Abstract
Objective
In a secondary analysis of a randomized controlled trial (RCT) of diabetes reciprocal peer support (RPS), we examined characteristics of peers associated with improvements in their partner’s glycemic control.
Methods
102 adults with diabetes were randomized to the RPS arm (vs. a nurse care management arm). The primary outcome was change in A1c over 6 months. Intermediate outcomes were insulin initiation and peer engagement. A number of baseline characteristics of peers were hypothesized to influence outcomes for their peer, and concordant characteristics of peer dyads were hypothesized that would influence outcomes for both peer partners.
Results
Improvement in A1c was associated with having a peer older than oneself (P<.05) or with higher diabetes-related distress (P<.01). Participants with peers who reported poorer health at baseline had worse glycemic control at follow up (P<.01). Hypothesized concordant characteristics were not associated with A1c improvements. Participants whose peers had a more controlled self-regulation style were more likely to initiate insulin (P<.05).
Discussion
The improved outcomes of peers whose partners were older and reported more diabetes distress at baseline supports the need for further research into the peer characteristics that lead to improved outcomes. This could allow for better matching and more effective partnerships.
Keywords: Peer support, peer characteristics, self management, diabetes, randomized controlled trial
Introduction
Ongoing diabetes self-management support (DSMS) can improve patients’ diabetes outcomes.1,2 Yet, these programs are often costly and challenging in practice. Peer support models – interventions in which patients who have diabetes provide mentorship to or join in reciprocal partnerships with others with diabetes – are a promising strategy for ongoing disease self-management support.3–7 Particularly in low-resource settings, models utilizing peer support have been shown to improve glycemic control among patients with diabetes.3,8–11
However, not all participants benefit equally from these programs. Moreover, it remains unclear which components of successful peer support interventions might be most effective. There may be particular characteristics of peers or similarities between peers associated with greater success in peer DSMS interventions. Success of peer support may depend, for example, on concordance in demographic characteristics, such as age, education level, or race; or concordance of severity of illness, shared self-management challenges, or educational level. It may also depend on other factors related to their chronic condition, such as whether or not they both are on insulin or their peer’s level of confidence in managing their health condition. For example, in a previous study examining the effectiveness of peer coaches in diabetes, coaches who had lower self-efficacy scores for their own diabetes self-management, lower depression scores (less depression), and higher levels of diabetes-related distress were associated with greater improvement in the glycemic control10 of the adults with diabetes they were coaching. Other studies have suggested the importance of peer communication skills such as being autonomy supportive (providing emotional support and options for health behavior change) rather than being bossy or overly directive.4,12 However, to date there is limited evidence on what makes effective reciprocal peers.
Identifying characteristics that affect the success of peer partnerships would improve matching of peers in future interventions, which could lead to improved patient outcomes. Accordingly, the goal of this study was to investigate peer characteristics associated with reductions in participants’ hemoglobin A1c (A1c) in a reciprocal peer support intervention that was found to be more effective than nurse care management in improving participants’ glycemic control in a randomized controlled trial.3 As secondary outcomes, we also examined insulin initiation and level of peer engagement.
Methods
Research Design
To identify whether certain peer characteristics led to significantly greater reductions in A1c, we conducted a secondary analysis on previously collected data from a reciprocal peer support randomized controlled trial (RCT). Full details of the RCT are reported elsewhere.3 Briefly, the study compared reciprocal peer support (RPS) to nurse care management (NCM) for DSMS. The focus of this secondary analysis is on the participants who were randomized to the RPS arm. RPS participants attended an initial 3-hour group session where they were paired with another age-matched participant in the program. The range in age was from 47 to 75 years, and peers were age-matched as closely as possible, with the mean age gap between partners being 3.2 years. Both peer partners were provided brief training in peer communication skills and in ‘action planning’.13 Peer pairs were encouraged to contact their peer at least once per week using a computer-facilitated telephone platform that noted frequency and duration of calls and allowed participants to call each other without sharing personal telephone information. Of note, peers were encouraged to both give and receive support to each other—it was not a hierarchical model in which one peer served as a peer coach or mentor to the other. If no peer calls were attempted for 7 days, an automated reminder phone call was sent to the participants. In addition, participants were offered 3 optional 1.5 hour group sessions at 1, 3, and 6 months that were participant-driven. Participants in the RPS arm of the RCT had significantly greater improvement in glycemic control after 6 months compared to the NCM arm (change in A1c of −0.29% (RPS) vs +0.29% (NCM), difference in change between groups of −0.58% (P=0.004) and were more likely to initiate insulin use (8 RPS patients compared to 1 NCM patient (P=.02)).
Setting and Participants
The study was based in 2 Midwestern U.S. Department of Veterans Affairs health care facilities. Patients were identified from electronic medical records using a validated algorithm.14 Participants in the secondary analysis included men with A1c levels greater than 7.5% who were randomized to the RPS arm, completed baseline and 6 month follow up surveys. The RPS participants’ baseline characteristics are presented in Table 1. Of the 113 RPS participants, included table 1, 11 had partners who did not complete both surveys, and therefore were excluded from the secondary analysis, resulting in the 102 participants included in table 1.
Table 1.
Reciprocal Peer Support Participant Baseline Characteristics* (N = 102)
| N or Mean | % or SD | |
|---|---|---|
| Age in years | 62 | 6 |
| Race or Ethnicity | ||
| White, non-Hispanic | 82 | 81.2% |
| Black, non-Hispanic | 8 | 7.9% |
| Hispanic | 3 | 3.0% |
| Other | 8 | 7.9% |
| Education | ||
| Less than high school/GED | 5 | 4.9% |
| High school/GED | 24 | 23.5% |
| Some technical/vocational school | 10 | 9.8% |
| Some college or more | 63 | 61.8% |
| Low health literacy | 50 | 49.0% |
| Diabetes Social Support† | 56 | 24.2 |
| Diabetes Distress‡ | 25 | 15.3 |
| Autonomous self-regulation style | 84 | 15.9 |
| Controlled self-regulation style | 41 | 27.4 |
| Diabetes self-efficacy | 4 | 0.9 |
| Fair/poor in SRHS++ | 46 | 45.1% |
Baseline characteristics are the results from surveys and blood draws taken during the first group session.
“Diabetes Support” was assessed using 6 questions from the Diabetes Support Scale.18 Each question had 6 answer choices, ranging from “Strongly Disagree” to “Strongly Agree” The answers were scored from 0 to 5 points, with higher scores indicating higher levels of diabetes social support, and the total score was calculated as a percentage of possible points.
”Diabetes Distress” was assessed using 14 questions from the Diabetes Distress Scale.15 Each question had 5 answer choices, ranging from “Not a problem” to “Serious problem”. The answers were scored from 0 to 4 points, with higher scores indicating higher levels of distress, and the total score was calculated as a percentage of possible points.
“Self Reported Health Status (SRHS)” was assessed using 1 question, “In general how would you report your health”, and 5 answer choices, scored from 1 to 5 points, with a lower score indicating better self-reported health. This 5 category variable was dichotomized into 2 categories for this analysis: either “Fair/poor” or Good/very good/excellent”
Peer Characteristics
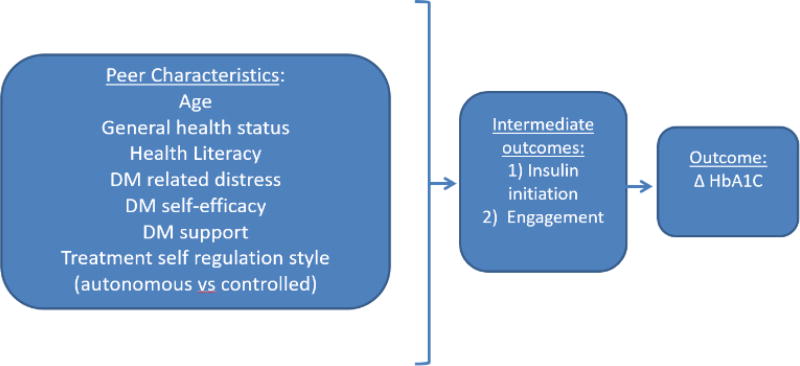
Based on previous literature10 and clinical experience, characteristics of each peer that we hypothesized might be associated with improvements in their partners’ A1c levels included age, self-reported health status, health literacy, diabetes care self-efficacy, diabetes-specific distress, perceived diabetes social support (DSS), and level of autonomous motivation for diabetes care (see figure 1). To determine peer characteristics, we used the diabetes distress scale,15 the self-efficacy scale,16,17 the diabetes support scale,18 the health literacy scale,19 and self-reported health status. We hypothesized that concordant (similar) characteristics between the two matched peers (concordant characteristics) that might improve outcomes for both peer partners included age, race/ethnicity, and education. More specifically, they were considered concordant if the age gap was less than five years, if race/ethnicity was the same, and if education level was the same. We also examined the intermediate outcomes of the influence of peer characteristics on insulin initiation by 6 months (gathered through self-report and confirmed by medical review) and peer engagement (total calls connected and numbers of minutes talked), as both of these intermediate outcomes had been associated with improved glycemic control in the RCT.20
Figure 1.
Hypothesized model: relationship between peer characteristics and change in HbA1c
Statistical Analysis
Using our pre-specified independent variables, for the primary analysis to examine the extent to which peer characteristics and concordance of peer dyad characteristics were significantly associated with change in A1c, we used multivariate linear regression by adjusting for patients’ own age, race/ethnicity, and education. To assess whether peer characteristics were significantly related to our intermediate outcomes (insulin initiation and peer engagement), we used bivariate logistic regression. Coefficients were obtained from the linear regressions, and risk ratios were obtained from the estimations in the logistic regressions.21 We clustered residual variance structures at the dyad level to obtain robust standard errors.22
Results
Characteristics of the RPS participants are presented in Table 1. The associations between peer characteristics and changes in A1c are presented in Table 2. Having a peer older than oneself (P<.05) or a peer with high levels of diabetes-related distress (P<.01) was associated with significant improvements in participants’ A1c. Participants with peers who reported poorer health at baseline had worse glycemic control at follow up (P<.01). Concordance in the hypothesized characteristics between peers was not associated with significant improvements in A1c. In 16 of the 51 pairs, 1 person experienced an improvement in A1c; in 11 of the pairs both participants experienced an improvement in A1c, and in 24 pairs neither person experienced an improvement in A1c. For intermediate outcomes, participants who had peers with a controlled self-regulation style were significantly more likely to initiate insulin (P<.05).
Table 2.
Peer Characteristics Associated with 6-month Change in HBA1C (6 month - baseline), N=1021
| N=102 | ||
|---|---|---|
|
| ||
| Coefficient | 95%CI | |
| Peer characteristics | ||
| Age | −0.065* | (−0.124, −0.006) |
| Fair/Poor in SRHS2 | 0.552** | (0.151, 0.953) |
| Health Literacy | −0.178 | (−0.569, 0.214) |
| Diabetes Distress (DDS) | −0.016** | (−0.028, −0.004) |
| Diabetes Self-efficacy | 0.149 | (−0.019, 0.317) |
| Diabetes Support | 0.002 | (−0.010, 0.013) |
| Autonomous Self-regulation Style | 0.003 | (−0.013, 0.018) |
| Controlled Self-regulation Style | 0.002 | (−0.007, 0.011) |
11 participants excluded from analysis because they did not have peer data
SRHS (Self-Reported Health Status): dichotomized outcome (0- good/excellent; 1- fair/poor)
p<.05
p<.01
Discussion
This secondary analysis examined the influence of peer characteristics and concordance between peer characteristics on A1c to fill the gap in knowledge of what makes a successful peer partnership for diabetes interventions utilizing peer support. We found that participants with peers who were older or reported higher levels of diabetes distress experienced improved primary A1c outcomes. While poorer health status of peers was associated with worse glycemic control at follow-up of their partners, higher levels of diabetes-specific distress at baseline were associated with improvements in their partner’s A1c. None of our hypothesized concordant characteristics were associated with improved A1c levels. To our knowledge, this is the first study to look at the influence of peer characteristics with reciprocal peer partners instead of peer coaches.
Our study builds on prior research in several key ways. Our findings that peers matched with another peer who reported higher diabetes-specific distress had greater improvements in model, diabetes patients with coaches who reported higher baseline diabetes distress also achieved greater improvements in A1c levels over the six-month intervention than patients with coaches with less diabetes distress.10 In their RCT of a peer coach model, diabetes patients with coaches who reported higher baseline diabetes distress also achieved greater improvements in A1c levels over the six-month intervention than patients with coaches with less diabetes distress.10 In their study, participants coached by coaches with lower reported self-efficacy than other coaches also had greater improvements in A1c, a finding that was not replicated in our study. In our study the baseline self-efficacy of one’s peer partner was not associated with significant A1c gains. Rogers et al hypothesized that peer coaches who had themselves struggled with diabetes self-management were more approachable, empathetic and open to patients developing their own solutions to manage their diabetes, which is a core technique of motivational interviewing. In our intervention in which both peers were giving and receiving support unlike the hierarchical peer coach model tested in Rogers’ et al’s RCT, peers who had less diabetes distress than their partners may have gained increased motivation to manage their diabetes from being able to help their peer with more distress from managing their diabetes. This hypothesis is supported by the large body of research on how helping others can help inspire improvements in one’s own health behaviors and outcomes, a hypothesis that underpinned the design of this mutual peer support RCT.3,23,24
Our intermediate outcomes showed that participants with peers who had a controlled self-regulation style were more likely to initiate insulin. This merits further study to better understand the influence that a peer’s self-regulation style may have on a participant’s health behaviors, and whether peers with a controlled self-regulation style should be strategically paired with participants who would benefit from insulin initiation.
While this study found that concordance of hypothesized peer characteristics had no effect on A1c, this area merits further exploration using other characteristics and diabetes-related outcomes, such as the influence of mutual areas of interest or other shared experiences on peer rapport building and subsequent intervention outcomes. In the case of Veteran peers, it is possible that shared military service, deployment location or concordance of other service-related experiences may influence the effectiveness of peer partnerships and contribute to improved outcomes. Moreover, language and race concordance between peer navigators and their assigned patients led to more timely care among women who had breast or cervical cancer screening abnormalities.25
Our study had limitations. Among these is the small sample size of the population. Moreover, in this VA study, the sample included only male Veterans who were receiving care in the VA Health System. Therefore, our findings may not generalize to other more diverse populations.
This study supports the value of conducting further investigation into the characteristics and the concordance in characteristics between peer dyads that lead to the biggest improvements in diabetes-related outcomes. A better understanding of the characteristics that make a successful peer relationship may improve peer matching and lead to greater improvements in participant outcomes in peer support DSMS programs.
Acknowledgments
Support: Supported by the Michigan Center for Diabetes Translation Research (NIDDK) P30DK092926).
Footnotes
Conflict of interest statement
The authors have no conflicts to disclose.
Prior presentations: None
Contributor Information
Elizabeth Kaselitz, Victor Vaughn Building, 1111 Catherine St., Ann Arbor, MI 48104, phone number: (248) 343-8841, fax number: 734-615-6300, emaccorm@umich.edu.
Megha Shah, 4500 N. Shallowford Rd., Dunwoody, GA 30338, 404-778-6920, meghakshah@gmail.com.
Hwajung Choi, 2800 Plymouth Rd., Ann Arbor, MI 48109, 734-647-0183, hwajungc@med.umich.edu.
Michele Heisler, 2800 Plymouth Rd., Ann Arbor, MI 48109, 734-845-3504, mheisler@umich.edu.
References
- 1.Qi L, Liu Q, Qi X, Wu N, Tang W, Xiong H. Effectiveness of peer support for improving glycaemic control in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. BMC public health. 2015;15(1):471. doi: 10.1186/s12889-015-1798-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang TS, Funnell MM, Noorulla S, Oh M, Brown MB. Sustaining short-term improvements over the long-term: results from a 2-year diabetes self-management support (DSMS) intervention. Diabetes research and clinical practice. 2012;95(1):85–92. doi: 10.1016/j.diabres.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Heisler M, Vijan S, Makki F, Piette JD. Diabetes control with reciprocal peer support versus nurse care management: a randomized trial. Ann Intern Med. 2010;153(8):507–515. doi: 10.7326/0003-4819-153-8-201010190-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fisher EB, Ballesteros J, Bhushan N, et al. Key Features Of Peer Support In Chronic Disease Prevention And Management. Health affairs. 2015;34(9):1523–1530. doi: 10.1377/hlthaff.2015.0365. [DOI] [PubMed] [Google Scholar]
- 5.Long JA, Jahnle EC, Richardson DM, Loewenstein G, Volpp KG. Peer mentoring and financial incentives to improve glucose control in African American veterans: a randomized trial. Ann Intern Med. 2012;156(6):416–424. doi: 10.1059/0003-4819-156-6-201203200-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dale J, Caramlau IO, Lindenmeyer A, Williams SM. Peer support telephone calls for improving health (review) The Cochrane Database of Systematic Reviews. 2008 doi: 10.1002/14651858.CD006903.pub2. The Cochrane Library(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Small N, Blickem C, Blakeman T, Panagioti M, Chew-Graham CA, Bower P. Telephone based self-management support by 'lay health workers' and 'peer support workers' to prevent and manage vascular diseases: a systematic review and meta-analysis. BMC health services research. 2013;13:533. doi: 10.1186/1472-6963-13-533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thom DH, Ghorob A, Hessler D, De Vore D, Chen E, Bodenheimer TA. Impact of peer health coaching on glycemic control in low-income patients with diabetes: a randomized controlled trial. Annals of family medicine. 2013;11(2):137–144. doi: 10.1370/afm.1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang TS, Funnell M, Sinco B, et al. Comparative effectiveness of peer leaders and community health workers in diabetes self-management support: results of a randomized controlled trial. Diabetes care. 2014;37(6):1525–1534. doi: 10.2337/dc13-2161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogers EA, Hessler DM, Bodenheimer TS, Ghorob A, Vittinghoff E, Thom DH. Diabetes peer coaching: do "better patients" make better coaches? The Diabetes educator. 2014;40(1):107–115. doi: 10.1177/0145721713513178. [DOI] [PubMed] [Google Scholar]
- 11.Moskowitz D, Thom DH, Hessler D, Ghorob A, Bodenheimer T. Peer coaching to improve diabetes self-management: which patients benefit most? Journal of general internal medicine. 2013;28(7):938–942. doi: 10.1007/s11606-013-2367-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leahey TM, Wing RR. A randomized controlled pilot study testing three types of health coaches for obesity treatment: Professional, peer, and mentor. Obesity. 2013;21(5):928–934. doi: 10.1038/oby.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Handley M, MacGregor K, Schillinger D, Sharifi C, Wong S, Bodenheimer T. Using action plans to help primary care patients adopt healthy behaviors: a descriptive study. J Am Board Fam Med. 2006;19(3):224–231. doi: 10.3122/jabfm.19.3.224. [DOI] [PubMed] [Google Scholar]
- 14.Heisler M, Bouknight RR, Hayward RA, Smith DM, Kerr EA. The relative importance of physician communication, participatory decision making, and patient understanding in diabetes self-management. Journal of general internal medicine. 2002;17(4):243–252. doi: 10.1046/j.1525-1497.2002.10905.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polonsky WH, Fisher L, Earles J, et al. Assessing psychosocial distress in diabetes: development of the diabetes distress scale. Diabetes care. 2005;28(3):626–631. doi: 10.2337/diacare.28.3.626. [DOI] [PubMed] [Google Scholar]
- 16.Williams GC, McGregor HA, King D, Nelson CC, Glasgow RE. Variation in perceived competence, glycemic control, and patient satisfaction: relationship to autonomy support from physicians. Patient education and counseling. 2005;57(1):39–45. doi: 10.1016/j.pec.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 17.Schwarzer R, Jerusalem M. Generalized Self-Efficacy Scale. In: Weinman J, Wright S, Johnson M, editors. Measures in health psychology: A user's portfolio, Causal and control beliefs. Windsor, England: NFER-NELSON; 1995. pp. 35–37. [Google Scholar]
- 18.Gallant MP. The influence of social support on chronic illness self-management: a review and directions for research. Health education & behavior : the official publication of the Society for Public Health Education. 2003;30(2):170–195. doi: 10.1177/1090198102251030. [DOI] [PubMed] [Google Scholar]
- 19.Chew LD, Griffin JM, Partin MR, et al. Validation of screening questions for limited health literacy in a large VA outpatient population. Journal of general internal medicine. 2008;23(5):561–566. doi: 10.1007/s11606-008-0520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Piette JD, Resnicow K, Choi H, Heisler M. A diabetes peer support intervention that improved glycemic control: mediators and moderators of intervention effectiveness. Chronic illness. 2013;9(4):258–267. doi: 10.1177/1742395313476522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norton EC, Miller MM, Kleinman LC. Computing adjusted risk ratios and risk differences in Stata. 2001;1(1):1–17. The Stata Journal. [Google Scholar]
- 22.Rogers W. Regression standard errors in clustered samples. Vol Bull 3. 1994;(13) Stata Tech. [Google Scholar]
- 23.Brown SL, Smith DM, Schulz R, et al. Caregiving behavior is associated with decreased mortality risk. Psychological science. 2009;20(4):488–494. doi: 10.1111/j.1467-9280.2009.02323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morrow-Howell N, Hong SI, Tang F. Who benefits from volunteering? Variations in perceived benefits. The Gerontologist. 2009;49(1):91–102. doi: 10.1093/geront/gnp007. [DOI] [PubMed] [Google Scholar]
- 25.Charlot M, Santana MC, Chen CA, et al. Impact of patient and navigator race and language concordance on care after cancer screening abnormalities. Cancer. 2015;121(9):1477–1483. doi: 10.1002/cncr.29221. [DOI] [PMC free article] [PubMed] [Google Scholar]