Abstract
The objective of our study was to examine the neurobiological support for an interoceptive sensory processing model of bulimia nervosa (BN). To do so, we conducted a systematic review of interoceptive sensory processing in BN, using the PRISMA guidelines. We searched PsychInfo, Pubmed, and Web of Knowledge databases to identify biological and behavioral studies that examine interoceptive detection in BN. After screening 390 articles for inclusion and conducting a quality assessment of articles that met inclusion criteria, we reviewed 41 articles. We found that global interoceptive sensory processing deficits may be present in BN. Specifically there is evidence of abnormal brain function, structure and connectivity in the interoceptive neural network, in addition to gastric and pain processing disturbances. These results suggest that there may be a neurobiological basis for global interoceptive sensory processing deficits in BN that remain after recovery. Data from taste and heart beat detection studies were inconclusive, yet some studies suggest interoceptive disturbances in these sensory domains. Discrepancies in findings appear to be due to methodological differences. In conclusion, interoceptive sensory processing deficits may directly contribute to and explain a variety of symptoms present in those with BN. Further examination of interoceptive sensory deficits could inform the development of treatments for those with BN.
Keywords: Interoception, bulimia nervosa, pain, gastric distention, taste, heartbeat
Introduction
Bulimia nervosa (BN) is a psychiatric disorder characterized by binge eating, purging and emotional dysregulation (APA, 2013). Patients with BN eat excessively large amounts of food in a short period of time, exhibit a loss of control and engage in compensatory behaviors in response to binge eating (APA, 2013). Compensatory behaviors include self-induced vomiting, excessive exercise and laxative, diuretic and diet pill use (APA, 2013). Comorbid behaviors are often sensory-seeking, such as substance abuse and self-harm (Koutek et al., 2016). These symptoms and behaviors may suggest a need for enhanced stimulation for detection of internal states and homeostatic processes, as well as difficulties in maintaining internal homeostasis. Symptoms of BN may function to regulate internal states. Additionally, patients with BN exhibit difficulties with emotion regulation (Lavender et al., 2014a). Thus, patients with BN may demonstrate difficulties maintaining both physical and emotional homeostasis.
Recent neurobiology research suggests that interoception plays a role in physical and emotional homeostasis (Craig, 2009). Interoception is defined as the “the sense of the physiological condition of the body” (Craig, 2002). By mapping spinal-thalamic-cortical afferents, Craig identified pathways projecting to the insula that are involved in interoceptive processing and he claimed that all physiological senses that obtain signals from C-fiber and A-delta afferents – such as pain, hunger, satiety, taste, sensual touch, heartbeat, breathlessness and the need to urinate and defecate – are considered interoceptive senses (Craig, 2002; Kanazawa et al., 2010; Yoshimura et al., 2006). Subsequent interoceptive research demonstrates the insula’s critical role in the previously mentioned interoceptive senses by its activation during fMRI (Bjornsdotter et al., 2014; Critchley et al., 2004; Greenspan and Winfield, 1992; Kanazawa et al., 2010; Ladabaum et al., 2001; Mayer et al., 2006; Ogawa et al., 1994; Olausson et al., 2008; Yoshimura et al., 2006). Although other definitions of interoception exist (Cameron, 2001), this review follows a neurobiological approach that examines interoception using Craig’s definition and focuses on the insula’s role in interoception.
Sensory processing, detection, regulation, awareness and prediction are important aspects of interoception. Interoceptive sensory processing, in particular, occurs automatically and may occur without conscious awareness. It assesses how well the body processes stimuli from interoceptive senses. Interoceptive awareness, on the other hand, as described within the eating disorder’s field, requires metacognitive (conscious and intentional) reflection upon one’s internal state. Interoceptive awareness may be impacted by numerous factors: interoceptive and exteroceptive integration, classical and operant conditioning, cognitive patterns, prediction accuracy, and defense mechanisms such as intentionally ignoring internal cues (consciously or unconsciously). Because interoceptive awareness is significantly influenced by higher order cortical processing (Garfinkel et al., 2015) and occurs secondary to interoceptive sensory processing, before making assumptions about the accuracy of one’s interoceptive awareness, it is critical to first examine how one processes interoceptive sensory cues.
Interoceptive sensory processing is important to explore in eating disorders since deficits in interoceptive neural networks may contribute to widespread disturbances in one’s ability to track his or her body. Specifically, difficulties with detecting hunger and satiety cues may lead to overeating, increased pain tolerance may make over-exercise and purging behaviors tolerable and abnormal taste processing could delay habituation to food rewards and could lead to binge eating behaviors. These widespread disturbances may not only impact interoceptive senses that are commonly related to eating disorder symptoms, but they may also impact interoceptive senses less directly associated with eating disorders such as pain, heartbeat detection, sensual touch, breathlessness and also the need to urinate. Interoceptive tracking abilities are also significant in ascertaining one’s sense of physical self and the ability to regulate one’s physiological self and emotions. Since BN is characterized by body image (Kaye, 2007; Mohr et al., 2011) and regulation disturbances (Svaldi et al., 2012), it is important to examine whether dysfunction within the insula and the interoceptive neural network could globally impact all interoceptive senses in BN, as compared to only impacting those specific to eating behaviors. These differentiations have numerous treatment implications, as global deficits may suggest a need for treatments that target broad interoceptive circuitry while specific interoceptive deficits may suggest targeting the specific mechanisms related to interoceptive senses of concern.
Recent research suggests that the insula contains distinct sub-divisions with different functions (Kurth et al., 2010; Shaw et al., 2008) which may contribute to different aspects of interoceptive processing. Specifically, the posterior/mid insula may be implicated in unconscious interoceptive processes while anterior regions may play important roles in conscious interoceptive processing such as interoceptive accuracy, awareness, sensibility, and anxiety sensitivity (Garfinkel et al., 2015). Additionally, although Craig described the insula and the ACC as the interoceptive cortex, it is important to consider that brain regions do not work in isolation and that insula subdivisions connect to distinct groupings of brain regions, thus forming networks that underlie various aspects of interoceptive processing (Simmons et al., 2013). A better understanding of the details involved in interoceptive circuitry may help us determine neurobiological mechanisms underlying symptoms in BN and improve treatments by addressing interoceptive dysfunction.
Classically, within the eating disorder field, interoception has been studied by examining conscious awareness of hunger and satiety in addition to also assessing emotion awareness. Bruch was the first to describe disturbed perception of body cues in eating disorders (Bruch, 1962) and later Garfinkel and colleagues labeled these deficits as poor interoception (Garfinkel et al., 1978). Although, a few studies from the 1960s and 1970s intentionally assessed these interoceptive deficits by examining hunger, satiety and body sense in eating disorders (Garfinkel et al., 1978), more recently, studies have relied on self-report These studies found elevated scores on the interoceptive awareness scale of the Eating Disorder Inventory (Garner et al., 1983) and suggest that those with BN report disturbed interoceptive processing (Fassino et al., 2004; Lilenfeld et al., 2006). Few studies, however, have examined whether perceived disturbances in BN are due to interoceptive sensory processing abnormalities or whether those with BN demonstrate disturbances in higher order processes. Studying interoceptive sensory processing in BN critical, as the results may elucidate whether interoceptive disturbances in BN are cognitively driven (and can be modified by changing cognitions) or whether interoceptive disturbances may be rooted in biological interoceptive sensory deficits. Our findings may ascertain whether self-report interoceptive awareness defects in BN are accurate reflections of one’s perceived internal state.
In this review, we propose and examine the existing support for a potential interoceptive model of BN. Our hypothesized model proposes that interoceptive sensory processing deficits drive symptoms and associated behaviors present in BN. Specifically, we hypothesize that global interoceptive sensory processing deficits are present in BN, and that abnormalities within the interoceptive neural network –and specifically the insula- impact numerous interoceptive senses in BN. These deficits then lead to greater susceptibility of binge eating and purging. We examine support for this model by systematically reviewing the neurobiological literature underlying interoceptive sensory processing in BN. We aggregated and synthesized studies in BN that examine brain regions involved in interoceptive processing and also various interoceptive senses. We examined interoceptive senses that were originally defined by Bud Craig and involved c-fiber or A-δ afferents that project to the insula (Craig, 2002). In BN, these studies include gastric, pain, taste processing and heart beat detection; no known studies to date have documented other interoceptive senses such as sensual touch, breathlessness and the urge to urinate in BN. To conduct this Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) compliant systematic review, as recommended by the PRISMA guidelines, we modified the literature search strategy, PICOS (patient population, intervention, comparison, outcomes and setting), to fit the requirements of this review. Study objectives were to examine case-controlled studies of interoceptive sensory processing in women with BN compared to controls.
2. Materials and Methods
2.1 Study eligibility criteria
We included original articles that examined interoceptive sensory processing in BN. In order to focus exclusively on BN, we excluded those in our search results only about anorexia nervosa (AN), binge eating disorder or obesity, animal studies, and case studies. Only original research articles were considered; reviews were excluded. We excluded all studies that did not include a control group. Within studies of BN, we included studies that examined current BN, BN purging type only and also those recovered from BN.
For neuroimaging studies, we reviewed imaging studies that used magnetic resonance imaging (MRI) and specifically described insula function in BN, as well as studies that directly examine interoceptive sensory processing in BN. In regards to gastric motility studies, we focused on cue detection and excluded articles exclusively focused on gastric emptying, which emphasized hormones and were outside the scope of this review on interoceptive sensory processing. With regard to taste studies, we included studies that assessed taste thresholds. We also reviewed studies using the heart beat detection task.
We assessed the quality of the included studies with a revised version of the Newcastle-Ottawa Quality Assessment Scale (Wells, et al., 2009). A detailed description of the quality criteria is included in figure 1. After rating each article using the revised Newcastle-Ottawa Assessment Scale, we included the means of these ratings in table 1.
Figure 1.

PRISMA 2009 Flow diagram.
From: Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group (2009). P referred R eporting I tems for S ystematic Reviews and M eta- A nalyses: The PRISMA Statement. PLoS Med 6(7): e1000097. doi:10.1371/journal.pmed1000097. For more information, visit www.prisma-statement.org.
Table 1.
Assessment of the quality of interoceptive sensory processing studies in bulimia nervosa using a modified version of the Newcastle-Ottawa Quality Assessment Scale.
| Author | Samples | Quality | Study Tasks | Presence of significant differences in insula or processing of interoceptive stimuli when comparing BN to HC? |
|---|---|---|---|---|
| Insula Studies | ||||
| Bohon & Stice 2012 | 26BN & 26HC | 4 | Functional connectivity as measured by fMRI when viewing food stimuli | Y |
| Brooks et al., 2011 | 8BN, 18AN & 24HC | 3.5 | fMRI food >non-food images | Y |
| Frank et al., 2013 | 19BN, 19AN, 19BN & 24HC | 3.5 | sMRI | Y |
| Kim et al., 2012 | 20BN, 18AN & 20HC | 2 | fMRI functional connectivity food >non-food images | Y |
| Mettler et al., 2013 | 20BN & 21HC | 4 | DTI measurement of white matter axon integrity | Y |
| Mohr et al., 2011 | 15BN & 16HC | 1 | fMRI body size estimation | Y |
| Schienle et al., 2009 | 24BN, 17BED, 19HC, 17 overweig ht HC | 3.5 | fMRI during food images, disgusting images and neutral images | Y |
| Van den Eynde et al., 2013 | 21BN & 23HC | 3.5 | fMRI during food pictures & self and thin other body picture comparison | Y-for body image only |
| Gastric | Gastric | |||
| Diamanti et al., 2003 | 10BN, 18AN & 16HC | 0.5 | EGG, Gastric Emptying Scintigraphy | Y |
| Geliebter et al., 1992 | 9BN & 19HC | 3 | Endoscopy of the upper GI tract, gastric capacity, gastric emptying, test-meal intake | Y |
| Geliebter et al., 2001 | 10BN, 10HC and 10 Obese Patients | 1 | Gastric capacity and self report ratings | Y |
| Koch et al., 1998 | 12BN & 13HC | 2 | Gastric emptying and visceral perception test | Y |
| Walsh et al., 2003 | 16BN & 16HC | 1 | Gastric capacity and gastric relaxation | Y-for gastric relaxation only |
| Zimmerli et al., 2006 | 19BN & 19HC | 3 | Gastric capacity and sensitivity to gastric relaxation | N-for gastric capacity; Y-for sensitivity to gastric distention |
| Pain | ||||
| de Zwaan et al., 1996 | 18BN, 22AN & 32HC | 3 | Mechanical and thermal pain threshold | Y |
| Girdler et al., 1998 | 14BN & 14HC | 4 | Pain threshold and its relationship to arterial blood pressure | Y |
| Lautenbacher et al., 1991 | 20BN, 21HC & 19AN | 2 | Heat pain threshold | Y |
| Papezova et al., 2005 | 18BN, 21AN & 17HC | 2 | Heat pain threshold | Y |
| Pauls et al., 1991 | 10BN, 9AN, 10HC | 2 | Heat and cold pain threshold and vibration pain threshold | Y for heat and cold pain; no for vibration pain threshold |
| Stein et al., 2003 | 11RAN, 15HC | 3 | Thermal pain and submaximal effort tourniquet test (SETT) | Y for SETT and near significance for thermal pain |
| Yamamotova et al., 2009 | 21BN & 21HC | 3 | Thermal pain threshold, blood pressure and heart rate under six different conditions | Y |
| Heart Beat Perception | ||||
| Eshkevari et al., 2014 | 22BN, 32AN, 20EDNOS & 60HC | 3.5 | Heartbeat tapping version of the HBPT | N |
| Klabunde et al., 2013 | 9RBN & 10HC | 2.5 | Heartbeat counting of the HBPT | Y |
| Pollatos & Georgiou 2016 | 23BN & 23HC | 3 | Heartbeat counting of the HBPT while controlling for BMI, depression, alexithymia and anxiety | N |
| Taste | ||||
| Aschenbrenner et al., 2008 | 24BN, 16AN & 23HC | 4 | Sniffin' Sticks olfactory test and Taste Strips gustatory test | N |
| Blazer et al., 2008 | 26BN & 26HC | 2.5 | Salivary and taste profile analysis | Y |
| Dazzi et al., 2013 | 19BN, 18AN & 19HC | 1.25 | Sniffin' Sticks olfactory test and Taste Strips gustatory test | Y |
| Drewnowki et al., 1987 | 7BN, 12AN-R, 13AN-BP & 16HC | 2.5 | Sensory estimates of sweetness and fat content in milk substances | N |
| Drewnowski et al., 1987 | 16BN & 16HC | 2.5 | Sensory estimates of sweetness and fat content in three cheeses | Y |
| Franko et al., 1994 (45) | 15 pure BN, 5BN with past history of AN, 20HC | 4 | Sweetness intensity | Y in BN-pure only |
| Frank et al., 2011 | 20BN & 23HC | 3 | Groups rated sweetness of taste solutions | N |
| Klein et al., 2009 | 11BN & 10HC | 4 | Consumption of varying levels of sweetened solutions | Y |
| Rodin et al., 1990 | 16BN & 16HC | 1 | Intensity of sweet, salty, sour and bitter solutions before and after ingesting a glucose load | Y |
| Sunday & Halmi 1990 | 29BN, 32AN-R, 28AN B-P, 16HC | 3 | Hunger and fullness ratings before and after a liquid meal and changes in hedonic preference for the liquid meal after an overnight fast and after completing the meal | N-rating of sweetness; Y- intensity of low fat and no sugar solutions |
| Wisniewski et al., 1997 | 18BN & 18CW | 2.5 | Salivation amount and desire to binge after consumption of a series of receiving 8 yogurt stimuli and a lemon juice trial | Y |
| Wöckel et al., 2007 | 18ED (BN and AN) & 15HC | 3.5 | Fungiform papillaie measured with PROP | N |
| Neurobiology Taste | ||||
| Bohon & Stice 2011 | 26BN & 26HC | 4 | fMRI taste anticipation and receipt of milkshake vs tasteless solution | Y |
| Frank et al., 2006 | 10BN & 6HC | 2 | fMRI taste processing of glucose > artificial saliva | N |
| Frank et al., 2011 | 20BN & 23HC | 3 | fMRI taste processing of expected and unexpected sucrose and artificial saliva administration | Y |
| Frank et al., 2016 | 20BN, 21AN, 19RAN & 27HC | 2.5 | fMRI classification accuracy of diagnosis during taste processing of sucrose, control solutions and no taste stimulation | N-but classification accuracy within the insula was negatively related with sweetness in BN |
| Radeloff et. al., 2014 | 14RBN, 15RAN & 18HC | 4 | fMRI taste of high fat crème, non-caloric substance viscous solution and water | N |
| Oberndorfer et al., 2013 | 14RBN, 14RAN & 14HC | 3 | fMRI taste processing of sucrose to sucralose | Y |
| Wagner et al., 2015 | 15BN, 15AN & 13HC | 3 | fMRI taste sensitization during sucrose vs sucralose administration | N |
2.2 Information sources
We used Pubmed, PsychINFO and Web of Knowledge databases and included articles up to June 2016. We also conducted a manual search on reference lists from relevant articles, reviews and editorials.
2.3 Search strategy
In order to identify all relevant studies published until June 2016, we searched PsychInfo, Pubmed, and Web of Knowledge databases according to the PRISMA guidelines. Our pre-defined search terms were influenced by interoceptive processes specified by Craig 2002 and included: “insula” and “bulimia nervosa”, “gastric distention” and “bulimia nervosa”, “pain threshold” and “bulimia nervosa”, “pain sensitivity” and “bulimia nervosa”, “pain tolerance” and “bulimia nervosa”, “taste” and “bulimia nervosa”, “interoceptive sensitivity” and “bulimia nervosa” and “interoception” and “bulimia nervosa”. We supplemented this list with references cited in reviewed articles that were specific to interoceptive sensory processing. Only studies in the English language, original case controlled studies and ‘human’ studies were included.
2.4 Data extraction
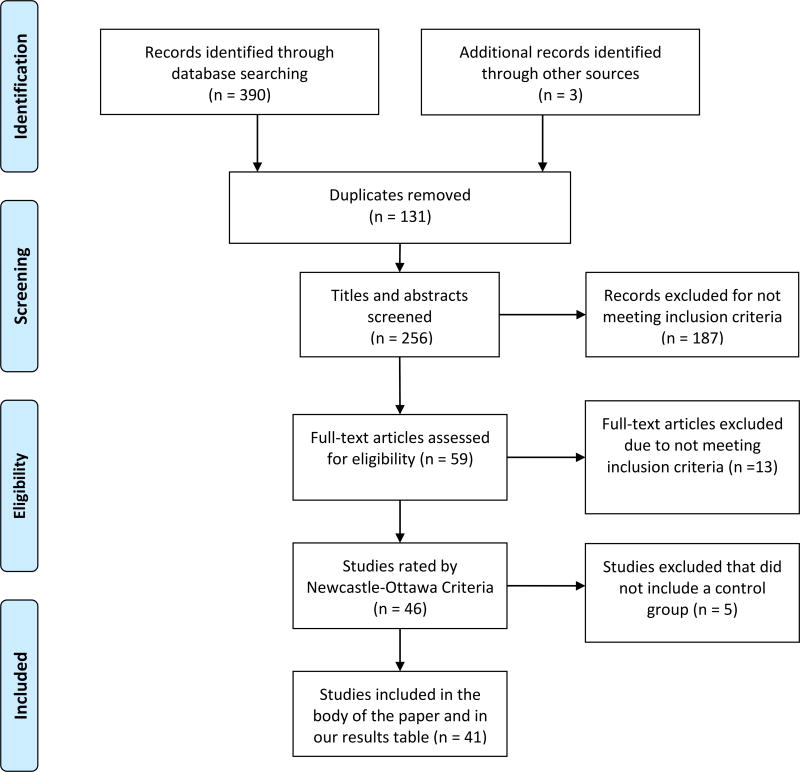
The relevant data were extracted from the included studies (see table 1) and briefly summarized. For this systematic review of case controlled studies, we modified and reported PICOS characteristics (O’Connor et al., 2008). To identify studies, authors (DC or MK) conducted a literature search using the keywords specified in section 2.3 and examined the titles and abstracts of the 390 records for their eligibility (see figure 2). They excluded 131 duplicate articles and excluded an additional 187 articles that did not meet eligibility criteria (as specified in section 2.1). The full texts of all potentially relevant studies were reviewed by either MK or CB, who excluded an additional 13 articles that did not meet inclusion criteria. The remaining 46 studies were reviewed by MK and CB who assessed (unblinded) the quality of the studies using the revised Newcastle Ottawa Quality Assessment Criteria (see figure 1). At this stage, five articles without control groups were excluded; thus a total of 41 articles were reviewed within this manuscript. An intraclass correlation coefficient (ICC) was calculated using a two-way random effects model with absolute agreement in order to assess our inner-rater reliability for each item of the revised Newcastle-Ottawa Quality Assessment Criteria (the mean Chronbach alpha for all items = .77).
Figure 2.
PRISMA flow chart for systematic review of interoceptive sensory processing in bulimia nervosa.
2.5 Risk of bias across studies
There was the potential risk of the following bias across studies: Language bias: we only considered articles in English, Database bias: we only included articles that were found in databases that were written in the English language, Study quality bias: we only included case-controlled studies and studies with clearly defined control groups. No randomized control trials were available on this topic in the literature. Publication bias: we only reviewed articles that were published in peer-reviewed journals, Selective reporting within studies: we did not present on the results of analyses that were not reported within the articles examined.
3. Results
We reviewed 41 articles that described various aspects of interoceptive sensory processing in BN. We examined each domain of interoceptive sensory processing in BN as identified by the literature search, present fMRI findings that have examined the insula in BN and describe the weaknesses of the studies reviewed.
3.1 Gastric Processing
Hunger and satiety regulate eating behaviors and can be investigated through gastric processing studies. Within the BN literature, these studies examine stomach distention and capacity with balloons and barostats, medical devices used to maintain constant pressure in a closed chamber. The barostat is also the gold standard used to evaluate the stomach’s volume, filling and emptying (Geliebter et al., 1992; Geliebter and Hashim, 2001; Walsh et al., 2003; Zimmerli et al., 2006) and is frequently used in distention procedures.
Two gastric distention studies using small sample sizes demonstrated that participants with BN have larger stomach capacities than both healthy controls and obese participants (Geliebter et al., 1992; Geliebter and Hashim, 2001). Additionally, when assessing gastric relaxation, how quickly the stomach relaxes after food intake, participants with BN have slower relaxation rates than controls (Walsh et al., 2003). When asked to rate upper abdominal sensation, participants with BN reported having lower sensation rates on a scale of 0–10 when the barostat’s pressure was increased (Zimmerli et al., 2006).
Another way to examine gastric processing is to measure gastric muscle activity with a noninvasive method called electrogastrography. When comparing AN and BN, the two groups differ significantly on gastric muscle activity, with BN showing decreased gastric muscle activity (Diamanti et al., 2003; Koch et al., 1998). Lower gastric relaxation rates and sensation rates could be attributed to reduced muscle activity; thus gastric muscle activity may be related to gastric capacity measures.
Despite the fact that two studies demonstrated low scores on the Newcastle Ottawa Quality Assessment Scale (Diamanti et al., 2003; Geliebter and Hashim, 2001), gastric studies demonstrate that females with BN have significantly decreased stomach sensitivity to fullness and decreased stomach relaxation compared to controls (Diamanti et al., 2003; Geliebter et al., 1992; Geliebter and Hashim, 2001). It is uncertain, however, whether the larger gastric capacities in those with BN are caused by prior binge eating or are due to interoceptive deficits that existed prior to the development of binge eating behaviors.
3.2 Pain
Several studies indicate impaired pain processing in BN. Studies have examined heat pain in BN and AN (Lautenbacher et al., 1991; Papezova et al., 2005). One found that participants with either BN or binge eating and purging type AN demonstrated significantly higher heat pain thresholds than both restricting type AN and healthy controls (Papezova et al., 2005). The second study also found that participants with BN and AN (not separated by type) demonstrated significantly higher heat pain thresholds than controls (Lautenbacher et al., 1991). A third study found significant differences in heat pain thresholds between BN, AN, and controls, with the greatest difference between BN and AN. Increased pain thresholds in AN and BN were also detected during cold pain stimuli (Pauls et al., 1991). Higher thermal pain thresholds in BN versus controls persisted across several different conditions (a mental stressor test, eating sweet food, rest, and a cold pressor test; (Yamamotova et al., 2009); pain thresholds correlated significantly with illness severity in BN. Several studies have also detected increased mechanical pain thresholds in BN (de Zwaan et al., 1996; Girdler et al., 1998)
High pain thresholds in females with BN persist after recovery. Significantly higher mechanical pain thresholds persisted in women recovered from BN (for over a year) in comparison to controls; there was a trend toward significant differences between groups on a heat pain threshold test (Stein et al., 2003). Overall, studies suggest that high pain thresholds occur in females with BN when administered a variety of pain stimuli and appear to remain after recovery. Several studies have attempted to further understand abnormal pain thresholds in BN. These studies again demonstrate pain deficits in BN, however, they reveal no definitive explanation for this abnormal pain processing in BN (Girdler et al., 1998; Lautenbacher et al., 1991; Papezova et al., 2005; Stein et al., 2003).
3.3 Heart Beat Perception
The heart beat perception task (HBPT) is a behavioral task used to examine interoceptive accuracy –“the sensitivity or accuracy in perceiving internal signals” (Garfinkel et al., 2015) by measuring the precision of the ability to detect one’s own heartbeats. Although the HBPT demonstrates some notable weaknesses as an interoceptive measure (Brener and Ring, 2016), it is commonly used to measure interoceptive accuracy in healthy and clinical populations. The HBPT also frequently demonstrates the role of the insula in interoception when administered during fMRI (Schulz, 2016).
Specifically, three studies have used the HBPT to examine interoceptive accuracy in BN. In comparison to controls, the first study examined a small number of women recovered from BN who had also never met prior criteria for AN (Klabunde et al., 2013), the second study examined participants with current AN and BN diagnoses (Eshkevari et al., 2014), while the third study examined women with a current BN diagnosis (Pollatos and Georgiou, 2016). The first and third studies used the heartbeat counting version of the HBPT (Schandry, 1981), which required participants to count how many heartbeats they could detect within specific time intervals. The second study used the heartbeat tapping version of the HBPT, which requires participants to indicate whether they felt their heartbeat when they heard a tone, which was presented at a frequency similar to the participant’s heart rate (Eshkevari et al., 2014). The heartbeat tapping version of the HBPT requires the integration of exteroceptive and interoceptive attention (Farb et al., 2013). The heartbeat tapping and counting versions of the HBPT use different algorithms for calculating accuracy scores.
The first study found that women recovered from diagnostically “pure” BN (no participants had a prior diagnosis of AN) demonstrate significantly lower interoceptive accuracy in comparison to controls (Klabunde et al., 2013); these participants did not have physiological complications that go alongside a BN diagnosis, which may make it easier to examine the neurobiology that may lead one to developing an eating disorder. The second study compared the BN and control groups during post-hoc comparisons and did not detect significant findings between groups (Eshkevari et al., 2014). In fact, for the second study, interoceptive accuracy was no higher than chance for either group. The third study found no significant differences in interoceptive accuracy when also covarying alexithymia, depressive symptoms and anxiety, which are all significantly associated with interoceptive deficits and are frequently associated with a BN diagnosis (Pollatos and Georgiou, 2016). Therefore, results differed across the three studies. Differences may be due to the covariates included during analyses, differences in the BN populations examined and the varying neurocognitive demands required of each version of the HBPT.
Thus, studies using the heart beat perception task in BN are inconclusive in determining whether there are interoceptive accuracy deficits in BN, none-the-less there is some evidence of heart beat perception deficits in BN.
3.4 Taste
Taste processing is one of the interoceptive senses most frequently examined in BN. It plays a significant role in the homeostasis of feeding behaviors, driving food consumption and refusal. BN studies of taste detection include administrations of sweet, sour, bitter, and salt tastes that vary in texture and intensity. When assessing taste detection in BN as compared to controls, several studies found no differences (Aschenbrenner et al., 2008; Drewnowski et al., 1987a, 1987b; Frank et al., 2011; Franko et al., 1994; Klein et al., 2009). Others found poor salt (Blazer et al., 2008), olfactory (Dazzi et al., 2013), and taste (specifically bitter) detection (Dazzi et al., 2013), abnormalities and abnormal perception of low fat solutions (Sunday and Halmi, 1990), non-sugar solutions (Sunday and Halmi, 1990), and taste solutions when they were administered directly to the palate (Rodin et al.,1990). A study by Wisniewski and colleagues found that BN participants failed to habituate when they consumed repeated taste stimuli (Wisniewski et al., 1997). To determine whether taste abnormalities are due to the number of taste receptors in BN, one study found no differences between BN an controls, but it did reveal differences across those with restrictive and binge purge behaviors (Wockel et al., 2007).
Two taste studies demonstrated low clinical assessment scores on the Adapted Newcastle-Ottawa Quality Scale (Dazzi et al., 2013; Wisniewski et al., 1997), and one included small sample sizes (Klein et al., 2009). Overall, studies indicate mixed findings when examining measures of taste detection and perception in BN.
3.5 Neurobiology Underlying Taste Processing in BN
The neurobiology underlying interoceptive sensory processing in BN has focused on examining taste by comparing BN participants to controls. Studies have implicated the insular cortex as the gustatory cortex (Ogawa et al., 1994) and the role of the ACC, the orbital frontal cortex (OFC) and the anteromedial temporal cortex in taste processing (Pritchard et al., 2007) and interoception (Schulz, 2016).
When examining how the brain responds to tasting glucose, one study revealed decreased activation in the right ACC and left cuneus when BN participants were administered tastes of glucose (Frank et al., 2006). Another glucose study found similar hypoactivation but within the insula, ventral putamen, amygdala, and the orbitofrontal cortex (Frank et al., 2011). In a sucrose as compared to sucralose study, women recovered from BN demonstrated decreased sensitization to sucrose in the left thalamus and the left medial frontal gyrus (Wagner et al., 2015). For another sucrose and sucralose study, using uncorrected region of interest analyses focused on the insula, another study demonstrated that women recovered from BN demonstrated increased activation in the right anterior insula when tasting sucrose compared to sucralose (Oberndorfer et al., 2013). In a study looking at patterns of activation during the sucrose > artificial saliva contrast, women with BN demonstrated no significant differences when self-reported interoceptive awareness deficits, taste perception, and comorbid depression and anxiety diagnoses were included in the model (Frank et al., 2016). When consuming crème as compared to water, women recovered from BN demonstrated higher activation within the left ventral anterior striatum (Radeloff et al., 2014). During chocolate milkshake administration, decreased activation in the left middle frontal gyrus, right insula, and the right precentral cortex was found in BN although this study used uncorrected analyses (Bohon and Stice, 2011).
In sum, those with BN display abnormal brain activation in response to tastes within regions that are typically involved in both interoceptive and taste processing. Insula dysfunction was frequently noted during BN taste studies as were other regions that are implicated during interoception (ACC, thalamus and OFC) and reward (thalamus, basal ganglia). Inconsistent findings across studies could be related to interoceptive neural network connectivity abnormalities.
3.6 Insula abnormalities in BN
In BN, several studies demonstrate abnormal insula structure, function and also abnormal functional connectivity involving the insula. When measured with structural MRI, BN participants were characterized by increased gray matter volumes within the ventral anterior insula (Frank et al., 2013) and when measured by DTI, BN participants displayed disturbed white matter integrity within the right sub-insula (Mettler et al., 2013).
Functional magnetic resonance imaging (fMRI) studies also reveal abnormalities within the insula in BN in comparison to controls. These studies did not specifically examine interoception, rather they primarily examined brain function in BN by presenting food and non-food images and also by showing the participants body images. For the BN participants, one study demonstrated decreased activation within the bilateral superior temporal gyrus/insula and the visual cortex (Brooks et al., 2011), another did not detect significant differences in activation (Van den Eynde et al., 2013) and a third study demonstrated increased insula activation when participants viewed pictures after an overnight fast. The third study did not detect any whole brain differences between groups, but uncorrected region of interest (ROI) analyses displayed increased activation within the ACC and insula (Schienle et al., 2009). Two studies of functional connectivity studies were also conducted. One study using uncorrected analyses found a significant interaction between the medial-frontal cortex and the anterior insula while viewing food compared to non-food pictures in BN when the insula was entered in as a seed region (Kim et al., 2012) while another study found that participants with BN demonstrated weaker functional connectivity between the amygdala, insula and putamen than controls during the perception of visual food cues (Bohon and Stice, 2012).
Visual body image stimuli have also been utilized during fMRI when comparing BN to controls. Van den Eynde and colleagues found activation in the insula for the BN group and increased activation in the fusiform for the control group (Van den Eynde et al., 2013) when participants compared their body size to pictures of slimmer women. Another study found a significant relationship between insula activation and higher body satisfaction ratings in BN when participants were shown pictures of thinner bodies and then rated their body satisfaction (Mohr et al., 2011); note that this study displayed low scores on the Newcastle Ottawa Quality Assessment Scale.
In summary, neuroimaging studies demonstrate disturbed insula functioning in BN. Although the insula is involved in other functions outside of interoception and insula activation does not equate interoceptive processing, the insula is considered a hub of the interoceptive neural network. It receives interoceptive afferent signals from numerous sensory functions throughout the body and sends interoceptive signals via efferents to be processed by higher-level cortical regions. Thus, insula disturbance may impact multiple sensory domains in BN and could contribute to widespread interoceptive disturbances. Thus, additional studies examining other aspects of interoceptive sensory function (besides taste) are needed in BN and should be examined.
4 Discussion
4.1 Explanation of Results
In BN, according to fMRI studies, abnormal function of the interoceptive neural network – and specifically insula function - is present. Since disturbed insula function is likely to impact all senses examined in BN, these findings support our hypothesis that evidence of abnormal interoceptive function is present, which could impact all interoceptive domains covered in this review (Craig, 2002). Our examination of numerous interoceptive senses in BN provides more support for this hypothesis. Although we find conflicting evidence from taste and heart beat detection studies, conflicting findings may be explained by methodology differences, the extent that interoceptive sensory processes are directly probed, the extent of the higher order cortical processing (such as aspects of interoceptive awareness and also the integration of interoceptive and exteroceptive stimuli) required to complete the interoceptive task, the populations examined (e.g. participants with pure BN vs participants with BN who had a prior AN diagnosis) and the statistical analyses, including the covariates used to examine the data. Nevertheless, despite inconsistencies across each of the domains measured, there is evidence of interoceptive sensory processing disturbances across multiple sensory domains in BN.
Self-report studies suggest that those with BN may be aware of their interoceptive and emotion processing difficulties (Fassino et al., 2004; Lilenfeld et al., 2006), this review’s findings add to this literature by demonstrating that interoceptive processing deficits at the sensory level appear, in-fact, to be present in those with BN. Thus, those with BN may be relatively accurate in their self-reports of interoceptive difficulties and may have intact metacognition of their interoceptive state.
4.2 Interoceptive Sensory Processing and BN symptoms
Results suggest that interoceptive disturbances are not exclusive to eating behaviors in BN. Taste disturbances, for example, may impact food preferences, while the combination of taste, pain and gastric distention disturbances may influence cycles of food restriction and binge eating behaviors in BN. Pain and gastric distention disturbances may allow objectively large amounts of food consumption and the capacity to withstand the pain that is associated with having a distended gut. Additionally, high pain thresholds may also contribute to over-exercising and tolerating vomiting when having a distended gut eventually becomes intolerable. Interoceptive sensory deficits may also contribute to other body cue-tracking problems in BN. Problems with body cue tracking may make it difficult to obtain and regulate a sense of self and could contribute to the problems with body image, self-disturbances, sensation/thrill seeking behaviors and substance abuse, in addition to eating regulation problems in BN (Brand-Gothelf et al., 2016; Favaro et al., 2008; Kaye, 2008; Kruschwitz et al., 2014). Specifically, body checking behaviors may be attempts to use external stimuli to obtain a sense of and track one’s body, self harm and risky thrill seeking behaviors may be attempts to “feel” the physical state of the body, while calorie counting may be an attempts to use cognitive abilities and external stimuli to compensate for hunger and satiety deficits by manually adjusting intake.
Emotion regulation difficulties in BN may also occur in response to interoceptive sensory processing disturbances. Studies demonstrate that interoceptive deficits contribute to alexithymia, emotional processing deficits, disturbed empathy (Brewer et al., 2016; Ernst et al., 2013; Lamm et al., 2007; Quattrocki and Friston, 2014; Shah et al., 2016; Zaki et al., 2012), poor mood regulation and comorbid mood/anxiety diagnoses (Paulus and Stein, 2010; Wiebking et al., 2010), which overlap with BN symptoms (Beales and Dolton, 2000; de Zwaan et al., 1996; Lavender et al., 2014b) and often persist after recovery (Lavender et al., 2014b; Schmidt et al. 1993). Interoceptive deficits may also influence various temperaments demonstrated by those with BN, such as high novelty seeking (Fassino et al., 2002). Novelty seeking is associated with interoception and oxytocin -a neural modulator that plays a significant role in interoceptive and social emotional development- and demonstrates disturbances in BN (Monteleone et al., 2016; Quattrocki and Friston, 2014). Thus, the role of oxytocin on interoceptive processing, novelty seeking and social interactions in BN should be further examined.
Since interoception plays a crucial role in emotional processing and regulation, interoceptive deficits may have a cross-diagnostic influence on emotion processing and mentalization deficits across a variety of psychiatric and neurogenetic syndromes. Because the neural networks studied overlap with emotion processing and mentalization, researchers may miss specific interoception effects by statistically controlling for the effects of emotion processing deficits and related comorbid diagnoses when examining interoception. Future studies should examine the functions of networks related to interoceptive and emotion processing across psychiatric diagnoses.
Ultimately, seemingly unrelated symptoms and behaviors in BN may be explained by global difficulties in interoceptive sensory processing; these deficits may predispose one to developing an eating disorder. This claim is supported by genetic studies which suggest that self-report deficits in interoceptive awareness may contribute to the development of eating disorders (Frieling et al., 2006; Gamero-Villarroel et al., 2015), findings from a self-report study that indicates that interoceptive awareness deficits are found in teenage girls who later develop eating disorder symptoms (Leon et al., 1993) and findings from studies that examine women recovered from BN. Deficits present after recovery may indicate either that interoceptive deficits existed prior to the development of an eating disorder or that interoceptive deficits are a scar from having BN.
4.3 Interoception and Treatment Development
Interoceptive sensory processing deficits may be a valuable focus for treatment innovation for people with BN. Since there is evidence that interoceptive deficits may exist after recovery and could be a trait of those who develop BN, it is possible that interoceptive deficits may not be changeable. Thus, prior to developing treatments aimed at improving interoceptive sensory processing deficits in BN, additional studies should further examine how interoceptive deficits develop and whether deficits are unchangeable traits or reflect a modifiable, temporary and treatable state in those with BN. If unchangeable, it may be helpful to teach patients with BN compensatory strategies for monitoring or modulating internal states. Treatments that help those with BN adaptively increase their internal stimulation and rely on exteroceptive cues in order to compensate for poor internal sensory processing may also be beneficial. If studies demonstrate that interoception is modifiable, then behavioral, biological and brain-based interoceptive measures should be examined and targeted in longitudinal studies. Additionally, our knowledge of interoceptive deficits in BN indicate that the assumption that all people have the physiological ability to accurately detect and appropriately respond to hunger and satiety cues and are able to “intuitively eat” may be a false assumption. Our results, in conjunction with studies demonstrating disturbed hormones related to hunger and satiety in BN and the relationship between interoceptive accuracy and the ability to eat intuitively (Herbert et al., 2013), suggest that intuitive eating might not be possible for all people, and specifically those with BN. Thus, intuitive eating interventions may be counterproductive in patients with interoceptive deficits. Also, because interoceptive sensory processing plays an integral role in emotion processing and social cognition, results also suggest that it may be more effective to target interoceptive sensory disturbances in those with BN prior to addressing emotion processing and regulation skills. Helping patients track, regulate their physiological state and compensate for these disturbances may have downstream effects that may help one improve emotion processing, regulation and mentalizing abilities. Lastly, given our findings that those with BN may demonstrate accurate assessment of interoceptive awareness (as evidenced by prior studies which substantially detail self-report interoceptive disturbances in BN), it may be unproductive to focus on challenging cognitions related to interoception functioning in treating those with BN.
4.4 Limitations and Future Directions
Despite the fact that this review suggests that global interoceptive sensory processing deficits may be present in BN, there are limitations inherent to some of the sensory domains examined. Within the interoceptive field, definitions of interoception are variable. For the purpose of this review, interoception was defined according to Craig (2002 and 2009). It is important to note that other interoceptive definitions exist and would likely change the scope of the interoceptive domains measured in other interoceptive reviews (such as including hormones or studies of heart rate variability).
This review focused on the interoceptive senses that have been previously examined in BN. Although studies suggest that interoceptive measures may generalize to other interoceptive processes (Garfinkel and Critchley, 2013; Herbert et al., 2012), since there were some inconsistencies in our findings, future studies should also examine whether those with BN demonstrate abnormal processing of sensual touch, itch, the need to breathe, defecate, and urinate. Additionally, although several studies have examined the neurobiology underlying interoceptive processing in AN (Kerr et al., 2016; Strigo et al., 2013), neuroimaging studies that examine various domains of interoceptive processing are also needed in BN. These studies should compare interoceptive processing in diagnostically pure AN and BN and in those who move between AN and BN diagnoses. Moreover, this review takes a relatively “insula centric” view of interoceptive processing in BN. Future imaging studies should examine networks associated with interoceptive processing in BN, the relationship between interoceptive processing and other domains such as reward, cognition and motivated behavior, and the influence of other brain networks (such as the salience and executive control network) on interoception in BN.
Another limitation of this review is the lack of precise and reliable interoception measurement tools and the inherent complications of interoceptive studies. The development of improved interoceptive measurement tools will further enhance our understanding of interoception in BN. Lastly, we acknowledge that it is difficult to directly compare results from multiple biological studies, which differ in their procedures, analyses and measurement tools. Our review attempts to make these comparisons, however we acknowledge that these attempts include a degree of subjectivity since procedures greatly differed from one project to the next.
4.5 Conclusion
In conclusion, we found evidence that there are interoceptive sensory processing deficits across several domains in BN, which supports our hypothesized model that interoceptive sensory processing deficits may be driving symptoms and associated behaviors present in BN. Results also suggest that brain disturbances in interoceptive circuitry may contribute to these deficits. Interoceptive deficits appear to play a significant role in the way that symptoms are present in BN. Because existing research focuses on cross-sectional data comparing interoception in patients compared to controls, in order to further evaluate this model, future studies should examine how interoceptive sensory processing deficits predict and influence the development and course of BN symptoms. If the proposed model is supported via longitudinal research, it will be important to examine whether it is possible to change interoceptive deficits in BN, as these results may have important treatment implications for BN.
References
- Aschenbrenner K, Scholze N, Joraschky P, Hummel T. Gustatory and olfactory sensitivity in patients with anorexia and bulimia in the course of treatment. J Psychiatr Res. 2008 doi: 10.1016/j.jpsychires.2008.03.003. [DOI] [PubMed] [Google Scholar]
- Association, A.P. Diagnostic and statistical manual of mental disorders. 5. American Psychiatric Association; Washington, DC: 2013. [Google Scholar]
- Barrett LF, Simmons WK. Interoceptive predictions in the brain. Nat. Rev. Neurosci. 2015;16:419–429. doi: 10.1038/nrn3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beales DL, Dolton R. Eating disordered patients: personality, alexithymia, and implications for primary care. Br J Gen Pr. 2000;50:21–26. [PMC free article] [PubMed] [Google Scholar]
- Bjornsdotter M, Gordon I, Pelphrey KA, Olausson H, Kaiser MD. Development of brain mechanisms for processing affective touch. Front Behav Neurosci. 2014;8:24. doi: 10.3389/fnbeh.2014.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer T, Latzer Y, Nagler RM. Salivary and gustatory alterations among bulimia nervosa patients. Eur J Clin Nutr. 2008;62:916–922. doi: 10.1038/sj.ejcn.1602801. doi:1602801 [pii]10.1038/sj.ejcn.1602801 [doi] [DOI] [PubMed] [Google Scholar]
- Bohon C, Stice E. Negative affect and neural response to palatable food intake in bulimia nervosa. Appetite. 2012;58:964–970. doi: 10.1016/j.appet.2012.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohon C, Stice E. Reward abnormalities among women with full and subthreshold bulimia nervosa: a functional magnetic resonance imaging study. Int J Eat Disord. 2011;44:585–595. doi: 10.1002/eat.20869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand-Gothelf A, Parush S, Eitan Y, Admoni S, Gur E, Stein D. Sensory modulation disorder symptoms in anorexia nervosa and bulimia nervosa: A pilot study. Int J Eat Disord. 2016;49:59–68. doi: 10.1002/eat.22460. [DOI] [PubMed] [Google Scholar]
- Brener J, Ring C. Towards a psychophysics of interoceptive processes: the measurement of heartbeat detection. Philos. Trans. R. Soc. B. 2016;371 doi: 10.1098/rstb.2016.0015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer R, Cook R, Bird G. Alexithymia: a general deficit of interoception. R. Soc. Open Sci. 2016;3:150664. doi: 10.1098/rsos.150664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks SJ, O’Daly OG, Uher R, Friederich HC, Giampietro V, Brammer M, Williams SC, Schioth HB, Treasure J, Campbell IC. Differential neural responses to food images in women with bulimia versus anorexia nervosa. PLoS One. 2011;6:e22259. doi: 10.1371/journal.pone.0022259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruch H. Perceptual and conceptual disturbances in anorexia nervosa. Psychosom Med. 1962;24:187–194. doi: 10.1097/00006842-196203000-00009. [DOI] [PubMed] [Google Scholar]
- Cameron OG. Interoception: the inside story--a model for psychosomatic processes. Psychosom Med. 2001;63:697–710. doi: 10.1097/00006842-200109000-00001. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel — now? The anterior insula and human awareness. Neuroscience. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nat Neurosci. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Dazzi F, Nitto SD, Zambetti G, Loriedo C, Ciofalo A. Alterations of the olfactory-gustatory functions in patients with eating disorders. Eur Eat Disord Rev. 2013;21:382–385. doi: 10.1002/erv.2238. [DOI] [PubMed] [Google Scholar]
- de Zwaan M, Biener D, Bach M, Wiesnagrotzki S, Stacher G. Pain sensitivity, alexithymia, and depression in patients with eating disorders: are they related? J Psychosom Res. 1996;41:65–70. doi: 10.1016/0022-3999(96)00088-8. [DOI] [PubMed] [Google Scholar]
- Diamanti A, Bracci F, Gambarara M, Ciofetta GC, Sabbi T, Ponticelli A, Montecchi F, Marinucci S, Bianco G, Castro M. Gastric electric activity assessed by electrogastrography and gastric emptying scintigraphy in adolescents with eating disorders. J Pediatr Gastroenterol Nutr. 2003;37:35–41. doi: 10.1097/00005176-200307000-00006. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Bellisle F, Aimez P, Remy B. Taste and bulimia. Physiol Behav. 1987a;41:621–626. doi: 10.1016/0031-9384(87)90320-9. [DOI] [PubMed] [Google Scholar]
- Drewnowski A, Halmi KA, Pierce B, Gibbs J, Smith GP. Taste and eating disorders. Am J Clin Nutr. 1987b;46:442–450. doi: 10.1093/ajcn/46.3.442. [DOI] [PubMed] [Google Scholar]
- Ernst J, Northoff G, Boker H, Seifritz E, Grimm S. Interoceptive awareness enhances neural activity during empathy. Hum. Brain Mapp. 2013;34:1615–1624. doi: 10.1002/hbm.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshkevari E, Rieger E, Musiat P, Treasure J. An investigation of interoceptive sensitivity in eating disorders using a heartbeat detection task and a self-report measure. Eur Eat Disord Rev. 2014;22:383–388. doi: 10.1002/erv.2305. [DOI] [PubMed] [Google Scholar]
- Farb NA, Segal ZV, Anderson AK. Attentional modulation of primary interoceptive and exteroceptive cortices. Cereb. cortex. 2013;23:114–126. doi: 10.1093/cercor/bhr385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fassino S, Piero A, Daga GA, Leombruni P, Mortara P, Rovera GG. Attentional biases and frontal functioning in anorexia nervosa. Int J Eat Disord. 2002;31:274–283. doi: 10.1002/eat.10028. [DOI] [PubMed] [Google Scholar]
- Fassino S, Piero A, Gramaglia C, Abbate-Daga G. Clinical, psychopathological and personality correlates of interoceptive awareness in anorexia nervosa, bulimia nervosa and obesity. Psychopathology. 2004;37:168–174. doi: 10.1159/000079420. [DOI] [PubMed] [Google Scholar]
- Favaro A, Santonastaso P, Monteleone P, Bellodi L, Mauri M, Rotondo A, Erzegovesi S, Maj M. Self-injurious behavior and attempted suicide in purging bulimia nervosa: associations with psychiatric comorbidity. J Affect Disord. 2008;105:285–289. doi: 10.1016/j.jad.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Frank GK, Reynolds JR, Shott ME, O’Reilly RC. Altered temporal difference learning in bulimia nervosa. Biol. Psychiatry. 2011;70:728–735. doi: 10.1016/j.biopsych.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GK, Shott ME, Hagman JO, Mittal VA. Alterations in brain structures related to taste reward circuitry in ill and recovered anorexia nervosa and in bulimia nervosa. Am J Psychiatry. 2013;170:1152–1160. doi: 10.1176/appi.ajp.2013.12101294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GK, Shott ME, Keffler C, Cornier MA. Extremes of eating are associated with reduced neural taste discrimination. Int J Eat Disord. 2016;49:603–612. doi: 10.1002/eat.22538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank GK, Wagner A, Achenbach S, McConaha C, Skovira K, Aizenstein H, Carter CS, Kaye WH. Altered brain activity in women recovered from bulimic-type eating disorders after a glucose challenge: a pilot study. Int J Eat Disord. 2006;39:76–79. doi: 10.1002/eat.20210. [DOI] [PubMed] [Google Scholar]
- Franko DL, Wolfe BE, Jimerson DC. Elevated sweet taste pleasantness ratings in bulimia nervosa. Physiol Behav. 1994;56:969–973. doi: 10.1016/0031-9384(94)90331-x. [DOI] [PubMed] [Google Scholar]
- Frieling H, Romer KD, Wilhelm J, Hillemacher T, Kornhuber J, de Zwaan M, Jacoby GE, Bleich S. Association of catecholamine-O-methyltransferase and 5-HTTLPR genotype with eating disorder-related behavior and attitudes in females with eating disorders. Psychiatr Genet. 2006;16:205–208. doi: 10.1097/01.ypg.0000218620.50386.f1. [DOI] [PubMed] [Google Scholar]
- Gamero-Villarroel C, Gonzalez LM, Gordillo I, Carrillo JA, Garcia-Herraiz A, Flores I, Rodriguez-Lopez R, Gervasini G. Impact of NEGR1 genetic variability on psychological traits of patients with eating disorders. Pharmacogenomics J. 2015;15:278–283. doi: 10.1038/tpj.2014.53. [DOI] [PubMed] [Google Scholar]
- Garfinkel PE, Moldofsky H, Garner DM, Stancer HC, Coscina DV. Body awareness in anorexia nervosa: disturbances in “body image” and “satiety”. Psychosom Med. 1978;40:487–498. doi: 10.1097/00006842-197810000-00004. [DOI] [PubMed] [Google Scholar]
- Garfinkel SN, Critchley HD. Interoception, emotion and brain: new insights link internal physiology to social behaviour. Commentary on:: “Anterior insular cortex mediates bodily sensibility and social anxiety” by Terasawa et al. (2012) Soc. Cogn. Affect. Neurosci. 2013;8:231–234. doi: 10.1093/scan/nss140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel SN, Seth AK, Barrett AB, Suzuki K, Critchley HD. Knowing your own heart: Distinguishing interoceptive accuracy from interoceptive awareness. Biol. Psychol. 2015;104:65–74. doi: 10.1016/j.biopsycho.2014.11.004. [DOI] [PubMed] [Google Scholar]
- Garner DM, Olmstead MP, Polivy J. Development and validation of a multidimensional eating disorder inventory for anorexia nervosa and bulimia. Int J Eat Disord. 1983;2:15–19. [Google Scholar]
- Geliebter A, Hashim SA. Gastric capacity in normal, obese, and bulimic women. Physiol Behav. 2001;74:743–746. doi: 10.1016/s0031-9384(01)00619-9. [DOI] [PubMed] [Google Scholar]
- Geliebter A, Melton PM, McCray RS, Gallagher DR, Gage D, Hashim SA. Gastric capacity, gastric emptying, and test-meal intake in normal and bulimic women. Am J Clin Nutr. 1992;56:656–661. doi: 10.1093/ajcn/56.4.656. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Koo-Loeb J, Pedersen CA, Brown HJ, Maixner W. Blood pressure-related hypoalgesia in bulimia nervosa. Psychosom Med. 1998;60:736–743. doi: 10.1097/00006842-199811000-00016. [DOI] [PubMed] [Google Scholar]
- Greenspan JD, Winfield JA. Reversible pain and tactile deficits associated with a cerebral tumor compressing the posterior insula and parietal operculum. Pain. 1992;50:29–39. doi: 10.1016/0304-3959(92)90109-O. [DOI] [PubMed] [Google Scholar]
- Herbert BM, Blechert J, Hautzinger M, Matthias E, Herbert C. Intuitive eating is associated with interoceptive sensitivity. Effects on body mass index. Appetite. 2013;70:22–30. doi: 10.1016/j.appet.2013.06.082. [DOI] [PubMed] [Google Scholar]
- Herbert BM, Muth ER, Pollatos O, Herbert C. Interoception across modalities: on the relationship between cardiac awareness and the sensitivity for gastric functions. PLoS One. 2012;7:e36646. doi: 10.1371/journal.pone.0036646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa M, Hamaguchi T, Watanabe S, Terui T, Mine H, Kano M, Fukudo S. Site-specific differences in central processing of visceral stimuli from the rectum and the descending colon in men. Neurogastroenterol Motil. 2010;22:173–80. e53. doi: 10.1111/j.1365-2982.2009.01417.x. [DOI] [PubMed] [Google Scholar]
- Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiol Behav. 2008;94:121–135. doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye W. Neurobiology of anorexia and bulimia nervosa. Physiol Behav. 2007 doi: 10.1016/j.physbeh.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr KL, Moseman SE, Avery JA, Bodurka J, Zucker NL, Simmons WK. Altered Insula Activity during Visceral Interoception in Weight-Restored Patients with Anorexia Nervosa. Neuropsychopharmacology. 2016;41:521–528. doi: 10.1038/npp.2015.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KR, Ku J, Lee JH, Lee H, Jung YC. Functional and effective connectivity of anterior insula in anorexia nervosa and bulimia nervosa. Neurosci Lett. 2012;521:152–157. doi: 10.1016/j.neulet.2012.05.075. [DOI] [PubMed] [Google Scholar]
- Klabunde M, Acheson DT, Boutelle K, Matthews SC, Kaye WH. Interoceptive sensitivity deficits in women recovered from bulimia nervosa. Eat Behav. 2013;14:488–492. doi: 10.1016/j.eatbeh.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DA, Schebendach JE, Brown AJ, Smith GP, Walsh BT. Modified sham feeding of sweet solutions in women with and without bulimia nervosa. Physiol Behav. 2009;96:44–50. doi: 10.1016/j.physbeh.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch KL, Bingaman S, Tan L, Stern RM. Visceral perceptions and gastric myoelectrical activity in healthy women and in patients with bulimia nervosa. Neurogastroenterol Motil. 1998;10:3–10. doi: 10.1046/j.1365-2982.1998.00080.x. [DOI] [PubMed] [Google Scholar]
- Koutek J, Kocourkova J, Dudova I. Suicidal behavior and self-harm in girls with eating disorders. Neuropsychiatr Dis Treat. 2016;12:787–793. doi: 10.2147/NDT.S103015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruschwitz JD, Lueken U, Wold A, Walter H, Paulus MP. High Thrill and adventure seeking is associated with reduced interoceptive sensitivity: evidence for an altered sex-specific homeostatic processing in high sensation seekers. Eur J Pers. 2014;28:472–481. doi: 10.1002/per.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct. Funct. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladabaum U, Minoshima S, Hasler WL, Cross D, Chey WD, Owyang C. Gastric distention correlates with activation of multiple cortical and subcortical regions. Gastroenterology. 2001;120:369–376. doi: 10.1053/gast.2001.21201. doi:S0016508501699906 [pii] [DOI] [PubMed] [Google Scholar]
- Lamm C, Nusbaum HC, Meltzoff AN, Decety J. What are you feeling? Using functional magnetic resonance imaging to assess the modulation of sensory and affective responses during empathy for pain. PLoS One. 2007;2:e1292. doi: 10.1371/journal.pone.0001292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenbacher S, Pauls AM, Strian F, Pirke KM, Krieg JC. Pain sensitivity in anorexia nervosa and bulimia nervosa. Biol Psychiatry. 1991;29:1073–1078. doi: 10.1016/0006-3223(91)90249-l. [DOI] [PubMed] [Google Scholar]
- Lavender JM, Wonderlich SA, Peterson CB, Crosby RD, Engel SG, Mitchell JE, Crow SJ, Smith TL, Klein MH, Goldschmidt AB, Berg KC. Dimensions of emotion dysregulation in bulimia nervosa. Eur Eat Disord Rev. 2014a;22:212–216. doi: 10.1002/erv.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavender JM, Wonderlich SA, Peterson CB, Crosby RD, Engel SG, Mitchell JE, Crow SJ, Smith TL, Klein MH, Goldschmidt AB, Berg KC. Dimensions of emotion dysregulation in bulimia nervosa. Eur Eat Disord Rev. 2014b;22:212–216. doi: 10.1002/erv.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon GR, Fulkerson JA, Perry CL, Cudeck R. Personality and behavioral vulnerabilities associated with risk status for eating disorders in adolescent girls. J Abnorm Psychol. 1993;102:438–444. doi: 10.1037//0021-843x.102.3.438. [DOI] [PubMed] [Google Scholar]
- Lilenfeld LR, Wonderlich S, Riso LP, Crosby R, Mitchell J. Eating disorders and personality: a methodological and empirical review. Clin Psychol Rev. 2006;26:299–320. doi: 10.1016/j.cpr.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Naliboff BD, Craig AD. Neuroimaging of the brain-gut axis: from basic understanding to treatment of functional GI disorders. Gastroenterology. 2006;131:1925–1942. doi: 10.1053/j.gastro.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Mettler LN, Shott ME, Pryor T, Yang TT, Frank GK. White matter integrity is reduced in bulimia nervosa. Int J Eat Disord. 2013;46:264–273. doi: 10.1002/eat.22083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohr HM, Roder C, Zimmermann J, Hummel D, Negele A, Grabhorn R. Body image distortions in bulimia nervosa: investigating body size overestimation and body size satisfaction by fMRI. Neuroimage. 2011;56:1822–1831. doi: 10.1016/j.neuroimage.2011.02.069. [DOI] [PubMed] [Google Scholar]
- Monteleone AM, Scognamiglio P, Volpe U, Di Maso V, Monteleone P. Investigation of Oxytocin Secretion in Anorexia Nervosa and Bulimia Nervosa: Relationships to Temperament Personality Dimensions. Eur. Eat. Disord. Rev. 2016;24:52–56. doi: 10.1002/erv.2391. [DOI] [PubMed] [Google Scholar]
- O’Connor Green S, Higgins JPT, D Defining the review question and developing criteria for including studies. Cochrane Handb. Syst. Rev. Interv 2008 [Google Scholar]
- Oberndorfer TA, Frank GK, Simmons AN, Wagner A, McCurdy D, Fudge JL, Yang TT, Paulus MP, Kaye WH. Altered insula response to sweet taste processing after recovery from anorexia and bulimia nervosa. Am J Psychiatry. 2013;170:1143–1151. doi: 10.1176/appi.ajp.2013.11111745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H, Hasegawa K, Ohgushi M, Murayama N. Changes in properties of neuronal responses in two cortical taste areas in rats of various ages. Neurosci. Res. 1994;19:407–417. doi: 10.1016/0168-0102(94)90082-5. [DOI] [PubMed] [Google Scholar]
- Olausson HW, Cole J, Vallbo A, McGlone F, Elam M, Kramer HH, Rylander K, Wessberg J, Bushnell MC. Unmyelinated tactile afferents have opposite effects on insular and somatosensory cortical processing. Neurosci. Lett. 2008;436:128–132. doi: 10.1016/J.Neulet.2008.03.015. [DOI] [PubMed] [Google Scholar]
- Papezova H, Yamamotova A, Uher R. Elevated pain threshold in eating disorders: physiological and psychological factors. J Psychiatr Res. 2005;39:431–438. doi: 10.1016/j.jpsychires.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Pauls AM, Lautenbacher S, Strian F, Pirke KM, Krieg JC. Assessment of somatosensory indicators of polyneuropathy in patients with eating disorders. Eur Arch Psychiatry Clin Neurosci. 1991;241:8–12. doi: 10.1007/BF02193748. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Stein MB. Interoception in anxiety and depression. Brain Struct. Funct. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollatos O, Georgiou E. Normal interoceptive accuracy in women with bulimia nervosa. Psychiatry Res. 2016;240:328–332. doi: 10.1016/j.psychres.2016.04.072. [DOI] [PubMed] [Google Scholar]
- Pritchard TC, Schwartz GJ, Scott TR. Taste in the medial orbitofrontal cortex of the macaque. Ann N Y Acad Sci. 2007;1121:121–135. doi: 10.1196/annals.1401.007. [DOI] [PubMed] [Google Scholar]
- Quattrocki E, Friston K. Autism, oxytocin and interoception. Neurosci. Biobehav. Rev. 2014;47:410–430. doi: 10.1016/j.neubiorev.2014.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radeloff D, Willmann K, Otto L, Lindner M, Putnam K, Leeuwen SV, Kaye WH, Poustka F, Wagner A. High-fat taste challenge reveals altered striatal response in women recovered from bulimia nervosa: A pilot study. World J Biol Psychiatry. 2014;15:307–316. doi: 10.3109/15622975.2012.671958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodin J, Bartoshuk L, Peterson C, Schank D. Bulimia and taste: possible interactions. J Abnorm Psychol. 1990;99:32–39. doi: 10.1037//0021-843x.99.1.32. [DOI] [PubMed] [Google Scholar]
- Schandry R. Heart beat perception and emotional experience. Psychophysiology. 1981;18:483–488. doi: 10.1111/j.1469-8986.1981.tb02486.x. [DOI] [PubMed] [Google Scholar]
- Schienle A, Schafer A, Hermann A, Vaitl D. Binge-eating disorder: reward sensitivity and brain activation to images of food. Biol Psychiatry. 2009;65:654–661. doi: 10.1016/j.biopsych.2008.09.028. [DOI] [PubMed] [Google Scholar]
- Schmidt U, Jiwany A, Treasure J. A controlled study of alexithymia in eating disorders. Compr Psychiatry. 1993;34:54–58. doi: 10.1016/0010-440x(93)90036-4. [DOI] [PubMed] [Google Scholar]
- Schulz SM. Neural correlates of heart-focused interoception: a functional magnetic resonance imaging meta-analysis. Philos. Trans. R. Soc. B. 2016;371 doi: 10.1098/rstb.2016.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah P, Hall R, Catmur C, Bird G. Alexithymia, not autism, is associated with impaired interoception. Cortex. 2016;81:215–220. doi: 10.1016/j.cortex.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Evans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J. Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons WK, Avery JA, Barcalow JC, Bodurka J, Drevets WC, Bellgowan P. Keeping the body in mind: insula functional organization and functional connectivity integrate interoceptive, exteroceptive, and emotional awareness. Hum Brain Mapp. 2013;34:2944–2958. doi: 10.1002/hbm.22113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein D, Kaye WH, Matsunaga H, Myers D, Orbach I, Har-Even D, Frank G, Rao R. Pain perception in recovered bulimia nervosa patients. Int J Eat Disord. 2003;34:331–336. doi: 10.1002/eat.10164. [DOI] [PubMed] [Google Scholar]
- Strigo IA, Matthews SC, Simmons AN, Oberndorfer T, Klabunde M, Reinhardt LE, Kaye WH. Altered insula activation during pain anticipation in individuals recovered from anorexia nervosa: Evidence of interoceptive dysregulation. Int. J. Eat. Disord. 2013;46:23–33. doi: 10.1002/eat.22045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunday SR, Halmi KA. Taste perceptions and hedonics in eating disorders. Physiol Behav. 1990;48:587–594. doi: 10.1016/0031-9384(90)90196-b. [DOI] [PubMed] [Google Scholar]
- Svaldi J, Griepenstroh J, Tuschen-Caffier B, Ehring T. Emotion regulation deficits in eating disorders: a marker of eating pathology or general psychopathology? Psychiatry Res. 2012;197:103–111. doi: 10.1016/j.psychres.2011.11.009. [DOI] [PubMed] [Google Scholar]
- Van den Eynde F, Giampietro V, Simmons A, Uher R, Andrew CM, Harvey PO, Campbell IC, Schmidt U. Brain responses to body image stimuli but not food are altered in women with bulimia nervosa. BMC Psychiatry. 2013;13:302. doi: 10.1186/1471-244X-13-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner A, Simmons AN, Oberndorfer TA, Frank GK, McCurdy-McKinnon D, Fudge JL, Yang TT, Paulus MP, Kaye WH. Altered sensitization patterns to sweet food stimuli in patients recovered from anorexia and bulimia nervosa. Psychiatry Res. 2015;234:305–313. doi: 10.1016/j.pscychresns.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh BT, Zimmerli E, Devlin MJ, Guss J, Kissileff HR. A disturbance of gastric function in bulimia nervosa. Biol. Psychiatry. 2003;54:929–933. doi: 10.1016/s0006-3223(03)00176-8. [DOI] [PubMed] [Google Scholar]
- Wells GA, O’Connell D, Peterson J, Welch V, Losos M, et al. S B. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses 2009 [Google Scholar]
- Wiebking C, Bauer A, de Greck M, Duncan NW, Tempelmann C, Northoff G. Abnormal body perception and neural activity in the insula in depression: an fMRI study of the depressed “material me”. World J. Biol. Psychiatry. 2010;11:538–549. doi: 10.3109/15622970903563794. [DOI] [PubMed] [Google Scholar]
- Wisniewski L, Epstein LH, Marcus MD, Kaye W. Differences in salivary habituation to palatable foods in bulimia nervosa patients and controls. Psychosom Med. 1997;59:427–433. doi: 10.1097/00006842-199707000-00013. [DOI] [PubMed] [Google Scholar]
- Wockel L, Hummel T, Zepf FD, Jacob A, Poustka F. Changed taste perception in patients with eating disorders. Z Kinder Jugendpsychiatr Psychother. 2007;35:423–434. doi: 10.1024/1422-4917.35.6.423. [DOI] [PubMed] [Google Scholar]
- Yamamotova A, Papezova H, Uher R. Modulation of thermal pain perception by stress and sweet taste in women with bulimia nervosa. Neuro Endocrinol Lett. 2009;30:237–244. [PubMed] [Google Scholar]
- Yoshimura N, Bennett NE, Hayashi Y, Ogawa T, Nishizawa O, Chancellor MB, de Groat WC, Seki S. Bladder overactivity and hyperexcitability of bladder afferent neurons after intrathecal delivery of nerve growth factor in rats. J Neurosci. 2006;26:10847–10855. doi: 10.1523/JNEUROSCI.3023-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaki J, Davis JI, Ochsner KN. Overlapping activity in anterior insula during interoception and emotional experience. Neuroimage. 2012;62:493–499. doi: 10.1016/j.neuroimage.2012.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli EJ, Walsh BT, Guss JL, Devlin MJ, Kissileff HR. Gastric compliance in bulimia nervosa. Physiol Behav. 2006;87:441–446. doi: 10.1016/j.physbeh.2005.11.010. [DOI] [PubMed] [Google Scholar]