Abstract
Purpose
This study sought to examine whether near total gastrectomy (nTG) confers a long-term nutritional benefit when compared with total gastrectomy (TG) for the treatment of gastric cancer.
Materials and Methods
Patients who underwent nTG or TG for gastric cancer were included (n=570). Using the 1:2 matched propensity score, 25 patients from the nTG group and 50 patients from the TG group were compared retrospectively for oncologic outcomes, including long-term survival and nutritional status.
Results
The length of the proximal resection margin, number of retrieved lymph nodes and tumor nodes, metastasis stage, short-term postoperative outcomes, and long-term survival were not significantly different between the groups. The body mass index values, and serum total protein and hemoglobin levels of the patients decreased significantly until postoperative 6 months, and then recovered slightly over time (P<0.05); however, there was no difference in the levels between the groups. The prognostic nutritional index values and serum albumin levels decreased significantly until postoperative 6 months and then recovered (P<0.05); the levels decreased more in the nTG group than in the TG group (P<0.05). The mean corpuscular volumes and serum transferrin levels increased significantly until postoperative 1 year and then recovered slightly over time (P<0.05); however, there was no difference between the groups. Serum vitamin B12, iron, and ferritin levels of the patients did not change significantly over time, and no difference existed between the groups.
Conclusions
A small remnant stomach after nTG conferred no significant nutritional benefits over TG.
Keywords: Stomach neoplasms, Nutritional status, Gastrectomy, Gastric stump
INTRODUCTION
With increasing cases of early gastric cancer (EGC), the survival rate of patients with gastric cancer has increased, consequently leading to an increased interest in long-term nutritional maintenance after surgery [1]. Most patients with gastric cancer experience weight loss and anemia after gastrectomy [2]. A recent study reported that maintenance of the body mass index (BMI) after gastrectomy is important to improve the long-term survival of patients with gastric cancer [3]. Thus, nutritional support after gastrectomy is important not only for better quality of life but also for improved long-term survival.
Treatment of gastric cancer involves a distal, total, or proximal gastrectomy, according to the location of the tumor [4]. Total gastrectomy (TG) is known to have oncologic advantages, such as no risk of remnant gastric cancer, more lymph node (LN) dissection, and a wider safety margin; however, it has nutritional disadvantages such as greater weight loss and anemia than that observed after subtotal gastrectomy [5,6]. A recent study reported that distal gastrectomy has a better outcome than proximal gastrectomy or TG, in terms of nutritional status [7]. Moreover, TG has a relatively higher rate of complications such as anastomosis leakage or stenosis, which could be life threatening [8,9,10]. Thus, near total gastrectomy (nTG) is sometimes performed to obtain the nutritional and oncologic advantages and to avoid the surgical complications associated with the treatment of upper- or middle-third gastric cancer. However, few studies have analyzed the benefit of a small remnant stomach in terms of nutrition [7].
The present study analyzed the long-term nutritional benefit of a small remnant stomach after nTG compared with TG for the treatment of gastric cancer.
MATERIALS AND METHODS
Patients and data collection
Patients who underwent curative radical TG or nTG for gastric cancer from 2009 to 2014 at Seoul St. Mary's Hospital were included in the present study. Patients with stage IV gastric cancer who had a synchronous malignancy were excluded. The 570 enrolled patients included 25 patients who underwent nTG (nTG group) and 545 patients who underwent TG (TG group). The demographics, clinical and pathological characteristics, operative details, short-term postoperative outcomes, long-term survival data, and nutrition data, which were collected retrospectively from the hospital's gastric cancer patient registry, were compared between the two groups. The nutritional parameters, including BMI, prognostic nutritional index (PNI), serum levels of albumin and total protein, hemoglobin, and mean corpuscular volume (MCV) of the patients were collected preoperatively and at 6 months, 1 year, and 2 years after surgery. Serum levels of vitamin B12, iron, ferritin, and transferrin were determined at 6 months, 1 year, and 2 years after surgery. The PNI was calculated using the formula [11]:
| PNI=10×serum albumin value (g/dL)+0.005×peripheral lymphocyte count |
Surgical procedures were performed according to the Japanese Gastric Cancer Treatment Guidelines [4]. nTG was defined as a remnant stomach 2–3 cm from the gastroesophageal junction [12,13]. Roux-en-Y esophagojejunostomy was performed after TG, and Billroth-II or Roux-en-Y gastrojejunostomy was performed after nTG.
The preoperative clinical characteristics of the patients were classified according to the criteria of the Eastern Cooperative Oncology Group (ECOG). Pathological stage was classified according to the Seventh American Joint Cancer Committee tumor, node, metastasis (TNM) classification system [14]. Postoperative complications within 30 postoperative days were classified according to the Clavien-Dindo system.
This study was approved by the Institutional Review Board of the Ethics Committee of the College of Medicine, Catholic University of Korea (KC18RESI0179). All patient records were anonymized and de-identified prior to analysis.
Postoperative follow-up schedule
Patients with advanced gastric cancer were followed up every 3 months after surgery, and those with early gastric cancer were followed up every 6 months until postoperative 5 years, and annually thereafter. Laboratory examinations, including the measurement of nutritional parameters and tumor markers, and abdominal computed tomography, were performed at each follow-up, and endoscopy and a bone scan were performed annually. During the postoperative follow-up, vitamin B12 was administered via an intramuscular injection and iron was administered orally or via an intravenous injection when their serum levels were lower than the normal values (160 pg/mL, and 60 mcg/dL for men and 50 mcg/dL for women, respectively).
Propensity score matching and statistical analysis
Propensity score matching was conducted to adjust for differences between the groups in the clinicopathological characteristics that were directly related to nutritional outcomes. Propensity scores were obtained using binary logistic regression with covariates of age, sex, ECOG, BMI, surgical approach, depth of invasion, LN metastasis, and pathological stage. Subsequently, the nTG group was 1:2 matched to the TG group based on the top 50 propensity scores.
χ2 or Fisher's exact test was used to compare categorical variables between the groups. Student's t-test and Mann-Whitney U test were used to compare continuous variables. A Kaplan-Meier survival curve was used to analyze the survival rates. A repeated measures analysis of variance and the Greenhouse-Geisser method were used to compare postoperative changes in body weight, PNI, and nutritional parameters between the groups. All statistical analyses were performed using SPSS for Windows (ver. 21.0; IBM Corp., Armonk, NY, USA); P-values <0.05 were deemed to indicate statistical significance.
RESULTS
Overall, 570 patients were included in this study; of these, 75 well-matched patients were selected. Their clinicopathological characteristics are shown in Table 1. After the matching, there was no significant difference between the groups in terms of clinicopathological characteristics, such as age, sex, BMI, ECOG, and comorbidities. Tumor diameter and location were not matched. Although the PNI was significantly higher in the nTG group (50.8±5.6 vs. 54.6±6.0, respectively, P=0.009), there were no initial differences in the values of the other nutritional parameters, including serum albumin, total protein, hemoglobin, and MCV (P=0.070, 0.237, 0.187, and 0.776, respectively). Other nutritional parameters such as the serum levels of vitamin B12, iron, ferritin, and transferrin were collected at 6 postoperative months; thus, the initial levels are not shown in Table 1. The tumor diameter was significantly greater in the TG group, and the location of the tumor was significantly different. However, no differences were noted between the groups in the number of tumors, length of the proximal resection margin, number of retrieved lymph nodes, number of metastatic LNs, and TNM stage (Table 1).
Table 1. Clinicopathological characteristics of patients.
| Variables | TG (n=50) | nTG (n=25) | P-value | |
|---|---|---|---|---|
| Age (years) | 57.1±12.4 | 57.6±12.3 | 0.864 | |
| Sex | 0.323 | |||
| Male | 19 (38) | 13 (52) | ||
| Female | 31 (62) | 12 (48) | ||
| BMI (kg/m2) | 24.4±3.2 | 24.6±3.2 | 0.820 | |
| ECOG | 0.826 | |||
| 0 | 32 (64) | 14 (56) | ||
| 1 | 14 (28) | 9 (36) | ||
| 2 | 3 (6) | 1 (4) | ||
| 3 | 1 (2) | 1 (4) | ||
| Comorbidity | ||||
| DM | 8 (16) | 1 (4) | 0.257 | |
| Hypertension | 16 (32) | 11 (44) | 0.321 | |
| Hepatitis | 0 (0) | 1 (4) | 0.333 | |
| Tuberculosis | 3 (6) | 1 (4) | 1.000 | |
| Initial nutritional parameters | ||||
| PNI | 50.8±5.6 | 54.6±6.0 | 0.009 | |
| Serum albumin (g/dL) | 4.2±0.4 | 4.4±0.3 | 0.070 | |
| Serum total protein (g/dL) | 7.1±0.6 | 7.2±0.5 | 0.237 | |
| Hemoglobin (g/dL) | 13.1±1.7 | 13.7±2.0 | 0.187 | |
| MCV (fL) | 90.0±5.3 | 90.3±2.8 | 0.776 | |
| Number of tumor | 1.000 | |||
| 1 | 49 (98) | 24 (96) | ||
| 2 | 1 (2) | 1 (4) | ||
| Tumor diameter (cm) | 5.4±3.1 | 3.2±2.5 | 0.001 | |
| Tumor location | 0.012 | |||
| Cardia | 3 (6) | 0 (0) | ||
| Upper 1/3rd | 20 (40) | 2 (8) | ||
| Middle 1/3rd | 22 (44) | 18 (72) | ||
| Lower 1/3rd | 5 (10) | 5 (20) | ||
| Length of proximal resection margin (cm) | 3.7±2.1 | 3.7±2.0 | 0.987 | |
| PRM | 0.632 | |||
| 0–2 | 10 (20) | 3 (12) | ||
| 2–5 | 29 (58) | 17 (68) | ||
| >5 | 11 (22) | 5 (20) | ||
| Number of retrieved LNs | 51.0±17.7 | 47.4±20.6 | 0.429 | |
| Number of metastatic LNs (cm) | 3.5±10.4 | 1.6±3.7 | 0.363 | |
| Depth of invasion | 0.623 | |||
| T1 | 27 (54) | 13 (52) | ||
| T2 | 5 (10) | 5 (20) | ||
| T3 | 12 (24) | 4 (16) | ||
| T4 | 6 (12) | 3 (12) | ||
| LN metastasis | 0.457 | |||
| N0 | 31 (62) | 18 (72) | ||
| N1 | 8 (16) | 2 (8) | ||
| N2 | 3 (6) | 3 (12) | ||
| N3 | 8 (16) | 2 (8) | ||
| Pathological stage (7th AJCC) | 0.228 | |||
| I | 31 (62) | 15 (60) | ||
| II | 7 (14) | 7 (28) | ||
| III | 12 (24) | 3 (12) | ||
Values are presented as number (%) or mean±standard deviation.
TG = total gastrectomy; nTG = near total gastrectomy; BMI = body mass index; ECOG = European Cooperative Oncology Group; DM = diabetes mellitus; PNI = prognostic nutritional index; MCV = mean corpuscular volume; PRM = proximal resection margin; LN = lymph node; AJCC = American Joint Committee on Cancer.
The operative details and short-term postoperative outcomes are shown in Table 2. No differences were found in the surgical approach, combined resection, extent of LN dissection, operation time, estimated blood loss, duration to flatus and soft diet, hospital stay, and complications within 30 postoperative days. However, there was a difference in the reconstruction method, because TG and nTG entail fundamentally different anastomotic procedures (P=0.000, Table 2).
Table 2. Operative details and short-term postoperative outcomes.
| Variables | TG (n=50) | nTG (n=25) | P-value | |
|---|---|---|---|---|
| Approach | 0.347 | |||
| Open | 38 (76) | 19 (76) | ||
| Laparoscopy | 12 (24) | 5 (20) | ||
| Robot | 0 (0) | 1 (4) | ||
| Combined resection | 0.258 | |||
| Yes | 4 (8) | 1 (4) | ||
| No | 46 (92) | 24 (96) | ||
| Extent of LN dissection | 0.152 | |||
| D1 or D1+ | 29 (58) | 10 (40) | ||
| D2 or more | 21 (42) | 15 (60) | ||
| Reconstruction | 0.000 | |||
| Billroth-II | 0 (0) | 22 (88) | ||
| Roux-en-Y | 50 (100) | 3 (12) | ||
| OP time (min) | 221.1±64.7 | 206.8±51.2 | 0.338 | |
| EBL (mL) | 229.6±207.8 | 198.6±167.5 | 0.519 | |
| Duration to flatus (days) | 3.3±0.6 | 3.3±0.6 | 0.891 | |
| Duration to soft diet (days) | 6.0±2.5 | 5.6±5.3 | 0.619 | |
| Duration to discharge (days) | 9.3±3.7 | 10.0±9.6 | 0.721 | |
| Complications*, CDC | 0.657 | |||
| 0–2 | 45 (90) | 24 (96) | ||
| ≥3 | 5 (10) | 1 (4) | ||
| Number of vitamin B12 injections† | 1.6±4.2 | 0.4±1.2 | 0.061 | |
Values are presented as number (%) or mean±standard deviation.
TG = total gastrectomy; nTG = near total gastrectomy; LN = lymph node; OP = operation; EBL = estimated blood loss; CDC = Clavien-Dindo classification.
*Within postoperative 1 month; †Within postoperative 2 years.
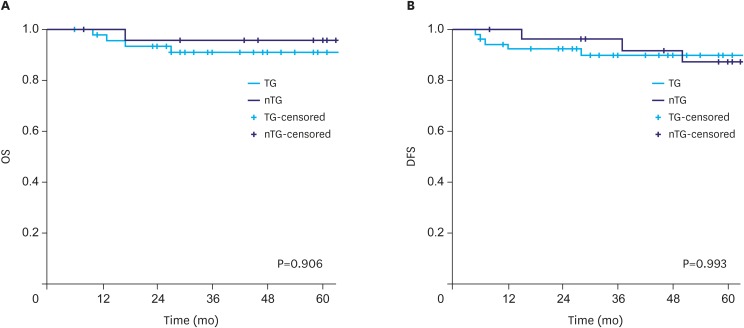
Overall survival (OS) and disease-free survival (DFS) were analyzed using Kaplan-Meier curves to compare the long-term oncologic outcomes between the groups. No difference was detected in the OS and DFS rates according to the extent of gastrectomy between the groups (P=0.906 and 0.993, respectively, Fig. 1A and B).
Fig. 1. Survival rate according to the extent of gastrectomy. (A) OS and (B) disease-free survival.
OS = overall survival; DFS = disease-free survival; TG = total gastrectomy; nTG = near total gastrectomy.
BMI decreased significantly until postoperative 6 months and then recovered slightly over time (P=0.000); there was no difference between the groups (P=0.457). PNI decreased significantly until postoperative 6 months and then recovered (P=0.024); the PNI in the nTG group decreased more than that in the TG group (P=0.019, Fig. 2A and B).
Fig. 2. Changes of nutritional status according to the extent of gastrectomy. (A) BMI and (B) PNI.
BMI = body mass index; PNI = prognostic nutritional index; TG = total gastrectomy; nTG = near total gastrectomy.
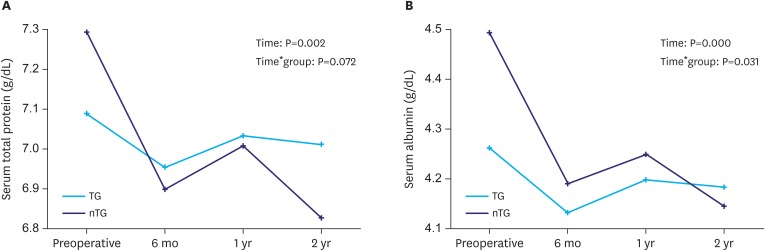
The serum total protein level decreased significantly until postoperative 6 months and then recovered (P=0.002); however, there was no difference between the groups (P=0.072). The serum albumin level decreased significantly until postoperative 6 months and then recovered over time (P=0.000); however, the level in the nTG group decreased more than that in the TG group (P=0.031, Fig. 3A and B).
Fig. 3. Changes of nutritional indices according to the extent of gastrectomy. (A) Serum total protein level and (B) serum albumin level.
TG = total gastrectomy; nTG = near total gastrectomy.
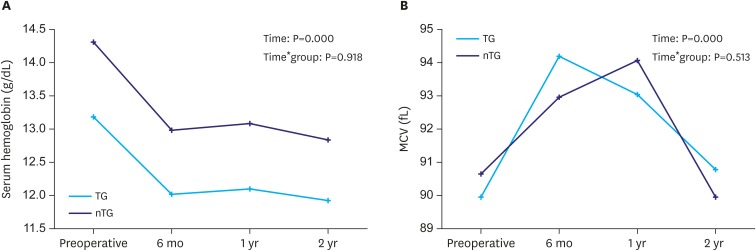
To measure the degree of anemia, the hemoglobin level of the patients was assessed. The hemoglobin level decreased significantly until postoperative 6 months, and then persisted over time (P=0.000); there was no difference between the groups (P=0.918). The MCV was checked to evaluate cobalamin deficiency anemia. The MCV increased significantly until postoperative 6 months and then recovered over time (P=0.000); there was no difference between the groups (P=0.513, Fig. 4A and B).
Fig. 4. Changes of anemia indices according to the extent of gastrectomy. (A) Serum hemoglobin level and (B) MCV.
MCV = mean corpuscular volume; TG = total gastrectomy; nTG = near total gastrectomy.
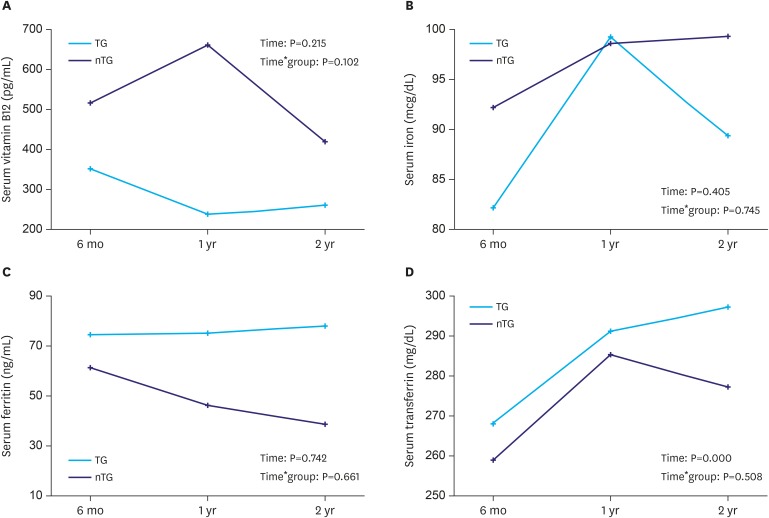
Although the mean vitamin B12 level was lower in the TG group, the serum vitamin B12 level did not change significantly over time (P=0.215), and there was no difference between the groups (P=0.102, Fig. 5A). The mean number of vitamin B12 injections was not significantly different between the groups, but a higher frequency was noted in the TG group (1.6±4.2 vs. 0.4±1.2, respectively; P=0.061, Table 2). To measure the degree of iron deficiency, the serum levels of iron, ferritin, and transferrin were assessed. The serum iron and ferritin levels showed no significant change over time (P=0.405 and 0.742, respectively), with no difference between the groups (P=0.745 and 0.661, respectively, Fig. 5B and C). The serum transferrin level increased significantly over time (P=0.000); however, there was no difference between the groups (P=0.508, Fig. 5D). A summary of the results of the nutritional parameters is shown in Table 3.
Fig. 5. Changes of vitamin B12 and iron profiles after gastrectomy. (A) Serum vitamin B12 level; (B) serum iron level; (C) serum ferritin level; and (D) serum transferrin level.
TG = total gastrectomy; nTG = near total gastrectomy.
Table 3. Summary of nutritional parameters.
| Variable | P-value of each item | |
|---|---|---|
| Effect of time | Interaction between time and group | |
| BMI | 0.000 | 0.457 |
| PNI | 0.024 | 0.019 |
| Albumin | 0.000 | 0.031 |
| Total protein | 0.002 | 0.072 |
| Hemoglobin | 0.000 | 0.918 |
| MCV | 0.000 | 0.513 |
| Vitamin B12 (pg/mL) | 0.215 | 0.102 |
| Iron (mcg/dL) | 0.405 | 0.745 |
| Ferritin (ng/mL) | 0.742 | 0.661 |
| Transferrin (mg/dL) | 0.000 | 0.508 |
BMI = body mass index; PNI = prognostic nutritional index; MCV = mean corpuscular volume.
DISCUSSION
Several studies have reported that the perioperative nutritional status of patients is important not only for improved quality of life but also for cancer prognosis after gastrectomy [3,15]. In case of distal gastrectomy, the type of reconstruction method used (e.g., Billroth-I, Billroth-II, or Roux-en-Y) could affect nutrition [16,17,18]. In addition, several special reconstruction methods, including jejunal interposition, duodenal transit, and pouch formation, have been introduced to improve nutrition and quality of life after TG [19,20]. However, these studies did not show a long-term nutritional benefit, and the nutritional function of the small reservoir after TG is controversial.
In addition to the reconstruction method, the extent of gastric resection is an important factor in nutrition. The extent of gastric resection is mainly determined by the location, size, and depth of tumor invasion [4]. In case of lower-third gastric cancer, distal gastrectomy has typically been applied. However, in case of upper- or middle-third gastric cancer, surgeons might be concerned about the difference between nTG and TG in some cases. The most important factor after oncologic surgery is the survival outcome. TG seems to have several advantages from an oncologic perspective. More radical LN dissection, including LN stations 2, 4sa, 10, and 11d, could be performed, and a proximal safety margin could be more easily secured in TG than in nTG [4]. Moreover, nTG seems to have the risk of remnant gastric cancer. In addition, in the present study, there was no significant difference in terms of long-term survival between the groups. Further, the number of retrieved LNs and proximal safety margin showed no statistical difference. None of the patients in the nTG group was diagnosed with remnant gastric cancer. This result shows that both TG and nTG have oncologic feasibility.
From a nutritional perspective, cobalamin deficiency anemia could easily occur after TG, because intrinsic factors are markedly decreased by the removal of parietal cells [21]. A cobalamin deficiency could cause not only anemia but also fatigue, depression, or headache [22]. Moreover, hormones such as ghrelin and gastrin would be removed after gastrectomy [23,24]. A cobalamin deficiency and loss of hormones are known causes of pernicious anemia and malnutrition [25]. In the present study, the serum hemoglobin level decreased after gastrectomy until 6 months and then recovered slightly, with statistical significance. In addition, the serum hemoglobin level was lower in the TG group, without statistical significance. The MCV of both groups was increased after gastrectomy and recovered slightly; however, there was no significant difference between the groups. The serum vitamin B12 level after gastrectomy was generally lower in the TG group than in the nTG group; however, there was no significant difference between the groups. In fact, we administered vitamin B12 via an intramuscular injection when the level was lower than 160 pg/mL during the follow-up period. However, only the number of intramuscular injections of vitamin B12 within 2 years in the TG group was higher than that in the nTG group, without statistical significance. These results indicate that compared with TG, nTG is insufficient to prevent a decrease in vitamin B12.
Another cause of anemia after gastrectomy is iron-deficiency anemia, because the change in the acidic environment after gastrectomy inhibits iron absorption [26]. We assessed the iron profiles, including the serum levels of iron, ferritin, and transferrin, from postoperative 6 months, and found no significant difference between the groups. These results show that compared with TG, nTG could not prevent the decrease in iron absorption.
The entire reservoir must be lost after TG, while a small remnant stomach could be preserved after nTG. However, the function of the small remnant stomach is controversial. Several studies have reported that the small reservoir (e.g., pouch formation) improved short-term post-gastrectomy symptoms, eating capability, body weight changes, and quality of life. However, the procedure was relatively complicated, and there were no significant long-term benefits [19,20,27]. In the present study, BMI and serum total protein level decreased until 6 months after gastrectomy and then recovered slightly, with statistical significance; however, no significant differences were observed between the groups. The PNI and serum albumin level decreased until 6 months after gastrectomy and then recovered slightly; in addition, more recovery was observed in the TG group than in the nTG group, with statistical significance. These results indicate that compared with TG, nTG with a small remnant stomach conferred no significant nutritional benefits.
In terms of the postoperative short-term outcomes, compared to subtotal gastrectomy, TG is associated with more postoperative complications such as leakage of the esophagojejunostomy [28,29]. In addition, patients who underwent a TG had a poorer quality of life than those who underwent a subtotal gastrectomy, in terms of their long-term outcomes [30]. In the present study, there was no significant difference between the groups in terms of short-term postoperative outcomes such as operation time, estimated blood loss, duration to flatus and soft diet, hospital stay, and complications within 30 postoperative days. This result indicates that compared with TG, nTG was unable to reduce postoperative complications.
The present study has several limitations. First, the study analyzed a small sample size, was retrospective, and was conducted at a single center. Thus, we used propensity score matching analysis to minimize these biases. Second, only postoperative changes in vitamin B12 and iron profiles could be analyzed because the data were not collected before surgery. In addition, supplementation with vitamin B12 or iron when their levels decreased during the follow-up period might have led to a bias. However, the maintenance of vitamin B12 or iron levels did not show a significant difference between the groups. Finally, gastric hormones and post-gastrectomy syndromes such as dumping and stasis were not analyzed in the present study. Despite these limitations, to the best of our knowledge, the present study is the first to analyze the long-term nutritional outcomes and variable nutritional parameters between nTG and TG using propensity score matching.
In conclusion, compared with TG, nTG with a small remnant stomach during the treatment of upper- or middle-third gastric cancer confers no nutritional benefit. However, further well-designed long-term prospective studies in this regard are warranted.
Footnotes
Funding: This research was supported by grants from the National Research Foundation of Korea (grant nos. 2012R1A1A1043576, 2015R1A1A1A05028000 and 2018R1D1A1B07045486), the Research Fund of Seoul St. Mary's Hospital, The Catholic University of Korea.
- Conceptualization: L.H.H.
- Data curation: S.H.S., J.Y.J.
- Formal analysis: S.H.S., K.J.H., L.H.H.
- Funding acquisition: L.H.H.
- Investigation: S.H.S.
- Methodology: S.H.S., L.H.H.
- Project administration: S.H.S., L.H.H.
- Resources: L.H.H.
- Software: S.H.S.
- Supervision: P.C.H., L.H.H.
- Validation: K.I.H., L.H.H.
- Visualization: S.H.S.
- Writing - original draft: S.H.S.
- Writing - review & editing: S.H.S., L.H.H.
Conflict of Interest: The authors have no potential conflicts (financial, professional, or personal), and no company has provided any financial arrangements for this study.
References
- 1.Jung KW, Won YJ, Kong HJ, Oh CM, Cho H, Lee DH, et al. Cancer statistics in Korea: incidence, mortality, survival, and prevalence in 2012. Cancer Res Treat. 2015;47:127–141. doi: 10.4143/crt.2015.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SS, Yu W, Chung HY, Kwon OK, Lee WK. Using quality of life scales with nutritional relevance after gastrectomy: a challenge for providing personalized treatment. J Gastric Cancer. 2017;17:342–353. doi: 10.5230/jgc.2017.17.e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pyo JH, Lee H, Min BH, Lee JH, Choi MG, Lee JH, et al. Long-term outcome of endoscopic resection vs. surgery for early gastric cancer: a non-inferiority-matched cohort study. Am J Gastroenterol. 2016;111:240–249. doi: 10.1038/ajg.2015.427. [DOI] [PubMed] [Google Scholar]
- 4.Pyo JH, Lee H, Min BH, Lee JH, Choi MG, Lee JH, et al. Early gastric cancer with a mixed-type Lauren classification is more aggressive and exhibits greater lymph node metastasis. J Gastroenterol. 2017;52:594–601. doi: 10.1007/s00535-016-1254-5. [DOI] [PubMed] [Google Scholar]
- 5.Kosuga T, Ichikawa D, Komatsu S, Okamoto K, Konishi H, Shiozaki A, et al. Feasibility and nutritional benefits of laparoscopic proximal gastrectomy for early gastric cancer in the upper stomach. Ann Surg Oncol. 2015;22(Suppl 3):S929–S935. doi: 10.1245/s10434-015-4590-4. [DOI] [PubMed] [Google Scholar]
- 6.Sasako M, Sano T, Yamamoto S, Sairenji M, Arai K, Kinoshita T, et al. Left thoracoabdominal approach versus abdominal-transhiatal approach for gastric cancer of the cardia or subcardia: a randomised controlled trial. Lancet Oncol. 2006;7:644–651. doi: 10.1016/S1470-2045(06)70766-5. [DOI] [PubMed] [Google Scholar]
- 7.Furukawa H, Kurokawa Y, Takiguchi S, Tanaka K, Miyazaki Y, Makino T, et al. Short-term outcomes and nutritional status after laparoscopic subtotal gastrectomy with a very small remnant stomach for cStage I proximal gastric carcinoma. Gastric Cancer. 2017;21:500–507. doi: 10.1007/s10120-017-0755-0. [DOI] [PubMed] [Google Scholar]
- 8.Migita K, Takayama T, Matsumoto S, Wakatsuki K, Enomoto K, Tanaka T, et al. Risk factors for esophagojejunal anastomotic leakage after elective gastrectomy for gastric cancer. J Gastrointest Surg. 2012;16:1659–1665. doi: 10.1007/s11605-012-1932-4. [DOI] [PubMed] [Google Scholar]
- 9.Kim DJ, Lee JH, Kim W. Comparison of the major postoperative complications between laparoscopic distal and total gastrectomies for gastric cancer using Clavien-Dindo classification. Surg Endosc. 2015;29:3196–3204. doi: 10.1007/s00464-014-4053-1. [DOI] [PubMed] [Google Scholar]
- 10.Fukagawa T, Gotoda T, Oda I, Deguchi Y, Saka M, Morita S, et al. Stenosis of esophago-jejuno anastomosis after gastric surgery. World J Surg. 2010;34:1859–1863. doi: 10.1007/s00268-010-0609-y. [DOI] [PubMed] [Google Scholar]
- 11.Nozoe T, Ninomiya M, Maeda T, Matsukuma A, Nakashima H, Ezaki T. Prognostic nutritional index: a tool to predict the biological aggressiveness of gastric carcinoma. Surg Today. 2010;40:440–443. doi: 10.1007/s00595-009-4065-y. [DOI] [PubMed] [Google Scholar]
- 12.Takagi H, Morimoto T. Near-total gastrectomy. J Surg Oncol. 1984;26:14–16. doi: 10.1002/jso.2930260105. [DOI] [PubMed] [Google Scholar]
- 13.Salo JA, Saario I, Kivilaakso EO, Lempinen M. Near-total gastrectomy for gastric cancer. Am J Surg. 1988;155:486–489. doi: 10.1016/s0002-9610(88)80119-3. [DOI] [PubMed] [Google Scholar]
- 14.Washington K. 7th edition of the AJCC cancer staging manual: stomach. Ann Surg Oncol. 2010;17:3077–3079. doi: 10.1245/s10434-010-1362-z. [DOI] [PubMed] [Google Scholar]
- 15.Park KB, Park JY, Lee SS, Kwon OK, Chung HY, Yu W. Impact of body mass index on the quality of life after total gastrectomy for gastric cancer. Cancer Res Treat. 2017 doi: 10.4143/crt.2017.080. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee MS, Ahn SH, Lee JH, Park DJ, Lee HJ, Kim HH, et al. What is the best reconstruction method after distal gastrectomy for gastric cancer? Surg Endosc. 2012;26:1539–1547. doi: 10.1007/s00464-011-2064-8. [DOI] [PubMed] [Google Scholar]
- 17.Lee J, Hur H, Kim W. Improved long-term quality of life in patients with laparoscopy-assisted distal gastrectomy with jejunal pouch interposition for early gastric cancer. Ann Surg Oncol. 2010;17:2024–2030. doi: 10.1245/s10434-010-1095-z. [DOI] [PubMed] [Google Scholar]
- 18.Lee HH, Song KY, Lee JS, Park SM, Kim JJ. Delta-shaped anastomosis, a good substitute for conventional Billroth I technique with comparable long-term functional outcome in totally laparoscopic distal gastrectomy. Surg Endosc. 2015;29:2545–2552. doi: 10.1007/s00464-014-3966-z. [DOI] [PubMed] [Google Scholar]
- 19.Lehnert T, Buhl K. Techniques of reconstruction after total gastrectomy for cancer. Br J Surg. 2004;91:528–539. doi: 10.1002/bjs.4512. [DOI] [PubMed] [Google Scholar]
- 20.Gertler R, Rosenberg R, Feith M, Schuster T, Friess H. Pouch vs. no pouch following total gastrectomy: meta-analysis and systematic review. Am J Gastroenterol. 2009;104:2838–2851. doi: 10.1038/ajg.2009.456. [DOI] [PubMed] [Google Scholar]
- 21.Scott JM, Molloy AM. The discovery of vitamin B12 . Ann Nutr Metab. 2012;61:239–245. doi: 10.1159/000343114. [DOI] [PubMed] [Google Scholar]
- 22.Couderc AL, Camalet J, Schneider S, Turpin JM, Bereder I, Boulahssass R, et al. Cobalamin deficiency in the elderly: aetiology and management: a study of 125 patients in a geriatric hospital. J Nutr Health Aging. 2015;19:234–239. doi: 10.1007/s12603-014-0525-1. [DOI] [PubMed] [Google Scholar]
- 23.Ariyasu H, Takaya K, Tagami T, Ogawa Y, Hosoda K, Akamizu T, et al. Stomach is a major source of circulating ghrelin, and feeding state determines plasma ghrelin-like immunoreactivity levels in humans. J Clin Endocrinol Metab. 2001;86:4753–4758. doi: 10.1210/jcem.86.10.7885. [DOI] [PubMed] [Google Scholar]
- 24.Castañeda TR, Tong J, Datta R, Culler M, Tschöp MH. Ghrelin in the regulation of body weight and metabolism. Front Neuroendocrinol. 2010;31:44–60. doi: 10.1016/j.yfrne.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Stabler SP. Clinical practice. Vitamin B12 deficiency. N Engl J Med. 2013;368:149–160. doi: 10.1056/NEJMcp1113996. [DOI] [PubMed] [Google Scholar]
- 26.Lim CH, Kim SW, Kim WC, Kim JS, Cho YK, Park JM, et al. Anemia after gastrectomy for early gastric cancer: long-term follow-up observational study. World J Gastroenterol. 2012;18:6114–6119. doi: 10.3748/wjg.v18.i42.6114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaiyasate K, Jacobs M, Brooks SE, del Rosario G, Andrus L, Kestenberg W, et al. The uncut Roux-en-Y with jejunal pouch: a new reconstruction technique for total gastrectomy. Surgery. 2007;142:33–39. doi: 10.1016/j.surg.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 28.Nagai E, Ohuchida K, Nakata K, Miyasaka Y, Maeyama R, Toma H, et al. Feasibility and safety of intracorporeal esophagojejunostomy after laparoscopic total gastrectomy: inverted T-shaped anastomosis using linear staplers. Surgery. 2013;153:732–738. doi: 10.1016/j.surg.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Kong L, Yang N, Shi L, Zhao G, Wang M, Zhang Y. Total versus subtotal gastrectomy for distal gastric cancer: meta-analysis of randomized clinical trials. Onco Targets Ther. 2016;9:6795–6800. doi: 10.2147/OTT.S110828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee SS, Chung HY, Kwon OK, Yu W. Long-term quality of life after distal subtotal and total gastrectomy: symptom- and behavior-oriented consequences. Ann Surg. 2016;263:738–744. doi: 10.1097/SLA.0000000000001481. [DOI] [PubMed] [Google Scholar]