Abstract
An age younger than 60 years, a body weight of 180 lb (82 kg) or more, performing heavy work, having chondrocalcinosis and having exposed bone in the patellofemoral (PF) joint are not contraindications for unicompartmental knee arthroplasty (UKA).
Severe wear of the lateral facet of the PF joint with bone loss and grooving is a contraindication for UKA.
Medial UKA should only be performed in cases of severe osteoarthritis (OA) as shown in pre-operative X-rays, with medial bone-on-bone contact and a medial/lateral ratio of < 20%.
The post-operative results of UKA are generally good. Medium-term and long-term studies have reported acceptable results at 10 years, with implant survival greater than 95% for UKAs performed for medial OA or osteonecrosis and for lateral UKA, especially when fixed-bearing implants are used.
When all implant-related re-operations are considered, the 10-year survival rate is 94%, and the 15-year survival rate is 91%.
Aseptic loosening is the principal failure mechanism in the first few years in mobile-bearing implants, whereas OA progression causes most failures in later years in fixed-bearing implants.
The overall complication rate and the comprehensive re-operation rate are comparable in both mobile bearings and fixed bearings.
The survival likelihood of the all-polyethylene UKA implant is similar to that of metal-backed modular designs for UKA.
Notable cost savings of approximately 50% can be achieved with an outpatient UKA surgery protocol. Outpatient surgery for UKA is efficacious and safe, with satisfactory clinical results thus far.
Cite this article: EFORT Open Rev 2018;3:363-373. DOI: 10.1302/2058-5241.3.170048
Keywords: arthroplasty, knee, unicompartmental
Introduction
Knee osteoarthritis (OA) is the most common form of lower-limb OA.1 It is estimated that 6% of those aged 30 years and older and 15% of those aged 45 years and older experience the condition,2 with a lifetime risk of 45%.3 For most patients with knee OA, the disease is restricted to the medial compartment.4 In the 1950s, MacIntosh first used a metal spacer in single tibiofemoral compartment cases.5 In the 1960s and 1970s, the St Georg and Marmor prostheses were introduced, with good outcomes.6,7 Both of these designs had polycentric metal femoral condyles that articulated on flat, fixed polyethylene tibial components, with the femoral and tibial components cemented to the bone. In 1974, the first mobile-bearing unicompartmental knee arthroplasty (UKA), the Oxford Knee (OUKA), was introduced, and in 1988 it was first reported.8
UKA surgery has gained interest in recent years because it can diminish post-operative pain and has a shorter recuperation time than a total knee arthroplasty (TKA). In the last two to three years, research has been conducted in this field. Several authors have reported on the safety of outpatient UKA9–13 and have concluded that, in general, this approach is safe. It is important, however, to adhere to a clear standardized protocol.9 Important financial savings to the healthcare system can be accomplished with such a protocol for outpatient UKA.
UKA is a surgical procedure in which the degenerated articular surfaces are replaced to alleviate OA in one of the knee compartments. Election of either TKA or UKA is a matter of debate. UKA has some published advantages over TKA, but it also seems to possess important disadvantages in terms of revision rates. The aim of this article is to analyse the indications, technical issues, and results of UKA.
Indications for UKA
The best indication for UKA is painful OA in an isolated tibiofemoral compartment (medial or lateral). Lateral osteophytes have been suggested to be related to lateral compartment disease. However, it is difficult to determine whether medial UKA should be performed in the presence of lateral osteophytes. Hamilton et al found that the presence of lateral osteophytes is not a contraindication for medial meniscal-bearing UKA.14 The clinical significance of this report was that it emphasized the importance of an adequate pre-operative evaluation of the lateral compartment, given that, in the context of full-thickness cartilage loss at surgery, lateral osteophytes did not compromise long-term functional results or implant survival.
Knifsund et al analysed the impact of the pre-operative grade of OA on the risk of re-operation after UKA.15 They suggested that UKA should only be performed in cases exhibiting severe OA in pre-operative X-rays, with medial bone-on-bone contact, and a medial/lateral ratio of < 20% (Fig. 1).
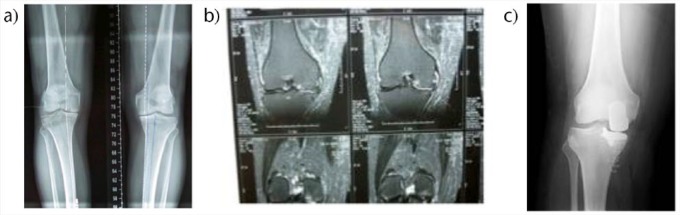
Fig. 1.
Painful osteoarthritis of the medial compartment of the right knee (varus deformity) in a 57-year-old man. Unicompartmental knee arthroplasty (UKA) was indicated. (a) Pre-operative radiograph and (b) MRI images showing advanced osteoarthritis of the medial compartment of the knee; (c) final X-ray appearance of the knee with the prosthesis in position.
Hamilton et al analysed the long-term results of a group of patients, some of whom had anterior knee pain and patellofemoral (PF) joint OA managed with UKA.16 Severe impairment to the lateral facet of the PF joint with osseous loss and grooving is a contraindication for mobile-bearing UKA. Less severe impairment of the lateral facet of the PF joint and involvement of the medial side, no matter how severe, did not affect the comprehensive function or implant survival, so should not be considered a contraindication. If a patient presents with full-thickness cartilage loss on the lateral facet of the PF joint, however, they may have a problem with their ability to walk down stairs. Pre-operative anterior knee pain also did not affect the functional result or implant survival and should not be considered a contraindication.
PF chondromalacia has historically been suggested to be a contraindication for UKA. Adams et al evaluated the effect of medial patellar and/or medial trochlear PF chondromalacia on comprehensive and PF-related results at two years following fixed-bearing medial UKA.17 Functional results of fixed-bearing medial UKA were not unfavourably impacted by the presence of PF chondromalacia affecting the medial patellar side and/or the medial or central trochlea. Table 1 summarizes the most important data on the current indications for UKA.14–18
Table 1.
Indications of unicompartmental knee arthroplasty in the literature
| Author | Year | Comments |
|---|---|---|
| Hamilton et al14 | 2017 | According to these authors, the presence of lateral osteophytes was not a contraindication for medial meniscal-bearing UKA. The clinical relevance of this study was that it highlighted the importance of an appropriate pre-operative assessment of the lateral compartment, given that at the setting of full-thickness cartilage at operation, lateral osteophytes did not compromise long-term functional outcome or implant survival. |
| Knifsund et al15 | 2017 | These authors suggested that UKA should only be performed in cases showing severe OA in pre-operative radiographs, with medial bone-on-bone contact, and a medial/lateral ratio of < 20%. Surgery was performed on 294 knees in 241 patients between 2001 and 2012 at a single institute, using cemented Oxford phase III UKA. The mean age at the time of operation was 67 years, and the mean follow-up time was 8.7 years. The knees with a pre-operative Kellgren-Lawrence grade of 0–2 osteoarthritis had a higher risk of re-operation than those with a Kellgren-Lawrence grade of 3–4. In addition, the knees with a medial joint space width of more than 1 mm or a high medial/lateral joint space width ratio had an increased risk of re-operation. |
| Hamilton et al16 | 2017 | Severe damage to the lateral side of the PF joint with bone loss and grooving remains a contraindication for mobile-bearing UKA. Less severe damage to the lateral side of the PF joint and damage to the medial side, however severe, does not compromise the overall function or survival, so should not be considered to be a contraindication. However, if a patient does have full-thickness cartilage loss on the lateral side of the PF joint they might have a slight problem with their ability to descend stairs. Pre-operative anterior knee pain also does not compromise the functional outcome or survival and should not be considered to be a contraindication. |
| Adams et al17 | 2017 | Functional results of fixed-bearing medial UKA were not adversely impacted by the presence of PF chondromalacia involving the medial patellar facet and/or the medial or central trochlea. |
| Hamilton et al18 | 2017 | The indications for UKA remain controversial. Previously recommended contraindications included the following: age younger than 60 years, weight 180 lb (82 kg) or over, patients undertaking heavy labour, chondrocalcinosis, and exposed bone in the PF joint. This study provided evidence that patients with the previously reported contraindications did as well as, or even better than, those without contraindications. Therefore, these contraindications should not apply to UKA. |
Notes: UKA, unicompartmental knee arthroplasty; OA, osteoarthritis; PF, patellofemoral.
Technical issues
The role of coronal alignment in the improvement of functional results after UKA is controversial. Most reports on the control of coronal alignment and implant positioning observed no influence on functional results and quality of life at one-year follow-up. However, the influence of implant positioning on failure rate and durability is better supported in the literature (Fig. 2).
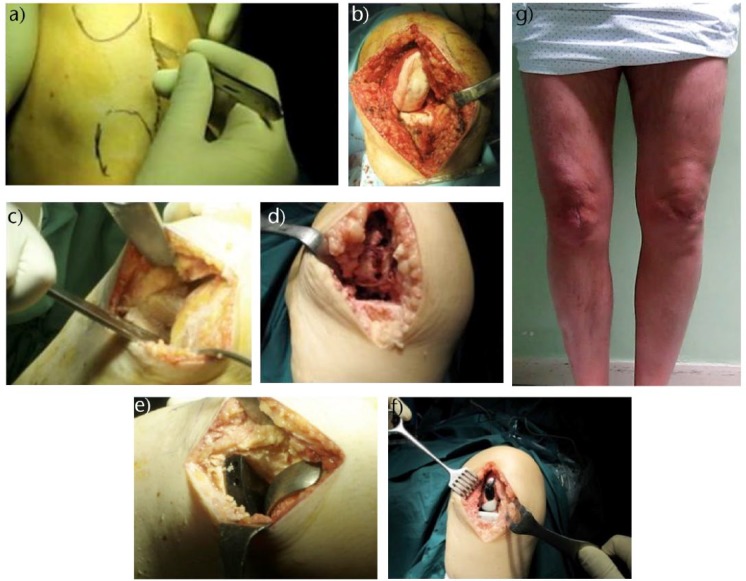
Fig. 2.
Fixed-bearing cemented medial unicompartmental knee arthroplasty (UKA). (a) Drawing of anatomical landmarks before skin incision; (b) intra-operative view of the medial compartment showing severe degeneration of the articular cartilage; (c) intra-operative view after having performed femoral and tibial cuts; (d) checking of femoral and tibial cuts; (e) trial components in place; (f) final implant in place; (g) clinical view of the surgical scar after implantation of the medial UKA.
Some authors have reported that robotics or patient-specific instrumentation (PSI) can increase the likelihood of achieving good alignment during surgery.
Patient-specific instrumentation
In 2016, Ollivier et al reported that PSI might provide little, if any, benefit in alignment, pain, or function following UKA.19 They also stated that this assertion could therefore not be used to justify the extra cost and uncertainty associated with this surgical technique.
Ng et al have reported that PSI can improve the capacity of orthopaedic surgeons in training to reproduce a pre-operative plan.20 However, their results suggested the necessity for larger-scale clinical studies to ascertain the role of PSI in this surgical technique.
In 2017, Alvand et al reported a prospective randomized controlled study to compare the precision of implantation and the functional result of mobile-bearing medial UKAs implanted with and without PSI by expert UKA orthopaedic surgeons.21 They found that PSI was equivalent to standard instrumentation based on Oxford Knee Score ameliorations at a short period of follow-up of 12 months. Table 2 shows the most recent reports on the role of PSI in UKA.19–21
Table 2.
Patient-specific instrumentation in unicompartmental knee arthroplasty in the literature
| Author | Year | Comments |
|---|---|---|
| Ollivier et al19 | 2016 | Ollivier et al stated that PSI might provide little, if any, benefit in alignment, pain, or function following UKA. |
| Ng et al20 | 2017 | This study offered some evidence that PSI can improve the capacity of orthopaedic surgeons in training to reproduce a pre-operative plan. |
| Alvand et al21 | 2017 | Although PSI was equivalent to standard instrumentation based on Oxford Knee Score improvements at 12 months, these authors continued to use standard instrumentation for UKA at their centre until further ameliorations to the PSI guides were shown. |
Notes: PSI, patient-specific instrumentation; UKA, unicompartmental knee arthroplasty.
Robot-assisted UKA
Robot-assisted systems are robotic appliances that perform specific tasks according to pre-operative data. There are three main categories of robot-assisted systems: passive systems, semi-active robotic systems, and active robotic systems.22 Passive systems perform part of the surgical procedure under the continuous and direct control of the orthopaedic surgeon. A semi-active robotic system is a tactile feedback system that increases the surgeon’s ability to control the tool, typically by restricting the cut volume by defining constraints of the cut motion in space; however, the system still requires the surgeon to manipulate the cutter. Finally, an active robotic system performs a surgical task without direct intervention from the orthopaedic surgeon, such as permitting the robotic arm to cut the bone without direct manipulation of the cutter by the surgeon.23
Many of these types of systems have been developed and prototyped. However, only the following have been used successfully in clinical settings throughout the world.23 The ROBODOC System (Curexo Technology Corporation, Fremont, CA, USA), the CASPAR system (URS Ortho Rastatt, Germany), the Robotic Arm Interactive Orthopaedic System (RIO; MAKO Surgical Corporation, Fort Lauderdale, FL, USA), and the Stanmore Sculptor Robotic Guidance Arm (RGA) System (Stanmore Implants, Elstree, UK), formerly known as the Acrobot System. MAKO’s RIO and the Stanmore Sculptor RGA System are semi-active systems, whereas the CASPAR and ROBODOC systems are active robotic systems.23 Table 3 summarizes primary data on robot-assisted UKA in the orthopaedic literature.24–30
Table 3.
Robot-assisted unicompartmental knee arthroplasty in the literature
| Author | Year | Comments |
|---|---|---|
| Moschetti et al24 | 2016 | In 2016, these authors devised a Markov decision analysis to assess the costs, results, and incremental cost-effectiveness of robot-assisted UKA in 64-year-old patients with advanced unicompartmental knee OA. The system was cost-effective when case volume exceeded 94 cases per year, two-year failure rates were below 1.2%, and total system costs were < $1.426 million. |
| Song et al25 | 2016 | These authors studied whether the use of imageless navigation can improve implant positioning and clinical results of UKA at a long-term follow-up compared with the standard surgical technique. Their results showed that the use of navigation significantly improved component placement as compared with the standard technique. |
| Bell et al26 | 2016 | Bell et al assessed the precision of component positioning in UKA, comparing robot-assisted techniques using the MAKO RIO system and standard implantation techniques. They observed that robotic-assisted surgical procedures with the use of the MAKO RIO led to improved precision of implant positioning compared with standard UKA surgical techniques. |
| Van der List et al27 | 2016 | Results in this systematic review and meta-analysis implied that computer navigation or robotic assistance could improve results. |
| Pearle et al28 | 2017 | Pearle et al reported a prospective multicentre study that evaluated results of robot-assisted UKA. In this analysis, robot-assisted UKA was found to have high survivorship and satisfaction rate at short-term follow-up. |
| Chowdhry et al29 | 2017 | These authors observed that computer-assisted UKA, to manage medial tibiofemoral joint arthritis, yielded five-year survival rates that were comparable with TKA. |
| Gaudiani et al30 | 2017 | Gaudiani et al stated that changing posterior tibial slope, while keeping PCOR, was paramount in accomplishing native kinematics and optimising range of motion in the sagittal plane. This could be best achieved using robotic techniques for UKA. |
Notes: UKA, unicompartmental knee arthroplasty; OA, osteoarthritis; RIO, Robotic Interactive Orthopaedic; TKA, total knee arthroplasty; PCOR, posterior condylar offset ratio.
Mobile-bearing versus fixed-bearing UKA
There is controversy in the literature regarding which type of bearing is preferable: mobile or fixed. In 2015, Ko et al reported a systematic review of comparative studies between fixed and mobile bearings focussing on complications.31 The overall re-operation rate per 100 component years was comparable between mobile and fixed bearings. Nevertheless, the mobile bearings were more prone to re-operations in patients from aseptic loosening, progression of OA, and implant dislocation. The comprehensive frequency of complications was analogous for fixed- and mobile-bearing designs in UKA.
In 2017, Choy et al analysed the results of minimally invasive mobile-bearing medial UKA for Korean patients.32 Their hypothesis was that because Asian patients have distinct lifestyles from those of Western patients, such as squatting and sitting on the floor, it was plausible that the clinical outcomes and survival rate of UKA for Asian patients could be distinct. A total of 164 knees were treated with mobile-bearing UKAs in 147 patients. The mean follow-up period was 12 years. The clinical results demonstrated statistically significant improvement from pre-operative to final follow-up. A total of 26 UKAs (15.8%) needed revision; the most common cause was bearing dislocation. The implant survival rate at 12 years with revision for any reason as the end point was 84.1%. Minimally invasive mobile-bearing UKA in Asian patients who needed high ranges of knee flexion demonstrated rapid recovery and good clinical results. Nonetheless, they also had relatively high percentages of bearing dislocation and aseptic loosening.
All-polyethylene UKA
Whether all-poly tibial components give similar results to metal-backed modular components during UKA remains debatable. In 2016, Hawi et al found that an all-polyethylene tibial component had a similar survivorship to modular designs.33 Implant selection did not appear to have great impact on the result, but, rather, success depended on adequate indications and surgical technique. One hundred patients with indications for UKA for isolated medial knee compartment OA were analysed. The survival likelihood of the all-polyethylene UKA implant was 95.4% after a mean follow-up of eight years, which is similar to reports from studies utilizing metal-backed modular designs for UKA. The reasons for failure were progression of OA in contiguous compartments (2%) and loosening of the tibial component (2%).
In 2017, Koh et al compared the results between all-poly and metal-backed modular components in UKA.34 All-poly tibial component use during UKA augmented the risk of initial failure, which could have been due to a failure in tibial loading distribution. Some 101 UKAs were analysed. Overall, 51 UKAs were performed using all-poly tibial components; 50 others used metal-backed modular components. Despite the lack of group differences in clinical and radiographic results, adaptive bone remodelling at two years after surgery of all-poly UKAs was more progressive compared with metal-backed UKAs (1.2 in the all-poly UKA group vs. 0.9 in the metal-backed UKA group). In addition, 6 of 51 all-poly UKAs failed post-operatively within two years, whereas no metal-backed UKAs failed (11% in the all-poly UKA group vs. 0% in the metal-backed UKA group).
Inpatient versus outpatient UKA
The demand for TKA and UKA is increasing rapidly due to the established success of these surgical techniques and an aging population. However, resources are limited and healthcare budgets are restricted. Recently, some care providers have begun performing these surgical techniques on an outpatient basis, with the patients discharged from the hospital on the day of surgery. Table 4 summarizes the most important data on inpatient vs. outpatient surgery in patients undergoing UKA.9–13
Table 4.
Inpatient vs. outpatient surgery in patients undergoing unicompartmental knee arthroplasty in the literature
| Author | Year | Comments |
|---|---|---|
| Pollock et al9 | 2016 | This systematic review showed that in selected patients, outpatient UKA can be performed safely and effectively. The included studies lacked sufficient internal validity, sample size, methodological consistency, and standardization of protocols and outcomes. Pollock et al found a need for high-quality prospective cohort and randomised trials to definitively assess the safety and effectiveness of outpatient UKA. |
| Kort et al10 | 2017 | The results of this study illustrated that an OS pathway for UKA was effective and safe, with acceptable clinical outcomes. Well-established and adequate standardized protocols, inclusion and exclusion criteria, and a change in mindset for both the patient and the multidisciplinary team were the key factors for the implementation of an OS pathway. |
| Richter et al11 | 2017 | This study demonstrated that significant cost savings of roughly 50% can be achieved with an outpatient UKA protocol performed at an outpatient surgical facility. |
| Hoorntje et al12 | 2017 | The results of this study emphasized the feasibility of an OS pathway in carefully selected UKA patients. The OS pathway was safe, and clinical outcome, including levels of anxiety and depression, satisfaction, and pain, was similar in OS patients compared with the standard fast-track patients. |
| Bovonratwet et al13 | 2017 | These authors stated that outpatient UKA can be appropriately considered in carefully selected patients based on the lack of differences in rates of 30-day peri-operative complications and readmissions between the outpatient and matched inpatient groups. |
Notes: UKA, unicompartmental knee arthroplasty; OS, outpatient surgery
Discharge on the day of surgery
Bradley et al have reported that patients can be safely and efficaciously discharged on the day of surgery after UKA, with high satisfaction.35 This plainly offers improved management of assets and financial savings to the healthcare system. The most common causes of failure were logistical (the operation was too late in the day), inappropriate control of pain, and leaking wounds. No re-admissions were found. All patients had a high level of satisfaction.
Outcomes of UKA
The major advantage of UKA compared with TKA appears to be the higher rate of satisfaction and meeting expectations (return to work and return to sports) in young patients.
Results reported after UKA are generally favourable in the literature.36–65 In 2015, Parratte et al stated that medium- and long-term studies indicated acceptable results at 10 years with survival greater than 95% in UKA performed for medial OA or osteonecrosis, and also for lateral UKA, especially when fixed-bearing implants were used.37 Walker et al reported that patients aged 60 years or younger after medial UKA were able to return to their regular physical activities, with approximately two-thirds of the patients attaining a high activity level.39
A study by Pandit et al also supported the continued use of minimally invasive UKA for the advised indications.40 There were some implant-related re-operations at a mean of 5.5 years. The most common causes for re-operation were OA in the lateral compartment (2.5%), bearing dislocation (0.7%) and unelucidated pain (0.7%). When all implant-related re-operations were considered failures, the 10-year rate of implant survival was 94% and the 15-year rate 91%. When failure of the implant was the end point, the 15-year survival rate was 99%. In a systematic review reported by Howieson et al on UKA in the elderly, it was found that in patients over the age of 70 years there was no peri-operative mortality and the 10-year prosthesis survival rate was 87.5%–98.0%. In addition, revision due to periprosthetic infection was low at 0.13%–0.30%.41
In 2016, Ali et al reported that high activity did not jeopardize the result of the OUKA and might improve it.43 Activity should not be limited nor considered to be a contraindication. Forster-Horvárth et al observed that fixed-bearing Uniglide UKA with an all-polyethylene tibial component is a useful tool in the treatment of medial compartment OA, providing good short-term survivorship.51 The five-year survival rate was 94.1%, with implant revision surgery as an end point. The predicted 10-year implant survival rate is 91.3%.
The systematic review reported in 2017 by Campi et al showed that cementless fixation was a safe and efficacious alternative to cementation in medial UKA.52 Clinical results, failures, re-operation percentages, and implant survival were analogous to those reported for cemented implants. In 2017, Kerens et al compared cementless OUKA with cemented OUKA.55 Implant survival percentages were 90% at 34 months for the cementless UKA and 84% at 54 months for the cemented UKA. Clinical results were not significantly different. In a systematic review published in 2017 by Hamilton et al, the authors stated that to achieve optimal results, surgeons, whether high or low caseload, should follow the advised indications such that ⩾ 20%, or ideally > 30% of their knee arthroplasties are UKA.56 If they take this into account, then they can anticipate outcomes comparable to those of the long-term series, all of which had high usage (> 20%) and an average 10-year implant survival of 94%.
In 2017, Blaney et al supported the use of the cementless Oxford UKAs outside the design centre.58 The number of patients needing revision at five years was lower than that typically published for UKA. The accumulated implant survival at five years was 98.8%, and the survival time was 5.8 years on average. Table 5 summarizes primary data regarding outcomes and prosthetic survival following UKA.36–65
Table 5.
Results of unicompartmental knee arthroplasty in the literature
| Author | Year | Comments |
|---|---|---|
| Liddle et al36 | 2015 | UKA provided better early patient-reported outcomes than TKA; these differences were most marked for the very best outcomes. Complications and readmission were more likely after TKA. |
| Parratte et al37 | 2015 | Medium- and long-term studies suggested reasonable outcomes at 10 years, with implant survival greater than 95% in UKA performed for medial OA or osteonecrosis, and similarly for lateral UKA, particularly when fixed-bearing implants were used. |
| Vasso et al38 | 2015 | This study demonstrated excellent outcomes and implant survivorship for the ZUK UKA. |
| Walker et al39 | 2015 | The results of this study demonstrated that patients aged 60 years or younger following medial UKA were able to return to regular physical activities, with almost two-thirds of the patients reaching a high activity level. |
| Pandit et al40 | 2015 | The results of this study supported the continued use of minimally invasive UKA for the recommended indications. There were some implant-related re-operations at a mean of 5.5 years. When all implant-related re-operations were considered as failures, the 10-year rate of survival was 94% and the 15-year survival rate 91%. When failure of the implant was the end point, the 15-year survival rate was 99%. |
| Howieson et al41 | 2015 | This systematic review on UKA in the elderly showed that there was no peri-operative mortality, and the 10-year prosthesis survival rate was 87.5%–98.0%. Revision for peri-prosthetic infection was low at 0.13%–0.30%. |
| Iacono et al42 | 2016 | These authors stated that UKA was a viable option for treating unicompartmental knee OA. With the proper indications and an accurate technique, UKA might also be indicated for very elderly patients with reduced complications and morbidity, and excellent implant survivorship. |
| Ali et al43 | 2016 | High activity levels did not compromise the outcome of the Oxford UKA. Activity should not be restricted nor considered to be a contraindication. The study included the first 1000 Phase 3 cemented Oxford UKAs implanted between 1998 and 2010. |
| Zuiderbaan et al44 | 2016 | This study suggested that greater pain relief can be expected in patients aged < 65 years and that a post-operative lower limb alignment of 1°–4° varus should be pursued. Taking these factors into consideration will help to maximize clinical outcomes, fulfil patient expectations after medial UKA, and subsequently minimize revision rates. |
| Lee et al45 | 2016 | The study included 724 UKAs. Minimum duration of follow-up was two years, with an overall patient satisfaction rate of 92.2%. |
| Konan and Haddad46 | 2016 | Topographical location and severity of cartilage damage of the patella can significantly influence function after successful Oxford medial UKA. |
| Bottomley et al47 | 2016 | This study demonstrated that good results can be achieved by a heterogeneous group of surgeons, including trainees, if performed within a high-volume centre with considerable experience with the procedure. It was an implant survival analysis of 1084 knees of the Oxford UKA (a comparison between consultant and trainee surgeons). |
| Emerson et al48 | 2016 | This 10-year follow-up study of the Oxford UKA undertaken in the United States showed good implant survival and excellent function in a wide selection of patients with anteromedial OA and avascular necrosis. It included 213 knees (173 patients). |
| Lisowski et al49 | 2016 | This study supported the use of UKA in medial compartment OA, with excellent long-term functional and radiological outcomes and an excellent 15-year implant survival rate. |
| van der List et al50 | 2016 | This meta-analysis critique showed that findings of increased revision risk in younger patients and increased revision risk with inferior outcomes in females gave a more nuanced perspective on historical criteria, such that surgical decision-making can be based on UKA outcome data for subgroups rather than strict exclusion criteria. |
| Forster-Horváth et al51 | 2016 | Fixed bearing Uniglide UKA with an all-polyethylene tibial component was a valuable tool in the management of medial compartment OA, affording good short-term implant survival. The five-year survival rate was 94.1%, with implant revision surgery as an end point. The estimated 10-year survival rate is 91.3%. |
| Campi et al52 | 2017 | This systematic review demonstrated that cementless fixation was a safe and effective alternative to cementation in medial UKA. Clinical outcome, failures, re-operation rate, and implant survival were similar to those reported for cemented implants with lower incidence of RLL. |
| Streit et al53 | 2017 | Minimally invasive Oxford medial UKA was reliable and effective in a young and active patient cohort, providing high patient satisfaction at the mid-term follow-up. |
| Pandit et al54 | 2017 | This study included 512 cementless Phase 3 Oxford UKAs. The clinical results of this study were as good as or better than those previously reported for cemented fixation. The radiographic results were better, with secure bony attachment to the implants in every case. There were eight re-operations of which six were revisions, giving a five-year implant survival of 98%. |
| Kerens et al55 | 2017 | In this multicentre retrospective study, a cohort of 60 consecutive cases of cementless Oxford UKA was compared with a cohort of 60 consecutive cases of cemented Oxford UKA. Survival rates were 90% at 34 months for the cementless group and 84% at 54 months for the cemented group. Mean operation time was 10 min shorter in the cementless group, and clinical results were not significantly different. |
| Hamilton et al56 | 2017 | Medial UKA should be reserved for patients with full-thickness cartilage loss on both the femur and tibia. |
| Hamilton et al57 | 2017 | In this systematic review the authors stated that to achieve optimum results, surgeons, whether high or low caseload, should adhere to the recommended indications such that ⩾ 20%, or ideally > 30% of their knee arthroplasties are UKA. If they do this, then they can expect to achieve results similar to those of the long-term series, which all had high usage (> 20%) and an average 10-year survival of 94%. |
| Blaney et al58 | 2017 | The findings of this report added support for the use of the cementless Oxford UKAs outside the design centre. The cumulative survival at five years was 98.8% and the mean survival time was 5.8 years. A total of seven Oxford UKAs (2.7%) were revised; three within five years and four thereafter, between 5.1 and 5.7 years postoperatively. Five (1.9%) had re-operations within five years. |
| Kleeblad et al59 | 2017 | This was the first study showing that physiological femoral RLL occur later than tibial RLL. A total of 352 patients were included who underwent robotic-assisted medial UKA surgery and received a fixed-bearing metal-backed cemented medial UKA. |
| van der List et al60 | 2017 | This systematic review showed that good to excellent extrapolated implant survival and functional outcomes are observed following modern cementless UKA, with a low incidence of aseptic loosening. |
| Kim et al61 | 2017 | Oxford medial UKA was reliable and effective in young, active Asian patients, providing good clinical results and implant survival rates in the mid-term follow-up. Including three bearing dislocations, one medial tibial collapse and one lateral osteoarthritis, the total complication rate was 6.1% (5/82). The 10-year cumulative survival rate using the Kaplan-Meier survival method was 94.7%. |
| Panzram et al62 | 2017 | Cementless fixation showed good implant survival rates and clinical outcome compared with cemented fixation. The five-year survival rate of the cementless group was 89.7% and of the cemented group 94.1%. Both groups showed excellent post-operative clinical scores. |
| Xue et al63 | 2017 | This study demonstrated that Oxford UKA was a good option for the treatment of anteromedial OA and spontaneous osteonecrosis of the knee in Asian patients. |
| Mohammad et al64 | 2018 | The annual revision rate was 0.74% corresponding to a 10-year survival of 93% and 15-year survival of 89%. The non-revision re-operation rate was 0.19%. The re-operation rate was 0.89%. The most common causes of revision were lateral disease progression (1.42%), aseptic loosening (1.25%), bearing dislocation (0.58%), and pain (0.57%). The incidence of medical complications was 0.83%. |
| Tadros et al65 | 2018 | The two-year short-term functional outcome, revision rates and satisfaction of UKA in the octogenarian population did not differ statistically from other age groups. No significant difference in implant survival was found between the groups. The overall revision rate was 28/395 (7%). The 90-day mortality in this series was one patient. |
Notes: UKA, unicompartmental knee arthroplasty; TKA, total knee arthroplasty; OA, osteoarthritis; MRI, magnetic resonance imaging; RLL, radiolucent lines.
Complications of UKA
In 2016, Kim et al analysed the causes and types of complications following UKA, and determined appropriate prevention and management methods.66 The most common complication after UKA was dislocation of the mobile bearing. The authors concluded that when a complication happens after UKA, adequate treatment should be performed after a proper analysis of the cause of the complication.
In 2016, van der List et al reported a level III systematic review.67 They recognized aseptic loosening and OA progression as the dominant failure forms. Aseptic loosening was the principal failure form in the early years and in mobile-bearing implants, whereas OA progression produced the majority of failures in later years and in fixed-bearing implants. In 2016, Inui et al reported two cases of snapping pes syndrome following UKA.68 Conservative treatment was efficacious in one case, whereas surgical excision of the gracilis tendon was needed to alleviate painful snapping in the other case. The main reason for the first case was probably posteromedial overhang of the tibial tray, which reached up to 5 mm. The potential cause of the second case was posteromedial overhang of the mobile bearing.
In 2016, Chen et al studied the amount of post-operative fixed flexion deformity that is clinically appropriate following UKA.69 Their data suggested that post-operative fixed flexion deformity of > 10° following UKA was associated with significantly poorer functional results.
Ahn et al noted the likelihood of post-operative malalignment during medial UKA in patients with a greater varus angle in pre-operative distal femoral varus angle (DFVA), tibial bone varus angle (TBVA), and valgus stress angle, particularly with a greater varus DFVA, which was the strongest predictor for malalignment.70
Inclining of the mobile bearing relative to the tibial tray in the flexion position could be the consequence of implanting the femoral components more laterally relative to the tibial components during UKA using the Oxford Knee. Inui et al compared femoral component positions after UKA using the phase 3 device and a novel device.71 They also assessed the placement of the femoral components with the new device in the flexion position to define the association with short-term prognosis. They observed that to prevent implantation of the femoral component too laterally using a new device during UKA, knee surgeons should set the drill guide more medially, such that the centre of the drill is aligned with the middle of the medial femoral condyle. Impingement of the mobile bearing on the lateral wall of the tibial tray in UKA must be avoided (Fig. 3).
Fig. 3.
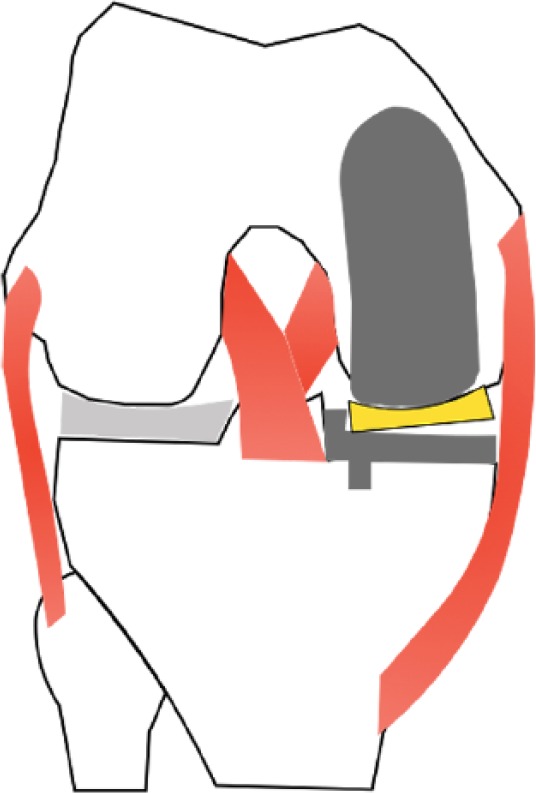
Illustration showing impingement of the mobile bearing on the lateral wall of the tibial tray in UKA. This complication must be avoided by preventing implantation of the femoral component too laterally. Surgeons should set the drill guide more medially, such that the centre of the drill is aligned with the middle of the medial femoral condyle.
Van der List et al performed a systematic review to evaluate failure mechanisms in lateral UKA and compared failure mechanisms in cohort studies with those encountered in registry-based studies.72 The most common failure forms in lateral UKA were progression of OA (29%), aseptic loosening (23%), and bearing dislocation (10%). In cohort studies, progression of OA was more common (36%) than bearing dislocation (17%) and aseptic loosening (16%), whereas in the registry-based studies, aseptic loosening (28%) was more common than progression of OA (24%) and bearing dislocation (5%). These authors concluded that progression of OA is the most common failure mechanism in lateral UKA. They also recommended that in the future, both cohort studies and registry-based studies should report the failure mechanisms of medial and lateral UKA independently. Table 6 summarizes the primary complications of UKA in the orthopaedic literature.66–72
Table 6.
Complications of unicompartmental knee arthroplasty in the literature
| Author | Year | Comments |
|---|---|---|
| Kim et al66 | 2016 | A total of 1576 UKAs were performed for OA of the knee. These authors retrospectively analysed complications after UKA and investigated proper methods of treatment. A total of 89 complications (5.6%) occurred after UKA. Regarding the type of complications after UKA, there were 42 cases of dislocation of the mobile bearing, 23 cases of loosening of the prosthesis, six cases of periprosthetic fracture, three cases of polyethylene wear, three cases of progression of OA in the contralateral compartment, two cases of medial collateral ligament injury, two cases of impingement, five cases of infection, one case of arthrofibrosis, and two cases of failure due to unexplained pain. The most common complication after UKA was mobile-bearing dislocation in the mobile-bearing knees and loosening of the prosthesis in the fixed-bearing knees, but polyethylene wear and progression of OA were relatively rare. The complications were treated with conversion to TKA in 58 cases and simple bearing change in 21 cases. |
| van der List et al67 | 2016 | This level III systematic review identified aseptic loosening and OA progression as the major failure modes. Aseptic loosening was the main failure mode in early years and in mobile-bearing implants, whereas OA progression caused most failures in later years and in fixed-bearing implants. Aseptic loosening (36%) and OA progression (20%) were the most common failure mechanisms. Aseptic loosening (26%) was the most common early failure mechanism, whereas OA progression was more commonly seen in mid-term and late failures (38% and 40%, respectively). Polyethylene wear (12%) and instability (12%) were more common in fixed-bearing implants, whereas pain (14%) and bearing dislocation (11%) were more common in mobile-bearing implants. |
| Inui et al68 | 2016 | These authors reported two cases of snapping pes syndrome after UKA. Conservative treatment was effective in one case, while surgical excision of the gracilis tendon was necessary to relieve painful snapping in the other case. The main cause of the first case might have been posteromedial overhang of the tibial tray that reached up to 5 mm. The probable cause of the second case was posteromedial overhang of the mobile bearing. |
| Chen et al69 | 2016 | These authors studied the amount of post-operative FFD that is clinically appropriate following UKA. Their data suggested that post-operative FFD of > 10° following UKA was associated with significantly poorer functional results. |
| Ahn et al70 | 2016 | These authors analysed 92 patients who had 127 medial UKAs. According to post-operative limb mechanical axis (HKA), 127 enrolled knees were sorted into acceptable alignment with HKA angle within the conventional ± 3-degree range from a neutral alignment (n = 73) and outlier with HKA angle outside ± 3-degree range (n = 54) groups. Multivariate logistic regression was used to analyse risk factors including age, sex, body mass index, thickness of polyethylene tibial insert, pre-operative HKA, DFVA, FBA, TBVA, mechanical distal femoral and proximal tibial angles, varus and valgus stress angles, size of femoral and tibial osteophytes, and femoral and tibial component alignment angles. Pre-operative DFVA, TBVA and valgus stress angle were identified as significant risk factors. |
| Inui et al71 | 2016 | Inclining of the mobile bearing relative to the tibial tray in the flexion position could be the consequence of implanting the femoral components more laterally relative to tibial components during UKA using the Oxford Knee. These authors compared femoral component positions after UKA using the phase 3 device and a novel device. They also assessed the placement of the femoral components with the new device in the flexion position to define the association with short-term prognosis. They observed that to prevent implantation of the femoral component too laterally using a new device during UKA, knee surgeons should set the drill guide more medially such that the centre of the drill is aligned with the middle of the medial femoral condyle. |
| van der List et al72 | 2016 | These authors performed a systematic review to evaluate failure mechanisms in lateral UKA. Progression of OA was the most common failure mechanism in lateral UKA. |
Notes: UKA, unicompartmental knee arthroplasty; OA, osteoarthritis; FFD, fixed flexion deformity; HKA, hip-knee angle; DFVA, distal femoral varus angle; FBA, femoral bowing angle; TBVA, tibial bone varus angle.
Optimal usage of UKA
According to Liddle et al, UKA has advantages over TKA; however, national joint registries communicate a significantly higher revision rate for UKA.73 As a consequence, the majority of surgeons are highly selective, proposing UKA only to a small proportion (up to 5%) of patients needing arthroplasty of the knee, and accordingly performing few procedures each year. Nevertheless, surgeons with large UKA practices have the lowest percentages of revision. The comprehensive size of the practice is frequently beyond the surgeon’s control; thus, case volume might only be augmented by broadening the indications for surgery and proposing UKA to a greater proportion of patients needing arthroplasty of the knee.
Liddle et al stated that UKA usage has a complicated, non-linear relationship with the rate of revision.73 Reasonable outcomes are obtained with 20% usage or more. Optimal results are accomplished with usage between 40% and 60%. Surgeons with the smallest usage (up to 5%) have the highest rates of revision. Revision rates per 100 implant years, according to Liddle et al, ranged from 1% to 4.5%, depending on UKA usage (expressed as % UKA).73
Conclusions
UKA has considerable advantages, including lower peri-operative morbidity and earlier recovery, compared with TKA. The traditionally stringent indications for UKA have been called into question by reports that extended the indications based on a diagnosis of anteromedial OA of the knee and showed successful results. Both fixed- and mobile-bearing UKA implants show excellent clinical results at more than 10 years post-operatively but continue experiencing distinct forms of long-term implant failure. Appropriate patient selection and execution of surgical technique are paramount to optimizing patient results.
Footnotes
ICMJE Conflict of interest statement: None declared.
Funding statement
No benefits in any form have been received or will be received from a commercial party related directly or indirectly to the subject of this article.
References
- 1. Oliveria SA, Felson DT, Reed JI, Cirillo PA, Walker AM. Incidence of symptomatic hand, hip, and knee osteoarthritis among patients in a health maintenance organization. Arthritis Rheum 1995;38:1134–1141. [DOI] [PubMed] [Google Scholar]
- 2. Felson DT, Zhang Y. An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum 1998;41:1343–1355. [DOI] [PubMed] [Google Scholar]
- 3. Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum 2008;59:1207–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wise BL, Niu J, Yang M, et al. Multicenter Osteoarthritis (MOST) Group: patterns of compartment involvement in tibiofemoral osteoarthritis in men and women and in whites and African Americans. Arthritis Care Res (Hoboken) 2012;64:847–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. MacIntosh DL. Hemiarthroplasty of the knee using a space occupying prosthesis for painful varus and valgus deformities. J Bone Joint Surg Am 1958;40:1431. [Google Scholar]
- 6. Broughton NS, Newman JH, Baily RA. Unicompartmental replacement and high tibial osteotomy for osteoarthritis of the knee: a comparative study after 5–10 years’ follow-up. J Bone Joint Surg Br 1986;68:447–452. [DOI] [PubMed] [Google Scholar]
- 7. Marmor L. Marmor modular knee in unicompartmental disease: minimum four-year follow-up. J Bone Joint Surg Am 1979;61:347–353. [PubMed] [Google Scholar]
- 8. Goodfellow JW, Kershaw CJ, Benson MK, O’Connor JJ. The Oxford Knee for unicompartmental osteoarthritis: the first 103 cases. J Bone Joint Surg Br 1988;70:692–701. [DOI] [PubMed] [Google Scholar]
- 9. Pollock M, Somerville L, Firth A, Lanting B. Outpatient total hip arthroplasty, total knee arthroplasty, and unicompartmental knee arthroplasty: a systematic review of the literature. JBJS Rev 2016;27:01874474-201612000-00004. [DOI] [PubMed] [Google Scholar]
- 10. Kort NP, Bemelmans YFL, Schotanus MGM. Outpatient surgery for unicompartmental knee arthroplasty is effective and safe. Knee Surg Sports Traumatol Arthrosc 2017;25:2659–2667. [DOI] [PubMed] [Google Scholar]
- 11. Richter DL, Diduch DR. Cost comparison of outpatient versus inpatient unicompartmental knee arthroplasty. Orthop J Sports Med 2017;5:2325967117694352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoorntje A, Koenraadt KLM, Boevé MG, van Geenen RCI. Outpatient unicompartmental knee arthroplasty: who is afraid of outpatient surgery? Knee Surg Sports Traumatol Arthrosc 2017;25:759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bovonratwet P, Ondeck NT, Tyagi V, Nelson SJ, Rubin LE, Grauer JN. Outpatient and inpatient unicompartmental knee arthroplasty procedures have similar short-term complication profiles. J Arthroplasty 2017;32:2935–2940. [DOI] [PubMed]
- 14. Hamilton TW, Choudhary R, Jenkins C, et al. Lateral osteophytes do not represent a contraindication to medial unicompartmental knee arthroplasty: a 15-year follow-up. Knee Surg Sports Traumatol Arthrosc 2017;25:652–659. [DOI] [PubMed] [Google Scholar]
- 15. Knifsund J, Hatakka J, Keemu H, Mäkelä K, Koivisto M, Niinimäki T. Unicompartmental knee arthroplasties are performed on the patients with radiologically too mild osteoarthritis. Scand J Surg 2017;106:338–341. [DOI] [PubMed] [Google Scholar]
- 16. Hamilton TW, Pandit HG, Maurer DG, et al. Anterior knee pain and evidence of osteoarthritis of the patellofemoral joint should not be considered contraindications to mobile-bearing unicompartmental knee arthroplasty: a 15-year follow-up. Bone Joint J 2017;99-B:632–639. [DOI] [PubMed] [Google Scholar]
- 17. Adams AJ, Kazarian GS, Lonner JH. Preoperative patellofemoral chondromalacia is not a contraindication for fixed-bearing medial unicompartmental knee arthroplasty. J Arthroplasty 2017;32:1786–1791. [DOI] [PubMed] [Google Scholar]
- 18. Hamilton TW, Pandit HG, Jenkins C, Mellon SJ, Dodd CAF, Murray DW. Evidence-based indications for mobile-bearing unicompartmental knee arthroplasty in a consecutive cohort of thousand knees. J Arthroplasty 2017;32:1779–1785. [DOI] [PubMed] [Google Scholar]
- 19. Ollivier M, Parratte S, Lunebourg A, Viehweger E, Argenson JN. The John Insall award: no functional benefit after unicompartmental knee arthroplasty performed with patient-specific instrumentation: a randomized trial. Clin Orthop Relat Res 2016;474:60–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ng CTJ, Newman S, Harris S, Clarke S, Cobb J. Patient-specific instrumentation improves alignment of lateral unicompartmental knee replacements by novice surgeons. Int Orthop 2017;41:1379–1385. [DOI] [PubMed] [Google Scholar]
- 21. Alvand A, Khan T, Jenkins C, et al. The impact of patient-specific instrumentation on unicompartmental knee arthroplasty: a prospective randomised controlled study. Knee Surg Sports Traumatol Arthrosc 2017. 10.1007/s00167-017-4677-5 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
- 22. Picard F, Moody J, DiGioia AM, III, Jaramaz B. Clinical classifications of CAOS systems. In: DiGioia AM, Jaramaz B, Picard F, Nolte LP, eds. Computer and robotic assisted hip and knee surgery. New York, NY: Oxford University Press, 2004:43–48. [Google Scholar]
- 23. Netravali NA, Shen F, Park Y, Bargar WL. A perspective on robotic assistance for knee arthroplasty. Adv Orthop 2013;2013:970703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Moschetti WE, Konopka JF, Rubash HE, Genuario JW. Can robot-assisted unicompartmental knee arthroplasty be cost-effective? A Markov decision analysis. J Arthroplasty 2016;31:759–765. [DOI] [PubMed] [Google Scholar]
- 25. Song EK, N M, Lee SH, Na BR, Seon JK. Comparison of outcome and survival after unicompartmental knee arthroplasty between navigation and conventional techniques with an average 9-year follow-up. J Arthroplasty 2016;31:395–400. [DOI] [PubMed] [Google Scholar]
- 26. Bell SW, Anthony I, Jones B, MacLean A, Rowe P, Blyth M. Improved accuracy of component positioning with robotic-assisted unicompartmental knee arthroplasty: data from a prospective, randomized controlled study. J Bone Joint Surg Am 2016;98:627–635. [DOI] [PubMed] [Google Scholar]
- 27. van der List JP, Chawla H, Joskowicz L, Pearle AD. Current state of computer navigation and robotics in unicompartmental and total knee arthroplasty: a systematic review with meta-analysis. Knee Surg Sports Traumatol Arthrosc 2016;24:3482–3495. [DOI] [PubMed] [Google Scholar]
- 28. Pearle AD, van der List JP, Lee L, Coon TM, Borus TA, Roche MW. Survivorship and patient satisfaction of robotic-assisted medial unicompartmental knee arthroplasty at a minimum two-year follow-up. Knee 2017;24:419–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chowdhry M, Khakha RS, Norris M, Kheiran A, Chauhan SK. Improved survival of computer-assisted unicompartmental knee arthroplasty: 252 cases with a minimum follow-up of 5 years. J Arthroplasty 2017;32:1132–1136. [DOI] [PubMed] [Google Scholar]
- 30. Gaudiani MA, Nwachukwu BU, Baviskar JV, Sharma M, Ranawat AS. Optimization of sagittal and coronal planes with robotic-assisted unicompartmental knee arthroplasty. Knee 2017;24:837–843. [DOI] [PubMed] [Google Scholar]
- 31. Ko YB, Gujarathi MR, Oh KJ. Outcome of unicompartmental knee arthroplasty: a systematic review of comparative studies between fixed and mobile bearings focusing on complications. Knee Surg Relat Res 2015;27:141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Choy WS, Lee KW, Kim HY, Kim KJ, Chun YS, Yang DS. Mobile bearing medial unicompartmental knee arthroplasty in patients whose lifestyles involve high degrees of knee flexion: a 10–14year follow-up study. Knee 2017;24:829–836. [DOI] [PubMed] [Google Scholar]
- 33. Hawi N, Plutat J, Kendoff D, et al. Midterm results after unicompartmental knee replacement with all-polyethylene tibial component: a single surgeon experience. Arch Orthop Trauma Surg 2016;136:1303–1307. [DOI] [PubMed] [Google Scholar]
- 34. Koh IJ, Suhl KH, Kim MW, Kim MS, Choi KY, In Y. Use of all-polyethylene tibial components in unicompartmental knee arthroplasty increases the risk of early failure. J Knee Surg 2017;30:807–815. [DOI] [PubMed] [Google Scholar]
- 35. Bradley B, Middleton S, Davis N, et al. Discharge on the day of surgery following unicompartmental knee arthroplasty within the United Kingdom NHS. Bone Joint J 2017;99-B:788–792. [DOI] [PubMed] [Google Scholar]
- 36. Liddle AD, Pandit H, Judge A, Murray DW. Patient-reported outcomes after total and unicompartmental knee arthroplasty: a study of 14,076 matched patients from the National Joint Registry for England and Wales. Bone Joint J 2015;97-B:793–801. [DOI] [PubMed] [Google Scholar]
- 37. Parratte S, Ollivier M, Lunebourg A, Abdel MP, Argenson JN. Long-term results of compartmental arthroplasties of the knee: long term results of partial knee arthroplasty. Bone Joint J 2015;97-B:9–15. [DOI] [PubMed] [Google Scholar]
- 38. Vasso M, Del Regno C, Perisano C, D’Amelio A, Corona K, Schiavone Panni A. Unicompartmental knee arthroplasty is effective: ten year results. Int Orthop 2015;39:2341–2346. [DOI] [PubMed] [Google Scholar]
- 39. Walker T, Streit J, Gotterbarm T, Bruckner T, Merle C, Streit MR. Sports, physical activity and patient-reported outcomes after medial unicompartmental knee arthroplasty in young patients. J Arthroplasty 2015;30:1911–1916. [DOI] [PubMed] [Google Scholar]
- 40. Pandit H, Hamilton TW, Jenkins C, Mellon SJ, Dodd CA, Murray DW. The clinical outcome of minimally invasive Phase 3 Oxford unicompartmental knee arthroplasty: a 15-year follow-up of 1000 UKAs. Bone Joint J 2015;97-B:1493–1500. [DOI] [PubMed] [Google Scholar]
- 41. Howieson A, Farrington W. Unicompartmental knee replacement in the elderly: a systematic review. Acta Orthop Belg 2015;81:565–571. [PubMed] [Google Scholar]
- 42. Iacono F, Raspugli GF, Akkawi I, et al. Unicompartmental knee arthroplasty in patients over 75 years: a definitive solution? Arch Orthop Trauma Surg 2016;136:117–123. [DOI] [PubMed] [Google Scholar]
- 43. Ali AM, Pandit H, Liddle AD, et al. Does activity affect the outcome of the Oxford unicompartmental knee replacement? Knee 2016;23:327–330. [DOI] [PubMed] [Google Scholar]
- 44. Zuiderbaan HA, van der List JP, Chawla H, Khamaisy S, Thein R, Pearle AD. Predictors of subjective outcome after medial unicompartmental knee arthroplasty. J Arthroplasty 2016;31:1453–1458. [DOI] [PubMed] [Google Scholar]
- 45. Lee M, Huang Y, Chong HC, Ning Y, Lo NN, Yeo SJ. Predicting satisfaction for unicompartmental knee arthroplasty patients in an Asian population. J Arthroplasty 2016;31:1706–1710. [DOI] [PubMed] [Google Scholar]
- 46. Konan S, Haddad FS. Does location of patellofemoral chondral lesion influence outcome after Oxford medial compartmental knee arthroplasty? Bone Joint J 2016;98-B:11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bottomley N, Jones LD, Rout R, et al. A survival analysis of 1084 knees of the Oxford unicompartmental knee arthroplasty: a comparison between consultant and trainee surgeons. Bone Joint J 2016;98-B:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Emerson RH, Alnachoukati O, Barrington J, Ennin K. The results of Oxford unicompartmental knee arthroplasty in the United States: a mean ten-year survival analysis. Bone Joint J 2016;98-B:34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lisowski LA, Meijer LI, Bekerom MP, Pilot P, Lisowski AE. Ten- to 15-year results of the Oxford Phase III mobile unicompartmental knee arthroplasty: a prospective study from a non-designer group. Bone Joint J 2016;98-B:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. van der List JP, Chawla H, Zuiderbaan HA, Pearle AD. The role of preoperative patient characteristics on outcomes of unicompartmental knee arthroplasty: A meta-analysis critique. J Arthroplasty 2016;31:2617–2627. [DOI] [PubMed] [Google Scholar]
- 51. Forster-Horváth C, Artz N, Hassaballa MA, et al. Survivorship and clinical outcome of the minimally invasive Uniglide medial fixed bearing, all-polyethylene tibia, unicompartmental knee arthroplasty at a mean follow-up of 7.3 years. Knee 2016;23:981–986. [DOI] [PubMed] [Google Scholar]
- 52. Campi S, Pandit HG, Dodd CAF, Murray DW. Cementless fixation in medial unicompartmental knee arthroplasty: a systematic review. Knee Surg Sports Traumatol Arthrosc 2017;25:736–745. [DOI] [PubMed] [Google Scholar]
- 53. Streit MR, Streit J, Walker T, et al. Minimally invasive Oxford medial unicompartmental knee arthroplasty in young patients. Knee Surg Sports Traumatol Arthrosc 2017;25:660–668. [DOI] [PubMed] [Google Scholar]
- 54. Pandit HG, Campi S, Hamilton TW, et al. Five-year experience of cementless Oxford unicompartmental knee replacement. Knee Surg Sports Traumatol Arthrosc 2017;25:694–702. [DOI] [PubMed] [Google Scholar]
- 55. Kerens B, Schotanus MGM, Boonen B, et al. Cementless versus cemented Oxford unicompartmental knee arthroplasty: early results of a non-designer user group. Knee Surg Sports Traumatol Arthrosc 2017;25:703–709. [DOI] [PubMed] [Google Scholar]
- 56. Hamilton TW, Pandit HG, Inabathula A, et al. Unsatisfactory outcomes following unicompartmental knee arthroplasty in patients with partial thickness cartilage loss: a medium-term follow-up. Bone Joint J 2017;99-B:475–482. [DOI] [PubMed] [Google Scholar]
- 57. Hamilton TW, Rizkalla JM, Kontochristos L, Marks BE, Mellon SJ, Dodd CAF, Pandit HG, Murray DW. The interaction of caseload and usage in determining outcomes of unicompartmental knee arthroplasty: A meta-analysis. J Arthroplasty 2017;32:3228-3237.e2. [DOI] [PubMed]
- 58. Blaney J, Harty H, Doran E, et al. Five-year clinical and radiological outcomes in 257 consecutive cementless Oxford medial unicompartmental knee arthroplasties. Bone Joint J 2017;99-B:623–631. [DOI] [PubMed] [Google Scholar]
- 59. Kleeblad LJ, van der List JP, Zuiderbaan HA, Pearle AD. Regional femoral and tibial radiolucency in cemented unicompartmental knee arthroplasty and the relationship to functional outcomes. J Arthroplasty 2017;32:3345–3351. [DOI] [PubMed] [Google Scholar]
- 60. van der List JP, Sheng DL, Kleeblad LJ, Chawla H, Pearle AD. Outcomes of cementless unicompartmental and total knee arthroplasty: a systematic review. Knee 2017;24:497–507. [DOI] [PubMed] [Google Scholar]
- 61. Kim YJ, Kim BH, Yoo SH, Kang SW, Kwack CH, Song MH. Mid-term results of Oxford medial unicompartmental knee arthroplasty in young Asian patients less than 60 years of age: a minimum 5-year follow-up. Knee Surg Relat Res 2017;29:122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Panzram B, Bertlich I, Reiner T, Walker T, Hagmann S, Gotterbarm T. Cementless Oxford medial unicompartimental knee replacement: an independent series with a 5-year-follow-up. Arch Orthop Trauma Surg 2017;137:1011–1017. [DOI] [PubMed] [Google Scholar]
- 63. Xue H, Tu Y, Ma T, Wen T, Yang T, Cai M. Up to twelve year follow-up of the Oxford phase three unicompartmental knee replacement in China: seven hundred and eight knees from an independent centre. Int Orthop 2017;41:1571–1577. [DOI] [PubMed] [Google Scholar]
- 64. Mohammad HR, Strickland L, Hamilton TW, Murray DW. Long-term outcomes of over 8,000 medial Oxford Phase 3 unicompartmental knees: a systematic review. Acta Orthop 2018;89:101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tadros BJ, Dabis J, Twyman R. Short-term outcome of unicompartmental knee arthroplasty in the octogenarian population. Knee Surg Sports Traumatol Arthrosc 2018;26:1571–1576. [DOI] [PubMed] [Google Scholar]
- 66. Kim KT, Lee S, Lee JI, Kim JW. Analysis and treatment of complications after unicompartmental knee arthroplasty. Knee Surg Relat Res 2016;28:46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. van der List JP, Zuiderbaan HA, Pearle AD. Why do medial unicompartmental knee arthroplasties fail today? J Arthroplasty 2016;31:1016–1021. [DOI] [PubMed] [Google Scholar]
- 68. Inui H, Taketomi S, Yamagami R, Tahara K, Tanaka S. Snapping pes syndrome after unicompartmental knee arthroplasty. Knee Surg Relat Res 2016;28:172–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Chen JY, Loh B, Woo YL, Chia SL, Lo NN, Yeo SJ. Fixed flexion deformity after unicompartmental knee arthroplasty: how much is too much. J Arthroplasty 2016;31:1313–1316. [DOI] [PubMed] [Google Scholar]
- 70. Ahn JH, Kang HW, Yang TY, Lee JY. Risk factors of post-operative malalignment in fixed-bearing medial unicompartmental knee arthroplasty. Int Orthop 2016;40:1455–1463. [DOI] [PubMed] [Google Scholar]
- 71. Inui H, Taketomi S, Yamagami R, Sanada T, Shirakawa N, Tanaka S. Impingement of the mobile bearing on the lateral wall of the tibial tray in unicompartmental knee arthroplasty. J Arthroplasty 2016;31:1459–1464. [DOI] [PubMed] [Google Scholar]
- 72. van der List JP, Zuiderbaan HA, Pearle AD. Why do lateral unicompartmental knee arthroplasties fail today? Am J Orthop (Belle Mead NJ) 2016;45:432–462. [PubMed] [Google Scholar]
- 73. Liddle AD, Pandit H, Judge A, Murray DW. Optimal usage of unicompartmental knee arthroplasty: a study of 41,986 cases from the National Joint Registry for England and Wales. Bone Joint J 2015;97-B:1506–1511. [DOI] [PubMed] [Google Scholar]