Abstract
Considerable advancements in cochlear implant technology (e.g., electric acoustic stimulation) and assessment materials have yielded expanded criteria. Despite this, it is unclear whether individuals with better audiometric thresholds and speech understanding are being referred for cochlear implant workup and pursuing cochlear implantation. The purpose of this study was to characterize the mean auditory and demographic profile of adults presenting for preoperative cochlear implant workup. Data were collected prospectively for all adult preoperative workups at Vanderbilt from 2013 to 2015. Subjects included 287 adults (253 postlingually deafened) with a mean age of 62.3 years. Each individual was assessed using the minimum speech test battery, spectral modulation detection, subjective questionnaires, and cognitive screening. Mean consonant-nucleus-consonant word scores, AzBio sentence scores, and pure-tone averages for postlingually deafened adults were 10%, 13%, and 89 dB HL, respectively, for the ear to be implanted. Seventy-three individuals (25.4%) met labeled indications for Hybrid-L and 207 individuals (72.1%) had aidable hearing in the better hearing ear to be used in a bimodal hearing configuration. These results suggest that mean speech understanding evaluated at cochlear implant workup remains very low despite recent advancements. Greater awareness and insurance accessibility may be needed to make cochlear implant technology available to those who qualify for electric acoustic stimulation devices as well as individuals meeting conventional cochlear implant criteria.
Keywords: cochlear implants, speech understanding, implant candidacy, adult, hearing loss
Introduction
In 2014, approximately 37.5 million Americans or 15% of the U.S. population reported difficulty hearing without hearing aids (Blackwell, Lucas, & Clarke, 2014). It is further estimated that of these 37.5 million individuals, over 750,000 have severe-to-profound hearing loss (National Institute on Deafness and Other Communication Disorders, 2004, 2016). As of December 2012, roughly 58,000 cochlear implants had been implanted in adults in the United States. Thus, the market penetration for cochlear implantation was just 7.7% in the adult population of individuals with severe-to-profound sensory hearing loss. Based on these statistics, it appears that there are many potential cochlear implant (CI) candidates who are not being identified due to a variety of reasons including lack of initial consult for hearing loss, lack of appropriate referral from other health-care providers, lack of education about CIs, and exclusion based on labeled criteria, or some combination.
Labeled CI indications for adult recipients are based on audiometric thresholds—consistent with moderate-to-profound sensory hearing loss—and open-set sentence recognition (Centers for Medicare and Medicaid Services [CMS], 2005; Cochlear Americas, 2016; Gifford, Dorman, Shallop, & Sydlowski, 2010). The current minimum speech test battery (MSTB; 2011) for adult CI recipients serves as the best-practices document for the assessment of adult CI performance. Specifically, the MSTB recommends the use of the AzBio sentences (Spahr et al., 2012) for determining adult CI candidacy. The MSTB for adult CI users also recommends administration of consonant-nucleus-consonant (CNC; Peterson & Lehiste, 1962) monosyllabic words and the Bamford–Kowal–Bench Speech in Noise (BKB-SIN, Etymotic Research Inc, 2005) tests.
In addition to the MSTB, other assessments have proven to be valuable additions to the CI candidacy evaluation. In particular, many CI clinics routinely administer subjective questionnaires, cognitive screenings, and some centers even administer more complex assessments of auditory function such as spectral resolution or spectral–temporal resolution. Subjective questionnaires can be used to assess the functional impact of hearing loss and the chosen intervention to capture a more holistic picture of patient outcomes and resultant quality of life (Capretta & Moberly, 2016). Spectral resolution can be quickly assessed in a clinical setting via tasks of spectral modulation detection (SMD; e.g., Gifford, Hedley-Williams, & Spahr, 2014) or spectral ripple discrimination (e.g., Drennan, Anderson, Won, & Rubinstein, 2014). SMD tasks require the listener to discriminate a flat spectrum noise from one with spectral peaks and valleys. Spectral ripple discrimination tasks require the listener to discriminate a stimulus in which the temporal location of spectral peaks and valleys has been time-reversed. Tasks of spectral–temporal modulation detection (e.g., Bernstein, Iyer, & Brungart, 2013) impose modulation to both frequency and amplitude and thus may be of even greater value given the spectral and temporal complexity of speech.
Evolution of Adult Implant Candidacy
Gifford et al. (2010) advocated for a revision and expansion of adult CI candidacy criteria based upon results documenting that implant recipients with higher levels of preoperative speech understanding than traditional patients still demonstrated significant benefit from cochlear implantation. Since that article was published 8 years ago, there have been many advancements in CI technology including the Food and Drug Administration (FDA) approval and subsequent market release of electric acoustic stimulation (EAS) CI systems for adults with thresholds in the normal sloping to moderate-to-severe hearing loss range. Candidates for this type of EAS/Hybrid implant can have up to 60% word recognition in the ear to be implanted and up to 80% correct in the contralateral ear. Aside from the market release of the Hybrid-L24, MED-EL EAS, and Advanced Bionics EAS systems, adult implant indications have not changed since 2005 when the CMS expanded the national coverage determination for cochlear implantation to include individuals with moderate-to-profound sensory hearing loss with aided sentence recognition scores up to 40% correct (CMS, 2005). The new indications for EAS devices have made qualifying for cochlear implantation possible for more individuals. It is unclear, however, whether the market availability of EAS/Hybrid CI systems has influenced clinical practice. In other words, can we identify a change in the typical patient auditory profile for those presenting for a preoperative evaluation to determine CI candidacy? To make such comparisons in the future, we must first describe the typical auditory profile for an aggregated clinical dataset against which future comparisons can be benchmarked.
Although there are no known studies in the peer-reviewed literature describing the typical auditory profile of adult preoperative patients for a large-scale clinical population, there are published reports resulting from several CI clinical trials that report preimplant audiometric thresholds and speech recognition scores. Zwolan et al. (2001) reported the results of the Clarion (Advanced Bionics) HiFocus clinical trial for 56 postlingually deafened adults. The mean preoperative CNC monosyllabic word recognition was 3% with a range of 0% to 22% correct. Parkinson et al. (2002) reported the results of the Nucleus 24 multicenter trial for 56 postlingually deafened adults. The mean preoperative CNC monosyllabic word recognition for the ear to be implanted was 4% with a range of 0% to 30% correct. Balkany et al. (2007) reported the results of the Nucleus Freedom (CI24RE) clinical trial for 71 postlingually deafened adult patients. Although the inclusion criteria were broader than for previous FDA clinical trials allowing for higher levels of aided speech understanding, the mean preoperative, best-aided CNC performance was just 3% with a range of 0% to 19% correct. Roland, Gantz, Waltzman, and Parkinson (2016) reported the results of the Nucleus Hybrid-L24 clinical trial in the United States for 50 postlingually deafened adults with significantly greater residual acoustic hearing than traditional CI candidates. Inclusion criteria for this device were the broadest to date, allowing up to 60% CNC word recognition in the ear to be implanted; however, even in this study, mean preoperative word recognition was just 18% correct.
Study Aims
Despite the evolution of adult implant criteria from the mid-to-late 1990s through the mid-2000s, clinical trials completed over that time period recruited similar study populations including patients with little-to-no auditory only, open-set speech understanding. This begs the question of whether we are truly expanding our reach to adults experiencing communicative difficulty who have moderate-to-profound sensory hearing losses with better speech understanding for traditional implants or those with better low-frequency hearing and up to 60% sentence or word recognition for EAS/Hybrid implants. Alternatively, perhaps we are simply reaching a greater number of traditional CI candidates with severe-to-profound hearing losses and little-to-no speech understanding who had not been previously evaluated for CI candidacy.
The primary goal of this research project is to provide a thorough description of the adult population seeking audiological evaluation for CI candidacy so that we may ultimately improve access to CI technology for individuals who do not receive hearing aid benefit. In light of this primary goal, our specific research objectives were as follows: (a) to provide a thorough description of the current audiometric thresholds, auditory function (i.e., spectral resolution), and patient demographics (i.e., age, racial and ethnic composition, subjective communication difficulty, and cognitive status) of adults presenting for preoperative CI candidacy evaluation in a large academic medical center and (b) to examine the relationship between preoperative measures of auditory function (e.g., Drennan et al., 2014; Drennan, Won, Timme, & Rubinstein, 2016; Gifford et al., 2014), subjective reports of communication difficulty, and cognitive status. Although there are no published papers describing aggregate clinical datasets on which to base a data-driven hypothesis, we have been closely following our clinical data for a number of years on which we drafted the following working hypothesis: We are currently seeing preoperative candidates across a significantly broader distribution of preoperative audiometric thresholds and speech understanding performance than that reported previously in the literature.
The secondary goal of this study was to define the proportion of adults presenting for preoperative CI evaluation who are candidates for either bimodal hearing (CI plus contralateral hearing aid) or bilateral cochlear implantation. Dorman and Gifford (2010) reported that 59.8% of adult CI candidates at two large academic medical centers (n = 276) had “aidable” low-frequency hearing in the nonimplanted ear—defined as a 250-Hz threshold ≤ 85 dB HL. Although we are not suggesting that individuals with thresholds up to 85 dB HL are ideal bimodal candidates, we used the 85-dB-HL criterion described by Dorman and Gifford (2010) as a benchmark against which we could compare the current dataset. Our working hypothesis was that the percentage of potential bimodal candidates has increased since 2010. Fulfilling the objectives aligned with our primary and secondary goals will allow us to determine whether additional outreach efforts are needed to improve access to CI technology for individuals with bilateral moderate-to-profound sensorineural hearing loss who could derive benefit from cochlear implantation.
Methods
Participants
Data were collected prospectively for all adult preoperative workups at Vanderbilt from August 2013 through July 2015 in conjunction with a National Institute of Health-funded study investigating SMD in pre- and postimplant adult recipients for whom we had institutional review board approval. The participants were adult patients presenting to the clinic for CI candidacy evaluations. Data are reported for every adult patient presenting between the dates of August 2013 to August 2015. The only subjects excluded from this study were those who had been previously implanted, explanted, and were seeking reimplantation.
Candidacy for cochlear implantation was determined by individual ear scores on the AzBio sentence test presented in noise at a level of +5 dB signal-to-noise ratio (SNR). For participants with private insurance, they met candidacy criteria if their score fell below 60%. For participants with Medicare, they met candidacy criteria if their score fell below 40%. The ear selected for implantation was based on several factors including, but not limited to patient preference, duration of deafness, speech recognition scores, preoperative imaging, and surgeon recommendation. The choice to pursue bilateral implantation was generally patient driven assuming that both ears met candidacy criteria; no patients with interest in pursuing bilateral implantation were excluded from receiving bilateral implants due to insurance coverage. That is, no patients in the current sample were denied insurance coverage for bilateral implantation. Our rationale for basing candidacy criteria on performance at +5 dB SNR stems from (a) patients’ greatest complaint is speech understanding in noise (e.g., Kochkin, 2010), (b) there are a number of sound ecological studies demonstrating that +5 dB is the most common real-world SNR (Pearsons, Bennett, & Fidell, 1977; Smeds, Wolters, & Rung, 2015), and (c) both our own clinical data and that reported by Mudery, Francis, McCrary, and Jacob (2017) have demonstrated that even older listeners for whom candidacy was determined at +5 dB demonstrate significant improvement both in quiet and noise following implantation.
CI candidacy evaluation results are reported for 287 patients. Two hundred fifty-three patients were postlingually deafened adults and 34 were prelingually deafened adults. The average age of the subjects was 62.3 years. One hundred thirty subjects were female and 157 subjects were male. The racial and ethnic representation of the participants was as follows: 87% Caucasian, 7% African American, and 6% other (i.e., Asian, American Indian). There are no published reports of racial or ethnic composition of adults pursuing cochlear implantation. Of interest here is that the racial and ethnic composition of this sample is not directly representative of neither the state of Tennessee nor of the greater Nashville metropolitan area during this time period, which was 78.7% White/Caucasian, 17.1% Black/African American, 1.8% Asian, 0.4% American Indian or Alaska Native, 0.1% Native Hawaiian and Other Pacific Islander, and 1.9% two or more races (United States Census Bureau, 2016). It should be noted that “two or more races” was not given as a possible option for Vanderbilt patients as it is by the U.S. Census Bureau, and thus, these racial distributions may not be directly comparable.
Etiology of the participants’ hearing losses was highly variable. Etiology was based on patient report when physician diagnosis was not present and should therefore be interpreted with caution. An overwhelming majority of the participants reported that the etiology of their hearing loss was unknown, noise induced, hereditary (not linked to a specific gene), or “other.”
Procedures
Each participant underwent preoperative audiological assessment according to Vanderbilt’s standard CI workup protocol as outlined later. Study data were collected and managed using the Research Electronic Data Capture (REDCap) secure data management tool (Harris et al., 2009).
Audiometric Thresholds
Audiometric thresholds were completed in a double-walled sound treated booth. Air-conduction thresholds were obtained for 125, 250, 500, 750, 1000, 1500, 2000, 3000, 4000, 6000, and 8000 Hz using insert earphones. Bone conduction thresholds were obtained for 500, 1000, 2000, and 4000 Hz using the bone oscillator placed on the mastoid. Contralateral masking was implemented when appropriate.
Speech Recognition
Speech recognition was assessed as recommended by the revised MSTB (2011) for adult CI recipients. All speech recognition testing was completed in a double-walled sound treated booth. Stimuli were presented at 60 dBA through a single loudspeaker positioned at 0° azimuth approximately 1 m from the listener. All clinic booths were equipped with a Larson Davis LxT sound level meter located in the clinician control room with the pre-amp and microphone suspended from the ceiling at the location of the patient’s head in the booth. This setup allowed for calibration prior to assessment for every patient seen in the clinic. All speech recognition testing was completed using either the listener’s personal hearing aids or clinic stock hearing aids. Hearing aids were verified for all patients using test box measurements with simulated real-ear measures (using an average real-ear to coupler difference), and adjustments were made when necessary to match to NAL-NL2 target audibility for 60-dB-SPL speech. If the patient’s personal hearing aids did not match prescriptive targets, either the patients’ hearing aids were reprogrammed, if possible, or clinic stock aids were programmed for testing purposes. Only 32 of 110 (29.1%) patients’ own hearing aids achieved NAL-NL2 target audibility for 60-dB-SPL speech; thus, the remaining patients’ hearing aids were reprogrammed or they were fitted with clinic stock aids. In all cases for which prescriptive targets were not met, hearing aid output was lower than recommended by 5 dB or greater for at least one potentially audible frequency. Of interest is that 177 of 287 patients reported to the preoperative appointment without hearing aids. All patients had hearing aid experience, but those who failed to bring their aids to the appointment cited that they did not wear the devices due to lack of perceived benefit.
CNC word recognition was assessed using a full 50-word list for which the target word is preceded by the carrier word ready such as, “Ready, bird” (Peterson & Lehiste, 1962). Participants were instructed to repeat as much of each word as possible and encouraged to guess. Each phoneme and word was scored individually resulting in an overall word and phoneme percentage correct score. Participants completed one list per ear and one list in the bilaterally aided condition.
AzBio sentence recognition was assessed using a full 20-sentence list. All lists comprise sentences spoken by four talkers, two male and two female (e.g., “He drank to excess after the hamster’s death”; Spahr et al., 2012). These sentences were presented in quiet and in continuous 20-talker babble at +10 and +5 SNR.1 Participants were instructed to repeat as much of each sentence as possible and encouraged to guess. The listener was given credit for each word she or he accurately repeated, resulting in an overall percentage correct score. Participants completed one list per ear as well as one list in the bilaterally aided condition.
The BKB-SIN test consists of sentences spoken by a single male talker in a background of four-talker babble (e.g., “The football player lost a shoe.”; Etymotic Research Inc, 2005). These paired lists contain 20 sentences each with three to four key words per sentence. The stimuli are initially presented at an SNR of +21 dB decreasing in 3-dB steps per sentence to 0 dB SNR or −6 dB SNR, depending on the list. Assessment included one paired BKB-SIN list per ear and one paired list in the bilaterally aided condition. The result of the test is an SNR at which the listener correctly recognizes 50% of the key words, expressed as the SNR-50, in dB.
Assessment of Spectral Resolution
Spectral resolution was assessed via the quick spectral modulation detection (QSMD) task. The QSMD task (Gifford et al., 2014) is a three-interval forced choice procedure for which two intervals contain a flat spectrum of noise and the third contains spectrally modulated noise. Spectral modulation was achieved by applying logarithmically spaced, sinusoidal modulation to the broadband carrier stimulus (125–5600 Hz). There were six trials presented for each of the 10 modulation depths (4 to 22 dB, in 2-dB steps) with a fixed modulation frequency or rate of 1.0 cycle/oct for a total of 60 trials. This version of the QSMD designed for assessment with acoustic hearing differs somewhat from the version described in previous studies, which was designed for assessment with CI or electric hearing (e.g., Gifford et al., 2014; Noble, Gifford, Hedley-Williams, Dawant, & Labadie, 2014; Noble, Labadie, Gifford, & Dawant, 2013). In either case, the QSMD task provides an overall percentage correct score averaged across all modulation depths, with 33% representing chance. The QSMD task was not used to determine CI candidacy. Rather, this measure was included as part of a larger study investigating longitudinal outcomes for CI recipients in an effort to evaluate whether this measure may hold clinical utility for (a) implant ear selection and (b) correlation with postoperative outcomes. The purpose of its inclusion here is to quantify preoperative spectral resolution for a large clinical population of CI candidates so that this could serve as a benchmark against which future data will be compared. A number of recent studies have reported postoperative outcomes on abbreviated, clinically feasible measures of SMD (Gifford et al., 2014) and spectral ripple discrimination (Drennan et al., 2014, 2016); however, up until this point, there have been no preoperative data with which to compare postoperative outcomes.
Perceived Hearing Handicap and Quality of Life
The Abbreviated Profile of Hearing Aid Benefit (APHAB) is a 24-item self-assessment inventory in which patients report the amount of difficulty they are having with communication or noises in various everyday situations. It produces scores for four subscales: Ease of Communication, Reverberation, Background Noise, and Aversiveness (Cox & Alexander, 1995). The scores for each subscale reflect a percentage of problems that the patient is experiencing. Patients completed this assessment according to their perception of sound with hearing aids only; that is, we did not probe the unaided condition.
The Speech Spatial Qualities (SSQ) questionnaire employs a visual analog scale, which gauges hearing ability across three listening domains: speech understanding in various listening conditions; spatial hearing associated with distance, movement, and direction; and the overall quality of speech including clarity and naturalness of sound (Gatehouse & Noble, 2004). Patients completed this assessment according to their perception of sound with hearing aids. Higher scores on this metric are correlated with better speech understanding, spatial hearing, and sound quality.
Both APHAB and SSQ are used by our hearing aid program successfully. These measures were not designed or validated for use with CI recipients, but we currently use them in the clinical CI program at Vanderbilt. From a clinical perspective, we believe that using the same qualitative assessments across rehabilitative programs allows us to gauge subjective outcomes for large clinical populations and for comparison across a range of auditory profiles, ages, and intervention strategies (e.g., hearing aids, bone anchored implants, middle ear implants, CIs).
Screening for Cognitive Impairments
The Mini-Mental State Exam (MMSE) is a validated screening tool designed to screen for risk of cognitive impairment. It includes tasks that evaluate orientation, attention, memory, language, and visual-spatial skills (Folstein, Folstein, & McHugh, 1975). The questionnaire produces a numerical score with 30 points as maximum. It is generally accepted that a score of less than 25 is indicative of cognitive impairment, but a slight decline in MMSE score should be expected in the typically aging population (Bleecker, Bolla-Wilson, Kawas, & Agnew, 1988). It should be noted that this screener was administered to all subjects regardless of age or mental status, and the resulting score did not exclude the patient from obtaining a CI.
Results
Cochlear Implantation Decisions
Of the 287 patients who underwent CI candidacy evaluations, 236 or 82.2% went on to receive a CI. Of the 51 individuals (17.8%) who did not receive an implant, 27.4% (n = 14) exceeded criteria for implantation, 13.7% (n = 7) postponed surgery due to other medical complications, 17.6% (n = 9) elected not to proceed with implantation despite meeting FDA and CMS labeled indications, and 1.9% (n = 1) passed away prior to the surgery date. The remaining 39.2% (n = 20) of patients who did not receive an implant were lost to follow-up.
Audiometric Thresholds
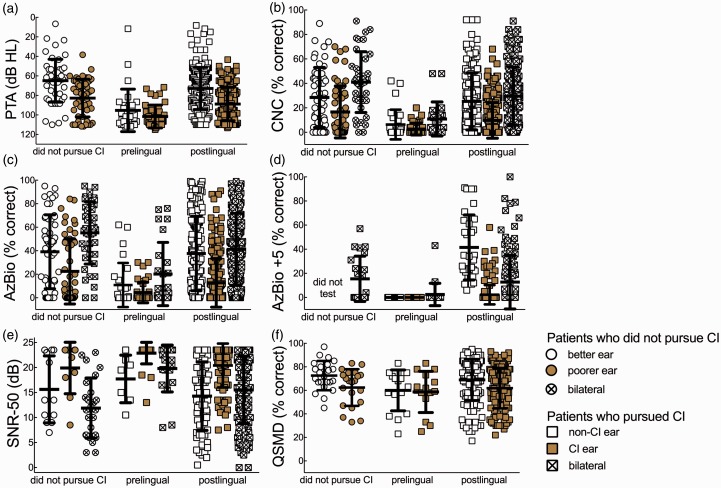
Figure 1(a) shows individual and mean pure-tone averages (PTA), the average of thresholds at 500, 1000, and 2000 Hz, for patients who did not get an implant in either ear (76.1 dB HL), implanted ears (90.7 dB HL), and contralateral nonimplanted (75.6 dB HL) ears. As expected, when separated by onset of deafness, prelingually deafened patients’ PTA was higher at 99.2 dB HL compared to 80.2 dB HL for postlingually deafened patients. The average PTA for nonimplanted ears was 75.6 dB HL; however, the range was 6.7 to 115 dB HL. The low end of this range represented six patients who presented for CI workup and were implanted for single-sided deafness.
Figure 1.
(a) Individual and mean pure-tone averages (PTA), the average of thresholds at 500, 1000, and 2000 Hz, for patients who did not get an implant in either ear (76.1 dB HL), implanted ears (90.7 dB HL), and contralateral nonimplanted (75.6 dB HL) ears. (b–f) Individual and mean scores for the CNC, AzBio, AzBio at +5 dB SNR, BKB-SIN, and QSMD, respectively. The data are arranged by implanted and nonimplanted ears as unfilled and shaded symbols and bilateral aided as crosshatched symbols. The data for individuals ultimately not pursuing implantation are displayed separately in each figure as better ear, poorer ear, and bilateral.
Speech Recognition
Figure 1(b) to (e) displays the individual and mean scores for the CNC, AzBio, AzBio at +5 dB, and BKB-SIN, respectively. The data are arranged by implanted and nonimplanted ears as unfilled and shaded symbols and bilateral aided as crosshatched symbols. The data for individuals ultimately not pursuing implantation are displayed separately in each figure as better ear, poorer ear, and bilateral. Table 1 displays mean scores and standard deviations for all measures with postlingually and prelingually deafened patients combined. Ear-specific data are presented here given that all three FDA-approved CI manufacturers are moving toward an ear-specific criterion for CI candidacy (NCT01337076, 2011; NCT03052920, 2017; NCT02811549, 2017). The data included in Table 1 include all individuals who were seen for preoperative workup, irrespective of whether or not the patient pursued cochlear implantation. Although the distributions of scores were similar across the groups, best-aided speech understanding was significantly better for individuals who did not pursue cochlear implantation as compared to postlingually deafened patients who did pursue implantation for all measures except AzBio sentences at +5 dB SNR (CNC: 41.0% vs. 24.3 %, t = 4.0, p < .001; AzBio: 55.2% vs. 35.6%, t = 3.7, p < .0001; AzBio + 5: 15.4% vs. 11.1%, t = 0.97, p = .36; BKB-SIN: 12.2 vs. 15.6 dB, t = 2.4, p = .017). Considering the ear to be implanted (or poorer ear for those who did not pursue CI), speech understanding performance was significantly different across the two groups for all speech measures presented in quiet (CNC: 16.6% vs. 8.8%, t = 3.3, p = .01; AzBio: 22.5% vs. 11.6%, t = 3.4, p = .008). However, for speech understanding in noise, there was no difference in performance across the groups for the ear to be implanted or the poorer ear (AzBio +5: did not analyze due to few data points for this measure; BKB-SIN: 19.9 dB vs. 21.1 dB, t = 0.90, p = .39).
Table 1.
Means and Ranges of All Ears Tested, Ears That Went on to Receive a Cochlear Implant, and Ears That Were Not Implanted for All Included Subjects (n = 287).
| All ears (n = 535) | Implanted ears (n = 279) | Nonimplanted ears (n = 256) | |
|---|---|---|---|
| PTA (dB HL) | 82.5 (6.7–115.0) | 90.7 (43.3–115.0) | 75.6 (6.7–115.0) |
| CNC (% Correct) | 16.5 (0.0–92.0) | 8.7 (0.0–68.0) | 22.4 (0.0–92.0) |
| AzBio (% Correct) | 23.3 (0.0–99.0) | 11.9 (0.0–91.0) | 33.1 (0.0–99.0) |
| BKB-SIN (SNR-50) | 17.9 (0.5–23.5) | 21.1 (7.5–23.5) | 11.2 (0.0–23.5) |
| QSMD (% correct) | 64.8 (3.0–97.0) | 61.2 (3.0–93.0) | 65.4 (10.5–95.0) |
| SSQ | 2.6 (0.0–8.4) | n = 287 34 prelingually deafened 253 postlingually deafened Mean age = 62.3 years | |
| APHAB (% problems) | 71.2 (27.0–99.0) | ||
| MMSE | 29.0 (17.0–30.0) | ||
Note. Note that these data include all individuals who were seen for preoperative workup, irrespective of whether or not the patient pursued cochlear implantation. PTA = pure-tone averages; CNC = consonant-nucleus-consonant; QSMD = quick spectral modulation detection; SSQ = Speech Spatial Qualities; APHAB = Abbreviated Profile of Hearing Aid Benefit; MMSE = Mini-Mental State Exam; BKB-SIN = Bamford–Kowal–Bench Speech in Noise.
Quick Spectral Modulation Detection
Figure 1(f) displays individual and mean QSMD scores for the implanted and nonimplanted ears as unfilled and shaded symbols, respectively. The data for individuals not pursuing implantation are displayed separately as in Figure 1(b) to (e). Mean QSMD scores were 61.2% for the ear to be implanted and 66.6% for nonimplanted ears. An unpaired t-test was completed for QSMD spectral resolution for the implanted versus nonimplanted ears of all patients who pursued implantation. There was a significant difference between QSMD across the implanted and nonimplanted ears (t = 3.2, p = .0016) with the poorer ear, on average, targeted for intervention.
Questionnaires and Cognitive Screener
The mean MMSE score was 29 with a range of 17 to 30. The mean overall SSQ score was 2.6 with a range of 0 to 8.4 of 10. The mean global APHAB score was 71.2% problems with a range of 27.0% to 99.0% problems. The means and standard deviations for the SSQ and APHAB scores were broken out into their individual subsections and separated by patient populations as shown in Table 2. We completed an unpaired t-test for global APHAB score for those who did and did not pursue cochlear implantation. There was no significant difference between the global APHAB score across the two groups of patients (t = 2.0, p = .05). Similarly, we completed an unpaired t-test for overall SSQ score for those who did and did not pursue cochlear implantation. There was no difference between the overall SSQ score for patients who did and did not pursue cochlear implantation (t = 0.90, p = .37).
Table 2.
Mean Data and Standard Deviations (in Parentheses) of the APHAB and SSQ Questionnaires.
| All patients | Prelingual patients | Postlingual patients | Implanted patients | Nonimplanted patients | ||
|---|---|---|---|---|---|---|
| APHAB | Global | 71.3 (15.3) | 69.1 (15.6) | 71.6 (15.2) | 70.3 (15.1) | 76.3 (15.1) |
| EC | 63.2 (23.8) | 61.2 (25.2) | 63.5 (23.6) | 62.0 (23.4) | 69.4 (24.8) | |
| RV | 75.9 (16.9) | 74.8 (19.1) | 76.1 (16.5) | 75.5 (17.2) | 78.2 (14.8) | |
| BN | 77.2 (14.7) | 73.6 (16.4) | 77.6 (14.4) | 75.9 (14.7) | 83.4 (13.3) | |
| AV | 36.7 (27.5) | 26.7 (24.9) | 38.0 (27.5) | 36.4 (26.6) | 37.7 (31.1) | |
| SSQ | Overall | 2.6 (1.7) | 2.8 (1.8) | 2.6 (1.7) | 2.6 (1.7) | 2.9 (1.7) |
| Speech | 2.1 (1.7) | 2.6 (1.9) | 2.1 (1.6) | 2.1 (1.8) | 2.1 (1.4) | |
| Spatial | 2.7 (2.1) | 2.7 (2.0) | 2.7 (2.1) | 2.6 (2.1) | 3.2 (2.2) | |
| Qualities | 3.1 (2.1) | 3.4 (2.3) | 3.1 (2.1) | 3.1 (2.1) | 3.3 (2.0) | |
Note. Mean scores for the APHAB are shown in % problems and in numerical units on the visual analog scale for the SSQ. SSQ = Speech Spatial Qualities; APHAB = Abbreviated Profile of Hearing Aid Benefit; EC = Ease of Communication; RV = Reverberation, BN = Background Noise, AV = Aversiveness.
Correlation Analysis
Given that multiple assessments are completed for each patient in a clinical setting, it is important for clinicians to understand which measures are correlated with one another. This can provide us with information regarding a “checks and balances” approach as well as potentially help streamline our clinical protocols. Thus, we completed a first pass set of Pearson product moment correlation analyses for each measure related to audiometric threshold (PTA and low-frequency PTA [LFPTA]), speech understanding (CNC, AzBio, and BKB-SIN), and auditory function (QSMD) in the ear to be implanted along with our subjective questionnaire data (APHAB and SSQ) and cognitive state (MMSE). We did not include AzBio +5 in the correlation given that this was not available for all patients (n = 50); AzBio +5 was added to our required clinical protocol in mid-2014 and is now the metric by which we determine candidacy—prior to that time it was optional based on clinician judgment and allowable time. Furthermore, it is our clinical protocol that should a patient score less than 20% correct for AzBio sentences in quiet, we do not require preoperative assessment at +5 dB SNR, though some clinicians may choose to complete this testing to obtain a baseline score in noise. This resulted in 36 individual correlations as shown in Table 3. To control for Type I error with multiple comparisons, we have imposed a Bonferroni correction bringing the alpha level for significance to .0014.
Table 3.
Pearson Product Moment Correlation Coefficients, r, for Each Measure Related to Audiometric Threshold (PTA and LFPTA), Speech Understanding (CNC, AzBio, AzBio +5, and BKB-SIN), and Auditory Function (QSMD) in the Ear to be Implanted as Well as Subjective Questionnaire Data (APHAB and SSQ) and Cognitive State (MMSE).
| PTA | LFPTA | CNC | AzBio | BKB-SIN | QSMD | APHAB | SSQ | MMSE | |
|---|---|---|---|---|---|---|---|---|---|
| LFPTA | 0.8143* < 0.0001 | X | X | X | X | X | X | X | X |
| CNC | −0.7191* | −0.6060* | X | X | X | X | X | X | X |
| <0.0001 | <0.0001 | ||||||||
| AzBio | −0.7132* | −0.6325* | 0.9157* | X | X | X | X | X | X |
| <0.0001 | <0.0001 | <0.0001 | |||||||
| BKB-SIN | 0.3787* | 0.3602* | −0.8771* | −0.9059* | X | X | X | X | X |
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | ||||||
| QSMD | −0.3020* | −0.2959* | 0.3354* | 0.4112* | −0.4200* | X | X | X | X |
| <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||
| APHAB | 0.1411 | 0.1095 | 0.0162 | 0.0007 | −0.0679 | 0.0110 | X | X | X |
| 0.0089 | 0.0427 | 0.7687 | 0.9893 | 0.4828 | 0.8610 | ||||
| SSQ | −0.2854* | −0.2930* | 0.0982 | 0.0910 | 0.0114 | 0.1692 | 0.6084* | X | X |
| <0.0001 | <0.0001 | 0.1342 | 0.1682 | 0.9291 | 0.0286 | <0.0001 | |||
| MMSE | −0.0098 | −0.0486 | 0.0494 | 0.0926 | 0.0088 | 0.2378* | −0.0574 | 0.1570 | X |
| 0.8407 | 0.3185 | 0.3171 | 0.0595 | 0.9139 | <0.0001 | 0.3343 | 0.0243 |
Note. Bonferroni correction was applied to control for Type I error with a resultant alpha level of.0014 for significance. Significant correlations are indicated via asterisk and in bold text. PTA = pure-tone averages; CNC = consonant-nucleus-consonant; QSMD = quick spectral modulation detection; SSQ = Speech Spatial Qualities; APHAB = Abbreviated Profile of Hearing Aid Benefit; MMSE = Mini-Mental State Exam; LFPTA = low-frequency pure-tone averages; BKB-SIN = Bamford–Kowal–Bench Speech in Noise.
As displayed in Table 3, all measures of speech understanding were significantly correlated with one another, which is an expected finding and documented previously (e.g., Gifford, Shallop, & Peterson, 2008). All speech measures were also significantly correlated with PTA and LFPTA (mean of thresholds for 125, 250, and 500 Hz)—also an expected finding and documented extensively (e.g., Erber, 1974; Verschuure & van Benthem, 1992). Spectral resolution via QSMD was significantly correlated with PTA and LFPTA as well as all measures of speech understanding. PTA and LFPTA were both significantly and inversely correlated with SSQ. SSQ was also significantly correlated with APHAB. APHAB, conversely, was only correlated with SSQ.
Stepwise Regression
From a clinical standpoint, we would like to know which, if any, variables might contribute to a patient’s perceived hearing handicap and quality of life. Separate stepwise regressions were completed to predict APHAB or SSQ (dependent variables) based on independent variables including age, gender, prelingual/postlingual status, MMSE, subjective measure (APHAB or SSQ), as well as LFPTA, PTA, QSMD, CNC, and AzBio—with the latter two variables being included for both the ear to be implanted as well as the bilateral aided condition. For the global APHAB score, the model excluded all variables with the exception of overall SSQ as being a significant predictor of global APHAB percentage of problems reported at pre-CI workup. A significant regression equation was determined, F(1. 114) = 98.7, p = .000, with a correlation coefficient of .68 and a corresponding r2 value of .46. For the overall SSQ score, the model excluded all variables with the exception of global APHAB, bilateral AzBio, bilateral CNC, AzBio in the ear to be implanted, and LFPTA as being significant predictors of overall SSQ score reported at pre-CI workup. A significant regression equation was determined, F(5. 110) = 27.1, p = .000, with a correlation coefficient of .74 and a corresponding r2 value of .55. These data suggest that the SSQ questionnaire provides greater cross-correlational value in the preoperative period as it was predicted by commonly used metrics of patient hearing and speech understanding performance. Global APHAB scores, on the other hand, were only explained by the outcomes of another subjective questionnaire, the SSQ.
Stepwise regression was also completed for QSMD (dependent variable) based on age, gender, prelingual/postlingual status, APHAB global, SSQ overall, and MMSE as well as the following variables for the ear to be implanted: LFPTA, PTA, CNC, and AzBio. The model excluded all variables with the exception of PTA, MMSE, and age at implantation as being significant predictors of SMD at pre-CI workup. A significant regression equation was determined, F(3,140) = 12.4, p = .000, with a correlation coefficient of .46 and a corresponding r2 value of .21. Although this was significant, the effect size was small-to-medium (Cohen, 1988) with preoperative PTA, MMSE, and age at implantation accounting for just 21% of the variance in QSMD.
Bilateral CI, Bimodal Hearing, and Unilateral CI
Those pursuing cochlear implantation were distributed such that 19.1% (n = 45) received bilateral implants and the remaining 80.9% (n = 191) received unilateral implants as of August 2015. We further analyzed unilaterally implanted patients by separating them into two groups—bimodal candidates and bilateral CI candidates. Given that Dorman and Gifford (2010) reported bimodal candidacy for an aggregate clinical population, we first used their definition for “aidable” hearing as an audiometric threshold up to 85 dB HL at 250 Hz in the nonimplanted ear. Although one could argue that their definition of aidable hearing may have been a bit too generous, we have applied that same criterion (≤85 dB HL at 250 Hz) to the current dataset and found that 162 of 190 nonimplanted ears—or 85.3% of the population—had potentially “aidable” hearing in the nonimplanted ear. Although we are not suggesting that this criterion defines an ideal bimodal candidate, we did apply this same criterion for the implanted ear to determine whether our newer dataset represented a different distribution of “potential” bimodal listeners as compared to previous reports. We found that 179 of 279 implanted ears—64.2% of the population—had preoperative thresholds ≤ 85 dB HL at 250 Hz in the implanted ear.
For the purposes of this study, we have chosen to define a bimodal candidate as a patient with an audiometric threshold ≤70 dB HL at 250 Hz and a bilateral CI candidate as a patient with audiometric thresholds >70 dB HL at 250 Hz. This criterion was chosen since we expect diminishing returns from amplification for thresholds greater than 70 dB HL (Amos & Humes, 2007; Ching, Dillon, & Byrne, 1998; Hogan & Turner, 1998; Hornsby, Johnson, & Picou, 2011; Hornsby & Ricketts, 2006; Turner, 2006; Turner & Cummings, 1999; Vickers, Moore, & Baer, 2001). Furthermore, 250 Hz was chosen for two reasons: (a) it is the lowest frequency for which we are able to verify hearing aid output for various prescriptive fitting targets, thus serving as a marker for whether the hearing is functionally useful and (b) previous research has shown that significant bimodal benefit is observed with just 250 Hz aided in the non-CI ear (Sheffield & Gifford, 2014; Zhang, Dorman, & Spahr, 2010). Of the 191 unilaterally implanted patients, 137 (71.7%) were bimodal candidates (as defined by our limited criteria) and 54 (28.3%) were categorized as bilateral candidates.
Nontraditional CI Candidates
Seventy-three patients or 25.4% of the CI workup population met preoperative candidacy criteria for the Nucleus Hybrid-L24. This is an important statistic to track as it can provide us with information about public awareness of current implant indications including appropriate referrals from various sources. Of the 73 patients meeting Hybrid-L24 criteria, 50 (68.5%) ultimately pursued cochlear implantation; however, only 5 received a Hybrid-L24 device. The reason is that many patients meeting Hybrid-L24 criteria also met conventional CI indications, for the ear to be implanted. Thus, some of these patients pursued implantation with other atraumatic electrodes including MED-EL FLEX24 (n = 6), FLEX28 (n = 13), or Nucleus CI422/522 (n = 10).
Device Selection
At the Vanderbilt Bill Wilkerson Center, CI patients are generally charged with selecting the manufacturer unless there are extenuating circumstances such as anatomical anomalies or medical conditions that would cause the surgeon or audiologist to recommend a particular device. Of the 236 adult patients who received implants, 24.6% selected Advanced Bionics, 40.8% selected Cochlear Americas, and 34.5% selected MED-EL.
Discussion
This study was designed with two primary goals: (a) to provide a thorough description of the adult population seeking audiological evaluation for CI candidacy so that we may ultimately improve access to CI technology, and (b) to define the proportion of adults presenting for preoperative CI evaluation who are candidates for either bimodal hearing or bilateral cochlear implantation. Providing a thorough description of the demographics of this population is an important step in our progress toward making CI technology accessible to more individuals with hearing loss who are struggling to communicate despite being appropriately fitted with acoustic amplification.
Pre-CI Workup: Typical Audiometric Profile
The majority of our current adult patient population presenting to Vanderbilt for pre-CI workup have severe-to-profound sensory hearing loss (64.8%, n = 186) despite the fact that since 2005, candidacy indications have included those with moderate-to-profound sensory hearing loss (CMS, 2005; Cochlear Americas, 2016). This feature of the workup patient profile remains largely unchanged since the N24 (2002) and Freedom (2007) clinical trials. Our patients are, however, performing significantly better than the earlier clinical trial patients on measures of CNC word recognition by 5.7 percentage points in the ear to be implanted. It should be noted that this is not a perfect comparison due to the inherent differences between a clinical trial and a large clinical population; however, there are currently no other studies in the literature that would provide a more accurate comparison. Despite inherent differences between our aggregate clinical data and previous clinical trials, given that the majority of our adult preoperative population has severe-to-profound sensorineural hearing loss and a mean preoperative CNC score of just 8.7%, we are not expanding our reach to individuals with bilateral moderate-to-profound hearing losses who have aided speech understanding near the upper range of candidacy—currently 60% monosyllabic word recognition for Hybrid-L24 labeled indications. These data provide evidence that we have the potential to expand our outreach efforts to individuals with less severe hearing losses who still experience significant communication difficulty despite the use of appropriately fitted hearing aids.
Why Are Patients Not Pursuing Cochlear Implantation?
In our population, 51 of 287 patients (17.8%) did not receive a CI in either ear; those not pursuing cochlear implantation did demonstrate significantly better speech recognition and audiometric thresholds as compared to the individuals who did pursue implantation. Despite having better speech recognition and audiometric thresholds, the majority of those not pursuing implantation (37 of 51 or 72.5%) did, in fact, meet FDA and CMS criteria. These data suggest that patients may need additional education, counseling, and follow-up after the initial pre-CI workup to ensure that they are equipped with the tools needed to follow through with recommendations for audiologic or otologic intervention. For example, should a patient not pursue cochlear implantation despite meeting criteria for fear of surgery, we may need to provide greater education regarding the surgical procedure and associated complications and discuss the needs for adding remote microphone technology (e.g., FM/DM or Bluetooth microphones) to existing hearing aids to further aid speech understanding in challenging listening environments.
These data also suggest that we are seeing very few (n = 14 or 5%) patients for preoperative CI workup who are scoring near or above the candidacy criteria for implantation. Based on the historically low market penetration of CIs with estimates ranging from 0.97% to 7.7% (Gifford et al., 2010; Hoffman et al., 2015; iData Research Inc., 2010; Kochkin, 2005; Sorkin, 2013; Wilson & Dorman, 2008a), low numbers of preoperative patients exceeding candidacy criteria, and the significant yet small increase in preoperative word recognition scores despite expansion of labeled indications, it is reasonable to conclude that there are still many hearing aid users that could benefit from a CI who are not being referred.
Another possible reason potential CI candidates are not presenting for preoperative workup may be because patients and clinicians may be unaware of how severe their hearing loss is or how hearing loss is affecting their lives. In this study, only the SSQ was found to be related to preoperative measure of hearing and speech understanding (LFPTA in the ear to be implanted, CNC and AzBio in the bilateral aided condition, and AzBio in the ear to be implanted). The APHAB questionnaire is the more commonly used measure for assessing communication difficulty with and without hearing aids in audiology clinics; however, the global APHAB score was not correlated with any of the hearing or speech understanding measures administered in this population. In other words, patients with greater degrees of hearing loss and poorer aided speech understanding did not report greater communication difficulty on the APHAB questionnaire. Although the APHAB was not intended to be used with CI candidates, these data suggest that (a) the SSQ may be a more clinically useful tool for CI programs (and also possibly HA programs) being better able to gauge preoperative hearing difficulties in various communicative environments and significantly related to commonly used speech understanding measures and (b) our field may require a more sensitive questionnaire designed specifically for our CI patient population.
Another possible reason that potential CI candidates are not presenting for preoperative workup may be related to lack of education among general otolaryngologists and audiologists regarding current labeled indications for adult cochlear implantation. Indeed, many self-referring patients report that hearing health-care professionals had reported that the patient was not a CI candidate. While these reports are anecdotal in nature, it is reasonable to assume that not all otolaryngologists and audiologists are knowledgeable about current CI indications. This requires ongoing continuing education regarding CI candidacy and outcomes as well as proactive outreach by professionals working in clinical CI programs so that we are effectively communicating with potential referral sources.
Aside from correlations found within the MSTB such as PTA and CNC and PTA and AzBio, correlations between MSTB measures and subjective questionnaires and MSTB measures and QSMD were generally found to be small or not significant. While several previous studies have demonstrated a significant postimplantation correlation (.86–.92) between auditory-only speech understanding and SMD or discrimination (Drennan et al., 2014; Gifford et al., 2014; Henry, Turner, & Behrens, 2005; Henry & Turner, 2003; Jung et al., 2012; Saoji, Litvak, Spahr, Eddins, 2009; Won, Drennan, & Rubinstein, 2007; Zhang, Spahr, Dorman, & Saoji, 2013), this high correlation was not observed in this preoperative population. This study did, however, demonstrate a significant relationship between QSMD and the following variables: PTA, MMSE, and age at implantation. This suggests that patients with better preoperative thresholds, cognition, and younger age at implantation are more likely to demonstrate better spectral resolution in the ear to be implanted. The relationship between SMD and PTA makes sense given the known relationship between auditory sensitivity and spectral resolution (e.g., Davies-Venn, Nelson, & Souza, 2015; Kortlang, Mauermann, & Ewert, 2016; Moore, Vickers, Plack, & Oxenham, 1999; Peters, 1992), but the relationship between spectral resolution and both cognitive status and age is somewhat puzzling. We theorize that older patients or those with poorer cognitive resources may not fully understand the QSMD task, or perhaps, the QSMD task taxes higher levels of cognitive processing that when degraded, adversely affects measures of spectral resolution without proportionally affecting speech understanding—at least not for the measures currently used for assessment. We are continuing to investigate the relationship between cognitive status and spectral resolution on hearing outcomes in both the preoperative and postoperative periods. Furthermore, we are continuing to investigate the relationship between pre-CI QSMD as well as aided speech understanding, audiometric thresholds, cognitive status, and subjective questionnaires in a prospective manner to determine whether any combination yields predicted clinical utility for postoperative outcomes. The fields of audiology and otology are in need of a viable clinical tool that could assist with guiding clinical decisions regarding implant ear selection as well as expectations management for postoperative outcomes.
Bimodal Candidates
As reported previously, Dorman and Gifford (2010) reported that 59.8% of adult CI candidates at two large academic medical centers (n = 276) had “aidable” low-frequency hearing in the nonimplanted ear defined as a threshold in the non-CI ear ≤85 dB HL at 250 Hz. Applying that criterion to the current dataset, we found that 85.3% of the population had potentially “aidable” hearing in the nonimplanted ear. Although differing geographical locations may impact referral trends, this simple comparison provides indirect evidence for an expansion of acceptable or perceived CI criteria among professionals referring or the patient self-referrals for cochlear implantation. That is, from 2010 to present, we have demonstrated a higher proportion of patients with “better than profound” hearing in the non-CI ear, typically the better hearing ear, increasing from 59.8% to 85.3% of the patient population at a large academic medical center.
As mentioned in the Results section, the hearing aid literature has numerous reports of diminishing amplification benefit for spectral regions corresponding to audiometric thresholds ≥ 70 dB HL (Amos & Humes, 2007; Baer, Moore, & Kluk, 2002; Ching et al., 1998; Hogan & Turner, 1998; Hornsby et al., 2011; Turner, 2006; Turner & Cummings, 1999; Vickers et al., 2001). Researchers theorize that the lack of value-added amplification benefit for spectral regions with thresholds above 70 dB HL could be due to cochlear dead regions (Moore et al., 1999; Vickers et al., 2001; Zhang et al., 2010), suprathreshold distortion related to high output levels required for audibility (Ching et al., 1998; Dorman & Dougherty, 1981; Hornsby & Ricketts, 2001; Studebaker, Sherbecoe, McDaniel, & Gwaltney, 1999), and negative effects from high input compression ratios (Boike & Souza, 2000; Chung, Killion, & Christensen, 2007; Hohmann & Kollmeier, 1995; Hornsby & Ricketts, 2001). Thus, considering only those patients with thresholds up to 70 dB HL in the non-CI ear, we found that 72.1% of the entire preoperative CI population (n = 207) had aidable hearing at 250 Hz and 70.4% (n = 202) had aidable hearing when considering the LFPTA (125, 250, and 500 Hz). Considering the 191 patients who ultimately pursued unilateral implantation, 137 (71.7%) had thresholds up to 70 dB HL at 250 Hz in the non-CI ear. Thus, even using a more conservative criterion for aidable low-frequency hearing than that defined by Dorman and Gifford (2010), the majority of adult CI candidates have aidable acoustic hearing in the low-frequency region to be used in a bimodal hearing configuration. Further investigation is needed, however, to determine which individuals may fare better with a second CI and which would be better served from a bimodal hearing configuration. A number of patient-specific variables including audiometric thresholds, speech understanding, music appreciation, auditory function, neurocognitive, and demographical may prove useful in making clinical recommendations for bimodal versus bilateral CI candidacy. Thus, it is critically important that clinicians continue to assess both ear-specific and bimodal speech understanding in the postoperative period to document bimodal benefit or lack thereof for determining bilateral CI candidacy.
Racial and Ethnic Disparities in Hearing Health Care
Although not a specific aim of this study, we demonstrated that the racial and ethnic distribution of our aggregate clinical population did not match that of the greater Nashville metropolitan area or the state of Tennessee—both of which largely represent the geographical area from which we draw our clinical population. Thus, this raises significant concern regarding racial, ethnic, and likely socioeconomic disparities associated with access to cochlear implantation for adults—issues that have been previously raised for pediatric CI candidates (e.g., Chang, Ko, Murray, Arnold, & Megerian, 2010; Jones, 2009; Stern, Yueh, Lewis, Norton, & Sie, 2005). Nieman et al. (2016) reported racial/ethnic and socioeconomic disparities in access to hearing health care based on reports of recent hearing evaluations and hearing aid use. A large proportion of the adult CI population is 65 years or older and hence covered by Medicare; because Medicare is essentially universal, Nieman et al. (2016) argued that Medicare coverage protects against disparities in health-care access for covered services. Although cochlear implantation is a covered service, there are some possible reasons explaining this disparity. First, Medicare approved indications for cochlear implantation are more stringent than the FDA-labeled indications and do not currently provide universal coverage for EAS/Hybrid systems. Should Medicare expand criteria to be comparable to the indications for individuals under 65 years of age, the effect of racial/ethnic disparities associated with cochlear implantation may be less prevalent and more similar to what has been seen with universal Medicare coverage of hearing and vision evaluations (e.g., Chou et al., 2012). Second, because Medicare part A covers 80% of the costs associated with CI surgery, should a candidate not have a supplemental policy (part B) to cover the remaining 20%, access to cochlear implantation is not be possible for those of lower socioeconomic status. Third, geographical access to hearing centers knowledgeable about and offering cochlear implantation may play a role, as there are fewer CI centers than those offering standard hearing assessments and hearing aid services. Fourth, it is possible that such disparities do not exist in other geographical areas as Vanderbilt is one of only a few CI centers in the state of TN and one of even fewer that will accept Medicare patients. Tennessee is also quite vast spanning 440 miles and two time zones; furthermore, much of the state’s population is located in rural areas. Thus, we cannot be certain that our experience is representative of other large CI programs in the United States. For this reason, we would encourage other programs investigate the demographic details of their aggregate clinical population so that we may have a better understanding of the access to cochlear implantation for all adults meeting candidacy who are struggling with communication. We will continue to investigate this issue in an attempt to ensure that CI technology is available to those meeting candidacy irrespective of race, ethnicity, or socioeconomic status.
Limitations
There were some obvious limitations to the current study. First, these data represent those presenting to a large academic medical center, and as a result, may not be representative of the greater U.S. population pursuing CI. Second, because these data represent our aggregate clinical population from 2013 to 2015, we may already expect even more changes in this population in the last year due to the release of the MED-EL EAS processor and heightened CI awareness. Third, the comparison presented previously in the manuscript between the current data and previous clinical trials is imperfect; however, there are currently no other studies in the literature describing an aggregated clinical dataset that would provide a more accurate comparison. In future research, we hope to make more accurate comparisons to this dataset as well as others that may arise. Finally, despite the tremendous benefit that can be derived from EAS/Hybrid implant systems, this technology is not appropriate for everyone who meets candidacy criteria. It is important to note that individuals with EAS/Hybrid-qualifying audiograms who derive significant benefit from conventional or nonlinear frequency compression hearing aids are not candidates for a cochlear implantation.
Conclusion
CIs have been termed the most successful neural prosthesis to date (Wilson & Dorman, 2008b). Despite the documented success of CIs and expanded criteria, the present data suggest that while preoperative word recognition scores are significantly higher than previous clinical trial populations, mean preoperative speech understanding is still extremely low (<10% in the ear to be implanted) and over 60% of adults pursuing evaluation for CI candidacy have severe-to-profound sensorineural hearing loss. These data reinforce the continued need for appropriate education, outreach, and referral for CI candidacy evaluation if we are to achieve our primary goal of improving access to CI technology for individuals experiencing significant communication difficulties despite appropriately fitted acoustic amplification.
With respect to the secondary goal of this study, the proportion of individuals meeting candidacy for bimodal hearing has increased dramatically since 2010—from 60% to over 85%. Thus, we may, indeed, be heading in the right direction, albeit achieving slow progress toward increased CI access for individuals with less severe hearing losses. We have entered a new era of rehabilitative audiology as hearing aid and CI technologies are rapidly merging. Audiologists, otolaryngologists, and all hearing health-care providers—as well as primary care providers and perhaps other medical specialists such as neurologists—should be informed regarding current CI candidacy criteria so that the appropriate patient population is referred for CI evaluation. Only then will more individuals be able to take advantage of this technology.
Note
We have based candidacy on performance both in quiet and in noise (at +5 dB SNR) since 2011. Prior to 2013, we would assess aided speech understanding in quiet, +10 and +5 dB SNR; however, our team made a decision to eliminate testing at +10 dB in an effort to reduce assessment time and associated patient fatigue particularly given that there are numerous studies demonstrating ecological validity of +5 dB SNR for assessment purposes (Pearsons et al., 1977; Smeds et al., 2015), and normal-hearing listeners achieve ceiling level performance at +5 dB SNR (Dorman & Gifford, 2017)—further demonstrating the deleterious effects of sensorineural hearing loss on communication).
Authors’ Note
A portion of this dataset was presented at the 2016 American Auditory Society meeting in Scottsdale, AZ, March 3–5, 2016.
Declaration of Conflicting Interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Health [R01 DC13117 Clinical application of spectral envelope perception: cochlear implant evaluation] and approved by Vanderbilt’s Institutional Review Board (#140152).
References
- Amos N. E., Humes L. E. (2007) Contribution of high frequencies to speech recognition in quiet and noise in listeners with varying degrees of high-frequency sensorineural hearing loss. Journal of Speech, Language, and Hearing Research: JSLHR 50(4): 819–834. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17675588. doi: 10.1044/1092-4388(2007/057). [DOI] [PubMed] [Google Scholar]
- Baer T., Moore B. C. J., Kluk K. (2002) Effects of low pass filtering on the intelligibility of speech in noise for people with and without dead regions at high frequencies. The Journal of the Acoustical Society of America 112(3): 1133–1144. Retrieved from http://scitation.aip.org/content/asa/journal/jasa/112/3/10.1121/1.1498853. doi: 10.1121/1.1498853. [DOI] [PubMed] [Google Scholar]
- Balkany T., Hodges A., Menapace C., Hazard L., Driscoll C., Gantz B., Payne S. (2007) Nucleus Freedom North American clinical trial. Otolaryngology - Head and Neck Surgery 136(5): 757–762. Retrieved from http://oto.sagepub.com/lookup/doi/10.1016/j.otohns.2007.01.006. [DOI] [PubMed] [Google Scholar]
- Bernstein J. G., Iyer N., Brungart D. S. (2013) Spatial release from masking in simulations of cochlear implants for single-sided deafness. The Journal of the Acoustical Society of America 133(5): 3383 doi: 10.1080/14992027.2016.1197426. [Google Scholar]
- Blackwell D. L., Lucas J. W., Clarke T. C. (2014) Summary health statistics for U.S. adults: National health interview survey, 2012. Vital and Health Statistics. Series 10, Data from the National Health Survey 260: 1–161. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24819891. [PubMed] [Google Scholar]
- Bleecker M. L., Bolla-Wilson K., Kawas C., Agnew J. (1988) Age-specific norms for the Mini-Mental State Exam. Neurology 38(10): 1565–1568. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/3419600. [DOI] [PubMed] [Google Scholar]
- Boike K. T., Souza P. E. (2000) Effect of compression ratio on speech recognition and speech-quality ratings with wide dynamic range compression amplification. Journal of Speech, Language, and Hearing Research: JSLHR 43(2): 456–468. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10757696. [DOI] [PubMed] [Google Scholar]
- Capretta N. R., Moberly A. C. (2016) Does quality of life depend on speech recognition performance for adult cochlear implant users? The Laryngoscope 126(3): 699–706. Retrieved from http://doi.wiley.com/10.1002/lary.25525. doi: 10.1002/lary.25525. [DOI] [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services (2005) CMS Manual System, Pub 100-03, Medicare National Coverage Determination, Subject: Cochlear Implantation Transmittal 42, Baltimore, MD: Department of Health & Human Services, Center for Medicare and Medicaid Services. [Google Scholar]
- Chang D. T., Ko A. B., Murray G. S., Arnold J. E., Megerian C. A. (2010) Lack of financial barriers to pediatric cochlear implantation impact of socioeconomic status on access and outcomes. Arch Otolaryngol Head Neck Surg 136(7): 648–657. doi: 10.1001/archoto.2010.90. [DOI] [PubMed] [Google Scholar]
- Ching T. Y., Dillon H., Byrne D. (1998) Speech recognition of hearing-impaired listeners: Predictions from audibility and the limited role of high-frequency amplification. The Journal of the Acoustical Society of America 103(2): 1128–1140. doi: 10.1121/1.421224. [DOI] [PubMed] [Google Scholar]
- Chou C. F., Barker L. E., Crews J. E., Primo S. A., Zhang X., Elliott A. F., Saaddine J. B. (2012) Disparities in eye care utilization among the United States adults with visual impairment: Findings from the behavioral risk factor surveillance system 2006-2009. American Journal of Ophthalmology 154(6 SUPPL.doi: 10.1016/j.ajo.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Chung K., Killion M. C., Christensen L. A. (2007) Ranking hearing aid input-output functions for understanding low-, conversational-, and high-level speech in multitalker babble. Journal of Speech, Language, and Hearing Research: JSLHR 50(2): 304–322. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/17463231. doi: 10.1044/1092-4388(2007/022). [DOI] [PubMed] [Google Scholar]
- Cochlear Americas (2016). Determining candidacy: Cochlear implants. Retrieved from http://www.cochlear.com/wps/wcm/connect/us/for-professionals/products/cochlear-implants/Candidacy.
- Cohen J. (1988) Statistical power analysis for the behavioral sciences, 2nd ed Hillsdale, NJ: Lawrence Erlbaum Associates. [Google Scholar]
- Cox R. M., Alexander G. C. (1995) The abbreviated profile of hearing aid benefit. Ear and Hearing 16(2): 176–186. [DOI] [PubMed] [Google Scholar]
- Davies-Venn E., Nelson P., Souza P. (2015) Comparing auditory filter bandwidths, spectral ripple modulation detection, spectral ripple discrimination, and speech recognition: Normal and impaired hearing. The Journal of the Acoustical Society of America 138(1): 492 Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/26233047. doi: 10.1121/1.4922700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman M. F., Dougherty K. (1981) Shifts in phonetic identification with changes in signal presentation level. The Journal of the Acoustical Society of America 69(5): 1439–1440. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7240575. [DOI] [PubMed] [Google Scholar]
- Dorman M. F., Gifford R. H. (2010) Combining acoustic and electric stimulation in the service of speech recognition. International Journal of Audiology 49(12): 912–919. doi: 10.3109/14992027.2010.509113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorman M. F., Gifford R. H. (2017) Speech understanding in complex listening environments by listeners fit with cochlear implants. Journal of Speech, Language, and Hearing Research: JSLHR 60(10): 3019–3026. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/29049602. doi: 10.1044/2017_JSLHR-H-17-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drennan W. R., Anderson E. S., Won J. H., Rubinstein J. T. (2014) Validation of a clinical assessment of spectral-ripple resolution for cochlear implant users. Ear and Hearing 35(3): e92–e98. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24552679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drennan W. R., Won J. H., Timme A. O., Rubinstein J. T. (2016) Nonlinguistic outcome measures in adult cochlear implant users over the first year of implantation. Ear and Hearing 37(3): 354–364. Retrieved from http://insights.ovid.com/crossref?an=00003446-201605000-00012. doi: 10.1097/AUD.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erber N. P. (1974) Pure-tone thresholds and word-recognition abilities of hearing-impaired children. Journal of Speech and Hearing Research 17(2): 194–202. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/4836040. [DOI] [PubMed] [Google Scholar]
- Etymotic Research Inc. (2005) BKB-SIN Test, Elk Grove Village, IL: Etymotic Research, Inc. [Google Scholar]
- Folstein M. F., Folstein S. E., McHugh P. R. (1975) ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 12(3): 189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Gatehouse S., Noble W. (2004) The speech, spatial and qualities of Hearing Scale (SSQ). International Journal of Audiology 43(2): 85–99. doi: 10.1080/14992020400050014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford R. H., Shallop J. K., Peterson A. M. (2008) Speech recognition materials and ceiling effects: Considerations for cochlear implant programs. Audiology and Neurotology 13(3): 193–205. doi: 10.1159/000113510. [DOI] [PubMed] [Google Scholar]
- Gifford R. H., Dorman M. F., Shallop J. K., Sydlowski S. A. (2010) Evidence for the expansion of adult cochlear implant candidacy. Ear and Hearing 31(2): 186–194. doi: 10.1097/AUD.0b013e3181c6b831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford R. H., Hedley-Williams A., Spahr A. J. (2014) Clinical assessment of spectral modulation detection for adult cochlear implant recipients: A non-language based measure of performance outcomes. International Journal of Audiology 53(3): 159–164. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/24456178. doi: 10.3109/14992027.2013.851800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris P. A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J. G. (2009) Research electronic data capture (REDCap) – A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics 42(2): 377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry B. A., Turner C. W. (2003) The resolution of complex spectral patterns by cochlear implant and normal-hearing listeners. The Journal of the Acoustical Society of America 113(5): 2861–2873. Retrieved from http://asa.scitation.org/doi/10.1121/1.1561900. doi: 10.1121/1.1561900. [DOI] [PubMed] [Google Scholar]
- Henry B. A., Turner C. W., Behrens A. (2005) Spectral peak resolution and speech recognition in quiet: Normal hearing, hearing impaired, and cochlear implant listeners. The Journal of the Acoustical Society of America 118(2): 1111–1121. Retrieved from http://asa.scitation.org/doi/10.1121/1.1944567. doi: 10.1121/1.1944567. [DOI] [PubMed] [Google Scholar]
- Hoffman H. J., Li C.-M., Losonczy K. G., Chiu M. S., Themann C. L., Steiner C. (2015, October 31–November 4, 2015) Hearing impairment rehabilitation through use of hearing aids and cochlear implants: Monitoring trends in age-specific prevalence for US Healthy People 2020. In: 2015 APHA Annual Meeting & Expo, Chicago, IL. [Google Scholar]
- Hogan C. A., Turner C. W. (1998) High-frequency audibility: Benefits for hearing-impaired listeners. The Journal of the Acoustical Society of America 104(1): 432–441. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/9670535. doi: 10.1121/1.423247. [DOI] [PubMed] [Google Scholar]
- Hohmann V., Kollmeier B. (1995) The effect of multichannel dynamic compression on speech intelligibility. The Journal of the Acoustical Society of America 97(2): 1191–1195. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/7876441. [DOI] [PubMed] [Google Scholar]
- Hornsby B. W., Ricketts T. A. (2001) The effects of compression ratio, signal-to-noise ratio, and level on speech recognition in normal-hearing listeners. The Journal of the Acoustical Society of America 109(6): 2964–2973. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/11425138. doi: 10.1121/1.1369105. [DOI] [PubMed] [Google Scholar]
- Hornsby B. W. Y., Ricketts T. A. (2006) The effects of hearing loss on the contribution of high- and low-frequency speech information to speech understanding. II. Sloping hearing loss. The Journal of the Acoustical Society of America 119(3): 1752–1763. Retrieved from http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=2735822&tool=pmcentrez&rendertype=abstract. doi: 10.1121/1.2161432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornsby B. W. Y., Johnson E. E., Picou E. (2011) Effects of degree and configuration of hearing loss on the contribution of high- and low-frequency speech information to bilateral speech understanding. Ear and Hearing 32(5): 543–555. Retrieved from http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00003446-201109000-00001. doi: 10.1097/AUD.0b013e31820e5028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- iData Research Inc. (2010) US market for hearing aids and audiology devices. Retrieved from www.idataresearch.net. [Google Scholar]
- Jones R. (2009) Acquisition of information and the utilization of hearing health care services and related hearing aid technologies by parents of deaf and severely hard of hearing children. ECHO 4(1): 6–23. [PMC free article] [PubMed] [Google Scholar]
- Jung K. H., Won J. H., Drennan W. R., Jameyson E., Miyasaki G., Norton S. J., Rubinstein J. T. (2012) Psychoacoustic performance and music and speech perception in prelingually deafened children with cochlear implants. Audiology and Neurotology 17(3): 189–197. doi: 10.1159/000336407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochkin S. (2005) MarkeTrak VII: Customer satisfaction with hearing instruments in the digital age. The Hearing Journal 58(9): 30 doi: 10.1097/01.HJ.0000286545.33961.e7. [Google Scholar]
- Kochkin S. (2010) MarkeTrak VIII: Consumer satisfaction with hearing aids is slowly increasing. Hearing Journal 63(1): 19–27. Retrieved from http://www.betterhearing.org/sites/default/files/hearingpedia-resources/MarkeTrak VIII Customer satisfaction with hearing aids is slowly increasing.pdf. [Google Scholar]
- Kortlang S., Mauermann M., Ewert S. D. (2016) Suprathreshold auditory processing deficits in noise: Effects of hearing loss and age. Hearing Research 331: 27–40. doi: 10.1016/j.heares.2015.10.004. [DOI] [PubMed] [Google Scholar]
- Moore B. C. J., Vickers D. A., Plack C. J., Oxenham A. J. (1999) Inter-relationship between different psychoacoustic measures assumed to be related to the cochlear active mechanism. The Journal of the Acoustical Society of America 106(5): 2761 Retrieved from http://scitation.aip.org/content/asa/journal/jasa/106/5/10.1121/1.428133. doi: 10.1121/1.428133. [DOI] [PubMed] [Google Scholar]
- MSTB. (2011). Minimum Speech test battery for adult cochlear implant users. Retrieved from http://www.auditorypotential.com/MSTBfiles/MSTBManual2011-06-20%20.pdf.
- Mudery, J. A., Francis, R., McCrary, H., & Jacob, A. (2017). Older individuals meeting medicare cochlear implant candidacy criteria in noise but not in quiet: Are these patients improved by surgery? Otology & Neurotology: Official Publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology, 38(2), 187–191. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/27832005. doi: 10.1097/MAO.0000000000001271. [DOI] [PubMed]
- NCT01337076 - ClinicalTrials.gov. (2011, April 18) Evaluation of a Revised Indication for determining adult cochlear implant candidacy. Bethesda, MD: National Library of Medicine. Retrieved from https://clinicaltrials.gov/ct2/show/NCT01337076.
- NCT03052920 - ClinicalTrials.gov. (2017, February 14). Cochlear implantation in adults with asymmetric hearing loss clinical trial. Bethesda, MD: National Library of Medicine. Retrieved from https://clinicaltrials.gov/ct2/show/NCT03052920.
- NCT02811549 - ClinicalTrials.gov. (2017, February 14). Benefits of the HiResolution bionic ear system in adults with asymmetric hearing loss. Bethesda, MD: National Library of Medicine. Retrieved from https://clinicaltrials.gov/ct2/show/NCT02811549.
- National Institute on Deafness and Other Communication Disorders. (2004). Healthy hearing 2010: Where are we now? DATA2010, the healthy people 2010 database. Retrieved from https://www.nidcd.nih.gov/healthy-people-2010/hearing-health-progress-review-october-2004#ref2.
- National Institute on Deafness and Other Communication Disorders (2016) Cochlear implants, Bethesda, MD: NIH Public. [Google Scholar]
- Nieman C. L., Marrone N., Szanton S. L., Thorpe R. J., Jr, Lin F. R. (2016) Racial/ethnic and socioeconomic disparities in hearing health care among older Americans. Journal of Aging and Health 28(1): 68–94. Retrieved from http://jah.sagepub.com/cgi/doi/10.1177/0898264315585505. doi: 10.1177/0898264315585505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble J. H., Labadie R. F., Gifford R. H., Dawant B. M. (2013) Image-guidance enables new methods for customizing cochlear implant stimulation strategies. IEEE Transactions on Neural Systems and Rehabilitation Engineering 21(5): 820–829. Retrieved from http://ieeexplore.ieee.org/document/6482251/. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble J. H., Gifford R. H., Hedley-Williams A. J., Dawant B. M., Labadie R. F. (2014) Clinical evaluation of an image-guided cochlear implant programming strategy. Audiology and Neurotology 19(6): 400–411. Retrieved from http://www.karger.com?doi=10.1159/000365273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson A. J., Arcaroli J., Staller S. J., Arndt P. L., Cosgriff A., Ebinger K. (2002) The nucleus 24 contour cochlear implant system: Adult clinical trial results. Ear and Hearing 23(1 Suppl): 41S–48S. [DOI] [PubMed] [Google Scholar]
- Pearsons K., Bennett R., Fidell S. (1977) Speech levels in verious noise environments. (Report No. EPA-600/1-77-025). Washington, DC: United States Environmental Protection Agency. [Google Scholar]
- Peters R. W. (1992) Auditory filter shapes at low center frequencies in young and elderly hearing-impaired subjects. The Journal of the Acoustical Society of America 91(1): 256 Retrieved from http://scitation.aip.org/content/asa/journal/jasa/91/1/10.1121/1.402769. doi: 10.1121/1.402769. [DOI] [PubMed] [Google Scholar]
- Peterson G. E., Lehiste I. (1962) Revised CNC lists for auditory tests. Journal of Speech and Hearing Disorders 27(1): 62 Retrieved from http://jshd.pubs.asha.org/article.aspx?doi=10.1044/jshd.2701.62. [DOI] [PubMed] [Google Scholar]
- Roland J. T., Gantz B. J., Waltzman S. B., Parkinson A. J. (2016) Multicenter Clinical Trial Group. United States multicenter clinical trial of the cochlear nucleus hybrid implant system. Laryngoscope 126(1): 175–181. doi: 10.1002/lary.25451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saoji A. A., Litvak L., Spahr A. J., Eddins D. A. (2009) Spectral modulation detection and vowel and consonant identifications in cochlear implant listeners. The Journal of the Acoustical Society of America 126(3): 955–958. Retrieved from http://asa.scitation.org/doi/10.1121/1.3179670. doi: 10.1121/1.3179670. [DOI] [PubMed] [Google Scholar]
- Sheffield S. W., Gifford R. H. (2014) The benefits of bimodal hearing: Effect of frequency region and acoustic bandwidth. Audiology and Neurotology 19(3): 151–163. doi: 10.1159/000357588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeds K., Wolters F., Rung M. (2015) Estimation of signal-to-noise ratios in realistic sound scenarios. Journal of the American Academy of Audiology 26(2): 183–196. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/25690777. doi: 10.3766/jaaa.26.2.7. [DOI] [PubMed] [Google Scholar]
- Sorkin D. L. (2013) Cochlear implantation in the world’s largest medical device market: Utilization and awareness of cochlear implants in the United States. Cochlear Implants International 14(supp1): S4–S12. Retrieved from http://www.tandfonline.com/doi/full/10.1179/1467010013Z.00000000076. doi: 10.1179/1467010013Z.00000000076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahr A. J., Dorman M. F., Litvak L. M., Van Wie S., Gifford R. H., Loizou P. C., Cook S. (2012) Development and validation of the AzBio sentence lists. Ear and Hearing 33(1): 112–117. doi: 10.1097/AUD.0b013e31822c2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern R. E., Yueh B., Lewis C., Norton S., Sie K. C. (2005) Recent epidemiology of pediatric cochlear implantation in the United States: Disparity among children of different ethnicity and socioeconomic status. Laryngoscope the Department of Health Services 115: 125–131. doi: 10.1097/01.mlg.0000150698.61624.3c. [DOI] [PubMed] [Google Scholar]
- Studebaker G. A., Sherbecoe R. L., McDaniel D. M., Gwaltney C. A. (1999) Monosyllabic word recognition at higher-than-normal speech and noise levels. The Journal of the Acoustical Society of America 105(4): 2431–2444. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/10212424. doi: 10.1121/1.426848. [DOI] [PubMed] [Google Scholar]
- Turner C. W. (2006) Hearing loss and the limits of amplification. Audiology and Neurotology 11: 2–5. doi: 10.1159/000095606. [DOI] [PubMed] [Google Scholar]
- Turner C. W., Cummings K. J. (1999) Speech audibility for listeners with high-frequency hearing loss. American Journal of Audiology 8(1): 47–56. [DOI] [PubMed] [Google Scholar]
- United States Census Bureau. (2016). QuickFacts. Retrieved from https://www.census.gov/quickfacts/fact/table/TN/PST040217.
- Verschuure J., van Benthem P. P. (1992) Effect of hearing aids on speech perception in noisy situations. Audiology 31(4): 205–221. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/1444932. [DOI] [PubMed] [Google Scholar]
- Vickers D. A., Moore B. C., Baer T. (2001) Effects of low-pass filtering on the intelligibility of speech in quiet for people with and without dead regions at high frequencies. The Journal of the Acoustical Society of America 110(2): 1164–1175. [DOI] [PubMed] [Google Scholar]
- Wilson B. S., Dorman M. F. (2008. a) Cochlear implants: A remarkable past and a brilliant future. Hearing Research 242(1–2): 3–21. doi: 10.1016/j.heares.2008.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson B. S., Dorman M. F. (2008. b) Cochlear implants: Current designs and future possibilities. Journal of Rehabilitation Research and Development 45(5): 695–730. doi: 10.1682/JRRD.2007.10.0173. [DOI] [PubMed] [Google Scholar]
- Won J. H., Drennan W. R., Rubinstein J. T. (2007) Spectral-ripple resolution correlates with speech reception in noise in cochlear implant users. JARO - Journal of the Association for Research in Otolaryngology 8(3): 384–392. doi: 10.1007/s10162-007-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Dorman M. F., Spahr A. J. (2010) Information from the voice fundamental frequency (F0) region accounts for the majority of the benefit when acoustic stimulation is added to electric stimulation. Ear and Hearing 31(1): 63–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T., Spahr A. J., Dorman M. F., Saoji A. (2013) Relationship between auditory function of nonimplanted ears and bimodal benefit. Ear and Hearing 34(2): 133–141. Retrieved from http://content.wkhealth.com/linkback/openurl?sid=WKPTLP:landingpage&an=00003446-201303000-00001. doi: 10.1097/AUD.0b013e31826709af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwolan T., Kileny P. R., Smith S., Mills D., Koch D., Osberger M. J. (2001) Adult cochlear implant patient performance with evolving electrode technology. Otology & Neurotology 22(6): 844–849. doi: 10.1097/00129492-200111000-00022. [DOI] [PubMed] [Google Scholar]