Abstract
Targeting different members of the Akt pathways is a promising therapeutic chance in solid tumors including breast cancer. The variable expression levels of Akt isoforms with opposite effects on tumor growth and metastasis, however, make it difficult to select the inhibitors to be used for specific breast tumor subtypes. Using in vitro and in vivo models, we demonstrated here that Vav1, ectopically expressed in invasive breast tumors derived cells, downmodulates Akt acting at expression and/or activation levels depending on tumor subtype. The decreased p‐Akt1 (Ser473) levels are a common effect of Vav1 upmodulation, suggesting that, in breast tumor‐derived cells and independently of their phenotype, Vav1 interferes with signaling pathways ended to specifically recruit Akt1. Only in ER‐negative cell lines, the silencing of Vav1 induced the expression but not the activation of Akt2. A retrospective analysis of early invasive breast tumors allowed to establish the prognostic significance of the p‐Akt/Vav1 relationship. In particular, low Vav1 levels negatively influence the follow‐up of patients with low p‐Akt in their primary tumors and subjected to adjuvant chemotherapy. As the use of specific or pan Akt inhibitors may not be sufficient or may even be detrimental, increasing the levels of Vav1 could be a new approach to improve breast cancer outcomes. This might be particularly relevant for tumors with a triple‐negative phenotype, for which target‐based therapies are not currently available.
Keywords: Akt1, breast cancer, Vav1
Abbreviations
- DMEM
Dulbecco's modified Eagle's medium
- DRFS
distant relapse‐free survival
- FBS
fetal bovine serum
- GEF
guanosine exchange factor
- TMAs
tissue microarrays
- TNBC
triple‐negative breast cancers
1. Introduction
The multidomain protein Vav1 is physiologically expressed solely in the hematopoietic system in which it participates to cytoskeleton reorganization and gene transcription (Romero and Fischer, 1996; Tybulewicz, 2005). In recent years, aberrant expression of Vav1 has been reported in nonhematopoietic cancers (Bartolome et al., 2006; Fernandez‐Zapico et al., 2005; Hornstein et al., 2003; Lazer et al., 2009; Qi et al., 2015; Wakahashi et al., 2013; Zhu et al., 2017), in which this protein is involved in signal transduction processes correlated with tumor phenotype (Fernandez‐Zapico et al., 2005; Qi et al., 2015; Zhu et al., 2017). Vav1 is expressed in the majority of breast carcinomas (Sebban et al., 2013), in which we have previously demonstrated its peculiar localization inside the cell nucleus (Grassilli et al., 2014). In early‐stage invasive breast tumors, high levels of nuclear Vav1 were positively correlated with low risk of relapse, constituting a positive prognostic factor independent of molecular subtypes. Accordingly, the upmodulation of Vav1 in invasive breast tumor‐derived cells reduced their invasiveness in vitro and their metastatic efficiency in vivo (Grassilli et al., 2014).
The malignancy‐associated role of Vav1 has been variously linked to its best known cytoplasmic function as guanosine exchange factor (GEF) for Rho/Rac GTPases (Bustelo, 2002), mainly devoted to the rearrangement of actin cytoskeleton at the basis of the functional changes that characterize tumor cells (Bartolome et al., 2006; Fernandez‐Zapico et al., 2005; Lazer et al., 2009). However, additional roles of Vav1, correlated to its nuclear localization, were clearly established in cells from myeloid leukemia (Bertagnolo et al., 2012; Houlard et al., 2002), in which it plays an antitumor role by collaborating in gene expression (Brugnoli et al., 2010; Grassilli et al., 2016) and in mRNA production and stability (Bertagnolo et al., 2011).
In breast tumor‐derived cell lines, divergent effects exerted by Vav1 on Rac1 activation (Sebban et al., 2013) suggest the existence of signaling pathways involving Vav1 alternative to guanosine exchange activity. According to its role in modulating nucleic acid metabolism, in mammary tumor cells Vav1 accumulates in subnuclear structures and its downmodulation induces a phenotype‐related expression of genes variously involved in malignant progression (Grassilli et al., 2014). They include Akt1 and the gene encoding for the PI3K/Akt activator PDGFRB (Zhang et al., 2007), suggesting that Vav1 may affect the Akt‐related machinery in mammary tumors.
Abnormal activation of Akt pathways is the most common aberrations of signal transduction in solid tumors, including breast cancer (Mundi et al., 2016; Yang et al., 2016) in which the three known Akt isoforms (Akt1, Akt2, and Akt3) show a phenotype‐related expression (Clark and Toker, 2014; Iacovides et al., 2013). Epidemiological and preclinical studies confirmed that activation of Akt is implicated in the pathogenesis of breast cancer also by conferring resistance to systemic treatments (Yang et al., 2016) and a number of molecules have been generated to selectively or nonselectively inhibit the three isoforms (Dey et al., 2017; Mundi et al., 2016). Therefore, despite a promising chance, emerging data revealing different or even opposite functional roles of Akt isoforms in the regulation of proliferation, migration, and invasion (Dillon et al., 2009; Li et al., 2016; Riggio et al., 2017) opened the issue of the reliability of the use of pan or isoform‐specific Akt inhibitors in the different tumor subtypes and of the combined use of inhibitors and cytotoxic chemotherapy. The development of Akt inhibitors is particularly problematic in triple‐negative breast cancers (TNBC) that express all three Akt isoforms and show the highest activation of the Akt downstream pathways (Chin et al., 2014; Grottke et al., 2016; Massihnia et al., 2016).
This work was aimed to establish whether Vav1 has a role in modulating the Akt signaling in breast cancer cell lines belonging to different tumor phenotypes. The study included both in vitro and in vivo models in which the effects of forcedly modulated Vav1 on the main Akt1‐related pathways were investigated primarily in breast tumor cells with a triple‐negative phenotype. Archived formalin‐fixed breast tumor samples allowed to establish the prognostic significance of the Vav1/p‐Akt relationship in patients with early breast cancer.
2. Materials and methods
2.1. Antibodies and reagents
All reagents were from Sigma (St Louis, MO, USA) unless otherwise indicated.
For immunochemical analysis, antibodies against Vav1 (sc‐132), Akt1 (sc‐1618), Akt2 (sc‐5270), Akt3 (sc‐11520), p‐Akt1/2/3 (sc‐14032), Bcl‐2 (sc‐509), Caspase‐3 (sc‐371), and IkBα (sc‐7148) were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti‐p‐Akt1 (Ser473) (#4060), anti‐p‐Akt2 (Ser474) (#8599), anti‐p‐P70S6K (Thr389) (#9205), and anti‐P70S6K (#9202) were from Cell Signaling Technology (Danvers, MA, USA). Anti‐Bax (#610983) was from BD Biosciences (Milan, Italy), anti‐Cyclin D1 (#04‐1151) was from Merck Millipore (Milan, Italy), and anti‐β‐Tubulin (#T4026) was from Sigma.
For immunohistochemical analysis, the anti‐Vav1 (sc‐132) and the anti‐Akt1 (sc‐377457) antibodies were from Santa Cruz Biotechnology, anti‐p‐Akt (Ser473) (#3787) antibody was from Cell Signaling Technology, and the anti‐Cyclin D1 (MCP511) antibody was from YLEM (Rome, Italy).
2.2. Cell culture
MDA‐MB‐231, MCF7, and MDA‐MB‐453 cell lines were from the American Type Culture Collection (Rockville, MD, USA) and were maintained in Dulbecco's modified Eagle's medium (DMEM, Gibco Laboratories, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (FBS, Gibco Laboratories). The BT‐474 cell line was from ICLC (Genova, Italy) and was cultured in RPMI 1640 growth medium (Gibco Laboratories) supplemented with 10% FBS, 1 mm Na pyruvate, and 0.01 mg·mL−1 bovine insulin. Cells were grown at 37 °C in a humidified atmosphere of 5% CO2 in air.
MDA‐MB‐231 cells stably expressing Vav1 were obtained by transfection with a construct expressing the human full‐length Vav1, as previously reported (Grassilli et al., 2014).
All cell lines were monthly tested for mycoplasm and other contaminations and quarterly subjected to cell identification by single nucleotide polymorphism.
2.3. Modulation of Vav1 expression and cell analysis
Vav1 overexpression was obtained by transient transfection with a pEF plasmid expressing the human full‐length Vav1 (Grassilli et al., 2014), and the downmodulation of Vav1 was performed by silencing the protein with specific siRNA (Santa Cruz Biotechnology), following previously described procedures (Grassilli et al., 2014).
For western blot analysis, total lysates (50 μg protein) and Akt3 immunoprecipitates, obtained as previously reported (Bertagnolo et al., 2007), were separated on polyacrylamide denaturing gels, blotted to nitrocellulose membranes (GE Healthcare Life Science, Little Chalfont, UK), and reacted with primary antibodies. Membranes were then incubated with peroxidase‐conjugated secondary antibodies and revealed with the ECL system (PerkinElmer, Boston, MA, USA). The chemiluminescence of bands was acquired with ImageQuant™ LAS 4000 biomolecular imager (GE Healthcare), and the densitometric analysis was performed with image quant tl software (GE Healthcare).
Proliferation of MDA‐MB‐231 cells subjected to forced modulation of Vav1 was evaluated with the xCELLigence Real‐Time Cell Analyzer System (Acea Biosciences Inc., San Diego, CA, USA), as previously reported (Brugnoli et al., 2013).
To determine the levels of apoptosis, one‐step staining procedure with the Annexin V‐FITC kit was performed, following manufacturer's protocol (Immunotech, Beckman Coulter Company, Marseille, France). Data collected from 20 000 cells were analyzed using a FACSCalibur flow cytometer (BD Biosciences) with cellquest pro 6.0 analysis software (BD Biosciences).
2.4. MDA‐MB‐231‐derived xenografts
All procedures for animal experiments were approved by the Committee on the Use and Care of Animals and carried out in strict accordance with the institution guidelines (CEISA—Interuniversity Ethics Committee for Animal Experimentation, Chieti, Italy). 6 × 106 MDA‐MB‐231 cells, stably overexpressing human Vav1 or an empty vector, were injected subcutaneously into BALB/c nude mice (female, age six weeks, n = 10 per group, Charles River Laboratories Italia, Lecco, Italy), anesthetized with ethyl ether. Tumors were weakly measured with caliper, and tumor volume was determined (width2 × length)/2).
Throughout the experiments, mice were maintained with free access to pellet food and water. After 8 weeks, the mice were euthanized with inhalation of CO2 and the tumor xenografts were removed, photographed, and fixed with 4% paraformaldehyde.
Paraffin‐embedded xenografts were sliced into 5‐μm sections using a Leica microtome (Leica Biosystems, Wetzlar, Germany). Tissue sections were deparaffinized, stained with hematoxylin and eosin (HE) dyes (Bio‐Optica, Milano, Italy), and observed by an optical microscope (Carl Zeiss Axiophot 100, Zeiss, Göttingen, Germany) equipped with a Nikon Digital Sight DS Vi1 camera (Nikon Instruments S.p.A., Florence, Italy).
2.5. Patients and tumors
The current study included a cohort of 126 primary unilateral infiltrating breast cancers from N0 patients with T1/T2 tumors and diagnosed between 1994 and 2001 at the Regina Elena National Cancer Institute (Rome, Italy). The study was reviewed and approved by the ethics committee of the ‘Regina Elena’ National Cancer Institute, and written informed consent was obtained from all patients, according to the Helsinki Declaration of 1975. Tumors were evaluated for their proliferation levels and receptors status as previously reported (Lattanzio et al., 2013). Radiotherapy was offered to all patients, 37 of them were treated exclusively with hormonal therapy and 76 received adjuvant chemotherapy followed or not by hormonal therapy. Patients with HER‐2‐positive tumors did not receive trastuzumab, because it was unavailable during the study period. The median follow‐up was of 94 months (range 19–171 months). Follow‐up data were obtained from institutional records or by the referring physicians. Patients and tumor characteristics are summarized in Table 1. Tissue microarrays (TMAs) were constructed as described by Lattanzio et al. (2013).
Table 1.
Patients and tumor characteristics (n = 126)
| Variable | Value (%) |
|---|---|
| Age at diagnosis (years) | |
| Median | 55.7 |
| <50 | 42 (33.3) |
| 50–65 | 50 (39.7) |
| >65 | 34 (27.0) |
| Menopausal status | |
| Pre/perimenopausal | 44 (34.9) |
| Postmenopausal | 82 (65.1) |
| Tumor size | |
| 2 cm | 81 (64.3) |
| >2 cm | 45 (35.7) |
| Histotypes | |
| Ductal carcinoma | 100 (79.4) |
| Lobular carcinoma | 17 (13.5) |
| Other | 9 (7.1) |
| Molecular subtypes | |
| Luminal A‐like | 56 (44.4) |
| Luminal B‐like (HER2 negative) | 31 (24.6) |
| Luminal B‐like (HER2 positive) | 11 (8.7) |
| HER2 positive (non‐luminal) | 6 (4.8) |
| Triple negative (ductal) | 22 (17.5) |
| Tumor grade | |
| 1 | 16 (12.7) |
| 2 | 68 (54.0) |
| 3 | 42 (33.3) |
| Ki‐67 | |
| Low | 68 (54.0) |
| High | 58 (46.0) |
| Patient outcome | |
| Without recurrence | 103 (81.7) |
| Local recurrence | 8 (6.4) |
| Distant recurrence | 15 (11.9) |
2.6. Immunohistochemical analysis and statistical methods
Immunohistochemical analysis of Vav1 in TMAs and of Akt1 and p‐Akt (Ser473) in xenografts was performed as previously reported (Grassilli et al., 2014). For evaluation of Vav1 levels, the cellular staining intensity was estimated with the imagescope software (Aperio, Vista, CA, USA) by analysis of acquired images, as previously described (Grassilli et al., 2014). For quantification of Akt1 and p‐Akt (Ser473) staining, optical microscope images containing at least 100 cells were analyzed using the Aperio Positive Pixel Count algorithm, embedded in the aperio imagescope software.
Immunohistochemical analysis of p‐Akt (Ser473) and Cyclin D1 in TMAs was performed as reported by Lattanzio et al. (2013). p‐Akt (Ser473) score was based on the number of stained cells: 0, no staining; 1+, < 20% of positive cells; 2+, 20–50% of positive cells; 3+, >50% of positive cells. For statistical purposes, scores of 2+ or 3+ in either the nucleus or cytoplasm were considered high staining (Huang et al., 2013). The cutoff value used for high Cyclin D1 expression was >10% (Ravikumar and Ananthamurthy, 2014).
Comparisons of immunostaining (immunohistochemistry, western blot, flow cytometry) values, proliferation rate, and tumor growth between groups were performed using the two‐tailed t‐test for unpaired data. The statistical analysis was performed with the graphpad prism 6.0 statistical package (GraphPad Software, San Diego, CA, USA). P < 0.05 was considered statistically significant.
The relationship between Vav1 status and the expression of p‐Akt (Ser473) and/or Cyclin D1 in patients’ samples, as well as the correlation of Vav1 with clinicopathological parameters, was evaluated by Pearson's chi‐square test. Kaplan–Meier plots were used to show the survival in specified cohorts and the log‐rank test to check for equality of survival curves. Distant relapse‐free survival (DRFS) was the time from surgery to the occurrence of relapse at a distant site. spss version 15.0 (SPSS, Chicago, IL, USA) was used throughout.
3. Results
3.1. Modulation of Vav1 affects the activation status of Akt1 in breast cancer‐derived cells with a triple‐negative phenotype
To assess whether Vav1 affects expression and/or activation of Akt1 in breast tumor cells with a triple‐negative phenotype, MDA‐MB‐231 cells were transiently transfected with siRNA specific for Vav1 or with a construct expressing the human protein (Fig. 1A). While immunochemical analysis showed unchanged Akt1 amount, the use of a specific phospho‐antibody revealed, in MDA‐MB‐231 cells subjected to Vav1 downmodulation, an increased phosphorylation of Akt1 on Ser473, known to be required for its maximal activity (Guo et al., 2014). Accordingly, a significant decrease in p‐Akt1 (Ser473) was detected as a consequence of Vav1 overexpression (Fig. 1B).
Figure 1.
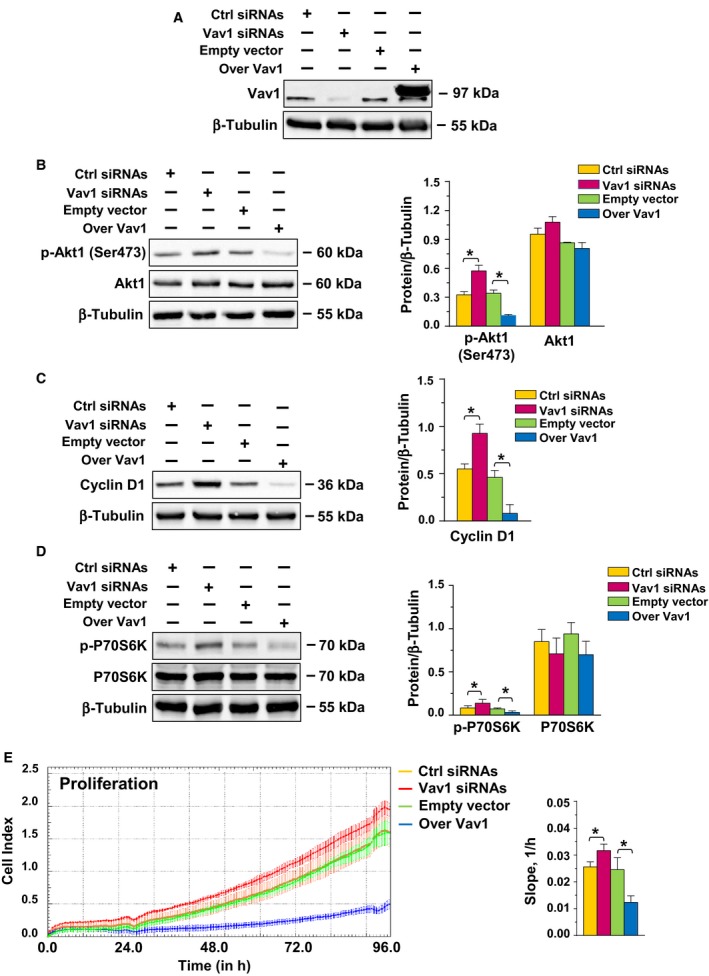
Vav1‐dependent regulation of Akt1 in triple‐negative breast cancer‐derived cells. (A, B, C, D) Representative western blot analysis, performed with the indicated antibodies, of total lysates from MDA‐MB‐231 cells transfected with siRNA specific for Vav1 (Vav1 siRNA) or with a plasmid expressing human Vav1 (Over Vav1). Scramble siRNA (Ctrl siRNA) and an empty vector were used as control of the experiment. β‐Tubulin was blotted as an internal control of loaded proteins. Right histograms show the levels of the indicated proteins normalized to β‐tubulin. (E) MDA‐MB‐231 cells under the same experimental conditions were subjected to dynamic monitoring of proliferation using the impedance‐based xCELLigence Real‐Time Cell analysis (RTCA) system. Cell Index (CI) is reported, and error bars indicate ±SD. The correspondent slope analysis that describes the steepness, incline, gradient, and changing rate of the CI curves over time is shown on the right. All the data are the mean of three separate experiments ±SD. *P < 0.05.
As activated Akt1 exerts its role in tumor progression mainly by inducing cell proliferation or preventing apoptosis (Guerrero‐Zotano et al., 2016), the effects of the forced modulation of Vav1 on these cellular events were investigated. Immunochemical analysis revealed a significant increase or decrease in Cyclin D1 as a consequence of silencing or overexpression of Vav1, respectively (Fig. 1C). Lysates from MDA‐MB‐231 cells in which the expression of Vav1 was forcedly modulated were also subjected to immunochemical analysis of the serine/threonine kinase P70S6K. While the total amount of the protein was not affected, its phosphorylation level on Thr389 was significantly increased or decreased, respectively, by silencing or overexpression of Vav1 (Fig. 1D). The real‐time proliferation assay demonstrated a small but significant increase in growth of Vav1‐silenced cells and a substantial decrease in cell proliferation as a consequence of Vav1 overexpression (Fig. 1E).
Immunochemical analysis did not show changes of Bcl‐2, Bax, pro‐Caspase 3, and IkBα (Fig. S1A,B) as a consequence of Vav1 modulation. Accordingly, no significant modification of the number of apoptotic cells was observed (Fig. S1C).
As in vitro data suggested that dysregulation of the Akt1 pathway induced by Vav1 is mainly reflected by cell proliferation, the role of Vav1 in affecting the in vivo proliferation of MDA‐MB‐231 cells was investigated. Xenografted mice were obtained with MDA‐MB‐231 cells stably overexpressing Vav1 (Fig. 2A), and the solid tumors formed in the subcutaneous skin layer were evaluated. Tumor masses, detected starting from the second week after injection, reached a significantly lower dimension in mice receiving MDA‐MB‐231 cells overexpressing Vav1 compared to those derived from MDA‐MB‐231 transfected with an empty vector (Fig. 2B).
Figure 2.
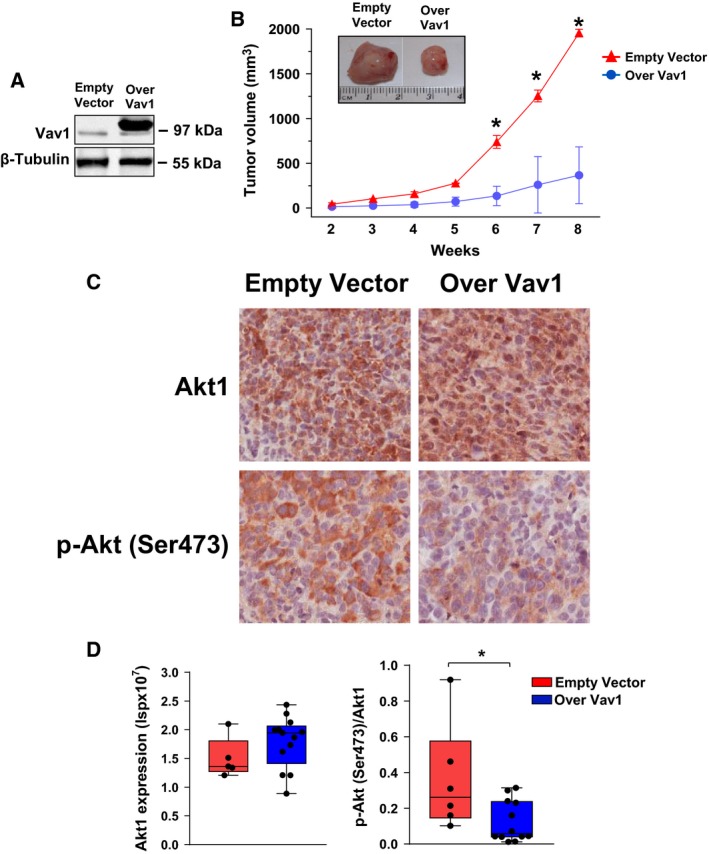
Effects of Vav1 on growth of MDA‐MB‐231‐derived xenografts. (A) Immunochemical analysis with specific antibodies of MDA‐MB‐231 cells stably expressing an empty vector or human Vav1 (Over Vav1) that were injected into 6‐week‐old female nude mice. (B) Xenograft volumes measured from the second week after the injection of MDA‐MB‐231 cells whose Vav1 expression is shown in (A). In (C), representative images of xenograft sections subjected to immunohistochemical analysis with the indicated antibodies (Bar = 50 μm). Positive pixel analysis of Akt1 and p‐Akt (Ser473) staining was carried out using Aperio Positive Pixel Count algorithm and is reported as intensity of strong positive pixels (Isp) (D). *P < 0.05.
Immunohistochemical analysis of xenografts revealed a similar expression of total Akt1 and a significantly lower level of p‐Akt (Ser473) in tumors derived from mice injected with MDA‐MB‐231 stably overexpressing Vav1 (Fig. 2C,D).
3.2. Modulation of Vav1 induces phenotype‐related changes of Akt1 in breast cancer‐derived cells
The question of whether Vav1 affects Akt1 activation in breast tumor cells with phenotypes other than triple negative was addressed in the breast cancer‐derived cell lines BT‐474, MCF7, and MDA‐MB‐453, which represent the most frequent subtypes of breast tumors and express Vav1 to a variable extent (Grassilli et al., 2014; Fig. 3A). As reported in Fig. 3, the silencing of Vav1 induced increase in Akt1 expression only in BT‐474 cells (Fig. 3A, 3B), consistent with our previous data in the same cell model (Grassilli et al., 2014) and in parallel with the increased p‐Akt1 (Ser473) levels (Fig. 3A,C). Silencing of Vav1 also induced increase in p‐Akt1 (Ser473) in MDA‐MB‐453 cells, while no effects were observed in MCF7, according to the very low basal expression of the protein in this cell line (Fig. 3A,C). The total protein amount remained unchanged (Fig. 3A,B), while a significant decrease in the p‐Akt1 (Ser473) levels was induced by Vav1 overexpression in both MCF7 and MDA‐MB‐453 cell lines (Fig. 3A,C).
Figure 3.
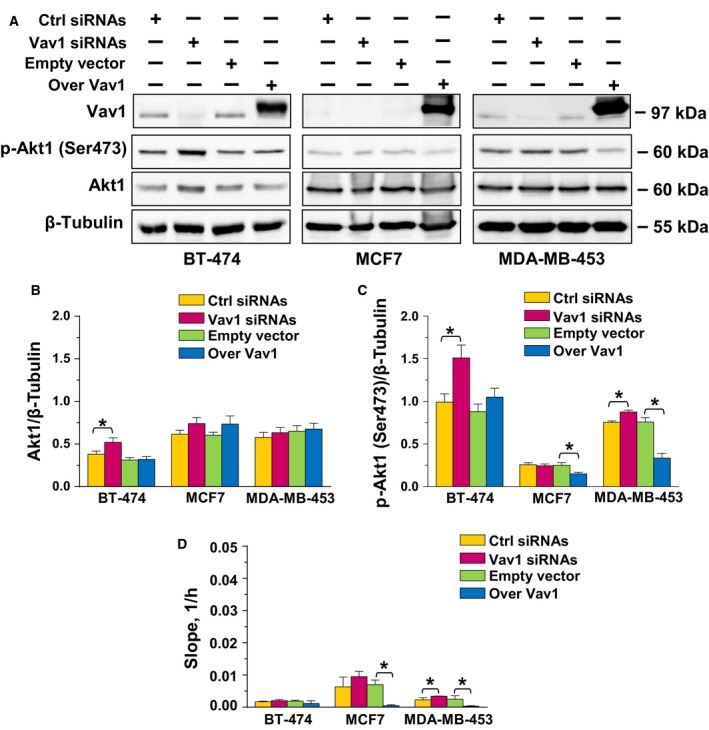
Vav1‐dependent regulation of Akt1 in breast cancer‐derived cells with different phenotypes. (A) Representative western blot analysis, performed with the indicated antibodies, of lysates from BT‐474, MCF7, and MDA‐MB‐453 cells transfected for 48 h with siRNA specific for Vav1 (Vav1 siRNA) or with a construct expressing human Vav1 (Over Vav1). Scramble siRNA (Ctrl siRNA) and an empty vector were used as controls of the experiment. β‐Tubulin was used as internal control for equivalence of loaded proteins. (B, C) Histograms, as deduced from the densitometry of western blot bands, reporting the levels of proteins normalized to β‐tubulin. (D) Slope analysis of the dynamic monitoring of proliferation of BT‐474, MCF7, and MDA‐MB‐453 cells, under the above reported experimental conditions. All the data are the mean of three separate experiments ±SD. *P < 0.05.
The analysis of cell proliferation failed to reveal changes in growth of BT‐474 subjected to forced modulation of Vav1 but indicated that silencing of this protein is sufficient to increase the growth of MDA‐MB‐453 and that Vav1 overexpression induced a decline of proliferation in both MCF7 and MDA‐MB‐453 cells (Fig. 3D), paralleling the effects observed on p‐Akt1 (Ser473) levels.
3.3. Silencing of Vav1 induced the expression but not the activation of Akt2 in breast tumors cells with different phenotypes
Concerning the possible involvement of Vav1 in expression and/or activation of Akt isoforms other than Akt1, the use of specific antibodies revealed a significant increase in Akt2 expression in the MDA‐MB‐453 and MDA‐MB‐231 cells in which Vav1 was silenced, while the protein level remained unchanged in BT‐474 and MCF7 cells (Fig. 4A,B). No significant levels of Akt2 phosphorylated on Ser474 were revealed in all cell lines grown in control conditions or subjected to forced modulation of Vav1 (Fig. 4A,C).
Figure 4.
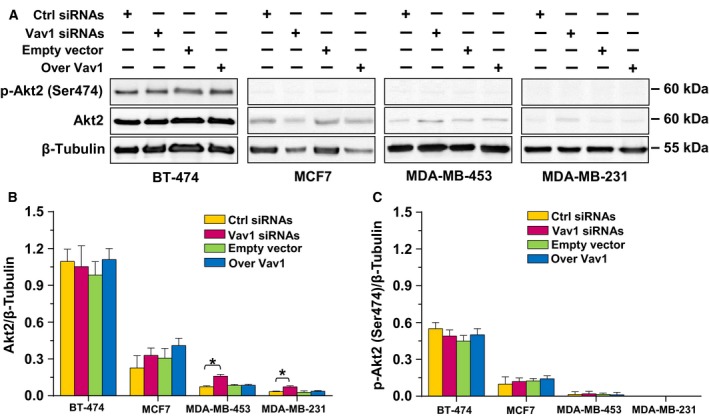
Vav1‐dependent regulation of Akt2 in breast cancer‐derived cells with different phenotypes. (A) Representative western blot analysis, performed with the indicated antibodies, of lysates from BT‐474, MCF7, MDA‐MB‐453, and MDA‐MB‐231 cells transfected for 48 h with siRNA specific for Vav1 (Vav1 siRNA) or with a construct expressing human Vav1 (Over Vav1). Scramble siRNA (Ctrl siRNA) and an empty vector were used as controls of the experiment. β‐Tubulin was used as internal control for equivalence of loaded proteins. (B, C) Histograms, as deduced from the densitometry of western blot bands, reporting the levels of proteins normalized to β‐tubulin. All the data are the mean of three separate experiments ±SD. *P < 0.05.
Concerning Akt3, literature data excluded its expression (Mundi et al., 2016) and our results failed to reveal effects of Vav1 modulation on this Akt isoform in BT‐474, MCF7, and MDA‐MB‐453 cell lines (data not shown). In MDA‐MB‐231 cells, known to express Akt3, no difference in protein expression and no phosphorylation were revealed as a consequence of forced modulation of Vav1 (Fig. 5).
Figure 5.
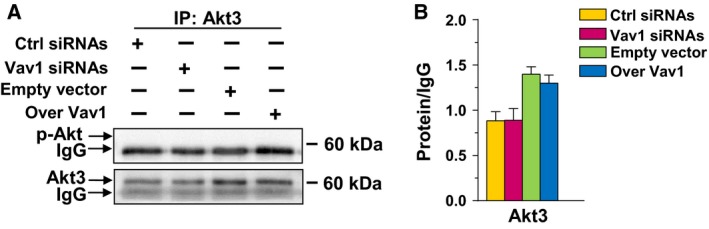
Vav1 and Akt3 in MDA‐MB‐231 cells. (A) Representative western blot analysis of Akt3 immunoprecipitated from MDA‐MB‐231 cells transfected for 48 h with siRNA specific for Vav1 (Vav1 siRNA) or with a construct expressing human Vav1 (Over Vav1) and revealed with an anti‐p‐Akt or with a specific anti‐Akt3 antibody. IgG was blotted as a control for equal antibody addition to samples. The normalized values of Akt3 are shown in the right (B). All the data are the mean of three separate experiments performed in triplicate ±SD.
3.4. The relationship between Vav1 and phosphorylation status of Akt has a prognostic significance in invasive breast tumors
Once established that Vav1 modulates the activation of Akt1 in breast tumor‐derived cells, we investigated the association between the Vav1/p‐Akt (Ser473) levels and the clinical outcome of breast cancer patients. The study group consisted of 126 patients with primary unilateral breast carcinoma (T1/T2), with no evidence of nodal involvement and distant metastases (Table 1), in which both Vav1 and phosphorylated Akt were evaluated by immunohistochemistry on TMAs. The selection of the cutoff value for Vav1 was based on the results of a previously reported study (Grassilli et al., 2014). Based on this dichotomization, 60 cases of 126 (47.6%) were included in the group of low Vav1‐expressing tumors and 66 (52.4%) were included in the group of high Vav1‐expressing tumors. The levels of activated Akt were dichotomized as reported in Materials and Methods, and the percentage of cases with low or high levels of p‐Akt (Ser473) was 69.8% and 30.2%, respectively.
By Pearson's chi‐square analysis, Vav1 expression was found to be inversely correlated with p‐Akt staining (P = 0.011; R = –0.239), with 80.3% of Vav1high tumors that displayed low levels of phosphorylated Akt (Table 2). A strong inverse correlation (P < 0.001; R = –0.544) was also found between Cyclin D1 expression and Vav1/p‐Akt (Ser473) status. In particular, 74.5% of tumors with Vav1high/p‐Aktlow expressed low levels of Cyclin D1, while 83.3% of Vav1low/p‐Akthigh cases showed high Cyclin D1 staining (Table 3).
Table 2.
Correlation between Vav1 expression and p‐Akt (Ser473) status in breast cancer patients (n = 126)
| Variable | Vav1low, n (%) | Vav1high, n (%) | P a | R b |
|---|---|---|---|---|
| p‐Aktlow | 35 (58.3) | 53 (80.3) | 0.011 | −0.239 |
| p‐Akthigh | 25 (41.7) | 13 (19.7) |
Pearson's chi‐square test.
Pearson's R.
Table 3.
Correlation between Vav1/p‐Akt (Ser473) and Cyclin D1 expressions in breast cancer patients (n = 75)
| Variable | Vav1low/p‐Akthigh n (%) | Vav1high/p‐Aktlow n (%) | P a | R b |
|---|---|---|---|---|
| Cyclin D1Low | 4 (16.7) | 38 (74.5) | <0.001 | −0.544 |
| Cyclin D1High | 20 (83.3) | 13 (25.5) |
Pearson's chi‐square test.
Pearson's R; °Cutoff value for Cyclin D1 > 10%.
At Kaplan–Meier analysis, the p‐Akt (Ser473) status was not associated with distant relapse in patients of the whole population (Fig. 6A). Only in patients harboring p‐Aktlow tumors, we found that low levels of Vav1 were significantly associated with higher incidence of distant relapse (P = 0.009) (Fig. 6B).
Figure 6.
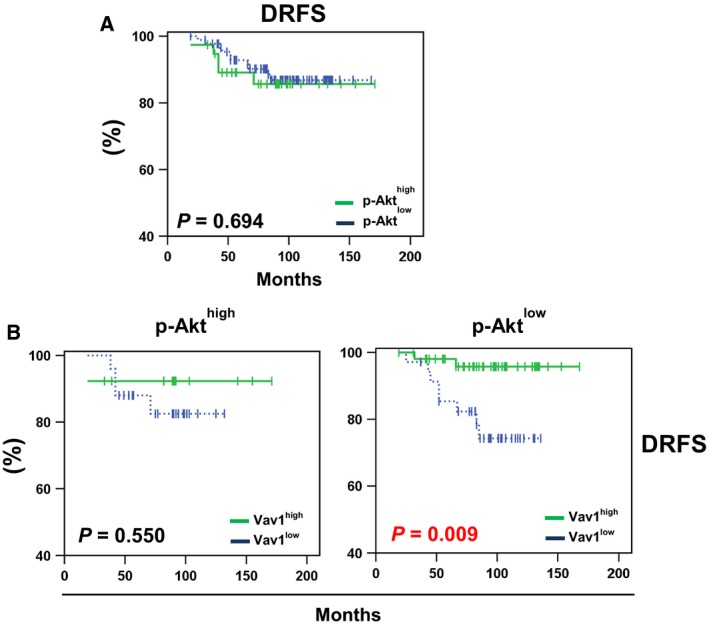
Distant relapse‐free survival of breast cancer patients according to p‐Akt and/or Vav1 status of their primary tumors. (A) Kaplan–Meier estimates of distant relapse‐free survival (DRFS) in patients (n = 126) according to p‐Akt status. (B) Kaplan–Meier estimates of DRFS in patients with p‐Akthigh tumors (n = 38) (left panel), and in patients with p‐Aktlow tumors (n = 88) (right panel), according to high (green solid line) and low (blue dashed line) expression of Vav1. In these cohort of patients, a distant relapse occurred in the 11% (10/88) p‐Aktlow and 13% (5/38) p‐Akthigh tumors, in the 16% (4/25) Vav1low and 8% (1/13) Vav1high of patients with p‐Akthigh tumors, and in the 23% (8/35) Vav1low and 4% (2/53) Vav1high of patients with p‐Aktlow tumors.
To explore the possible predictive value of Vav1 in patients with p‐Aktlow tumors, analyses of DRFS were conducted on the subset of 88 patients receiving systemic adjuvant treatment (chemotherapy or hormonal). At univariate analyses, low expressions of Vav1 were significantly associated with higher risk of metastases (HR = 8.2; 95% CI = 1.0–66.8; P = 0.049) only in patients treated with chemotherapy (Table 4). In the cohort of patients with p‐Aktlow tumors, we also searched for possible correlations among the expression of Vav1 and the analyzed clinic‐pathological variables. As reported in Table 5, Vav1 was significantly less expressed in tumors from patients with age <50 at diagnosis (P = 0.018), while Vav1 status did not differ significantly for the distribution of the other considered clinic‐pathological variables.
Table 4.
Risk of distant relapse associated with Vav1 status in p‐Akt (Ser473)low breast cancer cases (n = 88) according to adjuvant therapy
| Adjuvant therapy | HRa | 95% CI | P b |
|---|---|---|---|
| Radiotherapy (n = 10) | 1.4 | 0.1–1.0 | 0.582 |
| Hormonal (n = 31) | 1.0 | 0.1–2.0 | 0.608 |
| Chemotherapy (n = 47) | 8.2 | 1.0–66.8 | 0.049 |
Hazard ratio of low versus high Vav1 expression.
Pearson's chi‐square test.
Table 5.
Vav1 status according to clinicopathological features of breast cancer patients with p‐Aktlow tumors (n = 88)
| Variable | Vav1 | P a | |
|---|---|---|---|
| Low, n (%) | High, n (%) | ||
| Age at diagnosis (years) | |||
| <50 | 15 (42.9) | 10 (18.9) | 0.018 |
| 50–65 | 9 (25.7) | 28 (52.8) | |
| >65 | 11 (31.4) | 15 (28.3) | |
| Menopausal status | |||
| Pre/perimenopausal | 15 (42.9) | 12 (22.6) | 0.054 |
| Postmenopausal | 20 (57.1) | 41 (77.4) | |
| Tumor size | |||
| ≤2 cm | 25 (71.4) | 35 (66.0) | 0.646 |
| >2 cm | 10 (28.6) | 18 (34.0) | |
| Histotypes | |||
| Ductal carcinoma | 27 (77.1) | 46 (86.8) | 0.466 |
| Lobular carcinoma | 5 (14.3) | 5 (9.4) | |
| Other | 3 (8.6) | 2 (3.8) | |
| Molecular subtypes | |||
| Luminal A‐like | 18 (51.4) | 23 (43.4) | 0.260 |
| Luminal B‐like (HER2 negative) | 6 (17.1) | 15 (28.3) | |
| Luminal B‐like (HER2 positive) | 1 (2.9) | 4 (7.5) | |
| HER2 positive (nonluminal) | 2 (5.7) | 0 (0.0) | |
| Triple negative (ductal) | 8 (22.9) | 11(20.8) | |
| Tumor grade | |||
| 1 | 6 (17.1) | 6 (11.3) | 0.478 |
| 2 | 16 (45.8) | 31 (58.5) | |
| 3 | 13 (37.1) | 16 (30.2) | |
| Ki‐67 | |||
| Low | 22 (62.9) | 31 (58.5) | 0.824 |
| High | 13 (37.1) | 22 (41.5) | |
Pearson's chi‐square test.
4. Discussion
Currently, breast cancer treatments are highly dependent on tumor phenotypes and mainly developed to target ER, PR, or HER2. As a result, systemic adjuvant chemotherapy is the only option for TNBC patients who, although highly responsive, show a greater recurrence and poorer prognosis than those bearing other types of breast cancers (Fleisher et al., 2016). The PI3K/Akt/mTOR pathway is an exciting objective for developing anticancer strategies, and a number of agents targeting different members of this signaling pathway have been developed (Chia et al., 2015; Ma, 2015; Nagini, 2017). Unfortunately, frequent mutations or copy number changes in genes important for cell cycle arrest or apoptosis limit the antitumor activity of inhibitors of the PI3K/Akt pathway (Ma, 2015). The scenario is further complicated by the expression of all three Akt isoforms in cells with a triple‐negative phenotype, with dissimilar effects on the metastatic process (Dillon et al., 2009; Grottke et al., 2016; Riggio et al., 2017), raising the problem of the identification of isoform‐specific inhibitors to be selectively used in the different breast tumor subtypes.
Our previous data revealed that Vav1, ectopically expressed in breast tumors in which positively correlates with follow‐up, regulates the transcription levels of genes encoding for Akt1 in breast tumor‐derived cells with a luminal B phenotype and for activators of the PI3K/Akt signaling in TNBC‐derived cells (Grassilli et al., 2014). To elucidate whether modulation of the complex pathways triggered by Akt may be at the basis of the role of Vav1 in breast cancer cells, we explored here the ability of this protein to affect Akt expression and/or activation in breast tumor cells with different phenotypes. We firstly investigated the role of Vav1 in regulating Akt1 in TNBC‐derived MDA‐MB‐231 cells, confirming our previous data indicating that the forced modulation of Vav1 is unable to affect the expression of Akt1 (Grassilli et al., 2014). Remarkably, we found that silencing of Vav1 increased the phosphorylation level of Akt1 at Ser473, known to be required for its maximal activity (Guo et al., 2014). By investigating in vitro the main events triggered by activated Akt1, we demonstrated that the downmodulation of the p‐Akt1 (Ser473) level induced by Vav1 in MDA‐MB‐231 cells correlates with their reduced proliferation rate, possibly mediated by the Akt/S6K pathway, a well‐described mechanism in breast tumor cells (Riggio et al., 2017). The reduced size of xenografts derived from MDA‐MB‐231 stably overexpressing Vav1 confirmed the in vivo role of this protein as a strong suppressor of Akt1 activity in cells with a TNBC phenotype.
The ability of Vav1 to downmodulate p‐Akt1 is not restricted to cells with a triple‐negative phenotype, as we revealed by modulating Vav1 in cell lines representing the most frequent breast tumor subtypes. Apart from BT‐474 cells, showing a luminal B phenotype, in which we confirmed the increase in Akt1 expression as a consequence of Vav1 downmodulation (Grassilli et al., 2014), overexpression of Vav1 reduced p‐Akt1 (Ser473) levels in MCF‐7 cells, showing a luminal A phenotype, and in the HER2‐positive MDA‐MB‐453, allowing to conclude that, in breast tumor‐derived cells and regardless their phenotype, Vav1 is part of signaling pathways ended to modulate the activation status of Akt1.
We further explored the possible role of Vav1 in modulating Akt2, whose expression is phenotype‐dependent and whose role in breast tumors seems to be different from that of Akt1 and mainly associated with migration and metastasis (Chin et al., 2014; Dillon et al., 2009). Although all used cell lines express Akt2, we found that silencing of Vav1 induced a slight expression of Akt2 only in MDA‐MB‐453 and MDA‐MB‐231 cells, without affecting its phosphorylation at the Ser474 residue and, therefore, its activation status (Vadlakonda et al., 2013). As no effects on Akt3 expression and/or phosphorylation were observed by modulating Vav1, a phenotype‐related mechanism in which the removal of Vav1 releases transcriptional machineries that regulate the expression of specific Akt isoforms may be postulated. This may involve the estrogen receptors, already reported to influence the expression of the different Akt isoforms (Sun et al., 2001; Yndestad et al., 2017).
Overall, our data indicate that, in breast tumor‐derived cells with different phenotypes, the sole modulation of Vav1 is sufficient to affect the activation status of Akt1, involved in the insurgence and growth of breast tumors, but not of Akt2, mainly responsible of tumor metastasis (Dillon et al., 2009; Riggio et al., 2017). These data also suggest that, only in breast tumor cells with an ER‐negative phenotype, low Vav1 levels promote high expression of Akt2 that may be recruited and activated by specific environmental events.
The relationship between cellular staining of Vav1 and activated Akt was also investigated in the tumor tissue of 126 patients with completely resected (T1‐T2, N0) breast cancer, showing that more than 80% of tumors expressing high levels of Vav1 displayed low levels of phosphorylated Akt. Also in tumor tissues, we found that high levels of Vav1 and low levels of p‐Akt correlated with low Cyclin D1 staining, suggestive of the inverse relationship between Vav1 and activated Akt1. By performing a retrospective analysis, we found that, at variance with the sole p‐Akt status, the Vav1/p‐Akt relationship may have a prognostic significance. Remarkably, Vav1 levels significantly influence the follow‐up of patients with low p‐Akt in their primary tumors, in which low expression of Vav1 is associated with the highest probability of distant relapses. These results may be explained considering that Akt1, the only Akt isoform activated by low Vav1 in cell lines with different phenotypes, may inhibit EMT (Li et al., 2016) and acts as an invasion suppressor (Riggio et al., 2017). Accordingly, p‐Aktlow tumors, lacking these protective mechanisms, show the greatest dependence on Vav1 levels that we demonstrated to inversely correlate with the expression of EMT‐related genes (Grassilli et al., 2014). In addition, we revealed that the higher incidence of distant relapses only occurs in patients with p‐Aktlow/Vav1low tumors treated with adjuvant chemotherapy, indicative of the critical role of the balance between expression of Akt and p‐Akt (Ser473) in breast cancer chemo‐resistance and metastasis (Xu et al., 2018). Finally, we found that tumors with p‐Aktlow/Vav1low, showing the poorer prognosis, were correlated with a significantly lower age at diagnosis, allowing to identify in younger patients the main potential beneficiaries of the evaluation of p‐Akt/Vav1 relationship.
5. Conclusions
On the basis of our results, we can conclude that, in breast tumor‐derived cells, Vav1 is responsible of downmodulating Akt, acting at expression and/or activation levels of the different isoforms depending on tumor subtype. The p‐Akt/Vav1 relationship may have a prognostic role, and low Vav1 is a negative prognostic parameter in node‐negative breast cancer with low p‐Akt. As in breast cancer, the use of specific or pan Akt inhibitors may not be sufficient or may even be detrimental as it might promote tumor invasiveness and cancer dissemination, protocols devised to increase the levels of Vav1 could be an option to improve breast cancer outcomes. This might be particularly relevant for tumors with a triple‐negative phenotype, for which target‐based therapies are not currently available.
Authors’ contributions
VB was responsible for the study concept, supervised all the experiments, and integrated the results. SG and FB performed in vitro experiments and assisted with the data analysis and interpretation. AB provided technical and material support. MM performed in vivo experiments. SG and RL performed tumor staining. RL performed survival and statistical analysis. LP provided patients samples. MP provided patients’ associated clinical data. SC critically revised the manuscript for important intellectual content. VB drafted the manuscript with input and approval from all authors.
Supporting information
Fig. S1. Effects of Vav1 on Akt mediated apoptosis of MDA‐MB‐231 cells.
Acknowledgements
This work was supported by grants from Italian MIUR (FIRB RBAP10Z7FS_002) to SC and from University of Ferrara (Italy) to V.B. We thank Mr. Cosmo Rossi for his technical expertise in producing xenografts.
References
- Bartolome RA, Molina‐Ortiz I, Samaniego R, Sánchez‐Mateos P, Bustelo XR and Teixidó J (2006) Activation of Vav/Rho GTPase signaling by CXCL12 controls membrane‐type matrix metalloproteinase‐dependent melanoma cell invasion. Cancer Res 66, 248–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertagnolo V, Benedusi M, Brugnoli F, Lanuti P, Marchisio M, Querzoli P and Capitani S (2007) Phospholipase C‐beta 2 promotes mitosis and migration of human breast cancer‐derived cells. Carcinogenesis 28, 1638–1645. [DOI] [PubMed] [Google Scholar]
- Bertagnolo V, Brugnoli F, Grassilli S, Nika E and Capitani S (2012) Vav1 in differentiation of tumoral promyelocytes. Cell Signal 24, 612–620. [DOI] [PubMed] [Google Scholar]
- Bertagnolo V, Grassilli S, Petretto A, Lambertini E, Astati L, Bruschi M, Brugnoli F, Nika E, Candiano G, Piva R et al (2011) Nuclear proteome analysis reveals a role of Vav1 in modulating RNA processing during maturation of tumoral promyelocytes. J Proteomics 75, 398–409. [DOI] [PubMed] [Google Scholar]
- Brugnoli F, Grassilli S, Piazzi M, Palomba M, Nika E, Bavelloni A, Capitani S and Bertagnolo V (2013) In triple negative breast tumor cells, PLC‐beta2 promotes the conversion of CD133high to CD133low phenotype and reduces the CD133‐related invasiveness. Mol Cancer 12, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugnoli F, Lambertini E, Varin‐Blank N, Piva R, Marchisio M, Grassilli S, Miscia S, Capitani S and Bertagnolo V (2010) Vav1 and PU.1 are recruited to the CD11b promoter in APL‐derived promyelocytes: role of Vav1 in modulating PU.1‐containing complexes during ATRA‐induced differentiation. Exp Cell Res 316, 38–47. [DOI] [PubMed] [Google Scholar]
- Bustelo XR (2002) Regulation of Vav proteins by intramolecular events. Front Biosci 7, 24–30. [DOI] [PubMed] [Google Scholar]
- Chia S, Gandhi S, Joy AA, Edwards S, Gorr M, Hopkins S, Kondejewski J, Ayoub JP, Califaretti N, Rayson D et al (2015) Novel agents and associated toxicities of inhibitors of the pi3k/Akt/mtor pathway for the treatment of breast cancer. Curr Oncol 22, 33–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin YR, Yoshida T, Marusyk A, Beck AH, Polyak K and Toker A (2014) Targeting Akt3 signaling in triple‐negative breast cancer. Cancer Res 74, 964–973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark AR and Toker A (2014) Signalling specificity in the Akt pathway in breast cancer. Biochem Soc Trans 42, 1349–1355. [DOI] [PubMed] [Google Scholar]
- Dey N, De P and Leyland‐Jones B (2017) PI3K‐AKT‐mTOR inhibitors in breast cancers: from tumor cell signaling to clinical trials. Pharmacol Ther 175, 91–106. [DOI] [PubMed] [Google Scholar]
- Dillon RL, Marcotte R, Hennessy BT, Woodgett JR, Mills GB and Muller WJ (2009) Akt1 and akt2 play distinct roles in the initiation and metastatic phases of mammary tumor progression. Cancer Res 69, 5057–5064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez‐Zapico ME, Gonzalez‐Paz NC, Weiss E, Savoy DN, Molina JR, Fonseca R, Smyrk TC, Chari ST, Urrutia R and Billadeau DD (2005) Ectopic expression of VAV1 reveals an unexpected role in pancreatic cancer tumorigenesis. Cancer Cell 7, 39–49. [DOI] [PubMed] [Google Scholar]
- Fleisher B, Clarke C and Ait‐Oudhia S (2016) Current advances in biomarkers for targeted therapy in triple‐negative breast cancer. Breast Cancer (Dove Med Press) 8, 183–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassilli S, Brugnoli F, Lattanzio R, Rossi C, Perracchio L, Mottolese M, Marchisio M, Palomba M, Nika E, Natali PG et al (2014) High nuclear level of Vav1 is a positive prognostic factor in early invasive breast tumors: a role in modulating genes related to the efficiency of metastatic process. Oncotarget 5, 4320–4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassilli S, Nika E, Lambertini E, Brugnoli F, Piva R, Capitani S and Bertagnolo V (2016) A network including PU.1, Vav1 and miR‐142‐3p sustains ATRA‐induced differentiation of acute promyelocytic leukemia cells – a short report. Cell Oncol (Dordr) 39, 483–489. [DOI] [PubMed] [Google Scholar]
- Grottke A, Ewald F, Lange T, Nörz D, Herzberger C, Bach J, Grabinski N, Gräser L, Höppner F, Nashan B et al (2016) Downregulation of AKT3 increases migration and metastasis in triple negative breast cancer cells by upregulating S100A4. PLoS One 11, e0146370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrero‐Zotano A, Mayer IA and Arteaga CL (2016) PI3K/AKT/mTOR: role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev 35, 515–524. [DOI] [PubMed] [Google Scholar]
- Guo H, Gao M, Lu Y, Liang J, Lorenzi PL, Bai S, Hawke DH, Li J, Dogruluk T, Scott KL et al (2014) Coordinate phosphorylation of multiple residues on single AKT1 and AKT2 molecules. Oncogene 33, 3463–3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornstein I, Pikarsky E, Groysman M, Amir G, Peylan‐Ramu N and Katzav S (2003) The haematopoietic specific signal transducer Vav1 is expressed in a subset of human neuroblastomas. J Pathol 199, 526–533. [DOI] [PubMed] [Google Scholar]
- Houlard M, Arudchandran R, Regnier‐Ricard F, Germani A, Gisselbrecht S, Blank U, Rivera J and Varin‐Blank N (2002) Vav1 is a component of transcriptionally active complexes. J Exp Med 195, 1115–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Chen T, Chen C, Chen S, Liu Y, Wu J and Shao Z (2013) Prognostic and predictive value of Phospho‐p44/42 and pAKT in HER2‐positive locally advanced breast cancer patients treated with anthracycline‐based neoadjuvant chemotherapy. World J Surg Oncol 11, 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacovides DC, Johnson AB, Wang N, Boddapati S, Korkola J and Gray JW (2013) Identification and quantification of AKT isoforms and phosphoforms in breast cancer using a novel nanofluidic immunoassay. Mol Cell Proteomics 12, 3210–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattanzio R, Marchisio M, La Sorda R, Tinari N, Falasca M, Alberti S, Miscia S, Ercolani C, Di Benedetto A, Perracchio L et al (2013) Overexpression of activated phospholipase Cgamma1 is a risk factor for distant metastases in T1‐T2, N0 breast cancer patients undergoing adjuvant chemotherapy. Int J Cancer 132, 1022–1031. [DOI] [PubMed] [Google Scholar]
- Lazer G, Idelchuk Y, Schapira V, Pikarsky E and Katzav S (2009) The haematopoietic specific signal transducer Vav1 is aberrantly expressed in lung cancer and plays a role in tumourigenesis. J Pathol 219, 25–34. [DOI] [PubMed] [Google Scholar]
- Li CW, Xia W, Lim SO, Hsu JL, Huo L, Wu Y, Li LY, Lai CC, Chang SS, Hsu YH et al (2016) AKT1 inhibits epithelial‐to‐mesenchymal transition in breast cancer through phosphorylation‐dependent Twist1 degradation. Cancer Res 76, 1451–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CX (2015) The PI3K pathway as a therapeutic target in breast cancer. Am J Hematol Oncol 11, 23–29. [Google Scholar]
- Massihnia D, Galvano A, Fanale D, Perez A, Castiglia M, Incorvaia L, Listì A, Rizzo S, Cicero G, Bazan V et al (2016) Triple negative breast cancer: shedding light onto the role of pi3k/akt/mtor pathway. Oncotarget 7, 60712–60722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundi PS, Sachdev J, McCourt C and Kalinsky K (2016) AKT in cancer: new molecular insights and advances in drug development. Br J Clin Pharmacol 82, 943–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagini S (2017) Breast cancer: current molecular therapeutic targets and new players. Anticancer Agents Med Chem 17, 152–163. [DOI] [PubMed] [Google Scholar]
- Qi Y, Kong FM, Deng Q, Li JY, Cui R, Pu YD, Zhai QL, Jia YJ and Li YM (2015) Clinical significance and prognostic value of Vav1 expression in non‐small cell lung cancer. Am J Cancer Res 5, 2491–2497. [PMC free article] [PubMed] [Google Scholar]
- Ravikumar G and Ananthamurthy A (2014) Cyclin D1 expression in ductal carcinoma of the breast and its corelation with other prognostic parameters. J Cancer Res Ther 10, 671–675. [DOI] [PubMed] [Google Scholar]
- Riggio M, Perrone MC, Polo ML, Rodriguez MJ, May M, Abba M, Lanari C and Novaro V (2017) AKT1 and AKT2 isoforms play distinct roles during breast cancer progression through the regulation of specific downstream proteins. Sci Rep 7, 44244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero F and Fischer S (1996) Structure and function of Vav. Cell Signal 8, 545–553. [DOI] [PubMed] [Google Scholar]
- Sebban S, Farago M, Gashai D, Ilan L, Pikarsky E, Ben‐Porath I and Katzav S (2013) Vav1 fine tunes p53 control of apoptosis versus proliferation in breast cancer. PLoS One 8, e54321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun M, Paciga JE, Feldman RI, Yuan Z, Coppola D, Lu YY, Shelley SA, Nicosia SV and Cheng JQ (2001) Phosphatidylinositol‐3‐OH Kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor alpha (ERalpha) via interaction between ERalpha and PI3K. Cancer Res 61, 5985–5991. [PubMed] [Google Scholar]
- Tybulewicz VL (2005) Vav‐family proteins in T‐cell signalling. Curr Opin Immunol 17, 267–274. [DOI] [PubMed] [Google Scholar]
- Vadlakonda L, Dash A, Pasupuleti M, Anil Kumar K and Reddanna P (2013) The paradox of Akt‐mTOR interactions. Front Oncol 3, 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakahashi S, Sudo T, Oka N, Ueno S, Yamaguchi S, Fujiwara K, Ohbayashi C and Nishimura R (2013) VAV1 represses E‐cadherin expression through the transactivation of Snail and Slug: a potential mechanism for aberrant epithelial to mesenchymal transition in human epithelial ovarian cancer. Transl Res 162, 181–190. [DOI] [PubMed] [Google Scholar]
- Xu H, Lin F, Wang Z, Yang L, Meng J, Ou Z, Shao Z, Di G and Yang G (2018) CXCR2 promotes breast cancer metastasis and chemoresistance viasuppression of AKT1 and activation of COX2. Cancer Lett 412, 69–80. [DOI] [PubMed] [Google Scholar]
- Yang SX, Polley E and Lipkowitz S (2016) New insights on PI3K/AKT pathway alterations and clinical outcomes in breast cancer. Cancer Treat Rev 45, 87–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yndestad S, Austreid E, Svanberg IR, Knappskog S, Lønning PE and Eikesdal HP (2017) Activation of Akt characterizes estrogen receptor positive human breast cancers which respond to anthracyclines. Oncotarget 8, 41227–41241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Bajraszewski N, Wu E, Wang H, Moseman AP, Dabora SL, Griffin JD and Kwiatkowski DJ (2007) PDGFRs are critical for PI3K/Akt activation and negatively regulated by mTOR. J Clin Invest 117, 730–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Jin H, Xia Z, Wu X, Yang M, Zhang H, Shang X, Cheng R, Zhan Z and Yu Z (2017) Vav1 expression is increased in esophageal squamous cell carcinoma and indicates poor prognosis. Biochem Biophys Res Commun 486, 571–576. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Effects of Vav1 on Akt mediated apoptosis of MDA‐MB‐231 cells.


