Abstract
Background
Major depressive disorder (MDD) is characterized by dysfunction in cognitive and emotional systems. However, the neural network correlates of cognitive control (cold cognition) and emotion processing (hot cognition) during the remitted state of MDD (rMDD) remain unclear and not fully probed, which has important implications for identifying intermediate phenotypes of depression risk.
Methods
43 young adults with rMDD and 33 healthy controls (HCs) underwent fMRI while completing separate tasks of cold cognition (Parametric Go/No-Go test) and hot cognition (Facial Emotion Processing Test). Two 2 group (rMDD, HC) x 2 event (sad/fearful faces, correct rejections) factorial models of activation were calculated in SPM8. Functional activation was evaluated in the salience and emotional network (SEN) and the cognitive control network (CCN), including hypothesized interaction between group and task within the CCN.
Results
Individuals with rMDD demonstrated greater spatial extent of suprathreshold activation within the SEN during sad faces relative to HCs. There were several regions within the CCN in which HCs showed greater activation than rMDD during correct rejections of lures, whereas individuals with rMDD showed greater activation than HCs during sad or fearful faces.
Limitations
Results were not directly compared with active MDD.
Conclusions
These results provide evidence of deficient CCN engagement during cognitive control in rMDD (dysfunctional cold cognition). Elevated SEN activation during sad faces could represent heightened salience of negative emotional faces in rMDD; elevated CCN activation during emotional faces in rMDD could represent compensatory regulatory control. These group differences may represent vulnerability factors, scars of prior depressive episodes, or processes maintaining wellness.
Introduction
Major depressive disorder (MDD) often is conceptualized within a framework of emotion dysregulation (Aldao et al., 2010; Hofmann et al., 2012; Phillips et al., 2003; Gross and Muñoz, 1995). Individuals with MDD often demonstrate an impaired ability to successfully modulate emotional responses, resulting from abnormal interactions between emotional and cognitive processes (Langenecker et al., 2014; Phillips et al., 2003; Robinson et al., 2016). Two primary, synergistic hypotheses about dysfunctional emotion regulation in MDD have been proposed. First, individuals with MDD may demonstrate excessive emotional reactivity to affective stimuli, as evidenced by greater limbic activation than healthy controls during emotion processing tasks (e.g., Sheline et al., 2001), often described as dysfunctional “hot cognition” (Roiser and Sahakian, 2013). Second, individuals with MDD may have deficits in the ability to recruit cognitive control, exhibiting decreased activation in relevant prefrontal cortical regions (Gotlib et al., 2005) even when emotion regulation demands are not present (“cold cognition”). Hot and cold cognition may have distinct but interactive roles in the depression phenotype (Roiser and Sahakian, 2013), especially considering the adaptive functions of these two processes. Determining the extent to which deficits in these functions are present during the remitted state of MDD (rMDD) may facilitate the identification of candidate intermediate phenotypes or risk markers for disease or recurrence (IPs; Meyer-Lindenberg and Weinberger, 2006; Gottesman and Gould, 2003) of MDD.
Hot cognition involves affective stimuli that have survival implications, such as identification of faces with fearful or sad emotional expressions that might indicate the need for action. Hot cognition is considered a “bottom-up” process that originates subcortically in limbic regions of the brain and subsequently directs prefrontal cortical resources toward emotional processing that facilitates survival (Banich et al., 2009). One neural network involved in detecting, integrating, and filtering relevant emotionally salient information relevant to hot cognition is the salience and emotional network (SEN), which includes the amygdala, anterior insula, and subgenual anterior cingulate (Menon, 2011; Seeley et al., 2007). Individuals with active MDD show deficits in hot cognition, including poorer facial emotion recognition than healthy individuals (Langenecker et al., 2005; Kohler et al., 2011), perhaps due to a negative processing bias, and show greater SEN activation than controls when presented with negative emotional stimuli (Briceno et al., 2013; Sheline et al., 2001; Surguladze et al., 2005; Siegle et al., 2007; Fu et al., 2004, 2008a,b; Gotlib et al., 2005). Most extant studies on hot cognition have involved individuals with active MDD (e.g., Etkin and Schatzberg, 2011; Ladouceur et al., 2006), showing that these individuals have excessive focus on negative information at the expense of positive information (Robinson et al., 2016). Fewer studies have attempted to disentangle state versus trait factors by studying individuals with rMDD; some evidence has suggested the presence of selective attention to sad faces in rMDD in addition to active MDD (Joormann and Gotlib, 2007). These results support the hypothesis that hot cognition deficits could reflect trait-like vulnerabilities underlying MDD.
In contrast with hot cognition, cold cognition refers to the exertion of cognitive control in affectively neutral tasks (Roiser and Sahakian, 2013). Although non-affective itself, cold cognition facilitates the effective implementation of emotion regulation strategies and the inhibition of unhelpful forms of thought such as rumination (Langenecker et al., 2014; Joormann and Quinn, 2014; Joormann and Vanderlind, 2014; Ochsner, Silvers, and Buhle, 2012; Pe et al., 2013a; Malooly et al., 2013). Cold cognition is considered a top-down, cortically-mediated process (Roiser and Sahakian, 2013). A cognitive control network (CCN) has been identified and includes the dorsolateral prefrontal cortex (dlPFC), inferior parietal lobule, and dorsal cingulate (Menon, 2011; Seeley et al., 2007; Yeo et al., 2011; Derrfuss et al., 2005). The CCN subserves cold cognition and the control of negative affect (Disner et al., 2011; Langenecker et al., 2014; Ochsner et al., 2012; Wang et al., 2016; Cromheeke and Mueller, 2014; Seeley et al., 2007). CCN function is particularly relevant for MDD. For example, our prior work has demonstrated poor integrity of the CCN in rMDD, which was associated with behavioral phenotypes of depression risk (Stange et al., 2017). Furthermore, the CCN is integral in the cognitive regulation of negative affect and in the reduction of risk for depression (Disner et al., 2011; Banks et al., 2007; Johnstone et al., 2007; Moses-Kolko et al., 2010; Versace et al., 2010; Banks et al., 2007; Perlman et al., 2012). CCN measures, at the performance and imaging level, are also predictive of short- and long-term treatment response (Crane et al., 2017; Dawson et al., 2017). Together, there is evidence that deficits in cold cognition (and its CCN correlates) and the cognitive control of emotion may persist into remission (Robinson et al., 2016), and thus may represent a potential IP for MDD.
Previous studies have observed behavioral deficits in both hot and cold cognition in the same individuals with active MDD (e.g., Langenecker et al., 2005). Some even have attempted to study hot and cold cognition simultaneously within the same paradigm (e.g., “emotional” versions of go/no-go, stroop, flanker, set-shifting, and n-back tasks; Cromheeke and Mueller, 2014; Etkin et al., 2015; Etkin and Schatzberg, 2011; Pe et al., 2013b; Bertocci et al., 2012; Mitterschiffthaler et al., 2008; Joormann and Gotlib, 2008; Joormann et al., 2011; de Lissnyder et al., 2010, 2012; Murphy et al., 2011; Malooly et al., 2013). This approach has utility in determining whether cognition in MDD is impaired specifically in the context of emotional stimuli (i.e., hot cognition), as several (e.g., Etkin and Schatzberg, 2011; Bertocci et al., 2012; Mitterschiffthaler et al., 2008; Joormann and Gotlib, 2008; Joormann et al., 2011) but not all (de Lissnyder et al., 2010, 2012; Murphy et al., 2011) studies have shown in active MDD. However, because these tasks require a combination of hot and cold cognition simultaneously, the specific neural processes engaged during these tasks may be ambiguous, leaving interpretation of results unclear. Instead, perhaps the simplest method for parsing the degree to which relevant networks are differentially engaged or disengaged during hot and cold cognition in rMDD would be to evaluate patterns of activation across tasks that separately assess each of these constructs. This approach may facilitate conclusions about how deficits in these two systems may jointly contribute to current MDD (Siegle et al., 2007) or IPs of MDD that would be detectable even in rMDD (e.g., Meyer-Lindenberg and Weinberger, 2006; Gottesman and Gould, 2003).
We used a triple network-based approach (Menon, 2011) to evaluate the height and spatial extent of SEN and CCN activity (e.g., Briceno et al., 2013) during separate tasks reflecting hot and cold cognition. Spatial extent analysis is complementary to height analysis – both degree (height) and extent convey important information about neural system engagement. To evaluate possible trait-like IPs of MDD risk while avoiding effects of current symptoms, we recruited a sample of young adult individuals with rMDD and healthy controls (HCs). We hypothesized (Table 1) that individuals with rMDD would demonstrate hyperactivation of the SEN during hot cognition (Hypothesis 1), consistent with past findings among individuals with active MDD. Given that individuals with MDD often experience emotional activation in contexts in which such activation is inappropriate (Disner et al., 2011), we hypothesized that individuals with rMDD also would show greater SEN activation than HCs during cold cognition (Hypothesis 2). Based on prior work in active MDD showing hyperactivation of prefrontal cortical regions during hot cognition (e.g., Johnstone et al., 2007), we expected individuals with rMDD to show hyperactivation of the CCN during hot cognition (Hypothesis 3). We hypothesized that relative to HCs, individuals with rMDD would show deficiencies in CCN activity during cold cognition (Hypothesis 4).
Table 1.
Hypotheses about group differences in activation within SEN and CCN during hot and cold cognition tasks.
| SEN | CCN | |
|---|---|---|
|
Hot Cognition (Sad or Fearful Faces) |
rMDD > HC (Hyp. 1) |
rMDD > HC (Hyp. 3) |
|
Cold Cognition (Go/No-Go Correct Rejections) |
rMDD > HC (Hyp. 2) |
rMDD < HC (Hyp. 4) |
Method
Participants
Participants were recruited using flyers and internet postings and were tested at the University of Michigan (UM) and the University of Illinois at Chicago (UIC). The rMDD group comprised 57 (13 UM, 30 UIC) individuals with a history of MDD who were in full remission at the time of the study, as defined by DSM-IV-TR criteria. The HC group comprised 33 individuals (10 UM, 23 UIC) who not meet current or past criteria for MDD or any other Axis I psychiatric disorder. The proportion of individuals in each diagnostic group did not differ by site, X2 = 0.08, p = .77. Participants were between 18 and 23 years of age (63% Female). Participants were required to be medication free for 30 days prior to the scan. Individuals with substance abuse or dependence within the past six months were excluded. Participant demographics and clinical characteristics are presented in Table 2.
Table 2.
Demographic comparisons between groups.
| HC (n = 33) | rMDD (n = 43) | |
|---|---|---|
|
| ||
| M (SD) / N (%) | M (SD) / N (%) | |
| Female | 58% | 65% |
| Age | 21.15 (1.58) | 21.40 (1.50) |
| Race | ||
| African American/Black | 1 (3%) | 3 (7%) |
| Asian or Indian | 7 (21%) | 5 (12%) |
| Caucasian/White | 24 (73%) | 30 (70%) |
| Latino(a) | 1 (3%) | 2 (5%) |
| Middle Eastern | 0 (0%) | 1 (2%) |
| More than One Race | 0 (0%) | 2 (5%) |
| Hispanic | 3 (9%) | 5 (12%) |
| HDRS* | 0.33 (0.89) | 2.51 (4.81) |
| Age at onset | n/a | 16.33 (3.43) |
| Number of depressive episodes | n/a | 1.88 (1.25) |
| Education | 14.85 (1.28) | 14.60 (1.40) |
| Estimated Verbal IQ | 106.61 (9.08) | 106.27 (9.64) |
p < .05.
Note. HC, Healthy control; rMDD, remitted major depressive disorder; HDRS, Hamilton Rating Scale for Depression.
Procedures
Written informed consent was obtained according to the guidelines of the Institutional Review Boards of UM and UIC and consistent with the Declaration of Helsinki. All participants completed a battery of cognitive and diagnostic measures, followed by an MRI scan. Participants completed practice trials of fMRI tasks prior to scanning and completed the fMRI tasks again inside the scanner.
Measures
Cold cognition
The Parametric Go/No-Go (PGNG) task provided a measure of behavioral and neural cognitive control. This task (described in detail in Supplemental Methods and elsewhere; Langenecker et al., 2007a,b; Votruba and Langenecker, 2013) was administered to all participants early in the scan session to eliminate effects of fatigue and to assess sustained inhibitory control (Langenecker et al., 2010). Neural activation was evaluated during correct rejection trials.
Hot cognition
The Facial Emotion Perception Test (FEPT) provided a measure of emotion perception and processing. In the FEPT (see Supplemental Methods; Rapport et al., 2002; Langenecker et al., 2005, 2007), participants view rapidly presented sad and fearful faces (given the relevance of sadness and fear to MDD, as discussed above) and must categorize them by emotion. The FEPT was presented after the PGNG task to avoid carry-over effects of emotion on cold cognition. Neural activation was evaluated during correct trials for identification of sad and fearful stimuli.
fMRI acquisition and processing
Whole-brain imaging was performed at two sites, each using a 3T GE scanner. These details are included in the Supplementary Materials. SPM8 (http://www.fil.ion.ucl.ac.uk/spm/), AFNI (http://afni.nimh.nih.gov/afni/) and FSL (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/) were used to preprocess fMRI data.
Defining the CCN and SEN
To define the masks for the CCN and SEN, we selected key networks identify by Yeo et al. (2011). From Yeo et al.’s seven-network parcellation, the CCN was created by combining the dorsal attention and frontoparietal network masks, and the SEN was created by combining ventral attention and limbic networks. Our definition of networks was consistent with prior work (Seeley et al., 2007; Menon, 2011; Stange et al., 2017).
Statistical Analyses
For behavioral performance analyses, a 2 group (rMDD, HC) x 3 condition (rejections, sad faces, fearful faces) repeated measures ANCOVA was calculated for accuracy (percent correct inhibition on PGNG, percent correct for sad and fearful faces on FEPT), with diagnostic group as the independent variable, and sex and site as covariates. To evaluate the four hypotheses about neural activation, we integrated these conditions into combined models to evaluate group differences in activation across tasks and networks. This approach that has been suggested by prior work that has used affective versions of cold cognitive tasks (e.g., Siegle et al., 2007; Fitzgerald et al., 2008; Cromheeke and Mueller, 2014; Etkin et al., 2015; Etkin and Schatzberg, 2011; Pe et al., 2013b; Bertocci et al., 2012; Mitterschiffthaler et al., 2008; Joormann and Gotlib, 2008; Joormann et al., 2011; de Lissnyder et al., 2010, 2012; Murphy et al., 2011; Malooly et al., 2013) but to our knowledge has not been directly tested, particularly in rMDD where it can satisfy one of the criteria for IPs (state independence; Meyer-Lindenberg and Weinberger, 2006; Gottesman and Gould, 2003).
Two diagnostic group x condition factorial models were built in SPM8. In the first model, group served as one factor and condition (rejections and sad faces) served as the other factor. In the second model, group served as one factor and condition (rejections and fearful faces) served as the other factor. Sex, imaging site (UM/UIC), and head motion (x, y and z translation; which did not differ between groups for PGNG [t = −0.76, p = .45] or FEPT [t = −0.30, p = .77]) were included as covariates. The threshold of significance reported for the fMRI analyses was p < 0.005 and k = 57 (3dClustsim with whole brain corrected p value of .01 per analysis). The use of whole brain correction thresholds despite focused hypotheses that utilized of only a subset of voxels (i.e., CCN and SEN masks, for clarity in interpretation) represents a conservative approach. Analyses used a gray matter mask, and were repeated with the CCN and SEN masks for interpreting regions of activation. The main effect of group contrast was interpreted with the SEN mask to evaluate Hypotheses 1 and 2 (rMDD > HC across hot and cold cognition conditions). To evaluate Hypotheses 3 and 4 (rMDD > HC during hot cognition, but rMDD < HC during cold cognition), the group x condition interaction contrasts were interpreted within the CCN mask.
In addition to the primary analysis of the height of regions of network activation, as a secondary analysis we evaluated the spatial extent of activation within each network mask by group and condition. This involved summing the number of voxels active for each person using a more lenient threshold of p < .05 and k = 15 for each event type, network, and individual, to allow for optimal variability. Group differences in the number of activated voxels per network, per event type, per person were evaluated with independent samples t-tests and effect sizes, given that comparing groups across large networks is likely to obscure specific regions that differ between groups.
Results
Although not a hypothesis for the study, we did test performance effects. For behavioral performance (accuracy), the 2 group x 3 condition ANCOVA was not significant for main effect of condition (F(1, 70) = 0.01, p = .60, ηp2 < .01), group (F(1, 70) = 0.11, p = .74, ηp2 < .01), or condition x group interaction (F(1, 70) = 1.92, p = .17, ηp2 = .03).
Primary Analyses: Height Extent of Group Differences in Network Activation
Given the hypotheses outlined in the introduction, we anticipated a main effect of group on activation within the SEN, with rMDD demonstrating greater activation than HCs across both conditions. We also anticipated a group-by-condition interaction for activation within the CCN, such that HCs would show activation of the CCN during rejections and relative deactivation while interpreting emotional faces, whereas rMDD would show lack of CCN activation during rejections and excessive CCN activation while interpreting faces.
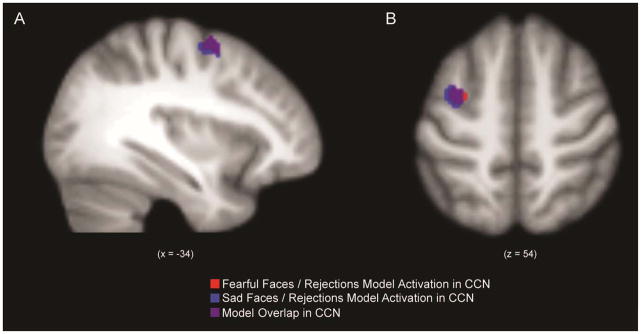
We observed a main effect of group for the CCN (Figure 1), such that HCs demonstrated greater activation than individuals with rMDD in the left middle frontal gyrus across both conditions. However, there was no main effect of group in the SEN.
Figure 1.
Spatial map of main effect of group within the cognitive control network (CCN) for factorial models of fearful faces and rejections, and sad faces and rejections (Panel A: sagittal view; Panel B: axial view).
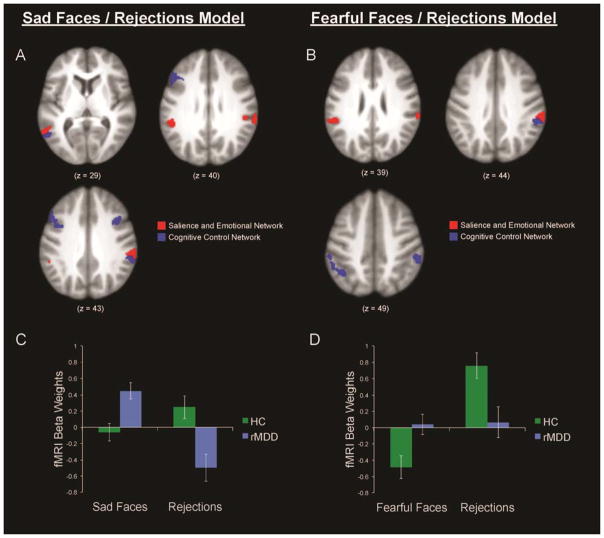
The hypothesized group x event interaction contrasts yielded several regions of group differences within the CCN (Table 3; Figure 2). In the sadness and rejections model, significant interactions between group and event type were associated with several regions within the CCN mask, including the middle and inferior frontal gyri, medial and inferior parietal lobule (IPL), precuneus, middle and inferior temporal gyri, and fusiform gyrus. The activation height values were extracted from these clusters of significant interaction using Marsbar to illustrate and quantify the nature of group interactions in these regions within the CCN (Figure 2C). During rejections, HCs demonstrated greater activation in these CCN regions than individuals with rMDD, who showed deactivation (d = −0.77). Also as hypothesized, while identifying sad faces, individuals with rMDD showed greater activation in these CCN regions than HCs (d = 0.80). In addition to the CCN, significant interactions between group and event type were associated with several regions within the SEN mask, including middle, inferior, and superior temporal gyri. Several of these regions were spatially proximal (i.e., contiguous) to regions of the CCN that showed a similar pattern, particularly in bilateral IPL.
Table 3.
fMRI Results of Main Effects of Group and Group x Task Interactions.
| Model/Contrast | Lobe/Gyrus | BA | MNI Coordinates | Peak Z | Cluster mm3 | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Sad / Rejections Model | |||||||
| Main Effect of Group (CCN only) | Frontal | ||||||
| Middle Frontal | 6 | −34 | −2 | 56 | 3.44 | 640 | |
| Group x Task Interaction (CCN) | Frontal | ||||||
| Middle Frontal | 9 | −50 | 24 | 30 | 3.72 | 2408 | |
| 10 | 38 | 48 | −4 | 3.41 | 824 | ||
| Inferior Frontal | 9 | 40 | 12 | 32 | 3.26 | 684 | |
| Parietal | |||||||
| Inferior Parietal | 40 | 62 | −40 | 32 | 3.74 | 1496 | |
| 40 | −46 | −48 | 40 | 3.22 | 872 | ||
| Medial/Inferior (Precuneus) | 7 | 28 | −68 | 42 | 3.10 | 504 | |
| Temporal | |||||||
| Middle Temporal | 37 | −60 | −58 | −2 | 4.22 | 2152 | |
| 37 | 50 | −54 | −12 | 3.63 | 1000 | ||
| Fusiform | 37 | −40 | −42 | −14 | 3.57 | 680 | |
| Group x Task Interaction (SEN) | Temporal | ||||||
| Superior Temporal | 40 | 64 | −36 | 30 | 4.69 | 1560 | |
| 40 | −50 | −38 | 28 | 4.05 | 1976 | ||
| Middle Temporal | 39 | −54 | −48 | 10 | 3.75 | 1096 | |
| Inferior Temporal | 20 | 48 | 0 | −46 | 3.82 | 712 | |
| 20 | 38 | −16 | −38 | 3.45 | 808 | ||
| Fear / Rejections Model | |||||||
| Main Effect of Group (CCN only) | Frontal | ||||||
| Middle Frontal | 6 | −36 | −2 | 58 | 3.35 | 528 | |
| Group x Task Interaction (CCN) | Parietal | ||||||
| Inferior Parietal | 40 | 60 | −34 | 38 | 3.73 | 1824 | |
| 40 | −38 | −54 | 44 | 3.15 | 1008 | ||
| Group x Task Interaction (SEN) | Parietal | ||||||
| Supramarginal | 40 | 64 | −36 | 30 | 4.40 | 1336 | |
| Supramarginal | 3 | −52 | −38 | 26 | 3.41 | 704 | |
| Temporal | |||||||
| Middle Temporal | 22 | −54 | −50 | 10 | 3.59 | 728 | |
Note. BA, Brodmann area. x, y, z = MNI (Montreal Neurological Institute) coordinates of significant effects.
Figure 2.
Axial views of group x condition interactions within the cognitive control network (CCN) and the salience and emotional network (SEN) in models of sad faces and rejections (panel A), and fearful faces and rejections (panel B), along with bar graphs representing degree of activation in these CCN regions by group and condition in sad faces and rejections model (panel C), and fearful faces and rejections model (panel D).
In the fear and rejections model (Figure 2), two CCN regions were identified by the hypothesized group x condition interaction in bilateral IPL, such that during rejections, HCs demonstrated greater CCN activation in these bilateral IPL regions than individuals with rMDD (d = 0.65); in contrast, while interpreting fearful faces, HCs experienced greater deactivation in these regions relative to individuals with rMDD (d = 0.65; Figure 2D). Several regions within the SEN mask also were identified with the group x condition interaction contrast, including the supramarginal and middle temporal gyri. Similar to the sadness and rejections model, these regions were spatially contiguous to bilateral IPL regions of the CCN that showed a similar pattern.
Secondary Analyses: Spatial Extent of Group Differences in Network Activation
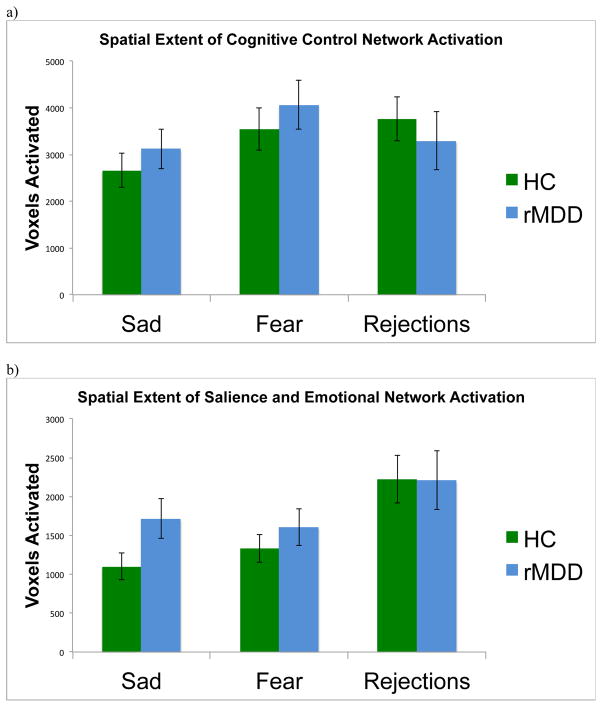
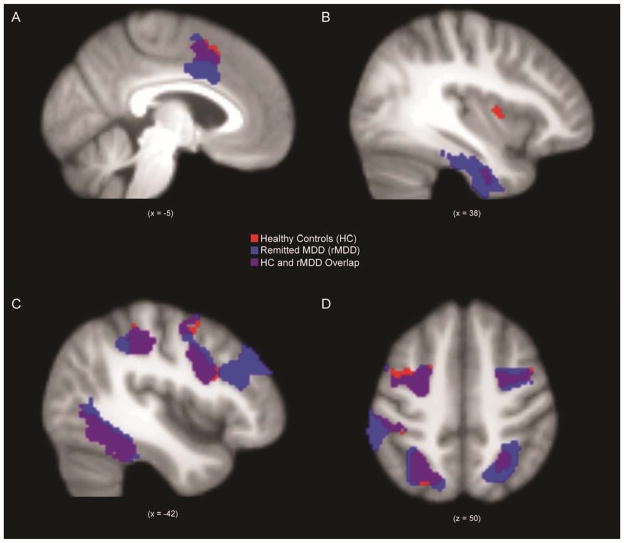
While interpreting sad faces, individuals with rMDD demonstrated greater spatial extent of suprathreshold activation within the SEN than HCs (t = 2.01, p < .05, d = 0.45; Figure 3). While interpreting fearful faces, individuals with rMDD did not demonstrate significantly greater spatial extent of activation in the SEN compared to HCs (t = 0.94, p = .35, d = 0.21). There were no group differences in spatial extent of SEN activation during rejections (t = −0.03, p = .98, d = 0.14). For general comparison, suprathreshold activation within the SEN while interpreting sad faces is displayed for each group in Figure 4 (panels A and B), p < .005, k > 57 whole brain corrected threshold (panels A and B).
Figure 3.
Extent of spatial activation within (a) the cognitive control network (CCN) and (b) the salience and emotional network (SEN) in healthy control individuals (HC) and individuals with remitted major depressive disorder (rMDD) during sad faces, fearful faces, and correct rejection trials.
Figure 4.
Spatial map of activation within salience and emotional network (panels A, B) and cognitive control network (panels C, D) in healthy control individuals (HC) and individuals with remitted major depressive disorder (rMDD) during sad faces.
There were no significant group differences in spatial extent of activation within the CCN while interpreting sad faces (t = 0.79, p = .43, d = 0.19), fearful faces (t = 0.74, p = .46, d = 0.19), or rejections (t = −0.60, p = .55, d = 0.01). For general comparison of groups, activation within the CCN while interpreting sad faces is displayed for each group in Figure 4 (panels C and D).
Discussion
We examined factorial models of hot and cold cognition using a network-based approach, with the goal of taking a step toward identifying IPs of dysfunctional cognitive and emotional systems in depression. In studying individuals in the remitted state of MDD, we hoped to illuminate trait-like characteristics rather than effects of current illness. We identified several regions within the CCN in which individuals with rMDD showed differential patterns of network activation, including over-activity in both the SEN and CCN during hot cognition (supporting Hypotheses 1 and 3), and under-activity in the CCN during cold cognition (supporting Hypothesis 4). Surprisingly, we did not observe the hypothesized over-activity of the SEN during cold cognition in rMDD (failing to support Hypothesis 2). Individuals with rMDD also had greater spatial activation of the SEN than HCs in response to sad faces (supporting Hypothesis 1).
Although there were no group differences in behavioral performance on hot and cold cognition tasks, individuals with rMDD showed attenuated activation within the CCN (e.g., in bilateral middle frontal gyrus and IPL) during cold cognition, consistent with prior work in active MDD (Roiser and Sahakian, 2013; Langenecker et al., 2014; Phillips et al., 2003). However, individuals with rMDD recruited similar CCN regions during hot cognition (while viewing sad faces) as HCs recruited during cold cognition, perhaps to facilitate emotion regulation or to overcome emotional reactivity in response to sad faces (e.g., Kudinova et al., 2016; Phan et al., 2005). Together, this suggests that individuals with rMDD demonstrate an atypical pattern of activation during hot and cold cognition in rMDD. The finding that individuals with rMDD did not recruit the CCN in a context in which it is expected to be helpful (go/no-go) may suggest a deficiency within cognitive control circuitry. This could be problematic when difficult emotional contexts arise that require CCN activation, such as when attempting to down-regulate negative affect, or when trying to inhibit excessive focus on negative experiences (e.g., rumination) (Langenecker et al., 2014; Phan et al., 2005; Phillips et al., 2003).
Prior work has demonstrated that individuals with active MDD show hypersensitivity to emotional stimuli such as negative social signals (Gur et al., 1992; Surguladze et al., 2004; Etkin and Schatzberg, 2011; Ladouceur et al., 2006). Most extant studies on hot cognition have involved individuals with active MDD, showing that individuals with MDD have excessive focus on negative information at the expense of positive information (Robinson et al., 2016). This has included SEN hyperactivity during negative autobiographical recall (Young et al., 2016), as well as behavioral biases toward negative stimuli in an emotional Stroop task with faces (Strand et al., 2013) and recognition memory for emotional faces (Mikhailova et al., 1996). Such group differences have been shown to be particularly pronounced when processing sad faces (Gotlib et al., 2004; Joormann and Gotlib, 2007; Gibb et al., 2016; Victor et al., 2010) and fearful faces (Ohman, 2002; Monk et al., 2008), which may be interpreted as especially personally relevant and most likely to elicit a need for regulation. Thus, in the present study of individuals with MDD in remission, the elevated activation observed in the SEN during hot cognition, particularly when processing sad faces, could indicate elevated salience of (or emotional reactivity toward) negative affective stimuli. Although we are not able to disentangle directionality in the present study, individuals with rMDD who experience excessive SEN activation also may require additional compensatory recruitment of CCN regions (e.g., to inhibit excessive processing of salient stimuli, focusing attention instead on performing the task) to attain performance similar to HCs.
Given that the results from the rMDD sample in the present study parallel those in prior studies of active MDD, the group differences detected here may represent a step toward identifying trait-like IPs that are consistent across phases of illness. The effects observed in the rMDD group might confer vulnerability for depressive relapse, or alternatively, could represent neural scars of past episodes (Rohde et al., 1990). Conversely, recruitment of CCN regions during hot cognition could represent a mechanism of remission maintenance, and hence could be adaptive. For instance, recruiting CCN regions could help individuals to effectively down-regulate excessive negative affect that might be elicited by negative affective stimuli. However, longitudinal studies are necessary in order to determine the stability of these effects across phases of illness and to disentangle these possible alternative explanations.
Strengths and Limitations
A major strength of our study lies in the design. Specifically, we are one of the first to investigate neural network correlates of hot and cold cognition in individuals with rMDD. The study used a narrow, young age range to capture individuals early in the course of depressive illness to minimize the potential accumulation of scarring effects of recurrent episodes. The study also evaluated individuals at a point when developmental variability and age degeneration effects were minimized. However, limitations and directions for future research should be noted. First, we did not directly compare individuals with rMDD to those with active MDD, so it is not possible to determine with certainty whether or how network activation might change during active illness. Second, although the FEPT and PGNG tasks were designed to elicit network-specific activation (i.e., SEN and CCN, respectively), both tasks engaged a significant spatial extent of voxels within the “unintended” network, even amongst healthy controls. We note that these networks identified as “intrinsic” via resting state analyses, are not so simplistic as to have an “off or on” characterization. Third, the FEPT was always administered after the PGNG, to avoid emotional contamination of the PGNG. Unfortunately, this could result in network/task order effects due to fatigue. Future studies of rMDD could complement these results by using an interactive, explicit emotion regulation task that would directly require engaging cognitive control over negative affective material (e.g., Phan et al., 2005; Wager et al., 2008), which could provide additional information about individual differences in trait-like cognitive vulnerability factors, particularly in a dynamic context. In addition, future work should evaluate these processes in at-risk individuals prior to the onset of illness to better distinguish vulnerability markers (e.g., Stange et al., 2016) from scar effects.
In conclusion, the present results suggest that individuals with a history of depression may be characterized by over-activity in both the SEN and CCN during hot cognition, and under-activity in the CCN during cold cognition. Exploring how networks underlying cognition, emotion, and their interactions go awry in depression will improve our mechanistic understanding of the pathophysiology of depression and facilitate the identification of IPs. Understanding individual differences in these processes, and the change in relation to state changes of illness, could be used to identify targets for behavioral or pharmacological treatments, thereby reducing vulnerability to relapse.
Supplementary Material
Acknowledgments
This work was supported by NIMH grant MH 091811 (SAL), and UIC Clinical and Translational Science Awards Program NCATS UL1TR000050 and 1S10RR028898. Jonathan P. Stange was supported by grant 5T32MH067631-12 from NIMH.
References
- Aldao A, Nolen-Hoeksema S, Schweizer S. Emotion-regulation strategies across psychopathology: A meta-analytic review. Clin Psychol Rev. 2010;30:217–237. doi: 10.1016/j.cpr.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Alexopoulos GS, Murphy CF, Gunning-Dixon FM, Kalayam B, Katz R, Kanellopoulos D, Etwaroo GR, Klimstra S, Foxe JJ. Event-related potentials in an emotional go/no-go task and remission of geriatric depression. Neuroreport. 2007;18:217–221. doi: 10.1097/WNR.0b013e328013ceda. [DOI] [PubMed] [Google Scholar]
- Banich MT, Mackiewicz KL, Depue BE, Whitmer AJ, Miller GA, Heller W. Cognitive control mechanisms, emotion and memory: a neural perspective with implications for psychopathology. Neurosci Biobehav Rev. 2009;33:613–630. doi: 10.1016/j.neubiorev.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2:303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertocci MA, Bebko GM, Mullin BC, Langenecker SA, Ladouceur CD, Almeida JR, Phillips ML. Abnormal anterior cingulate cortical activity during emotional n-back task performance distinguishes bipolar from unipolar depressed females. Psychol Med. 2012;42:1417–1428. doi: 10.1017/S003329171100242X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briceño EM, Weisenbach SL, Rapport LJ, Hazlett KE, Bieliauskas LA, Haase BD, Ransom MT, Brinkman ML, Pecina M, Schteingart DE, Starkman MN, Giordani B, Noll DC, Zubieta JK, Langenecker SA. Shifted inferior frontal laterality in women with major depressive disorder is related to emotion-processing deficits. Psychol Med. 2013;43:1433–1445. doi: 10.1017/S0033291712002176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucholz KK, Cadoret R, Cloninger CR, Dinwiddie SH, Hesselbrock VM, Nurnberger JI, Jr, Reich T, Schmidt I, Schuckit MA. A new, semi-structured psychiatric interview for use in genetic linkage studies: a report on the reliability of the SSAGA. J Stud Alcohol Drugs. 1994;55:149–158. doi: 10.15288/jsa.1994.55.149. [DOI] [PubMed] [Google Scholar]
- Crane NA, Jenkins LM, Bhaumik R, Dion C, Gowins JR, Mickey BJ, Zubieta JK, Langenecker SA. Multidimensional prediction of treatment response to antidepressants with cognitive control and functional MRI. Brain. 2017;140:472–486. doi: 10.1093/brain/aww326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cromheeke S, Mueller SC. Probing emotional influences on cognitive control: an ALE metaanalysis of cognition emotion interactions. Brain Struct Func. 2014;219:995–1008. doi: 10.1007/s00429-013-0549-z. [DOI] [PubMed] [Google Scholar]
- Dawson EL, Caveney AF, Meyers KK, Weisenbach SL, Giordani B, Avery ET, Schallmo MP, Bahadori A, Bieliauskas LA, Mordhorst M, Marcus SM. Executive Functioning at Baseline Prospectively Predicts Depression Treatment Response. Prim Care Companion CNS Disord. 2017:19. doi: 10.4088/PCC.16m01949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lissnyder E, Koster EH, Everaert J, Schacht R, Van den Abeele D, De Raedt R. Internal cognitive control in clinical depression: General but no emotion-specific impairments. Psychiatry Res. 2012;199:124–130. doi: 10.1016/j.psychres.2012.04.019. [DOI] [PubMed] [Google Scholar]
- De Lissnyder E, Koster EH, Derakshan N, De Raedt R. The association between depressive symptoms and executive control impairments in response to emotional and non- emotional information. Cogn Emot. 2010;24:264–280. [Google Scholar]
- Derrfuss J, Brass M, Neumann J, von Cramon DY. Involvement of the inferior frontal junction in cognitive control: Meta–analyses of switching and Stroop studies. Hum Brain Mapp. 2005;25:22–34. doi: 10.1002/hbm.20127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EA, Beck AT. Neural mechanisms of the cognitive model of depression. Nat Rev Neurosci. 2011;12:467–477. doi: 10.1038/nrn3027. [DOI] [PubMed] [Google Scholar]
- Ekman P, Friesen P. Pictures of Facial Affect. Consulting Psychologists Press; Palo Alto, CA: 1976. [Google Scholar]
- Etkin A, Büchel C, Gross JJ. The neural bases of emotion regulation. Nat Rev Neurosci. 2015;16:693–700. doi: 10.1038/nrn4044. [DOI] [PubMed] [Google Scholar]
- Etkin A, Schatzberg AF. Common abnormalities and disorder-specific compensation during implicit regulation of emotional processing in generalized anxiety and major depressive disorders. Am J Psychiatry. 2011;168:968–78. doi: 10.1176/appi.ajp.2011.10091290. [DOI] [PubMed] [Google Scholar]
- Fitzgerald PB, Laird AR, Maller J, Daskalakis ZJ. A meta-analytic study of changes in brain activation in depression. Hum Brain Mapp. 2008;29:683–95. doi: 10.1002/hbm.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu CH, Mourao-Miranda J, Costafreda SG, Khanna A, Marquand AF, Williams SC, Brammer MJ. Pattern classification of sad facial processing: toward the development of neurobiological markers in depression. Biol Psychiatry. 2008;63:656–662. doi: 10.1016/j.biopsych.2007.08.020. [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Scott J, Mitterschiffthaler MT, Walsh ND, Donaldson C, Suckling J, Andrew C, Steiner H, Murray RM. Neural responses to sad facial expressions in major depression following cognitive behavioral therapy. Biol Psychiatry. 2008;64:505–512. doi: 10.1016/j.biopsych.2008.04.033. [DOI] [PubMed] [Google Scholar]
- Fu CH, Williams SC, Cleare AJ, Brammer MJ, Walsh ND, Kim J, Andrew CM, Pich EM, Williams PM, Reed LJ, Mitterschiffthaler MT, Suckling J, Bullmore ET. Attenuation of the neural response to sad faces in major depression by antidepressant treatment: a prospective, event-related functional magnetic resonance imaging study. Arch Gen Psychiatry. 2004;61:877–889. doi: 10.1001/archpsyc.61.9.877. [DOI] [PubMed] [Google Scholar]
- Gibb BE, McGeary JE, Beevers CG. Attentional biases to emotional stimuli: Key components of the RDoC constructs of sustained threat and loss. Am J Med Genet B Neuropsychiatr Genet. 2016;171:65–80. doi: 10.1002/ajmg.b.32383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–45. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Muñoz RF. Emotion regulation and mental health. Clin Psychol (New York) 1995;2:151–64. [Google Scholar]
- Gotlib IH, Krasnoperova E, Yue DN, Joormann J. Attentional biases for negative interpersonal stimuli in clinical depression. J Abnorm Psychol. 2004;113:121–135. doi: 10.1037/0021-843X.113.1.121. [DOI] [PubMed] [Google Scholar]
- Gotlib IH, Sivers H, Gabrieli JD, Whitfield-Gabrieli S, Goldin P, Minor KL, Canli T. Subgenual anterior cingulate activation to valenced emotional stimuli in major depression. Neuroreport. 2005;16:1731–1734. doi: 10.1097/01.wnr.0000183901.70030.82. [DOI] [PubMed] [Google Scholar]
- Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC. Facial emotion discrimination: II. Behavioral Findings in Depression. Psychiatry Res. 1992;42:241–51. doi: 10.1016/0165-1781(92)90116-k. [DOI] [PubMed] [Google Scholar]
- Hofmann SG, Sawyer AT, Fang A, Asnaani A. Emotion dysregulation model of mood and anxiety disorders. Depress Anxiety. 2012;29:409–416. doi: 10.1002/da.21888. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Selective attention to emotional faces following recovery from depression. J Abnorm Psychol. 2007;116:80–85. doi: 10.1037/0021-843X.116.1.80. [DOI] [PubMed] [Google Scholar]
- Joormann J, Gotlib IH. Updating the contents of working memory in depression: interference from irrelevant negative material. J Abnorm Psychol. 2008;117:182–192. doi: 10.1037/0021-843X.117.1.182. [DOI] [PubMed] [Google Scholar]
- Joormann J, Levens SM, Gotlib IH. Sticky thoughts: Depression and rumination are associated with difficulties manipulating emotional material in working memory. Psychol Sci. 2011;22:979–983. doi: 10.1177/0956797611415539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Quinn ME. Cognitive processes and emotion regulation in depression. Depress Anxiety. 2014;31:308–315. doi: 10.1002/da.22264. [DOI] [PubMed] [Google Scholar]
- Joormann J, Vanderlind WM. Emotion Regulation in Depression: The Role of Biased Cognition and Reduced Cognitive Control. Clinical Psychol Sci. 2014;2:402–421. [Google Scholar]
- Kohler CG, Hoffman LJ, Eastman LB, Healey K, Moberg PJ. Facial emotion perception in depression and bipolar disorder: a quantitative review. Psychiatry Res. 2011;188:303–309. doi: 10.1016/j.psychres.2011.04.019. [DOI] [PubMed] [Google Scholar]
- Kudinova AY, Burkhouse KL, Siegle G, Owens M, Woody ML, Gibb BE. Pupillary reactivity to negative stimuli prospectively predicts recurrence of major depressive disorder in women. Psychophysiology. 2016;53:1836–1842. doi: 10.1111/psyp.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladouceur CD, Dahl RE, Williamson DE, Birmaher B, Axelson DA, Ryan ND, Casey BJ. Processing emotional facial expressions influences performance on a Go/NoGo task in pediatric anxiety and depression. J Child Psychol Psychiatry. 2006;47:1107–1115. doi: 10.1111/j.1469-7610.2006.01640.x. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Bieliauskas LA, Rapport LJ, Zubieta JK, Wilde EA, Berent S. Face emotion perception and executive functioning deficits in depression. J Clin Exp Neuropsychol. 2005;27:320–33. doi: 10.1080/13803390490490515720. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Caveney AF, Giordani B, Young EA, Nielson KA, Rapport LJ, Bieliauskas LA, Mordhorst MJ, Marcus S, Yodkovik N, Kerber K, Berent S, Zubieta JK. The sensitivity and psychometric properties of a brief computer-based cognitive screening battery in a depression clinic. Psychiatry Res. 2007;152:143–154. doi: 10.1016/j.psychres.2006.03.019. [DOI] [PubMed] [Google Scholar]
- Langenecker SA, Jacobs R, Passarotti HAM. Current Neural and Behavioral Dimensional Constructs across Mood Disorders. Curr Behav Neurosci Rep. 2014;1:144–153. doi: 10.1007/s40473-014-0018-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Kennedy SE, Guidotti LM, Briceno EM, Own LS, Hooven T, Young EA, Akil H, Noll DC, Zubieta JK. Frontal and limbic activation during inhibitory control predicts treatment response in major depressive disorder. Biol Psychiatry. 2007;62:1272–1280. doi: 10.1016/j.biopsych.2007.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langenecker SA, Zubieta JK, Young EA, Akil H, Nielson KA. A task to manipulate attentional load, set-shifting, and inhibitory control: convergent validity and test-retest reliability of the Parametric Go/No-Go Test. J Clin Exp Neuropsychol. 2007;29:842–853. doi: 10.1080/13803390601147611. [DOI] [PubMed] [Google Scholar]
- Malooly AM, Genet JJ, Siemer M. Individual differences in reappraisal effectiveness: the role of affective flexibility. Emotion. 2013;13:302–313. doi: 10.1037/a0029980. [DOI] [PubMed] [Google Scholar]
- Maxwell ME. Clinical Neurogenetics Branch, Intramural Research Program. National Institute of Mental Health; Bethesda, MD: 1992. Family Interview for Genetic Studies (FIGS): a manual for FIGS. [Google Scholar]
- Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci. 2011;15:483–506. doi: 10.1016/j.tics.2011.08.003. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Weinberger DR. Intermediate phenotypes and genetic mechanisms of psychiatric disorders. Nat Rev Neurosci. 2006;7:818–27. doi: 10.1038/nrn1993. [DOI] [PubMed] [Google Scholar]
- Mikhailova ES, Vladimirova TV, Iznak AF, Tsusulkovskaya EJ, Sushko NV. Abnormal recognition of facial expression of emotions in depressed patients with major depression disorder and schizotypal personality disorder. Biol Psychiatry. 1996;40:697–705. doi: 10.1016/0006-3223(96)00032-7. [DOI] [PubMed] [Google Scholar]
- Mitterschiffthaler MT, Williams SC, Walsh ND, Cleare AJ, Donaldson C, Scott J, Fu CH. Neural basis of the emotional Stroop interference effect in major depression. Psychol Med. 2008;38:247–56. doi: 10.1017/S0033291707001523. [DOI] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, 3rd, Guardino M, Masten CL, McClure-Tone EB, Fromm S, Blair RJ, Pine DS, Ernst M. Amygdala and nucleus accumbens activation to emotional facial expressions in children and adolescents at risk for major depression. Am J Psychiatry. 2008;165:90–98. doi: 10.1176/appi.ajp.2007.06111917. [DOI] [PubMed] [Google Scholar]
- Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT, Phillips ML. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. Am J Psychiatry. 2010;167:1373–1380. doi: 10.1176/appi.ajp.2010.09081235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy FC, Michael A, Sahakian BJ. Emotion modulates cognitive flexibility in patients with major depression. Psychol Med. 2012;42:1373–1382. doi: 10.1017/S0033291711002418. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy, Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training NIMH Genetics Initiative. Arch Gen Psychiatry. 1994;51:849–59. doi: 10.1001/archpsyc.1994.03950110009002. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Silvers JA, Buhle JT. Functional imaging studies of emotion regulation: a synthetic review and evolving model of the cognitive control of emotion. Ann N Y Acad Sci. 2012;1251:E1–24. doi: 10.1111/j.1749-6632.2012.06751.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Öhman A. Automaticity and the amygdala: Nonconscious responses to emotional faces. Current Directions in Psychol Sci. 2002;11:62–66. [Google Scholar]
- Pe ML, Raes F, Koval P, Brans K, Verduyn P, Kuppens P. Interference resolution moderates the impact of rumination and reappraisal on affective experiences in daily life. Cogn Emo. 2013;27:492–501. doi: 10.1080/02699931.2012.719489. [DOI] [PubMed] [Google Scholar]
- Pe ML, Vandekerckhove J, Kuppens P. A diffusion model account of the relationship between the emotional flanker task and rumination and depression. Emotion. 2013;13:739–747. doi: 10.1037/a0031628. [DOI] [PubMed] [Google Scholar]
- Perlman SB, Almeida JR, Kronhaus DM, Versace A, Labarbara EJ, Klein CR, Phillips ML. Amygdala activity and prefrontal cortex-amygdala effective connectivity to emerging emotional faces distinguish remitted and depressed mood states in bipolar disorder. Bipolar Disord. 2012;14:162–174. doi: 10.1111/j.1399-5618.2012.00999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters AT, Jacobs RH, Crane NA, Ryan KA, Weisenbach SL, Ajilore O, Lamar M, Kassel MT, Gabriel LB, West AE, Zubieta JK, Langenecker SA. Domain-specific impairment in cognitive control among remitted youth with a history of major depression. Early Interv Psychiatry. 2015 doi: 10.1111/eip.12253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biol Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: Implications for major psychiatric disorders. Biol Psychiatry. 2003;54:515–528. doi: 10.1016/s0006-3223(03)00171-9. [DOI] [PubMed] [Google Scholar]
- Rapport LJ, Friedman SL, Tzelepis A, Van Voorhis A. Experienced emotion and affect recognition in adult attention-deficit hyperactivity disorder. Neuropsychology. 2002;16:102–110. doi: 10.1037//0894-4105.16.1.102. [DOI] [PubMed] [Google Scholar]
- Rohde P, Lewinsohn PM, Seeley JR. Are people changed by the experience of having an episode of depression? A further test of the scar hypothesis. J Abnorm Psychol. 1990;99:264. doi: 10.1037//0021-843x.99.3.264. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Roiser JP, Sahakian BJ. Hot and cold cognition in depression. In: McIntyre RS, editor. Cognitive Impairment in Major Depressive Disorder. Cambridge University Press; Cambridge, UK: 2016. [Google Scholar]
- Roiser JP, Sahakian BJ. Hot and cold cognition in depression. CNS Spectr. 2013;18:139–149. doi: 10.1017/S1092852913000072. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheline YI, Barch DM, Donnelly JM, Ollinger JM, Snyder AZ, Mintun MA. Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study. Biol Psychiatry. 2001;50:651–658. doi: 10.1016/s0006-3223(01)01263-x. [DOI] [PubMed] [Google Scholar]
- Siegle GJ, Thompson W, Carter CS, Steinhauer SR, Thase ME. Increased amygdala and decreased dorsolateral prefrontal BOLD responses in unipolar depression: related and independent features. Biol Psychiatry. 2007;61:198–209. doi: 10.1016/j.biopsych.2006.05.048. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, Bessette KL, Jenkins L, Peters AT, Feldhaus C, Crane NA, Ajilore O, Jacobs RH, Watkins ER, Langenecker SA. Attenuated intrinsic connectivity within the cognitive control network among individuals with remitted depression is associated with cognitive control deficits and negative cognitive styles. Hum Brain Mapp. 2017;38:2939–2954. doi: 10.1002/hbm.23564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stange JP, Connolly SL, Burke TA, Hamilton JL, Hamlat EJ, Abramson LY, Alloy LB. Inflexible cognition predicts first onset of major depressive episodes in adolescence. Depress Anxiety. 2016;33:1005–1012. doi: 10.1002/da.22513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strand M, Oram MW, Hamma Å. Emotional information processing in major depression remission and partial remission: faces come first. Appl Neuropsychol Adult. 2013;20:110–119. doi: 10.1080/09084282.2012.670159. [DOI] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML. A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry. 2005;57:201–209. doi: 10.1016/j.biopsych.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Surguladze SA, Young AW, Senior C, Brébion G, Travis MJ, Phillips ML. Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology. 2004;18:12–18. doi: 10.1037/0894-4105.18.2.212. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey BJ, Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168:242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versace A, Thompson WK, Zhou D, Almeida JR, Hassel S, Klein CR, Kupfer DJ, Phillips ML. Abnormal left and right amygdala-orbitofrontal cortical functional connectivity to emotional faces: state versus trait vulnerability markers of depression in bipolar disorder. Biol Psychiatry. 2010;67:422–431. doi: 10.1016/j.biopsych.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor TA, Furey ML, Fromm SJ, Ohman A, Drevets WC. Relationship between amygdala responses to masked faces and mood state and treatment in major depressive disorder. Arch Gen Psychiatry. 2010;67:1128–1138. doi: 10.1001/archgenpsychiatry.2010.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Votruba KL, Langenecker SA. Factor structure, construct validity, and age-and education-based normative data for the Parametric Go/No-Go Test. J Clin Exp Neuropsychol. 2013;35:132–146. doi: 10.1080/13803395.2012.758239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron. 2008;59:1037–1050. doi: 10.1016/j.neuron.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Öngür D, Auerbach RP, Yao S. Cognitive Vulnerability to Major Depression: View from the Intrinsic Network and Cross-network Interactions. Harv Rev Psychiatry. 2016;24:188–201. doi: 10.1097/HRP.0000000000000081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL. The organization of the human cerebral cortex estimated by intrinsic functional connectivity. J Neurophysiol. 2011;106:1125–1165. doi: 10.1152/jn.00338.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young KD, Siegle GJ, Bodurka J, Drevets WC. Amygdala Activity During Autobiographical Memory Recall in Depressed and Vulnerable Individuals: Association With Symptom Severity and Autobiographical Overgenerality. Am J Psychiatry. 2016;173:78–89. doi: 10.1176/appi.ajp.2015.15010119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.