Abstract
The present publication surveys several applications of in silico (i.e., computational) toxicology approaches across different industries and institutions. It highlights the need to develop standardized protocols when conducting toxicity-related predictions. This contribution articulates the information needed for protocols to support in silico predictions for major toxicological endpoints of concern (e.g., genetic toxicity, carcinogenicity, acute toxicity, reproductive toxicity, developmental toxicity) across several industries and regulatory bodies. Such novel in silico toxicology (IST) protocols, when fully developed and implemented, will ensure in silico toxicological assessments are performed and evaluated in a consistent, reproducible, and well-documented manner across industries and regulatory bodies to support wider uptake and acceptance of the approaches. The development of IST protocols is an initiative developed through a collaboration among an international consortium to reflect the state-of-the-art in in silico toxicology for hazard identification and characterization. A general outline for describing the development of such protocols is included and it is based on in silico predictions and/or available experimental data for a defined series of relevant toxicological effects or mechanisms. The publication presents a novel approach for determining the reliability of in silico predictions alongside experimental data. In addition, we discuss how to determine the level of confidence in the assessment based on the relevance and reliability of the information.
Keywords: In silico, in silico toxicology, computational toxicology, predictive toxicology, QSAR, expert alert, expert review
Graphical Abstract
1. Introduction
In silico toxicology (IST) methods are computational approaches that analyze, simulate, visualize, or predict the toxicity of chemicals. IST encompasses all methodologies for analyzing chemical and biological properties generally based upon a chemical structure that represents either an actual or a proposed (i.e., virtual) chemical. Today, in silico approaches are often used in combination with other toxicity tests; however, the approaches are starting to be used to generate toxicity assessments information with less need to perform any in vitro or in vivo studies depending on the decision context. IST uses models which can be encoded within software tools to predict the potential toxicity of a chemical and in some situations to quantitatively predict the toxic dose or potency. These models are based on experimental data, structure-activity relationships, and scientific knowledge (such as structural alerts reported in the literature).
There are a number of different situations where in silico methods serve an important role in the hazard assessment of existing chemicals or new substances under development that would benefit from the development of in silico toxicology protocols. These include:
emergency situations where rapid understanding of potential toxicological consequences from exposure is needed in the absence of existing toxicological testing data;
cases where there is only a limited supply of a test material available;\
scenarios where there are challenges to conduct laboratory studies;
instances where synthesis of a complex test material is not feasible; and
situations where a less time-consuming and less expensive high-throughput approach than an experimental test is needed.
IST methods are one approach to generating additional information for complementing and ultimately enhancing the reliability or supporting a risk assessment, including an understanding of the structural and/or mechanistic basis that may contribute ideas for the rational design of new chemicals, development of a testing strategy or an overall weight-of-evidence evaluation. IST inherently supports the principle of the 3Rs (replacement, refinement and reduction) relating to the use of animals in research (Russell and Burch, 1959; Ford 2016). Table 1 outlines fifteen specific uses of IST to illustrate the diversity of applications that currently can benefit from in silico methods. Stanton and Kruszewski (2016) quantified the benefits of using in silico and read-across methods where they determined that the approach used across two voluntary high-production-volume (HPV) chemical programs for 261 chemicals obviated the use of 100,000 – 150,000 test animals and saved 50,000,000 US$ to 70,000,000 US$.
Table 1.
Applications of in silico toxicology
| In silico toxicology application | Discussion |
|---|---|
| 1. Alternative to test data. | The use of non-animal alternative methods including in silico approaches, may substitute for other types of tests in regulatory submissions in certain cases. Acceptable alternative methods for filling data gaps are outlined in Annex XI of the European Union’s REACH regulation (EU 2006). In the United States, Frank R. Lautenberg Chemical Safety for the 21st Century Act revised the Toxic Substances Control Act (TSCA) to include predictive models and expert review as part of an overall assessment (TSCA 2016). The United States Food and Drug Administration (US FDA) Center for Devices and Radiological Health (CDRH) issued a guidance for industry and FDA staff. This guidance is on the use of International Standard ISO 10993-1 for biological evaluation of medical devices and indicates in the absence of experimentally derived carcinogenicity information, structure activity relationship modeling for these materials may be needed (CDRH 2016). The FDA draft guidance on Electronic Nicotine Delivery Devices (ENDS) also discusses the use of computational toxicology models in the absence of toxicological data for potential toxicants created by the aerosolization process (PMTA/FDA 2016). When chemicals with limited toxicity data are required to be classified and labeled for shipping or other purposes, in silico toxicology provides an alternative method for quickly filling the data gaps in the toxicity/safety information, such as predictions of acute toxicity to support assignment to the Globally Harmonized System of Classification and Labelling category (Freidig et al., 2007; ECHA 2015). |
| 2. As part of the weight-of-evidence in regulatory submissions. | There are currently several regulatory frameworks where only specific laboratory tests for an endpoint of concern may be submitted (such as for drugs or food additives). However, in such cases, in silico predictions can be submitted alongside standard toxicological data to complement the assessment. This may include in silico assessments provided as supporting data or adjuncts to the primary in vivo or in vitro studies to give a mechanistic understanding of the observed results and/or allow a better definition of experimental needs. Additionally, in silico methods may be used to guide or prioritize in vitro testing (EU 2012). The European Union’s Cosmetics Regulation (EU 2009a) prohibits the use of animal testing for products or ingredients and a complete marketing ban of such products tested as a whole or containing tested ingredients. This requires the use of alternative methods, such as IST, in the assessment of new cosmetics ingredients. In a recent memorandum, the European Commission’s Scientific Committee for Consumer Safety (SCCS), which is responsible for the risk assessment of cosmetic ingredients, acknowledged the importance and limitations of in silico methods; the SCCS recommended that in silico methods be used either for internal decision making or as part of a weight-of-evidence (WOE) approach to estimate toxicity risks before embarking on any experimental testing (SCCS 2016). |
| 3. Mixtures assessment. | Most exposures are not to a single chemical but rather to complex mixtures of chemicals that may be found in food, beverages, the environment, cigarette smoke, electronic nicotine delivery systems (ENDS) aerosols, botanical drugs or natural products. In certain situations, it may be possible to use in silico methods to assess individual components since today’s in silico analysis can only be performed on discrete identifiable chemicals. While preliminary analytical work is required to identify all chemicals in the mixture above appropriate Analytical Evaluation Thresholds (AET) (Ball and Norwood 2012), leveraging in silico approaches may avoid having to synthesize or purify each of the potentially large number of mixture components to perform standard toxicological tests (Mumtaz et al., 2010). Careful consideration is required for mixtures when there are multiple chemicals for interactions, such as synergistic or additive effects that may have the same, similar or different mechanisms of action (MOA). |
| 4. Assessment of impurities and degradation products. | Chemicals, such as pharmaceuticals or plant protection products, may contain low levels of impurities produced during manufacturing and degradation. Many such substances, when present at levels above accepted thresholds, need to be assessed. In most cases, mutagenicity evaluation of the impurity under question is required as a first step of the risk assessment. (Harvey et al., 2017) The ICH M7 guideline provides specific recommendations for assessing drug impurities (ICH M7, 2017(R1)), including the use of two complementary computational toxicology methodologies (i.e., statistical-based and expert rule-based models) to predict bacterial mutagenicity. |
| 5. Residues of plant protection products. | Residues of plant protection products may be evaluated as a part of residue definition for dietary risk assessment of plant protection products (EU 2009b). In this context, in silico methods provide a useful alternative approach. (EFSA 2016) |
| 6. Assessment of extractables and leachables. | Medical devices, such as inhaled aerosols, food-contact substances, and consumer product packaging materials may pose a risk for human health due to release of potentially harmful chemicals that are used in the production of the components (Bossuyt et al., 2017). These include plasticizers, copolymers, vulcanization additives, etc. for which toxicological data is often lacking but where a risk assessment must be performed. A migration or leachables study supports the discovery, identification, and quantification of any leachables. An in silico toxicological assessment, in certain situations, can provide sufficient data for the risk assessment. |
| 7. Workers’ safety and occupational health. | Chemicals used in the manufacture of a product are assessed for mutagenicity, carcinogenicity, skin and respiratory sensitization, irritation (skin, eye and respiratory), and reproductive and developmental toxicity and possibly acute toxicity. In silico assessments make it possible to estimate the potential toxicity of chemicals and adopt proper engineering controls and personal protective equipment usage to protect workers who could be exposed to these substances during production, transfer, storage, and delivery processes (EU 2006). In silico approaches have been utilized to assess these major toxicological endpoints in the occupational safety setting. In silico methods to predict respiratory sensitization potential of industrial chemicals have recently been reviewed by Seed and Agius (2017). |
| 8. Metabolite analysis. | Metabolites can present an increased or decreased risk of local or systemic toxicity compared with the parent chemical (Mumtaz and Durkin, 1992). While reactive or toxic metabolites may be formed by an organism, their identification, separation as well as possible synthesis for testing purposes may be challenging. In silico methods provide a practical alternative approach to understanding the safety profiles of this potentially large number of chemicals as well as to support the prediction of metabolites. |
| 9. Ecotoxicology. | Various chemicals are discharged into the environment that may cause harm. Furthermore, the parent compounds can be transformed by hydrolysis, redox-reactions, or photolysis into numerous additional chemicals. IST methods often provide the most practical approach to assess the potential effects on the environment and wildlife species of the many chemicals that are discharged. Prediction of physicochemical parameters supports assessment of potential environment exposure to the chemical (e.g., persistence and distribution). As an example, Chen at al., 2015 describes the use of in silico assessment of potentially hazardous contaminants present in water. |
| 10. Green chemistry and safer alternatives. | In silico methods can play an important role when identifying alternative chemicals that may have a safer profile than existing chemicals (Rastogi et al., 2014). This includes, for example, alternatives for use in manufacturing processes, alternative packaging/delivery materials and the use of specific additives. In silico methods can provide insights about structural features responsible for the toxicity of different groups of chemicals and thereby allow for the rational design of intrinsically safer chemicals. |
| 11. Selection of product development candidates. | In early product discovery or development, many thousands of compounds may be evaluated. In silico methods may provide a helpful approach to selecting candidates, since in silico methods are inexpensive, rapid to perform, and high throughput. In addition, in silico methods can suggest which molecular substructures (toxicophores) are responsible for the predicted toxic activity, thereby supporting the optimization of future compounds (Hillisch et al., 2015; Myatt et al., 2016). Later in the product development process, a smaller number of chemicals may be selected as candidates to take forward for further development; in normal situations, preference would be given to the candidate(s) with the most advantageous safety profile(s) (Myatt et al., 2016). |
| 12. Emergency response situations. | When one or more chemicals are unexpectedly released into the environment (e.g., the West Virginia chemical spill (NTP 2016)) or into a production process, it is important to quickly evaluate the potential effects on humans, wildlife, and the environment. In such emergency situations the toxicological profile of the released chemicals needs to be established as quickly as possible to support the proper emergency response and to protect emergency services staff and bystanders (Hochstein et al., 2008; Schilter, et al., 2014). In such a limited timeframe and in the absence of previously generated data, in silico approaches may be a practical option for rapid hazard identification. |
| 13. Prioritizing testing of chemicals. | In silico approaches can help prioritize in vitro and in vivo toxicology testing, based upon the chemical’s exposure and prediction of toxicity; they are an important aspect of the work at several organizations such as the US EPA, National Toxicology Program, Environment and Climate Change Canada and ECHA (Schwetz 1995). In silico methods may be used to prioritize (based on potential toxicological liabilities) the order in which a series of toxicological studies will be performed (Myatt et al., 2016). |
| 14. Rationalization of in vivo or in vitro study results. | As mentioned previously in the description of the in silico application titled “As part of the weight-of-evidence in regulatory studies”, results from quantitative structure-activity relationship (QSAR) models (toxicophore information, chemical fragments or physicochemical properties) may be used in conjunction with biological data to infer a mechanism of action (MOA), molecular initiating event (MIE), or mode of toxicity as part of an adverse outcome pathway (AOP) (Martin et al., 2015; Ellison et al., 2016). Information from in silico methods can also be used to tailor an in vivo study, e.g., by inclusion of additional endpoints. When existing experimental data on a compound are equivocal or when not all relevant safety information are available or accessible, in silico data may be used as additional information as part of the weight- of-evidence approach in reaching a more informed decision (Kruhlak et al., 2012). |
The increased interest and acceptance of in silico methods for regulatory data submission and chemicals evaluation is driving the adoption of its use for regulatory purposes. Several guidance documents have been drafted to improve standardization, harmonization, and uptake of in silico methods by regulatory authorities including the International Council for Harmonization (ICH) M7 guideline (assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk) (ICH M7, 2017(R1)), the European Union’s Registration, Evaluation, Authorization, and restriction of Chemicals (REACH) regulation (EU 2006; ECHA 2008; ECHA 2015), European Food Safety Authority (EFSA) residue guidance (EFSA 2016), Canada’s Chemicals Management Plan (CMP) assessments for new and existing substances under the Canadian Environmental Protection Act (CEPA 1999) (Canada 2016), and the Toxic Substances Control Act (TSCA) (TSCA 2016). A number of national and international initiatives have focused on developing specific documents supporting the use of in silico tools. The OECD has published a series of (Quantitative) Structure-Activity Relationship (Q)SAR validation principles that are discussed in detail in Section 2.3.2. (OECD 2004; OECD 2007) Other initiatives include the North American Free Trade Agreement pesticides Quantitative Structure-Activity Relationship (QSAR) guidance (NAFTA 2012), considerations on the use of in silico approaches for assessing cosmetics ingredients (Amaral et al., 2014), European Food Safety Agency report (EFSA 2014), European Chemicals Agency REACH supporting documentation (ECHA 2008; ECHA 2016, 2017b), Organization for Economic Co-operation and Development (OECD) documentation (OECD 2007; OECD 2014; OECD 2015), and the ICH M7 guideline (previously mentioned) along with complementary peer reviewed publications outlining the process for implementation of such computational assessments (e.g., Amberg et al., 2016; Barber et al., 2015; Powley et al., 2015; Schilter et al., 2014). Certain projects have provided substantial guidance on the documentation of the models and prediction results (JRC 2014; Patlewicz et al., 2016) as well as principles and workflows to support safety assessments (Bassan and Worth, 2008; ECHA 2015; Worth et al., 2014; Berggren et al., 2017; Amaral et al., 2017).
These prior initiatives provide a robust foundation for the current project to establish the IST protocols described here; however, several issues have hindered the general acceptance and use of in silico methods on a larger scale. In particular, there remains a lack of generally accepted procedures for performing in silico assessments for the toxicological endpoints. The lack of such procedures or protocols has led to inconsistency in the application and use of in silico tools across different organizations, industries, and regulatory agencies (e.g., searching databases, applying predictive models and alerts, performing an expert review/assessment, documenting and communicating the results and associated uncertainties). The use of traditional experimental evidence coupled with in silico information to support hazard identification and risk assessment also varies both across, and often within, organizations. Although not always, such ad hoc approaches may be time-consuming and the results poorly accepted. Standardization of protocols will enhance the acceptability of the methods and their results by end users. Additionally, there are misconceptions about when in silico predictions are appropriate to use as well as a lack of defined consensus processes for interpreting the result(s) of such predictions (Bower et al., 2017; SCCS 2016). Some scientists view in silico methods as a “black box” that inhibits their ability to critically assess the predictions and their reliability. (Alves et al., 2016) Others lack expertise to interpret the results of in silico predictions, and some have an unrealistic expectation that an in silico prediction can always provide an unerring definitive assessment.
Standardization of in silico tool use and interpretation of results would greatly reduce the burden on both industry and regulators to provide confidence in or justification for the use of these approaches. The objective of developing IST protocols is to define in silico assessment principles so the results can be generated, recorded, communicated, archived and then evaluated in a uniform, consistent and reproducible manner. Incorporating these principles routinely into the use of in silico methods will support a more transparent analysis of the results and serves to mitigate “black box” concerns1. This approach is similar to guideline studies that provide a framework for the proper conduct of toxicological studies and assurance in the validity of the results (such as OECD Guidelines for the Testing of Chemicals) (OECD 2017). The development of these protocols is driven by consensus amongst leading scientists representing industry, private sector and governmental agencies. Consequently, this project provides an important step towards a quality-driven science for IST or good in silico practice.
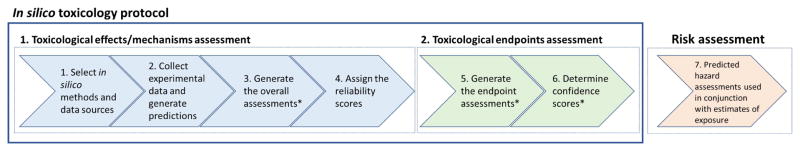
Herein, we provide a framework to develop a series of procedures for performing an in silico assessment to foster greater acceptance. These IST protocols are being created for a number of toxicological endpoints (e.g., genetic toxicity, carcinogenicity, acute toxicity, reproductive toxicity, developmental toxicity) as well as other related properties (e.g., biodegradation and bioaccumulation) that could impact the chemical hazard classification. Throughout this publication, these toxicological and related endpoints are referred to as “major endpoints” and the protocols are referred to as IST protocols. These protocols will support the assessment of hazards and in some cases the prediction of quantitative values, such as a No Observed Adverse Effect Levels (NOAELs); however, these protocols do not define how a risk assessment will be performed. This publication outlines the components of an IST protocol, including schematics to describe how a prediction could be performed, approaches to assess the reliability and confidence of the results, and items that may be considered as part of an expert review. This publication also outlines the process for creating the IST protocols through an international consortium comprising representatives across regulatory agencies, government research agencies, different industrial sectors, academia and other stakeholders. Specific endpoint-dependent considerations will be described in future separate publications and IST protocols (developed as a result of this process) will also be published for widespread use and for incorporation into different technology platforms.
2. In silico toxicology protocols
2.1 Overview
Each IST protocol describes the prediction process in a consistent, transparent, and well-documented manner. This includes recommendations on how to:
plan the in silico analyses including identifying what toxicological effects or mechanisms to predict (Section 2.2), what in silico methodologies to use (Section 2.3.1), and other selection criteria for the in silico methods (Section 2.3.2),
conduct the appropriate individual software predictions (Section 2.3.3) and further database searches (Section 2.5),
perform and document the in silico analysis (Sections 2.6 and 2.7) including expert review (Section 2.4), and
report and share the information and assessment results, including information about uncertainties (Section 2.9).
Section 2.8 provides a template for the individual IST protocols for major toxicological endpoints. IST protocols could be applicable for use with several in silico programs, including different in silico models and databases.
2.2 Toxicological effects and mechanisms
In an experimental approach, hazard is evaluated based on specific observations (toxicological effects) during toxicity studies. Often, toxicity of a chemical involves a biological event: a non-specific or specific interaction with a vital biological structure, which causes sequential perturbation of a physiological pathway at a cellular, tissue, organ and/or system level, leading to a toxicological effect observed at the organism level. Experiments evaluating the potential of a chemical to cause such a biological event (e.g., in vitro analysis of specific interaction with a cellular receptor or inhibition of an enzyme or non-specific cytotoxicity), may support hazard assessment and provide information about the mechanism of toxicity. Such an approach is utilized in the Adverse Outcome Pathway (AOP), where identification of a molecular initiating event supports assessment of the related adverse outcome at the organism level (Bell et al., 2016; OECD 2016a; OECD 2016b). A computational approach to hazard assessment may address the two complementary levels of hazard identification in a similar way (i.e., predicting the resulting manifestation (effect) or the molecular perturbation (mechanism) that led to the toxicological effect).
Each IST protocol defines a series of known toxicological effects and mechanisms relevant to the assessment of the major toxicological endpoint. For example, in the reproductive toxicity IST protocol, the list of toxicological effects/mechanisms may include reduced sperm count, androgen signaling disruption in vitro, and so on. Within each IST protocol, these effects/mechanisms may be species and/or route of administration specific.
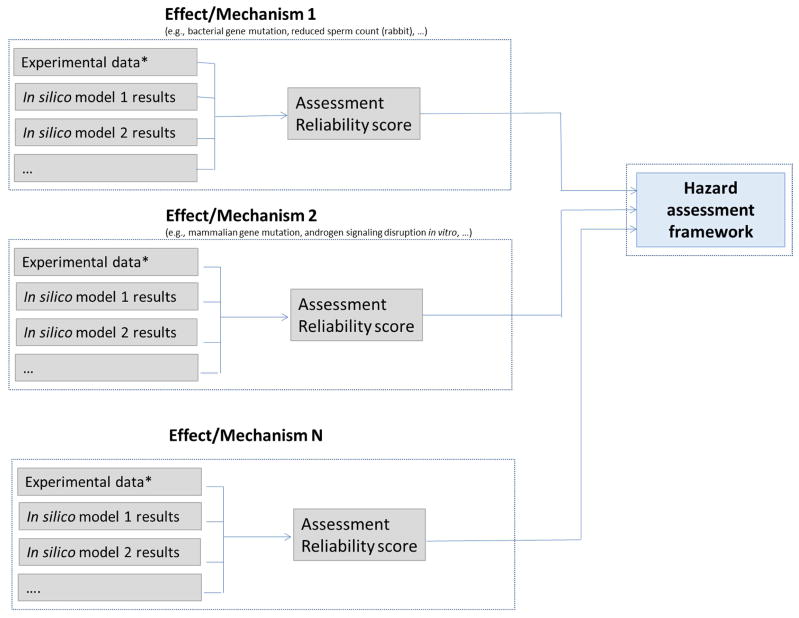
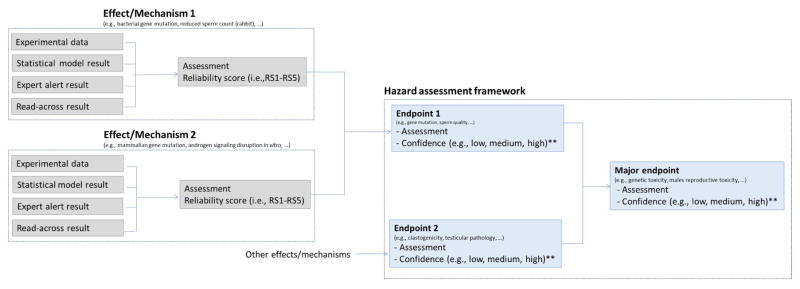
Figure 1 outlines a general approach to performing an in silico assessment. For each toxicological effect/mechanism, relevant information (as defined in the IST protocol) is collected, including any available experimental data as well as in silico predictions. The experimental data and/or in silico results are then analyzed and an overall assessment of the toxicological effect or mechanism is generated alongside a reliability score (defined in Section 2.6.2) that reflects the quality of the results. The assessment results and reliability scores for a range of relevant toxicological effects/mechanisms are then used to support a hazard assessment within the hazard assessment framework.
Figure 1.
Overview of the IST protocol framework, showing how experimental data or in silico model(s) for each defined toxicological effect/mechanism are assessed and used to support a hazard assessment. (Note Effect/Mechanism N is used to illustrate that there can be any number of effects/mechanisms in each protocol)
* From the literature, database or study report
2.3 In silico predictions
2.3.1 In silico methodologies
Several organizations develop and make available computer software packages for predicting toxicity or physicochemical properties of query chemical(s). These systems generally contain one or more models, where each model predicts the compound’s putative toxicological effect or mechanism of action. For example, a model may predict the results for bacterial gene mutation using data generated from the bacterial reverse mutation test or Ames test. These models may be revised over time as more data become available, structure-activity relationships are better characterized, and any data set used is updated. Each new or updated model is given a different version number because the results from different model versions may vary and it is important to track the source of the results. (Amberg et al., 2016)
All IST protocols will identify the toxicological effects or mechanisms to be predicted as discussed in Section 2.2. These predictions may be dichotomous (e.g., predict mutagenic or non-mutagenic compounds), quantal (e.g., Globally Harmonized System [GHS] Classification and Labeling2 scheme) or quantitative/continuous (e.g., prediction of median toxic dose [TD50] values). The specific IST protocols will detail the type of prediction(s) ideally generated.
The major in silico prediction methodologies include the following:
Statistical-based (or QSAR). This methodology uses a mathematical model that was derived from a training set of example chemicals. The training set includes the chemicals that were found to be positive and negative in a given toxicological study (e.g., the bacterial reverse mutation assay) or to induce a continuous response (e.g., NOAEL in teratogenicity) that the model will predict. As part of the process to generate the model, physicochemical property- based descriptors (e.g., molecular weight, octanol water partition coefficient [log P]), electronic and topological descriptors (e.g., quantum mechanics calculations), or chemical structure-based descriptors (e.g., the presence or absence of different functional groups) are generated and used to describe the training set compounds. The model encodes the relationship between these descriptors and the (toxicological) response. After the model is built and validated (OECD 2007; Myatt et al., 2016), it can be used to make a prediction. The (physico)chemical descriptors incorporated into the model are then generated for the test compound and are used by the model to generate a prediction. This prediction is only accepted when the test compound is sufficiently similar to the training set compounds (i.e., it is considered within the applicability domain of the QSAR model, often considering the significance of descriptors). (Netzeva et al., 2005; Carrió et al., 2014; Patlewicz et al., 2016) This applicability domain analysis may be performed automatically by some software to determine whether the training set compounds share similar chemical and/or biological properties with the test chemical.
Expert rule-based (or expert/structural alerts). This methodology uses structural rules or alerts to make predictions for specific toxicological effects or mechanisms of toxicity. These rules are derived from the literature or from an analysis of data sets generated by scientists. Structural alerts are defined as molecular substructures that can activate the toxicological effect or mechanism. The rules may also encode situations where the alert is deactivated. Expert rule-based models often include a description of the toxic mechanism and examples from the literature or other reference sources to justify the structural alert. A positive prediction is generally made when a structural alert is present (without deactivating structural features or properties) in the test compound. When no alerts are triggered for a test chemical, a negative prediction may be generated for well investigated endpoints; however, additional analysis is generally required to make this assessment as discussed further in Section 2.4.3.
Read-across: Read-across uses data on one or more analogs (the “source”) to make a prediction about a query compound or compounds (the “target”). Source compounds are identified that have a structurally or toxicologically meaningful relationship to the target compound, often underpinned by an understanding of a plausible biological mechanism shared between the source and target compounds. The toxicological experimental data from these source compounds can then be used to “read-across” to the specific target compound(s). Read-across is an intellectually-derived endpoint-specific method that provides justification for why a chemical is similar to another chemical (with respect to chemical reactivity, toxicokinetics, mechanism/mode of action, structure, physicochemical properties, and metabolic profile). (Wu et al., 2010; ECETOC 2012; Patlewicz et al., 2013a; Patlewicz et al., 2013b; OECD 2014; Blackburn and Stuard, 2014; Patlewicz (2014); Patlewicz et al., 2015; Schultz et al., 2015; Ball et al., 2016; ECHA 2017b)
Other approaches: In certain cases, other in silico methodologies may be appropriate. Examples include the use of molecular dynamics (e.g., simulating interactions of a query chemical with a metabolic enzyme) and receptor binding as an indication of a possible Molecular Initiating Event (e.g., estrogen receptor-ligand docking).
Each IST protocol will include an assessment of key computational aspects and specific issues to consider. For example, when performing read-across, issues such as the data quality of the source compound(s), how to perform an assessment of non-reactive chemical features and selection of grouping approaches used to form categories will be discussed to ensure source compound(s) are sufficiently similar, both chemically and biologically, for the endpoint being considered.
Each methodology has its strengths and weaknesses, which often depend on the type of toxicological effect or mechanism being predicted. This will be discussed in the individual IST protocols. In addition, there may be cases of unique or novel compounds for which it is not possible to make a prediction or for which confidence in the predictions is so low as to render it meaningless or unhelpful.
2.3.2 In silico methods selection criteria
In silico methods selection may include the following five considerations:
Relevant toxicological effects or mechanisms. As discussed in Section 2.2, each IST protocol will define a series of toxicological effects or mechanisms relevant to a specific endpoint and appropriate in silico models need to be selected that predict these specific effects or mechanisms.
Model validity. Best practices for validation of (Q)SAR in silico models have been documented in a number of publications (Cherkasov et al.; 2014, Raies and Bajic, 2016; Myatt et al., 2016), and models built using these best practices may be preferred. The OECD has published a series of validation principles for in silico models (OECD 2004; OECD 2007) and valid statistical-based or expert rule-based in silico methods. Such (Q)SAR methods have: 1) a defined endpoint; 2) an unambiguous algorithm; 3) a defined domain of applicability; 4) appropriate measures of goodness-of-fit, robustness and predictivity; and 5) a mechanistic interpretation, if possible. Any in silico model must include documentation that supports an assessment of the model’s scientific validity, including the toxicological effect or mechanism being predicted, version number, type of methodology, training set size and content, as well as any predictive performance information. Validation performance is documented in report formats such as the QSAR Model Reporting Format (QMRF) (JRC 2014). The level of adherence to the OECD principles and the performance statistics need to be appropriate for the purpose of the assessment.
Chemical space. Often, in silico models will only make predictions for specific classes of chemicals, the so called “applicability domain”. The chosen in silico model(s) may report the applicability domain assessment to demonstrate its proficiency for this class of compounds. Vice versa, only models are ideally chosen where the query compound is in the applicability domain. (Netzeva et al., 2005; Carrió et al., 2014; Patlewicz et al., 2016)
Model combinations. Complementary or independent in silico models may be selected, as concurring results increase the reliability of the prediction (as discussed in Section 2.6.2).
Supporting an expert review. For QSAR models, tools to help the expert review (see Section 2.4) include the ability to allow examination of the descriptors and weightings used in the model, underlying training set data, and how the applicability domain assessment was defined. For expert rule-based systems, this could include how the alert was defined (including any factors that activate or deactivate the alert), any mechanistic understanding associated with the alert, citations, and any relevant known examples of alerting chemicals.
Read across may be used when there are experimental data from high quality databases for one or more substances which are similar enough to the target chemical of interest. The Read-Across Assessment Framework (RAAF), or similar published and established frameworks, may be used to document the read-across assessment and to support its scientific plausibility (ECHA 2017b; Patlewicz et al., 2013b; Blackburn & Stuard 2014; Schultz et al., 2015; Patlewicz et al., 2015). The OECD has also produced guidance on the process of grouping chemicals and other considerations as part of a read-across assessment (OECD 2014), and ECHA has generated guidelines on the process of performing a valid read-across assessment (ECHA 2008).
2.3.3 Running the in silico models
All in silico systems require an electronic representation of the chemical structure and any errors in this representation will result in invalid predictions. Therefore, it is important to ensure that the chemical structure is properly curated and entered following conventions set out by the model’s developer, including appropriate representations for tautomers, aromaticity, salt forms, stereochemistry, charges, and specific functional groups (e.g., nitro or carboxylic acid groups). It is possible that different formats (i.e., SMILES vs. MOL files) may be processed differently. It is also important to verify that the software correctly interprets the structural representation during processing, particularly for complex molecules. For some types of chemicals, in silico models may not be applicable due to the structural representation or the unsuitability of the experiment assay for the specific chemical class. Some in silico models cannot distinguish cis- and trans- isomers. Examples include non-discrete chemical substances, UVCBs (unknown/variable composition, complex reaction products and biologicals), metals, inorganics, polymers, mixtures, organometallics and nano-materials. (Mansouri et al., 2016)
Some models, such as statistical-based models, allow for prediction settings to be adjusted or turned off (e.g., they report “positive” when a value is greater than a predetermined threshold). The settings are ideally selected in a way that does not compromise the model’s validity (such as changing the validation statistics of the model) and appropriately reported.
A thorough documentation of all selected models and computer software packages including, version numbers, and any parameters set, is needed as part of the materials and methods in sufficient detail to assess and potentially repeat the analysis (discussed in Section 2.9). In addition, the results need to be presented in enough detail to fully understand how they were generated and to critically assess the findings.
2.4 In silico expert review
2.4.1 Overview
As with in vitro or in vivo study data, in silico predictions may be critically assessed and an expert review of the output is often prudent (Dobo et al., 2012; Sutter et al., 2013). Frameworks for conducting an expert review ensure that it is performed in a consistent and transparent manner. Examples of such a review framework include the Office of Health Assessment and Translation (OHAT) systematic review and evidence integration (Rooney et al., 2014), weight-of-evidence assessments (ECHA 2017a), and Integrated Approaches to Testing and Assessment (IATA) (OECD 2016a; OECD 2016b).
The purpose of an in silico expert review is to evaluate the reliability of the prediction. The outcome of the review provides information to include in the assessment of the toxicological effect or mechanism. As part of this review, the expert might agree with, or refute, individual in silico predictions. In addition, these reviews might support cases when a chemical is out of the applicability domain of the model, support the use of an equivocal prediction (i.e., there is evidence both for and against the supposition), or support cases where multiple predictions do not agree. A checklist of items to consider and report will help to ensure such reviews are performed in a consistent manner (as illustrated in Tables 2 and 3). This review may include knowledge from proprietary information available within an organization from the testing of related chemicals.
Table 2.
Checklist of elements to consider as part of an expert review of a QSAR model result
| Expert review elements | Considerations |
|---|---|
| A. Inspection of model output |
|
| B. Analysis of structural descriptors and corresponding training set data (see Note A) |
|
| C. Analysis of physicochemical descriptors used by model (see Note B) |
|
| D. Assessment of other information |
|
(Note A: items to consider when the QSAR model includes structure-based descriptions; Note B: items to consider when the QSAR model includes physicochemical descriptors)
Table 3.
Checklist of elements to consider as part of an expert review of results from expert rule-based
| Expert 4review elements | Considerations |
|---|---|
| A. Alert score or qualitative output |
|
| B. Justification of negative prediction |
|
| C. Reliability of the mechanism of toxicity |
|
| D. Inspection of chemicals and experimental data matching the alert |
|
When an expert review assesses multiple predictions from different in silico systems, it is important to justify how they complement each other with regard to the training set (i.e., the use of relevant guideline studies plus relevant chemical classes), methodology (e.g., expert rule-based vs. statistical-based vs. read-across), or QSAR descriptor sets.
It is essential to document the reasoning and decisions of the expert review steps so they can be retraced at any time, including the information used as the basis for the review.
2.4.2 Expert review of statistical models
An expert review of a statistical-based model involves a critical assessment of how the model generated the prediction. This includes examining the weightings of the model descriptors (e.g., structural features or physicochemical properties related to toxicity), underlying data, chemical space of the training set of the model, and the experimental results for analog compounds and model performance for these analogs (e.g., nearest-neighbor list of compounds) (Amberg et al., 2016). This may also incorporate an understanding of the mechanism of toxicity or knowledge of factors that activate or deactivate the toxicity. The items described in Table 2 provide a checklist of elements to consider as part of any QSAR expert review to ensure such a review is as objective as possible, transparent and based on a consistent set of considerations. An expert review may increase the reliability of statistical model results based on one or more elements defined in Table 2.
Individual IST protocols will outline specific points to consider when performing an expert review, such as how the similarity of analogs could be assessed.
2.4.3 Expert review of expert rule-based (structural) alert systems
An expert review of the results from an expert rule-based alert system may involve inspection of the underlying information as well as external knowledge. Special emphasis needs to be placed on the assessment of chemicals where no alerts are identified in the expert alert system. When no alert is fired (i.e., it is not predicted active), it is often not reported if the prediction is negative, equivocal, or out of the applicability domain of the model and often no prediction is generated. An expert review may increase the reliability of the results based on one or more elements defined in Table 3.
2.4.4 Read-across expert review
Read-across contains an expert assessment by its nature: it requires expert judgment of the analogs, their data and extrapolation to the query chemical. For example, read-across assessments performed and documented according to the RAAF (i.e., following the detailed RAAF Assessment Elements), or similar frameworks, as discussed earlier, incorporate an expert review as part of the assessment. This type of assessment includes a strong justification for biological plausibility of any analogs selected (including an assessment of the structural differences and similarities to the target structure, and an analysis of potential metabolism). It also includes an expert assessment when a read-across prediction concludes there is an absence of effects. In addition, an assessment of supporting evidence (including the reliability of the source data), any weight-of-evidence considerations, and an assessment of any possible bias in the selection of source chemicals is required.
2.5 Assessment of available experimental data
Experimental data may have been previously generated and reported for a chemical being assessed, for example, in the literature or through a public or proprietary database. To support the identification of experimental data, each IST protocol will identify a series of relevant study types and specific result(s) corresponding to the identified toxicological effects or mechanisms, as discussed in Section 2.2. To illustrate, in the assessment of the toxicological effect/mechanism bacterial gene mutation (part of the genetic toxicity IST protocol), the overall mutagenic or non-mutagenic results from a bacterial reverse mutation assay may be used. A more complex example is in the assessment of the toxicological effect/mechanism of sperm morphology (part of the reproductive IST protocol). Here, specific results from potentially different study types, such as one- or two- generation reproductive studies, repeated dose toxicity studies or segment I (fertility) studies, and possibly also from different species (rat, mouse, rabbit) will be applicable.
The selection of experimental study types need focus on those that have general value based on scientific justification. This includes study types that have widespread use in risk assessments, regulatory acceptance and that follow internationally recognized test guidelines. In addition, other types of data may be considered relevant on a case-by-case basis. Numerous guidance documents discuss acceptable studies, their relevancy, and their use in hazard identification, hazard characterization and risk assessment. These include guidance documents from the ICH (ICH 2017), OECD (OECD 2017), European Food Safety Authority (EFSA) (EFSA 2017a), Scientific Committee on Consumer Safety (SCCS) (SCCS 2017), REACH/ECHA (ECHA 2008; ECHA 2015), United States Environmental Protection Agency (EPA) Office of Chemical Safety and Pollution Prevention (OCSPP 2017), and National Institute of Environmental Health Sciences (NIEHS) (NIEHS 2017) guidance documents. Such guidance documents provide a useful basis for test considerations but may not always be harmonized across legislation, industrial sector or geographical regions, as requirements may differ across guidance documents.
The IST protocols will discuss how to assess and document the experimental data and uncertainties to ensure the proper justification of the experimental results’ reliability, including defining what specific elements or fields are important to document. With older studies pre-dating existing guidelines, it will often still be possible to perform an expert review to determine the adequacy of the data, but it will be important to document specifically why the study results were considered acceptable or dismissed as unacceptable. The IST protocols will also provide recommendations on how to select a result when multiple studies (with potentially conflicting results) for the same effect or mechanism are reported.
Klimisch scores are a widely used approach adopted to support an assessment of experimental data reliability (Table 4; Klimisch et al., 1997). The Klimisch score (1 to 4) is based on factors including whether the test was compliant with the OECD principles of Good Laboratory Practices (GLP) or Good In Vitro Methods Practices (GIVIMP) standards (OECD 2016c), whether the data were generated using accepted test guidelines, whether the data are available for independent inspection, and the quality of the report. ECHA uses this score, for example, as part of its data submission process (ECHA 2011), and there are tools to support the assignment of Klimisch scores (ECVAM 2017; Schneider et al., 2009). Another approach to the assessment of the reliability of the experimental data is the Science in Risk Assessment and Policy (SciRAP) application, a web-based reporting and evaluation resource created to help understand how academic toxicity-related studies can be used as part of any regulatory assessment (Molander et al., 2014). An approach proposed by EFSA is a detailed analysis of different parameters of the study (e.g. statistical power; verification of measurement methods and data; control of experimental variables that could affect measurements; universality of the effects in validated test systems using relevant animal strains and appropriate routes of exposure, etc.) with detailed documentation of the process (EFSA, 2011).
Table 4.
Summary of Klimisch scores for data reliability (adapted from Klimisch et al., 1997) (Note “restriction”, as part of scores 1 and 2, implies restricted quality)
| Score | Description | Summary |
|---|---|---|
| 1 | Reliable without restriction |
|
| 2 | Reliable with restriction |
|
| 3 | Not reliable |
|
| 4 | Not assignable |
|
2.6 Combined assessment of experimental data and in silico predictions
2.6.1 Toxicological effect or mechanism assessment
Reliable data, generally defined by Klimisch scores 1 or 2 reviewed by an expert (see Table 4), is ideally used for the toxicological effect or mechanism (shown in Figure 1) whenever available3. In the absence of adequate experimental data, results from one or more in silico models can be used to support assessment of the toxicological effect or mechanism. When multiple in silico model results, from potentially different methodologies, or QSAR models using different descriptors and/or training sets, are generated per toxicological effect or mechanism, the individual results need to be compiled to provide one overall assessment, as shown in Figure 1. This assessment may take into consideration information from any expert review of the in silico results, as certain results may need to be refuted. Similarly, when there are data assigned Klimisch 3 or 4 and/or there are in silico results, this information needs to be compiled into an overall assessment. Individual IST protocols will document such procedures.
There are multiple approaches to compile results. A cautious approach is to use the most conservative data or prediction for this assessment. For example, when predicting the results of the bacterial reverse mutation test using two models, if either model’s prediction result is mutagenic then the overall assessment is mutagenic. Other options include a weight-of-evidence or consensus approach or selection of the prediction with the highest confidence (e.g., predictive probability score and relevance of analogous structures). Specific considerations per endpoint may be addressed in the individual IST protocols and may be dependent on the problem formulation.
2.6.2 Reliability scores
Reliability, in this context, is defined as the inherent quality of the experimental study (Klimisch et al., 1997) and/or in silico analysis. It is used to support any hazard assessment, in combination with other information. A reliability score (RS) is associated with the toxicological effect or mechanism assessment (as shown in Figure 1). As noted earlier, when data from the literature or other sources are considered, Klimisch scores can be used to assess the reliability of the results. However, the Klimisch framework was never intended to assess the reliability of in silico predictions. It is also important to note that regardless of the approach taken, reliability assessments will contain subjective decisions.
A number of general factors can affect the reliability of in silico results:
Multiple in silico results: Combining results from multiple complementary or independent in silico tools which use different methodologies or QSAR descriptors and/or training sets, has been shown to improve overall sensitivity, but it can lower specificity by increasing false positive rates (Myatt et al., 2016). In the case of quantitative predictions, such process are overly conservative estimates. Hence, consistency across several different models can increase the reliability of the results.
Expert review: A plausible and well-documented read-across (consistent with the RAAF or similar frameworks) may be acceptable as part of a REACH regulatory submission as an alternative to experimental data. A structured expert review is implicit in any read-across assessment (as discussed in Section 2.4.4). Similarly, an explicit expert review (following the elements described in Sections 2.4.2 and 2.4.3) of the in silico predictions can improve the reliability of the final results, especially for negative predictions. (Dobo et al., 2012)
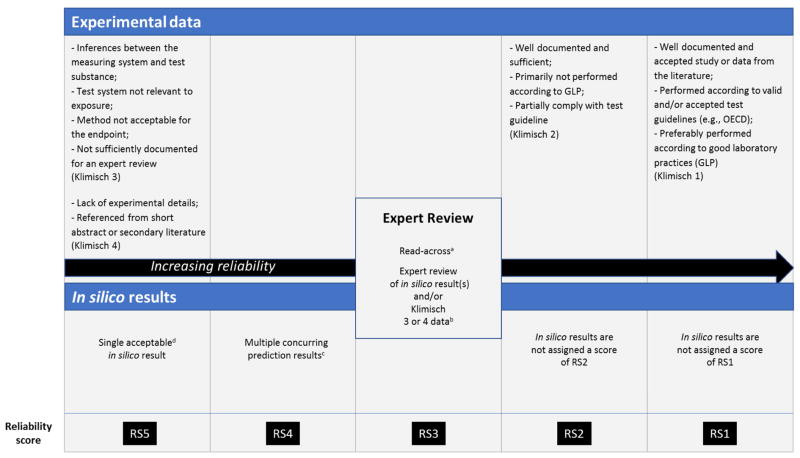
To generate an overall reliability score for assessments based on experimental data and/or in silico predictions, the Klimisch score has been adapted (as shown in Figure 2) to include an assessment of in silico prediction results.
Figure 2.
Reliability of toxicity assessments based on computational models and experimental data
a. Read-across performed according to the RAAF or similar
b. Expert review resulting in increased confidence
c. 2+ concurring results from different methodologies or QSAR descriptor sets or training sets
d. Based on the selection criteria provided in Section 2.3.2
Experimental data assigned a Klimisch score of 1 or 2 is assigned a score of RS1 and RS2, respectively, in this revised scheme. In silico results are not assigned a score of RS1 or RS2 since adequate experimental data is preferred over in silico predictions. Since in silico results may be used directly as part of certain regulatory submissions, whereas experimental data with a Klimisch score of 3 or 4 would not (or only as supporting data under REACH, for example), the next two categories (RS3 and RS4) represent, in part, in silico predictions. The following may be acceptable as part of a regulatory submission: (1) an adequately performed read-across prediction (EU 2006), or (2) an expert review of in silico and/or other experimental data (ICH M7, 2017(R1); EU 2006); they are assigned a reliability score of RS3. A score of RS4 would be assigned when two or more predictive models are available that are complementary, with concurring results (with no expert review), and no supporting literature data are available. Examples include those predictive models that use either substantially different QSAR descriptors and/or QSAR training sets or different in silico methodologies. If two or more in silico model results do not agree, then an expert review would be required to assess the results. This review might increase the confidence in the assessment, resulting in an increased reliability score of RS3. A single acceptable (as discussed in Section 2.3.2) in silico model result, without further expert review, is afforded the same reliability score of RS5 as an actual test result of lowest reliability (Klimisch 3 or 4). The in silico result is placed in the same category as low reliability data because such models inform decisions based on a series of compounds or trends. However, this reliability score may be increased following expert review. This reliability score closely follows the ICH M7 guideline, where submissions corresponding to reliability scores RS1–RS4 would be accepted according to the guideline. In addition to this score, it may be helpful to document any additional considerations that may be important to the overall assessment. Individual IST protocols may deviate from this scheme with appropriate justification.
2.6.3 Worked examples
Three examples from Amberg et al. (2016) illustrate how the framework described in this publication can be used for determining a toxicological effect or mechanism assessment and reliability score, based on experimental data and/or in silico predictions. Assessing reliability is an initial step in the overall assessment of hazard, where it will be combined with other information, including an evaluation of the relevance of the information, to support decision making.
In the example in Figure 3, no experimental data were identified. Two in silico models were run; the statistical-based model prediction was negative and the expert rule-based alert prediction was negative. The initial score would be RS4 based on multiple concurring prediction results; however, an expert review was performed on the results from both methodologies and the negative result was confirmed with increased reliability. The review concluded there were no potentially reactive features in the chemical. This resulted in a negative overall assessment and a reliability score of RS3 (as a result of the expert review increasing the reliability).
Figure 3.
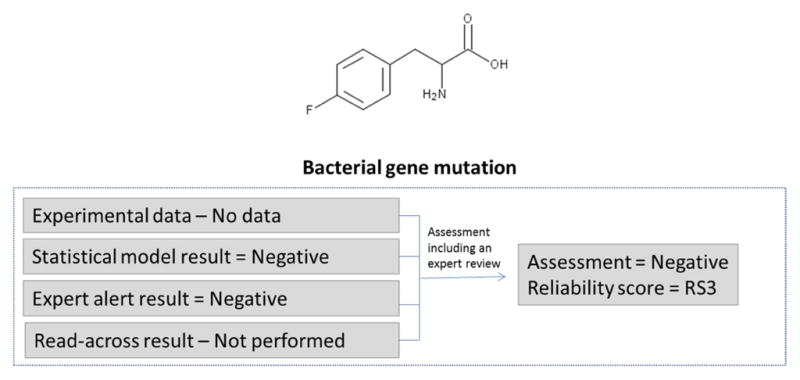
Determining the bacterial gene mutation assessment and reliability score for two concurring in silico results with expert review
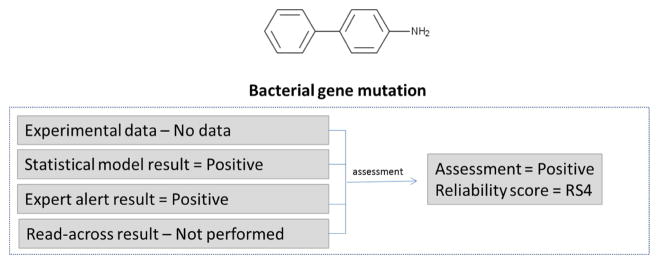
In the example in Figure 4, no experimental data were identified. Two in silico models were run; the statistical model prediction was positive and the expert alert prediction was positive. No expert review of the results was performed. The overall assessment was therefore positive and a reliability score of RS4 was assigned as a result of two concurring positive predictions using complementary in silico methodologies but without expert review.
Figure 4.
Determining the bacterial gene mutation assessment and reliability score for two concurring in silico results with no expert review
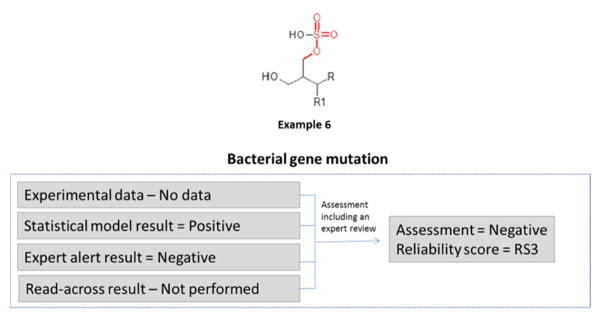
In the example in Figure 5, no experimental data were identified. Two in silico models were run; the statistical model prediction was positive and the expert alert prediction was negative. An expert review was performed on the results from both methodologies, refuting the statistical model’s positive prediction. This review was based on an analysis of the test chemical’s potential to react with DNA and the highlighted structural feature was determined to be irrelevant for the mechanism of interaction with DNA. This resulted in a negative overall assessment and a reliability score of RS3 (as a result of the expert review increasing the reliability).
Figure 5.
Determining the bacterial gene mutation assessment and reliability score where there is no experimental data available and conflicting in silico results
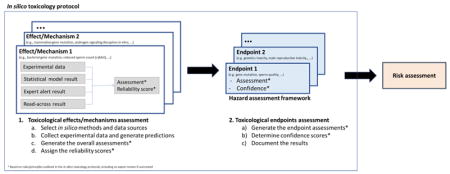
2.7 Hazard assessment framework
2.7.1 Toxicological endpoints
Figure 6 illustrates a general scheme for the prediction of a major toxicological endpoint. In this scheme, the specific toxicological effects or mechanisms are used to support the assessment of a series of toxicological endpoints. These toxicological endpoint assessments are, in turn, used in the overall assessment of the major toxicological endpoint. In Figure 6, effect/mechanism 1 is identified as being relevant to an assessment of a specific toxicological endpoint (Endpoint 1). For example, bacterial gene mutation (effect/mechanism 1) is relevant to the assessment of gene mutation (endpoint 1). Endpoint 1 is, in turn, one of the endpoints that are relevant to the major toxicological endpoint (e.g., genetic toxicity). Other identified toxicological effects or mechanisms are associated with toxicological endpoints as shown in Figure 6. For example, the mammalian gene mutation (effect/mechanism 2) is also relevant to the assessment of gene mutations (endpoint 1) and clastogenicity (endpoint 2) is another endpoint to be used in the assessment of genetic toxicity (a major toxicological endpoint). Figure 6 also includes another example to illustrate how this scheme might be used to assess male reproductive toxicity.
Figure 6.
Hazard assessment framework
* From the literature, database or study report
** Function of the associated reliability, relevance and completeness
The hazard assessment framework scheme for each IST protocol will contain different numbers of toxicological endpoints as needed to support the assessment of each major toxicological endpoint in a complete and transparent manner.
It is noteworthy that only the toxicological endpoints required to support a particular problem formulation need to be assessed. For example, in certain applications only an assessment of gene mutation may be needed (i.e., it may not be necessary to compute clastogenicity or the genetic toxicity major toxicological endpoint).
2.7.2 Relevance
Relevance, in this context, is defined as the scientific predictivity of the each toxicological effect or mechanism for the purpose of assessing a specific toxicological endpoint. As shown in Figure 6, the assessment of toxicological endpoints may be based on the associated toxicological effects or mechanisms. To support a transparent overall analysis, the relevance of the toxicological effect/mechanism information in support of the assessment of the associated toxicological endpoint will be defined in the IST protocols. This relevance will be based on the collective experience of the consortium and available validation information.
2.7.3 Toxicological endpoint assessment
The assessment of each toxicological endpoint (as shown in Figure 6) is a function of all associated toxicological effects or mechanisms and, in some cases, other toxicological endpoints. For example, in Figure 6, bacterial gene mutation and mammalian gene mutation (toxicological effects or mechanisms) are associated with gene mutation, whereas gene mutation and clastogenicity (both toxicological endpoints) are associated with genetic toxicity. Rules or general principles for combining all associated results for each endpoint will be defined in the upcoming IST protocols. For example, a rule may state that if one of the associated effects/mechanisms is positive then the endpoint assessment is positive. These rules or principles will take into consideration how combinations of different toxicological effects/mechanisms are evaluated to generate an assessment for any toxicological endpoint which may include a sequence of steps and incorporate Boolean logic.
2.7.4 Toxicological endpoint confidence
Confidence, in this context, is defined as a score that combines the reliability and relevance of the associated toxicological effects or mechanisms. This is an additional score associated with toxicological endpoints. The score may, in some cases, use other toxicological endpoint confidence scores (as shown in Figure 6). This score will also take into consideration the completeness of the information available; for example, the confidence score may be lowered when information on an effect or mechanism is missing. It will also include complementary effects or mechanisms that need to be considered. This score will be generated based on a series of general principles and/or rules defined in each IST protocol. Each protocol will outline the different confidence values to generate, such as high, medium or low.
A confidence score is one of the most important items to generate. Different decision contexts tolerate a different level of confidence in the assessment result as exemplified in the following two scenarios.
Scenario 1. The decision is to prioritize a large number of chemicals to screen as part of product development. In this scenario, selecting a small subset of compounds using in silico methods supports strategic resource utilization with the eventual goal of reducing overall costs.
Scenario 2. A regulatory submission for a new cosmetic ingredient is being prepared based on results from in silico methods.
Although in both scenarios, toxicological endpoint assessments generated at the highest level of confidence would be preferable, Scenario 1 could still make beneficial use of lower confidence predictions because the safety consequences of a false negative is lower than in Scenario 2. Therefore, a risk assessment which takes into account the acceptable tolerance for a wrong prediction can be used to evaluate the necessity for high confidence.
The assignment of the confidence score for each toxicological endpoint has to support the decision context(s), regulatory framework and the type of product being assessed. Minimum confidence scores for regulatory purposes may need to be set; however for other applications, the use of these scores may be based on the individual organization’s risk tolerance or based on the context, a decision on the maximum permitted effort to be expended (since higher confidence score may be generated with additional resources), or an organization’s internal policy for using the confidence scores for specific tasks.
2.7.5 Expert review of toxicological endpoints
In certain situations, an expert review of the toxicological endpoint assessment and/or confidence may be warranted, and specific points to consider as part of such an expert review will be detailed in the individual IST protocols. This review may take into consideration the context of the assessment, that is, the type of product being assessed and any potential regulatory framework. It may be helpful to document any additional considerations concerning the assessment and confidence to support an overall assessment.
2.8 In silico toxicology protocol components
Ongoing efforts are concentrated on the development of individual IST protocols for major endpoints including genetic toxicity, carcinogenicity, acute toxicity, repeated dose toxicity, reproductive toxicity, and developmental toxicity. Table 5 outlines proposed common components for these IST protocols.
Table 5.
Common components of an IST protocol (IATA = Integrated Approaches to Testing and Assessment; AOP = Adverse Outcome Pathways)
| Introduction |
|
| In silico methodologies and models |
|
| Experimental data |
|
| Toxicological effects or mechanisms assessment and reliability scores |
|
| Toxicological endpoint assessment and confidence |
|
| Reporting |
|
| Other considerations |
|
2.9 Reporting formats
Standardized reporting of the results and expert review is good scientific practice and assures that when such information is communicated to regulatory authorities, it is complete, consistent and transparent; this may avoid requests for additional information and maintain a consistent, expedient, and streamline regulatory review process. Table 6 outlines a proposed structure for the report format.
Table 6.
Elements of an in silico toxicology report (QMRF = QSAR Model Reporting Format)
| Section | Content |
|---|---|
| Title page |
|
| Executive summary |
|
| Purpose |
|
| Materials and methods |
|
| Results of Analysis |
|
| Conclusion |
|
| References |
|
| Appendices (optional) |
|
The proposed report format is more comprehensive than existing data formats by including information on overall assessment and expert reviews. For example, the “QSAR prediction reporting format” (QPRF; JRC 2014) could be used to report the individual model results (as shown in Section D of Table 6), or “QSAR model reporting format” (QMRF) can be used to report the QSAR model’s details (as shown in Section H of Table 6).
The new proposed report format collects enough details on how the predictions were generated to enable another expert to repeat the process. It is also important that the reasoning and decisions of the expert review steps are transparently documented and can be retraced at any time, including the information used as their basis for conclusions.
3. Summary and outlook
IST is poised to play an increasingly significant role in the assessment of chemicals in a range of chemical exposure scenarios that have the potential to impact public health. Thus, this is an opportune time for the development of IST protocols. As expected, the quality and quantity of experimental data will vary as will the available in silico methods. For example, experimental data could be from a variety of sources, studies, protocols and laboratories using or not using GLP standards. Similarly, several in silico methods and approaches are available for assessment of toxicity. Thus, accepted selection criteria have to be defined for experimental data and in silico methods, for consistent and uniform use. The development of IST protocols will support the use and adoption of in silico methods in the same manner in which in vitro and in vivo test guidelines support the use and adoption of those assays.
Figure 7 summarizes the steps to perform an in silico assessment consistent with the framework defined in this publication. The key elements needed for the development of IST protocols are outlined in this publication, including: 1) how to select, assess and integrate in silico predictions alongside experimental data for defined toxicological effects or mechanisms, including a new methodology for establishing the reliability of this assessment, 2) a hazard assessment framework for systematic assessment of these toxicological effects or mechanisms to predict specific endpoints and assess the confidence in the results. Wherever possible, this is based on mechanistic knowledge on different biological levels of organization. (Bell et al., 2016; OECD 2016a; OECD 2016b) Overall, the IST protocols will contain information to ensure predictions are performed in a consistent, repeatable, transparent and ultimately accepted manner and will include a checklist (as defined in Section 2.4) to guide an expert review of the information. Each individual IST protocol will address how predictions will be performed in alignment with the framework discussed in this publication. These new protocols will provide specific guidance for each toxicological endpoint, including situations where no AOP or IATA is currently available. These protocols build on and fully incorporate wherever possible the considerable work previously reported, such as the OECD validation principles (see Sections 2.3.2), IATAs (see Sections 2.2), AOPs (see Sections 2.2), read-across frameworks (see Sections 2.3.2, 2.6.2), the Klimisch score (see Sections 2.5, 2.6.1, 2.6.2) and the QMRF/QPRF (see Sections 2.3.2, 2.9).
Figure 7.
Summary of the IST protocol process
* Based on rules/principles outlined in the IST protocols, including an expert review if warranted
The IST protocols do not define how a risk assessment will be performed; they solely define the process which will lead to the prediction of the potential toxicity (hazard) of a chemical. Risk analysis depends on the exposure scenario, industry, regulatory framework and decision context based on the level of tolerated uncertainty and is performed in the hands of an expert.
The process of developing IST protocols requires an understanding of the best practices and science across various organizations, different industries and regulatory authorities. To develop such protocols, an international consortium was established comprising regulators, government agencies, industry, academics, model developers, and consultants across many different sectors. This consortium initially developed the overall strategy outlined in this publication. Working subgroups will develop individual IST protocols for major endpoints including genetic toxicity, carcinogenicity, acute toxicity, reproductive toxicity, and developmental toxicity. As each IST protocol is established, it will be reviewed internally within each organization and published. This process will evolve over time, as computational technology progresses, as will the assays and other information relevant to assessing these major endpoints emerges. Hence, similar to other test guidelines, the IST protocols will need to be periodically reviewed and updated. The implementation of IST protocols will also require user-friendly tools for performing such analyses and reporting the results, education, as well as further collaboration with organizations to support global adoption.
Supplementary Material
Highlights.
General outline of in silico toxicology protocols is described
A reliability score for predictions alongside experimental data is discussed
A checklist for performing an expert review of the in silico results is outlined
A hazard assessment framework is proposed that includes in silico results
Acknowledgments
Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number R43ES026909. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to thank Sachin Bhusari and George Pugh from the Coca-Cola Company for their input into the manuscript.
Footnotes
FDA CDER Disclaimer: The findings and conclusions in this manuscript have not been formally disseminated by the FDA and should not be construed to represent any agency determination or policy. The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services.
NIEHS Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the National Institutes of Health (NIH), NIEHS. The mention of commercial products, their sources, or their use should not be construed as either an actual or implied endorsement of such products by the NIH/NIEHS.
MHRA Disclaimer: Any opinions expressed in this document are the author’s and are not necessarily shared by other assessors at the Medicines and Healthcare products Regulatory Agency (MHRA). As such, they cannot be considered to be UK policy. The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the UK’s MHRA.
CDC/ATSDR Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention or the Agency for Toxic Substances and Disease Registry. Mention of trade names is not an endorsement of any commercial product.
EPA Disclaimer: The views expressed in this article are those of the author(s) and do not necessarily reflect the views or policies of the U.S. Environmental Protection Agency. Mention of trade names or commercial products does not constitute endorsement or recommendation for use.
EFSA Disclaimer: This paper reflects Dr. Serafimova’s personal view and is not endorsed by the European Food Safety Authority
Health Canada Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of Health Canada. The mention of commercial products, their sources, or their use should not be construed as either an actual or implied endorsement of such products by Health Canada.
DHS Disclaimer: The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the U.S. Government, Department of Homeland Security (DHS), DHS Science and Technology (S&T) Homeland Security Advanced Research Projects Agency (HSARPA), or the Chemical Security Analysis Center (CSAC). In no event shall either the U.S. Government, DHS, HSARPA, CSAC, or the author(s) have any responsibility or liability for any consequences of any use, misuse, inability to use, or reliance upon the information contained herein, nor do any of the parties set forth in this disclaimer warrant or otherwise represent in any way the accuracy, adequacy, efficacy, or applicability of the contents hereof. The use of trade, firm, company, or corporation names including product descriptions does not constitute an official DHS endorsement of any service or product.
European Commission Disclaimer: The views expressed are solely those of the authors and the content does not necessarily represent the views or position of the European Commission.
It should be noted that black box models may be acceptable in certain situations, such as compound filtering and virtual screening, as long as they show acceptable performance in validation studies; however, for most applications the acceptance of this class of models is low.
A chemical is assigned to a category (e.g., 1, 2, 3, 4, or 5) based on distinct ranges of quantitative values (e.g., LD50). Examples of such ranges include LD50 <5mg/kg (i.e., category 1) or 50–300mg/kg (i.e., category 3).
As mentioned in Section 2.5, where high quality experimental data are available (as shown in Figure 1), it may not be necessary to run in silico models. However, generating in silico predictions for chemicals with known values is sometimes performed to verify experimental results because an unexpected positive or negative experimental result in a physical assay may be explained by the presence of an active impurity or to provide additional weight-of-evidence or for other reasons.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alves V, Muratov E, Capuzzi S, Politi R, Low Y, Braga R, Zakharov AV, Sedykh A, Mokshyna E, Farag S, Andrade C, Kuz’min D, Fourches D, Tropsha A. Alarms about structural alerts. Green Chem. 2016;18(16):4348–4360. doi: 10.1039/C6GC01492E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral RTD, Ansell J, Aptula N, Ashikaga T, Chaudhry Q, Hirose A, Jaworska J, Kojima H, Lafranconi M, Matthews E, Milstein S, Roesler C, Vaillancourt E, Verma R, Worth A, Yourick J. Report for the International Cooperation on Cosmetics Regulation. Silico Approaches for Safety Assessment of Cosmetic Ingredients. 2014 https://www.pharmamedtechbi.com/~/media/Supporting%20Documents/The%20Rose%20Sheet/36/ICCR%20In%20Silico%20Report.pdf.
- Amaral R, Amores Da Silva P, Ansell J, Boisleve F, Dent M, Hatao M, Hirose A, Kasai Y, Kojima H, Kern P, Kreiling R, Milstein S, Oliveira J, Richarz A, Taalman R, Vaillancourt E, Verma R, Vieira NC, Weiss C. Report for the International Cooperation on Cosmetics Regulation, Joint Regulators-Industry Working Group: Integrated Strategies for Safety Assessments of Cosmetic Ingredients - Part I. 2017 http://www.iccr-cosmetics.org/files/4715/0824/0761/ICCR-11_JWG_Integrated_Strategies_Integrated_Strategies_for_Safety_Assessments_of_Cosmetic_Ingredients_-_Part_I.pdf.
- Amberg A, Harvey JS, Czich A, Spirkl H-P, Robinson S, White A, Elder DP. Do Carboxylic/Sulfonic acid halides really present a mutagenic and carcinogenic risk as impurities in final drug products? Org Process Res Dev. 2015;19:1495–506. doi: 10.1021/acs.oprd.5b00106. [DOI] [Google Scholar]
- Amberg A, Beilke L, Bercu J, Bower D, Brigo A, Cross KP, Custer L, Dobo K, Dowdy E, Ford KA, Glowienke S, Gompel JV, Harvey J, Hasselgren C, Honma M, Jolly R, Kemper R, Kenyon M, Kruhlak N, Leavitt P, Miller S, Muster W, Nicolette J, Plaper A, Powley M, Quigley DP, Reddy MV, Spirkl HP, Stavitskaya L, Teasdale A, Weiner S, Welch DS, White A, Wichard J, Myatt GJ. Principles and procedures for implementation of ICH M7 recommended (Q)SAR analyses. Regulatory Toxicology and Pharmacology. 2016;77:13–24. doi: 10.1016//j.yrtph.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Ball N, Cronin MT, Shen J, Blackburn K, Booth ED, Bouhifd M, Donley E, Egnash L, Hastings C, Juberg DR, Kleensang A, Kleinstreuer N, Kroese ED, Lee AC, Luechtefeld T, Maertens A, Marty S, Naciff JM, Palmer J, Pamies D, Penman M, Richarz AN, Russo DP, Stuard SB, Patlewicz G, van Ravenzwaay B, Wu S, Zhu H, Hartung T. Toward Good Read-Across Practice (GRAP) guidance. ALTEX. 2016;33:149–66. doi: 10.14573/altex.1601251. http://dx.doi.org/10.14573/altex.1601251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball D, Norwood D. Leachables and Extractables Handbook. Wiley; 2012. pp. 58–79. [Google Scholar]
- Barber C, Amberg A, Custer L, Dobo KL, Glowienke S, Gompel JV, Gutsell S, Harvey J, Honma M, Kenyon MO, Kruhlak N, Muster W, Stavitskaya L, Teasdale A, Vessey J, Wichard J. Establishing best practise in the application of expert review of mutagenicity under ICH M7. Regulatory Toxicology and Pharmacology. 2015;73:367–377. doi: 10.1016/j.yrtph.2015.07.018. [DOI] [PubMed] [Google Scholar]
- Bassan A, Worth AP. The Integrated Use of Models for the Properties and Effects of Chemicals by means of a Structured Workflow. QSAR & Combinatorial Science. 2008;27:6–20. doi: 10.1002/qsar.200710119. [DOI] [Google Scholar]
- Bell SM, Angrish MM, Wood CE, Edwards SW. Integrating Publicly Available Data to Generate Computationally Predicted Adverse Outcome Pathways for Fatty Liver. Toxicological Sciences. 2016;150:510–520. doi: 10.1093/toxsci/kfw017. [DOI] [PubMed] [Google Scholar]
- Berggren E, White A, Ouedraogo G, Paini A, Richarz AN, Bois FY, Exner T, Leite S, Grunsven LAV, Worth A, Mahony C. Ab initio chemical safety assessment: A workflow based on exposure considerations and non-animal methods. Computational Toxicology. 2017;4:31–44. doi: 10.1016/j.comtox.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn K, Stuard SB. A framework to facilitate consistent characterization of read across uncertainty. Regulatory Toxicology and Pharmacology. 2014;68:353–362. doi: 10.1016/j.yrtph.2014.01.004. [DOI] [PubMed] [Google Scholar]
- Bossuyt MV, Hoeck EV, Raitano G, Manganelli S, Braeken E, Ates G, Vanhaecke T, Miert SV, Benfenati E, Mertens B, Rogiers V. (Q)SAR tools for priority setting: A case study with printed paper and board food contact material substances. Food and Chemical Toxicology. 2017;102:109–119. doi: 10.1016/j.fct.2017.02.002. [DOI] [PubMed] [Google Scholar]
- Bower D, Cross KP, Esche S, Myatt GJ, Quigley DP. In silico Toxicology: An Overview of Toxicity Databases, Prediction Methodologies, and Expert Review. In: Richardson RJ, Johnson DE, editors. Computational Systems Pharmacology and Toxicology. Royal Society of Chemistry; 2017. [DOI] [Google Scholar]
- Canada. Chemicals Management Plan. Government of Canada; 2016. https://www.canada.ca/en/health-canada/services/chemical-substances/chemicals-management-plan.html. [Google Scholar]
- Carrió P, Pinto M, Ecker G, Sanz F, Pastor M. Applicability Domain Analysis (ADAN): A Robust Method for Assessing the Reliability of Drug Property Predictions. Journal of Chemical Information and Modeling. 2014;54:1500–1511. doi: 10.1021/ci500172z. [DOI] [PubMed] [Google Scholar]
- CDRH. Use of International Standard ISO 10993-1. Biological evaluation of medical devices - Part 1: Evaluation and testing within a risk management process. Guidance for Industry and Food and Drug Administration Staff. 2016 Jun 16; 2016. https://www.fda.gov/downloads/medicaldevices/deviceregulationandguidance/guidancedocuments/ucm348890.pdf.
- Chen B, Zhang T, Bond T, Gan Y. Development of quantitative structure activity relationship (QSAR) model for disinfection byproduct research: A review of methods and resources. J Hazard Mater. 2015;299:260–279. doi: 10.1016/j.jhazmat.2015.06.054. [DOI] [PubMed] [Google Scholar]
- Cherkasov A, Muratov EN, Fourches D, Varnek A, Baskin II, Cronin M, Dearden J, Gramatica P, Martin YC, Todeschini R, Consonni V, Kuz’min VE, Cramer R, Benigni R, Yang C, Rathman J, Terfloth L, Gasteiger J, Richard A, Tropsha A. QSAR modeling: where have you been? Where are you going to? J Med Chem. 2014;57:4977–5010. doi: 10.1021/jm4004285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobo KL, Greene N, Fred C, Glowienke S, Harvey JS, Hasselgren C, Jolly R, Kenyon MO, Munzner JB, Muster W, Neft R, Reddy MV, White AT, Weiner S. In silico methods combined with expert knowledge rule out mutagenic potential of pharmaceutical impurities: an industry survey. Regul Toxicol Pharmacol. 2012;62:449–455. doi: 10.1016/j.yrtph.2012.01.007. [DOI] [PubMed] [Google Scholar]
- ECETOC. ECETOC Technical Report No. 116: Category approaches, Read-across, (Q)SAR. 2012 http://www.ecetoc.org/wp-content/uploads/2014/08/ECETOC-TR-116-Category-approaches-Read-across-QSAR.pdf.
- ECHA. Guidance on information requirements and chemical safety assessment Chapter R.6: QSARs and grouping of chemicals. 2008 https://echa.europa.eu/documents/10162/13632/information_requirements_r6_en.pdf/77f49f81-b76d-40ab-8513-4f3a533b6ac9.
- ECHA. Guidance on information requirements and chemical safety assessment Chapter R.4: Evaluation of available Information. 2011 https://echa.europa.eu/documents/10162/13643/information_requirements_r4_en.pdf/d6395ad2-1596-4708-ba86-0136686d205e.
- ECHA. Guidance on the Application of the CLP Criteria Guidance to Regulation (EC) No 1272/2008 on classification, labelling and packaging (CLP) of substances and mixtures. 2015 https://echa.europa.eu/documents/10162/23036412/clp_en.pdf/58b5dc6d-ac2a-4910-9702-e9e1f5051cc5.
- ECHA. Practical guide: How to use and report (Q)SARs. 2016 https://echa.europa.eu/documents/10162/13655/pg_report_qsars_en.pdf.
- ECHA. Weight of evidence. 2017a https://echa.europa.eu/support/registration/how-to-avoid-unnecessary-testing-on-animals/weight-of-evidence.
- ECHA. Read-Across Assessment Framework (RAAF) 2017b https://echa.europa.eu/documents/10162/13628/raaf_en.pdf.
- ECVAM. ToxRTool - Toxicological data Reliability Assessment Tool. 2017 https://eurl-ecvam.jrc.ec.europa.eu/about-ecvam/archive-publications/toxrtool/toxrtool-toxicological-data-reliability-assessment-tool.
- EFSA. European Food Safety Authority; Submission of scientific peer-reviewed open literature for the approval of pesticide active substances under Regulation (EC) No 1107/2009. EFSA Journal. 2011;9:1–49. doi: 10.2903/j.efsa.2011.2092.. https://www.efsa.europa.eu/en/efsajournal/pub/2092. [DOI] [Google Scholar]
- EFSA. Modern methodologies and tools for human hazard assessment of chemicals. EFSA Journal. 2014;2014:121–87. doi: 10.2903/j.efsa.2014.3638.. https://www.efsa.europa.eu/de/efsajournal/pub/3638. [DOI] [Google Scholar]
- EFSA. Guidance on the establishment of the residue definition for dietary risk assessment: EFSA Panel on Plant Protection Products and their Residues (PPR) EFSA Journal. 2016;14:1–12. http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2016.4549/epdf. [Google Scholar]
- EFSA. European Food Safety Authority. 2017a http://www.efsa.europa.eu/
- Ellison CM, Piechota P, Madden JC, Enoch SJ, Cronin MTD. Adverse Outcome Pathway (AOP) Informed Modeling of Aquatic Toxicology: QSARs, Read-Across, and Interspecies Verification of Modes of Action. Environmental Science & Technology. 2016;50:3995–4007. doi: 10.1021/acs.est.5b05918. [DOI] [PubMed] [Google Scholar]
- EU. Regulation (EC) No 1907/2006 of the European Parliament and of the council of 18 December 2006 concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) 2006 http://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02006R1907-20161011&from=EN.
- EU. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products (recast) (Text with EEA relevance) 2009a http://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2009:342:0059:0209:en:PDF.
- EU. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. 2009b http://eur-lex.europa.eu/legal-content/en/TXT/?uri=CELEX%3A32009R1107.
- EU. Guidance Document on the Assessment of the Equivalence of Technical Materials of Substances Regulated UNDER Regulation (EC) No 1107/2009 SANCO/10597/2003 –rev. 10.1 13. 2012 https://ec.europa.eu/food/sites/food/files/plant/docs/pesticides_guidance_equivalence-chem-substances_en.pdf.
- Ford KA. Refinement, Reduction, and Replacement of Animal Toxicity Tests by Computational Methods. ILAR J. 2016;57(2):226–233. doi: 10.1093/ilar/ilw031. [DOI] [PubMed] [Google Scholar]
- Freidig A, Dekkers S, Verwei M, Zvinavashe E, Bessems J, Sandt JVD. Development of a QSAR for worst case estimates of acute toxicity of chemically reactive compounds. Toxicology Letters. 2007;170:214–222. doi: 10.1016/j.toxlet.2007.03.008. [DOI] [PubMed] [Google Scholar]
- Harvey J, Fleetwood A, Ogilvie R, Teasdale A, Wilcox P, Spanhaak S. Management of organic impurities in small molecule medicinal products: Deriving safe limits for use in early development. Regulatory Toxicology and Pharmacology. 2017;84:116–123. doi: 10.1016/j.yrtph.2016.12.011. [DOI] [PubMed] [Google Scholar]
- Hillisch A, Heinrich N, Wild H. Computational Chemistry in the Pharmaceutical Industry: From Childhood to Adolescence. ChemMedChem. 2015;10:1958–1962. doi: 10.1002/cmdc.201500346. [DOI] [PubMed] [Google Scholar]
- Hochstein C, Arnesen S, Goshorn J, Szczur M. Selected Resources for Emergency and Disaster Preparedness and Response from the United States National Library of Medicine. Medical Reference Services Quarterly. 2008;27:1–20. doi: 10.1300/j115v27n01_01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ICH M7. (R1). Assessment and control of DNA reactive (mutagenic) impurities in pharmaceuticals to limit potential carcinogenic risk. 2017 http://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Multidisciplinary/M7/M7_R1_Addendum_Step_4_31Mar2017.pdf.
- ICH. International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use. 2017 http://www.ich.org/products/guidelines.html.
- JRC. JRC QSAR Model Database and QSAR Model Reporting Formats. 2014 https://eurl-ecvam.jrc.ec.europa.eu/laboratories-research/predictive_toxicology/qsar_tools/qrf and http://qsardb.jrc.it/qmrf/
- Klimisch HJ, Andreae M, Tillmann U. A Systematic Approach for Evaluating the Quality of Experimental Toxicological and Ecotoxicological Data. Regulatory Toxicology and Pharmacology. 1997;25:1–5. doi: 10.1006/rtph.1996.1076. [DOI] [PubMed] [Google Scholar]
- Kruhlak NL, Benz RD, Zhou H, Colatsky TJ. (Q)SAR modeling and safety assessment in regulatory review. Clin Pharmacol Ther. 2012;91:529–34. doi: 10.1038/clpt.2011.300. [DOI] [PubMed] [Google Scholar]
- Mansouri K, Grulke CM, Richard AM, Judson RS, Williams AJ. An automated curation procedure for addressing chemical errors and inconsistencies in public datasets used in QSAR modelling. SAR and QSAR in Environmental Research. 2016;27:911–937. doi: 10.1080/1062936x.2016.1253611. [DOI] [PubMed] [Google Scholar]
- Martin T, Young D, Lilavois C, Barron M. Comparison of global and mode of action-based models for aquatic toxicity. SAR and QSAR in Environmental Research. 2015;26:245–262. doi: 10.1080/1062936x.2015.1018939. [DOI] [PubMed] [Google Scholar]
- Molander L, Ågerstrand M, Beronius A, Hanberg A, Rudén C. Science in Risk Assessment and Policy (SciRAP): An Online Resource for Evaluating and Reporting In Vivo(Eco)Toxicity Studies. Human and Ecological Risk Assessment: An International Journal. 2014;21:753–762. doi: 10.1080/10807039.2014.928104. [DOI] [Google Scholar]
- Mumtaz MM, Suk WA, Yang RSH. Introduction to Mixtures Toxicology and Risk Assessment. In: Mumtaz M, editor. Principles and Practice of Mixtures Toxicology. Wiley-VCH; Weinheim, Germany: 2010. pp. p1–25. [DOI] [Google Scholar]
- Mumtaz MM, Durkin PR. A weight of evidence scheme for assessing interactions in chemical mixtures. Toxicol Indus Health. 1992;8:377–406. [PubMed] [Google Scholar]
- Myatt GJ, Beilke LD, Cross KP. In Silico Tools and their Application. In: Reedijk J, editor. Reference Module in Chemistry, Molecular Sciences and Chemical Engineering. Elsevier; 2016. doi: https://doi.org/10.1016/B978-0-12-409547-2.12379-0. [Google Scholar]
- NAFTA. TWG Quantitative Structure Activity Relationships [(Q)SAR] Guidance Document. 2012 https://archive.epa.gov/pesticides/news/web/pdf/qsar-guidance.pdf.
- Netzeva TI, Worth AP, Aldenberg T, Benigni R, Cronin MTD, Gramatica P, Jaworska JS, Kahn S, Klopman G, Marchant CA, Myatt G, Nikolova-Jeliazkova N, Patlewicz GY, Perkins R, Roberts DW, Schultz TW, Stanton DT, van de Sandt JJM, Tong W, Veith G, Yang C. Current Status of Methods for Defining the Applicability Domain of (Quantitative) Structure-Activity Relationships. The Report and Recommendations of ECVAM Workshop 52. ATLA. 2005;33:155–173. doi: 10.1177/026119290503300209. [DOI] [PubMed] [Google Scholar]
- NIEHS. National Institute of Environmental Health Sciences. 2017 https://www.niehs.nih.gov/index.cfm.
- NTP. West Virginia Chemical Spill: NTP Studies. 2016 https://ntp.niehs.nih.gov/results/areas/wvspill/studies/
- OCSPP. Office of Chemical Safety and Pollution Prevention (OCSPP) 2017 https://www.epa.gov/aboutepa/about-office-chemical-safety-and-pollution-prevention-ocspp.
- OECD. The report from the expert group on (Quantitative) Structure-Activity Relationships [(Q)SARs] on the principles for the validation of (Q)SARs, No. 49 (ENV/JM/MONO(2004)24) 2004 http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2004)24&doclanguage=en.
- OECD. Guidance Document on the Validation of (Quantitative) Structure-activity Relationships [(Q)SAR] Models, OECD Environment Health and Safety Publications Series on Testing and Assessment No. 69 (ENV/JM/MONO(2007)2) 2007 http://www.oecd.org/env/guidance-document-on-the-validation-of-quantitative-structure-activity-relationship-q-sar-models-9789264085442-en.htm.
- OECD. Guidance on grouping of chemicals, second edition. OECD Environment Health and Safety Publications Series on Testing & Assessment. No. 194 ENV/JM/MONO(2014)4. 2014 http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2014)4&doclanguage=en.
- OECD. Fundamental And Guiding Principles For (Q)SAR Analysis Of Chemical Carcinogens with Mechanistic Considerations, Monograph 229 (ENV/JM/MONO(2015)46), Series on Testing and Assessment No. 229. 2015 http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2015)46&doclanguage=en.
- OECD. Guidance document on the reporting of defined approaches to be used within integrated approaches to testing and assessment, Series on Testing & Assessment No. 255, ENV/JM/MONO(2016)28. 2016a http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2016)28&doclanguage=en.
- OECD. Guidance document on the reporting of defined approaches and individual information sources to be used within integrated approaches to testing and assessment (IATA) for skin sensitation. ENV/JM/MONO(2016)29. Series on Testing & Assessment No. 256. 2016b http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2016)29&doclanguage=en.
- OECD. Draft guidance document on good in vitro methods Practices (GIVIMP) for the development and implementation of in vitro methods for regulatory use in human safety assessment. 2016c http://www.oecd.org/env/ehs/testing/OECD_Draft_GIVIMP_in_Human_Safety_Assessment.pdf.
- OECD. OECD Guidelines for the Testing of Chemicals. 2017 http://www.oecd.org/chemicalsafety/testing/oecdguidelinesforthetestingofchemicals.htm.
- Patlewicz G, Ball N, Booth ED, Hulzebos E, Zvinavashe E, Hennes C. Use of category approaches, read-across and (Q)SAR: General considerations. Regulatory Toxicology and Pharmacology. 2013a;67:1–12. doi: 10.1016/j.yrtph.2013.06.002. [DOI] [PubMed] [Google Scholar]
- Patlewicz G, Roberts DW, Aptula A, Blackburn K, Hubesch B. Workshop: Use of “read-across” for chemical safety assessment under REACH. Regulatory Toxicology and Pharmacology. 2013b;65:226–228. doi: 10.1016/j.yrtph.2012.12.004. [DOI] [PubMed] [Google Scholar]
- Patlewicz G. Read-across approaches - misconceptions, promises and challenges ahead. Altex. 2014;31:387–396. doi: 10.14573/altex.1410071. [DOI] [PubMed] [Google Scholar]
- Patlewicz G, Ball N, Boogaard P, Becker R, Hubesch B. Building scientific confidence in the development and evaluation of read-across. Regulatory Toxicology and Pharmacology. 2015;72:117–133. doi: 10.1016/j.yrtph.2015.03.015. [DOI] [PubMed] [Google Scholar]
- Patlewicz G, Worth AP, Ball N. Validation of Computational Methods. Advances in Experimental Medicine and Biology Validation of Alternative Methods for Toxicity Testing. 2016:165–187. doi: 10.1007/978-3-319-33826-2_6. [DOI] [PubMed] [Google Scholar]
- PMTA/FDA. Guidance for Industry. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Tobacco Products; May, 2016. Premarket Tobacco Product Applications for Electronic Nicotine Delivery Systems. 2016. https://www.fda.gov/downloads/tobaccoproducts/labeling/rulesregulationsguidance/ucm499352.pdf. [Google Scholar]
- Powley MW. (Q)SAR assessments of potentially mutagenic impurities: a regulatory perspective on the utility of expert knowledge and data submission. Regul Toxicol Pharmacol. 2015;71:295–300. doi: 10.1016/j.yrtph.2014.12.012. [DOI] [PubMed] [Google Scholar]
- Raies AB, Bajic VB. In silico toxicology: computational methods for the prediction of chemical toxicity. Wiley Interdiscip Rev Comput Mol Sci. 2016;6:147–172. doi: 10.1002/wcms.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rastogi T, Leder C, Kümmerer K. Designing green derivatives of β-blocker Metoprolol: A tiered approach for green and sustainable pharmacy and chemistry. Chemosphere. 2014;111:493–499. doi: 10.1016/j.chemosphere.2014.03.119. [DOI] [PubMed] [Google Scholar]
- Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA. Systematic review and evidence integration for literature-based environmental health science assessments. Environ Health Perspect. 2014;122:711–8. doi: 10.1289/ehp.1307972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell WMS, Burch RL. The principles of humane experimental technique. Methuen; London: 1959. [Google Scholar]
- SCCS. Memorandum on the Use of In Silico Methods for Assessment of Chemical Hazard. SCCS/1578/16. 2016 https://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_200.pdf.
- SCCS. Scientific Committee on Consumer Safety. 2017 https://ec.europa.eu/health/scientific_committees/consumer_safety_en.
- Schilter B, Benigni R, Boobis A, Chiodini A, Cockburn A, Cronin MT, Piparo EL, Modi S, Thiel A, Worth A. Establishing the level of safety concern for chemicals in food without the need for toxicity testing. Regulatory Toxicology and Pharmacology. 2014;68:275–296. doi: 10.1016/j.yrtph.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Schneider K, Schwarz M, Burkholder I, Kopp-Schneider A, Edler L, Kinsner-Ovaskainen A, Hartung T, Hoffmann S. “ToxRTool”, a new tool to assess the reliability of toxicological data. Toxicology Letters. 2009;189:138–144. doi: 10.1016/j.toxlet.2009.05.013. [DOI] [PubMed] [Google Scholar]
- Schultz T, Amcoff P, Berggren E, Gautier F, Klaric M, Knight D, Mahony C, Schwarz M, White A, Cronin M. A strategy for structuring and reporting a read-across prediction of toxicity. Regulatory Toxicology and Pharmacology. 2015;72:586–601. doi: 10.1016/j.yrtph.2015.05.016. [DOI] [PubMed] [Google Scholar]
- Schwetz B. Use of mechanistic and pharmacokinetic data for risk assessment at the National Institute of Environmental Health Sciences (NIEHS) Toxicology Letters. 1995;79:29–32. doi: 10.1016/0378-4274(95)03354-n. [DOI] [PubMed] [Google Scholar]
- Seed MJ, Agius RM. Progress with Structure-Activity Relationship modelling of occupational chemical respiratory sensitizers. Curr Opin Allergy Clin Immunol. 2017;17:64–71. doi: 10.1097/ACI.0000000000000355. [DOI] [PubMed] [Google Scholar]
- Stanton K, Kruszewski FH. Quantifying the benefits of using read-across and in silico techniques to fulfill hazard data requirements for chemical categories. Regulatory Toxicology and Pharmacology. 2016;81:250–259. doi: 10.1016/j.yrtph.2016.09.004. [DOI] [PubMed] [Google Scholar]
- Sutter A, Amberg A, Boyer S, Brigo A, Contrera JF, Custer LL, Dobo KL, Gervais V, Glowienke S, Gompel JV, Greene N, Muster W, Nicolette J, Reddy MV, Thybaud V, Vock E, White AT, Müller L. Use of in silico systems and expert knowledge for structure-based assessment of potentially mutagenic impurities. Regul Toxicol Pharmacol. 2013;67:39–52. doi: 10.1016/j.yrtph.2013.05.001. [DOI] [PubMed] [Google Scholar]
- TSCA. Toxic Substances Control Act (TSCA) 2016 https://www.congress.gov/bill/114th-congress/senate-bill/697/all-info.
- Worth A, Barroso J, Bremer S, Burton J, Casati S, Coecke S, Corvi R, Desprez B, Dumont C, Gouliarmou V, Goumenou M, Gräpel R, Griesinger C, Halder M, Janusch Roi A, Kienzler A, Madia F, Munn S, Nepelska M, Paini A, Price A, Prieto P, Rolaki A, Schäffer M, Triebe J, Whelan M, Wittwehr C, Zuang V. Alternative methods for regulatory toxicology – a state-of-the-art review. JRC report EUR 26797 EN. Publications Office of the European Union. 2014 http://publications.jrc.ec.europa.eu/repository/bitstream/JRC91361/echa_jrc_sla_report_public_05-09-14_withcover%20ipo.pdf.
- Wu S, Blackburn K, Amburgey J, Jaworska J, Federle T. A framework for using structural, reactivity, metabolic and physicochemical similarity to evaluate the suitability of analogs for SAR-based toxicological assessments. Regulatory Toxicology and Pharmacology. 2010;56:67–81. doi: 10.1016/j.yrtph.2009.09.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.