Abstract
Background
EGFR‐tyrosine kinase inhibitors play an important role in the treatment of advanced non‐small cell lung cancer (NSCLC). EGFR mutations in advanced NSCLC occur in approximately 35% of Asian patients and 60% of patients with adenocarcinoma. However, the frequency and type of EGFR mutations in early‐stage lung adenocarcinoma remain unclear.
Methods
We retrospectively collected data on patients diagnosed with lung adenocarcinoma tested for EGFR mutation. Early stage was defined as pathological stage IA–IIIA after radical lung cancer surgery, and advanced stage was defined as clinical stage IIIB without the opportunity for curative treatment or stage IV according to the American Joint Committee on Cancer Staging Manual, 7th edition.
Results
A total of 1699 patients were enrolled in this study from May 2014 to May 2016; 750 were assigned to the early‐stage and 949 to the advanced‐stage group. Baseline characteristics of the two groups were balanced, except that there were more smokers in the advanced‐stage group (P < 0.001). The total EGFR mutation rate in the early‐stage group was similar to that in the advanced‐stage group (53.6% vs. 51.4%, respectively; P = 0.379). There was no significant difference in EGFR mutation type between the two groups. In subgroup analysis of smoking history, there was no difference in EGFR mutation frequency or type between the early‐stage and advanced‐stage groups.
Conclusion
Early‐stage and advanced‐stage groups exhibited the same EGFR mutation frequencies and types.
Keywords: EGFR mutation, lung adenocarcinoma, mutation frequency, mutation type, staging
Introduction
With the development of precision medicine, targeted therapies are playing an increasingly significant role in advanced non‐small cell lung cancer (NSCLC). EGFR is the most important driver gene in NSCLC, especially in Asians. As the first‐line therapy for advanced EGFR‐mutant NSCLC, EGFR‐tyrosine kinase inhibitors (TKIs), including gefitinib, erlotinib, afatinib, and osimertinib, prolong progression‐free survival (PFS) to 9–18 months and have become standard first‐line treatment.1, 2, 3, 4, 5, 6, 7
In addition to advanced‐stage NSCLC, several studies have indicated that EGFR‐TKIs play a role in early‐stage NSCLC. Two recent clinical trials, SELECT and ADJUVENT, demonstrated that adjuvant EGFR‐TKI treatment is feasible in patients with EGFR‐mutant early‐stage NSCLC.8, 9
EGFR mutation status can predict the effects of EGFR‐TKIs.10, 11 EGFR mutations in advanced NSCLC occur in approximately 30% of Asian patients and 60% of female non‐smokers with adenocarcinoma.12, 13, 14 However, the frequency and type of EGFR mutations in early‐stage lung adenocarcinoma remain unclear. In this study, we retrospectively reviewed the clinical characteristics and EGFR status of patients with lung adenocarcinoma to evaluate the differences in EGFR mutation rates and subtypes between early‐stage and advanced‐stage lung adenocarcinoma.
Methods
Patients and study design
All treatment‐naїve patients treated at the Gongdong Lung Cancer Institute/Guangdong General Hospital over the last 10 years signed informed consent permitting a query of their clinical information for the purpose of research.
We retrospectively collected data on patients diagnosed with adenocarcinoma (treatment‐naïve) and tested for EGFR mutations from May 2014 to May 2016 at Guangdong General Hospital. Patients with non‐adenocarcinoma NSCLC, those without EGFR mutations, and those who previously received anti‐tumor treatment or underwent re‐biopsy were excluded. The patients were divided into two groups: early‐stage, defined as pathological stage IA–IIIA (pT1‐3N0‐2M0 or T4N0‐1M0) after radical lung cancer surgery; and advanced‐stage, defined as stage IIIB without the opportunity for curative treatment or stage IV by clinical examination. Tumor stage was categorized according to the American Joint Committee on Cancer (AJCC) Staging Manual, 7th edition. Patients who received concurrent or sequential chemoradiotherapy were excluded.
The EGFR mutation type was categorized into five subgroups: exon 19 deletion, exon 21 L858R mutation, de novo exon 20 T790M mutation, compound mutations, and uncommon mutations (including G719X, L861Q, S768I, and 20 insertions). The definition of a compound mutation was two coexisting EGFR‐sensitive mutations, including exon 19 deletion, L858R, S768I, L861Q, and G719X, in the same patient.
Data collection
The baseline characteristics of all patients, including age, gender, smoking history, pathology, EGFR mutation type, and clinical or pathological stage, were collected from the electronic medical record system of the Gongdong Lung Cancer Institute. In early‐stage NSCLC, T and N staging was based on the results of surgical resection, and in advanced‐stage NSCLC, tumor node metastasis (TNM) staging was based on comprehensive imaging results. EGFR mutations were detected using an amplification refractory mutation system (ARMS) (AmoyDx, XiaMen, China), as previously described.15
Statistical analysis
Differences among subgroups stratified by gender, age, and smoking status were analyzed by chi‐square or Fisher's exact tests, where appropriate. All analyses were performed using SPSS version 22.0 (IBM Corp., Armonk, NY, USA). Two‐sided P values of < 0.05 were considered statistically significant.
Results
Patient characteristics
A flow chart of patient enrolment into the study is shown in Figure 1. A total of 3396 patients underwent EGFR mutation screening. Reasons for exclusion from the study were as follows: non‐adenocarcinoma pathology (n = 786), history of EGFR‐TKI treatment (n = 559), and no EGFR mutation screening (n = 249). Thus, a total of 1699 patients were included in the subsequent analyses. Of the 1699 patients, 750 were assigned to the early‐stage and 949 to the advanced‐stage group. The baseline characteristics of all patients are listed in Table 1. There were more smokers in the advanced‐stage group (P < 0.001), but there was no difference in gender or age.
Figure 1.
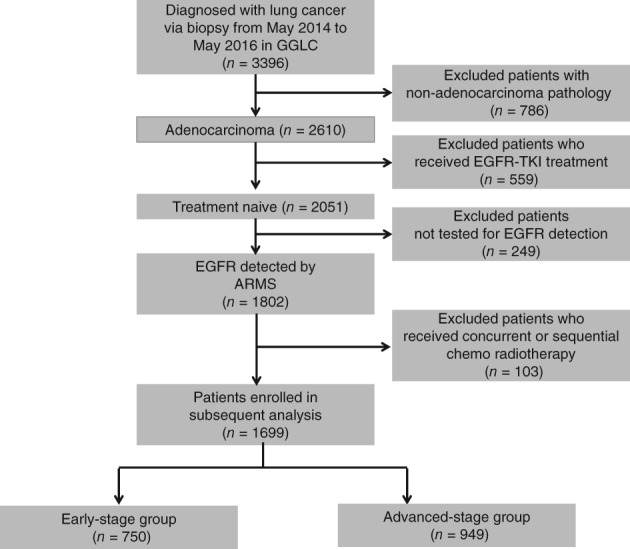
Flow chart of study enrolment. ARMS, amplification refractory mutation system.
Table 1.
Baseline patient characteristics
| Characteristic | Number | P | |
|---|---|---|---|
| Early‐stage (n = 750) |
Advanced‐stage (n = 949) |
||
| Gender | |||
| Male | 406 (54.1%) | 557 (58.7%) | 0.061 |
| Female | 344 (45.9%) | 392 (41.3%) | |
| Age | |||
| ≤ 60 years | 434 (57.9%) | 527 (55.5%) | 0.349 |
| > 60 years | 316 (42.1%) | 422 (44.5%) | |
| Smoking | |||
| Never‐smoker | 527 (70.3%) | 402 (53.7%) | < 0.001 |
| Ever‐smoker | 223 (29.7%) | 347 (46.3%) | |
| EGFR mutation type | |||
| 19DEL | 174 (23.2%) | 217 (22.9%) | 0.908 |
| L858R | 186 (24.8%) | 224 (23.6%) | 0.569 |
| De novoT790M | 10 (1.3%) | 11 (1.2%) | 0.826 |
| Double mutation | 12 (1.6%) | 4 (0.4%) | 0.02 |
| Uncommon mutation | 20 (2.7%) | 32 (3.4%) | 0.479 |
| G719X | 9 | 12 | |
| S768I | 1 | 1 | |
| L861Q | 3 | 3 | |
| 20 insertion | 7 | 16 | |
| Total | 402(53.6%) | 488(51.4%) | P = 0.379 |
Comparison of the EGFR mutation rate between early‐stage and advanced‐stage adenocarcinoma
The EGFR mutation rate was 53.6% (402/750) in the early‐stage and 51.4% (488/949) in the advanced‐stage (P = 0.379) group. The mutation subtypes and rates in the early‐stage group were: exon 19 deletion (23.2%), L858R mutation (24.8%), uncommon mutation (1.6%), de novo T790M mutation (1.3%), and compound mutations (1.6%). There were no significant differences in EGFR mutation subtypes between the two groups, except for compound mutations (early‐stage 1.6% vs. advanced‐stage 0.4%; P = 0.02). The rates of the different EGFR mutation subtypes are listed in Figure 2. We further compared the differences in EGFR mutations between ever‐smokers and never‐smokers within the early‐stage and advanced‐stage groups. No significant differences in EGFR mutation frequency or subtype between the groups were found (Tables S1 and S2).
Figure 2.
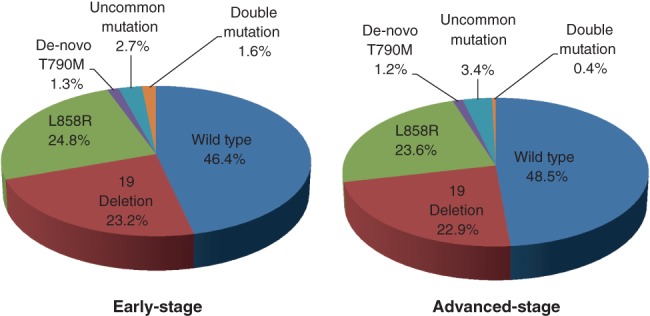
EGFR mutation types and rates in early‐stage and advanced‐stage groups.
EGFR mutation in early‐stage lung adenocarcinoma
The EGFR mutation status at each NSCLC stage is listed in Table 2. The mutation rate ranged from 32.4% (12/37, stage IIB) to 60.2% (171/284, stage IA). The EGFR mutation status was significantly higher in stage IA than in stage IIB (P = 0.002) and stage IIIA (P < 0.001), but there was no difference between stage IA and IB (P = 0.256) or stage IIA (P = 0.107) The EGFR mutation rate in patients with stage IIA–IIIA in the potential adjuvant targeted therapy population was 42.5% (114/268). We further explored the EGFR mutation rates according to lymph node metastasis status (N0, N1 and N2). The EGFR mutation rate was similar among N0, N1, and N2 NSCLC patients (N0: 55.2%, N1: 45.5%, N2: 44.8%; P = 0.391).
Table 2.
EGFR mutation rate at different stages
| Clinical stage | EGFR mutation | EGFR wild type | Mutation rate |
|---|---|---|---|
| IA | 171 | 113 | 60.2% |
| IB | 105 | 87 | 54.7% |
| IIA | 35 | 36 | 49.3% |
| IIB | 12 | 25 | 32.4% |
| IIIA | 67 | 93 | 41.9% |
| Total (IIA–IIIA) | 114 | 154 | 42.5% |
| Lymph node metastasis | |||
| N0 | 288 | 234 | 55.2% |
| N1 | 35 | 42 | 45.5% |
| N2 | 65 | 80 | 44.8% |
Discussion
Research on the differences in EGFR mutation status between early‐stage and advanced‐stage NSCLC is lacking. Herein, we retrospectively analyzed the records of 750 early‐stage and 949 advanced‐stage patients diagnosed with lung adenocarcinoma who received EGFR mutation screening at Guangdong General Hospital from May 2014 to May 2016. The clinical characteristics and EGFR mutation rates and types of these patients were compared. There were no significant differences in EGFR mutation frequency or subtype between early‐stage and advanced‐stage lung adenocarcinoma.
Previous research indicated that EGFR mutation is an “early event,” occurring during the initiation of lung cancer.16 Our research suggests that the EGFR mutation rate and type are similar between early‐stage and advanced‐stage adenocarcinoma patients (53.6% vs. 51.4%, respectively; P = 0.379). There were more never‐smokers in the early‐stage than in the advanced‐stage group (P < 0.001). To eliminate any error caused by smoking status, we compared the never‐smoker and ever‐smoker subgroups within the early‐stage and advanced‐stage groups, respectively. The EGFR mutation rate and type were similar between the never‐smoker and ever smoker subgroups. When considering the whole population, the rate of compound mutations differed significantly between the early‐stage and advanced‐stage groups (P = 0.02). However, in the never‐smoker and ever‐smoker subgroups, the compound mutation rate did not differ significantly between the early‐stage and advanced‐stage groups. This result may have been caused by the small sample size of the compound mutation subgroup. Thus, our results indicate that EGFR mutations detected during the early stage of tumor growth may be an important treatment target, similar to advanced‐stage NSCLC.
The IGNITE study is the largest analysis of real‐world EGFR mutations, with 3382 advanced NSCLC patients from Asia‐Pacific and Russia enrolled.17 The EGFR mutation rate was 49.3% in adenocarcinoma patients. A high EGFR mutation rate in tumors in Asian patients with adenocarcinoma was also reported in the PIONEER prospective study.18 In total, 1482 patients from seven Asian regions were enrolled, and the EGFR mutation rate was 51.4%, consistent with the EGFR mutation rate in advanced adenocarcinoma in our study. Previous studies have reported varying EGFR mutation rates in early‐stage NSCLC patients. A retrospective study enrolled 311 patients with resected lung adenocarcinoma (high‐risk stage IB–IIIA), and the EGFR mutation rate was only 28.3%.19 Another study enrolled 230 patients with stage I–III NSCLC, and the EGFR mutation rate was only 16.9% (39/230).20 Similarly, an EGFR mutation rate of only 20% was detected among 1118 patients with stage I–III lung adenocarcinoma, enrolled from 2002 to 2009.21 Yet another study reported an EGFR mutation rate of 34.5% in 754 patients with stage I–III NSCLC; according to subgroup analysis, the EGFR mutation rate was 38.7% in patients with adenocarcinoma.22 However, in our study, the EGFR mutation rate in stage I–IIIA lung adenocarcinoma was 53.6%. The difference in our EGFR mutation rate from those of previous studies may be explained by the following. First, the study population in the majority of previous studies was non‐Asian, and the EGFR mutation rate is significantly higher in Asians than non‐Asians. Additionally, we focused exclusively on patients with adenocarcinoma. Second, most of the previous studies used a small sample size. Finally, the method of EGFR detection may also have affected the results.
In our study, we used ARMS to detect EGFR mutations. ARMS can be used to detect EGFR mutations in tumor tissue at a frequency as low as 0.1%.23 Other methods, such as direct DNA sequencing, COBAS, and droplet digital PCR are also commonly used worldwide; however, ARMS is the only method approved by the China Food and Drug Administration for EGFR detection in tumor tissue.
Our study has a few limitations. First, this was a respective, single‐center study and thus may not be representative of the general population. Data from multiple centers would be more comprehensive. Second, the number of patients who underwent EGFR mutation screening during the early stages of their disease, especially stage IIA and IIB, was small, which may have affected our results. Finally, we only analyzed EGFR mutation status, while other driver genes such as ALK, ROS1, BRAF, and MET may also be adjuvant treatment targets.
In our study, early‐stage and advanced‐stage groups exhibited the same EGFR mutation frequencies and types.
Disclosure
No authors report any conflict of interest.
Supporting information
Table S1. Baseline characteristics of never‐smokers in early‐stage and advanced‐stage groups.
Table S2. Baseline characteristics of ever‐smokers in early‐stage and advanced‐stage groups.
Acknowledgments
This study was supported by the National Key R&D Program of China (No. 2016YFC1303800), the National Natural Science Foundation of China (No.81572282), the Guangzhou Science and Technology Bureau (No.2014Y2‐00545), the Guangdong Provincial Key Laboratory of Lung Cancer Translational Medicine (No. 2012A061400006), and the Natural Science Foundation of Guangdong Province (No.2015A030313539).
References
- 1. Mok TS, Wu YL, Thongprasert S et al Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–57. [DOI] [PubMed] [Google Scholar]
- 2. Maemondo M, Inoue A, Kobayashi K et al Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–8. [DOI] [PubMed] [Google Scholar]
- 3. Mitsudomi T, Morita S, Yatabe Y et al Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010; 11: 121–8. [DOI] [PubMed] [Google Scholar]
- 4. Zhou C, Wu YL, Chen G et al Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): A multicentre, open‐label, randomised, phase 3 study. Lancet Oncol 2011; 12: 735–42. [DOI] [PubMed] [Google Scholar]
- 5. Rosell R, Carcereny E, Gervais R et al Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–46. [DOI] [PubMed] [Google Scholar]
- 6. Popat S. Osimertinib as first‐line treatment in EGFR‐mutated non‐small‐cell lung cancer. N Engl J Med 2018; 378: 192–3. [DOI] [PubMed] [Google Scholar]
- 7. Park K, Tan EH, O'Byrne K et al Afatinib versus gefitinib as first‐line treatment of patients with EGFR mutation‐positive non‐small‐cell lung cancer (LUX‐Lung 7): A phase 2B, open‐label, randomised controlled trial. Lancet Oncol 2016; 17: 577–89. [DOI] [PubMed] [Google Scholar]
- 8. Pennell NA, Neal JW, Chaft JE et al SELECT: A multicenter phase II trial of adjuvant erlotinib in resected early‐stage EGFR mutation‐positive NSCLC. J Clin Oncol 2014; 32 (5 Suppl): Abstract 7514. [Google Scholar]
- 9. Zhong WZ, Wang Q, Mao WM et al Gefitinib versus vinorelbine plus cisplatin as adjuvant treatment for stage II‐IIIA (N1‐N2) EGFR‐mutant NSCLC (ADJUVANT/CTONG1104): A randomised, open‐label, phase 3 study. Lancet Oncol 2018; 19: 139–48. [DOI] [PubMed] [Google Scholar]
- 10. Lynch TJ, Bell DW, Sordella R et al Activating mutations in the epidermal growth factor receptor underlying responsiveness of non‐small‐cell lung cancer to gefitinib. N Engl J Med 2004; 350: 2129–39. [DOI] [PubMed] [Google Scholar]
- 11. Paez JG, Jänne PA, Lee JC et al EGFR mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science 2004; 304: 1497–500. [DOI] [PubMed] [Google Scholar]
- 12. Wu YL, Zhong WZ, Li LY et al Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non‐small cell lung cancer: A meta‐analysis based on updated individual patient data from six medical centers in mainland China. J Thorac Oncol 2007; 2: 430–9. [DOI] [PubMed] [Google Scholar]
- 13. Shigematsu H, Lin L, Takahashi T et al Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst 2005; 97: 339–46. [DOI] [PubMed] [Google Scholar]
- 14. Rosell R, Moran T, Queralt C et al Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009; 361: 958–67. [DOI] [PubMed] [Google Scholar]
- 15. Zhou Q, Zhang XC, Chen ZH et al Relative abundance of EGFR mutations predicts benefit from gefitinib treatment for advanced non‐small‐cell lung cancer. J Clin Oncol 2011; 29: 3316–21. [DOI] [PubMed] [Google Scholar]
- 16. McGranahan N, Swanton C. Biological and therapeutic impact of intratumor heterogeneity in cancer evolution. Cancer Cell 2015; 27: 15–26 (Published erratum appears in Cancer Cell 2015;28:141). [DOI] [PubMed] [Google Scholar]
- 17. Han B, Tjulandin S, Hagiwara K et al EGFR mutation prevalence in Asia‐Pacific and Russian patients with advanced NSCLC of adenocarcinoma and non‐adenocarcinoma histology: The IGNITE study. Lung Cancer 2017; 113: 37–44. [DOI] [PubMed] [Google Scholar]
- 18. Shi Y, Au JS, Thongprasert S et al A prospective, molecular epidemiology study of EGFR mutations in Asian patients with advanced non‐small‐cell lung cancer of adenocarcinoma histology (PIONEER). J Thorac Oncol 2014; 9: 154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kim IH, Lee IH, Lee JE et al Clinical significance of C‐MET overexpression and epidermal growth factor receptor mutation in platinum‐based adjuvant chemotherapy outcome in surgically resected lung adenocarcinoma. Ann Surg Oncol 2017; 24: 770–7. [DOI] [PubMed] [Google Scholar]
- 20. Ragusa M, Vannucci J, Ludovini V et al Impact of epidermal growth factor receptor and KRAS mutations on clinical outcome in resected non‐small cell lung cancer patients. Am J Clin Oncol 2014; 37: 343–9. [DOI] [PubMed] [Google Scholar]
- 21. D'Angelo SP, Janjigian YY, Ahye N et al Distinct clinical course of EGFR‐mutant resected lung cancers: Results of testing of 1118 surgical specimens and effects of adjuvant gefitinib and erlotinib. J Thorac Oncol 2012; 7: 1815–22. [DOI] [PubMed] [Google Scholar]
- 22. Zhao J, Gao J, Guo L et al [EGFR and KRAS gene mutations in 754 patients with resectable stage I‐IIIa non‐small cell lung cancer and its clinical significance]. Zhongguo Fei Ai Za Zhi 2017; 20: 617–22 (In Chinese). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ellison G, Donald E, McWalter G et al A comparison of ARMS and DNA sequencing for mutation analysis in clinical biopsy samples. J Exp Clin Cancer Res 2010; 29: 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline characteristics of never‐smokers in early‐stage and advanced‐stage groups.
Table S2. Baseline characteristics of ever‐smokers in early‐stage and advanced‐stage groups.


