Summary
Background
Several trials have evaluated the effect of prostate-specific antigen (PSA)-based screening on prostate cancer (PC) mortality, with conflicting results. We report on the mortality in the European Randomized Trial of Screening for Prostate Cancer (ERSPC) with two added years of follow-up.
Methods
The ERSPC is a randomized screening trial in men aged 50 – 74 years (N=182,160) at entry, with a predefined core age group of 55 – 69 years (N=162,388) conducted in eight European countries Men randomized to the intervention arm were offered prostate specific antigen (PSA)-based screening while those in the control arm were not offered screening. The primary outcome is PC mortality.
Results
After a median follow-up of 11 years the relative risk reduction for PC death in the intention to screen analysis was 21% (risk ratio 0.79, 95%CI 0.68 – 0.91, p=0.001), and 29% for screened men after correction for non-compliance in the core age group. The absolute difference in mortality amounted to 0.10 per 1000 person years or 1.07 per 1000 men randomized. The rate ratio of PC mortality during the follow-up years 10 -11 was 0.62 (95% CI 0.45 – 0.85, P=0.003). The numbers needed invite (NNI) and detect (NND) to prevent one PC death amounted to 1055 and 37 at 11 years of follow-up and 936 and 33 for the entire follow-up. There was no difference in all-cause mortality.
Conclusions
Two added years of follow-up consolidate our previous finding that PSA-based screening reduces PC mortality but does not affect all cause mortality. (The trial is registered in the ISRCTN registry under number 49127736.)
Introduction
Screening for prostate cancer (PC) has remained controversial, despite results demonstrating a significant 20% reduction in mortality from PC in men offered prostate-specific antigen (PSA)-based screening [1]. In this report, mortality results from the European randomized screening trial at 11 years of follow-up are reported, adding two more years to the initial analysis.
Methods
The European Randomized study of Screening for Prostate Cancer (ERSPC) is a randomized multicenter trial initiated in 1991 in the Netherlands and in Belgium, with five more European countries (Sweden, Finland, Italy, Spain, Switzerland) joining in 1994-1998. Recruitment was completed in these centers between 1995 and 2003. Later, France also joined (enrolment in 2000-2005), but due to short follow-up (median 4.6 years) is not yet included in the analysis.
The trial protocol has been described [1, 2]. A core age group of 55-69 years at entry was defined in the trial protocol in 1994 [3]. Screening was carried out with an interval of four years and of two years in Sweden
The principal screening test was the serum PSA concentration measured using the Hybritech/Beckman-Coulter Tandem-R/Tandem-E/Access assay. A PSA value 3.0 ng/ml was the biopsy indication in most centers. Sextant prostatic biopsies were recommended for all test-positive men (lateralized sextant biopsies [4] were adopted in June 1996. Some exceptions to these procedures are described in [1].
The primary endpoint of the trial is PC mortality. Deaths among men diagnosed with PC in both the intervention and control arms (including those first diagnosed at autopsy) were evaluated regardless of the official cause of death as described earlier [1,5]. Data on overall mortality were collected by linkage to the national registries. Sample size was estimated as sufficient to show a statistically significant (p<0.05) reduction in mortality in men actually screened if the true effect would be 25% with a power of 80% at a follow-up of 10 years [6]. Hence, the primary analysis was planned at the outset based on follow-up of at least ten years, which was reached with data through 2008.
Each trial center followed the common core protocol and provided key data to the joint independent data center every six months. The independent Data Monitoring Committee (DMC) received six-monthly updates with a pre-defined monitoring and evaluation plan [7].
Statistical analysis
The present analysis includes follow-up through 2008 and follows the third interim monitoring analysis, which initially showed a significant reduction in PC mortality [1]. The analysis was based on the core age group 55-69 years at randomization. Besides the intention-to-screen analysis, a hypothesis- generating secondary analysis limited to attendees and correction for selection bias [8] was performed to show the effect among screened men. The cumulative hazard of prostate cancer specific and overall mortality was calculated using the Nelson-Aalen method [9]. A Forest plot and Kaplan Meier curves of prostate cancer specific survival were made according to standard techniques. All p-values are two-sided, no adjustment for significance due to prior analyses was used because the present analysis was not driven by statistical significance but was protocol based [10,11]. Poisson regression analysis was used to calculate rate ratios, adjusted by center. The number needed to invite (NNI) to prevent one PC death was calculated as the inverse of the absolute risk reduction in men randomized and truncated for follow-up at both 9 and 11 years. Where applicable, results were calculated with the control population for Finland weighted by 1:1.5 to account for the allocation ratio. The number needed to detect (NND) was calculated as the inverse absolute risk reduction multiplied by excess incidence in the screening arm for the same time periods, as well as all available follow-up as in [1]. The terminology was changed from number needed to screen (NNS) and number needed to treat (NNT), because the definitions differ from the previous report and more correctly reflect the choice of data included in the calculations. NNI is calculated from the intention to screen analysis and involves also men invited but not screened, and NND is different from NNT in treatment trials. The NNI and NND were also calculated considering the total cumulative follow-up [1].
Ethical review has been conducted in each center (see appendix). The trial is registered in the ISRCTN under number 49127736.
Results
The recruitment of the ERSPC trial was completed by 2003 in the centers included in the mortality analysis, and hence the number of subjects remained almost unchanged (182,160 overall, of whom 162,388 in the core age group) since the first mortality analysis [1] (Figure 1). During the two additional years of follow-up, screening has continued in the Netherlands, Sweden, Italy, Switzerland, and France, but discontinued after three screening rounds in Belgium, Finland and Spain (Table 1, Supplementary Table 1A for all ages).
Figure 1. Flow diagram of the ERSPC trial.
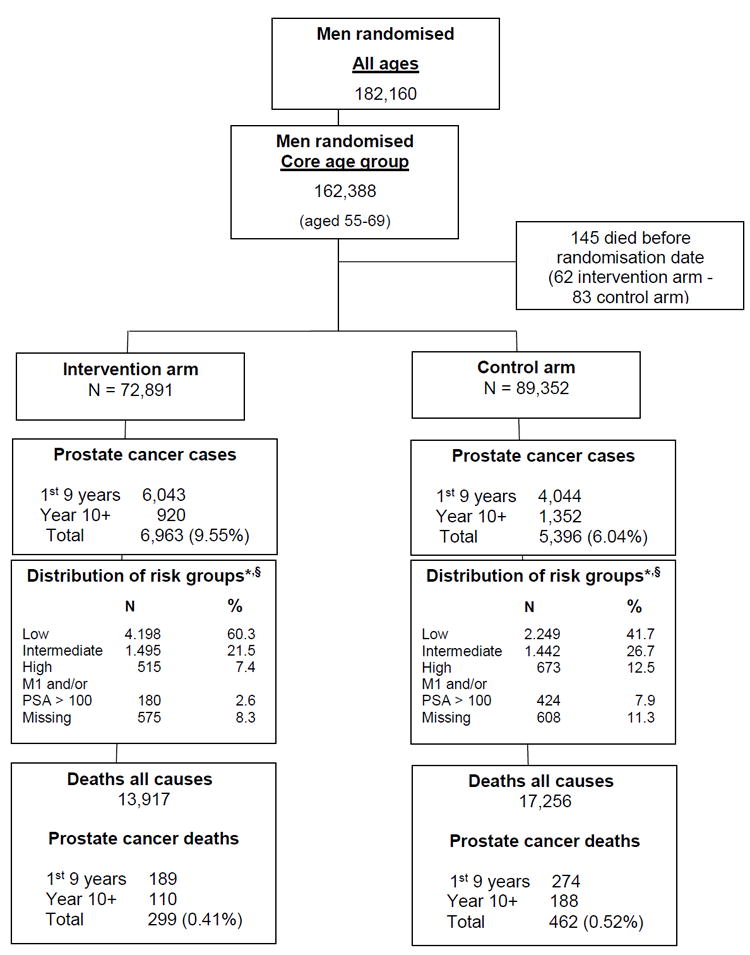
* Low risk= T1,T2 with Gleason score (GS) <= 6; Intermediate risk = T1,T2 with GS 7 and T3 with GS <=7; High risk = T1,T2,T3 with GS 8-10 and T4 with any GS; M1 and/or PSA > 100 = any T stage or GS with M1 and/or PSA > 100.
§ More detailed data on prognostic factors are provided in tables 5A, 5B and 5C of the supplementary appendix
Table 1.
Randomization, participants and results of screening per centre (core ages, cut-off date December 31, 2008).
| ERSPC – Randomization, participants and results of screening per centre (core ages, cut-off date December 31, 2008) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Netherlands | Belgium | Sweden | Finland | Italy | Spain | Switzerland | Total1 Excluding France |
France- Herault |
France- Tarn |
Total | |
|
|
|
||||||||||
| Period of randomization |
Nov 1993 – March 2000 |
June 1991- Dec 2003 |
31 Dec 2004 |
Jan 1996- Jan 1999 |
Oct 1996 – Oct 2000 |
Feb 1996 – June 1999 |
Sep 1998 - Aug 2003 |
June 2003 – Mar 2005 |
Dec 2000 – June 2004 |
||
|
| |||||||||||
| Randomized – N | 34,833 | 8,562 | 11,852 | 80,379 | 14,517 | 2,197 | 9,903 | 162,243 | 57,658 | 21,356 | 241,257 |
| - screening | 17,443 (50.1%) | 4,307 (50.3%) | 5,901 (49.9%) | 31,970 (39.8%) | 7,266 (50.1%) | 1,056 (48.1%) | 4,948 (50.0) | 72,891 (44.9) | 28,792 (49.9) | 10,886 (51.0) | 112,569 (46.7) |
| - control | 17,390 (49.9%) | 4,255 (49.7%) | 5,951 (50.1%) | 48,409 (60.2%) | 7,251 (49.9%) | 1,141 (51.9%) | 4,955 (50.0) | 89,352 (55.1) | 28,866 (50.1) | 10,470 (49.0) | 128,688 (53.3) |
|
| |||||||||||
| Screened at least once N (%) | 16502 (94.6) | 3908 (90.7) | 4484 (76.0) | 23771 (74.4) | 5730 (78.9) | 1056 (100) | 4793 (96.9) | 60244 (82.6) | 7164 (24.9) | 4005 (36.8) | 71413 (63.4) |
| Screen tests done – N | 37375 | 6438 | 15474 | 52142 | 12731 | 1846 | 10683 | 136689 | 7164 | 4005 | 147858 |
| Positive tests – N (%) | 8892 (23.8%) | 1055 (16.4%) | 2897 (18.7%) | 5925 (11.4) | 1443 (11.3) | 354 (19.2) | 2299 (21.5) | 22865 (16.7) | 1091 (15.2) | 614 (15.3) | 24570 (16.6) |
| Biopsies – N (% of screen positive) | 7989 (89.8%) | 750 (71.1%) | 2509 (86.6%) | 5397 (91.1) | 902 (62.5) | 263 (74.3) | 1836 (79.9) | 19646 (85.9) | 315 (28.9) | 352 (57.3) | 20313 (82.7) |
|
| |||||||||||
| Prostate cancers | |||||||||||
| Screening cohort total – N | 2028 | 420 | 759 | 2838 | 374 | 69 | 475 | 6963 | 885 | 497 | 8345 |
| Screen detected N | 1730 | 187 | 576 | 1631 | 197 | 60 | 376 | 4757 | 163 | 112 | 5032 |
| Interval and Non attender N | 298 | 233* | 183 | 1207 | 177 | 9 | 99 | 2206 | 722 | 385 | 3313 |
| PPV (S det cancers/biopsy, %) | 21.7 | 24.9 | 23.0 | 30.2 | 21.8 | 22.8 | 20.5 | 24.2 | 51.7 | 31.8 | 24.8 |
| Cumulative incidence (total cancers / all rand. To S arm, %) | 11.6 | 9.8 | 12.9 | 8.9 | 5.1 | 6.5 | 9.6 | 9.6 | 3.1 | 4.6 | 7.4 |
|
| |||||||||||
| Prostate cancers | |||||||||||
| Control group – N | 896 | 311 | 507 | 3175 | 257 | 24 | 226 | 5396 | 782 | 443 | 6621 |
| Cumulative incidence (%) | (5.2) | (7.3) | (8.5) | (6.6) | (3.5) | (2.1) | (4.6) | (6.0) | (2.7) | (4.2) | (5.1) |
|
| |||||||||||
| Mean follow up (years) | 10.7 | 11.1 | 12.5 | 10.4 | 9.9 | 10.4 | 7.9 | 10.5 | 4.3 | 5.5 | 8.6 |
| Median follow-up (years) | 11.1 | 12.1 | 14.0 | 11.0 | 10.7 | 10.7 | 8.2 | 11.0 | 4.4 | 5.5 | 9.8 |
median screening interval of 6 years between rounds 1 and 2
Interval PC are cases that were clinically detected during the screening interval. Non-attender PC are clinically detected cases in men who refused screening.
Excludes the 145 men who died before randomisation
Within the core age group, 136,689 screen tests were performed, on average 2.27 per participant, 16.7% were positive and 85.9% of the screen-positive men underwent prostate biopsy. The median screening interval was 4.02 years. A total of 6963 prostate cancers were diagnosed in the intervention arm (cumulative incidence 9.6%) and 5396 in the control arm (6.0%). with approximately 1000 additional cases in each arm compared to our earlier analysis [1]. With follow-up through 2008, the mean and median duration of follow-up for the core age group were 10.5 and 11.0 years respectively. PC incidence during the entire follow-up was 9.66 per 1000 person-years in the screening and 5.95 in the control arm, rate ratio (RR) 1.63, (95% CI 1.57-1.69), with a rate difference 3.71 per 1000 person-years (95% CI 3.44-3.99) (Table 2a). Data shown for the years 0-9 are not identical with the previous publication, because of continued follow-up in the centers with late entry contributing to this period. The excess incidence in the screening arm was largely due to small, well-differentiated tumors, and the incidence of advanced (T3-4, M1) and aggressive (Gleason 8-10) cancers was lower in the screening than control arm (Supplementary tables 2A,B,C)
Table 2.
Prostate-Cancer Incidence in Men 55 to 69 Years of Age, According to Study Period
| Intervention (n= 72,891) | Control (n=89,352) | Rate Ratio1 (95% CI) | Rate difference per 1000 persons year (95% CI) | Rate difference per 1000 men | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Prostate Cancer N | Person years | Rate per 1000 person years | Prostate Cancer N | Person years | Rate per 1000 person years | ||||
|
| |||||||||
| Years 1-9 | 6043 | 580502 | 10.41 | 4044 | 731204 | 5.53 | 1.88 (1.81 – 1.96) | 4.80 (4.49 – 5.12) | 37.6 |
| 4.89 (4.56 – 5.21)* | 38.7* | ||||||||
|
| |||||||||
| Years 8-9 | 1410 | 113850 | 12.38 | 1174 | 145293 | 8.08 | 1.56 (1.44 – 1.69) | 4.30 (3.51 – 5.10) | 6.2 |
| 4.54 (3.72 – 5.36)* | 6.7* | ||||||||
|
| |||||||||
| Years 10-11 | 541 | 88999 | 6.08 | 916 | 114462 | 8.00 | 0.78 (0.70 – 0.87) | -1.92 (-2.65 – -1.20) | -2.8 |
| -1.76 (-2.52 – -0.99)* | -2.5* | ||||||||
|
| |||||||||
| Years 1-11 | 6584 | 669501 | 9.83 | 4960 | 845666 | 5.87 | 1.68 (1.62-1.75) | 3.97 (3.68-4.26) | 34.8 |
| 4.10 (3.80-4.40)* | 36.2* | ||||||||
|
| |||||||||
| Years 12+ | 379 | 51141 | 7.41 | 436 | 61726 | 7.06 | 1.03 (0.90 – 1.19) | 0.35 (-0.65 – 1.35) | 0.3 |
| 0.35 (-0.68 – +1.39)* | 0.1* | ||||||||
|
| |||||||||
| Total | 6963 | 720643 | 9.66 | 5396 | 907392 | 5.95 | 1.63 (1.57 – 1.69) | 3.71 (3.44 – 3.99) | 35.1 |
| 3.84 (3.55 – 1.72)* | 36.3* | ||||||||
Adjusted by centre
Finland control population weighted by 2/3
There were a total of 299 deaths from PC in the screening arm and 462 in the control arm, with mortality rates of 0.39 and 0.50 per 1000 person-years, respectively (Table 2b). Overall, a RR of 0.79 (95% CI 0.68-0.91, P=0.001), corresponding to a relative risk reduction of 21% in favor of screening was found. The absolute difference in mortality amounted to 0.10 per 1000 person years or 1.07 per 1000 men randomized. After correction for selection bias and non-attendance, an adjusted RR of 0.71 (95% CI 0.58-0.86, P=0.001) was obtained for screened men, translating to a relative risk reduction of 29%. Rate ratios for the periods years 1-9 and 1-11 were 0.85 (95% CI 0.71-1.03) and 0.79 (95% CI 0.67 – 0.92), respectively. The absolute effect of screening expressed as number needed to invite (NNI) to prevent one death from PC over 11 years was 1055 and the number needed to detect (NND) was 37. For the non-truncated analysis (including all available follow-up), the NND amounted to 936 and the NNI to 33. The NNI and NND varied considerably with the period of follow-up (NNI 2111 – 936 and NND 80 – 33 for all centers) and among the three largest centers (NNI 194 – 1825 and NND 8 – 42 for total follow-up) (Supplementary Table 3A). In tables 2 and 3 the effect of weighting the control population of Finland by 1:1.5 is also shown.
Table 3.
Mortality from Prostate Cancer among Men 55 to 69 Years of Age, According to Study Period
| Intervention | Control | Rate Ratio1 (95% CI) | Rate difference per 1000 persons year (95% CI) | Rate difference per 1000 men | |||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Prostate Cancer deaths N | Person years | Rate per 1000 person years | Prostate Cancer deaths N | Person years | Rate per 1000 person years | ||||
|
| |||||||||
| Years 1-9 | 189 | 608852 | 0.31 | 274 | 745912 | 0.37 | 0.85 (0.71 – 1.03) P=0.09 | -0.06 (-0.12 – +0.005) | -0.47 |
| -.0.06 (-0.12 – + 0.000009)* | -0.46* | ||||||||
|
| |||||||||
| Years 8-9 | 71 | 122867 | 0.58 | 118 | 151319 | 0.78 | 0.74 (0.55-0.99) P = 0.04 | -0.20 (-0.40 – -0.000007) | -0.35 |
| -0.20 (-0.40 – + 0.00001)* | -0.33* | ||||||||
|
| |||||||||
| Years 10-11 | 56 | 97994 | 0.57 | 111 | 120900 | 0.92 | 0.62 (0.45 – 0.85) P=0.003 | -0.35 (-0.57 – -0.12) | -0.47 |
| -0.37 (-0.61 – -0.12)* | -0.48* | ||||||||
|
| |||||||||
| Year 1-11 | 245 | 706846 | 0.35 | 385 | 866812 | 0.44 | 0.79 (0.67-0.92) P = 0.003 | -0.10 (-0.16 – -0.04) | -0.95 |
| -0.10 (-0.17 – -0.03)* | -0.94* | ||||||||
|
| |||||||||
| Years 12+ | 54 | 57387 | 0.94 | 77 | 66241 | 1.16 | 0.80 (0.56 – 1.13) P=0.21 | -0.22 (-0.58 – +0.14) | -0.12 |
| -0.26 (-0.64 – + 0.12)* | -0.19* | ||||||||
|
| |||||||||
| Total | 299 | 764233 | 0.39 | 462 | 933052 | 0.50 | 0.79 (0.68 – 0.91) P=0.001 | -0.10 (-0.17 – -0.04) | -1.07 |
| -0.11 (-0.18 – 0.04)* | -1.13* | ||||||||
Adjusted by centre
Finland control population weighted by 2/3
The Nelson-Aalen cumulative PC mortality curves for the two arms separate gradually, starting approximately 7 years after randomization (Figure 2). For PC mortality, a steadily increasing mortality with follow-up was found for both arms over the three periods chosen (Table 2b). The RR for the two year follow-up period of years 10-11 was 0.62 (0.45-0.85), a relative risk reduction of 38%. The mortality results per center are shown in the Forest plot (Supplementary Figure 1A) and the Appendix table 4A. The Kaplan Meier analysis by arm and Gleason score is included as supplementary Figure 2A.
Figure 2.
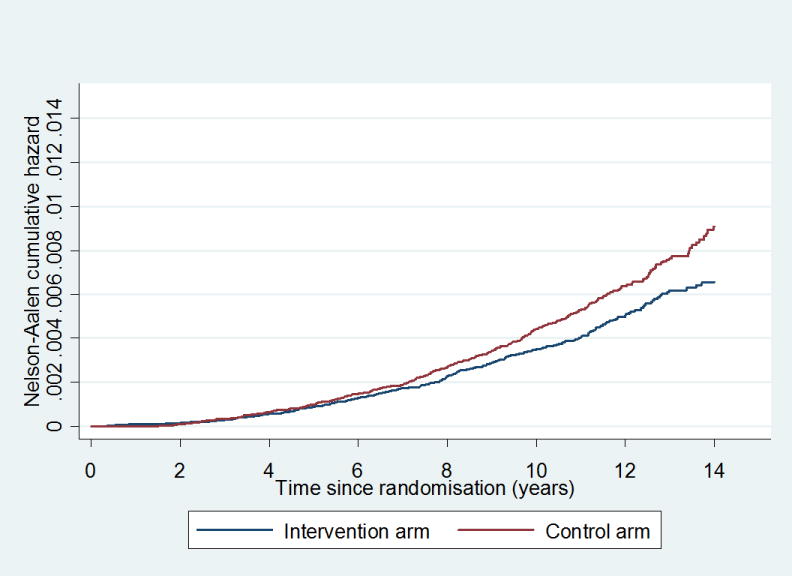
Cumulative mortality from prostate cancer in the core age group, excluding France
The RR of PC mortality was significantly below one in the core age group and for all ages, but only in the age group 65-69 in the sub-group analyses. The study was powered upfront for the core age group analysis (Supplementary Table 5A). Only three centers (Finland, the Netherlands and Sweden) had more than 100 PC deaths and the RR for PC mortality (for the core age group) ranged from 0.56 in Sweden to 0.89 in Finland, with significant reduction in Sweden and the Netherlands (Supplementary table 4A). Supplementary Figure 3A shows the distribution of the 299 deaths in the screen arm between screen detected cancers, interval cases and non attendees. Nearly half of the deaths in the screening arm occurred in men with screen-detected cancers, and of them 74% were diagnosed at the first round. Roughly a quarter of the deaths were among interval cases and a similar number in non-attendees.
An analysis of influence by center was carried out by calculating the RR’s of PC mortality omitting each center one at a time (Supplementary Table 6A). The overall RR’s remained significant with a point estimate of RR close to 0.8 regardless of the exclusion of any of the seven centers. With the omission of Finland, however, the RR approached 0.7. For details on PC mortality per center and time period see Supplementary Table 7A1,7A2.
Overall mortality was similar in both arms (18.2 in the screening and 18.5 per 1000 person-years in the control arm, RR=0.99, 95% CI 0.97-1.01, Supplementary Table 6A). Data on all cause mortality by age groups are supplied in Supplementary Table 8A.
Data on stage and grade distribution and treatment per study arm are given in Supplementary Tables 2A,B,C and 9A,B.
Discussion
The controversy around screening for prostate cancer has recently been renewed by the publication of the draft report of the United States Preventive Services Task Force (USPSTF) which, after a literature-based analysis of benefits and harms, recommends against the use of PSA in asymptomatic men [12]. The report has been discussed in several “Perspectives” published in NEJM [13]. Clearly, the issue can only be resolved by evidence that considers both the advantages and disadvantages of screening, which is not available at this time.
Our study shows that the absolute effect of screening on PC mortality increased in the intention-to-screen analysis from 0.71 to one of 1.07 per 1000 men at a median of 11 years of follow-up compared to the initial results with a shorter follow-up [1]. Correspondingly, the numbers needed to invite and to detect to avert one PC death decreased from 1410 to 936 and from 48 to 33. These numbers are expected to decrease further with longer follow-up [14,15]. In contrast, the relative risk reduction remained practically unchanged at 21%. After correction for non-compliance, a relative difference of 29% for screened men resulted.
During the years of follow-up 10 to 11, a relative risk reduction of 38% was seen. The reduced mortality needs to be balanced, however, against the downsides of early detection of PC, most importantly over-diagnosis estimated to be in the range of 50% [16]. Loeb et al [17] showed in a review that septic complications of biopsies increased in line with increasing resistance of large bowel bacteria to antibiotics. Another important issue is the small effect of radical prostatectomy versus watchful waiting in the SPG 4 trial [18] of only 6% absolute mortality reduction and the absence of an effect in the PIVOT trial after 12 years of follow–up [19]. There is no effect on all cause mortality, and an evaluation of the effect on quality of life is pending.
The effect of the extended follow-up is best assessed by comparing the figures in follow-up truncated at 9 versus 11 years. Both NNI and NND were reduced by approximately half when based on follow-up of 11 years compared to 9 years. These results are not directly comparable with our earlier analysis based on all available data through 2006 (not truncated by follow-up time). The absolute risk reduction is a concrete measure of effect of screening but depends on underlying risk in the population and therefore cannot be directly generalized [20].
The mortality effect of screening was significant for the core age group and for all ages. There was, however, no indication of benefit for men aged 70 or older, though the confidence interval was wide. Upper age limit and life expectancy warrant careful consideration in future screening programs. The Kaplan Meier analyses of PC specific mortality per arm and Gleason scores show significant differences for both prognostic groups. This analysis is considered inadequate in the evaluation of randomized screening trials because of lead-time, length and over diagnosis biases [21].
The overall screening effect (in terms of RR reduction) was not driven by any single center, as indicated by consistency in the analysis of influence, despite some variation in screening protocol. Yet, the RR by center was not constant, as also shown in the Forest plot. The screening effect depends on the proportion of cancers diagnosed at a potentially curable stage, which may differ by center due to differences in screening procedures and in underlying risk. However, the screening effect can also be attenuated by contamination, i.e. screening in the control arm. The Göteborg screening trial [23] has shown a larger mortality effect and a more favorable NNI and NND with biannual screening and at a longer follow-up of 14 years with a higher background mortality from PC.
Some biases could affect the mortality results of the screening trial. Also, similar treatment needs to be applied for similar disease to ensure that the difference between the trial arms is attributable to screening, and not superior management of screen-detected cases. Earlier analyses have shown comparable treatment modalities between the trial arms by stage [22,23,24]. Second, assigning causes of death is prone to error, which is minimized by the standardized measures and blinded assignments [5]. Finally, the potential attenuating effect of contamination is considered to be approximately 20% of men per year in the control arm in the early follow-up [25,26].
The reasons why the effect of screening did not increase more during the extended follow-up remain unclear at this time. The majority of the PC deaths among screen-detected cases (100 out of 136 or 74%) occurred in men whose cancer was diagnosed at the first screen. Natural history studies confirm the need for very long observation periods. Johansson et al. [27] found a large increase of PC mortality in confined cancers 15-20 years after inclusion. The high prevalence of 25.8% of all PC deaths occurring in interval cancers necessitates optimization of screening procedures. This confirms that despite the exclusion of clinically evident PC at entry, a large number of men probably harbored latent, but aggressive disease, which subsequently turned out to be deadly even after prolonged follow-up.
In summary, our trial showed a relative risk reduction of 21% in favor of screening in the intention-to-screen analysis and 29% among screened men after adjustment for non-compliance, while the absolute risk reduction amounted to 1.07 per 1000 men randomized at a median follow-up of 11 years. This corresponds to an NNI to avert one PC death of 936 and NND of 33. During the years of follow-up 10 - 11 the relative risk reduction amounted to 32%. It is important to note that there was no effect of screening on all-cause mortality. More information on the balance of benefits and adverse effects, as well as cost-effectiveness of screening is needed before general recommendations can be made.
Supplementary Material
References
- 1.Schröder FH, Hugosson J, Roobol MJ, Tammela TLJ, Ciatto S, Nelen V, Kwiatkowski M, Lujan M, Lilja H, Zappa M, Denis LJ, Recker F, Berenguer A, Määttänen L, Bangma CH, Aus G, Villers A, Rebillard X, van der Kwast TH, Blijenberg BG, Moss SM, de Koning HJ, Auvinen A for the ERSPC Investigators. Screening and prostate-cancer mortality in a randomized European Study. N Engl J Med. 2009 Mar 26;360(13):1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 2.Roobol MJ, Schröder FH. The European Randomized study of Screening for Prostate Cancer (ERSPC): rationale, structure and preliminary results 1994-2003. BJU Int. 2003 Dec;92(Suppl 2) doi: 10.1111/j.1464-410x.2003.4698x.x. [DOI] [PubMed] [Google Scholar]
- 3. [1.12.2011]; http://www.erspc.org/publist.php / study protocol 1994, admission criteria and minimal data set. Section 4.0 definitions, 4.1 age groups.
- 4.Stamey TA. Making the most out of six systematic sextant biopsies. Urology. 1995;45:2–12. doi: 10.1016/s0090-4295(95)96168-2. [DOI] [PubMed] [Google Scholar]
- 5.De Koning HJ, Blom J, Merkelbach JW, Raaijmakers R, Verhaegen H, Van Vliet P, Nelen V, Coebergh JWW, Hermans A, Ciatto S, Mäkinen T. Determining the cause of death in randomized screening trial(s) for prostate cancer. BJU Int. 2003 Dec;92(Suppl 2):71–8. doi: 10.1111/j.1465-5101.2003.04402.x. [DOI] [PubMed] [Google Scholar]
- 6.De Koning HJ, Liem MK, Baan CA, Boer R, Schröder FH, Alexander FE. Prostate cancer mortality reduction by screening: power and time frame with complete enrollment in the European Randomized Screening for Prostate Cancer (ERSPC) trial. Int J Cancer. 2002 Mar 10;98(2):268–73. doi: 10.1002/ijc.10188. [DOI] [PubMed] [Google Scholar]
- 7.De Koning HJ, Hakulinen T, Moss SM, Adolfsson J, Smith PH, Alexander FE for the ERSPC. Monitoring the ERSPC trial. BJU Int. 2003 Dec;92(Suppl 2):112–4. doi: 10.1111/j.1464-410x.2003.4410x.x. [DOI] [PubMed] [Google Scholar]
- 8.Cuzick J, Edwards R, Segnan N. Adjusting for non-compliance and contamination in randomized clinical trials. Stat Med. 1997;16:1017–29. doi: 10.1002/(sici)1097-0258(19970515)16:9<1017::aid-sim508>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 9.Peto R, Pike MC, Armitage P, Breslow NE, Cox DR, Howard SV, Mantel N, McPherson K, Peto J, Smith PG. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. I. Introduction and design. Br J Cancer. 1976 Dec;34(6):585–612. doi: 10.1038/bjc.1976.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeMets DL, Lan KK. Interim analysis: the alpha spending function approach. Stat Med. 1994 Jul 15-30;13(13-14):1341–52. doi: 10.1002/sim.4780131308. discussion 1353-6. [DOI] [PubMed] [Google Scholar]
- 11.Aalen OO. Nonparametric inference for a family of counting processes. Ann Stat. 1978;6:701–27. [Google Scholar]
- 12. [1.12.2011]; http://www.uspreventiveservicestaskforce.org/uspstf/uspsprca.htm.
- 13. [1.12.2011]; http://www.nejm.org/toc/nejm/365/21.
- 14.Gulati R, Mariotto AB, Chen S, Gore JL, Etzioni R. Long-term projections of the harm-benefit trade-off in prostate cancer screening are more favorable than previous short-term estimates. J Clin Epidemiol. 2011 Dec;64(12):1412–7. doi: 10.1016/j.jclinepi.2011.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loeb S, Vonesh EF, Metter EJ, Carter HB, Gann PH, Catalona WJ. What is the true number needed to screen and treat to save a life with prostate-specific antigen testing? J Clin Oncol. 2011 Feb 1;29(4):464–7. doi: 10.1200/JCO.2010.30.6373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Draisma G, Boer R, Otto SJ, van der Cruijsen IW, Damhuis RA, Schröder FH, de Koning HJ. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003 Jun 18;95(12):868–78. doi: 10.1093/jnci/95.12.868. [DOI] [PubMed] [Google Scholar]
- 17.Loeb S, Carter HB, Berndt SI, Ricker W, Schaeffer EM. Complications after prostate biopsy: data from SEER-medicare. J Urol. 2011 Nov;186(5):1830–4. doi: 10.1016/j.juro.2011.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bill-Axelson A, Holmberg L, Ruutu M, Garmo H, Stark JR, Busch C, Nordling S, Häggman M, Andersson SO, Bratell S, Spångberg A, Palmgren J, Steineck G, Adami HO, Johansson JE. SPCG-4 Investigators. Radical prostatectomy versus watchful waiting in early prostate cancer. N Engl J Med. 2011 May 5;364(18):1708–17. doi: 10.1056/NEJMoa1011967. [DOI] [PubMed] [Google Scholar]
- 19.Wilt T. The VA/NCI/AHRQ CSP #407, AUA 2011: Prostate Cancer Intervention Versus Observation Trial (PIVOT): Main Results From a Randomized Trial Comparing Radical Prostatectomy to Watchful Waiting in Men With Clinically Localized Prostate Cancer [Google Scholar]
- 20.Rembold CM. Number needed to screen: development of a statistic for disease screening. BMJ. 2008;317:307–312. doi: 10.1136/bmj.317.7154.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu GH, Auvinen A, Yen AM, Hakama M, walter SD, Chen H. A stochastic model for urvival of early prostate cancer with adjustments for leadtime, length bias, and overdetection. Biometrical Journal. 2012 doi: 10.1002/bimj.201000107. in press. [DOI] [PubMed] [Google Scholar]
- 22.Hugosson J, Carlsson S, Aus G, Bergdahl S, Khatami A, Lodding P, Pihl CG, Stranne J, Holmberg E, Lilja H. Mortality results from the Göteborg randomized population-based prostate-cancer screening trial. Lancet Oncol. 2010 Aug;11(8):725–32. doi: 10.1016/S1470-2045(10)70146-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wolters T, Roobol MJ, Steyerberg EW, van den Bergh RC, Bangma CH, Hugosson J, Ciatto S, Kwiatkowski M, Villers A, Lujan M, Nelen V, Tammela TL, Schröder FH. The effect of study arm on prostate cancer treatment in the large screening trial ERSPC. Int J Cancer. 2010 May 15;126(10):2387–93. doi: 10.1002/ijc.24870. [DOI] [PubMed] [Google Scholar]
- 24.Boevee SJ, Venderbos LD, Tammela TL, Nelen V, Ciatto S, Kwiatkowski M, Paez A, Malavaud B, Hugosson J, Roobol MJ. Change of tumour characteristics and treatment over time in both arms of the European Randomized study of Screening for Prostate Cancer. Eur J Cancer. 2010 Nov;46(17):3082–9. doi: 10.1016/j.ejca.2010.09.033. [DOI] [PubMed] [Google Scholar]
- 25.Otto S, van der Cruijsen IW, Liem MK, Korfage IJ, Lous JJ, Schroder FH, de Koning HJ. Effective PSA contamination in the Rotterdam section of the European Randomised Study of Screening for Prostate Cancer. Int J Cancer. 2003;105:394–9. doi: 10.1002/ijc.11074. [DOI] [PubMed] [Google Scholar]
- 26.Ciatto S, Zappa M, Villers A, Paez A, Otto SJ, Auvinen A. Contamination by opportunistic screening in the European Randomized Study of Prostate Cancer Screening. BJU Int. 2003 Dec;92(Suppl 2):97–100. doi: 10.1111/j.1464-410x.2003.04407.x. [DOI] [PubMed] [Google Scholar]
- 27.Johansson J-E, Andren O, Andersson SO, Dickman PW, Holmberg L, Magnuson A, Adami H-O. Natural history of early, localised prostate cancer. JAMA. 2004;291:2713–19. doi: 10.1001/jama.291.22.2713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


