Abstract
The use of NADH- and NADPH-dependent ketoreductases to access enantioenriched pharmaceutical building blocks is reported. Seven structurally diverse synthons are obtained, including those for atomoxetine (KRED 132), talampanel (RS1-ADH and CPADH), Dolastatin (KRED 132) and fluoxetine (KRED 108/132). Ethanol may be used as stoichiometric reductant, regenerating both nicotinamide cofactors, particularly under four-electron redox conditions. Its favorable thermodynamic and economic profile, coupled with its advantageous dual cosolvent role, suggests a new application for biomass-derived ethanol.
As has been pointed out in a recent overview from the Merck Process Group,1 advances in ketoreductase (KRED or alcohol dehydrogenase = ADH) technology have increased their potential for process chemistry. Asymmetric enzymatic reductions, ex vivo, are now more easily investigated in the research laboratory, and may be optimized there, under controlled conditions, offering a viable and complementary alternative to in vivo approaches, for example, in genetically engineered yeast2 or E. coli.3 The ex vivo system circumvents issues of substrate, product and cosolvent toxicity, provided that enzyme activity and enantioselectivity are preserved.
We have a standing interest in the use of enzymes in asymmetric synthesis, for example, to access enantiomerically enriched podophyllum lignans4 or quaternary, α-vinyl amino acids.5 More recently, that focus has turned to ADH’s, as catalytic reporting enzymes to facilitate the evaluation of organometallic catalysts via ISES (In Situ Enzymatic Screening).5, 6 Parallel to these studies, we have undertaken to exploit ketoreductases in target-directed asymmetric synthesis. Indeed, the repertoire of enzymes in modern asymmetric synthesis continues to expand, including lipases,7 amidases,8 amine oxidases,9 alcohol10 and amine DH’s,11 epoxide hydrolases12 and aldolases,13 among others.14
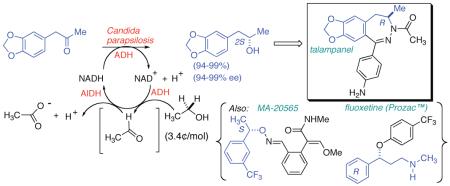
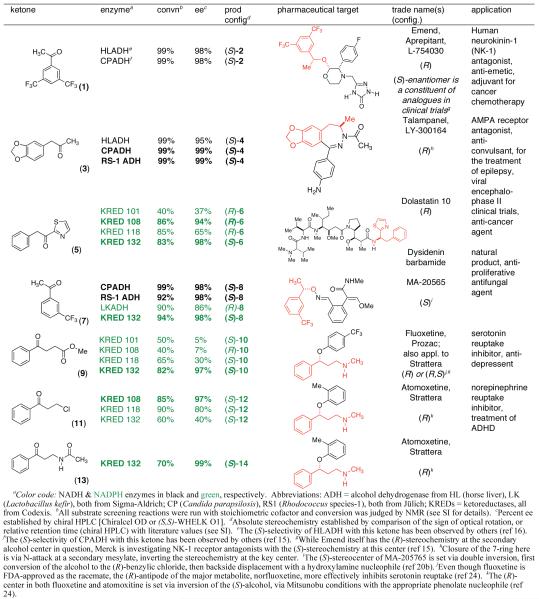
In this work, we have focused upon an array of ketones, the asymmetric reduction of which provides valuable pharmaceutical building blocks. In Table 1, each chiral secondary alcohol product is mapped (red shading) onto the pharmaceutical for which it is a synthon. The Aprepitant-leading ketone 1, served as a model for our ex vivo conditions, giving high (S)-selectivity with CPADH and HLADH, consistent with reports from Merck15 and Rhodia,16 respectively. The second ketone screened serves as the substrate for a classic biocatalytic process (Z. rouxi whole cell route-Zmijewski group at Lilly17) for the production of Talampanel. Our screen identified two new DH’s here, CPADH and RS-1 ADH, each of which also gives the correct antipode (S)-4, with high selectivity.
Table 1.
Asymmetric Ketoreductase-Mediated Access to Pharmaceutical Building Blocks
Ketones 5 and 7, respectively, are precursors to building blocks for the promising chemotherapeutic candidate, Dolastatin 10, and Mitsubishi’s broad spectrum fungicide MA-20565, respectively. In the former case, Genet has reported the use of stoichiometric DIP-Cl (92% ee),18 whereas Masui employs a diphenylprolinol-ligated borane reagent (92% ee).19 The highly enantioselective reductions seen here (KREDs 108 and 132) open up alternative “green” processes. Similarly, while both Ru(II)-diamine20 and Rh-diamine-based21 asymmetric hydrogenations of 7 have been reported, reductions with CPADH, RS-1 ADH and KRED 132, uncovered in these studies, provide viable biocatalytic alternatives.
The final three entries (9, 11, 13) in Table 1 are precursors to either (R)-Strattera or (R)-Fluoxetine. While there are isolated reports of whole cell procedures for the asymmetric carbonyl reduction of 11, either with Saccharomyces22 or Rhodotorula23 species, we find no previous literature descriptions of asymmetric biocatalytic reductions of either 9 or 13. In this regard, the success we have had with KRED 132, in both cases, is quite notable. The ee’s are certainly competitive with those seen using Itsuno-Corey oxazaborolidine reduction (Senanayake),24 in the former case, or Pd(II)-sparteine-mediated oxidative kinetic resolution (Stoltz),25 in the latter.
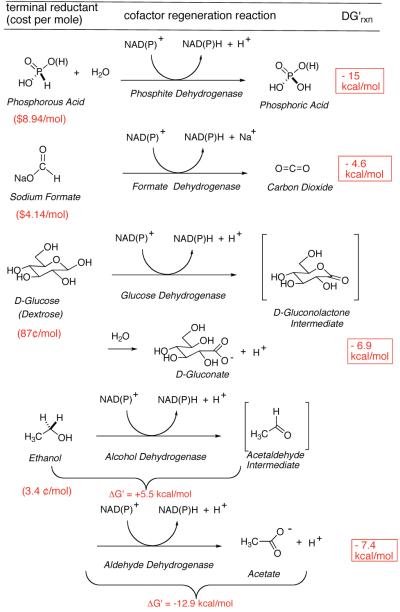
With a half dozen promising new DH-based asymmetric reductions, in hand, we next set about to examine cofactor regeneration. The most commonly used nicotinamide-regenerating reagents, with favorable thermodynamics, are collected in Figure 1, and compared with EtOH. Note that van der Donk and Zhao26 have recently opened the door to phosphite-based reductions, with the most favorable redox potential of the group. While Wong and Whitesides27 established the potential for using EtOH in biocatalytic reductions with water soluble substrates, use of this reductant for chemoenzymatic synthesis has lagged behind. However, EtOH is attactive here in (a) having a favorable redox potential, (b) being economically priced and readily available from the biomass fermentation stream, and (c) potentially serving a dual role as organic cosolvent. Regarding the first point, employing EtOH as a four electron reductant provides for more favorable thermodynamics, which result from the highly exergonic reduction of NAD(P) with acetaldehyde, provided that aldehyde DH (AlDH) activity is present.
Figure 1.
Thermodynamics of nicotinamide cofactor regeneration – Tunability of the ethanol reductant.
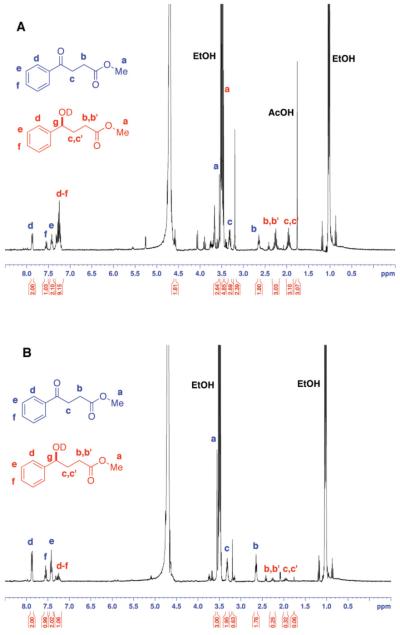
This tunability of the EtOH reductant was examined in a model NMR experiment (Figure 2), with KRED 132 and ketone 9. KRED 132 requires NADPH. We have found that LKADH can effectively be used to oxidize EtOH with NADP. In our hands, yeast AlDH also efficiently utilizes NADP. So, this LKADH/YAlDH couple was employed to access the full four electron reducing capacity of EtOH (panel A), and compared with the reaction under two electron redox conditions (no YAlDH, panel B, Le Chatelier effect alone). In fact, the reduction run under four electron reducing conditions proceeds much more rapidly. As expected, one sees the clear AcOH signature in the former case, attesting to the four electron redox cycle in play. Table 2 illustrates the use of these four electron conditions across three different substrates and four different DH’s at the mmol scale.
Figure 2.
Comparison of the KRED-132-mediated reduction of ketone 9 with NADPH (@ 2 mol %) regeneration using LKADH (50 mM KPO4 in D2O, pD 7.5; 300 rpm, 30 °C, 3 h), both with (panel A) and without (panel B) YAlDH (see Supporting Information for details). Note the increased conversion and AcOH production under four electron reduction conditions.
Table 2. Biocatalytic Reductions @ the mmol Scale – Ethanol as Four Electron Reductant.
| chiral product | ADH | regen system |
cofactor (mol %) |
yield | ee |
|---|---|---|---|---|---|
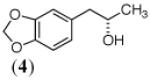
|
CP- ADH |
YADH/ YAlDH |
NAD+ (0.4) |
89% | 94% (S) |
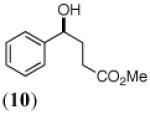
|
KRED 132 |
LK ADH/ YAlDH |
NADP+ (1) |
86% | 96% (S) |
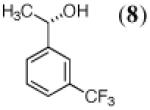
|
RS-1 ADH |
YADH/ YAlDH |
NAD+ (1) |
98% | 99% (S) |
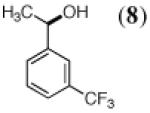
|
LK ADH |
(LK ADH)/ YAlDH |
NADP+ (2) |
64% | 86% (R) |
All reductions were performed on a 1 mmol scale at 30 °C, 300 rpm, pH 7.5 with the cofactor regeneration systems shown. See Supporting Information for details.
In summary, the first viable ketoreductase-based entries into secondary alcohol building blocks for Dolastatin 10 (5), Prozac (9) and Strattera (13) are presented here, as are new biocatalytic entries into building blocks for Talampanel (3) and MA-20565 (7). The viability of using biomass-derived EtOH for cofactor regeneration is examined, and the advantage of using four electron redox cycles in such processes is demonstrated. Future studies will further probe the scope, limitations and optimal conditions for such “green” alternatives to transition metal- or boron hydride-based chiral carbonyl reductants for asymmetric process chemistry.
Supplementary Material
Acknowledgment
Support from the NSF (CHE-0616840), Nebraska Center for Energy Sciences Research and Nebraska UCARE (fellowship to RWC) is gratefully acknowledged. Thomas Daußmann and Pascal Dünkelmann (Jülich Chiral Solutions) are thanked for providing CPADH and RS1-ADH.
Footnotes
Supporting Information Available. Details of the synthetic and enzymatic chemistry, and spectroscopic and chiral HPLC characterization of products. This material is available free of charge via the internet at http://pubs.acs.org.
References
- (1). Moore JC Pollard DJ Kosjek B Devine PN Acc. Chem. Res 2007. 40 1412–1419 [DOI] [PubMed] [Google Scholar]
- (2). Kaluzna IA Feske BD Wittayanan W Ghiviriga I Stewart JD J. Org. Chem 2005. 70 342–345 [DOI] [PubMed] [Google Scholar]
- (3) (a). Li W Xie D Frost JW Niu W J. Am. Chem. Soc 2005. 127 2874–2882 [DOI] [PubMed] [Google Scholar]; (b) Draths KM Frost JW Biotechnol. Progress 2002. 18 201–211 [DOI] [PubMed] [Google Scholar]
- (4) (a). Berkowitz DB Choi S Maeng J-H J. Org. Chem 2000. 65 847–860 [DOI] [PubMed] [Google Scholar]; (b) Berkowitz DB Hartung RE Choi S Tetrahedron: Asymmetry 1999. 10 4513–4520 [Google Scholar]; (c) Berkowitz DB Maeng J-H Dantzig AH Shepard RL Norman BH J. Am. Chem. Soc 1996. 118 9426–9427 [Google Scholar]
- (5). Berkowitz DB Pumphrey JA Shen Q Tetrahedron Lett 1994. 35 8743–8746 [Google Scholar]
- (6) (a). Dey S Powell DR Hu C Berkowitz DB Angew. Chem., Int. Ed 2007. 46 7010–7014 [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Dey S Karukurichi KR Shen W Berkowitz DB J. Am. Chem. Soc 2005. 127 8610–8611 [DOI] [PubMed] [Google Scholar]; (c) Berkowitz DB Maiti G Org. Lett 2004. 6 2661–2664 [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Berkowitz DB Bose M Choi S Angew. Chem., Int. Ed 2002. 41 1603–1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7) (a). Fluxa VS Wahler D Reymond J-L Nat. Protoc 2008. 3 1270–1277 [DOI] [PubMed] [Google Scholar]; (b) Boettcher D Bornscheuer UT Nat. Protoc 2006. 1 2340–2343 [DOI] [PubMed] [Google Scholar]; (c) Reetz MT Bocola M Carballeira JD Zha D Vogel A Angew. Chem., Int. Ed 2005. 44 4192–4196 [DOI] [PubMed] [Google Scholar]; (c) Qian Z Lutz S J. Am. Chem. Soc 2005. 127 13466–13467 [DOI] [PubMed] [Google Scholar]
- (8). Savile CK Magloire VP Kazlauskas RJ J. Am. Chem. Soc 2005. 127 2104–2113 [DOI] [PubMed] [Google Scholar]
- (9). Dunsmore CJ Carr R Fleming T Turner NJ J. Am. Chem. Soc 2006. 128 2224–2225 [DOI] [PubMed] [Google Scholar]
- (10) (a). Zhu D Yang Y Majkowicz S Pan TH-Y Kantardjieff K Hua L Org. Lett 2008. 10 525–528 [DOI] [PubMed] [Google Scholar]; (b) Zhu D Ankati H Mukherjee C Yang Y Biehl ER Hua L Org. Lett 2007. 9 2561–2563 [DOI] [PubMed] [Google Scholar]; (c) Voss CV Gruber CC Kroutil W Angew. Chem., Int. Ed 2008. 47 741–745 [DOI] [PubMed] [Google Scholar]; (d) Kalaitzakis D Rozzell JD Kambourakis S Smonou I Org. Lett 2005. 7 4799–4801 [DOI] [PubMed] [Google Scholar]
- (11). Hummel W Kuzu M Geueke B Org. Lett 2003. 5 3649–3650 [DOI] [PubMed] [Google Scholar]
- (12) (a). Bottalla A-L Ibrahim-Ouali M Santelli M Furstoss R Archelas A Adv. Synth. Catal 2007. 349 1102–1110 [Google Scholar]; (b) Reetz MT Wang L-W Bocola M Angew. Chem., Int. Ed 2006. 45 1236–1241 [DOI] [PubMed] [Google Scholar]; (c) Edegger K Mayer SF Steinreiber A Faber K Tetrahedron 2004. 60 583–588 [Google Scholar]
- (13). Dean SM Greenberg WA Wong C-H Adv. Synth. Catal 2007. 349 1308–1320 [Google Scholar]
- (14). Voelkert M Koul S Mueller GH Lehnig M Waldmann H J. Org. Chem 2002. 67 6902–6910 [DOI] [PubMed] [Google Scholar]
- (15). Pollard D Truppo M Pollard J Chen C-Y Moore J Tetrahedron: Asymmetry 2006. 17 554–559 [Google Scholar]
- (16). Gelo-Pujic M Le Guyader F Schlama T Tetrahedron: Asymmetry 2006. 17 2000–2005 [Google Scholar]
- (17). Anderson BA Hansen MM Harkness AR Henry CL Vicenzi JT Zmijewski MJ J. Am. Chem. Soc 1995. 117 12358–12359 [Google Scholar]
- (18). Mordant C Reymond S Tone H Lavergne D Touati R Ben Hassine B Ratovelomanana-Vidal V Genet J-P Tetrahedron 2007. 63 6115–6123 [Google Scholar]
- (19). Masui M Shioiri T Synlett 1997. 273–274 [Google Scholar]
- (20) (a). Ahlford K Zaitsev AB Ekstroem J Adolfsson H Synlett 2007. 2541–2544 [Google Scholar]; (b) Tanaka K Katsurada M Ohno F Shiga Y Oda M Miyagi M Takehara J Okano K J. Org. Chem 2000. 65 432–437 [DOI] [PubMed] [Google Scholar]
- (21). Matharu DS Morris DJ Clarkson GJ Wills M Chem. Commun 2006. 3232–3234 [DOI] [PubMed] [Google Scholar]
- (22) (a). Ou Z Wu J Yang L Cen P Kor. J. Chem. Enj 2008. 25 124–128 [Google Scholar]; (b) Fronza G Fuganti C Grasselli P Mele A J. Org. Chem 1991. 56 6019–6023 [DOI] [PubMed] [Google Scholar]
- (23). Yang W Xu J-H Xie Y Xu Y Zhao G Lin G-Q Tetrahedron: Asymmetry 2006. 17 1769–1774 [Google Scholar]
- (24). Hilborn JW Lu Z-H Jurgens AR Fang QK Byers P Wald SA Senanayake CH Tetrahedron Lett 2001. 42 8919–8921 [Google Scholar]
- (25). Caspi DD Ebner DC Bagdanoff JT Stoltz BM Adv. Synth. Catal 2004. 346 185–189 [Google Scholar]
- (26). Woodyer R Zhao H van der Donk WA FEBS J 2005. 272 3816–27 [DOI] [PubMed] [Google Scholar]
- (27). Wong CH Whitesides GM J. Am. Chem. Soc 1983. 105 5012–14 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.