Abstract
microRNAs (miRNAs) play a key role in regulating inflammation, vascular health and in-turn, cardiovascular disease. Specifically, altered circulating expression of miR-17, miR-21, miR-34a, miR-92a, miR-126, miR-145, miR-146a and miR-150 has been linked with the pathogenesis and progression of cardiovascular disease. The aim of this study was to determine whether the circulating profile of these vascular-related miRNAs is disrupted with hypertension. Thirty sedentary, middle-aged adults were studied: 15 normotensive (10M/5F; age: 56±1 yr; BP: 114/71±2/1 mmHg) and 15 hypertensive (10M/5F; 56±2 yr; 140/87±2/2 mmHg). All subjects were non-obese and free of other cardiometabolic disorders. Circulating miRNAs were determined in plasma using standard RT-PCR techniques with miRNA primers of interest. Expression was normalized to exogenous C. elegans miR-39 and reported as relative expression in arbitrary units (AU). Circulating expression of miR-34a (9.18±0.94 vs 5.33±0.91 AU) was higher (~170%; P<0.01) whereas the expression of miR-21 (1.32±0.25 vs 2.50±0.29 AU), miR-126 (0.85±0.10 vs 1.74±0.27 AU) and miR-146a (1.50±0.20 vs 3.10±0.50 AU) were markedly lower (~50%, ~55% and ~55% respectively; P<0.05) in the hypertensive vs normotensive groups. Moreover, circulating levels of miR-34a, miR-21 and miR-126 were significantly related to systolic blood pressure (r=0.48, r=−0.38; r=−0.48); whereas, miR-146a was significantly related to both systolic (r=−0.58) and diastolic (r=−0.55) blood pressure. There were no significant group differences in circulating miR-17, miR-92a, miR-145 and miR-150. In summary, these results suggest that hypertension, independent of other cardiometabolic risk factors, adversely affects the circulating profile of a subset of vascular-related miRNAs that have been link to CVD risk and development.
INTRODUCTION
Hypertension, defined as a systolic blood pressure (SBP) greater than 130 mmHg and/or diastolic blood pressure (DBP) greater than 80 mmHg, is an independent risk factor for atherosclerotic cardiovascular disease (CVD). Adults with hypertension are two to four times more prone to develop vascular disease and suffer a myocardial infarction or stroke compared with their normotensive counterparts of similar age1. It is estimated that ~40% of adults in the United States have blood pressure in the hypertensive range and this number is expected to increase as the population ages 2. The mechanisms responsible the increased risk and prevalence of CVD risk with elevated blood pressure are diverse and involve a myriad of vascular abnormalities such as, impaired vasomotor and fibrinolytic function as well as elevated vascular inflammation3,4.
It is now recognized that microRNAs (miRNAs) play a central role in regulating vascular health and function5. miRNAs are short (18–22 nt), single stranded, noncoding RNAs that are involved in the regulation of a number of physiological and pathological processes5. Circulating concentrations of a subset of miRNAs, specifically miR-17, miR-21, miR-34a, miR-92a, miR-126, miR-145, miR-146a and miR-150, have garnered significant clinical interest due to their regulatory influence on inflammatory burden, vascular function and, in turn, cardiovascular risk and disease5–8. Indeed, levels of these specific vascular-related miRs have been shown to be dysregulated with CVD5 and, more importantly, may be involved in the development and progression of vascular disease and its clinical consequences6,8. The influence of hypertension on the circulating profile of vascular-related miRNAs is not well understood. miRNA’s may reflect not only a biomarker of vascular risk but also a therapeutic target to improve vascular health in adults with elevated blood pressure.
Accordingly, the aim of the present study was to determine whether hypertension, independent of other cardiometabolic risk factors, is associated with a disrupted circulating miRNA signature profile. Specifically, we tested the hypothesis that circulating levels of miR-17, miR-21, miR-34a and miR-92a would be higher and miR-126, miR-145, miR-146a and miR-150 lower in hypertensive compared with normotensive adults. The rationale for the postulated blood-pressure related differences in circulating miRNA profile is based on the directional-association of each circulating miRNA with CVD risk and outcome5,7.
METHODS
Subjects
Thirty sedentary middle-aged adults (51–65 years) were studied: 15 normotensive (10M/5F; blood pressure: 97–119/ 58–79 mmHg) and 15 hypertensive (10M/5F; 130–151/80–95 mmHg). All subjects were sedentary, non-obese, non-smokers, normolipidemic, non-medicated and free of overt cardiovascular, metabolic, renal, and hematologic disease, as assessed by medical history, resting and exercise electrocardiograms, and fasting blood chemistries. Female subjects were at least 1 year postmenopausal and had never taken or had discontinued use of hormone replacement therapy at least 1 year before the start of the study. Prior to participation in the study, all subjects had the research study and its potential risk and benefits explained before providing written informed consent according to the guidelines of the University of Colorado Boulder. This study was approved by the University of Colorado Institutional Review Board.
Blood Pressure Measurement
Resting blood pressure measurements were performed in the sitting position on at least two separate days at least one week apart. Subjects were instructed not to ingest caffeine-containing beverages prior to all BP measurements. The recordings were made under quiet, comfortable ambient (~24°C) laboratory conditions. To avoid the possibility of investigator bias, measurements were made with a semi-automated device (Dinamap, Crtikon, FL) that uses an oscillometric technique over the brachial artery. Recordings were made in triplicate in the upright sitting position. All measurements conformed to American Heart Association guidelines as established by the Council for High Blood Pressure Research.
Body Composition and Metabolic Measures
Body mass was measured to the nearest 0.1 kg using a medical beam balance. Percent body fat was determined by dual energy X-ray absorptiometry (Lunar Corp., Madison, WI, USA). Body mass index (BMI) was calculated as weight (kg) divided by height (m) squared. Fasting plasma lipid, lipoprotein, glucose, and insulin concentrations were determined using standard techniques.
MicroRNA isolation and Reverse Transcription Quantitative Polymerase Chain Reaction Analysis (RT-qPCR)
Blood samples were collected from the antecubital vein after an overnight fast into EDTA containing tubes (BD Bioscience, Franklin Lakes, NJ, USA). Blood was centrifuged at 600 x g for 20 minutes at 4°C. Plasma was removed and centrifuged at 1500 x g for 15 minutes at 4°C to remove any additional cellular debris. Platelet poor plasma was aliquoted and stored at −80°C for batch analysis.
Total RNA was isolated from platelet poor plasma using the miRNeasy Serum/Plasma Kit (Qiagen, Hilden, Germany)9. Briefly, RNA was isolated from 100μL of plasma using the QIAsol lysis reagent, washed and eluted in RNAse free water. To normalize between samples 3.5μL (1.6x108 copies/μL) Canorhabditis elegans miR-39 (cel-miR-39) was added to each sample9. Immediately after RNA isolation, 12μL of RNA was reverse transcribed using the miScript Reverse Transcription Kit (Qiagen, Hilden, German)9,10. cDNA was PCR-amplified (BioRad CFX96 Touch Real Time System) using the miScript SYBR green PCR kit (Qiagen, Hilden, Germany) and miRNA specific primers for miR-17, miR-21, miR-34a, miR-92a, miR-126, miR-145, miR-146a and miR-150 (Qiagen, Hilden, Germany)10. All samples were assayed in duplicate. Relative expression level for a given miR was normalized to cel-miR-39, calculated as ΔCt =2−(Ct[miR]-Ct[cel-miR-39]) and expressed as arbitrary units (AU)9,10.
Statistical Analysis
Group differences in subject characteristics and circulating miRNA levels were determined by ANOVA. There were no significant group x sex interactions, therefore the data were pooled and presented together. Pearson correlations were determined between variables of interest. All values are expressed as mean±SE. Statistical significance was set a priori at P<0.05.
RESULTS
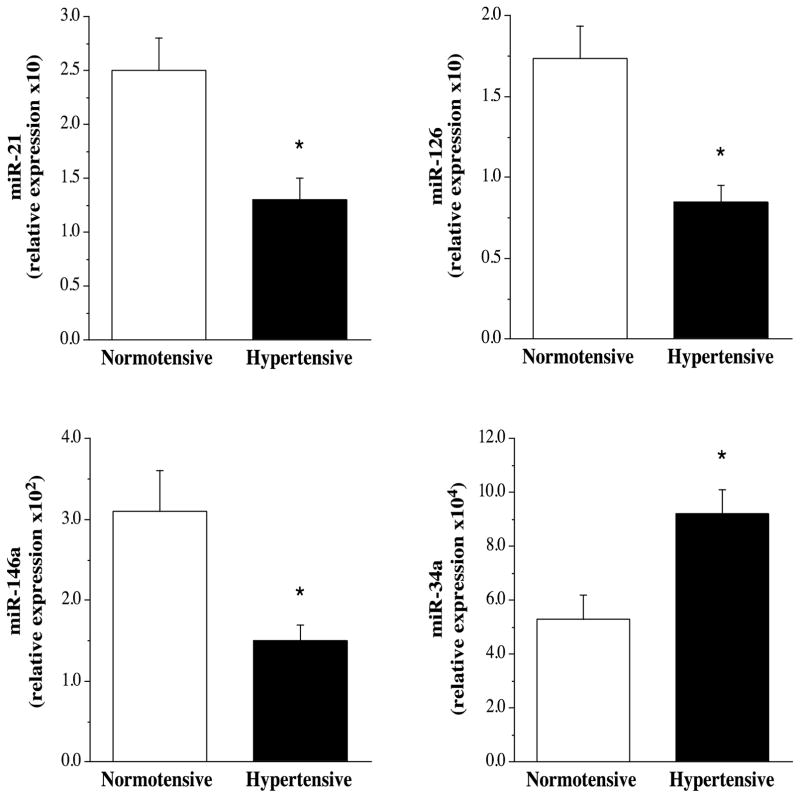
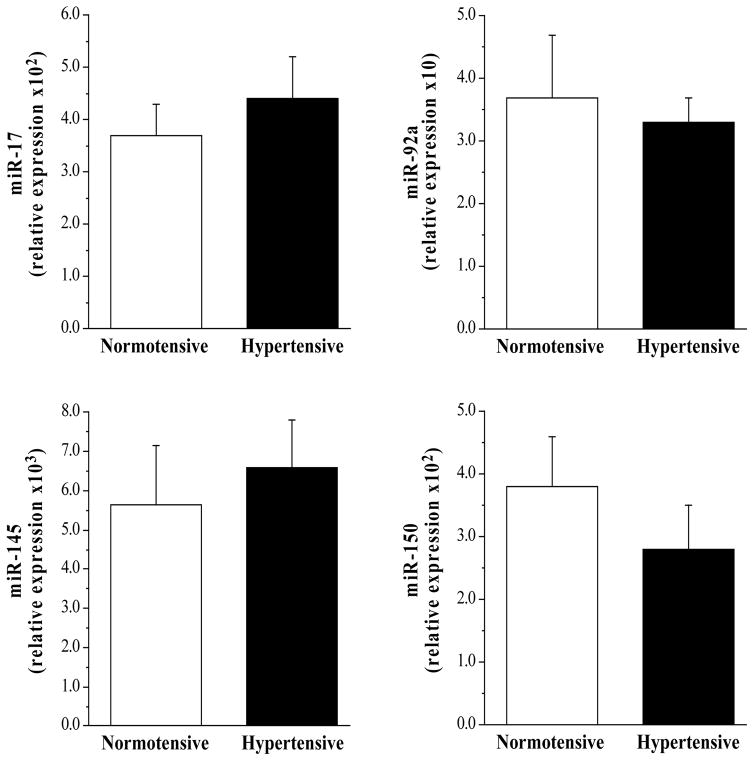
Selected subject characteristics are presented in the Table. Other than blood pressure, there were no significant differences between groups in anthropometric or metabolic variables. Circulating levels of miR-21 (1.32±0.25 vs 2.50±0.29 AU), miR-126 (0.85±0.10 vs 1.74±0.27 AU) and miR-146a (1.50±0.20 vs 3.10±0.50 AU) were significantly lower (~50%, ~55%, and ~55% respectively) in the hypertensive compared with the normotensive group (Figure 1); whereas, miR-34a levels were ~170% higher (P<0.01) in the hypertensive (9.18±0.94 AU) than normotensive (5.33±0.91 AU) group (Figure 1). There were no significant differences in circulating miR-17 (4.40±0.81 vs 3.75±0.64 AU), miR-92a (3.32±0.42 vs 3.72±1.01 AU), miR-145 (5.54±1.10 vs 6.40±0.85 AU) and miR-150 (2.91±0.67 vs 3.22±0.57 AU) between the hypertensive and normotensive groups (Figure 2).
Table.
Selected subject characteristics
| Variable | Normotensive (n=15) | Hypertensive (n=15) |
|---|---|---|
| Age (yr) | 56±1 | 59±2 |
| Sex (M/F) | 10/5 | 10/5 |
| Body Mass (kg) | 76.9±3.4 | 80.8±3.4 |
| BMI (kg m−2) | 25.2±0.7 | 25.8±0.7 |
| Body Fat (%) | 29.1±2.0 | 30.5±2.3 |
| Systolic Blood Pressure, (mmHg) | 113±2 | 140±2* |
| Diastolic Blood Pressure (mmHg) | 71±2 | 87±2* |
| Total Cholesterol (mmol·l−1) | 4.9±0.2 | 5.2±0.2 |
| LDL-C (mmol·l−1) | 3.3±0.1 | 3.4±0.2 |
| HDL-C (mmol·l−1) | 1.4±0.1 | 1.4±0.1 |
| Triglycerides (mmol·l−1) | 2.6±0.2 | 2.9±0.3 |
| Glucose (mmol·l−1) | 4.8±0.1 | 4.9±0.1 |
| Insulin (μU·ml−1) | 7.0±0.7 | 8.3±0.7 |
Values expressed as Mean±SE. BMI: body mass index. LDL-C: low-density lipoprotein. HDL-C: high-density lipoprotein.
P<0.05 vs. normotensive.
Figure 1.
Circulating miR-21, miR-126, miR-146 and miR-34a in the normotensive and hypertensive adults. *P<0.05 vs normotensive.
Figure 2.
Circulating miR-17, miR-92a, miR-145 and miR-150 in the normotensive and hypertensive adults.
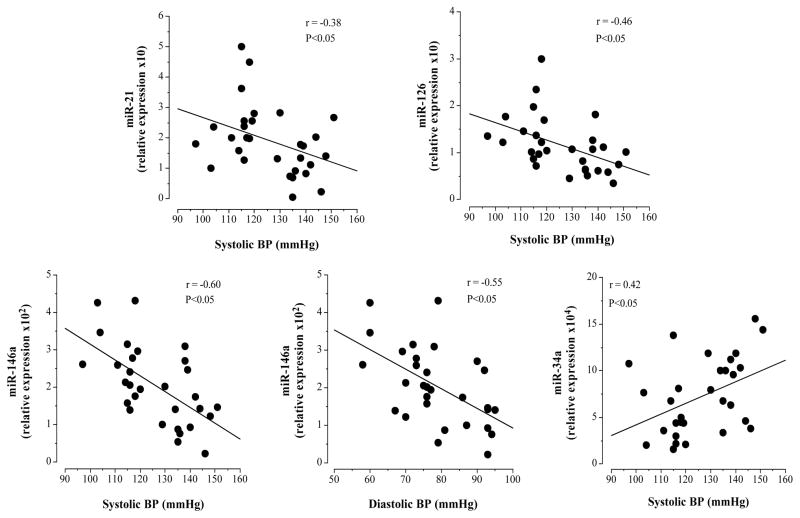
In the overall study population, miR-21 (r=−0.38; P<0.05) and miR-126 (r=−0.48; P<0.05) levels were inversely related to systolic blood pressure (Figure 3). Circulating miR-146a was inversely related with both systolic (r=−0.58; P<0.05) and diastolic (r=−0.55 P<0.05) blood pressure; and circulating miR-34a (r=0.48; P<0.05) was positively related with systolic blood pressure (Figure 3). No other miRNAs were associated with blood pressure; nor were there any significant correlations between any miRNA and other anthropometric or metabolic variables.
Figure 3.
Relation between circulating miR-21, miR-126, miR-34 and miR-146a and blood pressure.
DISCUSSION
Over the past decade, interest in circulating miRNAs has increased due to the role of miRNAs as important regulators of vascular function and their potential use as biomarkers and mediators of CVD5,7. The key finding of the present study is that circulating levels of miR-21, miR-34a, miR-126 and miR-146a are significantly altered in hypertensive compared with normotensive adults. Circulating concentrations of miR-21, miR-126 and miR-146a were significantly lower, whereas miR34a was significantly higher in the hypertensive adults. Disruption in the circulating profile of these vascular-related microRNAs may be a contributing factor to the increased CVD risk and prevalence associated with hypertension1,7. To our knowledge, this is the first study to determine the influence of hypertension per se, on circulating miRNAs.
Previous studies have clearly demonstrated independent links between circulating miRNAs (including: miR-21, miR-34a, miR-126 and miR-146a) and both the prevention and development of vascular dysfunction and disease as well as its clinical consequences11,12. From a cardioprotective perspective, miR-126 is recognized to be critical for the maintenance of vascular homeostasis11,13,14. Predominately expressed in endothelial cells, miR-126 reduces atherosclerotic lesion development by lowering the expression of proteins involved in endothelial cell activation and inflammation (e.g.: HMGB1, vascular cell adhesion molecule (VCAM)-1, CXCL-12 and SPREAD-1) thereby reducing endothelial inflammation and arresting lesion development and progression11,14. Thus, lower cellular expression of miR-126 promotes inflammation, endothelial cell dysfunction and reduced capacity for vascular repair11,13. In addition, lower circulating levels of miR-126 has been shown to be predictive of the development of CVD15 as well as diabetes16. Herein, we demonstrate that the circulating level of miR-126 is markedly lower (~55%) in adults with hypertension. Given the primary source of miR-126 expression, hypertension-related inflammation and endothelial dysfunction may be a consequence of miR-126 dysregulation14.
Similar to miR-126, miR-146a plays a central role in vascular inflammation and reduced expression has been linked with atherogenesis17. Indeed, lower expression of miR-146a promotes vascular inflammation and the development of CVD in animal models17,18. Moreover, in a seminal study, Ma et al. 18, demonstrated that exogenously increasing circulating miR-146a levels blunts the development and progression of atherosclerotic lesions in a rat model of CVD, indicating that higher circulating levels of miR-146a is cardioprotective. miR-146a is a major regulator of the inflammatory mediator, nuclear factor-kB (NF-kB). NF-kB activates the transcription of proinflammatory genes including interluekin-6 and tumor necrosis factor-α, increasing inflammatory burden17. In addition to inflammation, NF-κB activity contributes to the pathogenesis of atherosclerosis and has been shown to be elevated with hypertension19. miR-146a directly suppresses the NF-kB activation cascade17 thereby reducing inflammatory mediators. Reduction in miR-146a may underlie the escalation in systemic inflammation associated with hypertension18.
In addition to miR-126 and miR-146a, circulating miR-21 levels were also significantly lower (~50%) in the hypertensive compared with normotensive adults. miR-21 has been linked with both favorable and unfavorable vascular effects. For example, through the regulation of PTEN, a phosphatase involved in the suppression of cell cycling and activation of the Akt pathway20, miR-21 has been shown to promote cardiovascular fibrosis and myointimal hyperplasia accelerating atherosclerotic lesion formation and progression 20,21. Conversely, miR-21 neutralizes inhibitors of endothelial nitric oxide synthase (eNOS) protecting eNOS activity and, in turn, nitric oxide production 22. Lower miR-21 levels in our hypertensive adults are certainly consistent with previous findings from our laboratory4 and others23 demonstrating that nitric oxide-mediated endothelium-dependent vasodilation is severely diminished in adults with elevated blood pressure. Interestingly, sheer stress has been shown to down-regulate miR-21 expression22. Thus, it is possible that lower miR-21 levels associated with elevated blood pressure may be due to a shear stress related phenomenon.
Contrary to miR-21, miR-126 and miR-146a, circulating levels of miR-34a were significantly higher in the hypertensive than normotensive adults. Higher expression of miR-34a is associated with endothelial cell senescence and dysfunction and has been linked with the development of CVD24,25. miR-34a promotes cellular senescence and dysfunction through the regulation of sirtuin-1 (SIRT-1) and the apoptotic protein BCL-224. Importantly, in addition to tissue levels, circulating concentrations of miR-34a have been shown to be elevated with disease25. For example, circulating miR-34a has been reported to be two-fold higher in adults with coronary artery disease compared with healthy controls25 and is thought to play a contributing role in the development of heart failure and cardiac death24,25. In fact, reduction in circulating levels of miR-34a in animal models has been shown to limit ventricular remodeling and cell death and improve cardiac function after myocardial infarction24. In the present study, circulating miR-34a was markedly higher (~170%) in hypertensive adults. Considering that elevations in blood pressure induce a prosenescent endothelial phenotype, miR-34a dysregulation may play a part in this pathology and the deleterious cardiac effects of hypertension24–26.
In stark contrast to miR-21, miR-126, miR-146 and miR-34a, we observed no blood pressure-related differences in circulating concentrations of miR-17, miR-92a, miR-145 and miR-150. Elevations in miR-17 and miR-92a and reductions in miR-145 and miR-150 have been linked to CVD events and outcome7. For example, miR-92a targets Kruppel like factor (KLF)-2 and -4 resulting in diminished endothelial repair capacity, eNOS expression and endothelial function 6,8,27. Increased cellular expression of miR-92a is etiologically involved in the formation of atherosclerotic lesions27 and elevations in circulating levels of miR-92 have been shown to be associated with CVD6. Whereas, miR-145 and miR-150 confer cardioprotective effects. Indeed, miR-145 mediated reductions in vascular smooth muscle cell proliferation has been shown to blunt atherosclerotic lesion enlargement8,28 and miR-150 limits atherogenesis by moderating immune cell activation and secretion of cytokines as well as enhancing endothelial progenitor cell function and vascular repair 29. Lower circulating levels of both miR-145 and miR-150 are associated with a myriad of CVD risk factors as well as vascular events5. Given the putative roles of these miRNAs in the etiology of CVD, it is somewhat surprising that their circulating levels were not altered in the hypertensive adults. It is possible that the level of hypertension (stage 1 hypertension) may not have been severe enough or that other cardiometabolic risk factors (such as dyslipidemia and diabetes) or disease may have to accompany hypertension in order to induce changes in circulating miR-17, miR-92, miR-145 and miR-1507,15,16,30,31.
There are a few experimental considerations regarding the present study that deserve mention. Firstly, inherent with all cross-sectional human studies we cannot dismiss the possibility that genetic and/or lifestyle behaviors may have influenced the results of our group comparisons. However, to minimize the effects of other lifestyle behaviors, all subjects were similar in age, sedentary, nonsmokers, and not taking medication. Moreover, all subjects were carefully screened to eliminate the confounding effects of clinically overt metabolic or cardiovascular disease. The presence of other cardiometabolic risk factors, or disease, would undoubtedly influence circulating miRNAs making it difficult, if not impossible, to tease out the primary influence of hypertension15,16,30,31. This distinction highlights the novelty of the present study. Previous studies addressing elevated blood pressure and circulating miRNAs have involved patients varying in adiposity, on various blood pressure medications, and presenting with numerous comorbidities making it difficult to discern the impact of hypertension, per se, on circulating miRNA signature patterns30,32–34. Secondly, we are unable to identify which cells or tissues are involved in the production and release of miRNAs into the circulation. However, circulating miRNA levels, regardless of cell origin or mechanism of release, are highly correlated with disease35 and disruption of the circulating miRNA milieu has been shown to provide mechanistic insight into the pathogenesis and progression of disease 5–7. Thirdly, although we observed no significant main effect or interaction of sex on the blood pressure-related differences in circulating miRNAs, the small number of women in the present study precludes us from definitively dismissing possible sex differences with respect to the effect of hypertension on circulating miRNAs. Finally, although our study design does not allow elucidation of mechanisms, the strict inclusion/exclusion criteria employed to isolate the potential independent influence of blood pressure as well as the observed univariate correlations does suggest a primary effect of elevated blood pressure on circulating miRNAs. Future studies are needed to identify the mechanistic links between blood pressure and miRNA expression.
In conclusion, the results of the present study indicate that hypertension, independent of other cardiometabolic risk factors, adversely affects the circulating profile of a subset of vascular-related miRNAs. Blood pressure-related changes in circulating concentrations of miR-21, miR-34a, miR-126 and miR-146a may play a role in the increased risk of vascular disease and associated events in adults with hypertension. To be sure, the observed disruption in these miRNAs is consistent with the aberrant circulating miRNA signature noted to be associated with CVD pathogenesis, progression and prognosis5.
Summary Table.
What is known about topic
Hypertension is an independent risk factor for atherosclerotic cardiovascular disease (CVD). The mechanisms responsible the increased risk and prevalence of CVD risk with elevated blood pressure are diverse and involve a myriad of vascular abnormalities as well as elevated vascular inflammation.
It is now recognized that microRNAs (miRNAs) play a central role in regulating vascular health and function as well as disease pathogenesis.
Clinical interest in circulating concentrations of a subset of miRNAs, specifically miR-17, miR-21, miR-34a, miR-92a, miR-126, miR-145, miR-146a and miR-150, has increased due to their regulatory influence on inflammatory burden and CVD risk.
What this study adds
The key finding of the present study is that hypertension, independent of other cardiometabolic risk factors, adversely affects the circulating profile of a subset of vascular-related miRNAs. Specifically, circulating levels of miR-21, miR-34a, miR-126 and miR-146a are significantly altered in hypertensive compared with normotensive adults.
The hypertension-related dysregulation in miR-21, miR-34a, miR-126 and miR-146a is consistent with the aberrant circulating miRNA signature noted to be associated with CVD pathogenesis, progression and prognosis.
Increased vascular risk with hypertension may be mediated, in-part, by changes in miRNA expression.
Acknowledgments
The authors would like to thank all subjects who participated in this study and the University of Colorado Boulder, Clinical and Translational Research Center clinical staff for their assistance. This study was supported by National Institutes of Health awards HL131458, HL135598 and NIH/NCATS UL1 TR001082.
Funding: National Institutes of Health awards HL131458, HL135598 and NIH/NCATS UL1 TR001082.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to disclose.
References
- 1.Rahimi K, Emdin CA, MacMahon S. The Epidemiology of Blood Pressure and Its Worldwide Management. Circ Res. 2015;116:925–936. doi: 10.1161/CIRCRESAHA.116.304723. [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DE, Collins KJ, Himmelfarb CD, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2017 HYP.0000000000000065. [Google Scholar]
- 3.Diehl KJ, Weil BR, Greiner JJ, Wright KP, Stauffer BL, DeSouza CA. Impaired endogenous fibrinolytic capacity in prehypertensive men. J Hum Hypertens. 2015 doi: 10.1038/jhh.2014.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weil BR, Stauffer BL, Greiner JJ, DeSouza CA. Prehypertension Is Associated With Impaired Nitric Oxide-Mediated Endothelium-Dependent Vasodilation in Sedentary Adults. Am J Hypertens. 2011;24:976–981. doi: 10.1038/ajh.2011.88. [DOI] [PubMed] [Google Scholar]
- 5.Viereck J, Thum T. Circulating Noncoding RNAs as Biomarkers of Cardiovascular Disease and Injury. Circ Res. 2017;120:381–399. doi: 10.1161/CIRCRESAHA.116.308434. [DOI] [PubMed] [Google Scholar]
- 6.Economou EK, Oikonomou E, Siasos G, Papageorgiou N, Tsalamandris S, Mourouzis K, et al. The role of microRNAs in coronary artery disease: From pathophysiology to diagnosis and treatment. Atherosclerosis. 2015;241:624–633. doi: 10.1016/j.atherosclerosis.2015.06.037. [DOI] [PubMed] [Google Scholar]
- 7.Wronska A, Kurkowska-Jastrzebska I, Santulli G. Application of microRNAs in diagnosis and treatment of cardiovascular disease. Acta Physiol. 2015;213:60–83. doi: 10.1111/apha.12416. [DOI] [PubMed] [Google Scholar]
- 8.Andreou I, Sun X, Stone PH, Edelman ER, Feinberg MW. miRNAs in atherosclerotic plaque initiation, progression, and rupture. Trends Mol Med. 2015;21:307–318. doi: 10.1016/j.molmed.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kroh EM, Parkin RK, Mitchell PS, Tewari M. Analysis of circulating microRNA biomarkers in plasma and serum using quantitative reverse transcription-PCR (qRT-PCR) Methods. 2010;50:298–301. doi: 10.1016/j.ymeth.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elbehidy RM, Youssef DM, El-Shal AS, Shalaby SM, Sherbiny HS, Sherief LM, et al. MicroRNA–21 as a novel biomarker in diagnosis and response to therapy in asthmatic children. Mol Immunol. 2016;71:107–114. doi: 10.1016/j.molimm.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 11.Chamorro-Jorganes A, Araldi E, Suárez Y. microRNAs as Pharmacological Targets in Endothelial Cell Function and Dysfunction. Pharmacol Res Off J Ital Pharmacol Soc. 2013;75:15–27. doi: 10.1016/j.phrs.2013.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paterson MR, Kriegel AJ. MiR-146a/b: a family with shared seeds and different roots. Physiol Genomics. 2017;49:243–252. doi: 10.1152/physiolgenomics.00133.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Wang J, Wang B, Yang J, Gong Z, Zhao X, et al. MiR-126 inhibits vascular endothelial cell apoptosis through targeting PI3K/Akt signaling. Ann Hematol. 2016;95:365–374. doi: 10.1007/s00277-015-2567-9. [DOI] [PubMed] [Google Scholar]
- 14.Tang S, Wang F, Shao M, Wang Y, Zhu H. MicroRNA-126 suppresses inflammation in endothelial cells under hyperglycemic condition by targeting HMGB1. Vascul Pharmacol. 2017;88:48–55. doi: 10.1016/j.vph.2016.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Fukushima Y, Nakanishi M, Nonogi H, Goto Y, Iwai N. Assessment of Plasma miRNAs in Congestive Heart Failure. Circ J. 2011;75:336–340. doi: 10.1253/circj.cj-10-0457. [DOI] [PubMed] [Google Scholar]
- 16.Zhang T, Li L, Shang Q, Lv C, Wang C, Su B. Circulating miR-126 is a potential biomarker to predict the onset of type 2 diabetes mellitus in susceptible individuals. Biochem Biophys Res Commun. 2015;463:60–63. doi: 10.1016/j.bbrc.2015.05.017. [DOI] [PubMed] [Google Scholar]
- 17.Cheng HS, Besla R, Li A, Chen Z, Shikatani EA, Nazari-Jahantigh M, et al. Paradoxical Suppression of Atherosclerosis in the Absence of microRNA-146a. Circ Res. 2017;121:354–367. doi: 10.1161/CIRCRESAHA.116.310529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma S, Tian XY, Zhang Y, Mu C, Shen H, Bismuth J, et al. E-selectin-targeting delivery of microRNAs by microparticles ameliorates endothelial inflammation and atherosclerosis. Sci Rep. 2016:6. doi: 10.1038/srep22910. [DOI] [PMC free article] [PubMed]
- 19.Singh MV, Abboud FM. Toll-like receptors and hypertension. Am J Physiol - Regul Integr Comp Physiol. 2014;307:R501–R504. doi: 10.1152/ajpregu.00194.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maegdefessel L, Azuma J, Toh R, Deng A, Merk DR, Raiesdana A, et al. MicroRNA-21 Blocks Abdominal Aortic Aneurysm Development and Nicotine-Augmented Expansion. Sci Transl Med. 2012;4:122ra22–122ra22. doi: 10.1126/scitranslmed.3003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDonald RA, White KM, Wu J, Cooley BC, Robertson KE, Halliday CA, et al. miRNA-21 is dysregulated in response to vein grafting in multiple models and genetic ablation in mice attenuates neointima formation. Eur Heart J. 2013;34:1636–1643. doi: 10.1093/eurheartj/eht105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weber M, Baker MB, Moore JP, Searles CD. MiR-21 is induced in endothelial cells by shear stress and modulates apoptosis and eNOS activity. Biochem Biophys Res Commun. 2010;393:643–648. doi: 10.1016/j.bbrc.2010.02.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.John S, Schmieder RE. Potential mechanisms of impaired endothelial function in arterial hypertension and hypercholesterolemia. Curr Hypertens Rep. 2003;5:199–207. doi: 10.1007/s11906-003-0021-1. [DOI] [PubMed] [Google Scholar]
- 24.Boon RA, Iekushi K, Lechner S, Seeger T, Fischer A, Heydt S, et al. MicroRNA-34a regulates cardiac ageing and function. Nature. 2013;495:107–110. doi: 10.1038/nature11919. [DOI] [PubMed] [Google Scholar]
- 25.Han H, Qu G, Han C, Wang Y, Sun T, Li F, et al. MiR-34a, miR-21 and miR-23a as potential biomarkers for coronary artery disease: a pilot microarray study and confirmation in a 32 patient cohort. Exp Mol Med. 2015;47:e138. doi: 10.1038/emm.2014.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yin H, Pickering JG. Cellular Senescence and Vascular Disease: Novel Routes to Better Understanding and Therapy. Can J Cardiol. 2016;32:612–623. doi: 10.1016/j.cjca.2016.02.051. [DOI] [PubMed] [Google Scholar]
- 27.Daniel J-M, Penzkofer D, Teske R, Dutzmann J, Koch A, Bielenberg W, et al. Inhibition of miR-92a improves re-endothelialization and prevents neointima formation following vascular injury. Cardiovasc Res. 2014;103:564–572. doi: 10.1093/cvr/cvu162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lovren F, Pan Y, Quan A, Singh KK, Shukla PC, Gupta N, et al. MicroRNA-145 Targeted Therapy Reduces Atherosclerosis. Circulation. 2012;126:S81–S90. doi: 10.1161/CIRCULATIONAHA.111.084186. [DOI] [PubMed] [Google Scholar]
- 29.Desjarlais M, Dussault S, Dhahri W, Mathieu R, Rivard A. MicroRNA-150 Modulates Ischemia-Induced Neovascularization in Atherosclerotic Conditions. Arterioscler Thromb Vasc Biol. 2017;37:900–908. doi: 10.1161/ATVBAHA.117.309189. [DOI] [PubMed] [Google Scholar]
- 30.Park MY, Herrmann SM, Saad A, Widmer RJ, Tang H, Zhu X-Y, et al. Circulating and renal vein levels of microRNAs in patients with renal artery stenosis. Nephrol Dial Transplant. 2015;30:480–490. doi: 10.1093/ndt/gfu341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jones Buie JN, Goodwin AJ, Cook JA, Halushka PV, Fan H. The role of miRNAs in cardiovascular disease risk factors. Atherosclerosis. doi: 10.1016/j.atherosclerosis.2016.09.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cengiz M, Karatas OF, Koparir E, Yavuzer S, Ali C, Yavuzer H, et al. Differential Expression of Hypertension-Associated MicroRNAs in the Plasma of Patients With White Coat Hypertension. Medicine (Baltimore) 2015;94 doi: 10.1097/MD.0000000000000693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cengiz M, Yavuzer S, Avcı BK, Yürüyen M, Yavuzer H, Dikici SA, et al. Circulating miR-21 and eNOS in subclinical atherosclerosis in patients with hypertension. Clin Exp Hypertens. 2015;37:643–649. doi: 10.3109/10641963.2015.1036064. [DOI] [PubMed] [Google Scholar]
- 34.Marques FZ, Campain AE, Tomaszewski M, Zukowska-Szczechowska E, Yang YHJ, Charchar FJ, et al. Gene Expression Profiling Reveals Renin mRNA Overexpression in Human Hypertensive Kidneys and a Role for MicroRNAs. Hypertension. 2011;58:1093–1098. doi: 10.1161/HYPERTENSIONAHA.111.180729. [DOI] [PubMed] [Google Scholar]
- 35.Nagy ZB, Barták BK, Kalmár A, Galamb O, Wichmann B, Dank M, et al. Comparison of Circulating miRNAs Expression Alterations in Matched Tissue and Plasma Samples During Colorectal Cancer Progression. Pathol Oncol Res. 2017:1–9. doi: 10.1007/s12253-017-0308-1. [DOI] [PubMed] [Google Scholar]