Abstract
We have developed a duplex ultrasound simulator for training and assessment of scanning skills. We used the simulator to test examiner performance in the measurement of flow velocities in dialysis access fistulas. Test cases were created from three-dimensional (3D) ultrasound scans of two dialysis access fistulas by reconstructing 3D blood vessel models and simulating blood flow velocity fields within the lumens. The simulator displays a two-dimensional B-mode or color Doppler image corresponding to transducer position on a mannequin; a spectral waveform is generated according to Doppler sample volume location and system settings. Examiner performance was assessed by comparing the measured peak systolic velocity (PSV) with the true PSV provided by the computational flow model. The PSV measured by four expert examiners deviated from the true value by 7.8 ± 6.1%. The results demonstrate the ability of the simulator to objectively assess an examiner’s measurement accuracy in complex vascular targets.
Keywords: Duplex ultrasound, Medical simulation, Dialysis access fistula, Color Doppler, Doppler spectral waveform, Peak systolic velocity
INTRODUCTION
Simulation for training in ultrasound provides such advantages as immediate availability of a wide range of clinical scenarios or pathology, and avoidance of patient comfort and safety concerns associated with having trainees perform clinical examinations. Medical ultrasound simulators have been developed for a variety of diagnostic and point-of-care applications, including echocardiography, obstetrics, trauma, and critical care (Parks et al. 2013, Ferrero et al. 2014, Liu et al. 2015, Paddock et al. 2015). These simulators, however, focus on two-dimensional (2D) B-mode images, while vascular ultrasound examinations rely primarily on Doppler assessment of blood velocities and flow patterns. In duplex ultrasound scanning, blood flow is characterized by Doppler spectral waveforms and color Doppler imaging (Beach et al. 2010). The peak systolic velocity (PSV) obtained from the Doppler spectral waveform is the principal criterion for the classification of arterial stenosis (Beach et al. 2012). A simulator for training and assessment in vascular ultrasound examinations requires realistic representations of the color Doppler images and Doppler spectral waveforms that must be acquired during performance of a complete duplex scan.
We have developed a computer-based duplex ultrasound simulator that incorporates real-time color Doppler images and Doppler spectral waveforms along with corresponding B-mode images. Testing results with the simulator for normal and stenotic carotid artery models have been previously reported (Zierler et al. 2016). This paper describes testing results for more complex vascular models: dialysis access fistula cases that include flow simulations for the arterial inflow, the arterial-venous anastomosis, and the venous return.
Hemodialysis treatments for patients with end-stage renal failure (ESRD) require cannula access to blood flow at two sites along a vessel segment, one for withdrawing blood and the other for returning it to the patient. A dialysis access fistula is a surgical connection between an artery and a vein that produces higher than normal blood flow through the venous return segment. This segment can then be cannulated to supply the high flow volume flow rates required for effective hemodialysis. Flow rates through a dialysis access fistula are of clinical importance because these fistulas are susceptible to stenosis development, which can reduce blood flow and eventually compromise the dialysis procedure (Kohler and Mraz 2015). Doppler ultrasound examination of dialysis access fistulas can be challenging because of the non-standard anatomy produced by the surgical connection of an artery and a vein, the unique and often complex vascular configuration at the arterial-venous anastomosis, and flow velocities that can be significantly higher than those observed in normal blood vessels (Teodorescu et al. 2012).
MATERIALS AND METHODS
Doppler Simulator Design
The duplex ultrasound simulator hardware (Figure 1) consists of a personal computer, a mannequin, and a mock transducer whose spatial location and orientation are measured using a tracking device (Patriot, Polhemus Inc., Colchester, VT, USA). As the examiner manipulates the mock transducer over the mannequin, the computer displays ultrasound images in a 2D B-mode view that changes in real-time according to the transducer’s position and orientation. These images are extracted in real time from a three-dimensional (3D) volume of image data previously generated from scans of patients or normal volunteers (Sheehan et al. 2013). In addition, computational flow modeling is used to populate a 3D computer model of the blood vessel with time-varying velocity vectors that define the blood flow at all points within the vessel (McGah et al. 2011, 2012, 2013). This velocity field is sampled along with the image data to create a spectral waveform display that responds in real-time to the control panel settings selected by the examiner. The steps from 3D ultrasound scanning to simulated spectral waveform display are described in the following sections.
Figure 1.
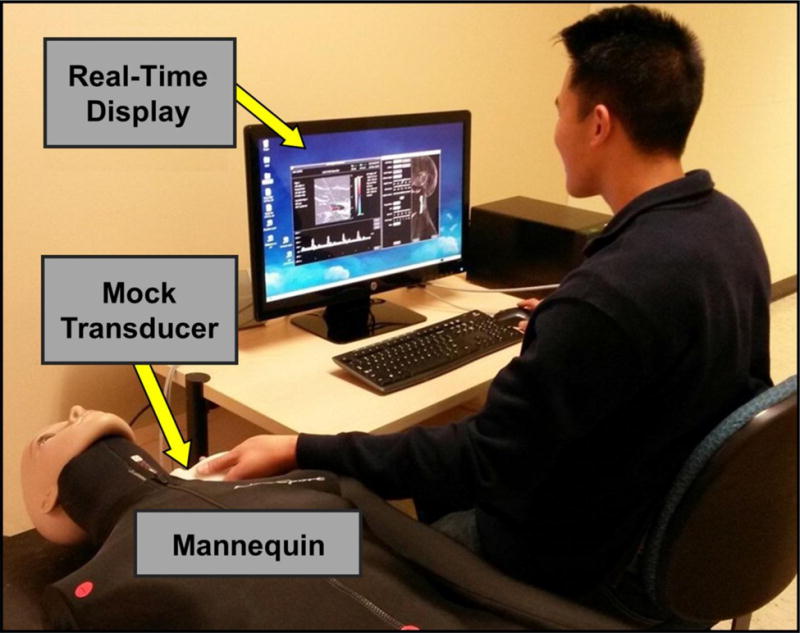
Photograph of the Doppler ultrasound simulator system. A spatial tracking system records the position and orientation of the mock transducer in real-time: a magnetic field transmitter is located inside the mannequin and a receiver is fixed inside the plastic transducer housing. As the examiner moves the mock transducer over the mannequin, a 2D B-mode image derived from a saved 3D data set is displayed. A Doppler spectral waveform display is generated in real-time from the velocity database as the examiner positions the Doppler sample volume and adjusts the control panel settings.
Patient Scanning and 3D Image Reconstruction
3D ultrasound scans were performed on two patients with dialysis access fistulas. The imaging procedure was approved by the University of Washington IRB and the subjects gave informed consent. Case 1 was a mature and normally-functioning end-to-side fistula in the lower arm near the wrist. Case 2 was a recent (10 months post-surgery) end-to-side fistula in the lower arm near the elbow, with a narrowed region in the venous return segment. End-to-side fistulas are created by surgically transecting the vein and attaching the proximal end to the side of the artery and tying off the distal end of the vein.
The 3D ultrasound data sets included gray-scale (B-mode) images of the dialysis access proximal inflow artery, arterial-venous anastomosis, distal artery, and proximal return vein. An ultrasound transducer with an attached tracking device (Flock of Birds, Ascension Technology Corp., Burlington, VT, USA) was used to acquire closely-spaced B-mode images along the length of the vessels of interest (Leotta and Martin 2000a, 2000b) (Figure 2a). Custom software developed using the LabVIEW engineering software package (National Instruments, Austin, TX, USA) was used to synchronously capture 2D ultrasound images and 3D tracking data at a rate of 30 frames/second. For each dialysis access case 900 images were captured during a 30-second continuous scan along the length of the target vessels. These images were reformatted into a regular 3D grid using a volume reconstruction algorithm (Leotta and Martin 2000a, 2000b) (Figure 2b). A 3D surface model of the blood vessel was then generated from traced borders of the vessel lumens in parallel planes extracted from the reconstructed volume data sets (Leotta et al. 2001a, 2001b) (Figure 2c).
Figure 2.
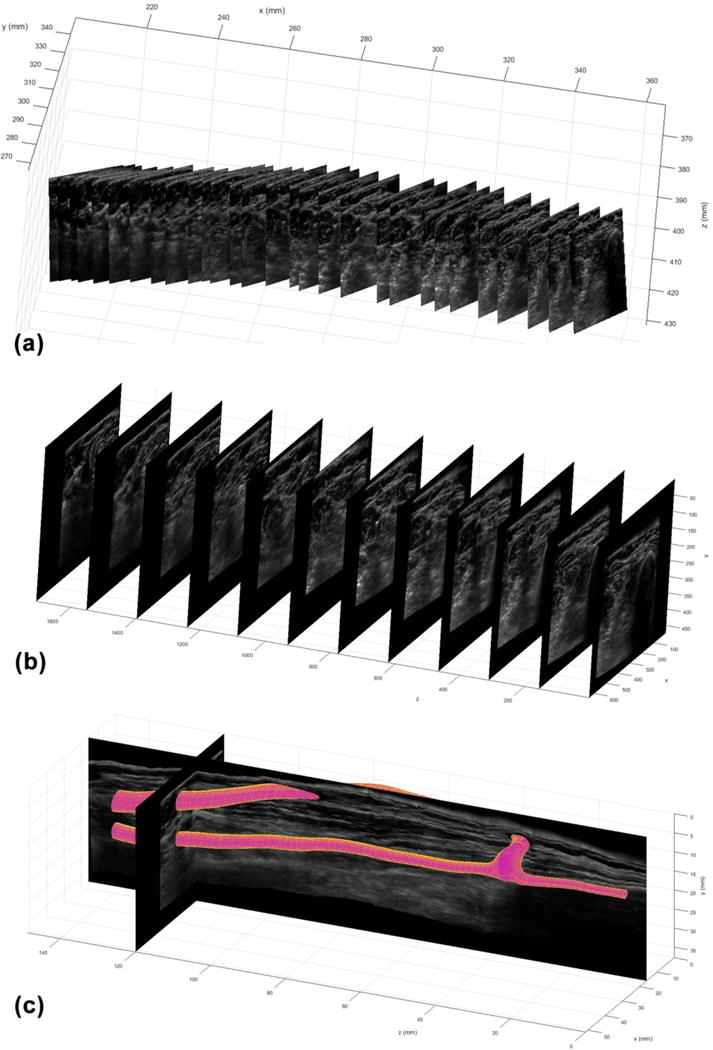
3D ultrasound data acquisition and vessel reconstruction. (a) 2D ultrasound images of a dialysis access fistula are acquired while the 3D location and orientation of the transducer are tracked and recorded. Every 30th image from a continuous scan of 900 images is shown in 3D space. (b) The 2D images are reformatted in a 3D gray-scale volume. Planes in the x-y direction are shown at steps of 155 voxels along the z-axis for a reconstructed volume size of 642 × 473 × 1860 voxels (51.4 × 37.8 × 148.8 mm, voxel size = 0.08 mm). (c) Mesh reconstruction from manual outlines produces a 3D surface model of the arterial and venous segments of the dialysis access fistula.
Calculation of Blood Flow Velocity
Computational fluid dynamics (CFD) modeling was applied to the 3D vessel surface models to calculate four-dimensional (4D) flow velocity fields (spatially 3D and temporally resolved) inside the vascular lumen (McGah et al. 2011, 2012, 2013). The Navier-Stokes equations that describe the flowing blood are solved by a commercial CFD software package (Fluent, ANSYS Inc., Cannonsburg, PA, USA) under the assumptions of an incompressible Newtonian fluid. Although blood is a non-Newtonian fluid, it has been shown that the Newtonian fluid assumption works well for arteries over 3 mm in diameter and under normal hematocrit conditions (Sarrami-Foroushani et al. 2017). Therefore, for the specific vessels under consideration in our simulation, blood is treated as a Newtonian fluid without loss of accuracy in the hemodynamic results.
The computation discretizes the equations of motion into a volumetric mesh that fills the patient-specific lumen geometry. For boundary conditions, one fluid velocity Doppler spectral waveform is used at the model inlet, and the peripheral capacitance-resistance conditions are used downstream of the anastomosis. The fluid velocity and pressure are solved inside the vessel of interest with spatial resolution on the order of 100 microns and temporal resolution of 1 ms. Ten cardiac cycles are simulated to allow for chaotic flow to develop, and then phase-averaged to obtain a velocity field representative of the blood flow in the vessel, including cycle-to-cycle variability inherent to the complex flow within dialysis access fistulas. The 4D velocity database is exported to the Doppler simulator as three components of velocity at each node of the computational mesh and for each time step of the cardiac cycle (Figure 3). These data establish the true velocities for every point within the 3D vessel surface models, providing the reference values to which the examiner’s measurements will be compared.
Figure 3.
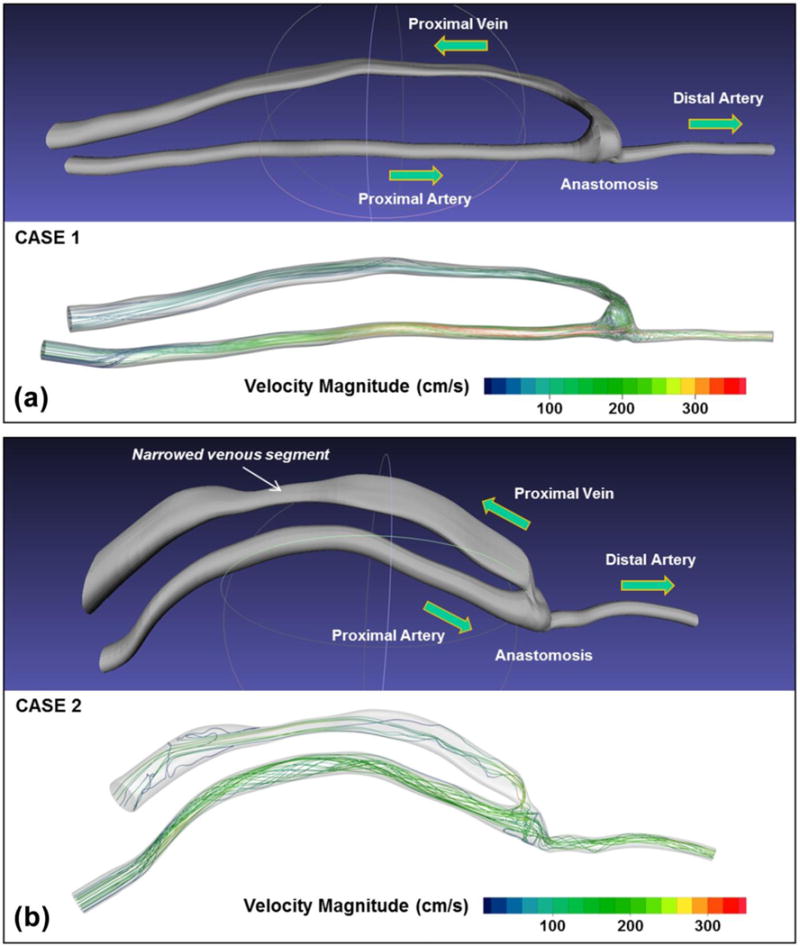
3D vessel lumen models and blood velocity flow simulation data for the two dialysis access fistula cases used in this study. Case 1 is a fistula between the radial artery and cephalic vein; Case 2 is a fistula between the brachial artery and basilic vein. For each case the surface model of the dialysis access fistula is shown at the top; arrows indicate the direction of flow in each segment. The lower panel for each case shows representative velocity pathlines (trajectories of a blood particle from the inlet to the outlet of the artery segment) calculated by the computational fluid dynamics simulation at peak systole; the pathlines are color-coded by velocity magnitude (color bar).
Examiner Interface
The Doppler ultrasound simulator examiner interface is shown in Figure 4. When the mock transducer is moved along the mannequin surface, the computer displays B-mode images in views corresponding to the transducer position and orientation, as reported by the magnetic tracking receiver mounted within the model transducer (Figure 4a). To simulate a clinical ultrasound examination, a control panel on the computer display (Figure 4c) shows settings analogous to a clinical duplex ultrasound system, and the examiner is required to steer the Doppler beam, select the size and depth of the Doppler sample volume within the B-mode/color Doppler image, specify the Doppler angle relative to the vessel axis, and adjust the baseline and pulse repetition frequency (PRF) to avoid aliasing. After acquiring and saving a spectral waveform on the display, the examiner measures blood flow velocity by positioning a cursor at a selected point on the waveform (Figure 4b).
Figure 4.
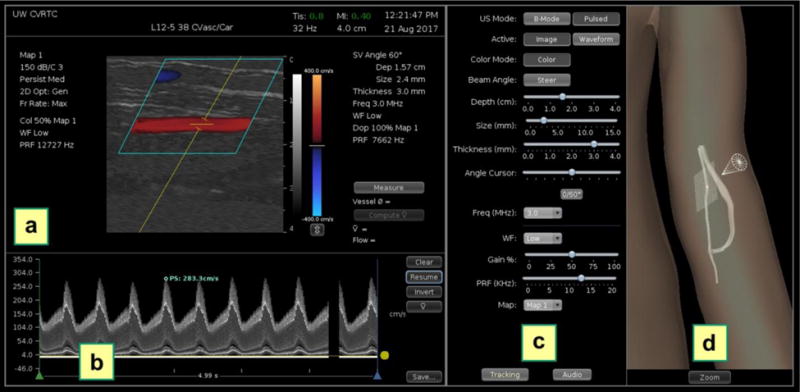
Duplex ultrasound simulator examiner interface. (a) Color Doppler image showing blood flow velocity superimposed on a B-mode image. (b) Doppler spectral waveform recorded from the Doppler sample volume location shown in (a). A peak systolic velocity measurement has been made by placing a cursor (blue circle) at a selected point on the waveform. (c) System controls for the ultrasound image display and Doppler examination settings (including beam angle, sample volume depth, sample volume size, angle correction cursor and pulse repetition frequency). (d) 3D display showing the location of the vessel model, the transducer (cone) and the 2D image plane on the mannequin. This display is intended for training; it can be disabled for competency testing.
Doppler Waveform and Image Simulation
Doppler spectral waveforms are simulated by retrieving the velocities calculated within the 3D model of the vessel lumen and projecting the three components of the velocity within the Doppler sample volume onto the Doppler beam (Figure 5). Based on the examiner’s settings for beam steering, sample volume depth and size, Doppler angle correction and PRF, the stored 4D velocity field within the 3D sample volume is converted to a spectral waveform display (Figure 4b). Frequency shifts are calculated by the Doppler equation using the incident beam angle relative to the blood flow direction provided by the CFD modeling. The simulator waveform display uses the angle correction and PRF specified by the examiner to convert the frequencies to velocities. The spectral waveform display is updated in real-time according to the beam angle and the Doppler sample volume size and location specified. The sample volume size includes dimensions in the lateral and elevational beam directions (Figure 5); spectral broadening in the displayed waveform is related directly to the variation in the blood flow vectors within the sample volume.
Figure 5.
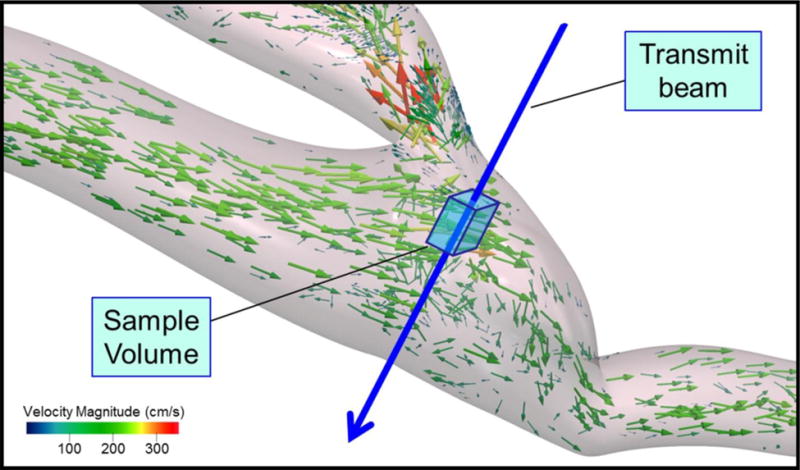
Spectral waveform synthesis from a flow velocity field. The size and position of the Doppler sample volume in the 2D image (Figure 4a) specifies a location within the 3D flow velocity field from which the Doppler spectral waveform is extracted. The sample volume includes beam width in both the lateral and elevation directions to capture flow vectors within a 3D region around the transmit beam. A spectral waveform is synthesized by compiling the Doppler shifts calculated for all vectors within the sample volume, based on the angle between the transmit beam and each flow vector. A subset of vectors is shown for clarity; a typical sample volume contains between 500 and 1000 flow vectors. The Doppler shifts are converted to velocities using the Doppler equation with the angle correction term specified by the examiner. The calculations are updated in real time to create a waveform display of blood velocity over the cardiac cycle.
Color Doppler imaging is also included to enhance the realism of the simulator (Figure 4a). At the intersection of the 2D virtual image plane of the Doppler simulator with the calculated flow field in the 3D vascular models, the CFD velocity vector fields are projected onto the Doppler beam and converted to a color map, animated in real-time, within a color box specified by the examiner.
Validation
The accuracy of Doppler velocity measurements obtained by four different experts performing a duplex ultrasound scan of the dialysis access fistulas was determined by comparing the blood flow velocities measured from the simulated spectral waveforms to the true velocities in the computational flow model data sets used to populate the artery models. The deviation is expressed as a percent of the true velocity.
Four experienced examiners (two vascular sonographers, one vascular surgeon, and one ultrasound engineer) measured PSV in the proximal artery, distal artery, and proximal return vein of the two dialysis access cases (Figure 6). The examiner was free to choose sites in each of the designated segments where, in their judgement, accurate measurements of PSV could be made. The examiner was also free to make multiple measurements on a waveform and to delete or save any measurement during the scanning session. For each velocity measurement made and saved by the examiner, the true PSV was computed as the maximum of the velocity magnitude from the CFD-generated database for those voxels within the sample volume at that specific time and location.
Figure 6.
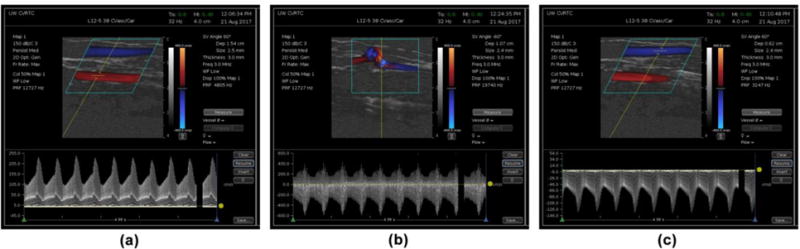
Doppler spectral waveform examples for three segments of dialysis access fistula Case 1. (a) Inflow artery. (b) Anastomosis. (c) Venous return. In each case the arterial flow is from left to right and the venous return flow is from right to left.
Statistical Analysis
The mean absolute deviation from the true velocity magnitude was computed for each PSV measurement made by the examiners and expressed as a percent of the true velocity. Measurements made by the four examiners on each of the arterial and venous fistula segments were compared using ANOVA. Bias in PSV measurement was assessed using the paired t-test.
RESULTS
The four examiners made a total of 43 velocity measurements for the two dialysis access cases. Twenty-four PSV measurements were made in arterial segments, and 19 PSV measurements were made in venous segments. The PSV measured by the examiners deviated from the true value by 7.8 ± 6.1% overall, and there was no significant difference in performance between examiners (Figure 7a). There was a bias toward overestimation by examiners: the true velocity averaged 273 ± 94 cm/s, and the velocity measured by the examiners averaged 283 ± 103 cm/s (p = 0.023). There was also no significant difference in the mean absolute deviation of PSV measurements between the arterial and venous segments (Figure 7b).
Figure 7.
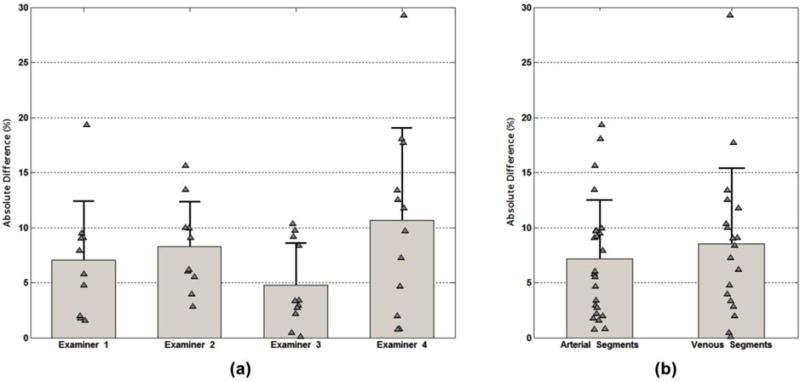
Comparison of measured versus true peak systolic velocity (PSV) for two dialysis access fistulas examined by four experts. (a) Percent absolute deviation from the true PSV for each of the four examiners. (b) Percent absolute deviation for measurements in the arterial and venous segments. Bar height: mean absolute deviation. Error bar length: standard deviation. Triangles: individual data points.
DISCUSSION
Simulation-based medical training allows learners to acquire knowledge and skills in an educationally focused environment without concerns regarding patient access, safety, comfort, or privacy (Aggarwal et al. 2010). Such training also lets learners progress at their own pace, and it can provide a wide range of clinical experience in a relatively short period of time. A variety of medical simulator types are available, including standardized patients, computer-based virtual patients, interactive mannequins, and task trainers for procedural skills (Cook et al. 2011, 2013).
Simulators are now available for many non-vascular ultrasound applications that require only 2D B-mode imaging (Liu et al. 2015, Paddock et al. 2015). However, duplex ultrasound for vascular applications combines B-mode imaging with Doppler flow detection in the form of both spectral waveforms and color Doppler images. The acquisition and interpretation of Doppler velocity information, particularly the arterial PSV, plays a key role in the classification of vascular disease severity (Beach et al. 2010, 2012). There are commercially-available physical flow models that can provide some examples of vascular flow abnormalities; these flow phantoms, however, offer a limited range of cases and true fluid velocities are not accurately known.
While simulation of 2D B-mode images can be accomplished by using computer-generated images or images from patient scans, simulation of Doppler information presents a significant technical challenge, given the complexity of creating flow information in real-time throughout a simulated volume of tissue. In addition, a realistic simulation of the duplex scanning process includes manipulation of the ultrasound transducer to obtain the appropriate B-mode image of a vessel of interest, and interactive control of a number of highly variable ultrasound parameters, including Doppler sample volume size and depth, beam angle, and angle correction. The spectral waveforms must then be acquired and a cursor placed for measurement of velocity. Each step in this complex task must be simulated in real-time.
The Doppler ultrasound simulator described here provides real-time Doppler spectral waveforms and color Doppler images, which to the best of our knowledge are not available on any commercial ultrasound simulators (Zierler et al. 2016). In addition to a realistic scanning experience, this simulator can provide an absolute measure of examiner performance because the CFD velocity database provides the true flow velocity values for all locations in the patient vessel model at all instants of time. This gives the simulator the unique ability to evaluate trainees based on a set of standardized flow velocities. The dialysis access models tested in the current study demonstrate the ability of the Doppler simulator to present unique clinical cases with both complex flow and vessel anatomy, which may not be available or easily assessed in standard clinical training.
There is one limitation to note in the present study: although a button labeled ‘Audio’ is shown in the simulator control panel (Figure 4c), audio was not available on the Doppler simulator at the time of this study. Real-time audio output of the Doppler shifts corresponding to the spectral waveforms was implemented on the simulator after this study was completed.
The results described here establish the capability of the duplex ultrasound simulator to realistically reproduce complex dialysis access cases. It also shows that expert examiners can use the simulator to measure PSV with accuracy consistent with clinical practice at multiple sites on these models. Directions for future work include creating a library of additional dialysis access sites, in addition to carotid artery and peripheral artery cases. The goal is to populate the library with enough cases to enable trainees to acquire experience with both commonly encountered and rare vascular pathologies and abnormalities. We will also quantitatively assess the validity of the duplex ultrasound simulator for competency testing and its efficacy as a training tool.
SUMMARY
We have developed a computer-based duplex ultrasound simulator that provides real-time color Doppler images and Doppler spectral waveforms. The examiner manipulates a mock transducer along a region of interest on a mannequin, and the computer displays a 2D B-mode image of the scanned anatomy. Velocity measurements can then be made in a realistic manner with the examiner setting the appropriate Doppler ultrasound parameters. This application of the Doppler simulator with dialysis access fistulas showed that an expert examiner can accurately measure PSV from the spectral waveforms provided in real time by the simulator with a mean absolute error of less than 10%. With the addition of cases representing a range of vascular targets and pathologies, this Doppler ultrasound simulator could be a useful tool for training as well as for assessment of skills in vascular ultrasound.
Acknowledgments
This work was supported by funding from the National Institute of Biomedical Imaging and Bioengineering and the National Institute of Environmental Health Sciences, National Institutes of Health, Bethesda, MD (Grant Numbers 1R41EB018124-01A1, 1R42EB018124).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Conflicting Interests
Dr. Sheehan is the founder and President of Sheehan Medical, LLC, which markets a transthoracic echocardiography (TTE) simulator that she and co-investigators developed and validated at the University of Washington (UW). Dr. Sheehan supports research in medical education by lending TTE simulators from her laboratory at the UW to investigators for up to six months. Neither the co-authors of this manuscript nor the UW have involvement in Sheehan Medical, LLC and none receive any benefit from simulator sales.
References
- Aggarwal R, Mytton OT, Derbrew M, Hananel D, Heydenburg M, Issenberg B, MacAulay C, Mancini ME, Morimoto T, Soper N, Ziv A, Reznick R. Training and simulation for patient safety. Qual Saf Health Care. 2010;19(Suppl 2):i34–i43. doi: 10.1136/qshc.2009.038562. [DOI] [PubMed] [Google Scholar]
- Beach KW, Bergelin RO, Leotta DF, Primozich JF, Sevareid PM, Stutzman ET, Zierler RE. Standardized ultrasound evaluation of carotid stenosis for clinical trials: University of Washington Ultrasound Reading Center. Cardiovasc Ult. 2010;8:39. doi: 10.1186/1476-7120-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beach KW, Leotta DF, Zierler RE. Carotid Doppler velocity measurements and anatomic stenosis: Correlation is futile. Vasc Endovascular Surg. 2012;46:466–474. doi: 10.1177/1538574412452159. [DOI] [PubMed] [Google Scholar]
- Cook DA, Hatala R, Brydges R, Zendejas B, Szostek JH, Wang AT, Erwin PJ, Hamstra SJ. Technology-enhanced simulation for health professions education: a systematic review and meta-analysis. JAMA. 2011;306:978–988. doi: 10.1001/jama.2011.1234. [DOI] [PubMed] [Google Scholar]
- Cook DA, Brydges R, Zendajas B, Hamstra SJ, Hatala R. Technology-enhanced simulation to assess health professionals: a systematic review of validity evidence, research methods, and reporting quality. Acad Med. 2013:872–883. doi: 10.1097/ACM.0b013e31828ffdcf. [DOI] [PubMed] [Google Scholar]
- Ferrero NA, Bortsov AV, Arora H, Martinelli SM, Kolarczyk LM, Teeter EC, Zvara DA, Kumar PA. Simulator training enhances resident performance in transesophageal echocardiography. Anesthesiology. 2014;120:149–159. doi: 10.1097/ALN.0000000000000063. [DOI] [PubMed] [Google Scholar]
- Kohler TR, Mraz BA. Dialysis access procedures. In: Zierler RE, Dawson DL, editors. Strandness’s duplex scanning in vascular disorders. 5th. Philadelphia, PA: LWW; 2015. pp. 453–470. [Google Scholar]
- Leotta DF, Martin RW. Three-dimensional spatial compounding of ultrasound scans with weighting by incidence angle. Ultrasonic Imaging. 2000a;22:1–9. doi: 10.1177/016173460002200101. [DOI] [PubMed] [Google Scholar]
- Leotta DF, Martin RW. Three-dimensional ultrasound imaging of the rotator cuff: spatial compounding and tendon thickness measurement. Ultrasound Med Biol. 2000b;26:509–525. doi: 10.1016/s0301-5629(99)00173-8. [DOI] [PubMed] [Google Scholar]
- Leotta DF, Primozich JF, Beach KW, Bergelin RO, Strandness DE., Jr Serial measurement of cross-sectional area in peripheral vein grafts using three-dimensional ultrasound. Ultrasound Med Biol. 2001;27:61–68. doi: 10.1016/s0301-5629(00)00296-9. [DOI] [PubMed] [Google Scholar]
- Leotta DF, Paun M, Beach KW, Kohler TR, Zierler RE, Strandness DE., Jr Measurement of abdominal aortic aneurysms using three-dimensional ultrasound imaging: preliminary report. J Vasc Surg. 2001;33:700–707. doi: 10.1067/mva.2001.112812. [DOI] [PubMed] [Google Scholar]
- Liu L, Kutarnia J, Belady P, Pedersen PC. Obstetric ultrasound simulator with task-based training and assessment. IEEE Trans Biomed Eng. 2015;62:2480–2497. doi: 10.1109/TBME.2015.2433679. [DOI] [PubMed] [Google Scholar]
- McGah P, Leotta DF, Beach KW, Zierler RE, Aliseda A. Incomplete restoration of homeostatic shear stress within arteriovenous fistulae. J Biomech Eng. 2013;135:1–9. doi: 10.1115/1.4023133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGah P, Leotta DF, Beach KW, Riley JJ, Aliseda A. The impact of flow stresses on remodeling of peripheral artery bypass graft: intimal hyperplasia at the stenosis and vessel wall dilation in the post-stenotic region. J Vasc Surg. 2012;56:403–409. doi: 10.1016/j.jvs.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGah PM, Leotta DF, Beach KW, Riley JJ, Aliseda A. A longitudinal study of remodeling in a revised peripheral artery bypass graft using 3D ultrasound imaging and computational hemodynamics. J Biomech Eng. 2011;133:041008. doi: 10.1115/1.4003622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddock MT, Bailitz J, Horowitz R, Khishfe B, Cosby K, Sergel MJ. Disaster response team FAST skills training with a portable ultrasound simulator compared to traditional training: pilot study. Western J Emerg Med. 2015;16:325–320. doi: 10.5811/westjem.2015.1.23720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks AR, Atkinson P, Verheul G, Leblanc-Duchin D. Can medical learners achieve point-of-care ultrasound competency using a high-fidelity ultrasound simulator?: a pilot study. Crit Ultrasound J. 2013;5:9. doi: 10.1186/2036-7902-5-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarrami-Foroushani A, Lassila T, Frangi AF. Virtual endovascular treatment of intracranial aneurysms: models and uncertainty. WIREs Syst Biol Med. 2017;9:e1385. doi: 10.1002/wsbm.1385. [DOI] [PubMed] [Google Scholar]
- Sheehan FH, Otto CM, Freeman RV. Echo simulator with novel training and competency testing tools. Stud Health Technol Inform. 2013;184:397–403. [PubMed] [Google Scholar]
- Teodorescu V, Gustavson S, Schanzer H. Duplex ultrasound evaluation of hemodialysis access: a detailed protocol. Int J Nephrol. 2012;508956 doi: 10.1155/2012/508956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zierler RE, Leotta DF, Sansom K, Aliseda A, Anderson MA, Sheehan FH. Development of a duplex ultrasound simulator and preliminary validation of velocity measurements in carotid artery models. Vasc Endovasc Surgery. 2016;50:309–316. doi: 10.1177/1538574416647502. [DOI] [PMC free article] [PubMed] [Google Scholar]


