Abstract
A growing body of evidence has shown that neighborhood characteristics have significant effects on quality metrics evaluating health plans or health care providers. Using a data set of an urban teaching hospital patient discharges, this study aimed to determine whether a significant effect of neighborhood characteristics, measured by the Area Deprivation Index, could be observed on patients’ readmission risk, independent of patient-level clinical and demographic factors. We found that patients residing in the more disadvantaged neighborhoods had significantly higher 30-day readmission risks, compared to those living in the less disadvantaged neighborhoods, even after accounting for individual-level factors. Those living in the most extremely socioeconomically challenged neighborhoods were 70 percent more likely to be readmitted than their counterparts who lived in the less disadvantaged neighborhoods. Our findings suggest that neighborhood-level factors should be considered along with individual-level factors in future work on adjustment of quality metrics for social risk factors.
INTRODUCTION
The continuing expansion of “pay for performance” programs in health care, and a parallel expansion of public reporting of quality metrics, have led to a concern about whether “safety net” providers are being treated fairly in these programs. A significant body of evidence indicates that safety net hospitals,1 Medicare Advantage plans with relatively large numbers of dual-eligible members,2 and physicians or physician groups serving patients with a disproportionate share of social risk factors3 tend to score below the average on quality metrics in such programs, and therefore are more likely to face financial penalties or not receive financial rewards.4
The question of whether “safety net” plans or providers are being treated fairly depends on the nature of the relationship between social risk factors and quality measures, particularly measures reflecting outcomes of care. If the effects of social risk factors such as poverty, illiteracy, homelessness, or lack of social support are mediated by quality of care (i.e., poor people are served by low-quality plans or providers), then a relatively lower ranking in “pay for performance” or public reporting programs may seem fair. On the other hand, if the effects of social risk factors are independent of quality of care (e.g., patients cannot afford medications or do not have transportation after hospital discharge) and outside of plans’ or providers’ control, then the differences in “performance” reflect characteristics of the patients or communities served rather than quality of care.5
Previous policy of the National Quality Forum (NQF) prohibited risk adjustment for socioeconomic status in its endorsed performance measures.6 In 2014, after reviewing the policy and analyzing the issue, an expert panel report to the NQF7 recommended adjustment of quality measures on the basis of social and economic characteristics in specific situations. Two more recent reports, one by the National Academy of Medicine (NAM)8 and the other by the Associate Secretary for Planning and Evaluation (ASPE)9 of the Department of Health and Human Services made similar recommendations. As a result of the NQF panel recommendation, the policy against adjustment at NQF was changed to allow and encourage adjustment, with a two-year trial period established to allow for evaluation of the change.10 The policy change was recently confirmed for an additional three years.11 In the two-year trial period, some measures have been proposed and approved by NQF with some element of adjustment for social risk factors,12 but the majority of measures coming through the NQF endorsement process have been approved without adjustment.
Most of these efforts, and many of the previous analyses of the effects of social risk factors on outcome measures,2,3,13 have focused on the individual patient or plan member characteristics, or in some instances their individual characteristics inferred from their addresses,14–16 as the unit of data collection and analysis. However, there are important influences of social risk factors on key health care quality metrics that are inherently felt at the community or neighborhood level instead of only at the individual level. Poor public transportation, crime, absence of social support programs or services such as Meals on Wheels, or absence of accessible primary care are characteristics of geographic areas. These factors have causal effects separate from those of similar factors at the individual level. For example, being poor and not owning a car may be a minor problem in a community with excellent public transportation, but may present a major challenge to access to care in a community with poor or non-existent public transportation. The Moving to Opportunity (MTO) study confirmed that positive effects in a number of domains of health and educational achievement could be obtained by low-income families’ moving from relatively more disadvantaged to relatively less disadvantaged neighborhoods.17
There have been some recent examples of analyses pointing to important effects of community-level variables, such as county- or city-level poverty and employment rates.18–21 However, the distinction between individual-level and community-level social risk factors has not yet been explored in detail in the context of quality measurement and risk adjustment.
The Area Deprivation Index (ADI) is a promising measure of neighborhood-disadvantage that could influence health care outcomes, including readmission risk. The ADI was originally created by the US federal government over two decades ago from long-form Census data and primarily used at the county level to assess mortality and disease prevalence. To make the metric more applicable to the modern era, the ADI has been refined to the census block group (i.e., “neighborhood”) level and adapted to updated data sources from the American Community Survey by Kind and team at the University of Wisconsin School of Medicine and Public Health. This updated, refined ADI has been validated using a number of known neighborhood disadvantage-linked outcomes, and is being actively used by the Centers for Medicare and Medicaid Services (CMS) in one of its programs.22, 23 A previous national study20 using ADI as the neighborhood disadvantage measurement found that readmission rates increased significantly among Medicare patients residing in the top 15% most disadvantaged neighborhoods.
The analyses reported here were designed to determine whether an independent effect of the ADI on hospital readmission could be observed in a data set of hospital discharges, when patient clinical and demographic characteristics were taken into account. Our hypothesis was that we would see a similar relationship between neighborhood disadvantage level and readmission risk among our study cohort as had been observed in the older national study, even with patient-level clinical and sociodemographic factors included in the models. Finding such an effect would suggest that community-level factors should be considered along with individual-level factors in future work on adjustment of quality metrics for social risk factors.
METHODS
DATA
We used two major data files. Our readmission data file was obtained from a “dry run report” distributed by CMS to the hospital for review for the purpose of public reporting. This file contained all qualified index admissions and 30-day readmissions (to any hospital) for Medicare fee-for-service patients aged 65 years and above, who were discharged from Henry Ford Hospital (HFH) in 2010. A detailed description of the study setting, patient population, and inclusion/exclusion criteria can be found in our previous study.24 We extracted patients’ demographics, street addresses, and clinical data from Corporate Data Store, the hospital’s central repository for patient encounter data. Michigan ADI dataset (block group files V1.2) was obtained through the University of Wisconsin-Madison School of Medicine and Public Health. This file provided ADI scores for 8205 census block groups in Michigan. We geocoded patients’ street addresses to census block groups, linked the readmission to the Michigan ADI file, and assigned each patient a neighborhood ADI value according to the census block group in which he or she resided.
KEY VARIABLES
Our dependent variable was 30-day readmission, which was constructed following the relevant definitions and specifications of the CMS hospital-wide all-cause unplanned 30-day readmission (HWR) measure. Our key explanatory variable was a patient’s neighborhood ADI score, using methods previously described. A larger ADI score indicates a higher level of disadvantage.
Primary diagnosis at time of discharge was defined as the principal discharge diagnosis of the index admission; comorbidities were assessed using additional diagnosis data from the index admission and any admissions in the prior year. The top ten primary diagnoses present at discharge and comorbidities with a prevalence among study patients of >10% were included in our model; all other comorbidities were grouped into an “other” category. Appendices A and B show the detailed ICD-9 codes and CMS risk variable groups we used to identify the clinical factors.
STATISTICAL APPROACHES
We first examined the distribution of the ADI values among our study cohort, and compared it with the national distribution, considering that the study area (Detroit Metropolitan Area) is one of the areas with the highest concentration of socially and economically disadvantaged neighborhoods in the nation. Second, since previous national studies showed a nonlinear relationship between patient outcomes and ADI,20 we explored whether this nonlinear relationship also existed among our study population. We sorted all admissions according to the patients’ ADI scores into 20 equally-sized groups and observed the unadjusted relationship between patient ADI groups and the readmission rates of the groups.
Previous national analyses have demonstrated a marked increase in readmission risk amongst the top 15% most disadvantaged neighborhoods by ADI.20 This corresponds to an ADI value of 116.2, which falls close to the HFH cohort ADI median (117.6). Guided by these findings and comparisons of the national and local ADI distributions, we grouped the HFH neighborhoods into two disadvantage levels: neighborhoods with ADI values in the top 50% of the overall HFH ADI distribution were classified as more disadvantaged while those in the lower 50% were classified as less disadvantaged.
Multivariate regression analysis was used to examine the relationship between neighborhood disadvantage level and readmission risk. Our multivariate models followed the clinical risk adjustment methods for the HWR measure, and adjusted for the most common primary diagnoses at time of discharge and comorbidities among the patients. We also included the following patient-level social and demographic characteristics: age, sex, race, and marital status. Definitions and details of the specifications of these covariates can be found in our previous study.24 We selected all the independent variables in the multivariate model based on relevant literature25 and conceptual models of readmission.
Finally, given our observation of the relationship between readmission and the highest ADI values at the right tail of the ADI distribution, we did an exploratory analysis to examine this most extremely socioeconomically challenged group by identifying patients living in the top 5% highest HFH ADI neighborhoods (the “most disadvantaged” group) and comparing their readmission risk with that of the “less disadvantaged” group. All statistical analyses were performed using STATA/SE version 13 (StataCorp).
RESULTS
We started with a data set containing 6832 qualified admissions for 4646 unique patients. Among those, 187 admissions for 137 unique patients could not be mapped to census block groups with high confidence point mapping and therefore could not be linked to the ADI file, leaving us a final cohort of 6645 hospital admissions for 4509 unique patients. ADI scores for the study cohort ranged from 27.4 to 129.2, with a mean of 114.7 and a median of 117.6 (Figure 1A). Compared to national ADI distribution (range 2.1 – 129.3, mean 100.0, median 105.4), more of our study patients lived in higher ADI (more disadvantaged) neighborhoods. More than half of HFH patients lived in the most disadvantaged neighborhoods as defined by the top 15% highest ADI neighborhoods in the nation (ADI > 116.2) (Figure 1B).
Figure 1A.
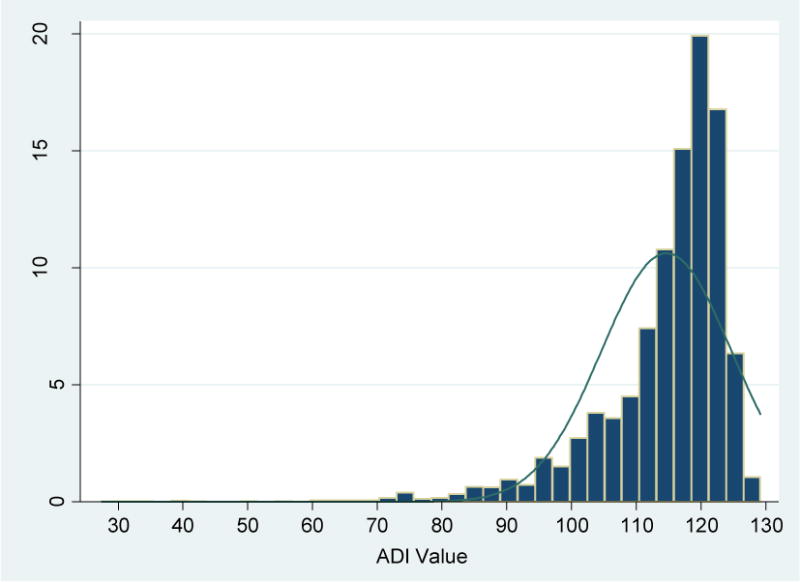
Distribution of ADI Values among Henry Ford Hospital Medicare Fee-For-Service Beneficiaries 65+, 2010
Figure 1B.
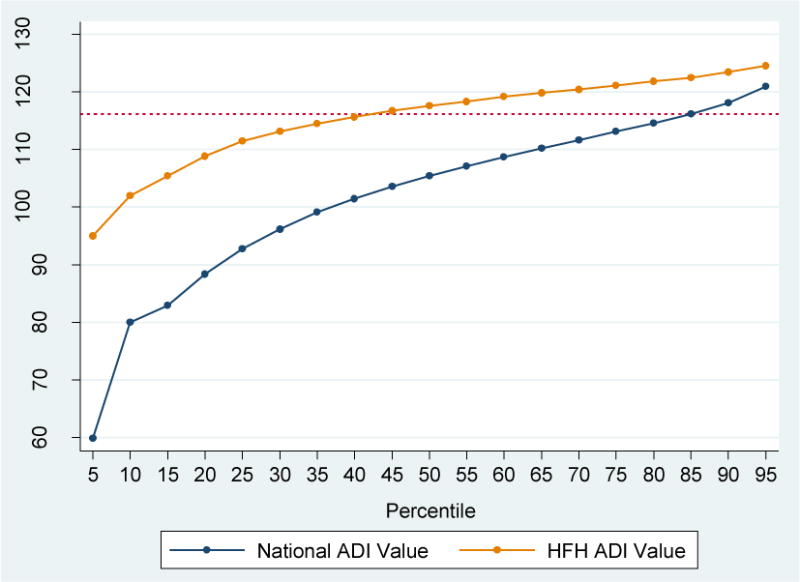
Comparison of the ADI Values by Percentiles between National and Henry Ford Hospital Patients
Note: The horizontal dotted reference line indicates the ADI value of 116.2, the 85% of the national ADI distribution.
Figure 2 shows the relationship between the HFH ADI scores (in 20 HFH-specific percentile groups) and the readmission rates, unadjusted for any covariates and clinical factors. The trend of readmission rates across the 20 HFH ADI groups was not linear, and in general showed a significant increase after the HFH ADI median point (corresponding roughly to the most disadvantaged neighborhood cut point nationwide). At the very high end of the ADI distribution, among the top 5% “most disadvantaged” HFH neighborhoods, the readmission rate increased substantially to 25.9%, from an average of 16.1% across the first half of all HFH neighborhoods (“less advantaged”) and an average of 21.9% across the 45% neighborhoods in between.
Figure 2.
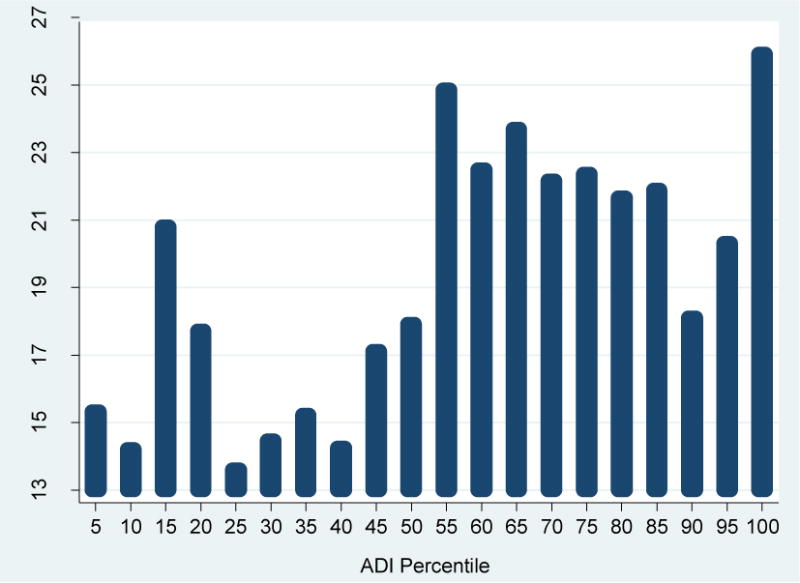
Unadjusted Relationship between ADI Value (in Percentiles) and Readmission Rate: Henry Ford Hospital Medicare Fee-For-Service Beneficiaries 65+, 2010
Table 1 describes the patient characteristics by their residing neighborhood ADI score groups (“less disadvantaged” vs. “more disadvantaged”) for HFH. Compared to those living in the “less disadvantaged” HFH neighborhoods (bottom 50%; below the HFH ADI median point), patients in the “more disadvantaged” neighborhoods (top 50%) were older, predominantly female, of Black race, and unmarried. There were larger proportions of primary diagnosis at time of discharge of congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD) and bronchiectasis, urinary tract infections (UTI), and fluid and electrolyte disorders among those living in the “more disadvantaged” neighborhoods, compared to their “less disadvantaged” counterparts. As for clinical comorbidities, a larger proportion of those living in the “more disadvantaged” neighborhoods had diabetes, CHF, COPD, acute renal failure, and any other comorbidity, but a smaller proportion of them had specified arrhythmias and drug/alcohol disorders and psychiatric conditions, compared to their “less disadvantaged” counterparts.
Table 1.
Demographic and Clinical Characteristics and 30-Day Readmission Rates, Stratified by Neighborhood Disadvantage Levels: Henry Ford Hospital Medicare Fee-For-Service Beneficiaries 65+, 2010
| Characteristic | Overall (N=6,645) |
Less Disadvantaged (N=3,327) |
More Disadvantaged (N=3,318) |
p-value |
|---|---|---|---|---|
| Age (in years) | 77.1 | 76.7 | 77.5 | *** .000 |
| Sex: male (%) | 45 | 48 | 41 | *** .000 |
| Marital Status: married (%) | 43 | 52 | 34 | *** .000 |
| Race: Black (%) | 65 | 45 | 84 | *** .000 |
| Primary Diagnosis at Time of Discharge (%) | ||||
| Congestive heart failure | 7.2 | 6.0 | 8.4 | *** .000 |
| Septicemia | 3.9 | 3.5 | 4.3 | .072 |
| Acute myocardial infarction | 3.8 | 3.7 | 3.9 | .549 |
| Acute cerebrovascular disease | 3.8 | 3.9 | 3.7 | .671 |
| Complication of device | 3.6 | 3.8 | 3.3 | .217 |
| Chronic obstructive pulmonary disease and bronchiectasis | 3.3 | 2.3 | 4.3 | ***.000 |
| Urinary tract infections | 3.5 | 2.7 | 4.2 | ***.000 |
| Cardiac dysrhythmias | 3.1 | 3.2 | 3.0 | .491 |
| Pneumonia | 2.8 | 2.6 | 3.0 | .255 |
| Fluid and electrolyte disorders | 2.8 | 2.3 | 3.3 | *.014 |
| Comorbidities (%) | ||||
| Diabetes mellitus | 37.4 | 35.7 | 39.1 | **.004 |
| Congestive heart failure | 10.4 | 7.7 | 13.1 | ***.000 |
| Coronary atherosclerosis/angina, cerebrovascular disease | 47.6 | 46.6 | 48.6 | .093 |
| Specified arrhythmias | 24.2 | 26.2 | 22.1 | ***.000 |
| Chronic obstructive pulmonary disease | 15.3 | 14.5 | 16.2 | *.047 |
| Acute renal failure | 13.8 | 10.7 | 16.9 | ***.000 |
| Cancers | 10.9 | 11.6 | 10.2 | .059 |
| Drug/alcohol disorders and psychiatric conditions | 12.8 | 13.7 | 12.0 | *.034 |
| Other | 39.9 | 37.9 | 42.0 | **.001 |
| Readmission (%) | 19.2 | 16.1 | 22.3 | ***.000 |
Notes:
The ADI cut point (50% or median) used to classify the neighborhoods (less disadvantaged vs. more disadvantaged) was based on the HFH ADI distribution, rather than the national distribution which was referred to for the purpose of comparison only.
Other comorbid risk factors include: severe infection, other infectious disease & pneumonias, protein-calorie malnutrition, end-stage liver disease, other hematological disorders, hemiplegia, paraplegia, paralysis, functional disability, seizure disorders and convulsions, fibrosis of lung or other chronic lung disorders, dialysis status, ulcers, septicemia/shock, disorders of fluid, electrolyte, acid-base, iron deficiency, cardiorespiratory failure or cardiorespiratory shock, pancreatic disease, rheumatoid arthritis and inflammatory connective tissue, respirator dependence/tracheostomy status, transplants, coagulation defects/other specified hematological disorders, hip fracture/dislocation.
p<.001
p<.01
p<.05
Table 2 reports the results of the multivariate regression examining the relationship between patient’s neighborhood disadvantage level (measured by the two HFH ADI groups) and readmission risk, controlling for patient-level demographic and clinical factors. Older (OR=1.27, P=.001) and male (OR=1.38, P=.000) patients had a higher readmission risk compared to their younger and female counterparts; married patients were less likely to have 30-day readmissions (OR=.79, P=.001). All other factors held constant, patients residing in the “more disadvantaged” neighborhoods had significantly higher risks of being readmitted, compared to those living in the “less disadvantaged” neighborhoods (OR=1.42, P=.000).
Table 2.
Multivariate Logistic Regression Results of Relationship between Patient Characteristics and Readmission Risk: Comparing Patients Living in “More Disadvantaged” Neighborhoods and Patients Living in “Less Disadvantaged” Neighborhoods
| Characteristics | OR | 95% CI | p-value | |
|---|---|---|---|---|
| Neighborhood | ||||
| Less disadvantaged (bottom 50%): reference | ||||
| More disadvantaged (top 50%) | 1.42 | 1.23 | 1.63 | ***.000 |
| Age (in years) | 1.27 | 1.10 | 1.47 | **.001 |
| Sex: male | 1.38 | 1.21 | 1.58 | ***.000 |
| Marital Status: married | 0.79 | 0.69 | 0.90 | **.001 |
| Race: Black | 0.95 | 0.81 | 1.10 | .456 |
| Primary Diagnosis at Time of Discharge | ||||
| Congestive heart failure | 1.42 | 1.12 | 1.79 | **.004 |
| Septicemia | 1.17 | 0.86 | 1.59 | .322 |
| Acute myocardial infarction | 1.43 | 1.05 | 1.97 | *.026 |
| Acute cerebrovascular disease | 0.63 | 0.43 | 0.92 | *.016 |
| Complication of device | 1.07 | 0.76 | 1.49 | .702 |
| Chronic obstructive pulmonary disease and bronchiectasis | 1.14 | 0.81 | 1.61 | .443 |
| Urinary tract infections | 1.16 | 0.83 | 1.62 | .392 |
| Cardiac dysrhythmias | 0.89 | 0.60 | 1.31 | .551 |
| Pneumonia | 1.19 | 0.83 | 1.70 | .357 |
| Fluid and electrolyte disorders | 1.02 | 0.71 | 1.48 | .909 |
| Comorbidities | ||||
| Diabetes mellitus | 1.11 | 0.97 | 1.26 | .121 |
| Congestive heart failure | 1.22 | 0.99 | 1.51 | .067 |
| Coronary atherosclerosis/angina, cerebrovascular disease | 0.95 | 0.83 | 1.09 | .476 |
| Specified arrhythmias | 1.13 | 0.98 | 1.31 | .098 |
| Chronic obstructive pulmonary disease | 1.15 | 0.97 | 1.36 | .109 |
| Acute renal failure | 1.51 | 1.24 | 1.83 | ***.000 |
| Cancers | 1.52 | 1.26 | 1.84 | ***.000 |
| Drug/alcohol disorders and psychiatric conditions | 1.12 | 0.93 | 1.35 | .235 |
| Other | 1.56 | 1.36 | 1.79 | ***.000 |
Notes:
The ADI cut point (50% or median) used to classify the neighborhoods (less disadvantaged vs. more disadvantaged) was based on the HFH ADI distribution, rather than the national distribution which was referred to for the purpose of comparison only.
Other comorbid risk factors include: severe infection, other infectious disease & pneumonias, protein-calorie malnutrition, end-stage liver disease, other hematological disorders, hemiplegia, paraplegia, paralysis, functional disability, seizure disorders and convulsions, fibrosis of lung or other chronic lung disorders, dialysis status, ulcers, septicemia/shock, disorders of fluid, electrolyte, acid-base, iron deficiency, cardiorespiratory failure or cardiorespiratory shock, pancreatic disease, rheumatoid arthritis and inflammatory connective tissue, respirator dependence/tracheostomy status, transplants, coagulation defects/other specified hematological disorders, hip fracture/dislocation.
OR is odds ratio; CI is confidence interval.
p<.001
p<.01
p<.05
Results of our exploratory analysis of the top 5% “most disadvantaged” HFH ADI neighborhoods (Table 3) show that patients living in these most extremely socioeconomically challenged HFH neighborhoods were 70 percent more likely to be readmitted than those living in the “less disadvantaged” neighborhoods (OR=1.70, P=.000).
Table 3.
Multivariate Logistic Regression Results of Relationship between Patient Characteristics and Readmission Risk: Comparing Patients Living in “Most Disadvantaged” Neighborhoods and Patients Living in “Less Disadvantaged” Neighborhoods
| Characteristics | OR | 95% CI | p-value | |
|---|---|---|---|---|
| Neighborhood | ||||
| Less disadvantaged (bottom 50%): reference | ||||
| Disadvantaged (45% in between) | 1.39 | 1.21 | 1.60 | ***.000 |
| Most disadvantaged (top 5%) | 1.70 | 1.28 | 2.25 | ***.000 |
| Age (in years) | 1.27 | 1.10 | 1.47 | **.001 |
| Sex: male | 1.39 | 1.22 | 1.58 | ***.000 |
| Marital Status: married | 0.80 | 0.70 | 0.91 | **.001 |
| Race: Black | 0.94 | 0.81 | 1.09 | .408 |
| Primary Diagnosis at Time of Discharge | ||||
| Congestive heart failure | 1.42 | 1.12 | 1.79 | **.004 |
| Septicemia | 1.17 | 0.86 | 1.58 | .330 |
| Acute myocardial infarction | 1.43 | 1.04 | 1.96 | *.027 |
| Acute cerebrovascular disease | 0.63 | 0.43 | 0.92 | *.017 |
| Complication of device | 1.07 | 0.77 | 1.50 | .680 |
| Chronic obstructive pulmonary disease and bronchiectasis | 1.15 | 0.81 | 1.62 | .434 |
| Urinary tract infections | 1.15 | 0.82 | 1.61 | .415 |
| Cardiac dysrhythmias | 0.88 | 0.60 | 1.31 | .535 |
| Pneumonia | 1.19 | 0.83 | 1.70 | .354 |
| Fluid and electrolyte disorders | 1.02 | 0.71 | 1.48 | .904 |
| Comorbidities | ||||
| Diabetes mellitus | 1.11 | 0.97 | 1.26 | .133 |
| Congestive heart failure | 1.22 | 0.99 | 1.52 | .065 |
| Coronary atherosclerosis/angina, cerebrovascular disease | 0.96 | 0.84 | 1.09 | .507 |
| Specified arrhythmias | 1.13 | 0.98 | 1.31 | .100 |
| Chronic obstructive pulmonary disease | 1.15 | 0.97 | 1.37 | .101 |
| Acute renal failure | 1.51 | 1.24 | 1.83 | ***.000 |
| Cancers | 1.53 | 1.27 | 1.84 | ***.000 |
| Drug/alcohol disorders and psychiatric conditions | 1.12 | 0.93 | 1.35 | .234 |
| Other | 1.56 | 1.36 | 1.79 | ***.000 |
Notes:
The ADI cut points (50% or median, 45% in between, and top 5%) used to classify the neighborhoods were based on the HFH ADI distribution, rather than the national distribution which was referred to for the purpose of comparison only.
The “most disadvantaged” neighborhoods were defined as the top 5% highest HFH ADI neighborhoods.
Other comorbid risk factors include: severe infection, other infectious disease & pneumonias, protein-calorie malnutrition, end-stage liver disease, other hematological disorders, hemiplegia, paraplegia, paralysis, functional disability, seizure disorders and convulsions, fibrosis of lung or other chronic lung disorders, dialysis status, ulcers, septicemia/shock, disorders of fluid, electrolyte, acid-base, iron deficiency, cardiorespiratory failure or cardiorespiratory shock, pancreatic disease, rheumatoid arthritis and inflammatory connective tissue, respirator dependence/tracheostomy status, transplants, coagulation defects/other specified hematological disorders, hip fracture/dislocation.
OR is odds ratio; CI is confidence interval.
p<.001
p<.01
p<.05
DISCUSSION
The finding of greatest policy importance is the significant predictive power of the ADI for hospital readmission, in a data set and an analytic model in which clinical predictors and individual-level demographics had already been considered. This finding strongly suggests that area-level or community-level variables can influence health care outcomes such as hospital readmission through causal pathways that are independent of individual-level characteristics. This finding is consistent with other work supporting the important impact neighborhood disadvantage can have on health outcomes.18–21 For example, Joynt and Jha found patients discharged from hospitals in low-income counties had a higher readmission risk compared with those discharged from hospitals in high-income counties.18 Herrin and colleagues extended the study by including more county-level characteristics and concluded that as much as 60% of the variance in hospital readmission rates could be explained by these county-level characteristics.19 Kind and colleagues demonstrated that living in a highly disadvantaged neighborhood increased risk of 30-day readmission as much as having chronic lung disease.20 Hu and Nerenz found that approximately 20% of the variance on CMS “star” ratings for hospitals could be explained by a set of city-level characteristics including poverty, employment, and crime.21 Additional investigation in this area is underway.
Similar to the findings of Kind and colleagues, 20 the effect of neighborhood disadvantage on readmission was not linear. Across the lower part of the HFH ADI distribution, there was no detectable effect on readmission rates. In the upper tail of the distribution reflecting the highest degree of disadvantage, though, the effect was significant. It does not seem to matter whether one lives in a relatively affluent area compared to a middle-class area; the likelihood of readmission is not different. This probably reflects a sort of “minimum necessity” phenomenon. There may be a certain necessary level of supportive services or characteristics in a neighborhood for patients who have been discharged from a hospital. If that minimum level is met, readmission is relatively unlikely and may be driven primarily by individual-level clinical or social factors and/or by actual variation in quality of care. If the minimum level is not met, though, then there is a higher likelihood of readmission even with the other factors taken into account.
The ADI distribution found in this study was quite different from the national distribution, with a significant effect on readmission noted in the top half of the HFH distribution, corresponding to the top 15% of the national ADI distribution. This reflects the “safety net” status of HFH and that it cares for a much more disadvantaged population than the national average. The higher readmission rate in the top half of the HFH distribution replicates the earlier finding about poorer outcomes in the top 15% of the national distribution.20 An additional significant effect was noted in the top 5% of the HFH distribution, suggesting that the most challenged neighborhoods carry even more risk.
Our study had several limitations. First, the data set used in the study is from just one hospital, so the findings reported here may not be directly generalizable to other hospitals or to other communities. The ability to detect an effect of ADI, and the specific shape of the effect across a local ADI distribution, may differ across study contexts. On the other hand, the focus on one hospital eliminates some potential confounding variables that have to be managed in larger multi-hospital studies, such as characteristics of the hospitals’ physician staff or state-level variation in Medicaid eligibility and scope of services (e.g., transportation) paid for by Medicaid.24 Second, even though we considered a full range of variables in our analyses based on the existing literature, we were unable to completely disentangle the effects of patient- and community-level characteristics, due to the complex interplay between them. For example, the effects of clinical factors/comorbidities (and their day-to-day management) and neighborhood characteristics were likely to intertwine. Third, we did not examine the patients who died within 30 days following discharge and how it would affect our study findings. We did not have that information from the CMS dry run data file. The relationship between mortality and readmission has been controversial and studies have mixed findings.26,27 The overall conclusions are that mortality and readmission are not correlated or only weakly but significantly correlated,26 and the correlation can be either positive or negative.27 Fourth, data were unavailable to allow us to examine the specific reasons for readmissions, especially those linked to social factors, either at patient or at community level, such as lack of transportation for follow-up visits. This is an important question to be investigated in future work but is beyond the scope of the present study. And finally, we could have missed some unobserved variables, or some important clinical factors that were unavailable in our data files or we did not identify that could affect patients’ readmission risk.
An accurate and unbiased measure of quality, whether in the context of hospital readmission or any other context in health care, requires careful adjustment of confounding factors that influence the measure but do not have anything to do with the actual quality of care provided. Recent efforts to identify and adjust for those confounders have focused heavily on individual-level patient characteristics, with varying levels of success.2,3,13 The findings of this study suggest that the scope of analysis should expand to include neighborhood- or community-level variables, such as ADI, that may have effects on health care quality metrics that are not only independent of quality of care, but also independent of patient-level factors. Being poor in a high-disadvantage neighborhood or community is not the same experience as being poor in a low-disadvantage neighborhood or community. The effects of both individual-level and community-level variables should be considered in developing risk-adjustment models for health care quality metrics.
Acknowledgments
Drs. Hu and Nerenz’s work received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors; Dr. Kind’s work was supported by the National Institute on Minority Health and Health Disparities of the National Institutes of Health under award number R01MD010243 (PI: Kind). The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the NIH.
Footnotes
Declaration of Conflicting Interests statement: The Authors declare that there is no conflict of interest.
Contributor Information
Jianhui Hu, Research Associate, Center for Health Policy & Health Services Research, Henry Ford Health System, 1 Ford Place, Suite 3A, Detroit, MI 48202, Phone: (313)874-5454, Fax: (313)874-7137, jhu1@hfhs.org
Amy J.H. Kind, Division of Geriatrics, Department of Medicine, University of Wisconsin School of Medicine and Public Health & VA Geriatrics Research Education and Clinical Center (GRECC), William S. Middleton Veteran’s Affairs Hospital, ajk@medicine.wisc.edu
David Nerenz, Henry Ford Health System, dnerenz1@hfhs.org
References
- 1.Joynt K, Jha A. Characteristics of Hospitals Receiving Penalties Under the Hospital Readmissions Reduction Program. JAMA. 2013;309(4):342–343. doi: 10.1001/jama.2012.94856. [DOI] [PubMed] [Google Scholar]
- 2.Centers for Medicare & Medicaid Services. Examining the Potential Effects of Socioeconomic Factors on Star Ratings. Baltimore, MD: September 8, 2015. https://www.cms.gov/Medicare/Prescription-Drug-Coverage/PrescriptionDrugCovGenIn/Downloads/Research-on-the-Impact-of-Socioeconomic-Status-on-Star-Ratingsv1-09082015.pdf (accessed September 11, 2017) [Google Scholar]
- 3.Chen L, Epstein A, Orav E, Filice C, Samson L, Joynt Maddox K. Association of Practice-Level Social and Medical Risk With Performance in the Medicare Physician Value-Based Payment Modifier Program. JAMA. 2017;318(5):453–461. doi: 10.1001/jama.2017.9643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Joynt K, De Lew N, Sheingold S, Conway P, Goodrich K, Epstein A. Should Medicare Value-Based Purchasing Take Social Risk into Account? N Engl J Med. 2017;376(6):510–513. doi: 10.1056/NEJMp1616278. [DOI] [PubMed] [Google Scholar]
- 5.Lipstein S, Dunagan W. The Risks of Not Adjusting Performance Measures for Sociodemographic Factors. Ann Intern Med. 2014;161(8):594–596. doi: 10.7326/M14-1601. [DOI] [PubMed] [Google Scholar]
- 6.Risk adjustment for socioeconomic status or other sociodemographic factors. National Quality Forum. http://www.qualityforum.org/Publications/2014/08/Risk_Adjustment_for_Socioeconomic_Status_or_Other_Sociodemographic_Factors.aspx.
- 7.National Quality Forum. Risk adjustment for socioeconomic status or other sociodemographic factors. http://www.qualityforum.org/Publications/2014/08/Risk_Adjustment_for_Socioeconomic_Status_or_Other_Sociodemographic_Factors.aspx (accessed September 11, 2017)
- 8.National Academies of Sciences, Engineering, and Medicine. Accounting for social risk factors in Medicare payment. Washington, DC: National Academies Press; 2017. [PubMed] [Google Scholar]
- 9.United States Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation. Social Risk Factors and Performance Under Medicare’s Value-Based Purchasing Programs. Washington, DC: December, 2016. https://aspe.hhs.gov/system/files/pdf/253971/ASPESESRTCfull.pdf (accessed September 11, 2017) [Google Scholar]
- 10.Fiscella K, Burstin H, Nerenz D. Quality Measures and Sociodemographic Risk Factors. JAMA. 2014;312(24):2615–2616. doi: 10.1001/jama.2014.15372. [DOI] [PubMed] [Google Scholar]
- 11.National Quality Forum. NQF Statement on Board of Directors Decision Regarding Social Risk Trial. Washongtin, DC: NQF; July 24, 2017. https://www.qualityforum.org/News_And_Resources/Press_Releases/2017/NQF_Statement_on_Board_of_Directors_Decision_Regarding_Social_Risk_Trial.aspx (accessed September 11, 2017) [Google Scholar]
- 12.National Quality Forum. Final Report. Washingon, DC: NQF; Evaluation of the NQF trial period for risk adjustment for social risk factors. July 18, 2017. http://www.qualityforum.org/Publications/2017/07/Social_Risk_Trial_Final_Report.aspx (accessed September 11, 2017) [Google Scholar]
- 13.Barnett M, Hsu J, McWilliams J. Patient Characteristics and Differences in Hospital Readmission Rates. JAMA Internal Medicine. 2015;175(11):1803–1812. doi: 10.1001/jamainternmed.2015.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernheim S, Parzynski C, Horwitz L, et al. Accounting For Patients Socioeconomic Status Does Not Change Hospital Readmission Rates. Health Aff (Millwood) 2016;35(8):1461–1470. doi: 10.1377/hlthaff.2015.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaslavsky AM, Epstein AM. How patients’ sociodemographic characteristics affect comparisons of competing health plans in California on HEDIS quality measures. Int J Qual Health Care. 2005;17(1):67–74. doi: 10.1093/intqhc/mzi005. [DOI] [PubMed] [Google Scholar]
- 16.Young G, Rickles N, Chou C, Raver E. Socioeconomic characteristics of enrollees appear to influence performance scores for Medicare Part D contractors. Health Aff (Millwood) 2014;33(1):140–146. doi: 10.1377/hlthaff.2013.0261. [DOI] [PubMed] [Google Scholar]
- 17.Halfon N. Socioeconomic Influences on Child Health: Building New Ladders of Social Opportunity. JAMA. 2014;311(9):915–917. doi: 10.1001/jama.2014.608. [DOI] [PubMed] [Google Scholar]
- 18.Joynt K, Jha A. Who Has Higher Readmission Rates for Heart Failure, and Why? Implications for Efforts to Improve Care Using Financial Incentives. Circulation: Cardiovascular Quality and Outcomes. 2010;4(1):53–59. doi: 10.1161/CIRCOUTCOMES.110.950964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Herrin J, St Andre J, Kenward K, Joshi M, Audet A, Hines S. Community Factors and Hospital Readmission Rates. Health Serv Res. 2014;50(1):20–39. doi: 10.1111/1475-6773.12177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kind A, Jencks S, Brock J, et al. Neighborhood Socioeconomic Disadvantage and 30-Day Rehospitalization: A retrospective cohort study. Ann Intern Med. 2014;161(11):765–774. doi: 10.7326/M13-2946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu J, Nerenz D. Relationship Between Stress Rankings and the Overall Hospital Star Ratings. JAMA Internal Medicine. 2017;177(1):136–137. doi: 10.1001/jamainternmed.2016.7068. [DOI] [PubMed] [Google Scholar]
- 22.Dennis Wagner, Director, Quality Improvement & Innovation Group at Centers for Medicare and Medicaid Services quoted in his blog: “Exciting news for Everyone with Diabetes Counts (EDC)! A few weeks ago, University of Wisconsin-Madison announced new research findings that will help the Centers for Medicare & Medicaid Services identify and target disparities in diabetes care. This improved population database will aid us in finding underserved populations with high rates of diabetes across the United States. Special thanks to Dr. Amy Kind, associate professor of medicine at University of Wisconsin-Madison School of Medicine and Public Health, for leading the charge in refining the way we can identify neighborhoods of socioeconomic disadvantage.” …. posted March 7, 2017 (https://www.linkedin.com/feed/update/urn:li:activity:6244899965945024512)
- 23.University of Wisconsin-Madison. UW Research To Help Feds Target Diabetes Outreach. 2017 Available at: https://www.med.wisc.edu/news-events/uw-research-to-help-feds-target-diabetes-outreach/50262. Accessed November 1, 2017.
- 24.Hu J, Gonsahn M, Nerenz D. Socioeconomic Status And Readmissions: Evidence From An Urban Teaching Hospital. Health Aff (Millwood) 2014;33(5):778–785. doi: 10.1377/hlthaff.2013.0816. [DOI] [PubMed] [Google Scholar]
- 25.Calvillo–King L, Arnold D, Eubank K, et al. Impact of Social Factors on Risk of Readmission or Mortality in Pneumonia and Heart Failure: Systematic Review. J Gen Intern Med. 2012;28(2):269–282. doi: 10.1007/s11606-012-2235-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krumholz H, Lin Z, Keenan P, et al. Relationship Between Hospital Readmission and Mortality Rates for Patients Hospitalized With Acute Myocardial Infarction, Heart Failure, or Pneumonia. JAMA. 2013;309(6):587–593. doi: 10.1001/jama.2013.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dharmarajan K, Wang Y, Lin Z, et al. Association of Changing Hospital Readmission Rates With Mortality Rates After Hospital Discharge. JAMA. 2017;318(3):270–278. doi: 10.1001/jama.2017.8444. [DOI] [PMC free article] [PubMed] [Google Scholar]


