Abstract
BACKGROUND
Cancer survivors are at increased risk of sepsis, possibly attributed to weakened physiologic conditions. The aims of this study to were to examine the mediation effect of indicators of frailty on the association between cancer survivorship and sepsis incidence, and whether these differences are varied by race.
METHODS
We performed a prospective analysis using data from the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort from years 2003 – 2012. We categorized frailty as the presence of ≥ 2 frailty components (weakness, exhaustion, and low physical activity). We categorized participants as “cancer survivors” or “no cancer history” derived from self-reported responses of being diagnosed with any cancer. We examined the mediation effect of frailty on the association between cancer survivorship and sepsis incidence using Cox regression. We repeated analysis stratified by race.
RESULTS
Among 28,062 eligible participants, 2773 (9.88%) were cancer survivors, and 25,289 (90.03%) were no cancer history participants. Among a total 1315 sepsis cases, cancer survivors were more likely to develop sepsis (12.66% vs. 3.81%, p value <0.01) when compared to participants with no cancer history (HR: 2.62, 95% CI: 2.31 – 2.98, p value <0.01). The mediation effects of frailty on the log-hazard scale were very small: weakness (0.57%), exhaustion (0.31%), low physical activity (0.20%), frailty (0.75%), and total number of frailty indicators (0.69%). Similar results were observed when stratified by race.
CONCLUSION
Cancer survivors had more than a two-fold increased risk of sepsis and indicators of frailty contributed to less than one percent of this disparity.
Keywords: Frailty, Mediation, Sepsis, Cancer, Racial Disparities
INTRODUCTION
Sepsis is a life-threatening condition, characterized by severe infection and organ dysfunction, and is responsible for more than 200,000 annual deaths in the United States1–3. Patients with cancer are nearly 10 times more likely to develop sepsis when compared with no cancer history patients4. A diagnosis of sepsis among patients with cancer has been shown to increase the risk of mortality up to 2 to 3- fold, making sepsis a significant, but modifiable, threat to survivorship4–7. Cancer care and treatment have improved over the past decades, with average 5-year survival approaching 70%8, 9. However, there are marked differences in survival rates by race and socio-economic status, a trend that mirrors disparities in sepsis rates among US adults4, 10–27.
Frailty is a state of increased vulnerability to stressors resulting from age-related decline in reserve and function across multiple physiologic systems and has been associated with a host of health risks including increased hip fracture, disability, hospitalization, and death28, 29. Frailty is associated with age and chronic medical conditions, similarly to both cancer and sepsis30–33. Studies have shown that cancer survivors are associated with higher odds of frailty32, 33. Further, in analysis among the REasons for Geographic and Racial Differences in Stroke (REGARDS) cohort we reported that frailty is associated with a 44% increased risk of sepsis34. However, less is known of the effect of frailty on the risk of sepsis after cancer. For example, Mohile et al (2009) observed that cancer diagnosis was significantly associated with a 46% increased odds of low self-rated health, 19% increased odds of limitations in activities of daily living, and a 46% increased odds of frailty32.
Nevertheless, less is known on the mediating effects of frailty indicators on the association between cancer and sepsis among a national large longitudinal cohort of community-dwelling adults. Therefore, the objective of this study is to determine the mediating effects of frailty on the risk of sepsis after cancer survivorship, and whether these effects are modified by race. We hypothesize that due to weakened physical condition and function, sepsis incidence will be higher in cancer survivors compared to participants with no cancer history and partially explained by measured indicators of frailty.
METHODS
Study Participants
We analyzed data from the prospective REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort study, one of the nation’s largest ongoing cohorts of community-dwelling adults (i.e., participants considered healthy at study baseline). REGARDS investigators designed the cohort to evaluate the origins for racial and geographic differences in stroke mortality, and this cohort includes 30,239 participants aged ≥ 45 years at baseline. REGARDS recruited participants between January 2003 and October 2007 and contacted participants by telephone to identify any hospitalizations at six-month intervals until December 31 2012. REGARDS cohort consists of participants that are 45% male, 41% black race, and 69% >60 years old after recruitment. REGARDS recruited participants between January 2003 and October 2007 and contacted participants by telephone to identify any hospitalizations at six-month intervals until December 31 2012. Further details related to REGARDS study methods are described elsewhere35.
Data Collection
At baseline, trained REGARDS personnel conducted computer-assisted telephone interviews among all REGARDS participants to collect information regarding participant demographics, health behaviors (e.g., physical activity, smoking status, alcohol use), cognitive impairment, exhaustion, impaired mobility, and self-report of prior physician-diagnosis chronic medical conditions. Following the computer-assisted telephone interviews, REGARDS personnel performed in-home visits to obtain physiologic measurements (e.g. height, weight, blood pressure, and heart rate), collect blood and urine samples, perform an electrocardiogram, and a pill bottle review. During the pill bottle review, technicians collected the names of all medications that participants reported taking during the 2 weeks prior to the in-home study visit. Medication dosages were not collected.
Primary Outcome – Community Acquired Sepsis
Our primary outcome of interest in this study was first sepsis events. This analysis focused on community-acquired sepsis derived from vital signs and laboratory findings within the first 28-hours of hospitalization that included care from the Emergency Department care and up to one full day of inpatient care. We included hospitalization events reported from January 1, 2003 through December 31, 2012 to align with prior REGARD-sepsis investigations. Using the taxonomy of Angus et al (2001), we identified all hospitalizations (Emergency Department visits and/or hospital admission) attributed by participants to a serious infection (i.e., all hospitalizations with a bacterial, fungal, or viral infectious process)1. REGARDS investigators defined a sepsis event as a hospital admission for serious infection with the presence of at least two Systemic Inflammatory Response Syndrome (SIRS) criteria, including heart rate >90 beats/minute, fever (temperature >38.3°C or <36°C), tachypnea (>20 breaths/min) or PCO2<32 mmHg, and leukocytosis (white blood cells >12,000 or <4,000 cells/mm3 or >10% band forms)1. Initial review of 1,329 hospital records indicated excellent inter-rater consensus for the presence of serious infection (kappa=0.92) and the presence of sepsis (kappa=0.90) at the time of hospital presentation. In this analysis we elected to focus on the SIRS-based sepsis definition as the primary analysis because of its common use in prior sepsis epidemiology studies instead of the international consensus conferences “Sepsis-3” definition ” (defined as the presence of serious infection in addition to sequential organ failure assessment (SOFA) score ≥2)36. In sensitivity analysis (data not shown) we observed very similar effect measures (adjusted HR: 2.84, 95% CI: 2.15 – 3.76) when analyzing the total effect of cancer survivorship on “Sepsis-3” after controlling for age, sex, race, region, education, income, tobacco and alcohol use, comorbidity score, Cystatin-C, and aspirin use.
Primary Exposure – Cancer Survivors
Our primary cancer exposure was defined as cancer survivorship at baseline (i.e., participants that reported a history of cancer at baseline). We classified those with a history of cancer as “cancer survivors” and those without cancer as “no cancer history.” REGARDS investigators identified participants with self-reported cancer survivors during baseline interview using the following baseline question: “Have you ever been diagnosed with cancer?” If the participant answered “yes”, then they were asked the following follow-up question regarding the date of their last treatment: “Have you been treated with chemotherapy or radiation in the past two years?” If the participant had been treated within past two years, they were excluded from participation in the study. Due to the focus on community-dwelling participants, REGARDS investigators excluded participants receiving treatment for cancer within past two years in order to study participants considered “healthy” at baseline. Therefore, participants defined as cancer survivors at baseline were those that had cancer remission for at least two years before entrance into REGARDS cohort. Self-reported cancer survivorship in prospective cohort studies have been previously shown to have sensitivity values of 0.90 and positive predictive values of 0.7537.
Definition of Frailty – Mediator Variables
Various measures have been used to define frailty28, 38–40. We focused on indicators of frailty that could be identified using existing data collected on REGARDS participants from the in-home survey questionnaire at baseline. We utilized methods from our prior REGARDS investigation and adopted methods by Johansen et al. (2014) that approximated components of frailty from self-reported measures41, 42. Prior studies have reported that the specificity and overall accuracy for identifying frailty using self-reported measures are 90% and 72%, respectively41.
We defined frailty as the presence of two of the three factors: 1) weakness, 2) exhaustion, and 3) low physical activity41, 42. Using participant self-reported responses to the 12-Item Short Form Survey (SF-12), we defined weakness as a physical composite score of <7529, 40. Similarly using the SF-12, we defined exhaustion as responses of “a little of the time” or “none of the time” to the question “How much of the time during the past 4 weeks did you have energy?” Lastly, we defined low physical activity as responses of “almost never” or “never exercising enough to work up a sweat” to the question: “How many times per week do you engage in intense physical activity, enough to build up a sweat?”29, 40. For statistical analyses we presented all frailty indicators as dichotomous with either “yes” for the presence, or “no” for the absence of each frailty indicator. We additionally summed the total number of frailty indicators for a number of frailty indicators variable.
Participant Characteristics
We examined demographic variables available from REGARDS baseline interview that included self-reported age, race, sex, household income, education, and geographic region. Health behaviors included tobacco, and alcohol use. We defined alcohol use as moderate (one drink per day for women or two drinks per day for men) and heavy alcohol use (>1 drink per day for women and >2 drinks per day for men), per the National Institute on Alcohol Abuse and Alcoholism classification43. We examined medical conditions self-reported by participants during REGARDS baseline interview that included atrial fibrillation, chronic lung disease, coronary artery disease, deep vein thrombosis, diabetes, dyslipidemia, hypertension, myocardial infarction, obesity, peripheral artery disease, and stroke. Previous REGARDS investigations have observed that the total number of chronic medical conditions is associated with increased risk of sepsis (e.g., HRs for total number of chronic medical conditions: two = 2.65, three = 3.11, four = 3.81, p-value for trend <0.001)30. Therefore, we additionally created an individual level comorbidity score based on the sum of total number of baseline medical conditions, and those with missing information for an individual medical conditions were included as having no presence of a medical condition. Prior epidemiologic studies within the REGARDS cohort have reported inflammatory biomarkers, biomarkers of renal function, chronic aspirin use, steroid use, and statin use to be associated with long-term risk for sepsis44–48. Therefore, to account for these potential biomarkers of inflammation and chronic disease we included the biomarkers high sensitivity C-reactive protein, albumin-creatinine ratio (ACR), and Cystatin-C in sensitivity analysis. In addition, we analyzed self-reported baseline medication usage of aspirin, statins, and steroids as potential confounders in sensitivity analysis. We provide detailed information regarding participant characteristics in Supplemental Table 1.
Clinical Characteristics of Sepsis Hospitalizations
We identified clinical characteristics among sepsis events. Clinical characteristic variables included infection type, Sequential Organ Failure Assessment (SOFA) score for the respiratory, renal, hepatic, cardiovascular, hematologic, and neurologic systems, intensive care unit (ICU) admission, and in-hospital sepsis case-fatality. Based on medical record and/or death certificate, we defined sepsis case fatality as in-hospital death attributed to sepsis of a physician-adjudicated sepsis event.
Statistical Analysis
We compared differences in demographic, substance use, comorbidities, frailty indicators, clinical characteristics, and sepsis incidence between cancer survivors and no cancer history participants using Chi-square, ANOVA, and Wilcoxon tests as appropriate. We estimated the mean survival times and associated 95% confidence limits using the product-limit method of the Kaplan-Meier survivor function. We additionally estimated the hazard for time to first sepsis event between cancer survivors and participants with no cancer history using a Cox proportional hazard model adjusted for age, sex, race, and comorbidity score. We a priori decided to adjust models for sociodemographic and comorbidities, however we performed sensitivity analyses further adjusting models for Cystatin-C and chronic aspirin use.
Mediation Analysis
The objective of our analysis was to test for the mediation effect of frailty indicators on the association between cancer and sepsis risk. In a mediation model, the indirect (or mediation) effect represents the causal pathway in which an exposure affects an outcome indirectly through mediator(s)49–55. Therefore, we examined the mediating effects of indicators of frailty (i.e., weakness, low physical activity, and exhaustion) on the association between cancer survivors compared to participants with no cancer history and risk of sepsis using Cox proportional hazard models. We determined the mediating effects of frailty indicators on the association between cancer and sepsis incidence using SAS macros for mediation with survival data developed by Valeri and VanderWeele (2015)54–56. We presented results from mediation analysis as the 1) natural direct effects (NDE) (i.e., the effect of cancer on sepsis outcome not through the mediator), 2) natural indirect effect (NIE) (i.e., the effect of cancer on sepsis outcome through the mediator), 3) total effects (i.e., total association between cancer and sepsis risk), 4) and proportions mediated (i.e., the percent of the total association (on the log hazard scale) that was mediated by frailty indicators).56 We present the direct and indirect effects as the hazard ratios (HRs) and associated 95% confidence intervals, determined using bootstrapping technique with 500 resamples and with replacement.54, 55 We computed the proportion mediated on the log hazard scale using the formula 1 − (lnHRnde/lnHRtotal) where nde represents the natural direct effect and total represents total effect54–56. We additionally stratified mediation models by race to determine whether there are any differences in mediation possibly attributed to effect modification of race. We decided a priori to adjust all mediation models for age, sex, race (not in race stratified models), and comorbidity score. Further, we performed mediation analysis excluding participants that died from a cancer-related death within three years of follow-up time. We used SAS version 9.4 for all statistical analyses.
Ethical Statement
The Institutional Review Board of the University of Alabama at Birmingham approved this study.
RESULTS
Among 30,239 REGARDS participants, we excluded 546 due to missing cancer and sepsis information, 962 due to missing frailty information, and 669 due to missing covariates, corresponding to a total of 28,062 participants included in study analysis (Figure 1). Among the study participants 2773 (9.88%) were categorized as cancer survivors, and 25,289 (90.12%) were categorized as no cancer history participants. We compared cancer survivors and no cancer history participants (Table 1), and cancer survivors had older age (mean age 69.55 vs. 64.32, p value <0.01), were more likely male (56.65% vs. 43.36%, p value <0.01), more likely White race (69.82% vs. 58.11%, p value <0.01). Additionally, cancer survivors were more likely to have income less than $20,000 per year (18.10% vs. 17.77%, p value <0.01), reside in the Stroke Belt (36.35% vs. 34.56%, p value <0.01), and less likely to be current tobacco users (10.71% vs. 14.76%, p value <0.01). Cancer survivors had a greater prevalence of atrial fibrillation (11.47% vs. 8.40%), chronic lung disease (11.03% vs. 9.06%), coronary artery disease (23.88% vs. 17.17%), deep vein thrombosis (7.87% vs. 4.93%), hypertension (63.17% vs. 58.61%), myocardial infarction (17.16% vs. 12.19%), and stroke (8.80% vs. 6.05%) when compared with participants with no cancer history (p values <0.01). Compared with no cancer history participants, cancer survivors had higher baseline Cystatin-C (0.98 mg/dL vs. 0.94 mg/dL, p value <0.01) and were more likely to be chronic aspirin users (47.39% vs. 42.90%, p value <0.01).
Figure 1.
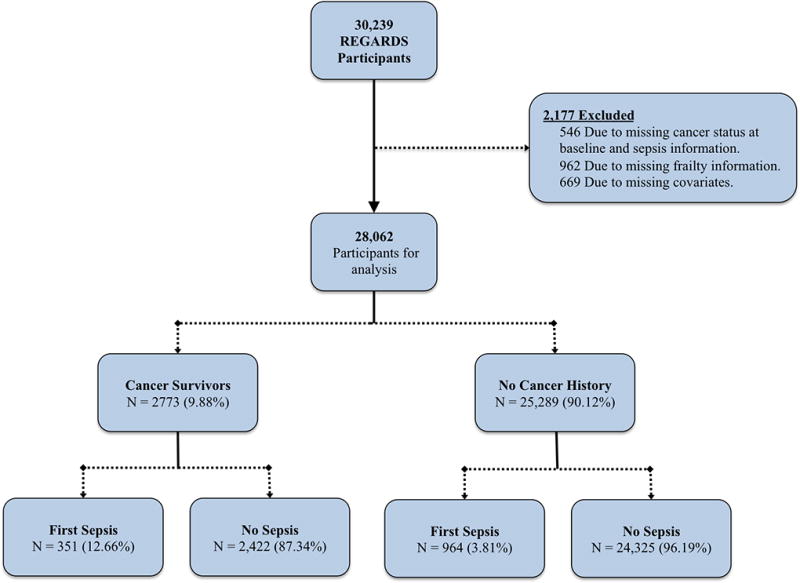
Flowchart of REGARDS study participants used in study analysis for mediation effect of frailty on cancer and sepsis.
Table 1.
Comparison of demographic, substance use, comorbidity characteristics, frailty, and sepsis incidence by cancer survivorship status. Among 28,062 REGARDS participants.
| Cancer Survivors (N = 2773) |
No Cancer History (N = 25,289) |
||
|---|---|---|---|
|
| |||
| N (%) Mean (SD)† |
N (%) Mean (SD)† |
p value** | |
| Age, Mean (SD) | 69.55 (8.67) | 64.32 (9.33) | <0.01 |
| Male Gender | 1571 (56.65) | 10965 (43.36) | <0.01 |
| Race | |||
| Black | 837 (30.18) | 10593 (41.89) | <0.01 |
| White | 1936 (69.82) | 14696 (58.11) | |
| < High School Education | 353 (12.73) | 3081 (12.18) | 0.02 |
| Income ≤ $20 000 | 502 (18.10) | 4493 (17.77) | <0.01 |
| Stroke Belt Residence | 1008 (36.35) | 8740 (34.56) | <0.01 |
| Current Tobacco Use | 297 (10.71) | 3733 (14.76) | <0.01 |
| Heavy Alcohol Use | 95 (3.43) | 1044 (4.13) | 0.01 |
| Baseline Medical Condition | |||
| Atrial fibrillation | 312 (11.47) | 2077 (8.40) | <0.01 |
| Chronic lung disease | 306 (11.03) | 2290 (9.06) | <0.01 |
| Coronary artery disease | 650 (23.88) | 4262 (17.17) | <0.01 |
| Chronic kidney disease | 320 (11.54) | 2748 (10.87) | 0.28 |
| Deep vein thrombosis | 218 (7.87) | 1241 (4.93) | <0.01 |
| Diabetes | 639 (23.09) | 5656 (22.45) | 0.44 |
| Dyslipidemia | 1637 (61.63) | 14394 (59.08) | 0.01 |
| Hypertension | 1748 (63.17) | 14786 (58.61) | <0.01 |
| Myocardial infarction | 467 (17.16) | 3025 (12.19) | <0.01 |
| Obesity | 1412 (50.99) | 13568 (53.74) | 0.01 |
| Peripheral artery disease | 77 (2.78) | 542 (2.14) | 0.03 |
| Stroke | 243 (8.80) | 1526 (6.05) | <0.01 |
| Comorbidity Score, Mean (SD) | 2.27 (1.58) | 1.97 (1.48) | <0.01 |
| Biomarkers, Median (P25, P75)‡ | |||
| hs-CRP mg/dL | 2.18 (0.98, 4.85) | 2.22 (0.96, 5.06) | 0.85 |
| ACR mcg/mg | 7.73 (4.82, 18.67) | 7.33 (4.62, 15.56) | 0.22 |
| Cystatin-C mg/dL | 0.98 (0.85, 1.18) | 0.94 (0.82, 1.10) | <0.01 |
| Baseline Medication Use | |||
| Aspirin | 1314 (47.39) | 10848 (42.90) | <0.01 |
| Statins | 911 (32.85) | 7956 (31.46) | 0.13 |
| Steroids | 113 (4.08) | 875 (3.46) | 0.10 |
| Frailty Variables | |||
| Weakness | 889 (32.06) | 7495 (29.64) | 0.01 |
| Exhaustion | 422 (15.22) | 3453 (13.65) | 0.02 |
| Low Physical Activity | 1007 (36.31) | 8577 (33.92) | 0.01 |
| Frailty | 638 (23.01) | 5132 (20.29) | <0.01 |
| Number of Frailty Indicators | 0.84 (0.93) | 0.77 (0.89) | <0.01 |
Mean (Standard deviation)
Estimated using χ2, ANOVA, and Wilcoxon rank sums tests as appropriate.
Comorbidity score is total of comorbidities, presented as mean and standard deviation (SD).
Number of frailty components presented as mean and standard deviation (SD).
Biomarkers presented as median and 25th and 75th percentiles.
The most common infection types among the 1315 sepsis hospitalizations were pneumonia (39.16%), urinary tract infections (17.19%), and abdominal infections (15.29%; Table 2). There were no statistically significant differences in infection types between cancer survivors and no cancer history participants. The majority of sepsis hospitalizations had SOFA scores of 0 (69.43%), however cancer survivors were more likely to have more severe SOFA scores compared to no cancer history participants (SOFA Score ≥2: 24.22% vs. 18.88, p value = 0.04). In addition, cancer survivors were more likely to have in-hospital sepsis case-fatality (7.98% vs. 3.94%, p value <0.01) when compared to no cancer history participants.
Table 2.
Clinical characteristics of 1315 sepsis case hospitalizations.
| Variable | All Participants (N = 1315) |
Cancer Survivors (N = 351) |
No Cancer History (N = 964) |
p value1 |
|---|---|---|---|---|
| Infection Type (%) | ||||
| Pneumonia | 515 (39.16) | 141 (40.17) | 374 (38.80) | 0.85 |
| Urinary tract infections | 226 (17.19) | 60 (17.09) | 166 (17.22) | |
| Abdominal | 201 (15.29) | 58 (16.52) | 143 (14.83) | |
| Bronchitis | 121 (9.20) | 25 (7.12) | 96 (9.96) | |
| Skin | 100 (7.60) | 26 (7.41) | 74 (7.68) | |
| Sepsis | 87 (6.62) | 25 (7.12) | 62 (6.43) | |
| Fever of unknown origin | 27 (2.05) | 6 (1.71) | 21 (2.18) | |
| Catheter/Other | 38 (2.89) | 10 (2.85) | 28 (2.90) | |
| SOFA (%) | ||||
| 0 | 913 (69.43) | 225 (64.10) | 688 (71.37) | 0.04 |
| 1 | 135 (10.27) | 41 (11.68) | 94 (9.75) | |
| ≥2 | 267 (20.30) | 85 (24.22) | 182 (18.88) | |
| ICU Admission (%) | 61 (4.64) | 20 (5.70) | 41 (4.25) | 0.27 |
| Hospital Case-Fatality2 (%) | 66 (12.09) | 28 (7.98) | 38 (3.94) | <0.01 |
% - Represents the column percentages.
Significance for comparison between cancer survivors and no history of cancer participants with sepsis; determined using Chi-square tests.
Defined as in-hospital death attributed to sepsis.
SOFA=Sequential Organ Failure Assessment Score. ICU=Intensive Care Unit
Mediation Results
Cancer survivors were more likely to develop sepsis (12.66% vs. 3.81%) when compared to participants with no cancer history (HR: 2.62, 95% CI: 2.31 – 2.98, p value <0.01) (Table 3). We examined whether frailty indicators mediation the association between cancer survivorship and risk of sepsis (Figure 2). Among 1315 sepsis events, weakness (percent mediated on log-hazard scale = 0.57%), exhaustion (percent mediated = 0.31%), and low physical activity (percent mediated = 0.20%) were very weak mediators on the association between cancer and sepsis risk, after adjustments for sex, age, race, and total number of comorbidities. Frailty, as defined by 2 of 3 indicators of frailty, was the strongest mediator (percent mediated = 0.75%, natural indirect effect (NIE) = 1.007, 95% CI: 1.005 – 1.013). The total number of frailty components was a weak mediator (percent mediated = 0.69%).
Table 3.
Mediating effects1 of indicators of frailty on the association between cancer and sepsis. Among 28,062 REGARDS participants with 1315 first sepsis events.
| Mediation Analysis | |||||
|
| |||||
| Natural Indirect Effect2 (Mediation Effect) | Natural Direct Effect3 | Percent Mediated4 (%) (Log Hazard Scale) | |||
|
| |||||
| HR | 95% CI5 | HR | 95% CI5 | ||
|
| |||||
| Mediators | |||||
| Weakness | 1.006 | 1.003 – 1.011 | 2.640 | 2.314 – 2.900 | 0.57% |
| Exhaustion | 1.003 | 1.001 – 1.006 | 2.644 | 2.317 – 2.900 | 0.31% |
| Low Physical Activity | 1.002 | 0.999 – 1.005 | 2.644 | 2.316 – 2.895 | 0.20% |
| Frailty | 1.007 | 1.005 – 1.013 | 2.631 | 2.307 – 2.891 | 0.75% |
| # Frailty Indicators | 1.007 | 1.005 – 1.012 | 2.631 | 2.307 – 2.891 | 0.69% |
|
| |||||
| Total Effect (Risk of Sepsis) | |||||
|
| |||||
| No. Sepsis Events (%) | Mean Survival Time (95% CI)6 | Hazard Ratio (95% CI) | |||
|
| |||||
| Cancer Survivors | 351 (12.66) | 8.56 (8.48 – 8.64) | 2.62 (2.31 – 2.98) | ||
| No Cancer History | 964 (3.81) | 9.19 (9.17 – 9.20) | Ref | ||
Models adjusted for age, sex, race, and comorbidity score.
Natural Indirect Effect (i.e., the effect of the cancer on sepsis incidence through the mediator)
Natural Direct Effect (i.e., the effect of the cancer on sepsis incidence NOT through mediator)
Percent Mediated = Percent of the total association between the cancer and sepsis incidence that was mediated on the log hazard scale.
95% Confidence intervals (CIs) estimated using 500 bootstrapped resamples.
Mean survival time in years.
Figure 2.
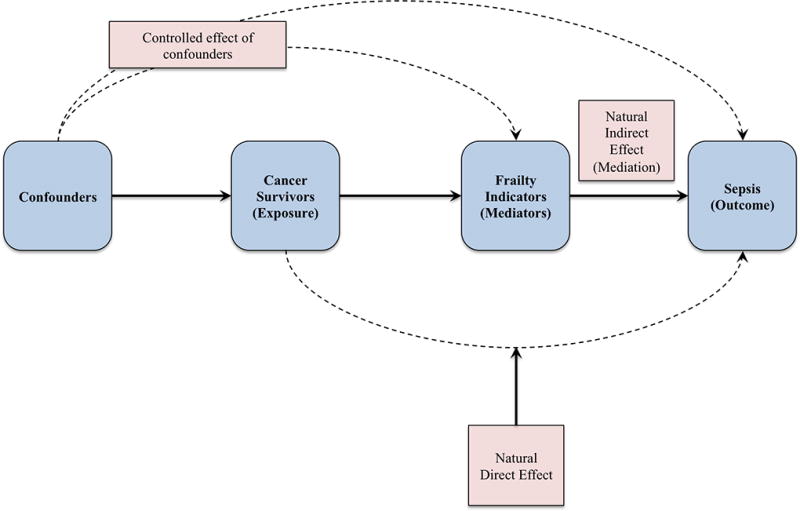
Overview of mediation analysis performed using VanderWeele mediation methodology54–56. Analysis controlled for confounding variables while examining the mediation effect (natural indirect effect) of frailty indicators on the association (natural direct effect) between cancer survivorship and risk of sepsis.
Similarly, when limited to the 442 sepsis events among Blacks, weakness (percent mediated = 0.25%), exhaustion (percent mediated = 0.35%), low physical activity (percent mediated = 0.25%), composite frailty (percent mediated = 0.25%), and number of frailty components (percent mediated = 0.25%) were all weak mediating effects on the association between cancer and sepsis, after adjustments for sex, age, and total number of comorbidities (Table 4). When limited to the 873 sepsis events among White participants, weakness (percent mediated = 0.79%), exhaustion (percent mediated = 0.28%), low physical activity (percent mediated = 0.47%), composite frailty (percent mediated = 0.47%), and total number of frailty components (percent mediated = 0.45%) weakly mediated the association between cancer survivorship and risk of sepsis after adjustments for confounders (Table 5). It is possible that cancer survivors were not-cancer free at baseline; therefore we excluded 184 participants that died from cancer related deaths within three years of follow-time. From these analyses, we observed very weak mediating effects for weakness (percent mediated = 0.77%), exhaustion (percent mediated = 0.50%), low physical activity (percent mediated = 0.26%), composite frailty (percent mediated = 0.92%), and total number of frailty components (percent mediated = 0.85%) on the association between cancer and sepsis (Supplemental Table 2). We further adjusted all models for Cystatin-C (biomarker of inflammation) and chronic aspirin use and results were much similar to less adjusted models (Supplemental Tables 3 through 5).
Table 4.
Mediating effects1 of indicators of frailty on the association between cancer and sepsis. Among 11,430 Black participants with 442 first sepsis events.
| Natural Indirect Effect2 (Mediation Effect) | Natural Direct Effect3 | Percent Mediated4 (%) (Log Hazard Scale) | |||
|
| |||||
| Mediators | HR | 95% CI5 | HR | 95% CI5 | |
|
| |||||
| Weakness | 1.002 | 0.999 – 1.013 | 2.677 | 2.118 – 3.476 | 0.25% |
| Exhaustion | 1.004 | 0.999 – 1.009 | 2.668 | 2.111 – 3.463 | 0.35% |
| Low Physical Activity | 1.002 | 0.999 – 1.006 | 2.669 | 2.114 – 3.449 | 0.25% |
| Frailty | 1.002 | 0.999 – 1.006 | 2.669 | 2.114 – 3.449 | 0.25% |
| # Frailty Indicators | 1.002 | 0.999 – 1.006 | 2.669 | 2.114 – 3.449 | 0.25% |
|
| |||||
| Total Effect (Risk of Sepsis) | |||||
|
| |||||
| No. Sepsis Events (%)6 | Mean Survival Time (95% CI)7 | Hazard Ratio (95% CI)8 | |||
|
| |||||
| Cancer Survivors | 93 (11.11) | 8.13 (8.01 – 8.26) | 2.84 (2.25 – 3.60) | ||
| No Cancer History | 349 (3.29) | 9.21 (9.19 – 9.24) | Ref | ||
Models adjusted for age, sex, and comorbidity score.
Natural Indirect Effect (i.e., the effect of the cancer on sepsis incidence through the mediator)
Natural Direct Effect (i.e., the effect of the cancer on sepsis incidence NOT through mediator)
Percent Mediated = Percent of the total association between the cancer and sepsis incidence that was mediated on the log hazard scale.
95% Confidence intervals (CIs) estimated using 500 bootstrapped resamples.
% represents the proportion within cancer group with sepsis event.
Mean survival time in years.
Estimated from Cox proportional hazards model.
Table 5.
Mediating effects1 of indicators of frailty on the association between cancer and sepsis. Among 16,632 White participants with 873 first sepsis events.
| Natural Indirect Effect2 (Mediation Effect) | Natural Direct Effect3 | Percent Mediated4 (%) (Log Hazard Scale) | |||
|
| |||||
| Mediators | HR | 95% CI5 | HR | 95% CI5 | |
|
| |||||
| Weakness | 1.007 | 1.002 – 1.015 | 2.552 | 2.395 – 2.764 | 0.79% |
| Exhaustion | 1.003 | 1.000 – 1.007 | 2.563 | 2.401 – 2.767 | 0.28% |
| Low Physical Activity | 1.004 | 1.000 – 1.008 | 2.555 | 2.401 – 2.758 | 0.47% |
| Frailty | 1.004 | 1.001 – 1.008 | 2.555 | 2.401 – 2.756 | 0.47% |
| # Frailty Indicators | 1.004 | 1.001 – 1.008 | 2.555 | 2.401 – 2.758 | 0.45% |
|
| |||||
| Total Effect (Risk of Sepsis) | |||||
|
| |||||
| No. Sepsis Events (%) | Mean Survival Time (95% CI)7 | Hazard Ratio (95% CI)8 | |||
|
| |||||
| Cancer Survivors | 258 (13.33) | 8.52 (8.42 – 8.62) | 2.52 (2.17 – 2.93) | ||
| No Cancer History | 615 (4.18) | 8.82 (8.80 – 8.84) | Ref | ||
Models adjusted for age, sex, and comorbidity score.
Natural Indirect Effect (i.e., the effect of the cancer on sepsis incidence through the mediator)
Natural Direct Effect (i.e., the effect of the cancer on sepsis incidence NOT through mediator)
Percent Mediated = Percent of the total association between the cancer and sepsis incidence that was mediated on the log hazard scale.
95% Confidence intervals (CIs) estimated using 500 bootstrapped resamples.
% represents the proportion within cancer group with sepsis event.
Mean survival time in years.
Estimated from Cox proportional hazards model.
DISCUSSION
In this large prospective cohort of REGARDS participants we examined whether frailty indicators mediated the association between cancer survivorship and future risk of sepsis episodes. Cancer survivors were at a more than a two-fold increased risk of sepsis and were more likely to be frail when compared with their no cancer history counterparts. Further, we observed that frailty indicators were associated with no more than a one percent mediation effect on the association between cancer survivorship and risk of sepsis after controlling for confounders. These results suggest that the observed increased risk of sepsis after cancer may not be through frailty mediators, but can be explained mostly by the underlying physiologic conditions of cancer.
To date, this is first prospective analysis to examine whether frailty and components of frailty mediate the association between cancer and sepsis risk. Conversely, several studies have examined the association between cancer and risk of frailty32, 33, 57. Firstly, using data from more than 12,000 community-dwelling older adults from the Medicare Current Beneficiary Survey, Mohile et al (2009) reported that cancer survivors were at a 19% increased odds of limitations for activities of daily living and 46% increased odds of frailty32. Similarly to our study, the authors defined frailty using survey response information based on physical and cognitive decline; participants were considered frail if they were either 85 years or older, had a limitation in an activity of daily living, any geriatric syndrome, or three or more chronic medical conditions)32. In another cross-sectional study among 8,022 older adults from the Mexican Health and Aging Study, Perez-Zepeda et al (2016) observed that cancer survivors with cancers diagnosed within the prior 10 years were at a 74% increased odds of having frailty when compared with no cancer history participants33. Perez-Zepeda et al (2016) reported that more recent cancers were even more strongly associated with frailty33, and hence a future study examining the mediating effect of frailty on the association between cancer and sepsis examining varying time epochs since cancer diagnosis (e.g., <1 year, 1 – 5 years, and 5+ years since cancer diagnosis). In addition, the effect of frailty on sepsis risk has been studied in large cohorts42, 58. For example, in a recent analysis among the REGARDS cohort we reported that frailty was associated with 44% increased risk of sepsis (HR: 1.44, 95% CI: 1.26 – 1.64)42. Similarly, Brummel et al (2017) observed that greater clinical frailty scale scores were associated with a 40% greater risk of death at three months (HR: 1.4, 95% CI: 1.1 – 1.8, p value = 0.01) and 50% greater risk of death one year (HR: 1.5, 95% CI: 1.2 – 1.8, p value <0.001) among critically ill patients58. Furthermore, the results of this study contribute to the limited knowledge on the pathway between cancer, frailty, and sepsis.
Cancer and sepsis have a biologically plausible association and prior cross-sectional studies report infections as common complications among cancer patients59, 60. Nevertheless, there exists limited epidemiologic evidence to support long-term sepsis risk among cancer survivors. Initially, we hypothesized that frailty was a potential mediator on the association between cancer survivorship and long-term risk of sepsis. However, we observed that indicators of frailty represented only a small mediation effect. Further, while prior studies have reported that indicators of frailty such as weakness and exhaustion are associated with higher inflammatory biomarkers such as CRP and interleukin-659, 60., in this study we did not observe major differences in baseline inflammatory biomarkers (i.e., CRP) between our cancer survivors and non-cancer participants. As a result, we suggest several potential pathways of mediation between cancer survivorship and sepsis. Possible biologic mechanisms that may explain the association between cancer and long-term risk of sepsis include: 1) cancer causing a chronic inflammatory state, and/or 2) cancer treatment and therapy causing degradation and necrosis of healthy tissues, both of which would lead cancer survivors to having a compromised immune system that would in turn increase their long-term risks for infection.
We intended to examine whether frailty mediated the association between cancer and sepsis in anticipation of illuminating a potential physiologic pathway that health care practitioners and cancer survivors could mitigate for increasing the overall quality of life after cancer diagnosis and treatment. Frailty is a clinical condition defined by a weakened physiologic state that advances with age in which there is an increase in an individual's vulnerability for developing increased dependency and/or mortality when performing otherwise normal tasks61. As suggested by Perez-Zepeda et al (2016) there are several biologic pathways between cancer survivorship and risk of frailty including genetic instability, DNA repair imbalance, telomere shortening, epigenetic alterations, changes in metabolic regulation, protein instability, and cellular senescence33. However, these underlying conditions are evident precursors of eventual disability and dependence, which in turn reduces the overall quality of life. Further, studies have shown that frailty among patients with cancer are associated with an 87% increased risk of all-cause mortality, more than a 2.5-fold increased risk of post-operative mortality, nearly 5-fold increased odds for intolerance to cancer treatment, and a three-fold increased risk of postoperative complications62. Nonetheless, while frailty accounted for less than one percent of a mediation effect on cancer and sepsis risk, we observed that frailty was associated with cancer survivorship. While prior studies indicate that patients with cancer have higher prevalence of frailty, the findings of our study suggest that this increased risk of frailty does not translate into an increased risk of infection among cancer survivors.
Limitations
While the REGARDS-sepsis cohort study has afforded a great opportunity to examine the risk of sepsis after cancer survivorship among a large prospective cohort of community-dwelling adults, the results of this study should be viewed in the light of certain limitations. First, the REGARDS cohort was intended to investigate stroke outcomes, not cancer incidence or sepsis outcomes. Since this study did not surveil for sepsis or cancer prospectively, we may have not achieved complete ascertainment of all sepsis cases, cancer survivors, and frailty prevalence. However, given that the REGARDS cohort was not intended to study the aforementioned variables of interest it is reasonable for us to assume non-differential disease misclassification. Moreover, in our study the potential for recall biases to lead to misclassification are very minimal because sepsis case identification and adjudication were determined by REGARDS investigators completely unrelated to the cancer survivorship exposure data. As a result, due to our non-differential misclassification it is plausible that our observed effects biased towards the null (i.e., underestimates of the true effects). Secondly, we limited the analysis to three indicators of frailty, and did not assess other frailty measures; however, there is no current consensus for frailty definitions in epidemiologic research28, 38–40. Further, because we relied on a self-reported history of cancer to identify cancer survivors we were unable to examine the effects (direct and indirect) by cancer subtype. Cancer is a heterogeneous disease that has different underlying risk factors, disease courses, treatments, and survival. Thus, a future study with more information regarding cancer types and antitumor treatments is warranted. In addition, there is potential for recall and information bias as the indicators of frailty were subjective measures reported by participants. That said, there is no evidence to suggest that there is differential misclassification of frailty between cancer survivors and no cancer history participants as all participants were considered “healthy” enough at baseline to participate in a longitudinal cohort. Conversely, the prevalence of frailty among cancer survivors in our study was lower when compared to other studies investigating the association between cancer and frailty; with the median frailty prevalence among cancer survivors being 42% (compared to our frailty prevalence of 23%) among a systematic review performed by Handforth et al (2015)62. There are two possible explanations for this observed difference: 1) we identified frailty using self-reported measures at baseline and thus we underestimated the true prevalence and/or 2) prior studies ascertained frailty prevalence from cross-sectional data among recently hospitalized patients with cancer and thus are subject to temporality biases. As a third limitation, although we adjusted for confounders, the associations between cancer and sepsis risk could still be biased due to residual confounding from other unmeasured variables such as access to health care.
Further, there is a possible concern for temporality as both definitions for frailty and cancer survivor status were determined at baseline; thus, it possible that frailty came after cancer development. In a sensitivity analysis, to account for participants with active cancers following frailty, we excluding individuals with a cancer death within 3 years of follow up. Results were very similar to the primary analysis, and therefore it is likely that our primary exposure (i.e., cancer survivors) preceded our mediators (i.e., indicators of frailty). In addition, there is no consensus on the definition of frailty as evidenced by the various criteria used in the aforementioned studies and therefore the usage of defining frailty from self-reported measures was the most opportunistic analysis possible61. As a result, there is possibility for recall biases depending on participants’ health status. For example, individuals with poorer health conditions at baseline may be more likely to self-report indicators of frailty (i.e., weakness, exhaustion, and physical activity). However, there is no evidence that suggest any differential misclassification of frailty status between cancer survivors and no cancer history participants as both groups were independently categorized into cancer groups, and were considered healthy community-dwelling adults at baseline. Altogether, the inference of the results from the current study are similar to prior studies; however, the observed increased frailty risk did not increase the subsequent risk of sepsis or the pathway between cancer and sepsis is not much explained by frailty. Nevertheless, it is unlikely that there is a differential recall bias between our exposure comparison groups (i.e., cancer survivors vs. no cancer history participants) because we classified participants into exposure groups completely unrelated to the frailty indicators and sepsis outcomes. Future studies using objective measures among a prospective cohort of community-dwelling adults could reduce potential of frailty misclassification.
Conclusion
Cancer survivors had more than a 2.5-fold increased risk of sepsis, and indicators of frailty contributed to less than one percent of this disparity. It is feasible that frailty, physiologic condition and functioning, plays a role in the overall risk of infection after cancer, however future studies should assess frailty conditions using physiologic measures over time to better assess the mediating effects. At this point, using frailty indicators as discriminatory predictors of sepsis risk for cancer survivors is not yet warranted. Cancer survivors are at a very high risk of sepsis infection and clinical practice should attempt to mitigate sepsis risk and subsequent morbidity and mortality by timely and appropriate treatment (e.g., antibiotic administration) when encountering cancer survivors with suspected infection regardless of the number of years since cancer remission.
Supplementary Material
Acknowledgments
FINANCIAL SUPPORT
This work was supported by award (grant number R01-NR012726) from the National Institute for Nursing Research, (grant number UL1-RR025777) from the National Center for Research Resources, as well as by grants from the Center for Clinical and Translational Science and the Lister Hill Center for Health Policy of the University of Alabama at Birmingham. The parent REGARDS study was supported by cooperative agreement (grant number U01-NS041588) from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies. Representatives of the funding agencies have been involved in the review of the manuscript but not directly involved in the collection, management, analysis or interpretation of the data. The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org and http://www.regardssepsis.org. Dr. Moore received grant support from (grant R25 CA47888), the Cancer Prevention and Control Training Program grant, funded by the National Cancer Institute, National Institutes of Health. Dr. Moore was additionally supported by grant T32190194 (Colditz) and by the foundation for Barnes Jewish Hospital and by Siteman Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Footnotes
DECLARATION OF CONFLICTING INTERESTS: The authors declare no conflicts of interests.
AUTHOR CONTRIBUTIONS
JXM, TA, AB, HEW, JW, and RG conceived and designed the study. JXM oversaw data collection and developed the dataset. JXM conducted the analysis. TA, AB, HEW, JW, and RG contributed epidemiologic expertise in the approach for statistical analyses. JXM drafted the manuscript and all authors contributed to its critical review.
References
- 1.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Critical Care Medicine. 2001;29(7):1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. New England Journal of Medicine. 2003;348:1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 3.Wang HE, Shapiro NI, Angus DC, Yealy DM. National estimates of severe sepsis in United States emergency departments. Critical Care Medicine. 2007;35(8):1928–1936. doi: 10.1097/01.CCM.0000277043.85378.C1. [DOI] [PubMed] [Google Scholar]
- 4.Danai PA, Moss M, Mannino DM, Martin GS. The epidemiology of sepsis in patients with malignancy. Chest. 2006;129:1432–1440. doi: 10.1378/chest.129.6.1432. [DOI] [PubMed] [Google Scholar]
- 5.Sammon JD, Klett DE, Sood A, Olugbade K, Schmid M, Kim SP, Menon M, Trinh Q. Sepsis after major cancer surgery. Journal of Surgical Research. 2015;193:788–794. doi: 10.1016/j.jss.2014.07.046. [DOI] [PubMed] [Google Scholar]
- 6.Williams MD, Braun LA, Cooper LM, Johnston J, Weiss RV, Qualy RL, Linde-Zwirble W. Hospitalized cancer patients with severe sepsis: analysis of incidence, mortality, and associated costs of care. Crit Care. 2004 Oct;8(5):R291–298. doi: 10.1186/cc2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Remick DG. Pathophysiology of sepsis. Am J Pathol. 2007 May;170(5):1435–1444. doi: 10.2353/ajpath.2007.060872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gloeckler Ries LA, Reichman ME, Lewis DR, Hankey BF, Edwards BK. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist. 2003;8(6):541–552. doi: 10.1634/theoncologist.8-6-541. [DOI] [PubMed] [Google Scholar]
- 9.Welch HG, Schwartz LM, Woloshin S. Are increasing 5-year survival rates evidence of success against cancer? JAMA. 2000 Jun 14;283(22):2975–2978. doi: 10.1001/jama.283.22.2975. [DOI] [PubMed] [Google Scholar]
- 10.Aizer AA, Wilhite TJ, Chen MH, Graham PL, Choueiri TK, Hoffman KE, Martin NE, Trinh QD, Hu JC, Nguyen PL. Lack of reduction in racial disparities in cancer-specific mortality over a 20-year period. Cancer. 2014 May 15;120(10):1532–1539. doi: 10.1002/cncr.28617. [DOI] [PubMed] [Google Scholar]
- 11.Akinyemiju TF, Soliman AS, Yassine M, Banerjee M, Schwartz K, Merajver S. Healthcare access and mammography screening in Michigan: a multilevel cross-sectional study. Int J Equity Health. 2012;11:16. doi: 10.1186/1475-9276-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akinyemiju TF, Soliman AS, Johnson NJ, Altekruse SF, Welch K, Banerjee M, Schwartz K, Merajver S. Individual and neighborhood socioeconomic status and healthcare resources in relation to black-white breast cancer survival disparities. J Cancer Epidemiol. 2013;2013:490472. doi: 10.1155/2013/490472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Akinyemiju TF, Soliman AS, Copeland G, Banerjee M, Schwartz K, Merajver SD. Trends in breast cancer stage and mortality in Michigan (1992–2009) by race, socioeconomic status, and area healthcare resources. PLoS One. 2013;8(4):e61879. doi: 10.1371/journal.pone.0061879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Akinyemiju TF, Vin-Raviv N, Chavez-Yenter D, Zhao X, Budhwani H. Race/ethnicity and socio-economic differences in breast cancer surgery outcomes. Cancer Epidemiol. 2015 Oct;39(5):745–751. doi: 10.1016/j.canep.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 15.Bradley CJ, Given CW, Roberts C. Race, socioeconomic status, and breast cancer treatment and survival. J Natl Cancer Inst. 2002;94(7):490–496. doi: 10.1093/jnci/94.7.490. [DOI] [PubMed] [Google Scholar]
- 16.Cross CK, Harris J, Recht A. Race, socioeconomic status, and breast carcinoma in the U.S: what have we learned from clinical studies. Cancer. 2002;95(9):1988–1999. doi: 10.1002/cncr.10830. [DOI] [PubMed] [Google Scholar]
- 17.Dai D. Black residential segregation, disparities in spatial access to health care facilities, and late-stage breast cancer diagnosis in metropolitan Detroit. Health & Place. 2010;16:1038–1052. doi: 10.1016/j.healthplace.2010.06.012. [DOI] [PubMed] [Google Scholar]
- 18.El-Tamer MB, Homel P, Wait RB. Is race a poor prognostic factor in breast cancer? J Am Coll Surg. 1999;189(1):41–45. doi: 10.1016/s1072-7515(99)00055-1. [DOI] [PubMed] [Google Scholar]
- 19.Gwyn K, Bondy ML, Cohen DS, Lund MJ, Liff JM, Flagg EW, Brinton LA, Eley JW, Coates RJ. Racial differences in diagnosis, treatment, and clinical delays in a population-based study of patients with newly diagnosed breast carcinoma. Cancer. 2004;100(8):1595–1604. doi: 10.1002/cncr.20169. [DOI] [PubMed] [Google Scholar]
- 20.Kim SH, Ferrante J, Won BR, Hameed M. Barriers to adequate follow-up during adjuvant therapy may be important factors in the worse outcome for Black women after breast cancer treatment. World Journal of Surgical Oncology. 2008;6(26):10. doi: 10.1186/1477-7819-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Laiyemo AO. Reducing racial disparity in colorectal cancer burden. Dig Dis Sci. 2014 Sep;59(9):2025–2027. doi: 10.1007/s10620-014-3238-8. [DOI] [PubMed] [Google Scholar]
- 22.Li CI, Malone KE, Daling JR. Differences in breast cancer stage, treatment, and survival by race and ethnicity. Arch Intern Med. 2003;163(1):49–56. doi: 10.1001/archinte.163.1.49. [DOI] [PubMed] [Google Scholar]
- 23.Sheppard VB, Oppong BA, Hampton R, Snead F, Horton S, Hirpa F, Brathwaite EJ, Makambi K, Onyewu S, Boisvert M, Willey S. Disparities in breast cancer surgery delay: the lingering effect of race. Ann Surg Oncol. 2015 Sep;22(9):2902–2911. doi: 10.1245/s10434-015-4397-3. [DOI] [PubMed] [Google Scholar]
- 24.Barnato AE, Alexander SL, Linde-Zwirble WT, Angus DC. Racial variation in the incidence, care, and outcomes or severe sepsis: analysis of population, patient, and hospital characteristics. Am J Respir Crit Care Med. 2008;177(279–284) doi: 10.1164/rccm.200703-480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 26.Mayr FB, Yende S, Linde-Zwirble WT, Peck-Pamler OM, Weissfeld LA, Angus DC. Infection rate and acute organ dysfunction risk as explanations for racial differences in severe sepsis. JAMA. 2010;303(24):2495–2503. doi: 10.1001/jama.2010.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayr FB, Yende S, Angus DC. Epidemiology of severe sepsis. Virulence. 2014;5(1):4–11. doi: 10.4161/viru.27372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fried LP, Tangen CM, Walston J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001 Mar;56(3):M146–156. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 29.Woods NF, LaCroix AZ, Gray SL, Aragaki A, Cochrane BB, Brunner RL, Masaki K, Murray A, Newman AB. Women's Health, Initiative. Frailty: emergence and consequences in women aged 65 and older in the Women's Health Initiative Observational Study. J Am Geriatr Soc. 2005 Aug;53(8):1321–1330. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang HE, Shapiro NI, Griffin R, Safford MM, Judd S, Howard G. Chronic medical conditions and risk of sepsis. PLoS One. 2012;7(10):e48307. doi: 10.1371/journal.pone.0048307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang HE, Baddley J, Griffin RL, et al. Physical inactivity and long-term rates of community-acquired sepsis. Prev Med. 2014 Aug;65:58–64. doi: 10.1016/j.ypmed.2014.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohile SG, Xian Y, Dale W, et al. Association of a cancer diagnosis with vulnerability and frailty in older Medicare beneficiaries. J Natl Cancer Inst. 2009 Sep 02;101(17):1206–1215. doi: 10.1093/jnci/djp239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perez-Zepeda MU, Cardenas-Cardenas E, Cesari M, Navarrete-Reyes AP, Gutierrez-Robledo LM. Cancer and frailty in older adults: a nested case-control study of the Mexican Health and Aging Study. J Cancer Surviv. 2016 Aug;10(4):736–742. doi: 10.1007/s11764-016-0519-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mahalingam M, Moore JX, Donnelly JP, Safford MM, Wang HE. Frailty Syndrome and Risk of Sepsis in the REasons for Geographic And Racial Differences in Stroke (REGARDS) Cohort. Journal of Intensive Care Medicine. 2017:1–9. doi: 10.1177/0885066617715251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The Reasons for Geographic and Racial Differences in Stroke Study: Objectives and Design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 36.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3) JAMA. 2016 Feb 23;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bergmann MM, Calle EE, Mervis CA, Miracle-McMahill HL, Thun MJ, Heath CW. Validity of self-reported cancers in a prospective cohort study in comparison with data from state cancer registries. Am J Epidemiol. 1998 Mar 15;147(6):556–562. doi: 10.1093/oxfordjournals.aje.a009487. [DOI] [PubMed] [Google Scholar]
- 38.Garcia-Garcia FJ, Carcaillon L, Fernandez-Tresguerres J, Alfaro A, Larrion JL, Castillo C, Rodriguez-Manas L. A new operational definition of frailty: the Frailty Trait Scale. J Am Med Dir Assoc. 2014 May;15(5):371 e377–371 e313. doi: 10.1016/j.jamda.2014.01.004. [DOI] [PubMed] [Google Scholar]
- 39.Johansen KL, Dalrymple LS, Delgado C, Kaysen GA, Kornak J, Grimes B, Chertow GM. Comparison of self-report-based and physical performance-based frailty definitions among patients recieving maintenance hemodialysis. Am J Kidney Dis. 2014;64(4):600–607. doi: 10.1053/j.ajkd.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnsen KL, Chertow GM, Jin C, Kutner NG. Significance of frailty among dialysis patients. J Am Soc Nephrol. 2007;18(11):2960–2967. doi: 10.1681/ASN.2007020221. [DOI] [PubMed] [Google Scholar]
- 41.Johansen KL, Dalrymple LS, Delgado C, et al. Comparison of self-report-based and physical performance-based frailty definitions among patients receiving maintenance hemodialysis. Am J Kidney Dis. 2014 Oct;64(4):600–607. doi: 10.1053/j.ajkd.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mahalingam M, Moore JX, Donnelly JP, Safford MM, Wang HE. Frailty Syndrome and Risk of Sepsis in the REasons for Geographic And Racial Differences in Stroke (REGARDS) Cohort. J Intensive Care Med. 2017 Jan 1;:885066617715251. doi: 10.1177/0885066617715251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Willenbring ML, Massey SH, Gardner MB. Helping patients who drink too much: an evidence-based guide for primary care clinicians. American family physician. 2009;80(1):44–50. [PubMed] [Google Scholar]
- 44.Powell TC, Donnelly JP, Gutierrez OM, Griffin RL, Safford MM, Wang HE. Cystatin C and long term risk of community-acquired sepsis: a population-based cohort study. BMC Nephrol. 2015 Apr 23;16:61. doi: 10.1186/s12882-015-0055-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang HE, Shapiro NI, Safford MM, et al. High-sensitivity C-reactive protein and risk of sepsis. PLoS One. 2013;8(7):e69232. doi: 10.1371/journal.pone.0069232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang HE, Griffin R, Shapiro NI, Howard G, Safford MM. Chronic Statin Use and Long-Term Rates of Sepsis: A Population-Based Cohort Study. J Intensive Care Med. 2016 Jul;31(6):386–396. doi: 10.1177/0885066614550280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaudhary NS, Donnelly JP, Moore JX, Baddley JW, Safford MM, Wang HE. Association of baseline steroid use with long-term rates of infection and sepsis in the REGARDS cohort. Crit Care. 2017 Jul 13;21(1):185. doi: 10.1186/s13054-017-1767-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang HE, Shapiro NI, Griffin R, Safford MM, Judd S, Howard G. Inflammatory and endothelial activation biomarkers and risk of sepsis: a nested case-control study. J Crit Care. 2013 Oct;28(5):549–555. doi: 10.1016/j.jcrc.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: Conceptual, strategic, and statistical considerations. Journal of Personality and Social Psychology. 1986;51(6):1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 50.Hayes AF, Scharkow M. The relative trustworthiness of inferential tests of the indirect effect in statistical mediation analysis: Does method really matter? Psychological Science. 2013;24(10):1918–1927. doi: 10.1177/0956797613480187. [DOI] [PubMed] [Google Scholar]
- 51.Hayes AF. Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York: Guilford Press; 2013. [Google Scholar]
- 52.Hayes AF, Preacher KJ. Statistical mediation analysis with a multicategorical independent variable. Br J Math Stat Psychol. 2014 Nov;67(3):451–470. doi: 10.1111/bmsp.12028. [DOI] [PubMed] [Google Scholar]
- 53.Vanderweele TJ, Vansteelandt S. Odds ratios for mediation analysis for a dichotomous outcome. Am J Epidemiol. 2010 Dec 15;172(12):1339–1348. doi: 10.1093/aje/kwq332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valeri L, Vanderweele TJ. Mediation analysis allowing for exposure-mediator interactions and causal interpretation: theoretical assumptions and implementation with SAS and SPSS macros. Psychol Methods. 2013 Jun;18(2):137–150. doi: 10.1037/a0031034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Valeri L, VanderWeele TJ. SAS macro for causal mediation analysis with survival data. Epidemiology. 2015 Mar;26(2):e23–24. doi: 10.1097/EDE.0000000000000253. [DOI] [PubMed] [Google Scholar]
- 56.Rice MS, Bertrand KA, VanderWeele TJ, et al. Mammographic density and breast cancer risk: a mediation analysis. Breast Cancer Res. 2016 Sep 21;18(1):94. doi: 10.1186/s13058-016-0750-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bennett JA, Winters-Stone KM, Dobek J, Nail LM. Frailty in older breast cancer survivors: age, prevalence, and associated factors. Oncol Nurs Forum. 2013 May 01;40(3):E126–134. doi: 10.1188/13.ONF.E126-E134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brummel NE, Bell SP, Girard TD, et al. Frailty and Subsequent Disability and Mortality among Patients with Critical Illness. Am J Respir Crit Care Med. 2017 Jul 1;196(1):64–72. doi: 10.1164/rccm.201605-0939OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schaap LA, Pluijm SM, Deeg DJ, Visser M. Inflammatory markers and loss of muscle mass (sarcopenia) and strength. Am J Med. 2006 Jun;119(6):526 e529–517. doi: 10.1016/j.amjmed.2005.10.049. [DOI] [PubMed] [Google Scholar]
- 60.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002 May;57(5):M326–332. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 61.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc. 2013 Jun;14(6):392–397. doi: 10.1016/j.jamda.2013.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Handforth C, Clegg A, Young C, et al. The prevalence and outcomes of frailty in older cancer patients: a systematic review. Ann Oncol. 2015 Jun;26(6):1091–1101. doi: 10.1093/annonc/mdu540. [DOI] [PubMed] [Google Scholar]
- 63.National Institute on Alcohol Abuse and Alcoholism. [Accessed February 13, 2012];Helping Patients Who Drink Too Much, a Clinician's Guide. Available at: http://pubs.niaaa.nih.gov/publications/Practitioner/CliniciansGuide2005/guide.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


