Abstract
Cancer immunotherapy aims to selectively target and kill tumor cells whilst limiting the damage to healthy tissues. Controlled delivery of plant, bacterial and human toxins or enzymes has been shown to promote the induction of apoptosis in cancerous cells. The 4th generation of targeted effectors are being designed to be as humanized as possible—a solution to the problem of immunogenicity encountered with existing generations. Granzymes are serine proteases which naturally function in humans as integral cytolytic effectors during the programmed cell death of cancerous and pathogen-infected cells. Secreted predominantly by cytotoxic T lymphocytes and natural killer cells, granzymes function mechanistically by caspase-dependent or caspase-independent pathways. These natural characteristics make granzymes one of the most promising human enzymes for use in the development of fusion protein-based targeted therapeutic strategies for various cancers. In this review, we explore research involving the use of granzymes as cytolytic effectors fused to antibody fragments as selective binding domains.
Keywords: cancer immunotherapy, granzyme B (GrB), human cytolytic fusion proteins (hCFPs), immunotoxins (ITs)
1. Introduction to Targeted Therapy and Humanization of Immunotoxins
An intervention that selectively repairs or kills cancerous cells, with no off-target related toxicity, represents the gold standard of targeted elimination of unwanted cells. One of the most promising approaches to date is based on protein engineering. Over the past 30 years, improvements in medical biotechnology have supported the development of four generations of therapeutic cell targeting ligand-toxin/enzyme named immunotoxins [1,2]. Immunotoxins (ITs) are bifunctional molecules consisting of a cell-specific binding ligand genetically fused or chemically conjugated to a cytotoxic component. The 1st and 2nd generations of ITs were produced by chemically conjugating native bacterial (Pseudomonas aeruginosa Exotoxin A) or plant toxins (ricin and gelonin) to full-length murine antibodies [3,4,5]. However, several challenges were encountered, ranging from poor specificity and stability to heterogeneity. Fortunately, through the use of recombinant DNA technology, the 3rd generation of ITs have been introduced, consisting primarily of a single-chain variable fragment (scFv) directly linked to bacterial toxins [6,7]. Such recombinant ITs exhibit better efficacy and tumor penetration. Though a step in the right direction, immune rejection remains a debilitating problem. Hence, this has led to the rise of the latest and 4th generation of ITs termed as human cytolytic fusion proteins (hCFPs). These hCFPs are designed by replacing existing toxins with human pro-apoptotic proteins capable of inducing cell death [8,9,10,11,12,13,14].
Therefore, the combined specificity of targeting the human ligand and the apoptosis-inducing effector protein offers a palpable impact, including reduced immunogenicity and toxicity, high selectivity and increased tumor penetration [15]. Of the various human apoptosis-inducing enzymes, granzyme B (GrB)-mediated apoptosis of target cells has been clinically associated with improved patient outcomes for various types of cancers. Human GrB belongs to a family of five serine proteases called granzymes, which were discovered in the cytoplasmic granules of natural killer (NK) cells and cytotoxic T lymphocytes (CTLs) in the 1980s [16,17,18]. Since their discovery, scientists have attempted to investigate the major role that they play in the destruction of malignant or virus-infected cells [19]. Additionally, in furtherance of the development of hCFPs, researchers have also explored the apoptosis-inducing mechanisms of GrB [20,21,22]. On that account, this review represents an updated understanding of the importance of GrB in the establishment of recent hCFPs.
2. Granzyme B and Its Anti-Tumor Activity
GrB (32-kDa) is reported to be the most potent of all the human granzymes produced by CTLs [22]. Due to its cytotoxic nature, it is expressed as an inactive prepro-enzyme and becomes functional by the removal of two pro-peptide residues (Gly-Glu dipeptide from its N-terminus) by lysosomal dipeptidyl peptidase I/cathepsin C [23]. Its biological activity during a CTL or NK cell-mediated immune response is dependent upon: (i) co-release with pore forming proteins called perforin towards target cells at intercellular spaces called immunological synapses [21,24]; (ii) successful entry into the cytosol of the cell (an event still broadly debated and hypothesized to be mediated by perforin, either through the formation of holes in the cell membrane or through destabilization of the ionic gradient to allow pore-formation in endosomal vesicles [25]); (iii) activation of several pro-apoptotic pathways by proteolytically attacking several intracellular protein substrates.
While up to about 300 intracellular proteins have been identified in humans as potential GrB substrates [22], only a few have been confirmed to be related to GrB-mediated apoptosis. For instance, the activation of multiple caspase family members (–3, –6, –7, –8, –9, –10) and cleavage of BH3-only pro-apoptotic protein (Bid) are well demonstrated in literature [26]. A nuclear pro-apoptotic pathway has also been reported for human GrB and involves cleavage of cell cycle regulatory proteins and/or kinase cell division cycle (CDC) activation. The potential of GrB to directly trigger post-caspase cytoplasmic apoptotic death pathway has also been described [23]. Therefore, the ability to activate multiple pro-apoptosis inducing pathways (including the induction of DNA fragmentation) in target cells, is what makes the development of GrB-based fusion proteins an attractive solution for cancer therapy.
Although highly efficient in its apoptosis-inducing mechanisms, the design of granzyme-based targeted therapeutics suffers an important hurdle: GrB possesses a number of basic amino acids on its surface, which results in a high isoelectric point [27]. This culminates in the unspecific binding or uptake of GrB by negatively charged cells. Reduced specificity increases the probability of immunogenicity, thus decreasing the therapeutic potential of the immunotoxin. Additionally, to prevent CTLs and by-standing cells from GrB mediated-cleavage, its enzymatic activity is tightly controlled by the presence of inhibitor Serpin B9 (PI-9, also known as proteinase inhibitor 9). PI-9 first forms a reversible Michaelis-like complex with GrB and then covalently binds to it with a stoichiometric ratio of 1:1 [28]. The up-regulation of PI-9 is useful for health cells, however tumor cells evade destruction by producing this inhibitor and making GrB-based targeted therapies ineffective.
To mitigate these shortcomings, continuous innovation in the design of GrB-based cytolytic fusion proteins has enabled steadily improved performance. The details of these are outlined in the following section.
Granzyme B in Targeted Therapy
The use of GrB in targeted therapy was pioneered simultaneously by the laboratories of Michael G. Rosenblum (M.D Anderson Cancer Center, Houston, Texas, USA) and Stefan Barth (IDM, Faculty of Health Sciences, University of Cape Town, South Africa) about a decade ago [29]. This novel concept involved the genetic fusion of an antibody moiety or derivative of a natural ligand targeting a surface protein or receptor on a diseased cell to the carboxyl end of GrB. Corresponding fusion proteins named immunoproteases allowed the selective ligand-mediated uptake and delivery of GrB into the target cells in the absence of perforin (Figure 1). Since then, a series of GrB-based hCFPs targeting different surface proteins on target cells have been developed for various diseases (Table 1). For instance, a GrB-H22(scFv) fusion protein has been shown to be effective in the killing of CD64+ U937 cells (lymphoblasts from human lung) and cells from acute myeloid leukaemia patients expressing CD64 as a surface receptor [30]. A significant activation of caspase-3 in the lysate of GrB-targeted treated cells when compared with the untreated control confirmed the potential of the GrB-H22(scFv) fusion protein to initiate apoptosis in target cells. Using a similar approach, early studies from the group of Rosenblum also demonstrated the therapeutic potential of GrB-based constructs. A GrB fusion to an anti-melanoma single chain antibody fragment (scFvMEL) targeting human A375-M melanoma cells induced apoptosis 8 h after treatment. Results showed both cleavage of caspase-3 and release of cytochrome C from the mitochondria into the cytosolic compartment as apoptosis pathways were mediated by the GrB-scFvMEL construct [31]. Consequently, several GrB-based cytolytic fusion proteins have been developed and evaluated against various carcinomas [31,32,33,34]. In recent years, newer versions of GrB-based hCFPs have emerged against existing clinical limitations and are reviewed below.
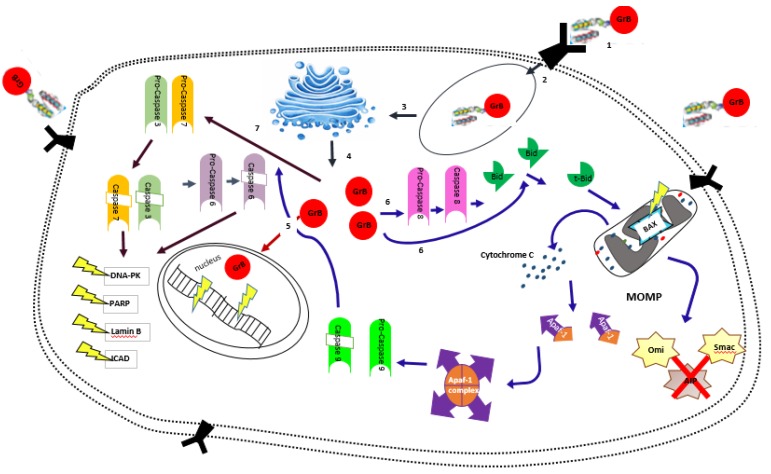
Figure 1.
Mechanism of action for the targeted delivery of GrB to the tumor cell. (1) Binding of GrB hCFP to receptor on cell membrane; (2) Take up of hCFP into the cell; (3) Routing and processing in ER and Golgi leading to endosomal escape of GrB; (4) Active GrB released into the cytosol; (5) Direct cleavage of nuclear machinery; (6) Initiation of the mitochondrial/caspase independent pathway. GrB cleaves procaspase-8 leading to caspase-8 activation. Caspase-8 cleaves the BH3 interacting domain death antagonist (BID) leading to the activation of truncated Bid (t-Bid). T-Bid translocates into the mitochondria and activates pro-apoptolyic protein BAX. This leads to mitochondrial outer membrane permealization (MOMP) and release of cytochrome C, Smac/DIABLO and OMI/HTRA2 which promote the blocking of inhibitor of apoptosis protein (IAP). Cytochrome C binds to the apoptotic protease activating factor (APAF-1) in the presence of dATP, leading to the formation of the apotosome which activates procaspase 9. Activated caspase-9 cleaves procaspase-3 and -7; (7) Initiation of the caspase dependent pathway. GrB directly cleaves procaspase-3 and -7 leading to the activation of the executioner caspases -3 and -7. These caspases cleave procaspase-6 leading to activated caspase-6. Caspase-3, -6 and -7 cleave downstream substrates such as PARP (poly(ADP-ribose) polymerase), DNA-PK (DNA-dependent protein kinase), Lamin B and ICAD (inhibitor of caspase activated DNase).
Table 1.
Granzyme B based human Cytolytic Fusion Proteins (hCFPs) resistant to serpin B9 inhibition, targeting surface proteins expressed in various cancers.
| Construct | Disease * | Target | Cell Line | P19 Expression in Cell Line | Cytotoxicity | Reference |
|---|---|---|---|---|---|---|
| GrB (wt)-H22(scFv) and GrBR201K-H22(scFv) | CMML | CD64+ | Cells from AMML and CMML patients. | Yes | Not specified | [35] |
| CD64+ HL60 | No | 4–7 nM | ||||
| GrB (wt)-ki4(scFv) and GrBR201K-Ki4(scFv) | cHL | CD30+ | L428 | Yes | [12] | |
| L540cy | No | |||||
| GrBR201K-scFv1711 | Epidermoid cancer cells | EGFR+ | A431 | Yes | 133.3 nM | [36] |
| RD target cells | Yes | 21.1 nM | ||||
| GrBR201K-αEpCAM(scFv) | TNBC | EpCAM+ | MDA-MB-231 | Yes | N/A | [37] |
| MDA-MB-468 | yes | 221 nM | ||||
| MDA-MB-453 | No | 307 nM |
* AMML, Acute myelomonocytic leukemia; CMML, Chronic myelomonocytic leukemia; cHL, Classical Hodgkin’s lymphoma; TNBC, Triple-negative breast cancer.
3. Reducing the Off-Target Toxicity of Granzyme B for Future Targeted Therapies
Over the years, clinical limitations of GrB-based constructs have driven research into the development of highly innovative constructs that can efficiently deliver active/cleaved GrB into the cytosol of target cells following systemic application without any therapeutic limitation. Below, we document data on highly improved versions of GrB-based hCFPs produced by Kurschus and Bird in the last decade (Table 2).
Table 2.
Granzyme B mutants for improved specific binding and reduced off-target toxicity.
| Granzyme B Variant | Mutation | Implication of Mutation | Result | Reference |
|---|---|---|---|---|
| GzmBFacD | The kktmrkry sequence at the C-terminus was replaced with the acidic peptide DSVLA derived from human complement factor D | This sequence motif is not positively charged and should have little immunogenic potential because complement factor D occurs at relatively high levels in human plasma | Binding to HL60 cells was completely abolished | [21] |
| GzmBKD | The region around K127 and K131 is known to function as a heparin binding site in thrombin. To stabilize this, both lysines were replaced with aspartate residues | Reduced HS binding | Reduced binding to HL60 cells compared to wild type GrB | [21] |
| GzmBKD-FacD | Double mutant consisting with aspartate replacement at position K127 and K131 and the acidic C-terminal peptide DSVLA | Combined effect of mutation | The binding and internalization efficiency was completely abolished | [21] |
| cs1 | Arginine in position 110, 114 and 116 (R110, R114, and R116) replaced with alanine. Constitutes an altered classical GAG-binding motif | Most proteins bind GAG. This is dependent on electrostatic interaction between the positively and negatively charged cells. Mutation in this region alters binding of GrB to negatively charged cells | Reduced cytotoxic activity. 20-fold less cytotoxic compared to wild type GrB. Abolished binding to Heparin region | [27] |
| cs2 | Lysine in position 239, 240, 243 and 244 (K239, K240, K243, and K244) replaced with alanine. Constitutes an altered C-terminal helix | Amphipathic C-terminal helix that has paired basic residues that bind GAGs. Mutation in this region alters binding of GrB to negatively charged cells. | Reduced cytotoxic activity. 2.5-fold less cytotoxic compared to wild type GrB. Reduced binding to Heparin region | [27] |
| cs1+2 | Combined mutation of cs1 and cs2 | Combined mutation of cs1 and cs3 | Reduced cytotoxic activity. 20-fold less cytotoxic compared to wild type GrB. Abolished binding to Heparin region | [27] |
Off-target toxicity and bioavailability of immunoconjugates are important topics in the clinical evaluation of targeted therapies. In general, the lack of disease specific surface target is the key reason for off-target toxicity in targeted therapy. The other possibility is the ability of immunoconjugates (recombinant GrB) to bind to various types of receptor in addition to the receptor of interest, say for example, GrB has the ability to bind and activate PAR-1 receptor and induce neuronal apoptosis in vitro and in vivo [38]. Jabulowsky stated that recombinant GrB binds to non-target tissues by forming electrostatic interactions between the heparin-binding motifs RKAKRTR and KKTMKR of GrB with glucosaminoglycans found on the cell surface of these non-target tissues. The author then intended to replace the positively charged amino acids at 116, 120, 122, 241, 242, 245 and 246 located in the heparin binding motifs to Alanine, and generated mutant GrBcs which show similar enzymatic activity and cytotoxicity, and reduced unspecific binding to non-targeted tissues, as compared to wild type GrB [39]. This will eventually not only reduce off-target toxicity, but also increase the bioavailability (increase the amount) of GrB in targeted cells, as non-specific binding could limit the amount of protein available for tumor-specific cell killing [39]. In natural GrB that are exocytosed by NK cells and T cells, the positively charged amino acids in heparin binding motifs and other motifs form complexes with negatively charged sulphate proteoglycan serglycin (SG), resulting in the shielding of its positively charged surface [39,40]. Thus, the use of GrB-SG complex as a therapeutic moiety can be used to attenuate the problem of off-target toxicity. Another method would be to encapsulate the recombinant GrB with another protein to prevent non-specific binding of GrB.
Moreover, as mentioned previously, endogenous GrB is in its inactive form when it bears the N-terminal Gly-Glu dipeptide, which is cleaved off by dipeptidyl peptidase cathepsin C (CatC) in lytic granules inside immune cell and frees the newly N-terminal Ile 16 residue to form a salt bridge with Asp 194 [23,41]. The resulting conformational change enables the simultaneous generation of an oxyanion hole and maturation of the active-site S1 pocket. This active form of endogenous GrB is released from lytic granules and has the ability to kill any target cell it enters. Based on this fundamental concept, scientists started to use the inactive form of GrB by filling the free NH2 terminus with an N-terminal inhibitory peptide (SUMO1 peptide) which can be selectively removed by cognate proteases, called sentrin-specific proteases (SENPs). These SENPs are highly expressed in tumor cells and can be used to activate GrB’s catalytic function. Such engineered GrB molecules would require both a surface-bound antigen and an intracellular protease to trigger target-cell apoptosis, thereby increasing targeting specificity and the safety profile of GrB-based therapeutics [42].
4. Overcoming Natural Granzyme Inhibitors
Cytotoxic T Lymphocytes (CTLs) and Natural Killer (NK) cells are the main immune effector cells responsible for killing infected and transformed cells. They do this by delivering cytotoxic granules (Granzyme B) stored in lysosome-related organelles (LROs), into the targeted cell cytoplasm [43]. The LRO fuses with the plasma membranes and releases GrB into a synapse or cleft between the effector and target cell [44]. Synaptic release of GrB from CTLs may cause fratricide and suicide of CTL/NK cells. This happens when GrB leaks back into the immune cell. In addition, secreted GrB can be endocytosed by CTLs or bystander cells and be released in the cytoplasm after endolysosomic stress [45], culminating in fratricide and/or suicide. To avoid this self-killing mechanism and to protect CTLs and NK cells from leaked GrB, the cell has its own natural nucleocytoplasmic protease inhibitor PI-9 which inactivates GrB proteases by acting as suicide substrates [46] through the formation of a 1:1 complex [47]. The release of GrB into cytosol in CTL/NK cells is tightly controlled and is not accidental. Here, the removal of immune cells that are under constant stress conditions becomes essential; repeated stimulation of activated CTLs and NK cells will lead to LRO damage and lysosomal membrane permeabilization, which releases the lysosomal contents (GrB) into the cytosol. During this process, the protective mechanism of PI-9 in CTLs is aborted and the cell undergoes activation-induced cell death (AICD). Thus, the survival of CTLs and NK cells depends on the balance between the amount of GrB released and the level of active PI-9 in the cytosol [43], which varies when cell is under stress.
Certain cancers of the lung [48], breast [49] and prostate [50] have increased levels of PI-9 in the cytosol that evade immune destruction, thereby hampering the therapeutic use of recombinant wild type GrB as a cytotoxic agent [37]. However, the findings from some studies beg to differ; in some, the endogenous presence of PI-9 has had no effect on the cytotoxic effect of their constructs [51,52]. In hypoxic conditions, cancer cells selectively degrade GrB by forming a fusion of amphisome (the fusion of enlarged endosomes containing GrB and autophagosomes) and lysosome [53]. To overcome this challenge, seven variants (R28A, R28E, R28K, R201A, R201E, R201K, K27A) of GrB were engineered by the Barth and Carloni group to be insensitive to PI-9 (Table 3). Their work was based primarily on using computational alanine scanning mutagenesis (CASM) to identify residues on GrB that might be crucial for its interaction with PI-9. Thereafter, some of these variants were selected and molecular dynamics simulations were carried out to determine the effect of these variants on the stability of the GrB-PI-9 complex in aqueous solution. Interestingly, the R201K variant was the most promising and resistant, as it maintained the same enzymatic activity in the presence and absence of recombinant PI-9 in our in vitro studies [54].
Table 3.
Granzyme B variants to improve cytotoxicity and bypass serpin B9 inhibition.
| Granzyme B Variant | Mutation | Implication of Mutation | References |
|---|---|---|---|
| R28A | Substitution of Arginine residue with Alanine (constitutes a neutral charge at position 28) | In the presence of PI-9 the GrBR28A mutant contains 54% activity | [54] |
| R28E | Substitution of Arginine residue with Glutamate (constitutes an opposite charge at position 28) | In the presence of PI-9 the GrBR28E mutant contains 25% activity | [54] |
| R28K | Substitution of Arginine residue with Lysine (constitutes an identical charge at position 28) | In the presence of PI-9, the GrBR28K and mutants retained 76% of their original activity | [54] |
| R201A | Substitution of Arginine residue with Alanine (constitutes a neutral charge at position 201) | In the presence of PI-9, the GrBR201A mutants retained 46% of their original activity | [54] |
| R201E | Substitution of Arginine residue with Glutamate (constitutes an opposite charge at position 201) | No activity in the presence of PI-9 | [54] |
| R201K | Substitution of Arginine residue with Lysine (constitutes an identical charge at position 28) | In the presence of PI-9, the GrBR201K mutant retained 94% of its activity | [54] |
| K27A | Substitution of Lysine residue with Alanine (constitutes a neutral charge at position 27) | Insensitive to P1-9 activity and K27A mutant showed a marked decrease in the ability to bind and cleave a substrate (substrate 3) containing P9 residues | [54] |
| R28A & R201A | Double mutant; Arginine replaced with Alanine at position 28 and 201 | In the presence of PI-9 the double mutant contains 0.5% activity | [54] |
| K27E & R28A (EA) | Double mutant; Lysine replaced with Glutamate at position 27 and Arginine replaced with Alanine at position 28 | In the presence of 50% human serum, the enzymatic activity of EA remained over 40% over 24 h | [41] |
| K27L & R28A (LA) | Double mutant; Lysine replaced with Leucine at position 27 and Arginine replaced with Alanine at position 28 | LA double mutant appeared to behave intermediate to the wild-type protein (GrB/VEGF121) and the EA construct | [41] |
More recently, Rosenblum and colleagues also reported similar mutations on the GrB primary sequence which reduced binding to PI-9. Two double mutants termed EA “K27E and R28A” and LA “K27L and R28A” respectively demonstrated insensitivity towards PI-9 without any effect on enzymatic activity [41]. Of note is that the enzymatic activity of EA was reported to remain over 40% in the presence of 50% human serum when compared to unmodified GrB-fused to VEGF121, whose enzymatic activity declined to less than 10% activity over the same time period [41].
5. Effective Cytosolic Delivery of Granzyme B to Target Cells: A Major Bottleneck
Lastly, a major limiting factor to the activity of existing GrB-based fusion proteins, is the effective translocation and delivery of the lethal GrB molecules from the endosomes to the cytosol of the target cell. In its natural state, GrB is secreted along with perforin, an endosomolytic agent that facilitates the cytosolic release of GrB from endocytic compartments [55]. However, the application of GrB as a human lead enzyme has led researchers to consider alternative methods to perforin-mediated cytosolic release. For instance, to allow the cytosolic uptake of GrB fusion proteins targeting EGFR/ErbB2, Dalken et al. used chloroquine to raise the pH value in endosomes and lysosomes, thereby culminating in the osmotic rupture of these vesicles [32]. In another study, the translocation domain of Pseudomonas exotoxin A (PE-II), was fused to a HER2(scFv) and GrB, demonstrating the effective killing of HER2-overexpressing cells both in vitro and in nude mice [56]. While acknowledging that this method induces tumor growth inhibition, Kurschus and colleague raise the possibility of immune rejection [57]. An alternative approach to consider would be the use of sophisticated protein transduction domains (PTD) flanked with cleavable adapters, which would allow the specific and efficient transport of human enzymes into the cytosol of target cells [58,59]. Further investigations are still required to determine whether other endosomolytic agents or adapter sequences can be used to increase the cytotoxic activity of GrB-based hCFPs.
6. Conclusions
Whilst GrB as the natural human apoptosis-inducing enzyme has clinical potential when delivered as a ligand-specific fusion protein, strict inhibition by PI-9 may cause resistance to therapy. As shown by the Barth and Carloni group [54], computational approaches can now be used to study enzyme-substrate interactions to greater depth. These might be a useful approach to consider for finding new mutations that might reduce PI-9 binding to GrB. Moreover, for effective translation of scientific research from bench to bedside, further in vitro and in vivo studies are urgently needed to assess the activity, efficacy and safety of promising mutant candidates described in this review. Such studies form the basis for the continued optimization of this effective human enzyme in the future of cancer therapy.
Acknowledgments
This work is partially based on the research supported by the South African Research Chairs Initiative of the Department of Science and Technology (DST) and National Research Foundation (NRF) of South Africa (Grant No. 47904). The NRF, the Cancer Association of South Africa, and the South African Research Chair in Cancer Biotechnology have provided bursaries to support students contributing to this work.
Author Contributions
P.H. and S.C. conceived and designed the paper; P.H., N.M., S.M. and O.A.A. wrote the paper; P.H., N.M., S.C. and S.B. contributed to the editing and narrative of the paper.
Funding
This research had no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Akbari B., Farajnia S., Ahdi Khosroshahi S., Safari F., Yousefi M., Dariushnejad H., Rahbarnia L. Immunotoxins in cancer therapy: Review and update. Int. Rev. Immunol. 2017;36:207–219. doi: 10.1080/08830185.2017.1284211. [DOI] [PubMed] [Google Scholar]
- 2.Liu W., Onda M., Lee B., Kreitman R.J., Hassan R., Xiang L., Pastan I. Recombinant immunotoxin engineered for low immunogenicity and antigenicity by identifying and silencing human B-cell epitopes. Proc. Natl. Acad. Sci. USA. 2012;109:11782–11787. doi: 10.1073/pnas.1209292109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao Y., Marks J.D., Huang Q., Rudnick S.I., Xiong C., Hittelman W.N., Wen X., Marks J.W., Cheung L.H., Boland K., et al. Single-Chain Antibody-Based Immunotoxins Targeting Her2/neu: Design Optimization and Impact of Affinity on Antitumor Efficacy and Off-Target Toxicity. Mol. Cancer Ther. 2012;11:143–153. doi: 10.1158/1535-7163.MCT-11-0519. [DOI] [PubMed] [Google Scholar]
- 4.Fuchs H., Weng A., Gilabert-Oriol R. Augmenting the efficacy of immunotoxins and other targeted protein toxins by endosomal escape enhancers. Toxins (Basel) 2016;8:200. doi: 10.3390/toxins8070200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X.-F., Xiang L., FitzGerald D.J., Pastan I. Antitumor Effects of Immunotoxins Are Enhanced by Lowering HCK or Treatment with Src Kinase Inhibitors. Mol. Cancer Ther. 2014;13:82–89. doi: 10.1158/1535-7163.MCT-13-0726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kessler C., Pardo A., Tur M.K., Gattenlöhner S., Fischer R., Kolberg K., Barth S. Novel PSCA targeting scFv-fusion proteins for diagnosis and immunotherapy of prostate cancer. J. Cancer Res. Clin. Oncol. 2017;143:2025–2038. doi: 10.1007/s00432-017-2472-9. [DOI] [PubMed] [Google Scholar]
- 7.Yu K., Hu Y., Tan Y., Shen Z., Jiang S., Qian H., Liang B., Shan D. Immunotherapy of lymphomas with T cells modified by anti-CD20 scFv/CD28/ CD3ζ recombinant gene. Leuk. Lymphoma. 2008;49:1368–1373. doi: 10.1080/10428190802064958. [DOI] [PubMed] [Google Scholar]
- 8.Bochicchio A., Jordaan S., Losasso V., Chetty S., Perera R., Ippoliti E., Barth S., Carloni P. Designing the Sniper: Improving Targeted Human Cytolytic Fusion Proteins for Anti-Cancer Therapy via Molecular Simulation. Biomedicines. 2017;5:9. doi: 10.3390/biomedicines5010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schiffer S., Hansen H.P., Hehmann-Titt G., Huhn M., Fischer R., Barth S., Thepen T. Efficacy of an adapted granzyme B-based anti-CD30 cytolytic fusion protein against PI-9-positive classical Hodgkin lymphoma cells in a murine model. Blood Cancer J. 2013;3:e106-7. doi: 10.1038/bcj.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akinrinmade O., Jordaan S., Hristodorov D., Mladenov R., Mungra N., Chetty S., Barth S. Human MAP Tau Based Targeted Cytolytic Fusion Proteins. Biomedicines. 2017;5:36. doi: 10.3390/biomedicines5030036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jordaan S., Chetty S., Mungra N., Koopmans I., van Bommel P., Helfrich W., Barth S. CSPG4: A Target for Selective Delivery of Human Cytolytic Fusion Proteins and TRAIL. Biomedicines. 2017;5:37. doi: 10.3390/biomedicines5030037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathew M., Verma R.S. Humanized immunotoxins: A new generation of immunotoxins for targeted cancer therapy. Cancer Sci. 2009;100:1359–1365. doi: 10.1111/j.1349-7006.2009.01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krauss J., Arndt M.A.E., Vu B.K., Newton D.L., Seeber S., Rybak S.M. Efficient killing of CD22+ tumor cells by a humanized diabody-RNase fusion protein. Biochem. Biophys. Res. Commun. 2005;331:595–602. doi: 10.1016/j.bbrc.2005.03.215. [DOI] [PubMed] [Google Scholar]
- 14.Arndt M.A.E., Vu B.K., Newton D.L., Rybak S.M. A Dimeric Angiogenin Immunofusion Protein Mediates Selective Toxicity Toward CD22+ Tumor Cells. J. Immunother. 2005;28:245–251. doi: 10.1097/01.cji.0000161396.96582.10. [DOI] [PubMed] [Google Scholar]
- 15.Nishimura Y., Tomita Y., Yuno A., Yoshitake Y., Shinohara M. Cancer immunotherapy using novel tumor-associated antigenic peptides identified by genome-wide cDNA microarray analyses. Cancer Sci. 2015;106:505–511. doi: 10.1111/cas.12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krzewski K., Coligan J.E. Human NK cell lytic granules and regulation of their exocytosis. Front. Immunol. 2012;3:1–16. doi: 10.3389/fimmu.2012.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Veugelers K., Motyka B., Frantz C., Shostak I., Sawchuk T., Bleackley R.C. The granzyme B-serglycin complex from cytotoxic granules requires dynamin for endocytosis. Blood. 2004;103:3845–3853. doi: 10.1182/blood-2003-06-2156. [DOI] [PubMed] [Google Scholar]
- 18.Martin S.J., Amarante-Mendes G.P., Shi L., Chuang T.H., Casiano C.A., O’Brien G.A., Fitzgerald P., Tan E.M., Bokoch G.M., Greenberg A.H., et al. The cytotoxic cell protease granzyme B initiates apoptosis in a cell-free system by proteolytic processing and activation of the ICE/CED-3 family protease, CPP32, via a novel two-step mechanism. EMBO J. 1996;15:2407–2416. [PMC free article] [PubMed] [Google Scholar]
- 19.Li P., Zheng G., Yang Y., Zhang C., Xiong P., Xu Y., Fang M., Tan Z., Zheng F., Gong F. Granzyme B is recovered by natural killer cells via clathrin-dependent endocytosis. Cell. Mol. Life Sci. 2010;67:3197–3208. doi: 10.1007/s00018-010-0377-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Afonina I.S., Tynan G.A., Logue S.E., Cullen S.P., Bots M., Lüthi A.U., Reeves E.P., McElvaney N.G., Medema J.P., Lavelle E.C., et al. Granzyme B-dependent proteolysis acts as a switch to enhance the proinflammatory activity of IL-1α. Mol. Cell. 2011;44:265–278. doi: 10.1016/j.molcel.2011.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurschus F.C., Fellows E., Stegmann E., Jenne D.E. Granzyme B delivery via perforin is restricted by size, but not by heparan sulfate-dependent endocytosis. Proc. Natl. Acad. Sci. USA. 2008;105:13799–13804. doi: 10.1073/pnas.0801724105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garcia-sanz J.A., Macdonald H.R., Jenne D.E., Nabholz M., Tschopp J. Cell specificity of granzyme gene expression. J. Immunol. 1990;145:3111–3118. [PubMed] [Google Scholar]
- 23.Cremer C., Hehmann-Titt G., Schiffer S., Melmer G., Carloni P., Barth S., Nachreiner T. Engineered Versions of Granzyme B and Angiogenin Overcome Intrinsic Resistance to Apoptosis Mediated by Human Cytolytic Fusion Proteins. In: Verma R.S., Bonavida B., editors. Resistance to Immunotoxins in Cancer Therapy. Springer; Cham, Switzerland: 2015. pp. 185–219. [Google Scholar]
- 24.Beseničar M.P., Metkar S., Wang B., Froelich C.J., Anderluh G. Granzyme B translocates across the lipid membrane only in the presence of lytic agents. Biochem. Biophys. Res. Commun. 2008;371:391–394. doi: 10.1016/j.bbrc.2008.04.071. [DOI] [PubMed] [Google Scholar]
- 25.Bots M., Medema J.P. Granzymes at a glance. J. Cell Sci. 2006;119:5011–5014. doi: 10.1242/jcs.03239. [DOI] [PubMed] [Google Scholar]
- 26.Phillips T., Opferman J.T., Shah R., Liu N., Froelich C.J., Ashton-Rickardt P.G. A role for the granzyme B inhibitor serine protease inhibitor 6 in CD8+ memory cell homeostasis. J. Immunol. (Baltimore, Md. 1950) 2004;173:3801–3809. doi: 10.4049/jimmunol.173.6.3801. [DOI] [PubMed] [Google Scholar]
- 27.Bird C.H., Sun J., Ung K., Karambalis D., Whisstock J.C., Trapani J.A., Bird P.I. Cationic Sites on Granzyme B Contribute to Cytotoxicity by Promoting Its Uptake into Target Cells. Mol. Cell. Biol. 2005;25:7854–7867. doi: 10.1128/MCB.25.17.7854-7867.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buzza M.S., Hirst C.E., Bird C.H., Hosking P., McKendrick J., Bird P.I. The granzyme B inhibitor, PI-9, is present in endothelial and mesothelial cells, suggesting that it protects bystander cells during immune responses. Cell. Immunol. 2001;210:21–29. doi: 10.1006/cimm.2001.1806. [DOI] [PubMed] [Google Scholar]
- 29.Rosenblum M.G., Barth S. Development of novel, highly cytotoxic fusion constructs containing granzyme B: Unique mechanisms and functions. Curr. Pharm. Des. 2009;15:2676–2692. doi: 10.2174/138161209788923958. [DOI] [PubMed] [Google Scholar]
- 30.Stahnke B., Thepen T., Stocker M., Rosinke R., Jost E., Fischer R., Tur M.K., Barth S. Granzyme B-H22(scFv), a human immunotoxin targeting CD64 in acute myeloid leukemia of monocytic subtypes. Mol. Cancer Ther. 2008;7:2924–2932. doi: 10.1158/1535-7163.MCT-08-0554. [DOI] [PubMed] [Google Scholar]
- 31.Liu Y., Cheung L.H., Thorpe P., Rosenblum M.G. Mechanistic studies of a novel human fusion toxin composed of vascular endothelial growth factor (VEGF)121 and the serine protease granzyme B: Directed apoptotic events in vascular endothelial cells. Mol. Cancer Ther. 2003;2:949–959. [PubMed] [Google Scholar]
- 32.Dälken B., Giesübel U., Knauer S.K., Wels W.S. Targeted induction of apoptosis by chimeric granzyme B fusion proteins carrying antibody and growth factor domains for cell recognition. Cell Death Differ. 2006;13:576–585. doi: 10.1038/sj.cdd.4401773. [DOI] [PubMed] [Google Scholar]
- 33.Kurschus F.C., Kleinschmidt M., Fellows E., Dornmair K., Rudolph R., Lilie H., Jenne D.E. Killing of target cells by redirected granzyme B in the absence of perforin. FEBS Lett. 2004;562:87–92. doi: 10.1016/S0014-5793(04)00187-5. [DOI] [PubMed] [Google Scholar]
- 34.Wang T., Zhao J., Ren J.L., Zhang L., Wen W.H., Zhang R., Qin W.W., Jia L.T., Yao L.B., Zhang Y.Q., et al. Recombinant immunoproapoptotic proteins with furin site can translocate and kill HER2-positive cancer cells. Cancer Res. 2007;67:11830–11839. doi: 10.1158/0008-5472.CAN-07-1160. [DOI] [PubMed] [Google Scholar]
- 35.Schiffer S., Rosinke R., Jost E., Hehmann-Titt G., Huhn M., Melmer G., Barth S., Thepen T. Targeted ex vivo reduction of CD64-positive monocytes in chronic myelomonocytic leukemia and acute myelomonocytic leukemia using human granzyme B-based cytolytic fusion proteins. Int. J. Cancer. 2014;135:1497–1508. doi: 10.1002/ijc.28786. [DOI] [PubMed] [Google Scholar]
- 36.Niesen J., Hehmann-Titt G., Woitok M., Fendel R., Barth S., Fischer R., Stein C. A novel fully-human cytolytic fusion protein based on granzyme B shows in vitro cytotoxicity and ex vivo binding to solid tumors overexpressing the epidermal growth factor receptor. Cancer Lett. 2016;374:229–240. doi: 10.1016/j.canlet.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 37.Amoury M., Kolberg K., Pham A.T., Hristodorov D., Mladenov R., Di Fiore S., Helfrich W., Kiessling F., Fischer R., Pardo A., et al. Granzyme B-based cytolytic fusion protein targeting EpCAM specifically kills triple negative breast cancer cells in vitro and inhibits tumor growth in a subcutaneous mouse tumor model. Cancer Lett. 2016;372:201–209. doi: 10.1016/j.canlet.2016.01.027. [DOI] [PubMed] [Google Scholar]
- 38.Wang T., Lee M.H., Choi E., Pardo-Villamizar C.A., Lee S.B., Yang I.H., Calabresi P.A., Nath A. Granzyme B-Induced Neurotoxicity Is Mediated via Activation of PAR-1 Receptor and Kv1.3 Channel. PLoS ONE. 2012;7 doi: 10.1371/journal.pone.0043950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jabulowsky R.A., Oberoi P., Bähr-Mahmud H., Dälken B., Wels W.S. Surface charge-modification prevents sequestration and enhances tumor-cell specificity of a recombinant granzyme B-TGFα fusion protein. Bioconjug. Chem. 2012;23:1567–1576. doi: 10.1021/bc3000657. [DOI] [PubMed] [Google Scholar]
- 40.Raja S.M., Metkar S.S., Höning S., Wang B., Russin W.A., Pipalia N.H., Menaa C., Belting M., Cao X., Dressel R., et al. A novel mechanism for protein delivery: Granzyme B undergoes electrostatic exchange from serglycin to target cells. J. Biol. Chem. 2005;280:20752–20761. doi: 10.1074/jbc.M501181200. [DOI] [PubMed] [Google Scholar]
- 41.Mohamedali K.A., Cheung L.H., Rosenblum M.G. Tumor-targeted fusion constructs containing engineered granzyme B variants with optimized stability and potency. Cancer Res. 2015;75:632 LP-632. doi: 10.1158/1538-7445.AM2015-632. [DOI] [Google Scholar]
- 42.Ho P., Ede C., Chen Y.Y. Modularly Constructed Synthetic Granzyme B Molecule Enables Interrogation of Intracellular Proteases for Targeted Cytotoxicity. ACS Synth. Biol. 2017;6:1484–1495. doi: 10.1021/acssynbio.6b00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bird C.H., Christensen M.E., Mangan M.S.J., Prakash M.D., Sedelies K.A., Smyth M.J., Harper I., Waterhouse N.J., Bird P.I. The granzyme B-Serpinb9 axis controls the fate of lymphocytes after lysosomal stress. Cell Death Differ. 2014;21:876–887. doi: 10.1038/cdd.2014.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cullen S.P., Martin S.J. Mechanisms of granule-dependent killing. Cell Death Differ. 2008;15:251–262. doi: 10.1038/sj.cdd.4402244. [DOI] [PubMed] [Google Scholar]
- 45.Kaiserman D., Bird P.I. Control of granzymes by serpins. Cell Death Differ. 2010;17:586–595. doi: 10.1038/cdd.2009.169. [DOI] [PubMed] [Google Scholar]
- 46.Azzi J., Ohori S., Ting C., Uehara M., Abdoli R., Smith B.D., Safa K., Solhjou Z., Lukyanchykov P., Patel J., et al. Serine protease inhibitor-6 differentially affects the survival of effector and memory alloreactive CD8-T cells. Am. J. Transplant. 2015;15:234–241. doi: 10.1111/ajt.13051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ashton-Rickardt P.G. An emerging role for Serine Protease Inhibitors in T lymphocyte immunity and beyond. Immunol. Lett. 2013;152:65–76. doi: 10.1016/j.imlet.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 48.Soriano C., Mukaro V., Hodge G., Ahern J., Holmes M., Jersmann H., Moffat D., Meredith D., Jurisevic C., Reynolds P.N., et al. Increased proteinase inhibitor-9 (PI-9) and reduced granzyme B in lung cancer: Mechanism for immune evasion? Lung Cancer. 2012;77:38–45. doi: 10.1016/j.lungcan.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 49.Jiang X., Ellison S.J., Alarid E.T., Shapiro D.J. Interplay between the levels of estrogen and estrogen receptor controls the level of the granzyme inhibitor, proteinase inhibitor 9 and susceptibility to immune surveillance by natural killer cells. Oncogene. 2007;26:4106–4114. doi: 10.1038/sj.onc.1210197. [DOI] [PubMed] [Google Scholar]
- 50.Ray M., Hostetter D.R., Loeb C.R.K., Simko J., Craik C.S. Inhibition of Granzyme B by PI-9 protects prostate cancer cells from apoptosis. Prostate. 2012;72:846–855. doi: 10.1002/pros.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao Y., Mohamedali K.A., Marks J.W., Cheung L.H., Hittelman W.N., Rosenblum M.G. Construction and characterization of novel, completely human serine protease therapeutics targeting Her2/neu. Mol. Cancer Ther. 2013;12:979–991. doi: 10.1158/1535-7163.MCT-13-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou H., Mohamedali K.A., Gonzalez-Angulo A.M., Cao Y., Migliorini M., Cheung L.H., LoBello J., Lei X., Qi Y., Hittelman W.N., et al. Development of human serine protease-based therapeutics targeting Fn14 and identification of Fn14 as a new target overexpressed in TNBC. Mol. Cancer Ther. 2014;13:2688–2705. doi: 10.1158/1535-7163.MCT-14-0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Baginska J., Viry E., Berchem G., Poli A., Noman M.Z., van Moer K., Medves S., Zimmer J., Oudin A., Niclou S.P., et al. Granzyme B degradation by autophagy decreases tumor cell susceptibility to natural killer-mediated lysis under hypoxia. Proc. Natl. Acad. Sci. USA. 2013;110:17450–17455. doi: 10.1073/pnas.1304790110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Losasso V., Schiffer S., Barth S., Carloni P. Design of human granzyme B variants resistant to serpin B9. Proteins Struct. Funct. Bioinform. 2012;80:2514–2522. doi: 10.1002/prot.24133. [DOI] [PubMed] [Google Scholar]
- 55.Froelich C.J., Orth K., Turbov J., Seth P., Gottlieb R., Babior B., Shah G.M., Bleackley R.C., Dixit V.M., Hanna W. New Paradigm for Lymphocyte Granule-mediated Cytotoxicity. J. Biol. Chem. 1996;271:29073–29079. doi: 10.1074/jbc.271.46.29073. [DOI] [PubMed] [Google Scholar]
- 56.Zhao J., Zhang L.H., Jia L.T., Zhang L., Xu Y.M., Wang Z., Yu C.J., Peng W.D., Wen W.H., Wang C.J., et al. Secreted antibody/granzyme B fusion protein stimulates selective killing of HER2-overexpressing tumor cells. J. Biol. Chem. 2004;279:21343–21348. doi: 10.1074/jbc.M312648200. [DOI] [PubMed] [Google Scholar]
- 57.Kurschus F.C., Jenne D.E. Delivery and therapeutic potential of human granzyme B. Immunol. Rev. 2010;235:159–171. doi: 10.1111/j.0105-2896.2010.00894.x. [DOI] [PubMed] [Google Scholar]
- 58.Hetzel C., Bachran C., Fischer R., Fuchs H., Barth S., Stöcker M. Small cleavable adapters enhance the specific cytotoxicity of a humanized immunotoxin directed against CD64-positive cells. J. Immunother. 2008;31:370–376. doi: 10.1097/CJI.0b013e31816a2d23. [DOI] [PubMed] [Google Scholar]
- 59.Fuchs H., Bachran C., Li T., Heisler I., Dürkop H., Sutherland M. A cleavable molecular adapter reduces side effects and concomitantly enhances efficacy in tumor treatment by targeted toxins in mice. J. Control. Release. 2007;117:342–350. doi: 10.1016/j.jconrel.2006.11.019. [DOI] [PubMed] [Google Scholar]