Abstract
A consensus for venous flow quantification using echo spectral Doppler sonography (DS) is lacking. DS data from 83 healthy individuals have been examined using manually traced transverse cross-sectional area (CSA) and diameter-derived CSA obtained in a longitudinal view measurements of the internal jugular vein (IJV). The time-averaged velocity (TAV) over a 4-second interval was obtained in longitudinal plane using manual tracing of waveform. Manual and computer generated blood flow volume calculations were also obtained for common carotid artery (CCA), for accuracy purposes. No differences in semiautomated vs. manual blood flow volume calculation was detected for CCA. The manual calculation method resulted in almost two-fold larger venous IJV flow when compared to the semi-automated. DS equipment does not provide accurate automated calculation of venous size and blood flow. Until further technological development occurs, manual calculation of the venous blood flow is warranted.
Keywords: Doppler sonography, IJV, blood flow, veins
Introduction
Doppler sonography (DS) is the most utilized imaging technique for evaluating the vascular system. The hemodynamic evaluations derived from the ultrasonography method are critical for correct diagnosis of emergency, cardiac, obstetric, hepatic and neurological conditions. (Ficial, et al. 2013, Naqvi, et al. 2013, Stankovic, et al. 2012) For an example, the measurement of the respiratory changes at the level of inferior vena cava are highly associated with invasively determined central venous pressure. (Nagdev, et al. 2010) The authors purposed that DS-derived respiratory changes of the inferior vena cava greater or equal than 50% correlated with an invasively-derived central venous pressure lower than 8mmHg. (Nagdev, et al. 2010) Similarly, measurement of the changes in the internal jugular vein (IJV) cross-sectional area (CSA) during the cardiac cycle, can estimate the jugular venous pulse. (Sisini, et al. 2015) Despite potential inaccuracies, the DS observation of the IJV pulsatility remains an acceptable method of central venous pressure monitoring. (Constant 2000)
Spectral DS analysis of the IJVs or the vertebral veins is an emerging new concept in examining their physiological hemodynamic ranges. Recent vascular studies show abnormal extracranial venous flow in variety of central nervous system disorders, such as Meniere syndrome, (Di Berardino, et al. 2015), migraine (Chung, et al. 2010) transient global amnesia, (Cejas, et al. 2010) multiple sclerosis, (Zivadinov, et al. 2011) Parkinson’s disease (Liu, et al. 2015), obstructive sleep apnea (Chi, et al. 2015), and cough headache (Chuang and Hu 2005), among others. Therefore, an accurate and reproducible DS venous blood flow volume (BFV) method is needed. Although extracranial venography is the imaging gold standard for detecting venous abnormalities in the neck, the DS does allow a portable, cost-efficient, non-invasive assessment of the neck veins, potentially allowing screening in larger number of subjects. (Zivadinov, et al. 2014)
In the past two decades, studies have attempted to calculate the venous BFV with various measurement methods (Table 1). Due to the thin and malleable venous wall, the shape of the vein does not always result in geometrically circular form. (Gill 1985) Therefore, the use of cross-sectional diameter as cross-sectional measure probably underestimates the size of the vessel. (Chambers, et al. 2014) In response to the limitation of DS use in venous flow quantification, a number of technical considerations have been proposed in order to rectify the discrepancies and improve the reproducibility of the scans. (Nicolaides, et al. 2011) Additionally, several groups have resorted to manual CSA segmentation and manual spectral waveform tracing. (Jakimovski, et al. 2017, Monti, et al. 2014) However, a general consensus on extracranial venous BFV measurement and calculation is currently lacking. Although the current Doppler units are equipped with automated measurement software packages, the DS physic principles for velocity, CSA, and BFV calculation are based on the previously validated arterial anatomy and physiology. The use of the aforementioned automated BFV calculation on the irregular and complex venous system would potentially provide erroneous data.
Table 1.
Review of the methods used for measurement of the cross-sectional area and the velocity of the internal jugular vein.
| Reference | CSA measurment | Velocity measurment |
|---|---|---|
| Sisini et al. (Sisini, et al. 2015) | manually drawn circumference | TAV over 4 cardiac cycles |
|
| ||
| Ozen et al. (Ozen, et al. 2014) | D/2*π in longitudinal plane | mean TAV over 2–3 cardiac cycles |
| Jakimovski et al. (Jakimovski, et al. 2017) | manually drawn circumference | mean TAV over 4 seconds |
| Yeoh et al. (Yeoh, et al. 2017) | manually drawn circumference | mean TAV over 3 seconds |
| Chambers et al. (Chambers, et al. 2014) | D/2*π in longitudinal plane | mean TAV over 4–5 cardiac cycles |
| Schreiber et al. (Schreiber, et al. 2003) | D/2*π in transverse plane | not described |
| Monti et al. (Monti, et al. 2011) | manually drawn circumference | TAV over 3 cardiac cycles at end of expiration |
| Ciuti et al. (Ciuti, et al. 2013) | D/2*π in transverse plane | Quality Doppler Profiles (QDP) velocity |
CSA – cross-sectional measurement, D – diameter, TAV – time averaged velocity
Based on this background, we aimed to compare the IJV BFV calculated manually versus using the automated DS unit methodology. As a bench-mark comparison, a manual and semi-automated DS-unit computed volumes of the common carotid artery (CCA) were obtained.
Materials and Methods
Clinical and demographic characteristics
The subjects used in this analysis were prospectively enrolled in the “Combined extra- and intra-cranial venous Doppler and MRI evaluation in healthy individuals” (CEIVD-MRI-HI study). The study inclusion criteria were: 1) having age between 18 and 89 years, and 2) qualifying on a health screen questionnaire. The exclusion criteria were: 1) having pre-existing medical conditions that are known to be associated with brain pathology (e.g. cerebrovascular disease, alcohol abuse and other neurological diseases); 2) history of cerebral vascular malformations and congenital malformations (i.e. Klippel-Trenaunay, Parkes-Weber, Servelle-Martorell, and Budd-Chiari syndrome); and 3) pregnant or nursing mothers. The study was approved by the local Institutional Review Board (IRB) and informed consent was obtained from all subjects enrolled in the study.
Doppler sonography acquisition
Echo-color DS (Biosound My Lab 25 Gold, Esaote, Genoa, Liguria, Italy) equipped with 2.5 and 7.5–10 MHz transducers was used for extra- and intra- cranial examination. For the purpose of examining the IJV and CCA, the 7.5–10 MHz linear probe was used. The DS-unit velocity, color flow direction, axial and vertical spatial resolution was tested regularly using a Mini-Doppler Phantom unit (Model 1430, Gammex, Middleton, WI, USA).
All sonographic examinations were performed by the same registered vascular technologist with 27 years of experience (KM). Subjects drank 16 oz of water within 1 hour before the study. The subjects were placed on a hydraulic chair and instructed to lay in supine position for minimum of 3 minutes before scanning started. Warm Aquasonic 100 water-soluble, hypoallergenic, transmission gel was placed on neck area with the head placed in neutral, straight forward position. The IJV was scanned in the transverse plane to assess for regions of interest. IJV volume were assessed at three levels bilaterally; just below the facial vein entry (J3), approximate mid thyroid region (J2) and approximately one cm above the IJV valve (J1). At each IJV level, a transverse CSA measurement followed by the longitudinal image were recorded. Manual waveform tracing over four second period was used to calculate the time-average velocity (TAV). In order to shorten the exam, the measurements in a longitudinal view were calculated after the exam completion. Angled color box during longitudinal image acquisition, angle of incident at or between 45° and 60° with angle correction bar parallel to vessel walls, and spectral gate size, adjusted accordingly within lumen per standard vascular protocol, was maintained. Sample gate was open to maximal size yet maintained within vessel lumen to avoid vessel wall artifact in waveform.
The left and right CCA blood flow was obtained in supine position approximately 1.5 cm before the bifurcation and at the proximal neck level of the CCA. Similarly to the vein measurements, the four second longitudinal TAV image was also recorded. The DS frequency, 55°– 60° angle of incident, and spectral gate size were adjusted according to standard vascular protocol.
Doppler sonography measurement
Semi-automated blood flow volume calculation
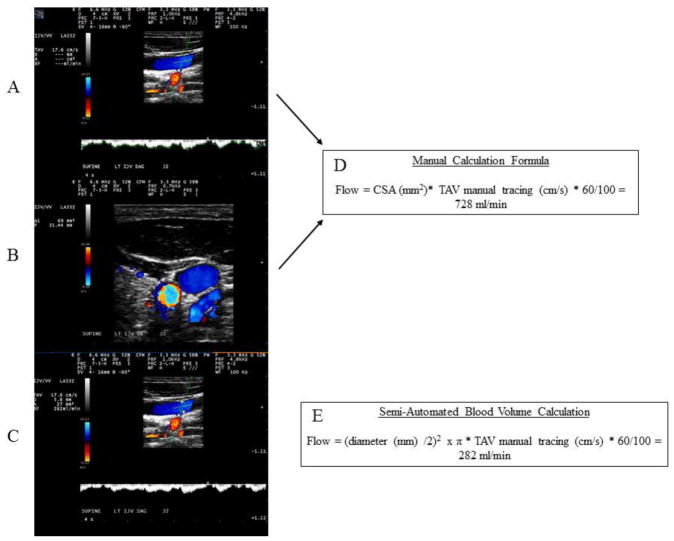
The mean semi-automated TAV (TAVmean) over a period of four seconds was utilized in both the manual and computer generated blood flow calculations. As the computer generated waveform tracings may render inconsistent and irregular waveforms, a manual 4 second tracing was utilized. The vessel diameter was obtained in a longitudinal plane, as measured from anterior wall to posterior wall on a 180° perpendicular plane alignment in respect to the vessel walls. The DS-unit’s computer system utilized these values to calculate BFV. The measurement protocol from the computer-generated calculation of BFV utilized manual tracing during time period of 4 seconds for the TAVmean and multiplied it by the manual anterior/posterior diameter measurement (Figure 1).
Figure 1.
Differences in the manual (top two panels) versus the semi-automated calculation of the venous blood flow volumes and the corresponding formulas.
Panels A and B demonstrate the manual method of calculation of the venous blood volume. A) Manual time averaged velocity detection. Angle of incident at or between 45° and 60° with angle correction bar parallel to vessel walls was used. B) Manual tracing of the cross-sectional area in transverse plane. Panel C depicts the semi-automated blood volume calculation with an anterior to posterior wall diameter obtained at 180° orthogonal plane alignment in regards to the vessel wall. Panel boxes D and E show the corresponding calculation formulas used to derive the internal jugular vein volume (manual versus semi-automated calculation, respectively). CSA – cross-sectional area, TAV – time average velocity, D – dimeter, ml – milliliters, min – minutes.
Manual blood flow volume calculation
The manual BFV calculations were obtained using manual IJV CSA tracing and manual tracing of the same 4 seconds of the TAVmean. Since the gray-scale mode does not depict the actual CSA of the blood flow, the manual CSA was performed in transverse plane using color DS mode. Adjustment of pulse repetition frequency was maintained to ensure no bleeding of the DS color that would artificially overestimate the measurement (Figure 1).
All measurements were repeated on the CCA and arterial BFV was obtained. To maintain consistency with the IJV method of calculation, the 4 second TAV of the carotid artery flow was calculated using manual area tracing in a longitudinal view. Anterior to posterior wall diameter (AP) obtained at 180° peprendicular plane alignment in respect to the vessel wall generated the CCA CSA. Additional manual tracing of the arterial CSA was performed in a transverse plane. Both methods of manual and semi-automated calculation of arterial BFV were performed.
Statistical analysis
Statistical analyses were performed using SPSS version 24.0 (IBM, Armonk, NY, USA). In order to assess the correlation between the semi-automated and manual calculation of the arterial and venous BFV, Pearson correlations coefficients were used. In all analyses, a minimum significance level of p≤0.05 based on a two-tailed test was considered significant. Scatter-plots were employed in order to visually demonstrate the differences of both methods used.
Results
The subjects used in this study had mean age of 42.5 years, mean body mass index of 27.3 and the sample consisted of 45 (54.2%) females. The clinical screening demonstrated the healthy status of the population used with only one individual having a history of heart attack 10 years prior to the study. Thirteen individuals had an official diagnosis of hypertension (15.7%). The mean systolic (131.6mmHg) and diastolic (79.1mmHg) pressures of the study cohort were within the normal limits. All clinical and demographic characteristics are shown in Table 2.
Table 2.
Demographic and clinical characteristics of the study subjects.
| Demographic and clinical characteristics | Healthy Individuals (n=83) |
|---|---|
| Female, n (%) | 45 (54.2) |
| Age, mean (SD) | 42.5 (19.5) |
| BMI, mean (SD) | 27.3 (6.1) |
| Hypertension, n (%) | 13 (15.7) |
| Heart disease, n (%) | 1 (1.2) |
| Smoking status, n (%) | 2 (2.4) |
| Systolic pressure, mean (SD) | 131.6 (20.1) |
| Diastolic pressure, mean (SD) | 79.1 (11.8) |
| Pulse, mean (SD) | 69.4 (12.4) |
n-number, SD- standard deviation, BMI – body mass index
Semi-automated versus manual blood flow volume calculations
The TAV, CSA and the semi-automated and manually calculated BFV are shown in Table 3. The difference in venous BFV was in range of double increase within the manual calculation when compared to the semi-automated one (R upper IJV, 890.2 ml/min vs. 390.9 ml/min; L upper IJV, 590.0 ml/min vs. 267.7 ml/min; R mid IJV, 1090.1 ml/min vs. 463.9 ml/min; L mid IJV 704.5 ml/min vs. 311.4 ml/min; R lower IJV 1179.3 ml/min vs. 620.9 ml/min; and L lower IJV 881.1 ml/min vs. 442.7 ml/min). On the other hand, the DS-programmed automated software calculated almost identical arterial BFV as did the manual one (R upper CCA, 685.7 ml/min vs. 685.0; L upper CCA, 706.5 ml/min vs. 706.2 ml/min; R lower CCA, 698.8 ml/min vs. 701.3 ml/min; and L lower CCA 776.9 ml/min vs. 777.4 ml/min).
Table 3.
Doppler characteristics of the measured vessels in 83 healthy individuals.
| Site of measurment | Diameter (mm2) | TAV (cm/sec) | Semi - automated CSA (mm2) | Manual CSA (mm2) | Semi-automated calculation of BFV | Manual calculation of BFV | Correlation between methods |
|---|---|---|---|---|---|---|---|
| Venous side | |||||||
| R upper IJV | 5.9 (2.1) | 22.6 (11.2) | 30.9 (21.4) | 69.5 (39.4) | 390.9 (249.5) | 890.2 (557.9) | 0.580 |
| L upper IJV | 4.9 (1.8) | 21.3 (12.4) | 21.1 (14.8) | 49.2 (35.6) | 267.7 (216.4) | 590.0 (473.9) | 0.580 |
| R mid IJV | 6.5 (2.6) | 25.2 (16.5) | 38.0 (29.6) | 88.6 (54.8) | 463.9 (280.6) | 1090.1 (538.0) | 0.565 |
| L mid IJV | 5.4 (2.1) | 23.6 (15.9) | 26.1 (20.2) | 56.6 (39.3) | 311.4 (235.6) | 704.5 (504.2) | 0.669 |
| R lower IJV | 7.5 (2.7) | 25.8 (16.6) | 49.3 (33.2) | 89.7 (51.7) | 620.9 (384.7) | 1179.3 (618.8) | 0.500 |
| L lower IJV | 5.8 (2.3) | 27.5 (20.4) | 30.7 (22.9) | 60.7 (44.9) | 442.7 (333.7) | 881.1 (698.2) | 0.444 |
| Arterial side | |||||||
| R upper CCA | 5.8 (1.2) | 41.4 (7.1) | 27.3 (7.9) | 27.9 (6.6) | 685.0 (151.9) | 685.7 (163.1) | 0.999 |
| L upper CCA | 5.9 (0.8) | 43.4 (7.4) | 27.6 (7.7) | 27.6 (7.7) | 706.2 (187.4) | 706.5 (188.0) | 0.999 |
| R lower CCA | 5.9 (1.2) | 39.7 (6.6) | 28.9 (8.2) | 29.7 (6.8) | 701.3 (183.0) | 698.8 (170.0) | 0.887 |
| L lower CCA | 6.1 (0.7) | 44.5 (8.3) | 29.3 (7.1) | 29.3 (7.1) | 777.4 (205.3) | 776.9 (205.1) | 0.999 |
TAV – time averaged velocity, CSA – cross-sectional area, R – right, L – left, IJV – internal jugular vein, CCA – common carotid artery, BFV – blood flow volume. All measures are shown as mean (standard deviation). The volume is represented in ml/min. The comparison between the methods was derived using Pearson correlation coefficients
Correlations between semi-automated versus manual blood flow volume calculations
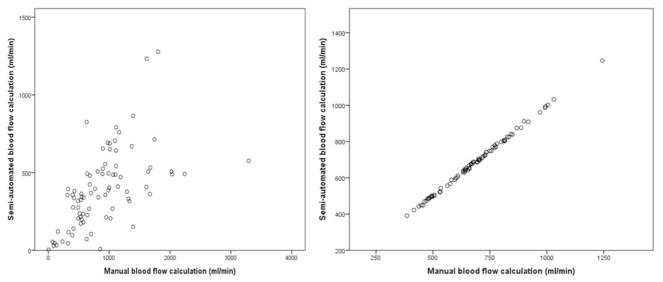
Correlations between semi-automated and manual venous BFV calculation are shown in Table 3. Additionally, the difference between the methods agreement in both arterial and venous vasculature are shown in Figure 2. The correlation between the manual and automated methods in measuring the venous BFV was generally low (r= 0.44–0.67). On the other hand, there was a strong correlation when the manual and automated methods were used for measurement of the arterial BFV (r= 0.99, with an exception of the right lower CCA segment of r=0.89).
Figure 2.
Scatter plot showing the difference between the semi-automated and manual calculation of the venous blood flow (left) versus the arterial blood flow (right)
For representation of the venous blood flow volume measurement, the right upper IJV was used. The blood flow volumes represented on both axis are depicted as ml/min. For the corresponding arterial blood flow representation, the right upper CCA was used. IJV – internal jugular vein, CCA – common carotid artery.
Discussion
In this study, we compared two different (manual and semi-automated) DS-based BFV calculations methods. Additionally, we applied the same type of measurements to the arterial BFV calculations. The main finding of the study is that the semi-automated DS method provided approximately two-fold underestimation of the venous BFV when compared to manual, while these differences were not found for the calculation of arterial BFV. The high differences in the flow measures are systematic and highly dependable on the method of the CSA measurement.
By publishing standardized protocols, the American Institute of Ultrasound in Medicine (AIUM) and American College of Radiology (ACR) are multidisciplinary agencies that promote safe and effective use of DS in medicine. Although protocols for peripheral venous examination have been previously published, there are no equivalent guidelines for extra- and intra-cranial venous BFV measurement at this time. (American Institute of Ultrasound in, et al. 2014, Guideline developed in collaboration with the American College of, et al. 2015) Recently, numerous studies were published on the extracranial venous BFV measurements, however only few of them are methodologically comparable (Table 1). For an example, the IJV BFV has been measured using CSA acquired from either longitudinal or transverse planes and measurement of the vessel diameter (D/2*π) or manually drawn circumference. (Ciuti, et al. 2013, Jakimovski, et al. 2017) In this paper we showed that the CSA derived as a diameter in a longitudinal plane significantly underestimates the real size of the of the vessel. Due to the naturally elliptic form of the jugular vein section, the relationship between the minor and major axis depend on the degree of eccentricity. Therefore, the employed semi-automated method (measuring the AP diameter) is represented by the minor axis and on average underestimates the size by half. Similarly, the TAV has been calculated over different periods of time (from 2–5 seconds or over 2–5 cardiac cycles) and in different stages of the respiratory cycle. (Gill 1985) More detailed methodological review of the literature examining the IJV BFV methods is shown in Table 1.
Using DS to measure the blood velocity within a vessel has multiple inherited limitations. The velocities recorded within the sample area are considerably affected by the flow profile. In comparison to unidirectional flow, a presence of complex flow that is commonly present in the veins, introduces non-longitudinal velocity vectors that contribute to the total velocity measured and can produce over- or under-estimation of the assumed BFV. (Van Canneyt, et al. 2013) Moreover, when assessing the velocity over a small sample of volume that is positioned in the center of the CSA, a correction factor β (shape of velocity profile) should be applied. (Van Canneyt, et al. 2013) Additionally, the flow within the veins does not usually have parabolic profile, thus the maximal intensity of the flow is not always situated in the center of the vessel. Due to the thicker arterial wall and the higher blood pressure within the arteries, the arterial vessel maintains a circular shape which in turn results in laminar blood flow. Since the laminar flow profile does not require extensive β factor correction, the AP diameter measure can be validated as correct CSA measurement.
Color cine-loop DS technique determines the maximum velocity of the entire CSA rather than small volume sample selected by the operator. (Gerada, et al. 2006) In methods that sample the velocity of all color flow pixels, some pixels might intersect the vessel wall or correspond to areas outside of the vessel. (Pinter, et al. 2015) Power-weighted surface integration of velocities can potentially correct for flow pixels that only partially intersect the vessel and only partially contribute to the flow estimate. (Pinter, et al. 2015) Furthermore, studies have shown that there are weak correlations between peak flow velocities and the corresponding venous BFV, indicating that determining peak velocity measure alone is not enough for accurate volumetric assessment. (Breen, et al. 2007) For more consistent velocity calculation, assessment of the TAV over certain period of seconds or heart cycles is preferred. (Gill 1985) In our study we used TAV calculated over the period of 4 seconds and a manual tracing of the spectral wave. Although the DS unit is capable of automatic tracing of the waveform, this is based on arterial flow physics. Measuring the scattered IJV flow pattern with this automatic setting will result in inaccurate and erroneous mean flow velocity measurement.
The location of the IJV assessment may also affect the IJV volume outcome. Further assessments should include additional calculation of the BFV from the tributary veins. Some of the aforementioned tributaries can contribute to considerable BFV that might be neglected if not systematically documented. (Jakimovski, et al. 2017) As there is no exact location of the entry level of these tributary in relation to the corresponding thyroid or cervical spinal cord anatomy, measurement locations should be explained in reference to the IJV vessel itself. Similarly, groups have used an ultrasound- and MRI-based calculation models that can derive the total brain blood outflow. (Utriainen, et al. 2012) (Zamboni, et al. 2013) By using arterial flow correction (difference between the inflow versus the outflow blood flow), the models can provide an estimation of the collateral venous index. (Zamboni, et al. 2013)
Due to respiration patterns, the cardiac function, and the thin muscular layer of the vein contribute in significant variation of the wall shape and size over time, devaluing the AP diameter measurement. Moreover, the IJV contains valve located near the IJV-subclavian vein junction that prevents the backflow of blood. Even with a normal function of the valve, the closure can cause some turbulent flow with possible distension of the IJV wall, followed by valve opening and elevated velocity rate.
Previous research has tried to classify the abnormal IJV flow into distinct five groups including: 1) markedly decreased flow with peak velocity below 10cm/s, 2) stasis due to thrombus formation resulting in very low or no flow detected, 3) reversed flow (reflux) 4) increased turbulent flow with abnormal spectral waveforms and 5) pulsatile flow related the arterial pulsations. (Lin, et al. 2009) Additionally, the authors showed IJV CSA changes in relation to the respiratory pulsations due to an increase and decrease in intrathoracic pressure. Furthermore, another study showed that when the IJV CSA measurement was performed during normal breathing versus apnea, the flow during apnea had visibly more regular pattern. (Sisini, et al. 2016) Although still subjective, acquiring the CSA during specific points of the breathing cycle is a step forward in acquiring reproducible measurements.
In addition to the respiratory dependency of the CSA and TAV of the IJV flow, the measurements also depend on the retrograde pressure changes caused by the heart. In order to eliminate the influence of respiration and cardiac pressure waves on the CSA measurement, authors have suggested use of corrections based on the simultaneously acquired ECG trace. (Sisini, et al. 2016) This changes in CSA over the cardiac cycle point to a significant measurement variation up to 30%. (Lagana, et al. 2017, Sisini, et al. 2016) Using a similar technique, the cardiac cycle induced changes in IJV CSA that were more than 50% in amplitude. (Nakamura, et al. 2016) Additionally, the study showed similarities of the IJV CSA waveforms and those of the jugular venous pressure. (Nakamura, et al. 2016)
The new volumetric 3D/4D probes acquire multivolume color Doppler datasets. (Badano, et al. 2007) Each multivolume dataset consists of many image volumes acquired sequentially. This allows volume calculations that are independent of the vessel geometry, the angle and flow profile. However, these images are not true real-time images, but only a result of superimposition of multiple small datasets that are acquired during apnea. Therefore, ECG-, respiration-gating and potential movement may affect the image quality that is more prone to artifacts occurrence. (Badano, et al. 2007) In cases of this particular 3D Doppler reconstruction, the pulsatile nature of the blood flow and the slower venous flow may induce reduction or absence of the recorded signal, an artifact that might mimic pathologies involved with no flow or stenosis. (Vicenzini, et al. 2012) The second generation of 3D Doppler systems uses transducers with higher frequency that allow true “real-time” image acquisition that eliminates the long scan time and the dependence of cardiac and respiratory gating. (von Ramm and Smith 1990)
Inaccurate IJV volume analysis may lead to misinterpretation of the magnitude and functional role of extracranial venous hemodynamic disturbances. Therefore, an established methodology on the use of Doppler ultrasound BFV measurements in the extracranial venous system is urgently needed. A joint effort between researchers and Doppler unit developers should determinate future protocols for IJV volume calculation. Similarly, concerns regarding the validity and consistency of the flow rate assessment has been discussed in previous publications. (Sisini, et al. 2014, Zamboni 2016) Until this is accomplished, the specific details on scan location, probe frequency, angle of incidence, and the formula of BFV calculation should be clearly documented and reported.
The study had some inherited limitations. The acquired CSA was derived from a single screenshot image obtained at the end of inspiration. In comparison to other breathing cycle periods, the end of inspiration phase can be easily and repetitively detected by the US technologist. Moreover, for further improvement of the overall BFV calculation, taking into account the pulsatile nature of the jugular vein CSA should be considered. Additionally, the study did not use simultaneous ECG and blood pressure measurement. Potential differences in the blood pressure during the scanning time might contribute to considerable changes in the venous flow.
In conclusion, current Doppler equipment does not provide accurate automated calculation of extracranial venous BFV. Until further technological development occurs, manual calculation of the venous BFV is warranted. Due to the elliptical form of the large major veins, the use of diameter-based CSA measurement is erroneous. Additionally, in order to provide consistent and reproducible research results, studies examining the venous BFV need to be methodologically scrutinized.
Footnotes
Study disclosure:
This study was funded in part by the The Annette Funicello Research Fund for Neurological Diseases and internal resources of the Buffalo Neuroimaging Analysis Center. In addition, we received support from the Jacquemin Family Foundation.
Research reported in this publication was also funded in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under award Number UL1TR001412. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Financial Relationships/Potential Conflicts of Interest:
Karen Marr, Dejan Jakimovski and Ellen Carl have nothing to disclose.
Marcello Mancini received personal compensation from SDN S.p.A.
Robert Zivadinov received personal compensation from EMD Serono, Genzyme-Sanofi, Claret Medical, Celgene and Novartis for speaking and consultant fees. He received financial support for research activities from Genenetech, Genzyme-Sanofi, Novartis, and Quintiles/IMS.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Institute of Ultrasound in M, American College of R, Society of Radiologists in U. AIUM practice guideline for the performance of peripheral arterial ultrasound examinations using color and spectral doppler imaging. J Ultrasound Med. 2014;33:1111–21. doi: 10.7863/ultra.33.6.1111. [DOI] [PubMed] [Google Scholar]
- Badano LP, Dall’Armellina E, Monaghan MJ, Pepi M, Baldassi M, Cinello M, Fioretti PM. Real-time three-dimensional echocardiography: technological gadget or clinical tool? J Cardiovasc Med (Hagerstown) 2007;8:144–62. doi: 10.2459/JCM.0b013e3280116b50. [DOI] [PubMed] [Google Scholar]
- Breen PP, Galvin O, Grace PA, Laighin GO. Doppler ultrasound measurements of venous return in the popliteal vein. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:978–81. doi: 10.1109/IEMBS.2007.4352457. [DOI] [PubMed] [Google Scholar]
- Cejas C, Cisneros LF, Lagos R, Zuk C, Ameriso SF. Internal jugular vein valve incompetence is highly prevalent in transient global amnesia. Stroke. 2010;41:67–71. doi: 10.1161/STROKEAHA.109.566315. [DOI] [PubMed] [Google Scholar]
- Chambers B, Chambers J, Churilov L, Cameron H, Macdonell R. Internal jugular and vertebral vein volume flow in patients with clinically isolated syndrome or mild multiple sclerosis and healthy controls: results from a prospective sonographer-blinded study. Phlebology. 2014;29:528–35. doi: 10.1177/0268355513505505. [DOI] [PubMed] [Google Scholar]
- Chi HY, Lin CS, Hsu MH, Chan PC, Hu HH. Chronic Influences of Obstructive Sleep Apnea on Cerebral Venous Flow. J Ultrasound Med. 2015;34:2043–8. doi: 10.7863/ultra.14.12065. [DOI] [PubMed] [Google Scholar]
- Chuang YM, Hu HH. Cough headache and thoracic inlet valvular competence in uremia. Eur Neurol. 2005;53:78–80. doi: 10.1159/000084651. [DOI] [PubMed] [Google Scholar]
- Chung CP, Chao AC, Hsu HY, Lin SJ, Hu HH. Decreased jugular venous distensibility in migraine. Ultrasound Med Biol. 2010;36:11–6. doi: 10.1016/j.ultrasmedbio.2009.08.007. [DOI] [PubMed] [Google Scholar]
- Ciuti G, Righi D, Forzoni L, Fabbri A, Pignone AM. Differences between internal jugular vein and vertebral vein flow examined in real time with the use of multigate ultrasound color Doppler. AJNR Am J Neuroradiol. 2013;34:2000–4. doi: 10.3174/ajnr.A3557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constant J. Using internal jugular pulsations as a manometer for right atrial pressure measurements. Cardiology. 2000;93:26–30. doi: 10.1159/000006998. [DOI] [PubMed] [Google Scholar]
- Di Berardino F, Alpini DC, Bavera PM, Cecconi P, Farabola M, Mattei V, Ambrosetti U, Cesarani A. Chronic cerebrospinal venous insufficiency in Meniere disease. Phlebology. 2015;30:274–9. doi: 10.1177/0268355514526871. [DOI] [PubMed] [Google Scholar]
- Ficial B, Finnemore AE, Cox DJ, Broadhouse KM, Price AN, Durighel G, Ekitzidou G, Hajnal JV, Edwards AD, Groves AM. Validation study of the accuracy of echocardiographic measurements of systemic blood flow volume in newborn infants. J Am Soc Echocardiogr. 2013;26:1365–71. doi: 10.1016/j.echo.2013.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerada M, Struijk PC, Stewart PA, Guerriero S, Melis GB, Wladimiroff JW. Comparison between color Doppler cineloop- and conventional spectral Doppler-derived maximum velocity and flow in the umbilical vein. Ultrasound Obstet Gynecol. 2006;28:156–61. doi: 10.1002/uog.2729. [DOI] [PubMed] [Google Scholar]
- Gill RW. Measurement of blood flow by ultrasound: accuracy and sources of error. Ultrasound Med Biol. 1985;11:625–41. doi: 10.1016/0301-5629(85)90035-3. [DOI] [PubMed] [Google Scholar]
- Guideline developed in collaboration with the American College of R, Society of Pediatric R, Society of Radiologists in U. AIUM Practice Guideline for the Performance of Peripheral Venous Ultrasound Examinations. J Ultrasound Med. 2015;34:1–9. doi: 10.7863/ultra.34.8.15.13.0002. [DOI] [PubMed] [Google Scholar]
- Jakimovski D, Marr K, Mancini M, Caprio MG, Gandhi S, Bergsland N, Paunkoski I, Hagemeier J, Chandra A, Weinstock-Guttman B, Zivadinov R. Global and regional brain atrophy is associated with low or retrograde facial vein flow in multiple sclerosis. Veins and Lymphatics. 2017:6. [Google Scholar]
- Lagana MM, Di Rienzo M, Rizzo F, Ricci C, D’Onofrio S, Forzoni L, Cecconi P. Cardiac, Respiratory and Postural Influences on Venous Return of Internal Jugular and Vertebral Veins. Ultrasound Med Biol. 2017;43:1195–204. doi: 10.1016/j.ultrasmedbio.2017.02.007. [DOI] [PubMed] [Google Scholar]
- Lin S-K, Chang Y-J, Yang F-Y. Hemodynamics of the Internal Jugular Vein: An Ultrasonographic Study. Tzu Chi Medical Journal. 2009;21:317–22. [Google Scholar]
- Liu M, Xu H, Wang Y, Zhong Y, Xia S, Utriainen D, Wang T, Haacke EM. Patterns of chronic venous insufficiency in the dural sinuses and extracranial draining veins and their relationship with white matter hyperintensities for patients with Parkinson’s disease. J Vasc Surg. 2015;61:1511–20. e1. doi: 10.1016/j.jvs.2014.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti L, Menci E, Piu P, Leonini S, Arrigucci U, Bellini M, Zandonella A, Galluzzi P, Casasco A. A sonographic quantitative cutoff value of cerebral venous outflow in neurologic diseases: a blinded study of 115 subjects. AJNR Am J Neuroradiol. 2014;35:1381–6. doi: 10.3174/ajnr.A3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti L, Menci E, Ulivelli M, Cerase A, Bartalini S, Piu P, Marotti N, Leonini S, Galluzzi P, Romano DG, Casasco AE, Venturi C. Quantitative ColourDopplerSonography evaluation of cerebral venous outflow: a comparative study between patients with multiple sclerosis and controls. PLoS One. 2011;6:e25012. doi: 10.1371/journal.pone.0025012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagdev AD, Merchant RC, Tirado-Gonzalez A, Sisson CA, Murphy MC. Emergency department bedside ultrasonographic measurement of the caval index for noninvasive determination of low central venous pressure. Ann Emerg Med. 2010;55:290–5. doi: 10.1016/j.annemergmed.2009.04.021. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Qian K, Ando T, Inokuchi R, Doi K, Kobayashi E, Sakuma I, Nakajima S, Yahagi N. Cardiac Variation of Internal Jugular Vein for the Evaluation of Hemodynamics. Ultrasound Med Biol. 2016;42:1764–70. doi: 10.1016/j.ultrasmedbio.2016.03.003. [DOI] [PubMed] [Google Scholar]
- Naqvi J, Yap KH, Ahmad G, Ghosh J. Transcranial Doppler ultrasound: a review of the physical principles and major applications in critical care. Int J Vasc Med. 2013;2013:629378. doi: 10.1155/2013/629378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolaides AN, Morovic S, Menegatti E, Viselner G, Zamboni P. Screening for chronic cerebrospinal venous insufficiency (CCSVI) using ultrasound: recommendations for a protocol. Funct Neurol. 2011;26:229–48. [PMC free article] [PubMed] [Google Scholar]
- Ozen O, Unal O, Avcu S. Flow volumes of internal jugular veins are significantly reduced in patients with cerebral venous sinus thrombosis. Curr Neurovasc Res. 2014;11:75–82. doi: 10.2174/1567202610666131209122928. [DOI] [PubMed] [Google Scholar]
- Pinter SZ, Rubin JM, Kripfgans OD, Novelli PM, Vargas-Vila M, Hall AL, Fowlkes JB. Volumetric blood flow in transjugular intrahepatic portosystemic shunt revision using 3-dimensional Doppler sonography. J Ultrasound Med. 2015;34:257–66. doi: 10.7863/ultra.34.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber SJ, Lurtzing F, Gotze R, Doepp F, Klingebiel R, Valdueza JM. Extrajugular pathways of human cerebral venous blood drainage assessed by duplex ultrasound. J Appl Physiol (1985) 2003;94:1802–5. doi: 10.1152/japplphysiol.00782.2002. [DOI] [PubMed] [Google Scholar]
- Sisini F, Gianesini S, Menegatti E, Taibi A, Tessari M, Di Domenico G, Malagoni AM, Gambaccini M. On the consistency of flow rate color Doppler assessment for the internal jugular vein. Veins and Lymphatics. 2014:3. [Google Scholar]
- Sisini F, Tessari M, Gadda G, Di Domenico G, Taibi A, Menegatti E, Gambaccini M, Zamboni P. An ultrasonographic technique to assess the jugular venous pulse: a proof of concept. Ultrasound Med Biol. 2015;41:1334–41. doi: 10.1016/j.ultrasmedbio.2014.12.666. [DOI] [PubMed] [Google Scholar]
- Sisini F, Tessari M, Menegatti E, Vannini ME, Gianesini S, Tavoni V, Gadda G, Gambaccini M, Taibi A, Zamboni P. Clinical Applicability of Assessment of Jugular Flow over the Individual Cardiac Cycle Compared with Current Ultrasound Methodology. Ultrasound Med Biol. 2016;42:1750–63. doi: 10.1016/j.ultrasmedbio.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Stankovic Z, Csatari Z, Deibert P, Euringer W, Blanke P, Kreisel W, Abdullah Zadeh Z, Kallfass F, Langer M, Markl M. Normal and altered three-dimensional portal venous hemodynamics in patients with liver cirrhosis. Radiology. 2012;262:862–73. doi: 10.1148/radiol.11110127. [DOI] [PubMed] [Google Scholar]
- Utriainen D, Feng W, Elias S, Latif Z, Hubbard D, Haacke EM. Using magnetic resonance imaging as a means to study chronic cerebral spinal venous insufficiency in multiple sclerosis patients. Tech Vasc Interv Radiol. 2012;15:101–12. doi: 10.1053/j.tvir.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Van Canneyt K, Swillens A, Lovstakken L, Antiga L, Verdonck P, Segers P. The accuracy of ultrasound volume flow measurements in the complex flow setting of a forearm vascular access. J Vasc Access. 2013;14:281–90. doi: 10.5301/jva.5000118. [DOI] [PubMed] [Google Scholar]
- Vicenzini E, Pro S, Sirimarco G, Pulitano P, Mecarelli O, Lenzi GL, Di Piero V. Threedimensional imaging of carotid arteries: Advantages and pitfalls of ultrasound investigations. Perspectives in Medicine. 2012;1:82–85. [Google Scholar]
- von Ramm OT, Smith SW. Real time volumetric ultrasound imaging system. J Digit Imaging. 1990;3:261–6. doi: 10.1007/BF03168124. [DOI] [PubMed] [Google Scholar]
- Yeoh TY, Venkatraghavan L, Fisher JA, Meineri M. Internal jugular vein blood flow in the upright position during external compression and increased central venous pressure: an ultrasound study in healthy volunteers. Can J Anaesth. 2017;64:854–59. doi: 10.1007/s12630-017-0903-3. [DOI] [PubMed] [Google Scholar]
- Zamboni P. Why Current Doppler Ultrasound Methodology Is Inaccurate in Assessing Cerebral Venous Return: The Alternative of the Ultrasonic Jugular Venous Pulse. Behav Neurol. 2016;2016:7082856. doi: 10.1155/2016/7082856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamboni P, Sisini F, Menegatti E, Taibi A, Malagoni AM, Morovic S, Gambaccini M. An ultrasound model to calculate the brain blood outflow through collateral vessels: a pilot study. BMC Neurol. 2013;13:81. doi: 10.1186/1471-2377-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinov R, Bastianello S, Dake MD, Ferral H, Haacke EM, Haskal ZJ, Hubbard D, Liasis N, Mandato K, Sclafani S, Siddiqui AH, Simka M, Zamboni P International Society for Neurovascular D. Recommendations for multimodal noninvasive and invasive screening for detection of extracranial venous abnormalities indicative of chronic cerebrospinal venous insufficiency: a position statement of the International Society for Neurovascular Disease. J Vasc Interv Radiol. 2014;25:1785–94. e17. doi: 10.1016/j.jvir.2014.07.024. [DOI] [PubMed] [Google Scholar]
- Zivadinov R, Ramanathan M, Dolic K, Marr K, Karmon Y, Siddiqui AH, Benedict RH, Weinstock-Guttman B. Chronic cerebrospinal venous insufficiency in multiple sclerosis: diagnostic, pathogenetic, clinical and treatment perspectives. Expert Rev Neurother. 2011;11:1277–94. doi: 10.1586/ern.11.117. [DOI] [PubMed] [Google Scholar]