Abstract
Background
S100A14 is a member of the S100 calcium‐binding protein family that exerts important phenotypic effects on cell proliferation, apoptosis, differentiation, and motility. However, the functional role and potential clinical significance of S100A14 in lung adenocarcinoma has not yet been clarified.
Methods
We analyzed genomic alterations of S100A14 using The Cancer Genome Atlas lung adenocarcinoma genomic dataset. S100A14 displayed significant copy number amplification in lung adenocarcinoma. We detected S100A14 expression in lung adenocarcinoma and analyzed the correlation between S100A14 expression and clinicopathological characteristics.
Results
Immunohistochemical analysis showed that S100A14 expression was obviously upregulated in lung adenocarcinoma tissues compared to matched normal counterparts. Statistical analysis revealed that S100A14 expression strongly correlated with poor differentiation, metastasis, stage, smoking, and EGFR mutation. Furthermore, our data indicated that S100A14 serum levels were higher in lung adenocarcinoma patients than healthy controls. Intriguingly, S100A14 serum levels were related to distant metastasis (P = 0.028). High S100A14 expression was significantly associated with overall (P = 0.0016) and post progression (P = 0.039) survival. In addition, we investigated the biological functions of S100A14 in lung adenocarcinoma cell lines. The results demonstrated that S100A14 promoted cell migration and invasion of SPCA1 and Glc‐82 cells.
Conclusions
S100A14 increases the motility of lung adenocarcinoma cells, and might be a diagnostic and prognostic serum biomarker and potential therapeutic target for lung adenocarcinoma.
Keywords: Diagnosis, lung adenocarcinoma, metastasis, S100A14
Introduction
Lung cancer is one of the most common malignant tumors in China with the highest morbidity and mortality in a variety of cancers.1 Lung adenocarcinoma is the main histological type of lung cancer. The current treatments for lung adenocarcinoma are mainly radiotherapy, chemotherapy, surgery, immunotherapy, and Chinese medicine; of these, surgery remains the most effective method. However, as patients tend to develop distant metastasis in the early stage, and thus surgery is no longer an option, the five‐year survival rate is < 15%.2 Therefore, identifying new biomarkers and exploring the molecular mechanisms of invasion and metastasis of lung adenocarcinoma for early detection are essential for the development of new diagnostic and treatment solutions.
The S100 protein family is a group of calcium‐dependent regulatory proteins with EF hand types including more than 20 proteins, many of which are located at the 1q21 site of chromosomes. The S100 family is involved in structural and numerical aberrations in various human tumors,3 exerting a wide range of biological functions and playing an important role in cell growth, differentiation, energy metabolism, and intracellular signal transduction. A large number of studies have shown that S100 protein expression is closely related to a variety of cancers.4, 5
S100 binding protein A14 (S100A14) is a member of the S100 family. Its N‐terminal contains the S100‐specific EF hand of 13 amino acids, with low affinity for calcium ions. The C‐terminal contains the S100‐common EF hand of 12 amino acids, with high affinity for calcium ions. It located at lq21.3 with four exons and three introns, encodes a protein of 104 amino acids, and shares 68% homologous to the S100A13 protein. In addition, there are N‐glycosylation, protein kinase C phosphorylation, five casein kinase II phosphorylation, and N‐myristoylation sites in this protein.6 S100A14 protein expression is tissue‐specific. Studies have shown that S100A14 is preferentially overexpressed in breast and liver cancers, and is mainly underexpressed in colon cancer.7, 8, 9 We examined the expression of S100A14 in lung adenocarcinoma tissues and cell lines in order to define its potential clinical significance. We then analyzed the relationship between S100A14 expression and clinicopathological parameters of lung adenocarcinoma and detected S100A14 levels in serum to provide a potential target for the diagnosis and treatment of lung adenocarcinoma.
Methods
Patients and samples
Tissue specimens from 208 patients with lung adenocarcinoma were analyzed, including 115 men and 93 women. Serum from 319 cases of lung adenocarcinoma and 79 healthy controls were collected. Patients were recruited from the National Cancer Center/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College. None of the patients underwent surgery, chemotherapy or targeted therapy before serum collection. The institutional review board of the Cancer Hospital, Chinese Academy of Medical Sciences, approved the study. All patients provided written informed consent prior to study enrollment.
Cell lines and cell culture
Lung adenocarcinoma cell lines (SPCA1 and Glc‐82) were purchased from the National Infrastructure of Cell Line Resource (Beijing, China). SPCA1 and Glc‐82 cells were grown in RPMI1640 medium supplemented with 10% fetal bovine serum and antibiotics. Cell cultures were maintained at 37 °C with 5% CO2.
Immunohistochemical analysis
Paraffin‐embedded tissues were cut into 4 μm sections, dewaxed, and then rehydrated with a graded alcohol series. To quench endogenous peroxidase activity, the sections were incubated with 3% H2O2 at room temperature for 20 minutes. The sections were then heated in ethylene diamine tetra acetic acid buffer (pH =6.0) for two minutes using a pressure cooker for antigen retrieval. The slides were blocked with serum at 37 °C for 20 minutes. Samples were incubated with monoclonal antibodies against S100A14 (1:100; Sigma‐Aldrich, St Louis, MO, USA) overnight at 4 °C. Secondary antibody incubation and staining was performed using an immunohistochemical staining kit (ZSGB‐BIO; OriGene Technologies, Inc., Beijing, China). The immunoreaction was visualized with diaminobenzidine‐tetrahydrochloride staining, followed by counterstaining with hematoxylin. Phosphate buffered saline replaced the primary antibody for the negative control, with S100A14 positive tablets as a positive control (Sigma‐Aldrich, USA).
Two experienced pathologists blinded to clinical information independently evaluated percentages of positive S100A14 tumor cells and staining intensity. They assessed 20 sequential high‐power fields (0.54 mm diameter per field) using Aperio ImageScope software (Leica Biosystems Inc., Wetzlar, Germany) to analyze images. Staining intensity was graded as: no staining, 0; weak positive staining, 1; positive staining, 2; and strong positive staining, 3. The staining percentage was automatically evaluated by ImageScope software. The expression score was determined by multiplying the staining intensity and percentage. The patients were divided into two groups according to the expression score ratio of matched cancer/normal tissues: ≤ 1 = non‐overexpression, > 1 = overexpression. A representative area was selected for each section.
Enzyme‐linked immunosorbent assay
Serum S100A14 protein levels in lung adenocarcinoma patients and healthy controls were examined using an S100A14 enzyme‐linked immunosorbent assay (ELISA) test kit (QiSong Co., Beijing, China). A level of S100A14 ≥ 4.503 ng/mL based on the receiver operating characteristic curve was defined as positive, and below this value as negative.
Plasmid construction
Full‐length complementary DNA of human S100A14 was cloned using Xba I and BamH I sites into pLVX‐IRES‐Neo to generate the lentiviral expression plasmid.
Lentivirus production and transduction
Lentiviral vector (pLVX‐IRES‐Neo or pLVX‐IRES‐Neo‐S100A14) and packaging plasmids (pCMV Δ8.91, VSVG, and PLP2) were co‐transfected into 293T cells using Lipofectamine 2000 (Thermo Fisher Scientific, Waltham, MA, USA). The recombinant lentiviruses were harvested 48 hours after transfection, and subsequently pre‐cleaned with a 3000 g centrifuge and 0.45 μm filtration (Millipore, Billerica, MA, USA). For lentiviral transduction, 2 × 105 cells/well were seeded into six‐well culture plates and infected the following day with lentiviruses plus 5 μg/mL Polybrene (Sigma‐Aldrich, USA). After the cells were infected with the lentivirus, 650 μg/mL geneticin was continuously added to the culture for two weeks. Overexpressing S100A14 stable clones were then obtained for further experiments.
RNA isolation and quantitative PCR
Total RNA was extracted with TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and complementary DNA was synthesized using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). S100A14 quantification was performed with SYBR Premix Ex Taq (Takara, Dalian, China). The primers were as follows: S100A14‐F: CTGACCCCTTCTGAGCTACG; S100A14‐R: TTCTCTTCCAGGCCACAGTT;GAPDH‐F: TGCACCACCAACTGCTTAGC;GAPDH‐R: GGCATGGACTGTGGTCATGAG.
Western blotting
Cells were lysed with cell lysis buffer on ice and protein extracts were then separated with 10% next gel (1B1724‐500mL, AMRESCO Inc., Solon, OH, USA) and transferred to polyvinylidene difluoride membrane. The membrane was blocked with 5% nonfat milk for two hours at room temperature. Membranes were then incubated with primary antibody overnight at 4 °C and secondary antibody for two hours at room temperature. The membranes were washed extensively with tris‐buffered saline plus tween 20 and S100A14 levels finally were detected by chemiluminescence detection (Pierce Biotechnology, Rockford, IL, USA) with the LAS4000 mini system (GE Healthcare, Piscataway, NJ, USA). Antibodies used to detect specific proteins were as follows: glyceraldehyde 3‐phosphate dehydrogenase (G8795; Sigma‐Aldrich, USA) and S100A14 (10489‐1‐AP; Proteintech, Chicago, IL, USA).
Migration and invasion assay
Migration and invasion assays were performed using a Boyden chamber with 8 μm pore polyethylene terephthalate (Costar, Cambridge, MA, USA) according to manufacturer protocol. The chambers were coated with Matrigel (BD Biosciences, San Jose, CA, USA) after cell invasion assay. Briefly, 5 × 104 cells were plated in the upper chamber in serum free media. The bottom chamber contained RPMI 1640 media with 10% fetal bovine serum. After 24 hours, the bottom of the chamber insert was fixed and stained with 0.5% crystal violet. Cells from four randomly selected fields were counted and data are represented as mean ± standard deviation.
Statistical analysis
SPSS version 22.0 (IBM Corp., Armonk, NY, USA) was used for statistical analysis. Count data were compared using Fisher's exact and chi‐squared (χ2) tests, whereas measurement data were compared using t and Mann–Whitney U tests. Survival data was analyzed using the log‐rank test. P < 0.05 was considered to indicate a statistically significant difference.
Results
S100A14 displayed copy number amplification and was overexpressed in lung adenocarcinoma
As one of the causes of gene deregulation in cancer is copy number alteration, we examined S100A14 copy number alterations in lung adenocarcinoma using data from The Cancer Genome Atlas. Analysis revealed that amplification of S100A14 was present in 67 (~13%) of 520 sequenced lung adenocarcinoma patients. Unfortunately, after integrating the analysis of copy number variation and the gene expression profiles, no correlation was found between genomic amplification and S100A14 messenger RNA expression (Fig 1a,b).
Figure 1.
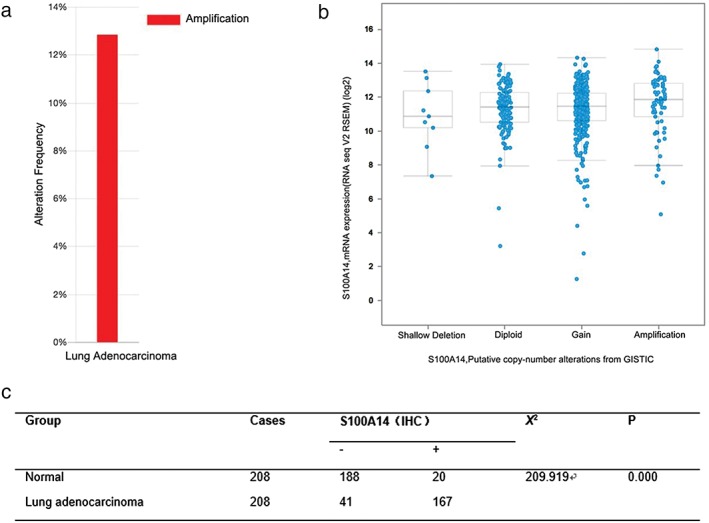
S100A14 amplification in The Cancer Genome Atlas database and overexpression in lung adenocarcinoma. (a) S100A14 copy number amplification was found in 67 (13%) out of 520 sequenced lung adenocarcinoma patients. (b) The correlation between genomic alteration and messenger RNA (mRNA) expression of S100A14. (c) S100A14 expression in lung adenocarcinoma and matched normal counterparts.
We then detected S100A14 expression in 208 lung adenocarcinoma specimens and adjacent normal tissues by immunohistochemical staining. S100A14 protein expression was mainly localized in lung adenocarcinoma tissues in the cell membrane and cytoplasm. S100A14 was overexpressed in lung adenocarcinoma tissues in 167 of 208 cases (80.3%) and non‐overexpressed in 41 (19.7%). By contrast, S100A14 exhibited high expression in 20 cases (9.6%) and low expression in 188 cases (90.4%) in the matched normal tissues (Fig 2). Statistical analysis showed that S100A14 expression was significantly increased in lung adenocarcinoma (Fig 1c). Together, these results clearly demonstrated that S100A14 is amplified and overexpressed in lung adenocarcinoma.
Figure 2.
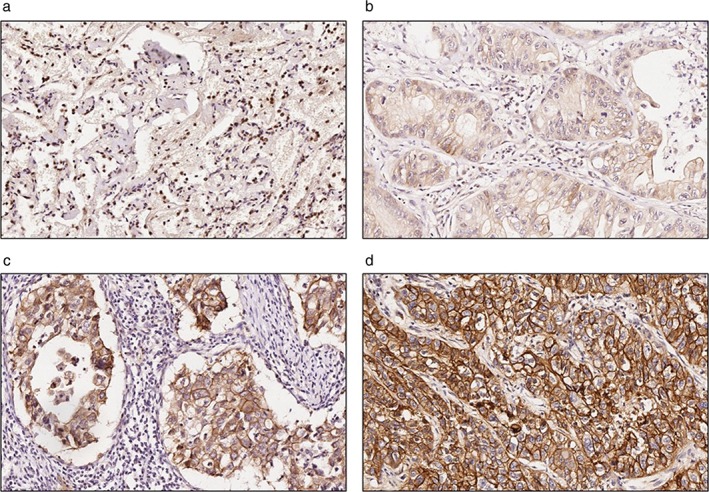
Representative images of immunohistochemical (IHC) staining of S100A14 in normal or lung adenocarcinoma tissues. Faint or no signals of S100A14 were detected in normal lung specimens, whereas S100A14 was mainly expressed in the cell membrane and cytoplasm in lung adenocarcinoma tissues. (a) Normal lung tissue, (b) weak positive staining (IHC = 1), (c) positive staining (IHC = 2), and (d) strong positive staining (IHC = 3) in lung adenocarcinoma tissue.
S100A14 overexpression was associated with differentiation and metastasis in lung adenocarcinoma
To further determine whether S100A14 overexpression is linked to clinicopathological parameters, lung adenocarcinoma specimens were grouped according to their histological type, differentiation, clinical stage, and metastasis. No significance was found between S100A14 expression level and gender or age. However, S100A14 upregulation was significantly correlated with lung adenocarcinoma differentiation (P = 0.033), metastasis (P = 0.035), smoking (P = 0.011), and EGFR mutation (P = 0.023) (Table 1).
Table 1.
The relationship between S100A14 expression and the clinicopathological features of lung adenocarcinoma
| Characteristics | IHC = 0 | IHC = 1 | IHC = 2 | IHC = 3 | χ2 | P |
|---|---|---|---|---|---|---|
| Gender | 7.613 | 0.055 | ||||
| Male | 30 | 38 | 43 | 4 | ||
| Female | 11 | 35 | 40 | 7 | ||
| Age | 3.869 | 0.276 | ||||
| > 60 | 20 | 43 | 36 | 6 | ||
| ≤ 60 | 21 | 30 | 47 | 5 | ||
| Differentiation | 22.416 | 0.033 | ||||
| High | 6 | 10 | 9 | |||
| High‐medium | 1 | 9 | 9 | 1 | ||
| Medium | 9 | 29 | 35 | 2 | ||
| Medium‐low | 10 | 14 | 18 | 5 | ||
| Low | 15 | 10 | 12 | 3 | ||
| Metastasis | 8.581 | 0.035 | ||||
| N | 30 | 39 | 40 | 4 | ||
| Y | 11 | 34 | 43 | 7 | ||
| Stage | 11.644 | 0.234 | ||||
| I | 14 | 34 | 33 | 4 | ||
| II | 11 | 18 | 16 | 1 | ||
| III | 13 | 11 | 22 | 6 | ||
| IV | 3 | 1 | 2 | 0 | ||
| Smoking status | 11.197 | 0.011 | ||||
| Never | 16 | 48 | 59 | 7 | ||
| Smoker | 26 | 27 | 28 | 4 | ||
| EGFR mutation (n = 76) | 9.555 | 0.023 | ||||
| Exon 21 | 1 | 11 | 11 | 3 | ||
| Exon 19 | 0 | 4 | 6 | 1 | ||
| No | 11 | 10 | 15 | 3 |
Bold values indicate: two‐sided t‐test: P < 0.05. Negative staining (immunohistochemical [IHC] = 0), weak positive staining (IHC = 1), positive staining (IHC = 2), strong positive staining (IHC = 3).
Increased S100A14 serum levels are correlated with distant metastasis of lung adenocarcinoma
As our previous study showed that S100A14 can be secreted into cancer cell culture medium, we examined S100A14 serum levels in 319 lung adenocarcinoma patients and 79 healthy controls using ELISA assay to assess the potential significance of S100A14. The S100A14 serum levels in lung adenocarcinoma patients were significantly higher than in healthy controls (P = 0.004) (Table 2, Fig 3.). Moreover, S100A14 was related to distant metastasis (P = 0.028) (Table 3).
Table 2.
S100A14 serum levels in lung adenocarcinoma patients and healthy controls
| Type | Cases | Median (ng/ml) | Mann–Whitney U | P |
|---|---|---|---|---|
| Lung adenocarcinoma | 319 | 4.3003 | 9959.000 | 0.004 |
| Normal | 79 | 3.8923 |
Figure 3.
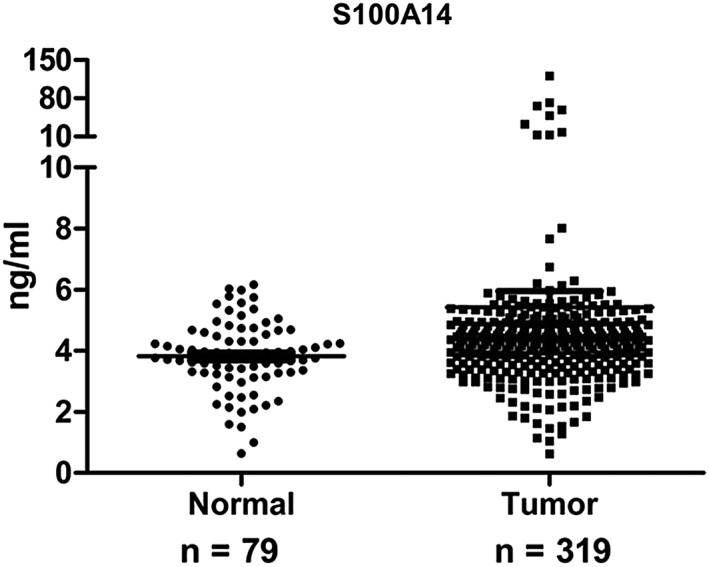
S100A14 levels in serum from lung adenocarcinoma patients and healthy controls.
Table 3.
S100A14 serum levels and clinicopathological features of lung adenocarcinoma
| Type | Cases | Median | χ2 | P |
|---|---|---|---|---|
| Differentiation | 3.246 | 0.197 | ||
| Well | 59 | 4.470 08 | ||
| Moderate | 110 | 4.285 31 | ||
| Poor | 131 | 4.226 88 | ||
| Stage | 5.098 | 0.165 | ||
| I | 95 | 4.3003 | ||
| II | 57 | 4.1143 | ||
| III | 78 | 4.1855 | ||
| IV | 60 | 4.4138 | ||
| T | 0.823 | 0.844 | ||
| I | 76 | 4.3353 | ||
| II | 154 | 4.2853 | ||
| III | 34 | 4.0204 | ||
| IV | 31 | 4.0484 | ||
| N | 4.923 | 0.178 | ||
| 0 | 150 | 4.3003 | ||
| 1 | 34 | 3.9375 | ||
| 2 | 76 | 4.3754 | ||
| 3 | 31 | 4.4004 | ||
| M | 4792.5† | 0.028 | ||
| 0 | 220 | 4.2049 | ||
| 1 | 54 | 4.4139 |
Bold values indicate: two‐sided t‐test: P < 0.05.
Mann–Whitney U test.
S100A14 expression is associated with lung adenocarcinoma prognosis
We next analyzed the relationship between S100A14 expression and survival in our lung adenocarcinoma samples, but found no obvious significance (data not shown). We investigated whether there was an association between S100A14 expression and patient survival using the Kaplan–Meier Plotter online tool. The results demonstrated that high S100A14 expression is significantly associated with overall survival (OS; P = 0.0016) and post progression survival (PPS; P = 0.039) (Fig 4). The OS and PPS in patients with high S100A14 expression were significantly lower than in patients with low S100A14 expression, suggesting that S100A14 expression in a large database sample correlates with lung adenocarcinoma prognosis. Overall, these results suggested that S100A14 may act as a potential biomarker for progression and poor prognosis of lung adenocarcinoma.
Figure 4.
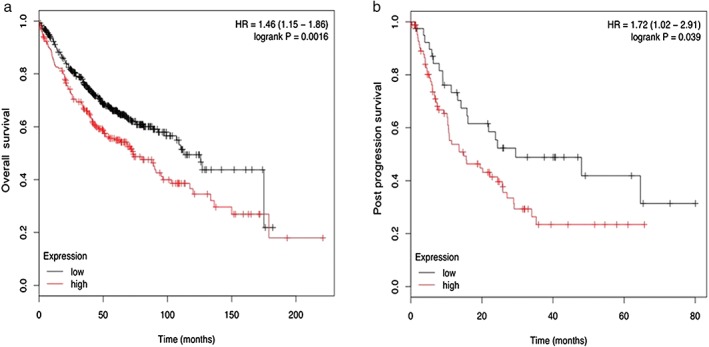
Kaplan–Meier survival curves: lung adenocarcinoma with (a) low (n = 498) and high (n = 222) S100A14 expression (P = 0.0016) for overall survival; and (b) low (n = 40) and high (n = 85) S100A14 expression (P = 0.039) for post progression survival.
Establishment of lentiviral‐mediated overexpression of S100A14 lung adenocarcinoma cell lines
Previous studies in our lab have shown that S100A14 is implicated in multiple processes, such as cell proliferation, migration, and invasion.6, 10 In order to fully understand the potential functional roles of S100A14 in the tumorigenesis and progression of lung adenocarcinoma we established stable lung adenocarcinoma cell lines of S100A14 overexpression. The messenger RNA and protein levels of S100A14 in SPCA1 and Glc‐82 cells were obviously increased in S100A14‐overexpressed cells (Fig 5a,b).
Figure 5.
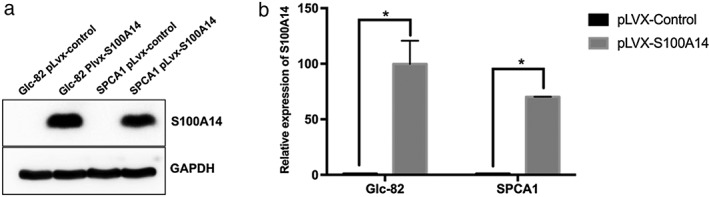
Establishment of lung adenocarcinoma stable cell lines of ectopic expression of S100A14. SPCA1 and Glc‐82 cells were infected with pLVX‐IRES‐NEO‐control and pLVX‐IRES‐NEO‐S100A14 lentivirus; stable cells were screened by Geneticin (G418) for two weeks. S100A14 expression was detected by (a) Western Blot and (b) quantitative real time‐PCR (*P < 0.05; mean ± standard deviation; two‐sided t‐test; normalized to glyceraldehyde 3‐phosphate dehydrogenase).
S100A14 promoted migration and invasion of lung adenocarcinoma cells
We detected the effect of S100A14 overexpression on cell cycle progression, proliferation, migration, and invasion. S100A14 overexpression did not impact cell cycle progression or proliferation. Transwell assay showed that S100A14 overexpression dramatically promoted migration and invasion of SPCA1 and Glc‐82 cells (Fig. 6a,c). The quantitative results of cell migration and invasion are shown (Fig. 6b,d). S100A14 significantly increased lung adenocarcinoma cell migration and invasion without affecting cell proliferation.
Figure 6.
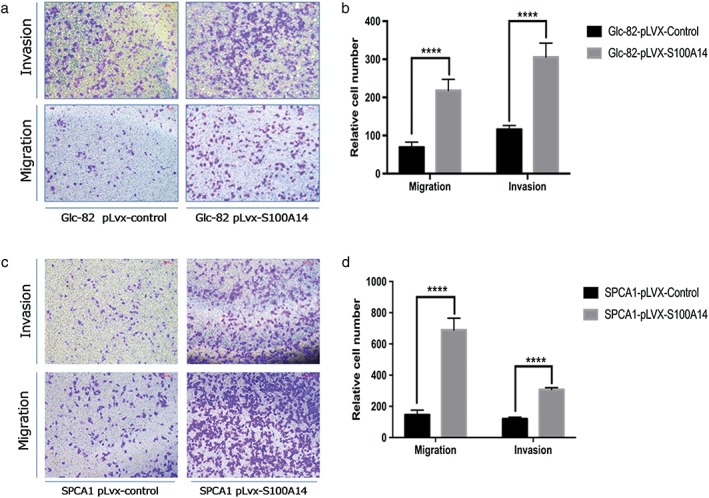
S100A14 promotes cell migration and invasion of lung adenocarcinoma cells. (a,c) Transwell assays were performed in S100A14‐overexpressed SPCA1, Glc‐82, and corresponding control cells. The stained cells were manually counted from four randomly selected fields; representative images are shown. (b,d) Quantitative results of cell migration and invasion (****P < 0.0001; two‐sided t‐test).
Discussion
Tumorigenesis is a very complex biological phenomenon of multiple factors: polygene, multi‐stage, and multi‐step. Research of the occurrence and development of lung adenocarcinoma at multiple levels to determine closely related protein and gene markers is important for early prevention, treatment, and assessment of prognosis.
S100A14 is a newly discovered member of the S100 family. It has the typical characteristics of the S100 family and has high homology with S100 family members, especially S100A13. Many studies have suggested that S100A14 is a new molecular marker closely related to the metastasis of malignant tumors.7 S100A14 is downregulated in esophageal carcinoma and decreased S100A14 is correlated with poor differentiation.10 Katono et al. and Hu et al. found that S100A14 overexpression is associated with tumor metastasis and S100A14 knockdown by RNA interference leads to dramatically reduced migration and invasion of these cells.11, 12 However, Zhu et al. found that S100A14 expression is negatively correlated to cell migration and invasion in gastric cancer. S100A14 blocks store‐operated Ca2+ influx by inhibiting Orai1 and STIM1 expression, leading to FAK activation, MMP downregulation, and focal adhesion assembly.13 S100A14 also promotes cell migration and invasion by regulating MMP2 expression in a p53‐dependent manner. Mechanistic investigation has shown that extracellular S100A14 binds to RAGE to activate the MAPK and NF‐κB pathway, regulating cell proliferation and apoptosis in a dose‐dependent manner.14 Moreover, S100A14 can act as a mediator of epithelial‐mesenchymal transformation, thereby promoting tumor metastasis.15, 16 Emerging evidence has demonstrated that S100A14 is regulated by KLF4, a zinc finger transcription factor. There is a KCNN4‐UBA52‐KLF4‐S100A14 regulatory axis in serous ovarian cancer.17 In addition, 12‐O‐tetradecanoylphorbol‐13‐acetate can increase the level of S100A14 in a KLF4‐dependent manner and promote the migration of breast invasive ductal carcinoma cells.18
Dysregulated expression of multiple members of the S100 protein family is considered a common feature in lung carcinoma,19 but the effects of S100A14 in lung adenocarcinoma have not been reported. In this project, we used immunohistochemical staining to detect expression of the S100A14 protein in 208 cases of lung adenocarcinoma and adjacent normal lung tissues. We found that S100A14 protein expression in lung adenocarcinoma was significantly higher than in normal lung tissues (P < 0.05). Moreover, high S100A14 expression was significantly correlated with tumor cell differentiation, metastasis, smoking, and EGFR mutation. Notably, high S100A14 expression can predict patient prognosis in lung adenocarcinoma.
Multiple members of the S100 family are secreted into the serum. A variety of S100 proteins, including S100A4, S100A8, and S100A9, have been used as serological tumor markers for tumor diagnosis and prognosis.20 Our former study showed that S100A14 overexpressing EC9706 cells secreted S100A14 into the supernatant and low extracellular concentrations of S100A14 can activate p‐ERK and NF‐κB dependent on glycosylated end products, which in turn promotes cell proliferation.21 In this study, we used ELISA to detect serum S100A14 protein levels in lung adenocarcinoma patients and healthy controls. The S100A14 serum levels in lung adenocarcinoma patients were higher than in the healthy controls (P = 0.004). Interestingly, high levels of S100A14 in serum led to greater incidence of distant metastasis, thus S100A14 might predict lung adenocarcinoma prognosis in clinical investigation.
Functional assays showed that S100A14 overexpression significantly enhanced the migration and invasion of lung adenocarcinoma cells. However, our study has some limitations. Firstly, the clear mechanism of S100A14 that promotes migration and invasion requires further elucidation to provide insight into lung adenocarcinoma metastasis. Secondly, additional animal model assays are required to confirm S100A14 function in metastasis in vivo.
In conclusion, we examined S100A14 expression in lung adenocarcinoma and evaluated the correlation between S100A14 and prognosis. S100A14 is overexpressed in lung adenocarcinoma and obviously enhances the invasion and migration of cancer cells. The S100A14 serum levels in lung adenocarcinoma patients were significantly higher than those in healthy controls and correlated with metastasis. High S100A14 expression is also associated with poor prognosis in lung adenocarcinoma. These results suggest that S100A14 could be a useful diagnostic and prognostic biomarker in lung adenocarcinoma.
Acknowledgment
This work was supported by the Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (Grant No.2017‐I2M‐1‐013 & No.2017‐I2M‐3‐005).
Disclosure
No authors report any conflict of interest.
References
- 1. Chen W, Zheng R, Baade PD et al Cancer statistics in China, 2015. CA Cancer J Clin 2016; 66: 115–32. [DOI] [PubMed] [Google Scholar]
- 2. Ettinger DS. Ten years of progress in non‐small cell lung cancer. J Natl Compr Canc Netw 2012; 10: 292–5. [DOI] [PubMed] [Google Scholar]
- 3. Donato R. S100: A multigenic family of calcium‐modulated proteins of the EF‐hand type with intracellular and extracellular functional roles. Int J Biochem Cell Biol 2001; 33: 637–68. [DOI] [PubMed] [Google Scholar]
- 4. Chen H, Xu C, Jin Q, Liu Z. S100 protein family in human cancer. Am J Cancer Res 2014; 4: 89–115. [PMC free article] [PubMed] [Google Scholar]
- 5. Bresnick AR, Weber DJ, Zimmer DB. S100 proteins in cancer. Nat Rev Cancer 2015; 15: 96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Xu C, Chen H, Wang X et al S100A14, a member of the EF‐hand calcium‐binding proteins, is overexpressed in breast cancer and acts as a modulator of HER2 signaling. J Biol Chem 2014; 289: 827–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Leth‐Larsen R, Terp MG, Christensen AG et al Functional heterogeneity within the CD44 high human breast cancer stem cell‐like compartment reveals a gene signature predictive of distant metastasis. Mol Med 2012; 18: 1109–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhao FT, Jia ZS, Yang Q, Song L, Jiang XJ. S100A14 promotes the growth and metastasis of hepatocellular carcinoma. Asian Pac J Cancer Prev 2013; 14: 3831–6. [DOI] [PubMed] [Google Scholar]
- 9. Wang HY, Zhang JY, Cui JT et al Expression status of S100A14 and S100A4 correlates with metastatic potential and clinical outcome in colorectal cancer after surgery. Oncol Rep 2010; 23: 45–52. [PubMed] [Google Scholar]
- 10. Chen H, Yu D, Luo A et al Functional role of S100A14 genetic variants and their association with esophageal squamous cell carcinoma. Cancer Res 2009; 69: 3451–7. [DOI] [PubMed] [Google Scholar]
- 11. Katono K, Sato Y, Kobayashi M et al Clinicopathological significance of S100A14 expression in lung adenocarcinoma. Oncol Res Treat 2017; 40: 594–602. [DOI] [PubMed] [Google Scholar]
- 12. Hu R, Huffman KE, Chu M, Zhang Y, Minna JD, Yu Y. Quantitative secretomic analysis identifies extracellular protein factors that modulate the metastatic phenotype of non‐small cell lung Cancer. J Proteome Res 2016; 15: 477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhu M, Wang H, Cui J et al Calcium‐binding protein S100A14 induces differentiation and suppresses metastasis in gastric cancer. Cell Death Dis 2017; 8: e2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Chen H, Yuan Y, Zhang C et al Involvement of S100A14 protein in cell invasion by affecting expression and function of matrix metalloproteinase (MMP)‐2 via p53‐dependent transcriptional regulation. J Biol Chem 2012; 287: 17109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ehmsen S, Hansen LT, Bak M, Brasch‐Andersen C, Ditzel HJ, Leth‐Larsen R. S100A14 is a novel independent prognostic biomarker in the triple‐negative breast cancer subtype. Int J Cancer 2015; 137: 2093–103. [DOI] [PubMed] [Google Scholar]
- 16. Wang X, Yang J, Qian J, Liu Z, Chen H, Cui Z. S100A14, a mediator of epithelial‐mesenchymal transition, regulates proliferation, migration and invasion of human cervical cancer cells. Am J Cancer Res 2015; 5: 1484–95. [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao H, Guo E, Hu T et al KCNN4 and S100A14 act as predictors of recurrence in optimally debulked patients with serous ovarian cancer. Oncotarget 2016; 7: 43924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. He H, Li S, Chen H et al 12‐O‐tetradecanoylphorbol‐13‐acetate promotes breast cancer cell motility by increasing S100A14 level in a Kruppel‐like transcription factor 4 (KLF4)‐dependent manner. J Biol Chem 2014; 289: 9089–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang T, Huo X, Chong Z, Khan H, Liu R, Wang T. A review of S100 protein family in lung cancer. Clin Chim Acta 2018; 476: 54–9. [DOI] [PubMed] [Google Scholar]
- 20. Hermani A, Hess J, De Servi B et al Calcium‐binding proteins S100A8 and S100A9 as novel diagnostic markers in human prostate cancer. Clin Cancer Res 2005; 11: 5146–52. [DOI] [PubMed] [Google Scholar]
- 21. Jin Q, Chen H, Luo A, Ding F, Liu Z. S100A14 stimulates cell proliferation and induces cell apoptosis at different concentrations via receptor for advanced glycation end products (RAGE). (Published erratum appears in PLoS One 2016; 11: e0147881). PLoS One 2011; 6: e19375. [DOI] [PMC free article] [PubMed] [Google Scholar]


