Abstract
Thymic carcinoma (TC) is a rare malignant tumor of the mediastinum with occult onset, rapid development, and poor prognosis. Surgery is the main treatment for early TC, but the majority of patients are diagnosed at Masaoka‐Koga stage III or IV with local invasion or distant metastasis. Platinum and anthracyclines are currently considered key components of first‐line chemotherapy for advanced TC; however, there are no standard treatment plans for patients who are refractory to first‐line and further chemotherapy. The clinical effect is also unsatisfactory. Apatinib has been successfully applied as third‐line treatment for advanced gastric cancer and has shown high efficacy in the treatment of various cancers, such as lung, liver, and colorectal cancers. Herein we report a case of advanced thymic squamous cell carcinoma harboring EGFR exon 20 insertion in which apatinib was administered after multi‐line chemotherapy and radiotherapy and a partial response was achieved after five months of treatment. To date, a five month overall response and 10 months of progression‐free survival have been achieved. Adverse reactions can be controlled and the patient's quality of life has improved. Apatinib provides a new option for clinicians to treat patients with advanced TC.
Keywords: Advanced thymic carcinoma (TC), anti‐angiogenic therapy, apatinib, chemotherapy, immunotherapy
Introduction
Thymic epithelial tumors (TETs) are rare cancers with an annual incidence of 1.3–1.7 per million, and are mainly composed of thymomas and thymic carcinomas (TC). Primary TCs account for 10% to 20% of TETs, can occur in all age groups, and incidence in men is slightly higher than in women.1, 2, 3 The histological behavior of TC is obviously malignant, which differs from thymomas. It features significant cell atypia, no organ‐like structure, strong invasion, and poor prognosis. The five‐year survival rate is 40%.4 According to histological differentiation, TC can be divided into squamous cell, basal cell, mucoepidermoid, sarcomatoid, papillary, and undifferentiated carcinomas, lymphoepithelioma, and other types. Most cases of TC are squamous cell carcinoma.5 Early TC has no specific symptoms; our case was detected in a mediastinal mass during physical examination. Tumors that increase to a certain size will oppress or invade the surrounding tissues and organs, causing chest tightness, chest pain, cough, and other symptoms. A small number of patients visit a doctor because of the emergence of superior vena cava syndrome. Comprehensive treatment methods include surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy.
The occurrence and development of a malignant tumor is closely related to its angiogenesis.6 The VEGF/VEGFR signaling pathway is the most important regulatory pathway that induces angiogenesis and is also the key target of a variety of anti‐angiogenic drugs.7 Apatinib is a novel small molecule tyrosine kinase inhibitor that acts highly selectively on VEGFR‐2, inhibits tumor angiogenesis, and thus plays an antitumor role.8 Clinical trial results of phase I–III trials of apatinib administered to patients with advanced gastric cancer that had experienced standard chemotherapy failure achieved improved progression‐free survival (PFS) and overall survival (OS). Therefore, the China Food and Drug Administration approved apatinib in October 2014 for third‐line treatment of advanced gastric cancer. Basic and clinical studies of apatinib alone or in combination with other drugs to treat various cancers, such as lung, liver, stomach, colorectal, and breast cancers, are underway. Herein we report a case of advanced thymic squamous cell carcinoma in which a response was achieved using apatinib after multi‐line chemotherapy and radiotherapy. To the best of our knowledge, this is the first report of successful treatment of advanced TC with apatinib.
Case report
A mediastinal mass was found during the physical examination of a 52‐year‐old Asian man with a 40‐year history of smoking 20 cigarettes per day. The patient presented to the Chinese People's Liberation Army General Hospital (Beijing 301 Hospital) four years ago. A positron emission tomography/computed tomography (PET‐CT) scan showed a high metabolic soft tissue mass shadow measuring 8.3 × 6.1 cm in the mediastinum, consistent with signs of TC, and multiple metastases in the lymph nodes of the right supraclavicular fossa and mediastinal, right pleura, and right lung. Histopathology combined with immunohistochemistry results showed non‐keratinizing squamous cell carcinoma and a diagnosis of Masaoka‐Koga stage IVB thymic squamous cell carcinoma was made. From November 2013 to March 2014, six cycles of first‐line chemotherapy of paclitaxel liposome (135 mg/m2 d1) and cisplatin (75 mg/m2 d1) were administered, and the best efficacy achieved was a partial response (PR). In the course of chemotherapy, volumetric intensity‐modulated arc therapy for metastasis was conducted (DT: 56Gy / 25F). A CT scan taken in October 2015 showed progressive disease (PD) with increased metastasis and five cycles of second‐line chemotherapy of paclitaxel (135 mg/m2 d1) and carboplatin (AUC 5 d1) were administered. A CT scan taken in July 2016 showed PD at the mediastinal tumor, with an increased diameter. Six cycles of third‐line pemetrexed (500 mg/m2 d1) and cisplatin (75mg/m2 d1) chemotherapy were administered up to December 2016.
A CT scan taken in January 2017 showed a trend of PD and the patient attended our hospital to obtain alternative treatment. The patient exhibited the characteristic facial appearance of a person with chronic disease, including a dull, dark and pale face, and dim eyes. His consciousness was clear, there was no yellow staining of the skin and mucous membrane, and no swelling of superficial lymph nodes. He had no history of heart disease, high blood pressure, or diabetes. We performed comprehensive imaging and a pathological examination. An emission computed tomography (ECT) scan showed bone metastases with abnormal radiation concentration on the right side of the seventh rib. Chest enhanced CT showed that the mediastinal mass was consistent with signs of malignant cancer and multiple metastases in the lymph nodes of the right hilar and mediastinal, right pleura, right intercostal muscle, and right lung (Fig 1a). Head CT and B‐mode ultrasound of the abdomen and neck revealed no obvious abnormalities. Histopathology combined with immunohistochemistry results (CD5‐, CD117+, TTF‐1‐, P63+, P40+, CK5/6+, CK7+, CK8/18+, Ki‐67+) showed non‐keratinizing squamous cell carcinoma (Fig 2a,c–h). We performed next generation sequencing and examined PD‐L1 expression using the same biopsy tissue sample, which showed EGFR exon 20 insertion (770‐771insGly) and JAK2 exon 17 missense (Lys752Thr) mutations, but PD‐L1 expression was negative (Table 1, Figs 2b, 3). An EGFR exon 20 insertion mutation is considered a primary drug resistant mutation of tyrosine kinase inhibitors (TKIs), such as gefitinib and erlotinib.9 There is currently no matched targeted therapy for JAK2 exon 17 missense mutations, but such mutations may be a simultaneous mechanism of acquired resistance to immunotherapy.10 Finally, we considered anti‐angiogenic therapy. The patient was administered oral apatinib at a dose of 850 mg/day. After five months of treatment with apatinib, the anterior mediastinum mass and the metastatic lesions became smaller compared to the baseline (Fig 1b). The patient achieved PR with a 31% reduction in tumor size. During the course of treatment, the dose was adjusted to 425 mg/day because of grade 2 hypertension and proteinuria, and grade 3 hand‐foot syndrome; the original dose was resumed after three weeks of remission. To date, the patient has achieved a five month overall response and 10 months of PFS (Fig 1c). He was in a good general condition after the initiation of apatinib (data lock, December 2017). The treatment timeline is shown in Figure 4.
Figure 1.
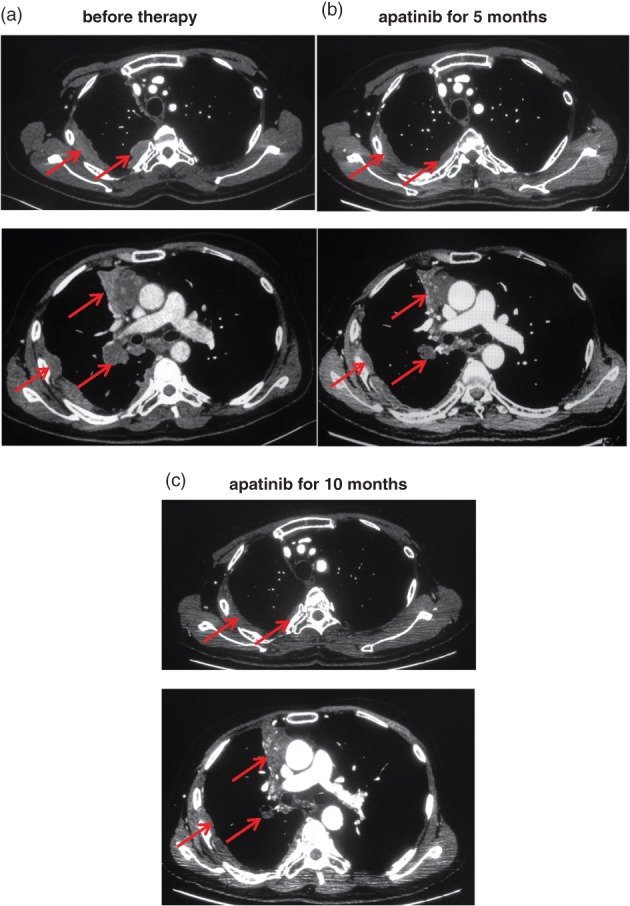
Chest enhanced computed tomography (CT) scans before and after apatinib therapy. (a) Chest enhanced CT of different layers taken before apatinib therapy revealed a soft tissue mass in the mediastinum and multiple metastases in the lymph nodes of the right hilar and mediastinal, right pleura, right intercostal muscle, and right lung. (b) After five months of apatinib treatment, chest enhanced CT showed that the mediastinal mass and the metastatic lesions had become smaller compared to the baseline. The patient achieved a partial response with a 31% reduction of the tumor. (c) After 10 months of apatinib treatment, the mediastinal mass and metastatic lesions reduced further and cavities formed. To date, a five month overall response and 10 months of PFS have been achieved.
Figure 2.
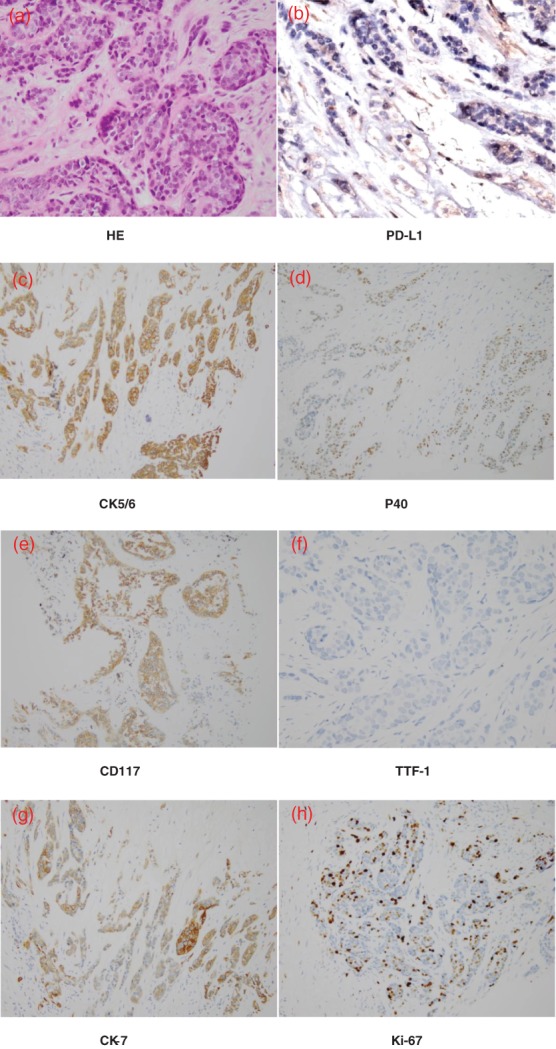
Biopsy pathology showed non‐keratinizing squamous cell carcinoma. (a) Hematoxylin and eosin (H&E); (b–h) immunohistochemistry: PD‐L1 (−); CK5/6(+); P40(+); CD117(+); TTF‐1(−); CK7(+); Ki‐67(+), (magnification 200×).
Table 1.
NGS and IHC results of biopsy tissue sample
| Item | Abundance | Result | Method |
|---|---|---|---|
| EGFR | 1.1% | C.2310_2311insGGT P.Asp770_Asn771insGly | NGS |
| ALK | 0 | –– | NGS |
| ERBB2 | 0 | –– | NGS |
| BRAF | 0 | –– | NGS |
| MET | 0 | –– | NGS |
| RET | 0 | –– | NGS |
| ROS1 | 0 | –– | NGS |
| KRAS | 0 | –– | NGS |
| JAK2 | 24.6% | C.2255A > C P.Lys752Thr | NGS |
| PD‐L1 | 0 | –– | IHC |
IHC, immunohistochemistry; NGS, next generation sequencing.
Figure 3.

The Integrative Genomics Viewer screenshots display next‐generation sequencing results. (a) EGFR exon 20 insertion mutation (C.2310_2311insGGT P.Asp770_Asn771insGly); (b) JAK2 exon 17 missense mutation (C.2255A > C P.Lys752Thr).
Figure 4.
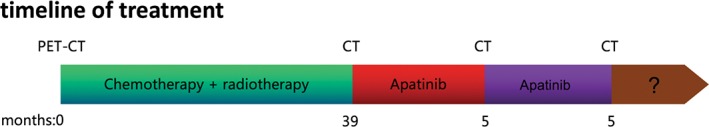
The various treatments the patient received and the duration of each treatment. PET‐CT, positron emission tomography‐computed tomography.
The patient provided informed consent and the hospital ethics committee approved the study.
Discussion
Thymic carcinoma is a clinically rare mediastinal malignant tumor with strong invasiveness and high malignancy. Local invasion and even distant metastasis have often occurred by the time of diagnosis. Masaoka stage III and IV TC cases account for 75% of all cancer cases at the Memorial Sloan Kettering Cancer Center.11 Surgery is the primary treatment for TC and offers the greatest chance of cure. However, in patients with advanced TC for whom resection is not an option, evidence suggests that concurrent chemoradiation may be more beneficial than sequential chemoradiation or chemotherapy alone.12 Platinum and anthracyclines are currently considered key components of first‐line chemotherapy for advanced TC and are thus the most common chemotherapy regimens applied.13 There is no standard second‐line chemotherapy for TC. In a study by Liang et al., pemetrexed achieved favorable results, with median PFS and OS of 6.5 and 12.7 months, respectively.14
In recent years, targeted therapy and immunotherapy for TC have drawn increasing attention. We tested gene mutation and PD‐L1 expression in this patient, which showed EGFR exon 20 insertion (770‐771insGly) and JAK2 exon 17 missense (Lys752Thr) mutations, but negative PD‐L1 expression. EGFR overexpression is common in thymoma and TC, but EGFR mutations are rare. Only three in a study of 158 patients had EGFR mutations, including two cases of L858R mutation and one case of G863D mutation in exon 21.15 Mutations in these two loci have been reported in cases of non‐small cell lung cancer (NSCLC) and TKI treatment has been effective.16 EGFR 20 exon insertion mutations can be divided into two subtypes: Ala767 to Cys775, and Glu761 to Met766 mutations. The former are considered primarily resistant to TKIs, while the latter are likely to be sensitive to TKI treatment.9 Anti‐PD‐1 or anti‐PD‐L1 are reported to exert a significant effect in various tumors, such as malignant melanomas, NSCLC, and ovarian and bladder cancers.17 In a phase II clinical trial of pembrolizumab (a PD‐1 inhibitor) of 40 TC patients refractory to at least one prior chemotherapy regimen, 1 (3%) achieved complete response, 8 (20%) had PR, and 21 (53%) had stable disease. The overall response rate was 22.5% and the most common grade 3 or 4 adverse events were increased transaminases. High PD‐L1 expression (defined as at least 50% of tumor cells with positive staining) was observed in 25% of the 40 TC patients. High PD‐L1 expression in tumor cells is currently the most relevant predictor of the therapeutic effect of anti‐PD‐1 and anti‐PD‐L1. Several studies have shown that patients with high PD‐L1 expression have significantly better therapeutic reactivity than those with no or low expression.18, 19
It has been reported that VEGF‐A, VEGFR‐1 and VEGFR‐2 are overexpressed in thymoma and TC patients.20 VEGFR‐2 is mainly expressed in endothelial cells and plays an important role in the regulation of cell mitosis, angiogenesis, and VEGF diffusion; thus VEGFR‐2 is considered most closely related to tumor angiogenesis.21 Apatinib competitively inhibits the binding of VEGF to VEGFR‐2 and subsequent autophosphorylation of VEGFR‐2 by binding to VEGFR‐2, thereby exerting potent antitumor effects. in vitro experiments showed that apatinib also moderately inhibited platelet‐derived growth factor receptor‐β, c‐Kit, Ret, and c‐src tyrosine kinase activity.8 A phase III study of 267 patients with advanced gastric cancer was randomized to either an apatinib‐treated (850 mg/day) or placebo‐controlled group, and showed significant prolonged median OS (6.5 vs. 4.7 months, P = 0.015; hazard ratio [HR] = 0.709; 95% confidence interval [CI] 0.537–0.937; P = 0.016) compared with the placebo, while PFS was also significantly prolonged (2.6 vs. 1.8 months, P < 0.001; HR = 0.444; 95% CI 0.331–0.595; P < 0.001). Common adverse reactions are grade 1–2 hypertension, proteinuria, and hand‐foot syndrome, most of which can be controlled.22 At present, apatinib has been successfully applied as third‐line treatment for advanced gastric cancer and has shown high efficacy in the treatment of various cancers, such as lung, liver, and colorectal cancers. However, because of the small number of samples in most studies, precise biological targets that predict clinical efficacy have not been identified and require more attention from researchers.
To the best of our knowledge, we report the first clinical evidence that apatinib has been used to successfully treat advanced thymic squamous cell carcinoma. After multi‐line chemotherapy and radiotherapy, apatinib was administered to treat advanced thymic squamous cell carcinoma harboring an EGFR exon 20 insertion and achieved PR after five months of treatment. To date, a five‐month duration of overall response and 10‐month PFS have been achieved. Adverse reactions can be controlled and the patient's quality of life is improved. Apatinib provides a new option for clinicians for the treatment of patients with advanced TC.
Disclosure
No authors report any conflict of interest.
Acknowledgments
This work was supported by funding from the Natural Science Foundation of Tianjin (No. 13JCYBJC23600) and the Science and Technique Foundation of Tianjin Public Health Bureau (No. 2015KZ085).
Contributor Information
Su Yudong, Email: suyudongdoc@foxmail.com.
Chen Peng, Email: pengchentj@163.com.
References
- 1. Siesling S, van der Zwan JM, Izarzugaza I et al Rare thoracic cancers, including peritoneum mesothelioma. Eur J Cancer 2012; 48: 949–60. [DOI] [PubMed] [Google Scholar]
- 2. Engels EA. Epidemiology of thymoma and associated malignancies. J Thorac Oncol 2010; 5(10 Suppl 4): S260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Weissferdt A, Moran CA. Thymic carcinoma, part 1: A clinicopathologic and immunohistochemical study of 65 cases. Am J Clin Pathol 2012; 138 (1): 103–14. [DOI] [PubMed] [Google Scholar]
- 4. de Jong WK, Blaauwgeers JL, Schaapveld M, Timens W, Klinkenberg TJ, Groen HJ. Thymic epithelial tumours: A population‐based study of the incidence, diagnostic procedures and therapy. Eur J Cancer 2008; 44: 123–30. [DOI] [PubMed] [Google Scholar]
- 5. Hayashi A, Fumon T, Miki Y, Sato H, Yoshino T, Takahashi K. The evaluation of immunohistochemical markers and thymic cortical microenvironmental cells in distinguishing thymic carcinoma from type B3 thymoma or lung squamous cell carcinoma. J Clin Exp Hematop 2013; 53: 9–19. [DOI] [PubMed] [Google Scholar]
- 6. Jayson GC, Kerbel R, Ellis LM, Harris AL. Antiangiogenic therapy in oncology: Current status and future directions. Lancet 2016; 388: 518–29. [DOI] [PubMed] [Google Scholar]
- 7. Koch S, Tugues S, Li X, Gualandi L, Claesson‐Welsh L. Signal transduction by vascular endothelial growth factor receptors. Biochem J 2011; 437: 169–83. [DOI] [PubMed] [Google Scholar]
- 8. Chen P, Iruela‐Arispe L, Lou L, Sun P, Yuan K. VEGFR inhibitor YN968D1 xenograft dose response studies against human colon cancer Ls174t and HT29. Proc Am Assoc Cancer Res 2006; 47(Suppl): Abstract 1764. [Google Scholar]
- 9. Pan Y, Zhang Y, Li Y et al Prevalence, clinicopathologic characteristics, and molecular associations of EGFR exon 20 insertion mutations in East Asian patients with lung adenocarcinoma. Ann Surg Oncol 2014; 21 (Suppl 4): S490–6. [DOI] [PubMed] [Google Scholar]
- 10. Zaretsky JM, Garcia‐Diaz A, Shin DS et al Mutations associated with acquired resistance to PD‐1 blockade in melanoma. N Engl J Med 2016; 375: 819–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huang J, Rizk NP, Travis WD et al Comparison of patterns of relapse in thymic carcinoma and thymoma. J Thorac Cardiovasc Surg 2009; 138: 26–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wang CL, Gao LT, Lv CX, Zhu L, Fang WT. Outcome of nonsurgical treatment for locally advanced thymic tumors. J Thorac Dis 2016; 8: 705–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Okuma Y, Saito M, Hosomi Y, Sakuyama T, Okamura T. Key components of chemotherapy for thymic malignancies: A systematic review and pooled analysis for anthracycline‐, carboplatin‐ or cisplatin‐based chemotherapy. J Cancer Res Clin Oncol 2015; 141: 323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liang Y, Padda SK, Riess JW, West RB, Neal JW, Wakelee HA. Pemetrexed in patients with thymic malignancies previously treated with chemotherapy. Lung Cancer 2015; 87: 34–8. [DOI] [PubMed] [Google Scholar]
- 15. Girard N. Thymic tumors: Relevant molecular data in the clinic. J Thorac Oncol 2010; 5 (10 Suppl 4): S291–5. [DOI] [PubMed] [Google Scholar]
- 16. Yamaguchi H, Soda H, Kitazaki T, Tsukamoto K, Hayashi T, Kohno S. Thymic carcinoma with epidermal growth factor gene mutations. Lung Cancer 2006; 52 (2): 261–2. [DOI] [PubMed] [Google Scholar]
- 17. Wang X, Bao Z, Zhang X et al Effectiveness and safety of PD‐1/PD‐L1 inhibitors in the treatment of solid tumors: A systematic review and meta‐analysis. Oncotarget 2017; 8: 59901–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Giaccone G, Kim C, Thompson J et al Pembrolizumab in patients with thymic carcinoma: A single‐arm, single‐centre, phase 2 study. Lancet Oncol 2018; 19: 347–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sunshine J, Taube JM. PD‐1/PD‐L1 inhibitors. Curr Opin Pharmacol 2015; 23: 32–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cimpean AM, Raica M, Encica S, Cornea R, Bocan V. Immunohistochemical expression of vascular endothelial growth factor A (VEGF), and its receptors (VEGFR1, 2) in normal and pathologic conditions of the human thymus. Ann Anat 2008; 190: 238–45. [DOI] [PubMed] [Google Scholar]
- 21. Lignet F, Benzekry S, Wilson S et al Theoretical investigation of the efficacy of antiangiogenic drugs combined to chemotherapy in xenografted mice. J Theor Biol 2013; 320: 86–99. [DOI] [PubMed] [Google Scholar]
- 22. Li J, Qin S, Xu J et al Randomized, double‐blind, placebo‐controlled phase III trial of apatinib in patients with chemotherapy‐refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016; 34: 1448–54. [DOI] [PubMed] [Google Scholar]


