Abstract
Liver dysfunction (LD) and liver failure are associated with poor outcome in critically ill patients. In patients with severe sepsis or septic shock, LD occurred in nearly 19% of patients. An early diagnosis of LD at time of initial damage of the liver can lead to a better prognosis of these patients because an early start of therapy is possible. We performed a second prospective study with septic patients to test a new cell-based cytotoxicity device (biosensor) to evaluate clinical relevance for early diagnosis of LD and prognostic capacity. In the clinical study, 99 intensive care unit patients were included in two groups. From the patients of the septic group (n = 51, SG), and the control (non-septic) group [n = 49, control group (CG)] were drawn 20 ml blood at inclusion, after 3, and 7 days for testing with the biosensor. Patients’ data were recorded for hospital survival, organ function, and demographic data, illness severity [acute physiology and chronic health evaluation (APACHE) II-, sepsis-related organ failure assessment (SOFA) scores], cytokines, circulating-free deoxyribonucleic acid/neutrophil-derived extracellular traps (cf-DNA/NETs), microbiological results, and pre-morbidity. For the developed cytotoxicity test, the human liver cell line HepG2/C3A was used. Patients’ plasma was incubated in a microtiter plate assay with the test cells and after 6 days incubation the viability (trypan blue staining, XTT-test) and functionality (synthesis of albumin, cytochrome 1A2 activity) was analyzed. An impairment of viability and functionality of test cells was only seen in the SG compared with the CG. The plasma of non-survivors in the SG led to a more pronounced impairment of test cells than the plasma of survivors at inclusion. In addition, the levels of cf-DNA/NETs were significantly higher in the SG at inclusion, after 3, and after 7 days compared with the CG. The SG showed an in-hospital mortality of 24% and the values of bilirubin, APACHE II-, and SOFA scores were markedly higher at inclusion than in the CG. Hepatotoxicity of septic plasma was already detected with the liver cell-based biosensor at inclusion and also in the course of disease. The biosensor may be a tool for early diagnosis of LD in septic patients and may have prognostic relevance.
Keywords: biosensing techniques, cytotoxicity, hepatocytes, inflammation, liver failure
Introduction
The development of liver dysfunction (LD) and liver failure in intensive care unit (ICU) patients have a relatively high incidence of 11% in all ICU patients and over 19% in patient with septic shock and is associated with increased in-hospital mortality (1–4).
Because physiological and online parameters are unable to diagnose LD early, laboratory parameters, like transaminases, albumin, and coagulation factors are commonly used, however, without convincing clinical data for detection of early LD (5). In addition, serum bilirubin is often utilized for diagnosis of (early) LD in critically ill patients (3); although an increase of bilirubin is seen late in patients, 2–3 days after initial impairment of the liver and other organ damages as displayed in sepsis-related organ failure assessment (SOFA)-, and SAPS scores (6).
LD, however, occurs as an early organ dysfunction in severe ill patients, e.g., in septic patients (3, 7). Experimental and clinical investigations have shown that impaired biliary secretion is the main component of early LD in systemic inflammatory response syndrome and sepsis (8–12).
Hepatotoxicity of inflammatory mediators like nitric oxide, chemokines and cytokines, endogen and exogen toxins like lipopolysaccharides, plasma cascade factors, and hepatic ischemia are the main pathophysiological factors for the development of LD leading to hyperbilirubinemia and intrahepatic cholestasis (3, 5, 10, 12–18).
In addition, activated neutrophils in response to infectious stimuli casting out their deoxyribonucleic acid DNA as main part of neutrophil-derived extracellular traps (NETs); so-called circulating-free deoxyribonucleic acid/neutrophil (derived) extracellular traps [cf-DNA/NETs; (19)]. NETs are emergency first-line defense mechanisms and kill microbiological pathogens in blood (19). Then again, high levels of NETs seem to be linked to multiorgan failure and sepsis (19, 20). Overwhelming NETs formation resulted in impaired microcirculation and organ damage (19).
To verify the clinical relevance, especially for (early) diagnosis of LD of a new cell-based test device [biosensor; (21)], we conducted a second prospective study with septic patients including cytokines-, and cf-DNA/NETs measurement. In a smaller first study, we showed that plasma of septic patients caused an impairment of functionality of hepatocytes in the cytotoxicity test compared with postoperative non-septic controls and healthy volunteers (22). The biosensor was actually also used for therapy monitoring of LD and liver failure in critically ill patients (23), for evaluation of experimental models of liver failure, and monitoring of hepatotoxicity of drugs and procalcitonin (PCT) (24–27).
Materials and Methods
Subjects and Procedures
Approval for the study from the responsible ethics committee (University of Rostock; II HV 16/2005) was obtained and for all included patients written informed consent was received. Furthermore, the study was carried out under the principles of the Declaration of Helsinki and good clinical practice.
Between June 2005 and May 2008, 51 septic patients were included in the study after screening in the two-perioperative ICUs of the University Hospital of Rostock for fulfilling the criteria of septic shock or severe sepsis (28). Organ dysfunction was defined according to the criteria of the PROWESS study (29); bilirubin levels >34.2 µmol/l (2 mg/dl) for at least 48 h was the criteria for LD (2, 3). The exclusion criteria were pre-existing liver disease, age under 18 years, pregnancy, HIV infection, and participation in another study. The control group (CG, n = 48) was included postoperative patients without signs of sepsis, without pre-described liver disease, and an ICU stay longer than 24 h were included.
From each patient, 20 ml blood was obtained for testing with the biosensor and screening for blood parameters, cytokines, and circulating-free deoxyribonucleic acid/neutrophil-derived extracellular traps (cf-DNA/NETs) at inclusion, after 3, and after 7 days. Patients were followed up to assess hospital survival and organ function; demographic data, illness severity, SOFA–acute physiology and chronic health evaluation (APACHE)-II, cytokines, microbiological results, and pre-morbidity were documented (Table 1).
Table 1.
Laboratory parameters and results of APACHE II-, and SOFA scores at inclusion, after 3, and 7 days in the septic- (SG, n = 51), septic survived- (SSG, n = 39), septic non-survived- (SNSG, n = 12), and CG (n = 48); (median/0.25–0.75 quartile).
| Values | At inclusion |
After 3 days |
After 7 days |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sepsis group (n = 51) |
CG (n = 48) | Sepsis group (n = 51) |
CG (n = 48) | Sepsis group (n = 51) |
CG (n = 48) | |||||||
| SG (all) | SSG | SNSG | SG (all) | SSG | SNSG | SG (all) | SSG | SNSG | ||||
| Lactate (mmol/l) | 1.7 (1.5–2.9)* | 1.7 (1.5–2.5)* | 2.4 (1.4–5.3)* | 1.1 (0.9–1.3) | 1.7 (1.2–2.4)* | 1.6 (1.2–2.2)* | 1.9 (1.8–3.4)* | 1.3 (0.9–1.6) | 1.4 (1.2–2.1) | 1.4 (1.1–2.0) | 2.2 (1.4–3.2) | 1.6 (1.3–2.8) |
| Bilirubin (μmol/l) | 19.9 (12.1–36.8)* | 19.2 (13.9–37.1)* | 21.4 (11.7–33) | 15.2 (11.3–18.8) | 15.7 (10.7–30.9)* | 15.0 (10.8–30.0)* | 21.5 (10.0–35.9)* | 11.0 (8.6–15.7) | 13.4 (9.1–24.3)* | 11.9 (8.6–23.9)* | 16.8 (12.3–27.8)* | 9.1 (7.6–10.8) |
| Ammonia (mmol/l) | 51.3 (34.5–63.9)* | 43.1 (30.9–60.4)* | 54.4 (43.0–68.7)* | 31.7 (22.4–39.4) | 49.3 (38.4–65.4)* | 48.1 (38.7–60.2)* | 50.4 (35.5–78.8)* | 31.8 (25.5–38.7) | 43.7 (37.3–59.2)* | 43.6 (36.8–59.8)* | 48.4 (38.3–80.2)* | 32.4 (24.0–43.5) |
| Creatinine (μmol/l) | 136 (94–202)* | 130 (93–193)* | 161 (103–245)* | 78 (66–94) | 130 (92–215)* | 127 (92–190)* | 148 (108–232)* | 77 (65–88) | 83 (64–165)* | 82 (62–155) | 95 (82–249)* | 79 (65–89) |
| Urea (mmol/l) | 10.5 (7.3–17.4)* | 9.4 (7.3–14.5)* | 17.3 (7.7–22.7)* | 4.4 (3.4–5.8) | 12.2 (7.9–18.1)* | 10.8 (7.0–15.1)* | 18 (11.4–26.7)* | 4.6 (3.3–5.5) | 11.3 (6.3–19.7)* | 11 (6.1–16.2)* | 18.6 (8.2–35.0)* | 4.5 (3.6–5.4) |
| PCT (ng/ml) | 12.2 (3.9–32.5)* | 10.5 (3.3–28.8)* | 18.2 (4.4–47.2)* | 0.5 (0.2–0.8) | 5.0 (2.0–15.7)* | 4.6 (2.0–12.5)* | 6.9 (2.2–24.0)* | 0.4 (0.1–0.6) | 0.7 (0.4–3.3)* | 0.7 (0.4–2.2)* | 4.6 (0.4–6.7)* | 0.2 (0.1–0.2) |
| Leukocytes (GpT/l) | 14.8 (9.1–23.0)* | 14.6 (8.2–22.1)* | 20.2 (11.8–51.5)* | 9.1 (7.6–11.7) | 12.6 (8.6–16.7)* | 11.6 (8.3–15.4)* | 14.6 (10.5–24.4)* | 8.9 (7.3–11.5) | 13.3 (11.0–18.3)* | 13.2 (10.4–17.7)* | 15.3 (12.4–25.3)* | 9.0 (8.0–10.9) |
| Thrombocytes (GpT/l) | 191 (106–254) | 201 (107–254) | 174 (65–248) | 184 (136–238) | 170 (100–244) | 187 (109–256) | 149 (71–189)* | 187 (149–252) | 229 (135–350)* | 272 (157–369)* | 184 (113–236)* | 334 (238–431) |
| Prothrombin time (%) | 71 (61–82)* | 73 (61–86)* | 66 (61–75)* | 90 (80–95) | 81 (70–98)* | 84 (72–102)* | 65 (50–80)* | 102 (92–112) | 88 (74–99)* | 88 (76–101)* | 84 (64–96)* | 99 (89–113) |
| APACHE II | 32 (26–36)* | 30 (25–35)* | 36 (28–42)* | 9 (7–12) | ||||||||
| SOFA | 13 (11–15)* | 12 (10–14)* | 15 (12–16)* | 2 (0–4) | 11 (9–14)* | 10 (8–13)* | 13 (11–17)* | 1 (0–2) | 9 (3–12)* | 7 (3–11)* | 13 (9–17)* | 0 (0–0) |
*p < 0.05 versus CG.
APACHE, acute physiology and chronic health evaluation; PCT, procalcitonin; SOFA, sepsis-related organ failure assessment; CG, control group.
Cell Cultures and Biosensor Methods
For the hepatocytes-based cytotoxicity assay, the human hepatocyte cell line HepG2/C3A (American Type Culture Collection CRL-10741) was used. The cells were cultivated at 37°C in a 5% CO2 humidified incubator with Dulbecco’s modified Eagle’s medium (GIBCO Life Technologies, Eggenstein, Germany), 10% fetal bovine serum (FBS, PAA Laboratories, Pasching, Germany), 1% antibiotics solution (penicillin G: 10,000 IE/ml/streptomycin: 10 mg/ml; PAA), and 1% 200 mM l-glutamine (PAA). Cell concentrations and vitality were determined by trypan blue (0.4%; Sigma, Seelze, Germany) staining technique using a C-Chip Neubauer improved hemocytometer (peqlab, Erlangen, Germany).
For testing the hepatotoxicity of patients’ plasma, the cells were seeded in 24-well microtiter plates in a density of 250,000 cells/well. Then, the cells were incubated for 3 days with 1 ml heparinized plasma from the subjects following a 3-day incubation period with fresh medium (1 ml). Cells and cell culture supernatants were obtained for the measurement of viability (XTT-test: dehydrogenases activity in the mitochondria, trypan blue staining: cell-count and vitality), cytochrome 1A2 activity, and synthesis of albumin. All test batches from test subjects and measurements were taken twice and a medium control was added.
The test of metabolism of ethoxyresorufine (Molecular Probes, Eugene, OR, USA) to resorufine was used for measurement of the activity of cytochrome P450 1A2; following the protocol of Kelly and Sussman (30). Resorufine concentration in the supernatants was determined at 530 nm (excitation), and 584 nm (emission) using a fluorescence multiwell plate reader (Fluoroskan Ascent Lab Systems, Vienna, VA, USA). Concentrations were estimated against a resorufine standard curve.
Albumin was measured from cell culture medium supernatant, carried out by a nephelometrical method (Immage 800, Beckman Coulter GmbH, Krefeld, Germany).
The XTT-test (Roche Diagnostics GmbH, Mannheim, Germany) was used following the describing of Scudiero et al. (31). At the start of XTT-determination 2 × 100 µl cell suspension as duplicate were transferred to a transparent 96-well plate. After adding 100 µl XTT-reaction reagent per well the absorbance of formazan was read at a wavelength of 450 nm on a microplate reader (Anthos Reader 2001, Anthos Labtec Instruments, Austria) after 1 h.
Cytokines and cfDNA/NETs Measurement
Interleukin (IL)-1 beta, IL-6, IL-10, and tumor necrosis factor (TNF)-alpha were measured in patients’ serum with commercial ELISA kits as described by the supplier (BioSource International, Camarillo, CA, USA).
The quantification of cf-DNA/NETs was performed with a fluorescent assay (Leukocare AG, Munich, Germany). A green fluorescent dye binds DNA and the intensity of fluorescence (emission at 530 nm wavelength; Fusion, PerkinElmer, Monza, Italy) correlates with the amounts of DNA. The measurement range was between 50 and 3,000 ng/ml (20, 32). A calibration curve was conducted with a defined calf thymus DNA (Sigma, Taufkirchen, Germany) in all measurements. Former studies have shown that healthy volunteers had cf-DNA/NETs levels less than 150 ng/ml (20).
Statistical Analysis
The results are expressed as the median with 0.25–0.75 quartile and are displayed as box plots in the figures. Nonparametric analyses were used after (negative) testing of normal distribution (with the Kolmogorov–Smirnov test; SPSS, Chicago, IL, USA). Statistical significance was analyzed with the Kruskal–Wallis one-way, the two-tailed Mann–Whitney U-test, the Friedman-test, and the Wilcoxon-test. Correlations between different parameters were tested with the Spearman’s-rho test. Statistical significance was assumed when the p-value was <0.05.
Results
Survival, Organ Functions, Laboratory Parameters, and Clinical Characteristics of Patients
The in-hospital mortality of the septic group (SG) was 23.5% (between day 3 and 20, n = 51). Two patients of the CG died in the hospital (4%, n = 48). All patients were surgical patients with the exception of three patients in the SG. In general, the patients of the SG fulfilled the criteria of septic shock; only 5.9% had severe sepsis. The septic patients were included in the study on average 0.5 ± 0.8 (0.2/0.8) days after beginning of severe sepsis or septic shock. In the SG, the median age (years) was 63.4 (53.2/72.9) and in the CG 68.3 (61.9/68.9); 24% of the SG- and 31% of the CG-patients were female.
Summaries of laboratory parameters and the results of the APACHE II-, and SOFA scores at inclusion, on days 3, and 7 of the CG, and the SG are displayed in Table 1. In the SG, the APACHE II was 32 at inclusion (CG: 9). The SOFA scores and the values of bilirubin, lactate, ammonia, creatinine, urea, PCT, leukocytes, and prothrombin time differed significantly between the SG and the CG at inclusion, and on day 3; the non-survivors of the SG had more pathologically values than the survivors.
Twenty-five patients of the SG (49%) developed acute kidney injury, and 31.4% (16 patients) needed renal replacement therapy (continuous methods). Criteria for LD (2, 3) were fulfilled in 17 patients (33.3%) of the SG at inclusion, in 16 patients (31.4%) after 3 days, and in 7 patients (13.7%) after 7 days. Five patients with LD at inclusion died during the hospital stay.
The sources of primary infection and results of microbiological analysis in the SG are displayed in Table 2. The predominant sources of infection were peritonitis, wound infections, and pneumonia; in only 68.6% of septic patients, the microbiological tests provided valuable findings.
Table 2.
Source of primary infection and results of microbiological analysis in the septic group (SG, n = 51).
| Source of primary infection | Peritonitis | Wound-infection/abscess | Pneumonia | Urogenital infection |
|---|---|---|---|---|
| Patients (n) | 24 | 7 | 20 | 1 |
| Microbiological results | Wound-/intraoperative-swap | Blood culture | Bronchial lavage/tracheal secrete | Urine-culture |
| Fungi (n) | 11 | 1 | 0 | 0 |
| Gram-positive bacteria (n) | 10 | 3 | 1 | 1 |
| Gram-negative bacteria (n) | 11 | 3 | 12 | 2 |
Cytokines and cfDNA/NETs Measurement
All values of IL-1-beta, TNF-alpha, and IL-10 were below 21 pg/ml (as median) in the SG and CG (Table 3). Higher levels of IL-6 were found in the SG; interestingly, the survivors had higher values of IL-6 than the non-survivors. A significant decrease of IL-6 and TNF-alpha was only seen in the SG between inclusion and day 3.
Table 3.
Cytokines values at inclusion, and after 3 days in the septic- (SG, n = 51), septic survived- (SSG, n = 39), septic none survived- (SNSG, n = 12), and CG (n = 48); (median/0.25–0.75 quartile).
| Cytokine (pg/ml) | At inclusion |
After 3 days |
||||||
|---|---|---|---|---|---|---|---|---|
| Sepsis group (n = 51) |
CG (n = 48) | Sepsis group (n = 51) |
CG (n = 48) | |||||
| SG (all) | SSG | SNSG | SG (all) | SSG | SNSG | |||
| IL-1 beta | 3.9 (2.4–4.5) | 5.8 (2.5–6.5) | 2.7 (2.5–2.9) | 2.5 (2.4–2.6) | 2.5 (2.3–2.6) | 2.6 (2.4–2.8) | 2.4 (2.2–2.9) | 2.6 (2.5–2.7) |
| IL-6 | 268 (106–557.5)+,° | 268 (116.5–557.5)#,° | 226 (98–501.5)§,° | 78 (42.1–143)° | 45.3 (25.9–90.5) | 48.2 (27–89) | 42.3 (25.4–155.9) | 30.5 (22.1–58) |
| IL-10 | 11.1 (2.4–25.9)+ | 12.3 (3.8–24.1)#,° | 4.6 (2.5–33.3) | 2.5 (2.3–8.6) | 7.5 (2.4–12)+ | 7.2 (2.3–11.9)# | 7.9 (2.5–17.5)§ | 2.5 (2.2–2.6) |
| TNF-alpha | 20.4 (12.9–32.2)+,° | 20.4 (13.9–29.8)#,° | 20.7 (11.2–41.9)§,° | 7.7 (5.7–9.7) | 14.7 (10.2–19.9)+ | 15.5 (11.2–19.9)# | 10.8 (7.1–23.2) | 6.6 (5.6–9.7) |
Statistically significant (p < 0.05): #between SSG and CG (U-test); §between SNSG and CG (U-test); +between SG and CG; °between inclusion and day 3.
IL, interleukin; TNF, tumor necrosis factor; CG, control group.
The values of cf-DNA/NETs were significantly higher in the SG than in the CG at inclusion, after 3, and after 7 days (Figure 1). A significant decrease of cf-DNA/NETs levels from inclusion to day 7 was observed in the survivors of the SG, but not in the non-survivors and in the CG.
Figure 1.
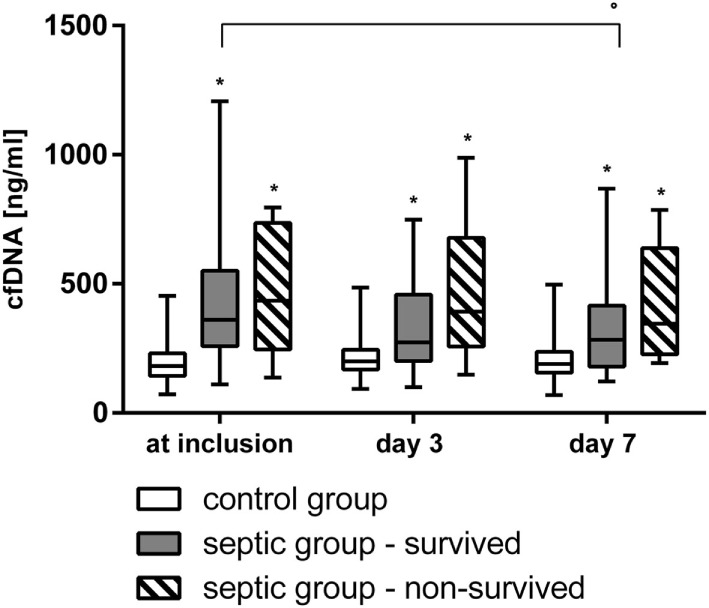
The values of circulating-free deoxyribonucleic acid (cf-DNA, median/0.25–0.75 quartile) at inclusion, after 3, and 7 days in the survivors (n = 39) and the non-survivors (n = 12) of the septic group and in the non-septic control group (CG) (n = 48). *p < 0.05 versus CG (Mann–Whitney U-test). °p < 0.05 between inclusion and day 7 (Wilcoxon-test).
Results of the Hepatocyte-Based Cytotoxicity Tests
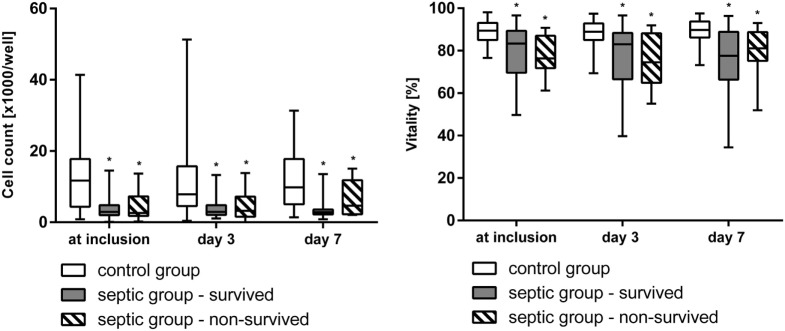
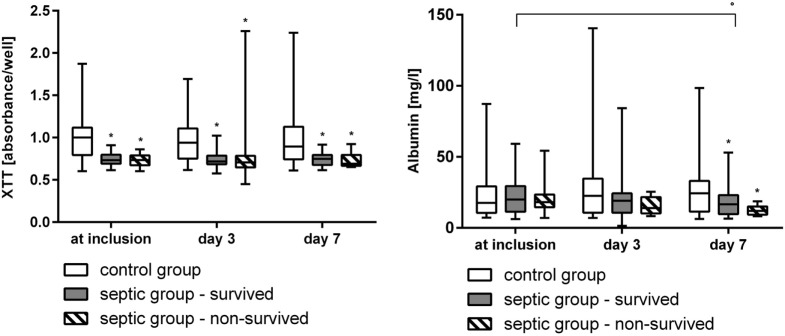
At inclusion, after 3, and 7 days the vitality and the cell count (Figure 2), the activity of mitochondrial dehydrogenase (XTT-test, Figure 3), and the metabolism of ethoxyresorufine (cytochrome 1A2 activity, Figure 4) were significantly decreased in the SG, compared to the test results of the CG. These impairments of viability and function of test cells were more pronounced in non-survivors of the SG compared with survivors only at inclusion (Figures 2–4). By contrast, the synthesis of albumin was impaired later and also pronounced in non-survivors of the SG after 3 and 7 days in SG compared to the CG (Figure 3).
Figure 2.
Cell count and vitality (trypan blue staining, median/0.25–0.75 quartile) of HepG2/C3A cells incubated with plasma from survived and non-survived septic patients and non-septic control patients at inclusion, after 3, and 7 days. *p < 0.05 versus control group (Mann–Whitney U-test).
Figure 3.
Results of the XTT-test (dehydrogenases activity in the mitochondria) and albumin synthesis of HepG2/C3A cells incubated with plasma from survived and non-survived septic patients and non-septic control patients at inclusion, after 3, and 7 days displayed as median/0.25–0.75 quartile. *p < 0.05 versus control group (Mann–Whitney U-test). °p < 0.05 between inclusion and day 7 (Wilcoxon-test).
Figure 4.
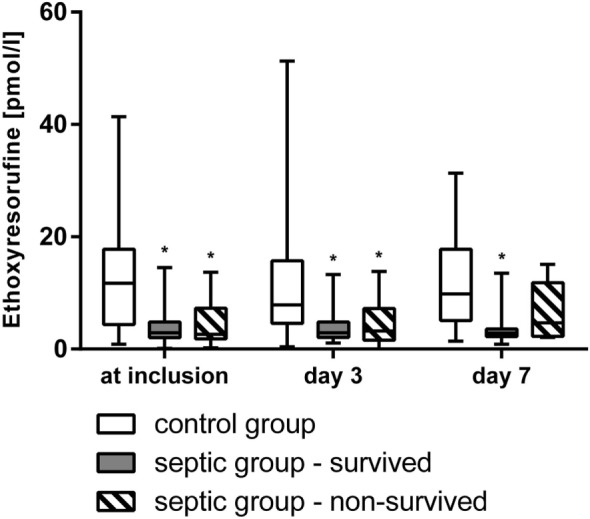
Metabolism of ethoxyresorufine to resorufine (activity of cytochrome 1A2, median/0.25–0.75 quartile) of HepG2/C3A cells incubated with plasma from survived and non-survived septic patients and non-septic control patients at inclusion, after 3, and 7 days. *p < 0.05 versus control group (Mann–Whitney U-test).
Correlation Analysis at Inclusion
Correlations between the hepatocyte-based cytotoxicity test parameters with bilirubin, alanine aminotransferase (ALAT), asparagine aminotransferase (ASAT), and ammonia were not observed. Significant negative correlations were found between the cytochrome 1A2 activity, the XTT-test, the vitality, and the cell count with the APACHE II-, and SOFA scores, lactate, and PCT (ρ between −0.3 and −0.5, p < 0.05). In addition, correlations were observed between the cytochrome 1A2 activity with TNF-alpha, IL-6, and IL-10 (ρ between −0.3 and −0.4, p < 0.001); the levels of TNF-alpha also correlated with the results of the XTT-test and the vitality (ρ = −0.3, p < 0.01).
The values of bilirubin correlated with the APACHE II-, SOFA scores, and lactate (ρ = +0.3, p < 0.01).
Significant correlations were found between the values of cf-DNA/NETs with the APACHE II-, and SOFA scores, and PCT (ρ between +0.4 and +0.6, p < 0.001) and with the cytochrome 1A2 activity, the XTT-test, the vitality, and the cell count (ρ between −0.3 and −0.5, p < 0.05).
The measured cytokines did not correlate with ALAT, ASAT, and ammonia; however, the cytokines IL-6, IL-10, and TNF-alpha correlated with bilirubin (ρ between +0.2 and +0.4, p < 0.05), PCT (ρ between +0.4 and +0.6, p < 0.001), lactate (ρ between +0.3 and +0.5, p < 0.01), APACHE II- (ρ between +0.4 and +0.6, p < 0.001), and SOFA- (ρ between +0.4 and +0.7, p < 0.001) scores.
Discussion
Clinical Characteristics of Study Cohort, Outcome, and Serum Bilirubin
In this study, we included 51 patients in general septic shock and observed a relatively low in-hospital mortality of 23.5% in comparison to other studies (33, 34). The time point of inclusion in the study after the beginning of septic shock was early in our mainly surgical patient cohort.
Liver dysfunction or liver failure occurred in 33.3% (n = 17) of the patients in the SG at inclusion; five patients with LD at inclusion died during the hospital stay. The rate of LD was much higher in our septic study cohort in comparison to other studies (3, 4). Interestingly, the seven other non-survivors in the SG did not show an increase of bilirubin at inclusion and we found only low correlations between morbidity and degree of multiorgan failure as displayed by the APACHE-II-, SOFA scores, and values of lactate with the results of serum bilirubin.
Hepatocyte-Based Cytotoxicity Tests and Role of Cytokines
In the state of severe sepsis or septic shock, the functionality of hepatocytes is partly decreased (5). The aim of this study was to test the direct hepatotoxicity of plasma from septic patients in a standardized hepatocyte-based cytotoxicity test in a second biosensor study (24–27).
In this study, we saw an impairment of viability and functionality of test cells after incubation with plasma from patients in general septic shock. The cytochrome 1A2 activity, the XTT values (activity of mitochondrial dehydrogenases), the cell count, and the vitality were significantly lower in the SG compared to the CG at inclusion, after 3, and after 7 days. In addition, the plasma of non-survivors in the SG led to a more pronounced impairment of test cells than the plasma of survivors at inclusion. These results support the aim of the study that the cell-based biosensor used may be a tool of early diagnosis for LD and has prognostic value. In addition to this thesis, we found significant negative correlation between the cytochrome 1A2 activity, the XTT-test, the vitality, and the cell count with the APACHE II- and SOFA scores, lactate, and PCT at inclusion. By contrast, the albumin synthesis in test cells seems to be a late changing parameter and is not valuable for early detection of LD.
In this study, correlations between static liver markers like bilirubin with the results of the hepatocyte cytotoxicity test parameters at inclusion were not seen. This may be due to the fact that bilirubin and other classical liver parameters increase late in LD or liver failure, especially in septic patients (6).
The impairment of cell function and viability seen in HepG2/C3A after incubation with septic plasma can be caused by endogenous and exogenous toxins, drugs, and metabolites (15, 35, 36). In addition, pro-inflammatory and anti-inflammatory cytokines modulate and impair the function of hepatocytes. We tested the patients’ plasma at inclusion, and after 3 days for IL-1 beta, TNF alpha, IL-6, and IL-10. The values of measured cytokines were relatively low, but higher in the SG than in the CG; only the values of IL-6 were markedly increased at inclusion in the SG but decreased after day 3.
Due to relative low values of cytokines with exception of IL-6, we observed only a few correlations between cytokines and parameters of the biosensor: between the cytochrome 1A2 activity with TNF-alpha, IL-6, and IL-10 at inclusion and results of the XTT-test, and the vitality with the levels of TNF-alpha also at inclusion.
Many cytokines, mainly the pro-inflammatory cytokines TNF-alpha, IL-1 beta, and IL-6, cause an impairment or dysregulation of the viability, the function, and apoptosis of human hepatocytes and hepatocyte cell lines, e.g., in HepG2/C3A cells [for review, see Ref. (35–48)]. These impairments of hepatocytes lead to dysfunction of mitochondria, to a decreased level of negative acute phase proteins like albumin, and a decreased activity of some P450 cytochromes including CYP 1A2 (35, 36, 40, 49). The lower values of biosensor parameters found in the cells incubated with septic plasma may be explained partly by the effects of pro-inflammatory cytokines on the sensor cells.
For our hepatocyte-based cytotoxicity test, we worked with the well-characterized cell line HepG2/C3A (50). The cell line is commonly used for toxicological studies (51, 52) and shows many functionalities after stimulation comparable with normal human hepatocytes [for review, see Ref. (30, 44, 48, 50, 53–60)]. Therefore, the HepG2/C3A cell line is a source for bioartficial liver support systems (61).
In a previous work from our research group, the testing of antimycotics (caspofungin, anidulafungin, and fluconazole) with HepG2/C3A cells compared with human primary isolated hepatocytes provided similar results regarding cytochrome 1A2 activity, vitality, and activity of mitochondrial dehydrogenase [for review, see Ref. (24)].
Role of cf-DNA/NETs in Sepsis and Liver Failure
NETs play a diametrical role in sepsis (19). As part of the innate immune system NETs quickly trap and neutralize microbes in tissues and blood (19). Then again, NETs also lead to damage of inflamed tissues as carrier of molecules with autodestructive immune effector functions, e.g., extracellular DNA, elastase, myeloperoxidase, lactoferrin, pentraxin, and bactericidal/permeability-increasing protein (19). During early stage sepsis, activated neutrophils traps and accumulate primarily in the sinusoids of the liver (38). The NETs in the liver sinusoids cause tissue- and endothelial damage resulting in denuding of the endothelium and allows platelets to enter the space of Disse (37). These mechanisms lead to extravasated platelet aggregation. NETs and platelet aggregation result in thrombosis and impaired blood flow in the liver sinusoids and seem to be an important cause of LD in sepsis (37).
In line with results of former studies (19, 20, 39), the values of NETs were increased in the septic patients, more pronounced in non-survivors and compared with the non-septic control patients in our study. We also saw correlations between morbidity and degree of multiorgan failure displayed in the APACHE-II, and SOFA scores and the values of cf-DNA/NETs in concordance with other studies (20, 39). Interestingly, we could also observe correlations of some parameters of the biosensor and the values of the cf-DNA/NETs at inclusion that may support the diagnostic capacity of the biosensor for LD in sepsis.
Limitations of the Study and Summary
The test time with the hepatocyte-based biosensor of 3–6 days in this study is not suitable for early diagnosis of LD. However, by optimization of the biosensor, the incubation time has meanwhile been able to have reduced to 20 h (unpublished data). By technical improvements of the cell culture system (shaking instead of a resting system) and the increase of the concentration of FBS in the cell culture medium, we achieved comparable viability and functionality of sensor cells in human plasma after incubation times of 6 days and of 20 h. The reduction of test time is an important condition for usability in clinical practice (Appendix).
Plasma of healthy volunteers was not tested in the presented analysis. In our former study, the values of the hepatocyte-based test were comparable and without significant difference between healthy volunteers and the postoperative non-septic CG (22).
In conclusion, hepatotoxicity of septic plasma was already detected with the liver cell-based cytotoxicity at inclusion and also in the course of disease. The causes of these cellular impairments need further basic science and clinical investigations. The influence of NETs on the development of liver failure in septic patients seems to be an interesting approach. Higher levels of cf-DNA/NETs and impairments in all parameters of the hepatocyte biosensor were associated with a worse outcome in this study. Since bilirubin is a late parameter in LD and only markedly increased in advanced liver damage (5, 6), cf-DNA/NETs- and hepatocyte-based biosensoring may help to detect “subclinical” liver damage with prognostic relevance; further clinical validation, especially for usefulness as early diagnostic tools for LD, are necessary.
Ethics Statement
The study received ethics approval from the local research ethics committee (University of Rostock; II HV 16/2005), which include compliance with the principles of good clinical practice. The study abided by the principles of the Declaration of Helsinki. Written informed consent was obtained from all subjects or from the patients’ representatives if direct consent could not be received.
Author Contributions
MS and GN-S participated in the design of the study. MS did the regulatory work and coordinated the study; wrote the manuscript; revising work was done from all other authors. MS, GR, CH, and SD did the data analysis. MS, CH, TM, JE, GN-S, and SMitzner were clinical investigators. SMargraf and JA analyzed the clinical probes for cf-DNA/NETs.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors want to thank Heike Potschka and Helga Weiss-Reining for valuable technical support. All the routine measurements were made at Institute of Clinical Chemistry and Laboratory Medicine, University Hospital of Rostock. Special thanks go to Johannes Brenner, Maria Brettschneider, and Swantje Kruspi for their great help in the clinical setting and collection of clinical data.
Footnotes
Funding. The study was supported by research grants of the University of Rostock.
Supplementary Material
The Supplementary Material for this article can be found online at https://www.frontiersin.org/articles/10.3389/fimmu.2018.01448/full#supplementary-material.
Abbreviations
ALAT, alanine aminotransferase; APACHE, acute physiology and chronic health evaluation; ASAT, asparagine aminotransferase; ATCC, American Type Culture Collection; cf-DNA, circulating-free deoxyribonucleic acid; CG, control group; ICU, intensive care unit; IL, interleukin; LPS, lipopolysaccharides; NETs, neutrophil-derived extracellular traps; PCT, procalcitonin; SG, septic group; SIRS, systemic inflammatory response syndrome; SOFA, sepsis-related organ failure assessment; TNF, tumor necrosis factor.
References
- 1.Harbrecht BG, Zenati MS, Doyle HR, McMichael J, Townsend RN, Clancy KD, et al. Hepatic dysfunction increases length of stay and risk of death after injury. J Trauma (2002) 53:517–23. 10.1097/00005373-200209000-00020 [DOI] [PubMed] [Google Scholar]
- 2.Brienza N, Dalfino L, Cinnella G, Diele C, Bruno F, Fiore T. Jaundice in critical illness: promoting factors of a concealed reality. Intensive Care Med (2006) 32:267–74. 10.1007/s00134-005-0023-3 [DOI] [PubMed] [Google Scholar]
- 3.Kramer L, Jordan B, Druml W, Bauer P, Metnitz PG. Incidence and prognosis of early hepatic dysfunction in critically ill patients – a prospective multicenter study. Crit Care Med (2007) 35:1099–104. 10.1097/01.CCM.0000259462.97164.A0 [DOI] [PubMed] [Google Scholar]
- 4.Bakker J, Grover R, McLuckie A, Holzapfel L, Andersson J, Lodato R, et al. Administration of the nitric oxide synthase inhibitor NG-methyl-L-arginine hydrochloride (546C88) by intravenous infusion for up to 72 hours can promote the resolution of shock in patients with severe sepsis: results of a randomized, double-blind, placebo-controlled multicenter study (study no. 144-002). Crit Care Med (2004) 32:1–12. 10.1097/01.CCM.0000105118.66983.19 [DOI] [PubMed] [Google Scholar]
- 5.Pastor CM, Suter PM. Hepatic hemodynamics and cell functions in human and experimental sepsis. Anesth Analg (1999) 89:344–52. 10.1097/00000539-199908000-00019 [DOI] [PubMed] [Google Scholar]
- 6.Moreno R, Vincent JL, Matos R, Mendonça A, Cantraine F, Thijs L, et al. The use of maximum SOFA score to quantify organ dysfunction/failure in intensive care. Results of a prospective, multicentre study. Working group on sepsis related problems of the ESICM. Intensive Care Med (1999) 25:686–96. 10.1007/s001340050931 [DOI] [PubMed] [Google Scholar]
- 7.Le Gall JR, Klar J, Lemeshow S, Saulnier F, Alberti C, Artigas A, et al. The logistic organ dysfunction system. A new way to assess organ dysfunction in the intensive care unit. ICU scoring group. JAMA (1996) 276:802–10. 10.1001/jama.1996.03540100046027 [DOI] [PubMed] [Google Scholar]
- 8.Bolder U, Ton-Nu HT, Schteingart CD, Frick E, Hofmann AF. Hepatocyte transport of bile acids and organic anions in endotoxemic rats: impaired uptake and secretion. Gastroenterology (1997) 112:214–25. 10.1016/S0016-5085(97)70238-5 [DOI] [PubMed] [Google Scholar]
- 9.Moseley RH. Sepsis and cholestasis. Clin Liver Dis (2004) 8:83–94. 10.1016/S1089-3261(03)00134-X [DOI] [PubMed] [Google Scholar]
- 10.Dhainaut JF, Marin N, Mignon A, Vinsonneau C. Hepatic response to sepsis: interaction between coagulation and inflammatory processes. Crit Care Med (2001) 29:S42–7. 10.1097/00003246-200107001-00016 [DOI] [PubMed] [Google Scholar]
- 11.Tu W, Satoi S, Zhang Z, Kitade H, Okumura T, Kwon A-H, et al. Hepatocellular dysfunction induced by nitric oxide production in hepatocytes isolated from rats with sepsis. Shock (2003) 19:373–7. 10.1097/00024382-200304000-00013 [DOI] [PubMed] [Google Scholar]
- 12.Bauer M, Press AT, Trauner M. The liver in sepsis: patterns of response and injury. Curr Opin Crit Care (2013) 19:123–7. 10.1097/MCC.0b013e32835eba6d [DOI] [PubMed] [Google Scholar]
- 13.Wiersinga WJ. Current insights in sepsis: from pathogenesis to new treatment targets. Curr Opin Crit Care (2011) 17:480–6. 10.1097/MCC.0b013e32834a4aeb [DOI] [PubMed] [Google Scholar]
- 14.Hillenbrand A, Knippschild U, Weiss M, Schrezenmeier H, Henne-Bruns D, Huber-Lang M, et al. Sepsis induced changes of adipokines and cytokines – septic patients compared to morbidly obese patients. BMC Surg (2010) 10:26. 10.1186/1471-2482-10-26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szabo G, Romics L, Frendl G. Liver in sepsis and systemic inflammatory response syndrome. Clin Liver Dis (2002) 6:1045–66. 10.1016/S1089-3261(02)00058-2 [DOI] [PubMed] [Google Scholar]
- 16.Raza H, John A, Shafarin J. Potentiation of LPS-induced apoptotic cell death in human hepatoma HepG2 cells by aspirin via ROS and mitochondrial dysfunction: protection by N-acetyl cysteine. PLoS One (2016) 11:e0159750. 10.1371/journal.pone.0159750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Assenat E, Gerbal-Chaloin S, Larrey D, Saric J, Fabre J-M, Maurel P, et al. Interleukin 1beta inhibits CAR-induced expression of hepatic genes involved in drug and bilirubin clearance. Hepatology (2004) 40:951–60. 10.1002/hep.20387 [DOI] [PubMed] [Google Scholar]
- 18.Strnad P, Tacke F, Koch A, Trautwein C. Liver – guardian, modifier and target of sepsis. Nat Rev Gastroenterol Hepatol (2017) 14:55–66. 10.1038/nrgastro.2016.168 [DOI] [PubMed] [Google Scholar]
- 19.Lögters T, Margraf S, Altrichter J, Cinatl J, Mitzner S, Windolf J, et al. The clinical value of neutrophil extracellular traps. Med Microbiol Immunol (2009) 198:211–9. 10.1007/s00430-009-0121-x [DOI] [PubMed] [Google Scholar]
- 20.Margraf S, Lögters T, Reipen J, Altrichter J, Scholz M, Windolf J. Neutrophil-derived circulating free DNA (cf-DNA/NETs): a potential prognostic marker for posttraumatic development of inflammatory second hit and sepsis. Shock (2008) 30:352–8. 10.1097/SHK.0b013e31816a6bb1 [DOI] [PubMed] [Google Scholar]
- 21.Sauer M. The use of human hepatocytes to determine liver function and liver regeneration (PCT/EP 2007/001047 Ed.). (2007).
- 22.Sauer M, Haubner C, Mencke T, Nöldge-Schomburg G, Mitzner S, Altrichter J, et al. Impaired cell functions of hepatocytes incubated with plasma of septic patients. Inflamm Res (2012) 61:609–16. 10.1007/s00011-012-0451-9 [DOI] [PubMed] [Google Scholar]
- 23.Sauer M, Altrichter J, Haubner C, Pertschy A, Wild T, Doß F, et al. Bioartificial therapy of sepsis: changes of norepinephrine-dosage in patients and influence on dynamic and cell based liver tests during extracorporeal treatments. Biomed Res Int (2016) 2016:7056492. 10.1155/2016/7056492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Doß S, Potschka H, Doß F, Mitzner S, Sauer M. Hepatotoxicity of antimycotics used for invasive fungal infections: in vitro results. Biomed Res Int (2017) 2017:9658018. 10.1155/2017/9658018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sauer M, Piel I, Haubner C, Richter G, Mann M, Nöldge-Schomburg G, et al. Rocuronium is more hepatotoxic than succinylcholine in vitro. Eur J Anaesthesiol (2017) 34:623–7. 10.1097/EJA.0000000000000666 [DOI] [PubMed] [Google Scholar]
- 26.Sombetzki M, Koslowski N, Doss S, Loebermann M, Trauner M, Reisinger EC, et al. Biosensor for hepatocellular injury corresponds to experimental scoring of hepatosplenic schistosomiasis in mice. Biomed Res Int (2016) 2016:1567254. 10.1155/2016/1567254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sauer M, Doß S, Ehler J, Mencke T, Wagner N-M. Procalcitonin impairs liver cell viability and function in vitro: a potential new mechanism of liver dysfunction and failure during sepsis? Biomed Res Int (2017) 2017:6130725. 10.1155/2017/6130725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS international sepsis definitions conference. Crit Care Med (2003) 31:1250–6. 10.1097/01.CCM.0000050454.01978.3B [DOI] [PubMed] [Google Scholar]
- 29.Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, et al. Efficacy and safety of recombinant human activated protein C for severe sepsis. N Engl J Med (2001) 344:699–709. 10.1056/NEJM200103083441001 [DOI] [PubMed] [Google Scholar]
- 30.Kelly JH, Sussman NL. A fluorescent cell-based assay for cytochrome P-450 isozyme 1A2 induction and inhibition. J Biomol Screen (2000) 5:249–54. 10.1177/108705710000500407 [DOI] [PubMed] [Google Scholar]
- 31.Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger TH, et al. Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res (1988) 48:4827–33. [PubMed] [Google Scholar]
- 32.Lögters T, Paunel-Görgülü A, Zilkens C, Altrichter J, Scholz M, Thelen S, et al. Diagnostic accuracy of neutrophil-derived circulating free DNA (cf-DNA/NETs) for septic arthritis. J Orthop Res (2009) 27:1401–7. 10.1002/jor.20911 [DOI] [PubMed] [Google Scholar]
- 33.Shankar-Hari M, Harrison DA, Rubenfeld GD, Rowan K. Epidemiology of sepsis and septic shock in critical care units: comparison between sepsis-2 and sepsis-3 populations using a national critical care database. Br J Anaesth (2017) 119:626–36. 10.1093/bja/aex234 [DOI] [PubMed] [Google Scholar]
- 34.Engel C, Brunkhorst FM, Bone H-G, Brunkhorst R, Gerlach H, Grond S, et al. Epidemiology of sepsis in Germany: results from a national prospective multicenter study. Intensive Care Med (2007) 33:606–18. 10.1007/s00134-006-0517-7 [DOI] [PubMed] [Google Scholar]
- 35.Regueira T, Lepper PM, Brandt S, Ochs M, Vuda M, Takala J, et al. Hypoxia inducible factor-1 alpha induction by tumour necrosis factor-alpha, but not by toll-like receptor agonists, modulates cellular respiration in cultured human hepatocytes. Liver Int (2009) 29:1582–92. 10.1111/j.1478-3231.2009.02109.x [DOI] [PubMed] [Google Scholar]
- 36.el-Saadany MA, Rawel HM, Raila J, el-Dashloty MS, Schweigert FJ. Antioxidants modulate the IL-6 induced inhibition of negative acute-phase protein secretion in HepG2 cells. Cell Biochem Funct (2008) 26:95–101. 10.1002/cbf.1405 [DOI] [PubMed] [Google Scholar]
- 37.Sakurai K, Miyashita T, Okazaki M, Yamaguchi T, Ohbatake Y, Nakanuma S, et al. Role for neutrophil extracellular traps (NETs) and platelet aggregation in early sepsis-induced hepatic dysfunction. In Vivo (2017) 31:1051–8. 10.21873/invivo.11169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Meng W, Paunel-Görgülü A, Flohé S, Hoffmann A, Witte I, MacKenzie C, et al. Depletion of neutrophil extracellular traps in vivo results in hypersusceptibility to polymicrobial sepsis in mice. Crit Care (2012) 16:R137. 10.1186/cc11442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Altrichter J, Zedler S, Kraft R, Faist E, Mitzner SR, Sauer M, et al. Neutrophil-derived circulating free DNA (cf-DNA/NETs), a potential prognostic marker for mortality in patients with severe burn injury. Eur J Trauma Emerg Surg (2010) 36:551–7. 10.1007/s00068-010-0013-1 [DOI] [PubMed] [Google Scholar]
- 40.Li T, Jahan A, Chiang JY. Bile acids and cytokines inhibit the human cholesterol 7 alpha-hydroxylase gene via the JNK/c-jun pathway in human liver cells. Hepatology (2006) 43:1202–10. 10.1002/hep.21183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwakkel J, Wiersinga WM, Boelen A. Interleukin-1beta modulates endogenous thyroid hormone receptor alpha gene transcription in liver cells. J Endocrinol (2007) 194:257–65. 10.1677/JOE-06-0177 [DOI] [PubMed] [Google Scholar]
- 42.Jakobs TC, Mentrup B, Schmutzler C, Dreher I, Köhrle J. Proinflammatory cytokines inhibit the expression and function of human type I 5’-deiodinase in HepG2 hepatocarcinoma cells. Eur J Endocrinol (2002) 146:559–66. 10.1530/eje.0.1460559 [DOI] [PubMed] [Google Scholar]
- 43.Mogilenko DA, Dizhe EB, Shavva VS, Lapikov IA, Orlov SV, Perevozchikov AP. Role of the nuclear receptors HNF4 alpha, PPAR alpha, and LXRs in the TNF alpha-mediated inhibition of human apolipoprotein A-I gene expression in HepG2 cells. Biochemistry (2009) 48:11950–60. 10.1021/bi9015742 [DOI] [PubMed] [Google Scholar]
- 44.Yang L, Shi W, Wang J, Jiang X, Feng W, Li Z. Comparison of cell deaths induced by transmembrane and secretory TNF-alpha. J Huazhong Univ Sci Technolog Med Sci (2007) 27:117–9. 10.1007/s11596-007-0201-3 [DOI] [PubMed] [Google Scholar]
- 45.Wang C, Qi R, Li N, Wang Z, An H, Zhang Q, et al. Notch1 signaling sensitizes tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human hepatocellular carcinoma cells by inhibiting Akt/Hdm2-mediated p53 degradation and up-regulating p53-dependent DR5 expression. J Biol Chem (2009) 284:16183–90. 10.1074/jbc.M109.002105 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Kassardjian A, Kreydiyyeh SI. JNK modulates the effect of caspases and NF-kappaB in the TNF-alpha-induced down-regulation of Na+/K+ATPase in HepG2 cells. J Cell Physiol (2008) 216:615–20. 10.1002/jcp.21436 [DOI] [PubMed] [Google Scholar]
- 47.Rosado JA, Rosenzweig I, Harding S, Sage SO. Tumor necrosis factor-alpha inhibits store-mediated Ca2+ entry in the human hepatocellular carcinoma cell line HepG2. Am J Physiol Cell Physiol (2001) 280:C1636–44. 10.1152/ajpcell.2001.280.6.C1636 [DOI] [PubMed] [Google Scholar]
- 48.Senn JJ, Klover PJ, Nowak IA, Zimmers TA, Koniaris LG, Furlanetto RW, et al. Suppressor of cytokine signaling-3 (SOCS-3), a potential mediator of interleukin-6-dependent insulin resistance in hepatocytes. J Biol Chem (2003) 278:13740–6. 10.1074/jbc.M210689200 [DOI] [PubMed] [Google Scholar]
- 49.Nakai K, Tanaka H, Hanada K, Ogata H, Suzuki F, Kumada H, et al. Decreased expression of cytochromes P450 1A2, 2E1, and 3A4 and drug transporters Na+-taurocholate-cotransporting polypeptide, organic cation transporter 1, and organic anion-transporting peptide-C correlates with the progression of liver fibrosis in chronic hepatitis C patients. Drug Metab Dispos (2008) 36:1786–93. 10.1124/dmd.107.020073 [DOI] [PubMed] [Google Scholar]
- 50.Kelly JH, Darlington GJ. Modulation of the liver specific phenotype in the human hepatoblastoma line Hep G2. In Vitro Cell Dev Biol (1989) 25:217–22. 10.1007/BF02626182 [DOI] [PubMed] [Google Scholar]
- 51.Dehn PF, White CM, Conners DE, Shipkey G, Cumbo TA. Characterization of the human hepatocellular carcinoma (hepg2) cell line as an in vitro model for cadmium toxicity studies. In Vitro Cell Dev Biol Anim (2004) 40:172–82. [DOI] [PubMed] [Google Scholar]
- 52.Modrianský M, Ulrichová J, Bachleda P, Anzenbacher P, Anzenbacherová E, Walterová D, et al. Human hepatocyte – a model for toxicological studies. Functional and biochemical characterization. Gen Physiol Biophys (2000) 19:223–35. [PubMed] [Google Scholar]
- 53.Babich H, Sardana MK, Borenfreund E. Acute cytotoxicities of polynuclear aromatic hydrocarbons determined in vitro with the human liver tumor cell line, HepG2. Cell Biol Toxicol (1988) 4:295–309. 10.1007/BF00058738 [DOI] [PubMed] [Google Scholar]
- 54.Roe AL, Snawder JE, Benson RW, Roberts DW, Casciano DA. HepG2 cells: an in vitro model for P450-dependent metabolism of acetaminophen. Biochem Biophys Res Commun (1993) 190:15–9. 10.1006/bbrc.1993.1003 [DOI] [PubMed] [Google Scholar]
- 55.Peppard JV, Knap AK. Effect of the selective and non-selective cysteine protease inhibitors on the intracellular processing of interleukin 6 by HEPG2 cells. In Vitro Cell Dev Biol Anim (1999) 35:459–64. 10.1007/s11626-999-0052-2 [DOI] [PubMed] [Google Scholar]
- 56.Dufresne M, Jane D, Theriault A, Adeli K. Expression of cathepsin B and aryl hydrocarbon hydroxylase activities, and of apolipoprotein B in human hepatoma cells maintained long-term in a serum-free medium. In Vitro Cell Dev Biol Anim (1993) 29A:873–8. 10.1007/BF02631366 [DOI] [PubMed] [Google Scholar]
- 57.Hahn SE, Parkes JG, Goldberg DM. Enzyme-linked immunosorbent assay to measure apolipoproteins AI and B secreted by a human hepatic carcinoma cell line (Hep G2). J Clin Lab Anal (1992) 6:182–9. 10.1002/jcla.1860060404 [DOI] [PubMed] [Google Scholar]
- 58.Krasteva N, Groth TH, Fey-Lamprecht F, Altankov G. The role of surface wettability on hepatocyte adhesive interactions and function. J Biomater Sci Polym Ed (2001) 12:613–27. 10.1163/156856201316883449 [DOI] [PubMed] [Google Scholar]
- 59.Rodríguez-Antona C, Donato MT, Boobis A, Edwards RJ, Watts PS, Castell JV, et al. Cytochrome P450 expression in human hepatocytes and hepatoma cell lines: molecular mechanisms that determine lower expression in cultured cells. Xenobiotica (2002) 32:505–20. 10.1080/00498250210128675 [DOI] [PubMed] [Google Scholar]
- 60.Grant MH, Duthie SJ, Gray AG, Burke MD. Mixed function oxidase and UDP-glucuronyltransferase activities in the human Hep G2 hepatoma cell line. Biochem Pharmacol (1988) 37:4111–6. 10.1016/0006-2952(88)90103-7 [DOI] [PubMed] [Google Scholar]
- 61.Ellis AJ, Hughes RD, Wendon JA, Dunne J, Langley PG, Kelly JH, et al. Pilot-controlled trial of the extracorporeal liver assist device in acute liver failure. Hepatology (1996) 24:1446–51. 10.1002/hep.510240625 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.