Abstract
Background
The risk of developing lung cancer is high in patients with interstitial lung disease (ILD), as few treatment options are available. Immune checkpoint inhibitors (ICI) are used for the treatment of non‐small cell lung cancer (NSCLC) in clinical practice; however, in patients with preexisting ILD, the risk of ICI‐related pneumonitis is unknown. We evaluated the efficacy and lung toxicity of nivolumab in patients with NSCLC and ILD.
Methods
We retrospectively reviewed the medical records of 216 NSCLC patients who had received nivolumab therapy. The existence of ILD in these patients was determined by lung computed tomography findings; 26 patients had ILD. We evaluated the efficacy of nivolumab by measuring the response rate (RR), progression‐free survival (PFS) duration, and lung toxicity by incidence, severity, and outcome of nivolumab‐related ILD.
Results
The RR and median PFS of the ILD and non‐ILD groups were 27% versus 13% (P = 0.078) and 2.7 (95% confidence interval [CI], 1.7–5.3) versus 2.9 months (95% CI 2.1–3.4; P = 0.919), respectively. The incidences of total and severe nivolumab‐related pneumonitis were significantly higher in the ILD group than in the non‐ILD group (31% vs. 12%, P = 0.014 and 19% vs. 5%, P = 0.022, respectively). No death from nivolumab‐related pneumonitis occurred. Over 50% of the patients in both groups with nivolumab‐related pneumonitis showed improvement over time.
Conclusion
Relative to the non‐ILD group, nivolumab‐related pneumonitis was observed more frequently in the ILD group; however, most cases were manageable.
Keywords: Chemotherapy, immune checkpoint inhibitor, immunotherapy, interstitial lung disease, non‐small cell lung cancer
Introduction
Patients with interstitial lung disease (ILD) have a twofold higher risk of developing lung cancer than smokers without ILD or chronic obstructive pulmonary disease (COPD).1, 2 Lung cancer patients with ILD have few treatment options because surgery, radiotherapy, and chemotherapy elevate the risk of exacerbation of ILD.3, 4, 5, 6, 7, 8, 9, 10
Treatment‐related pneumonitis is relatively common in Japanese patients with lung cancer.8, 11 In Japan, during treatment for advanced or recurrent lung cancer, the incidence of pneumonitis induced by cytotoxic chemotherapies and molecular targeted agents are reported to be 2–6% and 3.5–4.5%, respectively.7, 8, 11, 12, 13 The mortality rate of chemotherapy‐related pneumonitis is reported as approximately 30% and is believed to be the most common cause of treatment‐related death.14 Moreover, incidence and mortality rates of treatment‐related pneumonitis are higher in patients with preexisting ILD.7, 8, 15
In recent years, immune checkpoint inhibitors (ICIs) have been used for the treatment of non‐small cell lung cancer (NSCLC) in clinical practice.16, 17, 18, 19 The incidence of ICI‐related pneumonitis, including some fatal cases, is reported as 1–12%.16, 17, 18, 19, 20, 21, 22 In contrast to cytotoxic chemotherapy or molecular‐targeted agent‐related pneumonitis, most ICI‐related pneumonitis patients appear to be sensitive to treatment with corticosteroids; however, the risk of ICI‐related pneumonitis in patients with preexisting ILD is unknown.20
Thus, we evaluated the efficacy and lung toxicity of nivolumab in patients with NSCLC and preexisting ILD.
Methods
Patients
We retrospectively reviewed the electronic medical records of NSCLC patients who were treated with nivolumab at three institutions (the National Hospital Organization Kyoto Medical Center, Kyoto University Hospital, and the Japanese Red Cross Fukui Hospital) between 24 December 2015 and 31 December 2016. The study was initiated after approval from the Institutional Review Board of each institution and complied with the principles of the Declaration of Helsinki.
All of the patients were diagnosed with advanced or recurrent NSCLC after surgical resection or radiotherapy and were refractory to one or more chemotherapeutic regimens. The patients were administered at least one dose of nivolumab intravenously (3 mg/kg every two weeks or longer). The observation period was between 24 December 2015 and 30 June 2017. We identified patients with preexisting ILD (ILD group) by reviewing chest computed tomography (CT) images taken just before the initiation of first‐line chemotherapy. Patients with a history of drug‐induced pneumonitis or those with radiation‐related fibrotic lesions alone in the lung were excluded from the ILD group. We obtained clinical data of participating patients from the electronic medical records of each institution, including: age, gender, smoking history, Eastern Cooperative Oncology Group Performance Status (ECOG‐PS), histology, any existing driver gene mutations, prior therapies (including surgery and radiotherapy), and the comorbidity of autoimmune diseases.
Evaluation of preexisting interstitial lung disease (ILD)
We defined the ILD group of patients as those who exhibited at least one of the following CT findings in no less than two lobes of the lung: ground glass appearance, reticular opacity, consolidation (excluding infectious pneumonia), traction bronchiectasis, centrilobular nodularity, and honeycombing.20 As previously reported, the extent of preexisting ILD was measured using a five‐point scale for the upper, middle, and lower zones of the lung (0, none; 1 < 5%; 2, 5–25%; 3, 25–50%; and 4 > 50%).23 Radiological patterns of preexisting ILD were classified following the American Thoracic Society (ATS)/European Respiratory Society (ERS) International Multidisciplinary Classification of Interstitial Pneumonia: usual interstitial pneumonia (UIP), nonspecific interstitial pneumonia (NSIP), cryptogenic organizing pneumonia (COP), and acute interstitial pneumonia (AIP).24 The existence, extent, and radiographic patterns of ILD were assessed by two pulmonologists not directly involved in the treatment of patients and observed by a specialist in idiopathic interstitial pneumonia and another board certified radiologist. Interobserver disagreements were resolved by consensus.
Evaluation of efficacy
The effect of nivolumab treatment was evaluated by measuring the response rate (RR), disease control rate (DCR), and progression‐free survival (PFS) in patients. The RR and DCR were assessed using Response Evaluation Criteria in Solid Tumors version 1.1.25 We defined PFS as the duration between the initiation of nivolumab treatment and the date of confirmation of disease progression or death from any cause. We compared the RR, DCR, and PFS values between the ILD and non‐ILD groups.
Evaluation of toxicity
Nivolumab‐related pneumonitis was defined as the appearance of specific ILD‐related CT findings (as described in the previous section), and was observed between the date of initial administration and one month after the last administration of nivolumab.20 The following data were also reviewed in cases with newly developed nivolumab‐related pneumonitis: time until onset, toxicity grade, radiographic pattern, treatment, and outcome of nivolumab‐related pneumonitis. The toxicity grade of pneumonitis was evaluated according to the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (CTCAE) version 4.0.26 Radiographic patterns were classified into UIP, NSIP, COP, and AIP pattern groups following the ATS/ERS International Multidisciplinary Classification of Interstitial Pneumonia.24 Based on the association between the radiographic pattern of preexisting ILD and nivolumab‐related pneumonitis, the ILD patients were categorized into two types: (i) exacerbation type in which both radiographic patterns of ILD were similar; and (ii) de novo type in which radiographic patterns of nivolumab‐related pneumonitis differed from that of preexisting ILD. We compared the incidence, severity, time to onset, immunosuppressant treatment, and outcome of nivolumab‐related pneumonitis in patients with and without preexisting ILD who developed nivolumab‐related pneumonitis. We also compared the potential risk factors of pneumonitis (smoking, ECOG‐PS, and the radiographic pattern of preexisting ILD) between patients with and without nivolumab‐related pneumonitis in all patients and in the ILD group.8, 27
Statistics
Continuous data are presented as the mean and range, and comparisons were made using Student's t or Mann–Whitney U tests. Categorical data were obtained as counts and percentages, and Fisher's exact tests were used to compare variables. The PFS curves were generated by the Kaplan–Meier method and compared using log‐rank tests. A P value < 0.05 was considered statistically significant, with 95% confidence intervals (CIs). All statistical analyses were performed using R version 3.4.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Baseline characteristics
We reviewed the medical records of 216 NSCLC patients who had received treatment with nivolumab. None of the patients experienced drug‐induced pneumonitis, one patient was observed with radiation‐related fibrotic changes in the lung caused by treatment for esophageal cancer, and one patient developed COP after pulmonary resection but had been cured before the initial chemotherapy; these patients were classified in the non‐ILD group.
The patients were classified into ILD (26 patients, 12%) and non‐ILD (190 patients) groups. The baseline characteristics of all patients are summarized in Table 1. The median age was 69 years and the majority of patients were men (71%) and current or former smokers (79%). Fifty‐six patients (9 in the ILD, 47 in the non‐ILD group) had an ECOG‐PS score of ≥ 2. Eleven patients were comorbid with autoimmune diseases and all cases were rheumatoid arthritis (RA). There were significant differences between the groups regarding the number of patients carrying a positive EGFR mutation (0% vs. 22%; P = 0.003), with RA (27% vs. 2%; P < 0.001), or who had undergone prior thoracic radiotherapy (11% vs. 32%; P = 0.039). The median number of regimens received before nivolumab was administered was 1 in the ILD and 2 in the non‐ILD group (P = 0.001).
Table 1.
Baseline characteristics
| N | Total | ILD group | Non‐ILD group | ||
|---|---|---|---|---|---|
| 216 | 26 | 190 | P | ||
| Age | 69 (30–89) | 71 (55–85) | 69 (30–89) | 0.243 | |
| Gender (Male) | 154 (71) | 20 (77) | 134 (71) | 0.645 | |
| Current of former smoker | 170 (79) | 25 (96) | 145 (76) | 0.02 | |
| ECOG‐PS | 0, 1 | 160 (74) | 17 (65) | 143 (75) | 0.339 |
| ECOG‐PS | ≥ 2 | 56 (26) | 9 (35) | 47 (25) | 0.339 |
| Histology | Adenocarcinoma | 135 (62) | 13 (50) | 122 (64) | 0.424 |
| Squamous | 57 (27) | 11 (42) | 46 (24) | ||
| Other | 23 (1) | 2 (8) | 21 (2) | ||
| Positive EGFR mutation | 42 (19) | 0 (0) | 42 (22) | 0.003 | |
| Positive ALK translocation | 6 (3) | 0 (0) | 6 (3) | > 0.99 | |
| Comorbidity with RA | 11 (5) | 7 (27) | 4 (2) | < 0.001 | |
| Prior curable operation | 40 (19) | 1 (4) | 39 (21) | 0.055 | |
| Thoracic radiotherapy | 63 (29) | 3 (11) | 60 (32) | 0.039 | |
| No. of previous regimens | 2 (1–15) | 1 (1–8) | 2 (1–15) | < 0.001 | |
| Line of chemotherapy | Second | 82 (38) | 18 (69) | 64 (34) | 0.001 |
| Third or later | 134 (62) | 8 (31) | 126 (66) | ||
| Radiographic pattern | NSIP | 14 (54) | |||
| of preexisting ILD | UIP | 12 (46) | |||
Data are shown as median (range) or number (%). Radiographic patterns of interstitial lung disease (ILD) were categorized according to the American Thoracic Society/European Respiratory Society International Multidisciplinary Classification of Interstitial Pneumonia.
ECOG‐PS, Eastern Cooperative Oncology Group‐performance status; NSIP, non‐specific interstitial pneumonia; RA, rheumatoid arthritis; UIP, usual interstitial pneumonia.
Radiographic characteristics of preexisting ILD
The most common radiographic pattern of preexisting ILD, the NSIP pattern, was noted in 14 patients, followed by the UIP pattern in 12 patients. Other patterns were not observed in these patients. Based on the extent (or severity) and location of preexisting ILD, each patient was scored on a scale ranging from 0 to 4 and grouped in three lung zones (Fig 1). For the upper, middle, and lower zones, the median preexisting ILD extension scores were 1 (< 5%), 1 (< 5%), and 2 (5–25%), respectively. All patients in the ILD group had a high ILD extent score in the lower zone of the lung. Of the 26 patients, 20 (77%) had a score of ≤ 2 (5–25%) and 4 (15%) had a score of ≤ 1 (< 5%) in one of the zones. None of the patients achieved a score of 4 (< 50%) for any zone.
Figure 1.
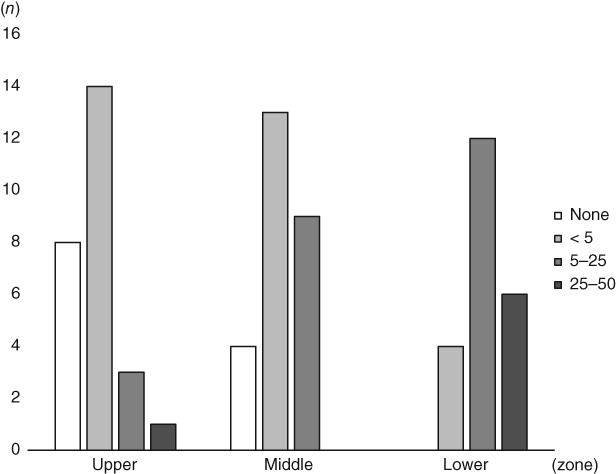
A bar graph indicating the number of patients with their extent scores for preexisting interstitial lung disease (ILD) in the upper, middle, and lower zones. None of the patients achieved a score of 4 (> 50%) in any zone, a score of 3 (25–50%) in the middle zone, or a score of 0 (none) in the lower zone. The proportion of patients with a score of 0 or 1 decreased in the order of the upper, middle, and lower zones.
Efficacy of nivolumab
A median number of five doses of nivolumab were administered to the ILD (range 1—37) and non‐ILD (range 1–33) groups (P = 0.564) (Table 2). Following nivolumab treatment, the RR of the ILD group was 27% versus 13% for the non‐ILD group (P = 0.078). The DCR was 58% versus 44% in the ILD and non‐ILD groups (P = 0.214), respectively. The median PFS rates of the ILD and non‐ILD groups were 2.7 (95% CI 1.7–5.3) and 2.9 (95% CI 2.1–3.4) months (P = 0.919), respectively (Fig 2). There were no significant differences in the RR, DCR, and PFS findings between the two groups.
Table 2.
Efficacy and lung toxicity of nivolumab
| N | Total | ILD group | Non‐ILD group | P | |
|---|---|---|---|---|---|
| 216 | 26 | 190 | |||
| Dose of nivolumab | 5 (1–37) | 5 (1–37) | 5 (1–33) | 0.564 | |
| Best response | CR | 7 (3) | 1 (4) | 6 (3) | 0.205 |
| PR | 25 (12) | 6 (23) | 19 (10) | ||
| SD | 67 (31) | 8 (31) | 59 (31) | ||
| PD | 117 (54) | 11 (42) | 106 (56) | ||
| Disease control rate | 99 (46) | 15 (58) | 84 (44) | 0.214 | |
| Response rate | 32 (15) | 7 (27) | 25 (13) | 0.078 | |
Data are shown as median (range) or number (%). The best response was evaluated according to Response Evaluation Criteria in Solid Tumors version 1.1.
CR, complete response; ILD, interstitial lung disease; PD, progressive disease; PR, partial response; SD, stable disease.
Figure 2.
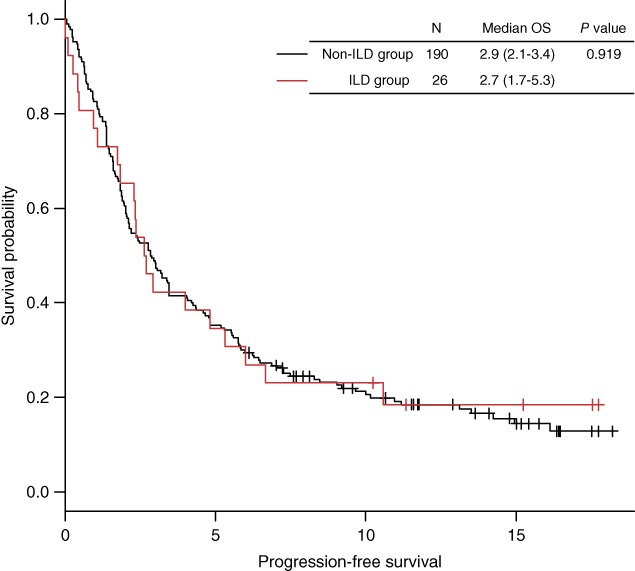
The progression‐free survival (PFS) curve was generated according to the Kaplan–Meier method. The median PFS of the patients with preexisting interstitial lung disease (ILD group) was comparable to that of patients without preexisting ILD (non‐ILD group). The P value was estimated using the log‐rank test.
Incidence, characteristics, treatment, and outcomes of nivolumab‐related pneumonitis
Of the 26 patients with preexisting ILD, eight patients developed nivolumab‐related pneumonitis. Four patients were categorized with the exacerbation type and the remaining four exhibited the de novo type (Table 3). The CT images in Figure 3 present examples of the exacerbation (Case 7) and de novo (Case 6) types of nivolumab‐related pneumonitis. The incidence of nivolumab‐related pneumonitis was significantly higher in the ILD than in the non‐ILD group (31% vs. 12%; P = 0.014) (Table 4). The frequency of severe nivolumab‐related pneumonitis (equivalent to grade ≥ 3, evaluated according to CTCAE v4.0) was significantly higher in the ILD than in the non‐ILD group (19% vs. 5%; P = 0.022). No pneumonitis‐related death was observed in this study. There were no significant differences in the median duration and number of doses of nivolumab until the onset of nivolumab‐related pneumonitis between the groups. COP was the major radiographic pattern of nivolumab‐related pneumonitis in both groups. Immunosuppressants, including systemic corticosteroids for the treatment of nivolumab‐related pneumonitis, were initiated for 5 (63%) patients in the ILD and 16 (73%) in the non‐ILD group (P = 0.666). Only one patient in the non‐ILD group was treated with cyclosporine A and infliximab for nivolumab‐related pneumonitis, while the others were treated with systemic corticosteroids alone or without any immunosuppressant. An improvement in symptoms was achieved in over half of the patients who developed nivolumab‐related pneumonitis, regardless of comorbidity with ILD. In all patients, the incidence of nivolumab‐related pneumonitis was significantly higher in men (Table 5). Patients with RA appeared to develop nivolumab‐related pneumonitis more frequently, but this result was not significant (P = 0.05). In the ILD group, the incidence of nivolumab‐related pneumonitis was not significantly influenced by risk factors of drug‐induced pneumonitis, such as age, gender, smoking history, ECOG‐PS, C‐reactive protein, and radiographic patterns of preexisting ILD (Table 5).
Table 3.
Characteristics of nivolumab‐related pneumonitis in patients with preexisting ILD
| Case | Age | Histology | ECOG‐PS | Radiographic pattern of | Category | Severity | Outcome | |
|---|---|---|---|---|---|---|---|---|
| Preexisting ILD | Pneumonitis | |||||||
| 1 | 72 | Squamous | 2 | UIP | AIP | Exacerbation | 3 | Improved |
| 2 | 76 | Adenocarcinoma | 1 | NSIP | AIP | Exacerbation | 4 | Improved |
| 3 | 72 | Adenocarcinoma | 0 | NSIP | COP | De novo | 1 | Improved |
| 4 | 69 | Squamous | 1 | UIP | COP | De novo | 3 | Improved |
| 5 | 62 | Squamous | 1 | UIP | AIP | Exacerbation | 3 | Progressed |
| 6 | 74 | Adenocarcinoma | 0 | NSIP | COP | De novo | 3 | Improved |
| 7 | 55 | Adenocarcinoma | 1 | NSIP | NSIP | Exacerbation | 1 | No change |
| 8 | 65 | Squamous | 0 | NSIP | COP | De novo | 1 | Improved |
All patients were male and current or former smokers. The severity of interstitial lung disease (ILD) was evaluated according to the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
AIP, acute interstitial pneumonia; COP, cryptogenic organizing pneumonia; NSIP, non‐specific interstitial pneumonia; UIP, usual interstitial pneumonia.
Figure 3.
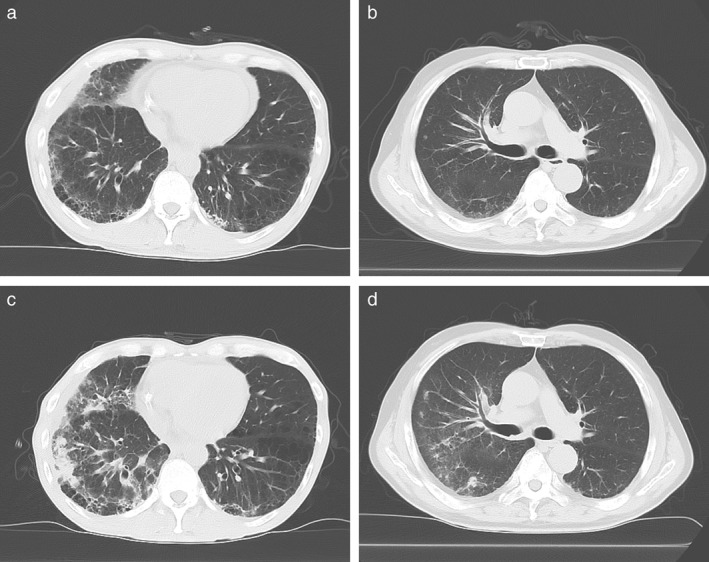
The computed tomography (CT) images of the patient's lung were acquired (a,b) before the initial administration of nivolumab and (c,d) at the onset of nivolumab‐related ILD. (a,c) An example of the exacerbation type (Case 7 in Table 3), in which new ground glass opacities and consolidation are distributed along the site of preexisting ILD. (b,d) An example of the de novo type (Case 6 in Table 3), in which ground glass appearance and consolidation are distributed around lung metastases that are not involved with the preexisting ILD.
Table 4.
Occurrence and treatment course of nivolumab‐induced pneumonitis
| ILD group | |||||||
|---|---|---|---|---|---|---|---|
| Factors | Total | All patterns | UIP pattern | NSIP pattern | Non‐ILD group | P | |
| n | 216 | 26 | 12 | 14 | 190 | ||
| ILD | 30 (14) | 8 (31) | 3 (25) | 5 (36) | 22 (12) | 0.014 | |
| ILD grade | 1, 2 | 15 (50) | 3 (38) | 0 (0) | 3 (60) | 12 (55) | 0.022 |
| 3, 4 | 15 (50) | 5 (62) | 3 (25) | 2 (14) | 10 (45) | ||
| Time to onset (months) | 2.3 (0.07–13.1) | 2.3 (0.7–4.4) | 0.9 (0.7–2.3) | 2.7 (1.1–4.4) | 2.3 (0.07–13.1) | 0.433 | |
| Dose of nivolumab until the onset of ILD | 5.5 (1–28) | 4 (1–10) | 2 (1–5) | 7 (3–10) | 6 (1–28) | 0.384 | |
| Radiographic pattern of | AIP | 5 (17) | 3 (37) | 2 (67) | 1 (20) | 2 (9) | |
| pneumonitis | COP | 18 (60) | 4 (50) | 1 (33) | 3 (60) | 14 (64) | 0.277 |
| NSIP | 7 (23) | 1 (13) | 0 (0) | 1 (20) | 6 (27) | ||
| UIP | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| Requirement of immunosuppressant | 21 (70) | 5 (63) | 3 (100) | 2 (40) | 16 (73) | 0.666 | |
| Outcome | 19 (63) | 6 (74) | 2 (67) | 4 (80) | 13 (59) | 0.851 | |
| 6 (20) | 1 (13) | 0 (0) | 1 (20) | 5 (23) | |||
| 5 (17) | 1 (13) | 1 (33) | 0 (0) | 4 (18) | |||
Data are shown as median (range) or number (%). The toxicity grade was evaluated according to National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0. A comparison between interstitial lung disease (ILD) and non‐ILD groups was performed using Fisher's exact or Mann–Whitney U tests where appropriate.
AIP, acute interstitial pneumonia; COP, cryptogenic organizing pneumonia; NSIP, non‐specific interstitial pneumonia; UIP, usual interstitial pneumonia.
Table 5.
Risk factors of nivolumab‐related pneumonitis in all the patients or ILD group
| N | Total | ILD group | |||||
|---|---|---|---|---|---|---|---|
| Pneumonitis − | Pneumonitis + | Pneumonitis − | Pneumonitis + | ||||
| 186 | 30 | P | 18 | 8 | P | ||
| Age ≥ 75 | 38 (20) | 4 (13) | 0.461 | 5 (28) | 1 (13) | 0.628 | |
| Gender (male) | 127 (68) | 27 (90) | 0.016 | 12 (67) | 8 (100) | 0.132 | |
| Current or former smoker | 143 (77) | 27 (90) | 0.148 | 17 (94) | 8 (100) | > 0.99 | |
| ECOG‐PS ≥ 2 | 51 (27) | 5 (17) | 0.265 | 8 (44) | 1 (13) | 0.19 | |
| Positive EGFR mutation | 38 (20) | 4 (13) | 0.461 | 0 (0) | 0 (0) | > 0.99 | |
| Comorbidity with RA | 7 (4) | 4 (13) | 0.05 | 4 (22) | 3 (38) | 0.635 | |
| Thoracic radiotherapy | 54 (29) | 9 (30) | >0.99 | 2 (11) | 1 (13) | > 0.99 | |
| Line of chemotherapy | Second | 69 (37) | 13 (43) | 0.547 | 13 (72) | 5 (63) | 0.667 |
| Third or later | 117 (63) | 17 (57) | 5 (28) | 3 (38) | |||
| CRP > 50 mg/l | 29 (16) | 3 (10) | 0.583 | 4 (22) | 0 (0) | 0.277 | |
| Total dose of nivolumab | 5.0 (1–30) | 6.0 (1–37) | 0.653 | 5.5 (1–30) | 5.0 (1–37) | 0.978 | |
| With preexisting ILD | 18 (10) | 8 (27) | 0.014 | ||||
| Radiographic pattern of | NSIP | 9 (50) | 5 (63) | 0.683 | |||
| preexisting ILD | UIP | 9 (50) | 3 (38) | ||||
P values were calculated by using Fisher's exact or Mann‐Whitney U tests where appropriate.
CRP, C‐reactive protein;
ECOG‐PS, Eastern Cooperative Oncology Group‐performance status; ILD, interstitial lung disease; NSIP, non‐specific interstitial pneumonia; RA, rheumatoid arthritis; UIP, usual interstitial pneumonia.
Discussion
In this study, we first evaluated the efficacy and incidence of nivolumab‐related pneumonitis in patients with NSCLC and preexisting ILD, and later compared them with patients without preexisting ILD under real‐world conditions. Our findings demonstrate that the RR, DCR, and PFS values associated with nivolumab therapy in patients with preexisting ILD were comparable to those without preexisting ILD. Although the incidences of nivolumab‐related pneumonitis and severe nivolumab‐related pneumonitis were significantly higher in patients with preexisting ILD, the treatment outcome of nivolumab‐related pneumonitis was comparable for patients with and without preexisting ILD. No death resulting from nivolumab‐related pneumonitis was observed.
The RR of nivolumab in the ILD group was higher than in the non‐ILD group but was not statistically significant. PFS in response to nivolumab was similar in both groups. The efficacy of nivolumab in the ILD group was remarkable. This observation contradicts the findings of a previous report demonstrating that the PFS of patients with preexisting ILD undergoing cytotoxic chemotherapies is significantly lower relative to those without preexisting ILD.10 Our result might be influenced by the significantly improved RR of 30% in patients who developed nivolumab‐related pneumonitis versus 12% in those who did not (P = 0.023). However, this finding may be biased because of the low proportion of patients with a poor ECOG‐PS score, which is reported to be associated with the efficacy of nivolumab treatment (17% in patients who developed pneumonitis vs. 27% in those who did not).28 Another pilot trial reported similar favorable efficacy of nivolumab in patients with NSCLC and ILD, and demonstrated a high RR of 50%.29 One reason for the efficacy of nivolumab in the ILD group may be the high proportion of patients without EGFR mutations or with a smoking history (as evidenced in patients with NSCLC and ILD),27 which appeared to be associated with a better treatment outcome in patients treated with ICIs.28, 30 Another explanation may be the tumor mutation burden, as a high tumor mutation burden is associated with a better response to nivolumab.31 Patients with idiopathic pulmonary fibrosis are known to exhibit DNA microsatellite instability, and this may account for the high mutation burden in these patients.32 For these reasons, we believe that treatment with nivolumab may exert favorable effects on the clinical outcomes of patients with NSCLC and ILD.
Although the incidences of total and severe nivolumab‐related pneumonitis were significantly higher in the ILD than in the non‐ILD group, the treatment outcome of nivolumab‐related pneumonitis was similar in both groups. Moreover, most patients who developed nivolumab‐related pneumonitis were sensitive to systemic corticosteroid therapy; only one patient from the non‐ILD group required cyclosporine A and infliximab treatment, but the patient later had a concurrent diagnosis of pneumonitis and tumor invasion.33 The benign outcome of nivolumab‐related pneumonitis in the ILD group was possibly a result of one of the following reasons. First, the majority of patients in the ILD group had a low preexisting ILD extent score; therefore more than two‐thirds of the patients in the ILD group could be treated with nivolumab as second‐line chemotherapy. Second, nivolumab‐related pneumonitis resulted in low mortality and was more responsive to immunosuppressive agents than the pneumonitis induced by cytotoxic chemotherapies or molecular‐targeted agents.8, 15, 20, 21 Finally, frequent chest radiographic examination of preexisting ILD might have led to the early detection of nivolumab‐related ILD, but may also have partially contributed to the higher incidence of nivolumab‐related pneumonitis in the ILD group. The low mortality and benign course of nivolumab‐related pneumonitis suggests that treatment with nivolumab may be tolerable in patients with NSCLC and those exhibiting mild symptoms of preexisting ILD.
In the total cohort, the incidence of nivolumab‐related ILD was significantly higher in men and patients with preexisting ILD. These factors are reported to be potential risk factors of pneumonitis in patients treated with cytotoxic chemotherapies.8, 27 In the ILD group, we could not determine the risk factor responsible for the incidence and severity of nivolumab‐related pneumonitis. We hypothesize that there are two etiologies of nivolumab‐related pneumonitis in the ILD group: (i) the exacerbation type, showing exacerbation of a preexisting ILD condition; and (ii) the de novo type, newly induced by nivolumab and independent of preexisting ILD. Moreover, based on the observation that patients with nivolumab‐related pneumonitis who present a typical radiographic pattern, specifically an AIP pattern, are reported to follow a poorer course of recovery than those with an atypical pattern, we hypothesized that there was an association between the severity and radiographic pattern (or category) of nivolumab‐related pneumonitis;20, 34, 35 however, we did not find any association.
We acknowledge four major limitations of this study. First, this study was retrospective, and some of the baseline characteristics were significantly different between the ILD and non‐ILD groups. Our cohort consisted of patients who were excluded from clinical trials: patients with a poor ECOG‐PS score, preexisting ILD, autoimmune diseases, or no measurable lesion. Second, our patient sample was insufficient to determine factors associated with the development of nivolumab‐related pneumonitis. Third, diagnoses of preexisting ILD and nivolumab‐related pneumonitis were largely dependent on CT findings; thus histological and physiological diagnoses of ILD are lacking. It is believed that CT scans may present an overestimation of the comorbidity rate of ILD. The ILD comorbidity rate in our study was 12%, which is comparable to the previously reported incidence of radiographic interstitial abnormality in patients with NSCLC.9 Considering the higher incidence of comorbidity with ILD in the Japanese population, the incidence of preexisting ILD was reasonable in this study.7, 8, 11 Finally, this cohort only consisted of Japanese patients. As Japanese people may be susceptible to drug‐induced pneumonitis, the results of this study may vary among different ethnic groups.8, 11 Despite these limitations, this multicenter study elucidated the efficacy and lung toxicity of nivolumab treatment in patients with preexisting ILD under real‐world conditions.
The results of our study suggest that the incidence of total and severe nivolumab‐related pneumonitis were higher in NSCLC patients with ILD than in those without ILD. However, nivolumab‐related pneumonitis was manageable in patients exhibiting a mild form of preexisting ILD. Considering the comparable efficacy of nivolumab therapy in patients with NSCLC and preexisting ILD, nivolumab therapy might become a promising treatment option for patients with these conditions; however, careful monitoring for early detection of pneumonitis is required. Additional prospective studies are needed to determine the efficacy and safety of nivolumab treatment in patients with NSCLC and preexisting ILD.
Disclosure
KO received personal fees from Bristol Myers Squibb and Taiho Pharma outside the submitted work. KYH received personal fees from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Chugai Pharmaceutical, Eli Lilly Japan K.K., MSD K.K., Ono Pharmaceutical, Pfizer, and Taiho Pharma outside the submitted work. FK received personal fees from AstraZeneca, Boehringer Ingelheim, and Bristol Myers Squibb outside the submitted work. MT received personal fees from AstraZeneca and Bristol Myers Squibb outside the submitted work. None of the remaining authors report any conflict of interest.
Acknowledgments
This study was supported in part by a grant from the National Hospital Organization's fiduciary funds (for English editing). We would like to thank Ms. Miki Koda for data management and American Journal Experts (http://www.aje.com) for their help with English language editing.
References
- 1. Choi WI, Park SH, Park BJ, Lee CW. Interstitial lung disease and lung cancer development: A 5‐year nationwide population‐based study. Cancer Res Treat 2018; 50: 374–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. de Torres JP, Marín JM, Casanova C et al Lung cancer in patients with chronic obstructive pulmonary disease: Incidence and predicting factors. Am J Respir Crit Care Med 2011; 184: 913–9. [DOI] [PubMed] [Google Scholar]
- 3. Sato T, Watanabe A, Kondo H et al Long‐term results and predictors of survival after surgical resection of patients with lung cancer and interstitial lung diseases. J Thorac Cardiovasc Surg 2015; 149: 64–9. [DOI] [PubMed] [Google Scholar]
- 4. Sato T, Teramukai S, Kondo H et al Impact and predictors of acute exacerbation of interstitial lung diseases after pulmonary resection for lung cancer. J Thorac Cardiovasc Surg 2014; 147: 1604–1611.e3. [DOI] [PubMed] [Google Scholar]
- 5. Chen H, Senan S, Nossent EJ et al Treatment‐related toxicity in patients with early‐stage non‐small cell lung cancer and co‐existing interstitial lung disease: A systematic review. Int J Radiat Oncol 2017; 98: 622–31. [DOI] [PubMed] [Google Scholar]
- 6. Ueki N, Matsuo Y, Togashi Y et al Impact of pretreatment interstitial lung disease on radiation pneumonitis and survival after stereotactic body radiation therapy for lung cancer. J Thorac Oncol 2015; 10: 116–25. [DOI] [PubMed] [Google Scholar]
- 7. Fujimoto D, Kato R, Morimoto T et al Characteristics and prognostic impact of pneumonitis during systemic anti‐cancer therapy in patients with advanced non‐small‐cell lung cancer. PLoS One 2016; 11: e0168465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kudoh S, Kato H, Nishiwaki Y et al Interstitial lung disease in Japanese patients with lung cancer: A cohort and nested case‐control study. Am J Respir Crit Care Med 2008; 177: 1348–57. [DOI] [PubMed] [Google Scholar]
- 9. Nishino M, Cardarella S, Dahlberg SE et al Interstitial lung abnormalities in treatment‐naïve advanced non‐small‐cell lung cancer patients are associated with shorter survival. Eur J Radiol 2015; 84: 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kinoshita T, Azuma K, Sasada T et al Chemotherapy for non‐small cell lung cancer complicated by idiopathic interstitial pneumonia. Oncol Lett 2012; 4: 477–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR‐TKI treatment for EGFR mutation‐positive non‐small cell lung cancer. Lung Cancer 2015; 88: 74–9. [DOI] [PubMed] [Google Scholar]
- 12. Ando M, Okamoto I, Yamamoto N et al Predictive factors for interstitial lung disease, antitumor response, and survival in non‐small‐cell lung cancer patients treated with gefitinib. J Clin Oncol 2006; 24: 2549–56. [DOI] [PubMed] [Google Scholar]
- 13. Nakagawa K, Kudoh S, Ohe Y et al Postmarketing surveillance study of erlotinib in Japanese patients with non–small‐cell lung cancer (NSCLC): An interim analysis of 3488 patients (POLARSTAR). J Thorac Oncol 2012; 7: 1296–303. [DOI] [PubMed] [Google Scholar]
- 14. Minami‐Shimmyo Y, Ohe Y, Yamamoto S et al Risk factors for treatment‐related death associated with chemotherapy and thoracic radiotherapy for lung cancer. J Thorac Oncol 2012; 7: 177–82. [DOI] [PubMed] [Google Scholar]
- 15. Sakurada T, Kakiuchi S, Tajima S et al Characteristics of and risk factors for interstitial lung disease induced by chemotherapy for lung cancer. Ann Pharmacother 2015; 49: 398–404. [DOI] [PubMed] [Google Scholar]
- 16. Brahmer J, Reckamp KL, Baas P et al Nivolumab versus docetaxel in advanced squamous‐cell non–small‐cell lung cancer. N Engl J Med 2015; 373: 123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borghaei H, Paz‐Ares L, Horn L et al Nivolumab versus docetaxel in advanced nonsquamous non–small‐cell lung cancer. N Engl J Med 2015; 373: 1627–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reck M, Rodríguez‐Abreu D, Robinson AG et al Pembrolizumab versus chemotherapy for PD‐L1‐positive non‐small‐cell lung cancer. N Engl J Med 2016; 375: 1823–33. [DOI] [PubMed] [Google Scholar]
- 19. Rittmeyer A, Barlesi F, Waterkamp D et al Atezolizumab versus docetaxel in patients with previously treated non‐small‐cell lung cancer (OAK): A phase 3, open‐label, multicentre randomised controlled trial. Lancet 2017; 389: 255–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nishino M, Ramaiya NH, Awad MM et al PD‐1 inhibitor‐related pneumonitis in advanced cancer patients: Radiographic patterns and clinical course. Clin Cancer Res 2016; 22: 6051–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kato T, Masuda N, Nakanishi Y et al Nivolumab‐induced interstitial lung disease analysis of two phase II studies patients with recurrent or advanced non‐small‐cell lung cancer. Lung Cancer 2017; 104: 111–8. [DOI] [PubMed] [Google Scholar]
- 22. Khunger M, Rakshit S, Pasupuleti V et al Incidence of pneumonitis with use of programmed death 1 and programmed death‐ligand 1 inhibitors in non‐small cell lung cancer: A systematic review and meta‐analysis of trials. Chest 2017; 152: 271–81. [DOI] [PubMed] [Google Scholar]
- 23. Nishino M, Boswell EN, Hatabu H, Ghobrial IM, Ramaiya NH. Drug‐related pneumonitis during mammalian target of rapamycin inhibitor therapy: Radiographic pattern‐based approach in Waldenström Macroglobulinemia as a paradigm. Oncologist 2015; 20: 1077–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Travis WD, Costabel U, Hansell DM et al An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med 2013; 188: 733–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eisenhauer EA, Therasse P, Bogaerts J et al New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009; 45: 228–47. [DOI] [PubMed] [Google Scholar]
- 26. National Cancer Institute . Common Terminology Criteria for Adverse Events (CTCAE). National Institute of Health, May 28 2009. [Cited 9 May 2018.] Available from URL: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf [Google Scholar]
- 27. Kanaji N, Tadokoro A, Kita N et al Impact of idiopathic pulmonary fibrosis on advanced non‐small cell lung cancer survival. J Cancer Res Clin Oncol 2016; 142: 1855–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bagley SJ, Kothari S, Aggarwal C et al Pretreatment neutrophil‐to‐lymphocyte ratio as a marker of outcomes in nivolumab‐treated patients with advanced non‐small‐cell lung cancer. Lung Cancer 2017; 106: 1–7. [DOI] [PubMed] [Google Scholar]
- 29. Fujimoto D, Morimoto T, Ito J et al A pilot trial of nivolumab treatment for advanced non‐small cell lung cancer patients with mild idiopathic interstitial pneumonia. Lung Cancer 2017; 111: 1–5. [DOI] [PubMed] [Google Scholar]
- 30. Lee CK, Man J, Lord S et al Checkpoint inhibitors in metastatic EGFR‐mutated non‐small cell lung cancer – A meta‐analysis. J Thorac Oncol 2017; 12: 403–7. [DOI] [PubMed] [Google Scholar]
- 31. Rizvi NA, Hellmann MD, Snyder A et al Cancer immunology. Mutational landscape determines sensitivity to PD‐1 blockade in non‐small cell lung cancer. Science 2015; 348: 124–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Demopoulos K, Arvanitis DA, Vassilakis DA, Siafakas N, Spandidos DA. MYCL1, FHIT, SPARC, p16INK4 and TP53 genes associated to lung cancer in idiopathic pulmonary fibrosis. J Cell Mol Med 2002; 6: 215–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kanai O, Nakatani K, Fujita K, Okamura M, Mio T. Concurrence of nivolumab‐induced interstitial lung disease and cancer invasion. Respirol Case Reports 2017; 5: e00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Nishino M, Ramaiya NH, Chambers ES et al Immune‐related response assessment during PD‐1 inhibitor therapy in advanced non‐small‐cell lung cancer patients. J Immunother Cancer 2016; 4: 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kato T, Sakai F, Baba T et al Nivolumab‐induced interstitial lung disease (ILD) in Japanese patients with non‐small cell lung cancer: A study on risk factors for fatal outcome. J Clin Oncol 2017; 35 (15 Suppl): Abstract 9077.


