Abstract
Hepatocellular carcinoma (HCC) is the fifth most common type of cancer and the second leading cause of cancer‐related deaths worldwide. Given that the rate of HCC recurrence 5 years after liver resection is as high as 70%, patient with HCC typically has a poor outcome. A biomarker or set of biomarkers that could predict disease recurrence would have a substantial clinical impact, allowing earlier detection of recurrence and more effective treatment. With the aim of identifying a new microRNA (miRNA) signature associated with HCC recurrence, we analyzed data on 306 patients with HCC for whom both miRNA expression profiles and complete clinical information were available from The Cancer Genome Atlas database. Through this analysis, we identified a six‐miRNA signature that could effectively predict patients’ recurrence risk; the high‐risk and low‐risk groups had significantly different recurrence‐free survival rates. Time‐dependent receiver operating characteristic analysis indicated that this signature had a good predictive performance. Multivariable Cox regression and stratified analyses demonstrated that the six‐miRNA signature was independent of other clinical features. Functional enrichment analysis of the gene targets of the six prognostic miRNA indicated enrichment mainly in cancer‐related pathways and important cell biological processes. Our results support use of this six‐miRNA signature as an independent factor for predicting recurrence and outcome of patients with HCC.
Keywords: biomarkers, disease recurrence, hepatocellular carcinoma, microRNA, risk score
Abbreviations
- AUC
area under the curve
- CI
confidence interval
- CRC
colorectal cancer
- GO
gene ontology
- HCC
hepatocellular carcinoma
- HR
hazard ratio
- KEGG
Kyoto Encyclopedia of Genes and Genomes Pathway
- OS
overall survival
- RFS
recurrence‐free survival
- ROC
receiver operating characteristic
- TCGA
The Cancer Genome Atlas
1. Introduction
Hepatocellular carcinoma (HCC), which accounts for 90% of liver cancers, is the fifth most common type of cancer and the second leading cause of cancer‐related deaths worldwide. In 2012, an estimated 782 500 new cases of HCC were diagnosed and 745 500 deaths were attributable to HCC worldwide (Ao et al., 2017; El‐Serag, 2011; Torre et al., 2015). The therapeutic approaches to HCC include liver resection, transplantation, ablation, chemoembolization, and targeted therapy with sorafenib (Mokdad et al., 2016). Liver resection is considered the first choice in HCC treatment, but the recurrence rate at 5 years after liver resection exceeds 70% (Vilarinho and Calvisi, 2014). Although postoperative chemotherapy is reported to be effective in some patients with recurrence, its efficacy remains controversial (Hashimoto et al., 2005).
Because of this high rate of recurrence (Vilarinho and Calvisi, 2014) and the challenge of treating recurrent HCC (Poon, 2011), the prognosis of HCC is still very poor. One or more molecular biomarkers that accurately predict recurrence would be of great clinical significance. Several studies have tried to identify such biomarkers (Jin et al., 2013; Kim et al., 2014; Yang et al., 2017), but an early and reliable predictor that can be applied readily to clinical practice is still lacking, and identifying patients at high risk for recurrence is currently beyond our reach. The ability to distinguish patients with HCC at high risk of recurrence from those at low risk may lead to development of more effective therapeutic regimens and personalized therapies, which hold the promise to prolong survival and improve overall outcome.
miRNA are small noncoding RNA 18–25 nucleotides in length. Like long noncoding RNA and protein‐coding genes, miRNA can function as tumor suppressors or oncogenes during tumor progression (Bartel, 2004; Kwak et al., 2010). The mechanism through which miRNA contribute to cancer development is direct binding to the 3′‐untranslated region of mRNA to inhibit their translation (Bartel, 2009). There are some reports that miRNA can bind to the 5′‐untranslated region of mRNA (Chaluvally‐Raghavan et al., 2016), suggesting their complicated biological function. Increasing evidence suggests that miRNA play important roles in various biological processes, such as cellular development, metabolism, and proliferation (Ambros, 2004; Guo et al., 2015). In particular, it is believed that miRNA drive the pathogenesis and progression of cancer. Our previous studies and findings from other investigators have demonstrated associations of miRNA with a variety of cancers (Bae et al., 2015; Li et al., 2016, 2017; Szabo and Bala, 2013; Zhang et al., 2016). This justifies a new approach of investigating miRNA as potential cancer biomarkers. The prognostic value of various miRNA signatures has been reported in different types of cancers, such as colon cancer (Zhang et al., 2013), malignant pleural mesothelioma (Kirschner et al., 2015), lung cancer (Yu et al., 2008), and ovarian cancer (Bagnoli et al., 2016). Recently, miRNA associated with prognosis were reported in HCC. For instance, miR‐139, miR‐26, and miR‐140 have been reported to be associated with overall survival (OS) of patients with HCC (Ji et al., 2009; Wong et al., 2011; Yang et al., 2013). However, the prognostic value of miRNA in predicting the risk of disease recurrence in patients with HCC has not been fully elucidated.
Our purpose in this study was to identify miRNA that might serve as clinical biomarkers of HCC recurrence. Using miRNA sequencing data on HCC patients from The Cancer Genome Atlas (TCGA), we constructed a novel six‐miRNA signature that effectively identified patients at high risk of recurrence. Such a signature might be helpful in guiding development of novel, more effective individualized therapies, and improving clinical outcome of patients with HCC.
2. Materials and methods
2.1. TCGA miRNA dataset and patient information
MiRNA expression profiles and clinical data on 377 patients with liver cancer (seven hepatocholangiocarcinoma [mixed], three fibrolamellar carcinoma, and 367 HCC) were downloaded from the TCGA data portal (https://tcga-data.nci.nih.gov/). Patients chosen for model building met the following criteria: (a) histologic diagnosis of HCC; (b) both miRNA expression profile and complete clinicopathological and follow‐up data available; and (c) recurrence‐free survival (RFS) of more than 30 days and less than 3000 days. After filtering, a total of 306 patients were enrolled for further analysis. In addition, miRNA expression data for 50 adjacent noncancer tissues were also retrieved, and the expression of the prognostic miRNA between nontumor tissues and tumor tissues was compared. Those miRNA with an average count > 1 across all patients were kept in the expression profiles and normalized using the R/Bioconductor package of edgeR (Robinson et al., 2010). Because the data were all publicly available through the TCGA project, approval by our institutional ethics committees was not required. This study meets the publication guidelines provided by TCGA.
2.2. Prognostic model construction and statistical analysis
In the training set, a univariable Cox analysis was first performed to screen for miRNA correlated with RFS (P < 0.01). A multivariable Cox analysis was performed, from which six miRNA (miR‐210, miR‐550a‐1, miR‐3199‐2, miR‐4732, miR‐22, and miR‐139) were selected as independently associated with the RFS of patients with HCC (P < 0.01). We then devised a risk score that was a linear combination of the expression of these six miRNA and the multivariable Cox regression coefficients. The risk score for each patient was calculated as follows: Risk score = 0.215 × log2(ExpmiR‐210) + 0.376 × log2(ExpmiR‐550a‐1) − 0.113 × log2(ExpmiR‐3199‐2) − 0.069 × log2(ExpmiR‐4732) − 0.469 × log2(ExpmiR‐22) − 0.311 × log2(ExpmiR‐139). The risk scores of patients in each set were ranked ascendingly. The median risk score derived from the training set was used as a cutoff to separate the patients in each set into high‐ and low‐risk groups. Differences in RFS and OS between the high‐ and low‐risk groups were demonstrated by Kaplan–Meier curves and the log‐rank test. The chi‐square test was used to compare the difference in recurrence status between high‐ and low‐risk groups. The prognostic performance was measured using the area under the curve (AUC) derived from time‐dependent receiver operating characteristic (ROC) analysis, and the accuracy of the risk score to predict RFS at 1, 3, and 5 years and OS at 3, 5, and 7 years was assessed. To evaluate the independence of the miRNA signature as a predictor, multivariable Cox proportional hazards regression analysis was performed, where the RFS or OS was set as the dependent variable and miRNA signature as covariable together with other clinical features, including patient sex and age at diagnosis, tumor stage and grade, and TNM stage. All statistical analyses were conducted using R language (Version 3.3.3). Survival curves and ROC curves were generated by the ‘survminer’ (Kassambara and Kosinski, 2017), ‘survival’ (Therneau, 1994), and ‘survivalROC’ (Heagerty et al., 2000) packages.
2.3. Bioinformatic analysis of miRNA target genes and pathways
Potential gene targets of the six prognostic miRNA were obtained from miRTarBase (Hsu et al., 2014), an experimentally validated miRNA‐target interactions database. For the analysis, 1113 target mRNA with experimental evidence indicating the targeting regulatory relations were chosen. The DAVID Bioinformatics Tool (Huang da et al., 2009) (https://david.ncifcrf.gov/, version 6.8) was used to analyze these mRNA for gene ontology (GO, biological processes) and KEGG pathway enrichment. In this study, GO terms and KEGG pathways with P < 0.05 were considered significantly enriched function annotations.
3. Results
3.1. HCC patients in training and testing sets
For this study, we analyzed data on a total of 306 patients from the TCGA HCC database for model construction. These patients were randomly and evenly divided into a training set (n = 153) and a testing set (n = 153). Baseline demographic and clinical characteristics of the two groups did not differ significantly (all P > 0.05). These characteristics are summarized in Table S1.
3.2. MiRNA associated with recurrence‐free survival of HCC patients in training set
To define the association of miRNA with HCC recurrence, we used univariable Cox regression analysis to identify 13 miRNA that were significantly associated with RFS of patients with HCC (P < 0.01). From these 13 miRNA, a multivariable Cox regression analysis identified six as exhibiting significant prognostic value (P < 0.01) (Table 1). Of the six, four (miR‐3199‐2, miR‐4732, miR‐22, and miR‐139) showed negative regression coefficients, implying that these miRNA play protective roles against recurrence and thus were considered to signal a low risk of recurrence. The remaining two miRNA (miR‐210 and miR‐550a‐1) showed positive regression coefficients, indicating that they are associated with high risk of recurrence. Expression of miR‐210 and miR‐550a‐1 was upregulated in HCC tissues compared to nontumor tissues, while miR‐3199‐2, miR‐4732, miR‐22, and miR‐139 were downregulated (Fig. 1).
Table 1.
MiRNA associated with recurrence of HCC in the training set
| Gene symbol | Coeffa | Typeb | HR | low95 | high95 | P‐valuec |
|---|---|---|---|---|---|---|
| has‐miR‐210 | 0.2150 | Risky | 1.2404 | 1.0658 | 1.4435 | 0.0054 |
| has‐miR‐550a‐1 | 0.3760 | Risky | 1.4564 | 1.0982 | 1.9313 | 0.0090 |
| has‐miR‐3199‐2 | −0.1130 | Protective | 0.8935 | 0.8213 | 0.9721 | 0.0089 |
| has‐miR‐4732 | −0.0690 | Protective | 0.9330 | 0.8863 | 0.9823 | 0.0082 |
| has‐miR‐22 | −0.4690 | Protective | 0.6256 | 0.4791 | 0.8170 | 0.0006 |
| has‐miR‐139 | −0.3110 | Protective | 0.7324 | 0.6035 | 0.8887 | 0.0016 |
aCoefficients derived from multivariable Cox regression analysis. bTypes included protective (low risk) and risky (high risk). c P‐values obtained from multivariable Cox regression analysis.
Figure 1.
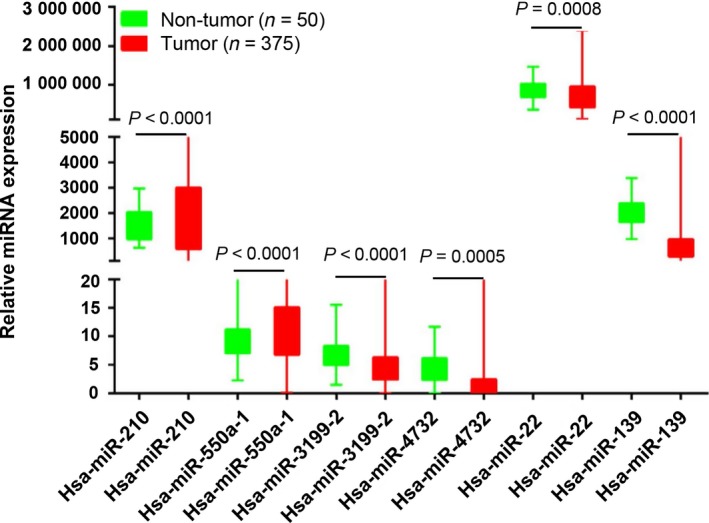
Expression of the six prognostic miRNA in HCC tumor and nontumor tissues. Expression of the six miRNA identified by multivariable Cox regression analysis was determined in tumor and corresponding nontumor tissues. Differences between tumor and nontumor tissues were analyzed by two‐sided Student's t‐test to determine significance. P < 0.05 was considered statistically significant. The boxplot shows the range (lower and upper whisker), the first quartile (lower hinge), and the third quartile (upper hinge). The result was graphed by graphpad prism 6.0 (GraphPad Software, Inc., San Diego, CA, USA).
3.3. Construction of miRNA signature and calculation of risk score in training set
To facilitate the application of these miRNA in clinical practice, we designed a risk prediction formula based on the six identified prognostic miRNA (see Section 2) and calculated the risk score for each patient in the training set. The 153 patients with HCC in the training set were assigned to the high‐risk group (n = 77) or the low‐risk group (n = 76) by their risk scores; the cutoff between the groups was the median risk score (−8.65). Kaplan–Meier analysis showed that patients in the high‐risk group had a worse outcome than those in the low‐risk group (median survival 1.05 years vs. 2.47 years, log‐rank test, P < 0.0001; Fig. 2A).
Figure 2.
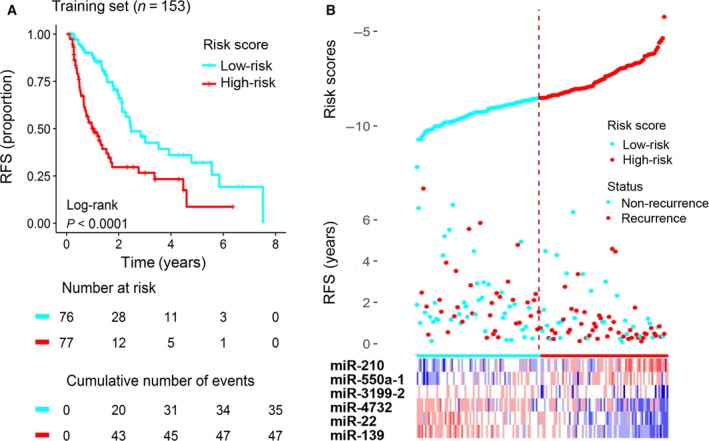
Construction of the six‐miRNA signature in the training set for determining HCC recurrence risk. (A) The training set was subjected to Kaplan–Meier analysis to compare RFS between patients in the high‐risk group and those in the low‐risk group. (B) The distribution of risk score, RFS, and recurrence status and the prognostic miRNA expression patterns for the 153 patients in the training set. The risk scores are arranged in ascending order from left to right. The number below the curve represents the number of the patients in the high‐ and low‐risk group. The ‘+’ symbols in the panel indicate censored data.
The distributions of the risk scores, RFS, recurrence status, and corresponding miRNA expression profiles of the 153 patients in the training set are shown in Fig. 2B. The protective miRNA (miR‐3199‐2, miR‐4732, miR‐22, and miR‐139) tended to be more highly expressed in the low‐risk group, while the remaining miRNA (miR‐210 and miR‐550a‐1) were more highly expressed in the high‐risk group. Moreover, the high‐risk group had more recurrences than the low‐risk group: the high‐risk group comprised 47 patients with recurrence and 30 patients without recurrence, whereas the low‐risk group comprised 35 patients with recurrence and 41 patients without recurrence. However, this difference in recurrence frequency is marginally significant (chi‐square test, P = 0.06).
3.4. Validation of six‐miRNA signature in the testing set and entire TCGA set
Using the same risk score formula and threshold value used in the training set, patients in the testing set (n = 153) and entire TCGA set (n = 306) were classified into high‐risk groups and low‐risk groups. Kaplan–Meier analysis showed that, in the testing set, patients in the high‐risk group (n = 73) had far shorter RFS than those in the low‐risk group (n = 80; median 0.95 years vs. 3.50 years; log‐rank test, P = 0.00045; Fig. 3A). Similar results were observed for the entire TCGA set (median 0.97 years vs. 2.99 years; log‐rank test, P < 0.0001; Fig. 3B).
Figure 3.
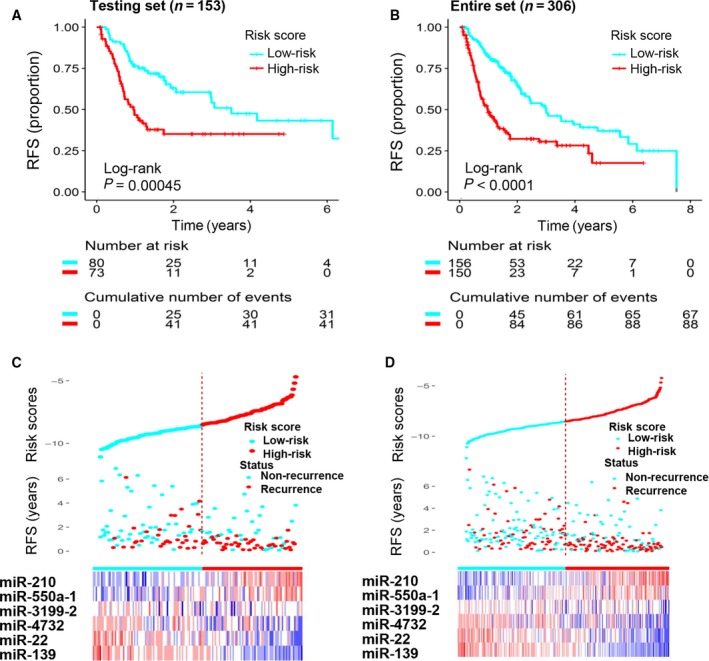
Validation of the prognostic value of the six‐miRNA signature in the testing and entire TCGA sets. (A) The testing set was subjected to Kaplan–Meier analysis to compare RFS between patients in the high‐risk group and patients in the low‐risk group. (B) The entire set was subjected to Kaplan–Meier analysis to compare RFS between patients in the high‐risk group and patients in the low‐risk group. (C) The distribution of risk scores, RFS, and recurrence status and the prognostic miRNA expression patterns for the 153 patients in the testing set. (D) The distribution of risk scores, RFS, and recurrence status and the prognostic miRNA expression patterns for the 306 patients in the entire set. The risk scores are arranged in ascending order from left to right. The number below the curve represents the number of the patients in the high‐ and low‐risk group. The ‘+’ symbols in the panel indicate censored data.
The distributions of the risk scores, RFS, recurrence status, and corresponding miRNA signature expression profiles of patients in the testing set and the entire set are shown in Fig. 3C,D (ranked according to increasing risk scores). The results are consistent with the results obtained from the training set: The protective miRNA were upregulated in the low‐risk group and downregulated in the high‐risk group, while the risky miRNA were downregulated in the low‐risk group and upregulated in the high‐risk group. Moreover, most of the patients with disease recurrence in both sets were clustered in the high‐risk group. In the testing set, the high‐risk group comprised 41 patients with recurrence and 32 patients without recurrence, whereas the low‐risk group comprised 32 with recurrence and 48 without recurrence. The difference in recurrence status is statistically significance (chi‐square test, P = 0.046). For the entire TCGA set, the high‐risk group comprised 88 patients with recurrence and 62 patients without recurrence, while the low‐risk group comprised 67 patients with recurrence and 89 patients without recurrence. The difference in recurrence status between the two groups is significant (chi‐square test, P = 0.006).
3.5. Evaluation of the predictive performance of the six‐miRNA signature
To evaluate the prognostic performance of the six‐miRNA signature, we performed a time‐dependent ROC curve analysis. The results showed that the six‐miRNA signature achieved AUC values of 0.744, 0.643, and 0.740 for predicting recurrence in the training set at 1, 3, and 5 years (Fig. 4A). The AUC values were 0.672, 0.625, and 0.641 for the testing set (Fig. 4B) and 0.706, 0.627, and 0.694 for the entire set (Fig. 4C). All of the AUC values exceed 0.6. These results suggested that our six‐miRNA signature performed well for prediction of disease course in patients with HCC.
Figure 4.
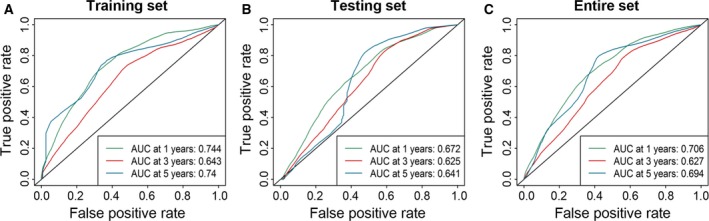
Performance assessment of the six‐miRNA signature by survival ROC analysis. ROC analysis of the six‐miRNA signature for prediction of recurrence risk at 1, 3, and 5 years in the training set (A), the testing set (B), and the entire set (C).
3.6. Multivariable Cox regression analysis and stratified survival analysis
We assessed whether the miRNA signature maintains its prognostic value within the context of other clinical features. The results of multivariable Cox regression analysis show that the miRNA signature maintained independence in predicting the RFS of patients with HCC in the training set (hazard ratio [HR] = 1.62, 95% confidence interval [CI] = 1.346–1.966, P < 0.0001), the testing set (HR = 1.38, 95%CI = 1.101–1.701, P = 0.004), and the entire set (HR = 1.48, 95%CI = 1.298–1.696, P < 0.0001; Table 2).
Table 2.
Multivariable Cox regression analysis of RFS in HCC patients in the training, testing, and entire sets
| Characteristic | Multivariable analysis | ||
|---|---|---|---|
| HR | 95% CI of HR | P‐value | |
| Training set (n = 153) | |||
| Risk score | 1.623 | 1.346–1.966 | < 0.0001 |
| Sex, female/male | 1.008 | 0.625–1.625 | 0.974 |
| Age (years), ≥ 65/< 65 | 0.767 | 0.4634–1.271 | 0.304 |
| Tumor stage, III.IV/I.II | 15.280 | 1.579–147.89 | 0.019 |
| Tumor grade, III.IV/I.II | 0.897 | 0.552–1.457 | 0.660 |
| T stage, T3.T4/T1.T2 | 0.124 | 0.013–1.178 | 0.069 |
| N stage, non‐N0/N0 | 1.665 | 0.906–3.059 | 0.101 |
| M stage, non‐M0/M0 | 0.952 | 0.505–1.792 | 0.878 |
| Testing set (n = 153) | |||
| Risk score | 1.378 | 1.101–1.701 | 0.004 |
| Sex, female/male | 0.580 | 0.333–1.011 | 0.055 |
| Age (years), ≥ 65/< 65 | 1.211 | 0.721–2.033 | 0.470 |
| Tumor stage, III.IV/I.II | 1.330 | 0.158–11.21 | 0.793 |
| Tumor grade, III.IV/I.II | 1.362 | 0.826–2.240 | 0.227 |
| T stage, T3.T4/T1.T2 | 2.468 | 0.211–16.073 | 0.581 |
| N stage, non‐N0/N0 | 0.843 | 0.376–1.582 | 0.479 |
| M stage, non‐M0/M0 | 1.022 | 0.631–2.298 | 0.574 |
| Entire set (n = 306) | |||
| Risk score | 1.484 | 1.298–1.696 | < 0.0001 |
| Sex, female/male | 0.739 | 0.514–1.061 | 0.101 |
| Age, years, ≥ 65/< 65 | 0.916 | 0.647–1.296 | 0.621 |
| Tumor stage, III.IV/I.II | 1.625 | 0.374–7.061 | 0.517 |
| Tumor grade, III.IV/I.II | 1.074 | 0.766–1.504 | 0.680 |
| T stage, T3.T4/T1.T2 | 1.230 | 0.278–5.441 | 0.785 |
| N stage, non‐N0/N0 | 1.028 | 0.661–1.598 | 0.904 |
| M stage, non‐M0/M0 | 1.239 | 0.803–1.910 | 0.332 |
Tumor grade: neoplasm histologic grade; Tumor stage: AJCC pathological stage; T stage: tumor size; N stage: lymph node involvement; M stage: metastasis status. Values in bold indicate they are statistically different.
Because tumor stage was significant in the multivariable analysis in the training set, we conducted a stratified analysis with this clinical parameter. All 306 patients were stratified by tumor stage into a stage I dataset (n = 155), a stage II dataset (n = 69), and a stage III/IV dataset (n = 82). Using the six‐miRNA signature, patients in the stage I dataset were classified into a high‐risk group (n = 54) or a low‐risk group (n = 101); these groups had significantly different RFS (median 1.69 years vs. 4.17 years; log‐rank test, P = 0.0320; Fig. 5A). Likewise, the patients in the stage II dataset also were classified into a high‐risk group (n = 38) and a low‐risk group (n = 31), which also differed significantly in RFS (median 0.73 years vs. 2.44 years; log‐rank test, P = 0.028; Fig. 5B). Similarly, patients in the stage III/IV dataset could be subdivided into a high‐risk group (n = 58) with shorter RFS and a low‐risk group (n = 24) with significantly longer RFS (median 0.71 years vs. 1.55 years; log‐rank test, P = 0.012; Fig. 5C).
Figure 5.
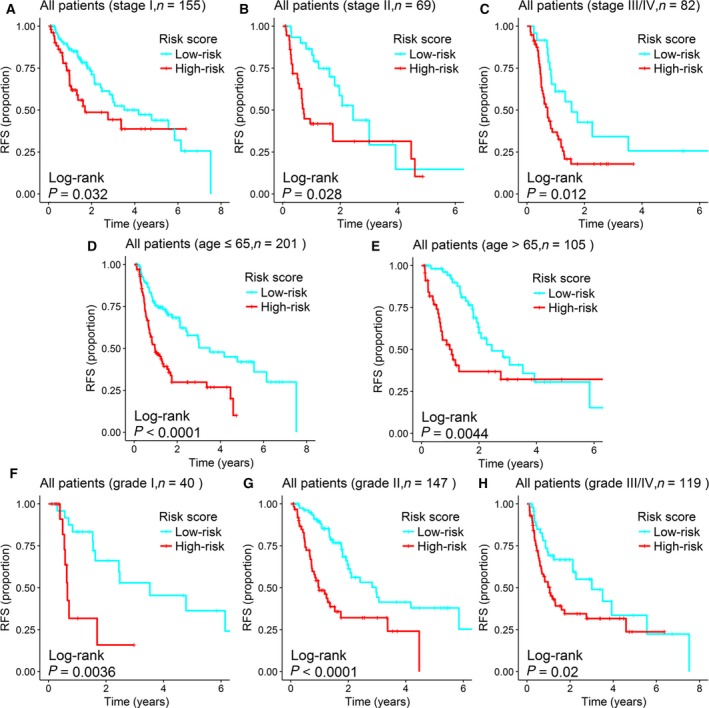
Survival analysis of all HCC patients stratified by stage, age, and tumor grade. Survival analysis compared RFS by recurrence risk (high vs. low) stratified by clinical characteristics. (A) Kaplan–Meier curves for patients with stage I HCC (n = 155). (B) Kaplan–Meier curves for the patients with stage II HCC (n = 69). (C) Kaplan–Meier curves for patients with stage III/IV HCC (n = 82). (D) Kaplan–Meier curves for patients aged 65 years or younger (n = 201). (E) Kaplan–Meier curves for patients aged older than 65 years (n = 105). (F) Kaplan–Meier curves for patients with grade I tumor (n = 40). (G) Kaplan–Meier curves for patients with grade II tumor (n = 147). (H) Kaplan–Meier curves for patients with grade III/IV tumor (n = 119). The ‘+’ symbols in the panel indicate censored data.
We also performed stratification analysis with the other two clinical features: age and tumor grade. All 306 patients were stratified by age into a younger dataset (age ≤ 65 years, n = 201) and an older dataset (age > 65 years, n = 105). Patients in the younger dataset could be stratified into a high‐risk group (n = 102) and a low‐risk group (n = 99) with significantly different RFS (median 0.97 years vs. 3.50 years; log‐rank test, P < 0.0001; Fig. 5D); a similar result was obtained in the older dataset (median 0.98 years vs. 2.44 years; log‐rank test, P = 0.0044; Fig. 5E). The prognostic performance of the six‐miRNA signature was similar when the entire set was stratified by tumor grade. The RFS of patients in the high‐risk group was significantly shorter than that of the low‐risk group in all three datasets (grade I dataset, median 0.66 years vs. 3.52 years, log‐rank test, P = 0.0036, Fig. 5F; grade II dataset, median 0.98 years vs. 2.97 years, log‐rank test, P < 0.0001, Fig. 5G; grade III/IV dataset, median 1.05 years vs. 3.01 years, log‐rank test, P = 0.02, Fig. 5H). When the same analysis with these three clinical variables was applied to the training set and the testing set, Kaplan–Meier curves of the high‐ and low‐risk groups were notably different, although the difference did not reach statistical significance in some subgroups. In those cases, a trend toward worse outcomes for patients with a high‐risk score was observed. The results of stratified analysis in the training and testing sets are shown in Figs S1 and S2.
3.7. Relationship between the miRNA signature and overall survival
Besides the association with RFS, we also analyzed the relationship between the miRNA signature and the OS of patients with HCC. Consistent with the RFS results, the results of this Kaplan–Meier analysis revealed that patients with a higher risk score had shorter OS than those with a lower risk score. The difference in OS between the two groups was statistically significant for the training set (P = 0.0062), the testing set (P < 0.0001), and the entire set (P < 0.0001; Fig. S3). Results of the multivariable Cox regression analysis (Table S2) and the data stratification analysis (Figs S4–S6) showed that the miRNA signature was independent of other clinical features in predicting OS. The AUC values of time‐dependent ROC curves were 0.658, 0.635, and 0.612 at 3, 5, and 7 years, respectively, for the training set. The AUC values were 0.752, 0.763, and 0.804 for the testing set and 0.709, 0.694, and 0.723 for the entire set; overall, the values ranged from 0.612 to 0.804 (Fig. S7).
3.8. Functional enrichment analysis of predicted target genes of prognostic miRNA
As a preliminary investigation of the function of the six miRNA most accurate in predicting HCC recurrence, a total of 1113 genes targeted by these miRNA were first identified. A functional enrichment analysis of these genes showed significant enrichment of 92 GO processes and 25 KEGG pathways. The top 15 enriched KEGG pathways are shown in Fig. 6A. The results demonstrated that several cancer‐related pathways were highly activated in these patients with HCC, such as pathway in cancer, transcriptional misregulation in cancer, and the p53 signaling pathway. The enriched GO terms mainly included regulation of transcription, regulation of cell proliferation, and apoptosis, which have long been recognized as functions of miRNA. The top 15 enriched GO terms are shown in Fig. 6B. These results suggested that the six prognostic miRNA might be tightly associated with regulation of gene expression and critical cell biological functions.
Figure 6.
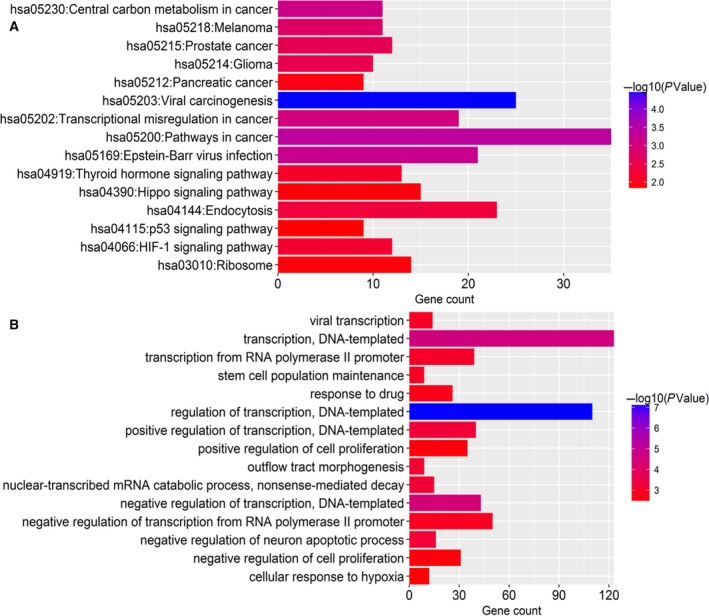
Functional enrichment analysis for predicted target genes of the six miRNA identified as independent predictors of HCC recurrence risk. (A) KEGG enrichment analysis. (B) GO enrichment analysis. The x‐axis represents the number of genes, and the y‐axis represents the GO terms and KEGG pathway names. The color indicates the P‐value.
4. Discussion
In this comprehensive analysis of miRNA sequencing data and its relation to recurrence in patients with HCC, we identified six miRNA that were closely associated with RFS in these patients. On the basis of the idea that a single miRNA is less sensitive and specific for prognostication than a combination of several miRNA (Xu et al., 2017), we developed a risk score formula based on these six miRNA. The six‐miRNA signature effectively divided patients in the training set into high‐ and low‐risk groups with significantly different RFS. We then successfully validated the signature in a testing set and in the entire set; the results indicated that the six‐miRNA signature had good reproducibility and reliability for prediction of RFS in patients with HCC. On further analysis, the six‐miRNA signature performed well in predicting recurrence at year 1, year 3, and year 5 and was an independent factor for predicting RFS in patients with HCC. The miRNA signature successfully classified patients with the same tumor stage into high‐ and low‐risk groups with significantly different RFS. A similar predictive ability was observed in patients with HCC stratified by age: Patients both in the younger dataset (age ≤ 65 years) and the older dataset (age > 65 years) were successfully divided into a high‐risk group and a low‐risk group with notably different prognoses. Similar results were observed when patients were stratified by tumor grade. Our six‐miRNA signature also was effective in stratifying patients with HCC into high‐ and low‐risk groups with significantly different OS. These results suggested that this miRNA signature might be helpful for clinical identification of patients who need more aggressive treatment, potentially prolonging their life.
It is well known that the initiation and progression of HCC is a long‐term process involving activation of key signaling pathways and dysregulation of cellular processes (Bupathi et al., 2015; Liu et al., 2014). Functional enrichment analysis of the six prognostic miRNA showed that most of the biological processes and pathways enriched are implicated in the development of HCC. Moreover, reports from other investigators indicate that four of the six miRNA play critical roles in human cancers. For example, miR‐139 has been reported to participate in a variety of biological processes, such as proliferation, apoptosis, invasion, and metastasis. Downregulation of miR‐139 was indicated to be associated with metastasis in HCC (Wong et al., 2011). A recent study showed that miR‐139‐5p could control cell growth, cell cycle, and apoptosis by targeting NOTCH1 in colorectal cancer (CRC) (Zhang et al., 2014). MiR‐139 could also function as a metastasis suppressor in CRC and may provide a therapeutic strategy for blocking CRC metastasis (Shen et al., 2012).
Several reports have revealed the mechanisms underlying the role of miR‐22 in development of cancers. MiR‐22 acts as a tumor suppressor through direct repression of MYCBP expression and subsequent reduction in oncogenic c‐Myc activities (Xiong et al., 2010). In gastric cancer, miR‐22 could inhibit tumor growth and metastasis by directly targeting MMP14 and Snail (Zuo et al., 2015). Interestingly, miR‐22 was reported to be downregulated in HCC, suppressing cell proliferation and tumorigenicity, and was correlated with more favorable prognosis of patients with HCC (Zhang et al., 2010), findings consistent with ours. A recent study indicated that vitamin D plays an anticancer role in CRC cells through suppressing proliferation, migration, and gene regulation by inducing the expression of miR‐22 (Alvarez‐Diaz et al., 2012), suggesting that this miRNA has a beneficial effect.
MiR‐210 has been widely investigated for the past few years and has most often been reported to be an oncomiR. In prostate cancer, miR‐210‐3p could promote cancer cell epithelial–mesenchymal transition and bone metastasis via the NF‐κB signaling pathway (Ren et al., 2017). Hypoxia‐induced miR‐210 enhances cancer cell viability by promoting proliferation and inhibiting apoptosis in epithelial ovarian cancer (Li et al., 2014). MiR‐210 was also identified as a biomarker or prognostic factor in breast cancer (Camps et al., 2008), clear cell renal cell carcinoma (Samaan et al., 2015), glioma (Lai et al., 2015), and head and neck cancer (Gee et al., 2010). MiR‐550a was implicated, by targeting RNF43, an inhibitor of Wnt/β‐catenin signaling, in promoting metastasis of CRC in vitro and in vivo (Wang et al., 2016). MiR‐550a also could act as a pro‐metastatic gene and directly targeted cytoplasmic polyadenylation element‐binding protein 4 in HCC (Tian et al., 2012).
Reports describing the function of miR‐3199‐2 and miR‐4732 in cancer are still rare. Our differential expression analysis indicates significant downregulation of these two miRNA in tumor tissues, suggesting their potential roles in tumorigenesis. Further investigation of the possible biological functions of these two miRNA at the cell level is warranted to expand our understanding of the molecular mechanism of HCC development. We have noticed that our findings are different from a recent study (Liu et al., 2017). The miRNA signature reported by Liu et al. is designed to predict the OS, but in our study, we focus on RFS. Moreover, the statistical analyses, P‐value setup, and inclusion criteria are different between the two studies. On the other hand, using Liu's methods, we have found a total of 24 miRNA (approximately 44% of all the miRNA identified in Liu's analysis) overlapped between the two studies.
These results, together with our own analysis, further strengthen the possibility that the six‐miRNA signature could be used effectively to predict disease course in HCC. Nevertheless, there may be some shortcomings to our study. First, there is a lack of experimental studies that might provide more convincing explanation of the biological implications and molecular mechanisms of these prognostic miRNA in liver cancer; second, a small proportion of results in the stratified survival analysis was not statistically significant but rather with trend difference, which may be attributed to the limited sample size after repeated grouping; third, independent cohorts from multicenter study in large population are required to validate the prognostic value of the miRNA signature before it can be applied to clinical practice.
5. Conclusion
In summary, after a comprehensive analysis, we have constructed a six‐miRNA signature that could serve as a reliable biomarker for stratifying risk of recurrence among patients with HCC. Further analysis revealed that the prognostic value of this miRNA signature was independent of other clinical features. Our study highlights the great potential of miRNA as tumor markers and therapeutic targets for patients with HCC.
Conflict of interest
All authors have no conflict of interests.
Author contributions
QHM, JXL, and FMB conceived and designed the study. FMB, HBZ, MNM, and CG accessed and analyzed the data. QHM, JXL, and FMB provided interpretation of the results. QHM, JXL, FMB, HBZ, and MNM wrote and revised the manuscript. All authors read and approved the final manuscript.
Supporting information
Fig. S1. Survival analysis of HCC patients in the training set stratified by stage, age and tumor grade.
Fig. S2. Survival analysis of HCC patients in the testing set stratified by stage, age and tumor grade.
Fig. S3. Association between six‐miRNA signature and OS of HCC patients in different sets.
Fig. S4. Stratified analysis of association between six‐miRNA signature and OS of all HCC patients.
Fig. S5. Stratified analysis of association between six‐miRNA signature and OS of HCC patients in the training set.
Fig. S6. Stratified analysis of association between six‐miRNA signature and OS of HCC patients in the testing set.
Fig. S7. Performance assessment of the six‐miRNA signature by survival ROC analysis.
Table S1. Baseline demographic and clinical features of HCC patients in training and testing set.
Table S2. Multivariable Cox regression analysis of OS in HCC patients in the training, testing, and entire sets.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (31670784, 31370795), Key Science and Technology Innovation Team of Zhejiang Province of China (2010R50048).
Contributor Information
Jianxin Lyu, Email: jxlu313@163.com.
Qing H. Meng, Email: qhmeng@mdanderson.org
References
- Alvarez‐Diaz S, Valle N, Ferrer‐Mayorga G, Lombardia L, Herrera M, Dominguez O, Segura MF, Bonilla F, Hernando E and Munoz A (2012) MicroRNA‐22 is induced by vitamin D and contributes to its antiproliferative, antimigratory and gene regulatory effects in colon cancer cells. Hum Mol Genet 21, 2157–2165. [DOI] [PubMed] [Google Scholar]
- Ambros V (2004) The functions of animal microRNAs. Nature 431, 350–355. [DOI] [PubMed] [Google Scholar]
- Ao L, Guo Y, Song X, Guan Q, Zheng W, Zhang J, Huang H, Zou Y, Guo Z, Wang X (2017) Evaluating hepatocellular carcinoma cell lines for tumour samples using within‐sample relative expression orderings of genes. Liver Int 37, 1688–1696. [DOI] [PubMed] [Google Scholar]
- Bae HJ, Jung KH, Eun JW, Shen Q, Kim HS, Park SJ, Shin WC, Yang HD, Park WS, Lee JY et al (2015) MicroRNA‐221 governs tumor suppressor HDAC6 to potentiate malignant progression of liver cancer. J Hepatol 63, 408–419. [DOI] [PubMed] [Google Scholar]
- Bagnoli M, Canevari S, Califano D, Losito S, Maio MD, Raspagliesi F, Carcangiu ML, Toffoli G, Cecchin E, Sorio R et al (2016) Development and validation of a microRNA‐based signature (MiROvaR) to predict early relapse or progression of epithelial ovarian cancer: a cohort study. Lancet Oncol 17, 1137–1146. [DOI] [PubMed] [Google Scholar]
- Bartel DP (2004) MicroRNAs: genomics, biogenesis, mechanism, and function. Cell 116, 281–297. [DOI] [PubMed] [Google Scholar]
- Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136, 215–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bupathi M, Kaseb A, Meric‐Bernstam F and Naing A (2015) Hepatocellular carcinoma: where there is unmet need. Mol Oncol 9, 1501–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camps C, Buffa FM, Colella S, Moore J, Sotiriou C, Sheldon H, Harris AL, Gleadle JM and Ragoussis J (2008) hsa‐miR‐210 Is induced by hypoxia and is an independent prognostic factor in breast cancer. Clin Cancer Res 14, 1340–1348. [DOI] [PubMed] [Google Scholar]
- Chaluvally‐Raghavan P, Jeong KJ, Pradeep S, Silva AM, Yu S, Liu W, Moss T, Rodriguez‐Aguayo C, Zhang D, Ram P et al (2016) Direct upregulation of STAT3 by microRNA‐551b‐3p deregulates growth and metastasis of ovarian cancer. Cell Rep 15, 1493–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El‐Serag HB (2011) Hepatocellular carcinoma. N Engl J Med 365, 1118–1127. [DOI] [PubMed] [Google Scholar]
- Gee HE, Camps C, Buffa FM, Patiar S, Winter SC, Betts G, Homer J, Corbridge R, Cox G, West CM et al (2010) hsa‐mir‐210 is a marker of tumor hypoxia and a prognostic factor in head and neck cancer. Cancer 116, 2148–2158. [DOI] [PubMed] [Google Scholar]
- Guo W, Qiu Z, Wang Z, Wang Q, Tan N, Chen T, Chen Z, Huang S, Gu J, Li J et al (2015) MiR‐199a‐5p is negatively associated with malignancies and regulates glycolysis and lactate production by targeting hexokinase 2 in liver cancer. Hepatology 62, 1132–1144. [DOI] [PubMed] [Google Scholar]
- Hashimoto K, Ikeda Y, Korenaga D, Tanoue K, Hamatake M, Kawasaki K, Yamaoka T, Iwatani Y, Akazawa K and Takenaka K (2005) The impact of preoperative serum C‐reactive protein on the prognosis of patients with hepatocellular carcinoma. Cancer 103, 1856–1864. [DOI] [PubMed] [Google Scholar]
- Heagerty PJ, Lumley T and Pepe MS (2000) Time‐dependent ROC curves for censored survival data and a diagnostic marker. Biometrics 56, 337–344. [DOI] [PubMed] [Google Scholar]
- Hsu SD, Tseng YT, Shrestha S, Lin YL, Khaleel A, Chou CH, Chu CF, Huang HY, Lin CM, Ho SY et al (2014) miRTarBase update 2014: an information resource for experimentally validated miRNA‐target interactions. Nucleic Acids Res 42, D78–D85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang da W, Sherman BT and Lempicki RA (2009) Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res 37, 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Shi J, Budhu A, Yu Z, Forgues M, Roessler S, Ambs S, Chen Y, Meltzer PS, Croce CM et al (2009) MicroRNA expression, survival, and response to interferon in liver cancer. N Engl J Med 361, 1437–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin GZ, Yu WL, Dong H, Zhou WP, Gu YJ, Yu H, Yu H, Lu XY, Xian ZH, Liu YK et al (2013) SUOX is a promising diagnostic and prognostic biomarker for hepatocellular carcinoma. J Hepatol 59, 510–517. [DOI] [PubMed] [Google Scholar]
- Kassambara A, Kosinski M (2017) survminer: Drawing Survival Curves using ‘ggplot2’. R package version 0.3.1. https://CRAN.R-project.org/package=survminer. [Google Scholar]
- Kim JH, Sohn BH, Lee HS, Kim SB, Yoo JE, Park YY, Jeong W, Lee SS, Park ES, Kaseb A et al (2014) Genomic predictors for recurrence patterns of hepatocellular carcinoma: model derivation and validation. PLoS Med 11, e1001770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner MB, Cheng YY, Armstrong NJ, Lin RC, Kao SC, Linton A, Klebe S, McCaughan BC, van Zandwijk N and Reid G (2015) MiR‐score: a novel 6‐microRNA signature that predicts survival outcomes in patients with malignant pleural mesothelioma. Mol Oncol 9, 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak PB, Iwasaki S and Tomari Y (2010) The microRNA pathway and cancer. Cancer Sci 101, 2309–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai NS, Wu DG, Fang XG, Lin YC, Chen SS, Li ZB and Xu SS (2015) Serum microRNA‐210 as a potential noninvasive biomarker for the diagnosis and prognosis of glioma. Br J Cancer 112, 1241–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Huang K, You Y, Fu X, Hu L, Song L and Meng Y (2014) Hypoxia‐induced miR‐210 in epithelial ovarian cancer enhances cancer cell viability via promoting proliferation and inhibiting apoptosis. Int J Oncol 44, 2111–2120. [DOI] [PubMed] [Google Scholar]
- Li C, Lyu J and Meng QH (2017) MiR‐93 promotes tumorigenesis and metastasis of non‐small cell lung cancer cells by activating the PI3K/Akt pathway via inhibition of LKB1/PTEN/CDKN1A. J Cancer 8, 870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Zhao L, Chen Y, He T, Chen X, Mao J, Li C, Lyu J and Meng QH (2016) MicroRNA‐21 promotes proliferation, migration, and invasion of colorectal cancer, and tumor growth associated with down‐regulation of sec23a expression. BMC Cancer 16, 605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Jiang L and Guan XY (2014) The genetic and epigenetic alterations in human hepatocellular carcinoma: a recent update. Protein Cell 5, 673–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Wang H, Fu JG, Liu JY, Yan AG, Guan YY (2017) A five‐miRNA expression signature predicts survival in hepatocellular carcinoma. APMIS 125, 614–622. [DOI] [PubMed] [Google Scholar]
- Mokdad AA, Singal AG and Yopp AC (2016) JAMA PATIENT PAGE. Treatment of liver cancer. JAMA 315, 100. [DOI] [PubMed] [Google Scholar]
- Poon RT (2011) Prevention of recurrence after resection of hepatocellular carcinoma: a daunting challenge. Hepatology 54, 757–759. [DOI] [PubMed] [Google Scholar]
- Ren D, Yang Q, Dai Y, Guo W, Du H, Song L and Peng X (2017) Oncogenic miR‐210‐3p promotes prostate cancer cell EMT and bone metastasis via NF‐kappaB signaling pathway. Mol Cancer 16, 117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MD, McCarthy DJ and Smyth GK (2010) edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samaan S, Khella HW, Girgis A, Scorilas A, Lianidou E, Gabril M, Krylov SN, Jewett M, Bjarnason GA, El‐said H et al (2015) miR‐210 is a prognostic marker in clear cell renal cell carcinoma. J Mol Diagn 17, 136–144. [DOI] [PubMed] [Google Scholar]
- Shen K, Liang Q, Xu K, Cui D, Jiang L, Yin P, Lu Y, Li Q and Liu J (2012) MiR‐139 inhibits invasion and metastasis of colorectal cancer by targeting the type I insulin‐like growth factor receptor. Biochem Pharmacol 84, 320–330. [DOI] [PubMed] [Google Scholar]
- Szabo G and Bala S (2013) MicroRNAs in liver disease. Nat Rev Gastroenterol Hepatol 10, 542–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therneau TM (1994) A Package for Survival Analysis in S. Mayo Clinic, Rochester, MN. [Google Scholar]
- Tian Q, Liang L, Ding J, Zha R, Shi H, Wang Q, Huang S, Guo W, Ge C, Chen T et al (2012) MicroRNA‐550a acts as a pro‐metastatic gene and directly targets cytoplasmic polyadenylation element‐binding protein 4 in hepatocellular carcinoma. PLoS One 7, e48958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre LA, Bray F, Siegel RL, Ferlay J, Lortet‐Tieulent J and Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65, 87–108. [DOI] [PubMed] [Google Scholar]
- Vilarinho S and Calvisi DF (2014) New advances in precision medicine for hepatocellular carcinoma recurrence prediction and treatment. Hepatology 60, 1812–1814. [DOI] [PubMed] [Google Scholar]
- Wang G, Fu Y, Yang X, Luo X, Wang J, Gong J and Hu J (2016) Brg‐1 targeting of novel miR550a‐5p/RNF43/Wnt signaling axis regulates colorectal cancer metastasis. Oncogene 35, 651–661. [DOI] [PubMed] [Google Scholar]
- Wong CC, Wong CM, Tung EK, Au SL, Lee JM, Poon RT, Man K and Ng IO (2011) The microRNA miR‐139 suppresses metastasis and progression of hepatocellular carcinoma by down‐regulating Rho‐kinase 2. Gastroenterology 140, 322–331. [DOI] [PubMed] [Google Scholar]
- Xiong J, Du Q and Liang Z (2010) Tumor‐suppressive microRNA‐22 inhibits the transcription of E‐box‐containing c‐Myc target genes by silencing c‐Myc binding protein. Oncogene 29, 4980–4988. [DOI] [PubMed] [Google Scholar]
- Xu F, Zhou W, Cao J, Xu Q, Jiang D and Chen Y (2017) A combination of DNA‐peptide Probes and Liquid Chromatography‐Tandem Mass Spectrometry (LC‐MS/MS): a quasi‐targeted proteomics approach for multiplexed microRNA quantification. Theranostics 7, 2849–2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Chen L, Gu J, Zhang H, Yuan J, Lian Q, Lv G, Wang S, Wu Y, Yang YT et al (2017) Recurrently deregulated lncRNAs in hepatocellular carcinoma. Nat Commun 8, 14421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Fang F, Chang R and Yang L (2013) MicroRNA‐140‐5p suppresses tumor growth and metastasis by targeting transforming growth factor beta receptor 1 and fibroblast growth factor 9 in hepatocellular carcinoma. Hepatology 58, 205–217. [DOI] [PubMed] [Google Scholar]
- Yu SL, Chen HY, Chang GC, Chen CY, Chen HW, Singh S, Cheng CL, Yu CJ, Lee YC, Chen HS et al (2008) MicroRNA signature predicts survival and relapse in lung cancer. Cancer Cell 13, 48–57. [DOI] [PubMed] [Google Scholar]
- Zhang CZ, Cao Y, Fu J, Yun JP and Zhang MF (2016) miR‐634 exhibits anti‐tumor activities toward hepatocellular carcinoma via Rab1A and DHX33. Mol Oncol 10, 1532–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Dong Y, Zhu N, Tsoi H, Zhao Z, Wu CW, Wang K, Zheng S, Ng SS, Chan FK et al (2014) microRNA‐139‐5p exerts tumor suppressor function by targeting NOTCH1 in colorectal cancer. Mol Cancer 13, 124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JX, Song W, Chen ZH, Wei JH, Liao YJ, Lei J, Hu M, Chen GZ, Liao B, Lu J et al (2013) Prognostic and predictive value of a microRNA signature in stage II colon cancer: a microRNA expression analysis. Lancet Oncol 14, 1295–1306. [DOI] [PubMed] [Google Scholar]
- Zhang J, Yang Y, Yang T, Liu Y, Li A, Fu S, Wu M, Pan Z and Zhou W (2010) microRNA‐22, downregulated in hepatocellular carcinoma and correlated with prognosis, suppresses cell proliferation and tumourigenicity. Br J Cancer 103, 1215–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo QF, Cao LY, Yu T, Gong L, Wang LN, Zhao YL, Xiao B and Zou QM (2015) MicroRNA‐22 inhibits tumor growth and metastasis in gastric cancer by directly targeting MMP14 and Snail. Cell Death Dis 6, e2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Survival analysis of HCC patients in the training set stratified by stage, age and tumor grade.
Fig. S2. Survival analysis of HCC patients in the testing set stratified by stage, age and tumor grade.
Fig. S3. Association between six‐miRNA signature and OS of HCC patients in different sets.
Fig. S4. Stratified analysis of association between six‐miRNA signature and OS of all HCC patients.
Fig. S5. Stratified analysis of association between six‐miRNA signature and OS of HCC patients in the training set.
Fig. S6. Stratified analysis of association between six‐miRNA signature and OS of HCC patients in the testing set.
Fig. S7. Performance assessment of the six‐miRNA signature by survival ROC analysis.
Table S1. Baseline demographic and clinical features of HCC patients in training and testing set.
Table S2. Multivariable Cox regression analysis of OS in HCC patients in the training, testing, and entire sets.


