Abstract
Background
Ginsenoside is the major bioactive component of ginseng, which has been proven to be a neuroprotective drug. The aim of this study was to evaluate the therapeutic effect of ginsenoside in a diabetic Goto-Kakizaki (GK) rat model.
Material/Methods
Twenty GK rats were randomly divided into a diabetic model (DM) group (n=10) and a ginsenoside + DM group (n=10); Wistar rats with the same age and body weight were used as the control (CON) group (n=10). Food and water intake, body weight, and blood fasting plasma glucose were measured. The Morris water maze test was used to detect learning and memory functions of the rats. Superoxide dismutase (SOD), malondialdehyde (MDA), and inflammatory cytokines (TNF-α, IL-1β, and IL-6) in the hippocampus were analyzed after ginsenoside treatment.
Results
The blood glucose, body weight, Morris correlation index, SOD, MDA, and other test results were increased in the diabetic rats. Ginsenoside ameliorated diabetic cognitive decline.
Conclusions
The possible mechanism was related to inhibiting brain oxidative/nitrosative damage and affecting the expression of the cytokines IL-1β, IL-6, and TNF-α.
MeSH Keywords: Cognitive Science; Ginsenosides; Receptors, N-Methyl-D-Aspartate
Background
Diabetes mellitus is a metabolic disorder with increasing incidence rates every year. In China, the incidence of diabetes is increasing year by year. Diabetes can cause damage to both the peripheral and central nervous system [1–3]. Central nervous system lesions mainly affect the white matter of the cerebral cortex, progression memory loss, and performance on spatiotemporal obstacles. Many studies have shown that diabetes is a risk factor for Alzheimer disease (AD) [4]. Diabetes is associated with changes in the central nervous system, including cognitive disorders, and with cerebrovascular disease [5–7]. Hyperglycemia can cause oxidative stress and an inflammatory response, which are important factors leading to dementia. Oxidative stress in the diabetic state can cause damage to the hippocampus or other brain regions. Many oxidative stress products can lead to learning and memory dysfunction. Inflammation is also an important factor in the progression of cognitive decline in diabetes.
Ginsenoside is the main component of ginseng, which has been proven to be a neuroprotective agent. It has been reported that ginsenoside has many pharmacological activities, including anti-cancer [8–10], anti-aging [11], anti-inflammatory [12], enhance immunity [13], etc. Many studies have shown that ginsenoside has neuroprotective effects [14], which may allow it to act as a potential novel agent to improve cognitive function in diabetes [15–17]. In this study, we assessed the probable therapeutic effect of ginsenoside and its underlying mechanisms in GK rats.
Material and Methods
Experimental animals
Twenty male 13-week-old diabetic GK rats and 10 age-matched male Wistar rats were obtained from the Laboratory Animal Center of North China University of Science and Technology (Tangshan, China) and raised in a thermostatically controlled room (25±2°C) with a 12-hour light/dark cycle and with free access to food and water. The experiment was approved by the Ethics Committee of the North China University of Science and Technology. After one week of adaptation, the GK rats were randomly divided into a DM group and a ginsenoside + DM group, and the Wistar rats were used as the control (CON) group (n=10 in each group). The rats in the ginsenoside + DM group were treated with ginsenoside (20 mg/kg) intragastrically once daily for 10 consecutive weeks. After 10 weeks of administration, food and water intake, fasting plasma glucose (FPG), behavioral tests, and biochemical experiments were performed in sequence.
FPG measurements
FPG was measured every week at 24 hours after last drug treatment; venous blood was collected for FPG measurements.
Food and water intake measurements
Body weights and water intake of rats were measured dynamically for 10 weeks (56 days) after ginsenoside administration.
Oral glucose tolerance test (OGTT)
Oral glucose tolerance test (OGTT) was performed in both control and GK rats after 10 weeks of ginsenoside administration. Prior to OGTT, rats were fasted for 4 to 6 hours. D-glucose was administered at 75 mg per rat by oral gavage, and blood was sampled from the saphenous vein at 0, 15, 30, 60, and 120 minutes. The samples were immediately analyzed for glucose using a blood glucose meter. Plasma samples were stored at −20°C until assayed for insulin via enzyme linked immunosorbent assays (ELISA).
Morris water maze
Ten weeks after ginsenoside treatments, spatial learning and memory of the rats was evaluated by the Morris water maze (MWM) [18] utilizing a pool 150 cm in diameter and 60 cm deep. The tank was filled with opaque water kept 24°C to 25°C. A platform (10 cm in diameter) was submerged 1 cm below the surface of the water. During training trials, the rats were placed in the pool and allowed to swim for 60 seconds, then they were led to the platform and kept there for 10 seconds. After adaptive training trials, the navigation experiment was carried out for 4 days. The rats were placed into the pool in each of the 4 different quadrants and allowed to find the platform within 120 seconds, and the time to locate the platform was recorded. Those who could not find the platform were placed on the platform and allowed to stand for 15 seconds and the time was recorded as 120 seconds. On day 5 of the spatial probe, the platform was removed, and the rats were placed into the water in any quadrant. The time that the rats stayed in the quadrant where the platform was previously placed was recorded. A number of behavioral tests were carried out using a computer-based video tracking system.
Hematoxylin and eosin (H&E) staining
After the MWM test, 5 rats were randomly selected for hematoxylin and eosin (H&E) staining in each group. Rats were sacrificed after anesthesia and the brains were rapidly obtained. Then, the brain tissues were perfused with 200 mL of 0.9% NaCl solution and subsequently with 4% paraformaldehyde in 0.1 mol/L phosphate buffer at pH 7.4. The brains were rapidly removed and fixed in formalin for 24 hours. The post-fixed tissues were embedded in paraffin wax, and 5-μm-thick serial coronal sections were obtained and mounted on poly-L-lysine-coated glass slices. For histological assessment of damage to the hippocampus, the paraffin-embedded sections were stained with H&E according to a standard protocol.
ELISA measurements of cytokines in the hippocampus
The rat hippocampus tissues were washed and then homogenized on ice in normal saline. The homogenates were centrifuged at 3000 g for 10 minutes at 4°C, and the supernatants (100 mL) were used for analysis. Expression levels of interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-α (TNF-α) were measured by ELISA kits (rat TNF alpha ELISA Kit (RAB0480) Roche, Switzerland; rat interleukin-1 beta ELISA Kit (RAB0311), Roche, Switzerland; rat IL-6 ELISA Kit (RAB0278) Roche, Switzerland) in triplicate according to the manufacturer’s recommended protocols.
Statistical analysis
Statistical analysis was performed in SPSS 17.0. All data are given as the mean ± SED. One-way analysis of variance (ANOVA) followed by Bonferroni post hoc testing for multiple comparisons was used to compare differences among 3 or more groups. Normality tests were used to ascertain that the data were normally distributed. The results of P<0.05 were regarded as significant.
Results
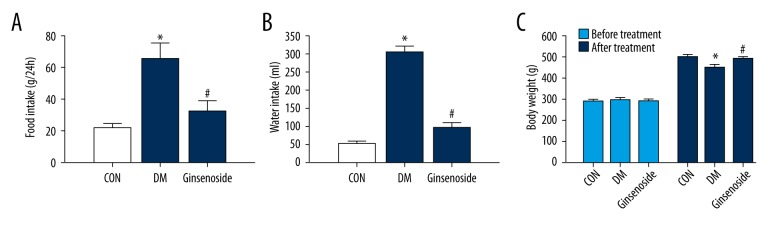
Ginsenoside influenced the body weight, water intake, and food intake in GK diabetic rats. There was a significant increase in food and water intake in the DM group (P<0.01). After ginsenoside administration, food and water intake measurements were much lower than those in the DM group (Figure 1A, 1B). The weight of the rats in the DM group decreased significantly (P<0.01), whereas ginsenoside administration reversed the body weights in the GK diabetic rats (Figure 1C).
Figure 1.
Food and water intake and body weight were measured (A–C). Statistical analysis was performed by repeated measures ANOVA followed by LSD post hoc analysis; * P<0.01 vs. the CON group, # P<0.01 vs. the DM group. LSD – least significant difference; CON – control; DM – diabetic model.
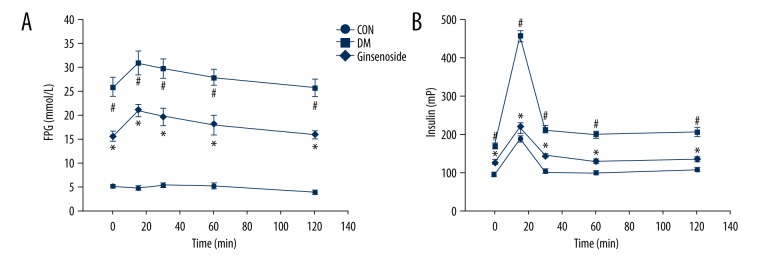
Ginsenoside decreased FPG levels in the GK rats. FPG was tested dynamically for 10 weeks. The blood glucose levels of the DM group were apparently higher than those of the CON group (P<0.01). After administration of ginsenoside for 10 weeks, blood glucose levels were obviously decreased compared to those in the DM group (P<0.01; Figure 2).
Figure 2.
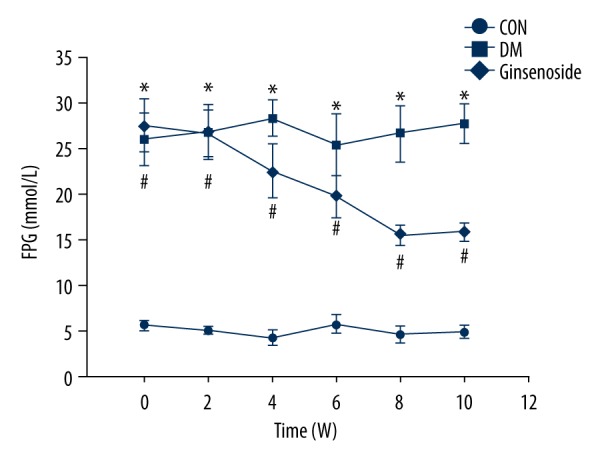
FPG levels of different groups at different time points. Statistical analysis was performed by repeated measures ANOVA followed by LSD post hoc analysis; * P<0.01 vs. the CON group, # P<0.01 vs. the DM group. FPG – fasting plasma glucose; LSD – least significant difference; CON – control; DM – diabetic model.
Ginsenoside influenced glucose and insulin levels in the GK diabetic rats. Rats on ginsenoside for 10 weeks had significantly lower blood glucose levels and insulin responses in vivo at all time-points measured compared to those in the DM group (Figure 3A, 3B).
Figure 3.
The in vivo parameters from rats in the CON, DM, and ginsenoside groups. (A) Blood glucose and (B) insulin levels of rats in the CON, the DM, and the ginsenoside groups after 10 weeks. Data are presented as the mean ±SEM. Statistical analysis was performed using one-way ANOVA with LSD post hoc test; * P<0.01 vs. the CON group, # P<0.01 vs. the DM group. SEM – standard error of the mean; LSD – least significant difference; CON – control; DM – diabetic model.
Effects of ginsenoside on cognitive deficits in the GK diabetic rats
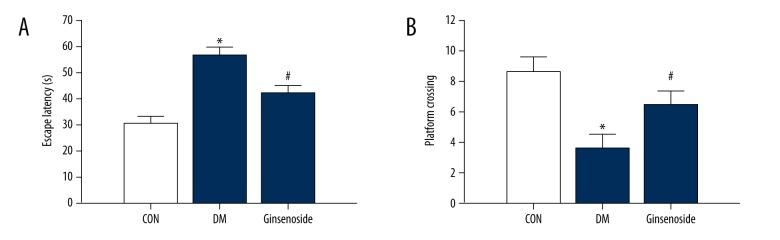
Ginsenoside improved the learning and memory abilities in the DM group. Compared with the CON group, the escape latency was significantly increased in the DM group (P<0.01). However, ginsenoside restored the escape latency (P<0.01 vs. the DM group; Figure 4A). In the probe test, compared with those in the CON group, the number of platform crossings was obviously decreased in the DM group (P<0.01), whereas ginsenoside administration reversed the decrease in platform crossings in the GK diabetic rats (Figure 4B).
Figure 4.
Effect of ginsenoside on diabetic GK rats in spatial learning and memory tested by the MWM. (A) Changes in the daily escape latencies; (B) time spent in the platform region during the probe trial without the platform. The results are shown as the mean ±SD. Statistical analysis was performed using one-way ANOVA with LSD post hoc test; * P<0.01 vs. the CON group, # P<0.01 vs. the DM group. GK – Goto-Kakizaki; MWM – Morris water maze; LSD – least significant difference; CON – control; DM – diabetic model.
Histopathological observations: H&E staining
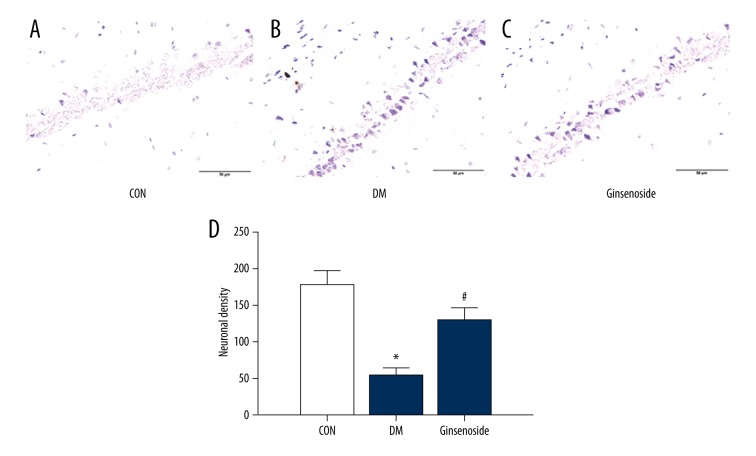
There were only a small number of necrotic cells in the CON group (Figure 5A). In the DM group, the cell size shrunk, the cell number decreased, and chromatin aggregation was shown by dense staining (Figure 5B). However, ginsenoside treatment dramatically restored the alterations (Figure 5C). Ginsenoside treatment significantly prevented neuronal cell loss in the hippocampal CA1 region.
Figure 5.
Histological analysis of the effects of ginsenoside on neuronal injury induced by diabetes in GK rats. H&E staining was performed on sections of the hippocampal CA1 region; magnification 40×. (A) The neurons in the hippocampal CA1 region of the rats in the CON group were neat and intact, and the cytoplasm and nuclei were full and clearly visible. (B) The neurons in the hippocampal CA1 region of were disturbed, loose, the number of cells was decreased, and the cells demonstrated nuclear pyknosis, chromatin aggregation, and cytoplasm reduction in the diabetic GK rat brains. (C) Ginsenoside treatment significantly prevented neuronal cell loss in the hippocampal CA1 region. (D) Neuronal density of each group. GK – Goto-Kakizaki; H&E – hematoxylin and eosin, CON – control.
Effects of ginsenoside on DM-induced changes in oxidative stress
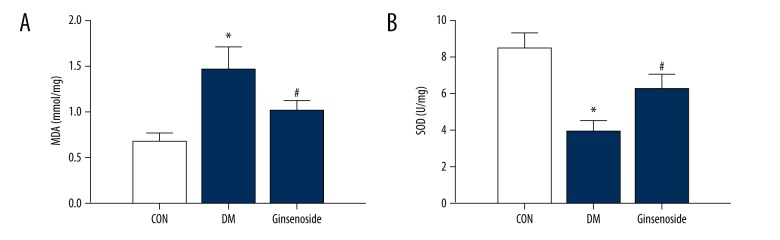
There was a significant increase in malondialdehyde (MDA) in the DM group. The oxidative production of MDA was significantly increased in the rats in the DM group. After ginsenoside administration, MDA activity was significantly decreased in the hippocampus compared with that in the DM group (Figure 6A). Additionally, superoxide dismutase (SOD) was obviously reduced in the hippocampus tissue from the DM group. However, administration of ginsenoside significantly increased SOD activity (Figure 6B).
Figure 6.
Hippocampal SOD and MDA levels in different groups (mean ±SD, n=5). Statistical analysis was performed using one-way ANOVA with LSD post hoc test; * P<0.05 vs. the CON group, # P<0.05 vs. the DM group. LSD, – least significant difference; SOD – superoxide dismutase; MDA – malondialdehyde, CON – control; DM – diabetic model.
Effects of ginsenoside on inflammatory cytokines in the hippocampus of GK rats
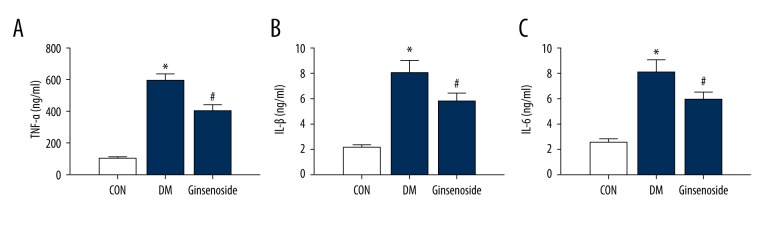
The expression levels of inflammatory factors including TNF-α, IL-1β, and IL-6 were obviously increased in the hippocampus of the GK rats (P<0.01) compared to those of the CON group (Figure 7A–7C). Ginsenoside treatment inhibited the inflammatory responses in the hippocampus of the diabetic GK rats.
Figure 7.
(A–C) TNF-α, IL-1β, and IL-6 levels in the hippocampus of the 3 groups. The results are expressed as the mean ±SD (n=5). Statistical analysis was performed using one-way ANOVA with LSD post hoc test; * P<0.05 vs. the CON group, # P<0.05 vs. the DM group. LSD – least significant difference; CON – control; DM – diabetic model.
Discussion
Diabetes is one of the most common chronic metabolic diseases, and its morbidity is positively correlated with age. In 2015, an epidemiological survey showed that the global incidence of diabetic patients aged 20 to 79 years old was approximately 8%, and the number is expected to reach to 9.9% by 2030 [19]. Cognitive dysfunction is an important complication of diabetes [20–22]. Recent studies have shown that the incidence of dementia increases with age in patients with diabetes mellitus. A prospective study supports this conclusion, stating that the risk of cognitive dysfunction in diabetic patients is 1.5 times higher than in non-diabetic patients.
Type 2 diabetes is associated with an increased risk of cognitive disfunction [23], mild cognitive impairment [24], and dementia. These results have been proven in middle-aged [25–27] and in old-age patients with diabetes. It is reported that even prediabetes stages have been related to an increased risk for cognitive impairment and an increased incidence of structural brain damage. Similarly, impaired fasting glucose is also related to cognitive decline. The pathogenesis of cognitive decline in diabetes is not clear. It is generally believed that it is closely related to apoptosis, neurotrophic factor abnormalities, and inflammatory factors.
SOD is an enzyme that catalyzes the partitioning of the superoxide radical into either non-toxic molecular oxygen or hydrogen peroxide. Hydrogen peroxide is continuously converted to H2O by glutathione peroxidase (GPx) using cytosolic GSH. When these constituents of the antioxidant system are reduced, ROS (reactive oxygen species) are not sufficiently scavenged, which causes oxidative stress and can induce lipid peroxidation and cell membrane deformation [28]. In addition, these stresses are known to continuously cause cell death by producing MDA and numerous free radicals. MDA is a secondary metabolite of lipid peroxidation, and it is used as a biomarker for oxidative stress during the onset of numerous diseases [28].
Ginseng is a traditional Chinese medicine which has a history of use of thousands of years. Ginsenoside is one of the active ingredients of ginseng, which has various pharmacological and physiological effects, such as life-extending, anti-fatigue, and anti-cancer effects. In addition, it is well known to people in most parts of the world, and now more and more people are paying attention to ginseng’s pharmacological action, especially in Asian countries such as Korea and Japan. Many studies have shown that it has been used to treat diabetes mellitus. Recent studies have demonstrated that ginsenoside has neuroprotective effects. In addition, it could play a major role in treating neurodegenerative diseases and central nervous system disorders. Increasing the number of newborn neurons, extending neurite growth and rescuing neurons have been proposed as possible mechanisms of ginsenoside by interacting with ligand-binding sites or channel pore sites in neuronal and heterologous expressed cells through regulating various types of ion channels and possibly inhibiting voltage-dependent Ca2+, K+, and Na+ channel activities [29]. It can also enhance cognitive performance and mood.
In this study, we found that the blood glucose levels of the diabetic GK rats were significantly increased, the rats’ weights were significantly reduced, and the water and food consumption increased significantly compared with those same measures of the Wistar rats in the CON group. After ginsenoside treatment, blood glucose was reduced, as well as water and food consumption. We also found that the GK rats with hyperglycemia showed a decline in learning and memory in the Morris maze test. Ginsenoside reversed cognitive decline. As shown in the H&E staining, the hippocampal neurons in the GK rats were shrunk and even necrotic, and ginsenoside reversed this phenomenon. We also tested the expression of inflammatory factors TNF-α, IL-1β, and IL-6 and the oxidative production of MDA and SOD. We found that the improvement in cognitive function by ginseng may be related to the expression of inflammatory factors and oxidative products.
In conclusion, the present study clearly demonstrated that ginsenoside improved cognitive decline in a diabetic GK rat model by decreasing oxidative stress and regulating the expression of inflammatory factors. Ginseng is a traditional Chinese medicine with few side effects and demonstrated safety, so it should be promoted clinically.
Conclusions
Ginsenoside could ameliorate diabetic cognitive decline. The possible mechanism was related to inhibiting brain oxidative/nitrosative damage and affecting the expression of cytokines such as IL-1β, IL-6, and TNF-α.
Footnotes
Source of support: Departmental sources
Conflicts of interest
None.
References
- 1.Biessels GJ, Staekenborg S, Brunner E, et al. Risk of dementia indiabetes mellitus: A systematic review. Lancet Neurol. 2006;5:64–74. doi: 10.1016/S1474-4422(05)70284-2. [DOI] [PubMed] [Google Scholar]
- 2.Katon W, Lyles CR, Parker MM, et al. Depression increases risk of dementia in patients with type 2 diabetes: The diabetes & aging study. Archives of General Psychiatry. 2011;69:410–17. doi: 10.1001/archgenpsychiatry.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure tocognitive function and dementia. Lancet Neurol. 2005;4:487–99. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 4.Kloppenborg RP, van den Berg E, Kappelle LJ, et al. Diabetes and othervascular risk factors for dementia: Which factor matters most? A systematicreview. Eur J Pharmacol. 2008;585:97–108. doi: 10.1016/j.ejphar.2008.02.049. [DOI] [PubMed] [Google Scholar]
- 5.Chatterjee A, Peters SA, Woodward M, et al. Type 2 diabetes as a risk factor for dementia in women compared with men: A pooled analysis of 2.3 million people comprising more than 100,000 cases of dementia. Diabetes Care. 2016;39:300–7. doi: 10.2337/dc15-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saedi E, Gheini MR, Faiz F, Arami MA. Diabetes mellitus and cognitive impairments. World J Diabetes. 2016;15:412–22. doi: 10.4239/wjd.v7.i17.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martín-Timón I, Sevillano-Collantes C, Segura-Galindo A, Del Cañizo-Gómez FJ, et al. Type 2 diabetes and cardiovascular disease: Have all risk factors the same strength? World J Diabetes. 2014;5:444–70. doi: 10.4239/wjd.v5.i4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Qi LW, Wang CZ, Yuan CS. American ginseng: Potential structure-function relationship in cancerchemoprevention. Biochem Pharmacol. 2010;l80:947–54. doi: 10.1016/j.bcp.2010.06.023. [DOI] [PubMed] [Google Scholar]
- 9.Wang CZ, Aung HH, Ni M, et al. Red American ginseng: Ginsenoside constituents and antiproliferative activities of heat-processed Panax quinquefolius roots. Planta Med. 2007;73:669–74. doi: 10.1055/s-2007-981524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang CZ, Li XL, Wang QF, et al. Themitochondrial pathway is involved in American ginseng-induced apoptosis of SW-480 coloncancer cells. Oncol Rep. 2009;21:577–54. doi: 10.3892/or_00000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu M, Zhang J. Effects of ginsenoside Rb1 and Rg1 on synaptosomal freecalcium level, ATP ase and calmodulin in rat hippecampus. Chin Med J (Eng J) 1995;108:544. [PubMed] [Google Scholar]
- 12.Pan MH, Chiou YS, Tsai ML, Ho CT. Anti-inflammatory activity of traditional Chinese medicinal herbs. J Tradit Complement Med. 2011;1:8–24. doi: 10.1016/s2225-4110(16)30052-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Du J, Cui C-H, Sung Park C, et al. Activation of multiple effector pathwaysof immune system by the antineoplastic immunostimulatoracidicpolysaccharideginsan isolated from panax ginseng. PLoS One. 2014;9:e96914. [Google Scholar]
- 14.Christensen LP. Ginsenosides chemistry, biosynthesis, analysis, and potential health effects. Adv Food Nutr Res. 2009;55:1–99. doi: 10.1016/S1043-4526(08)00401-4. [DOI] [PubMed] [Google Scholar]
- 15.Attele AS, Zhou YP, Xie JT, et al. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51:1851–58. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- 16.Dey L, Xie JT, Wang A, et al. Anti-hyperglycemic effects of ginseng: Comparison between root and berry. Phytomedicine. 2003;10:600–5. doi: 10.1078/094471103322331908. [DOI] [PubMed] [Google Scholar]
- 17.Xie JT, Zhou YP, Dey L, et al. Ginseng berry reducesblood glucose and body weight in db/db mice. Phytomedicine. 2002;9:254–58. doi: 10.1078/0944-7113-00106. [DOI] [PubMed] [Google Scholar]
- 18.Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature Protocols. 2011;1:848–58. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.International Diabetes Federation. IDF Diabetes Atlas. 5th edition. 2011. [Google Scholar]
- 20.Saczynski JS, Jónsdóttir MK, Garcia ME, et al. Cognitive impairment: an increasingly important complication of type 2 diabetes: The age, gene/environmentsusceptibility – Reykjavik study. Am J Epidemiol. 2008;168:1132–39. doi: 10.1093/aje/kwn228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fontbonne A, Berr C, Ducimetiere P, et al. Changes in cognitive abilitiesover a 4-year period are unfavorably affected in elderly diabetic subjects: Resultsof the epidemiology of vascular aging study. Diabetes Care. 2001;24:366–70. doi: 10.2337/diacare.24.2.366. [DOI] [PubMed] [Google Scholar]
- 22.Arvanitakis Z, Wilson R, Li Y, et al. Diabetes and function indifferent cognitive systems in older individuals without dementia. Diabetes Care. 2006;29:560–65. doi: 10.2337/diacare.29.03.06.dc05-1901. [DOI] [PubMed] [Google Scholar]
- 23.Arvanitakis Z, Wilson RS, Bienias JL, et al. Diabetes mellitus and risk ofAlzheimer disease and decline in cognitive function. Arch Neurol. 2004;61:661–66. doi: 10.1001/archneur.61.5.661. [DOI] [PubMed] [Google Scholar]
- 24.Luchsinger JA, Reitz C, Patel B, et al. Relation of diabetes to mildcognitive impairment. Arch Neurol. 2007;64:570–75. doi: 10.1001/archneur.64.4.570. [DOI] [PubMed] [Google Scholar]
- 25.Luchsinger JA, Tang MX, Stern Y, et al. Diabetes mellitus and risk of Alzheimer’sdisease and dementia with stroke in a multiethnic cohort. Am J Epidemiol. 2001;154:635–41. doi: 10.1093/aje/154.7.635. [DOI] [PubMed] [Google Scholar]
- 26.Luchsinger JA. Adiposity, hyperinsulinemia, diabetes and Alzheimer’s disease: An epidemiologicalperspective. Eur J Pharmacol. 2008;585:119–29. doi: 10.1016/j.ejphar.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schnaider Beeri M, Goldbourt U, Silverman JM, et al. Diabetes mellitus in midlife and the riskof dementia three decades later. Neurology. 2004;63:1902–7. doi: 10.1212/01.wnl.0000144278.79488.dd. [DOI] [PubMed] [Google Scholar]
- 28.Kim JM, Park CH, Park SK, et al. Ginsenoside re ameliorates brain insulin resistance and cognitive dysfunction in high-fat diet-induced C57BL/6 mice. J Agric Food Chem. 2017;65:2719–29. doi: 10.1021/acs.jafc.7b00297. [DOI] [PubMed] [Google Scholar]
- 29.Qi LW, Wang CZ, Yuan CS. Ginsenosides from American ginseng: Chemical andpharmacological diversity. Phytochemistry. 2011;72:689–99. doi: 10.1016/j.phytochem.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]