Abstract
Cellular junctions are critical for intercellular communication and for the assembly of cells into tissues. Cell junctions often consist of tight junctions, which form a permeability barrier and prevent the diffusion of lipids and proteins between cell compartments, and adherens junctions, which control the adhesion of cells and link cortical actin filaments to attachment sites on the plasma membrane. Proper tight junction formation and cell polarity require the function of membrane-associated guanylate kinases (MAGUKs) that contain the PDZ protein-protein interaction domain. In contrast, less is known about how adherens junctions are assembled. Here we describe how the PDZ-containing protein DLG-1 is required for the proper formation and function of adherens junctions in Caenorhabditis elegans. DLG-1 is a MAGUK protein that is most similar in sequence to mammalian SAP97, which is found at both synapses of the CNS, as well as at cell junctions of epithelia. DLG-1 is localized to adherens junctions, and DLG-1 localization is mediated by an amino-terminal domain shared with SAP97 but not found in other MAGUK family members. DLG-1 recruits other proteins and signaling molecules to adherens junctions, while embryos that lack DLG-1 fail to recruit the proteins AJM-1 and CPI-1 to adherens junctions. DLG-1 is required for the proper organization of the actin cytoskeleton and for the morphological elongation of embryos. In contrast to other proteins that have been observed to affect adherens junction assembly and function, DLG-1 is not required to maintain cell polarity. Our results suggest a new function for MAGUK proteins distinct from their role in cell polarity.
INTRODUCTION
Cell adhesion facilitates the assembly of individual cells into organized tissues, and several different cell adhesion mechanisms fulfill this role (Gumbiner, 1996). One of these adhesion mechanisms is the formation of the tight junction or zonula occludens, which functions to regulate the permeability of the cell layer and to polarize the cell surface into apical and basolateral compartments (Mitic and Anderson, 1998). Tight junctions (in vertebrates) and septate junctions (in invertebrates) maintain the separation between the apical and basolateral surfaces by hindering the diffusion of lipids and proteins (Powell, 1981; van Meer et al., 1986; van Meer and Simons, 1986; Gumbiner, 1987). In contrast to tight junctions, a second mechanism of cell adhesion is the adherens junction, which is responsible for maintaining adhesion between neighboring cells and is important for intercellular communication (Muller, 2000; Vasioukhin and Fuchs, 2001).
While many proteins have important roles in assembling these junctions, mutations in these proteins result in large defects in cell polarity as well. For example, mutations in the Drosophila genes bazooka (baz), crumbs (crb), discs lost (dlt), scribble (scrib), lethal (1) discs large (dlg), and lethal giant larvae (lgl), and the Caenorhabditis elegans gene let-413 disrupt epithelial cell polarity and prevent the formation of cell junctions (Legouis et al., 2000; Muller, 2000). Because of the large-scale defects in cell polarity caused by mutations in these genes, it is not clear whether they have a direct role in junction formation or if the failure to form junctions in mutants is due to general defects in cell polarity.
BAZ, DLG, SCRIB, LET-413, and DLT each contain one or more PDZ domains, which are stretches of approximately 90 amino acids that bind to a consensus sequence at the extreme carboxyl termini of other proteins (Doyle et al., 1996; Songyang et al., 1997). PDZ-containing proteins play a dual role in establishing polarity and assembling cell surface signaling molecules into protein complexes in a number of cell types and species, demonstrating the general importance of these proteins. For example, the C. elegans PDZ proteins LIN-2, LIN-7, and LIN-10 are thought to form a complex that is involved in mediating basolateral membrane localization of the LET-23 EGF receptor in vulval epithelial cells (Kaech et al., 1998; Rongo et al., 1998; Whitfield et al., 1999). In mammals, members of the membrane-associated guanylate kinase (MAGUK) family, which contain 3 PDZ domains, play an integral role in targeting glutamate receptors and effector molecules to excitatory synapses (Kim et al., 1995; Kornau et al., 1995; Brenman et al., 1996; Kim et al., 1996; Kornau et al., 1997). A better understanding of the functional role of PDZ proteins might elucidate universal mechanisms for cell polarity and the formation of cell junctions.
Bossinger and colleagues have recently shown that gene finder prediction C25F6.2 is similar in sequence to MAGUK family members, and they have named the sequence dlg-1 (Bossinger et al., 2001). Nematodes knocked down for dlg-1 function by RNAi fail to form proper adherens junctions and arrest as twofold stage embryos. To better understand the formation of adherens junction, we have cloned DLG-1 and show here that this C. elegans protein contains 3 PDZ domains, an SH3 domain, and a yeast guanylate kinase homology domain, most similar to the SAP97 MAGUK family member found at cell junctions and synapses in mammals (Muller et al., 1995). We find that this protein localizes to adherens junctions of the epidermis, intestine, and pharynx of embryos and adult nematodes, and that the first 186 amino acids are sufficient for this localization. Embryos deficient in DLG-1 show abnormal adherens junction formation at the ultrastructural level, and they fail to localize the adherens junction proteins AJM-1 and CPI-1 to cell junctions. Furthermore, the actin cytoskeleton in dlg-1(RNAi) embryos is disorganized, suggesting that like the cadherin-catenin system, DLG-1, also plays a role in coordinating the actin network with adherens junctions.
MATERIALS AND METHODS
Strains
The following strains were used: let-413(s128), hmp-1(zu278), hmp-2(zu364), zuEx24[hmp-1::gfp], hmr-1(zu389), mcEx[let-413::gfp], jcIs1[ajm-1::gfp].
DLG-1 cDNA
Based on Kohara expressed sequence tags, we sequenced several dlg-1 cDNA clones (a kind gift from Y. Kohara, National Institute of Genetics, Mishima, Japan), including yk333g2, yk435h12, yk481e2, yk348h11, and yk481c12. To identify the 5′ end of dlg-1, we isolated total RNA by homogenizing mixed stage N2 nematodes in 200 mM LiCl, 20 mM EDTA, 20 mM Tris Cl pH 7.8, and 2% SDS. The RNA was extracted several times with phenol and precipitated with ethanol. RT-PCR was conducted by reverse transcribing 5 μg total RNA with the use of AMV reverse transcriptase and oligo dT15 as a primer (Promega, Madison, WI). A single 1.2-kb PCR product was amplified from the cDNA pool with the use of a primer for the SL1 leader (5′-GGTTTAATTACCCAAGTTTGAG-3′) and a primer unique to dlg-1 (5′-GACCCGCCAAAGTTTCCTCCAATTGG-3′), cloned into pBluescript (Stratagene, LaJolla, CA), and sequenced. The cDNA sequence has been submitted to GenBank (Accession number AF406786)
DLG-1 Expression Constructs
The dlg-1::gfp transgenes were made by ligating an EcoRI/XhoI fragment from the cosmid C25F6 to sequences encoding GFP and the unc-54 3′UTR (pPD95.75, from A. Fire, Carnegie Institute of Washington, Baltimore, MD). Transgenic strains were isolated by microinjecting the resulting plasmid (50 ng/μl) with the use of rol-6(su1006dm) (C. Mello, University of Massachusetts Medical Center, Worchester, MA) as a cotransformation marker. Mutant versions of DLG-1::GFP were engineered using PCR. Three different lines were analyzed for each construct; all three had similar localization.
Fluorescent Microscopy
Immunohistochemistry of embryos and larvae was performed as described (Finney and Ruvkun, 1990). Anti-PKC-3 antibodies (Y. Tabuse, NEC Fundamental Research Laboratories, Ibaraki, Japan) were used as described (Tabuse et al., 1998). Actin filaments were visualized in embryos with rhodamine-phalloidin (Molecular Probes), as described (Costa et al., 1997). LET-413::GFP (M. Labouesse), AJM-1::GFP and HMP-1::GFP (J. Hardin), and DLG-1::GFP eggs and larvae were mounted and visualized on 2% agarose pads. Fluorescent images were observed with the use of a Zeiss (Oberkochen, Germany) Axioplan II and 63X1.4NA objective and captured with a SensiCam (Cooke, Auburn Hills, MI) with the use of ImagePro v4.1 (Media Cybernetics, Silver Spring, MD) and VayTek v6.2 software (VayTek, Inc., Fairfield, IA). Animals were optically sectioned (0.25 μm), and out-of-focus light was removed with a constrained interative deconvolution algorithm (VayTek).
RNA-mediated Interference
A dlg-1 cDNA in pBluescript, yk333g2 (Y. Kohara), was used as a template to generate dlg-1 sense and antisense transcripts. For cpi-1 RNAi, a 300 bp exon was PCR amplified from genomic DNA corresponding to sequence F38E11.3 and was ligated into pBluescript so that sense and antisense transcripts could be synthesized. Annealed transcripts were injected into worms to induce RNAi (Fire et al., 1998). An XhoI fragment from the dlg-1 cDNA and the cpi-1 PCR product were also ligated between two T7 promoters in the pPD129.36 vector (A. Fire), and the resulting plasmids (CR254 and CR255, respectively) were introduced into HT115(DE3) Escherichia coli. E. coli harboring CR254 or CR255 were fed to nematodes to induce RNAi as described (Kamath et al., 2000; Timmons and Fire, 1998). RNAi by injection and by feeding gave similar results; however, stronger phenotypic expressivity and penetrance were observed with the feeding method. RNAi data described in the text and figures reflects data from the feeding method. The empty pPD129.36 vector was used as a negative control in feeding experiments, and the resulting worms are shown as wild-type controls in all figures.
Electron Microscopy
Embryos were isolated from egg shells by digestion with chitinase (Sigma, St. Louis, MO) for 20 min in 120 mM NaCl, 40 mM KCl, 3 mM CaCl2, 3 mM MgCl2, and 5 mM HEPES pH7.2. Embryos were fixed in 4% paraformaldehyde/2% glutaraldehyde and postfixed in 1% osmium tetroxide and uranyl acetate. Twofold stage embryos were dehydrated, embedded in epon, and sectioned. Sections of 50 nm were contrasted with uranyl acetate and observed for transmission electron microscopy.
RESULTS
DLG-1 Is a PDZ Domain Protein that Is Localized to Adherens Junctions
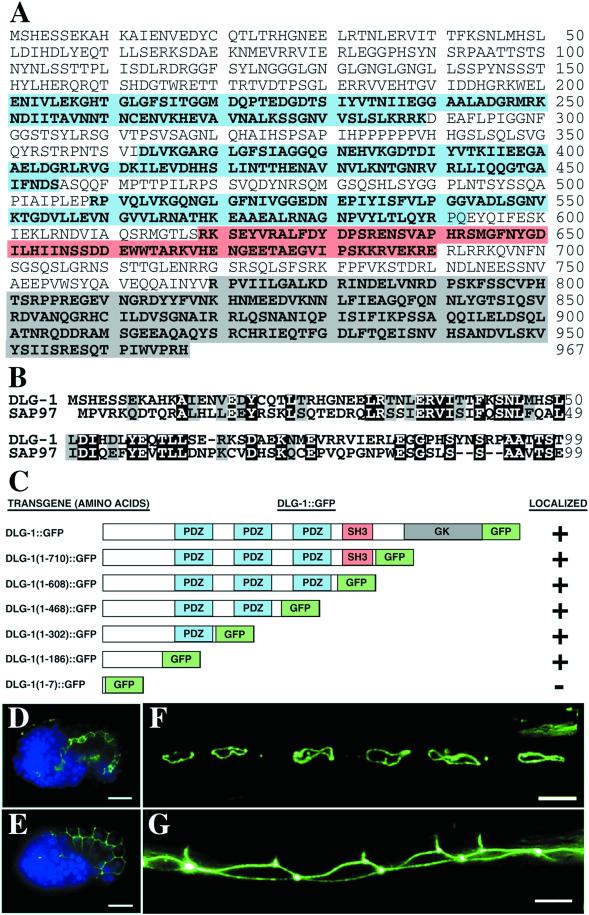
The C. elegans genome contains ∼50 genes predicted to contain at least 1 PDZ domain (Bargmann, 1998). Predicted gene C25F6.2 encodes the sole protein similar to MAGUK proteins of the disks large family and therefore has been named dlg-1 for discs large like protein (Bossinger et al., 2001). Genefinder algorithms often make incorrect predictions of 5′ and 3′ sequences; thus, to determine the correct protein sequence for dlg-1, we sequenced several dlg-1 cDNA clones from the Y. Kohara library(Kohara, 1996). We also isolated and reverse transcribed RNA from mixed stage nematodes. The dlg-1 5′end was amplified by RT-PCR from cDNA with the use of primers specific for dlg-1 sequences and the SL1 transsplice leader sequence. Our sequencing results of the Kohara clones and RT-PCR products suggest that dlg-1 generates a single mRNA product. DLG-1 protein is predicted to encode 967 amino acids with amino and carboxy-terminal sequences that differ from the original genefinder prediction that has been reported (Bossinger et al., 2001). DLG-1 contains three PDZ domains, an SH3 domain, and a yeast guanylate kinase homology domain (Figure 1A), and is 38%, 38%, 28%, and 34% identical to the MAGUK family members SAP97, SAP102, PSD-95, and Drosophila DLG, respectively (Woods and Bryant, 1991; Cho et al., 1992; Kistner et al., 1993; Muller et al., 1995; Muller et al., 1996). The amino terminal sequences of MAGUK proteins are thought to contain important sequences for their subcellular localization and regulation, and these sequences often diverge between family members. For example, the amino-terminus of PSD-95 contains cysteine residues that allow the palmitoylation and thus membrane association of this protein, the amino-terminus of SAP102 binds to zinc, and although the SAP97 amino terminus is important for localization, it is not palmitoylated and does not bind to zinc (Craven et al., 1999; El-Husseini et al., 2000a,b; Firestein et al., 2000; Wu et al., 1998a). Interestingly, the amino terminal sequence of DLG-1 is conserved (34% identity, 54% similarity) with the amino terminal 65 amino acids of SAP-97 (Figure 1B), a MAGUK protein found at mammalian synapses and epithelial cell junctions.
Figure 1.
DLG-1 is a MAGUK protein most similar to SAP97. (A) Deduced amino acid sequence of DLG-1 with PDZ domains in blue, SH3 domain in red, and guanylate kinase domain in gray. (B) Alignment of the amino-terminal 99 amino acids of DLG-1 with SAP97. Identical residues are indicated in black, whereas conserved residues are indicated in gray. (C) Schematic diagrams of the DLG-1:: GFP chimeric proteins. Embryos expressing DLG-1:: GFP (green) in intestine (D) and hypodermis (E) are shown with DAPI-stained nuclei (blue). Adult worms express DLG-1:: GFP in the Pnp epithelial cells (F) and lateral seam cells (G). Scale bar represents 10 μm.
The Conserved Amino-Terminus of DLG-1 Directs DLG-1 Localization to Adherens Junctions
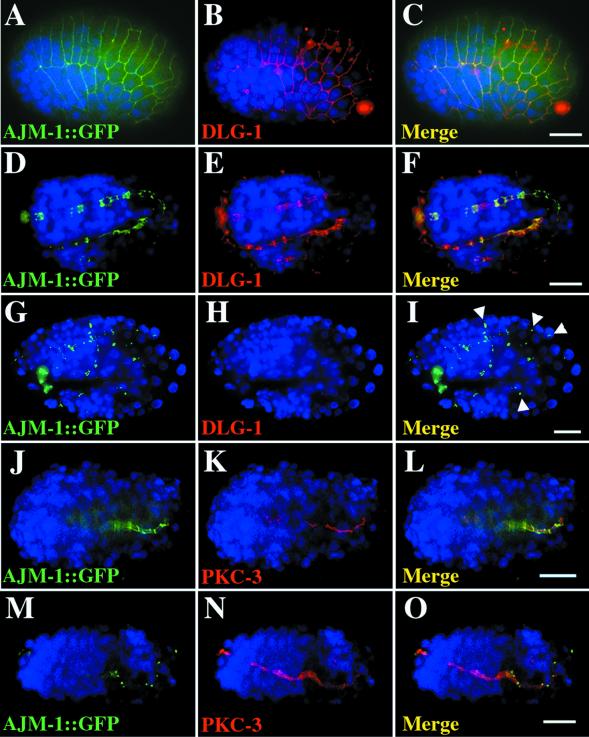
Because many of the MAGUK proteins like SAP97 are found both in neurons and epithelial cells, we wanted to visualize where DLG-1 was expressed and localized. Therefore, we constructed a GFP fusion gene by inserting GFP sequences at the carboxy terminus of DLG-1 (Figure 1C). Sequences for the resulting DLG-1::GFP chimeric protein were ligated to 5 kb of dlg-1 upstream promoter sequences. We introduced this construct into the C. elegans germline and found that the resulting fusion protein is situated at adherens junctions exclusively in epithelial cells of embryonic and adult epidermis, intestine, and pharynx (Figure 1D,E,F, n = 43/43). This pattern is also seen when embryos are stained with monoclonal antibodies that recognizes the mammalian PSD-95 (Figure 2, n = 30/30). DLG-1::GFP is not expressed in neurons, and we did not detect DLG-1 in the nervous system with the use of anti-PSD-95 antibodies.
Figure 2.
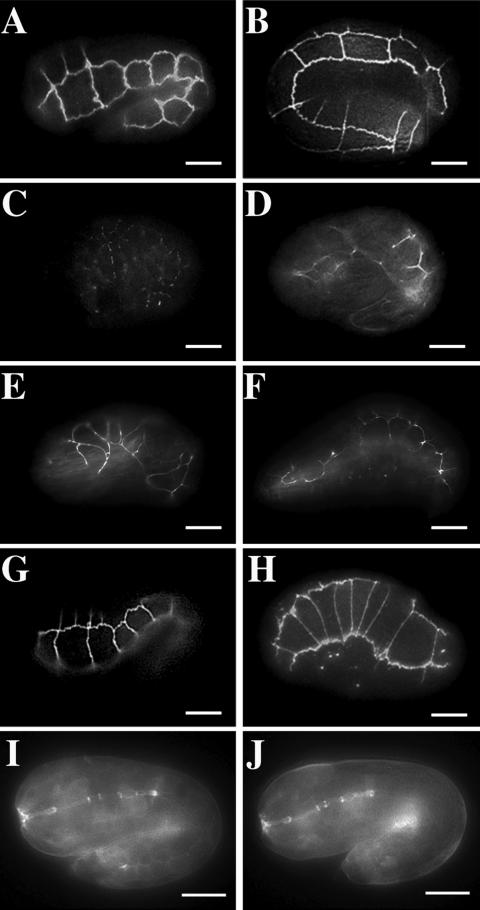
DLG-1 is localized to adherens junctions and required for AJM-1:: GFP localization. Immunostaining of embryos that express AJM-1:: GFP (green, A, D, G, J, M) with anti-PSD-95 antibodies (red, B, E, H) or with anti-PKC-3 antibodies (red, K, N). Nuclei are stained with DAPI (blue). Merged images (C, F, I, L, O) contain a 10 μm scale bar. AJM-1:: GFP and DLG-1 colocalize to adherens junction on the epidermis (A-C, lima bean stage) and in the intestine (D-F, tadpole stage) of wild-type embryos. (G-I, twofold stage) AJM-1:: GFP is found in small punctate structures between cells in dlg-1(RNAi) embryos No DLG-1 protein is detected in these embryos. (J-L, gastrulating embryo) AJM-1:: GFP is found at intestinal adherens junctions (green) whereas PKC-3 is localized to the apical surface of these cells (red) in wild-type embryos. (M-O, gastrulating embryo) AJM-1:: GFP is found in punctate structures, but PKC-3 is found at the apical surface of cells from dlg-1(RNAi) embryos.
To identify the domain of DLG-1 that is responsible for its localization to adherens junctions, we generated mutant versions of the DLG-1::GFP transgene that contained different domains of the DLG-1 protein (Figure 1C). The resulting transgenes were introduced into the C. elegans germline and the resulting chimeric proteins analyzed for their subcellular localization. DLG-1(1–7)::GFP, which contains the first seven amino acids of DLG-1 fused to GFP, fails to be localized to adherens junctions and is entirely cytosolic (n = 54/54). In contrast, the first 186 amino acids of DLG-1 are sufficient to direct localization of GFP to adherens junctions (Figure 1C, n = 18/18). This region of DLG-1 is conserved with SAP97, and recent results suggest that the same domain of SAP97 is important for cell junction localization when observed in colon carcinoma cells immortalized in culture (Wu et al., 1998a). Our results show that the 3 PDZ domains, the SH3 domain, and the guanylate kinase domain are not required for DLG-1 localization. Instead, we find that the amino-terminal domain functions as an adherens junction localization signal in vivo in epithelial cells of intact tissue.
DLG-1 Is Required for Adherens Junction Assembly but Is Not Essential for Polarized Protein Targeting
The pattern of DLG-1 expression highly resembles that of the adherens junction protein AJM-1, which encodes the MH27 antigen and was previously known as JAM-1 (Köppen et al., 2001; Mohler et al., 1998; Podbilewicz and White, 1994; Priess and Hirsh, 1986; Raich et al., 1999; Wood, 1988). Indeed, DLG-1 colocalizes with AJM-1::GFP as evidenced by immunostaining (Figure 2A-F, n = 18/18). Bossinger and colleagues tested the role of DLG-1 in adherens junction formation by injecting nematodes with double-stranded dlg-1 RNA to induce RNAi(Bossinger et al., 2001; Fire et al., 1998). They observed that dlg-1(RNAi) embryos form an irregular junction of AJM-1 around the apex of the cells and that, as morphogenesis proceeds, the junction begins to fragment. Their results suggest that DLG-1 might be needed to maintain the integrity of adherens junctions rather than for the initial formation of the junctions. Because dlg-1(RNAi) embryos still possess trace amounts of DLG-1 protein, particularly in their intestine and pharynx, an alternative explanation is that the phenotype of these embryos represents only a partial loss of dlg-1 function (Bossinger et al., 2001).
Although injection of double-stranded RNA can induce RNAi, recent studies have shown that RNAi induced by feeding nematodes bacteria that express double-stranded RNA for the gene of interest can induce stronger and more penetrant phenotypes by removing more gene product (Kamath et al., 2000). Thus, we examined the role of DLG-1 in adherens junction formation by removing DLG-1 with the use of the feeding method of double-stranded RNAi. RNAi with dlg-1 RNA abolishes DLG-1::GFP expression (n = 23/25, our unpublished results) and fluorescence from anti-PSD-95 antibody staining (Figure 2H) in all tissues examined (n = 42/44). Any DLG-1 protein that might remain in dlg-1(RNAi) embryos is below our limit of detection. We performed DLG-1 RNAi on nematodes that express AJM-1::GFP and found that dlg-1(RNAi) embryos do not form junctions of AJM-1 around the apex of cells even at the earliest developmental stages at which AJM-1::GFP can be detected (Figure 2G, n = 32/36, and our unpublished results). In most dlg-1(RNAi) animals, AJM-1::GFP is found in small punctate structures between cells (Figure 2G, n = 32/36) rather than showing a continuous ring of localization at cell boundaries as in wild-type embryos (Figure 2A,D, n = 22/22). Our results show that DLG-1 is needed for the initial localization of AJM-1 to adherens junctions, not just to maintain AJM-1 in continuous and unfragmented bands around the cells as originally suggested (Bossinger et al., 2001).
One simple explanation for AJM-1 mislocalization is that dlg-1(RNAi) embryos are incapable of localizing all cell surface proteins. For example, the dlg-1(RNAi) phenotype is similar to that found in let-413 mutants, which globally disrupt epithelial cell polarity (Legouis et al., 2000). Thus, we examined protein localization in the dlg-1(RNAi) embryos. Remarkably, PKC-3, an atypical PKC required for embryonic development (Wu et al., 1998b), is localized apically in the mutant embryos (Figure 2N, n = 22/24), as it is in wild-type embryos (Figure 2K, n = 24/28). Similarly, LET-413::GFP is correctly localized to the basolateral surface in dlg-1(RNAi) mutants (n = 30/31), as it is in wild-type embryos (n = 18/18, our unpublished results). These results demonstrate that at least two apical and basolateral proteins are properly localized in dlg-1(RNAi) embryos and suggest that DLG-1 plays a relatively specific role in adherens junction formation without disrupting gross aspects of cell polarity.
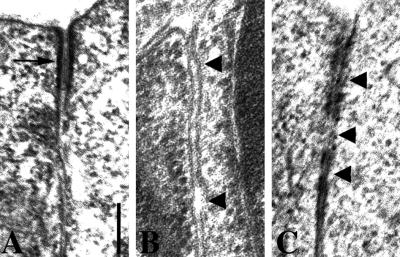
To determine whether there are ultrastructural changes to adherens junctions in dlg-1(RNAi) embryos, we examined the adherens junctions of hypodermal and intestinal cells by transmission electron microscopy. In wild-type embryos, adherens junctions form electron-dense structures between membranes of adjacent cells near their apical surfaces (Figure 3A, n = 20/20). In dlg-1(RNAi) embryos, the electron dense junctions are often missing (Figure 3B, n = 22/26) or broken into discontinuous structures (Figure 3C, n = 4/26). Gaps often form between adjacent cells (n = 13/26), suggesting that the adhesive properties of the junctions are diminished.
Figure 3.
Abnormal ultrastructure of adherens junctions in dlg-1(RNAi) embryos. (A) Electron microscopy of an adherens junction (arrow) in a wild-type embryo. (B) Electron-dense adherens junction material is often missing in dlg-1(RNAi) embryos. Arrowheads point to gaps that have formed between adjacent membranes. (C) When adherens junctions are visible in dlg-1(RNAi) embryos, they are often discontinuous (arrowheads). Scale bar is 200 nm.
DLG-1 Is Required for Proper Elongation
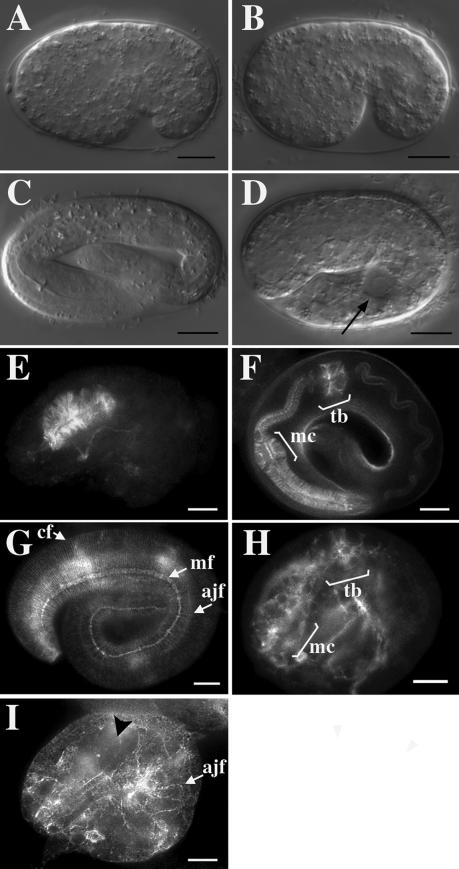
To determine if DLG-1 is involved in the function of adherens junctions during embryogenesis, we analyzed dlg-1(RNAi) embryos with the use of Nomarski interference microscopy. C. elegans embryos undergo two major morphogenetic movements during embryogenesis that require adherens junctions: ventral enclosure and elongation (Sulston et al., 1983; Priess and Hirsh, 1986; Williams-Masson et al., 1997; Costa et al., 1998). During ventral enclosure, the free edges of epidermis migrate to the ventral surface of the embryo, where they form new adherens junctions and thereby seal up the epithelial sheet (Williams-Masson et al., 1997). Mutations that prevent ventral closure fail to seal the ventral epithelia and result in embryos that rupture at elongation (Raich et al., 1999). After ventral enclosure, actin filament bundles at adherens junctions contract to change the shape of epithelial cells from circumferentially elongated to longitudinally elongated (Costa et al., 1997; Costa et al., 1998). These coordinated shape changes result in the elongation of the nematode body along the anteroposterior axis. Mutations that prevent elongation result in short embryos with a bulged appearance (Williams and Waterston, 1994; Costa et al., 1998). We found that dlg-1(RNAi) embryos undergo ventral enclosure (Figure 4B, n = 38/38), as do wild-type embryos (Figure 4A, n = 25/25), suggesting that DLG-1 is not required for that aspect of adherens junction function. However, unlike wild-type embryos (Figure 4C, n = 29/29), dlg-1(RNAi) embryos fail to elongate (Figure 4D, n = 33/33). Taken together, these results suggest that DLG-1 is not required for adherens junction-dependent filopodial priming and cell movement during ventral enclosure but is necessary instead for cell shape changes required for elongation.
Figure 4.
DLG-1 is required for elongation and proper organization of actin filaments. (A-D) Nomarski interference microscopy of embryos. Wild-type (A) and dlg-1(RNAi) (B) embryos undergo proper ventral enclosure during gastrulation. However, unlike wild-type threefold embryos (C), dlg-1(RNAi) embryos fail to elongate and often contain large vacuoles (arrowhead). (E-I) Rhodamine-phalloidin staining of filamentous actin. Wild-type twofold embryos (E) develop an unelongated pharynx with radially-oriented actin filaments, which elongates into a complete pharynx (F) in threefold embryos. (G) The hypodermis of wild-type embryos contains circumferential filaments (cf) that wrap around the nematode, and adherens junction filaments (ajf) that have contracted circumferentially. The label “mf” indicates actin filaments of the longitudinal muscles. (H) In contrast, the pharynx of a dlg-1(RNAi) embryo fails to elongate and contains disorganized actin filaments. The labels “mc” and “tb” indicate metacorpus and terminal bulb, respectively. (I) The hypodermis of dlg-1(RNAi) embryos contains regions that lack circumferential actin bundles (black arrowhead). Adherens junction filaments are found at the adherens junctions of lateral hypodermis but have failed to contract circumferentially. Scale bar is 10 μm.
dlg-1(RNAi) Embryos Show Abnormal Actin Bundles
Because dlg-1(RNAi) embryos show a defect in elongation, and because circumferential actin bundles within epidermal cells are thought to provide the contractile force for elongation of the embryo (Costa et al., 1997; Costa et al., 1998; Priess and Hirsh, 1986), we examined the actin pattern in wild-type and mutant embryos with the use of rhodamine-phalloidin to stain filamentous actin. The pharynges of dlg-1(RNAi) terminal-stage embryos, which appear arrested at the twofold stage (Figure 4H, n = 32/36), lack most of the radially-oriented actin filaments seen in their twofold stage (Figure 4E, n = 12/12) and threefold stage (Figure 4F, n = 35/35) wild-type counterparts. We also found that dlg-1(RNAi) worms have fewer than normal circumferential actin bundles, and the bundles are thicker and often not circumferentially oriented, with variable spacing between the bundles (Figure 4I, n = 24/36). These results suggest that DLG-1 plays an important role in coordinating actin filaments with the attachment sites at adherens junctions between cells.
HMR-1, HMP-1, and HMP-2 Are Not Required for DLG-1 Localization
What is the function of DLG-1 in the assembly of adherens junctions and the organization of actin filaments? One possibility is that DLG-1 interacts with the catenin-cadherin system, which mediates elongation in the C. elegans embryo and includes the proteins HMR-1 (cadherin), HMP-1 (α-catenin), and HMP-2 (β-catenin)(Costa et al., 1998). Mutants that lack HMR-1, HMP-1, or HMP-2 recruit AJM-1 to adherens junctions; however, mutant embryos are unable to elongate and possess disorganized actin filaments. To determine whether DLG-1 requires the catenin-cadherin system for localization, we introduced DLG-1::GFP into mutants that lack these proteins. In elongating and elongated wild-type embryos, DLG-1::GFP is localized to adherens junctions between cells (Figure 5A,B). Elongating and terminal-stage embryos that lack HMR-1, HMP-1, and HMP-2 form disorganized adherens junctions but that still recruit AJM-1 to these junctions(Costa et al., 1998). Similarly, we find that DLG-1::GFP is localized to adherens junctions in hmr-1 (n = 14/14), hmp-1 (n = 15/15), and hmp-2 (n = 28/28) embryos (Figure 5E-H,). In contrast, DLG-1::GFP fails to properly localize to adherens junctions in embryos that lack LET-413, which is required for proper apical-basolateral polarity (Figure 5C, n = 26/26).
Figure 5.
DLG-1:: GFP localization to adherens junctions does not require the cadherin-catenin system. Embryos expressing DLG-1:: GFP (A-H) and HMP-1:: GFP (I-J). Wild-type tadpole (A) and threefold stage (B) embryos localize DLG-1:: GFP to adherens junctions. (C) In contrast, let-413 mutant embryos localize DLG-1:: GFP to punctate structures between cells. (D) Tadpole stage hmr-1 embryos are disorganized but localize DLG-1:: GFP to adherens junctions. Tadpole (E) and terminal (threefold equivalent) stage (F) hmp-1 embryos localize DLG-1:: GFP to their disorganized adherens junctions. Tadpole (G) and terminal (threefold equivalent) stage (H) hmp-2 embryos localize DLG-1:: GFP to their disorganized adherens junctions. HMP-1:: GFP is found at adherens junctions of tadpole stage embryos from both wild-type (I) and dlg-1(RNAi) (J) embryos. Scale bar represents 10 μm.
HMR-1 cadherin is thought to recruit HMP-2 β-catenin to adherens junctions, and HMP-2 β-catenin is thought to recruit HMP-1 α-catenin to adherens junctions. Thus, mutants that lack HMR-1 or HMP-2 protein fail to localize a HMP-1::GFP fusion to adherens junctions(Costa et al., 1998). We examined the localization of HMP-1::GFP in dlg-1(RNAi) mutants to determine if DLG-1 is required for HMP-1 recruitment to adherens junctions. HMP-1::GFP is localized to adherens junctions both in hypodermis (our unpublished results) and intestinal epithelia (Figure 5I,J) in wild-type (n = 37/37) and dlg-1(RNAi) mutant (n = 25/25) embryos, demonstrating that DLG-1 is not required for HMP-1 localization. These results suggest that DLG-1 plays an important role in organizing underlying actin that might be independent of cadherin-catenin signaling.
DLG-1 Is Involved in Targeting Cypin to Adherens Junctions
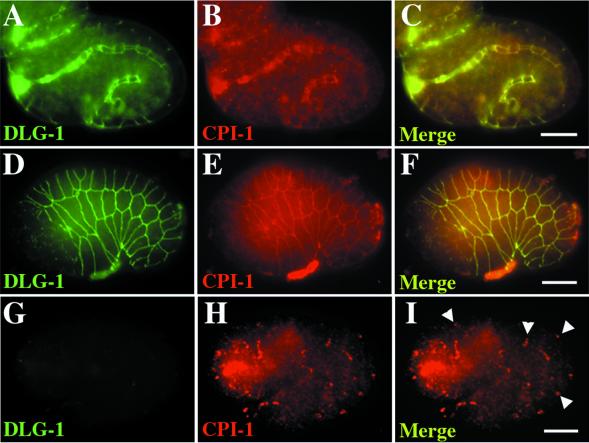
Recently, Firestein and colleagues (Firestein et al., 1999) reported the cloning of cypin, a cytosolic PSD-95 interactor, which is involved in MAGUK protein targeting at neuronal synapses. Mammalian cypin is also localized to the basolateral membrane of intestinal epithelial cells (Firestein et al., 1999), where it can act as an enzyme involved in guanine metabolism and, hence, uric acid production and excretion (Yuan and Atchison, 1999). Since cypin acts to regulate the targeting of MAGUK proteins in mammalian cells, we asked whether CPI-1, a cypin-like molecule in C. elegans (predicted sequence F38E11.3), plays a homologous role in the targeting of DLG-1 to adherens junctions. We found that CPI-1 localizes to the adherens junctions of pharynx (Figure 6B) and epidermis (Figure 6E), as evidenced by colocalization with DLG-1 in wild-type animals (n = 25/27). No CPI-1 staining was detected in cpi-1(RNAi) embryos, indicating that anticypin antibodies specifically detect CPI-1 (n = 17/17, our unpublished results). In contrast to wild-type embryos, CPI-1 was mislocalized in dlg-1(RNAi) embryos, where it was found in small clusters (Figure 6H, n = 36/38). This result is surprising, as cypin acts as a regulator of MAGUK targeting in mammalian cells. Instead, the MAGUK DLG-1 actually regulates CPI-1 targeting in vivo in C. elegans. As such, DLG-1 acts to assemble complexes at the adherens junction as well as to regulate proper adherens junction assembly itself.
Figure 6.
DLG-1 is required to localize CPI-1. Embryos immunostained with anti-PSD-95 antibodies to detect DLG-1 (green, A, D, G) and anticypin antibodies to detect CPI-1 (red, B, E, H). DLG-1 (green) and CPI-1 (red) colocalize to the adherens junctions of wild-type intestine (A-C) and epidermis (D-F). However, in dlg-1(RNAi) embryos, CPI-1 is found in small clusters (arrowheads) along the adherens junctions of intestine (not shown) and epidermis (G-I). Merged images (C, F, I, L, O) contain a 10 μm scale bar.
To assess the role of CPI-1 in adherens junction assembly, we removed CPI-1 expression with the use of RNAi. Surprisingly, these embryos showed normal adherens junction assembly (n = 19/19, our unpublished results) and DLG-1 localization. Thus, CPI-1 may act as a signaling molecule or enzyme at adherens junctions (Yuan and Atchison, 1999), but it is not required for proper adherens junction assembly under laboratory conditions.
DISCUSSION
Bossinger and colleagues have shown that the PDZ-domain protein DLG-1 is required for the formation of complete and continuous adherens junctions between epithelial cells (Bossinger et al., 2001). Embryos with reduced levels of DLG-1 form adherens junctions with discontinuous accumulations of the cell junction protein AJM-1. We have found that nematodes that are fed bacteria that express double-stranded dlg-1 RNA have undetectable levels of DLG-1 protein (Figure 2H), even when DLG-1 is overexpressed by a transgene (our unpublished results). Using this technique, we have extended the dlg-1(RNAi) studies by determining that DLG-1 is required for the initial localization of AJM-1 to adherens junctions. We do not believe that the defects in adherens junction assembly in dlg-1(RNAi) embryos are due to gross defects in cell polarity and protein trafficking because we find that representative apical and basolateral proteins are properly localized. Rather, we think that DLG-1 plays a relatively specific role in assembling adherens junction proteins. This is a novel observation for a MAGUK protein because other members of this family (like DLG and ZO-1) have a more general role in cell polarity and the formation of tight junctions.
Adherens junctions form belt-like structures around epithelial cells, thereby allowing cells to adhere to each other to form sheets. Some of the most well studied components of adherens junctions include cadherin, α-catenin, and β-catenin, encoded by the genes hmr-1, hmp-1, and hmp-2 in C. elegans. Mutations in these genes result in embryos that fail to elongate and fail to organize their actin cytoskeleton, a phenotype similar to that of dlg-1(RNAi) mutants(Costa et al., 1998). We have shown that circumferential actin bundles, which are required for nematode elongation, are not properly organized with respect to the adherens junctions in dlg-1(RNAi) embryos. Thus, embryos that lack DLG-1 cannot generate sufficient contractile force and therefore fail to elongate. Our results suggest that DLG-1 might function in a previously unknown mechanism (in addition to that of cadherins and catenins) by which actin filaments are attached to and regulated by adherens junctions during the morphological movements of cells. Indeed, other MAGUK proteins have been shown to bind to actin/spectrin-binding protein 4.1 family members or complex with cortactin through GKAP and Shank (Cohen et al., 1998; Jons et al., 1999; Marfatia et al., 1994; Marfatia et al., 1996; Naisbitt et al., 1999). Our results show that a MAGUK protein, DLG-1, is required for the proper organization of the actin cytoskeleton, and raise the possibility that the binding observed between MAGUK proteins and actin-binding proteins is important for cytoskeletal organization in other systems.
One simple explanation for the dlg-1(RNAi) phenotype could be that DLG-1 is required to recruit HMR-1, HMP-1, and HMP-2 to adherens junctions. Our results show that localization of α-catenin HMP-1, which requires HMR-1 and HMP-2, does not seem to require DLG-1 (Figure 4). Conversely, DLG-1 localization to adherens junctions does not require HMR-1, HMP-1, or HMP-2 (Figure 4). Could DLG-1 function redundantly with the cadherin-catenin system? Double mutant embryos of dlg-1(RNAi) with hmr-1, hmp-1, or hmp-2 arrest in development at the twofold stage and are indistinguishable from dlg-1(RNAi) single mutants; thus, DLG-1 and the cadherin-catenin system do not appear to be working synergistically, at least based on the current morphological and molecular markers available (our unpublished results). Rather, both DLG-1 and the cadherin-catenin system appear to be essential but independent components of epithelial adherens junctions.
We have sequenced the complete DLG-1 cDNA and have found that DLG-1 is most similar to SAP97, a MAGUK found at synaptic connections in the brain and cellular junctions of epithelia. In particular, we have found that the amino termini of DLG-1 and SAP97 are conserved and appear to play a pivotal role in directing DLG-1 localization to adherens junctions. This is in contrast to Drosophila DLG, which is localized to septate junctions through its second PDZ domain and a unique HOOK region found between the SH3 and GK domains (Thomas et al., 2000). The amino-terminus of other MAGUKs have also been found to act as subcellular localization signals; for example, the amino-terminus of PSD-95 is palmitoylated to allow its membrane association(Topinka and Bredt, 1998). Presumably by dedicating a unique localization domain independent of the other protein-protein interactions domains, MAGUKs can facilitate their own localization while maintaining the maximum number of valences for other binding partners.
It is currently unclear how the amino-terminus of DLG-1 and SAP97 direct their localization within cells. One possibility would be an interaction with elements of the actin cytoskeleton. However, mutants for hmr-1, hmp-1, and hmp-2 have severely disorganized actin networks but are able to localize DLG-1 to adherens junctions, making any role for actin in DLG-1 localization unclear at best. Presumably, further in vivo analysis of DLG-1 localization will shed light on this issue.
ACKNOWLEDGMENTS
We thank J. Hardin, M. Labouesse, A. Fire, Y. Kohara, Y. Tabuse, C. Mello, and T. Stiernagle/C. elegans Genetics Center for providing plasmids, antibodies, and strains. We thank the C. elegans genome sequencing consortium for providing sequence. We also thank V. Starovoytov and R. Triemer for assistance with the EM analysis. We are grateful to M. Driscoll for critical comments on the manuscript.
REFERENCES
- Bargmann CI. Neurobiology of the Caenorhabditis elegans genome. Science. 1998;282:2028–2033. doi: 10.1126/science.282.5396.2028. [DOI] [PubMed] [Google Scholar]
- Bossinger O, Klebes A, Segbert C, Theres C, Knust E. Zonula adherens formation in Caenorhabditis elegans requires dlg-1, the homologue of the Drosophila gene discs large. Dev Biol. 2001;230:29–42. doi: 10.1006/dbio.2000.0113. [DOI] [PubMed] [Google Scholar]
- Brenman JE, Chao DS, Gee SH, McGee AW, Craven SE, Santillano DR, Wu Z, Huang F, Xia H, Peters MF, Froehner SC, Bredt DS. Interaction of nitric oxide synthase with the postsynaptic density protein PSD-95 and alpha1-syntrophin mediated by PDZ domains. Cell. 1996;84:757–767. doi: 10.1016/s0092-8674(00)81053-3. [DOI] [PubMed] [Google Scholar]
- Cho K-O, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- Cohen AR, Woods DF, Marfatia SM, Walther Z, Chishti AH, Anderson JM, Wood DF. Human CASK/LIN-2 binds syndecan-2 and protein 4.1 and localizes to the basolateral membrane of epithelial cells. J Cell Biol. 1998;142:129–138. doi: 10.1083/jcb.142.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Draper BW, Priess JR. The role of actin filaments in patterning the Caenorhabditis elegans cuticle. Dev Biol. 1997;184:373–384. doi: 10.1006/dbio.1997.8530. [DOI] [PubMed] [Google Scholar]
- Costa M, Raich W, Agbunag C, Leung B, Hardin J, Priess JR. A putative catenin-cadherin system mediates morphogenesis of the Caenorhabditis elegans embryo. J Cell Biol. 1998;141:297–308. doi: 10.1083/jcb.141.1.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven SE, El-Husseini AE, Bredt DS. Synaptic targeting of the postsynaptic density protein PSD-95 mediated by lipid and protein motifs. Neuron. 1999;22:497–509. doi: 10.1016/s0896-6273(00)80705-9. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Lee A, Lewis J, Kim E, Sheng M, MacKinnon R. Crystal structure of a complexed and peptide-free membrane protein-binding domain: molecular basis of peptide recognition by PDZ. Cell. 1996;85:1067–1076. doi: 10.1016/s0092-8674(00)81307-0. [DOI] [PubMed] [Google Scholar]
- El-Husseini AE, Craven SE, Chetkovich DM, Firestein BL, Schnell E, Aoki C, Bredt DS. Dual palmitoylation of PSD-95 mediates its vesiculotubular sorting, postsynaptic targeting, and ion channel clustering. J Cell Biol. 2000a;148:159–172. doi: 10.1083/jcb.148.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Husseini AE, Topinka JR, Lehrer-Graiwer JE, Firestein BL, Craven SE, Aoki C, Bredt DS. Ion channel clustering by membrane-associated guanylate kinases. Differential regulation by N-terminal lipid and metal binding motifs. J Biol Chem. 2000b;275:23904–23910. doi: 10.1074/jbc.M909919199. [DOI] [PubMed] [Google Scholar]
- Finney M, Ruvkun G. The unc-86 gene product couples cell lineage and cell identity in C. elegans. Cell. 1990;63:895–905. doi: 10.1016/0092-8674(90)90493-x. [DOI] [PubMed] [Google Scholar]
- Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- Firestein BL, Brenman JE, Aoki C, Sanchez-Perez AM, El-Husseini AE, Bredt DS. Cypin: a cytosolic regulator of PSD-95 postsynaptic targeting. Neuron. 1999;24:659–672. doi: 10.1016/s0896-6273(00)81120-4. [DOI] [PubMed] [Google Scholar]
- Firestein BL, Craven SE, Bredt DS. Postsynaptic targeting of MAGUKs mediated by distinct N-terminal domains. Neuroreport. 2000;11:3479–3484. doi: 10.1097/00001756-200011090-00016. [DOI] [PubMed] [Google Scholar]
- Gumbiner B. Structure, biochemistry, and assembly of epithelial tight junctions. Am J Physiol. 1987;253:C749–758. doi: 10.1152/ajpcell.1987.253.6.C749. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Jons T, Heim HK, Kistner U, Ahnert-Hilger G. SAP 97 is a potential candidate for basolateral fixation of ezrin in parietal cells. Histochem Cell Biol. 1999;111:313–318. doi: 10.1007/s004180050362. [DOI] [PubMed] [Google Scholar]
- Kaech SM, Whitfield CW, Kim SK. The LIN-2/LIN-7/LIN-10 complex mediates basolateral membrane localization of the C. elegans EGF receptor LET-23 in vulval epithelial cells. Cell. 1998;94:761–771. doi: 10.1016/s0092-8674(00)81735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamath RS, Martinez-Campos M, Zipperlen P, Fraser AG, Ahringer J. Effectiveness of specific RNA-mediated interference through ingested double-stranded RNA in Caenorhabditis elegans. Genome Biol. 2000;2:1–10. doi: 10.1186/gb-2000-2-1-research0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Cho K, Rothschild A, Sheng M. Heteromultimerization and NMDA receptor-clustering activity of Chapsyn-110, a member of the PSD-95 family of proteins. Neuron. 1996;17:103–113. doi: 10.1016/s0896-6273(00)80284-6. [DOI] [PubMed] [Google Scholar]
- Kim E, Niethammer M, Rothschild A, Jan YN, Sheng M. Clustering of Shaker-type K+ channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- Kistner U, Wenzel BM, Veh RW, Cases-Langhoff C, Garner AM, Appeltauer U, Voss B, Gundelfinger ED, Garner CC. SAP90, a rat presynaptic protein related to the product of the Drosophila tumor suppressor gene. dlg-A. J Biol Chem. 1993;268:4580–4583. [PubMed] [Google Scholar]
- Kohara Y. Large scale analysis of C. elegans cDNA. Tanpakushitsu Kakusan Koso. 1996;41:715–720. [PubMed] [Google Scholar]
- Köppen, M., Simske, J.S., Sims, P.A., Firestein, B.L., Hall, D.H., Radice, A.D., Rongo, C., and Hardin, J.D. (2001). Cooperative regulation of AJM-1 by Discs large and LET-413 controls junctional tightness of Caenorhabditis elegans epithelia. Nat. Cell Biol. (in press). [DOI] [PubMed]
- Kornau H-C, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- Kornau HC, Seeburg PH, Kennedy MB. Interaction of ion channels and receptors with PDZ domain proteins. Curr Opin Neurobiol. 1997;7:368–373. doi: 10.1016/s0959-4388(97)80064-5. [DOI] [PubMed] [Google Scholar]
- Legouis R, Gansmuller A, Sookhareea S, Bosher JM, Baillie DL, Labouesse M. LET-413 is a basolateral protein required for the assembly of adherens junctions in Caenorhabditis elegans. Nat Cell Biol. 2000;2:415–422. doi: 10.1038/35017046. [DOI] [PubMed] [Google Scholar]
- Marfatia SM, Lue RA, Branton D, Chishti AH. In vitro binding studies suggest a membrane-associated complex between erythroid p55, protein 4.1, and glycophorin C. J Biol Chem. 1994;269:8631–8634. [PubMed] [Google Scholar]
- Marfatia SM, Morais Cabral JH, Lin L, Hough C, Bryant PJ, Stolz L, Chishti AH. Modular organization of the P.D.Z. domains in the human discs-large protein suggests a mechanism for coupling P.D.Z. domain-binding proteins to A.T.P. and the membrane cytoskeleton. J Cell Biol. 1996;135:753–766. doi: 10.1083/jcb.135.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitic LL, Anderson JM. Molecular architecture of tight junctions. Annu Rev Physiol. 1998;60:121–142. doi: 10.1146/annurev.physiol.60.1.121. [DOI] [PubMed] [Google Scholar]
- Mohler WA, Simske JS, Williams-Masson EM, Hardin JD, White JG. Dynamics and ultrastructure of developmental cell fusions in the Caenorhabditis elegans hypodermis. Curr Biol. 1998;8:1087–1090. doi: 10.1016/s0960-9822(98)70447-6. [DOI] [PubMed] [Google Scholar]
- Muller BM, Kistner U, Kindler S, Chung WJ, Kuhlendahl S, Fenster SD, Lau LF, Veh RW, Huganir RL, Gundelfinger ED, Garner CC. SAP102, a novel postsynaptic protein that interacts with NMDA receptor complexes in vivo. Neuron. 1996;17:255–265. doi: 10.1016/s0896-6273(00)80157-9. [DOI] [PubMed] [Google Scholar]
- Muller BM, Kistner U, Veh RW, Cases-Langhoff C, Becker B, Gundelfinger ED, Garner CC. Molecular characterization and spatial distribution of SAP97, a novel presynaptic protein homologous to SAP90 and the Drosophila discs-large tumor suppressor protein. J Neurosci. 1995;15:2354–2366. doi: 10.1523/JNEUROSCI.15-03-02354.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller HA. Genetic control of epithelial cell polarity: lessons from Drosophila. Dev Dyn. 2000;218:52–67. doi: 10.1002/(SICI)1097-0177(200005)218:1<52::AID-DVDY5>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Naisbitt S, Kim E, Tu JC, Xiao B, Sala C, Valtschanoff J, Weinberg RJ, Worley PF, Sheng M. Shank, a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin. Neuron. 1999;23:569–582. doi: 10.1016/s0896-6273(00)80809-0. [DOI] [PubMed] [Google Scholar]
- Podbilewicz B, White JG. Cell fusions in the developing epithelial of C. elegans. Dev Biol. 1994;161:408–424. doi: 10.1006/dbio.1994.1041. [DOI] [PubMed] [Google Scholar]
- Powell DW. Barrier function of epithelia. Am J Physiol. 1981;241:G275–288. doi: 10.1152/ajpgi.1981.241.4.G275. [DOI] [PubMed] [Google Scholar]
- Priess JR, Hirsh DI. Caenorhabditis elegans morphogenesis: the role of the cytoskeleton in elongation of the embryo. Dev Biol. 1986;117:156–173. doi: 10.1016/0012-1606(86)90358-1. [DOI] [PubMed] [Google Scholar]
- Raich WB, Agbunag C, Hardin J. Rapid epithelial-sheet sealing in the Caenorhabditis elegans embryo requires cadherin-dependent filopodial priming. Curr Biol. 1999;9:1139–1146. doi: 10.1016/S0960-9822(00)80015-9. [DOI] [PubMed] [Google Scholar]
- Rongo C, Whitfield CW, Rodal A, Kim SK, Kaplan JM. LIN-10 is a shared component of the polarized protein localization pathways in neurons and epithelia. Cell. 1998;94:751–759. doi: 10.1016/s0092-8674(00)81734-1. [DOI] [PubMed] [Google Scholar]
- Songyang Z, Fanning AS, Fu C, Xu J, Marfatia SM, Chishti AH, Crompton A, Chan AC, Anderson JM, Cantley LC. Recognition of unique carboxyl-terminal motifs by distinct PDZ domains. Science. 1997;275:73–77. doi: 10.1126/science.275.5296.73. [DOI] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Tabuse Y, Izumi Y, Piano F, Kemphues KJ, Miwa J, Ohno S. Atypical protein kinase C cooperates with PAR-3 to establish embryonic polarity in Caenorhabditis elegans. Development. 1998;125:3607–3614. doi: 10.1242/dev.125.18.3607. [DOI] [PubMed] [Google Scholar]
- Thomas U, Ebitsch S, Gorczyca M, Koh YH, Hough CD, Woods D, Gundelfinger ED, Budnik V. Synaptic targeting and localization of discs-large is a stepwise process controlled by different domains of the protein. Curr Biol. 2000;10:1108–1117. doi: 10.1016/s0960-9822(00)00696-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmons L, Fire A. Specific interference by ingested dsRNA. Nature. 1998;395:854. doi: 10.1038/27579. [DOI] [PubMed] [Google Scholar]
- Topinka JR, Bredt DS. N-terminal palmitoylation of PSD-95 regulates association with cell membranes and interaction with K+ channel Kv1.4. Neuron. 1998;20:125–134. doi: 10.1016/s0896-6273(00)80440-7. [DOI] [PubMed] [Google Scholar]
- van Meer G, Gumbiner B, Simons K. The tight junction does not allow lipid molecules to diffuse from one epithelial cell to the next. Nature. 1986;322:639–641. doi: 10.1038/322639a0. [DOI] [PubMed] [Google Scholar]
- van Meer G, Simons K. The function of tight junctions in maintaining differences in lipid composition between the apical and the basolateral cell surface domains of MDCK cells. EMBO J. 1986;5:1455–1464. doi: 10.1002/j.1460-2075.1986.tb04382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasioukhin V, Fuchs E. Actin dynamics and cell-cell adhesion in epithelia. Curr Opin Cell Biol. 2001;13:76–84. doi: 10.1016/s0955-0674(00)00177-0. [DOI] [PubMed] [Google Scholar]
- Whitfield CW, Benard C, Barnes T, Hekimi S, Kim SK. Basolateral localization of the Caenorhabditis elegans epidermal growth factor receptor in epithelial cells by the PDZ protein LIN-10. Mol Biol Cell. 1999;10:2087–2100. doi: 10.1091/mbc.10.6.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams BD, Waterston RH. Genes critical for muscle development and function in Caenorhabditis elegans identified through lethal mutations. J Cell Biol. 1994;124:475–490. doi: 10.1083/jcb.124.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Masson EM, Malik AN, Hardin J. An actin-mediated two-step mechanism is required for ventral enclosure of the C. elegans hypodermis. Development. 1997;124:2889–2901. doi: 10.1242/dev.124.15.2889. [DOI] [PubMed] [Google Scholar]
- Wood WB. The nematode C. elegans. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Wu H, Reuver SM, Kuhlendahl S, Chung WJ, Garner CC. Subcellular targeting and cytoskeletal attachment of SAP97 to the epithelial lateral membrane. J Cell Sci. 1998a;111:2365–2376. doi: 10.1242/jcs.111.16.2365. [DOI] [PubMed] [Google Scholar]
- Wu SL, Staudinger J, Olson EN, Rubin CS. Structure, expression, and properties of an atypical protein kinase C (PKC3) from Caenorhabditis elegans. PKC3 is required for the normal progression of embryogenesis and viability of the organism. J Biol Chem. 1998b;273:1130–1143. doi: 10.1074/jbc.273.2.1130. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Atchison WD. Comparative effects of methylmercury on parallel-fiber and climbing- fiber responses of rat cerebellar slices. J Pharmacol Exp Ther. 1999;288:1015–1025. [PubMed] [Google Scholar]