Abstract
The lasting behavioral changes elicited by social signals provide important adaptations for survival of organisms that thrive as a group. Such adaptations have been understudied unlike the rapid innate responses to social cues. Here, the rodent models of the lasting socially induced behavioral changes are presented as either modulations or reinforcements of the distinct forms of learning and memory or non-associative changes of affective state. The purpose of this categorization is to draw attention to the potential mechanistic links between the neuronal pathways that process social cues and the neuronal systems that mediate the well-studied forms of learning and memory.
Keywords: social signals, behavioral modulation, learning and memory
1. Introduction: behavioral responses to social signals
Social signals, broadly defined as information transmitted between conspecifics, are the strongest drivers and modulators of behaviors in many organisms, particularly in those which thrive as a group. In rodents, such signals control mating (Ferrero et al., 2013; Haga et al., 2010; Kimchi, Xu, & Dulac, 2007), territorial aggression (Chamero et al., 2011; Chamero et al., 2007; Mandiyan, Coats, & Shah, 2005), and rearing the progeny (Tachikawa, Yoshihara, & Kuroda, 2013). They also inform about threats (Kiyokawa, 2017), resources (B. G. Galef, Jr., 1986), and social status (Kaur et al., 2014). They elicit helping behaviors (Ben-Ami Bartal, Decety, & Mason, 2011; Sato, Tan, Tate, & Okada, 2015) and aid in learning the skills needed for survival (Aisner & Terkel, 1992; Zohar & Terkel, 1991, 1995). Animal models of behavioral modulation by social signals provide an invaluable tool for identifying basic neuronal mechanisms of social communication that are conserved among many species, including humans.
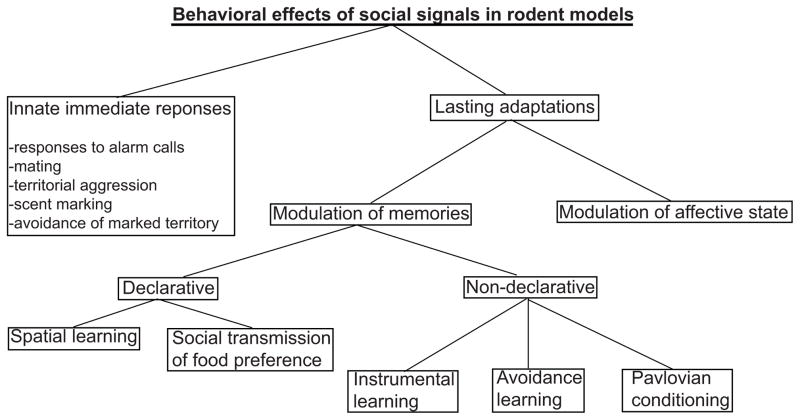
Based on when the socially induced behaviors emerge, they can be subdivided into two groups: immediate responses and lasting adaptations. The immediate responses are generally pre-programmed innate behaviors. These innate behaviors include defensive responses to alarm calls, mating with receptive female, aggression, retrieval of vocalizing pups, avoiding a territory marked by a male conspecific or scent marking. Many of these behaviors are regulated by specific olfactory and auditory signals or even by single molecules that activate distinct sensory pathways. For example, a mouse alarm pheromone, 2-sec-butyl-4,5-dihydrothiazole, activates the Grueneberg Ganglion neurons and thereby induces freezing in mice (Brechbuhl et al., 2013). The discoveries of several pheromones and their neuronal targets that trigger specific responses led to a rapid progress in the analyses of circuit mechanisms of the preprogrammed behaviors and this information is summarized in several excellent reviews (Fortes-Marco, Lanuza, & Martinez-Garcia, 2013; Martin-Sanchez et al., 2015; Stowers & Kuo, 2015; Stowers & Liberles, 2016). This review focuses on the second group of behaviors elicited by social signals - the lasting adaptations. Several forms of such adaptations have been described decades ago (Lubow & Moore, 1959; Zentall & Levine, 1972) but their mechanisms remain understudied, perhaps, because of a greater diversity and complexity than the mechanisms of the innate responses.
In this overview, such adaptations are presented as either modulations or reinforcements by social signals of the well-known forms of learning and memory (Fig. 1). This categorization is introduced to highlight the potential underlying neuronal mechanisms based on the extensive knowledge of the analogous forms of “asocial” learning and memory; however, it does not imply that the social learning phenomena involve exactly the same mechanisms that mediate the analogous forms of asocial learning (Heyes, 1994).
Fig. 1.
Summary of behavioral effects from social signals
The lasting adaptations discussed here include formation of declarative and non-declarative memories. The declarative memory paradigms include the spatial version of the Morris water maze, in which social signals enhance animals’ performance (Leggio et al., 2000), and the socially transmitted food preference, which is reinforced by social signals (B. G. Galef, Jr., 1986). Within the category of non-declarative learning, the instrumental conditioning and the avoidance learning, which involves both the Pavlovian and instrumental components, are modulated by social signals (Heyes, 1994; Kavaliers, Colwell, & Choleris, 2005; Masuda & Aou, 2009; Riess, 1972), whereas the classical Pavlovian conditioning can be either reinforced, as in the models of observational fear learning (Jeon et al., 2010) and fear conditioning by proxy (Bruchey, Jones, & Monfils, 2010), or modulated. The types of modulation include the social buffering, which attenuates fear learning, (Kikusui, Winslow, & Mori, 2006) and social facilitation, which enhances it (Ito, Erisir, & Morozov, 2015; Knapska, Mikosz, Werka, & Maren, 2010). Finally, social signals are powerful non-associative modulators of an affective state and can elicit anxiety-like or depression-like phenotypes in the rodent model of vicarious social defeat (Warren et al., 2013). The details and potential mechanisms of these effects are discussed below.
2. Modulation and reinforcement of explicit memories
2-1. Acceleration of spatial learning through observation
Spatial learning in the Morris water maze is strongly enhanced in rats that previously observed how other rats learned the task. Leggio et al., trained demonstrator rats in the spatial version of the maze while allowing the observer rats to watch 200 training trials during 10 daily sessions consisting of 4 trials each repeated 5 times over 5 consecutive days (Leggio et al., 2000). The observers had their cerebellum lesioned either before or after watching the training and were allowed to recover from surgery for 2 weeks prior to any tests; such lesions are known to impair the development of efficient search strategies (Petrosini, Molinari, & Dell’Anna, 1996). After watching the training of other rats, the observers themselves were trained in the Morris water maze. Despite watching how the demonstrators were escaping from water and sitting on the platform, the observers did not show any preference towards the platform location during initial training. Thus, the observation per se was not sufficient to learn the task. However, the observers who received the lesions after watching the demonstrators showed significantly faster decreases in the latency of finding the platform along the days of training than the animals lesioned prior to that. This experiment demonstrates that, by observing others, rats improve their performance in a spatial task via a cerebellum-dependent process. Since all the subjects in that study had cerebellar lesions, it remains to be determined whether the observational training improves the water maze learning in animals with intact brain. Interestingly, when a separate group of observers watched the training of the demonstrators that had cerebellar lesions and employed ineffective strategies in finding the platform, the observers’ performance was inferior to those watching the effective search. These findings suggest that the observers were learning how to solve the task rather than where the platform is located.
This notion that animals learn the strategy of solving the task is supported by a recent study on accelerated learning in the radial arms maze (Takano, Ukezono, Nakashima, Takahashi, & Hironaka, 2017). Takano et al. trained rats to search for sucrose pellets, which were placed in four arms of the 8 arm radial arms maze surrounded by spatial cues. The baited arms remained the same during the entire experiment, which consisted of 30 single daily trials. During each trial, a demonstrator rat was performing the task while an observer rat, placed in a small wire mesh cage in the center of the maze, was watching. Immediately after each demonstrator trial, the observer was trained in the same task. The performance of the observers always exceeded that of the demonstrators, indicating observational learning. Notably, the superior observers’ performance was found during the entire experiment including the very first trials when the demonstrators were using inefficient searching strategy. Thus, observing inefficient strategies does not always impair the observers’ performance, as seen in the water maze task (Petrosini et al., 1996), but may in some cases improve it.
While the described experiments argue that by watching another animal performing a spatial task, the observer is learning the strategy of how to solve the task rather than the spatial information about the target, a study by Mou and Ji (Mou & Ji, 2016) suggests that observing another animal can also form spatial maps in the form of hippocampal place cells. The observer rats were trained for 2 to 3 days by either watching from an observation box how the demonstrator rats were running for a food reward in a nearby linear track or by observing an empty track. During the following 7 to 15 days, the hippocampal CA1 cells were recorded from the observers while they were watching the demonstrators and also when they were running on that linear track themselves. In all rats, there were cells that were firing during the so called rotation events, when the observer, sitting in the observation box, was rotating his body clockwise or counterclockwise. Another group of cells were firing during the lap-running events when the observer was on the linear track. The authors found common cells between these two groups, but only in the animals that were trained by watching the demonstrators running the track. Interestingly, the identified cells were not specific to the demonstrator’s position on the track, but apparently, they did encode the space in the brain of the observer even before the observer was placed in that space. Notably, the effects could not be obtained when the live demonstrator was replaced with a toy-car running in the track, which indicates that social cues were required for creating those spatial maps. The findings strongly support the local enhancement theory, according to which the social cues from demonstrator enhance learning by increases animal attention to the task-relevant components of the environment (Heyes, 1994).
2-2. Social transmission of food preference
Acquiring preference to a particular food is an example of a highly adaptive form of social learning leading to explicit memory motivated by social cues. In this form of learning, social signals appear to act as a catalyst to learned safety or habituation of neophobia to the novel taste (Zentall, 2012). A series of studies have found that a naive observer rat can learn from a recently fed demonstrator rat the information about the food that was eaten by the demonstrator (B. G. Galef, 1983; B. G. Galef & Wigmore, 1983; Posadasandrews & Roper, 1983; Strupp & Levitsky, 1984).
In a set of experiments by Galef and colleagues, food preference was formed based entirely on the olfactory cues that were used to distinguish the foods (B. G. Galef, 1983; B. G. Galef & Wigmore, 1983; Posadasandrews & Roper, 1983). First, flavored diets were fed to demonstrator rats. During the exposure phase 30 min later, the observer rats were allowed to interact with demonstrators for 1 h. During the testing phase, the observers were offered a choice of two diets one of which was eaten by the demonstrator and another was completely unfamiliar. The observers exhibited high preference to the diet eaten by demonstrator and this preference remained for at least 12 h after the observer-demonstrator interaction.
In a slightly different paradigm developed by Strupp and Levitsky, the socially transmitted food preference lasted much longer - at least 5 days - and food was distinguished using both olfactory and visual cues (Strupp & Levitsky, 1984). During the exposure phase, the observer rats lived alongside the demonstrator separated by a transparent wire screen, which allowed for exchange of olfactory, visual and auditory cues. The exposure phase varied between 1 and 7 days. The demonstrator had continuous access to two differently flavored foods, one of which was adulterated with quinine sulfate, so the demonstrator did not eat it. Each food was always placed in a specific site in the cage - front or back. Meanwhile, the observer did not have an access to the food supplied to demonstrator and was fed with a non-flavored food, but it was able to receive olfactory cues from the demonstrator via the wired partition and see at which site the demonstrator was feeding. When offered the two flavored diets, the observer showed a clear preference for the food that their demonstrators were consuming. The preference was equally high after 1 or 7 days of exposure and persisted for 3 days when the exposure phase lasted 1 day and for 5 days when exposure phase lasted 2 days or longer. The same study revealed that visual cues were sufficient for the formation of diet preference. When olfactory cues distinguishing two diets were removed, the observer still formed a preference to the location where the demonstrator was feeding, although the preference was greater when both olfactory and visual differences between two diets were present.
The socially transmitted preferences are rather strong and can overcome earlier formed aversions and modify earlier formed preferences, which was demonstrated by Galef who, in one experiment, reversed a conditional taste eversion to a novel diet induced by intraperitoneal injection of LiCl, and, in another one, make rats choose less palatable and harder to handle diets (B. G. Galef, Jr., 1986).
The nature of social signals that cause these powerful effects have been determined by investigating the chemical composition of the rat’s breath, in which carbon disulfide was found to mediate the development of socially-induced diet preference in rats (B. G. Galef, Jr., Mason, Preti, & Bean, 1988). More recent studies revealed that a peptide uroguanilin, which is a ligand to the receptor guanylate cyclase type D and is present in urine and feces can promote food preference in mice (Arakawa, Kelliher, Zufall, & Munger, 2013). With the social transmission of food preference serving as an important adaptation, one could expect an opposing phenomenon when animals would learn from a poisoned conspecific not to ingest a toxic food. Several early studies suggested that LiCl-induced toxicosis could act as an unconditional stimulus in taste aversion (Lavin, Freise, & Coombes, 1980; Stierhoff & Lavin, 1982); however, the more recent works indicate that avoidance of potentially harmful foods cannot be transmitted between rodents (B. G. Galef, Jr., Wigmore, & Kennett, 1983; Jing, Zhou, & Xu, 2014).
3. Modulation and reinforcement of implicit memories
3-1. Social modulation of instrumental conditioning
3-1-1. Motivation vs observational learning
In their classical study of social facilitation of instrumental conditioning, Zental and Levine have found that water deprived rats learned to press a bar for a water reward more efficiently when they observed other rats performing the same task (Zentall & Levine, 1972). The enhanced learning theoretically could result from the motivational effect of the mere conspecific presence or from learning the behavior by observing it. To distinguish between these mechanisms, the subjects were trained without a demonstrator or using the demonstrators with one of the 3 behavioral patterns: 1) instrumental and consummatory (bar-pressing and drinking), 2) consummatory only (bar-pressing), 3) neither instrumental nor consummatory. The demonstrators with both instrumental and consummatory responses enhanced learning in the observers more strongly than the demonstrators with the consummatory responses only, whereas the presence of demonstrators that did not bar-press or drink, impaired the learning. Thus, “learning by rats was facilitated when response-relevant cues were provided by other rats” (Zentall & Levine, 1972), suggesting that social enhancement of instrumental learning is achieved via observation of the task-relevant responses. Meanwhile, the presence of another animal per se has an inhibitory effect on learning, possibly by distracting attention from the task.
3-1-2. Familiarity between demonstrator and observer inhibits observational learning
Possible effects of demonstrator on attention as a social modulator of instrumental learning was proposed by Saggerson and Honey who examined how the familiarity between the demonstrator and observer affects observational learning in an instrumental discrimination task, in which pulling a chain in response to one discriminative stimulus but not another (1 kHz tone vs 10 Hz clicker) was reinforced by food (Saggerson & Honey, 2006). In the first version of the task, demonstrator rats were trained to pull one of two chains, depending on the auditory stimulus presented. In the second version, rats learned to pull a single chain when one of the two auditory stimuli was presented. The observer rats were allowed to watch a demonstrator successfully perform the task during 8 to 10 daily sessions consisting of 10 trials each. When tested later in the same task, the observers that watched unfamiliar demonstrators, showed a better discrimination than those who watched familiar demonstrators (Saggerson & Honey, 2006). The authors attribute the inferior learning with the familiar demonstrator to the process akin latent inhibition (Lubow & Moore, 1959), in which a lower level of attention towards the familiar demonstrator results in the poor learning of its actions during the task.
3-1-3. Learning a procedural sequence can be socially facilitated in mice
While most rodent studies on social modulation of instrumental learning were conducted in rats, the ability of mice to learn a complex instrumental task was demonstrated by Carlier and Jamon (Carlier & Jamon, 2006). The task utilized a “puzzle box”, which required a conditional sequence of actions in specific locations in order to obtain a piece of dry cookie as a reward. First, the mouse had to go to the side of the box and push a metal tab, which made the food fall into a drawer. Next, the mouse had to go to the front of the box and open the drawer. The subjects watched successful performances of the task by trained demonstrators during 14 daily sessions repeated over 5 consecutive days. Prior to this training, none of the subjects could solve the task, whereas after the observational training, 6 out 15 observers solved the task and the remaining observers required fewer trials than naive mice to learn the same task via a non-observational training.
3-2. Social modulation and reinforcement of aversive learning
3-2-1. Social modulation of avoidance learning
The earliest evidence that the rat can learn passive avoidance through observation was provided by Lore et al. (Lore, Blanc, & Suedfeld, 1971). After the naive observer rats were exposed to the demonstrator rats learning to avoid a lighted candle, they learned to avoid the candle faster than control animals. However, later studies revealed that the effectiveness of social modulation in avoidance tasks is highly dependent on the specific paradigm and also on the animal’s experience.
3-2-1-a. A prior experience is required for social modulation of inhibitory avoidance in the shuttle box
Masuda and Aou examined social modulation of inhibitory avoidance as a function of the animal’s experience in the avoidance paradigm (Masuda & Aou, 2009). The “experienced” rats were, first, individually trained to avoid the dark compartment of the shuttle box. When tested for memory retention in the presence of a partner, they showed an attenuated avoidance memory (a shorter latency to cross into the dark compartment) than without a partner. The attenuation occurred even in the presence of experienced partners, who avoided the dark compartment, although it was higher with non-experienced partners. These experiments show that a mere presence of a partner, even experienced, reduces fear or entering the compartment where shock is administered, which can be explained by social buffering discussed in section 3-2-4.
The inexperienced subjects crossed to the dark compartment regardless the presence of a partner. Importantly, after witnessing the demonstrators being shocked after crossing, the inexperienced observers did not increase their crossing latencies. Thus, social information alone did not elicit avoidance. However, it did enhance it, when the subject had experience in passive avoidance. In a second experiment, the experienced subjects were habituated to the dark compartment to extinguish avoidance memories, and then were allowed to watch how their partners were shocked after crossing into dark compartment. It reinstated their avoidance behavior. Thus, social signals could both attenuate and reinstate avoidance behaviors and the anxiolytic effects of social signals from partner, even distressed, compete with the anxiogenic effects, while the interpretation of distress signals during inhibitory avoidance requires a prior experience.
3-2-1-b. Observational learning to avoid flies by burying does not require a prior experience and depends on the observer-demonstrator relationship
An ethologically relevant avoidance paradigm in which mice learn to defend themselves against natural micro-predators, stable flies Stomoxys calcitrans, have been used in deer mice and laboratory mice. Kavaliers and colleagues have shown that avoidance responses to the surgically altered flies, which no longer bite, do not occur unless the mouse had been conditioned to avoid the flies and that conditioning could be obtained vicariously, through observation of a demonstrator animal avoiding the bites (Kavaliers, Colwell, & Choleris, 2001, 2003; Kavaliers, Colwell, Choleris, & Ossenkopp, 1999). During vicarious training, the subject mice were observing another mouse been bitten by flies. To avoid the attacks, the demonstrators were burying themselves under the bedding. At that time, the observers did not show any defensive responses. During testing 1 to 3 days later, the observers were exposed to the biting flies that had their mouthpart removed, so they could no longer bite the mouse. The self-burying response, measured as the time the animal completely buried itself under the bedding, was taken as the measure of avoidance learning. The observers learned the task even though they never experienced biting and did not show defensive responses during training.
The relationship between the observer and demonstrator was found to be an important factor for observational learning (Kavaliers et al., 2005). The learning in the littermate observer-demonstrator pairs was better than in non-kin pairs, despite the animals were housed individually prior to the experiment for over 120 days. The dominance was found to be the critical parameter as well. In the observer-demonstrator pairs housed together, the subordinate observers learned better from dominant demonstrators than in the reverse configuration, white the effect of familiarity was less significant.
3-2-1-c. Extinction of avoidance is enhanced by social signals
Besides modulating acquisition if avoidance, social signals were also found to modulate its extinction (Baum, 1969). The subject rats were first trained to avoid electrical footshocks by jumping from a grid floor on a safety ledge. Each trail began with the subject rat placed on the ledge, which was retracted to drop the rat on the floor and then returned to allow escape. Next, the rats were trained to extinguish the learned jumping responses: the safety ledge was removed, so the rats were forced to remain on the floor in the absence of shocks. When a non-fearful companion rat was present on the floor, the extinction was more effective.
3-2-2. Fear conditioning via observation of fear learning
Most forms of learning described above, except for the social transmission of food preference, are either enhanced or attenuated by social signals, which act as modulators of association between non-social stimuli. Potential mechanisms of those effects include changing animal’s motivation, drawing subject’s attention to the place (local enhancement), object (stimulus enhancement) or reinforcing stimulus, and have been reviewed (B. G. Galef, 1988; Heyes, 1994; Zentall, 2012).
In another set of paradigms, the social signals from demonstrators act not only as a modulator of learning but as the unconditional stimulus. The term “observational conditioning” for such paradigms was originally introduced to describe experiments in monkeys, in which the subject acquired fear of snakes by observing fearful behavior of a conspecific exposed to a snake (Cook, Mineka, Wolkenstein, & Laitsch, 1985; Mineka, Davidson, Cook, & Keir, 1984). Yet, several studies indicate that in rodents social signals can also act as an aversive unconditional stimulus, although less effective than electrical footshocks. The formation of contextual fear memory after observing fear in mice was first demonstrated by Jeon et al. (Jeon et al., 2010). The subject observer mice were trained by watching, via a transparent partition, the demonstrator mouse receiving repeated electrical footshocks. When returned to the training box 24 h after the exposure, the observers exhibited freezing. When visual inputs were blocked during training by replacing the transparent partition with an opaque one, the contextual fear learning was attenuated but not abolished. It indicated that, in addition to vision, multiple sensory modalities convey the information about fear in the demonstrator. As in the vicarious learning to avoid biting flies, the social relationship between the animals was an important factor. The freezing was stronger in siblings than in stranger animals. Also, when a female mating partner was used as demonstrator, the freezing was stronger with the females that lived with the observer for a long time. Evidence that oxytocin participates in the perception of distress on conspecific were provided by Pisansky et al., who demonstrated that intranasal oxytocin administration and chemogenetic stimulation of oxytocin neurons made mice more sensitive to distress in a stranger conspecific (Pisansky, Hanson, Gottesman, & Gewirtz, 2017).
The formation of cued fear memories by observation of tone-footshock pairing administered to a conspecific has been demonstrated in mice where the memories were found to be more robust in the gregarious C57BL/6J strain than in the less social BALB/cJ mice (Chen, Panksepp, & Lahvis, 2009). In rats, such conditioning was found to be impaired by social isolation (Yusufishaq & Rosenkranz, 2013).
3-2-3. Fear conditioning by proxy (via observation of fear retrieval)
A paradigm, in which the observer rats form fear memory of an auditory signal reinforced exclusively by social cues from the previously conditioned demonstrator rat exposed to CS, was named “fear conditioning by proxy” (Bruchey et al., 2010). In contrast to the “observation of fear learning”, in which demonstrators express unconditioned responses to footshocks in front of the observers, in this paradigm the demonstrators express only the conditioned responses, which is expected to produce a different pattern of social cues.
At first, the demonstrator rats were conditioned in a classical Pavlovian paradigm, in which a tone was paired with an electrical footshock, repeated 3 times. Twenty four hours later, the observer subjects were exposed to the demonstrators while the three tones were playing and the demonstrators were expressing fear. When tested 24 h later in the presence of the same tones and in the same chamber, the subject exhibited, on average, a significantly higher level of freezing than the naive control rats. However, the mean freezing was very modest, about 10%, and half the animals did not freeze at all. The fear conditioning by proxy was replicated in females, which showed a freezing to the tone at above 60% (Jones, Riha, Gore, & Monfils, 2014), which was noticeably higher than in the earlier study with males (Bruchey et al., 2010). Interestingly, the levels of freezing during testing did not correlate with the amount of freezing in demonstrators. It suggests that the demonstrators that freeze less still express fear and emit social cues that act as a reinforcement contingency for the observer. Meanwhile, the freezing during testing did correlate with the duration of social interaction between subject and demonstrator during training, suggesting that social relationship between animals may influence the effectiveness of observational learning. This was confirmed in a follow-up study, in which the hierarchical relationship between animals defined by their dominance status was found to predict social fear transmission. Jones and Monfils found a robust transmission of fear from dominant to subordinate rat, but not in the opposite direction (Jones & Monfils, 2016).
3-2-4. Social buffering of learned fear
The phenomenon of “social buffering” in many species is broadly defined as a better recovery from stress (Hennessy, Kaiser, & Sachser, 2009; Kikusui et al., 2006). The types of social buffering and its dependency on the species’ social organization, sex and developmental stage and the status of the individual animal within a social unit are thoroughly reviewed elsewhere (Hennessy et al., 2009; Kiyokawa, 2017). The examples discussed here include social buffering of learned fear in adult rodents. They show how social signals can buffer fear when presented not only during the retrieval of fear memory, but also before fear learning and in the home cage during the time between fear training and testing.
The first evidence of social buffering of learned fear in rats was provided by Davitz and Mason, who observed the buffering effect during retrieval of fear memories (Davitz & Mason, 1955). Rats were fear conditioned by repeated exposures to a 2 sec blinking light followed a 3 sec footshock and then tested for fear of light either individually or in the presence of a companion rat. The amount of activity was used as an index of fear. Some companion rats were previously conditioned to be fearful of light whereas others were not. The non-fearful rats significantly attenuated fear expression in the subjects. Remarkably, the fearful rats influenced behavior in the same direction, although the effect did not reach significance. The authors explain the attenuation by distracting the subject from fearful context and triggering exploratory drive.
The role of fear expression by demonstrator in the buffering of contextual fear was examined by Kiyokawa et al. (Kiyokawa, Kikusui, Takeuchi, & Mori, 2004). The subject rats were tested for memory recall alone or in the presence of the fearful or non-fearful partners. Two indices of fear were used: freezing for behavioral response and hyperthermia for autonomic response. The presence of a partner decreased both measures of fear but the decreases were greater with the non-fearful partners. These findings are consistent with the earlier observations by Davitz and Mason that even fear-expressing partners buffer fear, but less effectively that the non-fearful ones.
A series of more recent studies by Kiyokawa and colleagues distinguished two forms of social buffering in experiment on auditory fear conditioning. Defined by the time of exposure to conspecific, they were named “pair-housing” and “pair-exposure” (Kiyokawa, 2017; Kiyokawa, Takeuchi, & Mori, 2007). In the pair housing, the subject is housed with a conspecific after fear conditioning training. In the pair exposure, the conspecific is present during fear memory retrieval. Both paradigms attenuated expression of fear but via different mechanisms: the pair-housing attenuated autonomic responses, measured as hypothermia but had no effect on freezing. Conversely, the pair-exposure eliminated freezing but had no effect on hypothermia. When pair-housing and pair-exposure were combined, both forms of fear expression were suppressed. Notably, in these experiments the buffering was achieved using unfamiliar rats (Kiyokawa et al., 2007). The selective effect of pair exposure on freezing appears specific only in the cued fear conditioning paradigm: during the recall of contextual fear, it also attenuated the autonomic responses (Kiyokawa et al., 2004).
The buffering effects of social cues presented prior to training was demonstrated by Guzman et al. in experiments on contextual fear conditioning (Guzman et al., 2009). The observer mice were pre-exposed to the training context in the presence of either fearful or non-fearful demonstrators or without another mouse for two consecutive days. The fearful demonstrators were previously conditioned to freeze in that context. One day after the pre-exposure, the observers were trained alone by a 3 min exposure to the same context followed by a single 2 s 0.7 mA footshock. The long-term memory test 24 h after conditioning revealed an attenuation of contextual freezing in the observers that were pre-exposed to the non-fearful demonstrators, but there was no attenuation of the short-term memory tested 30 min after conditioning. The buffering effect was long-lasting: the attenuation remained even when animals underwent contextual training 10 days after the pre-exposure. The buffering was also context-dependent: there was no attenuation of fear learning in a different context. These data suggest that an associative learning of safety rather than a non-specific fear reduction is responsible for this buffering effect. Notably, the pre-exposure to a fearful demonstrator neither enhanced contextual fear no attenuated it, which suggests that the social signals of distress and safety may cancel the effects of one another.
Search for the neuronal substrates of social buffering provided evidence that olfaction is its main sensory pathway: 1) an appeasing pheromone that decreases the heart rate in rats was found to be secreted by the neck region in male rats (Kiyokawa, Kikusui, Takeuchi, & Mori, 2005); the inactivation of olfactory epithelium with ZnSO4 was found to abolish social buffering of fear in rats (Kiyokawa, Takeuchi, Nishihara, & Mori, 2009); the disconnection of the olfactory peduncle and the amygdala also impaired social buffering (Kiyokawa, Wakabayashi, Takeuchi, & Mori, 2012). Besides olfaction, non-noxious tactile stimulation was proposed to mediate some effects of social buffering (Kikusui et al., 2006). The neuronal and endocrine mechanisms of social buffering were reviewed in (Hennessy et al., 2009; Kikusui et al., 2006) and involve reduction in the corticosterone response to stress and recruitment of oxytocin (Armario, Luna, & Balasch, 1983; Kikusui et al., 2006). At the level of neuronal activity in the basolateral amygdala, the buffering of cued fear conditioning coincided with a reduction in the CS-induced local field potentials, gamma oscillations (25–75 Hz) and high frequency oscillations (100–300 Hz) (Fuzzo et al., 2015).
3-2-5. Social facilitation of learned fear
Paradoxically, while distressed conspecifics can buffer fear in several paradigms discussed above, they can also facilitate fear learning. This paradox is further discussed in section 3-2-6. The immediate effects on learning in the two-way avoidance and contextual fear conditioning paradigms in rats were documented by Knapska et al. (Knapska et al., 2010). The demonstrators were distressed by electrical footshocks and then were allowed to interact with the observers for 10 minutes in the home cages. The control observers interacted with non-shocked demonstrators. Immediately afterwards, the observers were trained in a shuttle box to avoid electrical footshocks signaled by an auditory stimulus or underwent contextual fear conditioning. The efficiency of avoidance learning measured by the number of avoidance responses were higher in the observers that interacted with the fear conditioned demonstrators than with controls. Similarly, such observers showed enhanced retrieval of contextual fear memories.
The lasting effects of interaction with distressed conspecific on inhibitory avoidance in mice were found by Ito et al. (Ito et al., 2015). The observer animals were exposed to the cage-mate demonstrators while they were receiving repeated electrical footshocks for 4 minutes in the procedure, which was originally employed for the observational fear conditioning described in 3-2-2 (Jeon et al., 2010). During the exposure, the animals were separated by a transparent and perforated Plexiglas partition, which allowed transmission of visual, olfactory and auditory signals. In the control procedure, the observers were exposed to the non-shocked demonstrators. The next day, the observers were trained in the inhibitory avoidance paradigm and tested 24 h later. The observers exposed to shocked demonstrators shower a higher retention of the inhibitory avoidance memory and also formed silent synapses in the prefrontal-amygdala pathway, which may be responsible for the enhanced inhibitory avoidance learning (Ito et al., 2015). While there was no direct comparison of the social distress procedures employed by Knapska and Ito in the same species, the number and amounts of distress signals emitted by demonstrators during the footshocks are expected to be greater than immediately after the footshocks. This may explain the longer duration of the effect observed by Ito.
3-2-6. On competition between social buffering and social facilitation of fear
Social effects on fear include both buffering and facilitation. While only distressed demonstrators can facilitate fear in other animals, the buffering can be obtained by the exposure to either distressed or non-distressed animal. Then, why signals from distressed animals suppress fear in some paradigms, but enhance it in others? Studies on the nature of social signals indicate that rodents emit both the alarm pheromones, which is secreted by the perianal region in the rats, and an appeasing pheromone, which is secreted by the neck region of the rat skin but whose nature remains unknown (Kiyokawa et al., 2005). The interpretation of these opposing signals by the observer is likely determined by the context of the behavioral paradigm. In most cases of fear buffering, the demonstrator is introduced during acquisition or retention of fear memories (Davitz & Mason, 1955; Kiyokawa et al., 2004; Kiyokawa et al., 2007) or the interaction with demonstrator occurs before learning but in the learning context (Guzman et al., 2009). In such conditions, the buffering may result from a disruption of aversive learning via distraction of animals’ attention from the conditional stimuli or by a process in which appeasing signals from conspecific reinforce a safety perception of the training context. In contrast, the fear enhancement was obtained by exposure to demonstrator prior to fear learning and in a completely different context (Ito et al., 2015; Knapska et al., 2010). Such exposure does not distract from fear learning at a later time but the aversive social cues are likely to arouse the animal and thereby facilitate subsequent fear learning.
Another example of a possible competition between aversive and appeasing social signals is fear conditioning by proxy, which uses the same procedure as social buffering during testing (Bruchey et al., 2010; Jones & Monfils, 2016; Jones et al., 2014). Because of the buffering effects from the naive subject which include elimination of freezing and attenuation of autonomic responses to the CS in fear conditioned rats (Kiyokawa & Takeuchi, 2017; Kiyokawa et al., 2007), the expression of fear in the demonstrator during CS presentation must be strongly attenuated. This attenuation is expected to diminish the amount of distress signals emitted by the demonstrators and thereby interfere with the conditioning by proxy. However, the buffering effects seem variable: in another study, the presence of a naive rat did not abolish freezing to the CS (Kim, Kim, Covey, & Kim, 2010). Thus the low efficiency of fear conditioning by proxy can be explained in part by the buffering, whereas its high variability could result from the variance of the buffering effects among pairs of animals.
The neurons that mediate the competition between aversive and appeasing social signals remain largely unknown but a recent study possibly identified one group of such cells by demonstrating that glutamatergic inputs to the hypothalamic corticotropin-releasing hormone neurons acquire a short-term plasticity in mice that receive electrical footshocks or interact with a distressed conspecific whereas a subsequent interaction with a naive conspecific reverses those synaptic changes, although only in females (Sterley et al., 2018).
3-3. Modulation of affective states
Besides participation in the formation of specific memories, social signals are strong modulators of emotional state, which can be evaluated by quantifying behavioral traits relevant to depression and anxiety. A robust mouse model of such modulation is the vicarious social defeat introduced by Warren et al. (Warren et al., 2013). Once per day for 10 days, the observer C57BL/6J mouse is allowed to watch for 10 min how another C57BL/6J mouse is attacked by a stronger CD-1 aggressor, which is followed by continuous housing next to another CD1 male separated by a transparent perforated partition. The behavioral consequences included a reduction in sociability, or the preference to approach a mouse rather than empty cup, a decrease in % time spent in the open arms of elevated plus maze and an increased immobility in the forced swim test. These effects were long-lasting and detectable at 24 h and 1 month after the procedure. In addition, the animals exhibited anhedonia measured as a decrease in sucrose preference 1 month but not 24 h later. The study provided evidence that distress elicited by social signals is very similar to that from a physical trauma. Recently, the effectiveness of the vicarious defeat procedure was demonstrated in females, which were observing CD1 males attacking C57BL/6J males. The effects were tested at 24 h after the procedure and included reduced sociability, decreased time in the open arms of elevated plus maze, decreased sucrose preference and increased immobility in the tail suspension test (Iniguez et al., 2018).
4. Future directions
The circuit mechanisms of the long-lasting adaptations reviewed here remain to be determined. Since most of them involve formation of well-studied types of memory, one way to study the mechanisms of social modulation is to identify the links between the sensory pathways that receive and transmit social information and the memory circuits known from the well-studied models of “asocial” learning. Particularly interesting are potential links to the neuronal systems of reward and punishment, because many forms of socially-driven learning lack typical behavioral reinforcers like palatable food or electrical footshock. Instead, the social signals themselves act as reinforcers and it would be interesting to determine how they are encoded to become analogues of unconditional stimuli. Recent studies have identified neurons and molecules in the high brain structure that respond to social signals and modulate social behaviors (Brumback et al., 2017; Hung et al., 2017; Li et al., 2017; Nasanbuyan et al., 2017; Remedios et al., 2017; Yao, Bergan, Lanjuin, & Dulac, 2017). The next challenge would be to understand how those cells are recruited by social signals and what their effectors-targets are.
Significance statement.
Rodent models of social modulation of behaviors, some of which have been established decades ago, provide an invaluable resource for investigating what makes social organisms thrive as a group. This overview explores the behavioral paradigms in which social cues produce lasting effects on cognition and emotions by modulating the well-known memory systems. These models are understudied and are waiting to be interrogated by the recently developed tools for the functional analyses of neuronal circuits. They are expected to inform about mechanistic links between the sensory pathways that process social signals and the memory systems that underlie cognition and emotions.
Acknowledgments
This work was funded in part by NIH grant R21MH107970.
Literature Cited
- Aisner R, Terkel J. Ontogeny of Pine-Cone Opening Behavior in the Black Rat, Rattus-Rattus. Animal Behaviour. 1992;44(2):327–336. doi: 10.1016/0003-3472(92)90038-B. [DOI] [Google Scholar]
- Arakawa H, Kelliher KR, Zufall F, Munger SD. The Receptor Guanylyl Cyclase Type D (GC-D) Ligand Uroguanylin Promotes the Acquisition of Food Preferences in Mice. Chemical Senses. 2013;38(5):391. doi: 10.1093/chemse/bjt015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armario A, Luna G, Balasch J. The effect of conspecifics on corticoadrenal response of rats to a novel environment. Behav Neural Biol. 1983;37(2):332–337. doi: 10.1016/s0163-1047(83)91425-5. [DOI] [PubMed] [Google Scholar]
- Baum M. Extinction of an avoidance response motivated by intense fear: social facilitation of the action of response prevention (flooding) in rats. Behav Res Ther. 1969;7(1):57–62. doi: 10.1016/0005-7967(69)90049-7. [DOI] [PubMed] [Google Scholar]
- Ben-Ami Bartal I, Decety J, Mason P. Empathy and pro-social behavior in rats. Science. 2011;334(6061):1427–1430. doi: 10.1126/science.1210789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brechbuhl J, Moine F, Klaey M, Nenniger-Tosato M, Hurni N, Sporkert F, … Broillet MC. Mouse alarm pheromone shares structural similarity with predator scents. Proc Natl Acad Sci U S A. 2013;110(12):4762–4767. doi: 10.1073/pnas.1214249110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruchey AK, Jones CE, Monfils MH. Fear conditioning by-proxy: social transmission of fear during memory retrieval. Behav Brain Res. 2010;214(1):80–84. doi: 10.1016/j.bbr.2010.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brumback AC, Ellwood IT, Kjaerby C, Iafrati J, Robinson S, Lee AT, … Sohal VS. Identifying specific prefrontal neurons that contribute to autism-associated abnormalities in physiology and social behavior. Mol Psychiatry. 2017 doi: 10.1038/mp.2017.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier P, Jamon M. Observational learning in C57BL/6j mice. Behav Brain Res. 2006;174(1):125–131. doi: 10.1016/j.bbr.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Chamero P, Katsoulidou V, Hendrix P, Bufe B, Roberts R, Matsunami H, … Leinders-Zufall T. G protein G alpha o is essential for vomeronasal function and aggressive behavior in mice. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(31):12898–12903. doi: 10.1073/pnas.1107770108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamero P, Marton TF, Logan DW, Flanagan K, Cruz JR, Saghatelian A, … Stowers L. Identification of protein pheromones that promote aggressive behaviour. Nature. 2007;450(7171):899–U823. doi: 10.1038/nature05997. [DOI] [PubMed] [Google Scholar]
- Chen Q, Panksepp JB, Lahvis GP. Empathy is moderated by genetic background in mice. PLoS One. 2009;4(2):e4387. doi: 10.1371/journal.pone.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M, Mineka S, Wolkenstein B, Laitsch K. Observational conditioning of snake fear in unrelated rhesus monkeys. J Abnorm Psychol. 1985;94(4):591–610. doi: 10.1037//0021-843x.94.4.591. [DOI] [PubMed] [Google Scholar]
- Davitz JR, Mason DJ. Socially facilitated reduction of a fear response in rats. J Comp Physiol Psychol. 1955;48(3):149–151. doi: 10.1037/h0046411. [DOI] [PubMed] [Google Scholar]
- Ferrero DM, Moeller LM, Osakada T, Horio N, Li Q, Roy DS, … Liberles SD. A juvenile mouse pheromone inhibits sexual behaviour through the vomeronasal system. Nature. 2013;502(7471):368–371. doi: 10.1038/nature12579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortes-Marco L, Lanuza E, Martinez-Garcia F. Of Pheromones and Kairomones: What Receptors Mediate Innate Emotional Responses? Anatomical Record-Advances in Integrative Anatomy and Evolutionary Biology. 2013;296(9):1346–1363. doi: 10.1002/ar.22745. [DOI] [PubMed] [Google Scholar]
- Fuzzo F, Matsumoto J, Kiyokawa Y, Takeuchi Y, Ono T, Nishijo H. Social buffering suppresses fear-associated activation of the lateral amygdala in male rats: behavioral and neurophysiological evidence. Front Neurosci. 2015;9:99. doi: 10.3389/fnins.2015.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galef BG. Utilization by Norway Rats (R-Norvegicus) of Multiple Messages Concerning Distant Foods. Journal of Comparative Psychology. 1983;97(4):364–371. doi: 10.1037//0735-7036.97.4.364. [DOI] [Google Scholar]
- Galef BG. Imitation in animals: history, definition and interpretation of data from the psychological laboratory. In: Zentall TR, editor. Social Learning :Psychological and Biological Perspectives. Hillsdale, NJ: Erlbaum; 1988. pp. 3–28. [Google Scholar]
- Galef BG., Jr Social interaction modifies learned aversions, sodium appetite, and both palatability and handling-time induced dietary preference in rats (Rattus norvegicus) Journal of Comparative Psychology. 1986;100(4):432–439. [PubMed] [Google Scholar]
- Galef BG, Jr, Mason JR, Preti G, Bean NJ. Carbon disulfide: a semiochemical mediating socially-induced diet choice in rats. Physiol Behav. 1988;42(2):119–124. doi: 10.1016/0031-9384(88)90285-5. [DOI] [PubMed] [Google Scholar]
- Galef BG, Jr, Wigmore SW, Kennett DJ. A failure to find socially mediated taste aversion learning in Norway rats (R. norvegicus) Journal of Comparative Psychology. 1983;97(4):358–363. [PubMed] [Google Scholar]
- Galef BG, Wigmore SW. Transfer of Information Concerning Distant Foods - a Laboratory Investigation of the Information-Center Hypothesis. Animal Behaviour. 1983 Aug;31:748–758. doi: 10.1016/S0003-3472(83)80232-2. [DOI] [Google Scholar]
- Guzman YF, Tronson NC, Guedea A, Huh KH, Gao C, Radulovic J. Social modeling of conditioned fear in mice by non-fearful conspecifics. Behav Brain Res. 2009;201(1):173–178. doi: 10.1016/j.bbr.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haga S, Hattori T, Sato T, Sato K, Matsuda S, Kobayakawa R, … Touhara K. The male mouse pheromone ESP1 enhances female sexual receptive behaviour through a specific vomeronasal receptor. Nature. 2010;466(7302):118–122. doi: 10.1038/nature09142. [DOI] [PubMed] [Google Scholar]
- Hennessy MB, Kaiser S, Sachser N. Social buffering of the stress response: diversity, mechanisms, and functions. Front Neuroendocrinol. 2009;30(4):470–482. doi: 10.1016/j.yfrne.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Heyes CM. Social learning in animals: categories and mechanisms. Biol Rev Camb Philos Soc. 1994;69(2):207–231. doi: 10.1111/j.1469-185x.1994.tb01506.x. [DOI] [PubMed] [Google Scholar]
- Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, … Malenka RC. Gating of social reward by oxytocin in the ventral tegmental area. Science. 2017;357(6358):1406–1411. doi: 10.1126/science.aan4994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez SD, Flores-Ramirez FJ, Riggs LM, Alipio JB, Garcia-Carachure I, Hernandez MA, … Castillo SA. Vicarious Social Defeat Stress Induces Depression-Related Outcomes in Female Mice. Biol Psychiatry. 2018;83(1):9–17. doi: 10.1016/j.biopsych.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito W, Erisir A, Morozov A. Observation of Distressed Conspecific as a Model of Emotional Trauma Generates Silent Synapses in the Prefrontal-Amygdala Pathway and Enhances Fear Learning, but Ketamine Abolishes those Effects. Neuropsychopharmacology. 2015 doi: 10.1038/npp.2015.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon D, Kim S, Chetana M, Jo D, Ruley HE, Lin SY, … Shin HS. Observational fear learning involves affective pain system and Cav1.2 Ca2+ channels in ACC. Nat Neurosci. 2010;13(4):482–488. doi: 10.1038/nn.2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing L, Zhou QX, Xu L. Avoidance of potentially harmful food cannot be socially transmitted between rats. Dongwuxue Yanjiu. 2014;35(4):256–261. doi: 10.13918/j.issn.2095-8137.2014.4.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CE, Monfils MH. Dominance status predicts social fear transmission in laboratory rats. Anim Cogn. 2016;19(6):1051–1069. doi: 10.1007/s10071-016-1013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CE, Riha PD, Gore AC, Monfils MH. Social transmission of Pavlovian fear: fear-conditioning by-proxy in related female rats. Anim Cogn. 2014;17(3):827–834. doi: 10.1007/s10071-013-0711-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur AW, Ackels T, Kuo TH, Cichy A, Dey S, Hays C, … Stowers L. Murine pheromone proteins constitute a context-dependent combinatorial code governing multiple social behaviors. Cell. 2014;157(3):676–688. doi: 10.1016/j.cell.2014.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavaliers M, Colwell DD, Choleris E. NMDA-mediated social learning of fear-induced conditioned analgesia to biting flies. Neuroreport. 2001;12(4):663–667. doi: 10.1097/00001756-200103260-00009. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Colwell DD, Choleris E. Learning to fear and cope with a natural stressor: individually and socially acquired corticosterone and avoidance responses to biting flies. Horm Behav. 2003;43(1):99–107. doi: 10.1016/s0018-506x(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Kavaliers M, Colwell DD, Choleris E. Kinship, familiarity and social status modulate social learning about “micropredators” (biting flies) in deer mice. Behavioral Ecology and Sociobiology. 2005;58(1):60–71. doi: 10.1007/s00265-004-0896-0. [DOI] [Google Scholar]
- Kavaliers M, Colwell DD, Choleris E, Ossenkopp KP. Learning to cope with biting flies: rapid NMDA-mediated acquisition of conditioned analgesia. Behav Neurosci. 1999;113(1):126–135. [PubMed] [Google Scholar]
- Kikusui T, Winslow JT, Mori Y. Social buffering: relief from stress and anxiety. Philos Trans R Soc Lond B Biol Sci. 2006;361(1476):2215–2228. doi: 10.1098/rstb.2006.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim EJ, Kim ES, Covey E, Kim JJ. Social transmission of fear in rats: the role of 22-kHz ultrasonic distress vocalization. PLoS One. 2010;5(12):e15077. doi: 10.1371/journal.pone.0015077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi T, Xu J, Dulac C. A functional circuit underlying male sexual behaviour in the female mouse brain. Nature. 2007;448(7157):1009–1014. doi: 10.1038/nature06089. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y. Social Odors: Alarm Pheromones and Social Buffering. Curr Top Behav Neurosci. 2017;30:47–65. doi: 10.1007/7854_2015_406. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Partner’s stress status influences social buffering effects in rats. Behav Neurosci. 2004;118(4):798–804. doi: 10.1037/0735-7044.118.4.798. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Alarm pheromone that aggravates stress-induced hyperthermia is soluble in water. Chemical Senses. 2005;30(6):513–519. doi: 10.1093/chemse/bji044. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Takeuchi Y. Social buffering ameliorates conditioned fear responses in the presence of an auditory conditioned stimulus. Physiol Behav. 2017;168:34–40. doi: 10.1016/j.physbeh.2016.10.020. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Takeuchi Y, Mori Y. Two types of social buffering differentially mitigate conditioned fear responses. Eur J Neurosci. 2007;26(12):3606–3613. doi: 10.1111/j.1460-9568.2007.05969.x. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Takeuchi Y, Nishihara M, Mori Y. Main olfactory system mediates social buffering of conditioned fear responses in male rats. European Journal of Neuroscience. 2009;29(4):777–785. doi: 10.1111/j.1460-9568.2009.06618.x. [DOI] [PubMed] [Google Scholar]
- Kiyokawa Y, Wakabayashi Y, Takeuchi Y, Mori Y. The neural pathway underlying social buffering of conditioned fear responses in male rats. Eur J Neurosci. 2012;36(10):3429–3437. doi: 10.1111/j.1460-9568.2012.08257.x. [DOI] [PubMed] [Google Scholar]
- Knapska E, Mikosz M, Werka T, Maren S. Social modulation of learning in rats. Learn Mem. 2010;17(1):35–42. doi: 10.1101/lm.1670910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavin MJ, Freise B, Coombes S. Transferred flavor aversions in adult rats. Behav Neural Biol. 1980;28(1):15–33. doi: 10.1016/s0163-1047(80)93118-0. [DOI] [PubMed] [Google Scholar]
- Leggio MG, Molinari M, Neri P, Graziano A, Mandolesi L, Petrosini L. Representation of actions in rats: the role of cerebellum in learning spatial performances by observation. Proc Natl Acad Sci U S A. 2000;97(5):2320–2325. doi: 10.1073/pnas.040554297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Mathis A, Grewe BF, Osterhout JA, Ahanonu B, Schnitzer MJ, … Dulac C. Neuronal Representation of Social Information in the Medial Amygdala of Awake Behaving Mice. Cell. 2017;171(5):1176–1190. e1117. doi: 10.1016/j.cell.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lore R, Blanc A, Suedfeld P. Empathic Learning of a Passive-Avoidance Response in Domesticated Rattus-Norvegicus. Animal Behaviour. 1971 Feb;19:112. doi: 10.1016/S0003-3472(71)80143-4. [DOI] [Google Scholar]
- Lubow RE, Moore AU. Latent Inhibition - the Effect of Nonreinforced Pre-Exposure to the Conditional Stimulus. J Comp Physiol Psychol. 1959;52(4):415–419. doi: 10.1037/h0046700. [DOI] [PubMed] [Google Scholar]
- Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci. 2005;8(12):1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- Martin-Sanchez A, McLean L, Beynon RJ, Hurst JL, Ayala G, Lanuza E, Martinez-Garcia F. From sexual attraction to maternal aggression: When pheromones change their behavioural significance. Horm Behav. 2015;68:65–76. doi: 10.1016/j.yhbeh.2014.08.007. [DOI] [PubMed] [Google Scholar]
- Masuda A, Aou S. Social Transmission of Avoidance Behavior under Situational Change in Learned and Unlearned Rats. PLoS One. 2009;4(8) doi: 10.1371/journal.pone.0006794. ARTN e6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineka S, Davidson M, Cook M, Keir R. Observational Conditioning of Snake Fear in Rhesus-Monkeys. Journal of Abnormal Psychology. 1984;93(4):355–372. doi: 10.1037/0021-843x.93.4.355. [DOI] [PubMed] [Google Scholar]
- Mou X, Ji D. Social observation enhances cross-environment activation of hippocampal place cell patterns. Elife. 2016;5 doi: 10.7554/eLife.18022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasanbuyan N, Yoshida M, Takayanagi Y, Inutsuka A, Nishimori K, Yamanaka A, Onaka T. Oxytocin-oxytocin receptor systems facilitate social defeat posture in male mice. Endocrinology. 2017 doi: 10.1210/en.2017-00606. [DOI] [PubMed] [Google Scholar]
- Petrosini L, Molinari M, Dell’Anna ME. Cerebellar contribution to spatial event processing: Morris water maze and T-maze. Eur J Neurosci. 1996;8(9):1882–1896. doi: 10.1111/j.1460-9568.1996.tb01332.x. [DOI] [PubMed] [Google Scholar]
- Pisansky MT, Hanson LR, Gottesman II, Gewirtz JC. Oxytocin enhances observational fear in mice. Nat Commun. 2017;8(1):2102. doi: 10.1038/s41467-017-02279-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posadasandrews A, Roper TJ. Social Transmission of Food-Preferences in Adult-Rats. Animal Behaviour. 1983 Feb;31:265–271. doi: 10.1016/S0003-3472(83)80196-1. [DOI] [Google Scholar]
- Remedios R, Kennedy A, Zelikowsky M, Grewe BF, Schnitzer MJ, Anderson DJ. Social behaviour shapes hypothalamic neural ensemble representations of conspecific sex. Nature. 2017;550(7676):388–392. doi: 10.1038/nature23885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riess D. Vicarious conditioned acceleration: successful observational learning of an aversive Pavlovian stimulus contingency. J Exp Anal Behav. 1972;18(1):181–186. doi: 10.1901/jeab.1972.18-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggerson AL, Honey RC. Observational learning of instrumental discriminations in the rat: the role of demonstrator type. Q J Exp Psychol (Hove) 2006;59(11):1909–1920. doi: 10.1080/17470210600705032. [DOI] [PubMed] [Google Scholar]
- Sato N, Tan L, Tate K, Okada M. Rats demonstrate helping behavior toward a soaked conspecific. Anim Cogn. 2015;18(5):1039–1047. doi: 10.1007/s10071-015-0872-2. [DOI] [PubMed] [Google Scholar]
- Sterley TL, Baimoukhametova D, Fuzesi T, Zurek AA, Daviu N, Rasiah NP, … Bains JS. Social transmission and buffering of synaptic changes after stress. Nat Neurosci. 2018 doi: 10.1038/s41593-017-0044-6. [DOI] [PubMed] [Google Scholar]
- Stierhoff KA, Lavin MJ. The influence of rendering rats anosmic on the poisoned-partner effect. Behav Neural Biol. 1982;34(2):180–189. doi: 10.1016/s0163-1047(82)91562-x. [DOI] [PubMed] [Google Scholar]
- Stowers L, Kuo TH. Mammalian pheromones: emerging properties and mechanisms of detection. Curr Opin Neurobiol. 2015;34:103–109. doi: 10.1016/j.conb.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stowers L, Liberles SD. State-dependent responses to sex pheromones in mouse. Curr Opin Neurobiol. 2016;38:74–79. doi: 10.1016/j.conb.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strupp BJ, Levitsky DA. Social Transmission of Food Preferences in Adult Hooded Rats (Rattus-Norvegicus) Journal of Comparative Psychology. 1984;98(3):257–266. doi: 10.1037//0735-7036.98.3.257. [DOI] [Google Scholar]
- Tachikawa KS, Yoshihara Y, Kuroda KO. Behavioral transition from attack to parenting in male mice: a crucial role of the vomeronasal system. J Neurosci. 2013;33(12):5120–5126. doi: 10.1523/JNEUROSCI.2364-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano Y, Ukezono M, Nakashima SF, Takahashi N, Hironaka N. Learning of efficient behaviour in spatial exploration through observation of behaviour of conspecific in laboratory rats. R Soc Open Sci. 2017;4(9):170121. doi: 10.1098/rsos.170121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren BL, Vialou VF, Iniguez SD, Alcantara LF, Wright KN, Feng J, … Bolanos-Guzman CA. Neurobiological sequelae of witnessing stressful events in adult mice. Biol Psychiatry. 2013;73(1):7–14. doi: 10.1016/j.biopsych.2012.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao S, Bergan J, Lanjuin A, Dulac C. Oxytocin signaling in the medial amygdala is required for sex discrimination of social cues. Elife. 2017;6 doi: 10.7554/eLife.31373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusufishaq S, Rosenkranz JA. Post-weaning social isolation impairs observational fear conditioning. Behav Brain Res. 2013;242:142–149. doi: 10.1016/j.bbr.2012.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zentall TR. Perspectives on Observational Learning in Animals. Journal of Comparative Psychology. 2012;126(2):114–128. doi: 10.1037/a0025381. [DOI] [PubMed] [Google Scholar]
- Zentall TR, Levine JM. Observational learning and social facilitation in the rat. Science. 1972;178(4066):1220–1221. doi: 10.1126/science.178.4066.1220. [DOI] [PubMed] [Google Scholar]
- Zohar O, Terkel J. Acquisition of pine cone stripping behavior in black rats (Rattus rattus) International Journal of Comparative Psychology. 1991;5(1):1–6. [Google Scholar]
- Zohar O, Terkel J. Spontaneous learning of pine cone opening behavior by black rats (Rattus rattus) Mammalia. 1995;59(4):481–487. doi: 10.1515/mamm.1995.59.4.481. [DOI] [Google Scholar]