Abstract
Fertility awareness constitutes fundamental knowledge for every woman and is an important tool for health professionals. The objective of this review is to show how fertility awareness can be useful in the assessment of a woman's health. The main techniques for detecting ovulation are explained, and then the events that characterize a normal menstrual cycle are discussed. The relevance of cervical mucus from the perspective of female fertility is highlighted. Finally, the usefulness of fertility awareness 1) to identify fertile and infertile periods, 2) to help to detect several pathologies, and 3) in regards to how it exerts an important role in the success of programs in education for affectivity and sexuality are discussed.
I. Introduction
Menstruation is the most noticeable event during the female reproductive cycle; however, the most important event is ovulation. During a woman's life, the ovary passes through different stages with respect to its ovulatory and hormone secreting capacity. The concept of the ovarian cycle as a continuum–-known as the ovarian continuum1–-considers the different types of ovarian activity observed during a woman's reproductive life as normal responses to various environmental conditions, with the function of ensuring the mother and child's health in case of conception.2 The reproductive life of a female begins in the embryonic period: about two months after fertilization has occurred, the primordial germ cells, the future oogonia, leave the embryo and migrate to the yolk sac, thus avoiding the process of embryonic cell differentiation. Approximately four weeks later, the primordial germ cells migrate to the gonadal ridge, the region of the future ovary, settle and are surrounded by somatic cells to begin their process of differentiation, forming millions of rudimentary ovarian follicles. At this stage, about seven million primordial follicles are formed, most of which will suffer atresia (a degenerative process), reducing this number. When a girl is born, she will have between one and two million oocyte-containing follicles, only four to five hundred of which will complete folliculogenesis during ovulation.3
From a reproductive point of view, female puberty can be considered as the process leading to the hormonal changes that will enable mature oocytes to leave the ovary, making fertilization possible.4 In girls, puberty generally starts at age eight to ten, and ends with menarche, at age twelve to thirteen.5 It is known that the fundamental event leading to puberty is the activation of the hypothalamic-hypophyseal-gonadal axis when the hypothalamus starts its pulsatile release of gonadotrophin-releasing hormone (GnRH), subsequently stimulating release of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) by the adenohypophysis, which will exert their effects at the gonadal level, producing an increase in the biosynthesis of sex steroid hormones, among other changes.6 The complete endocrine mechanism underlying the activation of the hypothalamic-hypophyseal-gonadal axis is yet to be elucidated, but it has been proposed that it could be due to the rising levels of growth hormone, leptin, and kisspeptin,7 among other hormones. One of the main consequences of puberty at the reproductive level is gonadarche, evidenced by the activation of the ovulatory mechanism. Thus, for fertilization to be possible, ovulation has to take place first, and usually follows when LH reaches its peak value as a consequence of several highly synchronized and coordinated physiological events during the reproductive cycle.
During the first two years following menarche, occasional anovulatory cycles can occur. From then on, a healthy woman normally will have regular ovulations, characterized by twenty-four to thirty-six day cycles,8 but occasional infertile variants can occur.9 Ovulatory cycles are normally interrupted only by stages of pregnancy and lactation, at the end of which normal ovulatory activity and fertility are reestablished. Abnormalities in ovarian activity, such as short cycles, anovulation, or short luteal phases, take place close to menopause, or can also be due to endocrine-metabolic disorders and excessive physical activity,10 among other conditions.
Fertility awareness is the capacity of the woman to identify the occurrence or absence of ovulation. The presence of ovulation indicates that the woman is in good health, and thus the objective of this review is to show how fertility awareness is useful when assessing female health. This knowledge makes it possible to identify the fertile and infertile periods of a woman, gives information about the possible pathologies underlying reproductive disorders and proves to be very useful as part of the curriculum of programs in education for affectivity and sexuality.
II. Ovulation Monitoring during the Menstrual Cycle
The main aspect about a woman's fertility awareness that should be considered is the accurate monitoring of ovulation, in the understanding that this constitutes the main event in the menstrual cycle. The presence of ovulation in women can be understood as a sign of health; and, as such, every woman should be able to recognize it. For many people, being able to predict ovulation to achieve or avoid getting pregnant is also of great importance. Therefore, a method to evaluate whether a cycle is adequate on the basis of a given woman's condition, and if such a cycle is potentially fertile or not, is of utmost importance. It is known that normal menstrual cycles, as for several biological phenomena, show some degree of normal recurrent variability.11 For example, depending on the method of assessment, the length of a normal cycle may fluctuate between twenty-four and thirty-six days.12 Menstrual cycles also show considerable variability regarding fertility from one cycle to the next for a given woman, and also among women. Brown showed that menstrual cycles can be classified into six main categories, which follow a certain order during the development of fertility at the onset of her reproductive life13: 1) cycles with no ovarian activity (i.e., amenorrhea), 2) anovulatory cycles with fluctuating estrogen levels, 3) cycles with anovulatory ovarian activity with constantly increased estrogen levels, 4) cycles with a luteinized unruptured follicle, 5) cycles with ovulation followed by a deficient luteal phase, and 6) ovulatory cycles with adequate luteal phases, i.e., fertile.14 This constitutes a continuous progression encompassing almost all the types of cycles (i.e., the ovarian continuum), of which only the last stage, the fertile ovulatory cycle, can eventually lead to a viable pregnancy. Considering that these varying cycles can take place at any moment and in any cycle, a satisfactory understanding of the continuum requires cycle monitoring methods based on daily assessments.
There are several ways to monitor human fertility, among which the following are the major ones:
-
1.
Ultrasound, which allows visualization of the structures of the ovary and ovulation, and is of great usefulness in the monitoring of the changes undergone by the endometrium as a consequence of hormonal variations;
-
2.
Hormone tests;
-
3.
The signs and symptoms evidenced by the cervical mucus, as assessed by the Billings Ovulation Method15;
-
4.
Basal body temperature (BBT); and
-
5.
Pregnancy.
All these indicators are interrelated and reflect the action of the sex steroid hormones, estradiol, and progesterone.
FSH levels increase at the beginning of the cycle until a new cohort of follicles is recruited; when one of the follicles reaches a given level of maturity, it starts producing estradiol.16 The daily increasing levels of estradiol then suppress the levels of FSH and, eventually, trigger a large LH increase leading to ovulation. After ovulation, the developing corpus luteum starts producing large amounts of progesterone, which together with the high estradiol levels suppress further dominant follicle development until luteal cell apoptosis, a fact that constitutes the end of the cycle and the beginning of a new cycle. The serum levels of estradiol and progesterone, or their metabolites in urine, estrone glucuronide and pregnanediol glucuronide respectively, constitute the primary indicators of reproductive activity. On the other hand, cervical mucus characteristics and BBT are secondary symptoms, being controlled by the fluctuating levels of ovarian sex steroids.17
In order to provide point-of-care testing of fertility, the availability of a non-invasive and, at the same time, simple method to obtain real time information about ovarian activity is crucial; in addition, it must be thorough, accurate, and inexpensive. In this regard, the best two ways to monitor ovarian activity are ultrasound, which provides structural information, and hormone assays, which give functional information. Blood tests clearly do not constitute a viable alternative for daily monitoring, and thus urine analyses are more suited for clinical practice.18
Among the techniques that have been used for urinary assessment of ovarian hormones, the Ovarian Monitor, designed by Dr. James B. Brown (1919–2009), has been of great help to understand ovarian activity. This device has made it possible to monitor the daily production of estrone glucuronide and pregnanediol glucuronide in thousands of cycles of women of different characteristics. Moreover, it has established an association between the occurrence of these changes and the perception by the woman of dampness or dryness of cervical mucus in the vulvar area.19 The monitor is not currently available following the death of Dr. Brown, principal of the manufacturing company. However, the latter company is being restructured and the Ovarian Monitor soon should be in stock once more.
The methodology for the Ovarian Monitor has been widely described.20 The monitor measures estrone glucuronide and pregnanediol glucuronide levels in urine. The presence of a growing follicle in the ovary is distinctively proven by an increase in basal estrone glucuronide excretion, the growth rate of a follicle is parallel to an increasing level of estrone glucuronide, and ovulation is evidenced by increasing levels of pregnanediol glucuronide to equal or exceed a given defined level (or threshold).21
A study on the effective implementation of the Ovarian Monitor was published in 199122 and later corroborated by a World Health Organization initiative.23 These studies showed that the results obtained with the monitor are as accurate as those obtained in control laboratory tests and that women can obtain reliable results working at home.24 The monitor has been used to predict ovulation prospectively based on the first estrone glucuronide excretion increase25 to determine its exact time of occurrence by recognizing the ovulatory estrone glucuronide peak, and to confirm it with an associated pregnanediol glucuronide rise of up to 7 μmol per twenty-four hours26 and about 13.5 μmol per twenty-four hours within the six days following the estrone glucuronide peak.27 The data obtained with the monitor have also been correlated with the symptoms perceived by the woman, e.g., changes in the cervical mucus, as done when using the Billings Ovulation Method.28
Between the beginning of the estrone glucuronide increase and the ovulatory estrone glucuronide peak, there is normally a four to five day interval (sufficient to allow for spermatozoa survival in cervical mucus, which averages about three to four days). Since ovulation normally occurs in the twenty-six to forty-eight hour interval after the estrone glucuronide peak day, this gives five to six days’ warning of ovulation. The mucus symptoms are generally a response to the initial estradiol rise marking the beginning of the fertile window in the woman. The two hormonal markers give an average fertile period of about seven days.29
Observing the length of the menstrual cycle or the duration of the luteal phase is not enough. Figure 1 shows a cycle as determined by the Ovarian Monitor. The cycle has a length of thirty-two days and is considered to be normal as confirmed by the hormone profile. The key elements of a normal cycle identified by the daily monitor results are as follows:
Figure 1.
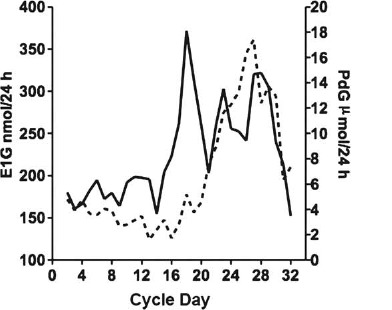
Normal menstrual cycle with a length of thirty-two days E1G: estrone glucuronide (solid line), PdG: pregnanediol glucuronide (broken line).
-
1.
The day when estrone glucuronide starts rising toward the hormone peak (day 15 of the cycle shown in figure 1).
-
2.
The day of estrone glucuronide excretion peak; this and the following day are the most fertile in the cycle (day 18 as shown in figure 1). Therefore, the pre-ovulatory fertile period comprises four to five days warning of impending ovulation.
-
3.
The first pregnanediol glucuronide increase on the day of the estrone glucuronide peak (day 18 in figure 1) or close to it, constituting a good sign of ovulation.
-
4.
The day when the pregnanediol glucuronide excretion rate reaches or exceeds 7 μmol per twenty-four hours–-i.e., the pregnanediol glucuronide threshold–-signaling the end of the fertile window (day 21 in the cycle in figure 1). Thus, the fertile window in this cycle includes days 15 to 20 (six days).
-
5.
The level of pregnanediol glucuronide excretion rate six days following the estrone glucuronide peak, 12.2 μmol per twenty-four hours. This should ideally reach 13.5 μmol per twenty-four hours for a fertile cycle30; even if the value is somewhat lower, this cycle is probably fertile, considering statistical variability.
-
6.
The maximum pregnanediol glucuronide value during the luteal phase (17 μmol per twenty-four hours), considering a normal luteal phase when the value is higher than 13.5 μmol per twenty-four hours.31
-
7.
The length of the luteal phase, fourteen days in figure 1, is considered normal.
Daily monitoring of estrone glucuronide levels is also of great importance to identify the midcycle peak as a reference point to begin monitoring pregnanediol glucuronide levels for six days. It has been shown that the peak mucus day takes place on average one day after the observed estrone glucuronide peak, therefore the peak day of cervical mucus can be used as an alternative and simpler marker.
In summary, daily monitoring of estrone glucuronide and pregnanediol glucuronide levels helps in the determination of the fertile phase of the cycle and provides women and health professionals with important prospective information regarding the normality of the cycles.
III. Events in the Normal Ovulatory Cycle
In order to understand female fertility regarding both physiological and pathophysiological aspects, it is necessary to identify each one of the sequential steps that occur in the menstrual cycle.32 The key events in the menstrual cycle are as follows:
-
1.
Increase in FSH levels, an event triggering follicle recruitment.33
-
2.
Development of the recruited follicles, leading to an increase in estradiol levels.34
-
3.
Selection of recruited follicles. These follicles produce increasing levels of estradiol, causing the proliferation of the endometrium and an increase in the amount of mucus secreted by the cervix, as well as a change in the type of secretion and the cervical os size.35
-
4.
Estrogens together with inhibin exert a negative feedback effect at the level of the hypothalamic-hypophyseal axis, thus decreasing FSH levels.36
-
5.
Dominance of one of the selected follicles is produced.37
-
6.
Estrogens, secreted in increasing levels by the dominant follicle, exert a change in the feedback system over the hypothalamichypophyseal axis, shifting from a negative to a positive system.38
-
7.
The positive feedback exerted by estrogens over the hypothalamic-hypophyseal axis causes an LH peak. This pre-ovulatory LH rise starts the process of follicle luteinization, and triggers ovulation. The oocyte is released from the follicle since its walls are degraded by prostaglandins (the life of the oocyte is limited, from twelve to twenty-four hours).39
-
8.
Parallel to follicle luteinization, follicular cells start secreting progesterone. This initial rise in progesterone maintains the plateau of LH during the peak of the hormone.40
-
9.
The LH release pattern helps in the formation of the corpus luteum, which promotes the later development and support of an adequate luteal phase.41
-
10.
The corpus luteum produces progesterone and also estrogens.42
-
11.
Progesterone modifies the endometrium lining from proliferative to secretory. This hormone also affects cervical secretion, which is no longer estrogenic but progestational, and thus not appropriate for migration of spermatozoa through the cervix. If fertilization has not taken place, after about six days the corpus luteum will undergo regression, with an approximate duration of eleven to sixteen days.43
-
12.
Estrogen and progesterone concentrations fall back to the levels observed in the early follicular phase approximately fourteen days following the initial formation of the corpus luteum.44
-
13.
This decrease in ovarian steroid concentration eliminates the endocrine suppression exerted over FSH and LH, triggering a new reproductive cycle.45
-
14.
Throughout the cycle the hypothalamus continues producing GnRH, secreting it in a continuous pulsatile pattern.46
Each of these sequential steps integrating the ovarian continuum can undergo substantial variations throughout a woman's reproductive life based on endocrine alterations, as has been documented in studies performed with the Ovarian Monitor.47 Ovulatory cycles can be interrupted by circumstances such as pregnancy and lactation.48 After these stages, the woman reestablishes her normal ovulatory level and, therefore, fertility. However, cycles can also be altered by other causes, such as psychological stress, excessive physical exercise, obesity, and sudden changes in body weight. These factors affect the ovarian cycle, leading to ovulatory dysfunction, which can become chronic and require medical treatment.
An example of a different ovarian continuum patterns occurs when the estrogen levels remain high over a long period, with values similar to those in the pre-ovulatory rise. In this case LH could be released, though not enough to cause the rupture of the follicle walls and thus ovulation. However, the secreted LH levels would be sufficient to initiate some degree of follicle luteinization, decreasing the production of progesterone to a lower level than that of the normal luteal phase.49 In this case, ultrasound will show an unruptured follicle.
IV. Cervical Mucus and Its Relevance for Female Fertility
Cervical mucus is a heterogeneous mixture of water, electrolytes, plasma, locally derived proteins, and mucous glycoproteins (mucins), among other compounds.50 Produced by the cervical secreting cells, mucus is considered a hydrogel due to its high water content (90 to 98 percent).51 During the menstrual cycle, two categories of cervical secretion can be identified: 1) estrogenic mucus (E), with a translucent appearance, which has higher elasticity and predominates in the periovulatory period, and 2) progestational mucus (gestagenic or G), with an opaque and sticky appearance, predominant in the luteal phase. Mucus E is divided into three types: mucus S, L, and P, each with a different function, and distinctive features regarding receptivity to spermatozoa.52
Even though the three types are secreted, one predominates over the others in the periovulatory period. The following is a detailed description of the types of E mucus:
-
•
Type S Mucus: Its function would be to transport the spermatozoa to the S-mucus secreting crypts located in the cervical canal towards the site of fertilization. This mucus is very fluid and spermatozoa can migrate quickly through it, reaching the crypts in a three to ten minute period.53 The pattern of crystallization of S mucus is arranged in fine parallel lines (figure 2A).
-
•
Type L Mucus: This mucus has medium viscosity and spermatozoa travel through it more slowly; the mucus would act as a filter, holding spermatozoa with morphological alterations.54 The pattern of crystallization of mucus L is characterized by its shape similar to fern fronds or palm leaves. A main axis is observed, from which branchings protrude to both sides in 90° angles (figure 2B).
-
•
Type P Mucus: This type is similar to type L since it also has a pattern of crystallization resembling fern fronds. It is more abundant on peak day, when the woman's feeling of lubrication reaches a high at the vulvar level. P mucus has five subtypes: Pt, Pa, P2, P4, and P6B. Pt mucus is characterized by crystallization formed by branches in a trigonal planar-like arrangement. Pa crystallization has a central nucleus of crystallization from which multiple branchings protrude in all directions. P2 mucus shows the closest resemblance to a fern in its crystallization pattern (figure 2C). A central axis is observed, with branches to either side, which are generally curved and gradually decreasing in length.55 Finally, P6B mucus crystallization is characterized by a distinctive stellate geometry: from the central crystallization nucleus, six well-defined axes protrude, forming a 60° angle with each other (figure 2D). This type of mucus would be frequent on the day of highest fertility.56
Figure 2.
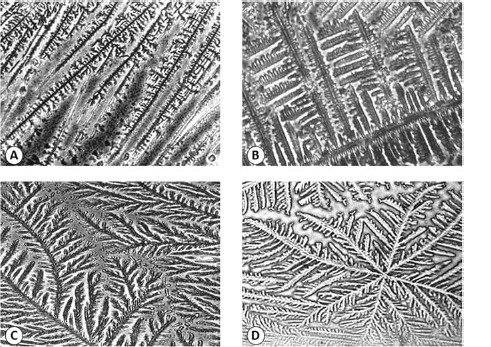
Some crystallization patterns of human periovulatory cervical mucus observed under light microscopy
A. Type S mucus crystallization: The crystals are arranged together forming rows (400×). B. Type L mucus crystallization: This crystalline pattern has a structure with a straight main axis and branchings protruding at a 90° angle from which smaller branches can originate, again at a 90° angle (400×). C. Subtype P2 crystallization: Characterized by an evident fern-like morphology. It consists of a well-defined main axis, from which branchings protrude to both sides, forming 60° angles with respect to the main axis (200×). D. Subtype P6B crystallization: Has a star-like geometry, with a central nucleus from which six well-defined axes protrude at a 60 ° angle with each other. From each of these axes, variable length branchings originate (200×).
According to Odeblad's schema, spermatozoa migrate to the cervical crypts specifically through S and P6B mucus, either to be stored57 or to migrate directly to the uterine cavity.58
It is worth noting that one of the main differences in the composition of S, L, and G mucus is based on their percentage of water content, being 98 percent for S, 95 percent for L, and 90 percent for G mucus.59 Differences in composition as well as the existence of different crystallization patterns for cervical mucus are mainly due to the heterogeneous nature of cervical mucus, which is composed by several types of cervical secretion produced in specific regions of the cervix. This heterogeneity is evidenced in the morphological differences observed in its crystallization.60
The ultrastructure of estrogenic mucus can be determined through scanning electron microscopy. M. Elstein and B. Daunter reported the presence of canals 30 μm in diameter through which spermatozoa, whose heads are approximately 5 μm in diameter, could easily migrate.61 Other scanning electron microscopy studies have found areas with a linear arrangement of estrogenic mucus fibers that allow spermatozoa migration, and also other areas resembling a filament mesh with multiple pores (figure 3A).62 The changes undergone by the ultrastructure as well as the biophysical properties of mucus along the cycle are mainly due to variations in sex steroid hormones, which affect mucus hydration and the expression of the glycoproteins (mucins) that comprise this hydrogel, among other features.63
Figure 3.
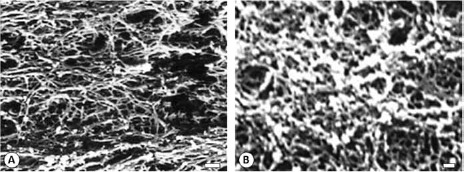
Scanning electron microscopy micrographs of human cervical mucus A. A network-shaped mesh of periovulatory (estrogenic) mucus. In this mesh there are a large number of variable size pores, scale bar = 10 μm. B. A compact network-shaped mesh of progestational (gestagenic) mucus, scale bar = 1 μm.
Mucins are mainly responsible for the rheological properties that characterize this hydrogel.64 To date, more than twenty mucin-encoding genes have been identified, and at least thirteen of them are expressed in the female genital tract.65 Of the many mucins, those found in mucus are gel-forming secreting mucins. It has been proposed that the characteristic structure of mucus, in which mucins exert an important role, appear when these glycoproteins form something like a mesh of interconnected molecules. Mucin 5B is the main gel-forming mucin expressed by the endocervical epithelium, and reaches its peak in midcycle.66 Similarly, there is evidence of other mucins, which are expressed in the cervix during the ovulatory phase, such as mucin 4.67
The amount of cervical mucus secreted during the cycle also varies on the basis of changes in ovarian sex hormones. During follicular development, when estradiol plasma levels reach average levels of 107 pg/mL, and the follicle is about eight to ten millimeters in diameter (about six to seven days prior to ovulation), cervical mucus secretion starts to increase until it reaches its maximum production of 500 mg/day in the periovulatory period68 After ovulation, there is an increase in progesterone, and estradiol plasma levels fluctuate between 130 and 200 pg/mL.69 The amount of mucus secreted by the endocervix decreases to 50 mg/day,70 and the mucus becomes opaque and more prone to breaking when stretched despite the high levels of estradiol. This is due to the antiestrogenic action of progesterone on mucus features.71
In addition, at the cervix, progesterone induces release of cathepsins from leucocytes and increases the action of sialyltransferase,72 favoring the appearance of a dense mesh formed by the mucin molecules that make up the cervical secretion. The mucus secreted during the luteal phase (G mucus) has a much lower capacity to crystallize, and its ultrastructure presents pores with a diameter of 3 to 5 μm, rendering it impenetrable for spermatozoa (figure 3B).73
Given the aforementioned features, mucus is expected to exert a series of very important biological functions. The main one is spermatozoa transport. Mucus constitutes the first barrier spermatozoa must go through in their journey toward the site of fertilization and serves as a selective barrier, allowing preferential passage of morphologically normal spermatozoa only and for only six to seven days of the menstrual cycle.74 Morphologically normal spermatozoa, which survive in cervical mucus, are known to keep their acrosome intact and probably their capacity to fertilize the oocyte.75 This is partly due to the high content of estradiol in periovulatory cervical mucus, since this hormone can delay the premature onset of the acrosome reaction.76 In addition, in midcycle the number of mucins expressed in the cervical canal increases, and being highly hydrophilic in character, these glycoproteins cause the mucus to acquire a high water retention capacity, hence facilitating the migration of spermatozoa and maintaining the moist and lubricated condition of the cervix. Finally, pathogenic agents and several noxious agents could be trapped by mucins, preventing their advance into the uterus and Fallopian tubes.77 Additionally, it has been shown that cervical mucus possesses antimicrobial activity.78
V. Usefulness of Fertility Awareness
The fluctuations in ovarian sex steroids during the menstrual cycle produce a characteristic pattern of mucus secretion, which can be observed and charted by the woman. Hence, this will enable her to recognize her fertile and infertile periods, to identify her ovarian response, and to identify ovulation. A woman who knows how to recognize her normal pattern of fertility will be able to identify a number of ovulatory and/or gynecological dysfunctions that might occur during her life.79 Being able to recognize when menstrual or mucus secretion pattern irregularities should be considered normal is relevant. Three or more cycles in a year with altered ovulation, as identified by alterations in the luteal phase, constitute a sign of abnormality in a woman. Such irregularity can be evidenced by a shorter luteal phase, or its absence in case of anovulation. Long or short cycles compared to the woman's average cycle length are also to be considered abnormal. The causes of menstrual irregularities and/or variations in the features of cervical secretion may be due to obstetric problems, endocrine dysfunctions (hypothalamichypophyseal, thyroid, adrenal, and ovarian disturbances), metabolic disorders, genitourinary tract infections, neoplasia, and iatrogenic causes, among others.80 Regarding endocrine dysfunctions, it is worth noting that there is no specific pattern of ovarian activity associated to each one of these. For this reason, each one has to be considered and discarded when making a differential diagnosis of menstrual irregularity in a woman.81 In order to do so definitively, a hormone assessment considering all the aforementioned conditions must be obtained. Women with persistent ovulatory dysfunctions associated with irregular cycles and abnormal cervical mucus patterns generally do not spontaneously regain normal cyclical activity without appropriate medical treatment. It has been shown that in the absence of an adequate diagnosis and treatment, menstrual disorders become more serious over time and, therefore, the underlying pathology worsens as well.
Endocrine and metabolic disorders are the most frequent cause of ovulatory dysfunction, which constitutes the most commonly diagnosed problem and is predominantly linked to menstrual irregularity.82Ovulatory dysfunction can be identified by means of fertility awareness by determining the presence or absence of fertility signs given by cervical mucus at the vulva, and can be characterized by abnormal ovulatory activity or by the lack of such activity. Abnormal ovulatory activity can be recognized by cycles with a short luteal phase (less than nine days in length) or with pregnanediol glucuronide levels below the fertility threshold,83 if home monitoring is available. It is also important to consider that young nulliparous women with regular cycles (between twenty-four and thirty-six days) can have an occasional ovulatory dysfunction event identifiable by means of their fertility awareness record.84
According to their origin, these endocrine disturbances can be classified into hypothalamic-hypophyseal, thyroid, or glucose metabolism disorders (including insulin resistance), and adrenal and/or ovarian alterations.
Hypothalamic disorders, e.g., anorexia nervosa, are characterized by hypoestrogenic cycles, with persistence of dry days. Persistent dryness is frequently associated with the low estradiol levels observed in these patients, who can also present with amenorrhea. This type of cycle is also observable among athletes,85 although this can be considered part of the ovarian continuum. In this case, regular ovarian activity is frequently regained in a three-month period when exercise becomes less exhausting. However, some of the young women in this category could also develop anorexia, and despite discontinuing physical exercise, fail to return to a normal ovarian cycle.86
Hypophyseal disorders, such as hyperprolactinemia, are characterized by amenorrhea, short cycles, short luteal phases with presence of intermenstrual spotting, and galactorrhea. In vitro studies have shown that there is an alteration in the steroidogenic activity of follicular cells when they are exposed to prolactin,87 a fact which could at least partially explain the abnormal luteal phases commonly observed in the record charts of women suffering from hyperprolactinemia.88 In these women, the interaction between the nervous, endocrine, and immune systems must always be taken into consideration, since they frequently present alterations such as allergies and warts, as well as a tendency to suffer from infections, such as human papillomavirus (HPV). The latter would be due to the presence of an alteration in the lymphocyte populations of such women.89 Stress is also worth taking into account as an important factor, which could relate to the high levels of prolactin.90
Adrenal and/or ovarian disorders are frequently associated with ovulatory dysfunction. Worldwide, the most prevalent of such disorders among reproductive age women is the polycystic ovary syndrome (PCOS), defined as an ovulatory dysfunction caused by hyperandrogenism and often associated with insulin resistance.91 The Androgen Excess and PCOS Society Task Force recommend the following diagnostic criteria of PCOS: presence of hyperandrogenism (clinical and/or biochemical), ovarian dysfunction (oligo-anovulation and/or polycystic ovaries), and the exclusion of related disorders. However, forms of PCOS may exist without overt evidence of hyperandrogenism.92 The menstrual cycles of women suffering from PCOS can be ovulatory (these women may have multiple periods of follicular development before the ultimately ovulatory follicle appears) or anovulatory. Current PCOS diagnostic criteria do not include insulin resistance, even though there is a growing consensus in the scientific community that hyperandrogenism and insulin resistance (and its co-morbidities) add both their effects and their risks in PCOS women. Studies carried out in the mid-nineties concluded that PCOS comprises two subpopulations: one with no insulin-resistance (i.e., insulin sensitive) and another resistant to insulin with different etiologies. These two subpopulations differ in their anthropometric and endocrine characteristics.93 More recently, the insulin-suppression test has shown that insulin sensitivity in PCOS women describes a discontinuous statistical distribution, implying that PCOS constitutes a heterogeneous disorder as regards insulin resistance.94 Thus, in these PCOS women, three subpopulations have been determined in relation to insulin sensitivity: the first corresponds to women with normal insulin sensitivity; the second group possesses a moderate alteration in their insulin sensitivity; i.e., a mild insulin resistance. Finally, the third subpopulation presents an evident alteration in the effects exerted by insulin on glucose homeostasis, with a severe insulin resistance.95 This last group would present the highest prevalence of co-morbidities such as obesity, dyslipidemia, heart disease, and, finally, the metabolic syndrome. Considering the last two subpopulations (those which present impaired insulin sensitivity), about 52 percent of the women studied suffering from PCOS presented with insulin resistance.96
It is known that the presence of PCOS may alter the characteristics of cervical mucus.97 PCOS cycles can be characterized by a hyperestrogenic state, identifiable with a pattern of continuously fertile mucus or the presence of fertile mucus patches on different days of the cycle.98 Compared with normal cycle women, PCOS women present alterations in the crystallization patterns of cervical mucus, which evidence a less orderly arrangement.99 In addition, scanning electron microscopy studies have revealed that in these women, cervical mucus presents ultrastructural alterations when compared to the mucus of women with normal menstrual cycles. PCOS women present a decrease in the symmetry in the glycoprotein filament mesh and a smaller average pore diameter.100 Taking into consideration that pore diameter is associated with spermatozoa ascent, the aforementioned changes which would partly explain the infertility observed in some PCOS women.
Appropriate management of PCOS women must include diet, exercise, and pharmacological treatment so that they regain normal ovulatory activity. Women who know how to recognize variations in their cervical mucus will be able to identify such abnormalities and also improvements in the treatment as regards recovery of their ovulatory function.
Thyroid disorders (hypo- and hyperthyroidism) constitute a less frequent cause of ovarian dysfunction, and are present in about 2 percent of the women with menstrual irregularities. Different patterns of ovarian dysfunction can be recognized among patients with thyroid disorders,101 menorrhagia being a frequent sign in women with these alterations.
Conditions such as premature ovarian failure can also constitute a cause of fertility alterations and are evidenced as irregular mucus patterns in response to fluctuating estrogen levels. With the progress of this condition, absence of fertile mucus prevails due to anovulation. In the perimenopausal period, there are also fluctuating estradiol and progesterone hormone patterns, with corresponding effects on mucus secretion patterns.102
Inflammatory processes constitute the second most frequent cause of fertility disorders, predominantly originating in sexually transmitted infections.103 Some pathogens causing sexually transmitted infections possess enzymes able to degrade the mucins that constitute cervical mucus. Such enzymes alter the beneficial cohabitation between hosts, such as Lactobacillus sp., which uses glycogen as an energy source and contributes to normal mucin recycling by producing the enzymes that degrade them, such as sialidase. Mucin molecules would be partly or totally degraded by microbial enzymes. As aforementioned, mucins are responsible for the rheological properties determining mucus amount, elasticity, and viscosity; therefore, these properties will change in response to the enzymes produced by microbial organisms present in the female genital tract.104 A woman who is aware of her mucus pattern will be able to recognize a genital infection early. The characteristics of the aqueous mucus pattern of a woman will depend partly on the pathogenic agent. In general, in the presence of infection or cervicitis, a mucus aqueous pattern with ovulatory features can be identified, but constantly associated with a yellowish secretion, and this mucus is less elastic and occasionally purulent.105 Fungal infections are generally associated with pruritus and a whitish, lumpy discharge. Bacterial infections, such as those caused by Gardnerella vaginalis, tend to occur associated with a strong smell. Parasitic cervicitis, such as that caused by Trichomonas vaginalis, is also linked to a strong odor and pruritus.106 In these cases, it is important to grow a microbiological culture and not base specific diagnosis on symptoms only. It is worth noting that pathogens often associate with other pathogens. For example, a mycosis may occur together with bacterial or parasitic infection. Another important cause of alteration in mucus secretion patterns, associated with variable degrees of pelvic pain, is due to Chlamydia trachomatis infections.107 It has been shown that these present at a 13 percent incidence in infertile couples, and are often associated with Fallopian tube pathologies.108 This infection can be asymptomatic or present a purulent secretion. Diagnosis and due treatment of C. trachomatis infection prevents fertility problems. These infections, which have been shown to lead to pelvic inflammatory processes, are also associated with spontaneous abortions.109 Previous use of intrauterine devices should lead to clinical suspicion of infection. HPV must also be considered among infectious agents. The space between mucin fibers is known to be large enough for small microorganisms, such as this virus, to go through the cervical mucus. HPV is a known cause for colonization of the cervical epithelium, sometimes associated with a continuous watery secretion. Oncogenic HPV types 16 and 18 as well as others have been identified as causative of carcinoma of the cervix.110 Self-recognition on the part of the woman of changes in her secretion patterns has been of help in the early diagnosis of this pathology. It is important to remember that the man has to undergo treatment in all the previously mentioned cases as well.111
Alterations in the ovarian cycle can also be of iatrogenic origins, mainly those caused by oral contraceptive pills (OCP) or different types of hormonal treatment. On suspending OCP treatment, the woman may present with short luteal phase cycles, absence of a well-defined mucus cycle (indicator of anovulation), and/or decrease in mucus secretion due to the changes undergone by the cervical epithelium as a consequence of the hormone therapy.112 A decrease in menstrual flow can also be observed. Menstrual cycle alterations frequently occur in women following OCP discontinuation. It has been shown that, compared to mechanical contraceptives, users of OCP present lower percentages of pregnancy during the first three months after interrupting intake. It has been found that pregnancy percentages are equal only after ten months following OCP discontinuation.113
Finally, it is worth noting that even though mucus features are a guide to the diagnosis of a health problem, in most cases additional information is required to confirm the diagnosis and to suggest a regime of treatment.
VI. Concluding Remarks
Fertility awareness is a valuable tool that allows the woman to recognize her health status.114 This knowledge makes it possible to detect disorders that may include the presence of conditions such as endocrine and metabolic disturbances, anatomical alterations, inflammatory pelvic diseases, or even certain neoplasias. Recording her fertility patterns provides the woman with highly relevant information about her ovarian function. Ovulatory dysfunction in a grown woman could have its origin in adolescence, and these conditions are not self-limited but deteriorate with adulthood.115 It has been shown that peri-menarcheal girls from a variety of ethnic and socioeconomic groups are able to learn how to recognize their cervical mucus patterns and to distinguish ovulatory from non-ovulatory cycles.116 This suggests that if young women were taught to keep a record of their fertility and infertility signals, they would be able to detect ovarian dysfunctions in their early stages. This finding could lead them to seek prompt medical help, which is crucial in the prevention of several diseases. Teaching young women about fertility awareness involves an effort that could prove useful all their lives.117 We have noted that those adolescents who learn to recognize their fertility as part of a sex education program curriculum show a greater appreciation for sexuality and affectivity, both their own and that of those in their age group.118 In addition, youngsters start perceiving fertility as a natural feature of their development, and show a better understanding of fertility and life, seeing them as important gifts that are to be understood, appreciated, and transmitted. Hence, we consider that the recognition of fertility should play a greater part in sex education programs, thereby becoming a very useful tool, both pedagogically and in health self-care. In addition, it has been shown that when learned during adolescence paired with personal formation involving every aspect of the person, this knowledge constitutes an important tool to strengthen girls’ sense of identity and prevent adolescent pregnancy.119
Notes
J.B. Brown, “Types of Ovarian Activity in Women and Their Significance: The Continuum (A Reinterpretation of Early Findings),” Human Reproduction Update 17 (2011): 141–158.
P. Vigil et al., “Usefulness of Monitoring Fertility from Menarche,” Journal of Pediatric and Adolescent Gynecology 19 (2006): 173–179.
B. Lunenfeld and V. Insler, “Follicular Development and Its Control,” Gynecological Endocrinology 7 (1993): 285–291.
Brown, “Types of Ovarian Activity in Women and Their Significance”; J.F. Roche, “Control and Regulation of Folliculogenesis: A Symposium in Perspective,” Reviews of Reproduction 1 (1996): 19–27.
L. Walter and D.M.S. Miller, “Female Puberty and Its Disorders,” in Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management, eds. S.C.C. Yen and R. Barbieri (Philadelphia: W.B. Saunders, 1999), 388–412.
E.J. Sigel, “Adolescent Growth and Development,” in Essential Adolescent Medicine, eds. D.E. Greydanus, D.R. Patel, and H.D. Pratt (New York: McGraw Hill Professional, 2005), 3–15.
G.A. Martos-Moreno, J.A. Chowen, and J. Argente, “Metabolic Signals in Human Puberty: Effects of Over and Undernutrition,” Molecular and Cellular Endocrinology 324 (2010): 70–81; R.V. García-Mayor et al., “Serum Leptin Levels in Normal Children: Relationship to Age, Gender, Body Mass Index, Pituitary-Gonadal Hormones, and Pubertal Stage,” Journal of Clinical Endocrinology and Metabolism 82 (1997): 2849–2855; M.G. Teles et al., “New Genetic Factors Implicated in Human GnRH-Dependent Precocious Puberty: The Role of Kisspeptin System,” Molecular Cellular Endocrinology 346 (2011): 84–90.
Vigil et al., “Usefulness of Monitoring Fertility from Menarche”; P. Vigil, La Fertilidad de la Pareja Humana. (Santiago, Chile: Ediciones Universidad Católica, 2004).
Brown, “Types of Ovarian Activity in Women and Their Significance.”
A.W. Diddle, “Athletic Activity and Menstruation,” Southern Medical Journal 76 (1983): 619–624; M.J. Mansfield and S.J. Emans, “Anorexia Nervosa, Athletics, and Amenorrhea,” Pediatric Clinics of North America 36 (1989): 533–549.
R.J. Fehring, M. Schneider, and K. Raviele, “Variability in the Phases of the Menstrual Cycle,” Journal of Obstetric, Gynecologic, and Neonatal Nursing 35 (2006): 376–384; L.A. Cole, D.G. Ladner, and F.W. Byrn, “The Normal Variabilities of the Menstrual Cycle,” Fertility and Sterility 91 (2009): 522–527.
Vigil et al., “Usefulness of Monitoring Fertility from Menarche”; Vigil, La Fertilidad de la Pareja Humana.
Brown, “Types of Ovarian Activity in Women and Their Significance.”
García-Mayor et al., “Serum Leptin Levels in Normal Children.”
E.L. Billings et al., “Symptoms and Hormonal Changes Accompanying Ovulation,” Lancet 1 (1972): 282–284; E.L. Billings, J.J. Billings, and M. Catarinich, Billings Atlas of the Ovulation Method: The Mucus Patterns of Fertility and Infertility (Melbourne, Australia: Advocate Press, 1989).
A.R. Baerwald et al., “Ovarian Antral Folliculogenesis During the Human Menstrual Cycle: A Review,” Human Reproduction Update 18 (2012): 73–91.
P. Vigil et al., “Reconocimiento de la Fertilidad: Un Signo de Salud Para la Mujer,” in Selected Members Communications, 18th General Assembly, ed. Pontifical Academy for Life (Vatican City: Pontifical Academy for Life, 2012), 19–39.
Ibid.
Ibid.
L.F. Blackwell et al., “Hormonal Monitoring of Ovarian Activity Using the Ovarian Monitor. Part I. Validation of Home and Laboratory Results Obtained During Ovulatory Cycles by Comparison with Radioimmunoassay,” Steroids 68 (2003): 465–476; D.G. Cooke, J.E. Binnie, and L.F. Blackwell, “Validation of a Reference ELISA for Estrone Glucuronide Using Urine Samples Normalized by Dilution to a Constant Rate of Urine Production,” Steroids 72 (2007): 580–591; L.F. Blackwell et al., “Monitoring of Ovarian Activity by Measurement of Urinary Excretion Rates of Oestrone Glucuronide and Pregnanediol Glucuronide Using the Ovarian Monitor, Part II: Reliability of Home Testing,” Human Reproduction 27 (2012): 550–557.
Brown, “Types of Ovarian Activity in Women and Their Significance”; L.F. Blackwell et al., “Definition of the Potentially Fertile Period from Urinary Steroid Excretion Rates. Part II. A Threshold Value for Pregnanediol Glucuronide as a Marker for the End of the Potentially Fertile Period in the Human Menstrual Cycle,” Steroids 63 (1998): 5–13.
J.B. Brown, J. Holmes, and G. Barker, “Use of the Home Ovarian Monitor in Pregnancy Avoidance,” American Journal of Obstetrics and Gynecology 165 (1991): 2008–2011.
Blackwell et al., “Hormonal Monitoring of Ovarian Activity Using the Ovarian Monitor, Part I”; Blackwell et al., “Monitoring of Ovarian Activity by Measurement of Urinary Excretion Rates, Part II.”
Blackwell et al., “Monitoring of Ovarian Activity by Measurement of Urinary Excretion Rates of Oestrone Glucuronide and Pregnanediol Glucuronide Using the Ovarian Monitor, Part II.”
L.F. Blackwell and J.B. Brown, “Application of Time-Series Analysis for the Recognition of Increases in Urinary Estrogens as Markers for the Beginning of the Potentially Fertile Period,” Steroids 57 (1992): 554–562.
Blackwell et al., “Definition of the Potentially Fertile Period from Urinary Steroid Excretion Rates. Part II.”
Brown, “Types of Ovarian Activity in Women and Their Significance.”
Billings et al., “Symptoms and Hormonal Changes Accompanying Ovulation”; Billings, Billings, and Catarinich, Billings Atlas of the Ovulation Method.
Blackwell and Brown, “Application of Time-Series Analysis for the Recognition of Increases in Urinary Estrogens.”
Brown, “Types of Ovarian Activity in Women and Their Significance.”
Ibid.
Vigil et al., “Usefulness of Monitoring Fertility from Menarche.”
F. Miro and L.J. Aspinall, “The Onset of the Initial Rise in Follicle-Stimulating Hormone During the Human Menstrual Cycle,” Human Reproduction 20 (2005): 96–100.
Blackwell et al., “Hormonal Monitoring of Ovarian Activity Using the Ovarian Monitor, Part I”; K.J. Catt and J.G. Pierce, “Gonadotropin Hormones of the Adenohypophysis,” in Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management, eds. S.S.C. Yen and R.B. Jaffe (Philadelphia: W.B. Saunders, 1978), 75–113.
R.W. Noyes, A.T. Hertig, and J. Rock, “Dating the Endometrial Biopsy,” Fertility and Sterility 1 (1950): 3–25; E. Johannisson et al., “Vascular Changes in the Human Endometrium Following the Administration of the Progesterone Antagonist RU 486,” Contraception 39 (1989): 103–117; C. Barros et al., “Scanning Electron Microscopy Study of Human Cervical Mucus,” Gamete Research 12 (1985): 85–89; P. Vigil et al., “Post-Partum Cervical Mucus: Biological and Rheological Properties,” Human Reproduction 6 (1991): 475–479; P. Morales, M. Rocco, and P. Vigil, “Human Cervical Mucus: Relationship Between Biochemical Characteristics and Ability to Allow Migration of Spermatozoa,” Human Reproduction 8 (1993): 78–83.
J.S. Laven and B.C. Fauser, “Inhibins and Adult Ovarian Function,” Molecular and Cellular Endocrinology 225 (2004): 37–44.
D.G. Armstrong and R. Webb, “Ovarian Follicular Dominance: The Role of Intraovarian Growth Factors and Novel Proteins,” Reviews of Reproduction 2 (1997): 139–146.
J.D. Hoff, M.E. Quiglel, and S.S. Yen, “Hormonal Dynamics at Midcycle: A Reevaluation,” Journal of Clinical Endocrinology and Metabolism 57 (1983): 792–796; M.D. Ferin, D. Van Vugt, and S. Warlaw, “The Hypothalamic Control of the Menstrual Cycle and the Role of Endogenous Opioid Peptides,” Recent Progress in Hormone Research 40 (1984): 441–480.
Ferin, Van Vugt, and Warlaw, “The Hypothalamic Control of the Menstrual Cycle and the Role of Endogenous Opioid Peptides”; J.F. Strauss and C.J. Williams, “The Ovarian Life Cycle,” in Yen and Jaffe's Reproductive Endocrinology. Physiology, Pathophysiology, and Clinical Management, eds. J.F. Strauss and R.L. Barbieri (Philadelphia: Elsevier Saunders, 2009).
Hoff, Quiglel, and Yen, “Hormonal Dynamics at Midcycle”; A. Miyake et al., “Changes in Plasma LRH During the Normal Menstrual Cycle in Women,” Acta Endocrinologica (Copenhagen) 93 (1980): 257–263.
Miro and Aspinall, “The Onset of the Initial Rise in Follicle-Stimulating Hormone”; Hoff, Quiglel, and Yen, “Hormonal Dynamics at Midcycle”; Miyake et al., “Changes in Plasma LRH During the Normal Menstrual Cycle.”
R. Misao et al., “Expression of Progesterone Receptor Isoforms in Corpora Lutea of Human Subjects: Correlation with Serum Estrogen and Progesterone Concentrations,” Molecular Human Reproduction 4 (1998): 1045–1052.
Brown, “Types of Ovarian Activity in Women and Their Significance”; Blackwell et al., “Monitoring of Ovarian Activity by Measurement of Urinary Excretion Ratesr, Part II.”
Miro and Aspinall, “The Onset of the Initial Rise in Follicle-Stimulating Hormone”; R.F. Vollman, “The Menstrual Cycle,” in Major Problems in Obstetrics and Gynecology, ed. E.A. Friedman (Toronto: W.B. Saunders, 1977), 11–193.
Miro and Aspinall, “The Onset of the Initial Rise in Follicle-Stimulating Hormone”; S.S.C. Yen and C.C. Tsai, “The Biphasic Pattern in the Feedback Action Ethynyl Estradiol on the Release of FSH and LH,” Journal of Clinical Endocrinology and Metabolism 33 (1971): 882–887.
D.W. Lincoln, H.M. Fraser, and G.A. Lincoln, “Hypothalamic Pulse Generators,” Recent Progress in Hormone Research 41 (1985): 369–419; S.S.C. Yen, “The Human Menstrual Cycle,” in Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management, eds. S.S.C. Yen and R.B. Jaffe (Philadelphia: W.B. Saunders, 1991), 273–308.
Brown, “Types of Ovarian Activity in Women and Their Significance”; Blackwell et al., “Hormonal Monitoring of Ovarian Activity Using the Ovarian Monitor, Part I”; Blackwell et al., “Monitoring of Ovarian Activity by Measurement of Urinary Excretion Rates, Part II.”
Vigil, La Fertilidad de la Pareja Humana; Vigil et al., “Post-Partum Cervical Mucus.”
J.B. Brown et al., “Natural Family Planning,” American Journal of Obstetrics and Gynecology 157 (1987): 1082–1089.
S.V. Nicosia, “Physiology of Cervical Mucus Production,” Seminars in Reproductive Endocrinology 4 (1986): 313–321.
E. Odeblad, “Sperm-Mucus Interaction and Cervical Mucus Penetration Test,” in Male Contraception. Advances and Future Prospects, eds. G. Zatuchni et al. (Philadelphia: Harper & Row Publishers, 1985), 134–137; E. Odeblad, “The Discovery of Different Types of Cervical Mucus and the Billings Ovulation Method,” Bulletin of the Natural Family Planning Council of Victoria (Australia) 21 (1994): 3–35.
Odeblad, “The Discovery of Different Types of Cervical Mucus and the Billings Ovulation Method.”
Ibid.
Odeblad, “Sperm-Mucus Interaction and Cervical Mucus Penetration Test”; E. Odeblad, “The Functional Structure of Human Cervical Mucus,” Acta Obstetricia et Gynecologica Scandinavica 47 (1968): 57–79; E. Odeblad, “Biophysical Techniques of Assessing Cervical Mucus and Microstructure of Cervical Epithelium,” in Cervical Mucus in Human Reproduction, World Health Organization Colloquium, Geneva, eds. M. Elstein, K.S. Moghissi, and R. Borth (Copenhagen: Scriptor, 1972): 58–64.
M. Menárguez, Caracterización Morfológica de Diversos Tipos de Moco Cervical Humano Mediante Microscopía de Luz y Microscopía Electrónica de Barrido (doctoral thesis; Murcia, Spain: Universidad de Murcia, 1998); M. Menárguez, L.M. Pastor, and E. Odeblad, “Morphological Characterization of Different Human Cervical Mucus Types Using Light and Scanning Electron Microscopy,” Human Reproduction 18 (2003): 1782–1789.
Menárguez, Caracterización Morfológica de Diversos Tipos de Moco Cervical Humano; Menárguez, Pastor, and Odeblad, “Morphological Characterization of Different Human Cervical Mucus Types”; P. Vigil et al., “Scanning Electron and Light Microscopy Study of the Cervical Mucus in Women with Polycystic Ovary Syndrome,” Journal of Electron Microscopy 58 (2009): 21–27.
E.S. Hafez, “Sperm Transport in the Human and Mammalian Cervix,” in The Cervix, eds. J.A. Jordan and A. Singer (London: W.B. Saunders, 1976), 164–175.
Odeblad, “The Functional Structure of Human Cervical Mucus.”
Ibid.
Menárguez, Caracterización Morfológica de Diversos Tipos de Moco Cervical Humano; Menárguez, Pastor, and Odeblad, “Morphological Characterization of Different Human Cervical Mucus Types.”
M. Elstein and B. Daunter, “The Structure of Cervical Mucus,” in The Cervix, eds. J.A. Jordan and A. Singer. (London: W.B. Saunders, 1976), 137–146.
Vigil et al., “Scanning Electron and Light Microscopy Study of the Cervical Mucus”; F. Ceric, D. Silva, and P. Vigil, “Ultrastructure of the Human Periovulatory Cervical Mucus,” Journal of Electron Microscopy 54 (2005): 479–484.
I.K. Gipson, “Human Endocervical Mucins,” Ernst Schering Research Foundation Workshop 52 (2005): 219–244.
J.L. Desseyn et al., “Evolutionary History of the 11p15 Human Mucin Gene Family,” Journal of Molecular Evolution 46 (1998): 102–106.
I.K. Gipson, “Mucins of the Human Endocervix,” Frontiers in Bioscience 6 (2001): D1245–1255; Gipson, “Human Endocervical Mucins”; S.J. Gendler and A.P. Spicer, “Epithelial Mucin Genes,” Annual Review of Physiology 57 (1995): 607–634; I.K. Gipson et al., “Mucin Genes Expressed by Human Female Reproductive Tract Epithelia,” Biology of Reproduction 56 (1997): 999–1011.
I.K. Gipson et al., “MUC4 and MUC5B Transcripts Are the Prevalent Mucin Messenger Ribonucleic Acids of the Human Endocervix,” Biology of Reproduction 60 (1999): 58–64; I.K. Gipson et al., “The Amount of MUC 5B Mucin in Cervical Mucus Peaks at Midcycle,” Journal of Clinical Endocrinology and Metabolism 86 (2001): 594–600.
Gipson et al., “MUC4 and MUC5B Transcripts Are the Prevalent Mucin Messenger Ribonucleic Acids”; Gipson et al., “The Amount of MUC 5B Mucin in Cervical Mucus Peaks”; Gipson, “Mucins of the Human Endocervix”; Gipson, “Human Endocervical Mucins.”
P. Vigil and E. Valdés, “Bases Científicas de la Planificación Natural de la Familia,” Actualizaciones en Ginecología y Obstetricia 3 (1989): 59–67.
R. Punnonen et al., “A Composite Picture of the Normal Menstrual Cycle,” Acta Obstetricia et Gynecologica Scandinavica 51 (1976): 63–70.
J.A. McCoshen, “The Role of Cervical Mucus in Reproduction,” Contemporary Obstetrics and Gynecology 26 (1987): 94–116.
R. Sitruk-Ware, Progesterone et Progestatifs en thérapeutique (Paris: Organon, 1982).
J.A. Jordan and A. Singer, “Effect of Oral Contraceptive Steroids upon Epithelium and Mucus,” in The Cervix, eds. J.A. Jordan and A. Singer (London: W.B. Saunders, 1976), 192–207.
Elstein and Daunter, “The Structure of Cervical Mucus.”
Barros et al., “Scanning Electron Microscopy Study of Human Cervical Mucus”; C. Barros et al., “Selection of Morphologically Abnormal Sperm by Human Cervical Mucus,” Archives of Andrology 12 (1984): 95–107.
Barros et al., “Scanning Electron Microscopy Study of Human Cervical Mucus”; Barros et al., “Selection of Morphologically Abnormal Sperm by Human Cervical Mucus.”
P. Vigil, A. Toro, and A. Godoy, “Physiological Action of Oestradiol on the Acrosome Reaction in Human Spermatozoa,” Andrologia 40 (2008): 146–151; P. Vigil, R.F. Orellana, and M.E. Cortés, “Modulation of Spermatozoon Acrosome Reaction,” Biological Research 44 (2011): 151–159.
S.S. Olmsted et al., “Diffusion of Macromolecules and Virus-like Particles in Human Cervical Mucus,” Biophysical Journal 81 (2001): 1930–1937.
H. Zuckerman, A. Kahana, and S. Carmel, “Antibacterial Activity of Human Cervical Mucus,” Gynecological Investigation 6 (1975): 265–271; W. Eggert-Kruse et al., “Antimicrobial Activity of Human Cervical Mucus,” Human Reproduction 15 (2000): 778–784.
Billings et al., “Symptoms and Hormonal Changes Accompanying Ovulation”; K.P. McNatty and R.S. Sawers, “Relationship Between the Endocrine Environment within the Graafian Follicle and the Subsequent Rate Secretion of Progesterone Secretion by Human Granulosa Cells in vitro,” Journal of Endocrinology 66 (1975): 391–400.
Vigil, La Fertilidad de la Pareja Humana; R.L. Barbieri, “Infertility,” in Reproductive Endocrinology: Physiology, Pathophysiology and Clinical Management, eds. S.S.C. Yen and R.B. Jaffe (Philadelphia: W.B. Saunders, 1999), 562–593.
D.A. Koutras, “Disturbances of Menstruation in Thyroid Disease,” Annals of the New York Academy of Sciences 816 (1997): 280–284.
P. Vigil et al., “Hiperandrogenismo e Irregularidades Menstruales en Mujeres Jóvenes,” Revista Chilena de Obstetricia y Ginecología 64 (1999): 389–394.
Brown, “Types of Ovarian Activity in Women and Their Significance.”
Billings et al., “Symptoms and Hormonal Changes Accompanying Ovulation”; Billings, Billings, and Catarinich, Billings Atlas of the Ovulation Method; E. Steinberger et al., “Consequences of Hyperandrogenism During Adolescence on the Ovarian Function of Adult Female,” in Reproductive Medicine, eds. G. Frajese, E. Steinberger, and L.J. Rodríguez-Rigau (New York: Raven Press, 1993), 253–264; P. Vigil et al., “Diagnosis of Menstrual Disorders in Adolescence,” in Reproductive Medicine, eds. G. Frajese, E. Steinberger and L.J. Rodríguez-Rigau (New York: Raven Press, 1993), 149–154.
Diddle, “Athletic Activity and Menstruation”; Mansfield and Emans, “Anorexia Nervosa, Athletics, and Amenorrhea.”
Ibid.
E. Cutie and N.A. Andino, “Prolactin Inhibits the Steroidogenesis in Midfollicular Phase Human Granulosa Cells Cultured in a Chemically Defined Medium,” Fertility and Sterility 49 (1988): 632–637.
M.L. Barron, “Proactive Management of Menstrual Cycle Abnormalities in Young Women,” Journal of Perinatal and Neonatal Nursing 18 (2004): 81–92.
P. Vigil et al., “Cellular Immunity Alterations in Hyperprolactinemia,” Acta Cytologica 51 (2007): 355.
G.G. Johansson, S.L. Karonen, and M.L. Laakso, “Reversal of an Elevated Plasma Level of Prolactin During Prolonged Psychological Stress,” Acta Physiologica Scandinavica 119 (1983): 463–464.
P. Vigil et al., “Síndrome de Ovario Poliquístico,” in Selección de Temas en Ginecoobstetricia, ed. E. Guzmán. (Santiago, Chile: Editorial Publimpacto, 2005), 833–842; P. Vigil et al., “Evidence of Subpopulations with Different Levels of Insulin Resistance in Women with Polycystic Ovary Syndrome,” Human Reproduction 22 (2007): 2974–2980.
R. Azziz et al., “The Androgen Excess and PCOS Society Criteria for the Polycystic Ovary Syndrome: The Complete Task Force Report,” Fertility and Sterility 91 (2009): 456–488.
D. Meirow et al., “Insulin Resistant and Non-Resistant Polycystic Ovary Syndrome Represent Two Clinical and Endocrinological Subgroups,” Human Reproduction 10 (1995): 1951–1956.
Vigil et al., “Evidence of Subpopulations with Different Levels of Insulin Resistance.”
Ibid.
Ibid.
Vigil et al., “Scanning Electron and Light Microscopy Study of the Cervical Mucus.”
Vigil et al., “Síndrome de Ovario Poliquístico.”
Vigil et al., “Scanning Electron and Light Microscopy Study of the Cervical Mucus.”
Ibid.
Koutras, “Disturbances of Menstruation in Thyroid Disease.”
Vigil et al., “Usefulness of Monitoring Fertility from Menarche”; Vigil et al., “Reconocimiento de la Fertilidad: un Signo de Salud para la Mujer.”
Vigil et al., “Usefulness of Monitoring Fertility from Menarche.”
L. Howe et al., “Mucinase and Sialidase Activity of the Vaginal Microflora: Implications for the Pathogenesis of Preterm Labour,” International Journal of STD and AIDS 10 (1999): 442–447.
Vigil et al., “Usefulness of Monitoring Fertility from Menarche.”
Ibid.
G.F. Gonzales et al., “Update on the Impact of Chlamydia trachomatis Infection on Male Fertility,” Andrologia 36 (2004): 1–23.
P. Vigil et al., “Chlamydia trachomatis Infection in Male Partners of Infertile Couples: Incidence and Sperm Function,” Andrologia 34 (2002): 155–161; P. Vigil et al., “First-Trimester Pregnancy Loss and Active Chlamydia trachomatis Infection: Correlation and Ultrastructural Evidence,” Andrologia 34 (2002): 373–378.
Vigil et al., “Chlamydia trachomatis Infection in Male Partners of Infertile Couples”; Vigil et al., “First-Trimester Pregnancy Loss and Active Chlamydia trachomatis Infection.”
H. zur Hausen, “Papillomaviruses and Cancer: From Basic Studies to Clinical Application,” Nature Reviews Cancer 2 (2002): 342–350.
Vigil et al., “Usefulness of Monitoring Fertility from Menarche.”
G.D. Pinkerton and H.M. Carey, “Post-pill Anovulation,” Medical Journal of Australia 8 (1976): 220–222; S. Linn et al., “Delay in Conception for Former ‘Pill’ Users,” Journal of the American Medical Association 247 (1982): 629–632; C. Gnoth et al., “Cycle Characteristics After Discontinuation of Oral Contraceptives,” Gynecological Endocrinology 16 (2002): 307–317.
Linn et al., “Delay in Conception for Former ‘Pill’ Users”; Gnoth et al., “Cycle Characteristics After Discontinuation of Oral Contraceptives.”
P. Vigil, “Every Woman Should Know Fertility Awareness so that Their Reproductive Health Can Be Monitored,” Bulletin of the Ovulation Method Research and Reference Centre of Australia 31 (2004): 8–9.
Steinberger et al., “Consequences of Hyperandrogenism During Adolescence.”
Barron, “Proactive Management of Menstrual Cycle Abnormalities in Young Women”; H. Klaus and J.L. Martin, “Recognition of Ovulatory/Anovulatory Cycle Pattern in Adolescents by Mucus Self Detection,” Journal of Adolescent Health Care 10 (1989): 93–96.
Klaus and Martin, “Recognition of Ovulatory/Anovulatory Cycle Pattern in Adolescents by Mucus Self Detection.”
H. Klaus, “Wie kann man die Verhaltensweisen Jugendlicher auf sexuellem Gebiet beeinflussen?” Kinderkrankenschwester 16 (1997): 32–35; P. Vigil, R. Riquelme, and A. Peirone, “Teen STAR: Opting for Maturity and Freedom,” in Natura e Dignità della Persona Umana a Fondamento del Diritto alla Vita. Le Sfide del Contesto Culturale Contemporaneo. Atti della VIII Assemblea della Pontificia Accademia per la Vita, eds. J.D. Vial Correa and E. Sgreccia (Vatican City: Libreria Editrice Vaticana, 2002), 101–113; C. Cabezón et al., “Adolescent Pregnancy Prevention: An Abstinence-Centered Randomized Controlled Intervention in a Chilean Public High School,” Journal of Adolescent Health 36 (2005): 64–69; P. Vigil et al., “Teen STAR: Una Opción de Madurez y Libertad. Programa de Educación Integral de la Sexualidad, Orientado a Adolescentes,” Revista Médica de Chile 133 (2005): 1173–1182; P. Vigil et al., “Effect of Teen STAR, an Abstinence-Only Sexual Education Program on Adolescent Sexual Behavior,” Journal of Pediatric and Adolescent Gynecology 18 (2005): 212; P. Vigil, M.E. Cortés, and H. Klaus, “A Randomized Control Trial of Teen STAR,” in Human Fertility: Where Faith and Science Meet, eds. R.J. Fehring and T. Notare (Milwaukee: Marquette University Press, 2007), 169–184; P. Vigil et al., “Educación en Afectividad y Sexualidad para Adolescentes: Resultados de la Implementación del Programa Teen STAR,” Ars Medica 17 (2008): 111–130; P. Vigil, C.T. Molina, and M.E. Cortés, “La Sexualidad de las Jóvenes Chilenas,” Ars Medica 18 (2009): 195–208; P. Vigil et al., Para Amar y ser Amado. Fundamentos para una Auténtica Educación en el Amor (Santiago: Conferencia Episcopal de Chile & RR Donelley, 2009); P. Vigil, J.P. del Río, and M.E. Cortés, “Educación Sexual. Educar para el Amor,” Revista de Pastoral Juvenil 470 (2011): 7–16.
Klaus, “Wie kann man die Verhaltensweisen Jugendlicher auf sexuellem Gebiet beeinflussen?”; Vigil, Riquelme, and Peirone, “Teen STAR”; Cabezón et al., “Adolescent Pregnancy Prevention”; Vigil et al., “Teen STAR”; Vigil et al., “Effect of Teen STAR”; Vigil, Cortés, and Klaus, “A Randomized Control Trial of Teen STAR”; Vigil et al., “Educación en Afectividad y Sexualidad para Adolescentes”; Vigil, Molina, and Cortés, “La Sexualidad de las Jóvenes Chilenas”; Vigil et al., Para Amar y Ser Amado; Vigil, del Río, and Cortés, “Educación Sexual.”