Summary
The gastrointestinal mucosae provide a critical barrier between the external and internal milieu. Thus, damage to the mucosa requires an immediate response to provide appropriate wound closure and healing. Metaplastic lineages with phenotypes similar to the mucous glands of the distal stomach or Brunner’s glands have been associated with various injurious scenarios in the stomach, small bowel and colon. These lineages have been assigned various names including pyloric metaplasia, pseudopyloric metaplasia, Ulcer-associated cell lineage (UACL) and spasmolytic polypeptide-expressing metaplasia (SPEM). A re-examination of the literature on these various forms of mucous cell metaplasia suggests that pyloric type mucosal gland lineages may provide a ubiquitous response to mucosal injury throughout the gastrointestinal tract as well as in the pancreas, esophagus and other mucosal surfaces. While the cellular origin of these putative reparative lineages likely varies in different regions of the gut, their final phenotypes may converge on a pyloric type gland dedicated to mucous secretion. In addition to their healing properties in the setting of acute injury, these pyloric type lineages may also represent precursors to neoplastic transitions in the face of chronic inflammatory influences. Further investigations are needed to determine how discrete molecular profiles relate to the origin and function of pyloric-type metaplasias previously described by histological characteristics in multiple epithelial mucosal systems in the setting of acute and chronic damage.
The gastrointestinal mucosal lining must withstand many luminal insults, including low pH in the stomach, luminal trauma, caustic contents and physical damage, in order to preserve the epithelial barrier separating the external and internal milieu. Given the components of the diet and the microbiome, this reparative process must be an ongoing function throughout gastrointestinal tract. Furthermore, pathological influences including viral and bacterial pathogens as well as alterations in mucosal immune tolerance, e.g. Crohn’s Disease, can lead to compromise of mucosal defenses requiring more active reparative responses to ulcerating influences. At the heart of many of these local responses to injury is the induction of reparative metaplastic lineages. These metaplastic lineages often take on the characteristics of mucin-secreting lineages of the distal stomach: antral/pyloric glands and Brunner’s glands (Figure 1). Here we examine the association of pyloric gland phenotype metaplasias with a broad spectrum of gastrointestinal mucosal injury scenarios. Central to this discussion is a need to re-examine over a century of histological observations by pathologists in light of more recent molecular insights that can provide greater detail for the characterization of reparative metaplastic lineages (Table 1).
Figure 1.
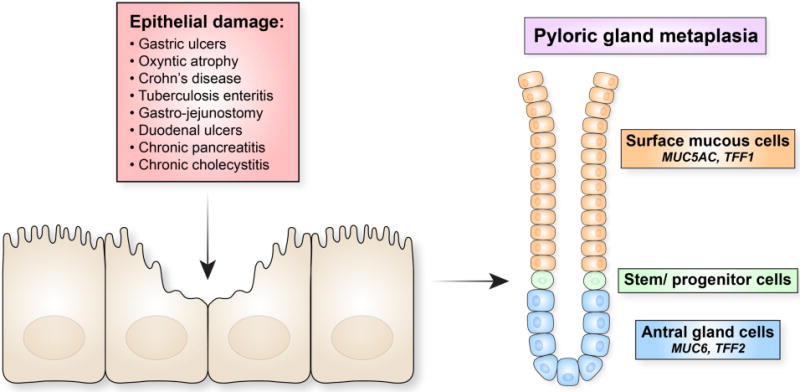
A generalized pattern for the induction of pyloric phenotype reparative metaplasias. Injury to the lining of gastrointestinal mucosae by various acute or chronic maladies leads to the evolution of metaplastic glands which recapitulate the structure of mucous-secreting glands of the distal stomach with TFF2/Muc6-expressing mucous cells at the bases and TFF1/Muc5AC-expressing cells towards the lumen. These reparative glands can be identified histologically and immunohistologically by various names, but all share a common pyloric gland phenotype.
Table 1.
Characteristics used to define metaplastic glands in the gastrointestinal tract.
| Metaplastic Lesion | Gut Region | Mode of Identification |
|---|---|---|
| Pseudopyloric metaplasia | Stomach Corpus | H&E, PAS, Immunostaining: Pepsinogen I |
| SPEM | Stomach Corpus | PAS, Immunostaining: TFF2, GSII lectin, Muc6, CD44v9, WFDC2 |
| Pyloric metaplasia | Duodenum | H&E, PAS |
| Ileum and colon | H&E, PAS | |
| UACL | Ileum and colon | H&E, PAS, Immunostaining: TFF1, TFF2, Muc5AC, Muc6 |
Pyloric metaplasia in the intestine
The presence of gastric pyloric type glands associated with injury in the small bowel has been noted by pathologists dating back to the nineteenth century. Early in the twentieth century, pathologists, physicians and surgeons identified “gastric metaplasia” or “pyloric metaplasia” associated with a number of chronic intestinal damage scenarios including regional enteritis (Crohn’s disease), tuberculosis enteritis, and following gastro-jejunostomy [1–3]. Liber [2] noted the association of pyloric gland metaplasia in the ileum of over half of patients with regional enteritis. Similar results in Crohn’s Disease patients were observed by Yokoyama [3]. In some cases of Crohn’s Disease a few glands with oxyntic gastric glands could also be observed within a field of pyloric metaplasia [3,4]. Pyloric gland metaplasia also has a strong association with Crohn’s Disease in patients with refractory pouchitis and correlates with attendant eosinophilic inflammatory infiltrates [5]. Interestingly, while pyloric metaplasia has been noted in Crohn’s colitis, it appears to be extremely rare in ulcerative colitis [3]. While these studies have established the connection of pyloric gland metaplasias with Crohn’s Disease, they do not address whether these metaplastic lineages directly contribute to resolution of mucosal injury.
Pyloric gland or gastric metaplasia, with characteristic diastase PAS-positive mucin staining [6], has long been recognized in association with duodenal ulcers [7]. Duodenal ulcers are often surrounded by gastric type epithelia with distinct microvillar patterns similar to the antrum [8]. The presence of pyloric gland metaplasia adjacent to duodenal ulcers is related to both H. pylori infection and acid production [9]. Pyloric metaplasia is often maintained in the duodenum after ulcer healing [10]. All of these studies based on histological examinations suggested that pyloric metaplasia represented a healing lineage in the ulcerated duodenal mucosa.
Ulcer-associated cell lineage (UACL)
In 1990, Sir Nicholas Wright first described the presence of a novel mucous cell lineage in the context of ulceration associated with Crohn’s Disease [11,12]. This lineage was associated with production of EGF and demonstrated expression of TFF2 in cells at the bases of glands with TFF1 expressed in mucous cells towards the lumen [13–16]. All of these characteristics are consistent with pyloric metaplasia associated with inflammatory bowel disease. More recently, Kaneko, et al. [17] reported that UACL is Pdx1-positive, again providing a molecular description of pyloric metaplasia. Furthermore, UACL glands also contain scattered TFF3-positive goblet cells [18]. Interestingly, UACL is observed in Crohn’s colitis, but not in the context of ulcerative colitis. Thus, overall, UACL serves as a more molecular description of pyloric metaplasia in the small intestine. It should also be noted that intrinsic goblet cells in the small and large intestine may also show alterations in response to ulcerative colitis or Crohn’s disease. Thus, colonic goblet cells in IBD patients show upregulation of TFF1 [19], but this phenomenon appears distinct from UACL.
Pseudopyloric metaplasia in the stomach
The term of pseudopyloric metaplasia has been utilized to described aberrant observation of antral type glands in both the body of stomach and in the intestine. The latter association appears to be synonymous with “pyloric metaplasia” in the intestines. The description of pseudopyloric metaplasia dates back at least to the beginning of the twentieth century, when it was associated with the healing of ulcers in the gastric corpus [20]. Bonne, et al. [21] described pseudopyloric glands as commonly associated with severe erosions in the body of the stomach in Chinese and Malay patients. Hebbel [22] described pseudopyloric glands in approximately 20% of patients with gastritis and also noted the presence of pseudopyloric glands adjacent to healing gastric ulcers. Magnus [23] also noted that gastric ulcerations were often completely surrounded by pyloric-type glands.
In the stomach, metaplastic lineages are most often associated with oxyntic atrophy or parietal cell loss, usually related to chronic infection with Helicobacter pylori [24]. Some authors have considered regions of pseudopyloric or pyloric metaplasia as extensions of the pyloric mucosa, often along the greater curvature into the oxyntic region [25,26]. This pattern of extension of pseudopyloric metaplasia from the distal stomach across the incisura has been described as “antralisation” and is also associated with aging [27]. These regions of atrophy and pseudopyloric metaplasia may in turn be associated with multi-focal intestinal metaplasia [26]. This type of pattern is consistent with the concept of field lineage changes or field cancerization, where metaplastic lineages are associated with an altered milieu that can pre-dispose to the development of neoplasia.
Spasmolytic polypeptide-expressing metaplasia (SPEM)
In 1999, Schmidt, et al. reported the presence of a metaplastic lineage adjacent to gastric cancers in the body of the stomach that expressed Trefoil Factor 2 (TFF2) which was then designated as spasmolytic polypeptide [28]. Spasmolytic polypeptide-expressing metaplasia (SPEM) was identified more than goblet cell-containing intestinal metaplasia in an initial cohort from the United States [28] and in association with early gastric cancers in Iceland [29]. This lineage showed many of the characteristics of deep antral gland cells or Brunner’s gland cells. Since its initial description, the SPEM lineage has been further-characterized as a diastase PAS-positive lineage that expresses TFF2 and Muc6 as well GSII-lectin binding, similar to the mucinous secreting cells of the deep antral gland [30]. This lineage has also expresses a number of other antral lineage markers including CD44 variant 9 (CD44v9) and clusterin [31–33]. One marker that is present uniquely in SPEM, but not in antrum, is the protease inhibitor HE4 (WFDC2) [34,35]. All of these studies show a strong similarity of SPEM lineages to those of deep antral glands. Indeed, since glands with SPEM lineages at their bases often show foveolar lineages expressing TFF1/Muc5AC luminal to SPEM, these reparative glands recapitulate the antral gland phenotype [36,37]. Thus, SPEM appears to represent a more molecular representation of the histological description of pseudopyloric or pyloric metaplasia.
While SPEM and pseudopyloric metaplasia can be identified by pink rather carmine diastase-resistant-PAS histological staining [38], one cannot determine the molecular nature of the lineage as reparative versus pre-neoplastic based on histological staining alone. SPEM arises in the setting of acute injury or ulceration [32,39–41]. The observation of SPEM emerging rapidly after acute parietal cell loss induced by DMP777, L635 or high dose tamoxifen is consistent with this lineage performing a role in the response to injury [41–43]. SPEM appears to resolve following resolution of injury and can often appear as a highly localized phenomenon [29,41,43]. Thus, acute ulceration injury in the stomach is associated with the appearance of SPEM around ulcers and SPEM contributes to the healing of these lesions [32,39,44]. As was noted decades ago for healing duodenal ulcers, local areas of gastric injury lead to the evolution of a pyloric type metaplasia, SPEM, that resolves after healing. Nevertheless, recent studies have shown that SPEM acquires more intestinalized phenotypes in the context of inflammatory influences, specifically alternatively activated macrophages [33,45,46]. Furthermore, increasing evidence indicates that SPEM likely represents an initial metaplastic response to gastric injury, while chronic injury and inflammation may lead to the further evolution of goblet-cell intestinal metaplasia from SPEM [30,47]. Thus, while pyloric type metaplasias in the stomach are initially reparative, their maintenance in the setting of chronic inflammation can lead to deleterious neoplastic scenarios.
Identification of pyloric metaplasia in other glandular mucosal tissues
Pyloric metaplastic lineages are not limited to the bowel lumen. Lineages with characteristics of pyloric glands (TFF2 and Muc6-expressing cells) are also observed in ductular metaplasias in the pancreas in the setting of chronic pancreatitis [11,48]. These metaplastic mucous cell lineages underlie the development of PanIN lesions in the pancreas. Pyloric metaplasia and intestinal metaplasia have also been noted in association with chronic cholecystitis and implicated in metaplasia to neoplasia progression similar to that in gastric carcinogenesis [49–51]. Gastric and intestinal metaplasia lineages are pathognomonic of Barrett’s epithelium and the organization of the metaplastic glands resembles the structure of pyloric glands [52]. Still Barrett’s epithelium metaplastic lineages arise in the setting of injured squamous epithelia, so their origin has been controversial [53]. It should also be noted that this pattern of reparative mucous cell metaplasia may be a more general phenomenon of columnar lined epithelia as pyloric and intestinal metaplasias have been observed in chronic injury within the female genital tract [54]. Similarly, mucinous adenocarcinomas in the lung have also been associated with pyloric metaplasia [55].
Lineage origins of pyloric-type reparative mucous cells
The origin of reparative mucous cell lineages has been the focus of a number of important investigations over the past several years. SPEM appears to arise in the setting of parietal cell loss or acute corpus mucosal damage, primarily through transdifferentiation of chief cells into mucous cell metaplasia [42,47,56]. While one group has suggested that SPEM arises from isthmal progenitor cells in the corpus [57], the vast majority of evidence from multiple groups indicates that chief cells are indeed the predominant origin of SPEM lineages in the stomach corpus.[42,47,56,58–60] Importantly, expression of pepsinogen I has been used as a marker of pseudopyloric metaplasia [61], consistent with the equivalence of pseudopyloric metaplasia with SPEM. Similarly, ductal metaplasia/PanIN lesions in the pancreas appear to evolve from reprogramming of zymogen-secreting pancreatic acinar cells [62]. These studies in stomach and pancreas have led to the suggestion that zymogen secreting cell lineages may serve as a general source of reparative mucous-secreting lineages. Far less is known about the possible origin of UACL/pyloric metaplasia in the small intestine. Still, the initial descriptions of UACL noted the origin of neo-glandular units arising from the bases of crypts [63]. This observation might implicate Paneth cells as an origin for the formation of UACL, but this requires further investigation. Of note, loss of Cdx2 in the intestines of mice leads to the development of a pyloric type metaplasia expressing antral mucins, TFF1 and TFF2 [64,65]. Thus, loss of the expression of the Cdx2 master-regulator transcription factor in stem cells may also account for colonic lineage reprogramming towards an antral mucous gland phenotype. Overall, these observations link induction of metaplastic lineages from the bases of glands and crypts in the gastrointestinal mucosa in response to significant injuries that may reach deep to the bases of the mucosa. More superficial injuries may not elicit metaplasia and are repaired by coverage and then expansion by surface mucous cells.
Conclusion
Acute and chronic injury to the mucosae of the gastrointestinal mucosa leads to the emergence of metaplastic lineages with similar phenotypes that recapitulate mucous-secreting glands of the gastric antrum (Figure 1). Pathological and molecular descriptions have given these metaplastic glands various names, including pyloric metaplasia, pseudopyloric metaplasia, SPEM and UACL. All of these reparative metaplasias provide protective mucins and growth factors that likely promote epithelial restitution. Although the cellular origins of these metaplastic lineages may vary, they all contribute to the resolution of acute and chronic damage to the lining of the gastrointestinal tract and other epithelia-lined organs.
Acknowledgments
J.R.G. is supported by grants from a Department of Veterans Affairs Merit Review Award (I01BX000930), NIH RO1 DK071590 and a grant from the Department of Defense (CA160479). I would like to thank David Graham, Massimo Rugge and Kentaro Sugano for critical discussions on this topic and Laura Otis and Volker Hess for location of historical material. I thank Eunyoung Choi and Anne Meyer for review of this manuscript and the artwork.
Footnotes
The author has no conflicts of interest.
References
- 1.Lee FD. Pyloric Metaplasia in the Small Intestine. J Pathol Bacteriol. 1964;87:267–277. doi: 10.1002/path.1700870207. [DOI] [PubMed] [Google Scholar]
- 2.Liber AF. Aberrant pyloric glands in regional ileitis. AMA Arch Pathol. 1951;51:205–212. [PubMed] [Google Scholar]
- 3.Yokoyama I, Kozuka S, Ito K, et al. Gastric gland metaplasia in the small and large intestine. Gut. 1977;18:214–218. doi: 10.1136/gut.18.3.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ming S-C, Simon M, Tandon BN. Gross Gastric Metaplasia of Ileum After Regional Enteritis. Gastroenterology. 1963;44:63–68. [Google Scholar]
- 5.Kariv R, Plesec TP, Gaffney K, et al. Pyloric gland metaplasia and pouchitis in patients with ileal pouch-anal anastomoses. Aliment Pharmacol Ther. 2010;31:862–873. doi: 10.1111/j.1365-2036.2010.04249.x. [DOI] [PubMed] [Google Scholar]
- 6.Morrissey SM, Ward PM, Jayaraj AP, et al. Histochemical changes in mucus in duodenal ulceration. Gut. 1983;24:909–913. doi: 10.1136/gut.24.10.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blomquist HE. Location of metaplasia in relation to duodenal and gastric ulcer. Ann Med Intern Fenn. 1954;43:241–248. [PubMed] [Google Scholar]
- 8.Steer HW. Surface morphology of the gastroduodenal mucosa in duodenal ulceration. Gut. 1984;25:1203–1210. doi: 10.1136/gut.25.11.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khulusi S, Badve S, Patel P, et al. Pathogenesis of gastric metaplasia of the human duodenum: role of Helicobacter pylori, gastric acid, and ulceration. Gastroenterology. 1996;110:452–458. doi: 10.1053/gast.1996.v110.pm8566592. [DOI] [PubMed] [Google Scholar]
- 10.Chang CC, Pan S, Lien GS, et al. Investigation of the extent of gastric metaplasia in the duodenal bulb by using methylene blue staining. J Gastroenterol Hepatol. 2001;16:729–733. doi: 10.1046/j.1440-1746.2001.02521.x. [DOI] [PubMed] [Google Scholar]
- 11.Wright NA, Poulsom R, Stamp GW, et al. Epidermal growth factor (EGF/URO) induces expression of regulatory peptides in damaged human gastrointestinal tissues. JPathol. 1990;162:279–284. doi: 10.1002/path.1711620402. [DOI] [PubMed] [Google Scholar]
- 12.Wright NA, Pike CM, Elia G. Ulceration induces a novel epidermal growth factor-secreting cell lineage in human gastrointestinal mucosa. Digestion. 1990;46(Suppl 2):125–133. doi: 10.1159/000200375. [DOI] [PubMed] [Google Scholar]
- 13.Wright NA, Poulsom R, Stamp G, et al. Trefoil peptide gene expression in gastrointestinal epithelial cells in inflammatory bowel disease. Gastroenterology. 1993;104:12–20. doi: 10.1016/0016-5085(93)90830-6. [DOI] [PubMed] [Google Scholar]
- 14.Poulsom R, Chinery R, Sarraf C, et al. Trefoil peptide gene expression in small intestinal Crohn’s disease and dietary adaptation. J Clin Gastroenterol. 1993;17(Suppl 1):S78–91. doi: 10.1097/00004836-199312001-00016. [DOI] [PubMed] [Google Scholar]
- 15.Hanby AM, Poulsom R, Elia G, et al. The expression of the trefoil peptides pS2 and human spasmolytic polypeptide (hSP) in gastric metaplasia ofthe proximal duodenum: implications for the nature of gastric metaplasia. JPathol. 1993;169:355–360. doi: 10.1002/path.1711690313. [DOI] [PubMed] [Google Scholar]
- 16.Roberts IS, Stoddart RW. Ulcer-associated cell lineage (‘pyloric metaplasia’) in Crohn’s disease: a lectin histochemical study. J Pathol. 1993;171:13–19. doi: 10.1002/path.1711710105. [DOI] [PubMed] [Google Scholar]
- 17.Kaneko Y, Nakamura T, Hayama M, et al. Altered expression of CDX-2, PDX-1 and mucin core proteins in “Ulcer-associated cell lineage (UACL)” in Crohn’s disease. Journal of molecular histology. 2008;39:161–168. doi: 10.1007/s10735-007-9149-7. [DOI] [PubMed] [Google Scholar]
- 18.Hauser F, Poulsom R, Chinery R, et al. hP1.B, a human P-domain peptide homologous with rat intestinal trefoil factor, is expressed also in the ulcer-associated cell lineage and the uterus. Proc Natl Acad Sci, USA. 1993;99:6961–6965. doi: 10.1073/pnas.90.15.6961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madsen J, Sorensen GL, Nielsen O, et al. A variant form of the human deleted in malignant brain tumor 1 (DMBT1) gene shows increased expression in inflammatory bowel diseases and interacts with dimeric trefoil factor 3 (TFF3) PLoS ONE. 2013;8:e64441. doi: 10.1371/journal.pone.0064441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalima T. Pathologisch-anatomische Studien iiber die Gastritis des Ulcusmagens nebst einigen Bemerkungen zur Pathogenese und pathologischen Anatomie des Magengeschwiirs. Arch Klin Chir. 1924;128:20–108. [Google Scholar]
- 21.Bonne C, Hartz PH, Klerks JV, et al. Morphology of the Stomach and Gastric Secretion in Malays and Chinese and the Different Incidence of Gastric Ulcer and Cancer in These Races. Am J Cancer. 1938;33:265–279. [Google Scholar]
- 22.Hebbel R. The topography of chronic gastritis in otherwise normal stomachs. Am J Pathol. 1949;25:125–141. [PMC free article] [PubMed] [Google Scholar]
- 23.Magnus HA. The pathology of peptic ulceration. Postgrad Med J. 1954;30:131–136. doi: 10.1136/pgmj.30.341.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham DY. History of Helicobacter pylori, duodenal ulcer, gastric ulcer and gastric cancer. World J Gastroenterol. 2014;20:5191–5204. doi: 10.3748/wjg.v20.i18.5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whitehead R, Truelove SC, Gear MWL. The histological diagnosis of chronic gastritis in fibreoptic gastroscope biopsy specimens. J Clin Pathol. 1972;25:1–11. doi: 10.1136/jcp.25.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Graham DY, Kato M, Asaka M. Gastric endoscopy in the 21st century: appropriate use of an invasive procedure in the era of non-invasive testing. Dig Liver Dis. 2008;40:497–503. doi: 10.1016/j.dld.2008.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xia HH, Yang Y, Lam SK, et al. Aberrant epithelial expression of trefoil family factor 2 and mucin 6 in Helicobacter pylori infected gastric antrum, incisura, and body and its association with antralisation. J Clin Pathol. 2004;57:861–866. doi: 10.1136/jcp.2003.015487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt PH, Lee JR, Joshi V, et al. Identification of a metaplastic cell lineage associated with human gastric adenocarcinoma. Lab Invest. 1999;79:639–646. [PubMed] [Google Scholar]
- 29.Halldorsdottir AM, Sigurdardottrir M, Jonasson JG, et al. Spasmolytic polypeptide-expressing metaplasia (SPEM) associated with gastric cancer in Iceland. Dig Dis Sci. 2003;48:431–441. doi: 10.1023/a:1022564027468. [DOI] [PubMed] [Google Scholar]
- 30.Lennerz JKM, Kim S, Oates EL, et al. The transcription factor MIST1 is a novel human gastric chief cell marker whose expression is lost in metaplasia, dysplasia and carcinoma. Amer J Pathol. 2010;177:1514–1533. doi: 10.2353/ajpath.2010.100328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wada T, Ishimoto T, Seishima R, et al. Functional role of CD44v-xCT system in the development of spasmolytic polypeptide-expressing metaplasia. Cancer science. 2013;104:1323–1329. doi: 10.1111/cas.12236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertaux-Skeirik N, Wunderlich M, Teal E, et al. CD44 variant isoform 9 emerges in response to injury and contributes to the regeneration of the gastric epithelium. J Pathol. 2017;242:463–475. doi: 10.1002/path.4918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen CP, Weis VG, Nam KT, et al. Macrophages Promote Progression of Spasmolytic Polypeptide-Expressing Metaplasia Following Acute Loss of Parietal Cells. Gastroenterology. 2014;146:1727–1738. doi: 10.1053/j.gastro.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nozaki K, Ogawa M, Williams JA, et al. A molecular signature of gastric metaplasia arising in response to acute parietal cell loss. Gastroenterology. 2008:511–521. doi: 10.1053/j.gastro.2007.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Neal RL, Nam KT, LaFleur BJ, et al. Human epididymis protein 4 is up-regulated in gastric and pancreatic adenocarcinomas. Human pathology. 2013;44:734–742. doi: 10.1016/j.humpath.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nomura S, Baxter S, Yamaguchi T, et al. Spasmolytic polypeptide expressing metaplasia (SPEM) to pre-neoplasia in H. felis-infected mice. Gastroenterology. 2004;127:582–594. doi: 10.1053/j.gastro.2004.05.029. [DOI] [PubMed] [Google Scholar]
- 37.Nomura S, Yamaguchi H, Wang TC, et al. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild type and gastrin deficient mice. AmerJPhysiol. 2004;288:G362–G375. doi: 10.1152/ajpgi.00160.2004. [DOI] [PubMed] [Google Scholar]
- 38.Goldenring JR, Nomura S. Differentiation of the gastric mucosa III. Animal models of oxyntic atrophy and metaplasia. Am J Physiol Gastrointest Liver Physiol. 2006;291:G999–1004. doi: 10.1152/ajpgi.00187.2006. [DOI] [PubMed] [Google Scholar]
- 39.Engevik AC, Feng R, Choi E, et al. The Development of Spasmolytic Polypeptide/TFF2-Expressing Metaplasia (SPEM) During Gastric Repair Is Absent in the Aged Stomach. Cell Molec Gastroent Hepatol. 2016;2:605–624. doi: 10.1016/j.jcmgh.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Goldenring JR, Ray GS, Coffey RJ, et al. Reversible drug-induced oxyntic atrophy in rats. Gastroenterology. 2000;118:1080–1093. doi: 10.1016/s0016-5085(00)70361-1. [DOI] [PubMed] [Google Scholar]
- 41.Nomura S, Yamaguchi H, Ogawa M, et al. Alterations in gastric mucosal lineages induced by acute oxyntic atrophy in wild-type and gastrin-deficient mice. Am J Physiol Gastrointest Liver Physiol. 2005;288:G362–375. doi: 10.1152/ajpgi.00160.2004. [DOI] [PubMed] [Google Scholar]
- 42.Nam KT, Lee H-J, Sousa JF, et al. Mature chief cells are cryptic progenitors for metaplasia in the stomach. Gastroenterology. 2010;139:2028–2037. doi: 10.1053/j.gastro.2010.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Huh WJ, Khurana SS, Geahlen JH, et al. Tamoxifen induces rapid, reversible atrophy, and metaplasia in mouse stomach. Gastroenterology. 2012;142:21–24.e27. doi: 10.1053/j.gastro.2011.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aihara E, Matthis AL, Karns RA, et al. Epithelial Regeneration After Gastric Ulceration Causes Prolonged Cell Type Alterations. Cell Molec Gastroent Hepatol. 2016;2:625–647. doi: 10.1016/j.jcmgh.2016.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weis VG, Sousa JF, LaFleur BJ, et al. Heterogeneity in mouse SPEM lineages identifies markers of metaplastic progression. Gut. 2013;62:1270–1279. doi: 10.1136/gutjnl-2012-302401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petersen CP, Meyer AR, De Salvo C, et al. A signalling cascade of IL-33 to IL-13 regulates metaplasia in the mouse stomach. Gut. 2017 doi: 10.1136/gutjnl-2016-312779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choi E, Hendley AM, Bailey JM, et al. Expression of Activated Ras in Gastric Chief Cells of Mice Leads to the Full Spectrum of Metaplastic Lineage Transitions. Gastroenterology. 2016;150:918–930. doi: 10.1053/j.gastro.2015.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamaguchi J, Mino-Kenudson M, Liss AS, et al. Loss of Trefoil Factor 2 From Pancreatic Duct Glands Promotes Formation of Intraductal Papillary Mucinous Neoplasms in Mice. Gastroenterology. 2016;151:1232–1244 e1210. doi: 10.1053/j.gastro.2016.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meirelles-Costa AL, Bresciani CJ, Perez RO, et al. Are histological alterations observed in the gallbladder precancerous lesions? Clinics (Sao Paulo) 2010;65:143–150. doi: 10.1590/S1807-59322010000200005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis JT, Talwalkar JA, Rosen CB, et al. Prevalence and risk factors for gallbladder neoplasia in patients with primary sclerosing cholangitis: evidence for a metaplasia-dysplasia-carcinoma sequence. Am J Surg Pathol. 2007;31:907–913. doi: 10.1097/01.pas.0000213435.99492.8a. [DOI] [PubMed] [Google Scholar]
- 51.Esendagli G, Akarca FG, Balci S, et al. A Retrospective Evaluation of the Epithelial Changes/Lesions and Neoplasms of the Gallbladder in Turkey and a Review of the Existing Sampling Methods: A Multicentre Study. Turk Patoloji Derg. 2018;34:41–48. doi: 10.5146/tjpath.2017.01404. [DOI] [PubMed] [Google Scholar]
- 52.Lavery DL, Nicholson AM, Poulsom R, et al. The stem cell organisation, and the proliferative and gene expression profile of Barrett’s epithelium, replicates pyloric-type gastric glands. Gut. 2014;6:1854–1863. doi: 10.1136/gutjnl-2013-306508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xian W, Ho KY, Crum CP, et al. Cellular origin of Barrett’s esophagus: controversy and therapeutic implications. Gastroenterology. 2012;142:1424–1430. doi: 10.1053/j.gastro.2012.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nicolae A, Goyenaga P, McCluggage WG, et al. Endometrial intestinal metaplasia: a report of two cases, including one associated with cervical intestinal and pyloric metaplasia. Int J Gynecol Pathol. 2011;30:492–496. doi: 10.1097/PGP.0b013e318211d586. [DOI] [PubMed] [Google Scholar]
- 55.Ota H, Langston C, Honda T, et al. Histochemical analysis of mucous cells of congenital adenomatoid malformation of the lung: insights into the carcinogenesis of pulmonary adenocarcinoma expressing gastric mucins. Am J Clin Pathol. 1998;110:450–455. doi: 10.1093/ajcp/110.4.450. [DOI] [PubMed] [Google Scholar]
- 56.Leushacke M, Tan SH, Wong A, et al. Lgr5-expressing chief cells drive epithelial regeneration and cancer in the oxyntic stomach. Nat Cell Biol. 2017;19:774–786. doi: 10.1038/ncb3541. [DOI] [PubMed] [Google Scholar]
- 57.Hayakawa Y, Ariyama H, Stancikova J, et al. Mist1 Expressing Gastric Stem Cells Maintain the Normal and Neoplastic Gastric Epithelium and Are Supported by a Perivascular Stem Cell Niche. Cancer Cell. 2015;28:800–814. doi: 10.1016/j.ccell.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Willet SG, Lewis MA, Miao ZF, et al. Regenerative proliferation of differentiated cells by mTORC1-dependent paligenosis. EMBO J. 2018 doi: 10.15252/embj.201798311. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Radyk MD, Burclaff J, Willet SG, et al. Metaplastic Cells in the Stomach Arise, Independently of Stem Cells, via Dedifferentiation or Transdifferentiation of Chief Cells. Gastroenterology. 2018 doi: 10.1053/j.gastro.2017.11.278. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Matsuo J, Kimura S, Yamamura A, et al. Identification of Stem Cells in the Epithelium of the Stomach Corpus and Antrum of Mice. Gastroenterology. 2017;152:218–231. doi: 10.1053/j.gastro.2016.09.018. [DOI] [PubMed] [Google Scholar]
- 61.El-Zimaity HMT, Ota H, Graham DY, et al. Patterns of gastric atrophy in intestinal type gastric carcinoma. Cancer. 2002;94:1428–1436. doi: 10.1002/cncr.10375. [DOI] [PubMed] [Google Scholar]
- 62.Means AL, Meszoely IM, Suzuki K, et al. Pancreatic epithelial plasticity mediated by acinar cell transdifferentiation and generation of nestin-positive intermediates. Development. 2005;132:3767–3776. doi: 10.1242/dev.01925. [DOI] [PubMed] [Google Scholar]
- 63.Wright NA, Pike C, Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990;343:82–85. doi: 10.1038/343082a0. [DOI] [PubMed] [Google Scholar]
- 64.Balbinot C, Armant O, Elarouci N, et al. The Cdx2 homeobox gene suppresses intestinal tumorigenesis through non-cell-autonomous mechanisms. J Exp Med. 2018 doi: 10.1084/jem.20170934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stringer EJ, Duluc I, Saandi T, et al. Cdx2 determines the fate of postnatal intestinal endoderm. Development. 2012;139:465–474. doi: 10.1242/dev.070722. [DOI] [PMC free article] [PubMed] [Google Scholar]


