Abstract
Objective
To evaluate the effect of overweight and obesity on outcomes and resource use among patients with sepsis in the pediatric intensive care unit (PICU).
Design
Retrospective analysis of clinical characteristics, resource use, and mortality among children 0 to 20 years of age admitted to the C.S. MottChildren’s Hospital PICU (University of Michigan) between January 2009 and December 2015, with a diagnostic code for sepsis at admission (based on International Classification of Diseases, Ninth Revision-Clinical Modification codes) and with weight and height measurements at PICU admission.
Measurements and Main Results
A total of 454 participants met the inclusion criteria. Seventy-six were categorized as underweight (body mass index [BMI] percentile <5th) and were excluded, which left a final sample size of 378 participants. Children with a BMI ≥5th and <85th percentiles for age were categorized as normal weight and those with a BMI ≥85th percentile as overweight/obese. After descriptive and bivariate analyses, multivariate regression methods were used to assess the independent effect of obesity status on mortality and the use of PICU technology after adjustment for patient age and illness severity at admission. Of the 378 patients studied, 41.3% were overweight/obese. There was no difference in microbiologic etiology of sepsis (P = .36), median PICU length of stay in days (5.4 vs 5.6; P = .61), or PICU mortality (6.4% vs 7.2%; P = .76) by weight status. The use of specialized PICU technology including extracorporeal membrane oxygenation (odds ratio [OR]: 2.77, 95% confidence interval [CI]:1.13–6.79) and continuous renal replacement therapy (OR: 4.58, 95% CI: 1.16–18.0) was higher among overweight/obese patients, compared with normal weight patients.
Conclusions
Although PICU mortality and length of stay were similar for obese–overweight patients and normal weight critically ill children with sepsis, there was significantly higher use of specialized organ-supportive technology among obese patients, likely indicating higher occurrence of multiple organ dysfunction.
Keywords: overweight, obesity, pediatrics, sepsis, intensive care unit, morbidity, mortality
Background
Rates of childhood obesity have reached epidemic proportions. In 2014, 17% of children and adolescents (2–19 years of age) in the United States were obese, with body mass index (BMI) ≥95th percentile.1,2 Given this high prevalence, it is important to evaluate the short- and long-term sequelae of childhood obesity. Although heightened risk of metabolic disease has been associated with obesity, there are multiple nonmetabolic diseases that obese children are at a risk of including infection,3 obstructive sleep apnea, cancer, orthopedic complications, psychosocial impairment, and autoimmunity.4–6 Obesity is associated with chronic low-grade inflammation, characterized by elevated levels of C-reactive protein, interleukin 6, tumor necrosis factor-α, and absolute neutrophil count.7,8 This inflammation is thought to be a maladaptive immune response and has been linked to diseases such as asthma, insulin resistance, nonalcoholic fatty liver disease, diabetes mellitus, and cardiovascular disease.9,10
Studies in both humans and animals have suggested that individuals with excess adiposity develop an exaggerated inflammatory response to infection,11,12 although conflicting results have been reported on the influence of obesity on sepsis incidence, morbidity, and mortality. Obese adults with sepsis may experience a different clinical course from normal weight adults, with some evidence for longer duration of mechanical ventilation, higher rates of nosocomial infections, skin infections, infections by gram-positive bacteria, and renal failure at admission but lower rates of bone marrow suppression.13–16
In the pediatric population, the impact of weight status on in-hospital outcomes and resource use in critically ill patients is an emerging area of study that similarly has yielded conflicting results. For instance, hospitalized obese children under 20 years of age with sepsis had higher occurrence of organ dysfunction and longer hospitalization compared to normal weight patients, with no difference in in-hospital mortality.17 Other pediatric studies have similarly not identified differences in mortality rate by obesity status,17–19 although there have been reported differences in resource use and morbidity, including longer hospital stay for obese children with status asthmaticus20 and higher rates of hospital-acquired infections in obese children undergoing mechanical ventilation.18
A protective effect of obese/overweight status, known as the “obesity paradox,” has been described in the critically ill adult population.14,21 Similarly, the “obesity paradox” was described among children with acute respiratory distress syndrome wherein the obese children with indirect lung injury (sepsis, trauma, transfusion-related, complete congenital cardiac repair, and vasculitis) had a lower risk of mortality than children with normal weight.22
Given the conflicting literature regarding their response to infection and the associated mortality, morbidity, and resource use among children with obesity, it was hypothesized that children with an elevated BMI experience a more complicated clinical course during sepsis admissions. This study was conducted to investigate the impact of an obesity/overweight status on the microbiology, length of stay, need for technology, and mortality among children admitted to the pediatric intensive care unit (PICU) with sepsis.
Methods
Study Sample
We reviewed the medical record charts of all patients between 0 and 20 years of age admitted to the PICU at the C.S. Mott Children’s Hospital—University of Michigan between January 2009 and December 2015 with a diagnostic code for sepsis at admission (based on International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] codes) and with weight and height measurements at PICU admission. The hospital-based Virtual PICU System database was queried to obtain study data. Seven hundred eighty-nine patients between 0 and 20 years of age and a diagnosis of sepsis at PICU admission had records available. Three hundred thirty-five were excluded due to missing height or weight values. A total of 454 participants met the inclusion criteria and were included in the final analysis. Body mass index z scores and BMI percentiles were calculated using the World Health Organization macro23 for individuals aged less than 24 months old. For individuals aged 24 months to less than 20 years, Centers for Disease Control and Prevention cutoffs24 were used for BMI. Seventy-six (16.7%) participants with a BMI percentile <5th were considered underweight and were excluded from the analysis due to the concern of other underlying illnesses. Three hundred seventy-eight participants were included in the final analysis. According to the BMI percentile, individuals at or above the 5th and below the 85th percentile were considered to have normal weight, those at or above the 85th and below the 95th percentile were considered overweight, and those at or above the 95th percentile were considered obese. The study was approved by the institutional review board of University of Michigan.
Study Variables
Patient weight status: normal weight, overweight, obese.
Patient demographics: age in months, sex.
Pediatric Risk of Mortality Score-III (PRISM III)25,26 score as a marker of illness severity assigned during the first day of PICU admission.
Pediatric intensive care unit technology and the duration of use in days: invasive mechanical ventilation (conventional ventilation or high-frequency oscillatory ventilation), noninvasive positive pressure ventilation (bilevel positive airway pressure [BiPAP], or continuous positive airway pressure [CPAP], tracheostomy, central venous catheterization, in-dwelling, or tunneled vascular access devices (portacath and broviac catheters), arterial catheterization, intermittent hemodialysis, continuous renal replacement therapy (CRRT), and extracorporeal membrane oxygenation (ECMO).
Pediatric Logistic Organ Dysfunction Score (PELOD)27,28 as a marker of organ dysfunction assigned on the first day of admission.
Pediatric intensive care unit mortality.
Pediatric intensive care unit length of stay.
Statistical Analysis
After descriptive analysis of patients by weight status, patient clinical characteristics, use of PICU technology, mortality, and length of PICU stay were compared between overweight/obese patients and normal weight patients using χ2 tests for categorical variables, and either student t test or Mann-Whitney U test for parametric or nonparametric continuous data, respectively, as appropriate. Given the presence of in-dwelling or tunneled vascular access devices in some patients prior to PICU admission, comparisons of the use of these devices were performed with and without these patients, and there was no statistically significant difference according to patient weight status. Median duration of PICU technology use was compared by weight status using Mann-Whitney U test.
Logistic regression models were fit to assess the independent effect of obesity/overweight status on the use of PICU technology, after adjusting for patient severity of illness and age. When evaluating outcomes related to CRRT or intermittent hemodialysis, models were run that were additionally adjusted for estimated glomerular filtration rate (eGFR) to control for the impact of eGFR on renal-related interventions. The eGFR was estimated using the revised Schwartz estimate with k = 0.413, calculated from height and serum creatinine measured at admission.29 Logistic regression models predicting odds of CRRT or intermittent hemodialysis included adjustment for patient age (represented as a 10-month change), PRISM III score, and eGFR. A P value of ≤.05 was considered statistically significant.
Results
Three hundred seventy-eight children (between 0 and 20 years of age) with a diagnostic ICD-9-CM code for sepsis at PICU admission, with weight and height measurements on PICU admission, and with a BMI percentile >5th were included in the final analysis.
Baseline Demographics
Of the 378 patients studied, 222 (58.7%) had a normal weight, while 156 (41.3%) were either overweight (15.4%) or obese (25.9%). There were no significant differences in age, sex, or PRISM III score on admission by obesity/overweight status (Table 1). This is consistent with the current overweight/obesity rates in the state of Michigan, where 32.0% of children between 10 and 17 years of age are overweight or obese.30
Table 1.
Patient Clinical Characteristics by Weight Status.
| Clinical Characteristics of Participants | Overweight/ Obese (n = 156) | Normal Weight (n = 222) | P |
|---|---|---|---|
| Age in months, median (IQR) | 63.5 (15.5–153.5) | 74.5 (18.0–173.0) | .40a |
| Gender (%) | |||
| Male | 82 (52.6%) | 109 (49.1%) | |
| Female | 74 (47.4%) | 113 (50.9%) | .51 |
| Admission PRISM III Score, median (IQR) | 6.0 (2.0–10.5) | 5.0 (0.0–10.0) | .21a |
| ICU length of stay in days, median (IQR) | 5.4 (2.0–14.5) | 5.6 (1.9–13.2) | .61a |
| Death (%) | 10 (6.4%) | 16 (7.2%) | .76 |
Abbreviation: IQR, interquartile range; PRISM III, Pediatric Risk of Mortality, version III.
P values are a result of Mann-Whitney U tests when indicated with a, otherwise P values are a result of χ2 tests.
Pediatric Intensive Care Unit Mortality and Length of Stay
Pediatric intensive care unit mortality and length of stay were similar between obese/overweight and normal weight patients (Table 1).
Organ Dysfunction: PELOD
One hundred ninety-one of 222 normal weight and 143 of 156 overweight/obese participants had PELOD scores reported at admission. Pediatric Logistic Organ Dysfunction Score ranged from 0 to 61 in the normal weight group (mean = 11.57, SD = 9.19) and from 0 to 40 in the overweight/obese group (mean = 10.90, SD = 8.06). These distributions were not different between the normal weight and overweight/obese individuals (P = .87).
Microbiologic Etiology of Sepsis
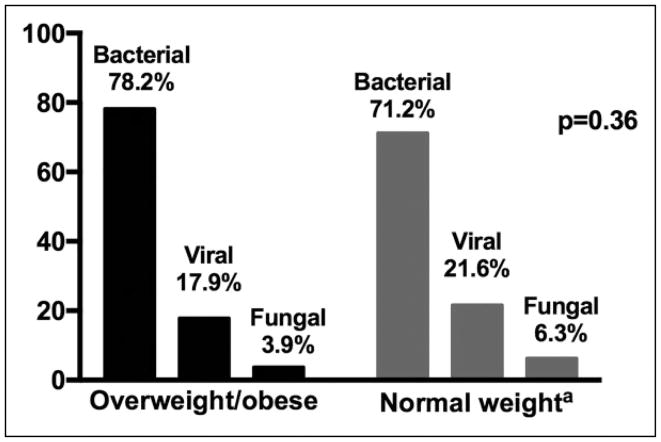
The distribution of bacterial, viral, and fungal infection frequency did not differ between overweight/obese patients and normal weight patients (P = .36; Figure 1).
Figure 1.
Microbiologic etiology of sepsis by weight status. a2 individuals (0.9%) from the normal weight group were categorized as having sepsis of other etiology different than bacterial, viral, or fungal infection.
Pediatric Intensive Care Unit Technology
Overweight/obese patients were more likely than patients with a normal weight to receive ECMO (5.8% vs 1.8%; P = .04) and CRRT (10.3% vs 4.5%; P = .03), while the frequency of use of invasive mechanical ventilation, BiPAP, CPAP, central venous catheters, arterial catheters, tracheostomy, and hemodialysis were similar between the 2 groups (Table 2). The duration of use of CRRT was also significantly longer in overweight/obese patients, although the duration of use of other PICU technologies did not differ by weight status (Table 3).
Table 2.
Frequency of Use of PICU Technology by Weight Status.
| PICU Technology | Overweight/ Obese (n = 156) | Normal Weight (n = 222) | P |
|---|---|---|---|
| BiPAP | 13 (8.3%) | 16 (7.2%) | .68 |
| CPAP | 8 (5.1%) | 19 (8.6%) | .20 |
| ECMO | 9 (5.8%) | 4 (1.8%) | .04 |
| Conventional invasive ventilation | 90 (57.7%) | 131 (61.4%) | .79 |
| HFOV | 8 (5.1%) | 13 (5.9%) | .76 |
| Tracheostomy | 32 (20.5%) | 39 (17.6%) | .47 |
| Broviac | 14 (9.0%) | 22 (9.9%) | .76 |
| PICC | 52 (33.3%) | 74 (33.2%) | 1.00 |
| Portacath | 23 (14.7%) | 29 (13.1%) | .64 |
| Arterial catheterization | 64 (41.0%) | 100 (45.0%) | .44 |
| Central venous catheterization | 61 (39.1%) | 85 (38.3%) | .87 |
| Continuous renal replacement therapy | 16 (10.3%) | 10 (4.5%) | .03 |
| Intermittent hemodialysis | 19 (12.2%) | 19 (8.6%) | .25 |
Abbreviations: BiPAP, Bilevel positive airway pressure; CPAP, continuous positive airway pressure; ECMO, extracorporeal membrane oxygenation; HFOV, high-frequency oscillatory ventilation; PICC, peripherally inserted central catheter; PICU, pediatric intensive care unit.
Table 3.
Median Duration of Use of PICU Technology by Weight Status.a
| PICU Technology Median (IQR) | Overweight/Obese | Normal Weight | P |
|---|---|---|---|
| Conventional invasive ventilation | 5.9 (1.8–12.8), n = 131 | 5.5 (2.0–10.7), n = 90 | .92 |
| HFOV | 2.8 (1.1–5.0), n = 8 | 3.6 (1.5–6.0), n = 13 | .34 |
| CRRT | 14.8 (4.9–26.0), n = 15 | 3.8 (3.0–5.5), n = 10 | .01 |
| ECMO | 7.1 (5.0–10.4), n = 9 | 7.2 (4.6–14.3), n = 4 | .71 |
| Intermittent hemodialysis | 6.6 (1.1–23.2), n = 19 | 7.3 (2.7–13.0), n = 19 | 1.0 |
| Arterial catheterization | 5.3 (1.8–15.1), n = 100 | 5.3 (2.2–10.3), n = 64 | .45 |
| Central venous catheterization | 7.8 (3.2–16.7), n = 61 | 7.2 (3.4–13.8), n = 85 | .78 |
Abbreviations: CRRT, continuous renal replacement therapy; ECMO, extracorporeal membrane oxygenation; HFOV, high frequency oscillatory ventilation; PICU, pediatric intensive care unit.
Median and interquartile range (IQR) in days. “n” represents the number of patients who received each PICU technology. P values are a result of Mann-Whitney U tests.
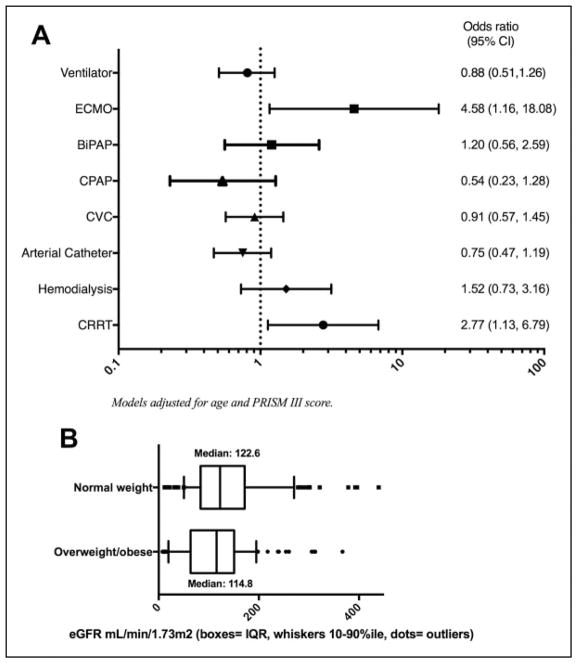
After multivariate adjustment, the relationships identified above persisted: Overweight/obese patients had elevated odds of ECMO use (odds ratio [OR]: 4.6; 95% confidence interval [CI]: 1.2–18.1; P = .03) and CRRT use (OR: 2.8; 95% CI: 1.1–6.8; P = .03) after adjustment for PRISM III score and age in months (Figure 2A). Odds of use of other PICU technologies did not differ by weight status.
Figure 2.
A, Adjusted odds of pediatric intensive care unit (PICU) technology use in overweight/obese patients compared to normal weight patients. B, Admission estimated glomerular filtration rate (eGFR) by weight status.
Kidney Function and Renal Replacement Therapies
Given the increased odds and duration of CRRT in overweight/obese patients, we sought to more closely examine kidney function and need for renal replacement therapies (CRRT and intermittent hemodialysis) in these patients. As depicted in Figure 2B, the median eGFR at admission was significantly lower in overweight/obese patients compared with normal weight patients: (114.8 mL/min/1.73m2 [range: 7.7–454.3, SD: 77.2] vs 122.6 mL/min/1.732 [range: 11.8–483.2, SD: 91.2]; P < .01). Interestingly, as shown in Table 4, among those who had eGFR available, after adjustment for eGFR at admission, overweight/obesity status remained significantly associated with elevated odds of CRRT use (OR: 3.00; 95% CI: 1.16–7.65).
Table 4.
Logistic Regression Models of Predictors of Renal Replacement Therapy.a
| Models of Predictors of Renal Replacement Therapy | CRRT (Model 1) | Dialysis (Model 2) | ||
|---|---|---|---|---|
|
|
|
|||
| OR (95% CI) | P | OR (95% CI) | P | |
| Obesity | 3.0 (1.16–7.65) | .02 | 1.20 (0.54–2.66) | .66 |
| Prism III Score | 1.14 (1.07–1.21) | <.0001 | 1.10 (1.04–1.16) | .001 |
| Age (months) | 1.04 (0.98–1.10) | .22 | 1.01 (0.96–1.06) | .73 |
Abbreviations: CI, confidence interval; CRRT, continuous renal replacement therapy; OR, odds ratio.
Models included obesity/overweight status, Prism III Score, age in months (per 10-month increase in age), and estimated glomerular filtration rate (per 5 unit increase). One hundred fifteen patients with missing values of estimated glomerular filtration rate were excluded from the model.
Discussion
Critically ill children with sepsis who were either obese/overweight or of normal weight had no difference in the microbiologic etiology of sepsis, duration of stay in the PICU, or the survival status at the end of the PICU stay. Importantly, there was a significantly higher use of specialized technology including CRRT and ECMO among the obese/overweight children, in comparison with the normal weight children.
Compared to prior studies among children with critical illness according to weight status, the current study incorporated several patient care process variables, such as the use of PICU technology, in the overall assessment of patient outcomes and resource use. By using a single center, many other variables are controlled for, and further participant data were available in this study compared to prior investigations. The higher frequency of use of CRRT and ECMO among overweight/obese patients raises the specter of greater occurrence of multiple organ dysfunction among these children in comparison with normal weight children. This increased resource use was independent of illness severity at PICU admission, a finding that might be related to the development of organ dysfunction over the course of the PICU stay which will not be captured using measures of illness severity assessed at the time of PICU admission. Future studies of the trajectory of illness among critically ill children with sepsis will need to incorporate existing longitudinal measures of organ dysfunction and illness severity27,28,31,32 that will permit continual assessment of risk of morbidity, need for specialized organ-supportive technology, overall resource use, and patient outcomes.
Given the previously described association of use of PICU technology with illness severity,33 it can be surmised that obese/overweight patients might have had progressively worsening multiple organ dysfunction that resolved with the use of highly specialized technology which in turn might have lowered the likelihood of death if the patients responded to treatment. Future study of the attributable cost burden that might result from the higher use of this specialized technology among the obese children is warranted.
Further, it appears that overweight/obese children with sepsis are more susceptible to severe acute kidney injury and cardiorespiratory decompensation requiring CRRT and ECMO, respectively. Given that the increased odds for requiring CRRT persisted despite accounting for admission eGFR, it is possible that overweight/obese pediatric patients with sepsis progress to renal failure more rapidly than their normal weight counterparts. It is noteworthy that a prior study in critically ill adult patients with sepsis also identified increased rates of renal failure on day 1 of ICU admission among obese patients, despite similar baseline rates of chronic kidney disease.14 It is possible that the chronic inflammatory state of obesity primes tissues such as the kidney to be at a higher risk of organ dysfunction during this state of illness.
The current study has limitations that need to be taken into account in interpreting the findings. The observational and retrospective nature of the study lends it to residual confounding given unobserved variables. Also, the data on weight and laboratory values were from the first day of PICU admission, making it impossible to assess the impact of daily changes in the data on patient outcomes and resource use. Another limitation is height assessment on critically ill patients; for patients 0 to 2 years of age, length is used to measure stature. Also, obtaining an accurate height in the pediatric critical care setting is often difficult and supine length might be the most common method used to evaluate the height in these patients. In addition, we did not have data to allow us to determine a preexisting history of renal disease. There were no mortality or morbidity data beyond PICU discharge, limiting the ability to assess the impact of weight status on clinical recidivism (hospital readmissions), late mortality, functional status, and long-term resource use, among survivors of critical illness.
This study describes a new association between obesity and kidney dysfunction in overweight and obese children with sepsis. It is possible that the chronic inflammatory state induced by obesity leads to this higher risk of organ dysfunction. Future research will need to investigate the mechanism by which elevated BMI may alter sepsis course in children with emphasis not only on clinical outcomes of mortality and length of stay but also on differences in longitudinal measures of organ dysfunction that might be associated with outcomes and resource use.
Acknowledgments
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Singer is supported by an NIH/NIDDK K08 award (K08DK101755).
Footnotes
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: united states, 2011–2014. NCHS Data Brief. 2015;(219):1–8. https://www.cdc.gov/nchs/products/databriefs.htm. [PubMed]
- 2.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kuperman EF, Showalter JW, Lehman EB, Leib AE, Kraschnewski JL. The impact of obesity on sepsis mortality: a retrospective review. BMC Infect Dis. 2013;13:377. doi: 10.1186/1471-2334-13-377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kelsey MM, Zaepfel A, Bjornstad P, Nadeau KJ. Age-related consequences of childhood obesity. Gerontology. 2014;60(3):222–228. doi: 10.1159/000356023. [DOI] [PubMed] [Google Scholar]
- 5.Gurnani M, Birken C, Hamilton J. Childhood obesity: causes, consequences, and management. Pediatr Clin North Am. 2015;62(4):821–840. doi: 10.1016/j.pcl.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Kumar S, Kelly AS. Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin Proc. 2017;92(2):251–265. doi: 10.1016/j.mayocp.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Singer K, Eng DS, Lumeng CN, Gebremariam A, Lee MJ. The relationship between body fat mass percentiles and inflammation in children. Obesity (Silver Spring) 2014;22(5):1332–1336. doi: 10.1002/oby.20710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schipper HS, Nuboer R, Prop S, et al. Systemic inflammation in childhood obesity: circulating inflammatory mediators and activated CD14++monocytes. Diabetologia. 2012;55(10):2800–2810. doi: 10.1007/s00125-012-2641-y. [DOI] [PubMed] [Google Scholar]
- 9.Murray PJ. Obesity corrupts myelopoiesis. Cell Metab. 2014;19(5):735–736. doi: 10.1016/j.cmet.2014.04.010. [DOI] [PubMed] [Google Scholar]
- 10.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Singer G, Stokes KY, Terao S, Granger DN. Sepsis-induced intestinal microvascular and inflammatory responses in obese mice. Shock. 2009;31(3):275–279. doi: 10.1097/SHK.0b013e3181834ab3. [DOI] [PubMed] [Google Scholar]
- 12.Trivedi V, Bavishi C, Jean R. Impact of obesity on sepsis mortality: a systematic review. J Crit Care. 2015;30(3):518–524. doi: 10.1016/j.jcrc.2014.12.007. [DOI] [PubMed] [Google Scholar]
- 13.Gaulton TG, Marshall MacNabb C, Mikkelsen ME, et al. A retrospective cohort study examining the association between body mass index and mortality in severe sepsis. Intern Emerg Med. 2015;10(4):471–479. doi: 10.1007/s11739-015-1200-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arabi YM, Dara SI, Tamim HM, et al. Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group. Clinical characteristics, sepsis interventions and outcomes in the obese patients with septic shock: an international multicenter cohort study. Crit Care. 2013;17(2):R72. doi: 10.1186/cc12680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papadimitriou-Olivgeris M, Aretha D, Zotou A, et al. The role of obesity in sepsis outcome among critically ill patients: a retrospective cohort analysis. Biomed Res Int. 2016;2016:5941279. doi: 10.1155/2016/5941279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genoni G, Prodam F, Marolda A, et al. Obesity and infection: two sides of one coin. Eur J Pediatr. 2014;173(1):25–32. doi: 10.1007/s00431-013-2178-1. [DOI] [PubMed] [Google Scholar]
- 17.Maley N, Gebremariam A, Odetola F, Singer K. Influence of obesity diagnosis with organ dysfunction, mortality, and resource use among children hospitalized with infection in the United States. J Intensive Care Med. 2017;32(5):339–345. doi: 10.1177/0885066616631325. [DOI] [PubMed] [Google Scholar]
- 18.Bechard LJ, Duggan C, Touger-Decker R, et al. Nutritional status based on body mass index is associated with morbidity and mortality in mechanically ventilated critically ill children in the PICU. Crit Care Med. 2016;44(8):1530–1537. doi: 10.1097/CCM.0000000000001713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kraft R, Herndon DN, Williams FN, Al-Mousawi AM, Finnerty CC, Jeschke MG. The effect of obesity on adverse outcomes and metabolism in pediatric burn patients. Int J Obes (Lond) 2012;36(4):485–490. doi: 10.1038/ijo.2011.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carroll CL, Bhandari A, Zucker AR, Schramm CM. Childhood obesity increases duration of therapy during severe asthma exacerbations. Pediatr Crit Care Med. 2006;7(6):527–531. doi: 10.1097/01.PCC.0000243749.14555.E8. [DOI] [PubMed] [Google Scholar]
- 21.Akinnusi ME, Pineda LA, El Solh AA. Effect of obesity on intensive care morbidity and mortality: a meta-analysis. Crit Care Med. 2008;36(1):151–158. doi: 10.1097/01.CCM.0000297885.60037.6E. [DOI] [PubMed] [Google Scholar]
- 22.Ward SL, Gildengorin V, Valentine SL, et al. Impact of weight extremes on clinical outcomes in pediatric acute respiratory distress syndrome. Crit Care Med. 2016;44(11):2052–2059. doi: 10.1097/CCM.0000000000001857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.WHO. Anthro for personal computers, version 3.2.2, 2011: Software for assessing growth and development of the world’s children. Geneva: WHO; 2010. [Accessed July 19, 2017]. http://www.who.int/childgrowth/software/en/ [Google Scholar]
- 24.Centers for Disease Control and Prevention. A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years) Atlanta, GA: Division of Nutrition, Physical Activity, and Obesity, National Center for Chronic Disease Prevention and Health Promotion, Centers for Disease Control and Prevention; 2014. [Accessed July 19, 2017]. http://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm. [Google Scholar]
- 25.Wang JN, Wu JM, Chen YJ. Validity of the updated Pediatric Risk of Mortality Score (PRISM III) in predicting the probability of mortality in a pediatric intensive care unit. Acta Paediatr Taiwan. 2001;42(6):333–337. [PubMed] [Google Scholar]
- 26.Pollack MM, Patel KM, Ruttimann UE. PRISM III: an updated pediatric risk of mortality score. Crit Care Med. 1996;24(5):743–752. doi: 10.1097/00003246-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 27.Leteurtre S, Duhamel A, Salleron J, et al. Groupe Francophone de Réanimation et d’Urgences Pédiatriques (GFRUP) PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med. 2013;41(7):1761–1773. doi: 10.1097/CCM.0b013e31828a2bbd. [DOI] [PubMed] [Google Scholar]
- 28.Leteurtre S, Martinot A, Duhamel A, et al. Validation of the Paediatric Logistic Organ Dysfunction (PELOD) score: prospective, observational, multicentre study. Lancet. 2003;362(9379):192–197. doi: 10.1016/S0140-6736(03)13908-6. [DOI] [PubMed] [Google Scholar]
- 29.Schwartz GJ, Munoz A, Schneider MF, et al. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. [Accessed December 18, 2017];The State of Obesity – Obesity Rates & Trends. 2017 Aug; https://stateofobesity.org/rates/
- 31.Leclerc F, Leteurtre S, Duhamel A, et al. Cumulative influence of organ dysfunctions and septic state on mortality of critically ill children. Am J Respir Crit Care Med. 2005;171(4):348–353. doi: 10.1164/rccm.200405-630OC. [DOI] [PubMed] [Google Scholar]
- 32.Matics TJ, Sanchez-Pinto LN. Adaptation and validation of a pediatric sequential organ failure assessment score and evaluation of the sepsis-3 definitions in critically ill children. JAMA Pediatr. 2017;170(10):e172352. doi: 10.1001/jamapediatrics.2017.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yeh TS, Pollack MM, Holbrook PR, Fields AI, Ruttiman U. Assessment of pediatric intensive care – application of the Therapeutic Intervention Scoring System. Crit Care Med. 1982;10(8):497–500. doi: 10.1097/00003246-198208000-00002. [DOI] [PubMed] [Google Scholar]