Abstract
Individuals with chronic HIV-1 infection have an increased prevalence of autoreactive Abs. Many of the isolated HIV broadly neutralizing Abs from these individuals are also autoreactive. However, the underlying mechanism(s) that produce these autoreactive broadly neutralizing Abs remains largely unknown. The highly regulated coordination among B cells, T follicular helper (TFH) cells, and T follicular regulatory (TFR) cells in germinal centers (GCs) of peripheral lymphatic tissues (LTs) is essential for defense against pathogens while also restricting autoreactive responses. We hypothesized that an altered ratio of TFH/TFR cells in the GC contributes to the increased prevalence of autoreactive Abs in chronic HIV infection. We tested this hypothesis using a rhesus macaque (RM) SIV model. We measured the frequency of TFH cells, TFR cells, and GC B cells in LTs and anti-dsDNA and anti-phospholipid Abs from Indian RMs, with and without SIV infection. We found that the frequency of anti-dsDNA and anti-phospholipid Abs was much higher in chronically infected RMs (83.3% [5/6] and 66.7% [4/6]) than in acutely infected RMs (33.3% [2/6] and 18.6% [1/6]) and uninfected RMs (0% [0/6] and 18.6% [1/6]). The increased ratio of TFH/TFR cells in SIV infection correlated with anti-dsDNA and anti-phospholipid autoreactive Ab levels, whereas the frequency of TFR cells alone did not correlate with the levels of autoreactive Abs. Our results provide direct evidence that the ratio of TFH/TFR cells in LTs is critical for regulating autoreactive Ab production in chronic SIV infection and possibly, by extension, in chronic HIV-1 infection.
Human immunodeficiency virus–1 infection of humans leads to immunodeficiency that is characterized by massive CD4+ T cell depletion. Importantly, HIV also causes B lymphocyte dysfunction (1–3) and an increased prevalence of autoreactive Abs (4–7). During chronic infection, HIV neutralizing Abs, including broadly neutralizing Abs (bNAbs), have enhanced polyreactive and autoreactive characteristics (8–12). For example, a previous study found that 101 of 134 monoclonal anti-HIV–gp140 neutralizing Abs isolated from HIV-infected individuals were polyreactive and likely to bind self-antigens (9).
To maintain humoral immunologic homeostasis, a highly regulated coordination among B cells, T follicular helper (TFH) cells, and T follicular regulatory (TFR) cells in germinal centers (GCs) of peripheral lymphatic tissues (LTs) is required. These interactions promote the development of protective Abs against pathogens (13–16); however, disruption of homeostatic GC reactions can result in the production of autoreactive Abs or even autoimmune disease (17–19). Regulation of GC reactions, in part, is dependent on the frequency of TFH cells. TFH cells are indispensable for Ab affinity maturation of B cells (15, 16), in which a stochastic process of somatic hypermutation results in a greater risk for development of autoreactive B cells (20, 21). Previous studies have shown that increased frequency of TFH cells in mice was associated with an increased frequency of GC B cells, and the mice were more prone to develop humoral-mediated autoimmunity (18, 22). Furthermore, increased frequency of TFH cells has been implicated in the pathogenesis of autoimmune disease in humans (23, 24).
TFR cells regulate GC reactions through interactions with GC B cells and TFH cells. TFR cells are an effector subset of regulatory T cells (TREGs) that can suppress TFH cell function, limit the frequency of TFH and B cells in GCs (14, 25–28), and prevent autoreactive Ab production (29–31). During chronic HIV infection of humans and SIV infection of rhesus macaques (RMs), TFH cells exhibit increased frequency (32, 33). Recent studies revealed that the frequency of TFR cells in the LTs of SIV-infected RMs declines postinfection (34, 35); however, the role of TFH and TFR cells in autoreactive Ab production and the frequency of GC autoreactive B cells in HIV-infected individuals remain largely unknown.
We hypothesized that an altered ratio of TFH/TFR cells in the GC contributes to the increased prevalence of autoreactive Abs in HIV infection. We tested this hypothesis using an RM SIV model, which is the best available model of HIV infection in humans. We measured autoreactive anti-dsDNA and anti-phospholipid Abs in peripheral blood and quantified the frequency of TFH, TFR, and B cells in the GC of LTs. We found that an increased ratio of TFH/TFR cells in SIV infection correlated strongly with anti-dsDNA and anti-phospholipid Ab levels, whereas the frequency of TFR cells alone did not correlate with autoreactive Ab levels. Our results provide direct evidence that the proper balance and adequate ratio of TFH/TFR cells are crucial in regulating the quality of GC reactions and autoreactive Ab production in SIV infection and possibly, by extension, HIV-1 infection.
Materials and Methods
Virus and animals
This study was reviewed and approved by the Institutional Animal Care and Use Committee at the University of Nebraska-Lincoln (protocol number 559) and BIOQUAL, Inc. (protocol number 10-0000-01). Adult male Indian RMs (Macaca mulatta) were housed and maintained in animal-housing facilities at BIOQUAL, in accordance with the Guide for the Care and Use of Laboratory Animals. All animals were free of simian retrovirus type D, simian T-lymphotropic virus type 1, and herpes B virus. Animals were sedated with ketamine or TELAZOL for all technical procedures and were fully anesthetized for SIV intrarectal inoculation with SIVmac251 (3.4 × 104 TCID50). Animals were euthanized by exsanguination, under deep (surgical plane) anesthesia using TELAZOL, which was performed under the direction of the attending veterinarian on a designated date. Macaques were euthanized before SIV infection, at days 28 and 180 d postinfection (n = 3 for each time point). We also included four additional lymph node tissue samples from RMs not infected with SIV. Longitudinal peripheral blood samples from three macaques were collected before infection, as well as at 28 and 180 d postinfection (dpi). Three additional peripheral blood specimens were included at 180 dpi.
Detection of total IgG, SIV Env Ab, and anti-phospholipid and anti-dsDNA Abs in plasma by ELISA
Total IgG in plasma was detected using a Monkey IgG ELISA kit (catalog number IGG-3; Life Diagnostics). SIV-specific Env Ab titers were determined using ELISA. SIVmac239 gp130 Env protein [from Dr. K. Uberla (36)] was obtained through the National Institutes of Health AIDS Reagent Program (catalog number 12797). High-binding flat-bottom 96-well plates (catalog number 3361; Corning, Kennebunk, ME) were coated with SIV-mac239 gp130 protein (1 µg/ml) and blocked with PBS containing 5% milk. A two-fold serial dilution of plasma sample (starting at 1:200) was added to each well. Plates were incubated at 37°C for 1 h. After incubation, plates were washed with washing buffer (0.5% Tween-20 in PBS), 0.16 µg/ml goat anti-human IgG-HRP (catalog number 627120; Invitrogen) was added, and plates were stored at 37°C for 1 h. Plates were washed and developed using OPD substrate (catalog number P9187-50SET; Sigma) and stopped with 1 M H2SO4. Absorbance was read at 490 nm with an ELx800 Microplate Reader (BioTek, Winooski, VT). The cutoff value was set as the mean OD of control plasma + 3 SD. Binding Ab titers were defined as the end point dilution with an OD value greater than the cutoff + 0.05.
Anti-phospholipid and anti-dsDNA IgG autoreactive Abs were detected in plasma using commercial kits (ORGENTEC Diagnostika, Mainz, Germany), and absorbance was detected at 450 nm (ELx800 Microplate Reader; BioTek) for samples and standards. Using known standard concentrations (provided by the ELISA manufacturer), a linear-regression analysis (Microsoft Excel) was used to calculate IgG concentrations for each sample. Cutoff values were determined based on recommendations from the manufacturer (2.5 times the OD value of the negative control).
Flow cytometry
Detection of TFH and TFR cells
A total of 3–4 × 106 cryopreserved cells isolated from lymph node tissues was stained for viability using a LIVE/DEAD Fixable Blue Dead Cell Stain Kit, following the manufacturer’s instructions (Life Technologies/Thermo Fisher Scientific, Waltham, MA). Cells were then stained with Brilliant Stain (BD Biosciences, Franklin Lakes, NJ) in FACS buffer with titrated amounts of the following surface Abs to detect T follicular cells: mouse anti-human CD3–Alexa Fluor 700 (clone SP34-2, 1:60; BD Biosciences), mouse anti–human CD4–Brilliant Violet 605 (clone OKT4, 1:60; BD Biosciences), mouse anti–human CXCR5-PE (clone MU5UBEE, 1:40; eBioscience, San Diego, CA), mouse anti–human PD-1–Brilliant Violet 785 (clone EH12.2H7, 1:30), mouse anti–human CD20–Brilliant Violet 421 (clone 2H7, 1:40), mouse anti–human CCR7–Brilliant Violet 711 (clone G043H7, 1:40), mouse anti–human CD95–PE–Cy7 (clone DX2, 1:30), and mouse anti–human ICOS–PerCP–Cy5.5 (clone C398.4A, 1:75) (all from Bio-Legend, San Diego, CA), and mouse anti–human CD25–FITC (clone 4E3, 1:15; Miltenyi Biotec, Bergisch Gladbach, Germany).
Foxp3 staining
After surface staining, cells were fixed and permeabilized for 30 min in 1× Foxp3 Fix/Perm Buffer (eBioscience) and then stained with Foxp3-allophycocyanin (clone PCH101; eBioscience). Cells were washed twice with 1× Perm Buffer (eBioscience). Foxp3 Ab was added to cells and allowed to incubate for 45 min, and cells were washed twice with 1× Perm Buffer and washed once in FACS buffer prior to running on a BD LSR II flow cytometer (BD Biosciences). A minimum of 700,000 live lymphocyte-gated events was detected for each sample. Gating strategy was determined based on fluorescence minus one and appropriate isotype controls.
Detection of GC B cells
Cryopreserved lymphocytes isolated from lymph node tissues were stained with the following Abs: mouse anti-human CD3–Alexa Fluor 700 (clone SP34-2, 1:60; BD Biosciences), mouse anti–human Ki67–Alexa Fluor 488 (clone B56, 1:50; BD Biosciences), mouse anti–human BCL-6–PE–Cy7 (clone K112-91, 1:50; BD Biosciences), and mouse anti–human CD20–allophycocyanin (clone 2H7, 1:40; BioLegend). BCL-6 staining was performed using the Foxp3 staining procedure described above. A minimum of 350,000 lymphocyte-gated events was collected for each sample on a BD FACSAria II Flow Cytometer (BD Biosciences).
Real-time RT-PCR to quantify SIV viral loads in plasma
Real-time RT-PCR assays were performed to determine the levels of SIVmac251 in plasma specimens using a previously reported method (37).
Immunofluorescence staining
To visualize TFH and TFR cells in lymph node tissues, immunofluorescence staining was conducted according to a previously described method (38). Goat polyclonal anti-human PD-1 Abs (catalog number AF1086, 1:100; R&D Systems), rabbit monoclonal anti-human CD4 Abs (clone EPR6855, 1:200; Abcam), and mouse monoclonal anti-human Foxp3 Abs (clone 236A/E, 1:200; Abcam) were incubated with tissues sections. Following primary Ab incubation and washing, donkey anti-goat IgG conjugated with Alexa Fluor 488 (catalog number A-11055, 1:100), donkey anti-rabbit IgG conjugated with Alexa Fluor 594 (catalog number R37119, 1:100), and donkey anti-mouse IgG conjugated with Alexa Fluor 647 (catalog number A-31571, 1:100; all from Thermo Fisher Scientific) were used. Cell nuclei were counterstained with DAPI. A Nikon A1R-TiE live cell imaging confocal system was used to visualize and capture images of stained samples.
Statistics
Statistical analyses of cell subset frequencies detected by flow cytometry were performed using a Mann–Whitney nonparametric t test (GraphPad Prism). Correlation analyses were performed using the Pearson r correlation method (GraphPad Prism). The p values < 0.05 were considered significant.
Results
Increased autoreactive Abs in chronic SIV infection
In individuals with chronic HIV infection, the prevalence of autoreactive Abs increases significantly (4–7). Previous studies reported that up to 40–50% of HIV-infected individuals produce Abs against the self-antigens cardiolipin and phosphatidylserine (6). An elevated frequency of autoreactive Abs has also been observed in SIV-infected RMs (39). We first sought to determine the frequency of autoreactive Abs in our cohort of SIV-infected RMs. SIV infection was confirmed by detection of SIV viral RNA in plasma using real-time quantitative PCR and in LTs using in situ hybridization (data not shown). Anti-dsDNA and anti-phospholipid autoreactive Abs were measured by ELISA in plasma specimens from RMs that were SIV naive or were in the acute (28 dpi) or chronic (180 dpi) stage of SIV infection.
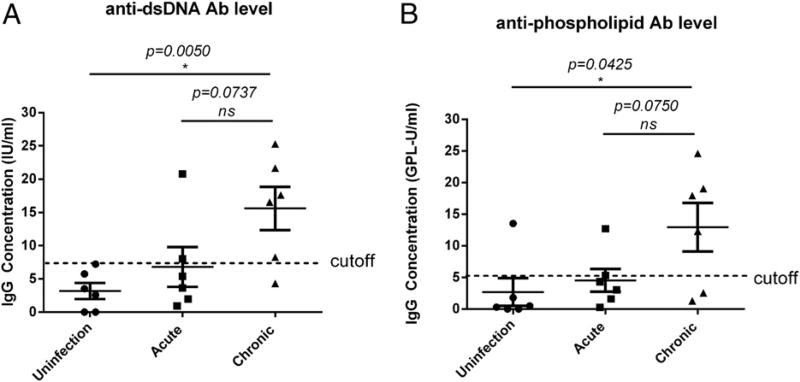
We found that the levels of anti-dsDNA Abs at the chronic stage of SIV infection were significantly increased compared with uninfected animals (p = 0.0050) (Fig. 1A). The level of anti-dsDNA Abs in chronically infected RMs was also higher than in animals in the acute stage of SIV infection (p = 0.0737), but the difference was not statistically significant. The percentage of RMs that had anti-dsDNA Abs was much higher in those that were chronically infected (83.3%, 5/6) compared with uninfected RMs (0%, 0/6) and acutely infected RMs (33.3%, 2/6).
FIGURE 1.
Increased autoreactive Abs during chronic SIV infection. Anti-dsDNA (A) and anti-phospholipid (B) autoreactive Abs increased in chronic SIV infection (180 dpi) compared with no infection (0 dpi). Cutoff value for anti-dsDNA Ab is 7.46 IU/ml, and the cutoff value for anti-phospholipid Ab is 5.04 GPL-U/ml. Cutoff values were determined based on recommendations from the manufacturer or 2.5 times the OD450 value of the negative control. *p < 0.05 indicates statistically significant differences between the compared groups, Mann–Whitney nonparametric t test. ns, not significant.
A similar trend was observed for anti-phospholipid (a mixture of cardiolipin, phosphatidyl serine, phosphatidyl inositol, phosphatidic acid, and human β-2-glycoprotein I) autoreactive Abs. Anti-phospholipid Ab levels in chronic SIV infection were increased significantly compared with uninfected animals (p = 0.0425) (Fig. 1B). The percentage of animals with anti-phospholipids Abs was much higher in association with chronic infection (66.7%, 4/6) than with no infection (18.6%, 1/6) or acute infection (18.6%, 1/6). Our results confirmed the increased prevalence of autoreactive Abs during chronic SIV infection.
To determine whether there is a correlation between the levels of autoreactive Abs and total IgG, we measured total IgG in plasma and performed a correlation analysis of total IgG levels and autoreactive Ab levels. We found an increased trend in total IgG in RMs with chronic SIV infection compared with acute and uninfected RMs; however, this increase was not statistically significant (Supplemental Fig. 1A). No significant correlation between total IgG levels and autoreactive Ab levels was found (Supplemental Fig. 1B, 1C).
Altered frequency of TFH and TFR cells in LTs following SIV infection
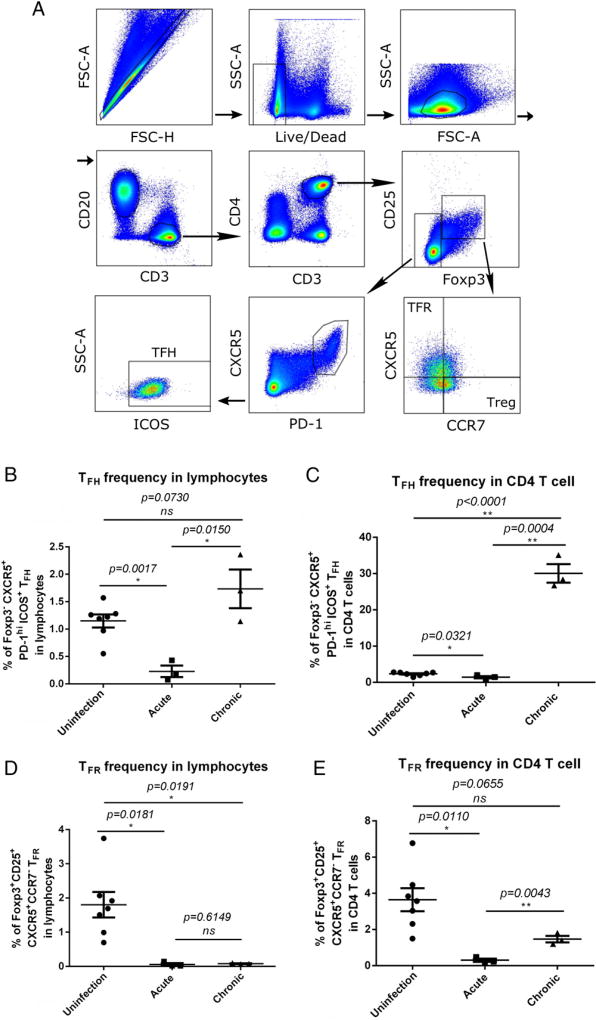
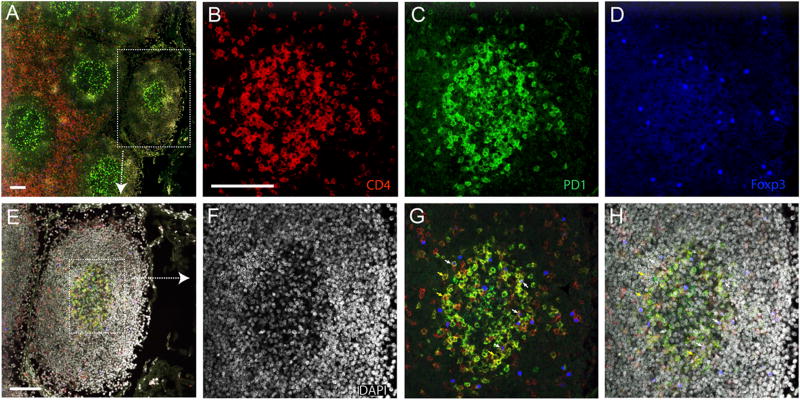
Next, we sought to examine the frequency of TFH and TFR cells in LTs during acute and chronic SIV infection using flow cytometry. We gated TFH and TFR subsets according to published methods (34) (Fig. 2A). The percentage of TFH cells (Foxp3− CXCR5+ PD-1hi ICOS+ CD4 T cells) within total lymphocytes and CD4 T cells decreased significantly in acute SIV infection compared with SIV-naive animals (Fig. 2B, 2C). During chronic infection, the TFH cell population in LTs expanded significantly, and their frequency increased significantly within CD4 T cells (Fig. 2C) and total lymphocytes (Fig. 2B), regardless of the decline in CD4 T cells in total lymphocytes (data not shown). During acute SIV infection, the percentage of TFR cells (Foxp3+ CD25+ CXCR5+ CCR7− CD4 T cells) within total lymphocytes and CD4 T cells was significantly decreased (Fig. 2D). However, TFR cell frequency in CD4 T cells also increased significantly during chronic infection compared with acute SIV infection (Fig. 2E). Furthermore, we determined the location of TFH and TFR cells in lymph node tissues using immunofluorescence staining (Fig. 3). TFH cells, defined as PD-1+ CD4+ Foxp3− (Fig. 3F, 3G, yellow arrows), and TFR cells, defined as PD-1+ CD4+ Foxp3+ (Fig. 3F, 3G, white arrows), were identified in GCs.
FIGURE 2.
Frequency of TFH and TFR cells in peripheral lymph node tissues following SIV infection. (A) Representative image of flow cytometry gating strategy for TFH and TFR cells. Frequency of TFH (Foxp3− PD−1hi CXCR5+ ICOS+) cells in total lymphocytes (B) and in CD4 T cells (C) following SIV infection. Frequency of TFR (Foxp3+ CD25+ PD-1hi CXCR5+ CCR7−) cells in total lymphocytes (D) and in CD4 T cells (E) following SIV infection. Data are mean ± SEM. *p < 0.05, **p < 0.001, Mann–Whitney nonparametric t test. ns, not significant.
FIGURE 3.
Confocal photomicrographs of TFH and TFR cell localization in lymph node tissues. (A) B cell and T cell zones in LTs of an SIV-naive macaque are characterized by the distinct density of CD4 T cells (red). The box highlights a B cell follicle. Immunofluorescence staining of CD4 [(B), red], PD1 [(C), green], FoxP3 [(D), blue], and nuclei [(F), DAPI] in the GC shown in the box in (E). (G and H) Merged images; white and yellow arrows indicate representative TFH and TFR cells, respectively. Scale bars, 100 µm.
Altered frequency of GC B cells in LTs following SIV infection
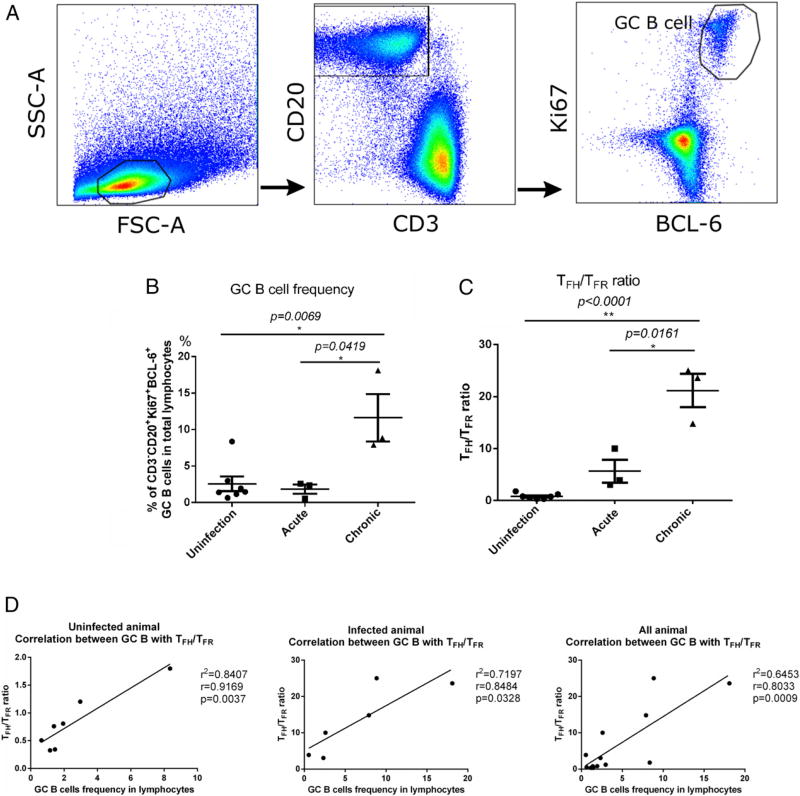
Next, we evaluated the frequency of GC B cells (CD3− CD20+ Ki67+ BCL-6+) in LTs of SIV-infected macaques using flow cytometry, according to previously published methods (32, 40) (Fig. 4A). The frequency of GC B cells did not change significantly during acute infection, but it increased significantly during chronic infection (Fig. 4B). Because TFH cells and TFR cells have been suggested to play a critical role in regulating the survival of GC B cells (13–16), we sought to determine whether there was an alteration in the TFH/TFR cell ratio and what role it played in the expansion of GC B cells during SIV infection. Consistent with the increased frequency of GC B cells, the TFH/TFR cell ratio was also increased significantly during chronic SIV infection (Fig. 4C). We found that the TFH/TFR cell ratio correlated strongly with GC B cells in uninfected and SIV-infected RMs (Fig. 4D); however, TFH cells alone correlated with GC B cells in SIV-infected RMs but not in uninfected RMs (Supplemental Fig. 2A). There was no correlation between TFR cells and GC B cells in SIV-infected or uninfected RMs (Supplemental Fig. 2B).
FIGURE 4.
Frequency of GC B cells following SIV infection. (A) Representative image of flow cytometry gating strategy for GC B (CD3− CD20+ Bcl-6+ Ki67+) cells. (B) Frequency of GC B cells. (C) TFH/TFR cell ratio. (D) Correlation between the frequency of GC B cells in LTs and the TFH/TFR cell ratio. Data are mean ± SEM. Pearson r correlation was performed for correlation between the frequency of GC B cells and the TFH/TFR cell ratio. *p < 0.05, **p < 0.001, Mann–Whitney nonparametric t test.
Autoreactive Ab levels correlated strongly with TFH/TFR cell ratio
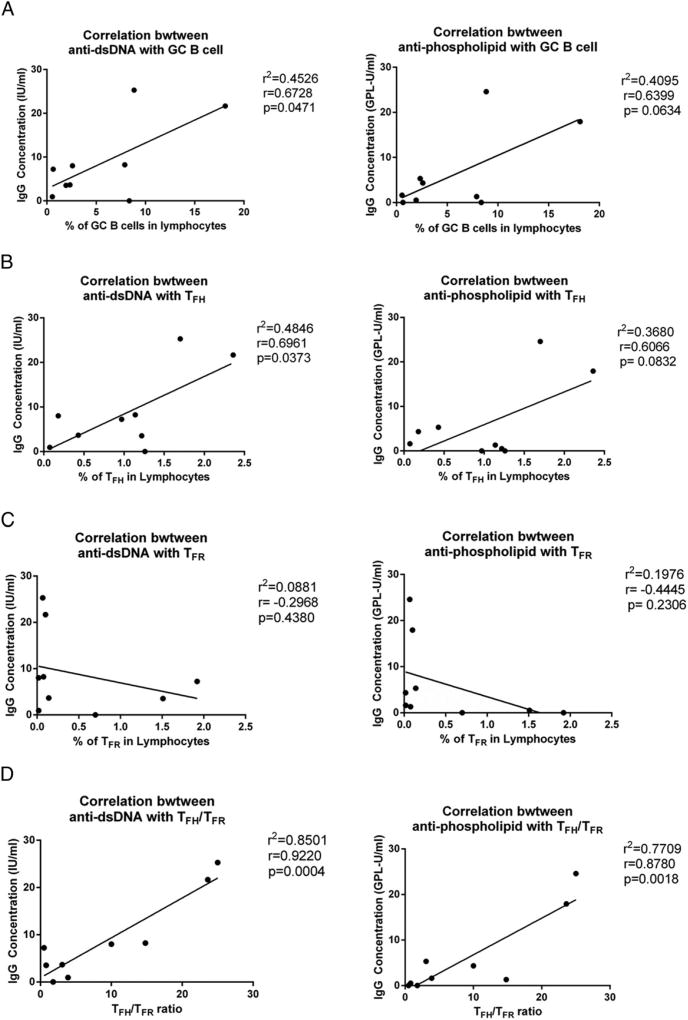
To examine the relationship between autoreactive Abs and TFH cells, TFR cells, and GC B cells, we performed a set of correlation analyses in SIV-infected and uninfected RMs. We found that the frequency of GC B cells had a weak correlation with anti-dsDNA autoreactive Abs (p = 0.0471) but not with anti-phospholipid autoreactive Abs (p = 0.0634) (Fig. 5A). The frequency of TFH cells correlated weakly with anti-dsDNA autoreactive Abs (p = 0.0373), but not with anti-phospholipid autoreactive Abs (p = 0.0832) (Fig. 5B). The frequency of TFR cells did not correlate with anti-dsDNA or anti-phospholipid autoreactive Abs (Fig. 5C); however, the TFH/TFR cell ratio correlated strongly with anti-dsDNA (p = 0.0004) and anti-phospholipid (p = 0.0018) autoreactive Abs (Fig. 5D). We also did a correlation analysis that excluded naive animals, and correlations remained significant (data not shown).
FIGURE 5.
Correlation analyses between autoreactive Abs and the frequency of TFH cells or TFR cells or the TFH/TFR cell ratio. Correlation between the levels of anti-dsDNA and anti-phospholipid autoreactive Abs and the frequency of GC B cells (A), the frequency of TFH cells (B), the frequency of TFR cells (C), and the TFH/TFR cell ratio (D), using Pearson r correlation.
We performed correlation analyses to determine the relationship between TREGs and autoreactive Abs. There was no association between TREGs (CD4+ Foxp3+ CD25+ CXCR5−), expressed as a percentage of total lymphocytes or CD4 T cells, and the levels of anti-dsDNA or anti-phospholipid Abs (Supplemental Fig. 3A). In addition, there was no association between CCR7+ TREGs (CD4+ Foxp3+ CD25+ CXCR5− CCR7+), expressed as a percentage of total lymphocytes or CD4 T cells, and the level of anti-dsDNA or anti-phospholipid Abs (Supplemental Fig. 3B).
To decipher the relationship between autoreactive Abs and SIV-specific Env Abs, we measured SIV-specific Abs against gp130 in plasma in the six chronically infected animals. We did not find a correlation between the level of SIV Env Abs and the level of autoreactive anti-dsDNA Abs (r2 = +0.3493, r = +0.5910, p = 0.2167) or anti-phospholipid Abs (r2 = +0.1151, r = +0.3393, p = 0.5106).
To investigate the relationship between viral loads and the levels of autoreactive Abs, TFH cells, TFR cells, TREGs, and GC B cells in peripheral blood, we performed a series of correlation analyses in chronically infected macaques and did not find any significant correlations (Supplemental Fig. 4).
Discussion
GCs of peripheral lymphoid tissues are a central hub of humoral immune responses. Within GCs, TFH cells and TFR cells regulate the survival and maturation of non-self-antigen–specific B cells while eliminating autoreactive B cells (13–16). GCs of LTs are also a major interaction site of the host immune system and HIV where TFH cells are productively infected (41, 42), follicular dendritic cells are deposited with abundant virions (43, 44), and B cells are dysfunctional (2, 3). A high prevalence of autoreactive Abs has been observed in individuals with chronic HIV infection (4–7) and in SIV-infected RMs (39); however, it remains largely unknown what role TFH and TFR cells play in the generation of autoreactive Abs in individuals who are chronically infected with HIV. In this study, we addressed this question using a well-established RM SIV model of human HIV infection.
Our study provides direct evidence that the ratio of TFH/TFR cells in LTs correlates with autoreactive Abs levels in naive RMs and in RMs in the acute or chronic stage of SIV infection. We measured plasma IgG autoreactive Abs (anti-dsDNA and anti-phospholipids), because it has been reported that there is an elevation in autoreactive Abs to dsDNA and phospholipids in macaques chronically infected with SIV (39). We found that the concentration of autoreactive Abs was significantly elevated in RMs chronically infected with SIV compared with SIV-naive or acutely infected RMs. The majority of chronically infected macaques had anti-dsDNA (83.3%) and anti-phospholipid (66.7%) Abs. We then determined the frequency of TFH cells, TFR cells, and GC B cells in LTs from uninfected RMs and in RMs in the acute and chronic stages of SIV infection. We found that there was an altered TFH/TFR cell ratio in LTs of chronically infected RMs, which is consistent with a previous study (35); however, that study did not investigate the relationship between the TFH/TFR cell ratio and autoreactive Ab production. In this study, we found that the frequency of GC B cells did not correlate with anti-phospholipid autoreactive Abs, and it correlated only weakly with anti-dsDNA autoreactive Abs, indicating that the production of autoreactive Abs is not a direct consequence of GC B cell expansion. The frequency of TFH cells alone did not correlate with anti-phospholipid autoreactive Abs, and it correlated only weakly with anti-dsDNA autoreactive Abs, whereas the frequency of TFR cells alone did not correlate with either autoreactive Ab tested; however, the ratio of TFH/TFR cells correlated strongly with anti-dsDNA and anti-phospholipid Abs. To our knowledge, our study is the first to demonstrate the potential role of the TFH/TFR cell ratio in regulating autoreactive Ab production in chronic SIV infection, which may be mediated through reduced peripheral tolerance for autoreactive B cells. TFR cells express CXCR5, PD-1, and ICOS, which are also expressed on TFH cells; however, TFR cells also express CD25, Foxp3, and Helios, which are characteristic of suppressive TREGs (25, 26). TFR cells have been shown to cooperate with TFH cells in regulating humoral immunity in GCs to fight against pathogens (14, 25–28) while also preventing the development of autoimmunity (29–31, 45). Therefore, an adequate ratio of TFH/TFR cells is important to maintain homeostasis of the humoral immune response.
Of note, HIV bNAbs are usually generated in chronically infected individuals and are often autoreactive to self-antigens (8–12). This production of autoreactive HIV bNAbs temporally coincides with an altered TFH/TFR cell ratio during chronic infection. The strong correlation that we observed between the emergence of autoreactive Abs and an increased ratio of TFH/TFR cells provides clues for future studies testing the possible enhanced induction of protective Abs by modulating the ratio of TFH/TFR cells during HIV-1 vaccination. We would like to point out that our TFH cell frequency in acute SIV infection compared with no infection differs from previous studies (32, 46), which may be due to the fact that the frequency was calculated based on different denominators and different dpi. There are several differences between the study by Petrovas et al. (32) and our study: their TFH cell frequency was calculated based on central memory CD4+ T cells as the denominator, whereas our TFH cell (CD4+ CXCR5+ PD-1hi CD25− FOXP3−) frequency was calculated based on total lymphocytes and CD4+ T cells; their acute cases were pooled samples from 3, 7, 10, 14, and 21 dpi, whereas we used 28 dpi; and our mean plasma viral load is 3.9 × 106 copies per milliliter, whereas they did not provide plasma viral load data. There are also differences between the study by Hong et al. (46) and our study: their TFH cell (CD4+ CXCR5+ PD-1+) frequency was calculated based on CD4+ CXCR5+ cells as the denominator, and their acute stage was 14 dpi, whereas we used 28 dpi.
Although this study revealed a direct association between autoreactive Abs and an altered TFH/TFR cell ratio in LTs of chronic SIV infection, our sample size was relatively limited, and the association is not direct causal evidence. Therefore, future studies are needed to elucidate the molecular mechanisms relating to an altered TFH/TFR cell ratio and its impact on autoreactive B cells.
In conclusion, we report that an altered TFH/TFR cell ratio in LTs correlates strongly with autoantibody levels in chronic SIV infection, and this altered TFH/TFR cell ratio could be one important mechanism leading to increased autoantibody production.
Supplementary Material
Acknowledgments
We thank the macaque care and veterinary staff at BIOQUAL, Inc., especially Dr. Mark Lewis. We also thank other members of the Li laboratory for technical assistance provided during this project, especially Lance Daharsh for proofreading this manuscript and Guobin Kang for immunofluorescence staining. We thank Phil Hexley and Victoria Smith (Flow Cytometry Core facility at the University of Nebraska Medical Center) and Danielle Shea (University of Nebraska-Lincoln Flow Cytometry Core Facility) for technical assistance in using the flow cytometer and Joe Zhou (University of Nebraska-Lincoln Microscopy Core Facility) for assistance with confocal imaging.
This work was supported by National Institutes of Health Grant R01 DK087625-01 (to Q.L.), National Center for Research Resources Grant 5P30RR031151-03, and National Institute of General Medical Sciences Grant 8 P30 GM103509-03.
W.F., A.J.D., and Q.L. designed the experiments; W.F., A.J.D., and Y.W. performed experiments and analyzed data; W.F. and A.J.D. wrote the manuscript; and Q.L. revised the manuscript. All authors commented on and approved the manuscript.
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Abbreviations used in this article
- bNAb
broadly neutralizing Ab
- dpi
d postinfection
- GC
germinal center
- LT
lymphatic tissue
- RM
rhesus macaque
- TFH
T follicular helper
- TFR
T follicular regulatory
- TREG
regulatory T cell
Footnotes
The online version of this article contains supplemental material.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.De Milito A, Nilsson A, Titanji K, Thorstensson R, Reizenstein E, Narita M, Grutzmeier S, Sönnerborg A, Chiodi F. Mechanisms of hypergammaglobulinemia and impaired antigen-specific humoral immunity in HIV-1 infection. Blood. 2004;103:2180–2186. doi: 10.1182/blood-2003-07-2375. [DOI] [PubMed] [Google Scholar]
- 2.Moir S, Ho J, Malaspina A, Wang W, DiPoto AC, O’Shea MA, Roby G, Kottilil S, Arthos J, Proschan MA, et al. Evidence for HIV-associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV-infected viremic individuals. J. Exp. Med. 2008;205:1797–1805. doi: 10.1084/jem.20072683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Moir S, Fauci AS. B cells in HIV infection and disease. Nat. Rev. Immunol. 2009;9:235–245. doi: 10.1038/nri2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Argov S, Schattner A, Burstein R, Handzel ZT, Shoenfeld Y, Bentwich Z. Autoantibodies in male homosexuals and HIV infection. Immunol. Lett. 1991;30:31–35. doi: 10.1016/0165-2478(91)90086-p. [DOI] [PubMed] [Google Scholar]
- 5.Massabki PS, Accetturi C, Nishie IA, da Silva NP, Sato EI, Andrade LE. Clinical implications of autoantibodies in HIV infection. AIDS. 1997;11:1845–1850. doi: 10.1097/00002030-199715000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Petrovas C, Vlachoyiannopoulos PG, Kordossis T, Moutsopoulos HM. Anti-phospholipid antibodies in HIV infection and SLE with or without anti-phospholipid syndrome: comparisons of phospholipid specificity, avidity and reactivity with β2-GPI. J. Autoimmun. 1999;13:347–355. doi: 10.1006/jaut.1999.0324. [DOI] [PubMed] [Google Scholar]
- 7.Leder AN, Flansbaum B, Zandman-Goddard G, Asherson R, Shoenfeld Y. Antiphospholipid syndrome induced by HIV. Lupus. 2001;10:370–374. doi: 10.1191/096120301669209574. [DOI] [PubMed] [Google Scholar]
- 8.Haynes BF, Fleming J, St Clair EW, Katinger H, Stiegler G, Kunert R, Robinson J, Scearce RM, Plonk K, Staats HF, et al. Cardiolipin polyspecific autoreactivity in two broadly neutralizing HIV-1 antibodies. Science. 2005;308:1906–1908. doi: 10.1126/science.1111781. [DOI] [PubMed] [Google Scholar]
- 9.Mouquet H, Scheid JF, Zoller MJ, Krogsgaard M, Ott RG, Shukair S, Artyomov MN, Pietzsch J, Connors M, Pereyra F, et al. Polyreactivity increases the apparent affinity of anti-HIV antibodies by heteroligation. Nature. 2010;467:591–595. doi: 10.1038/nature09385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bonsignori M, Wiehe K, Grimm SK, Lynch R, Yang G, Kozink DM, Perrin F, Cooper AJ, Hwang K-K, Chen X, et al. An autoreactive antibody from an SLE/HIV-1 individual broadly neutralizes HIV-1. J. Clin. Invest. 2014;124:1835–1843. doi: 10.1172/JCI73441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu M, Yang G, Wiehe K, Nicely NI, Vandergrift NA, Rountree W, Bonsignori M, Alam SM, Gao J, Haynes BF, Kelsoe G. Poly-reactivity and autoreactivity among HIV-1 antibodies. J. Virol. 2015;89:784–798. doi: 10.1128/JVI.02378-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moody MA, Pedroza-Pacheco I, Vandergrift NA, Chui C, Lloyd KE, Parks R, Soderberg KA, Ogbe AT, Cohen MS, Liao H-X, et al. Immune perturbations in HIV-1-infected individuals who make broadly neutralizing antibodies. Sci. Immunol. 2016;1:aag0851. doi: 10.1126/sciimmunol.aag0851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sage PT, Sharpe AH. T follicular regulatory cells in the regulation of B cell responses. Trends Immunol. 2015;36:410–418. doi: 10.1016/j.it.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wollenberg I, Agua-Doce A, Hernández A, Almeida C, Oliveira VG, Faro J, Graca L. Regulation of the germinal center reaction by Foxp3 + follicular regulatory T cells. J. Immunol. 2011;187:4553–4560. doi: 10.4049/jimmunol.1101328. [DOI] [PubMed] [Google Scholar]
- 15.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crotty S. Follicular helper CD4 T cells (TFH) Annu. Rev. Immunol. 2011;29:621–663. doi: 10.1146/annurev-immunol-031210-101400. [DOI] [PubMed] [Google Scholar]
- 17.Vinuesa CG, Tangye SG, Moser B, Mackay CR. Follicular B helper T cells in antibody responses and autoimmunity. Nat. Rev. Immunol. 2005;5:853–865. doi: 10.1038/nri1714. [DOI] [PubMed] [Google Scholar]
- 18.Mesquita D, Jr, Cruvinel WM, Resende LS, Mesquita FV, Silva NP, Câmara NO, Andrade LE. Follicular helper T cell in immunity and autoimmunity. Braz. J. Med. Biol. Res. 2016;49:e5209. doi: 10.1590/1414-431X20165209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Linterman MA, Rigby RJ, Wong RK, Yu D, Brink R, Cannons JL, Schwartzberg PL, Cook MC, Walters GD, Vinuesa CG. Follicular helper T cells are required for systemic autoimmunity. J. Exp. Med. 2009;206:561–576. doi: 10.1084/jem.20081886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Winkler TH, Fehr H, Kalden JR. Analysis of immunoglobulin variable region genes from human IgG anti-DNA hybridomas. Eur. J. Immunol. 1992;22:1719–1728. doi: 10.1002/eji.1830220709. [DOI] [PubMed] [Google Scholar]
- 21.Wellmann U, Letz M, Herrmann M, Angermüller S, Kalden JR, Winkler TH. The evolution of human anti-double-stranded DNA auto-antibodies. Proc. Natl. Acad. Sci. USA. 2005;102:9258–9263. doi: 10.1073/pnas.0500132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vinuesa CG, Cook MC, Angelucci C, Athanasopoulos V, Rui L, Hill KM, Yu D, Domaschenz H, Whittle B, Lambe T, et al. A RING-type ubiquitin ligase family member required to repress follicular helper T cells and autoimmunity. Nature. 2005;435:452–458. doi: 10.1038/nature03555. [DOI] [PubMed] [Google Scholar]
- 23.Romme Christensen J, Börnsen L, Ratzer R, Piehl F, Khademi M, Olsson T, Sørensen PS, Sellebjerg F. Systemic inflammation in progressive multiple sclerosis involves follicular T-helper, Th17- and activated B-cells and correlates with progression. PLoS One. 2013;8:e57820. doi: 10.1371/journal.pone.0057820. [Published erratum appears in 2013 PLoS One 8.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Le Coz C, Joublin A, Pasquali J-L, Korganow A-S, Dumortier H, Monneaux F. Circulating TFH subset distribution is strongly affected in lupus patients with an active disease. PLoS One. 2013;8:e75319. doi: 10.1371/journal.pone.0075319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, et al. Foxp3+ follicular regulatory T cells control the germinal center response. Nat. Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung Y, Tanaka S, Chu F, Nurieva RI, Martinez GJ, Rawal S, Wang Y-H, Lim H, Reynolds JM, Zhou XH, et al. Follicular regulatory T cells expressing Foxp3 and Bcl-6 suppress germinal center reactions. Nat. Med. 2011;17:983–988. doi: 10.1038/nm.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sage PT, Sharpe AH. T follicular regulatory cells. Immunol. Rev. 2016;271:246–259. doi: 10.1111/imr.12411. [DOI] [PubMed] [Google Scholar]
- 28.Sage PT, Francisco LM, Carman CV, Sharpe AH. The receptor PD-1 controls follicular regulatory T cells in the lymph nodes and blood. Nat. Immunol. 2013;14:152–161. doi: 10.1038/ni.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vaeth M, Müller G, Stauss D, Dietz L, Klein-Hessling S, Serfling E, Lipp M, Berberich I, Berberich-Siebelt F. Follicular regulatory T cells control humoral autoimmunity via NFAT2-regulated CXCR5 expression. J. Exp. Med. 2014;211:545–561. doi: 10.1084/jem.20130604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding Y, Li J, Yang P, Luo B, Wu Q, Zajac AJ, Wildner O, Hsu H-C, Mountz JD. Interleukin-21 promotes germinal center reaction by skewing the follicular regulatory T cell to follicular helper T cell balance in autoimmune BXD2 mice. Arthritis Rheumatol. 2014;66:2601–2612. doi: 10.1002/art.38735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Charbonnier L-M, Janssen E, Chou J, Ohsumi TK, Keles S, Hsu JT, Massaad MJ, Garcia-Lloret M, Hanna-Wakim R, Dbaibo G, et al. Regulatory T-cell deficiency and immune dysregulation, polyendocrinopathy, enteropathy, X-linked-like disorder caused by loss-of-function mutations in LRBA. J. Allergy Clin. Immunol. 2015;135:217–227. doi: 10.1016/j.jaci.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, Ambrozak DR, Sandler NG, Timmer KJ, Sun X, et al. CD4 T follicular helper cell dynamics during SIV infection. J. Clin. Invest. 2012;122:3281–3294. doi: 10.1172/JCI63039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, Flanders MD, Cutler S, Yudanin N, Muller MI, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J. Clin. Invest. 2012;122:3271–3280. doi: 10.1172/JCI64314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blackburn MJ, Zhong-Min M, Caccuri F, McKinnon K, Schifanella L, Guan Y, Gorini G, Venzon D, Fenizia C, Binello N, et al. Regulatory and helper follicular T cells and antibody avidity to simian immunodeficiency virus glycoprotein 120. J. Immunol. 2015;195:3227–3236. doi: 10.4049/jimmunol.1402699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chowdhury A, Del Rio Estrada PM, Tharp GK, Trible RP, Amara RR, Chahroudi A, Reyes-Teran G, Bosinger SE, Silvestri G. Decreased T follicular regulatory cell/T follicular helper cell (TFH) in simian immunodeficiency virus–infected rhesus macaques may contribute to accumulation of TFH in chronic infection. J. Immunol. 2015;195:3237–3247. doi: 10.4049/jimmunol.1402701. [Published erratum appears in 2015 J. Immunol. 195: 5843.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nabi G, Temchura V, Grossmann C, Kuate S, Tenbusch M, Überla K. T cell independent secondary antibody responses to the envelope protein of simian immunodeficiency virus. Retrovirology. 2012;9:42. doi: 10.1186/1742-4690-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salisch NC, Kaufmann DE, Awad AS, Reeves RK, Tighe DP, Li Y, Piatak M, Jr, Lifson JD, Evans DT, Pereyra F, et al. Inhibitory TCR coreceptor PD-1 is a sensitive indicator of low-level replication of SIV and HIV-1. J. Immunol. 2010;184:476–487. doi: 10.4049/jimmunol.0902781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Q, Duan L, Estes JD, Ma ZM, Rourke T, Wang Y, Reilly C, Carlis J, Miller CJ, Haase AT. Peak SIV replication in resting memory CD4+ T cells depletes gut lamina propria CD4+ T cells. Nature. 2005;434:1148–1152. doi: 10.1038/nature03513. [DOI] [PubMed] [Google Scholar]
- 39.Kuwata T, Nishimura Y, Whitted S, Ourmanov I, Brown CR, Dang Q, Buckler-White A, Iyengar R, Brenchley JM, Hirsch VM. Association of progressive CD4(+) T cell decline in SIV infection with the induction of autoreactive antibodies. PLoS Pathog. 2009;5:e1000372. doi: 10.1371/journal.ppat.1000372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vargas-Inchaustegui DA, Demers A, Shaw JM, Kang G, Ball D, Tuero I, Musich T, Mohanram V, Demberg T, Karpova TS, et al. Vaccine induction of lymph node-resident simian immunodeficiency virus env-specific T follicular helper cells in rhesus macaques. J. Immunol. 2016;196:1700–1710. doi: 10.4049/jimmunol.1502137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perreau M, Savoye AL, De Crignis E, Corpataux JM, Cubas R, Haddad EK, De Leval L, Graziosi C, Pantaleo G. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J. Exp. Med. 2013;210:143–156. doi: 10.1084/jem.20121932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Y, Weatherall C, Bailey M, Alcantara S, De Rose R, Estaquier J, Wilson K, Suzuki K, Corbeil J, Cooper DA, et al. Simian immunodeficiency virus infects follicular helper CD4 T cells in lymphoid tissues during pathogenic infection of pigtail macaques. J. Virol. 2013;87:3760–3773. doi: 10.1128/JVI.02497-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heath SL, Tew JG, Tew JG, Szakal AK, Burton GF. Follicular dendritic cells and human immunodeficiency virus infectivity. Nature. 1995;377:740–744. doi: 10.1038/377740a0. [DOI] [PubMed] [Google Scholar]
- 44.Haase AT, Henry K, Zupancic M, Sedgewick G, Faust RA, Melroe H, Cavert W, Gebhard K, Staskus K, Zhang ZQ, et al. Quantitative image analysis of HIV-1 infection in lymphoid tissue. Science. 1996;274:985–989. doi: 10.1126/science.274.5289.985. [DOI] [PubMed] [Google Scholar]
- 45.Paul WE. Fundamental Immunology. Wolters Kluwer Health/Lippincott; Williams & Wilkins, Philadelphia: 2013. [Google Scholar]
- 46.Hong JJ, Amancha PK, Rogers K, Ansari AA, Villinger F. Spatial alterations between CD4(+) T(FH), B and CD8(+)T cells during SIV infection: T/B cell homeostasis, activation and potential mechanism for viral escape. J. Immunol. 2012;188:3247–3256. doi: 10.4049/jimmunol.1103138. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.