Abstract
Developmental exposure to estrogenic chemicals is an established risk factor for cancer of the female reproductive tract. This increase in risk has been associated with disruption of normal patterns of cellular differentiation during critical stages of morphogenesis. The goal of this study was to document uterine epithelial phenotypes over time following neonatal treatment with the synthetic estrogen diethylstilbestrol (DES) or the soy phytoestrogen genistein (GEN) in female CD-1 mice. Both DES and GEN induced three distinct populations of abnormal endometrial epithelial cells: luminal (SIX1+/P63−/CK14−/CK18+), basal (SIX1+/P63+/CK14+/CK18−), and mixed/bipotential (SIX1+/P63−/CK14+/CK18+), which were all established by early adulthood. In older animals, DES and GEN resulted in uterine carcinomas with mixed glandular, basal, and squamous cell elements. All carcinomas were composed largely of the three abnormal cell types. These findings identify novel epithelial differentiation patterns in the uterus and support the idea that disruption of cellular programming in early development can influence cancer risk later in life.
Keywords: estrogen, uterine cancer, differentiation, early-life exposure, diethylstilbestrol, genistein, development, progenitor cell, mouse model
Introduction
Exposure to estrogenic chemicals during early development can result in adverse health effects later in life (Masse et al. 2009; Walker and Ho 2012; Ho et al. 2017; Herceg et al. 2018). The most infamous example occurred when the potent synthetic estrogen diethylstilbestrol (DES) was administered to an estimated 2-10 million pregnant women in the United States from 1938-1971 in a misguided effort to prevent miscarriages and premature delivery (Giusti, Iwamoto, and Hatch 1995; IARC 2012; Reed and Fenton 2013). The daughters of women treated with DES while pregnant were later found to have increased lifetime risk for a broad spectrum of reproductive health outcomes, including infertility, vaginal adenosis, high-grade cervical intraepithelial neoplasia, and vaginal and cervical adenocarcinoma (Hoover et al. 2011; Verloop et al. 2010; Hatch et al. 2001). Although DES has been contraindicated for use in pregnancy since 1971, this case study continues to highlight the importance of exposures that occur during sensitive windows of development and to serve as a classical model for developmental reprogramming, in which a shift in epigenetic processes early in life permanently alters physiological responses and disease risk later in life (FDA 1972; Jefferson et al. 2011; Jefferson et al. 2013; Laronda et al. 2012).
In the most widely used experimental model of early-life estrogen effects, female CD-1 mice are treated with an exogenous estrogen on postnatal day (PND) 1-5 (Newbold, Bullock, and McLachlan 1990; Newbold et al. 2001; Suen et al. 2016). This short-term exposure can result in a high incidence of uterine carcinoma by 18 months of age, as well as other changes including adenomyosis, basal and squamous cell metaplasia, and atypical hyperplasia of the endometrial glands (Newbold and McLachlan 1982; Newbold, Bullock, and McLachlan 1990; Suen et al. 2016). This spectrum of effects has been attributed to the timing of estrogen exposure, which corresponds with key periods of cellular differentiation and gland formation in the mouse reproductive tract (Kurita 2011; Cooke et al. 2013). The neonatal exposure mouse model has been used for over four decades, and carcinogenic effects have been reported across dozens of studies, but to date there has not been a detailed characterization of uterine epithelial phenotypes in relation to the development of neoplastic lesions.
Previous work has identified several different factors that contribute to the uterine effects of early-life estrogen exposure. In rodent models, for example, endogenous estrogens play a critical role in promoting DES-induced lesions, which are prevented by ovariectomy prior to puberty or conditional deletion of estrogen receptor alpha (ERα) (Ostrander, Mills, and Bern 1985; Newbold, Bullock, and McLachlan 1990; Hendry et al. 1999; Couse et al. 2001). Other evidence implicates disruption of signaling pathways related to differentiation, which leads to permanent shifts in epithelial cell fate patterns throughout the reproductive tract (Kurita and Cunha 2001; Kurita, Mills, and Cunha 2004; Kurita 2011; Jefferson et al. 2011). More recent findings point to early epigenetic modifications in key developmental proteins such as sine oculis-related homeobox 1 (SIX1), which may mediate persistent genomic and morphologic changes in the uterus (Jefferson et al. 2013; Suen et al. 2016; Ho et al. 2017). It is still unclear, however, how these different processes interact over time to drive carcinogenesis.
The goal of this study was to describe the histopathologic and immunohistochemical features of uterine epithelial changes following neonatal exposure to either DES or the soy phytoestrogen genistein (GEN) in female CD-1 mice up to 18 months of age. We show that both estrogens induce abnormal cell populations within endometrial glands by early adulthood. These cell types exhibit distinct differentiation profiles and predominate within atypical hyperplastic and neoplastic lesions that occur later in life.
Materials and Methods
Animals and exposure groups
All animal studies were conducted following the recommendations of the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. All procedures involving animals were performed at the National Institute of Environmental Health Sciences (NIEHS) according to an approved Institutional Animal Care and Use Committee protocol. Female CD-1 mouse pups were obtained from the NIEHS breeding colony and given daily subcutaneous injections (0.02 ml) of 1 mg/kg DES or 50 mg/kg GEN in corn oil or corn oil vehicle as negative control (CON) during PND 1-5, as described previously (Newbold, Bullock, and McLachlan 1990; Newbold et al. 2001; Jefferson et al. 2011; Suen et al. 2016). All mice were weaned at PND22 and housed 5 females/cage in static cages on a designated aging rack that was separated from (but in the same room as) male mice and breeding lines. Reproductive tracts were collected at 2-3, 6, 12, or 18 months (mo) of age. The age range of 2-3 mo old mice resulted from staggered availability of female pups from the NIEHS colony. Mice for the 6, 12, and 18 mo time points originated from a previous study by Suen et al. (2016). The total number of mice evaluated per exposure group was n=30-33, n=26-30, and n=30 for 6, 12, and 18 mo time points, respectively. Mice for the 2-3 mo time point were obtained from the same breeding colony and exposed to vehicle CON, DES, or GEN (total n=9-13 per exposure group) using the same dosing protocol as for the 6, 12, and 18 mo time points.
Histopathologic analysis
Mice were euthanized by CO2 asphyxiation, and uteri were collected and processed as previously described (Suen et al. 2016). The cranial half of each right uterine horn was frozen for molecular analysis and thus not available for histopathology. The remaining tract, including cervix and cranial vagina, was fixed flat in 10% neutral buffered formalin for 18 hours at 4°C and then transferred to 70% ethanol and stored at 4°C until processing. Tissues were processed using standard histologic procedures, paraffin-embedded, sectioned longitudinally at 6 μm, and either stained with hematoxylin and eosin (H&E) or left unstained for immunohistochemistry (IHC). IHC analysis was performed on the same mice used for histopathology. Reproductive tracts were sectioned until the central uterine lumen could be observed in plane with the cervicovaginal epithelium in order to have a continuous view of the epithelium from the uterus to the cranial vagina. Histology was performed by the Histology Core Laboratory of the NIEHS/National Toxicology Program (NTP).
A single H&E-stained section of uterus and cervix was examined for each mouse. Where applicable, histopathologic diagnoses were based on standard criteria and nomenclature for neoplastic and non-neoplastic lesions presented by the International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice (INHAND) Project (www.toxpath.org/inhand.asp) (Dixon et al. 2014). Any discrepancies with INHAND terminology are described in the results section. Severity of non-neoplastic lesions was qualitatively scored using a generic 0-4 scale (0=absent, 1=minimal, 2=mild, 3=moderate, 4=severe) based on lesion extent and complexity. All pathology data, tabulations, and observations were recorded by a board-certified veterinary pathologist (CEW).
Immunohistochemical analysis
All mice 2-3 mo of age (n=9-13/exposure group) and a subset of 18 mo mice (n=9-12/exposure group) were evaluated using a panel of IHC stains. The 18 mo subset included arbitrarily-selected CON mice (n=9) and all GEN (n=10) and DES (n=12) mice with uterine carcinomas, as determined previously (Suen et al. 2016). For the time-course evaluation, we also included descriptions of lesions and IHC labeling in mice at 6 and 12 mo of age. For each mouse, serial sections were stained for each of the following markers: SIX1 as a developmental differentiation factor and putative cancer progenitor cell marker; tumor protein 63 (P63) and cytokeratin 14 (CK14) as markers of basal cell differentiation; cytokeratin 18 (CK18) as a marker of luminal (glandular) cell differentiation; CD44 as a putative endometrial stem cell marker; terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) as a marker of apoptosis; and the hormone receptors estrogen receptor alpha (ERα) and progesterone receptor (PGR).
Immunohistochemical staining was performed at the NIEHS/NTP IHC core facility using standard protocols (Painter, Clayton, and Herbert 2010) (https://www.niehs.nih.gov/research/resources/protocols/protocols-immuno/index.cfm). Reagent preparations are listed in Table 1. Heat-induced epitope retrieval was performed using 1X Antigen Decloaker, pH 6.0 (Cat # CB910M, Biocare Medical, Concord, CA; SIX1, P63, CD44, ERα, PGR) or 1X Nuclear Decloaker, pH 9.5 (Cat # CB911M, Biocare Medical; CK18, Dual CK14/CK18) in the Decloaker pressure chamber for 5 min at 120°C. Labeling for CK14 alone did not require antigen retrieval. Endogenous peroxidase activity was quenched using 3% H2O2 for 15 min.
Table 1.
Antibodies used for immunohistochemistry
| Antibody | Provider | Cat # | Lot # | Clone | Concentration | Serum | |
|---|---|---|---|---|---|---|---|
|
Primary
| |||||||
| SIX1 | Sigma-Aldrich | HPA001893 |
C75776 C104244 |
Rabbit polyclonal | 0.2 μg/mL | Donkey | |
| P63 | Santa Cruz Biotechnology | sc-8343 | C2012 | Rabbit polyclonal | 0.4 μg/mL | Goat | |
| CK14 | Covance | PRB-155P | D13EF01483 | Rabbit polyclonal | 0.8 μg/mL | Donkey | |
| CK18 | Santa Cruz Biotechnology | sc-51582 | H2714 | Mouse monoclonal | 8 μg/mL | Horse | |
| Dual | CK14 CK18 |
Covance Santa Cruz Biotechnology |
PRB-155P sc-51582 |
D15LF02399 H2714 |
See above | 0.2 μg/mL 8 μg/mL |
Antibody blocker/diluent |
| CD44 | Lifespan Biosciences | LS-B5508 | 71714 | Mouse monoclonal | 2 μg/mL | Rabbit | |
| ERα | Biocare Medical | ACA054 | 011215 | Mouse monoclonal | 2 μg/mL | Horse | |
| PGR | Lifespan Biosciences | LS-B5236 | 38639 | Mouse monoclonal | 1 μg/mL | Horse | |
|
Secondary | |||||||
| Donkey anti-rabbit (SIX1, CK14) | Jackson Immunoresearch | 711-065-152 | 124459 | – | 2.4 μg/mL | – | |
| Goat anti-rabbit (P63) | Vector Laboratories | BA-1000 | ZA0924 | – | 3 μg/mL | – | |
| Horse anti-mouse (CK18, ERα, PGR) | Vector Laboratories | BA-2001 | Z0421 | – | 0.5 μg/mL | – | |
| Rabbit anti-rat (CD44) | Vector Laboratories | BA-4001 | Y0809 | – | 1 μg/mL | – | |
| Multiview Plus Kit Mouse-HRP and Rabbit-AP (Dual CK14/18) | Enzo Life Sciences | ENZ-KIT181 | 37CR13 | – | Ready-to-use | – | |
|
Negative Control | |||||||
| Rabbit IgG (P63, SIX1, CK14) | Calbiochem | NIO1 | D00168753 | – | Equivalent Dilution | – | |
| Mouse IgG1 (CK18, ERα, PGR) | BD Biosciences | 557273 | 4241584 | – | Equivalent Dilution | – | |
| Rat IgG2b (CD44) | BD Biosciences | 559478 | 3297579 | – | Equivalent Dilution | – | |
| Rabbit IgG and Mouse IgG1 (Dual CK14/18) | Calbiochem BD Biosciences | NIO1 557273 | D00168753 4241584 | – | Equivalent Dilution | – | |
For single antigen immunolabeling, non-specific sites were blocked using either 10% animal serum (Jackson ImmunoResearch, West Grove, PA) followed by the avidin/biotin blocking kit (Vector Laboratories, Burlingame, CA) or Rodent Block M (BioCare Medical). Sections were incubated with the appropriate primary antibody or negative control antibody for 1 hour at room temperature, followed by the secondary antibody for 30 min at room temperature. Sections were treated with the R.T.U. VECTASTAIN Universal ABC Kit (Vector Laboratories; SIX1, P63, CK14, CK18, CD44, and PGR) or the Streptavidin SS Label (Biogenex Laboratories, San Ramon, CA; ERα) and visualized using 3,3′-diaminobenzidine (DAB) chromogen. TUNEL staining was performed using the ApopTag Plus Peroxidase In Situ Apoptosis Detection Kit (Cat # S7101, Millipore, Billerica, MA) using the manufacturer’s instructions.
Dual immunolabeling was also performed for CK14 and CK18 to identify cells co-expressing these markers. For this staining, non-specific sites were blocked using the Antibody Blocker/Diluent (Enzo Life Sciences, Farmingdale, NY) for 10 min at room temperature. Sections were then incubated with a mixture of primary anti-CK14 and anti-CK18 antibodies or negative control antibodies for 1 hour at room temperature, followed by incubation with a 1:1 dual mix of MULTIVIEW PLUS mouse HRP/rabbit AP (Enzo Life Sciences, Farmingdale, NY). The HIGHDEF green AP and HIGHDEF DAB chromogens (Enzo Life Sciences, Farmingdale, NY) were used to visualize CK14 (rabbit antigen-antibody complex) and CK18 (mouse antigen- antibody complex), respectively.
All immunolabeled sections were counterstained with hematoxylin, dehydrated through graded ethanol, cleared in xylene, and cover-slipped. Appropriate positive control tissues (mouse skin and intestine) were stained with each experiment. Stratified squamous cervical and vaginal epithelium present on each slide was also used as an internal positive control for specific markers (e.g., SIX1, P63, and CK14).
Immunolabeling within endometrial and cervical epithelium was evaluated by a pathologist and assigned a qualitative labeling score from 0 to 4 based on the estimated percentage of labeled target cells and overall staining intensity. Corresponding H&E-stained sections were used as needed to confirm lesion- or cell-specific labeling. Dual labeling for CK14/18 was represented by a discrete forest green color and evaluated as for a single label; it was not possible to visually distinguish intensities of the component markers. Co-localization of IHC labels other than CK14 and CK18 was evaluated by comparing adjacent IHC-stained sections.
Imaging
All slides were scanned using the Aperio AT2 Scanner (Leica Biosystems Inc., Buffalo Grove, IL). Representative images were captured either from digital slides using Aperio ImageScope v. 12.3.0.5056 (Leica Biosystems Inc., Buffalo Grove, IL) or from glass slides using a Nikon Eclipse E600 light microscope (Nikon Instruments Inc., Melville, NY) and Lumenera Infinity 2-3C digital camera (Ottawa, ON).
Statistical Analysis
Statistical analyses were performed using GraphPad Prism, version 7.0 (https://www.graphpad.com/quickcalcs/contingency2/). Incidence data between age-matched treatment groups were compared using a two-tailed Fisher exact test.
Results
Uterine epithelial morphology
Normal
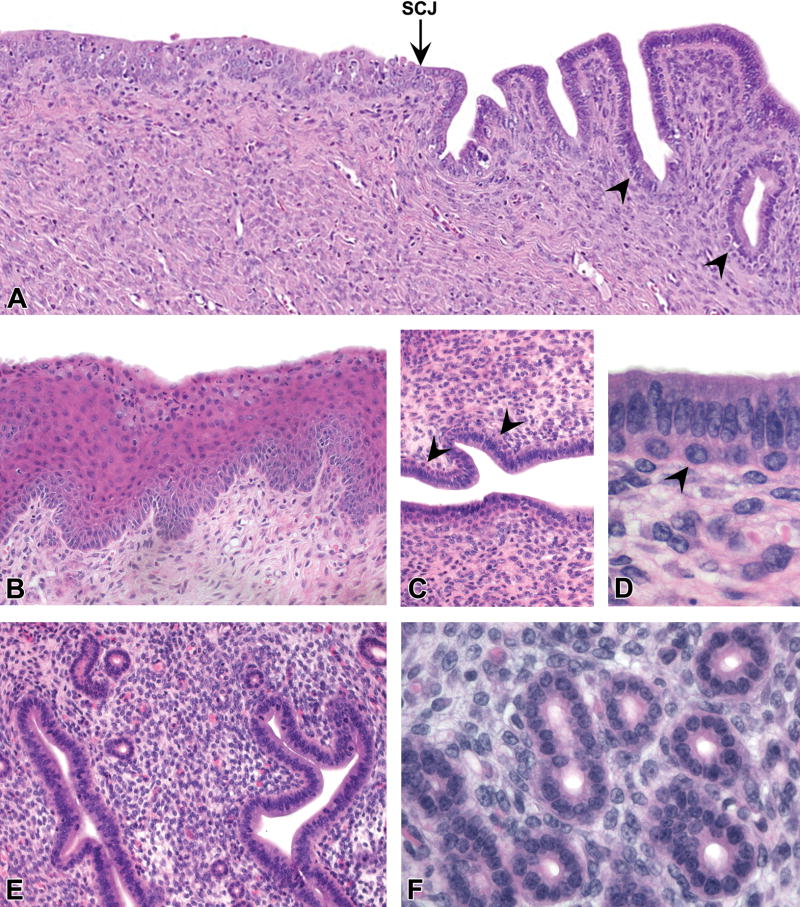
The caudal cervix was lined by stratified squamous epithelium (Fig. 1A and 1B) that varied based on normal features of estrous cycle stage (Dixon et al. 2014) in all age groups. These features included a superficial mucification layer extending from the vaginal epithelium along the squamous cervix in a subset of mice (Fig. 1B). Squamous epithelium was replaced by columnar glandular cells at or near the squamocolumnar junction (SCJ) (Fig. 1A). One to two layers of cuboidal basal cells (similar to “reserve cells” in the human cervix) were often present subjacent to the luminal columnar epithelium in this transition zone from cervix to uterine body (Fig. 1C and 1D). These basal cells varied in the degree to which they extended anteriorly past the SCJ. In some CON mice, they extended to the middle portion of the uterine body, either continuously from the squamous cervix or as scattered discontinuous aggregates. The latter cell populations were often indistinct on H&E staining and confirmed only with corresponding basal cell IHC markers.
Figure 1. Normal uterine epithelial morphology.
A, Transition zone between the uterine cervix (left) and body (right) showing stratified squamous epithelium, the squamocolumnar junction (SCJ, arrow), and columnar glandular epithelium with scattered underlying basal or “reserve” cells (arrowheads). B, Non-keratinized stratified squamous epithelium of posterior cervix showing maturation lineage of squamous cells with superficial mucification (proestrus). C and D, Basal cells (arrowheads) underlying glandular epithelium of central lumen near the SCJ. E and F, Normal simple tubular endometrial glands showing single layer of luminal epithelium. All images were taken from control mice. Slides for all images were stained with H&E. Original objective 10× (A), 20× (B), 20× (C), 60× (D), 20× (E), and 40× (F).
In the uterine body and horns, the central lumen and simple tubular glands of CON mice were lined by a single layer of cuboidal to columnar glandular epithelium (Fig. 1E and 1F). Glands lacked a peripheral layer of myoepithelial or basal cells, which is present in prostate, mammary, and other contractile glands. The normal endometrial stroma was clearly demarcated from the myometrium. Physiologic variations in endometrial morphology such as mitotic and apoptotic epithelial cells and stromal edema were evident based on estrous cycle stage, as described elsewhere (Dixon et al. 2014). Glandular cystic dilation and adenomyosis of endometrial glands were observed as age-related background findings (Suen et al. 2016).
Neonatal estrogen effects
Exposure to DES and GEN resulted in a spectrum of uterine abnormalities, consistent with those described previously in this model (e.g., Newbold, Bullock, and McLachlan 1990; Newbold et al. 2001; Suen et al. 2016). Effects on the squamous cervix included hyperkeratosis, reduced mucification, and a low incidence of adenosis at the fornices but no atypical hyperplastic or neoplastic lesions. Uteri from DES and GEN mice had reduced overall glandular complexity and loss of demarcation between the endometrial stroma and surrounding myometrium. Endometrial epithelial lesions induced by neonatal DES and GEN exposure included glandular cystic dilation, adenomyosis, basal cell metaplasia, squamous cell metaplasia, atypical hyperplasia, and carcinoma (Table 2). The overall pattern of effects was similar between the two types of estrogens. The following minor differences were observed in GEN compared to DES groups: lower incidence of basal cell metaplasia and atypical hyperplasia at 6 mo; higher incidence of glandular cystic dilation at 12 mo; and higher incidence of squamous metaplasia at 18 mo.
Table 2.
Incidence of uterine glandular epithelial lesions over time following neonatal exposure to GEN or DES.
| CON
|
GEN
|
DES
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (mo) | 2–3 | 6 | 12 | 18 | 2–3 | 6 | 12 | 18 | 2–3 | 6 | 12 | 18 |
|
|
|
|
||||||||||
| Diagnosis | Incidence (%) | Incidence (%) | Incidence (%) | |||||||||
| Glandular cystic dilation | 0/13 (0%) |
2/33 (6%) |
7/29 (24%) |
15/30 (50%) |
0/10 (0%) |
12/30b (40%) |
23/30c§ (77%) |
27/30b (90%) |
0/9 (0%) |
4/31 (13%) |
10/26 (38%) |
29/30c (97%) |
| Adenomyosis | 0/13 (0%) |
0/33 (0%) |
3/29 (10%) |
4/30 (13%) |
1/10 (10%) |
6/30b (20%) |
10/30 (33%) |
13/30a (43%) |
1/9 (11%) |
13/31c (42%) |
13/26b (50%) |
18/30c (60%) |
| Squamous metaplasia | 0/13 (0%) |
1/33 (3%) |
0/29 (0%) |
0/30 (0%) |
0/10 (0%) |
11/30c (37%) |
16/30c (53%) |
24/30c§ (80%) |
0/9 (0%) |
7/31a (23%) |
12/26c 46% |
14/30c (47%) |
| Severity grade | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.4 | 0.8 | 1.2 | 0.0 | 0.3 | 0.6 | 0.8 |
| Basal cell metaplasia | 1/13 (8%) |
3/33 (9%) |
2/29 (7%) |
0/30 (0%) |
7/10b (70%) |
19/30c‡ (63%) |
26/30c (87%) |
30/30c (100%) |
8/9c (89%) |
30/31c (97%) |
25/26c (96%) |
29/30c (97%) |
| Severity grade | 0.1 | 0.1 | 0.1 | 0.0 | 0.7 | 0.7 | 1.7 | 1.7 | 0.9 | 1.5 | 2.0 | 1.7 |
| Atypical hyperplasia | 0/13 (0%) |
0/33 (0%) |
0/29 (0%) |
0/30 (0%) |
0/10 (0%) |
3/30‡ (10%) |
9/30b (30%) |
15/30c (50%) |
0/9 (0%) |
14/31c (45%) |
14/26c (54%) |
18/30c (60%) |
| Carcinoma | 0/13 (0%) |
0/33 (0%) |
0/29 (0%) |
0/30 (0%) |
0/10 (0%) |
2/30 (7%) |
7/30a (23%) |
10/30c (33%) |
0/9 (0%) |
5/31a (16%) |
9/26b (35%) |
12/30c (40%) |
Incidence data for 6, 12, and 18 mo time points were previously reported by Suen et al. 2016.
Abbreviations: CON, control; GEN, genistein; DES, diethylstilbestrol
Severity values indicate average grade across the entire group (0-4).
Letters indicate
P<0.05,
P<0.01, and
P<0.001 compared with corresponding age-matched control group using a two-tailed Fisher exact test.
Symbols indicate
P<0.05 and
P<0.01 compared with corresponding age-matched DES group using a two-tailed Fisher exact test.
The earliest and most prevalent uterine effect of neonatal estrogen treatment was basal cell metaplasia (Fig. 2A and 2B, lower image). This change consisted of 1-2 layers of epithelial cells underlying morphologically normal columnar epithelium lining the central lumen and endometrial glands (Fig. 2A). Basal cells were cuboidal with round to oval nuclei, often with perinuclear clearing (Fig. 2B). This bilaminar appearance was often accompanied by rudimentary or multilocular luminal morphology, giving some glands an immature appearance. Basal cell metaplasia was most commonly identified in glands of the uterine body but was also found variably along the length of the uterine horns. At 2-3 mo of age, 70-89% of DES and GEN mice exhibited basal cell metaplasia, whereas basal cells were rarely observed in CON mice (beyond the transition zone of the cervix and uterine body).
Figure 2. Metaplastic changes in the uterine epithelium induced by neonatal exposure to DES or GEN.
A, Basal cell metaplasia of endometrial glands showing a distinct subluminal layer of cuboidal basaloid cells between the luminal cell layer and the basement membrane. B, Normal simple luminal epithelium overlying the basement membrane (upper image, from a control mouse) contrasted with bilaminar epithelium from a DES-treated mouse consisting of morphologically normal glandular epithelial cells and subjacent mature basal cells (lower image). C and D, Squamous cell metaplasia of endometrial glands. C, Multiple layers of squamoid cells with replacement of luminal layer (arrow). D, Metaplastic glands showing a full maturation lineage from basal cells (arrow) to sloughed squamous keratinocytes (arrowhead). Slides for all images were stained with H&E. Original objective 40× (A), 60× (B), 40× (C), and 20× (D).
Previous reports have included basal cell metaplasia under the diagnosis of squamous cell metaplasia (e.g., Newbold, Bullock, and McLachlan 1990; Newbold et al. 2001). Here, we split these terms based on stratification and maturation to keratinocytes. In squamous cell metaplasia, a lineage of stratified squamous epithelial cells typically extended from the basement membrane to the lumen, replacing the glandular epithelium and often including fully differentiated superficial keratinocytes (Fig. 2C and 2D). Basal cell metaplasia was characterized by compartmentalization of basal and luminal layers and did not show evidence of keratinocyte maturation, replace glandular epithelium, or border luminal spaces. Although basal cell metaplasia was highly associated with (and considered to be a likely precursor lesion for) squamous cell metaplasia, the vast majority of these lesions did not show clear squamous maturation.
Atypical focal glandular hyperplasia was defined by irregular endometrial glands with cytological and architectural pleomorphism (Fig. 3A). Features included increased epithelial cell numbers; abnormal growth patterns with mixed luminal, basal, squamous, and in rare cases mucous cells; small and irregular glandular lumens, often intraepithelial; and excessive crowding of glands. Cellular features included anisokaryosis, anisocytosis, and loss of polarity for luminal cells but no evidence of local or vascular invasion.
Figure 3. Proliferative uterine epithelial lesions induced by neonatal exposure to DES or GEN.
A, Atypical hyperplasia of uterine glands. Features include increased numbers of luminal and basal cells with loss of polarity and abnormal intraepithelial lumens but no evidence of local invasion. B-E, Uterine carcinomas. B, Multicentric location in uterine body and horn (arrowheads). C, Atypical glandular architecture with microcystic appearance. D, Areas of basement membrane disruption (arrowheads) and intraluminal secretory material and vacuolated cells (arrow). E, Neoplastic glands showing luminal (arrow), basal (arrowhead), and squamous (asterisk) differentiation patterns. Slides for all images were stained with H&E. Original objective 40× (A), 1× (B), 20× (C), 60× (D), and 20× (E).
Carcinomas induced by DES and GEN were characterized by the presence of atypical glandular structures with local invasion into the surrounding stroma or myometrium (Fig. 3B–3E). The most common tumor location was the uterine body near the bifurcation of uterine horns, although carcinomas also occurred in the uterine horns and were often multicentric (Fig. 3B). The growth pattern was highly infiltrative and did not typically result in mass lesions that could be observed grossly. The most common morphologic subtype consisted of small glandular structures with round lumens <50 μm in diameter (Fig. 3C), often containing secretory material and large vacuolated cells (Fig. 3D). Neoplastic cells along glandular margins were often difficult to distinguish from adjacent stromal cells (Fig. 3D), and vascular invasion was noted in rare cases. Carcinomas typically occurred on a background of atypical glandular hyperplasia (which was recorded as a distinct lesion). Fibrosis and inflammation were variably present along the margins of neoplastic glands.
All carcinomas contained acini with lumen formation, consistent with glandular differentiation. In rare cases, neoplastic glands contained luminal cells with abundant finely granular and basophilic cytoplasm, resembling mucous cells. However, none of the carcinomas had a pure luminal cell phenotype with expansile growth. All tumors showed some degree of basaloid differentiation, which in some cases progressed to squamous metaplasia with or without full keratinization (Fig. 3E). In these cases, it was difficult to distinguish adenocarcinoma with squamous differentiation from squamous cell carcinoma or adenosquamous carcinoma. According to INHAND terminology (Dixon et al. 2014), this latter term should be used for tumors with “at least 10% or more squamous differentiation.” Although a number of carcinomas in DES and GEN mice met this criterion, a diagnosis of adenosquamous carcinoma was not considered appropriate in many other cases given the distinction between basal and squamous cell differentiation described above. Based on these considerations, and the predominance of basal and luminal cell differentiation as the central feature, we propose calling these tumors adenobasal carcinomas with or without squamous differentiation.
Uterine epithelial IHC profile
To support morphologic observations and better understand the molecular phenotype of these metaplastic and neoplastic lesions, we applied a panel of IHC markers related to cellular differentiation, receptor expression, and apoptosis.
SIX1
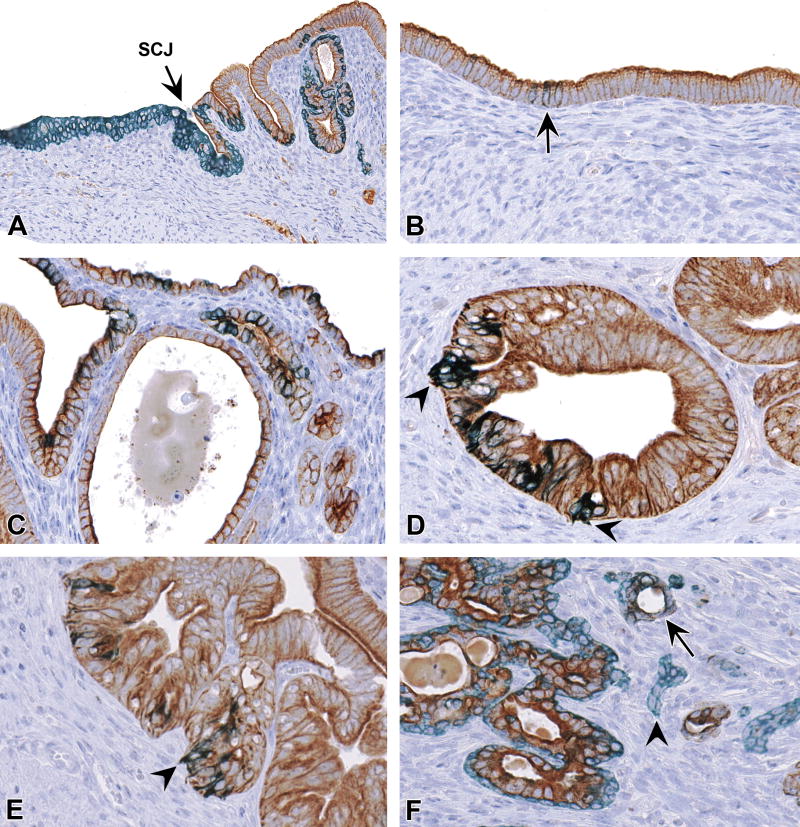
Nuclear expression of SIX1 was present in the stratified squamous epithelium of the ectocervix and transition zone in all mice (Fig. 4A, Table 3A). No differences were observed in SIX1 expression between CON and DES or GEN mice at these sites. SIX1 staining was most intense in basal/suprabasal layers but often extended full thickness to the superficial squamous layers (unlike the basal markers P63 and CK14, as described below). When present, basal cells in the transition zone of the cervix were also positive for SIX1, whereas overlying glandular cells showed variable labeling (Fig. 4A). In the uterine body and horns, SIX1 labeling was consistently absent throughout luminal/glandular epithelium of CON mice (Fig. 4B).
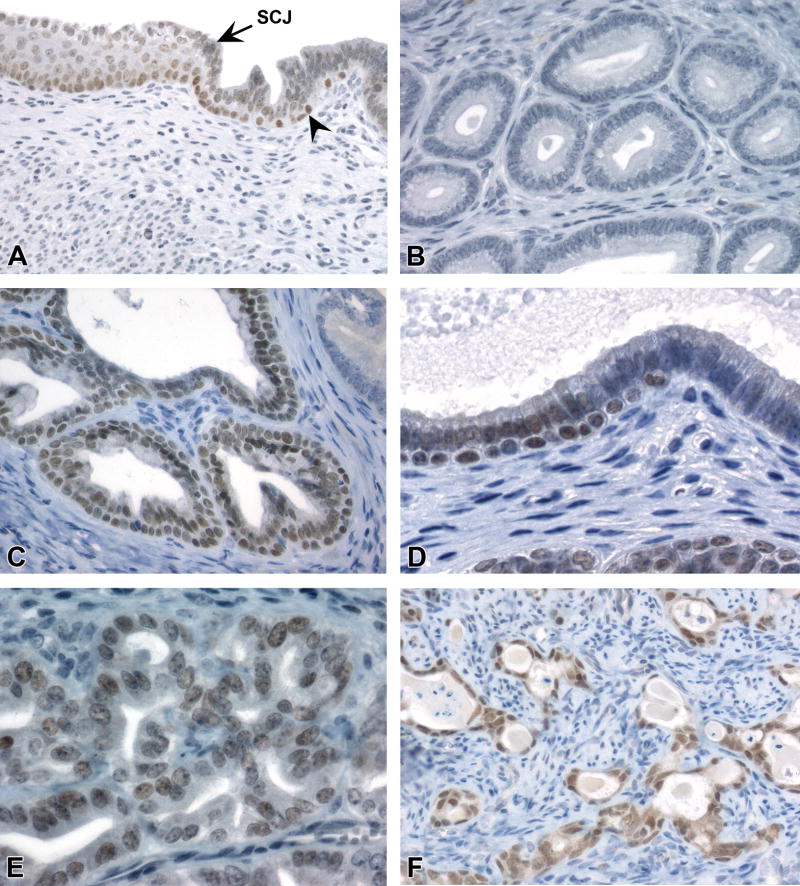
Figure 4. SIX1 IHC profile in the uterine epithelium.
A, Normal pattern of SIX1 expression in the stratified squamous epithelium of the cervix (left) and scattered luminal cells and basal cells (arrowhead) in the transition zone. B, Absence of SIX1 in normal luminal epithelium of endometrial glands. C and D, SIX1 expression in basal cells and adjacent luminal columnar epithelium of endometrial epithelium with basal cell metaplasia. E, Rare SIX1 expression in non-neoplastic luminal cells lacking subjacent basal cells (identified as glands with CK14+/CK18+ expression). F, SIX1 expression in luminal, basal, and squamoid cells of a uterine carcinoma in a DES mouse. Slides for all images were immunostained for SIX1 (A-G). Original objective 20× (A), 40× (B), 60× (C), 40× (D), 60× (E), and 40× (F and G).
Table 3A.
Immunohistochemical expression of differentiation markers in the uterine epithelium following neonatal exposure to GEN or DES
| Age (mo)
|
|||||||
|---|---|---|---|---|---|---|---|
| 2–3
|
18
|
||||||
| Marker | Site | CON | GEN | DES | CON | GEN | DES |
| SIX1 | Cervix: non-neoplastic | 13/13 (100%) |
10/10 (100%) |
9/9 (100%) |
9/9 (100%) |
10/10 (100%) |
12/12 (100%) |
| Labeling score | 2.2 | 1.9 | 2.0 | 1.9 | 1.4 | 1.6 | |
|
|
|
|
|||||
| Endometrium: non-neoplastic | 1/13 (8%) |
9/10c (90%) |
9/9c (100%) |
0/9 (0%) |
10/10c (100%) |
12/12c (100%) |
|
| Labeling score | 0.1 | 1.1 | 1.2 | 0.0 | 2.2 | 2.6 | |
|
|
|
|
|||||
| Endometrium: neoplastic | na | na | na | na | 10/10 (100%) |
12/12 (100%) |
|
| Labeling score | na | na | na | na | 2.0 | 2.5 | |
|
| |||||||
| P63 | Cervix: non-neoplastic | 13/13 (100%) |
10/10 (100%) |
9/9 (100%) |
9/9 (100%) |
10/10 (100%) |
12/12 (100%) |
| Labeling score | 2.7 | 3.0 | 2.7 | 2.6 | 2.7 | 2.8 | |
|
|
|
|
|||||
| Endometrium: non-neoplastic | 1/13 (8%) |
8/10c (80%) |
8/9c (89%) |
4/9 (44%) |
10/10a (100%) |
12/12b (100%) |
|
| Labeling score | 0.1 | 0.8 | 0.9 | 0.4 | 2.0 | 2.5 | |
|
|
|
|
|||||
| Endometrium: neoplastic | na | na | na | na | 10/10 (100%) |
12/12 (100%) |
|
| Labeling score | na | na | na | na | 2.1 | 1.8 | |
Abbreviations: CON, control; GEN, genistein; DES, diethylstilbestrol; na, not applicable. Cervix includes stratified squamous epithelium, mucification layer if present, and transition zone with uterine body; endometrium includes glands and epithelium lining central lumen. IHC labeling score represents a qualitative average (0-4) within each group.
Letters indicate
P<0.05,
P<0.01, and
P<0.001 compared with corresponding age- matched control group using a two-tailed Fisher exact test.
In DES and GEN mice, nuclear SIX1 expression occurred in basaloid and squamous cells of all metaplastic glands (Fig. 4C). Within the luminal compartment of these glands, SIX1 was typically present only in cells that had aberrant SIX1+ basal cells directly subjacent to them (Fig. 4D). SIX1 expression was also present in low numbers of scattered luminal cells within morphologically normal uterine glands (later identified as CK14+/CK18+ cells, as described below) (Fig. 4E). In hyperplastic and neoplastic glands, there was moderate to strong nuclear expression of SIX1 in basal and luminal cells (Fig. 4F), in contrast to other basal cell markers (P63 and CK14), which were only present in the basal compartment.
P63
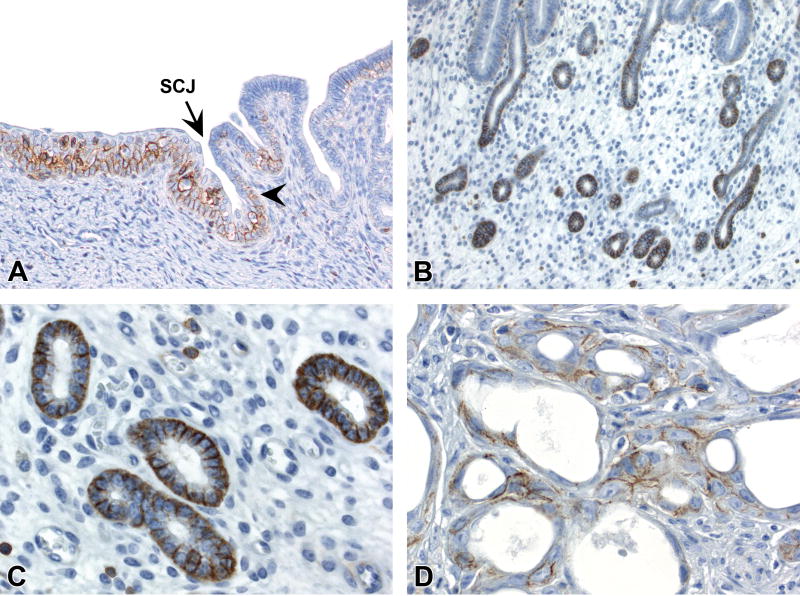
Strong nuclear P63 labeling was present in basal/suprabasal cells of the squamous cervix and basal (reserve) epithelial cells near the SCJ in all mice (Fig. 5A, Table 3A). Luminal/glandular endometrial epithelial cells in CON, GEN, and DES mice diffusely lacked P63 staining. Areas of basal cell metaplasia showed strong expression of P63 specifically within basal cells (but not overlying luminal cells) (Fig. 5B). Foci of squamous cell metaplasia also had P63 staining within basal/suprabasal cells, similar to normal squamous cervix (Fig. 5C). Endometrial epithelium with atypical hyperplasia and all uterine carcinomas showed moderate to strong expression of P63 in basal but not luminal cell populations (Fig. 5D).
Figure 5. P63 IHC profile in the uterine epithelium.
A, P63 expression in the basal layer of squamous cervix and scattered basal cells (arrowhead) in the transition zone. B, P63 expression in basal cells but not adjacent luminal columnar epithelial cells of glands with basal cell metaplasia. C, P63 expression in the basal and suprabasal layers of glands with squamous metaplasia. D, P63 expression specifically within basal compartment of neoplastic glands in a uterine carcinoma. Slides for all images were immunostained for P63 (A-D). Original objective 20× (A-D).
CK14
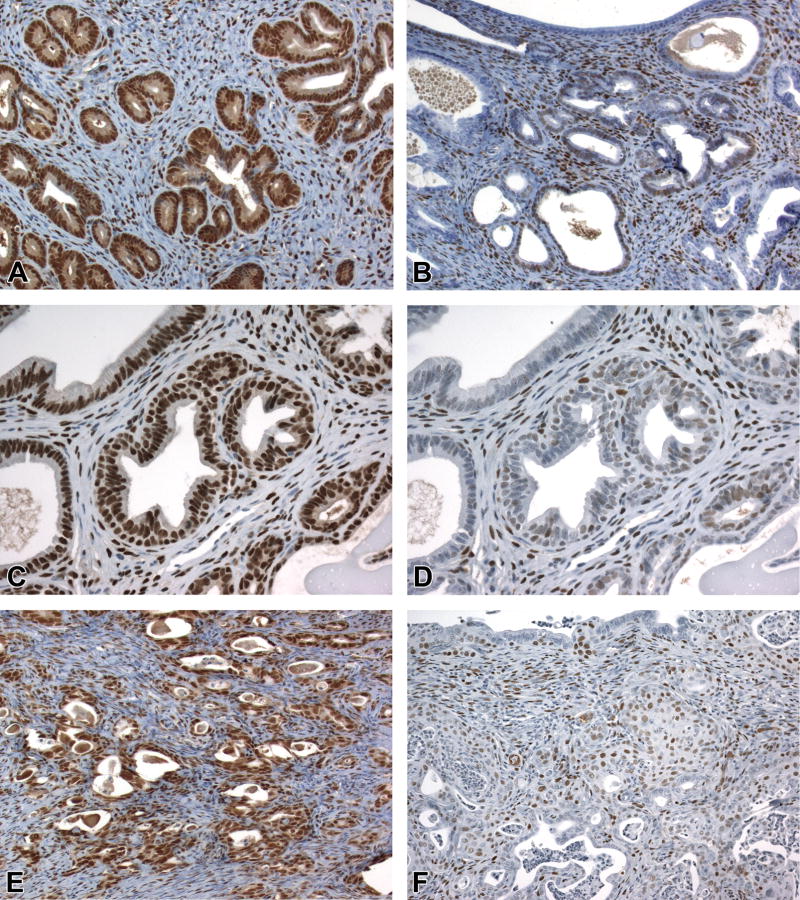
Strong cytoplasmic CK14 labeling was present diffusely within all layers of the cervical squamous epithelium and in basal/reserve cells near the SCJ of all mice (Fig. 6A, Table 3B). Endometrial expression of CK14 was most prominent within metaplastic glands (specifically within basal cells, as for P63) (Fig. 6B). Luminal epithelial cells of the endometrium diffusely lacked CK14 staining (Fig. 6C) with the exception of small populations of scattered cells not directly associated with basal or squamous cell metaplasia (Fig. 6D). These CK14+ luminal cells were present in very low numbers (<1% of total cells) in a subset of CON mice and appeared to co-express CK18 but not P63 based on comparison of serial sections; SIX1 labeling was either absent or equivocal. In DES and GEN mice, this type of CK14+ luminal cell was more prevalent (Table 3B) and consistently co-expressed both CK18 and SIX1. Hyperplastic and neoplastic lesions showed moderate to strong expression of CK14 in basaloid cells lining acinar structures, areas of squamous differentiation, and a small subset of luminal-type epithelial cells (Fig. 6E and 6F).
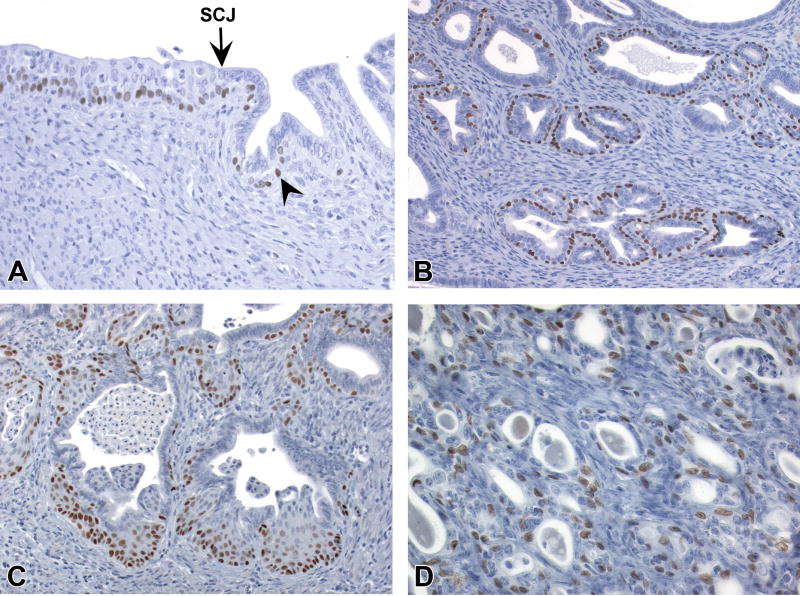
Figure 6. CK14 IHC profile in the uterine epithelium.
A, CK14 expression in all layers of squamous cervix and scattered basal cells (arrowhead) of the transition zone. B, CK14 expression in basal cells but not adjacent luminal epithelial cells of an endometrial gland with basal cell metaplasia. C, Lack of CK14 staining in morphologically normal epithelium of the endometrial central lumen and glands. D, Rare CK14 expression in luminal cells lacking basal morphology or subjacent basal cells (same cluster of glands as shown in Fig. 4E, subsequently identified as CK14+/CK18+ and SIX1+). E and F, CK14 expression in basal compartment and scattered luminal cells (arrows) within uterine carcinomas. Slides for all images were immunostained for CK14 (A-F). Original objective 20× (A), 60× (B), 20× (C), 40× (D), 20× (E), and 60× (F).
Table 3B.
Immunohistochemical expression of differentiation markers in the uterine epithelium following neonatal exposure to DES or GEN
| Age (mo)
|
|||||||
|---|---|---|---|---|---|---|---|
| 2–3
|
18
|
||||||
| Marker | Site | CON | GEN | DES | CON | GEN | DES |
| CK14 | Cervix: non-neoplastic | 13/13 (100%) |
10/10 (100%) |
9/9 (100%) |
9/9 (100%) |
10/10 (100%) |
12/12 (100%) |
| Labeling score | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | 4.0 | |
|
|
|
|
|||||
| Endometrium: non-neoplastic | 1/13 (8%) |
10/10c (100%) |
9/9c (100%) |
4/9 (44%) |
10/10a (100%) |
12/12b (100%) |
|
| Labeling score | 0.1 | 1.2 | 1.0 | 0.4 | 2.5 | 2.7 | |
|
|
|
|
|||||
| Endometrium: neoplastic | na | na | na | na | 10/10 (100%) |
12/12 (100%) |
|
| Labeling score | na | na | na | na | 3.5 | 3.2 | |
|
| |||||||
| CK18 | Cervix: non-neoplastic# | 9/13 (69%) |
0/10b (0%) |
2/9 (22%) |
1/9 (11%) |
5/10 (50%) |
5/12 (42%) |
| Labeling score | 0.9 | 0.0 | 0.2 | 0.1 | 0.5 | 0.4 | |
|
|
|
|
|||||
| Endometrium: non-neoplastic | 13/13 (100%) |
10/10 (100%) |
9/9 (100%) |
9/9 (100%) |
10/10 (100%) |
12/12 (100%) |
|
| Labeling score | 3.8 | 4.0 | 4.0 | 4.0 | 3.9 | 3.9 | |
|
|
|
|
|||||
| Endometrium: neoplastic | na | na | na | na | 10/10 (100%) |
12/12 (100%) |
|
| Labeling score | na | na | na | na | 3.1 | 2.8 | |
|
| |||||||
| Dual CK14/18 | Cervix: non-neoplastic#* | 6/10 (60%) |
0/10a (0%) |
1/9 (11%) |
4/9 (44%) |
0/10a (0%) |
2/11 (18%) |
| Labeling score | 0.8 | 0.0 | 0.1 | 0.4 | 0.0 | 0.2 | |
|
|
|
|
|||||
| Endometrium: non-neoplastic* | 4/9 (44%) |
10/10a (100%) |
9/9a (100%) |
2/9 (22%) |
10/10c (100%) |
10/11b (91%) |
|
| Labeling score | 0.4 | 1.2 | 1.1 | 0.2 | 1.0 | 0.9 | |
|
|
|
|
|||||
| Endometrium: neoplastic* | na | na | na | na | 9/9 (100%) |
11/11 (100%) |
|
| Labeling score | na | na | na | na | 2.1 | 2.1 | |
Abbreviations: CON, control; GEN, genistein; DES, diethylstilbestrol; na, not applicable. Cervix includes stratified squamous epithelium, mucification layer if present, and transition zone with uterine body; endometrium includes glands and epithelium lining central lumen. IHC labeling score represents average (0-4) within each group.
Tissues from some uteri were not present in section.
CK18 labeling in the cervix was most commonly observed in a superficial mucification layer, which was often not present in GEN or DES mice.
Letters indicate
P<0.05,
P<0.01, and
P<0.001 compared with corresponding age- matched control group using a two-tailed Fisher exact test.
CK18
Expression of CK18 was not present in normal squamous epithelium of the cervix (Fig. 7A) but was observed in the superficial mucification layer when present (Table 3B). The lower incidence of cervical CK18 expression in DES and GEN mice was due primarily to the lack of mucification. CK18 was also observed in small foci of adenosis at the fornices in low numbers of DES and GEN mice. In the endometrium, CK18 was expressed in all surface luminal/glandular epithelial cells of all mice. Cellular localization was predominantly membranous, to a lesser extent cytoplasmic, and most prominent along apical/luminal margins. In foci of basal metaplasia, CK18 staining was observed specifically within the luminal compartment (Fig. 7B). In hyperplastic and neoplastic glands, CK18 expression was present within luminal cells of all lesions evaluated (Fig. 7C and 7D). CK18 expression thus complemented CK14 and P63 expression and confirmed the two-compartment phenotype of these tumors.
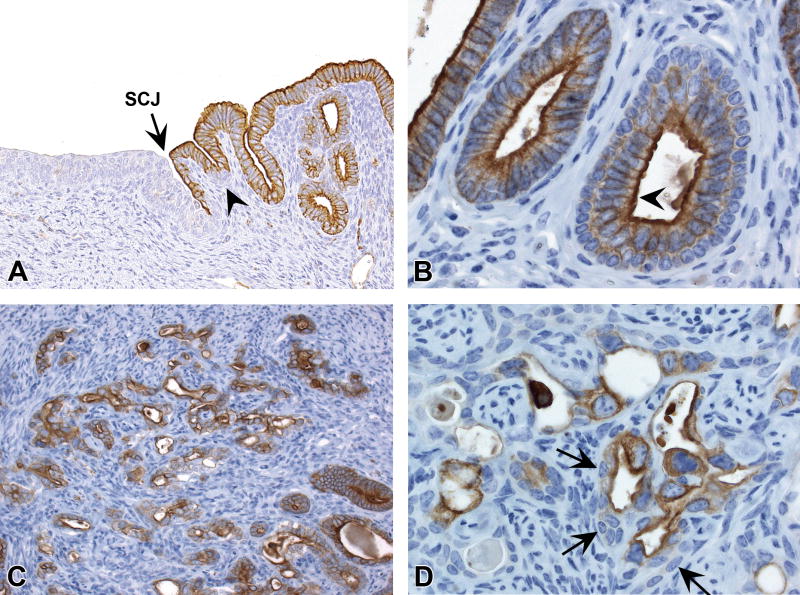
Figure 7. CK18 IHC profile in the uterine epithelium.
A, CK18 is expressed in the luminal epithelial cells within the transition zone but not in the scattered basal cells (arrowhead) or the stratified squamous epithelium. B, CK18 is expressed in luminal epithelial cells of morphologically normal glands (left side) and the luminal compartment of glands with basal cell metaplasia (arrowhead). C and D, CK18 expression in luminal cells within uterine carcinomas. Subjacent basal cells in carcinomas lacked CK18 expression (arrows). Slides for all images were immunostained for CK18 (A-D). Original objective 20× (A), 60× (B), 20× (C), and 60× (D).
CK14/CK18
Double-staining for CK14 and CK18 was performed to confirm co-expression of these markers in a distinct subset of uterine epithelial cells. Normal squamous cervical epithelium did not contain CK14+/CK18+ cells, whereas cells of the mucification layer, when present, were variably positive. Reduced mucification in DES and GEN mice accounted for the lower incidence of CK14/CK18 labeling in the cervix (Table 3B). Low numbers of these cells were also present within glandular epithelium near the SCJ in a subset of CON, DES, and GEN mice (Fig. 8A). In the endometrium, CK14+/CK18+ cells were either completely absent or limited to low numbers of scattered columnar cells lining the central lumen in a subset of CON mice (Fig. 8B). In DES and GEN mice, CK14+/CK18+ cells were observed within morphologically normal (non-neoplastic), hyperplastic, and neoplastic glands (Table 3B). In non-neoplastic epithelium, CK14+/CK18+ cells were luminal and did not include P63+ basal or squamous cells (Fig. 8C and 8D). In DES and GEN mice, dual-labeled cells were present in the epithelium of both the central lumen and smaller deeper glands, and some of these cells appeared to have processes expanding into the stroma (Fig. 8D and 8E).
Figure 8. Dual CK14/CK18 expression in the uterine epithelium.
A, CK14− only expression (teal blue) in all layers of stratified squamous epithelium in the squamous cervix and scattered basal cells (arrowhead) of the transition zone. CK18− only (brown) is expressed in luminal epithelial cells. B, Dual CK14/CK18 (forest green) expression in rare epithelial cells of the central lumen. C-E, Dual CK14/CK18 expression in luminal epithelium without associated basal cells. Some luminal CK14+/CK18+ cells have processes extending into the stroma that appear to disrupt the basement membrane (arrowheads). F, Uterine carcinoma showing glands with distinct CK14− only and CK18− only compartments, glands with CK14− only cells (arrowhead), and glands with dual CK14+/CK18+ cells (arrow). Slides for all images were double immunostained for CK14 and CK18 (A-F). Original objective 20× (A) and 40× (B-F).
Dual labeling was more prevalent in neoplastic compared to non-neoplastic epithelium (Table 3B). In neoplastic glands, CK14+/CK18+ cells were most common along the deep margins of glands and often associated with an invasive cellular morphology in the absence of basal metaplasia. The majority of neoplastic glands had distinct CK14+/CK18− and CK14−/CK18+ compartments, while a subset of glands, often with only a single epithelial layer, showed CK14+/CK18+ staining (Fig. 8F). Carcinomas thus consisted of a mosaic with luminal (SIX1+/P63−/CK14−/CK18+), basal (SIX1+/P63+/CK14+/CK18−), and mixed (SIX1+/P63−/CK14+/CK18+) cell types (Fig. 9A–9E). While small subsets of neoplastic glands (or portions of individual glands) showed basal-only, luminal-only, or mixed-only cells, the vast majority of glands within each uterine carcinoma showed a combination of cells with basal and luminal differentiation.
Figure 9. Composite IHC profile of uterine carcinomas.
Immunostaining for SIX1 (A), P63 (B), CK14 (C), CK18 (D), and dual CK14/18 (E) within neoplastic glands from the same uterine carcinoma. P63 and CK14 areexpressed in basal cells (arrows) and CK14 is expressed in luminal cells (arrowhead) in compartmentalized glands. Glands with a single luminal layer may have CK14− only, CK18− only, or dual CK14/18 expression (asterisks). Slides for all images were immunostained as labeled. Original objective 60× (A-D) and 40× (E).
CD44
Speckled membranous CD44 labeling was present in basal/suprabasal layers of the squamous cervical epithelium in all mice (Fig. 10A, Table 3C). In the endometrium, epithelial CD44 labeling was observed in some CON mice but not others, and no clear pattern could be discerned based on cycle stage. When present, staining of endometrial glandular epithelium in CON mice was sporadic, often scant, and limited to deeper glands (Fig. 10B). Strong CD44 labeling was also noted in immune cells. Cellular localization was membranous in cervical epithelium, cytoplasmic in stromal/immune cells, and membranous +/− cytoplasmic in glandular epithelium (Fig. 10C). In DES and GEN mice, positive membranous staining was often (but not always) present in metaplastic basal cells. Weak to moderate membranous CD44 staining was also observed within basal layers of neoplastic glands in a subset of tumors but was not a prominent feature (Fig. 10D).
Figure 10. CD44 IHC profile in the uterine epithelium.
A, CD44 expression in the basal and suprabasal layers of stratified squamous epithelium in the ectocervix and scattered basal cells (arrowhead) of the transition zone. B and C, CD44 expression in scattered glands. D, CD44 expression in neoplastic glands. Slides for all images were immunostained for CD44 (A-D). Original objective 20× (A), 20× (B), 60× (C), and 60× (D).
Table 3C.
Immunohistochemical expression of differentiation markers in the uterine epithelium following neonatal exposure to DES or GEN
| Age (mo)
|
|||||||
|---|---|---|---|---|---|---|---|
| 2–3
|
18
|
||||||
| Marker | Site | CON | GEN | DES | CON | GEN | DES |
| CD44 | Cervix: non-neoplastic | 13/13 (100%) |
10/10 (100%) |
9/9 (100%) |
9/9 (100%) |
10/10 (100%) |
12/12 (100%) |
| Labeling score | 2.3 | 3.0 | 3.0 | 2.8 | 2.7 | 2.8 | |
|
|
|
|
|||||
| Endometrium: non-neoplastic | 6/13 (46%) |
10/10b (100%) |
9/9a (100%) |
9/9 (100%) |
10/10 (100%) |
11/12 (92%) |
|
| Labeling score | 0.6 | 1.1 | 1.4 | 1.2 | 1.0 | 1.0 | |
|
|
|
|
|||||
| Endometrium: neoplastic | na | na | na | na | 9/10 (90%) |
8/12 (67%) |
|
| Labeling score | na | na | na | na | 1.3 | 1.0 | |
|
| |||||||
| TUNEL | Cervix: non-neoplastic* | 7/10 (70%) |
10/10 (100%) |
9/9 (100%) |
8/8 (100%) |
9/9 (100%) |
10/11 (91%) |
| Labeling score | 0.9 | 1.2 | 1.0 | 1.4 | 1.0 | 1.0 | |
|
|
|
|
|||||
| Endometrium: non-neoplastic* | 7/9 (78%) |
10/10 (100%) |
9/9 (100%) |
9/9 (100%) |
9/10 (90%) |
11/11 (100%) |
|
| Labeling score | 1.7 | 1.2 | 1.2 | 1.8 | 0.9 | 1.0 | |
|
|
|
|
|||||
| Endometrium: neoplastic* | na | na | na | na | 9/10 (90%) |
10/11 (91%) |
|
| Labeling score | na | na | na | na | 0.9 | 0.9 | |
Abbreviations: CON, control; GEN, genistein; DES, diethylstilbestrol; na, not applicable. Cervix includes stratified squamous epithelium, mucification layer if present, and transition zone with uterine body; endometrium includes glands and epithelium lining central lumen. IHC labeling score represents average (0-4) within each group.
Tissues from some uteri were not present in section.
Letters indicate
P<0.05,
P<0.01, and
P<0.001 compared with corresponding age- matched control group using a two-tailed Fisher exact test.
TUNEL
In cervical squamous epithelium, nuclear ± cytoplasmic TUNEL was present mainly in scattered cells within the superficial cornification layer and in cells that had already sloughed into the central lumen (Fig. 11A, Table 3C). In the endometrium, TUNEL+ cells were scattered throughout the luminal epithelium and tended to be more prevalent along the central lumen compared to deeper glands (Fig. 11B). No differences in cervical or endometrial epithelial TUNEL were noted between CON and DES or GEN mice at 2-3 or 18 mo of age. In metaplastic basal cells, TUNEL was completely absent or present in only a few scattered cells (estimated at <1% of total) (Fig. 11C). In areas of squamous metaplasia (non-neoplastic) or squamous differentiation (neoplastic), TUNEL was generally limited to the superficial layer and sloughed intraluminal cells, similar to normal squamous cervix (Fig. 11D). Neoplastic glands without full squamous differentiation (and not including sloughed cells) had either no TUNEL labeling or very few positive cells (<1%).
Figure 11. TUNEL profile in the uterine epithelium.
A, Sporadic TUNEL in the transition zone. B, Normal endometrium with TUNEL predominantly in central lumen epithelium and absent from deep glands. C, TUNEL+ nuclei in normal luminal epithelial cells (upper image, from a control mouse) and lack of staining in metaplastic basal cells (lower image, from a DES mouse). D, Uterine carcinoma showing TUNEL in sloughed cells within neoplastic glands. Slides for all images were stained for TUNEL (A-D). Original objective 20× (A), 40× (B), 60× (C), and 20× (D).
ERα and PGR
Nuclear expression of both ERα and PGR was present throughout normal cervical squamous and endometrial glandular epithelium of all mice (Table 3D). In the normal endometrium, ERα labeling was often present diffusely within glandular epithelium (Fig. 12A), whereas PGR expression varied from sporadic patchy labeling of basal and luminal cells to more diffuse labeling of all epithelial cells (Fig. 12B). No clear differences in epithelial ERα or PGR expression were observed between CON and DES or GEN mice (Table 3D). ERα and PGR were also expressed within all metaplastic, hyperplastic, and neoplastic uterine glands, including luminal and basal cells (Fig. 12B–F).
Table 3D.
Immunohistochemical expression of differentiation markers in the uterine epithelium following neonatal exposure to DES or GEN
| Age (mo)
|
|||||||
|---|---|---|---|---|---|---|---|
| 2–3
|
18
|
||||||
| Marker | Site | CON | GEN | DES | CON | GEN | DES |
| ERα | Cervix: non-neoplastic | 13/13 (100%) |
10/10 (100%) |
9/9 (100%) |
9/9 (100%) |
10/10 (100%) |
12/12 (100%) |
| Labeling score | 2.4 | 2.0 | 2.0 | 2.8 | 2.0 | 2.3 | |
|
|
|
|
|||||
| Endometrium: non-neoplastic* | 12/12 (100%) |
10/10 (100%) |
9/9 (100%) |
9/9 (100%) |
10/10 (100%) |
12/12 (100%) |
|
| Labeling score | 3.2 | 3.9 | 3.9 | 3.3 | 3.7 | 4.0 | |
|
|
|
|
|||||
| Endometrium: neoplastic | na | na | na | na | 10/10 (100%) |
12/12 (100%) |
|
| Labeling score | na | na | na | na | 3.3 | 3.5 | |
|
| |||||||
| PGR | Cervix: non-neoplastic | 13/13 (100%) |
10/10 (100%) |
9/9 (100%) |
9/9 (100%) |
10/10 (100%) |
12/12 (100%) |
| Labeling score | 2.5 | 3.5 | 3.4 | 2.1 | 2.7 | 2.4 | |
|
|
|
|
|||||
| Endometrium: non-neoplastic* | 12/12 (100%) |
10/10 (100%) |
9/9 (100%) |
9/9 (100%) |
10/10 (100%) |
12/12 (100%) |
|
| Labeling score | 1.3 | 1.6 | 1.8 | 2.0 | 2.2 | 2.1 | |
|
|
|
|
|||||
| Endometrium: neoplastic | na | na | na | na | 10/10 (100%) |
12/12 (100%) |
|
| Labeling score | na | na | na | na | 1.8 | 1.7 | |
Abbreviations: CON, control; GEN, genistein; DES, diethylstilbestrol; na, not applicable. Cervix includes stratified squamous epithelium, mucification layer if present, and transition zone with uterine body; endometrium includes glands and epithelium lining central lumen. IHC labeling score represents average (0-4) within each group.
Tissues from some uteri were not present in section.
Letters indicate
P<0.05,
P<0.01, and
P<0.001 compared with corresponding age-matched control group using a two-tailed Fisher exact test.
Figure 12. ERα and PGR IHC profile in the uterine epithelium.
A, Diffuse expression of ERα in the normal endometrial glands. B, Sporadic expression of PGR in normal endometrial glands. C and D, ERα and PGR labeling in basal and luminal epithelial cells of glands with squamous metaplasia. E, Diffuse ERα labeling in luminal and basal cells of neoplastic glands in a uterine carcinoma. F, PGR expression in luminal and basal compartments of a uterine carcinoma. Slides for all images were labeled for ERα (A, C, E) or PGR (B, D, F). Original objective 20× (A), 40× (B), and 20× (C-F).
Time-course of neoplastic changes
Previous studies have documented uterine changes in CD-1 mice induced by neonatal DES exposure up to PND 22 (Yoshida, Newbold, and Dixon 1999; Yoshida, Newbold, and Dixon 2000). Early effects included decreased glandular cell proliferation, abnormal expression of estrogen-related differentiation markers, and the development of fingerlike cytoplasmic processes in the intercellular basal regions of epithelial cells. Our goal here was to track DES and GEN effects from early to late adulthood. At 2-3 mo of age, all control mice showed evidence of estrous cyclicity based on uterine and vaginal histology. The only morphologic lesion in the uterine epithelium among DES and GEN mice at this time was basal cell metaplasia, which was represented by small foci scattered along the periphery of the primary endometrial lumen and endometrial glands within both the uterine body and horns (Table 2). All other glandular lesions induced by DES and GEN were present by 6 mo of age and increased in incidence with age. The incidence of uterine carcinoma in DES and GEN treated mice ranged from 0% at 2-3 mo to 7-16% at 6 mo, 29-35% at 12 mo, and 33-40% at 18 mo. No cases of uterine carcinoma were observed in CON mice at any time point assessed.
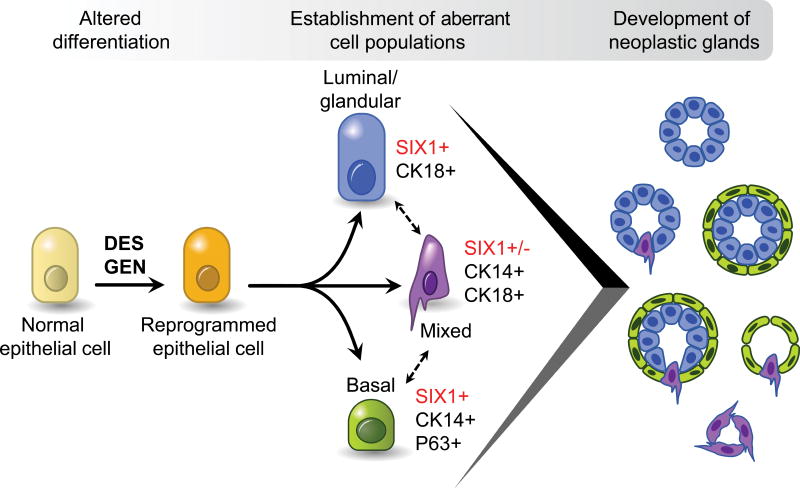
Morphologic and molecular characteristics of abnormal cell types and neoplastic lesions induced by DES and GEN are summarized in Fig. 13. At a cellular level, the SIX1+/P63+/CK14+/CK18− basal cells and associated SIX1+/P63−/CK14−/CK18+ luminal cells increased with age from small sparse foci at 2-3 mo of age to become widespread within endometrial glands in older DES and GEN mice. Accordingly, the IHC scores for basal cell markers SIX1, P63, and CK14 increased at least 2-fold between 2-3 mo and 18 mo of age (Table 3). These SIX1+ basal and luminal cells appeared at the same time, and there was no morphological evidence indicating a direct lineage between them (Fig. 13). A smaller population of mixed/bipotential cells co-expressing CK14 and CK18 was also present at both 2-3 and 18 mo of age. These cells were more prevalent in neoplastic compared to non-neoplastic glands but otherwise did not appear to increase with age in non-neoplastic glands. There were no clear age-related effects in the expression of CD44, TUNEL, ERα, or PGR in non-neoplastic endometrial glands in any of the groups (Table 3C and 3D). No differences in IHC marker expression were observed between DES and GEN groups at any of the observed time points (Table 3).
Figure 13. Lineage scheme for abnormal cell populations induced by neonatal exposure to estrogenic chemicals.
Diagram shows proposed differentiation patterns for cells present in uterine carcinomas.
Discussion
Exposure to estrogenic chemicals during key windows of development can increase risk of reproductive tract cancer. This carcinogenic effect has been associated with altered patterns of cellular differentiation (e.g., reviewed in Kurita 2011). The goal of this study was to characterize uterine epithelial phenotypes resulting from neonatal exposure to two different reference estrogens, DES and GEN. Both chemicals induced abnormal populations of luminal, basal, and mixed endometrial epithelial cells. These three distinct cell populations were established by early adulthood and later comprised the vast majority of cells within all uterine carcinomas. These findings support the idea that cancer risk later in life can be influenced by altered patterns of cellular differentiation and programming established during early development.
The most prominent early change induced by neonatal estrogen exposure was an increase in uterine basal cell metaplasia. This lesion was characterized by 1-2 layers of cuboidal cells distributed between the basal lamina and the glandular epithelium of the central endometrial lumen and along the perimeter of deeper uterine glands. Basal cells were rarely present in control mice beyond the junctional zone of the squamous cervix and uterine body. While this change may be seen sporadically as a background finding in other mouse strains, it is generally restricted to the uterine body and not associated with cancer (e.g., Couse et al. 1997). Endometrial basal cells in DES and GEN mice showed positive labeling for SIX1, P63, CK14, ERα, and PGR, low TUNEL expression, and sporadic CD44 expression, consistent with a mature basal cell phenotype. These cells increased with age so that by 18 mo they were a predominant feature of the endometrial epithelium (typically >50% of glands affected).
The second abnormal cell population induced by neonatal estrogen exposure consisted of columnar luminal cells overlying metaplastic basal cells. These cells were positive for CK18 and negative for P63 and CK14, similar to normal glandular epithelium. The distinguishing feature was nuclear labeling for SIX1, which was generally absent from normal endometrial luminal epithelium (beyond the junctional zone with cervix). SIX1 is a transcription factor widely expressed during development and involved in tissue differentiation (Christensen et al. 2008; Yajima et al. 2014). It is also overexpressed in various human cancers and has thus been considered an oncofetal protein because of its potential dual roles in development and cancer (Wu et al. 2015). Previously, we identified luminal SIX1+ “founder” cells at the time of DES and GEN exposure (PND5) and showed increasing SIX1 expression with age (Suen et al. 2016). Here, we found that SIX1 is expressed in both P63+/CK14+/CK18− basal cells and P63−/CK14−/CK18+ luminal cells when directly adjacent to basal cells. This distinctive localization suggests that SIX1 may mediate some type of cross-talk between abnormal basal and luminal cell populations.
The third cell population induced by neonatal estrogen exposure showed a mixed phenotype with both basal and luminal elements. These cells co-expressed CK14, CK18, and SIX1 but not P63 and could be identified only through IHC labeling. Morphologically, they had a columnar luminal phenotype and did not associate with P63+ basal cells. Low numbers of CK14+/CK18+ cells were present sporadically in the cervical and endometrial epithelium of the central lumen in control mice, but these cells (in the endometrium at least) had no or equivocal labeling for SIX1. In older DES and GEN mice, CK14+/CK18+ cells were present along the basal margin of endometrial glands. When present in neoplastic lesions, they often showed features of microinvasion including disruption of the basement membrane and extension of cytoplasmic processes into the adjacent stroma. To our knowledge, no prior studies have specifically identified CK14+/CK18+ cells in the endometrial epithelium. In other tissues, such as prostate and mammary gland (which normally have a layer of CK14+ basal/myoepithelial cells, unlike endometrium), several reports have described small fractions of CK14+/CK18+ cells and proposed that these represent a progenitor or stem cell population (e.g., Wang et al. 2001; Smith, Mehrel, and Roop 1990; Buono et al. 2006). Additional studies are needed to determine whether SIX1+/P63−/CK14+/CK18+ cells are specific to this mouse model or also present in human uterine cancers.
All uterine carcinomas and associated hyperplastic lesions in this study had a combination of basal and glandular components. We consider this to be a dual or mixed phenotype rather than a primary adenocarcinoma with squamous differentiation (or squamous cell carcinoma with glandular differentiation) based on the presence of all three abnormal cell types described above in all hyperplastic lesions and tumors evaluated. Still, it is not clear which of these cell types actually progresses to malignancy. Based on morphologic features of atypia and local invasion and the general lack of a differentiated state, we hypothesize that the population of mixed P63−/CK14+/CK18+/SIX1+ cells is the cell of origin for both P63+/CK14+/CK18−/SIX1+ basal cells and P63−/CK14−/CK18+/SIX1+ luminal cells and the most likely tumor progenitor candidate (Fig. 13). This idea is further supported by the observations that (1) P63+/CK14+/CK18− basal cells in other sites such as mammary gland or vagina are not promoted to cancer by neonatal estrogen exposure and (2) SIX1+/P63−/CK14−/CK18+ luminal cells do not show clear morphologic evidence of neoplastic progression. Glandular structures within carcinomas consisting of only SIX1+/P63−/CK14−/CK18+ luminal cells were rare (and when present, difficult to identify as neoplastic based on morphology), filling of glandular lumens to form solid tubules (as sometimes seen with other types of luminal cell adenocarcinomas) was not observed, and local invasion typically occurred along the basal margin of neoplastic glands by CK14+ cells. Future work in genetically-modified mouse models could be used to delineate the role of differentiation factors like SIX1 in regulating these distinct cell types and promoting uterine carcinogenesis.
A growing body of evidence supports the idea that chemical exposure during development can increase cancer risk later in life through epigenetic processes. However, there remains a critical need for mechanistic information and biomarkers that will enable identification of susceptible populations. Here we examined epithelial differentiation patterns over time following neonatal estrogen exposure and identified cell populations associated with endometrial cancer development. These results inform the relationship between early developmental exposures, aberrant cellular programming, and reproductive outcomes later in life. Identification of the molecular drivers of these differentiation patterns may provide insights into appropriate biomarkers and/or therapeutics for human endometrial cancer.
Acknowledgments
The authors would like to thank Elizabeth Padilla-Banks, Beth Mahler, Eli Ney, Heather Jensen, Geoffrey Hurlburt, Kyathanahalli Janardhan, Natasha Clayton, the staff at the NIEHS Histology and Immunohistochemistry Core, and the NIEHS Graphics Core for technical expertise and support. The authors would also like to thank internal reviewers for their detailed critiques of this manuscript.
Funding
This research was supported by the Intramural Research Program of the NIH, National Institute of Environmental Health Sciences, 1ZIAES102405 (CJW), the U.S. EPA Office of Research and Development (CEW), and the ORISE program (AAS).
Abbreviations
- CK
cytokeratin
- CON
control
- DAB
3,3′-diaminobenzidine
- DES
diethylstilbestrol
- EPA
U.S. Environmental Protection Agency
- ER
estrogen receptor
- FDA
U.S. Food and Drug Administration
- GEN
genistein
- H&E
hematoxylin and eosin
- IHC
immunohistochemistry
- INHAND
International Harmonization of Nomenclature and Diagnostic Criteria for Lesions in Rats and Mice
- mo
month
- NIEHS
National Institute of Environmental Health Sciences
- NIH
National Institutes of Health
- NTP
National Toxicology Program
- ORISE
Oak Ridge Institute for Science and Education
- PND
postnatal day
- PGR
progesterone receptor
- SCJ
squamocolumnar junction
- SIX1
sine oculis-related homeobox 1
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
Footnotes
Authors’ note
This article has been reviewed by the U.S. EPA and approved for publication. Approval does not signify that the contents necessarily reflect the views of the agency, and mention of trade names or commercial products does not constitute endorsement or recommendation for use.
Declaration of Conflicting Interests Statement
The author(s) declared no potential, real, or perceived conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Buono KD, Robinson GW, Martin C, Shi S, Stanley P, Tanigaki K, Honjo T, Hennighausen L. The canonical Notch/RBP-J. signaling pathway controls the balance of cell lineages in mammary epithelium during pregnancy. Dev Biol. 2006;293:565–80. doi: 10.1016/j.ydbio.2006.02.043. [DOI] [PubMed] [Google Scholar]
- Christensen KL, Patrick AN, McCoy EL, Ford HL. The six family of homeobox genes in development and cancer. Adv Cancer Res. 2008;101:93–126. doi: 10.1016/S0065-230X(08)00405-3. [DOI] [PubMed] [Google Scholar]
- Cooke PS, Spencer TE, Bartol FF, Hayashi K. Uterine glands: development, function and experimental model systems. Mol Hum Reprod. 2013;19:547–58. doi: 10.1093/molehr/gat031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couse JF, Dixon D, Yates M, Moore AB, Ma L, Maas R, Korach KS. Estrogen receptor-alpha knockout mice exhibit resistance to the developmental effects of neonatal diethylstilbestrol exposure on the female reproductive tract. Dev Biol. 2001;238:224–38. doi: 10.1006/dbio.2001.0413. [DOI] [PubMed] [Google Scholar]
- Couse JF, Davis VL, Hanson RB, Jefferson WN, McLachlan JA, Bullock BC, Newbold RR, Korach KS. Accelerated onset of uterine tumors in transgenic mice with aberrant expression of the estrogen receptor after neonatal exposure to diethylstilbestrol. Mol Carcinog. 1997;19:236–42. doi: 10.1002/(sici)1098-2744(199708)19:4<236::aid-mc4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Dixon D, Alison R, Bach U, Colman K, Foley GL, Harleman JH, Haworth R, et al. Nonproliferative and proliferative lesions of the rat and mouse female reproductive system. J Toxicol Pathol. 2014;27(3–4 Suppl):1S–107S. doi: 10.1293/tox.27.1S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA. Selected Item from the FDA Drug Bulletin—November 1971: Diethylstilbestrol Contraindicated in Pregnancy. Calif Med. 1972;116:85–6. [PMC free article] [PubMed] [Google Scholar]
- Giusti RM, Iwamoto K, Hatch EE. Diethylstilbestrol revisited: a review of the long-term health effects. Ann Intern Med. 1995;122:778–88. doi: 10.7326/0003-4819-122-10-199505150-00008. [DOI] [PubMed] [Google Scholar]
- Hatch EE, Herbst AL, Hoover RN, Noller KL, Adam E, Kaufman RH, Palmer JR, et al. Incidence of squamous neoplasia of the cervix and vagina in women exposed prenatally to diethylstilbestrol (United States) Cancer Causes Control. 2001;12:837–45. doi: 10.1023/a:1012229112696. [DOI] [PubMed] [Google Scholar]
- Hendry WJ, 3rd, DeBrot BL, Zheng X, Branham WS, Sheehan DM. Differential activity of diethylstilbestrol versus estradiol as neonatal endocrine disruptors in the female hamster (Mesocricetus auratus) reproductive tract. Biol Reprod. 1999;61:91–100. doi: 10.1095/biolreprod61.1.91. [DOI] [PubMed] [Google Scholar]
- Herceg Z, Ghantous A, Wild CP, Sklias A, Casati L, Duthie SJ, Fry R, et al. Roadmap for investigating epigenome deregulation and environmental origins of cancer. Int J Cancer. 2018;142:874–82. doi: 10.1002/ijc.31014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SM, Cheong A, Adgent MA, Veevers J, Suen AA, Tam NNC, Leung YK, Jefferson WN, Williams CJ. Environmental factors, epigenetics, and developmental origin of reproductive disorders. Reprod Toxicol. 2017;68:85–104. doi: 10.1016/j.reprotox.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover RN, Hyer M, Pfeiffer RM, Adam E, Bond B, Cheville AL, Colton T, et al. Adverse health outcomes in women exposed in utero to diethylstilbestrol. N Engl J Med. 2011;365:1304–14. doi: 10.1056/NEJMoa1013961. [DOI] [PubMed] [Google Scholar]
- IARC. Pharmaceuticals: Diethylstilbestrol. IARC Monographs on the evaluation of carcinogenic risks to humans. 2012:100A. [Google Scholar]
- Jefferson WN, Padilla-Banks E, Phelps JY, Gerrish KE, Williams CJ. Permanent oviduct posteriorization after neonatal exposure to the phytoestrogen genistein. Environ Health Perspect. 2011;119:1575–82. doi: 10.1289/ehp.1104018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson WN, Chevalier DM, Phelps JY, Cantor AM, Padilla-Banks E, Newbold RR, Archer TK, Kinyamu HK, Williams CJ. Persistently altered epigenetic marks in the mouse uterus after neonatal estrogen exposure. Mol Endocrinol. 2013;27:1666–77. doi: 10.1210/me.2013-1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita T, Cunha GR. Roles of p63 in differentiation of Müllerian duct epithelial cells. Ann N Y Acad Sci. 2001;948:9–12. doi: 10.1111/j.1749-6632.2001.tb03982.x. [DOI] [PubMed] [Google Scholar]
- Kurita T, Mills AA, Cunha GR. Roles of p63 in the diethylstilbestrol-induced cervicovaginal adenosis. Development. 2004;131:1639–49. doi: 10.1242/dev.01038. [DOI] [PubMed] [Google Scholar]
- Kurita T. Normal and abnormal epithelial differentiation in the female reproductive tract. Differentiation. 2011;82:117–26. doi: 10.1016/j.diff.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laronda MM, Unno K, Butler LM, Kurita T. The development of cervical and vaginal adenosis as a result of diethylstilbestrol exposure in utero. Differentiation. 2012;84:252–60. doi: 10.1016/j.diff.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masse J, Watrin T, Laurent A, Deschamps S, Guerrier D, Pellerin I. The developing female genital tract: from genetics to epigenetics. Int J Dev Biol. 2009;53:411–24. doi: 10.1387/ijdb.082680jm. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Banks EP, Bullock B, Jefferson WN. Uterine adenocarcinoma in mice treated neonatally with genistein. Cancer Res. 2001;61:4325–8. [PubMed] [Google Scholar]
- Newbold RR, Bullock BC, McLachlan JA. Uterine adenocarcinoma in mice following developmental treatment with estrogens: a model for hormonal carcinogenesis. Cancer Res. 1990;50:7677–81. [PubMed] [Google Scholar]
- Newbold RR, McLachlan JA. Vaginal adenosis and adenocarcinoma in mice exposed prenatally or neonatally to diethylstilbestrol. Cancer Res. 1982;42:2003–11. [PubMed] [Google Scholar]
- Ostrander PL, Mills KT, Bern HA. Long-term responses of the mouse uterus to neonatal diethylstilbestrol treatment and to later sex hormone exposure. J Natl Cancer Inst. 1985;74:121–35. [PubMed] [Google Scholar]
- Painter JT, Clayton NP, Herbert RA. Useful immunohistochemical markers of tumor differentiation. Toxicol Pathol. 2010;38:131–41. doi: 10.1177/0192623309356449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed CE, Fenton SE. Exposure to diethylstilbestrol during sensitive life stages: a legacy of heritable health effects. Birth Defects Res C Embryo Today. 2013;99:134–46. doi: 10.1002/bdrc.21035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith GH, Mehrel T, Roop DR. Differential keratin gene expression in developing, differentiating, preneoplastic, and neoplastic mouse mammary epithelium. Cell Growth Differ. 1990;1:161–70. [PubMed] [Google Scholar]
- Suen AA, Jefferson WN, Wood CE, Padilla-Banks E, Bae-Jump VL, Williams CJ. SIX1 oncoprotein as a biomarker in a model of hormonal carcinogenesis and in human endometrial cancer. Mol Cancer Res. 2016;14:849–58. doi: 10.1158/1541-7786.MCR-16-0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verloop J, van Leeuwen FE, Helmerhorst TJ, van Boven HH, Rookus MA. Cancer risk in DES daughters. Cancer Causes Control. 2010;21:999–1007. doi: 10.1007/s10552-010-9526-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CL, Ho SM. Developmental reprogramming of cancer susceptibility. Nat Rev Cancer. 2012;12:479–86. doi: 10.1038/nrc3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Hayward S, Cao M, Thayer K, Cunha G. Cell differentiation lineage in the prostate. Differentiation. 2001;68:270–9. doi: 10.1046/j.1432-0436.2001.680414.x. [DOI] [PubMed] [Google Scholar]
- Wu W, Ren Z, Li P, Yu D, Chen J, Huang R, Liu H. Six1: a critical transcription factor in tumorigenesis. Int J Cancer. 2015;136:1245–53. doi: 10.1002/ijc.28755. [DOI] [PubMed] [Google Scholar]
- Yajima H, Suzuki M, Ochi H, Ikeda K, Sato S, Yamamura K, Ogino H, Ueno N, Kawakami K. Six1 is a key regulator of the developmental and evolutionary architecture of sensory neurons in craniates. BMC Biol. 2014;12:40. doi: 10.1186/1741-7007-12-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida A, Newbold RR, Dixon D. Effects of neonatal diethylstilbestrol (DES) exposure on morphology and growth patterns of endometrial epithelial cells in CD-1 mice. Toxicol Pathol. 1999;27:325–33. doi: 10.1177/019262339902700308. [DOI] [PubMed] [Google Scholar]
- Yoshida A, Newbold RR, Dixon D. Abnormal cell differentiation and p21 expression of endometrial epithelial cells following developmental exposure to diethylstilbestrol (DES) Toxicol Pathol. 2000;28:237–45. doi: 10.1177/019262330002800203. [DOI] [PubMed] [Google Scholar]