Abstract
Calcium ion (Ca2+) serves as a second messenger for a variety of cell functions in trypanosomes. Several proteins in the plasma membrane, acidocalcisomes, endoplasmic reticulum, and mitochondria are involved in its homeostasis and in cell signaling roles. The plasma membrane has a Ca2+ channel for its uptake and a plasma membrane-type Ca2+-ATPase (PMCA) for its efflux. A similar PMCA is also located in acidocalcisomes, acidic organelles that are the primary Ca2+ store and that possess an inositol 1,4,5-trisphosphate receptor (IP3R) for Ca2+ efflux. Their mitochondria possess a mitochondrial calcium uniporter complex (MCUC) for Ca2+ uptake and a Ca2+/H+ exchanger for Ca2+ release. The endoplasmic reticulum has a sarcoplasmic-endoplasmic reticulum-type Ca2+-ATPase (SERCA) for Ca2+ uptake but no Ca2+ release mechanism has been identified. Additionally, the trypanosomatid genomes contain other membrane proteins that could potentially bind calcium and await further characterization.
Keywords: calcium, Trypanosoma, Leishmania, mitochondria, acidocalcisome
1. Introduction
Trypanosomatids are a group of unicellular parasitic organisms. This group includes two important genera: Trypanosoma and Leishmania, with several species that cause severe diseases in humans. Trypanosoma brucei and Trypanosoma cruzi cause African sleeping sickness and Chagas disease, respectively. Leishmania species are the causative agents for cutaneous, mucocutaneous and visceral leishmaniases. T. brucei is an extracellular parasite that replicates in the digestive system of tsetse flies as procyclic form (PCF) and in the tissue fluids and blood of mammals as bloodstream form (BSF). Similar to T. brucei, T. cruzi and Leishmania spp. replicate extracellularly in the midgut of their insect vectors as epimastigotes and promastigotes, respectively. However, once in the mammalian host, they both replicate intracellularly as amastigotes. While Leishmania amastigotes propagate further by infecting other macrophages, T. cruzi amastigotes convert to trypomastigotes before host cell lysis and enters the circulatory system to infect other nucleated cells.
Ca2+ signaling pathways in trypanosomatids are highly divergent from those in the mammalian hosts they infect. As a result, these pathways have been researched thoroughly for identification of potential targets for drugs, vaccines, and diagnostic tools. In the process, we have learned that these parasites possess many unique aspects that are central to their Ca2+ signaling network.
Both Trypanosoma and Leishmania species possess a single mitochondrion. Similar to mammalian cells, the mitochondrial Ca2+ uptake is mediated by a mitochondrial Ca2+ uniporter (MCU) complex. The discovery that trypanosomes have a MCU with similar physiological properties as the mammalian uniporter [1,2] was essential [3] for the discovery of the genes encoding the gatekeeper mitochondrial calcium uptake 1 (MICU1) [4] and the pore (MCU) [5,6] of the channel. MCU is indispensable for parasite growth and infectivity [7] while the channel is not essential in some mouse strains [8].
Moreover, while mammalian cells use the endoplasmic reticulum (ER) as a main Ca2+ storage site, trypanosomatids store most of their Ca2+ in an acidic store named the acidocalcisome [9]. Interestingly, while the Ca2+ export channel, inositol 1,4,5-trisphosphate receptor (IP3R), localizes to the ER in mammals, it localizes to acidocalcisomes in trypanosomatids [10,11]. In this review, we highlight differences in Ca2+ homeostasis and signaling pathways in Trypanosoma and Leishmania species as compared to those in their mammalian hosts. We indicate the specific proteins that mediate Ca2+ import and export in various organelles and identify proteins that have the potential to bind Ca2+ and participate in their overall Ca2+ homeostasis.
2. Significance of Ca2+ Signaling in Trypanosomatids
During evolution, two alkali metals, calcium, and magnesium, were present in high concentrations in the primordial soup. Both these elements had similar chemical properties and were chosen to perform many important functions in evolving cells and organisms. However, for inter and intracellular communication, calcium was chosen over magnesium as an important signaling regulator. Both calcium and magnesium can form stable cations by readily losing two electrons. However, calcium, with a larger ionic radius, loses its outermost electrons more easily than magnesium. This enabled calcium to readily participate in multiple chemical reactions that led to the evolution of life. The larger ionic radius also provided calcium with larger polarizability. This meant that calcium ions were highly flexible and therefore able to interact with sites of irregular geometry in various proteins [12]. Additionally, phosphate-based energetics, which also evolved early during evolution also preferred calcium as the main signaling ion due to its lower charge density [13]. Therefore, during evolution calcium emerged as an important cellular messenger. In eukaryotes, many important processes such as cell division, metabolism, motility, regulation of cell death, etc., are all governed through Ca2+ signaling. Similar to other eukaryotes, Ca2+ plays a ubiquitous role in trypanosomatids as well.
A variety of proteins that could be modulated by Ca2+ have been found in trypanosomatid genomes. Putative Ca2+-calmodulin dependent kinases have been identified [14]. In eukaryotes, calmodulin (CaM) kinases participate in gene transcription, translation, ion channel regulation as well as cell death processes [15]. In T. cruzi a CaM kinase II, which is activated by reactive oxygen species [16], was shown to play a role in heme-induced proliferation of parasites [17]. A CaM kinase from T. cruzi was also purified and its enzymatic characteristics were analyzed in detail [18,19].
Ca2+ activates the T. cruzi phosphoinositide phospholipase C (PI-PLC). This enzyme localizes to the plasma membrane and is upregulated during trypomastigote to amastigote conversion, indicating a potential role for Ca2+ in parasite differentiation [20]. This is supported by the observation that cytosolic Ca2+ concentration changes occur during differentiation of T. cruzi epimastigotes to metacyclic trypomastigotes [21]. Similarly, cytosolic Ca2+ concentration changes also occur during differentiation of T. brucei bloodstream forms to procyclic forms [22].
A T. cruzi adenylyl cyclase, which interacts with a paraflagellar rod protein is known to be activated by Ca2+ indicating a possible role for Ca2+ signaling in parasite motility [23]. The role for Ca2+ in parasite motility is further corroborated by the parasite centrins. Centrins are well known Ca2+ binding proteins. Out of the five centrins found in T. brucei, three localize to the flagellar basal body [24]. They play a role in flagellar motility [24] but are also necessary for organelle segregation [25] and cell division [26]. Similarly, a centrin in Leishmania donovani localizes to the basal body and is indispensable for basal body duplication and cell division [27,28] thereby highlighting how Ca2+ binding proteins could be playing a pivotal role in trypanosomatid motility and cell division.
Another protein that is activated by Ca2+ in trypanosomatids is the T. cruzi calcineurin [26,29]. Inhibition of calcineurin activity or downregulation of its expression inhibits parasite invasion of HeLa cells suggesting a role for Ca2+ in parasite invasion [29]. In support of its role in invasion, significant increase in intracellular Ca2+ is observed during parasites interaction with their host cells [30,31]. Prevention of this Ca2+ increase in the parasites results in decreased invasion for both T. cruzi [30] and Leishmania amazonensis [31]. Additionally, chelation of Ca2+ also impairs invasion of host cells [32]. Ca2+ has also been proposed to function in parasite osmoregulation [33], maintenance of cytoskeleton [34], and regulation of programmed cell death [35].
3. Calcium Transport Proteins in the Plasma Membrane
Several different types of transporters can mediate Ca2+ import through the plasma membrane. These include voltage gated Ca2+ channels (VGCCs), ligand gated Ca2+ channels (LGCCs), transient receptor potential channels (TRPs) and store-operated Ca2+ entry channels (SOCEs).
VGCCs are Ca2+ channels that respond to membrane depolarization events. Such channels are classified into high voltage (HVA) L, N, P/Q, R—type or low voltage T-type channels depending on their pharmacological and gating characteristics. Sequences of L, N, P/Q, and T type voltage channels from mammalian cells and sequence of the yeast VGCC named ScCch1p were used to search for VGCCs in parasite genomes. Such a search has identified at least one VGCC ortholog in T. brucei and T. cruzi whereas Leishmania spp. seem to harbor two such orthologs [36]. These orthologs from T. cruzi and Leishmania spp. have not been characterized yet. The T. brucei ortholog for VGCC has been shown to localize to the region where the flagellum attaches to the parasite cell body (known as the flagellar attachment zone) and appears to be necessary for flagellar attachment and proper parasite growth in T. brucei BSF parasites [37]. However, its role in Ca2+ signaling specifically has not been studied. Evidence has been presented on the presence of an sphingosine-stimulated Ca2+ channel in the plasma membrane of Leishmania mexicana [38] that is different from the nifedipine-inhibited L-type channel also present in these parasites [39].
LGCCs are ionotropic Ca2+ channels that open in response to a ligand and have not been reported to exist in protozoan parasites [40].
TRP channels open in response to either physical or chemical stimuli. They have been categorized into six families as TRPC (canonical), TRPM (melastatin), TRPV (vanniloid), TRPA (ankyrin), TRPML (mucolipin) and TRPP (polycystin) family. Among these, at least TRPC, TRPV, TRPA and TRPP have been shown to localize to plasma membrane and permeate Ca2+. Searching the parasite genome with either full-length or N-terminally truncated isoforms of human TRP proteins revealed two orthologs for TRPML/TRPP type in Leishmania spp. and T. brucei. Interestingly, this same search revealed four orthologs of TRPML/TRPP type in T. cruzi CL Brener strain, which has a hybrid genome [36]. Other strains have at least three distinct orthologs (Table 1). One of the TRPML ortholog from T. brucei has been shown to be involved in iron transport [41] while the orthologs from T. cruzi were shown to localize to acidic compartments such as reservosomes and lysosomes [42].
In mammalian cells, the SOCE is orchestrated by the activity of stromal interaction molecule (STIM) and ORAI proteins [43]. Both Trypanosoma and Leishmania spp. seem to lack orthologs for these proteins indicating that SOCE may be absent in trypanosomatids [36].
Ca2+ export through the plasma membrane of the mammalian cells is mediated by the co-operative action of a Na+/Ca2+ exchanger (NCX) and a plasma membrane Ca2+ ATPase (PMCA). Orthologs for the Na+/Ca2+ exchanger NCX are missing in the Excavata supergroup [44]. However, orthologs for PMCA can be found in both Trypanosoma and Leishmania spp. (Table 1). Four PMCA’s have been identified in T. cruzi genome. One of these has two copies and localizes to the plasma membrane as well as to the acidocalcisomes. The other two candidates remain to be characterized [45].
Table 1.
Organellar distribution of confirmed and putative Ca2+ signaling transporters in trypanosomatids.
| Organelle | Protein | Trypanosoma brucei | Trypanosoma cruzi | Leishmania major |
|---|---|---|---|---|
| Endoplasmic Reticulum (ER) | SERCA | Tb927.5.3400 | TcCLB.509777.70 | LmjF.04.0010 |
| Tb927.9.15460 | TcCLB.506241.70 | LmjF.35.2080 | ||
| PSEN | Tb927.9.4940 | TcCLB.508277.50 | LmjF.15.1530 | |
| Mitochondrion | VDAC | Tb927.2.2510 | TcCLB.504225.20 | LmjF.02.0460 |
| Tb927.2.2520 | TcCLB.508741.229 | |||
| TcCLB.509141.40 | ||||
| TcCLB.511687.10 | ||||
| TcCLB.511687.10 | ||||
| VDAC like 2 | Tb927.4.1610 | TcCLB504057 | LmjF.34.3100 | |
| MCU | Tb927.11.1350 | TcCLB.503893.120 | LmjF.27.0780 | |
| MICU1 | Tb927.8.1850 | TcCLB.511391.210 | LmjF.07.0110 | |
| MICU2 | Tb927.7.2960 | TcCLB.510525.130 | ||
| MCUb | Tb927.10.300 | TcCLB.504069 | LmjF.21.1690 | |
| LETM1 | Tb927.3.4920 | TcCLB.507951.270 | LmjF.29.0920 | |
| Acidocalcisome | Ca2+ ATPase | Tb927.8.1180 | TcCLB.508543.90 | LmjF.07.0630 |
| LmjF.07.0650 | ||||
| IP3R | Tb927.8.2770 | TcCLB.509461.90 | LmjF.16.0280 | |
| Golgi | Gdt1 | - | TcCLB.508895.70 | LmjF.19.0310 |
| Plasma membrane | PMCA | Tb927.8.1180 | TcCLB.506401.70 | LmjF.07.0630 |
| Tb927.8.1160 | TcCLB.508543.90 | LmjF.07.0650 | ||
| Tb927.8.1200 | TcCLB.510769.120 | LmjF.17.0600 | ||
| Tb927.10.11620 | TcCLB.509647.150 | LmjF.33.1010 | ||
| Cav channel (flagellum) | Tb927.10.2880 | TcCLB.504105.130 | LmjF.34.0480 | |
| LmjF.17.1440 | ||||
| Acidic stores | TRP | Tb927.8.850 | TcCLB.510861.94 | LmjF.07.0910. |
| TRPML | Tb927.7.950 | TcCLB.503463.20 | LmjF.26.0990 | |
| TcCLB.503735.30 |
In T. brucei, four orthologs for the PMCA can be found. TbPMC1 localizes to acidocalcisomes while TbPMC2 localizes to the plasma membrane. Loss of both these TbPMC proteins affects parasite growth and renders the parasite more sensitive to high levels of extracellular Ca2+ [46]. The PMCAs from both T. brucei (TbPMC1, TbPMC2) and T. cruzi (TcCa1) were able to complement the loss of a vacuolar PMC1 from yeast cells thereby demonstrating the functional capability of these orthologs. An earlier study with vesicles generated from Leishmania spp. plasma membrane has indicated the presence of a vanadate sensitive Ca2+-ATPase pump in the plasma membrane of these parasites [47]. In agreement with this finding, four orthologs to PMCA can be found in Leishmania spp. genomes [40]. However, characterization of these orthologs at the molecular level is still pending. Mammalian PMCAs harbor an auto-inhibitory C-terminal calmodulin-binding domain that regulates the activity of PMCA proteins. Although some trypanosomatids apparently lack this C-terminal calmodulin binding domain, it has been shown that calmodulin stimulates the plasma membrane ATPase activity in T. brucei [40], T. cruzi [48] and L. mexicana [49]. In this regard, the presence of a CaM-binding domain was recently demonstrated in the C-terminal region of the PMCA of Trypanosoma equiperdum [50,51], a subspecies of T. brucei [52].
4. Calcium Transport Proteins in the Membrane of the Endoplasmic Reticulum
Endoplasmic reticulum (ER) is the major Ca2+ store in mammalian cells. Uptake of Ca2+ into the ER is mediated by the sarco/endoplasmic reticulum Ca2+ATPase (SERCA). Orthologs for the SERCA protein have been identified and characterized in trypanosomatids (Table 1). Upon overexpression of the T. brucei SERCA ortholog, an increased ATPase activity was observed in the microsomal fractions. This activity was sensitive to thapsigargin [53], a specific inhibitor of the ER Ca2+ ATPase. Thapsigargin was also able to release Ca2+ from intracellular stores of Trypanosoma evansi [54], a subspecies of T. brucei [52]. Unlike the T. brucei protein, the T. cruzi SERCA ortholog is not affected by thapsigargin but clearly localizes to the parasite ER and exhibits Ca2+ dependent ATPase activity [55]. A SERCA ortholog has also been characterized in Leishmania spp. and appears to be overexpressed in virulent forms of the parasite [31]. Together these data indicate that Ca2+ uptake in the ER is a conserved process in trypanosomatids.
Ca2+ within the ER is buffered by calcium binding proteins such as calreticulin. T. brucei ortholog for calreticulin is yet to be studied. In T. cruzi the calreticulin localizing to the ER has been identified and its role in quality control of glycoprotein folding has been characterized in detail [56]. Interestingly, it was observed that upon infection of the mammalian host, this calreticulin translocates from the ER to the parasite surface thereby offering protection against the host complement system [57]. It has also been suggested that the translocated calreticulin binds the host complement component C1 and thereby facilitates parasite invasion [58]. Due to these properties, the T. cruzi calreticulin has also been regarded to possess an inhibitory effect on cancerous cells [59]. In Leishmania spp., loss of calreticulin affects the parasite secretory pathway and reduces parasite virulence indicating that ER calcium may have an important role in governing virulence of trypanosomatid parasites [60,61].
Ca2+ release from ER of mammalian cells is mediated by IP3R or ryanodine receptors (RyR). Trypanosomatids lack orthologs for ryanodine receptors but harbor an ortholog for the IP3R (Table 1). In Leishmania spp., this ortholog has not been characterized. However, in both T. brucei [10] and T. cruzi [11], the IP3R does not localize to the ER indicating that parasites employ a more divergent mechanism of Ca2+ release from the ER. Interestingly, another group of proteins termed presenilins have been identified and function as Ca2+ leak channels in the mammalian ER [62]. Trypanosomatids harbor orthologs for presenilins in their genome. However, their role in parasite Ca2+ signaling is still unconfirmed.
5. Calcium Transport Proteins in the Membranes of the Mitochondrion
Ca2+ regulates the activity of three dehydrogenases in the mitochondria of mammalian cells. Activation of these enzymes by Ca2+ results in increased oxidative phosphorylation and ATP production by the mitochondria [63,64,65]. Accumulation of Ca2+ in the mitochondria also acts as a signaling process for regulation of autophagic, apoptotic and necrotic pathways in the cell. A voltage dependent cation channel, VDAC in mammalian cells, mediates transfer of Ca2+ through the outer mitochondrial membrane [66]. Further, transfer through the inner mitochondrial membrane occurs by the activity of a mitochondrial Ca2+ uniporter complex (MCU complex). The MCU complex in mammalian cells comprises of the following components—(1) MCU, which is the pore forming subunit [5,6]; (2) MCUb which is a dominant negative regulator of the MCU complex [67]; (3) Mitochondrial calcium uptake 1 and 2 (MICU1 and MICU2), which are the gatekeepers of the channel [4,68] and (4) Essential MCU regulator (EMRE) which is indispensable for MCU complex mediated Ca2+ uptake [69]. Another protein termed MCUR1 [70] has also been suggested to be a part of the MCU complex and regulate its activity. However, it was proposed that this protein could modulate the mitochondrial membrane potential and therefore exert an indirect effect on the MCU complex [71]. As a result, their inclusion in the MCU complex is under debate [71,72].
Unlike mammalian cells, trypanosomatids harbor a single mitochondrion that runs the length of the cell body of the parasite and has many peculiar characteristics. Despite, such differences the mitochondrial Ca2+ uptake mechanism is well conserved between mammalian cells and trypanosomatids. An ortholog for the VDAC protein can be found in T. brucei, T. cruzi and Leishmania major (Table 1). The T. brucei VDAC (TbVDAC) has been studied in detail and is essential for parasite growth and mitochondrial ATP production. It has been suggested that TbVDAC acts as a conserved metabolic transporter in the outer mitochondrial membrane of the parasite. However, its direct role in Ca2+ import into the parasite mitochondrion was not tested [73]. Later, two other VDAC-like proteins were identified in T. brucei by bioinformatic analyses [74]. However, their role in Ca2+ import has not been explored either. Similar to the mammalian mitochondria, an MCU complex situated in the inner mitochondrial membrane is involved in driving Ca2+ into the trypanosomatid mitochondrial matrix. Orthologs of MCU, MICU1, MICU2 and MCUb have been identified and characterized in both T. brucei [10] and T. cruzi [75] (Table 1). MCU knockdown in T. brucei did not affect the mitochondrial membrane potential, but reduced the mitochondrial Ca2+ uptake, increased the AMP/ATP ratio and induced autophagy. Based on these results it has been conceived that the mitochondrial Ca2+ uptake is required for activation of dehydrogenases in the mitochondrion of the parasite [7]. Recent development of CRISPR/Cas9 mediated knockout generation has allowed the characterization of MCU and MCUb in T. cruzi. Analyses of these mutants indicate that both MCU and MCUb are necessary for Ca2+ uptake into the mitochondrion of T. cruzi. However, only MCUb was found to be indispensable for parasite growth, metacyclogenesis and infectivity. Additionally, MCUb in T. cruzi does not function as a dominant negative regulator of MCU indicating that this protein differs significantly from its mammalian counterpart [75]. MICU1 and MICU2 have not been characterized in these parasites yet. Similar to their proposed mechanism in mammals, they could be playing a regulatory role in trypanosomes as well. However, the absence of a MICU2 ortholog in Leishmania spp. indicates that a different and unique role for MICU proteins could exist in trypanosomatid parasites. Orthologs for EMRE and MCUR1 appear to be missing in both Trypanosoma and Leishmania spp [76].
Ca2+ release from mammalian mitochondria is mediated by the action of either a Na+/Ca2+ exchanger called NCLX [77] or a Ca2+/H+ exchanger. There is physiological evidence for the presence of a Ca2+/H+ exchanger in T. cruzi [1]. Trypanosomatids lack orthologs for NCLX but do contain orthologs for the leucine zipper EF hand-containing transmembrane protein 1 (Letm1) protein, a proposed Ca2+/H+ exchanger [78]. However, it has been proposed that the LETM1 ortholog functions as a K+/H+ exchanger in T. brucei [79]. Such a role for Letm1 in K+/H+ exchange has also been proposed in yeast and humans [80]. Recent work with LETM1 in mammalian cells suggests that Letm1 probably functions as a monovalent cation exchanger thereby functioning as a Na+/H+ or a K+/H+ exchanger. Changes in mitochondrial Ca2+ associated with Letm1 modulation may be due to its role in Na+/H+ exchange [81]. Whether this model holds true in trypanosomatids as well, remains to be determined.
6. Calcium Transport Proteins in the Membrane of Acidocalcisomes
Acidocalcisomes are electron dense acidic organelles that harbor a high concentration of phosphate, pyrophosphate, polyphosphate, magnesium and calcium [9]. Acidocalcisomes in trypanosomatids have received particular attention, as they are the largest calcium reservoir in these organisms [44]. The presence of Ca2+ in acidocalcisomes of T. cruzi was first detected by X-ray microanalysis [82]. The acidity of acidocalcisomes is maintained by the action of two proton pumps. A vacuolar type ATPase (V-ATPase) and a vacuolar pyrophosphatase (VP1) import H+ into these organelles by hydrolysis of ATP and pyrophosphate, respectively [83]. The H+ accumulated in the acidocalcisomes can then be used by either cation/H+ exchangers or a H+/Ca2+-ATPase [45,84,85] to import Ca2+ or other cations into the acidocalcisomes. For Ca2+ release, the trypanosomatid acidocalcisomes harbor an IP3R. In mammalian cells, this protein localizes to the ER. In T. brucei its unique localization in acidocalcisomes was initially observed by epitope tagging [10] and later confirmed by proteomic analysis and a IP3R specific antibody labeling [85] (Table 1). Furthermore, recent development of CRISPR/Cas9 based in situ epitope tagging in T. cruzi showed that the T. cruzi IP3R (TcIP3R) is also localized to the acidocalcisomes of the parasites [11]. In T. brucei, RNA interference (RNAi) mediated downregulation of IP3R reduces parasite growth in both procyclic and bloodstream forms. Additionally, it also reduces the ability of IP3 to release Ca2+ from permeabilized cells [10]. Deletion of the IP3R in T. cruzi was unsuccessful [86]. However, overexpression or reduced expression of TcIP3R results in reduced parasite proliferation, differentiation, and infectivity [86] indicating the significance of this protein in T. cruzi. This significance is further corroborated by the observation that reduced expression or activity of TcIP3R is associated with differentiation into trypomastigotes [87]. Moreover, functional studies with TbIP3R or TcIP3R in a chicken B lymphocyte cell line devoid of endogenous IP3Rs indicated that both these proteins could be stimulated by IP3 to release Ca2+ from permeabilized or intact cells [10,86]. Together these studies define a clear role for a functional IP3R in the acidocalcisomes of both T. brucei and T. cruzi.
7. An Emerging Role for Membrane Contact Sites in Ca2+ Signaling
The ER is the major Ca2+ storage site in mammalian cells [88]. As the ER ramifies throughout the entire cell body, it forms regions of close contact with various other organelles. Such regions of close contact wherein the organellar membranes are ≤ 30 nm apart are referred to as membrane contact sites (MCS). In mammalian cells such contact sites are often observed between ER-plasma membrane, ER-mitochondria and ER-endosomes, which facilitate direct transfer of Ca2+ and other biomolecules between these organelles without disturbing the overall cytosolic balance [89]. Similar membrane contact sites have also been observed in yeast [90] and plants [91]. However, their existence in trypanosomatids was not investigated until recently.
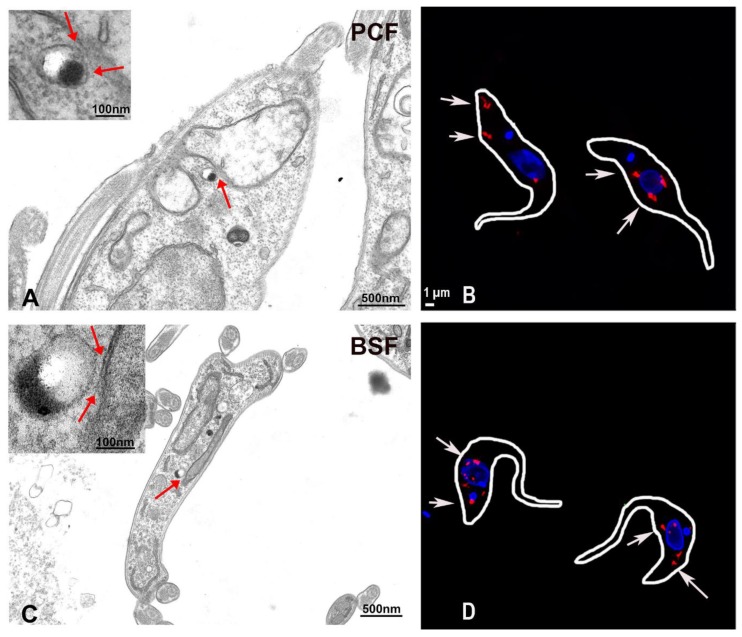
Among the various MCS, ER-mitochondria MCS are of particular interest. Ca2+ transfer to the mitochondria is necessary for cellular bioenergetics and for regulation of cell death processes [89]. Such significance of mitochondrial Ca2+ uptake has also been demonstrated in T. brucei wherein loss of the MCU affects the AMP/ATP ratio and stimulates autophagy [7]. Interestingly, acidocalcisomes, the main storage site for Ca2+ in trypanosomatids, also house the calcium release channel IP3R. Therefore, the role of mammalian ER-mitochondria MCS might be replaced by acidocalcisome-mitochondrion MCS in trypanosomes. In support of this hypothesis, our group recently demonstrated existence of such contact sites in T. brucei. Using high resolution microscopy and electron microscopy (Figure 1), close apposition between the parasite acidocalcisomes and mitochondrion could be observed. Additionally, such acidocalcisome-mitochondrion points of contact were clearly identified by using a proximity ligation assay [92].
Figure 1.
Representative transmission electron microscopy images of procyclic forms (PCF) (A) and bloodstream forms (BSF) (C) of Trypanosoma brucei showing contacts between acidocalcisomes and the mitochondria of the parasites. Acidocalcisomes appear as rounded organelles containing electron-dense material that adheres to one side of the membrane and are seen adjacent to the mitochondrion double membrane. The contact sites could be observed in both life cycle forms and are indicated by red arrows in the insets at higher magnification. Representative super resolution structured illumination images of T. brucei PCF (B) and BSF (D) trypanosomes subjected to proximity ligation assay. The red fluorescent signals indicated by the white arrows confirm the existence of membrane contact sites between acidocalcisomes and the mitochondria in these parasites. Only some of them are labeled with arrows. Scale bar in B also applies to D. Reproduced with permission from reference [92].
Whether these contact sites participate in T. brucei mitochondrial Ca2+ uptake remains to be evaluated. However, this hypothesis is supported by a previous study. In this study, genetically encoded Ca2+ indicators targeted to the T. brucei mitochondrion suggested the existence of a selective route for transfer of Ca2+ from acidic stores to the parasite mitochondrion [93]. It is highly likely that acidocalcisome-mitochondrion MCS provide this selective route for Ca2+ transfer in the parasite. Also, whether these contact sites exist in Leishmania spp. and T. cruzi remains to be seen as well. However, the existence of these contact sites in T. brucei provides sufficient basis to investigate their existence in other trypanosomatids and to characterize their role in trypanosomatid Ca2+ signaling.
8. A Potential Role for the Golgi Complex in Ca2+ Signaling
In mammals Ca2+ uptake into the Golgi is mediated by SERCA as well as by a Golgi-specific ATPase termed the secretory pathway Ca2+-ATPase (SPCA) [94,95]. An ortholog for this protein in yeast is also identified and termed as Pmr1p [96]. A Golgi-specific ortholog for SPCA/Pmr1p proteins have not been found in either Trypanosoma or Leishmania spp. Additionally, trypanosomatids seem to lack orthologs for Golgi specific Ca2+ binding proteins such as Calnuc, Cab45 and Calumenin [97]. Therefore, it is not known if the Golgi complex can even function as a Ca2+ store in trypanosomatids. However, new proteins that play a role in Golgi ion homeostasis are still being discovered in mammals that could also be conserved in trypanosomatid parasites.
Recently a Golgi resident protein belonging to the unknown protein family 0016 (UPF0016 family), termed transmembrane protein 165 (TMEM165) in humans and Gcr1 dependent translation factor 1 (Gdt1p) in yeast, was suggested to be a Ca2+/H+ exchanger. Gdt1p was shown to be indispensable for growth of Saccharomyces cerevisiae under high Ca2+ concentrations. The human ortholog TMEM165 was able to rescue the growth phenotype associated with the loss of Gdt1p indicating functional conservation between these proteins [98,99,100]. Mutations in TMEM165 are associated with human congenital glycosylation disorders [101]. Further, Gdt1p is required for protein glycosylation in yeast indicating that this Golgi resident Ca2+ transporter could play an important role in protein glycosylation. However, more recent studies have indicated that although Gdt1p has higher affinity for Ca2+, it is also able to transport Mn2+ and that its role in Mn2+ homeostasis may be key for efficient protein glycosylation in the Golgi [101]. An ortholog for this protein seems to be present in Leishmania spp. and T. cruzi but cannot be found in the T. brucei genome (Table 1). These orthologs also retain the amino acid residues from the pore domain, which were shown to be indispensable for Ca2+ tolerance in yeast [98]. It is possible that these orthologs of Gdt1 function in Golgi Ca2+ homeostasis of trypanosomatids. However, their localization and role in these parasites awaits characterization.
Golgi specific Ca2+ release channels have not been identified yet. It is believed that ER localized IP3R [102] or RyR [103] also localize to Golgi and facilitate Ca2+ export. Since RyR are absent in trypanosomatids and the IP3R appears to localize to acidocalcisomes instead of ER and Golgi, it is currently unknown how the Ca2+ stored in trypanosomatid Golgi could be exported.
9. Calcium Transport Proteins in the Flagellar Membrane
The role of Ca2+ in the development of trypanosome flagellum was realized early when it was reported that chelation of Ca2+ resulted in detachment of the flagellum from the cell body of newly formed daughter cells [104]. Further research on a different trypanosomatid, Crithidia oncopelti, suggested that alterations in the extracellular calcium concentration can alter the flagellum wave pattern of this trypanosomatid [105]. Based on these results it was believed that the trypanosomatid flagellum can sense extracellular Ca2+ concentrations. In support of its role in Ca2+ signaling, trypanosomatid flagellum harbors several Ca2+ binding proteins. Centrins are well-known calcium binding proteins. Three centrins of T. brucei localize to the flagellar basal body [25,106]. Among these, an RNAi mediated knockdown of TbCen3 compromises cell motility [24]. T. brucei also contain a family of Ca2+ binding proteins that localize to the flagellum and are termed calflagins [107]. Their localization to the parasite flagellum and their ability to bind Ca2+ has been demonstrated experimentally. RNAi mediated knockdown of entire calflagin family does not reduce parasite motility but results in reduced parasitemia and increased mice survival rate during in vivo infection [108]. A related Ca2+-binding protein that localizes to the flagellum has been identified in T. cruzi [109]. This protein termed the flagellar calcium binding protein (FCaBP) employs a N-terminal myristoylation and palmitoylation signals to facilitate its transport to the flagellum [110]. Another family of Ca2+ binding proteins called calmodulins can also be found in trypanosomatids. At least one such calmodulin has been localized to the T. brucei flagellum [111] and is required for flagellar attachment and cell motility [112]. In addition to this calmodulin, there are four other flagellar proteins named paraflagellar rod components (PFC): TbPFC1, TbPFC6, TbPFC7 and Tb5.20, which have been suggested to have EF-hand domains indicating their potential to bind Ca2+ and regulate the development or activity of the flagellum [113]. The plasma membrane PMCA-ATPase also localizes to the flagellum of T. brucei [46]. Additionally, a putative Ca2+ channel, which localizes to the flagellum of T. brucei, has been demonstrated to be essential for parasite growth and flagellar attachment to the cell body [37]. Overall these evidences clearly outline the indispensable role for Ca2+ signaling in the development and maintenance of the trypanosomatid flagellum.
10. Ca2+ Binding Proteins
A major portion of Ca2+ binding proteins belong to the EF-hand superfamily. More than 800 different proteins have been assigned to this family. Proteins from this family contain at least one EF-hand domain, which is approximately 30 amino acids long, and forms the Ca2+ binding loop. Among the EF-domain containing proteins, CaM is the most conserved Ca2+ binding proteins and is found in all eukaryotes [114,115,116]. CaM from T. cruzi has been purified and shown to activate the plasma membrane Ca2+-ATPase [48] and cyclic AMP phosphodiesterase enzyme [114]. A similar study also characterized the role of T. brucei CaM in activating the plasma membrane Ca2+-ATPase [49]. The T. brucei CaM has been localized to the paraflagellar rod. RNAi mediated loss of this protein affected the paraflagellar rod assembly and caused the flagellum to detach from the cell body [112].
Interestingly, the T. cruzi CaM does not localize to the flagellum but to the spongiome of the contractile vacuole [117,118]. Several other CaM-like proteins with EF-hand domains have also been identified in the genome of trypanosomatids [40] but they remain to be characterized (Table 2). Other proteins with low capacity and high affinity for binding Ca2+ include the FCaBP in T. cruzi [109] and the calflagins in T. brucei [107]. The calflagins have been shown to be important for T. brucei infection in mice [108]. In addition to these, the genomes of trypanosomatids also contain several hypothetical proteins with Ca2+ binding domains, which remain to be characterized.
Table 2.
Characterized and putative Ca2+ binding proteins in trypanosomatids.
| Protein | T. brucei | T. cruzi | L. major |
|---|---|---|---|
| Calreticulin | Tb927.8.7410 | TcCLB.509011.40 | LmjF.31.2600 |
| Flagellar Ca2+-binding protein | Tb927.8.5440 | TcCLB.509391.10 | LmjF.16.0910 |
| Tb927.8.5460 | TcCLB.509391.20 | LmjF.16.0920 | |
| Tb927.8.5465 | TcCLB.509391.30 | ||
| Tb927.8.5470 | TcCLB.506749.20 | ||
| Ca2+-binding protein | Tb927.6.2720 | TcCLB.507925.60 | LmjF.30.1240 |
| Tb927.4.1740 | TcCLB.510879.190 | LmjF.34.2950 | |
| Calmodulin (CaM) | Tb927.11.13020 | TcCLB.507483.30 | LmjF.09.0910 |
| Tb927.11.13030 | TcCLB.507483.39 | LmjF.09.0920 | |
| Tb927.11.13040 | LmjF.09.0930 | ||
| Tb927.11.13050 | |||
| CaM-like protein | Tb927.11.9790 | TcCLB.506963.90 | LmjF.36.3675 |
| Tb927.9.11230 | TcCLB.504075.3 | LmjF.35.3890 | |
| Tb927.11.3680 | TcCLB.508731.30 | LmjF.13.1160 | |
| Tb927.11.7940 | TcCLB.506933.89 | LmjF.28.0800 | |
| Tb927.9.6130 | TcCLB.508951.50 | LmjF.21.0220 | |
| Tb927.6.4710 | TcCLB.511729.9 | LmjF.15.0930 | |
| TcCLB.507483.50 | LmjF.30.3360 |
11. Conclusions and Future Directions
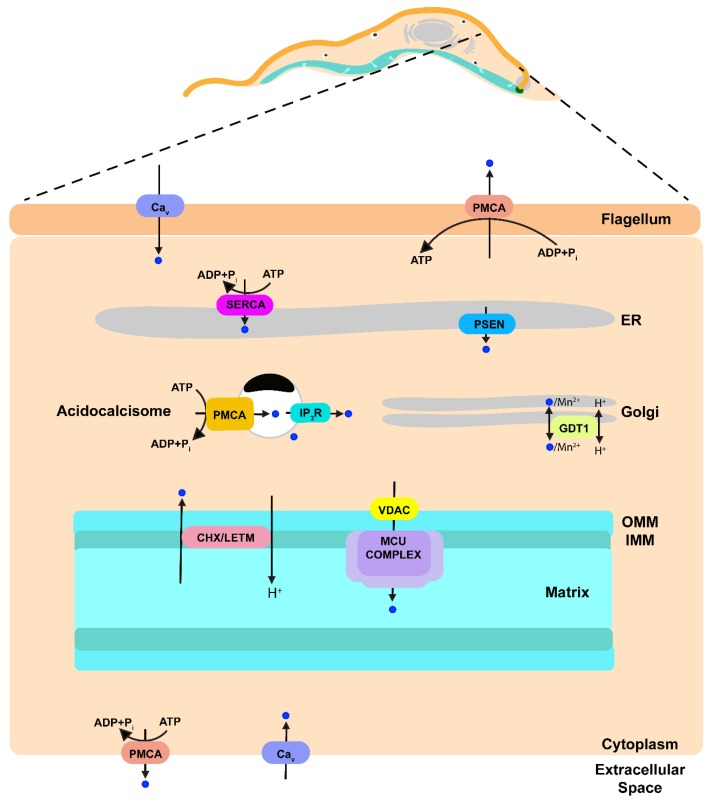
Trypanosomatids have some differences and similarities with mammalian cells regarding Ca2+ homeostasis and signaling (Figure 2). Some of the similarities were important for the discovery of the molecular nature of the MCU complex in mammalian cells [3]. Some channels and pumps are present but with peculiar localizations: an IP3R is in the acidocalcisomes instead of in the endoplasmic reticulum, a voltage-dependent Ca2+ channel is present in the flagellum, and a PMCA localizes to the acidocalcisomes in addition to the plasma membrane. Some transporters are missing, such as plasma membrane and mitochondrial Na+/Ca2+ exchangers, several subunits (MICU3, MCR1, EMRE) of the mitochondrial Ca2+ uniporter, and the components of the SOCE mechanism.
Figure 2.
Schematic representation of Ca2+ transport pathways within a model trypanosomatid parasite based on data published previously. Ca2+ is indicated as blue circles. Ca2+ upon entering the cell through a Ca2+ channel can be sequestered into the ER by the action of SERCA, into the mitochondrion by the action of MCU complex or into the acidocalcisomes by the action of a PMCA. Ca2+ release from the mitochondrion is through a Ca2+/H+ exchanger (CHX) that could be LETM1. Ca2+ from acidocalcisomes is released when IP3 stimulates the IP3R located in this organelle. A presenilin can be a leak channel in the ER. Ca2+ release through the parasite plasma membrane is achieved by a PMCA. PMCA, plasma membrane Ca2+-ATPase; Cav Voltage gated Ca2+ channel; IP3, inositol 1,4,5-trisphosphate; IP3R, IP3 receptor; GDT1, GCR1 dependent translation factor; MCU complex, mitochondrial calcium uniporter complex; VDAC, voltage-dependent anion-selective channel; Letm1 (LETM), leucine zipper-EF-hand containing transmembrane protein 1; CHX, Ca2+/H+ exchanger; PSEN presenilin; SERCA, sarcoplasmic-endoplasmic reticulum Ca2+-ATPase; ER, endoplasmic reticulum; OMM, outer mitochondrial membrane; IMM, inner mitochondrial membrane.
The presence of TRP in acidic stores [42] and the importance of acidic stores in Ca2+ signaling [119] suggest that more studies of these channels are needed. The essentiality of different membrane transporters involved in Ca2+ homeostasis and signaling also suggest that they could be exploited as drug, vaccines, or diagnostic targets. In this regard, trypanosomatids, which belong to the Excavata supergroup of eukaryotes, diverged early from the Ophistonkonta, containing animals, and fungi, and the differences pointed out in Ca2+ homeostasis and signaling suggest that the search for new targets is warranted.
Funding
This work was supported by grants from the U.S. National Institutes of Health (AI107663 an AI108222 to R.D.).
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
References
- 1.Docampo R., Vercesi A.E. Characteristics of Ca2+ transport by Trypanosoma cruzi mitochondria in situ. Arch. Biochem. Biophys. 1989;272:122–129. doi: 10.1016/0003-9861(89)90202-6. [DOI] [PubMed] [Google Scholar]
- 2.Docampo R., Vercesi A.E. Ca2+ transport by coupled Trypanosoma cruzi mitochondria in situ. J. Biol. Chem. 1989;264:108–111. [PubMed] [Google Scholar]
- 3.Docampo R., Lukes J. Trypanosomes and the solution to a 50-year mitochondrial calcium mystery. Trends Parasitol. 2012;28:31–37. doi: 10.1016/j.pt.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perocchi F., Gohil V.M., Girgis H.S., Bao X.R., McCombs J.E., Palmer A.E., Mootha V.K. MICU1 encodes a mitochondrial EF hand protein required for Ca2+ uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baughman J.M., Perocchi F., Girgis H.S., Plovanich M., Belcher-Timme C.A., Sancak Y., Bao X.R., Strittmatter L., Goldberger O., Bogorad R.L., et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Stefani D., Raffaello A., Teardo E., Szabo I., Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huang G., Vercesi A.E., Docampo R. Essential regulation of cell bioenergetics in Trypanosoma brucei by the mitochondrial calcium uniporter. Nat. Commun. 2013;4:2865. doi: 10.1038/ncomms3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan X., Liu J., Nguyen T., Liu C., Sun J., Teng Y., Fergusson M.M., Rovira I.I., Allen M., Springer D.A., et al. The physiological role of mitochondrial calcium revealed by mice lacking the mitochondrial calcium uniporter. Nat. Cell Biol. 2013;15:1464–1472. doi: 10.1038/ncb2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Docampo R., de Souza W., Miranda K., Rohloff P., Moreno S.N. Acidocalcisomes—Conserved from bacteria to man. Nat. Rev. Microbiol. 2005;3:251–261. doi: 10.1038/nrmicro1097. [DOI] [PubMed] [Google Scholar]
- 10.Huang G., Bartlett P.J., Thomas A.P., Moreno S.N., Docampo R. Acidocalcisomes of Trypanosoma brucei have an inositol 1,4,5-trisphosphate receptor that is required for growth and infectivity. Proc. Natl. Acad. Sci. USA. 2013;110:1887–1892. doi: 10.1073/pnas.1216955110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lander N., Chiurillo M.A., Storey M., Vercesi A.E., Docampo R. CRISPR/Cas9-mediated endogenous C-terminal tagging of Trypanosoma cruzi genes reveals the acidocalcisome localization of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 2016;291:25505–25515. doi: 10.1074/jbc.M116.749655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carafoli E., Krebs J. Why calcium? How calcium became the best communicator. J. Biol. Chem. 2016;291:20849–20857. doi: 10.1074/jbc.R116.735894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins K.D. Charge density-dependent strength of hydration and biological structure. Biophys. J. 1997;72:65–76. doi: 10.1016/S0006-3495(97)78647-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parsons M., Ruben L. Pathways involved in environmental sensing in trypanosomatids. Parasitol. Today. 2000;16:56–62. doi: 10.1016/S0169-4758(99)01590-2. [DOI] [PubMed] [Google Scholar]
- 15.Swulius M.T., Waxham M.N. Ca2+/calmodulin-dependent protein kinases. Cell. Mol. Life Sci. 2008;65:2637–2657. doi: 10.1007/s00018-008-8086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nogueira N.P., de Souza C.F., Saraiva F.M., Sultano P.E., Dalmau S.R., Bruno R.E., Goncalves Rde L., Laranja G.A., Leal L.H., Coelho M.G., et al. Heme-induced ros in Trypanosoma cruzi activates CaMKII-like that triggers epimastigote proliferation. One helpful effect of ROS. PLoS ONE. 2011;6:e25935. doi: 10.1371/journal.pone.0025935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Souza C.F., Carneiro A.B., Silveira A.B., Laranja G.A., Silva-Neto M.A., Costa S.C., Paes M.C. Heme-induced Trypanosoma cruzi proliferation is mediated by CaM kinase II. Biochem. Biophys. Res. Commun. 2009;390:541–546. doi: 10.1016/j.bbrc.2009.09.135. [DOI] [PubMed] [Google Scholar]
- 18.Ogueta S., Intosh G.M., Tellez-Iñon M.T. Regulation of Ca2+/calmodulin-dependent protein kinase from Trypanosoma cruzi. Mol. Biochem. Parasitol. 1996;78:171–183. doi: 10.1016/S0166-6851(96)02622-9. [DOI] [PubMed] [Google Scholar]
- 19.Ogueta S.B., Macintosh G.C., Tellez-Iñon M.T. Stage-specific substrate phosphorylation by a Ca2+/calmodulin-dependent protein kinase in Trypanosoma cruzi. J. Eukaryot. Microbiol. 1998;45:392–396. doi: 10.1111/j.1550-7408.1998.tb05089.x. [DOI] [PubMed] [Google Scholar]
- 20.Furuya T., Kashuba C., Docampo R., Moreno S.N. A novel phosphatidylinositol-phospholipase C of Trypanosoma cruzi that is lipid modified and activated during trypomastigote to amastigote differentiation. J. Biol. Chem. 2000;275:6428–6438. doi: 10.1074/jbc.275.9.6428. [DOI] [PubMed] [Google Scholar]
- 21.Lammel E.M., Barbieri M.A., Wilkowsky S.E., Bertini F., Isola E.L. Trypanosoma cruzi: Involvement of intracellular calcium in multiplication and differentiation. Exp. Parasitol. 1996;83:240–249. doi: 10.1006/expr.1996.0070. [DOI] [PubMed] [Google Scholar]
- 22.Stojdl D.F., Clarke M.W. Trypanosoma brucei: Analysis of cytoplasmic Ca2+ during differentiation of bloodstream stages in vitro. Exp. Parasitol. 1996;83:134–146. doi: 10.1006/expr.1996.0057. [DOI] [PubMed] [Google Scholar]
- 23.D’Angelo M.A., Montagna A.E., Sanguineti S., Torres H.N., Flawia M.M. A novel calcium-stimulated adenylyl cyclase from Trypanosoma cruzi, which interacts with the structural flagellar protein paraflagellar rod. J. Biol. Chem. 2002;277:35025–35034. doi: 10.1074/jbc.M204696200. [DOI] [PubMed] [Google Scholar]
- 24.Wei Y., Hu H., Lun Z.R., Li Z. Centrin3 in trypanosomes maintains the stability of a flagellar inner-arm dynein for cell motility. Nat. Commun. 2014;5:4060. doi: 10.1038/ncomms5060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Selvapandiyan A., Kumar P., Morris J.C., Salisbury J.L., Wang C.C., Nakhasi H.L. Centrin1 is required for organelle segregation and cytokinesis in Trypanosoma brucei. Mol. Biol. Cell. 2007;18:3290–3301. doi: 10.1091/mbc.e07-01-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Araya J.E., Cornejo A., Orrego P.R., Cordero E.M., Cortez M., Olivares H., Neira I., Sagua H., da Silveira J.F., Yoshida N., et al. Calcineurin B of the human protozoan parasite Trypanosoma cruzi is involved in cell invasion. Microbes Infect. 2008;10:892–900. doi: 10.1016/j.micinf.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 27.Selvapandiyan A., Debrabant A., Duncan R., Muller J., Salotra P., Sreenivas G., Salisbury J.L., Nakhasi H.L. Centrin gene disruption impairs stage-specific basal body duplication and cell cycle progression in Leishmania. J. Biol. Chem. 2004;279:25703–25710. doi: 10.1074/jbc.M402794200. [DOI] [PubMed] [Google Scholar]
- 28.Selvapandiyan A., Duncan R., Debrabant A., Bertholet S., Sreenivas G., Negi N.S., Salotra P., Nakhasi H.L. Expression of a mutant form of Leishmania donovani centrin reduces the growth of the parasite. J. Biol. Chem. 2001;276:43253–43261. doi: 10.1074/jbc.M106806200. [DOI] [PubMed] [Google Scholar]
- 29.Moreno V.R., Aguero F., Tekiel V., Sanchez D.O. The calcineurin a homologue from Trypanosoma cruzi lacks two important regulatory domains. Acta Trop. 2007;101:80–89. doi: 10.1016/j.actatropica.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 30.Moreno S.N., Silva J., Vercesi A.E., Docampo R. Cytosolic-free calcium elevation in Trypanosoma cruzi is required for cell invasion. J. Exp. Med. 1994;180:1535–1540. doi: 10.1084/jem.180.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu H.G., Zhong L., Chang K.P., Docampo R. Intracellular Ca2+ pool content and signaling and expression of a calcium pump are linked to virulence in Leishmania mexicana amazonesis amastigotes. J. Biol. Chem. 1997;272:9464–9473. doi: 10.1074/jbc.272.14.9464. [DOI] [PubMed] [Google Scholar]
- 32.Yakubu M.A., Majumder S., Kierszenbaum F. Changes in Trypanosoma cruzi infectivity by treatments that affect calcium ion levels. Mol. Biochem. Parasitol. 1994;66:119–125. doi: 10.1016/0166-6851(94)90042-6. [DOI] [PubMed] [Google Scholar]
- 33.Rohloff P., Rodrigues C.O., Docampo R. Regulatory volume decrease in Trypanosoma cruzi involves amino acid efflux and changes in intracellular calcium. Mol. Biochem. Parasitol. 2003;126:219–230. doi: 10.1016/S0166-6851(02)00277-3. [DOI] [PubMed] [Google Scholar]
- 34.Irigoin F., Inada N.M., Fernandes M.P., Piacenza L., Gadelha F.R., Vercesi A.E., Radi R. Mitochondrial calcium overload triggers complement-dependent superoxide-mediated programmed cell death in Trypanosoma cruzi. Biochem. J. 2009;418:595–604. doi: 10.1042/BJ20081981. [DOI] [PubMed] [Google Scholar]
- 35.Selzer P.M., Webster P., Duszenko M. Influence of Ca2+ depletion on cytoskeleton and nucleolus morphology in Trypanosoma brucei. Eur. J. Cell. Biol. 1991;56:104–112. [PubMed] [Google Scholar]
- 36.Prole D.L., Taylor C.W. Identification of intracellular and plasma membrane calcium channel homologues in pathogenic parasites. PLoS ONE. 2011;6:e26218. doi: 10.1371/journal.pone.0026218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oberholzer M., Langousis G., Nguyen H.T., Saada E.A., Shimogawa M.M., Jonsson Z.O., Nguyen S.M., Wohlschlegel J.A., Hill K.L. Independent analysis of the flagellum surface and matrix proteomes provides insight into flagellum signaling in mammalian-infectious Trypanosoma brucei. Mol. Cell. Proteom. 2011;10 doi: 10.1074/mcp.M111.010538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Benaim G., Garcia-Marchan Y., Reyes C., Uzcanga G., Figarella K. Identification of a sphingosine-sensitive Ca2+ channel in the plasma membrane of Leishmania mexicana. Biochem. Biophys. Res. Commun. 2013;430:1091–1096. doi: 10.1016/j.bbrc.2012.12.033. [DOI] [PubMed] [Google Scholar]
- 39.Pinto-Martinez A.K., Rodriguez-Duran J., Serrano-Martin X., Hernandez-Rodriguez V., Benaim G. Mechanism of action of miltefosine on Leishmania donovani involves the impairment of acidocalcisome function and the activation of the sphingosine-dependent plasma membrane Ca2+ channel. Antimicrob. Agents Chemother. 2018;62:e01614–e1617. doi: 10.1128/AAC.01614-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Docampo R., Huang G. Calcium signaling in trypanosomatid parasites. Cell Calcium. 2015;57:194–202. doi: 10.1016/j.ceca.2014.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor M.C., McLatchie A.P., Kelly J.M. Evidence that transport of iron from the lysosome to the cytosol in african trypanosomes is mediated by a mucolipin orthologue. Mol. Microbiol. 2013;89:420–432. doi: 10.1111/mmi.12285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cruz-Bustos T., Moreno S.N.J., Docampo R. Detection of weakly expressed Trypanosoma cruzi membrane proteins using high-performance probes. J. Eukaryot. Microbiol. 2018 doi: 10.1111/jeu.12517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Prakriya M., Lewis R.S. Store-operated calcium channels. Physiol. Rev. 2015;95:1383–1436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ulrich P., Cintron R., Docampo R. Calcium homeostasis and acidocalcisomes in Trypanosoma cruzi. In: Souza W.D., editor. Structures and Organelles in Pathogenic Protists. Springer; Berlin/Heidelberg, Germany: 2010. pp. 299–318. [Google Scholar]
- 45.Lu H.G., Zhong L., de Souza W., Benchimol M., Moreno S., Docampo R. Ca2+ content and expression of an acidocalcisomal calcium pump are elevated in intracellular forms of Trypanosoma cruzi. Mol. Cell. Biol. 1998;18:2309–2323. doi: 10.1128/MCB.18.4.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo S., Rohloff P., Cox J., Uyemura S.A., Docampo R. Trypanosoma brucei plasma membrane-type Ca2+-ATPase 1 (TbPMC1) and 2 (TbPMC2) genes encode functional Ca2+-ATPases localized to the acidocalcisomes and plasma membrane, and essential for Ca2+ homeostasis and growth. J. Biol. Chem. 2004;279:14427–14439. doi: 10.1074/jbc.M309978200. [DOI] [PubMed] [Google Scholar]
- 47.Mandal D., Mukherjee T., Sarkar S., Majumdar S., Bhaduri A. The plasma-membrane Ca2+-ATPase of Leishmania donovani is an extrusion pump for Ca2+ Pt 1Biochem. J. 1997;322:251–257. doi: 10.1042/bj3220251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Benaim G., Losada S., Gadelha F.R., Docampo R. A calmodulin-activated (Ca2+-Mg2+)-ATPase is involved in Ca2+ transport by plasma membrane vesicles from Trypanosoma cruzi. Pt 3Biochem. J. 1991;280:715–720. doi: 10.1042/bj2800715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benaim G., Cervino V., Hermoso T., Felibert P., Laurentin A. Intracellular calcium homeostasis in Leishmania mexicana. Identification and characterization of a plasma membrane calmodulin-dependent Ca2+-ATPase. Biol Res. 1993;26:141–150. [PubMed] [Google Scholar]
- 50.Ramirez-Iglesias J.R., Perez-Gordones M.C., Del Castillo J.R., Mijares A., Benaim G., Mendoza M. Identification and characterization of a calmodulin binding domain in the plasma membrane Ca2+-ATPase from Trypanosoma equiperdum. Mol. Biochem. Parasitol. 2018;222:51–60. doi: 10.1016/j.molbiopara.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Perez-Gordones M.C., Ramirez-Iglesias J.R., Cervino V., Uzcanga G.L., Benaim G., Mendoza M. Evidence of the presence of a calmodulin-sensitive plasma membrane Ca2+-ATPase in Trypanosoma equiperdum. Mol. Biochem. Parasitol. 2017;213:1–11. doi: 10.1016/j.molbiopara.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 52.Lai D.H., Hashimi H., Lun Z.R., Ayala F.J., Lukes J. Adaptations of Trypanosoma brucei to gradual loss of kinetoplast DNA: Trypanosoma equiperdum and Trypanosoma evansi are petite mutants of T. brucei. Proc. Natl. Acad. Sci. USA. 2008;105:1999–2004. doi: 10.1073/pnas.0711799105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nolan D.P., Reverlard P., Pays E. Overexpression and characterization of a gene for a Ca2+-ATPase of the endoplasmic reticulum in Trypanosoma brucei. J. Biol. Chem. 1994;269:26045–26051. [PubMed] [Google Scholar]
- 54.Mendoza M., Mijares A., Rojas H., Colina C., Cervino V., DiPolo R., Benaim G. Evaluation of the presence of a thapsigargin-sensitive calcium store in trypanosomatids using Trypanosoma evansi as a model. J. Parasitol. 2004;90:1181–1183. doi: 10.1645/GE-263R. [DOI] [PubMed] [Google Scholar]
- 55.Furuya T., Okura M., Ruiz F.A., Scott D.A., Docampo R. TcSCA complements yeast mutants defective in Ca2+ pumps and encodes a Ca2+-ATPase that localizes to the endoplasmic reticulum of Trypanosoma cruzi. J. Biol. Chem. 2001;276:32437–32445. doi: 10.1074/jbc.M104000200. [DOI] [PubMed] [Google Scholar]
- 56.Conte I., Labriola C., Cazzulo J.J., Docampo R., Parodi A.J. The interplay between folding-facilitating mechanisms in Trypanosoma cruzi endoplasmic reticulum. Mol. Biol. Cell. 2003;14:3529–3540. doi: 10.1091/mbc.e03-04-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ramirez G., Valck C., Aguilar L., Kemmerling U., Lopez-Munoz R., Cabrera G., Morello A., Ferreira J., Maya J.D., Galanti N., et al. Roles of Trypanosoma cruzi calreticulin in parasite-host interactions and in tumor growth. Mol. Immunol. 2012;52:133–140. doi: 10.1016/j.molimm.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 58.Rimoldi M.T., Tenner A.J., Bobak D.A., Joiner K.A. Complement component C1q enhances invasion of human mononuclear phagocytes and fibroblasts by Trypanosoma cruzi trypomastigotes. J. Clin. Investig. 1989;84:1982–1989. doi: 10.1172/JCI114388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ramirez-Toloza G., Abello P., Ferreira A. Is the antitumor property of Trypanosoma cruzi infection mediated by its calreticulin? Front. Immunol. 2016;7:268. doi: 10.3389/fimmu.2016.00268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joshi M., Pogue G.P., Duncan R.C., Lee N.S., Singh N.K., Atreya C.D., Dwyer D.M., Nakhasi H.L. Isolation and characterization of Leishmania donovani calreticulin gene and its conservation of the RNA binding activity. Mol. Biochem. Parasitol. 1996;81:53–64. doi: 10.1016/0166-6851(96)02676-X. [DOI] [PubMed] [Google Scholar]
- 61.Debrabant A., Lee N., Dwyer D.M., Nakhasi H.L. Role of calreticulin in Leishmania parasite secretory pathway and pathogenesis. In: Eggleton P.M.M., editor. Calreticulin. Springer; Boston, MA, USA: 2003. pp. 220–237. [Google Scholar]
- 62.Honarnejad K., Herms J. Presenilins: Role in calcium homeostasis. Int. J. Biochem. Cell Biol. 2012;44:1983–1986. doi: 10.1016/j.biocel.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 63.Denton R.M. Regulation of mitochondrial dehydrogenases by calcium ions. Biochim. Biophys. Acta. 2009;1787:1309–1316. doi: 10.1016/j.bbabio.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 64.Denton R.M., Randle P.J., Martin B.R. Stimulation by calcium ions of pyruvate dehydrogenase phosphate phosphatase. Biochem. J. 1972;128:161–163. doi: 10.1042/bj1280161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCormack J.G., Denton R.M. The effects of calcium ions and adenine nucleotides on the activity of pig heart 2-oxoglutarate dehydrogenase complex. Biochem. J. 1979;180:533–544. doi: 10.1042/bj1800533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gincel D., Zaid H., Shoshan-Barmatz V. Calcium binding and translocation by the voltage-dependent anion channel: A possible regulatory mechanism in mitochondrial function. Biochem. J. 2001;358:147–155. doi: 10.1042/bj3580147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raffaello A., De Stefani D., Sabbadin D., Teardo E., Merli G., Picard A., Checchetto V., Moro S., Szabo I., Rizzuto R. The mitochondrial calcium uniporter is a multimer that can include a dominant-negative pore-forming subunit. EMBO J. 2013;32:2362–2376. doi: 10.1038/emboj.2013.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Plovanich M., Bogorad R.L., Sancak Y., Kamer K.J., Strittmatter L., Li A.A., Girgis H.S., Kuchimanchi S., De Groot J., Speciner L., et al. MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling. PLoS ONE. 2013;8:e55785. doi: 10.1371/journal.pone.0055785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sancak Y., Markhard A.L., Kitami T., Kovacs-Bogdan E., Kamer K.J., Udeshi N.D., Carr S.A., Chaudhuri D., Clapham D.E., Li A.A., et al. EMRE is an essential component of the mitochondrial calcium uniporter complex. Science. 2013;342:1379–1382. doi: 10.1126/science.1242993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mallilankaraman K., Cardenas C., Doonan P.J., Chandramoorthy H.C., Irrinki K.M., Golenar T., Csordas G., Madireddi P., Yang J., Muller M., et al. MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism. Nat. Cell Biol. 2012;14:1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Paupe V., Prudent J., Dassa E.P., Rendon O.Z., Shoubridge E.A. Ccdc90a (MCUR1) is a cytochrome c oxidase assembly factor and not a regulator of the mitochondrial calcium uniporter. Cell Metab. 2015;21:109–116. doi: 10.1016/j.cmet.2014.12.004. [DOI] [PubMed] [Google Scholar]
- 72.Vais H., Tanis J.E., Muller M., Payne R., Mallilankaraman K., Foskett J.K. MCUR1, CCDC90A, is a regulator of the mitochondrial calcium uniporter. Cell Metab. 2015;22:533–535. doi: 10.1016/j.cmet.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pusnik M., Charriere F., Maser P., Waller R.F., Dagley M.J., Lithgow T., Schneider A. The single mitochondrial porin of Trypanosoma brucei is the main metabolite transporter in the outer mitochondrial membrane. Mol. Biol. Evol. 2009;26:671–680. doi: 10.1093/molbev/msn288. [DOI] [PubMed] [Google Scholar]
- 74.Flinner N., Schleiff E., Mirus O. Identification of two voltage-dependent anion channel-like protein sequences conserved in kinetoplastida. Biol. Lett. 2012;8:446–449. doi: 10.1098/rsbl.2011.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chiurillo M.A., Lander N., Bertolini M.S., Storey M., Vercesi A.E., Docampo R. Different roles of mitochondrial calcium uniporter complex subunits in growth and infectivity of Trypanosoma cruzi. MBio. 2017;8 doi: 10.1128/mBio.00574-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Docampo R., Vercesi A.E., Huang G. Mitochondrial calcium transport in trypanosomes. Mol. Biochem. Parasitol. 2014;196:108–116. doi: 10.1016/j.molbiopara.2014.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Palty R., Silverman W.F., Hershfinkel M., Caporale T., Sensi S.L., Parnis J., Nolte C., Fishman D., Shoshan-Barmatz V., Herrmann S., et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc. Natl. Acad. Sci. USA. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang D., Zhao L., Clapham D.E. Genome-wide RNAi screen identifies LETM1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hashimi H., McDonald L., Stribrna E., Lukes J. Trypanosome Letm1 protein is essential for mitochondrial potassium homeostasis. J. Biol. Chem. 2013;288:26914–26925. doi: 10.1074/jbc.M113.495119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Froschauer E., Nowikovsky K., Schweyen R.J. Electroneutral K+/H+ exchange in mitochondrial membrane vesicles involves YOL027/Letm1 proteins. Biochim. Biophys. Acta. 2005;1711:41–48. doi: 10.1016/j.bbamem.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 81.Austin S., Tavakoli M., Pfeiffer C., Seifert J., Mattarei A., De Stefani D., Zoratti M., Nowikovsky K. Letm1-mediated K+ and Na+ homeostasis regulates mitochondrial Ca2+ efflux. Front. Physiol. 2017;8:839. doi: 10.3389/fphys.2017.00839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dvorak J.A., Engel J.C., Leapman R.D., Swyt C.R., Pella P.A. Trypanosoma cruzi: Elemental composition heterogeneity of cloned stocks. Mol. Biochem. Parasitol. 1988;31:19–26. doi: 10.1016/0166-6851(88)90141-7. [DOI] [PubMed] [Google Scholar]
- 83.Scott D.A., Docampo R. Two types of H+-ATPase are involved in the acidification of internal compartments in Trypanosoma cruzi. Pt 2Biochem. J. 1998;331:583–589. doi: 10.1042/bj3310583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Docampo R., Scott D.A., Vercesi A.E., Moreno S.N. Intracellular Ca2+ storage in acidocalcisomes of Trypanosoma cruzi. Pt 3Biochem. J. 1995;310:1005–1012. doi: 10.1042/bj3101005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Huang G., Docampo R. Proteomic analysis of acidocalcisomes of Trypanosoma brucei uncovers their role in phosphate metabolism, cation homeostasis, and calcium signaling. Commun. Integr. Biol. 2015;8:e1017174. doi: 10.1080/19420889.2015.1017174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hashimoto M., Enomoto M., Morales J., Kurebayashi N., Sakurai T., Hashimoto T., Nara T., Mikoshiba K. Inositol 1,4,5-trisphosphate receptor regulates replication, differentiation, infectivity and virulence of the parasitic protist Trypanosoma cruzi. Mol. Microbiol. 2013;87:1133–1150. doi: 10.1111/mmi.12155. [DOI] [PubMed] [Google Scholar]
- 87.Hashimoto M., Morales J., Uemura H., Mikoshiba K., Nara T. A novel method for inducing amastigote-to-trypomastigote transformation in vitro in Trypanosoma cruzi reveals the importance of inositol 1,4,5-trisphosphate receptor. PLoS ONE. 2015;10:e0135726. doi: 10.1371/journal.pone.0135726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Koch G.L. The endoplasmic reticulum and calcium storage. Bioessays. 1990;12:527–531. doi: 10.1002/bies.950121105. [DOI] [PubMed] [Google Scholar]
- 89.Phillips M.J., Voeltz G.K. Structure and function of ER membrane contact sites with other organelles. Nat. Rev. Mol. Cell. Biol. 2016;17:69–82. doi: 10.1038/nrm.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lang A., John Peter A.T., Kornmann B. ER-mitochondria contact sites in yeast: Beyond the myths of ERMES. Curr. Opin. Cell. Biol. 2015;35:7–12. doi: 10.1016/j.ceb.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 91.Wang P., Hawes C., Hussey P.J. Plant endoplasmic reticulum-plasma membrane contact sites. Trends Plant Sci. 2017;22:289–297. doi: 10.1016/j.tplants.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 92.Ramakrishnan S., Asady B., Docampo R. Acidocalcisome-mitochondrion membrane contact sites in Trypanosoma brucei. Pathogens. 2018;7:33. doi: 10.3390/pathogens7020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xiong Z.H., Ridgley E.L., Enis D., Olness F., Ruben L. Selective transfer of calcium from an acidic compartment to the mitochondrion of Trypanosoma brucei. Measurements with targeted aequorins. J. Biol. Chem. 1997;272:31022–31028. doi: 10.1074/jbc.272.49.31022. [DOI] [PubMed] [Google Scholar]
- 94.Xiang M., Mohamalawari D., Rao R. A novel isoform of the secretory pathway Ca2+,Mn2+-ATPase, HsPCA2, has unusual properties and is expressed in the brain. J. Biol. Chem. 2005;280:11608–11614. doi: 10.1074/jbc.M413116200. [DOI] [PubMed] [Google Scholar]
- 95.Vanoevelen J., Dode L., Van Baelen K., Fairclough R.J., Missiaen L., Raeymaekers L., Wuytack F. The secretory pathway Ca2+/Mn2+-ATPase 2 is a Golgi-localized pump with high affinity for Ca2+ ions. J. Biol. Chem. 2005;280:22800–22808. doi: 10.1074/jbc.M501026200. [DOI] [PubMed] [Google Scholar]
- 96.Sorin A., Rosas G., Rao R. PMR1, a Ca2+-ATPase in yeast Golgi, has properties distinct from sarco/endoplasmic reticulum and plasma membrane calcium pumps. J. Biol. Chem. 1997;272:9895–9901. doi: 10.1074/jbc.272.15.9895. [DOI] [PubMed] [Google Scholar]
- 97.Pizzo P., Lissandron V., Capitanio P., Pozzan T. Ca2+ signalling in the Golgi apparatus. Cell Calcium. 2011;50:184–192. doi: 10.1016/j.ceca.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 98.Colinet A.S., Thines L., Deschamps A., Flemal G., Demaegd D., Morsomme P. Acidic and uncharged polar residues in the consensus motifs of the yeast Ca2+ transporter GDT1p are required for calcium transport. Cell. Microbiol. 2017;19 doi: 10.1111/cmi.12729. [DOI] [PubMed] [Google Scholar]
- 99.Colinet A.S., Sengottaiyan P., Deschamps A., Colsoul M.L., Thines L., Demaegd D., Duchene M.C., Foulquier F., Hols P., Morsomme P. Yeast GDT1 is a Golgi-localized calcium transporter required for stress-induced calcium signaling and protein glycosylation. Sci. Rep. 2016;6:24282. doi: 10.1038/srep24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Demaegd D., Foulquier F., Colinet A.S., Gremillon L., Legrand D., Mariot P., Peiter E., Van Schaftingen E., Matthijs G., Morsomme P. Newly characterized Golgi-localized family of proteins is involved in calcium and pH homeostasis in yeast and human cells. Proc. Natl. Acad. Sci. USA. 2013;110:6859–6864. doi: 10.1073/pnas.1219871110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Dulary E., Potelle S., Legrand D., Foulquier F. TMEM165 deficiencies in congenital disorders of glycosylation type II (CDG-II): Clues and evidences for roles of the protein in Golgi functions and ion homeostasis. Tissue Cell. 2017;49:150–156. doi: 10.1016/j.tice.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 102.Micaroni M. Calcium around the Golgi apparatus: Implications for intracellular membrane trafficking. Adv. Exp. Med. Biol. 2012;740:439–460. doi: 10.1007/978-94-007-2888-2_18. [DOI] [PubMed] [Google Scholar]
- 103.Cifuentes F., Gonzalez C.E., Fiordelisio T., Guerrero G., Lai F.A., Hernandez-Cruz A. A ryanodine fluorescent derivative reveals the presence of high-affinity ryanodine binding sites in the golgi complex of rat sympathetic neurons, with possible functional roles in intracellular Ca2+ signaling. Cell. Signal. 2001;13:353–362. doi: 10.1016/S0898-6568(01)00132-2. [DOI] [PubMed] [Google Scholar]
- 104.Vickerman K. On the surface coat and flagellar adhesion in trypanosomes. J. Cell Sci. 1969;5:163–193. doi: 10.1242/jcs.5.1.163. [DOI] [PubMed] [Google Scholar]
- 105.Sugrue P., Hirons M.R., Adam J.U., Holwill M.E. Flagellar wave reversal in the kinetoplastid flagellate Crithidia oncopelti. Biol. Cell. 1988;63:127–131. doi: 10.1016/0248-4900(88)90051-2. [DOI] [PubMed] [Google Scholar]
- 106.Selvapandiyan A., Kumar P., Salisbury J.L., Wang C.C., Nakhasi H.L. Role of centrins 2 and 3 in organelle segregation and cytokinesis in Trypanosoma brucei. PLoS ONE. 2012;7:e45288. doi: 10.1371/journal.pone.0045288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu Y., Deford J., Benjamin R., Lee M.G., Ruben L. The gene family of EF-hand calcium-binding proteins from the flagellum of Trypanosoma brucei. Pt 3Biochem. J. 1994;304:833–841. doi: 10.1042/bj3040833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Emmer B.T., Daniels M.D., Taylor J.M., Epting C.L., Engman D.M. Calflagin inhibition prolongs host survival and suppresses parasitemia in Trypanosoma brucei infection. Eukaryot. Cell. 2010;9:934–942. doi: 10.1128/EC.00086-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Engman D.M., Krause K.H., Blumin J.H., Kim K.S., Kirchhoff L.V., Donelson J.E. A novel flagellar Ca2+-binding protein in trypanosomes. J. Biol. Chem. 1989;264:18627–18631. [PubMed] [Google Scholar]
- 110.Wingard J.N., Ladner J., Vanarotti M., Fisher A.J., Robinson H., Buchanan K.T., Engman D.M., Ames J.B. Structural insights into membrane targeting by the flagellar calcium-binding protein (FCaBP), a myristoylated and palmitoylated calcium sensor in Trypanosoma cruzi. J. Biol. Chem. 2008;283:23388–23396. doi: 10.1074/jbc.M803178200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ridgley E., Webster P., Patton C., Ruben L. Calmodulin-binding properties of the paraflagellar rod complex from Trypanosoma brucei. Mol. Biochem. Parasitol. 2000;109:195–201. doi: 10.1016/S0166-6851(00)00246-2. [DOI] [PubMed] [Google Scholar]
- 112.Ginger M.L., Collingridge P.W., Brown R.W., Sproat R., Shaw M.K., Gull K. Calmodulin is required for paraflagellar rod assembly and flagellum-cell body attachment in trypanosomes. Protist. 2013;164:528–540. doi: 10.1016/j.protis.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 113.Portman N., Lacomble S., Thomas B., McKean P.G., Gull K. Combining RNA interference mutants and comparative proteomics to identify protein components and dependences in a eukaryotic flagellum. J. Biol. Chem. 2009;284:5610–5619. doi: 10.1074/jbc.M808859200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tellez-Inon M.T., Ulloa R.M., Torruella M., Torres H.N. Calmodulin and Ca2+-dependent cyclic AMP phosphodiesterase activity in Trypanosoma cruzi. Mol. Biochem. Parasitol. 1985;17:143–153. doi: 10.1016/0166-6851(85)90013-1. [DOI] [PubMed] [Google Scholar]
- 115.Wayne A Snedden H.F. Calmodulin as a versatile calcium signal transducer in plants. New Phytol. 2001;151:35–66. doi: 10.1046/j.1469-8137.2001.00154.x. [DOI] [PubMed] [Google Scholar]
- 116.Villalobo A., Ishida H., Vogel H.J., Berchtold M.W. Calmodulin as a protein linker and a regulator of adaptor/scaffold proteins. Biochim. Biophys. Acta. 2018;1865:507–521. doi: 10.1016/j.bbamcr.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 117.Rohloff P., Montalvetti A., Docampo R. Acidocalcisomes and the contractile vacuole complex are involved in osmoregulation in Trypanosoma cruzi. J. Biol. Chem. 2004;279:52270–52281. doi: 10.1074/jbc.M410372200. [DOI] [PubMed] [Google Scholar]
- 118.Ulrich P.N., Jimenez V., Park M., Martins V.P., Atwood J., 3rd, Moles K., Collins D., Rohloff P., Tarleton R., Moreno S.N., et al. Identification of contractile vacuole proteins in Trypanosoma cruzi. PLoS ONE. 2011;6:e18013. doi: 10.1371/journal.pone.0018013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Patel S., Docampo R. Acidic calcium stores open for business: Expanding the potential for intracellular Ca2+ signaling. Trends Cell Biol. 2010;20:277–286. doi: 10.1016/j.tcb.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]