Publisher's Note: There is a Blood Commentary on this article in this issue.
Key Points
Among adults with MRD-positive ALL in hematologic remission after chemotherapy, 78% achieved a complete MRD response with blinatumomab.
Complete MRD response after blinatumomab treatment in this population was associated with significantly improved OS.
Abstract
Approximately 30% to 50% of adults with acute lymphoblastic leukemia (ALL) in hematologic complete remission after multiagent therapy exhibit minimal residual disease (MRD) by reverse transcriptase–polymerase chain reaction or flow cytometry. MRD is the strongest predictor of relapse in ALL. In this open-label, single-arm study, adults with B-cell precursor ALL in hematologic complete remission with MRD (≥10−3) received blinatumomab 15 µg/m2 per day by continuous IV infusion for up to 4 cycles. Patients could undergo allogeneic hematopoietic stem-cell transplantation any time after cycle 1. The primary end point was complete MRD response status after 1 cycle of blinatumomab. One hundred sixteen patients received blinatumomab. Eighty-eight (78%) of 113 evaluable patients achieved a complete MRD response. In the subgroup of 110 patients with Ph-negative ALL in hematologic remission, the Kaplan-Meier estimate of relapse-free survival (RFS) at 18 months was 54%. Median overall survival (OS) was 36.5 months. In landmark analyses, complete MRD responders had longer RFS (23.6 vs 5.7 months; P = .002) and OS (38.9 vs 12.5 months; P = .002) compared with MRD nonresponders. Adverse events were consistent with previous studies of blinatumomab. Twelve (10%) and 3 patients (3%) had grade 3 or 4 neurologic events, respectively. Four patients (3%) had cytokine release syndrome grade 1, n = 2; grade 3, n = 2), all during cycle 1. After treatment with blinatumomab in a population of patients with MRD-positive B-cell precursor ALL, a majority achieved a complete MRD response, which was associated with significantly longer RFS and OS compared with MRD nonresponders. This study is registered at www.clinicaltrials.gov as #NCT01207388.
Introduction
Preemptive treatment of malignant disease after remission in patients with low but measurable disease may prolong overall survival (OS) compared with treatment of overt relapse.1 In acute lymphoblastic leukemia (ALL), minimal residual disease (MRD) is defined as the presence of leukemic cells not detectable by microscopy and may be measured by standardized methods with a sensitivity of 10−4 (ie, 0.01%).2
Despite intensive induction/consolidation chemotherapy with hematologic complete remission (CR) rates of 80% to 90%, approximately 30% to 50% of adult patients with ALL and 10% to 20% of pediatric patients with ALL in CR exhibit MRD.3-8 MRD persistence or recurrence indicates resistance to standard chemotherapy and is the most important risk factor for hematologic relapse in T-cell and B-cell ALL.9-13 For adult patients, 5-year hematologic relapse rates range from 56% to 100% for MRD positivity, compared with 18% to 33% for MRD negativity.4,5,14 Up to 70% of patients have an MRD level >10−3 after achieving CR, and their median duration of hematologic remission is 4.9 months.5 A meta-analysis of 16 studies, comprising 2076 adults with ALL, concluded that MRD negativity was associated with 10-year event-free survival of 64% vs 21% for MRD positivity (hazard ratio [HR], 0.28; 95% Bayesian credible interval, 0.24-0.33); MRD negativity was also associated with improved OS (HR, 0.28; 95% Bayesian credible interval, 0.20-0.39).13
No standard therapy has been defined for ALL with detectable MRD during or after intensive, multiagent chemotherapy.2,10 Study groups and expert guidelines recommend allogeneic hematopoietic stem-cell transplantation (HSCT).7,15 Patients with persistent MRD who undergo HSCT have better outcomes compared with those who do not undergo HSCT.5,8 However, many patients experience relapse while awaiting HSCT, and detectable MRD pretransplantation is associated with a higher relapse rate after HSCT.16,17
Targeted agents with alternative mechanisms of action may reduce MRD and delay or prevent hematologic relapse. Blinatumomab is a bispecific T cell–engager antibody construct that directs T cells to CD19+ cells.18 CD19 is expressed on blast cells in >95% of cases of B-cell precursor ALL.19 In a randomized phase 3 trial of patients with Ph-negative relapsed or refractory B-cell precursor ALL, 44% of 271 patients achieved hematologic CR with blinatumomab, compared with 25% of 134 patients with standard-of-care chemotherapy.20 On the basis of a phase 2 pilot study in MRD-positive ALL with an 80% MRD response rate,21 the single-arm, open-label study reported here evaluated efficacy and tolerability of single-agent blinatumomab in adult patients with ALL in hematologic CR with MRD ≥10−3. In contrast to the pilot study,22 the level of MRD had to be higher in our study (≥10−3 vs ≥10−4), and a relevant proportion of patients with MRD+ ALL after relapse were included.
Patients and methods
Study design
This open-label, single-arm phase 2 study was conducted at 46 centers in Europe and Russia (supplemental Table 1, available on the Blood Web site). Patients received blinatumomab 15 µg/m2 per day by continuous IV infusion for up to 4 cycles. Each cycle comprised 4 weeks of blinatumomab infusion followed by a 2-week treatment-free period. Patients could undergo HSCT any time after cycle 1 at the investigator’s discretion. Prophylaxis for central nervous system ALL was recommended before cycle 1 and after cycles 2 and 4. Corticosteroid pretreatment for prophylaxis of neurologic events and cytokine release syndrome was required (supplemental Methods B). Guidelines for supportive care, dose interruption and discontinuation for adverse events, and blinatumomab retreatment for MRD relapse within 18 months are provided in supplemental Methods C. Concurrent antileukemic therapy was prohibited.
MRD evaluation (supplemental Figure 1) for eligibility was performed mostly in the central reference laboratory (University of Kiel, Kiel, Germany) or in national reference laboratories using real-time quantitative polymerase chain reaction of clonally rearranged immunoglobulin and/or T-cell receptor gene rearrangements or using flow cytometry. The central reference laboratory exclusively performed MRD assessments at baseline, at the end of each treatment cycle, and during efficacy follow-up using real-time quantitative polymerase chain reaction.4 A complete MRD response was defined as no target amplification with a minimum sensitivity of 10−4.23 An MRD response was defined as either a complete MRD response or MRD response, which included patients with complete MRD response and those with detectable MRD <10−4.
Patients
Eligible patients were age ≥18 years with B-cell precursor ALL in first or later hematologic CR and with persistent or recurrent MRD ≥10−3 after a minimum of 3 blocks of intensive chemotherapy. Full eligibility criteria are provided in supplemental Methods A. Study procedures were approved by the investigational review board at each study center and by independent ethics committees per local regulations. Patients provided written informed consent. The study was registered with ClinicalTrials.gov (#NCT01207388) and EudraCT (#2010-018314-75). This analysis reflects all patients with a data cutoff date of 5 August 2015, when all patients had either completed at least 18 months of follow-up or discontinued the study.
Outcomes
Analysis sets are summarized in supplemental Table 2. For the analyses, different data sets were defined per protocol. This was necessary because some patients who were treated in the study fulfilled the inclusion criteria per local MRD testing, but these criteria were not confirmed after central review, or they had Ph+ disease or were not in remission (Figure 1). Reporting of patient characteristics and safety end points used the intent-to-treat full analysis set (N = 116). The primary end point was the rate of complete MRD response after cycle 1 among patients in the primary end point full analysis set (n = 113), which excluded patients from the full analysis set with either no central MRD assay results or a test sensitivity that did not reach 10−4. Overall MRD response was evaluated among patients in the primary end point efficacy set (n = 103), which excluded in addition patients from the primary end point full analysis set without hematologic CR or with MRD ≤10−3 at study entry. Thus, the primary end point efficacy set represents the originally intended study population. The key secondary end point, hematologic relapse-free survival (RFS) at 18 months after initiation of blinatumomab, was evaluated among patients in the key secondary end point full analysis set (n = 110), which excluded patients from the full analysis set who had either Ph-positive disease or ≥5% bone marrow blasts at study entry. For the primary analysis of RFS, patients were censored at the time of HSCT in continuous CR or at the time of chemotherapy after blinatumomab to focus on patients who were not eligible for HSCT. A sensitivity analysis of RFS not censored at HSCT or chemotherapy was also prespecified. All other analyses of secondary end points, such as landmark analysis of OS or duration of hematologic remission, were performed without censoring for HSCT or chemotherapy after blinatumomab.
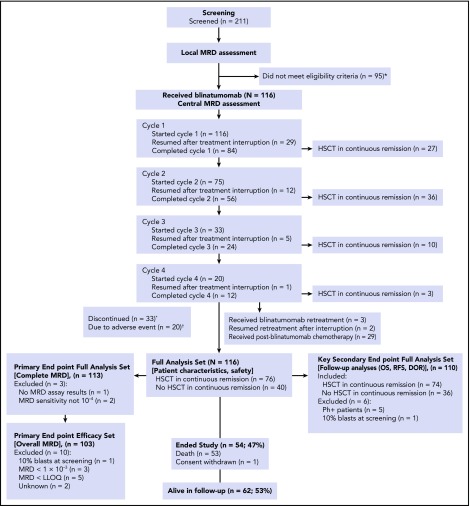
Figure 1.
Disposition of patients in the study. *Reasons for not meeting eligibility criteria included: MRD level lower than the required ≥10−3 (therefore, inclusion criterion not fulfilled because disease burden too low; n = 48); not in hematologic CR (ie, overt relapse; n = 31); technical (n = 5); central nervous system relapse (n = 2); active infection (n = 2); alternative therapy (n = 2); neurologic disorder (n = 2); CD19− (n = 1); hepatic disorder (n = 1); and consent withdrawn (n = 1). †Reasons for discontinuation (n = 33) included: adverse event (n = 20 [17.2%]), disease relapse (n = 10 [8.6%]), physician decision (n = 2 [1.7%]), and other (n = 1 [0.9%]). DOR, duration of hematologic remission; LLOQ, lower limit of quantitation; RFS, relapse-free survival.
Adverse events from the date of informed consent until 30 days after the last blinatumomab infusion were graded per Common Terminology Criteria for Adverse Events (version 4.0). For patients with HSCT within 30 days after the last dose, the final safety follow-up visit was performed as close as possible before initiation of transplantation conditioning. Adverse events occurring >30 days after treatment were recorded only if the investigator considered the adverse event possibly related to blinatumomab treatment.
Statistical analysis
Complete MRD response rates were calculated using 2-sided exact 95% confidence intervals (CIs) for all patients and in subgroups defined by baseline covariates. Kaplan-Meier estimates with 2-sided 95% CIs were used to describe RFS and OS; differences between subgroups were evaluated using log-rank test. Transplantation effects on long-term outcomes were evaluated in a post hoc analysis considering the waiting time for HSCT. Duration of hematologic remission was estimated by the 1 − cumulative incidence function of relapse, with death in CR as a competing event. Kaplan-Meier estimates of OS and RFS were repeated separately among patients who received >1 cycle or only 1 cycle of blinatumomab. For all Kaplan-Meier tests, censoring for HSCT or postblinatumomab chemotherapy was performed only if specifically stated.
Analysis of the primary and key secondary end points was hierarchical. One hundred evaluable patients would provide 90% power to demonstrate that a 97.5% 1-sided CI excluded 44% if the true unknown response rate was 61%.
For the key secondary end point, 100 patients provided 90% power to show that the lower boundary of the 95% CI for the Kaplan-Meier RFS rate (with censoring of patients at HSCT) would exceed 28%, assuming a true hematologic relapse rate at 18 months of 55% and an HSCT availability rate of ≤67%.
The statistical analysis plan predefined analysis sets that were based on availability of MRD data and patient population. The main outcome parameters (patient characteristics and safety) were analyzed in the intent-to-treat full analysis set (N = 116); the primary end point was analyzed in the primary end point full analysis set (n = 113); the secondary end points (OS, RFS, and duration of hematologic remission) were analyzed in the secondary end point full analysis set (n = 110). Overall MRD response was evaluated in the primary end point efficacy set (n = 103). Additional details on the definition and composition of data sets are given in Figure 1 and supplemental Table 2.
RFS, OS, and duration of hematologic remission were also calculated in landmark analyses beginning at day 45 for patients with or without a complete MRD response within cycle 1. Day 45 was selected for these analyses because it was the end of cycle 1, when the last MRD assessment was performed. RFS was defined as time from first blinatumomab dose to the earlier of first detection of hematologic/extramedullary relapse, secondary leukemia, death (resulting from any cause), or, if applicable, last date of continuous CR.
Role of the funding source
The trial was designed by Amgen Research (Munich), GmbH (formerly Micromet AG), in collaboration with the trial investigators. The first author prepared the first draft of the manuscript, with assistance from professional medical writers who were funded by Amgen. All authors had access to the data and provided contributions to subsequent drafts of the manuscript. All authors vouch for the integrity and completeness of the data and for the fidelity of the trial to the protocol. Statisticians employed by Amgen conducted the statistical analyses and contributed to the manuscript. An independent data and safety monitoring board met regularly to review safety and efficacy data to provide recommendations for safety oversight and protection of the scientific integrity of the study.
Results
Patient characteristics
The full analysis set for patient characteristics included 116 patients who received at least 1 blinatumomab infusion between November 2010 and February 2014 (Figure 1). Median age was 45 years (range, 18-76 years). Forty-seven percent of patients were enrolled with MRD ≥10−2, and 35% were in second or later hematologic CR (Table 1). All patients completed the treatment period (Figure 1). Overall, 76 patients underwent HSCT in continuous CR after 1 (n = 27), 2 (n = 36), or 3 to 4 cycles (n = 13; Figure 1). Treatment cycles, interruptions, and withdrawals are summarized in supplemental Results A.
Table 1.
Baseline demographics and disease characteristics (full analysis set)
| Characteristic | Patients (N = 116) |
|---|---|
| Sex, n (%) | |
| Male | 68 (59) |
| Female | 48 (41) |
| Median (range) age, years | 45.0 (18-76) |
| Age group, years, n (%) | |
| 18 to <35 | 36 (31) |
| 35 to <55 | 41 (35) |
| 55 to <65 | 24 (21) |
| ≥65 | 15 (13) |
| Cytogenetics/molecular genetics, n (%) | |
| t(9;22)/BCR-ABL+ | 5 (4) |
| t(4;11)/MLL-AF4+ | 5 (4) |
| Relapse history, n (%)* | |
| Patients in first CR | 75 (65) |
| Patients in second CR | 39 (34) |
| Patients in third CR | 2 (2) |
| Median (range) time from last prior treatment, months | 2.0 (0-55) |
| Baseline MRD levels, n (%)† | |
| ≥10−1 to <1 (≥10% to <1) | 9 (8) |
| ≥10−2 to <10−1 (≥1% to <10%) | 45 (39) |
| ≥10−3 to <10−2 (≥0.1% to <1%) | 52 (45) |
| <10−3 (<0.1%) | 3 (3) |
| Below LLOQ | 5 (4) |
| Unknown‡ | 2 (2) |
LLOQ, lower limit of quantification.
One patient was ineligible (hematologic relapse).
Nine patients had MRD levels ≥10−1 with bone marrow cytology in central reference laboratory showing <5% blasts.
Missing MRD assay results in central reference laboratory (n = 1); MRD quantification at baseline but with subsequent assessments (n = 1).
Primary end point: MRD response
The primary end point full analysis set for analysis of a complete MRD response included 113 patients with evaluable MRD markers (supplemental Table 2). Of these patients, 88 (78%) achieved a complete MRD response after cycle 1. The lower bound of the 95% CI (69% to 85%) was >44%, confirming the study hypothesis. Two additional patients achieved a complete MRD response after cycle 2; no additional patient achieved a complete MRD response after cycle 3 or cycle 4. Among 5 patients with Ph+ disease who had MRD evaluations, 3 (60%) had an MRD response during cycle 1.
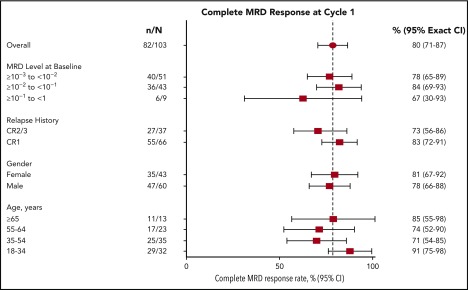
The primary end point efficacy set for analysis of overall MRD included 103 patients in hematologic CR and with MRD >10−3 at baseline. Of these patients, 91 (88%) achieved any MRD response, including 82 (80%; 95% CI, 71% to 87%) with a complete MRD response after cycle 1. Complete MRD response rates were similar between patients with MRD ≥10−2 and those with MRD <10−2 at baseline and between patients with first remission and those with later remission at baseline (Figure 2). Of 45 patients in this analysis set with treatment interruptions as a result of any cause during cycle 1, 37 (82%) achieved a complete MRD response.
Figure 2.
Complete MRD response after cycle 1 by clinical characteristics at baseline and conduct of therapy in cycle 1 (primary end point efficacy set). Three (75%) of 4 patients (95% CI, 19% to 99%) with Ph+ ALL and 2 (50%) of 4 patients (95% CI, 7% to 93%) with t(4;11) and/or MLL-AF4+ disease had a complete MRD response during cycle 1. MRD complete response rates were similar for patients with or without treatment interruptions during cycle 1. CR1, first CR; CR2/3, second or third CR.
Secondary efficacy end points: survival and transplantation
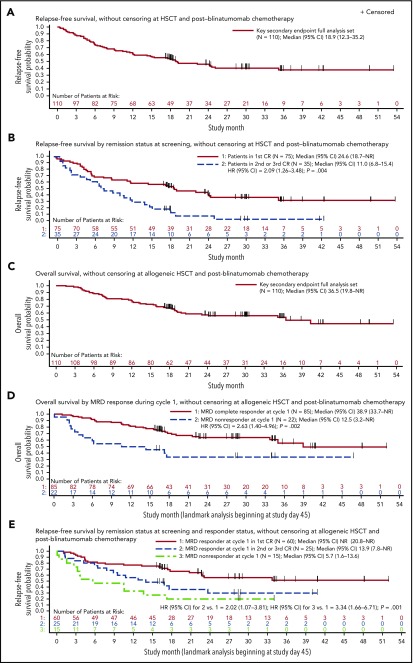
The key secondary end point full analysis set included 110 patients from the full analysis set who had Ph− disease and <5% blasts at baseline. Among these patients, the Kaplan-Meier estimate for RFS at 18 months was 54% (95% CI, 33% to 70%), exceeding the prespecified boundary of 28% and thereby meeting the key secondary end point. Estimates of RFS at 18 months were similar with or without censoring for postblinatumomab HSCT and chemotherapy. Median RFS was 18.9 months (95% CI, 12.3-35.2 months), with a median follow-up of 29.9 months (Figure 3A). Median RFS was 11.0 vs 24.6 months among patients treated within later CR vs first CR (unadjusted HR, 2.09; 95% CI, 1.26-3.48; P = .004; Figure 3B). Patients in first CR also had improved OS compared with those in later CR (supplemental Figure 2). The impact of other baseline covariates on OS, RFS, and duration of hematologic remission is summarized in supplemental Table 3.
Figure 3.
RFS and OS among Ph-positive patients in hematologic CR at start of treatment (key secondary end point full analysis set). (A) RFS without censoring at allogeneic HSCT or postblinatumomab chemotherapy. Median follow-up, 29.9 months. (B) RFS by remission status at screening without censoring at allogeneic HSCT or postblinatumomab chemotherapy. Complete MRD response was defined as MRD negativity with minimum sensitivity of 10−4. (C) OS without censoring at allogeneic HSCT or postblinatumomab chemotherapy. Median follow-up, 30.0 months. (D) OS by complete MRD responder status in cycle 1 among evaluable patients (landmark analysis, excluding patients who were censored or had relapsed or died within 45 days of beginning treatment), without censoring at allogeneic HSCT or postblinatumomab chemotherapy. (E) RFS without censoring at allogeneic HSCT or postblinatumomab chemotherapy by complete MRD responder status in cycle 1 and salvage status among evaluable patients (landmark analysis, excluding patients who were censored or had relapsed or died within 45 days of beginning treatment). NR, not reached.
Forty-eight of 110 patients remained in CR (36 after subsequent HSCT), 38 experienced relapse in CR, and 24 died in CR (20 after subsequent HSCT). Median duration of hematologic remission was not reached (supplemental Figure 3). Median OS was 36.5 months (95% CI, 19.8 months to not estimable), with a median follow-up of 30.0 months (Figure 3C). Among the entire study population of 116 patients, median OS was 36.5 months (95% CI, 19.2 months to not estimable; supplemental Figure 4).
Landmark analyses of RFS and OS by complete MRD response excluded 3 patients without MRD results (n = 1) or insufficient sensitivity of the assay (n = 2). The landmark of 45 days was used to represent the latest day of first MRD response assessment. Median RFS was 23.6 vs 5.7 months (P = .002) and median OS was 38.9 vs 12.5 months (P = .002) in patients with and without a complete MRD response in cycle 1, respectively (Table 2; Figure 3D). Median RFS was 13.9 months among patients in second or later CR who achieved a complete MRD response with blinatumomab; median RFS was not reached among patients in first CR who achieved a complete MRD response with blinatumomab (Figure 3E).
Table 2.
Overall long-term outcomes and by MRD complete response and nonresponse in cycle 1 (key secondary end point full analysis set)
| All patients | MRD responders* | MRD nonresponders* | |
|---|---|---|---|
| OS | |||
| Patients with events, n/N | 48/110 | 31/85 | 14/22 |
| Median† (95% CI) | 36.5 (19.8-NR) | 38.9 (33.7-NR) | 12.5 (3.2-NR) |
| Estimated probability at 18 months (95% CI)† | 0.67 (0.58-0.75) | 0.70 (0.59-0.79) | 0.34 (0.15-0.54) |
| P‡ | — | .002 | |
| Hematologic RFS | |||
| Patients with events, n/N | 62/110 | 40/85 | 12/15 |
| Median† (95% CI) | 18.9 (12.3-35.2) | 23.6 (17.4-NR) | 5.7 (1.6-13.6) |
| Estimated probability at 18 months (95% CI)† | 0.53 (0.44-0.62) | 0.58 (0.46-0.68) | 0.20 (0.05-0.42) |
| P‡ | — | .002 | |
| Duration of hematologic remission§ | |||
| Patients with events, n/N | 38/110 | 23/85 | 7/15 |
| Median† (95% CI) | NR (NR-NR) | NR (NR-NR) | NR (3.7-NR) |
| Estimated probability at 18 months (95% CI)† | 0.70 (0.61-0.78) | 0.77 (0.67-0.85) | 0.53 (0.30-0.80) |
| P¶ | — | .14 |
n, patients with events (deaths for OS, death in CR, or relapse for RFS and relapse for duration of hematologic remission); N, patients at risk; NR, not reached.
Landmark analysis includes patients in both the key secondary full analysis set and the primary end point analysis set and excludes patients with an event (death or relapse) or censored before day 45.
Kaplan-Meier estimate.
Log-rank test P value compared with MRD nonresponders.
Duration of hematologic remission is evaluated by 1 − cumulative incidence function of hematologic relapse with death in CR as a competing event.
Gray’s test P value compared with MRD nonresponders.
Seventy-four (67%) of 110 patients in the key secondary end point full analysis set underwent HSCT in continuous remission after blinatumomab: 55 in ongoing first CR and 19 in continuous second CR. Of the patients undergoing transplantation, 65% were age >35 years, and median age was 42.5 years (range, 18-67 years). Nine (25%) of 36 patients without HSCT or chemotherapy after blinatumomab remained in continuous CR, with a median follow-up of 24.0 months (range, 2.8-41.6 months), whereas 36 (49%) of 74 patients with HSCT remained in remission. Characteristics of patients who underwent postblinatumomab HSCT and a post hoc analysis of survival outcomes are summarized in supplemental Results B.
Adverse events
All 116 patients who started cycle 1 experienced at least 1 adverse event (supplemental Table 4). During cycles 2, 3, and 4, 85%, 79%, and 75% of treated patients, respectively, experienced adverse events. Overall, 33% and 27% of patients had grade 3 and 4 adverse events, respectively (Table 3), including 20% and 18%, respectively, in cycle 1 and 11% each in cycle 2. Investigators considered grade 3 and 4 adverse events to be treatment related for 29% and 22% of patients, respectively. Four patients (3%) had cytokine release syndrome (grade 1, n = 2; grade 3, n = 2), all during cycle 1 (supplemental Table 4).
Table 3.
Summary of adverse events (full analysis set)
| All patients (N = 116) | |||
|---|---|---|---|
| Any grade | Grade 3 | Grade 4 | |
| Any adverse event, n (%) | 116 (100) | 38 (33) | 31 (27) |
| Non-neurologic adverse events, worst grade ≥3 occurring in ≥3% of patients | |||
| Pyrexia | 103 (89) | 9 (8) | 0 (0) |
| Headache | 44 (38) | 4 (3) | 0 (0) |
| Neutropenia | 18 (16) | 2 (2) | 16 (14) |
| Leukopenia | 8 (7) | 5 (4) | 2 (2) |
| Anemia | 7 (6) | 4 (3) | 1 (1) |
| ALT increased | 7 (6) | 2 (2) | 4 (3) |
| Thrombocytopenia | 6 (5) | 2 (2) | 3 (3) |
| AST increased | 5 (4) | 1 (1) | 3 (3) |
| Any neurologic adverse event* | 61 (53) | 12 (10) | 3 (3) |
| Neurologic events, worst grade ≥3 | |||
| Tremor | 35 (30) | 6 (5) | 0 (0) |
| Aphasia | 15 (13) | 1 (1) | 0 (0) |
| Dizziness | 9 (8) | 1 (1) | 0 (0) |
| Confused state | 6 (5) | 1 (1) | 0 (0) |
| Encephalopathy | 6 (5) | 3 (3) | 2 (2) |
| Seizure | 3 (3) | 1 (1) | 1 (1) |
| Disorientation | 3 (3) | 1 (1) | 0 (0) |
| Depressed level of consciousness | 1 (1) | 1 (1) | 0 (0) |
| Generalized tonic-clonic seizure | 1 (1) | 1 (1) | 0 (0) |
All adverse events regardless of causality that occurred during the treatment period plus 30 days. Thirty-six patients (31%) had treatment interruptions because of treatment-emergent adverse events, mainly as a result of neurologic events and flu-like symptoms. Those occurring in ≥2% of patients included pyrexia (8%) and aphasia, encephalopathy, overdose, tremor, ALT increased, AST increased, and chills (3% each).
ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Among all patients. Multiple events may have occurred in some patients.
Sixty-one patients (53%) had neurologic events of any grade (Table 3), with decreasing incidence over cycles 1, 2, 3, and 4 of 47%, 24%, 15%, and 15%, respectively. Median duration from onset to resolution was 4 days (quartile 1, 2 days; quartile 3, 8 days). Twelve (10%) and 3 patients (3%) had grade 3 and 4 neurologic events, respectively, with 9%, 4%, 0%, and 0% reporting grade 3 and 4 events for cycles 1, 2, 3, and 4, respectively. Neurologic events resolved in 59 patients (97%) with any-grade events and in all patients with grade 3/4 events. Most patients who had grade 3/4 neurologic events resumed blinatumomab treatment after the event resolved.
Two fatal adverse events were reported during the treatment period (both in cycle 1): atypical pneumonitis with H1N1 influenza (considered treatment related by the investigator) and subdural hemorrhage (considered unrelated to treatment by the investigator). Investigators reported 4 fatal adverse events after blinatumomab treatment, including 2 in patients who underwent HSCT after blinatumomab (multifocal central nervous system lesions and graft-versus-host disease at 124 and 136 days posttreatment, respectively) and 2 (disease progression and multiorgan failure at 154 and 359 days posttreatment, respectively) after subsequent relapse in patients not undergoing transplantation.
Discussion
To date, most investigational therapies in ALL have been evaluated in patients with overt relapse. This was the first international, multicenter study in ALL to combine MRD-based inclusion criteria with an MRD-based primary end point assessed per the central reference laboratory. This patient group was selected based on poor prognosis as a result of a high relapse rate with continued chemotherapy, indicating resistance to chemotherapy. A targeted therapy with an alternative mechanism of action administered before overt relapse may improve the outcome in these patients.
The results confirm and extend those of the pilot study of blinatumomab in MRD+ ALL, in which 80% of patients achieved an MRD response.21,22 However, in contrast to the pilot study, this study enrolled patients with MRD ≥10−3 (vs ≥10−4) and included patients in second or later CR, both indicators of increased risk. Nevertheless, across all groups evaluated, complete MRD response rates were high, and no demographic/clinical characteristics were associated with MRD response after 1 cycle. In relapsed/refractory ALL, the only baseline feature that was predictive of response to blinatumomab was blast percentage, with a 73% composite CR or CR with partial hematologic recovery rate in patients with <50% blasts in bone marrow.24 This finding and the high response rates in MRD+ patients support the hypothesis that targeted single-drug immunotherapy has impressive antileukemic activity and that lower leukemia burden is a favorable prerequisite for this treatment principle.
Previous studies evaluating outcomes in adult patients with MRD+ ALL after conventional chemotherapy showed that MRD+ status was associated with shorter OS and RFS,5-7,16,25 and higher MRD burden was associated with shorter duration of hematologic remission and poorer outcomes despite continued chemotherapy.4 In this study, landmark analyses showed that achieving a complete MRD response was associated with prolonged OS and RFS compared with not achieving an MRD response, demonstrating a direct patient benefit for the immunotherapeutic conversion of MRD+ to MRD− disease. Although MRD response rates were high in patients treated in second or third CR, RFS, OS, and duration of hematologic remission were inferior compared with patients treated in first CR. MRD response may have different implications in relapsed/refractory ALL, presumably due to genetic instability and selection of more aggressive subclones, which are correlated to higher incidences of escape and resistance mechanisms.26 Thus, treatment with blinatumomab in MRD+ disease during an ongoing first CR may be beneficial.
In contrast to stepwise dosing of blinatumomab to prevent cytokine release syndrome in the relapsed/refractory setting (9 μg per day for 1 week followed by 28 μg per day for 3 weeks in cycle 1),24 we used a fixed blinatumomab dose of 15 µg/m2 per day × 28 days per cycle, corresponding to the fixed target dose of 28 µg per day for relapsed/refractory ALL. Despite implementation of a higher starting dose in this study, cytopenias, including those of grade 3/4, were less frequent compared with previous blinatumomab studies in relapsed/refractory ALL.18,22,24 The incidence of adverse events (including neurologic events) was greatest during cycle 1 and decreased over subsequent cycles. Neurologic events were mostly grade 1/2 and included tremor, aphasia, dizziness, encephalopathy, and seizure, with grade 1/2 tremor and aphasia occurring more frequently than in relapsed/refractory ALL.18,24 For those patients with adverse events leading to treatment interruptions, most events resolved rapidly after stopping blinatumomab, which has a serum half-life of ∼2 hours. Most patients who had grade 3/4 neurologic events resumed blinatumomab treatment after the event resolved. Similar neurologic events have been observed with other CD19-directed therapies,27-29 in other T cell–activating studies,30 and in blinatumomab studies in relapsed/refractory ALL and non-Hodgkin lymphoma.18,22,24 The pathogenesis of neurologic events is poorly understood and does not seem to be correlated to tumor burden, because rates of grade 3/4 neurologic events were similar for blinatumomab in relapsed/refractory ALL.24 In the MRD setting, adverse events associated with cytokine release syndrome were observed infrequently (n = 4), and no deaths were related to neurologic events or cytokine release syndrome.
A median OS of 36.5 months, and an equivalent estimate for patients treated in first CR in this study, compares favorably to that in published data for MRD-positive ALL5 and relapsed/refractory ALL.24 In this study, a median RFS of 18.9 months without censoring for HSCT was substantially greater than the median time to hematologic relapse of 7.6 months previously reported for patients in first CR with MRD >10−4 who did not undergo HSCT,5 suggesting that blinatumomab has the potential to improve the outcome of patients with chemotherapy-resistant disease. HSCT rates of 47% to 66% among patients with B-cell precursor ALL with MRD after standard frontline therapy have led to prolonged RFS.5,11 On the basis of these results, investigators offered HSCT in 67% of the patients in this study in remission after blinatumomab, because this became standard practice for MRD-positive patients in the countries participating in the trial. Because of changing practices, the proportion of patients undergoing HSCT was higher than expected. HSCT was also offered to older patients (65% age >35 years), and 34% of the transplantations used mismatched donors. This broad indication for HSCT may have contributed to transplantation-related mortality; nevertheless, mortality is in line with reports of HSCT in standard of care ranging between 20% to 30%.31-35 The proportion of patients not undergoing transplantation was small, and the study was neither planned nor powered to assess the impact of HSCT after blinatumomab treatment. Nevertheless, a number of patients with a complete MRD response remained in long-term remission without subsequent HSCT, which confirms the experience of the pilot study with long-term survivors without subsequent HSCT.21,22 This observation might be of relevance for the development of future treatment strategies, particularly for less fit and elderly patients. Additional studies need to determine the role of HSCT in this setting, particularly regarding indications for HSCT.
Treatment of patients with persistent chemotherapy-resistant disease after conventional treatment remains a major challenge in the management of pediatric and adult ALL, with high relapse rates during continued chemotherapy and even after subsequent HSCT. Among patients with chemotherapy-resistant MRD, targeted immunotherapy with blinatumomab resulted in a substantial molecular response rate and improved long-term outcomes among responders compared with nonresponders. Our results suggest that targeted treatment in early stages of MRD is a viable therapeutic strategy for patients with B-cell precursor ALL and that it should also be evaluated in other hematologic malignancies.36,37
Supplementary Material
The online version of this article contains a data supplement.
Acknowledgments
The authors acknowledge Ali Hassan and Miranda Tradewell of Complete Healthcare Communications, LLC (Chadds Ford, PA), as well as Jonathan Latham of PharmaScribe, LLC (Deerfield, MA), all of whose work was supported by Amgen, Inc., and Geoff Smith (Amgen, Inc., Thousand Oaks, CA) for providing medical writing support, including assistance with the initial draft (Ali Hassan and Miranda Tradewell) and draft revisions and editorial assistance (Jonathan Latham and Geoff Smith). Robert Dawson of CACTUS Communications, Inc., supported by Amgen, Inc., edited and formatted figures.
Footnotes
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: N.G., H. Dombret, M.S.T., M. Brüggemann, H.W., V. Haddad, G.Z., D.N., and R.C.B. designed the study; N.G., H. Dombret, M. Bonifacio, A.R., C.G., C.F., H. Diedrich, M.S.T., V. Havelange, and R.C.B. recruited patients, conducted patient follow-up, and collected clinical data; N.G., M. Brüggemann, H.-A.H., J.S., V. Haddad, J.E.B., G.Z., D.N., and R.C.B. analyzed the data; the manuscript was prepared by N.G., J.S., and J.E.B.; and all authors participated in data interpretation and drafting of the manuscript and read, revised, and approved the final manuscript.
Conflict-of-interest disclosure: R.C.B. is an advisor for Amgen, Novartis, AstraZeneca, GenMab, GeMoab, and Pfizer and reports patent royalties from Amgen. J.E.B. is a former Amgen employee and stockholder. M. Bonifacio reports consulting fees from Amgen, Pfizer, Bristol-Myers Squibb, and Ariad Pharmaceuticals (Incyte) and research funding from Novartis. M. Brüggemann reports consulting fees from Amgen, Incyte, and Roche and research funding from Affimed and Regeneron. H. Dombret is an advisor for, serves on a speakers’ bureau for, and reports research support, consultancy, honoraria, and travel/accommodation support from Amgen; is an advisor for and reports research support and honoraria from Roche/Genentech; is an advisor for, serves on a speakers’ bureau for, and reports honoraria and travel/accommodation support from Pfizer; is an advisor for, serves on a speakers’ bureau for, and reports research support, honoraria, and travel/accommodation support from Ariad (Incyte); is an advisor for and reports research support and honoraria from Jazz Pharma and Kite Pharma; is an advisor for and reports honoraria from Novartis, Agios, Sunesis, Ambit (Daiichi Sankyo), Karyopharm, Menarini, Astellas, Janssen, Servier, Seattle Genetics, and Cellectis; and is a consultant and advisor for, serves on a speakers’ bureau for, and reports honoraria from Celgene. C.F. serves on an advisory board for and reports research support associated with the present work from Amgen. N.G. serves on an advisory board and speakers’ bureau for and reports research support associated with the present work and travel support from Amgen and serves on an advisory board and speakers’ bureau for and reports travel support from Pfizer. V. Haddad is a former Amgen employee and stockholder. H.-A.H. reports research support associated with the present work from Amgen. D.N. is an employee and stockholder of and reports patent royalties from Amgen. J.S. is an Amgen employee and stockholder. M.S.T. serves on an advisory board for and reports travel support from Amgen, reports travel support from Roche, serves on an advisory board for and reports travel support from Affimed and Regeneron, and serves on an advisory board for Gilead and Jazz Pharma. H.W. is a former Amgen employee and former stockholder. G.Z. is an employee and stockholder of and reports patent royalties from Amgen. The remaining authors declare no competing financial interests.
Corresponding author: Nicola Gökbuget, Universitätsklinikum, Medizinische Klinik II, Hämatologie/Onkologie, Theodor Stern Kai 7, 60590 Frankfurt, Germany; e-mail: goekbuget@em.uni-frankfurt.de.
References
- 1.van der Velden VH, Hochhaus A, Cazzaniga G, Szczepanski T, Gabert J, van Dongen JJ. Detection of minimal residual disease in hematologic malignancies by real-time quantitative PCR: principles, approaches, and laboratory aspects. Leukemia. 2003;17(6):1013-1034. [DOI] [PubMed] [Google Scholar]
- 2.van Dongen JJ, van der Velden VH, Brüggemann M, Orfao A. Minimal residual disease diagnostics in acute lymphoblastic leukemia: need for sensitive, fast, and standardized technologies. Blood. 2015;125(26):3996-4009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bassan R, Spinelli O, Oldani E, et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood. 2009;113(18):4153-4162. [DOI] [PubMed] [Google Scholar]
- 4.Brüggemann M, Raff T, Flohr T, et al. ; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia. Clinical significance of minimal residual disease quantification in adult patients with standard-risk acute lymphoblastic leukemia. Blood. 2006;107(3):1116-1123. [DOI] [PubMed] [Google Scholar]
- 5.Gökbuget N, Kneba M, Raff T, et al. ; German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood. 2012;120(9):1868-1876. [DOI] [PubMed] [Google Scholar]
- 6.Holowiecki J, Krawczyk-Kulis M, Giebel S, et al. Status of minimal residual disease after induction predicts outcome in both standard and high-risk Ph-negative adult acute lymphoblastic leukaemia. The Polish Adult Leukemia Group ALL 4-2002 MRD study. Br J Haematol. 2008;142(2):227-237. [DOI] [PubMed] [Google Scholar]
- 7.Raff T, Gökbuget N, Lüschen S, et al. ; GMALL Study Group. Molecular relapse in adult standard-risk ALL patients detected by prospective MRD monitoring during and after maintenance treatment: data from the GMALL 06/99 and 07/03 trials. Blood. 2007;109(3):910-915. [DOI] [PubMed] [Google Scholar]
- 8.van Dongen JJ, Seriu T, Panzer-Grümayer ER, et al. Prognostic value of minimal residual disease in acute lymphoblastic leukaemia in childhood. Lancet. 1998;352(9142):1731-1738. [DOI] [PubMed] [Google Scholar]
- 9.Borowitz MJ, Devidas M, Hunger SP, et al. ; Children’s Oncology Group. Clinical significance of minimal residual disease in childhood acute lymphoblastic leukemia and its relationship to other prognostic factors: a Children’s Oncology Group study. Blood. 2008;111(12):5477-5485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brüggemann M, Gökbuget N, Kneba M. Acute lymphoblastic leukemia: monitoring minimal residual disease as a therapeutic principle. Semin Oncol. 2012;39(1):47-57. [DOI] [PubMed] [Google Scholar]
- 11.Dhédin N, Huynh A, Maury S, et al. ; GRAALL group. Role of allogeneic stem cell transplantation in adult patients with Ph-negative acute lymphoblastic leukemia. Blood. 2015;125(16):2486-2496, quiz 2586. [DOI] [PubMed] [Google Scholar]
- 12.Van der Velden VH, Corral L, Valsecchi MG, et al. ; Interfant-99 Study Group. Prognostic significance of minimal residual disease in infants with acute lymphoblastic leukemia treated within the Interfant-99 protocol. Leukemia. 2009;23(6):1073-1079. [DOI] [PubMed] [Google Scholar]
- 13.Berry DA, Zhou S, Higley H, et al. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: a meta-analysis. JAMA Oncol. 2017;3(7):e170580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beldjord K, Chevret S, Asnafi V, et al. ; Group for Research on Adult Acute Lymphoblastic Leukemia (GRAALL). Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood. 2014;123(24):3739-3749. [DOI] [PubMed] [Google Scholar]
- 15.Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29(5):532-543. [DOI] [PubMed] [Google Scholar]
- 16.Spinelli O, Peruta B, Tosi M, et al. Clearance of minimal residual disease after allogeneic stem cell transplantation and the prediction of the clinical outcome of adult patients with high-risk acute lymphoblastic leukemia. Haematologica. 2007;92(5):612-618. [DOI] [PubMed] [Google Scholar]
- 17.Schrappe M. Detection and management of minimal residual disease in acute lymphoblastic leukemia. Hematology Am Soc Hematol Educ Program. 2014(1):244-249. [DOI] [PubMed]
- 18.Bargou R, Leo E, Zugmaier G, et al. Tumor regression in cancer patients by very low doses of a T cell-engaging antibody. Science. 2008;321(5891):974-977. [DOI] [PubMed] [Google Scholar]
- 19.Raponi S, De Propris MS, Intoppa S, et al. Flow cytometric study of potential target antigens (CD19, CD20, CD22, CD33) for antibody-based immunotherapy in acute lymphoblastic leukemia: analysis of 552 cases. Leuk Lymphoma. 2011;52(6):1098-1107. [DOI] [PubMed] [Google Scholar]
- 20.Kantarjian H, Stein A, Gökbuget N, et al. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. N Engl J Med. 2017;376(9):836-847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Topp MS, Gökbuget N, Zugmaier G, et al. Long-term follow-up of hematologic relapse-free survival in a phase 2 study of blinatumomab in patients with MRD in B-lineage ALL. Blood. 2012;120(26):5185-5187. [DOI] [PubMed] [Google Scholar]
- 22.Topp MS, Kufer P, Gökbuget N, et al. Targeted therapy with the T-cell-engaging antibody blinatumomab of chemotherapy-refractory minimal residual disease in B-lineage acute lymphoblastic leukemia patients results in high response rate and prolonged leukemia-free survival. J Clin Oncol. 2011;29(18):2493-2498. [DOI] [PubMed] [Google Scholar]
- 23.Brüggemann M, Schrauder A, Raff T, et al. ; International Berlin-Frankfurt-Münster Study Group (I-BFM-SG). Standardized MRD quantification in European ALL trials: proceedings of the Second International Symposium on MRD assessment in Kiel, Germany, 18-20 September 2008. Leukemia. 2010;24(3):521-535. [DOI] [PubMed] [Google Scholar]
- 24.Topp MS, Gökbuget N, Stein AS, et al. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2015;16(1):57-66. [DOI] [PubMed] [Google Scholar]
- 25.Giebel S, Stella-Holowiecka B, Krawczyk-Kulis M, et al. ; Study Group for Adult ALL of the European Leukemia Net. Status of minimal residual disease determines outcome of autologous hematopoietic SCT in adult ALL. Bone Marrow Transplant. 2010;45(6):1095-1101. [DOI] [PubMed] [Google Scholar]
- 26.Choi S, Henderson MJ, Kwan E, et al. Relapse in children with acute lymphoblastic leukemia involving selection of a preexisting drug-resistant subclone. Blood. 2007;110(2):632-639. [DOI] [PubMed] [Google Scholar]
- 27.Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med. 2014;6(224):224ra25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kranick S, Phan G, Kochenderfer JN, Rosenberg SA, Nath A. Aphasia as a complication of CD19-targeted chimeric antigen receptor immunotherapy. Neurology. 2014;82(10 suppl 1):S52.006. [Google Scholar]
- 29.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371(16):1507-1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Parizel PM, Snoeck HW, van den Hauwe L, et al. Cerebral complications of murine monoclonal CD3 antibody (OKT3): CT and MR findings. AJNR Am J Neuroradiol. 1997;18(10):1935-1938. [PMC free article] [PubMed] [Google Scholar]
- 31.Pavlů J, Labopin M, Zoellner AK, et al. Allogeneic hematopoietic cell transplantation for primary refractory acute lymphoblastic leukemia: a report from the Acute Leukemia Working Party of the EBMT. Cancer. 2017;123(11):1965-1970. [DOI] [PubMed] [Google Scholar]
- 32.Marks DI, Wang T, Pérez WS, et al. The outcome of full-intensity and reduced-intensity conditioning matched sibling or unrelated donor transplantation in adults with Philadelphia chromosome-negative acute lymphoblastic leukemia in first and second complete remission. Blood. 2010;116(3):366-374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goldstone AH, Richards SM, Lazarus HM, et al. In adults with standard-risk acute lymphoblastic leukemia, the greatest benefit is achieved from a matched sibling allogeneic transplantation in first complete remission, and an autologous transplantation is less effective than conventional consolidation/maintenance chemotherapy in all patients: final results of the International ALL Trial (MRC UKALL XII/ECOG E2993). Blood. 2008;111(4):1827-1833. [DOI] [PubMed] [Google Scholar]
- 34.Wolach O, Stevenson KE, Wadleigh M, et al. Allogeneic transplantation is not superior to chemotherapy in most patients over 40 years of age with Philadelphia-negative acute lymphoblastic leukemia in first remission. Am J Hematol. 2016;91(8):793-799. [DOI] [PubMed] [Google Scholar]
- 35.Mohty M, Labopin M, Volin L, et al. ; Acute Leukemia Working Party of EBMT. Reduced-intensity versus conventional myeloablative conditioning allogeneic stem cell transplantation for patients with acute lymphoblastic leukemia: a retrospective study from the European Group for Blood and Marrow Transplantation. Blood. 2010;116(22):4439-4443. [DOI] [PubMed] [Google Scholar]
- 36.Paietta E. Minimal residual disease in acute myeloid leukemia: coming of age. Hematology Am Soc Hematol Educ Program. 2012;2012:35-42. [DOI] [PubMed] [Google Scholar]
- 37.Radich JP. How I monitor residual disease in chronic myeloid leukemia. Blood. 2009;114(16):3376-3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.