Abstract
Our aim was identify of the pandemic B2-ST131 Escherichia coli clone by to the Institute Pasteur and Achtman scheme, and investigate the resistance profile phenotypic-genotypic, with identification of class 1 integron. Of thirty-five ESBL-producing isolates recovered of patients with diagnosis of urinary tract infections (UTI), six E. coli strains serotype O25 were identified with resistance antimicrobial to several groups of antibiotics such as broad-spectrum cephalosporins, fluoroquinolones and aminoglycosides, harboring blaSHV, blaCTX-M genes in all isolates and blaTEM in two isolates. Sequencing of blaCTX-M revealed CTX-M-15 in all strains. The PMQR aac(6´)-Ib-cr and qnrB19 genes were presented in five and four isolates respectively, AMEs genes aac(6´)-Ib and aac(3)-IIa were presented in strain amikacin-gentamicin-resistant. Sequencing of the variable regions of the class 1 integron revealed dfrA and aadA genes cassette. The analysis of multilocus sequence typing (MLST) confirms the presence of the pandemic B2-ST131 E. coli clone by Achtman scheme in all ST43 isolates obtained by of the Institute Pasteur scheme. The results presented herein, reveal the presence of B2-ST131 E. coli clone in Ecuador, disseminated in hospitals and community settings.
Keywords: Escherichia coli, resistance, ST131, ESBL, MLST
A multi-resistant clonal group of Escherichia coli ESBL-producing isolates, O25b:H4/ST131, was identified in 2008 as a major clone linked to CTX-M-15. Since then, it has also been strongly associated with fluoroquinolone resistance and co-resistance to aminoglycosides and trimethoprim-sulfamethoxazole [1]. This clone presents multiple antimicrobial resistance patterns, including resistance genes such as blaCTX-M-15, blaTEM-1, blaOXA-1, aac(6’)-Ib-cr, qnrB, and aac(3)-II, which are mainly located at plasmids belonging to the IncF group [2]. Currently, the B2-ST131 Escherichia coli clone is spread worldwide among humans, but it is also frequently recovered from livestock, companion animals, and food [3]. The present report describes the first detection of the Escherichia coli B2-ST131 clone isolated from patients diagnosed with urinary tract infections (UTI) in Quito-Ecuador.
We investigated 35 non-duplicate Escherichia coli ESBL-producing isolates, recovered from patients with nosocomial/healthcare-acquired or community acquired UTIs, in the metropolitan area of Quito, Ecuador. Isolated between July and December 2012, six isolates of Escherichia coli serotype O25 were identified with resistance to several groups of antibiotics, and the molecular characteristics of resistance in these isolates were studied. The antimicrobial susceptibility profile was determined via the agar diffusion method in accordance with the 2015 Clinical & Laboratory Standards Institute, CLSI Guidelines. Following species identification and antimicrobial susceptibility testing, isolates were screened for ESBL-producing phenotypes using double-disc synergy. The minimum inhibitory concentrations (MICs) of ciprofloxacin, gentamicin, and cefotaxime were determined using Etest strips following the manufacturer’s recommendations. Polymerase chain reaction (PCR) testing was performed in order to determine the phylogenetic relationship of these isolates, as per the methodology used by Clermont [4], namely, the detection of the class 1 integrons and the β-lactamases genes blaSHV, blaTEM, blaCTX-M, blaAMPC; PMQR genes qnrA, qnrB, qnrS, and aac(6’)-Ib-cr; AMEs genes aac(3)-IIa, aac(6’)-Ib, and ant(2”)-Ia; and 16S rRNA methylase genes armA, rmtA, rmtB, rmtC, rmtD, and npmA. Amplification products were purified and sequenced in a 3730 DNA Analyzer (Applied Biosystems, Foster City, CA, USA), at the PDTIS-IOC DNA Sequencing Platform. Sequences were compared to those in the GenBank database. Multi-locus sequence typing (MLST), were determined using the platform for E. coli MLST maintained at the Institute Pasteur, Paris, France, (http://bigsdb.pasteur.fr/ecoli/ecoli.html) as well as the Achtman multi-locus sequence typing scheme (http://mlst.warwick.ac.uk/mlst/dbs/Ecoli/documents/primersColi_html).
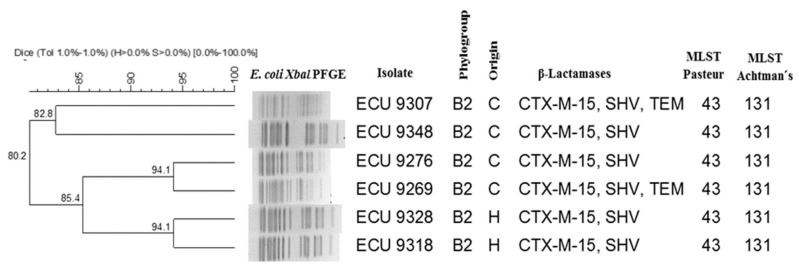
These six isolates of E. coli obtained from patients with UTIs, of which four were community-acquired and two were nosocomial, showed great resistance to broad-spectrum cephalosporins, fluoroquinolones, and aminoglycosides, confirmed by MICs. In addition, we detected co-resistance to various groups of antibiotics (Table 1). Sequence type 131 Escherichia coli clones were found to possess blaSHV and blaCTX-M in all isolates, with blaTEM present in two isolates. blaAMPC genes were not detected. Sequencing of blaCTX-M revealed CTX-M-15 in all isolates. The PMQR aac(6’)-Ib-cr and qnrB19 genes were present in five and four isolates, respectively. AMEs genes aac(6’)-Ib and aac(3)-IIa were present in amikacin-gentamicin-resistant strains. 16S rRNA methylase genes were not detected and sequencing of the variable regions of the class 1 integron presented dfrA and aadA genes in five isolates. The MICs of cefotaxime ranged from 2 to >32 µg/mL, those of gentamicin ranged from 8 to >16 µg/mL, and those of ciprofloxacin ranged from 2 to >32 µg/mL. The multi-locus sequence typing scheme, designated according to the Institute Pasteur, revealed the clone B2-ST43, and the Achtman scheme confirmed the presence of the B2-ST131 E. coli clone in all isolates. Several other sequence types were present (ST2, ST160, ST365, ST173, ST21, ST477, ST472, and ST38).
Table 1.
Phenotypic and genotypic features of uropathogenic E. coli ST131.
| Isolate | PG | Phenotypic Profile | Genetic Profile | |||
|---|---|---|---|---|---|---|
| Co-Resistance a | β-Lactamases | PMQR | AMEs | Integron 1 | ||
| ECU9269 | B2 | CTX-CAZ-ATM-AM-CF-AMC-CIP-NOR | CTX-M-15, SHV, TEM | aac(6’)-Ib-cr | ||
| ECU9276 | B2 | CTX-ATM-AM-CF-AMC SXT-AK-CIP-NOR | CTX-M-15, SHV | aac(6’)-Ib-cr | aac(6’)-Ib | dfrA17, aadA5 |
| ECU9307 | B2 | CTX-FEP-CAZ-ATM-AM-CF-AMC-SXT-CN-CIP-NOR | CTX-M-15, SHV, TEM | aac(6’)-Ib-cr/qnrB19 | aac(6’)-Ib/aac(3)-IIa | dfrA12, aadA2 |
| ECU9318 | B2 | CTX-AM-CF-AMC-SXT-AK- CIP-NOR | CTX-M-15, SHV | qnrB19 | aac(6’)-Ib | dfrA17, aadA5 |
| ECU9328 | B2 | CTX-AM-CF-AMC-SXT-CIP-NOR | CTX-M-15, SHV | aac(6’)-Ib-cr/qnrB19 | dfrA17, aadA5 | |
| ECU9348 | B2 | CTX-FEP-CAZ-ATM-AM-CF-AMC-SXT-CN-CIP-NOR | CTX-M-15, SHV | aac(6’)-Ib-cr/qnrB19 | aac(6’)-Ib/aac(3)-IIa | dfrA17, aadA5 |
AK, amikacin; CN, gentamicin; SXT, trimethoprim–sulphamethoxazole; CIP, ciprofloxacin; NOR, norfloxacin; AM, ampicillin; ATM, aztreonam; CF, cefalothin; FOX, cefoxitin; CTX, cefotaxime; CAZ, ceftazidime; FEP, cefepime; AMC, amoxicillin-clavulanic acid; PMQR, plasmid-mediated quinolone resistance determinant; AMEs, aminoglycoside-modifying enzymes; PG, phylogroup. a Antimicrobial non-susceptibility (i.e., resistant or intermediate).
The B2-ST131 E. coli clone has recently emerged, spreading out throughout the world, and is responsible for community and hospital-acquired urinary tract and bloodstream infections [1,2,3]. This clone represents a growing public health concern, primarily due to its resistance to various antimicrobial agents and its possession of high numbers of virulence factors, increasing morbidity and mortality [3]. The presence of several resistance genes in this clone has given it a broad antibiotic resistance profile, mainly against β-lactam antibiotics, aminoglycosides, and fluoroquinolones, all of which are widely used in the treatment of urinary tract infections. This study highlights the resistance of one strain to aminoglycosides and trimethoprim/sulphametoxazole, which lacks AMEs and dfrA/aadA genes, according to previous studies (Table 1). Epidemiological studies using pulsed-field gel electrophoresis (PFGE) have demonstrated that E. coli ST131 strains exhibit diverse pulsotypes [2]. In this research, we observed two clones with 94.1% similarity and one clone with less than 85% similarity (Figure 1). Our analysis of multi-locus sequence typing confirmed the presence of the pandemic B2-ST131 E. coli clone by the Achtman scheme in all ST43 isolates obtained by the Institute Pasteur scheme, in agreement with previous studies [5]. As such, it is important to maintain a surveillance system for the identification of clones representing a public health threat, particularly those that occur outside of the hospital environment.
Figure 1.
Dendrogram of pulsed-field gel electrophoresis (PFGE) patterns showing the genetic relatedness of the uropathogenic Escherichia Coli ST131 clone. C, community; H, Hospital.
Acknowledgments
This study was supported by Secretaria Nacional de Ciencia Tecnologia e Innovacion (SENESCYT)-Ecuador, Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Instituto Oswaldo Cruz, Brazil.
Author Contributions
C.G.C. conceived the design of the study, processed the samples, analyzed the results, and prepared a draft of the manuscript. E.P.J. processed the samples and corrected the draft of the manuscript. M.D.A. directed the research project, checked the analyzed results and corrected the draft of the manuscript
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Schembri M.A., Zakour N.L., Phan M.D., Forde B.M., Stanton-Cook M., Beatson S.A. Molecular characterization of the Multidrug Resistant Escherichia coli T131 Clone. Pathogens. 2015;4:422–430. doi: 10.3390/pathogens4030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodford N., Carattoli A., Karisik E., Underwood A., Ellington M.J., Livermore D.M. Complete Nucleotide Sequences of Plasmids pEK204, pEK499, and pEK516, Encoding CTX-M Enzymes in Three Major Escherichia coli Lineages from the United Kingdom, All Belonging to the International O25:H4-ST131 Clone. Antimicrob. Agents Chemother. 2009;53:4472–4482. doi: 10.1128/AAC.00688-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Platell J.L., Johnson J.R., Cobbold R.N., Trott D.J. Multidrug-resistant extraintestinal pathogenic Escherichia coli of sequence type ST131 in animals and foods. Vet. Microbiol. 2011;153:99–108. doi: 10.1016/j.vetmic.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 4.Clermont O., Bonacorsi S., Bingen E. Rapid and Simple Determination of the Escherichia coli Phylogenetic Group. Appl. Environ. Microbiol. 2000;66:4555–4558. doi: 10.1128/AEM.66.10.4555-4558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Suwantarat N., Rudin S.D., Marshall S.H., Hujer A.M., Perez F., Hujer K.M., Domitrovic T.N., Dumford D.M., Donskey C.J., Bonomo R.A. Infections caused by fluoroquinolone-resistant Escherichia coli following transrectal ultrasound-guided biopsy of the prostate. J. Glob. Antimicrob. Resist. 2014;2:71–76. doi: 10.1016/j.jgar.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]