ABSTRACT
Today's gold standard in HIV therapy is combined antiretroviral therapy (cART). It requires strict adherence by patients and lifelong medication, which can lower the viral load below detection limits and prevent HIV-associated immunodeficiency but cannot cure patients. The bispecific T cell-engaging (BiTE) antibody technology has demonstrated long-term relapse-free outcomes in patients with relapsed and refractory acute lymphocytic leukemia. Here, we generated BiTE antibody constructs that target the HIV-1 envelope protein gp120 (HIV gp120) using either the scFv B12 or VRC01, the first two extracellular domains (1 + 2) of human CD4 alone or joined to the single chain variable fragment (scFv) of the antibody 17b fused to an anti-human CD3ε scFv. These engineered human BiTE antibody constructs showed engagement of T cells for redirected lysis of HIV gp120-transfected CHO cells. Furthermore, they substantially inhibited HIV-1 replication in peripheral blood mononuclear cells (PBMCs) as well as in macrophages cocultured with autologous CD8+ T cells, the most potent being the human CD4(1 + 2) BiTE [termed CD(1 + 2) h BiTE] antibody construct and the CD4(1 + 2)L17b BiTE antibody construct. The CD4(1 + 2) h BiTE antibody construct promoted HIV infection of human CD4−/CD8+ T cells. In contrast, the neutralizing B12 and the VRC01 BiTE antibody constructs, as well as the CD4(1 + 2)L17b BiTE antibody construct, did not. Thus, BiTE antibody constructs targeting HIV gp120 are very promising for constraining HIV and warrant further development as novel antiviral therapy with curative potential.
IMPORTANCE HIV is a chronic infection well controlled with the current cART. However, we lack a cure for HIV, and the HIV pandemic goes on. Here, we showed in vitro and ex vivo that a BiTE antibody construct targeting HIV gp120 resulted in substantially reduced HIV replication. In addition, these BiTE antibody constructs display efficient killing of gp120-expressing cells and inhibited replication in ex vivo HIV-infected PBMCs or macrophages. We believe that BiTE antibody constructs recognizing HIV gp120 could be a very valuable strategy for a cure of HIV in combination with cART and compounds which reverse latency.
KEYWORDS: CD8+ T cells, HIV, bispecific T-cell-engaging antibodies, broadly neutralizing antibodies, macrophages
INTRODUCTION
With combined antiretroviral therapy (cART), most HIV patients experience a reduction in viral load below detection limits, shifting the HIV infection from a deadly disease toward a chronic infection. However, cART-based therapies are not curative, are dependent on strict adherence, expose patients to risk of long-term cART toxicity, and burden the health care systems with intensive costs due to lifelong treatment necessity. Studies on elite controllers demonstrated the critical role of the T cell response in the control of acute and chronic HIV-1 infection (1) Therefore, an attempt to improve the patients' cellular responses against HIV has emerged as a promising cure strategy. Indeed, genetically manipulated primary CD8+ T cells, expressing a chimeric antigen (Ag) receptor specific for the HIV gp120, effectively lysed HIV-infected CD4+ T cells in vitro (2). As an alternative to gene-engineered HIV-specific T cells, the BiTE technology (AMGEN, Inc.) redirects the cytotoxic potential of any T cell to the target cell expressing the corresponding antigen.
The BiTE approach has already been successfully applied in the clinic in patients suffering from non-Hodgkin's lymphoma or B-cell lymphoblastic leukemia (3, 4). Indeed, the FDA-licensed BiTE blinatumomab (Blincyto), targeting CD19+ cells results in shrinking of neoplastic lymph nodes (5) and in clearing bone marrow of blasts in those patients (6), respectively. Several other BiTE candidates are under clinical investigation in solid tumor indications, for example, AMG212/BAY2010112, which targets the prostate-specific membrane antigen (7), and MEDI-565/AMG211, which targets the carcinoembryonic antigen (8, 9).
In 1991, Traunecker and Berg independently published bispecific antibody constructs based on domains of the natural HIV receptor CD4 and an anti-CD3 binding moiety (10, 11). Shortly after, Okada et al. presented a novel bifunctional antibody consisting of a Fab part to HIV gp120 and anti-CD3 (12). All those bispecific antibody constructs showed in vitro lysis of HIV-infected T cell lines. Apart from work by Chamow et al. (13), who generated a bispecific antibody similar to the ones by Traunecker et al. and Berg et al., no further development of this concept took place for more than 20 years. In 2015, bispecific antibody constructs based on antibody fragments targeting HIV gp120 and CD3 were described to be active in vitro using patient samples (14, 15).
To further elucidate the naturally given broad potential of HIV gp120 as a target binding domain, here we generated BiTE antibody constructs by fusing either (i) the N-terminal domains 1 and 2 of human CD4 [CD4(1 + 2)], (ii) the scFv of broadly neutralizing antibody (bNAb) B12 or VRC01, or (iii) the human CD4(1 + 2), linked to the scFv of 17b (CD4L17b), to our proprietary human anti-human CD3ε scFv.
RESULTS
The human BiTE antibody constructs binding to HIV gp120-transfected cells resulted in redirected lysis using unstimulated PBMC or stimulated CD8+ T cells.
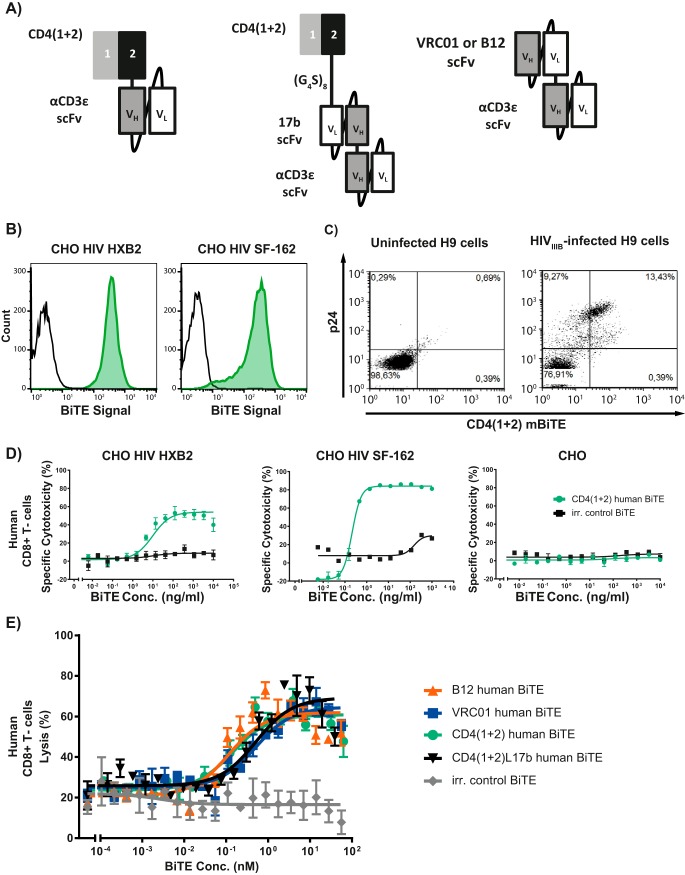
The two N-terminal domains of human CD4, the natural receptor for HIV, were fused to the proprietary human anti-human CD3ε scFv (Fig. 1A). This BiTE antibody construct separated very clearly CHO cells expressing the HIV gp120 of either the CXCR4-tropic strain, HXB2, or the CCR5-tropic strain, SF-162, from parental ones using flow cytometry (Fig. 1B).
FIG 1.
Various human BiTE antibody constructs are highly cytotoxic to HIV env gp120-expressing CHO cells when cocultured with CD8+ T cells. (A) Cartoon of the various BiTE antibody constructs generated. The first two N-terminal domains (1 + 2) of human CD4 (dark gray/black) are linked by a glycine/serine linker (G4S) to a proprietary anti-human CD3ε scFv (gray/white). Variable domains within an scFv are linked by a (G4S)3 linker. (B) Fluorescence-activated cell sorting (FACS) binding of the CD4(1 + 2) h BiTE antibody construct (green) and mouse anti-histidine tag MAb plus goat anti-mouse Fc MAb-phycoerythrin (PE) staining control (black) on HIV gp120 (HXB2/SF162)-transfected CHO cells. (C) Staining of HIVIIIB+ H9 T cells versus HIV− H9 T cells using the CD4(1 + 2)m BiTE. BiTE signal was measured using mouse anti-histidine MAb, followed by goat anti-mouse Fc MAb-PE. Fixed and lysed cells were stained using a mouse anti-HIV p24 MAb-allophycocyanin. (D and E) Prestimulated purified human CD8+ T cells were incubated with HIV gp120-transfected CHO cells (E:T ratio, 10:1) and BiTE dilution series of the CD4(1 + 2)h BiTE (green) BiTE versus irrelevant (irr.) control BiTE antibody construct (black) (D) or various BiTE antibody constructs (E).
To demonstrate the binding specificity of the two N-terminal domains of human CD4 for HIV-infected human T cells, the anti-CD3 domain from the human CD4(1 + 2) BiTE [CD4(1 + 2) h BiTE] was replaced by a murine counterpart, CD4(1 + 2) m BiTE, which does not recognize human CD3. As expected, binding of HIV gp120 on HIV+ T cells by the CD4(1 + 2) m BiTE occurred independently of binding to human CD3; in fact, HIV p24+ cells were bound by the CD4(1 + 2) m BiTE (Fig. 1C). These data with H9 cells clearly show that the BiTEs via the two N-terminal CD4 domains recognize and bind gp120 in cells infected with replication-competent HIV that approximates the in vivo situation more closely than data obtained with CHO cells genetically complemented with gp120. Notably, CHO cells of Chinese hamster origin are not per se permissive to HIV replication.
The BiTE antibody constructs redirected lysis of HIV gp120-transfected CHO cells when cocultured with prestimulated human CD8+ T cells in the pico- or low-nanomolar range (Fig. 1D). The other BiTE antibody constructs generated were equally cytotoxic in this assay (Fig. 1E). Untransfected CHO cells were not lysed when incubated with CD4(1 + 2) h BiTE and effector cells, proving the specificity of the BiTE antibody construct (Fig. 1D).
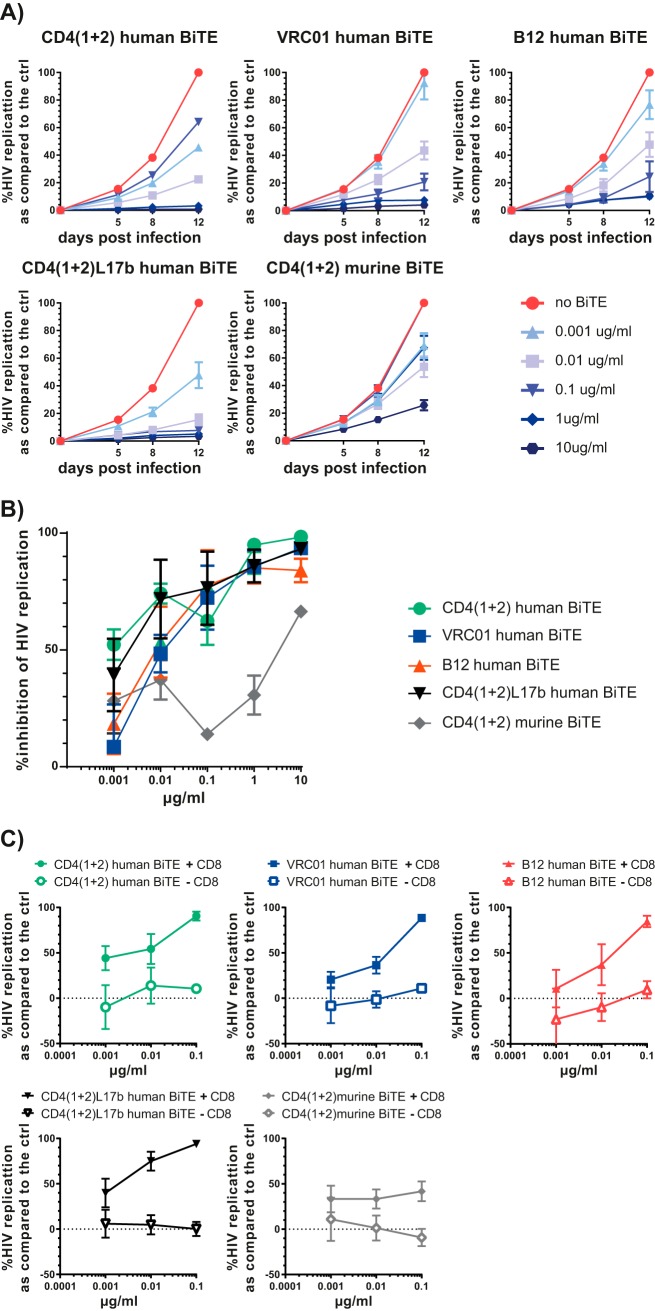
The human BiTE antibody constructs showed a dose-dependent inhibition of HIV replication in ex vivo HIV-infected PBMCs and monocyte-derived macrophages (MDMs) cocultured with CD8+ T cells.
Prestimulated PBMCs were infected with HIVNL4-3 and subsequently incubated with different concentrations of the various BiTE antibody constructs. All BiTE antibody constructs specifically targeting HIV gp120 showed a dose-dependent inhibition of HIV replication (Fig. 2A and B). The CD4(1 + 2), CD417b, and B12 BiTE antibody constructs were the most potent ones, with >90% HIV inhibition at 1 μg/ml; the CD417b BiTE antibody construct showed >90% HIV inhibition at 0.1 μg/ml. The effective inhibitory concentration 50 (IC50) was 0.048 μg/ml for the CD4(1 + 2) h BiTE, 0.037 μg/ml for the CD417b BiTE, 0.0038 μg/ml for the B12 BiTE, and 2.86 μg/ml for the VRC01 BiTE. The CD4(1 + 2) m BiTE also showed antiviral activity at 10 μg/ml, while at lower dosage it had a rather pro-HIV effect (Fig. 2B).
FIG 2.
Dose-dependent inhibition of HIV replication by the human BiTE antibody constructs tested in ex vivo HIV-infected PBMCs. (A) PBMCs were infected with the CCR5 HIV strain YU-2 overnight and washed, and the corresponding BiTE antibody constructs in dilution series were added. HIV replication was monitored by quantifying p24 Ag in the supernatant over time. (B) Compilation of the dose-dependent anti-HIV activity of all BiTE antibody constructs. The assays were performed using three independent donors, done in triplicates.
The viral resistance (Vres) to inhibition by maximal BiTE concentrations was also most prominent for the BiTE antibody constructs comprising the human CD4 domains 1 and 2 alone or coupled to the scFv 17b [percent (averages ± standard deviations) replication compared to the normalized control: human BiTE, 1.02 ± 0.31; CD4(1 + 2)L17b, 0.73 ± 0.4; VRCO1, 42.2 ± 30.8; B12, 1.34 ± 0.52; CD4(1 + 2) murine BiTE, 6.4 ± 5.5].
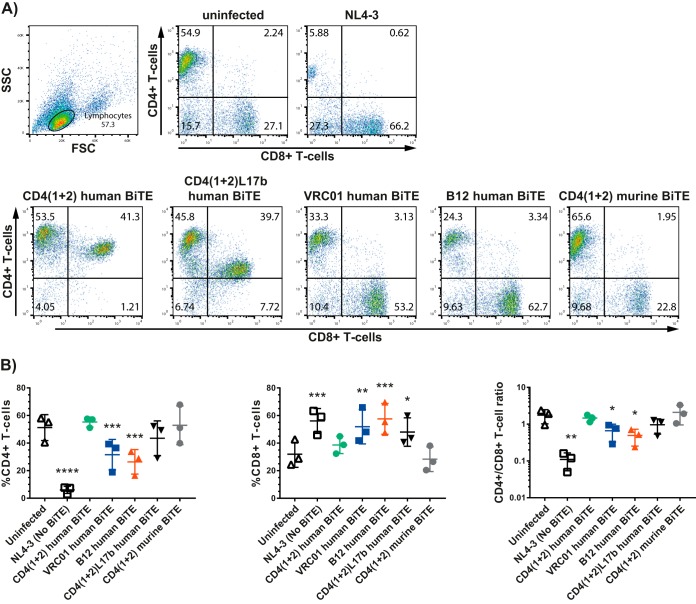
The HIV inhibition was paralleled by a preservation of CD4+ T cells and expansion of CD8+ T cells (Fig. 3A and B). Under the condition tested, the BiTE antibody constructs with the CD4 domains were the most beneficial ones for the preservation of CD4+ T cells.
FIG 3.
Preservation of the CD4+/CD8+ T-cell ratio in HIV-infected PBMCs treated with the human BiTE antibody constructs. (A) Representative FACS plots of ex vivo HIV-infected PBMCs treated with the BiTE antibody constructs at 10 μg/ml, which were harvested at the end of the experiment, i.e., at day 12, and which were stained for the human CD4 and CD8 cell surface marker. (B) Compilation of the experiments looking at the CD4+/CD8+ T-cell ratio (means ± standard errors of the means [SEM]; n = 3). Statistics were done using repeated analysis of variance (ANOVA) followed by Dunnett's multiple-comparison test comparing the BiTE-treated samples to the uninfected control (*, P < 0.05; **, P < 0.005; ***, P < 0.0005).
We observed very similar anti-HIV effects between HIV-infected cocultures of MDMs and autologous CD8+ T cells by the various BiTE antibody constructs and HIV-infected PBMCs. The BiTE antibody constructs comprising the CD4 domains excelled in their anti-HIV activity (Fig. 4A and B) with, at the limit, detectable HIV replication as judged by the Vres [percent (averages ± standard deviations) replication compared to normalized control: CD4(1 + 2) human BiTE, 0.75 ± 0.2; CD4(1 + 2)L17b, 3.3 ± 0.6; VRCO1, 4 ± 1.6; B12, 10.75 ± 5.64; CD4(1 + 2) murine BiTE, 24.2 ± 1.8]. As expected, the BiTE antibody constructs lost their anti-HIV effects in HIV-infected MDMs without the addition of CD8+ T cells (Fig. 4C).
FIG 4.
Dose-dependent inhibition of HIV replication by the human BiTE antibody constructs tested in ex vivo HIV-infected MDMs cocultured with autologous CD8+ T cells. (A) MDMs were infected overnight with the CCR5-tropic strain YU-2 and subsequently cocultured with autologous purified CD8+ T cells, and the corresponding BiTE antibody constructs in dilution series were added. HIV replication was monitored by quantifying p24 Ag in the supernatant over time (n = 3 in triplicates). (B) Compilation of the dose-dependent anti-HIV activity of all BiTE antibody constructs. (C) The human BiTE antibody constructs deploy their anti-HIV activities in ex vivo-infected MDMs only when cocultured with CD8+ T cells.
We again observed some anti-HIV activity with the CD4(1 + 2) murine BITE antibody construct but much less than that with the human counterpart. The HIV inhibition observed with the murine BiTE antibody construct points to the pure neutralization activity by the CD4 domains 1 and 2 as opposed to the human BiTE; notably, the scFv targeting the murine CD3 has no cross-reactivity to human CD3.
CD4(1 + 2) h BiTE led to HIV infection of CD4−/CD8+ T cells with the CXCR4-tropic strain NL4-3.
We know that soluble CD4 as well as CD4 presented in trans by another cell may promote a conformational change of the HIV gp120, resulting in infection of CD4− cells. Thus, we wanted to exclude that the CD4(1 + 2) h BiTE promotes HIV infection of CD4− T cells. However, we found that CD4(1 + 2) h BiTE treatment of isolated CD8+/CD4− T cells resulted in a higher p24 level than in the controls when using spinoculation. Notably, pretreatment with the CD4(1 + 2)17b-, B12-, and VRC01-based BiTE antibody constructs showed HIV p24 levels comparable to those of cells solely challenged with HIV (Fig. 5). Thus, only the CD4(1 + 2) BiTE antibody construct resulted in HIV infection of CD4−/CD8+ cells, pointing to its specific property to induce conformational changes of gp120, which in turn enables HIV to infect CD4− T cells.
FIG 5.
HIV replication in primary unstimulated CD4−/CD8+ T cells incubated with BiTE antibody constructs prior to HIV infection via spinoculation. The generated BiTE antibody constructs were incubated with primary CD8+ T cells prior to infection with NL4-3 or YU-2 via spinoculation. After washing and 7 days of incubation, the viral replication was determined by quantifying HIV p24 in the cell culture supernatant. The assay was performed using three independent donors in triplicates (averages ± SEM are shown). Statistics were calculated using ANOVA followed by Tukey's multiple-comparison test.
DISCUSSION
Here, we present the anti-HIV potency of BiTE antibody constructs with either CD4 domains 1 and 2, neutralizing scFv against gp120, or both together (Fig. 1A). All BiTE antibody constructs showed promising potency in constraining HIV replication in ex vivo HIV-infected PBMCs or MDMs cocultured with autologous CD8+ T cells. The CD4(1 + 2) human BiTE antibody construct resulted in very low levels infection of CD4− cells, prohibiting its clinical use. Notably, CD417b was the most potent BiTE antibody construct in a head-to-head comparison and did not show this disturbing infection of CD4− cells. Thus, this genre of BiTE is very promising for treating HIV and potentially also for targeting latently HIV-infected cells in a shock-and-kill approach.
We first verified the specificity of the CD4(1 + 2) human BiTE for binding HIV gp120: it bound firmly the HIV gp120 expressed on CHO cells irrespective of gp120's coreceptor selectivity (Fig. 1B). Furthermore, we found a correlation between the binding of the chimeric CD4(1 + 2) murine BiTE and intracellular HIV p24 Ag expression in HIVIIIB-infected H9 cells, pointing to CD4 domain specificity for HIV-infected cells; uninfected H9 cells did not bind the CD4(1 + 2) murine BiTE (Fig. 1C). The partial overlap observed between p24 Ag-positive and gp120-positive cells is a well-known phenomenon and is explained by a time-wise differential expression of HIV proteins. The CD4(1 + 2) human BiTE displayed its cytotoxicity on HIV gp120+ CHO cells when cocultured with CD8+ T cells. No cytotoxicity was observed with parental CHO cells (Fig. 1D). Furthermore, the data observed with H9 cells nicely demonstrate that the BiTE antibody constructs recognize and bind gp120 when naturally expressed in the context of a productive infection. Notably, productive infection results in a markedly lesser expression of gp120 than the one we had in the CHO cells genetically complemented with gp120, which speaks in favor of the high binding affinity of the two N-terminal domains of CD4 to gp120.
Since a BiTE comprising solely the CD4 domains may result in trans-infection of CD4-negative cells (16), we created additional BiTE antibody constructs combining the CD4 domains 1 and 2 with an scFV against HIV env gp120 or BiTE antibody constructs with an scFv against gp120 alone. The scFv were based on the 17B antibody, which binds to a CD4-induced antibody epitope (17), and on the VRCO1 or B12 binding site antibodies (18). These BiTE antibody constructs showed cytotoxicity against HIV gp120+ CHO cells cocultured with CD8+ T cells comparable to that of the human CD4(1 + 2) BiTE (17). All of the BiTE antibody constructs but the murine one showed a clear dose-dependent inhibition of HIV replication in ex vivo HIV-infected PBMCs as well as MDMs cocultured with autologous CD8+ T cells over time; in fact, the CD4(1 + 2) human BiTE as well as the CD417b BiTE were the most potent BiTE antibody constructs overall. In PBMCs at the highest dose tested and in MDMs cocultured with CD8+ T cells, even at all doses tested, the CD4(1 + 2) murine BiTE displayed some anti-HIV activity but much less than that of its human counterpart. This blocking effect occurs most likely via binding gp120 and is reminiscent of work showing virus neutralization by administering soluble CD4 domains (19). Notably, CD8+ T cells were mandatory for deploying the BiTE antibody constructs' anti-HIV activity in macrophages (Fig. 4C). As we purified CD8+ T cells using a CD8 microbead assay, we had a small number of CD8+ NKT+ cells in addition to CD8+ T cells in the cocultures of MDMs and CD8+ T cells. To what extent they contributed to the antiviral effect observed remains unknown. Notably, the antiviral effects of the BiTE antibody constructs went along with a preservation of the CD4+/CD8+ T-cell ratio.
The idea of bispecific antibodies retargeting T cells to constrain HIV has been around since the early nineties of the last century (10–13). In 2015, Sung et al. and Sloan et al. revived this idea by designing dual-affinity retargeting proteins comprising novel broadly neutralizing or nonneutralizing gp140 HIV antibodies (14, 15, 20). Notably, the CD4(1 + 2) human BiTE had a design, with the two N-terminal CD4 domains targeting gp120, similar to that of some of the older constructs (10–13). All the constructs reported had a very efficient cytotoxic activity against cells expressing HIV env gp120. The design of the constructs differs and most likely explains subtle differences in their antiviral potency. For example, we observed a very potent antiviral activity with the VRCO1 BiTE antibody construct, while Sloan et al. did not (15). Sloan et al. and Pegu et al. also showed that these retargeting molecules reverse HIV latency and lead to a decrease of the HIV latent reservoir (15, 20).
Similarly to plain broadly neutralizing antibodies, HIV may escape bispecific antibodies. Thus, there is a theoretical advantage of the human CD4(1 + 2) BiTE, since its efficacy is independent of the HIV gp120 sequence, i.e., the CD4(1 + 2) BiTE should be active as long as there is CD4-dependent HIV entry. It remains highly unlikely that one single bNAb will be sufficient to suppress HIV replication completely and prevent escape mutations (21). A potential downsize of the CD4(1 + 2) h BiTE antibody construct is its potential to mediate infection of CD4−/CD8+ T cells, as seen in the ex vivo experiment with HIV spinoculated PBMCs. Spinoculation is widely used in virology research to enhance viral infection. Spinoculation is coupled with spin-induced cytoskeletal dynamics that promote receptor mobilization, viral entry, and postentry processes (22). Thus, spinoculation-induced cellular permissiveness may be beyond the natural capacity of an infecting virus, and thus the data we generated of HIV infection of CD4−/CD8+ T cells need to be interpreted with caution.
While theoretically at least the CD4(1 + 2) h BiTE may trigger conformational changes on HIV gp120, allowing for HIV coreceptor binding and subsequent infection of CD4− cells (16, 23), the HIV gp120 neutralizing antibodies, such as B12 and VRC01, lock HIV gp120 prior to any conformational changes. In contrast, the scFv 17b targets a CD4-inducible HIV env gp120 epitope. Thus, the dual-targeting BiTE, CD417b BiTE, combines two very nice features, i.e., binding HIV env gp120 independent of its sequence via the two N-terminal CD4 domains, resulting in the exposure of the CD4-inducible epitope, which then will be accessible to the scFv 17b. This dual-targeting BiTE also prevented the CD4-dependent infection and therefore seems a promising approach to design broad and highly anti-HIV active BiTE antibody constructs. Notably, the 17b scFv might also enhance the specificity of a CD4(1 + 2)-based BiTE with respect to CD4-insensitive HIV strains (24). BiTE antibody constructs comprising the scFv of broadly neutralizing monoclonal antibody (MAb) B12 or VRC01 also show no induction of HIV infection but are prone to HIV mutational immune escape. Clearly, we do not know to what extent the phenomenon of BiTE-mediated HIV infection of CD4−/CD8+ cells is clinically relevant.
CD3-dependent internalization (25) of a BiTE that is bound to CD3 and to an HIV virion represents another potential mechanism of CD4-independent HIV infection. As HIV p24 levels are not increased in CD8+ T cells incubated with CD417b-, B12-, or VRC01-based BiTE antibody constructs compared to cells incubated with the control BiTE, potential uptake of virions probably does not occur, or if it occurs, virions will be degraded, not leading to productive infection.
Redirecting T cells to lyse HIV-infected cells warrants further development using the clinically validated BiTE platform technology. Since CD3/interleukin-2 (IL-2)-stimulated T cells increased viral replication in a small number of patients (26) and efficiently killed reactivated, latently infected CD4+ T cells in vitro (27), T cell-engaging BiTE antibodies via binding to the CD3 receptor could also reactivate viral replication in latently infected cells and subsequently induce their lysis, as discussed above (15, 20). Another approach of an anti-HIV-directed cytotoxic therapy was recently described in HIV+ BLT mice treated with an antibody-drug conjugate in addition to cART (28). Under ideal circumstances, BiTE antibodies might show curative potential, as has been demonstrated in prolonged relapse-free survival of leukemia patients (29).
In summary, the BiTE antibody constructs described redirected T cells to HIV gp120+ cells and inhibited viral replication in vitro. Notably, T-cell activation promotes viral replication in patients (26) and is also able to reverse HIV latency (27); thus, these BiTE antibody constructs could likewise mediate viral replication in latently infected cells via their activation, eventually resulting in their cell death. They might be especially valuable in patients with cART for eliminating the latent reservoir. cART in this scenario would in any case prevent spreading infection in the case of the unfortunate possibility of CD4-independent infection. Other T cell-engaging, bispecific antibodies, which bind to HIV gp120, also gave very promising data similar to ours (14, 15). Apart from the discussed bispecific antibodies, a rather impressive number of bispecific antibodies of any kind have been published in the field of HIV over the last couple of years, including, among others, bispecific antibodies for increasing the breadth and strength for neutralizing HIV gp120 (30) or bispecific antibodies recruiting NK cells for killing HIV-infected cells (31, 32). Thus, bispecific antibody constructs overall warrant further investigation for their potential to eliminate the HIV latent reservoir, preferentially in vivo using either humanized mice or nonhuman primate models. As of now, no clinical trials are registered on the ClinicalTrials.gov website.
MATERIALS AND METHODS
Cell lines.
Dihydrofolate reductase (DHFR)-deficient CHO cells were grown in suspension in a humidified incubator at 37°C and 5% CO2 with HyQ LS medium (HyClone, Logan) supplemented with 100 U/ml penicillin and 100 μg/ml streptomycin. For recombinant HIV envelope expression in CHO cells, the sequences of the extracellular domains of HIV HXB2 and HIV SF162 were fused to the transmembrane and intracellular domain of the human epithelial cell adhesion molecule (EpCAM; amino acids [aa] 266 to 314) using the pEF DHFR plasmid, and selection of transfected cells expressing HXB2 or SF162 was achieved by using nucleoside-free medium. The HPB-ALL cells (human T cell line) and the HIV+ and HIV− H9 cells (HIV IIIB+ H9 T cells [HIV+] and the H9 T cells [HIV−]; human T cell line) were grown in RPMI medium (Biochrom) supplemented with 10% fetal calf serum, 500 nM β-mercaptoethanol (Gibco), 100 U/ml penicillin, 100 μg/ml streptomycin, 1 mM sodium pyruvate, 1× nonessential amino acids, and 10 mM HEPES (RPMI complete medium; Biochrom).
Primary cells.
PBMCs obtained from the local blood bank or from healthy volunteers were Ficoll isolated and incubated in RPMI medium supplemented with 10 U/ml IL-2, 1% glutamine, and 1% penicillin. For the determination of BiTE activity, prestimulated CD8+ T cells were used as effector cells and CHO gp120+ cells as target cells. Prestimulated CD8+ T cells were isolated from PBMCs stimulated by anti-CD3 and anti-CD28 antibodies (1 μg/ml) coated on a petri dish and 20 U/ml IL-2 for 72 h, followed by an additional 24 h in a flask containing fresh medium and 20 U/ml IL-2. CD8+ T cells then were negatively isolated via magnetic cell labeling using microbeads (Miltenyi). To generate monocyte-derived macrophages (MDMs), monocytes were isolated using CD14 microbeads (catalog no. 130-050-201; Miltenyi), seeded at a cell count of 1.5 × 105 per cm2, and cultured in RPMI 1640 medium (BioWhittaker) supplemented with 10% human type AB serum (catalog no. H1513; Sigma), 2 mM l-glutamine, 1% penicillin-streptomycin, and 10 ng/ml M-CSF for 6 days. At this time point, medium was replaced with medium without cytokines and cultured for another 2 days.
Construction and production of BiTE antibody constructs.
The human CD4 protein domains 1 and 2 (CD4 aa 26 to 207) were fused to the proprietary anti-human CD3ε or anti-mouse CD3 scFv by SG4S linker. In case of the CD417b h BiTE, the CD4 domains 1 and 2 are linked by a (G4S)8 sequence to the scFv of MAb 17b [VL/VH order, linked by (G4S)3], which is fused to the anti-CD3ε scFv. The scFv of MAbs B12 and VRC01 [in VH/VL order, linked by (G4S)3 linker] are likewise fused to the anti-human CD3ε scFv. The target binding moieties were inserted in frame into a DHFR plasmid encoding a eukaryotic signal peptide for expression of the BiTE antibody constructs into the cell culture supernatant and a C-terminal histidine tag for protein purification and detection. A sequence-verified clone was used to transiently transfect HEK 293 F cells using 293-free transfection reagent (Novagen Inc.) for 72 h according to the manufacturer. The cell culture supernatant was filtered (0.2 μm) and stored at −20°C until purification. Proteins were purified by immobilized metal affinity chromatography (Fractogel loaded with ZnCl2) and eluted by 500 mM imidazole, followed by a concentration step and subsequent size exclusion chromatography in 10 mM citrate–75 mM lysine buffer as described by Kufer et al. (33). BiTE antibody construct monomers were collected and stored at −80°C.
Determination of in vitro cytotoxicity using HIV gp120+-transfected CHO cells.
HIV gp120-transfected CHO cells were used as target cells for in vitro cytotoxicity assays. Human PBMCs were isolated from blood of healthy volunteers by Ficoll (Biochrom) density gradient centrifugation using standard procedures. For the stimulated cytotoxicity assay, human PBMCs were stimulated by immobilized CD3 antibody (OKT-3; Janssen-Cilag), immobilized CD28 antibody (BD), and recombinant IL-2 (20 U/ml). After centrifugation, cells were washed with phosphate-buffered saline (PBS) solution and resuspended in RPMI 1640 complete medium. For chromium release assays with the various BiTE antibody constructs, target cells were labeled by incubation with approximately 7 MBq 51Cr for 1 h, followed by removal of free chromium with two washing steps and resuspension in RPMI 1640 complete medium (Gibco). T cells and labeled target cells were adjusted to an effector-to-target (E:T) ratio of 10:1. One hundred microliters of this suspension was transferred to each well of a 96-well plate prefilled with one hundred microliters of BiTE dilution or medium in the case of background controls. The BiTE antibody-mediated cytotoxic reaction proceeded for 18 h at 37°C in a 5% CO2 humidified incubator. After incubation, 100 μl of each well was removed, and cytotoxicity was measured as relative values of released chromium in the supernatant relative to the difference of maximum lysis (determined by addition of Triton X-100) and spontaneous lysis (in the absence of effector cells). All measurements were done in quadruplicate. Measurement of 51Cr activity in the supernatants was performed with a Wizard 3 γ-counter (Perkin-Elmer). For the CD4(1 + 2) h BiTE, target cells were labeled using Vybrant DiD (Life Technologies) dye as instructed by the manufacturer. Effector and target cells were adjusted and added to BiTE dilution or medium as described above. After the incubation period, cells were washed in PBS, stained with propidium iodide (PI; Sigma), and analyzed by flow cytometry for PI-positive/DiD-labeled target cells. Specific cytotoxicity was calculated using the formula (1 − viable cells [BiTE dilution]/viable cells [no BiTE control]) × 100. Data were plotted using sigmoidal dose-response fitting in GraphPad Prism 7.04.
HIV infection of primary cells.
The virus was generated by transfection of 293 T cells with proviral DNA from NL4-3 (p24 for the viral stock, 85 ng/ml) or YU-2 (30 ng/ml). PBMCs were infected overnight with supernatant diluted 1:3.
Coincubation of BiTE antibody constructs with HIV-infected PBMCs.
A total of 105 PBMC were incubated in 200 μl RPMI medium and incubated for 12 days with dilutions of the indicated BiTE antibody construct. On days 5, 8, and 12 postinfection (p.i.), 50 μl of the cell culture supernatant was sampled and replenished for further incubation. Aliquots of the cell culture supernatant were diluted for a final concentration of 1% Empigen detergent and stored at −20°C for later analysis. The HIV p24 antigen (Ag) levels were determined by a sandwich enzyme-linked immunosorbent assay (ELISA) as described by Moore et al. (34).
Coincubation of BiTE antibody constructs with HIV-infected MDMs cocultured with CD8+ T cells.
We first infected 5 × 104 MDMs overnight with YU-2, washed the MDMs extensively in the morning, and then added 2 × 105 autologous CD8+ cells together with the various BiTE antibody constructs to the coculture. Subsequently, we monitored HIV replication by quantifying the p24 Ag in the supernatant over time. In a subsequent experiment, we also compared the anti-HIV potential of the BiTE antibody constructs generated in ex vivo HIV-infected MDMs with or without CD8+ T cells. In these assays, we used purified CD8+ T cells, which we isolated using the MACS human CD8 microbeads assay from Miltenyi Biotech (130-045-201).
Flow cytometry.
Binding of BiTE antibody constructs (10 μg/ml) was visualized by a murine antihistidine antibody (10 μg/ml; AbD Serotec) and a fluorescently labeled anti-mouse Fc antibody (5 μg/ml; Jackson ImmunoResearch). Intracellular p24 was detected using a commercially available HIV gag p24 antibody (Kc57) (Beckman Coulter). We quantified the number of CD8+ T cells with the anti-CD8+ antibody Brilliant Violet 421 anti-human CD8a antibody from BioLegend (301036).
BiTE-mediated infection assay of CD4−/CD8+ T cells.
Unstimulated human CD8+ T cells were negatively isolated using microbeads after Ficoll purification and subsequently incubated with the indicated BiTE antibody construct (10 μg/ml) for 2 h. After washing with PBS, CD8+ T cells were challenged by spinoculation with HIV NL4-3 for 2 h, followed by washing and subsequent incubation in RPMI medium. Cell culture supernatants on day 7 p.i. were analyzed for HIV p24 Ag.
Statistics.
Statistical significance was calculated using GraphPad Prism, version 7.04, for Windows (www.graphpad.com; GraphPad Software, La Jolla, CA). The various test statistics used are described in the legends to the figures.
ACKNOWLEDGMENTS
We thank Annette Audigé for the provision of viral stocks and Jochen Pendzialek for the control BiTE.
The study was supported by the clinical research focus program Human Hemato-Lymphatic Diseases (R.F.S. and R.M.) of the University of Zurich, the Swiss National Science Foundation (31003A_153248/1) (R.F.S. and M.A.R.), and by AMGEN, a publically listed company.
M.M., J.B., T.R. and P.K. are employees of AMGEN Research Munich. M.M., J.B., T.R., P.K., and P.A.B. have equity positions in the company. AMGEN is focused on the development of BiTE antibodies for the treatment of malignant diseases. C.K.S.M., S.K.R., M.A.R., E.S., R.M., and R.F.S. have no conflict of interest to declare.
REFERENCES
- 1.Shasha D, Walker BD. 2013. Lessons to be learned from natural control of HIV–future directions, therapeutic, and preventive implications. Front Immunol 4:162. doi: 10.3389/fimmu.2013.00162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masiero S, Del Vecchio C, Gavioli R, Mattiuzzo G, Cusi MG, Micheli L, Gennari F, Siccardi A, Marasco WA, Palu G, Parolin C. 2005. T-cell engineering by a chimeric T-cell receptor with antibody-type specificity for the HIV-1 gp120. Gene Ther 12:299–310. doi: 10.1038/sj.gt.3302413. [DOI] [PubMed] [Google Scholar]
- 3.Nagorsen D, Kufer P, Baeuerle PA, Bargou R. 2012. Blinatumomab: a historical perspective. Pharmacol Ther 136:334–342. doi: 10.1016/j.pharmthera.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 4.Klinger M, Brandl C, Zugmaier G, Hijazi Y, Bargou RC, Topp MS, Gokbuget N, Neumann S, Goebeler M, Viardot A, Stelljes M, Bruggemann M, Hoelzer D, Degenhard E, Nagorsen D, Baeuerle PA, Wolf A, Kufer P. 2012. Immunopharmacologic response of patients with B-lineage acute lymphoblastic leukemia to continuous infusion of T cell-engaging CD19/CD3-bispecific BiTE antibody blinatumomab. Blood 119:6226–6233. doi: 10.1182/blood-2012-01-400515. [DOI] [PubMed] [Google Scholar]
- 5.Bargou R, Leo E, Zugmaier G, Klinger M, Goebeler M, Knop S, Noppeney R, Viardot A, Hess G, Schuler M, Einsele H, Brandl C, Wolf A, Kirchinger P, Klappers P, Schmidt M, Riethmuller G, Reinhardt C, Baeuerle PA, Kufer P. 2008. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science 321:974–977. doi: 10.1126/science.1158545. [DOI] [PubMed] [Google Scholar]
- 6.von Stackelberg A, Locatelli F, Zugmaier G, Handgretinger R, Trippett TM, Rizzari C, Bader P, O'Brien MM, Brethon B, Bhojwani D, Schlegel PG, Borkhardt A, Rheingold SR, Cooper TM, Zwaan CM, Barnette P, Messina C, Michel G, DuBois SG, Hu K, Zhu M, Whitlock JA, Gore L. 2016. Phase I/phase II study of blinatumomab in pediatric patients with relapsed/refractory acute lymphoblastic leukemia. J Clin Oncol 34:4381–4389. doi: 10.1200/JCO.2016.67.3301. [DOI] [PubMed] [Google Scholar]
- 7.Friedrich M, Raum T, Lutterbuese R, Voelkel M, Deegen P, Rau D, Kischel R, Hoffmann P, Brandl C, Schuhmacher J, Mueller P, Finnern R, Fuergut M, Zopf D, Slootstra JW, Baeuerle PA, Rattel B, Kufer P. 2012. Regression of human prostate cancer xenografts in mice by AMG 212/BAY2010112, a novel PSMA/CD3-bispecific BiTE antibody cross-reactive with non-human primate antigens. Mol Cancer Ther 11:2664–2673. doi: 10.1158/1535-7163.MCT-12-0042. [DOI] [PubMed] [Google Scholar]
- 8.Osada T, Hsu D, Hammond S, Hobeika A, Devi G, Clay TM, Lyerly HK, Morse MA. 2010. Metastatic colorectal cancer cells from patients previously treated with chemotherapy are sensitive to T-cell killing mediated by CEA/CD3-bispecific T-cell-engaging BiTE antibody. Br J Cancer 102:124–133. doi: 10.1038/sj.bjc.6605364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lutterbuese R, Raum T, Kischel R, Lutterbuese P, Schlereth B, Schaller E, Mangold S, Rau D, Meier P, Kiener PA, Mulgrew K, Oberst MD, Hammond SA, Baeuerle PA, Kufer P. 2009. Potent control of tumor growth by CEA/CD3-bispecific single-chain antibody constructs that are not competitively inhibited by soluble CEA. J Immunother 32:341–352. doi: 10.1097/CJI.0b013e31819b7c70. [DOI] [PubMed] [Google Scholar]
- 10.Traunecker A, Lanzavecchia A, Karjalainen K. 1991. Bispecific single chain molecules (Janusins) target cytotoxic lymphocytes on HIV infected cells. EMBO J 10:3655–3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg J, Lotscher E, Steimer KS, Capon DJ, Baenziger J, Jack HM, Wabl M. 1991. Bispecific antibodies that mediate killing of cells infected with human immunodeficiency virus of any strain. Proc Natl Acad Sci U S A 88:4723–4727. doi: 10.1073/pnas.88.11.4723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Okada H, Momota H, Azuma T, Hattori T, Okada N. 1992. Specific cytolysis of HIV-infected cells by lymphocytes armed with bifunctional antibodies. Immunol Lett 31:247–252. doi: 10.1016/0165-2478(92)90122-5. [DOI] [PubMed] [Google Scholar]
- 13.Chamow SM, Zhang DZ, Tan XY, Mhatre SM, Marsters SA, Peers DH, Byrn RA, Ashkenazi A, Junghans RP. 1994. A humanized, bispecific immunoadhesin-antibody that retargets CD3+ effectors to kill HIV-1-infected cells. J Immunol 153:4268–4280. [PubMed] [Google Scholar]
- 14.Sung JA, Pickeral J, Liu L, Stanfield-Oakley SA, Lam CY, Garrido C, Pollara J, LaBranche C, Bonsignori M, Moody MA, Yang Y, Parks R, Archin N, Allard B, Kirchherr J, Kuruc JD, Gay CL, Cohen MS, Ochsenbauer C, Soderberg K, Liao HX, Montefiori D, Moore P, Johnson S, Koenig S, Haynes BF, Nordstrom JL, Margolis DM, Ferrari G. 2015. Dual-affinity re-targeting proteins direct T cell-mediated cytolysis of latently HIV-infected cells. J Clin Investig 125:4077–4090. doi: 10.1172/JCI82314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sloan DD, Lam CY, Irrinki A, Liu L, Tsai A, Pace CS, Kaur J, Murry JP, Balakrishnan M, Moore PA, Johnson S, Nordstrom JL, Cihlar T, Koenig S. 2015. Targeting HIV reservoir in infected CD4 T cells by dual-affinity re-targeting molecules (DARTs) that bind HIV envelope and recruit cytotoxic T cells. PLoS Pathog 11:e1005233. doi: 10.1371/journal.ppat.1005233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Speck RF, Esser U, Penn ML, Eckstein DA, Pulliam L, Chan SY, Goldsmith MA. 1999. A trans-receptor mechanism for infection of CD4-negative cells by human immunodeficiency virus type 1. Curr Biol 9:547–550. [DOI] [PubMed] [Google Scholar]
- 17.Kwong PD, Wyatt R, Robinson J, Sweet RW, Sodroski J, Hendrickson WA. 1998. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature 393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burton DR, Hangartner L. 2016. Broadly neutralizing antibodies to HIV and their role in vaccine design. Annu Rev Immunol 34:635–659. doi: 10.1146/annurev-immunol-041015-055515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Daar ES, Li XL, Moudgil T, Ho DD. 1990. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency virus type 1 isolates. Proc Natl Acad Sci U S A 87:6574–6578. http://www.pnas.org/content/pnas/87/17/6574.full.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pegu A, Asokan M, Wu L, Wang K, Hataye J, Casazza JP, Guo X, Shi W, Georgiev I, Zhou T, Chen X, O'Dell S, Todd JP, Kwong PD, Rao SS, Yang ZY, Koup RA, Mascola JR, Nabel GJ. 2015. Activation and lysis of human CD4 cells latently infected with HIV-1. Nat Commun 6:8447. doi: 10.1038/ncomms9447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Halper-Stromberg A, Lu CL, Klein F, Horwitz JA, Bournazos S, Nogueira L, Eisenreich TR, Liu C, Gazumyan A, Schaefer U, Furze RC, Seaman MS, Prinjha R, Tarakhovsky A, Ravetch JV, Nussenzweig MC. 2014. Broadly neutralizing antibodies and viral inducers decrease rebound from HIV-1 latent reservoirs in humanized mice. Cell 158:989–999. doi: 10.1016/j.cell.2014.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo J, Wang W, Yu D, Wu Y. 2011. Spinoculation triggers dynamic actin and cofilin activity that facilitates HIV-1 infection of transformed and resting CD4 T cells. J Virol 85:9824–9833. doi: 10.1128/JVI.05170-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salzwedel K, Smith ED, Dey B, Berger EA. 2000. Sequential CD4-coreceptor interactions in human immunodeficiency virus type 1 Env function: soluble CD4 activates Env for coreceptor-dependent fusion and reveals blocking activities of antibodies against cryptic conserved epitopes on gp120. J Virol 74:326–333. doi: 10.1128/JVI.74.1.326-333.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin G, Baribaud F, Romano J, Doms RW, Hoxie JA. 2003. Identification of gp120 binding sites on CXCR4 by using CD4-independent human immunodeficiency virus type 2 Env proteins. J Virol 77:931–942. doi: 10.1128/JVI.77.2.931-942.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Telarico T, Perl A. 2012. The role of endocytic recycling in autoimmunity. Methods Mol Biol 900:91–107. doi: 10.1007/978-1-60761-720-4_5. [DOI] [PubMed] [Google Scholar]
- 26.Prins JM, Jurriaans S, van Praag RM, Blaak H, van Rij R, Schellekens PT, ten Berge IJ, Yong SL, Fox CH, Roos MT, de Wolf F, Goudsmit J, Schuitemaker H, Lange JM. 1999. Immuno-activation with anti-CD3 and recombinant human IL-2 in HIV-1-infected patients on potent antiretroviral therapy. AIDS 13:2405–2410. doi: 10.1097/00002030-199912030-00012. [DOI] [PubMed] [Google Scholar]
- 27.Shan L, Deng K, Shroff NS, Durand CM, Rabi SA, Yang HC, Zhang H, Margolick JB, Blankson JN, Siliciano RF. 2012. Stimulation of HIV-1-specific cytolytic T lymphocytes facilitates elimination of latent viral reservoir after virus reactivation. Immunity 36:491–501. doi: 10.1016/j.immuni.2012.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Denton PW, Long JM, Wietgrefe SW, Sykes C, Spagnuolo RA, Snyder OD, Perkey K, Archin NM, Choudhary SK, Yang K, Hudgens MG, Pastan I, Haase AT, Kashuba AD, Berger EA, Margolis DM, Garcia JV. 2014. Targeted cytotoxic therapy kills persisting HIV infected cells during ART. PLoS Pathog 10:e1003872. doi: 10.1371/journal.ppat.1003872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Topp MS, Gokbuget N, Zugmaier G, Degenhard E, Goebeler ME, Klinger M, Neumann SA, Horst HA, Raff T, Viardot A, Stelljes M, Schaich M, Kohne-Volland R, Bruggemann M, Ottmann OG, Burmeister T, Baeuerle PA, Nagorsen D, Schmidt M, Einsele H, Riethmuller G, Kneba M, Hoelzer D, Kufer P, Bargou RC. 2012. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood 120:5185–5187. doi: 10.1182/blood-2012-07-441030. [DOI] [PubMed] [Google Scholar]
- 30.Gardner MR, Farzan M. 2017. Engineering antibody-like inhibitors to prevent and treat HIV-1 infection. Curr Opin HIV AIDS 12:294–301. doi: 10.1097/COH.0000000000000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li W, Wu Y, Kong D, Yang H, Wang Y, Shao J, Feng Y, Chen W, Ma L, Ying T, Dimitrov DS. 2017. One-domain CD4 fused to human anti-CD16 antibody domain mediates effective killing of HIV-1-infected cells. Sci Rep 7:9130. doi: 10.1038/s41598-017-07966-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bardhi A, Wu Y, Chen W, Li W, Zhu Z, Zheng JH, Wong H, Jeng E, Jones J, Ochsenbauer C, Kappes JC, Dimitrov DS, Ying T, Goldstein H. 2017. Potent in vivo NK cell-mediated elimination of HIV-1-infected cells mobilized by a gp120-bispecific and hexavalent broadly neutralizing fusion protein. J Virol 91:e00937-17. doi: 10.1128/JVI.00937-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Topp MS, Kufer P, Gokbuget N, Goebeler M, Klinger M, Neumann S, Horst HA, Raff T, Viardot A, Schmid M, Stelljes M, Schaich M, Degenhard E, Kohne-Volland R, Bruggemann M, Ottmann O, Pfeifer H, Burmeister T, Nagorsen D, Schmidt M, Lutterbuese R, Reinhardt C, Baeuerle PA, Kneba M, Einsele H, Riethmuller G, Hoelzer D, Zugmaier G, Bargou RC. 2011. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol 29:2493–2498. doi: 10.1200/JCO.2010.32.7270. [DOI] [PubMed] [Google Scholar]
- 34.Moore JP, McKeating JA, Weiss RA, Sattentau QJ. 1990. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science 250:1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]