Abstract
Chelation complexes of the histidine-containing tripeptides HisAlaAla, AlaHisAla, and AlaAlaHis with Ni(II) and Cu(II) having a −1 net charge are characterized in the gas phase by infrared multiple-photon dissociation (IRMPD) spectroscopy and density functional theory calculations. We address the question of whether the gas-phase complexes carry over characteristics from the corresponding condensed-phase species. We focus particularly on three aspects of their structure: (i) square-planar chelation by the deprotonated amide nitrogens around the metal ion (low-spin for the Ni case), (ii) metal-ion coordination of the imidazole side chain nitrogen, and (iii) the exceptional preference for metal-ion chelation by peptides with His in the third position from the N-terminus, as in the amino terminal Cu and Ni (ATCUN) motif. We find that square-planar binding around the metal ion, involving bonds to both deprotonated backbone nitrogens, one of the carboxylate oxygens and the N-terminal nitrogen, is the dominant binding motif for all three isomers. In contrast to the condensed-phase behavior, the dominant mode of binding for all three isomers does not involve the imidazole side chain, which is instead placed outside the coordination zone. Only for the AlaAlaHis isomer, the imidazole-bound structure is also detected as a minority population, as identified from a distinctive short-wavelength IR absorption. The observation that this conformation exists only for AlaAlaHis correlates with condensed-phase behavior at neutral-to-basic pH, in the sense that the isomer with His in the third position is exceptionally disposed to metal ion chelation by four nitrogen atoms (4N) when compared with the other isomers. These results also emphasize the divergence between the conformational stabilities in the gas phase and in solution or crystalline environments: in the gas phase, direct metal binding of the imidazole is overall less favorable than the alternative of a remote imidazole that can act as an intramolecular H-bond donor enhancing the gas-phase stability.
Introduction
A principal question in the binding of metal ions to histidine-containing peptides is whether the binding does or does not involve direct metal-to-imidazole bonding. It is generally thought that the binding of copper, in particular in proteins, is largely related to histidine residues.1 Indeed, the binding of both Ni(II) and Cu(II) with histidine-containing peptides in the condensed phase is often (although not necessarily) associated with metal coordination to the imidazole side chain. It is of interest to consider whether the presence of histidine in gas-phase peptides also favors coordination to the imidazole moiety and, to the extent that this is the case, what the favorable configurations are.
For the condensed phases, there is extensive literature describing the binding of divalent transition metal ions to peptides and the successive degrees of deprotonation of the backbone amide nitrogens with concomitant binding of these sites to the metal ion, progressing as a function of increasing pH.2−10 The ability in electrospray ionization mass spectrometry (ESI-MS) to access corresponding gas-phase metal- ion–peptide complexes by explicit deprotonation at each chosen step along this progression—by MS selection of a specific charge state of the complex—enables us to investigate these systems in complete isolation. This is of interest because complexation is not encumbered by interactions with solvent molecules or a protein matrix. In the gas phase, the stage of triply deprotonated ligands complexed to divalent metal ions corresponds to an overall monoanionic species. For tripeptide ligands, these monoanionic complexes involve nearly obligatory deprotonation and metal-ion binding of the two amide nitrogens. Since this degree of backbone deprotonation is also normal for condensed-phase peptide complexation by Ni(II) and Cu(II), the present study of complexes in the monoanion charge state gives a particularly apt set of comparisons between condensed-phase and gas-phase structural features. The purpose is to assign the structures of the anionic gas-phase complexes and to compare these with their condensed-phase counterparts, as well as with previously reported nondeprotonated chelation complexes, with an overall positive charge.11−13 The discussion will focus on comparison of two modes of binding, differing primarily in whether the imidazole side chain is or is not bound to the metal ion, as is exemplified in Scheme 1 for the CuAAH complex (we shall use this simplified notation to refer to monoanionic complexes of the [Cu2+AlaAlaHis–3H]− type throughout this paper).
Scheme 1. Two Principal Binding Patterns Illustrated for the Anionic Complex of Cu(II) and AlaAlaHis.
Cu(II) and Ni(II) binding patterns and geometries to monohistidine peptides or histidine binding regions in peptides and proteins have been widely studied in the condensed phases, generating a large body of literature. Although we will not attempt to provide a comprehensive review, a few representative publications can be noted.5−8,14−23 The investigation of deprotonated peptide binding to metal ions, in particular Cu(II), has recently been stimulated by the strong binding that is characteristic of this ion to one or more of the four monohistidine regions in the “octarepeat” region of the prion protein PrP, as well as additional PrP sites outside this region.6,7,17,24 Prion proteins and their fragments are widely discussed as providing a variety of copper binding possibilities, including a 3N pattern with an additional water ligand at the fourth coordination site7 and a 4N pattern.17,25 Other copper-binding proteins of interest involving interaction of the metal ion with a histidine residue include albumin,15 Hpn protein,6,26 α-synuclein,17 and the amyloid-β peptide.7
One reason in particular for comparing spectra of all three of the sequence isomers in this study is to investigate whether the gas phase provides any evidence for the exceptional stability of complexes with histidine in the third position from the N-terminus. For Cu and Ni, such complexes have become well-known as the ATCUN (amino terminal Cu(II)- and Ni(II)) 4N-caged structure [NNNR], which is illustrated in Scheme 1 and continues to be the theme of many studies in a number of variations.1,3,6,15,16,22,23,27−34 This pattern was originally identified in human serum albumin, but it has been found to recur in a wide variety of proteins.1,6,32,35 An unexpected realization from the present results is that this 4N structure is not the most favorable structure for gas-phase CuAAH complexes. However, we show that this 4N structure does appear as a minor contribution, whereas it does not occur at all for gas-phase complexes with His in the first or second position.
In the gas phase, the coordination structures of the histidine amino acid with alkali metal cations36,37 and with Cd(II) and Zn(II), as singly deprotonated complexes,38 have been reported. In both Cd(II) and Zn(II) ground state complexes, His deprotonates at the carboxyl group, and a tridentate structure is formed involving metal ion binding at the imidazole N1 site.38 Complexes of Cu(II) with two His amino acid ligands have been investigated with IRMPD spectroscopy.39 Two gas-phase studies have investigated structures of histidine-containing dipeptide complexes, finding that iminol-type binding for Ni(II) is preferred over charge-solvation binding.11,40 For the dicationic (undeprotonated) complexes of HisGly with a number of metal ions including Ni(II), the imidazole nitrogen was found to be bound to the metal ion.11 Similarly, the monocationic (singly deprotonated) state of HisHis with Ca(II) and Ni(II), showed that the metal ion is chelated by both imidazoles along with the deprotonated amide nitrogen.40 The anionic NiHAA– complex was posed as an example illustrating the role of explicit deprotonation in promoting the switch from charge-solvation binding to iminol binding.12
In our previous report of the dicationic charge state of the same set of complexes,13 attention was directed at the question of how many of the backbone amide nitrogens were bound to the metal ion. In the monoanionic, triply deprotonated, charge state of interest here, this uncertainty is greatly reduced, since only one labile proton remains and the range of choices for its location are strongly constrained. All low-energy structures involve deprotonation and metal-coordination of both backbone amide nitrogens, so that the results are directly comparable to condensed-phase observations of complexes at the stage of double backbone deprotonation (pH > 6)3.
Experiments and Calculations
Nomenclature
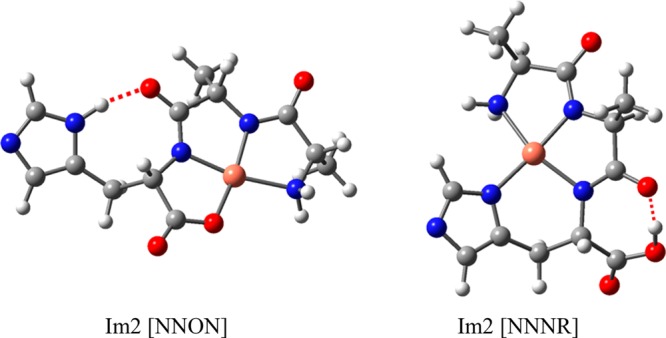
Coordination of the metal ion by an amide peptide linkage can occur through the carbonyl oxygen, which we refer to as charge-solvation (CS), or through a deprotonated nitrogen, which we refer to as iminol (Im). Consistent with our nomenclature for the cationic species,13 we use the following naming conventions: The format is for example Im2 [NNNR] or Im1CS1 [NOOR]. The prefix denotes the number of coordinate bonds with deprotonated amide nitrogens (Im) and with amide carbonyl oxygens (CS). All metal coordination points are then listed in square brackets following a fixed order: First are the deprotonated iminol amide nitrogens (N); next are the amide carbonyl oxygens (O); next is the C-terminal carboxylate or carboxyl oxygen (O); next is the N-terminal amino nitrogen (N); finally, the deprotonated imidazole ring nitrogen is listed with the symbol R.
Experimental Section
IR spectra of the gaseous metal-ion complexes in the 1000–1800 cm–1 spectral range were obtained using a modified quadrupole ion trap (QIT) mass spectrometer (Bruker, Amazon Speed ETD)41,42 coupled to the free electron laser for infrared experiments (FELIX).
Singly charged anionic metal-ion peptide complexes of copper and nickel with AAH, AHA, and HAA were generated by ESI from a solution containing ∼10–6 M of the peptide and metal nitrate salt in acetonitrile/H2O (4:1). Target ions were trapped and mass-selected before being irradiated by the wavelength-tunable infrared light from FELIX. A mass spectrum is recorded at each wavelength point and the extent of dissociation is determined as a yield, the summed intensity of all fragment ions ratioed by the intensity of all fragment plus precursor ions. The complexes showed rich IR induced dissociation mass spectra, with the main fragments corresponding to small neutral losses (H2O, CO2) as well as some sequence ions. A plot of this yield as a function of laser frequency is interpreted as the IR spectrum of the complex. DFT computed linear IR spectra of candidate ion structures were compared with the observed IRMPD spectra, with the calculated relative energetics providing additional guidance, to assign conformational and tautomeric structures.
Quantum-Chemical Calculations
All calculations were carried out using the Gaussian09 quantum chemistry package.43 The density functional theory (DFT) level used for initial calculations was B3LYP/6-31+g(d,p), but energies and spectra for all species within about 50 kJ mol–1 of the lowest-energy structure were optimized and computed with the 6-311++g(d,p) basis set, which never gave significant differences. Calculated electronic energies as well as free energies with corrections for zero-point energies and thermal and entropy effects at 300 K are given in Table 1 with the corresponding conformational structures displayed in Figures S1–S3 of the Supporting Information. Corrections to the simple zero-K energies were small in most cases and made no difference in the relative ordering of structures and hence to our interpretations. For weakly bound neutral/neutral complexes, there has been concern about the adequacy of B3LYP calculations to accurately determine the contribution of dispersion to the binding energy,44 but for ionic complexes like the present systems the binding is so strong compared with dispersion forces that such concerns were assumed to be insignificant.
Table 1. Relative Energies (in kJ/mol) for Different Complexes of Three His-Containing Tripeptides with Cu2+ and Ni2+ with an Overall 1– Chargea.
| peptide/conformer | Ni2+ | Cu2+ |
|---|---|---|
| AAH | ||
| Im2[NNON]a | 0 (0) | 0 (0) |
| Im2[NNON]b | 2 (2) | 3 (4) |
| Im2[NNNR] | 26 (29) | 13 (12) |
| Im2[NNON(R)] | 56 (52) | |
| Im1CS1[NOOR] | 159 (153) | |
| AHA | ||
| Im2[NNON]a | 0 (0) | 0 (1) |
| Im2[NNON]b | 5 (5) | 2 (0) |
| Im2[NNON(R)] | 56 (46) | 73 (69) |
| Im1CS1[NOOR] | 74 (70) | 83 (83) |
| HAA | ||
| Im2[NNON]a | 0 (0) | 0 (0) |
| Im2[NNON]b | 2 (2) | 31 (28) |
| Im2[NNOR] | 59 (59) | 36 (39) |
| Im1CS1[NOOR] | 109 (102) | 60 (68) |
| Im2[NNON(R)] | 114 (98) | b |
Values in parentheses are free energies (harmonic vibrations, free rotors) at 300 K.
Converges to Im2[NNON]b.
For comparison of DFT computed spectra to IRMPD spectra, the calculated frequencies were scaled by a factor of 0.975 (0.965 for the smaller basis set) in the fingerprint region (1000–1900 cm–1), which experience suggests to be appropriate at these levels of theory.45 Computed spectra were convoluted with a 20 cm–1 fwhm Gaussian line-shape function.
The conformations to be considered were developed by trial computation of structures known to be chemically reasonable. Not many conformations are possible which bind four or more Lewis-basic chelation sites to the metal ion for these highly deprotonated ligands, so manual searching was feasible. Alternative low energy structures are shown in the Supporting Information, Figures S1–S3.
Results and Discussion
Constraints on Ligand Deprotonation and Chelation Patterns
As the spectroscopic implications for the structures of these complexes are discussed below, it is important to have in mind the choices and constraints imposed by the removal of three protons to form the monoanionic complexes. The simplest case is GGG (or AAA) which has four potential metal-chelation points (the COOH, amino-N and two amide linkages) and three ionizable protons (COOH and two amide protons). Each amide linkage gives one ionizable proton and also gives one chelation site being either the amide carbonyl oxygen or the amide-nitrogen after iminol rearrangement (and deprotonation). The N-terminal amino nitrogen affords an additional binding site, but no ionizable proton. To form a four-coordinate complex of net charge −1 with a + 2 charge metal ion requires removing all three protons, and binding all four chelation points. This leaves no degrees of freedom, and the binding is an obligatory Im2 [NNON] pattern. As a useful reference point for identifying the [NNON] spectroscopic features in looking below at the spectra of the histidine tripeptides, we include in the figures the assigned spectrum of the previously reported12 anionic complex NiAAA. The geometry of this complex is essentially the same as that for the solution-phase Cu(II) complex of GGG, shown as structure 25 in ref (4), for example.
An additional (fifth) chelation point, along with an additional (fourth) ionizable proton, results from extending the peptide chain to AAAA, or as in the present case, from substituting a histidine residue into AAA, providing an additional ionizable proton on the imidazole moiety. Still within the requirements of removing three protons to give a −1 complex with a +2 metal ion, several possibilities now appear for three [H,A,A] isomers, since the remaining proton can potentially occupy one of the chelation sites. Questions of structure regarding these complexes thus revolve around the question whether the imidazole side chain does in fact ionize its proton with accompanying side-chain binding to the metal ion; if so, which of the other four chelation sites bind the metal ion and where does the free proton go?
Overview of the Spectra
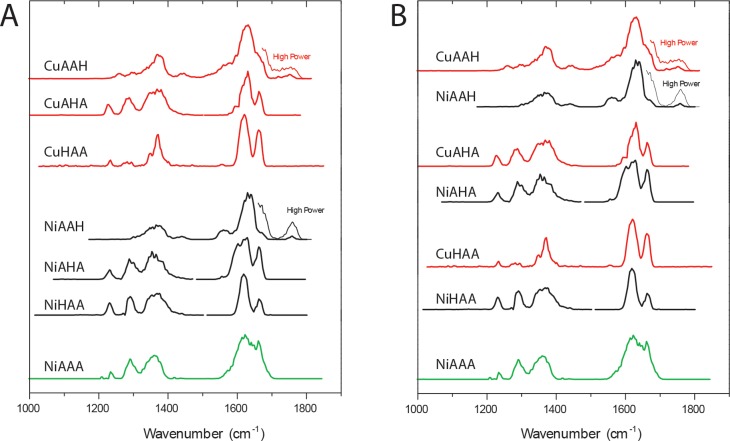
The experimental spectra are displayed in Figure 1, grouped both by ligand isomer and by metal ion. The features expected for the tripeptides in general can be highlighted by comparison with the NiAAA anion spectrum displayed at the bottom. Both displays suggest that the spectra for the complexes with Cu and Ni are similar, and these two metal ions will be analyzed together in much of the discussion. Figure 1B also suggests close similarity of the spectra for a given ligand isomer. A key feature that both spectra of the AAH complexes have in common is a small but reproducible peak near 1750 cm–1, which is absent for the HAA and AHA isomers (and also for the NiAAA reference spectrum). Moreover, the AHA spectra appear similar to the corresponding HAA spectra.
Figure 1.
(A) Experimental spectra of the monoanionic complexes of Ni(II) and Cu(II) with AAH, AHA, and HAA ligands. Spectra for copper complexes are displayed in red, those for nickel complexes in black, and that for NiAAA in green. (B) Same spectra as in part A, regrouped by metal ion.
Structure Assignments from the IR Spectra
More incisive structural analysis is based on comparison with calculated spectra. In Figure 2, the best-fitting computationally predicted spectra are displayed in green, overlaid on the experimental spectra. These best matches are selected from an extensive set of computed spectra for possible conformations displayed in Figures S4–S9 in the Supporting Information. Note that for CuAAH, NiAAH, CuAHA and NiAHA the best-match computed spectrum is a 50/50 average of two Im2 [NNON] isomers having similar calculated energies, see Figures S1 and S2; these conformers correspond to the lowest energy structures identified (see Table 1). Moreover, for CuAAH and NiAAH, a contribution of 25% has been added in for the higher-energy Im2 [NNNR] conformer shown in Figure S1. This fraction is solely based on a very qualitative matching of spectral intensities and does not (necessarily) correspond to actual fractional populations.
Figure 2.
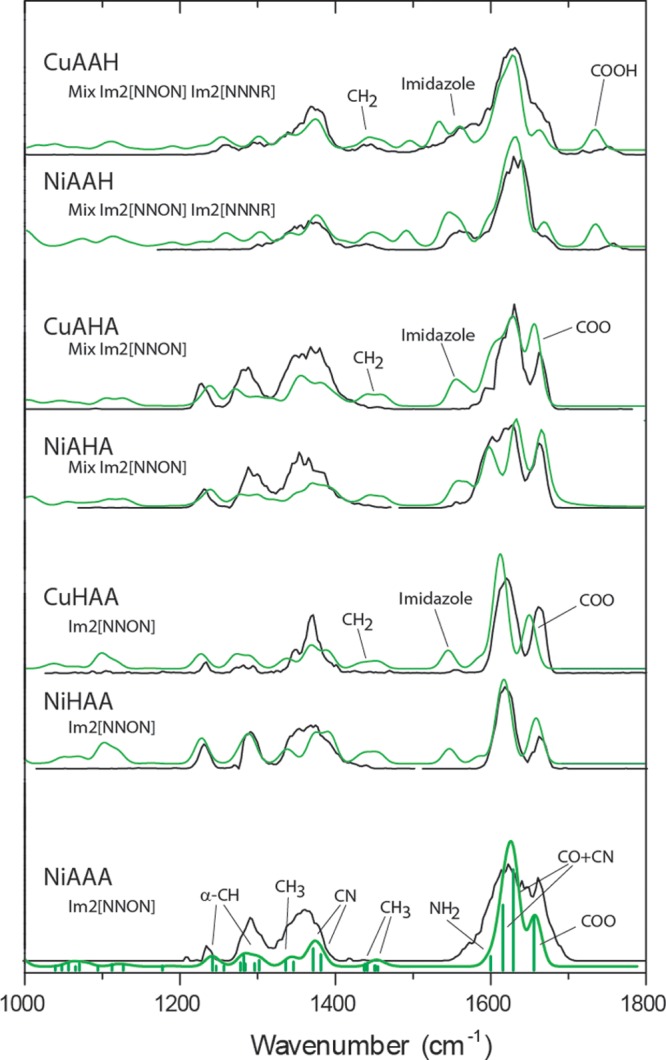
Comparison of calculated spectra (green) with experimental spectra (black) for the six complexes of Cu and Ni with each of the three [A,A,H] isomeric ligands. The COOH feature near 1750 cm–1 in the AAH complexes is assigned to the minority Im2 [NNNR] fraction of the ions, while the shoulder near 1660 cm–1 in the COO region of these two spectra is assigned to the majority Im2 [NNON] fraction. Main vibrational mode characters are indicated in the bottom spectrum (for AAA); for the His-containing ligands the mode characters are largely analogous to those in AAA and only where deviating mode characters are found the bands are labeled in the top three spectra.
The first conclusion is that the lowest-energy complex for all three ligand isomers does not have the imidazole moiety bound to the metal ion. This conclusion is supported by both the calculated relative energies and the spectroscopic data. Thus, the dominant structure for all six complexes in Figure 2 is the Im2 [NNON] structure, but we note that the spectra of the Cu and Ni complexes with AAH also definitely show an admixture of the Im2 [NNNR] structure having the imidazole ring bound to the metal ion.
For the HAA and AHA isomers, an Im2 [NNNR] structure having a square-planar 4N cage and a reasonable relative energy and geometry is not possible. Im2 [NNOR] structures with a metal coordinated imidazole are possible for the HAA isomer, but are about 40 to 60 kJ/mol higher in energy and their predicted spectra are poor matches to the observations (Figures S8 and S9).
However, the situation is different for AAH. Only for this isomer there is a thermochemically reasonable possibility of forming the ATCUN 4N-binding pattern (Im2 [NNNR] in our terminology or [NIm,2N–,NH2] in solution-phase terminology7). For both Cu and Ni, this is not the lowest-energy gas-phase structure (+13 and +26 kJ mol–1, respectively) and predicted spectra by themselves do not match the experimental spectra (Figures S4 and S5). However, both of these IRMPD spectra show a feature near 1750 cm–1, which we can attribute only to this structure, because of the unbound carboxyl CO stretching vibration. In contrast to the Im2 [NNON] conformers, the Im2 [NNNR] complexes are deprotonated at the imidazole ring instead of at the C-terminal COOH group. Observation of the 1750 cm–1 band is therefore strong evidence for a fraction of the population having these Im2 [NNNR] structures. A decrease in intensity in the 1200–1300 cm–1 range is also noted for AAH complexes with respect to AHA and HAA complexes.
The imidazole-coordinated conformations for AAH complexes are not expected to be thermally accessible (at least according to the B3LYP energies) and are probably kinetically trapped. The best-match calculated spectra shown in Figure 2 for both complexes (green traces) include a contribution of 25% from the Im2 [NNNR] 4N-structure, which gives a modest overall improvement in the fit and, most importantly, accounts for the short-wavelength feature. The distinctive 1750 cm–1 peak is not calculated at exactly the correct wavelength in either case: we attribute this to a previously noted deficiency of B3LYP vibrational calculations, which appear to consistently give slightly deviating values for high-frequency C=O stretching modes (>1700 cm–1) of unchelated C=O’s in for instance peptides and acetophenone.47
Previous work on complexes of the neutral peptides coordinated to the 2+ metal ion13 suggests the possibility of structures of the “charge-solvated” (CS) type having one or more amide linkages in the amido form with the carbonyl oxygen binding the metal. These previous results for the M(II)[H,A,A]2+ complexes indicated that CS binding of the metal ion was frequently observable along with Im-type binding. For the anionic complexes, CS2 configurations are not feasible, but Im1CS1 [NOOR] structures are potentially possible, though with rather high relative energies (Figures S1–S3). Neither the IR spectra nor the calculations suggest a likelihood of any CS2 or Im1CS1 contributions to these ion populations. Hence, we conclude that the extensive deprotonation of the present systems makes CS binding of the metal ion very unfavorable.
Correlation with Solution and Condensed Phases
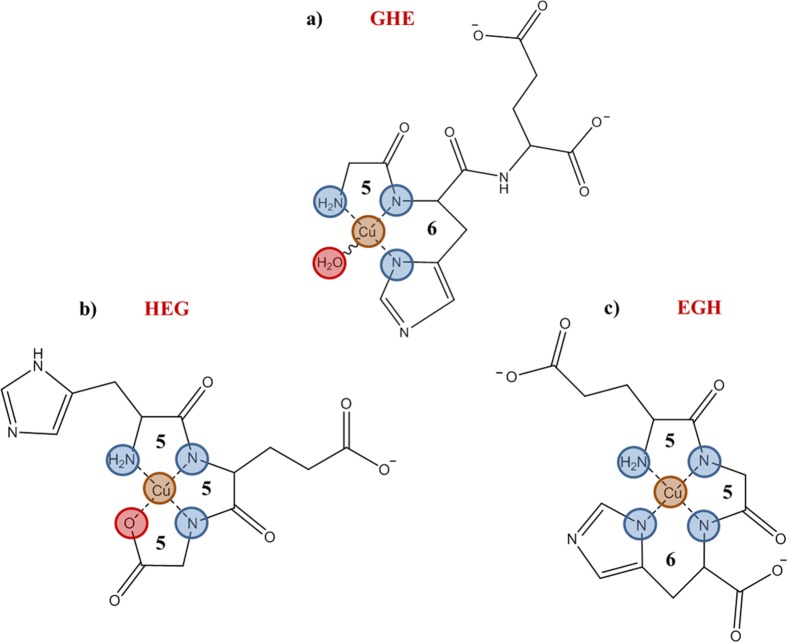
Our gas-phase experimental results in all six cases assign an Im2 [NNON] square-planar binding pattern with the imidazole side chain remote and hydrogen bonded, as the most energetically favored configuration. Unique to the AAH complexes, a minority presence of complexes with the Im2 [NNNR] binding motif has been established. How these results relate to condensed phase observations depends strongly on which of the three isomeric ligands is considered.7,18 Current condensed-phase understanding (see, for example, refs (6 and 7)) is exemplified in the recent report of Khoury et al.48 on Cu(II) complexes with three [H,E,G] isomers, with histidine occupying the three possible positions. For the purpose of comparison with our results, we can ignore the presence of the glutamic acid residue (E) in their study, since the Glu side chain in all three cases plays a spectator role, never coordinating to the metal ion. Their results can be considered a good representation of recent understanding of the histidine tripeptide complexes and the structure diagrams assigned in their study (reproduced here as Figure 3) provide an excellent framework for the present discussion.
Figure 3.
Solution-phase structures assigned by Khoury et al.48 for three [H,E,G] isomers coordinated to Cu(II). Note that the Glu residue is not coordinated to the Cu ion in any of the complexes and can be ignored in the present discussion. Reproduced with permission from ref (48). Copyright 2014 American Chemical Society.
1-His
In general the condensed-phase literature of binding to single-histidine peptide sequences considers the imidazole to be directly bound to the metal,7,16 but this is not necessarily the case for His positioned at an (unprotected) N-terminus as in HAA or HEG. Khoury et al. (see Figure 3b) assign a structure for CuHEG essentially similar to our Im2 [NNON] structure. Other examples of such observations can be noted: for the complex of the 1-His tetrapeptide HVGD with Cu(II), Myari et al.49 also report a structure analogous to our assigned Im2 [NNON] structure.
No planar 4N structure (Im2 [NNNR]) is feasible for 1-His tripeptides. With Ni2+ it is possible, but very unfavorable energetically (+114 kJ mol–1), to wrap the N-terminus around in a nonplanar Im2 [NNONR] complex (high-spin triplet), see Figure S3. However, this structure has not been observed in the present experiments nor has it been reported to our knowledge in the condensed-phase literature.
2-His
For tripeptides with His in the central position, it is essentially impossible for a 4-coordinate planar structure to have the imidazole and two deprotonated amide nitrogens bound simultaneously to the metal ion. In general,7,18,22,47 solution-phase structures retain the imidazole-metal bond, adopting a 3-coordinate peptide-binding pattern Im1 [NNR], involving only one deprotonated amide nitrogen site; a fourth external donor then coordinates to the metal center, see for example the structure proposed for GHE48 in Figure 3a and for GHG in ref (50). Addition of a second ligand to form a CuL2 complex is another possible route to finding sufficient ligation to satisfy the tetradentate Cu(II) requirement.18 In the gas phase with no H2O present, as with HAA, the favored structure takes a different course, giving up the imidazole coordination in order to achieve tetracoordinate binding via the Im2 [NNON] motif.
3-His
Tripeptides with C-terminal histidine, and in general peptides with histidine in the third position, chelating Cu(II) or Ni(II) ions have the immensely favorable Im2 [NNNR] square-planar 4N coordination available. This ATCUN configuration is so stable and dominant in condensed media that a surprise of the present study was finding that this is not the most stable, nor the most abundant, conformation for the gas-phase anionic complexes with AAH. Instead, this Im2 [NNNR] conformer is present in our ion population as a minority constituent, giving the small but reproducible vibrational band near 1750 cm–1.
Common to all the reported condensed-phase binding patterns in 3-His peptides is the direct binding of the imidazole ring to the metal. This highlights the interest of the present experimental and computational result that these three gas-phase tripeptides favor an [NNON] binding pattern having the imidazole ring remote from the metal ion. Hydrogen bonding between the imidazole ring and a carbonyl or carboxylate oxygen stabilizes this structure in the absence of water. However, the minority Im2 [NNNR] population is analogous to the common condensed-phase ATCUN configuration, as for instance the [NNNR] configuration for the CuGGH complex22,23 and the Cu2+DAHK complex15 (except that the loosely bound apical water in the X-ray structure as shown in Figure 2 of that paper is not present in the gas phase).
Conclusions
Compared with condensed-phase observations of deprotonated histidine-containing tripeptide systems, the gas phase reveals both parallels and contrasts. While the imidazole side chain is generally considered to enhance metal-ion binding, it is not true that a histidine residue inevitably coordinates to the metal ion, not even in solution. The present results show that the favored gas-phase structures avoid metal-imidazole binding for any of the three peptide isomers.
The chelation of both Cu and Ni ions in the −1 net-charge complexes follows the square-planar, four-coordinate low-spin pattern. In all cases, the dominant gas-phase structure is the Im2 [NNON] pattern, in which the metal ion is chelated by two deprotonated amide nitrogens, a carboxylate oxygen and the N-terminal nitrogen. The imidazole ring is remote from the metal ion, which deviates from the usual structures of histidine metal-complexing systems in condensed phase. For peptide ligands with His as the third residue (3-His), the 4N ATCUN chelation pattern Im2 [NNNR] with direct imidazole binding is highly favorable and is widely observed in condensed-phase systems. In our gas-phase results, however, this pattern is less stable than the alternative Im2 [NNON] and is observed as a minority constituent in the ion populations of the AAH complexes with Cu and Ni.
The present gas-phase triply deprotonated monoanionic complexes are shown to be model systems paralleling to some extent the solution-phase behavior in appropriate pH ranges. The contrast of the present systems, showing exclusive Cu and Ni binding in square planar Im binding patterns, with the corresponding dicationic complexes, which display competition between Im and CS binding modes,13 suggests the possibility of using explicit deprotonation in the gas phase to model pH variations in solution. Other charge states can model different degrees of deprotonation and this approach is being pursued in future studies. Finally, microhydration by water to fill vacant metal sites (as was investigated for NiGGG·H2O51) can be explored in gas-phase complexes like 2-His peptides, where peptide chelation alone does not saturate the square-planar sites in solution.
Acknowledgments
We wish to dedicate this manuscript to the memory of our collaborator, friend and mentor Robert C. Dunbar, who passed away on Oct. 31, 2017. We thank his wife Mary and sons Geoff and Bill for providing us with data files and lab notes, which were essential in preparing this manuscript. We gratefully acknowledge the Nederlandse Organisatie voor Wetenschappelijk Onderzoek (NWO) for the support of the FELIX Laboratory. Financial support for this project was provided by NWO Chemical Sciences under VICI Project No. 724.011.002. We thank SURFsara for their support in using the Lisa Compute Cluster and NWO Physical Sciences (EW) for access under Rekentijd Grant 16327.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jpca.8b02926.
The authors declare no competing financial interest.
Supplementary Material
References
- Neupane K. P.; Aldous A. R.; Kritzer J. A. Metal-Binding and Redox Properties of Substituted Linear and Cyclic ATCUN. J. Inorg. Biochem. 2014, 139, 65–76. 10.1016/j.jinorgbio.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garribba E.; Micera G. Complexation of Copper(II) Ion with Tetraglycine as Followed by Electronic Absorption Spectroscopy. A Bioinorganic Chemistry Experiment. J. Chem. Educ. 2007, 84, 832–835. 10.1021/ed084p832. [DOI] [Google Scholar]
- Kozlowski H.; Bal W.; Dyba M.; Kowalik-Jankowska T. Specific Structure-Stability Relations in Metallopeptides. Coord. Chem. Rev. 1999, 184, 319–346. 10.1016/S0010-8545(98)00261-6. [DOI] [Google Scholar]
- Sigel H.; Martin R. B. Coordinating Properties of the Amide Bond. Stability and Structure of Metal Ion Complexes of Peptides and Related Ligands. Chem. Rev. 1982, 82, 385–426. 10.1021/cr00050a003. [DOI] [Google Scholar]
- Kozlowski H.; Janicka-Klos A.; Stanczak P.; Valensin D.; Valensin G.; Kulon K. Specificity in the Cu2+ Interactions with Prion Protein Fragments and Related His-Rich Peptides from Mammals to Fishes. Coord. Chem. Rev. 2008, 252, 1069–1078. 10.1016/j.ccr.2007.08.006. [DOI] [Google Scholar]
- Kozlowski H.; Potocki S.; Remelli M.; Rowinska-Zyrek M.; Valensin D. Specific Metal Ion Binding Sites in Unstructured Regions of Proteins. Coord. Chem. Rev. 2013, 257, 2625–2638. 10.1016/j.ccr.2013.01.024. [DOI] [Google Scholar]
- Sovago I.; Varnagy K.; Lihi N.; Grenacs A. Coordinating Properties of Peptides Containing Histidyl Residues. Coord. Chem. Rev. 2016, 327–328, 43–54. 10.1016/j.ccr.2016.04.015. [DOI] [Google Scholar]
- Jakab N. I.; Gyurcsik B.; Koertvelyesi T.; Vosekalna I.; Jensen J.; Larsen E. Design of Histidine Containing Peptides for Better Understanding of Their Coordination Mode toward Copper(II) by CD Spectroscopy. J. Inorg. Biochem. 2007, 101, 1376–1385. 10.1016/j.jinorgbio.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Orfei M.; Alcaro M. C.; Marcon G.; Chelli M.; Ginanneschi M.; Kozlowski H.; Brasun J.; Messori L. Modeling of Copper(II) Sites in Proteins Based on Histidyl and Glycyl Residues. J. Inorg. Biochem. 2003, 97, 299–307. 10.1016/S0162-0134(03)00283-6. [DOI] [PubMed] [Google Scholar]
- Sovago I.; Sanna D.; Dessi A.; Varnagy K.; Micera G. EPR and Potentiometric Reinvestigation of Copper(II) Complexation with Simple Oligopeptides and Related Compounds. J. Inorg. Biochem. 1996, 63, 99–117. 10.1016/0162-0134(95)00185-9. [DOI] [Google Scholar]
- Dunbar R. C.; Berden G.; Oomens J. How Does a Small Peptide Choose How to Bind a Metal Ion? IRMPD and Computational Survey of CS Versus Iminol Binding Preferences. Int. J. Mass Spectrom. 2013, 354-355, 356–364. 10.1016/j.ijms.2013.07.017. [DOI] [Google Scholar]
- Dunbar R. C.; Martens J.; Berden G.; Oomens J. Complexes of Ni (II) and Cu (II) with Small Peptides: Deciding Whether to Deprotonate. Phys. Chem. Chem. Phys. 2016, 18, 26923–26932. 10.1039/C6CP03974J. [DOI] [PubMed] [Google Scholar]
- Dunbar R. C.; Martens J.; Berden G.; Oomens J. Transition Metal (II) Complexes of Histidine-Containing Tripeptides. Structures, and Infrared Spectroscopy by IRMPD. Int. J. Mass Spectrom. 2018, 429, 198–205. 10.1016/j.ijms.2017.10.004. [DOI] [Google Scholar]
- Bal W.; Christodoulou J.; Sadler P. J.; Tucker A. Multi-Metal Binding Site of Serum Albumin. J. Inorg. Biochem. 1998, 70, 33–39. 10.1016/S0162-0134(98)00010-5. [DOI] [PubMed] [Google Scholar]
- Bal W.; Sokolowska M.; Kurowska E.; Faller P. Binding of Transition Metal Ions to Albumin: Sites, Affinities and Rates. Biochim. Biophys. Acta, Gen. Subj. 2013, 1830, 5444–5455. 10.1016/j.bbagen.2013.06.018. [DOI] [PubMed] [Google Scholar]
- Kozlowski H.; Kowalik-Jankowska T.; Jezowska-Bojczuk M. Chemical and Biological Aspects of Cu2+ Interactions with Peptides and Aminoglycosides. Coord. Chem. Rev. 2005, 249, 2323–2334. 10.1016/j.ccr.2005.04.027. [DOI] [Google Scholar]
- Kozlowski H.; Luczkowski M.; Remelli M.; Valensin D. Copper, Zinc and Iron in Neurodegenerative Diseases (Alzheimer’s, Parkinson’s and Prion Diseases). Coord. Chem. Rev. 2012, 256, 2129–2141. 10.1016/j.ccr.2012.03.013. [DOI] [Google Scholar]
- Sovago I.; Kallay C.; Varnagy K. Peptides as Complexing Agents: Factors Influencing the Structure and Thermodynamic Stability of Peptide Complexes. Coord. Chem. Rev. 2012, 256, 2225–33. 10.1016/j.ccr.2012.02.026. [DOI] [Google Scholar]
- Remelli M.; Nurchi V. M.; Lachowicz J. I.; Medici S.; Zoroddu M. A.; Peana M. Competition between Cd(II) and Other Divalent Transition Metal Ions During Complex Formation with Amino Acids, Peptides, and Chelating Agents. Coord. Chem. Rev. 2016, 327–328, 55–69. 10.1016/j.ccr.2016.07.004. [DOI] [Google Scholar]
- Burns C. S.; Aronoff-Spencer E.; Dunham C. M.; Lario P.; Avdievich N. I.; Antholine W. E.; Olmstead M. M.; Vrielink A.; Gerfen G. J.; Peisach J.; et al. Molecular Features of the Copper Binding Sites in the Octarepeat Domain of the Prion Protein. Biochemistry 2002, 41, 3991–4001. 10.1021/bi011922x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrieri F.; Minicozzi V.; Morante S.; Rossi G.; Furlan S.; La Penna G. Modeling the Interplay of Glycine Protonation and Multiple Histidine Binding of Copper in the Prion Protein Octarepeat Subdomains. JBIC, J. Biol. Inorg. Chem. 2009, 14, 361–374. 10.1007/s00775-008-0454-8. [DOI] [PubMed] [Google Scholar]
- Aiba H.; Yokoyama A.; Tanaka H. Copper(II) Complexes of Glycyl-L-Histidine, Glycyl-L-Histidylglycine, and Glycylclycyl-L-Histidine in Aqueous Solution. Bull. Chem. Soc. Jpn. 1974, 47, 1437–1441. 10.1246/bcsj.47.1437. [DOI] [Google Scholar]
- Camerman N.; Camerman A.; Sarkar B. Molecular Design to Mimic the Copper(II) Transport Site of Human Albumin. The Crystal and Molecular Structure of Copper(II) - Glycylglycyl-L-Histidine-N-Methyl Amide Monoaquo Complex. Can. J. Chem. 1976, 54, 1309–1316. 10.1139/v76-185. [DOI] [Google Scholar]
- Csire G.; Nagy L.; Varnagy K.; Kallay C. Copper(II) Interaction with the Human Prion 103–112 Fragment - Coordination and Oxidation. J. Inorg. Biochem. 2017, 170, 195–201. 10.1016/j.jinorgbio.2017.02.018. [DOI] [PubMed] [Google Scholar]
- Dorlet P.; Gambarelli S.; Faller P.; Hureau C. Pulse EPR Spectroscopy Reveals the Coordination Sphere of Copper(II) Ions in the 1–16 Amyloid-Beta Peptide: A Key Role of the First Two N-Terminus Residues. Angew. Chem., Int. Ed. 2009, 48, 9273–9276. 10.1002/anie.200904567. [DOI] [PubMed] [Google Scholar]
- Witkowska D.; Politano R.; Rowinska-Zyrek M.; Guerrini R.; Remelli M.; Kozlowski H. The Coordination of NiII and CuII ions to the Polyhistidyl Motif of Hpn Protein: Is It as Strong as We Think?. Chem. - Eur. J. 2012, 18, 11088–11099. 10.1002/chem.201200780. [DOI] [PubMed] [Google Scholar]
- Agbale C. M.; Cardoso M. H.; Galyuon I. K.; Franco O. L. Designing Metallodrugs with Nuclease and Protease Activity. Metallomics 2016, 8, 1159–1169. 10.1039/C6MT00133E. [DOI] [PubMed] [Google Scholar]
- Harford C.; Sarkar B. Amino Terminal Cu(II)- and Ni(II)-Binding (ATCUN) Motif of Proteins and Peptides: Metal Binding, DNA Cleavage, and Other Properties. Acc. Chem. Res. 1997, 30, 123–130. 10.1021/ar9501535. [DOI] [Google Scholar]
- Miyamoto T.; Fukino Y.; Kamino S.; Ueda M.; Enomoto S. Enhanced Stability of Cu2+-ATCUN Complexes under Physiologically Relevant Conditions by Insertion of Structurally Bulky and Hydrophobic Amino Acid Residues into the ATCUN Motif. Dalton Trans. 2016, 45, 9436–9445. 10.1039/C6DT01387B. [DOI] [PubMed] [Google Scholar]
- Neupane K. P.; Aldous A. R.; Kritzer J. A. Macrocyclization of the ATCUN Motif Controls Metal Binding and Catalysis. Inorg. Chem. 2013, 52, 2729–2735. 10.1021/ic302820z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wende C.; Kulak N. Fluorophore ATCUN Complexes: Combining Agent and Probe for Oxidative DNA Cleavage. Chem. Commun. 2015, 51, 12395–12398. 10.1039/C5CC04508H. [DOI] [PubMed] [Google Scholar]
- Wiloch M. Z.; Ufnalska I.; Bonna A.; Bal W.; Wroblewski W.; Wawrzyniak U. E. Copper(II) Complexes with ATCUN Peptide Analogues: Studies on Redox Activity in Different Solutions. J. Electrochem. Soc. 2017, 164, G77–G81. 10.1149/2.1191706jes. [DOI] [Google Scholar]
- Sovago I.; Farkas E.; Bertalan C.; Lebkiri A.; Kowalik-Jankowska T.; Kozlowski H. Copper(II) Complexes of Dipeptides Containing Aspartyl, Glutamyl, and Histidyl Residues in the Side Chain. J. Inorg. Biochem. 1993, 51, 715–26. 10.1016/0162-0134(93)85004-R. [DOI] [PubMed] [Google Scholar]
- Eshelman M. R.; Aldous A. R.; Neupane K. P.; Kritzer J. A. Solution Structure of a Designed Cyclic Peptide Ligand for Nickel and Copper Ions. Tetrahedron 2014, 70, 7651–7654. 10.1016/j.tet.2014.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamariola G.; Watly J.; Gallerani E.; Gavioli R.; Guerrini R.; Kozlowski H.; Remelli M. AGHLDDLPGALSAL: A Hemoglobin Fragment Potentially Competing with Albumin to Bind Transition Metal Ions. J. Inorg. Biochem. 2016, 163, 301–310. 10.1016/j.jinorgbio.2016.04.001. [DOI] [PubMed] [Google Scholar]
- Citir M.; Hinton C. S.; Oomens J.; Steill J. D.; Armentrout P. B. Infrared Multiple Photon Dissociation Spectroscopy of Cationized Histidine: Effects of Metal Cation Size on Gas-Phase Conformation. J. Phys. Chem. A 2012, 116, 1532–1541. 10.1021/jp209636a. [DOI] [PubMed] [Google Scholar]
- Armentrout P. B.; Citir M.; Chen Y.; Rodgers M. T. Thermochemistry of Alkali Metal Cation Interactions with Histidine: Influence of the Side Chain. J. Phys. Chem. A 2012, 116, 11823–11832. 10.1021/jp310179c. [DOI] [PubMed] [Google Scholar]
- Hofstetter T. E.; Howder C.; Berden G.; Oomens J.; Armentrout P. B. Structural Elucidation of Biological and Toxicological Complexes: Investigation of Monomeric and Dimeric Complexes of Histidine with Multiply Charged Transition Metal (Zn and Cd) Cations Using IR Action Spectroscopy. J. Phys. Chem. B 2011, 115, 12648–12661. 10.1021/jp207294b. [DOI] [PubMed] [Google Scholar]
- Ziegler B. E.; Marta R. A.; Burt M. B.; McMahon T. B. Insight into the Gas-Phase Structure of a Copper(II) L-Histidine Complex, the Agent Used to Treat Menkes Disease. Inorg. Chem. 2014, 53, 2349–2351. 10.1021/ic402755q. [DOI] [PubMed] [Google Scholar]
- Peckelsen K.; Martens J.; Berden G.; Oomens J.; Dunbar R. C.; Meijer A. J. H. M.; Schäfer M. Gas-Phase Complexes of Ni2+ and Ca2+ with Deprotonated Histidylhistidine (HisHis): A Model Case for Polyhistidyl-Metal Binding Motifs. J. Mol. Spectrosc. 2017, 332, 38–44. 10.1016/j.jms.2016.10.008. [DOI] [Google Scholar]
- Martens J.; Berden G.; Gebhardt C. R.; Oomens J. Infrared Ion Spectroscopy in a Modified Quadrupole Ion Trap Mass Spectrometer at the FELIX Free Electron Laser Laboratory. Rev. Sci. Instrum. 2016, 87, 103108. 10.1063/1.4964703. [DOI] [PubMed] [Google Scholar]
- Munshi M. U.; Berden G.; Martens J.; Oomens J. Gas-Phase Vibrational Spectroscopy of Triphenylamine: The Effect of Charge on Structure and Spectra. Phys. Chem. Chem. Phys. 2017, 19, 19881–19889. 10.1039/C7CP02638B. [DOI] [PubMed] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.. et al. Gaussian 09, Rev. D.01, 2009.
- Grimme S. Density Functional Theory with London Dispersion Corrections. WIREs Comput. Mol. Sci. 2011, 1, 211–228. 10.1002/wcms.30. [DOI] [Google Scholar]
- Martens J.; Grzetic J.; Berden G.; Oomens J. Gas-Phase Conformations of Small Polyprolines and Their Fragment Ions by IRMPD Spectroscopy. Int. J. Mass Spectrom. 2015, 377, 179–187. 10.1016/j.ijms.2014.07.027. [DOI] [Google Scholar]
- Dunbar R. C.; Moore D. T.; Oomens J. IR-Spectroscopic Characterization of Acetophenone Complexes with Fe+, Co+, and Ni+ Using Free-Electron-Laser IRMPD. J. Phys. Chem. A 2006, 110, 8316–8326. 10.1021/jp0566921. [DOI] [PubMed] [Google Scholar]
- Khoury R. R.; Sutton G. J.; Ebrahimi D.; Hibbert D. B. Formation Constants of Copper(II) Complexes with Tripeptides Containing Glu, Gly, and His: Potentiometric Measurements and Modeling by Generalized Multiplicative Analysis of Variance. Inorg. Chem. 2014, 53, 1278–1287. 10.1021/ic4009575. [DOI] [PubMed] [Google Scholar]
- Myari A.; Malandrinos G.; Plakatouras J.; Hadjiliadis N.; Sovago I. Interaction of Cu(II) with His-Val-Gly-Asp and of Zn(II) with His-Val-His, Two Peptides at the Active Site of Cu,Zn-Superoxide Dismutase. Bioinorg. Chem. Appl. 2003, 1, 99–112. 10.1155/S1565363303000086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farkas E.; Sovago I.; Kiss T.; Gergely A. Studies on Transition-Metal-Peptide Complexes. Part 9. Copper(II) Complexes of Tripeptides Containing Histidine. J. Chem. Soc., Dalton Trans. 1984, 1984, 611–614. 10.1039/DT9840000611. [DOI] [Google Scholar]
- Dunbar R. C.; Martens J.; Berden G.; Oomens J. Water Microsolvation Can Switch the Binding Mode of Ni(II) with Small Peptides. J. Phys. Chem. Lett. 2017, 8, 2634–2638. 10.1021/acs.jpclett.7b00973. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.