton-gated ion channel from the nicotinic acetylcholine receptor family. Nature. 2007;445:116–119. doi: 10.1038/nature05371. [DOI] [PubMed] [Google Scholar]
- 28.Nury H, Van Renterghem C, Weng Y, Tran A, Baaden M, Dufresne V, Changeux JP, Sonner JM, Delarue M, Corringer PJ. X-ray structures of general anaesthetics bound to a pentameric ligand-gated ion channel. Nature. 2011;469:428–431. doi: 10.1038/nature09647. [DOI] [PubMed] [Google Scholar]
- 29.Hilf RJ, Dutzler R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature. 2008;452:375–379. doi: 10.1038/nature06717. [DOI] [PubMed] [Google Scholar]
- 30.Hilf RJ, Dutzler R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature. 2009;457:115–118. doi: 10.1038/nature07461. [DOI] [PubMed] [Google Scholar]
- 31.Hibbs RE, Gouaux E. Principles of activation and permeation in an anion-selective cys-loop receptor. Nature. 2011;474:54–60. doi: 10.1038/nature10139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Althoff T, Hibbs RE, Banerjee S, Gouaux E. X-ray structures of glucl in apo states reveal a gating mechanism of cys-loop receptors. Nature. 2014;512:333–337. doi: 10.1038/nature13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller PS, Aricescu AR. Crystal structure of a human gabaa receptor. Nature. 2014;512:270–275. doi: 10.1038/nature13293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Du J, Lu W, Wu S, Cheng Y, Gouaux E. Glycine receptor mechanism elucidated by electron cryo-microscopy. Nature. 2015;526:224–229. doi: 10.1038/nature14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Forman SA, Miller KW. Mapping general anesthetic sites in heteromeric gamma-aminobutyric acid type a receptors reveals a potential for targeting receptor subtypes. Anesth Analg. 2016;123:1263–1273. doi: 10.1213/ANE.0000000000001368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grosman C, Zhou M, Auerbach A. Mapping the conformational wave of acetylcholine receptor channel gating. Nature. 2000;403:773–776. doi: 10.1038/35001586. [DOI] [PubMed] [Google Scholar]
- 37.Mody I, Pearce RA. Diversity of inhibitory neurotransmission through gaba(a) receptors. Trends Neurosci. 2004;27:569–575. doi: 10.1016/j.tins.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 38.Zheleznova NN, Sedelnikova A, Weiss DS. Function and modulation of delta-containing gaba(a) receptors. Psychoneuroendocrinology. 2009;34(Suppl 1):S67–73. doi: 10.1016/j.psyneuen.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Egawa K, Fukuda A. Pathophysiological power of improper tonic gaba(a) conductances in mature and immature models. Frontiers in neural circuits. 2013;7:170. doi: 10.3389/fncir.2013.00170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whissell PD, Lecker I, Wang DS, Yu J, Orser BA. Altered expression of deltagabaa receptors in health and disease. Neuropharmacology. 2015;88:24–35. doi: 10.1016/j.neuropharm.2014.08.003. [DOI] [PubMed] [Google Scholar]
- 41.Belelli D, Peden DR, Rosahl TW, Wafford KA, Lambert JJ. Extrasynaptic gabaa receptors of thalamocortical neurons: A molecular target for hypnotics. J Neurosci. 2005;25:11513–11520. doi: 10.1523/JNEUROSCI.2679-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cope DW, Hughes SW, Crunelli V. Gabaa receptor-mediated tonic inhibition in thalamic neurons. J Neurosci. 2005;25:11553–11563. doi: 10.1523/JNEUROSCI.3362-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jones MV, Westbrook GL. Desensitized states prolong gabaa channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 44.Liu K, Jounaidi Y, Forman SA, Feng HJ. Etomidate uniquely modulates the desensitization of recombinant alpha1beta3delta gaba(a) receptors. Neuroscience. 2015;300:307–313. doi: 10.1016/j.neuroscience.2015.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eaton MM, Bracamontes J, Shu HJ, Li P, Mennerick S, Steinbach JH, Akk G. Gamma-aminobutyric acid type a alpha4, beta2, and delta subunits assemble to produce more than one functionally distinct receptor type. Mol Pharmacol. 2014;86:647–656. doi: 10.1124/mol.114.094813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Feng HJ, Jounaidi Y, Haburcak M, Yang X, Forman SA. Etomidate produces similar allosteric modulation in alpha1beta3delta and alpha1beta3gamma2l gabaa receptors. Br J Pharmacol. 2014;171:789–798. doi: 10.1111/bph.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Prinz H. Hill coefficients, dose-response curves and allosteric mechanisms. J Chem Biol. 2010;3:37–44. doi: 10.1007/s12154-009-0029-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stewart DS, Pierce DW, Hotta M, Stern AT, Forman SA. Mutations at beta n265 in gamma-aminobutyric acid type a receptors alter both binding affinity and efficacy of potent anesthetics. PloS one. 2014;9:e111470. doi: 10.1371/journal.pone.0111470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Feng HJ, Macdonald RL. Barbiturates require the n terminus and first transmembrane domain of the delta subunit for enhancement of alpha1beta3delta gabaa receptor currents. J Biol Chem. 2010;285:23614–23621. doi: 10.1074/jbc.M110.122564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baur R, Kaur KH, Sigel E. Diversity of structure and function of alpha1alpha6beta3delta gabaa receptors: Comparison with alpha1beta3delta and alpha6beta3delta receptors. J Biol Chem. 2010;285:17398–17405. doi: 10.1074/jbc.M110.108670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chang Y, Wang R, Barot S, Weiss DS. Stoichiometry of a recombinant gabaa receptor. J Neurosci. 1996;16:5415–5424. doi: 10.1523/JNEUROSCI.16-17-05415.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tretter V, Ehya N, Fuchs K, Sieghart W. Stoichiometry and assembly of a recombinant gabaa receptor subtype. J Neurosci. 1997;17:2728–2737. doi: 10.1523/JNEUROSCI.17-08-02728.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Farrar SJ, Whiting PJ, Bonnert TP, McKernan RM. Stoichiometry of a ligand-gated ion channel determined by fluorescence energy transfer. J Biol Chem. 1999;274:10100–10104. doi: 10.1074/jbc.274.15.10100. [DOI] [PubMed] [Google Scholar]
- 54.Baumann SW, Baur R, Sigel E. Forced subunit assembly in alpha1beta2gamma2 gabaa receptors. Insight into the absolute arrangement. J Biol Chem. 2002;277:46020–46025. doi: 10.1074/jbc.M207663200. [DOI] [PubMed] [Google Scholar]
- 55.Knoflach F, Benke D, Wang Y, Scheurer L, Luddens H, Hamilton BJ, Carter DB, Mohler H, Benson JA. Pharmacological modulation of the diazepam-insensitive recombinant gamma-aminobutyric acida receptors alpha 4 beta 2 gamma 2 and alpha 6 beta 2 gamma 2. Mol Pharmacol. 1996;50:1253–1261. [PubMed] [Google Scholar]
- 56.Bianchi MT. Context dependent benzodiazepine modulation of gaba(a) receptor opening frequency. Current neuropharmacology. 2010;8:10–17. doi: 10.2174/157015910790909467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Forman SA. Monod-wyman-changeux allosteric mechanisms of action and the pharmacology of etomidate. Curr Opin Anaesthesiol. 2012;25:411–418. doi: 10.1097/ACO.0b013e328354feea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wohlfarth KM, Bianchi MT, Macdonald RL. Enhanced neurosteroid potentiation of ternary gaba(a) receptors containing the delta subunit. J Neurosci. 2002;22:1541–1549. doi: 10.1523/JNEUROSCI.22-05-01541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Feng HJ, Bianchi MT, Macdonald RL. Pentobarbital differentially modulates alpha1beta3delta and alpha1beta3gamma2l gabaa receptor currents. Mol Pharmacol. 2004;66:988–1003. doi: 10.1124/mol.104.002543. [DOI] [PubMed] [Google Scholar]
- 60.Ziemba AM, Forman SA. Correction for inhibition leads to an allosteric co-agonist model for pentobarbital modulation and activation of alpha1beta3gamma2l gabaa receptors. PloS one. 2016;11:e0154031. doi: 10.1371/journal.pone.0154031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bianchi MT, Botzolakis EJ, Haas KF, Fisher JL, Macdonald RL. Microscopic kinetic determinants of macroscopic currents: Insights from coupling and uncoupling of gabaa receptor desensitization and deactivation. J Physiol. 2007;584:769–787. doi: 10.1113/jphysiol.2007.142364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bianchi MT, Haas KF, Macdonald RL. Structural determinants of fast desensitization and desensitization-deactivation coupling in gabaa receptors. J Neurosci. 2001;21:1127–1136. doi: 10.1523/JNEUROSCI.21-04-01127.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bianchi MT, Macdonald RL. Slow phases of gaba(a) receptor desensitization: Structural determinants and possible relevance for synaptic function. J Physiol. 2002;544:3–18. doi: 10.1113/jphysiol.2002.020255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Feng HJ, Macdonald RL. Multiple actions of propofol on alphabetagamma and alphabetadelta gabaa receptors. Mol Pharmacol. 2004;66:1517–1524. doi: 10.1124/mol.104.003426. [DOI] [PubMed] [Google Scholar]
- 65.Lagrange AH, Botzolakis EJ, Macdonald RL. Enhanced macroscopic desensitization shapes the response of alpha4 subtype-containing gabaa receptors to synaptic and extrasynaptic gaba. J Physiol. 2007;578:655–676. doi: 10.1113/jphysiol.2006.122135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhong H, Rusch D, Forman SA. Photo-activated azi-etomidate, a general anesthetic photolabel, irreversibly enhances gating and desensitization of gamma-aminobutyric acid type a receptors. Anesthesiology. 2008;108:103–112. doi: 10.1097/01.anes.0000296074.33999.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Feng HJ, Botzolakis EJ, Macdonald RL. Context-dependent modulation of alphabetagamma and alphabetadelta gaba a receptors by penicillin: Implications for phasic and tonic inhibition. Neuropharmacology. 2009;56:161–173. doi: 10.1016/j.neuropharm.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Feng HJ, Macdonald RL. Proton modulation of alpha 1 beta 3 delta gabaa receptor channel gating and desensitization. J Neurophysiol. 2004;92:1577–1585. doi: 10.1152/jn.00285.2004. [DOI] [PubMed] [Google Scholar]
- 69.Desai R, Ruesch D, Forman SA. Gamma-amino butyric acid type a receptor mutations at beta2n265 alter etomidate efficacy while preserving basal and agonist-dependent activity. Anesthesiology. 2009;111:774–784. doi: 10.1097/ALN.0b013e3181b55fae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Boileau AJ, Baur R, Sharkey LM, Sigel E, Czajkowski C. The relative amount of crna coding for gamma2 subunits affects stimulation by benzodiazepines in gaba(a) receptors expressed in xenopus oocytes. Neuropharmacology. 2002;43:695–700. doi: 10.1016/s0028-3908(02)00036-9. [DOI] [PubMed] [Google Scholar]
- 71.Boileau AJ, Li T, Benkwitz C, Czajkowski C, Pearce RA. Effects of gamma2s subunit incorporation on gabaa receptor macroscopic kinetics. Neuropharmacology. 2003;44:1003–1012. doi: 10.1016/s0028-3908(03)00114-x. [DOI] [PubMed] [Google Scholar]
- 72.Botzolakis EJ, Gurba KN, Lagrange AH, Feng HJ, Stanic AK, Hu N, Macdonald RL. Comparison of gamma-aminobutyric acid, type a (gabaa), receptor alphabetagamma and alphabetadelta expression using flow cytometry and electrophysiology: Evidence for alternative subunit stoichiometries and arrangements. J Biol Chem. 2016;291:20440–20461. doi: 10.1074/jbc.M115.698860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Baumann SW, Baur R, Sigel E. Subunit arrangement of gamma-aminobutyric acid type a receptors. J Biol Chem. 2001;276:36275–36280. doi: 10.1074/jbc.M105240200. [DOI] [PubMed] [Google Scholar]
- 74.Guitchounts G, Stewart DS, Forman SA. Two etomidate sites in alpha1beta2gamma2 gamma-aminobutyric acid type a receptors contribute equally and noncooperatively to modulation of channel gating. Anesthesiology. 2012;116:1235–1244. doi: 10.1097/ALN.0b013e3182567df3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patel B, Mortensen M, Smart TG. Stoichiometry of delta subunit containing gaba(a) receptors. Br J Pharmacol. 2014;171:985–994. doi: 10.1111/bph.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kaur KH, Baur R, Sigel E. Unanticipated structural and functional properties of delta-subunit-containing gabaa receptors. J Biol Chem. 2009;284:7889–7896. doi: 10.1074/jbc.M806484200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wongsamitkul N, Baur R, Sigel E. Toward understanding functional properties and subunit arrangement of alpha4beta2delta gamma-aminobutyric acid, type a (gabaa) receptors. J Biol Chem. 2016;291:18474–18483. doi: 10.1074/jbc.M116.738906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hadley SH, Amin J. Rat alpha6beta2delta gabaa receptors exhibit two distinct and separable agonist affinities. J Physiol. 2007;581:1001–1018. doi: 10.1113/jphysiol.2007.132886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tang X, Hernandez CC, Macdonald RL. Modulation of spontaneous and gaba-evoked tonic alpha4beta3delta and alpha4beta3gamma2l gabaa receptor currents by protein kinase a. J Neurophysiol. 2010;103:1007–1019. doi: 10.1152/jn.00801.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Feng HJ, Kang JQ, Song L, Dibbens L, Mulley J, Macdonald RL. Delta subunit susceptibility variants e177a and r220h associated with complex epilepsy alter channel gating and surface expression of alpha4beta2delta gabaa receptors. J Neurosci. 2006;26:1499–1506. doi: 10.1523/JNEUROSCI.2913-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zheleznova N, Sedelnikova A, Weiss DS. Alpha1beta2delta, a silent gabaa receptor: Recruitment by tracazolate and neurosteroids. Br J Pharmacol. 2008;153:1062–1071. doi: 10.1038/sj.bjp.0707665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type a receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing gabaa receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dibbens LM, Feng HJ, Richards MC, Harkin LA, Hodgson BL, Scott D, Jenkins M, Petrou S, Sutherland GR, Scheffer IE, Berkovic SF, Macdonald RL, Mulley JC. Gabrd encoding a protein for extra- or peri-synaptic gabaa receptors is a susceptibility locus for generalized epilepsies. Human molecular genetics. 2004;13:1315–1319. doi: 10.1093/hmg/ddh146. [DOI] [PubMed] [Google Scholar]
- 85.Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha(4)beta(3)delta gaba(a) receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Borghese CM, Harris RA. Studies of ethanol actions on recombinant delta-containing gamma-aminobutyric acid type a receptors yield contradictory results. Alcohol. 2007;41:155–162. doi: 10.1016/j.alcohol.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Karim N, Wellendorph P, Absalom N, Bang LH, Jensen ML, Hansen MM, Lee HJ, Johnston GA, Hanrahan JR, Chebib M. Low nanomolar gaba effects at extrasynaptic alpha4beta1/beta3delta gaba(a) receptor subtypes indicate a different binding mode for gaba at these receptors. Biochemical pharmacology. 2012;84:549–557. doi: 10.1016/j.bcp.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 88.Shu HJ, Bracamontes J, Taylor A, Wu K, Eaton MM, Akk G, Manion B, Evers AS, Krishnan K, Covey DF, Zorumski CF, Steinbach JH, Mennerick S. Characteristics of concatemeric gaba(a) receptors containing alpha4/delta subunits expressed in xenopus oocytes. Br J Pharmacol. 2012;165:2228–2243. doi: 10.1111/j.1476-5381.2011.01690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meera P, Olsen RW, Otis TS, Wallner M. Etomidate, propofol and the neurosteroid thdoc increase the gaba efficacy of recombinant alpha4beta3delta and alpha4beta3 gaba a receptors expressed in hek cells. Neuropharmacology. 2009;56:155–160. doi: 10.1016/j.neuropharm.2008.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.You H, Dunn SM. Identification of a domain in the delta subunit (s238-v264) of the alpha4beta3delta gabaa receptor that confers high agonist sensitivity. J Neurochem. 2007;103:1092–1101. doi: 10.1111/j.1471-4159.2007.04817.x. [DOI] [PubMed] [Google Scholar]
- 91.Wagoner KR, Czajkowski C. Stoichiometry of expressed alpha(4)beta(2)delta gamma-aminobutyric acid type a receptors depends on the ratio of subunit cdna transfected. J Biol Chem. 2010;285:14187–14194. doi: 10.1074/jbc.M110.104257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hevers W, Luddens H. The diversity of gabaa receptors. Pharmacological and electrophysiological properties of gabaa channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- 93.Akk G, Steinbach JH. Structural studies of the actions of anesthetic drugs on the gamma-aminobutyric acid type a receptor. Anesthesiology. 2011;115:1338–1348. doi: 10.1097/ALN.0b013e3182315d93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Forman SA, Miller KW. Anesthetic sites and allosteric mechanisms of action on cys-loop ligand-gated ion channels. Canadian journal of anaesthesia = Journal canadien d’anesthesie. 2011;58:191–205. doi: 10.1007/s12630-010-9419-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Olsen RW, Li GD, Wallner M, Trudell JR, Bertaccini EJ, Lindahl E, Miller KW, Alkana RL, Davies DL. Structural models of ligand-gated ion channels: Sites of action for anesthetics and ethanol. Alcohol Clin Exp Res. 2014;38:595–603. doi: 10.1111/acer.12283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dai S, Perouansky M, Pearce RA. Amnestic concentrations of etomidate modulate gabaa, slow synaptic inhibition in hippocampus. Anesthesiology. 2009;111:766–773. doi: 10.1097/ALN.0b013e3181b4392d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fu B, Wang Y, Yang H, Yu T. Effects of etomidate on gabaergic and glutamatergic transmission in rat thalamocortical slices. Neurochem Res. 2016;41:3181–3191. doi: 10.1007/s11064-016-2042-6. [DOI] [PubMed] [Google Scholar]
- 98.McDougall SJ, Bailey TW, Mendelowitz D, Andresen MC. Propofol enhances both tonic and phasic inhibitory currents in second-order neurons of the solitary tract nucleus (nts) Neuropharmacology. 2008;54:552–563. doi: 10.1016/j.neuropharm.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Koyanagi Y, Oi Y, Yamamoto K, Koshikawa N, Kobayashi M. Fast-spiking cell to pyramidal cell connections are the most sensitive to propofol-induced facilitation of gabaergic currents in rat insular cortex. Anesthesiology. 2014;121:68–78. doi: 10.1097/ALN.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 100.Gage PW, Robertson B. Prolongation of inhibitory postsynaptic currents by pentobarbitone, halothane and ketamine in ca1 pyramidal cells in rat hippocampus. Br J Pharmacol. 1985;85:675–681. doi: 10.1111/j.1476-5381.1985.tb10563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shin MC, Wakita M, Iwata S, Nonaka K, Kotani N, Akaike N. Comparative effects of pentobarbital on spontaneous and evoked transmitter release from inhibitory and excitatory nerve terminals in rat ca3 neurons. Brain Res Bull. 2013;90:10–18. doi: 10.1016/j.brainresbull.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 102.Schwabe K, Gavrilovici C, McIntyre DC, Poulter MO. Neurosteroids exhibit differential effects on mipscs recorded from normal and seizure prone rats. J Neurophysiol. 2005;94:2171–2181. doi: 10.1152/jn.01233.2004. [DOI] [PubMed] [Google Scholar]
- 103.Harrison NL, Vicini S, Barker JL. A steroid anesthetic prolongs inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurosci. 1987;7:604–609. doi: 10.1523/JNEUROSCI.07-02-00604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ruesch D, Neumann E, Wulf H, Forman SA. An allosteric coagonist model for propofol effects on alpha1beta2gamma2l gamma-aminobutyric acid type a receptors. Anesthesiology. 2012;116:47–55. doi: 10.1097/ALN.0b013e31823d0c36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Savechenkov PY, Chiara DC, Desai R, Stern AT, Zhou X, Ziemba AM, Szabo AL, Zhang Y, Cohen JB, Forman SA, Miller KW, Bruzik KS. Synthesis and pharmacological evaluation of neurosteroid photoaffinity ligands. Eur J Med Chem. 2017;136:334–347. doi: 10.1016/j.ejmech.2017.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Fisher JL, Macdonald RL. Single channel properties of recombinant gabaa receptors containing gamma 2 or delta subtypes expressed with alpha 1 and beta 3 subtypes in mouse 1929 cells. J Physiol. 1997;505(Pt 2):283–297. doi: 10.1111/j.1469-7793.1997.283bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steinbach JH, Akk G. Modulation of gaba(a) receptor channel gating by pentobarbital. J Physiol. 2001;537:715–733. doi: 10.1111/j.1469-7793.2001.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Belelli D, Lambert JJ, Peters JA, Wafford K, Whiting PJ. The interaction of the general anesthetic etomidate with the gamma-aminobutyric acid type a receptor is influenced by a single amino acid. Proc Natl Acad Sci U S A. 1997;94:11031–11036. doi: 10.1073/pnas.94.20.11031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hill-Venning C, Belelli D, Peters JA, Lambert JJ. Subunit-dependent interaction of the general anaesthetic etomidate with the gamma-aminobutyric acid type a receptor. Br J Pharmacol. 1997;120:749–756. doi: 10.1038/sj.bjp.0700927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bai D, Zhu G, Pennefather P, Jackson MF, MacDonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by gamma-aminobutyric acid(a) receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- 111.Bieda MC, MacIver MB. Major role for tonic gabaa conductances in anesthetic suppression of intrinsic neuronal excitability. J Neurophysiol. 2004;92:1658–1667. doi: 10.1152/jn.00223.2004. [DOI] [PubMed] [Google Scholar]
- 112.Caraiscos VB, Newell JG, You-Ten KE, Elliott EM, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Selective enhancement of tonic gabaergic inhibition in murine hippocampal neurons by low concentrations of the volatile anesthetic isoflurane. J Neurosci. 2004;24:8454–8458. doi: 10.1523/JNEUROSCI.2063-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Takahashi A, Mashimo T, Uchida I. Gabaergic tonic inhibition of substantia gelatinosa neurons in mouse spinal cord. Neuroreport. 2006;17:1331–1335. doi: 10.1097/01.wnr.0000230515.86090.bc. [DOI] [PubMed] [Google Scholar]
- 114.Jia F, Yue M, Chandra D, Homanics GE, Goldstein PA, Harrison NL. Isoflurane is a potent modulator of extrasynaptic gaba(a) receptors in the thalamus. J Pharmacol Exp Ther. 2008;324:1127–1135. doi: 10.1124/jpet.107.134569. [DOI] [PubMed] [Google Scholar]
- 115.Wang X. Propofol and isoflurane enhancement of tonic gamma-aminobutyric acid type a current in cardiac vagal neurons in the nucleus ambiguus. Anesth Analg. 2009;108:142–148. doi: 10.1213/ane.0b013e31818d8b79. [DOI] [PubMed] [Google Scholar]
- 116.Herd MB, Lambert JJ, Belelli D. The general anaesthetic etomidate inhibits the excitability of mouse thalamocortical relay neurons by modulating multiple modes of gabaa receptor-mediated inhibition. Eur J Neurosci. 2014;40:2487–2501. doi: 10.1111/ejn.12601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ando N, Sugasawa Y, Inoue R, Aosaki T, Miura M, Nishimura K. Effects of the volatile anesthetic sevoflurane on tonic gaba currents in the mouse striatum during postnatal development. Eur J Neurosci. 2014;40:3147–3157. doi: 10.1111/ejn.12691. [DOI] [PubMed] [Google Scholar]
- 118.Haas KF, Macdonald RL. Gabaa receptor subunit gamma2 and delta subtypes confer unique kinetic properties on recombinant gabaa receptor currents in mouse fibroblasts. J Physiol. 1999;514(Pt 1):27–45. doi: 10.1111/j.1469-7793.1999.027af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Akk G, Bracamontes J, Steinbach JH. Activation of gaba(a) receptors containing the alpha4 subunit by gaba and pentobarbital. J Physiol. 2004;556:387–399. doi: 10.1113/jphysiol.2003.058230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yamashita M, Marszalec W, Yeh JZ, Narahashi T. Effects of ethanol on tonic gaba currents in cerebellar granule cells and mammalian cells recombinantly expressing gaba(a) receptors. J Pharmacol Exp Ther. 2006;319:431–438. doi: 10.1124/jpet.106.106260. [DOI] [PubMed] [Google Scholar]
- 121.Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroids and gabaa receptor function. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- 122.Mitchell EA, Herd MB, Gunn BG, Lambert JJ, Belelli D. Neurosteroid modulation of gabaa receptors: Molecular determinants and significance in health and disease. Neurochem Int. 2008;52:588–595. doi: 10.1016/j.neuint.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 123.Belelli D, Harrison NL, Maguire J, Macdonald RL, Walker MC, Cope DW. Extrasynaptic gabaa receptors: Form, pharmacology, and function. J Neurosci. 2009;29:12757–12763. doi: 10.1523/JNEUROSCI.3340-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Smith SS. Alpha4betadelta gabaa receptors and tonic inhibitory current during adolescence: Effects on mood and synaptic plasticity. Frontiers in neural circuits. 2013;7:135. doi: 10.3389/fncir.2013.00135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maguire JL, Stell BM, Rafizadeh M, Mody I. Ovarian cycle-linked changes in gaba(a) receptors mediating tonic inhibition alter seizure susceptibility and anxiety. Nat Neurosci. 2005;8:797–804. doi: 10.1038/nn1469. [DOI] [PubMed] [Google Scholar]
- 126.Sanna E, Mostallino MC, Murru L, Carta M, Talani G, Zucca S, Mura ML, Maciocco E, Biggio G. Changes in expression and function of extrasynaptic gabaa receptors in the rat hippocampus during pregnancy and after delivery. J Neurosci. 2009;29:1755–1765. doi: 10.1523/JNEUROSCI.3684-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Smith SS, Aoki C, Shen H. Puberty, steroids and gaba(a) receptor plasticity. Psychoneuroendocrinology. 2009;34(Suppl 1):S91–S103. doi: 10.1016/j.psyneuen.2009.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shen H, Sabaliauskas N, Sherpa A, Fenton AA, Stelzer A, Aoki C, Smith SS. A critical role for alpha4betadelta gabaa receptors in shaping learning deficits at puberty in mice. Science. 2010;327:1515–1518. doi: 10.1126/science.1184245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Carver CM, Wu X, Gangisetty O, Reddy DS. Perimenstrual-like hormonal regulation of extrasynaptic delta-containing gabaa receptors mediating tonic inhibition and neurosteroid sensitivity. J Neurosci. 2014;34:14181–14197. doi: 10.1523/JNEUROSCI.0596-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sabaliauskas N, Shen H, Molla J, Gong QH, Kuver A, Aoki C, Smith SS. Neurosteroid effects at alpha4betadelta gabaa receptors alter spatial learning and synaptic plasticity in ca1 hippocampus across the estrous cycle of the mouse. Brain Res. 2015;1621:170–186. doi: 10.1016/j.brainres.2014.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Choi DS, Wei W, Deitchman JK, Kharazia VN, Lesscher HM, McMahon T, Wang D, Qi ZH, Sieghart W, Zhang C, Shokat KM, Mody I, Messing RO. Protein kinase cdelta regulates ethanol intoxication and enhancement of gaba-stimulated tonic current. J Neurosci. 2008;28:11890–11899. doi: 10.1523/JNEUROSCI.3156-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Adams JM, Thomas P, Smart TG. Modulation of neurosteroid potentiation by protein kinases at synaptic- and extrasynaptic-type gabaa receptors. Neuropharmacology. 2015;88:63–73. doi: 10.1016/j.neuropharm.2014.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bohnsack JP, Carlson SL, Morrow AL. Differential regulation of synaptic and extrasynaptic alpha4 gaba(a) receptor populations by protein kinase a and protein kinase c in cultured cortical neurons. Neuropharmacology. 2016;105:124–132. doi: 10.1016/j.neuropharm.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chang Y, Weiss DS. Allosteric activation mechanism of the alpha 1 beta 2 gamma 2 gamma-aminobutyric acid type a receptor revealed by mutation of the conserved m2 leucine. Biophys J. 1999;77:2542–2551. doi: 10.1016/s0006-3495(99)77089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rusch D, Zhong H, Forman SA. Gating allosterism at a single class of etomidate sites on alpha1beta2gamma2l gaba a receptors accounts for both direct activation and agonist modulation. J Biol Chem. 2004;279:20982–20992. doi: 10.1074/jbc.M400472200. [DOI] [PubMed] [Google Scholar]
- 136.Stewart DS, Hotta M, Desai R, Forman SA. State-dependent etomidate occupancy of its allosteric agonist sites measured in a cysteine-substituted gabaa receptor. Mol Pharmacol. 2013;83:1200–1208. doi: 10.1124/mol.112.084558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Scheller M, Forman SA. Coupled and uncoupled gating and desensitization effects by pore domain mutations in gaba(a) receptors. J Neurosci. 2002;22:8411–8421. doi: 10.1523/JNEUROSCI.22-19-08411.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mihic SJ, Ye Q, Wick MJ, Koltchine VV, Krasowski MD, Finn SE, Mascia MP, Valenzuela CF, Hanson KK, Greenblatt EP, Harris RA, Harrison NL. Sites of alcohol and volatile anaesthetic action on gaba(a) and glycine receptors. Nature. 1997;389:385–389. doi: 10.1038/38738. [DOI] [PubMed] [Google Scholar]
- 139.Jenkins A, Greenblatt EP, Faulkner HJ, Bertaccini E, Light A, Lin A, Andreasen A, Viner A, Trudell JR, Harrison NL. Evidence for a common binding cavity for three general anesthetics within the gabaa receptor. J Neurosci. 2001;21:RC136. doi: 10.1523/JNEUROSCI.21-06-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Estrada-Mondragon A, Lynch JW. Functional characterization of ivermectin binding sites in alpha1beta2gamma2l gaba(a) receptors. Front Mol Neurosci. 2015;8:55. doi: 10.3389/fnmol.2015.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Maldifassi MC, Baur R, Sigel E. Functional sites involved in modulation of the gabaa receptor channel by the intravenous anesthetics propofol, etomidate and pentobarbital. Neuropharmacology. 2016;105:207–214. doi: 10.1016/j.neuropharm.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 142.Serafini R, Bracamontes J, Steinbach JH. Structural domains of the human gabaa receptor 3 subunit involved in the actions of pentobarbital. J Physiol. 2000;524(Pt 3):649–676. doi: 10.1111/j.1469-7793.2000.00649.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nourmahnad A, Stern AT, Hotta M, Stewart DS, Ziemba AM, Szabo A, Forman SA. Tryptophan and cysteine mutations in m1 helices of alpha1beta3gamma2l gamma-aminobutyric acid type a receptors indicate distinct intersubunit sites for four intravenous anesthetics and one orphan site. Anesthesiology. 2016;125:1144–1158. doi: 10.1097/ALN.0000000000001390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Chiara DC, Gill JF, Chen Q, Tillman T, Dailey WP, Eckenhoff RG, Xu Y, Tang P, Cohen JB. Photoaffinity labeling the propofol binding site in glic. Biochemistry. 2014;53:135–142. doi: 10.1021/bi401492k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Zhang X, Miller KW. Dodecyl maltopyranoside enabled purification of active human gaba type a receptors for deep and direct proteomic sequencing. Molecular & cellular proteomics : MCP. 2015;14:724–738. doi: 10.1074/mcp.M114.042556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bali M, Akabas MH. Defining the propofol binding site location on the gabaa receptor. Mol Pharmacol. 2004;65:68–76. doi: 10.1124/mol.65.1.68. [DOI] [PubMed] [Google Scholar]
- 147.Husain SS, Ziebell MR, Ruesch D, Hong F, Arevalo E, Kosterlitz JA, Olsen RW, Forman SA, Cohen JB, Miller KW. 2-(3-methyl-3h–diaziren-3-yl)ethyl 1-(1-phenylethyl)-1h–imidazole-5-carboxylate: A derivative of the stereoselective general anesthetic etomidate for photolabeling ligand-gated ion channels. J Med Chem. 2003;46:1257–1265. doi: 10.1021/jm020465v. [DOI] [PubMed] [Google Scholar]
- 148.Siegwart R, Jurd R, Rudolph U. Molecular determinants for the action of general anesthetics at recombinant alpha(2)beta(3)gamma(2)gamma-aminobutyric acid(a) receptors. J Neurochem. 2002;80:140–148. doi: 10.1046/j.0022-3042.2001.00682.x. [DOI] [PubMed] [Google Scholar]
- 149.Li GD, Chiara DC, Sawyer GW, Husain SS, Olsen RW, Cohen JB. Identification of a gabaa receptor anesthetic binding site at subunit interfaces by photolabeling with an etomidate analog. J Neurosci. 2006;26:11599–11605. doi: 10.1523/JNEUROSCI.3467-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Husain SS, Stewart D, Desai R, Hamouda AK, Li SG, Kelly E, Dostalova Z, Zhou X, Cotten JF, Raines DE, Olsen RW, Cohen JB, Forman SA, Miller KW. P-trifluoromethyldiazirinyl-etomidate: A potent photoreactive general anesthetic derivative of etomidate that is selective for ligand-gated cationic ion channels. J Med Chem. 2010;53:6432–6444. doi: 10.1021/jm100498u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Stewart D, Desai R, Cheng Q, Liu A, Forman SA. Tryptophan mutations at azi-etomidate photo-incorporation sites on alpha1 or beta2 subunits enhance gabaa receptor gating and reduce etomidate modulation. Mol Pharmacol. 2008;74:1687–1695. doi: 10.1124/mol.108.050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chiara DC, Dostalova Z, Jayakar SS, Zhou X, Miller KW, Cohen JB. Mapping general anesthetic binding site(s) in human alpha1beta3 gamma-aminobutyric acid type a receptors with [(3)h]tdbzl-etomidate, a photoreactive etomidate analogue. Biochemistry. 2012;51:836–847. doi: 10.1021/bi201772m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Cestari IN, Uchida I, Li L, Burt D, Yang J. The agonistic action of pentobarbital on gabaa beta-subunit homomeric receptors. Neuroreport. 1996;7:943–947. doi: 10.1097/00001756-199603220-00023. [DOI] [PubMed] [Google Scholar]
- 154.Stewart DS, Hotta M, Li GD, Desai R, Chiara DC, Olsen RW, Forman SA. Cysteine substitutions define etomidate binding and gating linkages in the alpha-m1 domain of gamma-aminobutyric acid type a (gabaa) receptors. J Biol Chem. 2013;288:30373–30386. doi: 10.1074/jbc.M113.494583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Savechenkov PY, Zhang X, Chiara DC, Stewart DS, Ge R, Zhou X, Raines DE, Cohen JB, Forman SA, Miller KW, Bruzik KS. Allyl m-trifluoromethyldiazirine mephobarbital: An unusually potent enantioselective and photoreactive barbiturate general anesthetic. J Med Chem. 2012;55:6554–6565. doi: 10.1021/jm300631e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Chiara DC, Jayakar SS, Zhou X, Zhang X, Savechenkov PY, Bruzik KS, Miller KW, Cohen JB. Specificity of intersubunit general anesthetic-binding sites in the transmembrane domain of the human alpha1beta3gamma2 gamma-aminobutyric acid type a (gabaa) receptor. J Biol Chem. 2013;288:19343–19357. doi: 10.1074/jbc.M113.479725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kamiya Y, Andoh T, Furuya R, Hattori S, Watanabe I, Sasaki T, Ito H, Okumura F. Comparison of the effects of convulsant and depressant barbiturate stereoisomers on ampa-type glutamate receptors. Anesthesiology. 1999;90:1704–1713. doi: 10.1097/00000542-199906000-00028. [DOI] [PubMed] [Google Scholar]
- 158.Desai R, Savechenkov PY, Zolkowska D, Ge RL, Rogawski MA, Bruzik KS, Forman SA, Raines DE, Miller KW. Contrasting actions of a convulsant barbiturate and its anticonvulsant enantiomer on the alpha1 beta3 gamma2l gabaa receptor account for their in vivo effects. J Physiol. 2015;593:4943–4961. doi: 10.1113/JP270971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jayakar SS, Zhou X, Savechenkov PY, Chiara DC, Desai R, Bruzik KS, Miller KW, Cohen JB. Positive and negative allosteric modulation of an alpha1beta3gamma2 gamma-aminobutyric acid type a (gabaa) receptor by binding to a site in the transmembrane domain at the gamma+-beta- interface. J Biol Chem. 2015;290:23432–23446. doi: 10.1074/jbc.M115.672006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Krasowski MD, Koltchine VV, Rick CE, Ye Q, Finn SE, Harrison NL. Propofol and other intravenous anesthetics have sites of action on the gamma-aminobutyric acid type a receptor distinct from that for isoflurane. Mol Pharmacol. 1998;53:530–538. doi: 10.1124/mol.53.3.530. [DOI] [PubMed] [Google Scholar]
- 161.Jayakar SS, Zhou X, Chiara DC, Dostalova Z, Savechenkov PY, Bruzik KS, Dailey WP, Miller KW, Eckenhoff RG, Cohen JB. Multiple propofol-binding sites in a gamma-aminobutyric acid type a receptor (gabaar) identified using a photoreactive propofol analog. J Biol Chem. 2014;289:27456–27468. doi: 10.1074/jbc.M114.581728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Yip GM, Chen ZW, Edge CJ, Smith EH, Dickinson R, Hohenester E, Townsend RR, Fuchs K, Sieghart W, Evers AS, Franks NP. A propofol binding site on mammalian gabaa receptors identified by photolabeling. Nature chemical biology. 2013;9:715–720. doi: 10.1038/nchembio.1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Stern AT, Forman SA. A cysteine substitution probes beta3h267 interactions with propofol and other potent anesthetics in alpha1beta3gamma2l gamma-aminobutyric acid type a receptors. Anesthesiology. 2016;124:89–100. doi: 10.1097/ALN.0000000000000934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Eaton MM, Cao LQ, Chen Z, Franks NP, Evers AS, Akk G. Mutational analysis of the putative high-affinity propofol binding site in human beta3 homomeric gabaa receptors. Mol Pharmacol. 2015;88:736–745. doi: 10.1124/mol.115.100347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Li P, Bracamontes JR, Manion BD, Mennerick S, Steinbach JH, Evers AS, Akk G. The neurosteroid 5beta-pregnan-3alpha-ol-20-one enhances actions of etomidate as a positive allosteric modulator of alpha1beta2gamma2l gabaa receptors. Br J Pharmacol. 2014;171:5446–5457. doi: 10.1111/bph.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Li GD, Chiara DC, Cohen JB, Olsen RW. Neurosteroids allosterically modulate binding of the anesthetic etomidate to gamma-aminobutyric acid type a receptors. J Biol Chem. 2009;284:11771–11775. doi: 10.1074/jbc.C900016200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen ZW, Manion B, Townsend RR, Reichert DE, Covey DF, Steinbach JH, Sieghart W, Fuchs K, Evers AS. Neurosteroid analog photolabeling of a site in the third transmembrane domain of the beta3 subunit of the gaba(a) receptor. Mol Pharmacol. 82:408–419. doi: 10.1124/mol.112.078410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Miller PS, Scott S, Masiulis S, De Colibus L, Pardon E, Steyaert J, Aricescu AR. Structural basis for gabaa receptor potentiation by neurosteroids. Nature structural & molecular biology. 2017;24:986–992. doi: 10.1038/nsmb.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Laverty D, Thomas P, Field M, Andersen OJ, Gold MG, Biggin PC, Gielen M, Smart TG. Crystal structures of a gabaa-receptor chimera reveal new endogenous neurosteroid-binding sites. Nature structural & molecular biology. 2017;24:977–985. doi: 10.1038/nsmb.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hosie AM, Wilkins ME, da Silva HM, Smart TG. Endogenous neurosteroids regulate gabaa receptors through two discrete transmembrane sites. Nature. 2006;444:486–489. doi: 10.1038/nature05324. [DOI] [PubMed] [Google Scholar]
- 171.Akk G, Li P, Bracamontes J, Reichert DE, Covey DF, Steinbach JH. Mutations of the gaba-a receptor alpha1 subunit m1 domain reveal unexpected complexity for modulation by neuroactive steroids. Mol Pharmacol. 2008;74:614–627. doi: 10.1124/mol.108.048520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hosie AM, Clarke L, da Silva H, Smart TG. Conserved site for neurosteroid modulation of gaba a receptors. Neuropharmacology. 2009;56:149–154. doi: 10.1016/j.neuropharm.2008.07.050. [DOI] [PubMed] [Google Scholar]
- 173.Bracamontes JR, Li P, Akk G, Steinbach JH. A neurosteroid potentiation site can be moved among gabaa receptor subunits. J Physiol. 2012;590:5739–5747. doi: 10.1113/jphysiol.2012.237255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ziemba AM, Szabo A, Pierce DW, Haburcak M, Stern AT, Nourmahnad A, Halpin ES, Forman SA. Alphaxalone binds in inner transmembrane beta+-alpha- interfaces of alpha1beta3gamma2 gamma-aminobutyric acid type a receptors. Anesthesiology. 2017 doi: 10.1097/ALN.0000000000001978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ahring PK, Bang LH, Jensen ML, Strobaek D, Hartiadi LY, Chebib M, Absalom N. A pharmacological assessment of agonists and modulators at alpha4beta2gamma2 and alpha4beta2delta gabaa receptors: The challenge in comparing apples with oranges. Pharmacol Res. 2016;111:563–576. doi: 10.1016/j.phrs.2016.05.014. [DOI] [PubMed] [Google Scholar]
- 176.Barrera NP, Betts J, You H, Henderson RM, Martin IL, Dunn SM, Edwardson JM. Atomic force microscopy reveals the stoichiometry and subunit arrangement of the alpha4beta3delta gaba(a) receptor. Mol Pharmacol. 2008;73:960–967. doi: 10.1124/mol.107.042481. [DOI] [PubMed] [Google Scholar]
- 177.Li P, Akk G. Synaptic-type alpha1beta2gamma2l gabaa receptors produce large persistent currents in the presence of ambient gaba and anesthetic drugs. Mol Pharmacol. 2015;87:776–781. doi: 10.1124/mol.114.096453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Chiara DC, Jounaidi Y, Zhou X, Savechenkov PY, Bruzik KS, Miller KW, Cohen JB. General anesthetic binding sites in human alpha4beta3delta gamma-aminobutyric acid type a receptors (gabaars) J Biol Chem. 2016;291:26529–26539. doi: 10.1074/jbc.M116.753335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Roskoski R., Jr Guidelines for preparing color figures for everyone including the colorblind. Pharmacol Res. 2017;119:240–241. doi: 10.1016/j.phrs.2017.02.005. [DOI] [PubMed] [Google Scholar]