Abstract
In several cell types, specific membrane proteins are retained intracellularly and rapidly redistributed to the surface in response to stimulation. In fat and muscle, the GLUT4 glucose transporter is dynamically retained because it is rapidly internalized and slowly recycled to the plasma membrane. Insulin increases the recycling of GLUT4, resulting in a net translocation to the surface. We have shown that fibroblasts also have an insulin-regulated recycling mechanism. Here we show that GLUT4 is retained within the transferrin receptor-containing general endosomal recycling compartment in Chinese hamster ovary (CHO) cells rather than being segregated to a specialized, GLUT4-recycling compartment. With the use of total internal reflection microscopy, we demonstrate that the TR and GLUT4 are transported from the pericentriolar recycling compartment in separate vesicles. These data provide the first functional evidence for the formation of distinct classes of vesicles from the recycling compartment. We propose that GLUT4 is dynamically retained within the endosomal recycling compartment in CHO cells because it is concentrated in vesicles that form more slowly than those that transport TR. In 3T3-L1 adipocytes, cells that naturally express GLUT4, we find that GLUT4 is partially segregated to a separate compartment that is inaccessible to the TR. We present a model for the formation of this specialized compartment in fat cells, based on the general mechanism described in CHO cells, which may explain the increased retention of GLUT4 and its insulin-induced translocation in fat cells.
INTRODUCTION
Reversible retention in the endosomal system is a mechanism for cells to rapidly modulate levels of proteins on the cell surface. For any recycling protein in the endocytic pathway, the kinetics of internalization and recycling determine the distribution between the plasma membrane and intracellular compartments. A change in either of these rates causes a rapid redistribution. Several specialized cell types exploit this mechanism to move enzymes and transporters to the cell surface (Cannon et al., 1985; Bradbury and Bridges, 1994). Examples include translocation of aquaporin-2 in collecting duct cells of the kidney (Knepper and Inoue, 1997; Ward et al., 1999) and of an H+K+-ATPase in gastric parietal cells (Forte and Yao, 1996), which are important for water and acid secretion, respectively.
One of the most intensively studied examples of hormone-induced translocation is the effect of insulin on surface levels of the GLUT4 glucose transporter in muscle and fat cells (Czech, 1995; Rea and James, 1997). GLUT4 is dynamically retained inside cells because it is rapidly internalized and slowly recycled. Insulin stimulation reverses the retention by increasing the recycling of GLUT4 to the plasma membrane, thereby effecting an increase of GLUT4 on the surface and a concomitant increase of glucose transport (Jhun et al., 1992; Satoh et al., 1993; Yang and Holman, 1993). In fat and muscle, GLUT4 is believed to recycle back to the cell surface through a specialized, insulin-responsive compartment that is distinct from the general endosomal recycling system (reviewed by Rea and James, 1997; and Pessin et al., 1999). When GLUT4 is expressed in fibroblasts, it is also retained intracellularly and redistributes to the surface in response to insulin, which demonstrates that nonspecialized cells have the capacity for insulin-regulated recycling (Kanai et al., 1993; Lampson et al., 2000). Prominent differences between the behavior of GLUT4 expressed in fat and fibroblast-like cells are that a greater percentage of GLUT4 is sequestered intracellularly in fat cells in the basal state, and insulin induces a larger translocation of GLUT4 to the cell surface. Although the physiological significance of regulated endocytic traffic in fibroblasts is not clear, the insulin-regulated pathway may be functionally important in many cell types for translocation of the insulin-responsive aminopeptidase (IRAP), which is the only other protein known to traffic like GLUT4 in fat and muscle cells (Kandror and Pilch, 1994; Keller et al., 1995; Ross et al., 1996; Malide et al., 1997; Martin et al., 1997; Sumitani et al., 1997; Garza and Birnbaum, 2000). Unlike GLUT4, IRAP is expressed in many cell types. IRAP's physiological function is unknown, but one possibility is that insulin stimulation exposes IRAP to extracellular substrates.
To study the retention mechanism we have used a chimeric reporter, vpTR, which has the transmembrane and extracellular domains of the transferrin (Tf) receptor (TR) and the cytoplasmic domain of IRAP (Johnson et al., 1998; Subtil et al., 2000). Like GLUT4 and IRAP, vpTR is retained intracellularly and translocates to the cell surface in response to insulin. In this study we use the TR and vpTR as reporters for trafficking through the general endocytic pathway and the insulin-regulated retention pathway, respectively. The advantage of these reporters is that both bind Tf; therefore, they can be directly compared experimentally with the use of Tf conjugates as probes.
With this system we address two fundamental questions about the insulin-regulated retention pathway in Chinese hamster ovary (CHO) cells. The first is whether retention occurs within the general endosomal system or in a specialized compartment. With the use of a fluorescence-quenching assay, we show that GLUT4, vpTR, and the TR are all contained in the endosomal recycling compartment (ERC). The second question is how these proteins are delivered to the plasma membrane with different kinetics from a single compartment. Analysis of individual transport vesicles by total internal reflection fluorescence microscopy (TIR-FM) demonstrates that GLUT4 and vpTR traffic in the same vesicles, whereas the TR is in separate vesicles. This observation provides the first evidence of separate budding events from the ERC that generate two classes of vesicles directed to the plasma membrane. Together, these data support a single model for the reversible retention mechanism in undifferentiated cells.
In 3T3-L1 adipocytes we use the same quenching assay to demonstrate that GLUT4 is partially retained within a compartment that is inaccessible to the TR. These data are consistent with the results of previous biochemical studies that showed a fraction of GLUT4 is sequestered in a compartment inaccessible to TR (Livingstone et al., 1996; Martin et al., 1996; Millar et al., 1999). We propose that the specialized compartment in adipocytes develops, during differentiation, from the retention mechanism we describe in undifferentiated cells. The development of this specialized compartment may explain the greater sequestration and insulin-induced translocation of GLUT4 in fat cells.
MATERIALS AND METHODS
Ligands and Chemicals
Human Tf was purchased from Sigma (St. Louis, MO) and further purified by Sephacryl S-300 gel filtration. Iron-loaded diferric Tf and 125I-Tf were prepared as described previously (Yamashiro et al., 1984). Horseradish peroxidase (HRP) was conjugated to iron-loaded Tf as described previously (Mayor et al., 1998). A mouse anti-HA mAb (HA.11) was purchased from Berkeley Antibody (Richmond, CA). A mouse anti-Tac mAb was purified from ascites fluid prepared from the hybridoma cell line 2A3A1H (American Type Culture Collection, Manassas, VA). Tf and the anti-HA and anti-Tac antibodies were labeled with the fluorescent dye Cy3 (Biological Detection Systems, Pittsburgh, PA) according to the manufacturer's instructions. All chemicals were from Sigma unless otherwise specified.
Cell Lines and Cell Culture
The CHO cells expressing HA-GLUT4-green fluorescent protein (GFP) and either the human TR or the vpTR chimera have been described previously (Lampson et al., 2000). These cells are derived from TRVb CHO cells, which do not express any endogenous TR (McGraw et al., 1987). Cells were cultured in McCoy's 5A medium containing 5% fetal bovine serum, penicillin-streptomycin (Life Technologies, Gaithersburg, MD), 220 mM sodium bicarbonate, and 0.2 mg/ml G418 (Mediatech, Herndon, VA). Translocation of HA-GLUT4-GFP was demonstrated in the clonal lines, with the use of the assay described previously (Lampson et al., 2000). Translocation was shown to be the same after uptake of the Cy3-labeled anti-HA antibody, with the use of a similar assay with a Cy5-labeled anti-mouse antibody to measure surface levels of the anti-HA antibody. This result demonstrates that binding of the antibody does not alter the trafficking of HA-GLUT4-GFP. Expression of the TR and vpTR, measured by uptake of 125I-Tf, was similar in the clonal lines used here.
CHO cells expressing the TR and a chimeric Tac-furin construct were cultured in the same McCoy's medium described above with 200 U/ml hygromycin. These cells have been previously characterized (Mallet and Maxfield, 1999).
3T3-L1 fibroblasts were cultured in DMEM supplemented with 10% calf serum and penicillin-streptomycin. Cells were differentiated as described previously (Frost and Lane, 1985). Four days after beginning differentiation, cells were electroporated with 45 μg of each plasmid DNA (HA-GLUT4-GFP and either vpTR or the TR) at 180 V and 950 μF and plated onto coverslip bottom dishes. Cells were used for experiments 24 h after electroporation. Insulin-responsive translocation of vpTR and HA-GLUT4-GFP was tested and found to be normal following this procedure.
Recycling Kinetics
Recycling of the TR and vpTR was measured as described previously (Johnson et al., 2001). Cells were incubated with 3 μg/ml 125I-Tf in McCoy's 5A medium with 220 mM sodium bicarbonate, 20 mM HEPES, pH 7.4 (medium 1), for 3 h at 37°C to achieve steady-state occupancy of the receptors. Cells were washed with 150 mM NaCl, 20 mM HEPES, 1 mM CaCl2, 5 mM KCl, 1 mM MgCl2, pH 7.4 (medium 2), followed by a 2-min wash at 37°C in 200 mM NaCl, 50 mM 2-(N-morpholino)ethanesulfonic acid, pH 5.0, and two washes with medium 2 to remove surface-bound Tf. Cells were incubated with 3 μg/ml unlabeled Tf and 100 μM iron chelator desferroxamine in medium 1 (efflux medium) at 37°C. In some experiments, 170 nM insulin were added to the efflux medium. At each time point the efflux medium was collected, and the cells were solubilized. The radioactivity in the efflux medium is the 125I-Tf released from the cells, and the solubilized radioactivity is the 125I-Tf remaining inside the cell. Cells incubated with 200-fold excess unlabeled Tf were used to determine nonspecific binding of 125I-Tf.
To calculate the recycling rate constants, time points up to 10 min were used. The natural log of the percentage of radioactivity cell associated was plotted against time; the slope of this line is the rate constant for recycling. For the 50-min efflux experiment, each time point was corrected for the radioactivity that remained cell associated after 60 min (TR) or 120 min (vpTR), ∼10% of the total. After these times the cell-associated radioactivity no longer decreased.
To measure efflux of Cy3-Tf, cells expressing HA-GLUT4-GFP and either vpTR or the TR were grown on coverslip bottom dishes and treated as above, except that Cy3-Tf was used in place of 125I-Tf. At each time point the cells were washed with medium 2 and fixed with 3.7% formaldehyde in medium 2. Time points of 0, 5, 10, and 15 min were used, with duplicate dishes for each time point. Cells incubated with 200-fold excess unlabeled Tf were used to calculate nonspecific uptake of Cy3-Tf.
Fluorescence microscopy was performed with a DMIRB inverted microscope (Leica, Deerfield, IL), with a cooled CCD camera (Princeton Instruments, Trenton, NJ). Images were collected with a 40×1.25 NA oil immersion objective. Metamorph software (Universal Imaging, West Chester, PA) was used for image processing and quantification. The Cy3 and GFP fluorescence intensities were summed over all cells in a field, and nonspecific Cy3 was subtracted. The Cy3/GFP ratio was used as a measure of total Cy3-Tf inside the cells, normalized for the number of cells in the field. The perinuclear compartment was identified with the use of a threshold GFP intensity, chosen to include the concentrated GFP in this compartment while excluding peripheral compartments. The Cy3 fluorescence in this compartment was summed for all cells in a field and divided by the total GFP in the field to normalize for number of cells. The Cy3/GFP ratio (either total Cy3 or perinuclear Cy3) at each time point was divided by the Cy3/GFP at t = 0 to give the fraction of Cy3-Tf remaining inside the cell or inside the perinuclear compartment.
Fluorescence-quenching Assay
CHO cells grown on coverslip bottom dishes were incubated 40 min at 37°C with the Cy3-labeled anti-HA antibody in medium 1 with 1 mg/ml ovalbumin. Cells were washed with medium 1 and chased with 20 μg/ml HRP-Tf in medium 1. For control (unquenched) cells, 1 mg/ml unconjugated Tf was included to block specific binding of HRP-Tf to the receptor. The affinity of the unconjugated Tf is higher than that of the HRP-Tf, and a 50-fold excess of Tf is sufficient to bock specific binding of the HRP-Tf conjugate.
After the chase the cells were placed on ice and washed 2 times with medium 2. To strip surface-bound Tf, cells were incubated 5 min in ice-cold citrate buffer (20 mM sodium citrate, 150 mM NaCl, pH 5.0), with 1 exchange of buffer, followed by 2 5 min washes with ice-cold medium 2. For the quenching reaction, cells were incubated with 250 μg/ml diaminobenzidine (DAB) and 0.0025% H2O2 in the dark for 30 min on ice, followed by two 5-min washes with ice-cold medium 2. DAB (25 μg/ml) was used for some experiments with similar results. Finally, cells were fixed for 20 min with 3.7% formaldehyde in medium 2.
A similar experiment was performed with cells expressing the TR and a Tac-furin construct. Cells were incubated for 10 min at 37°C with the Cy3-labeled anti-Tac antibody in medium 1 with 1 mg/ml ovalbumin. Cells were washed and chased for 30 min with medium 1 and then for 20 min with 20 μg/ml HRP-Tf in medium 1. Unconjugated Tf (1 mg/ml) was included for control cells. Cells were processed for the DAB reaction as described above.
To test quenching of GFP in the lumen of the compartment, cells expressing the TR were transiently transfected with a vesicle-associated membrane protein-2 construct with a luminal GFP (a gift from Tim Ryan, Cornell University). Two days after transfection, cells were incubated with 20 μg/ml HRP-Tf in medium 1, with 1 mg/ml unconjugated Tf for control cells, and processed for the DAB reaction as described above.
Fluorescence microscopy and image processing were performed as described above. The GFP and Cy3 fluorescence intensities were summed over all cells in a field. Background fluorescence was subtracted and estimated from cells expressing the TR or vpTR, but not HA-GLUT4-GFP, treated identically in the experiment. The Cy3/GFP ratio was calculated for each field, which represents the unquenched fluorescence signal, normalized for total HA-GLUT4-GFP expressed.
For the quenching in fat cells, 3T3-L1 adipocytes electroporated with HA-GLUT4-GFP and either vpTR or the TR were used. Cells were incubated with 170 nM insulin for 20 min to increase recycling of HA-GLUT4-GFP, then washed with medium 2, incubated for 3 min with 200 mM NaCl, 50 mM 2-(N-morpholino)ethanesulfonic acid, pH 5.0, and washed three times with medium 2 to remove all insulin. After insulin stimulation, cells were incubated for 5 h with the Cy3-labeled anti-HA antibody, together with 20 μg/ml HRP-Tf for 5 h (cells expressing vpTR) or for the last 3 h (cells expressing the TR). The quenching reaction and fluorescence microscopy were performed as described above. For image processing, cells expressing HA-GLUT4-GFP were selected manually, and the GFP and Cy3 fluorescence intensities were summed over each cell. Background fluorescence was estimated from nonexpressing cells. The Cy3/GFP ratio was calculated for each cell and averaged over multiple cells.
TIR-FM
The TIR-FM apparatus has been described previously (Schmoranzer et al., 2000). For these experiments a 60×1.45 NA objective (Olympus America, Melville, NY) was used to perform “prism-less” TIR-FM. The evanescent field decay length was 100–250 nm with this objective, with a pixel size of 112 × 112 nm2 in the acquired images. For single-color TIR-FM the 488-nm line from an argon laser (Omnichrome, model 543-AP A01, Melles Griot, Carlsbad, CA) was reflected from a dichroic mirror (Q498LP) for excitation of GFP and Alexa488, and the emission was collected through a bandpass filter (HQ525/50 M). For dual-color TIR-FM, the 488-nm line was used for excitation of both Cy3 and GFP. In some experiments the 543-nm line from an HeNe laser (model 05-LGR-193, Melles Griot) was also used to excite Cy3 simultaneously, and both lines were reflected from a polychroic mirror (PC488/543). An emission splitter was used to simultaneously collect dual-color emissions (W-view, Hamamatsu, Bridgewater, NJ). Emissions were split with a dichroic mirror (550DCLP) and selected with a bandpass filter for GFP (HQ525/50 M) and a longpass filter for Cy3 (HQ570LP, all filters from Chroma Technologies, Brattleboro, VT).
Cells were incubated for at least 1 h at 37°C with Cy3-Tf to label the TR or vpTR. To strip Tf from the surface, cells were incubated for 2 min with 0.2 M NaCl, 50 mM 2-(N-morpholino)ethanesulfonic acid, pH 5.0, and washed with Hank's balanced salt solution with 10 mM HEPES, pH 7.4. Removal of surface-bound Tf ensured that we did not image Tf-containing vesicles internalized from the plasma membrane. The cells were incubated in the same buffered Hank's balanced salt solution at 32°C for imaging. Some experiments were done at 37°C with similar results. For experiments with insulin, cells were incubated with 170 nM insulin.
For two-color TIR-FM, videos were acquired at a rate of three to four frames per second with a cooled CCD camera (ORCA-1 or ORCA-ER, Hamamatsu, Bridgewater, NJ) at full resolution. The fluorescent signals from our probes were not bright enough for a higher rate. Because of this limited time resolution, we were unable to characterize fusion events as has been done previously (Schmoranzer et al., 2000; Toomre et al., 2000). In many cases vesicles appeared in the evanescent field and quickly disappeared. As endocytic vesicles may be smaller or less densely loaded with probes than post-Golgi carriers, we speculate that the diffusion of probes in the plasma membrane may be too fast for our time resolution. Consistent with this possibility, we found that resolution of many of the endocytic vesicles, but not of the post-Golgi carriers, was lost by 2 × 2 binning of the images, which changes the pixel size from 112 × 112 to 224 × 224 nm2. We selected only moving vesicles, assuming that these are transport intermediates, to analyze colocalization of the fluorescent probes. With single-color TIR-FM we were able to observe fusion of vesicles with the plasma membrane. Because of the increased signal intensity, videos were acquired at a rate of 6–10 frames/s.
Metamorph software was used for image analysis. First, a local background was subtracted from each pixel, with the use of the 20th percentile intensity in a 30- × 30-pixel area around that pixel. Vesicles moving either parallel or perpendicular to the membrane were selected manually, and the average pixel intensity of each probe was calculated over the vesicle area in a single image. To estimate the background intensity, spots of the same average size as the vesicles (5–10 pixels) were randomly placed throughout the image. Average red and green fluorescence was calculated for these spots. After removing obvious outliers (from cases in which the random spots overlapped with labeled structures), the 90th percentile from the population of random spots was used as a threshold for each color. Vesicles were classified as positive for red or green if the average fluorescence was above the threshold for that color. The same procedure was followed with the use of the 85th or 95th percentile from the random spots for the threshold, with little change in the results; therefore, the conclusions are not sensitive to this parameter.
RESULTS
We used three reporter molecules to investigate the retention mechanism in CHO cells. The first is the TR, a widely used marker for general endocytic trafficking, which recycles rapidly from the ERC. The second, vpTR, also binds Tf because it has the extracellular domain of the TR, but it recycles slowly because of the trafficking information encoded in the cytoplasmic domain of IRAP (Johnson et al., 1998). The third is a GLUT4 construct with an exofacial HA epitope and a C-terminal GFP (HA-GLUT4-GFP) (Lampson et al., 2000). We have previously shown that both vpTR and HA-GLUT4-GFP follow the reversible, insulin-regulated dynamic retention pathway in fibroblast-like cells, including CHO cells (Johnson et al., 1998, 2001; Lampson et al., 2000). In this study we used HA-GLUT4-GFP as a marker for the retention pathway and either the TR or vpTR to deliver Tf-conjugated probes.
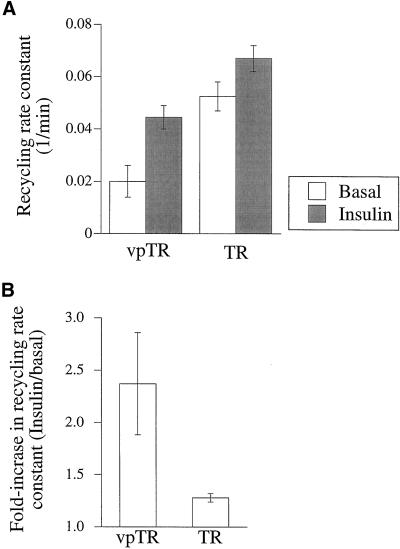
To ensure that the trafficking of TR and vpTR is not affected by expression of GLUT4, as might be expected if GLUT4 played a functional role in the retention mechanism, we measured the recycling kinetics in cells coexpressing HA-GLUT4-GFP. As shown in Figure 1, the recycling of vpTR was slow in the basal state and increased two- to threefold in response to insulin. In contrast, the TR was rapidly recycled and relatively unresponsive to insulin. These data are consistent with the rates measured previously in cells that do not express GLUT4 (Johnson et al., 1998). We conclude that although GLUT4 follows the retention pathway as “cargo,” its expression has no effect on the function of the pathway.
Figure 1.
Recycling of vpTR and the TR is not affected by GLUT4 expression. (A) Cells expressing HA-GLUT4-GFP and either vpTR or the TR were loaded to steady state with 125I-Tf and allowed to efflux for times up to 10 min, with or without insulin. The recycling rate constant of the TR or vpTR was calculated as the slope of the natural log of the percentage of radioactivity cell associated versus time. Each bar represents the average of two experiments. Error bars show the difference between the two. (B) The ratio of the recycling rates (insulin/basal) is plotted for the TR and vpTR, for the experiments shown in A.
TR and vpTR Recycle as Single Kinetic Pools from a Perinuclear Compartment
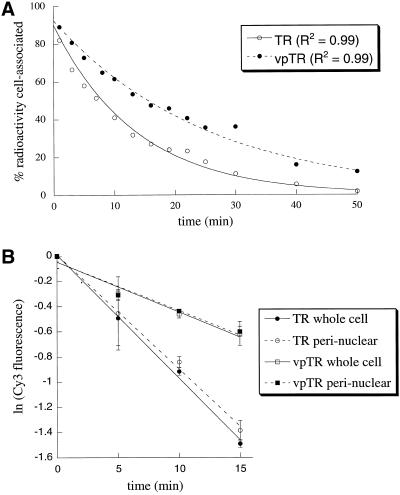
We have previously shown that vpTR and GLUT4 localize to a perinuclear retention compartment at steady state, which suggests that the rate-limiting step for recycling to the cell surface is exit from this compartment (Lampson et al., 2000). It is also possible, however, that some fraction of the proteins recycles rapidly from peripheral early endosomes, as has been shown for internalized lipids (Hao and Maxfield, 2000), whereas the rest recycles slowly from the perinuclear compartment. In this case, recycling would be composed of a fast and slow rate, with the overall recycling rate constant, determined by the relative amounts of probe trafficking through these two pathways. To test this possibility we measured the efflux of Tf from cells expressing either vpTR or the TR. The loss of Tf from cells followed first-order kinetics for 50 min for both vpTR and the TR, as shown by the single exponential fit, which implies a single rate-limiting step (Figure 2A). If a significant fraction recycled rapidly from early endosomes (i.e., with a t1/2 of 1–2 min; Hao and Maxfield, 2000), we would expect a slow and a fast phase, which is not consistent with the data.
Figure 2.
vpTR and the TR recycle as single kinetic pools from a perinuclear compartment. (A) Cells expressing either vpTR or the TR were loaded to steady state with 125I-Tf and allowed to efflux for times up to 50 min. The percentage of radioactivity remaining cell associated is plotted as a function of efflux time. Both curves are fit with single exponential decays. (B) Cells expressing HA-GLUT4-GFP and either vpTR or the TR were loaded to steady state with Cy3-Tf and allowed to efflux for times up to 15 min. The Cy3 fluorescence signal was summed either over the whole cell or in the perinuclear compartment only. At each time point, the Cy3 fluorescence intensity is normalized to the intensity at t = 0, and the natural log of this fraction is plotted versus time. Error bars are differences between duplicate dishes.
We also measured the efflux of fluorescently labeled Tf to confirm that the rate-limiting step is exit from the perinuclear compartment. Cells were incubated to achieve steady-state occupancy of vpTR or the TR with Cy3-Tf. The cells were washed and incubated at 37°C, and the amount of cell-associated Cy3-Tf was determined at the indicated times with the use of quantitative, digital microscopy. We measured both total cell fluorescence and the amount of Cy3-Tf fluorescence in the perinuclear compartment at each time point and found that the decrease in fluorescence over time was the same, regardless of which measurement was used (Figure 2B). These data demonstrate that exit from a perinuclear compartment is the rate-limiting step for recycling of both vpTR and the TR, as has been shown previously for the TR (Mayor et al., 1993). We conclude that recycling along the retention pathway occurs as a single kinetic pool from a perinuclear retention compartment.
GLUT4, vpTR, and the TR Are Contained in the Endocytic Recycling Compartment in CHO Cells
There are two general possibilities for the retention compartment: it could be a specialized compartment distinct from the general endosomal system, or retention could occur within the general endosomal system. By confocal microscopy, the vpTR and TR both colocalize well with GLUT4 and with fluorescently labeled sphingomyelin (C6-NBD-SM), a marker for the general endocytic recycling pathway (Lampson et al., 2000; Johnson et al., 2001). At the level of light microscopy, however, it is difficult to resolve compartments that are distinct but spatially close. In this study we used a quantitative fluorescence-quenching assay to determine the degree of colocalization more definitively.
In this assay HRP conjugated to Tf (HRP-Tf) is internalized by either the TR or vpTR, so that HRP is delivered to the intracellular compartments where these molecules are localized. On addition of DAB and H2O2, a dense reaction product is formed that fills the compartment containing HRP and quenches any fluorophores in the lumen. Because the reaction product does not extend beyond membranes, fluorophores in separate compartments are unaffected, regardless of spatial proximity. This assay has been used previously to test colocalization of fluorescently labeled proteins with the TR in the ERC (Ghosh et al., 1998; Mayor et al., 1998; Johnson et al., 2001).
Here we used HA-GLUT4-GFP as a specific marker for the retention compartment, and tested the ability of the TR or vpTR to deliver HRP-Tf to this compartment. To deliver a fluorophore to the compartment, we incubated the cells with a Cy3-labeled anti-HA antibody. This antibody binds the exofacial HA epitope on HA-GLUT4-GFP at the cell surface and is internalized with HA-GLUT4-GFP to the lumen of the retention compartment, and the GFP is on the cytoplasmic side of the membrane. Binding of the antibody did not affect the translocation of HA-GLUT4-GFP, indicating that its trafficking was not affected.
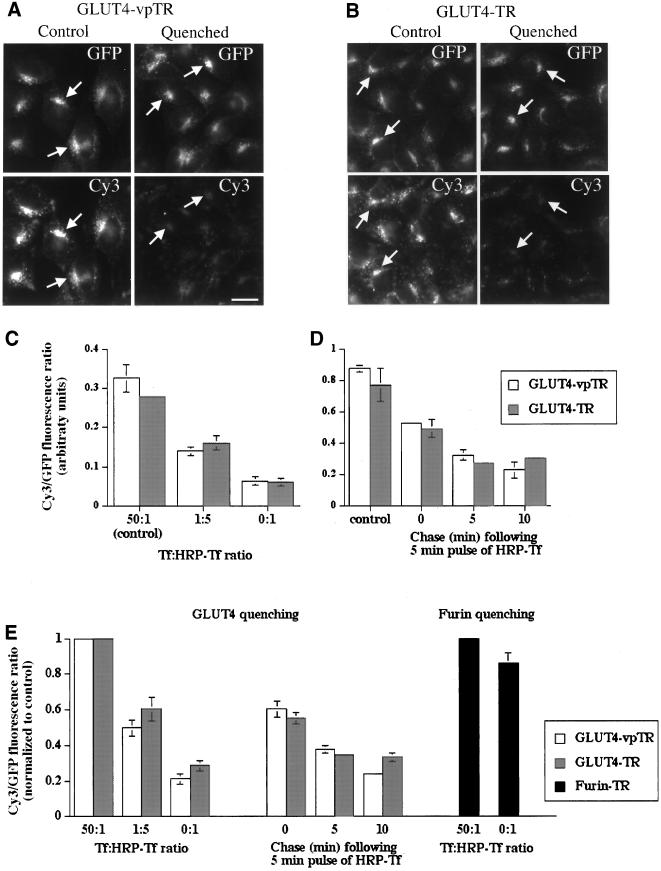
Cells expressing HA-GLUT4-GFP and either the TR or vpTR were allowed to take up HRP-Tf for 20 min and tested for quenching of the Cy3 fluorescence (Figure 3, A and B). The GFP labeled the perinuclear retention compartment (arrows), which is indistinguishable from the ERC by light microscopy. The Cy3 also labeled the retention compartment but on the luminal side. In both cell types, the Cy3 fluorescence was quenched relative to control cells, whereas the GFP fluorescence was unchanged because the GFP was on the cytoplasmic side of the membrane and inaccessible to the DAB reaction product. In control experiments, with the use of a transmembrane protein with a luminal GFP, the GFP fluorescence in endosomes was quenched by the reaction product (data not shown), which shows that GFP is sensitive to quenching in this assay. Therefore, because the GFP on the GLUT4 construct, which is on the cytoplasmic side of the membrane, was not quenched, we conclude that the reaction product does not extend beyond the lumen of the compartment. Because the GFP on HA-GLUT4-GFP was unaffected by the reaction, we used the GFP fluorescence as a normalization factor: the Cy3/GFP ratio is a measure of the Cy3 fluorescence signal normalized for total HA-GLUT4-GFP expressed.
Figure 3.
The GLUT4 retention compartment is accessible to both vpTR and the TR in CHO cells. (A and B) Cells expressing HA-GLUT4-GFP and either vpTR (A) or the TR (B) were incubated for 40 min with Cy3 anti-HA at 37°C, chased for 20 min with HRP-Tf, and processed for the DAB reaction. For control cells 1 mg/ml excess unconjugated Tf was included with the HRP-Tf to block specific binding of HRP-Tf. GFP and Cy3 fluorescence is shown. Arrows indicate the GLUT4 retention compartment in both control and quenched cells. Scale bar, 10 μm. (C) Cells were treated as in A and B and the Cy3/GFP ratio was calculated. Data are shown for a 50:1 ratio of unconjugated Tf/HRP-Tf (control), a 1:5 ratio of Tf/HRP-Tf, and for HRP-Tf only (maximal quenching). Each bar represents the mean of duplicate dishes, with eight fields averaged for each dish (>20 cells per field). Error bars represent the difference between the two dishes. (D) Cells were incubated for 40 min with Cy3 anti-HA, pulsed with HRP-Tf for 5 min, chased for the indicated times, and processed for the DAB reaction. The Cy3/GFP ratio was calculated and averaged over duplicate dishes as in C. The Cy3/GFP ratio is not comparable between the experiments in C and D. (E) Data were averaged over multiple experiments, performed as in C and D. To combine data from different experiments, the Cy3/GFP ratio was normalized to the control cells for each experiment. Each bar represents the mean (±SEM) of at least four experiments. Experiments with cells expressing the TR and furin were performed as in C.
In a quantitative comparison with the use of the Cy3/GFP ratio, 70–80% of the Cy3 fluorescence intensity was quenched, relative to control cells, in both cell types. These data demonstrate that both the TR and vpTR can deliver HRP-Tf to the retention compartment (labeled by HA-GLUT4-GFP), which suggests that this compartment is part of the general endosomal system. These data are consistent with HA-GLUT4-GFP, TR, and vpTR residing within the same ERC. However, small amounts of HRP in a compartment may be sufficient to catalyze the polymerization of DAB and thereby quench fluorescence. Thus, the possibility exists that there are two compartments, with the majority of the GLUT4 and vpTR in one compartment (i.e., the slow recycling compartment) and the majority of the TR in the other (i.e., the rapidly recycling compartment). To test this possibility we analyzed quenching of the Cy3-labeled anti-HA antibody as a function of HRP-Tf concentration. To titrate the amount of HRP-Tf taken up by cells, we incubated cells with varying ratios of HRP-Tf to unconjugated Tf. If the TR, vpTR, and GLUT4 are all in a single compartment, then changes in HRP-Tf uptake should affect quenching equally in both cell lines (i.e., TR/HA-GLUT4-GFP and vpTR/HA-GLUT4-GFP). If, on the other hand, the majority of the TR is in one compartment and vpTR and GLUT4 is in another, then the quenching as a function of HRP-Tf uptake should be different for both cell lines.
Quenching by the TR and vpTR was compared with different levels of HRP-Tf uptake (Figure 3D). In cells expressing either the TR or vpTR, the Cy3/GFP ratio was similar with no quenching (control) and the ratio decreased to similar levels with increased HRP-Tf uptake. For example, with a Tf:HRP-Tf ratio of 1:5, HRP-Tf uptake was reduced so that quenching was at an intermediate level, which demonstrates that the assay is sensitive to the amount of HRP delivered to the retention compartment. The finding that the quenching of the Cy3-labeled anti-HA antibody is the same regardless of whether Tf-HRP is taken up by the TR or vpTR provides strong evidence that the TR, vpTR, and GLUT4 are all localized to the pericentriolar ERC.
To determine the kinetics of delivery of the TR and vpTR to the ERC, we prelabeled with Cy3 anti-HA, pulsed with HRP-Tf for 5 min, and chased for varying times (Figure 3D). After a 5-min pulse with no chase, the level of quenching was intermediate, but a 5-min chase was sufficient to reach a maximal level for this protocol. As in the previous experiment, the quenching was similar in cells expressing either the TR or vpTR. The kinetics of delivery to the ERC are consistent with other measurements of TR trafficking.
These experiments were repeated multiple times either with different levels of HRP-Tf binding or with the pulse-chase protocol. In all cases, quenching was similar in cells expressing the TR or vpTR (Figure 3E). The results confirm the existence of a single compartment containing the TR, vpTR, and GLUT4 and demonstrate that the TR and vpTR traffic from the plasma membrane to the ERC with similar kinetics.
To confirm the specificity of the quenching assay, we used cells expressing the TR and a Tac-furin construct. This construct has the extracellular domain of the interleukin-2 receptor α chain (Tac), and the transmembrane and cytoplasmic domains of furin, which target it to the trans-Golgi network (TGN) (Bosshart et al., 1994; Mallet and Maxfield, 1999). Labeled anti-Tac antibodies are taken up and delivered to the TGN, a compartment that is spatially close but distinct from the ERC in these cells (Mallet and Maxfield, 1999). We measured quenching of <15% for Tac-furin (Figure 3E), which demonstrates that the quenching is specific to compartments containing the TR, as shown previously (Ghosh et al., 1998).
GLUT4 and the TR Recycle in Separate Vesicles
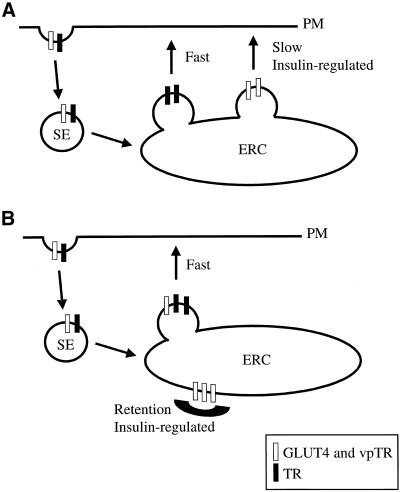
Given that the ERC is the retention compartment, there are two possible models for recycling of the TR and vpTR/GLUT4 at different rates from a single compartment. In the first model (Figure 4A), two distinct classes of transport vesicles bud from the ERC, one rapidly and one slowly. vpTR and GLUT4 are concentrated in the slowly budding vesicles, whereas the TR recycles in the rapidly budding vesicles. Insulin would increase the recycling of vpTR and GLUT4 by increasing the budding rate of the slow vesicles. This model does not require active concentration of the TR in the rapid vesicles. Because they bud more frequently, any molecule not actively excluded or concentrated elsewhere would recycle primarily in these vesicles. In the second model (Figure 4B) only one class of transport vesicles exists, and the slow recycling of vpTR and GLUT4 is achieved by excluding these molecules from the budding sites. The recycling rate of vpTR and GLUT4 would be determined by the efficiency of sequestration, and insulin would act by releasing the retention, allowing vpTR and GLUT4 to recycle with the TR.
Figure 4.
Models for retention in the ERC. (A) Two classes of vesicles bud from the ERC. The TR recycles in rapidly budding vesicles, and GLUT4 and vpTR are concentrated in separate, slowly budding vesicles. Insulin increases the budding of the slow vesicles. (B) Only one class of vesicles buds from the ERC. GLUT4 and vpTR are retained in the ERC by exclusion from these vesicles. The slow recycling of GLUT4 and vpTR is due to inefficient retention. Insulin releases the retention, allowing GLUT4 and vpTR to recycle with the TR. SE, sorting endosome; PM, plasma membrane.
The two models make different predictions about the contents of vesicles recycling from the ERC: either GLUT4 and the TR are separate (first model) or together (second model). To test these predictions, we used TIR-FM to analyze the contents of transport vesicles. With this technique only fluorophores within the evanescent field, which extends 100–200 nm from the glass surface, are excited; therefore, only compartments or transport intermediates close to the plasma membrane are observed (Axelrod et al., 1992). This method has been used previously to analyze fusion of post-Golgi transport intermediates with the plasma membrane (Schmoranzer et al., 2000; Toomre et al., 2000).
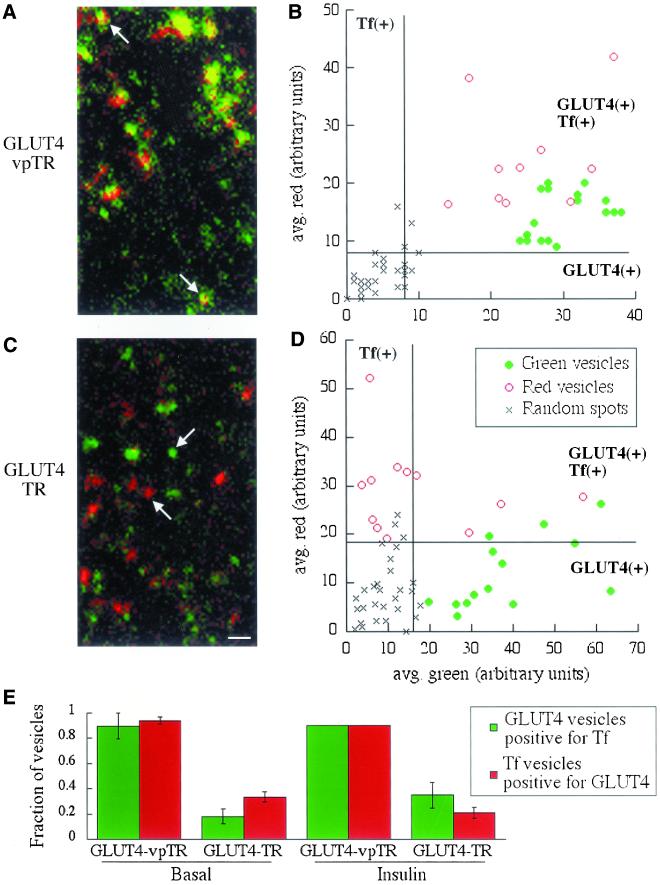
Cells expressing HA-GLUT4-GFP (green) and either the TR or vpTR were imaged by TIR-FM after uptake of Cy3-Tf (red). vpTR and GLUT4 are clearly colocalized near the plasma membrane, indicated by yellow/orange structures (Figure 5A). Because both of these molecules follow the slow recycling pathway, we expect colocalization in transport intermediates. There is no reason to expect the intensities of the two probes to be at a constant ratio; therefore, the structures do not all appear equally yellow. The TR and GLUT4, however, are in distinct red and green structures (Figure 5C). These data suggest that the TR and GLUT4 are delivered to the plasma membrane in separate transport intermediates.
Figure 5.
The TR is segregated from GLUT4 in transport vesicles imaged by TIR-FM. Cells expressing HA-GLUT4-GFP and either vpTR (A and B) or the TR (C and D) were loaded with Cy3-Tf and images were acquired by TIR-FM. (A and C) Images show structures containing GLUT4 (green), Tf (red), or both (yellow). Structures in A do not all contain the same ratio of vpTR to GLUT4; therefore, they do not all appear completely yellow. Scale bar, 1 μm. (B and D) Moving vesicles were selected for either GLUT4 content (solid green circles) or Tf content (open red circles). For each vesicle the average green intensity is plotted against the average red intensity, calculated over all the pixels in the vesicle. The intensity units are not comparable between the two plots. Spots randomly placed throughout the image represent background (×). Lines indicate threshold intensities, calculated from the random spots, to separate signal from background. Based on these thresholds, the quadrants are labeled as GLUT4(+) or Tf(+) to indicate where the intensities are higher than background. (E) The fraction of GLUT4 vesicles positive for Tf and Tf vesicles positive for GLUT4 is shown for the two cell types, both with and without insulin. The fractions are averaged over multiple videos in all cases, with at least 15 vesicles analyzed from each video. Error bars represent either the SEM of three or more determinations or the difference between two determinations.
TIR-FM videos showed static structures, vesicles and tubules, moving parallel to the plasma membrane, and vesicles appearing and disappearing. The static structures may either be docked vesicles or parts of the ERC that are within the evanescent field. To further analyze the data we quantified the codistribution of probes in transport intermediates, which we defined as moving vesicles.
Moving vesicles containing one of the fluorophores were selected by looking at a single color, so that the selection would not be biased by knowledge of the other color. Vesicles selected for GLUT4 (i.e., green) are designated here as GLUT4 vesicles, and those selected for Tf content (red) are Tf vesicles. The fluorescence intensity of each color was averaged over all the pixels in the vesicle in a single image. A population of these vesicles is shown graphically, plotted as average green versus average red fluorescence for each vesicle (Figure 5, B and D). To represent the background fluorescence, red and green intensities were also calculated for randomly placed spots of the same average size as the vesicles. In cells expressing vpTR (Figure 5B), the green and red intensities in all vesicles are higher than the background, by comparison to the random spots, which implies that the GLUT4 vesicles contain Tf and vice versa. In cells expressing the TR (Figure 5D), however, the GLUT4 vesicles generally have background levels of Tf, and the Tf vesicles have background levels of GLUT4. These data show graphically that vpTR and GLUT4 are delivered to the plasma membrane together, as expected, whereas the TR is segregated in separate vesicles. The segregation is not complete, because some vesicles contain both the TR and GLUT4.
We classified each vesicle as positive or negative for each fluorophore, based on a threshold intensity determined from the random spots (see MATERIALS AND METHODS). We calculated the percentage of GLUT4 vesicles positive for Tf and the percentage of Tf vesicles positive for GLUT4, and the percentages were averaged over multiple videos (Figure 5E). In cells expressing the TR, few of the GLUT4 vesicles contain Tf and few of the Tf vesicles contain GLUT4; but in cells expressing the vpTR, nearly all of the vesicles contain both probes. Finding a near-complete overlap of vpTR and GLUT4 vesicles is important because it demonstrates that the vast majority of GLUT4-containing vesicles we observe are derived from the endosomal system (i.e., labeled by Tf internalized from the plasma membrane) rather than from the biosynthetic pathway.
These data support the model proposed in Figure 4A, in which GLUT4 and vpTR are trafficked in vesicles distinct from those that transport the TR. These findings are inconsistent with the model in Figure 4B, which predicts that the slow recycling of GLUT4 is due to inefficient retention, so that some “leaks out” in vesicles with the TR. In this case, some of the TR vesicles could contain detectable GLUT4, as observed (Tf vesicles positive for GLUT4), but all the GLUT4 vesicles should contain TR (GLUT4 vesicles positive for Tf). Because only a small fraction of the GLUT4 vesicles contain TR, this model can be eliminated.
Insulin does not alter the distribution of GLUT4 and the TR (Figure 5E), which suggests that, although insulin increases the recycling of GLUT4 and vpTR, it does not do so by redistributing these proteins to the rapidly recycling TR-containing vesicles. These data also argue against the model in Figure 4B, which predicts that GLUT4 and the TR should recycle in the same vesicles after insulin stimulation.
In the two-color TIR-FM, we frequently observed vesicles appearing and disappearing in the evanescent field. However, we were unable to score these as fusion events if we applied the criteria for vesicle fusion previously developed (Schmoranzer et al., 2000). In the previous studies the transport vesicles were intensely labeled, allowing for analysis of the diffusion of fluorescence in the plasma membrane after fusion. In the current study, to avoid possibly saturating the retention mechanism for GLUT4 and vpTR, we have not expressed the proteins at very high levels. At the expression levels of the constructs and the current technical limitations of two-color TIR-FM imaging, we cannot distinguish fusion events from vesicles rapidly disappearing from the evanescent field.
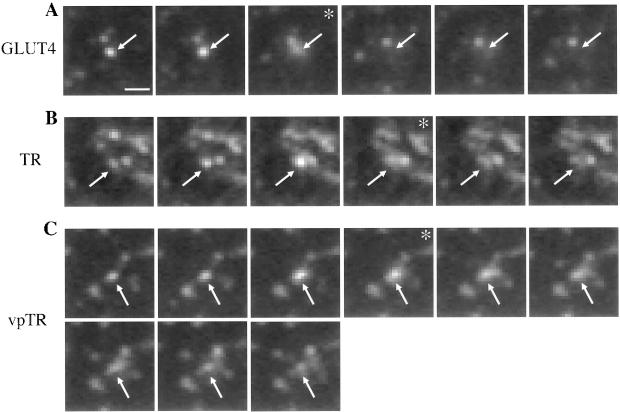
To establish that the TR, vpTR, and GLUT4 vesicles fuse with the plasma membrane, we used single-color TIR-FM. With single-color imaging, the fluorescence signal is brighter; therefore, we were able to acquire images at a higher rate and with a higher signal-to-noise ratio. We used GFP to examine the HA-GLUT4-GFP-containing vesicles, and Alexa488-Tf to label the TR or vpTR in CHO cells expressing only the TR or vpTR. In all three cases the intensity of the signal in single-color TIR-FM was sufficient to observe fusion. Fusing vesicles were identified by the increase in width and simultaneous decrease in peak intensity of the fluorescence as the probes diffused in the plasma membrane. This characteristic pattern was observed for each of the probes used in this study (Figure 6), and quantitative analysis showed that these fusion events were similar to those previously reported (Schmoranzer et al., 2000). These data indicate that at least a portion of the moving vesicles observed in the dual-color TIR-FM are transport intermediates between the ERC and the plasma membrane.
Figure 6.
Transport vesicles imaged by TIR-FM fuse with the plasma membrane. Cells expressing HA-GLUT4-GFP (A), the TR (B), or vpTR (C) were imaged by TIR-FM. Cells expressing the TR or vpTR were first loaded with Alexa488-Tf. Sequential frames, acquired at a rate of 6–10 frames/s, are shown for each probe. Arrows indicate vesicles that fuse in the sequence of frames shown. Asterisks mark frames showing the increase in area and decrease in peak intensity characteristic of fusion. Scale bar, 1 μm.
GLUT4 and vpTR Are Retained in a Specialized Compartment in 3T3-L1 Adipocytes
Our model for the insulin-regulated retention pathway in CHO cells raises the question of whether this pathway differs in fat cells, which express endogenous GLUT4 and are highly insulin responsive. Previous data suggest that GLUT4 is segregated from the TR in a specialized compartment in 3T3-L1 adipocytes, a widely used fat cell model (Livingstone et al., 1996; Martin et al., 1996; Millar et al., 1999). We used the fluorescence-quenching assay to test this model in 3T3-L1 adipocytes transfected with HA-GLUT4-GFP together with either vpTR or the TR.
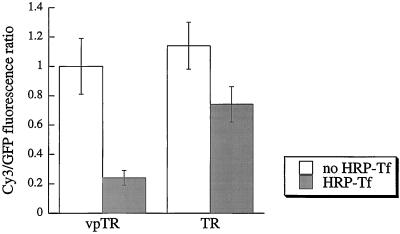
HRP-Tf taken up by vpTR quenched ∼80% of the Cy3 fluorescence associated with HA-GLUT4-GFP (Figure 7), as in CHO cells (Figure 3). The same extent of quenching was observed in cells incubated with HRP-Tf and Cy3-Tf simultaneously (data not shown), which indicates that this level of quenching is maximal. In cells expressing the TR, however, only 35% of the Cy3 fluorescence associated with HA-GLUT4-GFP was quenched (Figure 7). Because the cells were incubated for 3 hours with HRP-Tf, which is sufficient time for multiple cycles of endocytosis and recycling of the TR, all compartments along the TR pathway should contain HRP-Tf (Subtil et al., 2000). Our interpretation of the partial quenching is that 35% of the total pool of GLUT4 is in endosomes and therefore accessible to quenching, whereas the remainder is in a separate compartment containing very little or no TR. We conclude that 3T3-L1 adipocytes have a specialized retention compartment that is accessible to vpTR but not to the TR and, therefore, that the retention mechanism in differentiated and undifferentiated cell types is different.
Figure 7.
The GLUT4 retention compartment is only partially accessible to the TR in 3T3-L1 adipocytes. 3T3-L1 adipocytes expressing HA-GLUT4-GFP and either vpTR or the TR were incubated with Cy3 anti-HA, with or without HRP-Tf, and processed for the DAB reaction. The Cy3/GFP ratio (mean±SEM) was calculated and averaged over multiple cells. For both vpTR and the TR, the Cy3/GFP ratio with HRP-Tf is significantly different from the ratio with no HRP-Tf with p < 0.01 (heteroscedastic, two-tailed Student's t test).
DISCUSSION
We have tested several models that could, in principle, explain the kinetic differences observed between the general endocytic recycling pathway and the insulin-regulated recycling pathway in CHO cells. The first possibility is that molecules following the insulin-regulated pathway are targeted to a specialized retention compartment, separate from the general endosomal system. The results of the fluorescence-quenching experiments are not consistent with this model, because both the TR and vpTR deliver HRP-Tf to the compartment containing GLUT4. This finding establishes that molecules following both pathways recycle from the ERC, which suggests two possible models for the different recycling kinetics. In one case there are two classes of vesicles, one carrying the TR and one carrying vpTR and GLUT4, that transport cargo from the ERC to the plasma membrane at different rates. Alternatively, there is a single class of vesicles, but vpTR and GLUT4 are slowly recycled because they are sequestered in the ERC and excluded from these vesicles. Based on the TIR-FM data, we can eliminate the second possibility because we find the TR and GLUT4 in separate vesicles.
Taken together, the data are consistent with a single model in which the budding rates of distinct transport intermediates from the ERC determine the kinetic differences observed between the recycling of the TR and vpTR. In this model, the TR recycles in rapidly budding vesicles, whereas GLUT4 and vpTR are concentrated in distinct, slowly budding vesicles. Insulin would reverse the retention of GLUT4 and vpTR by increasing the budding rate of the slow vesicles.
Our data provide the first direct evidence for sorting of proteins into distinct transport intermediates in the ERC of nonpolarized cells. This process may be similar to the sorting that occurs in endosomes of polarized cells, in which proteins internalized from apical or basolateral membranes are delivered to a common endosomal compartment (Futter et al., 1998; Gibson et al., 1998; Orzech et al., 2000; Wang et al., 2000). In these cells, sorting into separate vesicles is required for delivery of recycling proteins to distinct membrane domains rather than for the kinetic purpose described here. Clathrin-coated pits and γ-adaptin have been implicated in this process (Futter et al., 1998; Gibson et al., 1998), but the mechanism has not been well characterized, and the required trafficking motifs are unknown.
We have recently identified the motifs required for dynamic retention of vpTR as a di-leucine sequence and a cluster of acidic residues (Johnson et al., 2001). These motifs serve as a specific signal for vpTR to escape the bulk-flow–recycling pathway followed by the TR (Mayor et al., 1993). We proposed that an adaptor protein recognizes the motifs and links the cargo, vpTR, to the appropriate coat proteins. The results reported here support a model in which this recognition occurs in the ERC and serves to concentrate vpTR in distinct vesicles. Several observations are consistent with the idea of specialized vesicles budding from a common endosomal compartment in nonpolarized cells. Both clathrin and COP-1 coat proteins, as well as the AP-1 complex, have been localized to endosomes (Whitney et al., 1995; Aniento et al., 1996; Le Borgne et al., 1996; Stoorvogel et al., 1996; Dell'Angelica et al., 1998). Different rab proteins, which are known to regulate trafficking, have also been localized to distinct endosome domains (Sonnichsen et al., 2000). The roles of these proteins are not well understood, but one possibility is that they regulate the formation and release of distinct classes of vesicles.
Sorting of proteins into separate transport vesicles has been extensively studied along the biosynthetic pathway. Polarized cells sort proteins for delivery from the TGN to the apical or basolateral plasma membrane (reviewed by Keller and Simons, 1997; and Ikonen and Simons, 1998), and a similar sorting process has been described in nonpolarized cells (Musch et al., 1996; Yoshimori et al., 1996; Keller et al., 2001). Segregation of proteins into distinct domains in the Golgi or TGN has recently been observed in live cells for proteins delivered to the plasma membrane in different vesicles (Keller et al., 2001). Our data suggest that the ERC may play an analogous role as a sorting station in the endocytic pathway by retaining vpTR and GLUT4 while allowing other proteins to recycle rapidly. Supporting this role of the ERC, there is evidence that proteins can be sorted to specific intracellular compartments, because TGN38 traffics from the ERC to the TGN (Ghosh et al., 1998). Further characterization of the adaptor and coat proteins involved in trafficking from the ERC and from the TGN will allow a more detailed comparison of the sorting events at these two compartments.
A recent study provides additional evidence for sorting of proteins along the endocytic pathway (Lim et al., 2001). In homogenized CHO cells, vesicles containing GLUT4 could be separated from vesicles containing the TR. The interpretation in this study was that the vesicles originate from early endosomes, but no direct evidence was presented to support this point. We propose that the vesicles originate instead from the ERC and are the same vesicles observed here by TIR-FM, because we also observe segregation of GLUT4 and the TR in these vesicles.
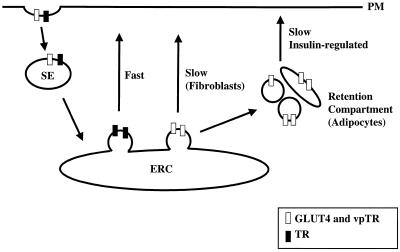
The presence of an insulin-regulated recycling pathway in CHO cells demonstrates that this specialized trafficking pathway is found in cell types other than fat and muscle, the classic insulin-target cells. As we have previously shown, the pathway in CHO cells is similar to that in fat cells in several respects: retention is achieved by slow recycling and rapid internalization, and insulin increases the recycling rate by a mechanism dependent on phosphatidylinositol 3′-kinase activity, which can be inhibited by wortmannin (Johnson et al., 1998). Fat cells are more highly responsive to insulin, however, as indicated by the observed 5- to 10-fold translocation versus 2- to 3-fold for CHO cells. Our data suggest a simple model by which the retention mechanism in fat cells might develop, during differentiation, from the more general mechanism present in undifferentiated cells. In fat cells we propose that GLUT4 vesicles, after budding from the ERC, do not directly fuse with the plasma membrane (as is the case in fibroblasts) but are retained in the cytosol to form a specialized retention compartment (Figure 8). GLUT4 in this compartment is separate from the TR and therefore inaccessible to quenching (Figure 7). Transport of GLUT4 from this compartment to the plasma membrane is stimulated by insulin. The specialized GLUT4 compartment could be a collection of tethered vesicles or the vesicles could fuse to form a new compartment.
Figure 8.
Model for the retention pathway in adipocytes. VpTR, the TR, and GLUT4 are internalized from the plasma membrane and delivered to the ERC. Two classes of vesicles bud from the ERC. The TR recycles rapidly in one class of vesicle. In fibroblasts, vesicles containing vpTR and GLUT4 recycle slowly from the ERC, whereas in adipocytes, these vesicles are tethered inside the cell, forming a separate retention compartment. SE, sorting endosome; PM, plasma membrane.
This model is consistent with a previous report that the GLUT4-containing membrane fraction from adipocytes can be separated into two peaks, the first depleted of TR and the second enriched in TR, whereas in CHO cells GLUT4 is primarily in the second peak (Hashiramoto and James, 2000). In agreement with this finding, our data demonstrate that adipocytes have a distinct GLUT4 compartment, which does not exist in CHO cells, that is accessible to IRAP (i.e., vpTR as a surrogate) but not to the TR. At steady-state GLUT4 is distributed between the ERC and the specialized retention compartment, so that only the fraction of GLUT4 that is in the ERC is accessible to the TR. In this model insulin would regulate both budding from the ERC, as in CHO cells, and translocation of GLUT4 from the specialized retention compartment to the plasma membrane. The additional regulated step (sequestering of GLUT4 in a specialized compartment) would make retention more efficient in fat cells and account for the difference in insulin-responsiveness between CHO cells and fat cells.
ACKNOWLEDGMENTS
We thank Fred Maxfield, Enrique Rodriguez-Boulan, Tim Ryan, and Bjorn Dittmer-Roche for helpful comments and discussions, Tim Ryan for the VAMP-2–GFP construct, and Yu Chen for writing the image acquisition software.. This work was supported by National Institutes of Health grants DK-52852 and DK-57689 to T.E.M., a fellowship from the WM Keck foundation (to M.A.L.), and by National Science Foundation grant BES-0110070 to S.M.S.
Abbreviations used:
- DAB
diaminobenzidine
- CHO
Chinese hamster ovary
- ERC
endosomal recycling compartment
- GFP
green fluorescent protein
- HRP
horseradish peroxidase
- IRAP
insulin-regulated aminopeptidase
- Tf
transferrin
- TGN
trans-Golgi network
- TIR-FM
total internal reflection fluorescence microscopy
- TR
transferrin receptor
- HA
influenza hemagglutinin epitope
REFERENCES
- Aniento F, Gu F, Parton R, Gruenberg J. An endosomal beta COP is involved in the pH-dependent formation of transport vesicles destined for late endosomes. J Cell Biol. 1996;133:29–41. doi: 10.1083/jcb.133.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D, Hellen EH, Fulbright RM. Total internal reflection fluorescence. In: Lakowicz JR, editor. Topics in Fluorescence Spectroscopy. 3: Biochemical Applications. New York: Plenum Press; 1992. pp. 289–343. [Google Scholar]
- Bosshart H, Humphrey J, Deignan E, Davidson J, Drazba J, Yuan L, Oorschot V, Peters PJ, Bonifacino JS. The cytoplasmic domain mediates localization of furin to the trans-Golgi network en route to the endosomal/lysosomal system. J Cell Biol. 1994;126:1157–1172. doi: 10.1083/jcb.126.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradbury NA, Bridges RJ. Role of membrane trafficking in plasma membrane solute transport. Am J Physiol. 1994;267:C1–24. doi: 10.1152/ajpcell.1994.267.1.C1. [DOI] [PubMed] [Google Scholar]
- Cannon C, van Adelsberg J, Kelly S, Al-Awqati Q. Carbon-dioxide-induced exocytotic insertion of H+ pumps in turtle-bladder luminal membrane: role of cell pH and calcium. Nature. 1985;314:443–446. doi: 10.1038/314443a0. [DOI] [PubMed] [Google Scholar]
- Czech MP. Molecular actions of insulin on glucose transport. Annu Rev Nutr. 1995;15:441–471. doi: 10.1146/annurev.nu.15.070195.002301. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC, Klumperman J, Stoorvogel W, Bonifacino JS. Association of the AP-3 adaptor complex with clathrin. Science. 1998;280:431–434. doi: 10.1126/science.280.5362.431. [DOI] [PubMed] [Google Scholar]
- Forte JG, Yao X. The membrane-recruitment-and-recycling hypothesis of gastric HCl secretion. Trends Cell Biol. 1996;6:45–48. doi: 10.1016/0962-8924(96)81009-9. [DOI] [PubMed] [Google Scholar]
- Frost SC, Lane MD. Evidence for the involvement of vicinal sulfhydryl groups in insulin-activated hexose transport by 3T3–L1 adipocytes. J Biol Chem. 1985;260:2646–2652. [PubMed] [Google Scholar]
- Futter CE, Gibson A, Allchin EH, Maxwell S, Ruddock LJ, Odorizzi G, Domingo D, Trowbridge IS, Hopkins CR. In polarized MDCK cells basolateral vesicles arise from clathrin-gamma-adaptin-coated domains on endosomal tubules. J Cell Biol. 1998;141:611–623. doi: 10.1083/jcb.141.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza L, Birnbaum M. Insulin-responsive aminopeptidase trafficking in 3T3–L1 adipocytes. J Biol Chem. 2000;275:2560–2567. doi: 10.1074/jbc.275.4.2560. [DOI] [PubMed] [Google Scholar]
- Ghosh RN, Mallet WG, Soe TT, McGraw TE, Maxfield FR. An endocytosed TGN38 chimeric protein is delivered to the TGN after trafficking through the endocytic recycling compartment in CHO cells. J Cell Biol. 1998;142:923–936. doi: 10.1083/jcb.142.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson A, Futter CE, Maxwell S, Allchin EH, Shipman M, Kraehenbuhl JP, Domingo D, Odorizzi G, Trowbridge IS, Hopkins CR. Sorting mechanisms regulating membrane protein traffic in the apical transcytotic pathway of polarized MDCK cells. J Cell Biol. 1998;143:81–94. doi: 10.1083/jcb.143.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao M, Maxfield FR. Characterization of rapid membrane internalization and recycling. J Biol Chem. 2000;275:15279–15286. doi: 10.1074/jbc.275.20.15279. [DOI] [PubMed] [Google Scholar]
- Hashiramoto M, James DE. Characterization of insulin-responsive GLUT4 storage vesicles isolated from 3T3–L1 adipocytes. Mol Cell Biol. 2000;20:416–427. doi: 10.1128/mcb.20.1.416-427.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E, Simons K. Protein and lipid sorting from the trans-Golgi network to the plasma membrane in polarized cells. Semin Cell Dev Biol. 1998;9:503–509. doi: 10.1006/scdb.1998.0258. [DOI] [PubMed] [Google Scholar]
- Jhun BH, Rampal AL, Liu H, Lachaal M, Jung CY. Effects of insulin on steady state kinetics of GLUT4 subcellular distribution in rat adipocytes: evidence of constitutive GLUT4 recycling. J Biol Chem. 1992;267:17710–17715. [PubMed] [Google Scholar]
- Johnson AO, Lampson MA, McGraw TE. A di-leucine sequence, and a cluster of acidic amino acids are required for dynamic retention in the endosomal recycling compartment of fibroblasts. Mol Biol Cell. 2001;12:367–381. doi: 10.1091/mbc.12.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson AO, Subtil A, Petrush R, Kobylarz K, Keller SR, McGraw TE. Identification of an insulin-responsive, slow endocytic recycling mechanism in Chinese hamster ovary cells. J Biol Chem. 1998;273:17968–17977. doi: 10.1074/jbc.273.28.17968. [DOI] [PubMed] [Google Scholar]
- Kanai F, Nishioka Y, Hayashi H, Kamohara S, Todaka M, Ebina Y. Direct demonstration of insulin-induced GLUT4 translocation to the surface of intact cells by insertion of a c-myc epitope into an exofacial GLUT4 domain. J Biol Chem. 1993;268:14523–14526. [PubMed] [Google Scholar]
- Kandror KV, Pilch PF. gp160, a tissue-specific marker for insulin-activated glucose transport. Proc Natl Acad Sci USA. 1994;91:8017–8021. doi: 10.1073/pnas.91.17.8017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P, Simons K. Post-Golgi biosynthetic trafficking. J Cell Sci. 1997;110:3001–3009. doi: 10.1242/jcs.110.24.3001. [DOI] [PubMed] [Google Scholar]
- Keller P, Toomre D, Diaz T, White J, Simons K. Multicolour imaging of post-Golgi sorting and trafficking in live cells. Nat Cell Biol. 2001;3:140–149. doi: 10.1038/35055042. [DOI] [PubMed] [Google Scholar]
- Keller SR, Scott HM, Mastick CC, Aebersold R, Lienhard GE. Cloning and characterization of a novel insulin-regulated membrane aminopeptidase from Glut4 vesicles [published erratum appears in J. Biol. Chem. 1995, Dec 15; 270(50):30236] J Biol Chem. 1995;270:23612–23618. doi: 10.1074/jbc.270.40.23612. [DOI] [PubMed] [Google Scholar]
- Knepper MA, Inoue T. Regulation of aquaporin-2 water channel trafficking by vasopressin. Curr Opin Cell Biol. 1997;9:560–564. doi: 10.1016/s0955-0674(97)80034-8. [DOI] [PubMed] [Google Scholar]
- Lampson MA, Racz A, Cushman SW, McGraw TE. Demonstration of insulin-responsive trafficking of GLUT4 and vpTR in fibroblasts. [record supplied by publisher] J Cell Sci. 2000;113:4065–4076. doi: 10.1242/jcs.113.22.4065. [DOI] [PubMed] [Google Scholar]
- Le Borgne R, Griffiths G, Hoflack B. Mannose 6-phosphate receptors and ADP-ribosylation factors cooperate for high affinity interaction of the AP-1 Golgi assembly proteins with membranes. J Biol Chem. 1996;271:2162–2170. doi: 10.1074/jbc.271.4.2162. [DOI] [PubMed] [Google Scholar]
- Lim SN, Bonzelius F, Low SH, Wille H, Weimbs T, Herman GA. Identification of discrete classes of endosome-derived small vesicles as a major cellular pool for recycling membrane proteins. Mol Biol Cell. 2001;12:981–995. doi: 10.1091/mbc.12.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone C, James DE, Rice JE, Hanpeter D, Gould GW. Compartment ablation analysis of the insulin-responsive glucose transporter (GLUT4) in 3T3–L1 adipocytes. Biochem J. 1996;315:487–495. doi: 10.1042/bj3150487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malide D, St-Denis JF, Keller SR, Cushman SW. Vp165 and GLUT4 share similar vesicle pools along their trafficking pathways in rat adipose cells. FEBS Lett. 1997;409:461–468. doi: 10.1016/s0014-5793(97)00563-2. [DOI] [PubMed] [Google Scholar]
- Mallet WG, Maxfield FR. Chimeric forms of furin and TGN38 are transported with the plasma membrane in the trans-Golgi network via distinct endosomal pathways. J Cell Biol. 1999;146:345–359. doi: 10.1083/jcb.146.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Rice JE, Gould GW, Keller SR, Slot JW, James DE. The glucose transporter GLUT4 and the aminopeptidase vp165 colocalise in tubulo-vesicular elements in adipocytes and cardiomyocytes. J Cell Sci. 1997;110:2281–2291. doi: 10.1242/jcs.110.18.2281. [DOI] [PubMed] [Google Scholar]
- Martin S, Tellam J, Livingstone C, Slot JW, Gould GW, James DE. The glucose transporter (GLUT-4) and vesicle-associated membrane protein-2 (VAMP-2) are segregated from recycling endosomes in insulin-sensitive cells. J Cell Biol. 1996;134:625–635. doi: 10.1083/jcb.134.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Presley JF, Maxfield FR. Sorting of membrane components from endosomes and subsequent recycling to the cell surface occurs by a bulk flow process. J Cell Biol. 1993;121:1257–1269. doi: 10.1083/jcb.121.6.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Sabharanjak S, Maxfield FR. Cholesterol-dependent retention of GPI-anchored proteins in endosomes. EMBO J. 1998;17:4626–4638. doi: 10.1093/emboj/17.16.4626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw TE, Greenfield L, Maxfield FR. Functional expression of the human transferrin receptor cDNA in Chinese hamster ovary cells deficient in endogenous transferrin receptor. J Cell Biol. 1987;105:207–214. doi: 10.1083/jcb.105.1.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millar CA, Shewan A, Hickson GR, James DE, Gould GW. Differential regulation of secretory compartments containing the insulin-responsive glucose transporter 4 in 3T3–L1 adipocytes. Mol Biol Cell. 1999;10:3675–3688. doi: 10.1091/mbc.10.11.3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musch A, Xu H, Shields D, Rodriguez-Boulan E. Transport of vesicular stomatitis virus G protein to the cell surface is signal mediated in polarized and nonpolarized cells. J Cell Biol. 1996;133:543–558. doi: 10.1083/jcb.133.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzech E, Cohen S, Weiss A, Aroeti B. Interactions between the exocytic and endocytic pathways in polarized Madin-Darby canine kidney cells. J Biol Chem. 2000;275:15207–15219. doi: 10.1074/jbc.275.20.15207. [DOI] [PubMed] [Google Scholar]
- Pessin JE, Thurmond DC, Elmendorf JS, Coker KJ, Okada S. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking: location! location! location! J Biol Chem. 1999;274:2593–2596. doi: 10.1074/jbc.274.5.2593. [DOI] [PubMed] [Google Scholar]
- Rea S, James DE. Moving GLUT4: the biogenesis and trafficking of GLUT4 storage vesicles. Diabetes. 1997;46:1667–1677. doi: 10.2337/diab.46.11.1667. [DOI] [PubMed] [Google Scholar]
- Ross SA, Scott HM, Morris NJ, Leung WY, Mao F, Lienhard GE, Keller SR. Characterization of the insulin-regulated membrane aminopeptidase in 3T3–L1 adipocytes. J Biol Chem. 1996;271:3328–3332. doi: 10.1074/jbc.271.6.3328. [DOI] [PubMed] [Google Scholar]
- Satoh S, Nishimura H, Clark AE, Kozka IJ, Vannucci SJ, Simpson IA, Quon MJ, Cushman SW, Holman GD. Use of bismannose photolabel to elucidate insulin-regulated GLUT4 subcellular trafficking kinetics in rat adipose cells: evidence that exocytosis is a critical site of hormone action. J Biol Chem. 1993;268:17820–17829. [PubMed] [Google Scholar]
- Schmoranzer J, Goulian M, Axelrod D, Simon SM. Imaging constitutive exocytosis with total internal reflection fluorescence microscopy. J Cell Biol. 2000;149:23–32. doi: 10.1083/jcb.149.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonnichsen B, De Renzis S, Nielsen E, Rietdorf J, Zerial M. Distinct membrane domains on endosomes in the recycling pathway visualized by multicolor imaging of Rab4, Rab5, and Rab11. J Cell Biol. 2000;149:901–914. doi: 10.1083/jcb.149.4.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoorvogel W, Oorschot V, Geuze HJ. A novel class of clathrin-coated vesicles budding from endosomes. J Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subtil A, Lampson MA, Keller SR, McGraw TE. Characterization of the insulin-regulated endocytic recycling mechanism in 3T3–L1 adipocytes using a novel reporter molecule. J Biol Chem. 2000;275:4787–4795. doi: 10.1074/jbc.275.7.4787. [DOI] [PubMed] [Google Scholar]
- Sumitani S, Ramlal T, Somwar R, Keller SR, Klip A. Insulin regulation and selective segregation with glucose transporter-4 of the membrane aminopeptidase vp165 in rat skeletal muscle cells. Endocrinology. 1997;138:1029–1034. doi: 10.1210/endo.138.3.5010. [DOI] [PubMed] [Google Scholar]
- Toomre D, Steyer JA, Keller P, Almers W, Simons K. Fusion of constitutive membrane traffic with the cell surface observed by evanescent wave microscopy. J Cell Biol. 2000;149:33–40. doi: 10.1083/jcb.149.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E, Brown PS, Aroeti B, Chapin SJ, Mostov KE, Dunn KW. Apical and basolateral endocytic pathways of MDCK cells meet in acidic common endosomes distinct from a nearly-neutral apical recycling endosome. Traffic. 2000;1:480–493. doi: 10.1034/j.1600-0854.2000.010606.x. [DOI] [PubMed] [Google Scholar]
- Ward DT, Hammond TG, Harris HW. Modulation of vasopressin-elicited water transport by trafficking of aquaporin2-containing vesicles. Annu Rev Physiol. 1999;61:683–697. doi: 10.1146/annurev.physiol.61.1.683. [DOI] [PubMed] [Google Scholar]
- Whitney JA, Gomez M, Sheff D, Kreis TE, Mellman I. Cytoplasmic coat proteins involved in endosome function. Cell. 1995;83:703–713. doi: 10.1016/0092-8674(95)90183-3. [DOI] [PubMed] [Google Scholar]
- Yamashiro DJ, Tycko B, Fluss SR, Maxfield FR. Segregation of transferrin to a mildly acidic (pH 6.5) para-Golgi compartment in the recycling pathway. Cell. 1984;37:789–800. doi: 10.1016/0092-8674(84)90414-8. [DOI] [PubMed] [Google Scholar]
- Yang J, Holman GD. Comparison of GLUT4 and GLUT1 subcellular trafficking in basal and insulin-stimulated 3T3–L1 cells. J Biol Chem. 1993;268:4600–4603. [PubMed] [Google Scholar]
- Yoshimori T, Keller P, Roth MG, Simons K. Different biosynthetic transport routes to the plasma membrane in BHK and CHO cells. J Cell Biol. 1996;133:247–256. doi: 10.1083/jcb.133.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]