Abstract
Background
The role of autophagy-related markers as the prognostic factor of post-operative hepatocellular carcinoma (HCC) recurrence remained controversial.
Methods
Overall, 535 consecutive HCC patients undergoing curative resection from 2010 to 2014 were followed and classified with early (ER, <2 years) or late recurrence (LR). Autophagy-related markers, LC3, Beclin-1, and p62 expression was immunohistochemically assessed in HCC and adjacent non-tumor (ANT) tissues.
Results
HCC recurred in 245 patients: 116 with ER and 129 with LR. The cumulative incidence of recurrence at 1, 3, 5, and 7 years was 9.7%, 33.9%, 53.3%, and 66.3%, respectively. In multivariate analysis, HCC recurrence was significantly associated with low LC3 expression in tumor and ANT tissues, HCC tissues only and ANT tissues only (hazard ratio/95% confidence interval: 6.12/2.473–17.53, 4.18/1.285–13.61, and 1.89/1.299–2.757) and macrovascular invasion (1.63/1.043–2.492) and cirrhosis (1.59/1.088–2.326). ER was significantly associated with low LC3 expression in tumor and ANT tissues, HCC tissues only and ANT tissues only (6.54/2.934–15.81, 3.26/1.034–10.27, and 2.09/1.313–3.321) and macrovascular and microvascular invasion (2.65/1.306–5.343 and 2.55/1.177–5.504). LR was significantly associated with low LC3 expression in tumor and ANT tissues, HCC tissues only and ANT tissues only (5.02/1.372–18.83, 3.19/1.13–12.09, and 1.66/1.051–2.620) and cirrhosis (1.66/1.049–2.631). Patients with low and high LC3 expression in tumor and ANT tissues showed a 5-year cumulative recurrence of 94.3% and 41.7%, respectively (p < 0.001).
Conclusions
The high LC3 expression in the tumor and liver microenvironments is significantly associated with lower HCC recurrence. Furthermore, tumor characteristics and liver microenvironment were also significantly associated with ER and LR, respectively.
Translational impact
The analysis for LC3 expression in both the HCC and ANT tissues could identify patients at risk of HCC recurrence.
Introduction
Hepatocellular carcinoma (HCC) is currently the fifth most common type of cancer and the third leading cause of cancer-related mortality worldwide1–3. In Taiwan, HCC is highly associated with viral- and alcoholic-associated cirrhosis4 and ranks as the second leading cause of cancer-related death5. Even after curative resection for HCC, the 5-year recurrence rate and survival rate remain as high as 60 and 50%, respectively6, 7. Identifying the factors associated with HCC recurrence after surgical resection could provide a promising strategy to improve the prognosis of HCC patients undergoing curative hepatectomy.
Autophagy is involved in the physiology and pathogenesis of human disease8, 9. Enhancement or inhibition of autophagy-related proteins has been reported to have therapeutic efficacy in cancer patients10, 11. However, studies of the involvement of autophagy in tumor recurrence have yielded controversial results12–15. In addition, the prognostic significance of autophagy-related markers such as LC3 and Beclin-1 in predicting the clinical outcome of HCC patients has been reported in previous studies13–18, but results have been conflicting due to the relatively limited case numbers19.
Recently, the molecular and histological changes that occur in the tumor microenvironment have become a main area of focus. The non-tumor liver microenvironment plays an important role in hepatocarcinogenesis20–23. The presence of autophagy in the non-tumor microenvironment was also found to promote tumor growth via the provision of nutrients24. These findings highlight the importance of studying both the tumor and non-tumor microenvironments to comprehensively understand the impact of autophagy in HCC development and progression. Hence, we conducted the current large-scale study to explore the impact of autophagy-related markers in both the tumor and adjacent non-tumor (ANT) microenvironments on HCC recurrence after surgical resection. Our results suggest that the low LC3 expression in both tumor and ANT microenvironments strongly predicts HCC recurrence in patients who have undergone curative resection.
Materials and methods
Patients and follow-up
This retrospective study included 535 consecutive, histologically proven HCC patients who underwent curative surgical resection between 2010 and 2014 at E-Da Hospital, I-Shou University, Kaohsiung, Southern Taiwan (n = 318) and Changhua Christian Hospital, Changhua, Central Taiwan (n = 217). All patients received regular follow-up every 3 months after surgery. The follow-up period was defined as the duration from the date of operation to the date of either death or the last follow-up. The last follow-up was on December 2016. Time to recurrence (TTR) was defined as the duration from the date of operation to the date of recurrence. Recurrent HCC was defined based on histological confirmation or highly elevated serum alpha-fetoprotein (AFP) in addition to diagnosis via at least two imaging methods according to the recommendations of the American Association for the Study of Liver Disease (AASLD)25. The patients were divided into four groups according to their TTR: patients experiencing recurrence within 2 years after operation (early recurrence group, ER, n = 116); patients experiencing recurrence 2–7 years after operation (late recurrence group, LR, n = 129); an all-patient recurrence group (AR, n = 245), consisting of both the ER and LR groups; and patients with no recurrence during the follow-up period after the first hepatectomy (non-recurrence group, NR, n = 290).
For the remaining materials and methods, please see Supporting information.
Results
Baseline demographic data
The demographic and clinicopathological factors of the 535 patients (73.1% male, mean age of 63 years) are shown in Table 1. Regarding the etiology of HCC, 46.7% of the patients had HBV, 28.4% had HCV, 3.9% had HBV/HCV co-infection, and 20.9% were not infected with HBV/HCV. One-third of the patients had liver cirrhosis, of which 22.8 and 9.5%, respectively, had a Child-Pugh score of A and B. One-tenth of the patients had an Edmondson-Steiner Grade of I–II, and approximately one-fifth of the patients had multiple tumors. Macrovascular and microvascular tumor invasion were observed in 20.7 and 46.0% of the patients, respectively. Regarding tumor stage, 16.4 and 36.1% of the patients were TNM stage III–IV and BCLC stage B-C, respectively. Regarding the expression of autophagy-related markers, 91.6% of the HCC tissues and 59.8% of the ANT tissues were high for LC3; 86.7% of the HCC tissues and 34.8% of the ANT tissues were high for Beclin-1; and 81.1% of the HCC tissues and 8.4% of the ANT tissues were high for p62.
Table 1.
Basic demographic data and univariate analysis of recurrence in all patients
| Characteristics | All patients (n = 535) | Without recurrence (n = 290) | With recurrence, all (n = 245) | p-value |
|---|---|---|---|---|
| Gender | ||||
| Female | 144 (26.9) | 69 (23.8) | 75 (30.6) | 0.076 |
| Male | 391 (73.1) | 221 (76.2) | 170 (69.4) | |
| Age (years) | 63.1 ± 11.5 | 62.3 ± 12.1 | 64.1 ± 12.7 | 0.076 |
| HTN | 101 (18.9) | 58 (20.0) | 43 (17.6) | 0.471 |
| DM | 59 (11.0) | 35 (12.1) | 24 (9.8) | 0.403 |
| Alcohol | 129 (24.9) | 67 (23.1) | 62 (25.3) | 0.553 |
| Smoking | 152 (28.4) | 83 (28.6) | 69 (28.2) | 0.907 |
| HCC etiology | ||||
| Non HBVHCV | 112 (20.9) | 60 (20.7) | 52 (21.2) | 0.826 |
| HBV | 250 (46.7) | 132 (45.5) | 118 (48.2) | |
| HCV | 152 (28.4) | 85 (29.3) | 67 (27.3) | |
| HBV + HCV | 21 (3.9) | 13 (4.5) | 8 (3.3) | |
| AST (IU/L) | 55 ± 38 | 56 ± 41 | 54 ± 34 | 0.412 |
| ALT (IU/L) | 50 ± 39 | 53 ± 40 | 48 ± 37 | 0.108 |
| Total bilirubin (mg/dl) | 0.79 ± 0.34 | 0.78 ± 0.36 | 0.81 ± 0.33 | 0.235 |
| Albumin (g/dl) | 3.9 ± 0.4 | 3.8 ± 0.5 | 3.9 ± 0.4 | 0.192 |
| Creatinine | 1.0 ± 0.7 | 1.1 ± 0.8 | 1.1 ± 0.9 | 0.676 |
| Platelet count (×103/ml) | 175 ± 71 | 171 ± 71 | 179 ± 72 | 0.254 |
| INR | 1.07 ± 0.10 | 1.07 ± 0.13 | 1.09 ± 0.14 | 0.091 |
| AFP (ng/dl) | 2797 ± 13215 | 2699 ± 11535 | 2913 ± 14985 | 0.856 |
| ICG (%) | 8.3 ± 5.3 | 7.8 ± 4.9 | 8.5 ± 5.8 | 0.466 |
| Liver cirrhosis | ||||
| Negative | 362 (67.7) | 211 (72.8) | 151 (61.6) | 0.006 |
| Positive | 173 (32.3) | 79 (27.2) | 94 (38.4) | |
| Child-Pugh score A | 122 (22.8) | 63 (21.7) | 59 (24.1) | |
| Child-Pugh score B | 51 (9.5) | 16 (5.5) | 35 (14.3) | |
| Antiviral therapy | ||||
| Negative | 185 (43.7) | 92 (40.0) | 93 (48.2) | 0.091 |
| Positive | 238 (56.3) | 138 (60.0) | 100 (51.8) | |
| Operative methods | ||||
| Minor LR | 412 (77.0) | 223 (76.9) | 189 (77.1) | 0.946 |
| Major LR | 123 (23.0) | 67 (23.1) | 56 (22.9) | |
| Operative margin ( > 1 cm) | ||||
| Negative | 150 (28.0) | 81 (27.9) | 69 (28.2) | 0.952 |
| Positive | 385 (72.0) | 209 (72.1) | 176 (71.8) | |
| Edmondson-Steiner grades | ||||
| I–II | 51 (9.5) | 34 (11.7) | 17 (6.9) | 0.060 |
| III–IV | 484 (90.5) | 256 (88.3) | 228 (93.1) | |
| Macrovascular invasion | ||||
| Negative | 424 (79.3) | 240 (82.8) | 184 (75.1) | 0.030 |
| Positive | 111 (20.7) | 50 (17.2) | 61 (24.9) | |
| Microvascular invasion | ||||
| Negative | 289 (54.0) | 158 (54.5) | 131 (53.5) | 0.815 |
| Positive | 246 (46.0) | 132 (45.5) | 114 (46.5) | |
| Tumor number | ||||
| Single | 438 (81.9) | 238 (82.1) | 200 (81.6) | 0.896 |
| Multiple | 97 (18.1) | 52 (17.9) | 45 (18.4) | |
| Tumor size | ||||
| <5 cm | 352 (65.8) | 191 (65.9) | 161 (65.7) | 0.971 |
| ≥5 cm | 183 (34.2) | 99 (34.1) | 84 (34.3) | |
| TNM stage | ||||
| I–II | 447 (83.6) | 241 (83.1) | 206 (84.1) | 0.761 |
| III–IV | 88 (16.4) | 49 (16.9) | 39 (15.9) | |
| BCLC stage | ||||
| 0-A | 342 (63.9) | 185 (63.8) | 157 (64.1) | 0.945 |
| B-C | 193 (36.1) | 105 (36.2) | 88 (35.9) | |
| LC3 in tumor tissues | ||||
| Low | 45 (8.4) | 11 (3.8) | 34 (13.9) | <.0001 |
| High | 490 (91.6) | 279 (96.2) | 211 (86.1) | |
| Beclin-1 in tumor tissues | ||||
| Low | 71 (13.3) | 40 (13.8) | 31 (12.7) | 0.699 |
| High | 464 (86.7) | 250 (86.2) | 214 (87.3) | |
| p62 in tumor tissues | ||||
| Low | 101 (18.9) | 58 (20.0) | 43 (17.6) | 0.471 |
| High | 434 (81.1) | 232 (80.0) | 202 (82.4) | |
| LC3 in ANT tissues | ||||
| Low | 215 (40.2) | 93 (32.1) | 122 (49.8) | <.0001 |
| High | 320 (59.8) | 197 (67.9) | 123 (50.2) | |
| Beclin-1 in ANT tissues | ||||
| Low | 349 (65.2) | 198 (68.3) | 151 (61.6) | 0.108 |
| High | 186 (34.8) | 92 (31.7) | 94 (38.4) | |
| p62 in ANT tissues | ||||
| Low | 490 (91.6) | 266 (91.7) | 224 (91.4) | 0.902 |
| High | 45 (8.4) | 24 (8.3) | 21 (8.6) | |
Data shown as mean ± standard deviation or number (%). Patients with the presence of liver cirrhosis were further sub-classified as those with a Child-Pugh score of A and B. Patients infected with HBV and/or HCV were further classified as those with and without antiviral therapy
HTN Hypertension, DM Diabetes Mellitus, HBV Hepatitis B virus, HCV Hepatitis C virus, AST aspartate aminotransferase, ALT alanine aminotransferase, INR International normalized ratio, AFP Alpha-fetoprotein, ICG Indocyanine green, Minor liver resection: ≤2 segmentectomy, Major liver resection: ≥3 segmentectomy, BCLC stage Barcelona clinic liver cancer, ANT adjacent non-tumor
The significance of bold enteries in tables 1, 2 and 3 is p-value < 0.005.
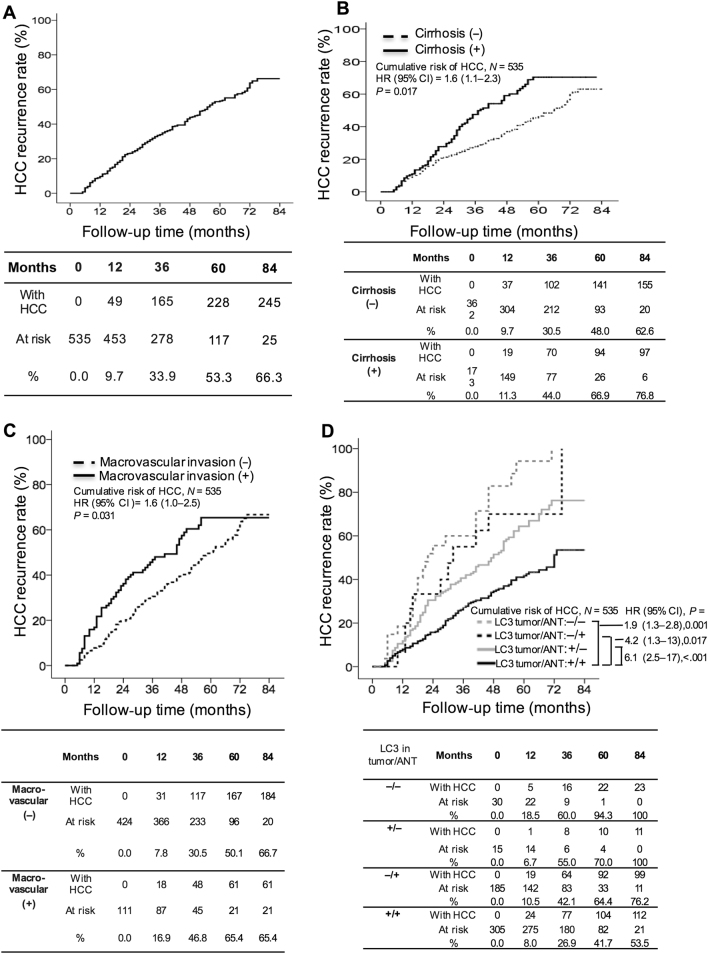
During the median follow-up of 42 months (range, 1–84 months), 245 patients experienced HCC recurrence, including 116 cases of ER and 129 cases of LR (incidence rate, 14.4% per person-year). Forty-two (17.1%) and 203 (82.9%) patients experienced extrahepatic recurrence and intrahepatic recurrence, respectively. The cumulative incidence of HCC recurrence at 1, 3, 5, and 7 years after HCC resection was 9.7%, 33.9%, 53.3%, and 66.3%, respectively (Fig. 1a).
Fig. 1. Cumulative incidence of HCC recurrence with respect to various clinicopathological factors.
The cumulative incidence of HCC in all patients (a). Patients with the presence of liver cirrhosis (b) and macrovascular invasion (c) were significantly more likely to develop HCC recurrence. Patients low LC3 expression in the adjacent non-tumor (ANT) tissues (+/−), HCC tissues (−/+) or both (−/−) had a significantly higher incidence of recurrence than patients with LC3 expression in both HCC and ANT tissues (+/+; d). HCC hepatocellular carcinoma, ANT adjacent non-tumor, HR hazard ratio, CI confidence intervals, + high, − low
Factors related to HCC recurrence in patients who underwent hepatectomy
In univariate analysis, the presence of liver cirrhosis and macrovascular invasion and the low LC3 expression in HCC tissues or ANT tissues were significantly associated with HCC recurrence (Table 1).
In multivariate analysis, the Cox proportional hazard model identified that patients with low LC3 expression in both the HCC and ANT tissues had the highest risk of HCC recurrence (−/−; hazard ratio [HR]: 6.12; 95% confidence interval [CI]: 2.473–17.53), followed by those low LC3 expression in HCC tissues only (−/+; HR: 4.18; 95% CI: 1.285–13.61), those low LC3 in ANT tissues only (+/−; HR: 1.89; 95% CI: 1.299–2.757), those with macrovascular invasion (HR: 1.63; 95% CI: 1.043–2.492) and those with the presence of liver cirrhosis (HR: 1.59; CI: 1.088–2.326) (Table 3).
Table 3.
Multivariate analyses of factors associated with all recurrence, early recurrence, and late recurrence
| Variables | Hazard ratio | 95% CI | p-value |
|---|---|---|---|
| All recurrence | |||
| Liver cirrhosis | |||
| Negative | 1 | ||
| Positive | 1.59 | 1.088–2.326 | 0.017 |
| Macrovascular invasion | |||
| Negative | 1 | ||
| Positive | 1.63 | 1.043–2.492 | 0.031 |
| LC3 in tumor/ANT tissues | |||
| +/+ | 1 | ||
| +/− | 1.89 | 1.299–2.757 | 0.001 |
| −/+ | 4.18 | 1.285–13.61 | 0.017 |
| −/− | 6.12 | 2.473–17.53 | <.0001 |
| Early recurrence | |||
| Macrovascular invasion | |||
| Negative | 1 | ||
| Positive | 2.65 | 1.306–5.343 | 0.017 |
| Microvascular invasion | |||
| Negative | 1 | ||
| Positive | 2.55 | 1.177–5.504 | 0.018 |
| Tumor size | |||
| <5 cm | 1 | ||
| ≥5 cm | 0.76 | 0.383–1.528 | 0.448 |
| BCLC stage | |||
| 0-A | 1 | ||
| B-C | 0.52 | 0.214–1.240 | 0.139 |
| LC3 in tumor/ANT tissues | |||
| +/+ | 1 | ||
| +/− | 2.09 | 1.313–3.321 | 0.002 |
| −/+ | 3.26 | 1.034–10.27 | 0.044 |
| −/− | 6.54 | 2.934–15.81 | <.0001 |
| Late recurrence | |||
| Liver cirrhosis | |||
| Negative | 1 | ||
| Positive | 1.66 | 1.049–2.631 | 0.031 |
| Microvascular invasion | |||
| Negative | 1 | ||
| Positive | 0.78 | 0.361–1.699 | 0.537 |
| BCLC stage | |||
| 0-A | 1 | ||
| B-C | 0.74 | 0.324–1.680 | 0.468 |
| LC3 in tumor/ANT tissues | |||
| +/+ | 1 | ||
| +/− | 1.66 | 1.051–2.620 | 0.030 |
| −/+ | 3.19 | 1.13–12.09 | 0.021 |
| −/− | 5.02 | 1.372–18.83 | 0.011 |
ANT adjacent non-tumor, BCLCstage Barcelona clinic liver cancer, + high, − low
The significance of bold enteries in tables 1, 2 and 3 is p-value < 0.005.
The 1-, 3-, 5-, and 7-year cumulative incidence of HCC recurrence was 11.3%, 44.0%, 66.9%, and 76.8%, respectively, in patients with liver cirrhosis and 16.9%, 46.8%, 65.4%, and 65.4%, respectively, in patients with macrovascular invasion, which were significantly higher levels than found in their counterparts (Fig. 1b, c, respectively). Next, LC3 expression was analyzed in parallel in HCC and ANT tissues. The results showed that patients with high LC3 expression in both tissues (+/+) had a 1-, 3-, 5-, and 7-year cumulative incidence of HCC recurrence of 8.0%, 26.9%, 41.7%, and 53.5%, respectively. Compared to this group, patients with low LC3 expression in both tissues (−/−; 18.5, 60.0, 94.3, and 100%, respectively), those low LC3 expression in HCC tissues only (−/+; 10.5%, 42.1%, 64.4%, and 76.2%, respectively) and those low LC3 expression in ANT tissues only (+/−; 6.7%, 55.0%, 70.0%, and 100%, respectively) were significantly more prone to HCC recurrence (Fig. 1d).
Factors related to early HCC recurrence in patients who underwent hepatectomy
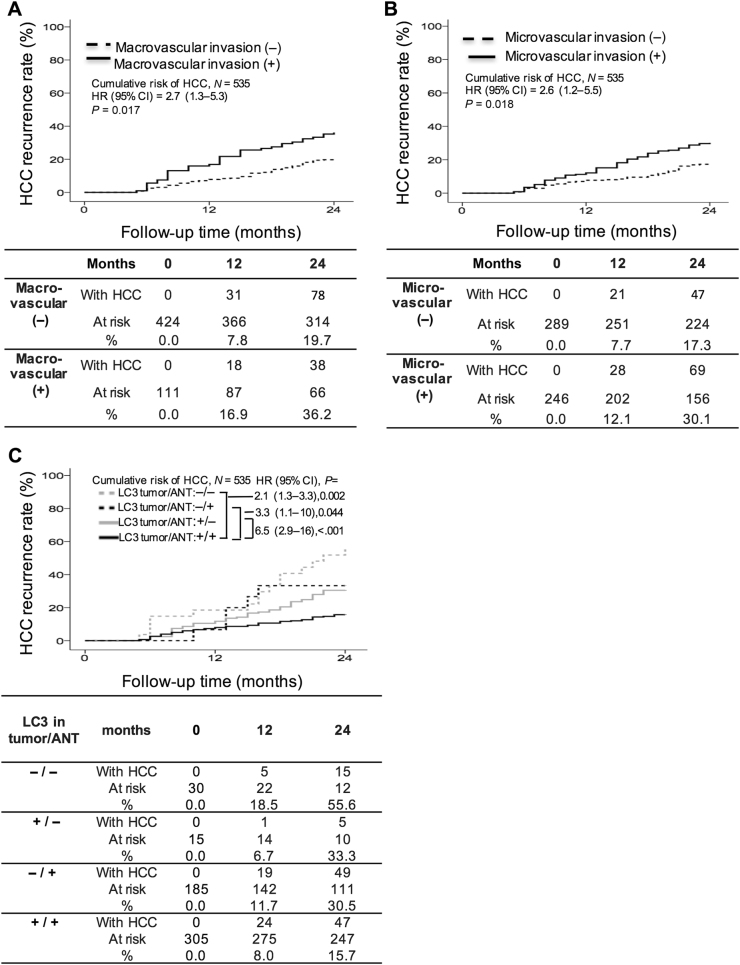
To identify factors associated with early HCC recurrence, clinicopathological features were compared between the ER group (n = 116) and patients with no recurrence within 24 months after operation (n = 419) (Table 2). The presence of macrovascular and microvascular invasion, tumor size ≥5 cm, advanced BCLC stage and low LC3 expression in HCC tissues or ANT tissues were significantly associated with the risk of early HCC recurrence.
Table 2.
Univariate analyses of early and late recurrences
| Early recurrence | Late recurrence | |||||
|---|---|---|---|---|---|---|
| Characteristics | Without (n = 419) | With (n = 116) | p-value | Without (n = 290) | With (n = 129) | p-value |
| Gender | ||||||
| Female | 107 (25.5) | 37 (31.9) | 0.172 | 69 (23.8) | 38 (29.5) | 0.220 |
| Male | 312 (74.5) | 79 (68.1) | 221 (76.2) | 91 (70.5) | ||
| Age (years) | 62.7 ± 11.9 | 64.3 ± 9.8 | 0.072 | 62.3 ± 12.1 | 63.7 ± 11.7 | 0.242 |
| HTN | 75 (17.9) | 26 (22.4) | 0.272 | 58 (20.0) | 17 (13.2) | 0.093 |
| DM | 47 (11.2) | 12 (10.3) | 0.791 | 35 (12.1) | 12 (9.3) | 0.407 |
| Alcohol | 99 (23.6) | 30 (25.9) | 0.619 | 67 (23.1) | 32 (24.8) | 0.705 |
| Smoking | 122 (29.1) | 30 (25.9) | 0.492 | 83 (28.6) | 39 (30.2) | 0.737 |
| HCC etiology | ||||||
| Non HBVHCV | 85 (20.3) | 27 (23.3) | 0.268 | 60 (20.7) | 25 (19.4) | 0.601 |
| HBV | 189 (45.1) | 61 (52.6) | 132 (45.5) | 57 (44.2) | ||
| HCV | 129 (30.8) | 23 (19.8) | 85 (29.3) | 44 (34.1) | ||
| HBV + HCV | 16 (3.8) | 5 (4.3) | 13 (4.5) | 3 (2.3) | ||
| AST (IU/L) | 56 ± 41 | 52 ± 34 | 0.372 | 56 ± 41 | 55 ± 37 | 0.704 |
| ALT (IU/L) | 53 ± 40 | 48 ± 38 | 0.056 | 53 ± 40 | 55 ± 42 | 0.970 |
| Total bilirubin (mg/dl) | 0.80 ± 0.35 | 0.79 ± 0.31 | 0.807 | 0.78 ± 0.36 | 0.84 ± 0.34 | 0.110 |
| Albumin (g/dl) | 3.8 ± 0.5 | 3.9 ± 0.4 | 0.349 | 3.8 ± 0.5 | 3.9 ± 0.4 | 0.332 |
| Creatinine | 1.1 ± 0.7 | 1.1 ± 0.9 | 0.275 | 1.1 ± 0.8 | 1.0 ± 0.8 | 0.815 |
| Platelet count (×103/ml) | 173 ± 68 | 180 ± 59 | 0.351 | 171 ± 71 | 175 ± 71 | 0.492 |
| INR | 1.08 ± 0.14 | 1.07 ± 0.10 | 0.618 | 1.07 ± 0.13 | 1.10 ± 0.15 | 0.083 |
| AFP (ng/dl) | 2625 ± 9736 | 2223 ± 10381 | 0.598 | 2699 ± 11535 | 3533 ± 16514 | 0.604 |
| ICG (%) | 8.0 ± 4.8 | 8.6 ± 5.9 | 0.686 | 8.2 ± 5.2 | 9.1 ± 7.8 | 0.372 |
| Liver cirrhosis | ||||||
| Negative | 291 (69.5) | 71 (61.2) | 0.093 | 212 (73.1) | 79 (61.2) | 0.015 |
| Positive | 128 (30.5) | 45 (38.8) | 78 (26.9) | 50 (30.2) | ||
| Antiviral therapy | ||||||
| Negative | 141 (42.2) | 44 (49.4) | 0.222 | 108 (49.5) | 42 (40.4) | 0.123 |
| Positive | 193 (57.8) | 45 (50.6) | 110 (50.5) | 62 (59.6) | ||
| Operative methods | ||||||
| Minor LR | 320 (76.4) | 92 (79.3) | 0.506 | 223 (76.9) | 97 (75.2) | 0.705 |
| Major LR | 99 (23.6) | 24 (20.7) | 67 (23.1) | 32 (24.8) | ||
| Operative margin ( > 1 cm) | ||||||
| Negative | 122 (29.1) | 28 (24.1) | 0.291 | 81 (27.9) | 41 (31.8) | 0.423 |
| Positive | 297 (70.9) | 88 (75.9) | 209 (72.1) | 88 (68.2) | ||
| Edmondson-Steiner grades | ||||||
| I–II | 45 (10.7) | 6 (5.2) | 0.0721 | 34 (11.7) | 11 (8.5) | 0.329 |
| III–IV | 374 (89.3) | 110 (94.8) | 256 (88.3) | 118 (91.5) | ||
| Macrovascular invasion | ||||||
| Negative | 346 (82.6) | 78 (67.2) | <.0001 | 240 (82.8) | 106 (82.2) | 0.884 |
| Positive | 73 (17.4) | 38 (32.8) | 50 (17.2) | 23 (17.8) | ||
| Microvascular invasion | ||||||
| Negative | 242 (57.8) | 47 (40.5) | 0.001 | 158 (54.5) | 84 (65.1) | 0.042 |
| Positive | 177 (42.2) | 69 (59.5) | 132 (45.5) | 45 (34.9) | ||
| Tumor number | ||||||
| Single | 339 (80.9) | 99 (85.3) | 0.272 | 238 (82.1) | 101 (78.3) | 0.364 |
| Multiple | 80 (19.1) | 17 (14.7) | 52 (17.9) | 28 (21.7) | ||
| Tumor size | ||||||
| <5 cm | 286 (68.3) | 66 (56.9) | 0.022 | 191 (65.9) | 95 (73.6) | 0.114 |
| ≥5 cm | 133 (31.7) | 50 (43.1) | 99 (34.1) | 34 (26.4) | ||
| TNM stage | ||||||
| I–II | 353 (84.2) | 94 (81.0) | 0.409 | 241 (83.1) | 112 (86.8) | 0.884 |
| III–IV | 66 (15.8) | 22 (19.0) | 49 (16.9) | 17 (13.2) | ||
| BCLC stage | ||||||
| 0-A | 280 (66.8) | 62 (53.4) | 0.008 | 185 (63.8) | 95 (73.6) | 0.048 |
| B-C | 139 (33.2) | 54 (46.6) | 105 (36.2) | 34 (26.4) | ||
| LC3 in tumor tissues | ||||||
| Low | 25 (6.0) | 20 (17.2) | <.0001 | 11 (3.8) | 14 (10.9) | 0.005 |
| High | 394 (94.0) | 96 (82.8) | 279 (96.2) | 115 (89.1) | ||
| Beclin-1 in tumor tissues | ||||||
| Low | 55 (13.1) | 16 (13.8) | 0.851 | 40 (13.8) | 15 (11.6) | 0.545 |
| High | 364 (86.9) | 100 (86.2) | 250 (86.2) | 114 (88.4) | ||
| p62 in tumor tissues | ||||||
| Low | 79 (18.9) | 22 (19.0) | 0.978 | 58 (20.0) | 21 (16.3) | 0.369 |
| High | 340 (81.1) | 94 (81.0) | 232 (80.0) | 108 (83.7) | ||
| LC3 in ANT tissues | ||||||
| Low | 151 (36.0) | 64 (55.2) | <.0001 | 95 (32.8) | 56 (43.4) | 0.036 |
| High | 268 (64.0) | 52 (44.8) | 195 (67.2) | 73 (56.6) | ||
| Beclin-1 in ANT tissues | ||||||
| Low | 260 (62.1) | 65 (56.0) | 0.067 | 186 (64.1) | 74 (57.4) | 0.075 |
| High | 159 (37.9) | 51 (44.0) | 104 (35.9) | 55 (42.6) | ||
| p62 in ANT tissues | ||||||
| Low | 381 (90.9) | 109 (94.0) | 0.297 | 266 (91.7) | 115 (89.1) | 0.397 |
| High | 38 (9.1) | 7 (6.0) | 24 (8.3) | 14 (10.9) | ||
Data shown as mean ± standard deviation or number (%). Patients in the antiviral therapy group were those infected with HBV and/or HCV
HTN Hypertension, DM Diabetes Mellitus, HBV Hepatitis B virus, HCV Hepatitis C virus, AST aspartate aminotransferase, ALT alanine aminotransferase, INR International normalized ratio, AFP Alpha-fetoprotein, ICG Indocyanine green, Minor liver resection: ≤2 segmentectomy, Major liver resection: ≥3 segmentectomy, BCLC stage Barcelona clinic liver cancer, ANT adjacent non-tumor
The significance of bold enteries in tables 1, 2 and 3 is p-value < 0.005.
In multivariate analysis, the Cox proportional hazard analysis identified that patients with low LC3 expression in both HCC and ANT tissues had the highest risk of HCC recurrence (−/−; HR: 6.54; 95% CI: 2.934–15.81), followed by those low LC3 expression in HCC tissues only (−/+; HR: 3.26; 95% CI: 1.034–10.27), those with the presence of macrovascular and microvascular invasion (HR: 2.65; 95% CI: 1.306–5.343 and HR: 2.55; 95% CI: 1.177–5.504, respectively) and those low LC3 expression in ANT tissues only (+/−; HR: 2.09; 95% CI: 1.313–3.321) (Table 3).
The 1- and 2-year cumulative incidence of HCC recurrence was 16.9% and 36.2%, respectively, in those with macrovascular invasion (Fig. 2a) and 12.1% and 30.1%, respectively, in those with microvascular invasion (Fig. 2b), which were significantly higher than those in their counterparts. To evaluate whether LC3 expression in HCC and ANT tissues has an effect on ER, LC expression patterns were analyzed in both tissue types. The results revealed a 1- and 2-year cumulative incidence of HCC recurrence of 8.0% and 15.7%, respectively, for patients with high LC3 expression in both tumor and ANT tissues (+/+). Compared to this group, patients with low LC3 in both tissues (−/−; 18.5% and 55.6%, respectively), those low LC3 in HCC tissues only (−/+; 11.7% and 30.5%, respectively) and those low LC3 in ANT tissues only (+/−; 6.7% and 33.3%, respectively) were significantly more prone to early recurrence (Fig. 2c).
Fig. 2. Cumulative incidence of early recurrence with respect to various clinicopathological factors.
Patients with macrovascular invasion (a) and microvascular invasion (b) were significantly more likely to develop early recurrence of HCC. Patients low LC3 expression in HCC tissues (−/+), adjacent non-tumor (ANT) tissues (+/−), or both (−/−) had a significantly higher incidence of early recurrence than patients with LC3 expression in both HCC and ANT tissues (+/+; c). HCC hepatocellular carcinoma, ANT adjacent non-tumor, HR hazard ratio, CI confidence intervals, + high, − low
Factors related to late HCC recurrence in patients who underwent hepatectomy
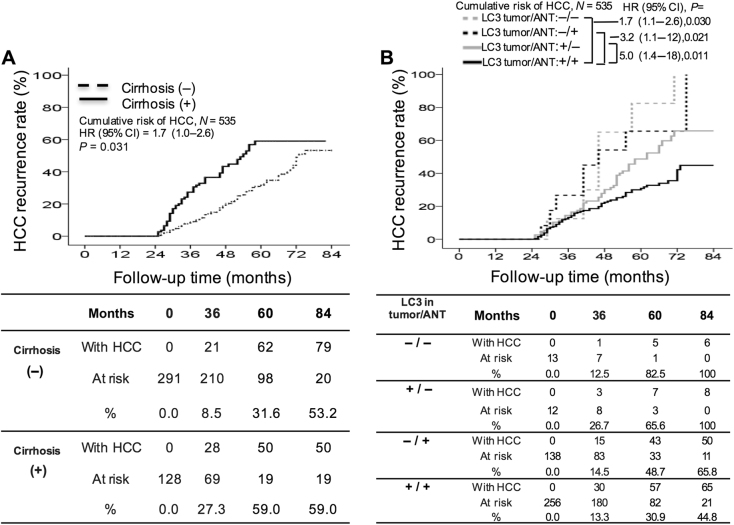
To identify factors associated with late HCC recurrence, clinicopathological factors were compared between the LR (n = 129) and the NR groups (n = 290) (Table 2). In univariate analysis, the presence of liver cirrhosis and microvascular invasion, early BCLC stage, and the low LC3 expression in HCC tissues or ANT tissues were significantly associated with late HCC recurrence.
In multivariate analysis (Table 3), the Cox proportional hazard model identified that patients with low LC3 expression in both HCC and ANT tissues had the highest risk of HCC recurrence (−/−; HR: 5.02; 95% CI: 1.372–18.83), followed by those low LC3 expression in HCC tissues only (−/+; HR: 3.19; 95% CI: 1.13–12.09), those low LC3 expression in ANT tissues only (+/−; HR: 1.66; 95% CI: 1.051–2.620) and those with the presence of liver cirrhosis (HR: 1.66; 95% CI: 1.049–2.631).
The 3-, 5-, and 7-year cumulative incidence of HCC recurrence was 27.3%, 59.0%, and 59.0%, respectively, for patients with liver cirrhosis, which was significantly higher than for those without liver cirrhosis (Fig. 3a). When LC3 expression was analyzed in parallel in tumor and ANT tissues, the 3-, 5-, and 7-year cumulative incidence of HCC recurrence was found to be 13.3%, 30.9%, and 44.8%, respectively, for patients with LC3 expression in both tissues (+/+). Compared to this group, patients with low LC3 expression in both tissues (−/−; 12.5%, 82.5%, and 100%, respectively), those low LC3 expression in HCC tissues only (−/+; 14.5%, 48.7%, and 65.8%, respectively) and those low LC3 expression in ANT tissues only (+/−; 26.7%, 65.6%, and 100%, respectively) were significantly more prone to late HCC recurrence (Fig. 3b).
Fig. 3. Cumulative incidence of late recurrence with respect to various clinicopathological factors.
Patients with the presence of liver cirrhosis (a) were significantly more likely to develop late recurrence of HCC. Patients low LC3 expression in HCC tissues (−/+), adjacent non-tumor (ANT) tissues (+/−), or both (−/−) had a higher incidence of late recurrence than patients with LC3 expression in both HCC and ANT tissues (+/+; b). HCC hepatocellular carcinoma, ANT adjacent non-tumor, HR hazard ratio, CI confidence intervals, + high, − low
LC3 staining of tumor and non-tumor tissues in re-operated patients
Having established the association between LC3 expression and HCC recurrence in patients who underwent first curative HCC hepatectomy, we next evaluated LC3 expression patterns in patients who underwent second (n = 32) and third (n = 5) operable HCC hepatectomy due to HCC recurrence (Table 4). The low LC3 expression in both tumor and ANT tissues was significantly associated with repeated HCC recurrence. Four and 3 patients showed a change from high to low LC3 staining in tumor tissues at the second and third surgical resection, respectively. Moreover, 5 and 4 patients showed a change from high to low LC3 staining in ANT tissues at the second and third surgical resection, respectively. In total, 100% of the patients showing a loss-of-LC3 staining in tumor and ANT tissues experienced HCC recurrence. This result further demonstrates that loss-of-LC3 expression in both HCC and ANT tissues is associated with a high risk of HCC recurrence, highlighting that LC3 expression significantly predicts HCC recurrence.
Table 4.
LC3 staining of the tumor and adjacent non-tumor tissues in 32 re-resected patients
| Characteristics | First resection (n = 32) | p-value | Second resection (n = 32) | p-value | Third resection (n = 5) | p-value |
|---|---|---|---|---|---|---|
| LC3 in tumor tissues | ||||||
| Low | 25 (78.1) | <0.001 | 29 (90.6) | <0.001 | 5 (100) | <0.001 |
| High | 7 (21.9) | 3 (9.4) | 0 (0) | |||
| LC3 in ANT tissues | ||||||
| Low | 22 (68.8) | <0.001 | 27 (84.4) | <0.001 | 4 (80) | <0.001 |
| High | 10 (31.2) | 5 (15.6) | 1 (20) | |||
ANT adjacent non-tumor
Discussion
Our study demonstrated that the high LC3 expression in both the tumor and non-tumor liver microenvironments is significantly associated with lower recurrence, regardless of early or late recurrence. This suggests that the measurement of LC3 expression in both tissues may serve as a predictor of HCC recurrence. The findings that the majority of the patients who underwent second and third operable hepatectomy also had a low LC3 expression in both tumor and ANT tissues and that the loss-of-LC3 expression in both HCC and ANT tissues led to a high risk of HCC recurrence further support the use of LC3 as a prognostic factor for HCC recurrence. In addition to LC3, vascular invasion was significantly associated with ER, and the presence of liver cirrhosis was significantly associated with LR. These results suggest that tumor characteristics have a higher impact on ER, whereas the liver microenvironment has a higher impact on LR.
Tumor risk factors such as tumor invasion into the portal vein and intrahepatic metastasis have been reported to be associated with ER26. In the current study, patients with vascular tumor invasion were at a significantly higher risk of developing ER. We postulate that oncogenes and tumor suppressor genes may have undergone genetic alterations during tumor progression27, hence allowing tumor cells to acquire invasive and metastatic potential. Intrahepatic metastasis might have occurred prior to hepatectomy or during tumor manipulation, contributing to ER26, 27. Cirrhosis is associated with carcinogenic potential and is also a predisposing factor for HCC recurrence28. Patients with pre-existing cirrhosis have been reported to have lower rates of recurrence-free survival at 3 years or later, suggesting that underlying liver status has an effect on LR29. This finding supports our observation that patients with liver cirrhosis, which denotes poor liver function, are more susceptible to LR. According to our results, the presence of vascular tumor invasion and liver cirrhosis may serve as prognostic factors for ER and LR, respectively.
Using a Drosophila melanogaster malignant tumor model, Katheder et al.24 recently demonstrated that dormant autophagy-deficient and growth-impaired tumors are capable of reactivating tumor growth when transplanted into an autophagy-efficient host, suggesting that autophagy in the microenvironment impacts tumor growth. Although many studies have revealed that LC3 expression in HCC tissues is higher than in non-tumor tissues and have associated LC3 expression in HCC tissues with tumor development and prognosis14, 18, 30, the relationship between LC3 expression in the non-tumor microenvironment and HCC progression has not been discussed in the literature. Here, we showed that the high LC3 expression in the tumor and ANT microenvironments have additional protective effects against HCC recurrence. Our results clearly demonstrate the importance and potential role of LC3 expression in the tumor and non-tumor liver microenvironments in the prognosis of HCC recurrence. This study is the first to demonstrate that LC3 expression in the non-tumor liver microenvironment is significantly associated with HCC recurrence and that the low LC3 expression in both tumor and ANT tissues significantly increases the risk of HCC recurrence.
Our findings indicate that patients with low LC3 expression in both tumor and ANT tissues are significantly more likely to experience first and second HCC recurrence. All the patients with a loss-of-LC3 staining in tumor and ANT tissues experienced recurrent HCC. The loss-of-LC3 in tumor and ANT tissues was also associated with a high risk of HCC recurrence. Regardless of any other HCC tumor characteristics or the status of ANT tissues in the liver, a low LC3 expression in tumor and ANT tissues at the time of surgery was associated with a significantly increased risk of HCC recurrence. This finding suggests that autophagy-related marker LC3 predicts HCC recurrence.
Our results revealed that the high LC3 in both the tumor and liver microenvironments provides patients who undergo curative hepatectomy with a survival advantage against HCC recurrence. However, there are some limitations to the current study. First, this study used a retrospective design, which could have resulted in unintended bias. Second, the findings of this study must still be validated in Western populations with different ethnicities. Third, the involvement of LC3 expression in the tumor and non-tumor microenvironment in limiting tumorigenesis related to HCC recurrence and its underlying mechanism related to HCC need further investigation in vivo and in vitro.
In summary, the low LC3 expression in both the tumor and non-tumor liver microenvironments was significantly associated with a very high risk of HCC recurrence in patients who underwent curative hepatectomy for HCC. Different factors were associated with early and late recurrence: while the presence of vascular tumor invasion was associated with ER, the liver microenvironment was associated with LR. This study is the first to demonstrate that, in addition to the tumor microenvironment, assessment of autophagy-related markers in the non-tumor liver microenvironment is very important for predicting HCC recurrence. The analysis of LC3 expression in tumor and ANT tissues, in conjunction with an assessment of the presence of vascular tumor invasion and liver cirrhosis, could identify patients at risk of HCC recurrence after curative resection. Our results indicated that autophagy-related marker LC3 is significantly associated with HCC recurrence and that LC3 may serve as a potential biomarker for predicting HCC recurrence.
Study highlights
What is current knowledge
Autophagy is involved in the physiology and pathogenesis of human disease, including HCC.
Autophagy-related markers, such as LC3 and Beclin-1 are used as prognostic factors of HCC.
The impact of autophagy-related markers on post-operative HCC recurrence is not documented.
What is new here
LC3 expression in the tumor and liver microenvironments is associated with risk of post-operative HCC recurrence.
The tumor characteristics and liver microenvironments have effects on early and late recurrence, respectively.
Translational impact
LC3 may serve as a potential biomarker for predicting post-operative HCC recurrence.
Electronic supplementary material
Acknowledgements
We thank Yu-Chan Li, Bao-Sheng Hou, and Shuting Lin for the collection and analysis of data. This work was financially and partly supported by the "Center for Intelligent Drug Systems and Smart Bio-devices" from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education in Taiwan. We also thank Liver Disease Prevention and Treatment Research Foundation, Taiwan for part financial support.
Conflict of interest
Guarantor of the article: Ming-Lung Yu.
Specific author contributions: Lin C.W. performed the experiments, collected the patient information and data, analyzed the data, and wrote the manuscript together with Chen Y.S., Lin C.C., Lee P.H., Lo G.H., Hsu C.C., Hsieh P.M., Koh K.W., Chou T.C., Dai C.Y., Huang J.F., Chuang W.L., and Chen Y.L. Yu M.L. designed the study and wrote the manuscript together with Lin C.W. All of the authors made important suggestions to the manuscript and had reviewed and approved the final version of the manuscript, including the authorship list.
Financial support: This study was supported by grants from MOST 103-2314-B-037-061-MY3, MOST 103-2314-B-650-005-MY2, MOST 105-2314-B-037-062-MY2, MOST 105-2314-B-650-004-MY3, and MOST 106-2314-B-037-074, E-Da Hospital (EDAHP106036, EDAHP106048, EDAHP106054, EDAHP107040, EDAHP107041, and EDAHP107064), Kaohsiung Medical University (107CM-KMU-06 and KMU-DK107004) and Kaohsiung Medical University Hospital (MOHW 107-TDU-B-212-123006).
Potential competing interests: None.
Footnotes
These authors contributed equally: Yao-Li Chen and Ming-Lung Yu
Electronic supplementary material
The online version of this article (10.1038/s41424-018-0033-4) contains supplementary material, which is available to authorized users.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.El-Serag HB. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology. 2012;142:1264–1273 e1. doi: 10.1053/j.gastro.2011.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forner A, Llovet JM, Bruix J. Hepatocellular carcinoma. Lancet. 2012;379:1245–1255. doi: 10.1016/S0140-6736(11)61347-0. [DOI] [PubMed] [Google Scholar]
- 3.Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat. Rev. Gastroenterol. Hepatol. 2010;7:448–458. doi: 10.1038/nrgastro.2010.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin CW, et al. Heavy alcohol consumption increases the incidence of hepatocellular carcinoma in hepatitis B virus-related cirrhosis. J. Hepatol. 2013;58:730–735. doi: 10.1016/j.jhep.2012.11.045. [DOI] [PubMed] [Google Scholar]
- 5.DOH. ROC. Report of Leading Cancer-related Death; 2012.
- 6.Roayaie S, et al. Resection of hepatocellular cancer </=2 cm: results from two Western centers. Hepatology. 2013;57:1426–1435. doi: 10.1002/hep.25832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forner A, et al. Diagnosis of hepatic nodules 20 mm or smaller in cirrhosis: prospective validation of the noninvasive diagnostic criteria for hepatocellular carcinoma. Hepatology. 2008;47:97–104. doi: 10.1002/hep.21966. [DOI] [PubMed] [Google Scholar]
- 8.Czaja MJ, et al. Functions of autophagy in normal and diseased liver. Autophagy. 2013;9:1131–1158. doi: 10.4161/auto.25063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schneider JL, Cuervo AM. Liver autophagy: much more than just taking out the trash. Nat. Rev. Gastroenterol. Hepatol. 2014;11:187–200. doi: 10.1038/nrgastro.2013.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Y, et al. The prognostic value of autophagy-related markers beclin-1 and microtubule-associated protein light chain 3B in cancers: a systematic review and meta-analysis. Tumor Biol. 2014;35:7317–7326. doi: 10.1007/s13277-014-2060-4. [DOI] [PubMed] [Google Scholar]
- 11.Rebecca VW, Amaravadi RK. Emerging strategies to effectively target autophagy in cancer. Oncogene. 2016;35:1–11. doi: 10.1038/onc.2015.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bao L, et al. Impaired autophagy response in human hepatocellular carcinoma. Exp. Mol. Pathol. 2014;96:149–154. doi: 10.1016/j.yexmp.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding ZB, et al. Association of autophagy defect with a malignant phenotype and poor prognosis of hepatocellular carcinoma. Cancer Res. 2008;68:9167–9175. doi: 10.1158/0008-5472.CAN-08-1573. [DOI] [PubMed] [Google Scholar]
- 14.Lee YJ, et al. The autophagy-related marker LC3 can predict prognosis in human hepatocellular carcinoma. PLoS ONE. 2013;8:e81540. doi: 10.1371/journal.pone.0081540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu DM, et al. The expression of beclin-1, an autophagic gene, in hepatocellular carcinoma associated with clinical pathological and prognostic significance. BMC Cancer. 2014;14:327. doi: 10.1186/1471-2407-14-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen KD, et al. Interconnections between autophagy and the coagulation cascade in hepatocellular carcinoma. Cell Death Dis. 2014;5:e1244. doi: 10.1038/cddis.2014.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi YH, et al. Prognostic significance of Beclin 1-dependent apoptotic activity in hepatocellular carcinoma. Autophagy. 2009;5:380–382. doi: 10.4161/auto.5.3.7658. [DOI] [PubMed] [Google Scholar]
- 18.Wu DH, et al. Autophagic LC3B overexpression correlates with malignant progression and predicts a poor prognosis in hepatocellular carcinoma. Tumour Biol. 2014;35:12225–12233. doi: 10.1007/s13277-014-2531-7. [DOI] [PubMed] [Google Scholar]
- 19.Lee YJ, Jang BK. The role of autophagy in hepatocellular carcinoma. Int. J. Mol. Sci. 2015;16:26629–26643. doi: 10.3390/ijms161125984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bissell MJ. Context matters. Trends Cancer. 2015;1:6–8. doi: 10.1016/j.trecan.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 21.Rice J. Metastasis: the rude awakening. Nature. 2012;485:S55–S57. doi: 10.1038/485S55a. [DOI] [PubMed] [Google Scholar]
- 22.Li H, Zhang L. Liver regeneration microenvironment of hepatocellular carcinoma for prevention and therapy. Oncotarget. 2016;8:1805–1813. doi: 10.18632/oncotarget.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshimoto S, et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature. 2013;499:97–101. doi: 10.1038/nature12347. [DOI] [PubMed] [Google Scholar]
- 24.Katheder NS, et al. Microenvironmental autophagy promotes tumour growth. Nature. 2017;541:417–420. doi: 10.1038/nature20815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bruix J, Sherman M. Practice guidelines committee AAftSoLD. Manag. Hepatocell. Carcinoma Hepatol. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 26.Pugh RNH, et al. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973;60:646–649. doi: 10.1002/bjs.1800600817. [DOI] [PubMed] [Google Scholar]
- 27.Yokota J. Tumor progression and metastasis. Carcinogenesis. 2000;21:497–503. doi: 10.1093/carcin/21.3.497. [DOI] [PubMed] [Google Scholar]
- 28.Poon TPR, Fan ST, Wong J. Risk factors, prevention, and management of postoperative recurrence after resection of hepatocellular carcinoma. Ann. Surg. 2000;232:10–24. doi: 10.1097/00000658-200007000-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sasaki Y, et al. Influence of coexisting cirrhosis on long-term prognosis after surgery in patients with hepatocellular carcinoma. Surgery. 1992;112:515–521. [PubMed] [Google Scholar]
- 30.Wu W, et al. Clinical significance of autophagic protein LC3 levels and its correlation with XIAP expression in hepatocellular carcinoma. Med. Oncol. 2014;31:108. doi: 10.1007/s12032-014-0108-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.