Abstract
BACKGROUND
The standard of burn treatment today reflects major advances. We sought to quantitate the impact of these advances on burn survival via age-stratified mortality ratios compared to other reported mortality analyses in burns.
STUDY DESIGN
Age, percent of the total body surface area (TBSA) burned, presence of inhalation injury, length of stay, and survival status were recorded at admission and at discharge for all new burn admissions between 1989 and 2017. The expected mortality probability was calculated using historical multiple regression techniques and compared with observed data. We developed a prediction model for our observed data.
RESULTS
Between 1989 and 2017, there were 10,384 consecutive new burn admissions with 355 mortalities (median age: 13 years; median percent TBSA burn: 11%). We observed a significant decrease in our observed mortality data compared to historical predictions (p<0.0001) and a 2% reduction per year in mortality over the three decades. The prediction model of mortality for the data is as follows: Pr(dying) = ex/(1 + ex) where x = −6.44 − 0.12 age + 0.0042 age2 − 0.0000283 age3 + 0.0499 TBSA + 1.21 Inhalation Injury + 0.015 third degree TBSA.
CONCLUSIONS
The reduction in mortality over time may be attributed to successful changes in standard of care protocols in the burn center that improved the outlook for burned individuals, including protocols for management of inhalation injury, nutrition, resuscitation, and early excision and grafting.
Keywords: prediction model, standard of care, age, total body surface area burn, inhalation injury
INTRODUCTION
Mortality from burns is determined by age, sex, burn size, and the presence or absence of inhalation injury. Severe burn injuries also produce a profound hypermetabolic stress response, which is characterized by excessive glucose production, protein catabolism, and an influx of oxidants (1–3). The stress response to burn causes a severe loss of lean body mass and muscle wasting (4, 5). Infection that occurs during the hospital course, immunological compromise (6), and growth delays in both muscle and bone (7) contribute to morbidity, mortality, and prolonged recovery.
The association between percent total body surface area (TBSA) burned and survival was first noted in 1902 (8). Beginning in 1949, age-stratified probit modeling was used to evaluate changes in the standard of burn care, although other methods have been occasionally used (9). Probit analysis converts a sigmoid dose-response curve into linear form and allows the evaluation of burn size in terms of mortality and other binary outcome data (10–12). Bull, Squire, and Fisher are credited with the first application of probit analysis for the quantitative assessment of advancements in burn care, and three analyses were separately published spanning the years 1942 to 1970. They selected the age categories of 0–14, 15–44, 45–64, and ≥65 years; for each, they reported the percent TBSA burned that resulted in 50% mortality (LA50) (13–15). Barnes reported data from Massachusetts General Hospital in 1957 (16). Schwartz (17), and later Pruitt, reported similar numbers for the Brooke Army Medical Center (18); additional reports of burn LA50 have used the four age categories established by Bull, Squire, and Fisher (Table 1). In 1980, Currerri, Luterman, Braun, and Shires predicted age-adjusted mortality in 937 burned patients (79% survival, median age of 29 years, median burn size of 18% TBSA) using a logistic regression formula to describe the standard of care at the time (19). Predicted mortality based on TBSA burn and age was used as the primary metric of progress in burn care in their model. There was an apparent decrease in mortality beginning in 1987, particularly in younger individuals, which may be attributed to the implementation of standardized protocols. To further explore mortality, we analyzed data from 1989 and onwards.
Table 1.
Historical Comparison of Percent Total Body Surface Area Burn Resulting in 50% Mortality (Lethal Area50)
| Year | First author (country) | 0–14 Years | 15–44 Years | 45–64 Years | ≥65 Years | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | ||
| 1949 | Bull (UK) | 342 | 51 | 311 | 43 | 95 | 23 | 46 | 9 |
| 1954 | Bull (UK) | 1,366 | 49 | 967 | 46 | 330 | 27 | 144 | 10 |
| 1956 | Schwartz (US) | -- | –– | 480 | 65 | -- | –– | -- | –– |
| 1957 | Barnes (US) | 217 | 39 | 221 | 65 | 219 | 39 | 128 | 26 |
| 1964 | Pruitt (US) | 238 | 49 | 806 | 56 | 56* | 29* | –– | –– |
| 1971 | Bull (UK) | 962 | 64 | 565 | 56 | 246 | 40 | 149 | 17 |
| 1980 | Curerri (US) | 232 | 63 | 413 | 63 | 178 | 38 | 114 | 23 |
| 1987 | Herndon (US) | 875 | 95 | 612 | 76 | 132 | 46 | 52 | 19 |
≥50 years.
The specific objectives of our study were to determine a regression model of mortality in all pediatric and adult burned patients who were admitted to Shriners Hospitals for Children—Galveston (SHC) or the Blocker Burn Unit (BBU) in Galveston from 1989 to 2017. All patients were treated according to standardized protocols of care at one burn center, including protocols for inhalation injury, nutrition, resuscitation strategies, and early excision and grafting. This retrospective chart and database review was approved by the University of Texas Medical Branch Institutional Review Board (Protocol No. 14-036 and 17-0036). The datasets analyzed during the current study are available from the corresponding author upon reasonable request.
We also compared our model with other prediction models from groups including Curreri et al.; Shirani, Pruitt, and Mason; and the revised Baux score from the National Burn Repository (19–21). We found that percentage of TBSA burned, patient age, and the presence of inhalation injury are primary determinants of mortality and that improvements in standardized protocols of burn care have resulted in a lower mortality compared to referenced prediction models from earlier periods.
METHODS
Subject Demographics and Injury Characteristics
A total of 10,384 patients were admitted to SHC and BBU between January 1989 and July 2017. All subjects regardless of age or TBSA burned were included in our analysis. Patients admitted for nonburns (toxic epidermal necrolysis, Stevens-Johnson syndrome, inhalation injury without burn, aggressive bacterial infections, reconstructive surgery only) were excluded from this study. Patient age, sex, percent TBSA burned, percent of TBSA with third-degree burns, length of stay, and presence of inhalation injury were recorded at the time of admission for patients. Age-appropriate diagrams were used to determine burn size (22). Survival status at the time of hospital discharge had been recorded. All subjects received our standard of care for wound treatment and nutrition as described previously (23, 24).
This study was approved by the Institutional Review Board of the University of Texas Medical Branch (Galveston, TX) and the Shriners Hospitals for Children’s Office for Clinical Research. Individual patient consents were not required for this retrospective review.
Inhalation Injury Diagnosis
The presence or absence of inhalation injury was confirmed by bronchoscopy in patients suspected to have inhalation injury. Presence was diagnosed by positive findings including edema, erythema, hemorrhage and bronchorrhea, mucosal blisters and erosion, and deposits of soot.
Statistical Modeling
The LA50 curve was produced by fitting a generalized nonlinear logistic model on age and TBSA burn (25). The curve corresponds to those values of TBSA burn by age for which 50% survival is expected. The confidence interval was generated by calibrating bootstrap confidence intervals on the fitted probability of mortality. To compare predicted mortality against actual mortality, a generalized smoothing spline was fit. The actual mortality risk estimate and standard errors were produced for each value of predicted mortality risk from the formulae of Curreri et al., Shirani et al., and Osler et al. The odds ratio was estimated by comparing the predicted mortality odds against the mortality odds of our cohort. The linear prediction model was constructed to minimize the Bayesian Information Criterion. Sensitivity, specificity, and accuracy were estimated using bootstrap smoothed cross-validation (26). Year-to-year mortality odds reduction was estimated based on a generalized additive model, adjusting for age, sex, TBSA burned, TBSA with third-degree burns, inhalation injury, and length of stay. The model for length of stay was calculated based on a parametric (exponential) time-to-event model, with death as the censoring mechanism.
With the exception of third-degree TBSA burn (for which values were missing), less than 10% of subjects had missing predictor or response values; thus, subjects with missing values were ignored. A sensitivity comparison was done to compare models with third-degree TBSA burn (and subjects with missing values ignored) against a fit model without third-degree TBSA burn; models were similar enough to conclude that the model with third-degree TBSA burn was not biased. All calculations were done in R (Version 3.4.0).
RESULTS
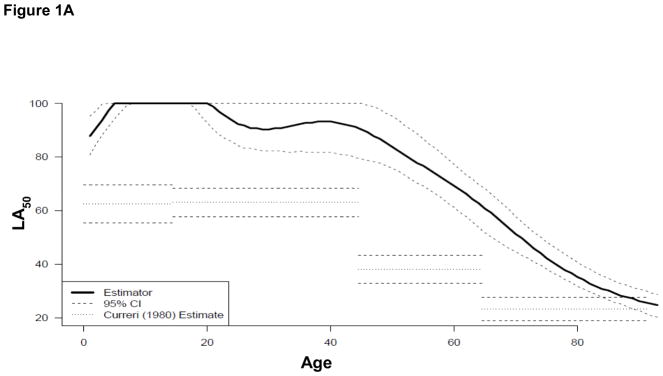
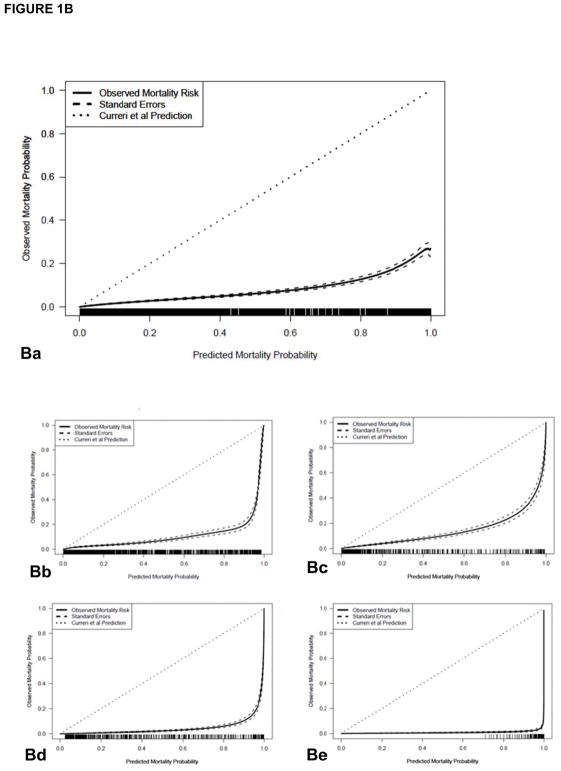
Figure 1A illustrates the LA50 of our prediction model (solid line) with 95% confidence intervals (CI, large dotted lines) compared to Curerri’s et al. probit model (small dotted lines). Figure 1B illustrates the Curreri et al. predicted and true survival rates overall (a) and among different age groups (0–14 years [b], 15–44 years [c], 45–64 years [d], >65 years [e]) compared to our data from 1989 to 2017 (Table 2; 10,029 survivors and 355 nonsurvivors [3.4% mortality]). The expected reciprocal odds ratio of mortality is 9.5 overall, 10.3 for 0–14 years, 4.7 for 15–44 years, 40 for 45–64 years, and 3030 for >65 years. Since the uncertainty in the Curreri et al. estimator is unknown, precise inference is not possible. On average, the Curreri et al. model overestimated the true mortality rate by an average of 12.4 standard errors (SE, overall), 6.0 SE for 0–14 years, 5.3 SE for 15–44 years, 11.9 SE for 45–64 years, and 31.8 SE for >65 years; in these cases, the comparisons are significantly different (p<0.05). Table 3 illustrates observed survival among all age-stratified groups of 10,384 burn patients from SHC from 1989 to 2017 compared with the Curerri et al. model expected mortality (via the sum predicted mortality probability).
Figure 1.
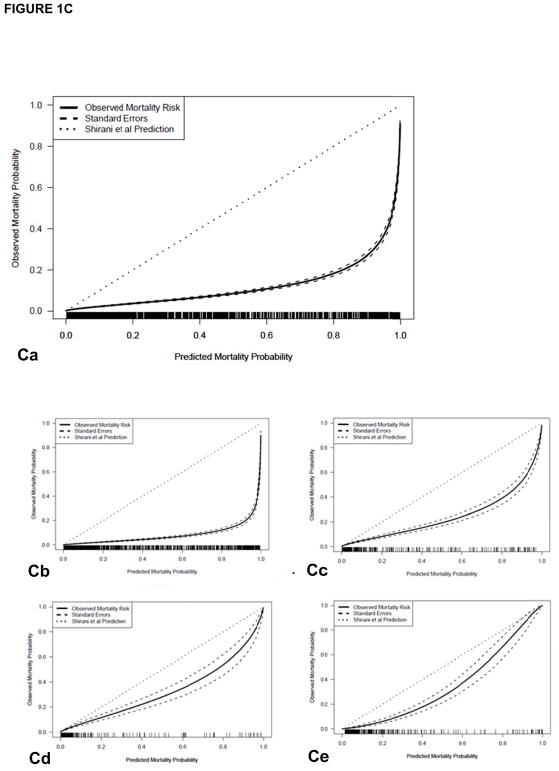
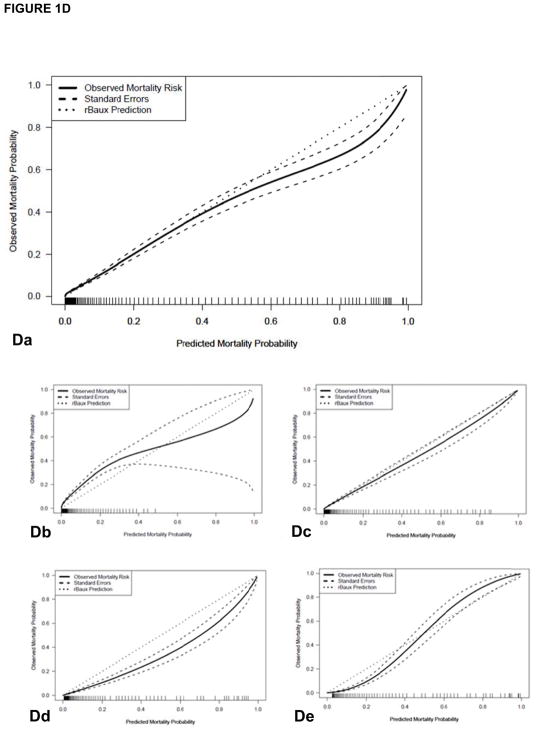
(A) The LA50 function of the nonlinear prediction model (solid line) with 95% confidence intervals (CI, dashed lines) compared to Curerri’s model (dotted lines). (B–D) shows a comparison of (B) Curreri, (C) Shirani, and (D) revised Baux prediction of probability of mortality (small dotted line at 45°) versus observed rate of mortality (solid line) along with standard errors, overall and divided by age groups. (Ba) The Curreri predicted and true survival rates overall and among different age groups: (Bb) 0 to 14 years, (Bc) 15 to 44 years, (Bd) 45 to 64 years, (Be) >65 years, from 1989 to 2017. Similar comparisons are illustrated with (Ca-e) Shirani and (Da-e) the revised Baux analysis. In both historical cases, the predicted fit falls below the line of agreement, indicating that these models predicted a greater number of mortalities than we observed in our dataset.
Table 2.
Demographics
| Parameter | Value |
|---|---|
| n | 10,384 |
| Age, y, mean ± SE (median, IQR) | 21 ± 0.21 (13, 3–35) |
| Male, % | 69 |
| TBSA burned, median, mean ± SE (median, IQR)* | 20 ± 0.21 (11, 4–30) |
| TBSA third-degree burned, mean ± SE (median, IQR)† | 13 ± 0.27 (1, 0–17) |
| Presence of inhalation injury, % | 12.2 |
| Length of stay, days, mean ± SE (median, IQR) | 12 ± 0.20 (5, 2–14) |
| Burn to admission, days, median, IQR | 1, 0–3 |
| Mortality, % | 3.4 |
TBSA burned, percent total body surface area burned.
TBSA third-degree burn, percent of total body surface area with third-degree burns.
TBSA, total body surface area.
Table 3.
Observed and Expected Survival in Overall and Age-Stratified Groups: Comparison with Curreri19
| Parameter | Total | Age group | |||
|---|---|---|---|---|---|
| 0–14 Years | 15–44 Years | 45–64 Years | ≥65 Years | ||
| SHC/BBU, n | 10,384 | 5,524 | 3,154 | 1,267 | 439 |
| Observed (actual) mortality at SHC/BBU, n | 355 | 133 | 93 | 57 | 72 |
| Expected mortality per Curreri’s model, n | 1,342 | 684 | 223 | 153 | 282 |
| Fold mortality reduction, reciprocal odds ratio | 4.2 | 5.7 | 2.7 | 2.9 | 9.2 |
| 95% CI | (3.7–4.7) | (4.7–6.9) | (2.1–3.5) | (2.1–4) | (6.7–12.6) |
| p Value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 |
SHC/BBU, Shriners Hospitals for Children—Galveston and Blocker Burn Unit.
Our observed mortality data were compared in a similar manner to other notable burn mortality prediction models including (1) Shirani et al.’s model (20), which accounts for the presence of inhalation injury and pneumonia, in addition to TBSA burn and age (Table 4; Figure 1C) and (2) the revised Baux Score (27), which is an updated version of the original Baux score that is calculated by adding patient age, TBSA burn, and 17 points for the presence of inhalation injury (Table 5, Figure 1D). Figure 1C illustrates the Shirani et al. predicted and true survival rates overall (a) and among different age groups (0–14 years [b], 15–44 years [c], 45–64 years [d], >65 years [e]) from 1989 to 2017; Table 4 illustrates that significantly lower mortality was observed overall and in all age groups except 45–64 years compared to the Shirani model prediction. Figure 1D illustrates significant differences between the revised Baux predicted and true survival rates overall (a) and among different age groups (0–14 years [b], 15–44 years [c], 45–64 years [d], >65 years [e]) from 1989 to 2017; Table 5 shows that there were significant differences between our observed data and the revised Baux prediction. However, the dataset used to generate the revised Baux was from 2000–2007, while our dataset includes patients from 1989–2017.
Table 4.
Comparison of Models: Shirani20
| Parameter | Total | Age group | |||
|---|---|---|---|---|---|
| 0–14 Years | 15–44 Years | 45–64 Years | ≥65 Years | ||
| SHC/BBU, n | 10,384 | 5,524 | 3,154 | 1,267 | 439 |
| Observed (actual) mortality at SHC/BBU, n | 355 | 133 | 93 | 57 | 72 |
| Expected mortality per Shirani’s model, n | 1,058 | 729 | 156 | 68 | 104 |
| Fold mortality reduction, reciprocal odds ratio | 3.2 | 6.2 | 1.7 | 1.2 | 1.6 |
| 95% CI | (2.8–3.6) | (5.1–7.4) | (1.3–2.2) | (0.8–17) | (1.1–2.2) |
| p Value | <0.0001* | <0.0001* | <0.0001* | 0.3 | 0.007* |
Significant.
SHC/BBU, Shriners Hospitals for Children—Galveston and Blocker Burn Unit
Table 5.
Comparison of Models: Revised Baux Score
| Parameter | Total | Age group | |||
|---|---|---|---|---|---|
| 0–14 Years | 15–44 Years | 45–64 Years | ≥65 Years | ||
| SHC/BBU, n | 10,384 | 5,524 | 3,154 | 1,267 | 439 |
| Observed (actual) mortality at SHC/BBU, n | 355 | 133 | 93 | 57 | 72 |
| Expected mortality per revised Baux, n | 412 | 112 | 114 | 89 | 97 |
| Fold mortality reduction, reciprocal odds ratio | 1.2 | 0.8 | 1.2 | 1.6 | 1.4 |
| 95% CI | (1.01–1.3) | (0.7–1.1) | (0.9–1.6) | (1.1–2.3) | (1–2) |
| p Value | 0.04* | 0.18 | 0.14 | 0.007* | 0.03* |
Significant.
SHC/BBU, Shriners Hospitals for Children—Galveston and Blocker Burn Unit.
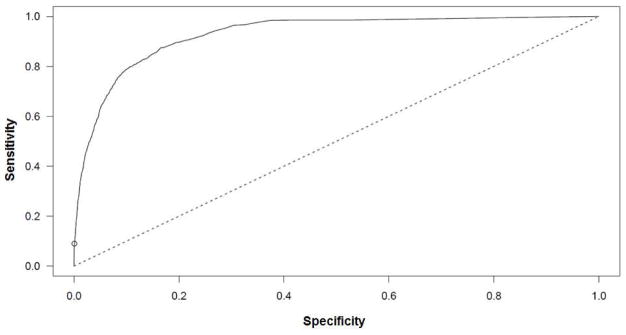
Of the nonsurvivors, 45% had concomitant inhalation injury (p<0.0001). We present a prediction model with accuracy of 97% (sensitivity: 9%, specificity: 99.9%; Figure 2). The following terms were included in the polynomial model: age, TBSA burned, presence of inhalation injury, and third-degree TBSA burned (Table 6). The prediction model of mortality for the data is as follows: logit(P(mortality)) = −6.44 – 0.12 age + 0.0042 age2 - 0.0000283 age3 + 0.0499 TBSA + 1.21 Inhalation Injury + 0.015 third-degree TBSA.
Figure 2.
The ROC curve for a nonlinear prediction model for 10,384 burn patients. The area underneath the ROC curve was calculated as 0.93.
Table 6.
Linear Logistic Prediction Model Coefficients for Mortality in Burn Patients
| Coefficient | Estimate | Std. Error | p Value |
|---|---|---|---|
| Intercept | −6.44 | 0.272 | <0.0001 |
| Age | −0.12 | 0.0274 | <0.0001 |
| Age squared | 0.0042 | 0.000884 | <0.0001 |
| Age cubed | −2.83 ×10−5 | 7.45 ×10−6 | 0.00015 |
| TBSA burn | 0.0499 | 0.00585 | <0.0001 |
| Inhalation injury | 1.21 | 0.192 | <0.0001 |
| TBSA burn third | 0.015 | 0.00482 | 0.002 |
TBSA, total body surface area.
Additionally, we illustrate that the relative odds of death decreased only slightly over the three-decade span from 1989 to 2017 (p<0.0001). Year-by-year reduction in the odds of mortality is 2.12% (p=0.03), with adjustments for sex, age, and TBSA burn. Probability of death increased as age increased (p<0.0001), as TBSA burned increased (p<0.0001), as length of stay increased (p<0.0001), and with the presence of inhalation injury (p<0.0001). Mortality for male patients was lower, with a 60% decreased odds of mortality compared to female patients (95% CI 44–81%, p<0.05).
Lastly, we present a prediction model of length of stay. The following terms were included in the polynomial model: age, TBSA burned, presence of inhalation injury, third-degree TBSA burn (Table 7). The prediction model of length of stay for the data is as follows: E(û) = (β0 + β1age + β2TBSA + β3(inhalation injury = “yes”) + β4TBSA 3rd)−1. Each percent increase of TBSA burn increases length of stay by 3.03%. Given that the average length of stay for survivors is 11.7 days, the average increase was 0.36 days per percent TBSA burn.
Table 7.
Linear Logistic Prediction Model Coefficients for Length of Stay in Burn Patients
| Coefficient | Estimate | Std. Error | p Value |
|---|---|---|---|
| Intercept | 1.13 | 0.0247 | <0.0001 |
| Age | 0.0106 | 0.000679 | <0.0001 |
| TBSA burn | 0.0342 | 0.00114 | <0.0001 |
| Inhalation injury | 0.309 | 0.0384 | <0.0001 |
| TBSA burn third | 0.0103 | 0.00124 | <0.0001 |
TBSA, total body surface area.
DISCUSSION
In 1980, Curreri, Shires, and colleagues reported improved survival after burn (19). In 1987, Abston, Barrow, and Herndon reported survival of a large cohort of children with burns covering more than 70% of the TBSA (28). In 2003, the same group reported greater than 50% survival in a cohort of children with burns covering over 88% of the TBSA (29).
Metrics that summarize field-specific improvements are warranted, and they can be used to determine whether care is improving universally and to evaluate how mortality at individual institutions performs compared to other institutions. Here, we present a generalized regression model based on a large consecutive patient cohort that illustrates the substantial increase in survival of burns. Overall, our data suggest that treatment by standard protocols, relative to other published datasets, may have contributed to decreases in mortality. Other variables include changes in public health and infrastructural changes allowing for more rapid transport of the critically ill. We compared our results to Curerri’s logistic prediction model (Table 3) because it reflected burn care in 1980 at an appropriate comparison time point; our results directly connect to their landmark probit studies in both mathematical and qualitative manners. Other notable burn mortality prediction models include (1) Pruitt et al.’s and Shirani et al.’s models (20, 30), which are based on TBSA burn and/or the presence of inhalation injury and pneumonia, and (2) the revised Baux Score (27). The revised Baux score is an updated version of the original Baux score that is calculated by adding patient age, TBSA burn, and 17 points for the presence of inhalation injury. Comparisons of Shirani’s model and the revised Baux score model are included in Tables 4 and 5; it is widely recognized that the revised Baux score underestimates mortality in the first decade of life.
Substantial advances in acute burn care occurred between 1980 and 1989, including early excision and grafting (23, 28, 31), early and standardized resuscitation (32–34), modulation of the hypermetabolic response (35–41), goal-directed nutrition and reversal of systemic catabolism (42, 43), prevention and support of organ failure syndromes (44, 45), and standardization of critical care (45, 46). The incorporation of these advances into the standard of burn care may have contributed to the reduction of postburn mortality observed. Additionally, all protocols were supervised by the last author consistently from 1989 to 2017 at our burn center.
Since age is included as a predictive variable, our models may be used to compare historically expected and observed mortality and length of stay across groups from different age cohorts. In clinical practice, the models can be used to gauge expected mortality and length of stay in an adjusted manner; thus, it allows for an individual prediction of mortality and length of stay at the time of admission for a burn patient treated with the current protocols. Furthermore, our model allows continuous analysis of the relationship between expected and observed mortality, as well as length of stay, in individual institutions.
Inhalation injury remains a contributor to morbidity and mortality in burn patients (47, 48). At our site, approximately 65% of all nonsurvivor pediatric burn patients had inhalation injury. The trauma caused by smoke inhalation injury in burn patients commonly results in an exaggerated inflammatory cascade and acute respiratory distress syndrome (49). The impact of inhalation injury is confounded by its difficulty of diagnosis and its spectrum of severity. However, the overall contribution of inhalation injury to mortality has decreased. The effects of inhalation injury are most seen in patients with burns covering 40–60% of the TBSA and between 18–60 years of age. Our findings show that, individually, percent TBSA burned and age are more powerful determinants of mortality than inhalation injury and become dominant at extremes of age and in the largest of burns (Table 6).
Limitations of our study include the unavailability of postdischarge follow-up information, including mortality, for all subjects. More importantly, it has been increasingly argued that using mortality as an endpoint to assess advances in burn care is losing validity because of the reduction in burn-related deaths (50). This reduced mortality poses a statistical problem owing to difficulty in devising interventions or achieving adequate enrollment to further impact this percentage positively. However, the three- to five-fold reduction, which we demonstrate in this analysis relative to 1980, leaves the actual absolute percentage of mortality at an all-time low. Thus, it is imperative that new metrics are established in a standardized manner over long periods of time to maintain the ability to quantify improvements in care and to define future research trajectories. In the future, long-term metrics that transcend survival, such as restoration of growth in children (35), mental and functional status, quality of life, or quality-adjusted life years, will likely gain even more traction as powerful endpoints (51–56). Second, our model has greater statistical power owing to patient number. We note that the median age of our cohort was 13 years (mean: 21 ± 0.21 years) with a median TBSA burn of 11% (mean: 20 ± 0.21) and that the cohort of Curerri et al. had a median age of 29 years with 18% TBSA burn, the cohort of Shirani et al. had an average age of 33 ± 20 years with 37 ± 22% TBSA burn, and the cohort for revised Baux had a mean age of 31 years with an average TBSA burn of 9.7%. Third, the retrospective nature of this study precludes inferences that could have been made in a prospective approach, which could have compared expected and actual mortality patient by patient. This concern is moderated by the inclusion of the entire cohort of burn admissions during this study period. Our present model is not able to directly assess the effectiveness of specific interventions or protocol changes. Last, several historically important prognostic models that were developed to predict mortality following burns were developed prior to widespread understanding of the importance of internal and external validation and therefore have an unknown generalizability (57). Because historically important models have unknown generalizability, their results are difficult to interpret when applied to modern data (58). Models that lack generalizability may give erroneously high or low estimates of mortality for reasons unrelated to changes in the quality of care. The various prediction models that have been developed, including our own, can best be validated against observed datasets that are either not widely available or suffer from variability. We also note that third-degree burn size reporting varies through hospital course because of progression of disease and inter-observer differences.
Future directions of our work include the inclusion of additional determinants such as resuscitation fluid, weight and body mass index, co-morbidities at admission, and the effect of infections such as pneumonia and sepsis during the hospital course. Also, stratifying the severity of inhalation injury rather than including a binary outcome of either presence or absence will more accurately describe its role in mortality. Lastly, the sexually dimorphic response to burn injuries observed in this large dataset encourages further study that may improve survival outcome, particularly in female patients.
CONCLUSION
Advances in burn care have significantly increased survival and raised the standard of care. Additional endpoints must be established to assess future advancements that focus on function and quality of life.
Acknowledgments
Support: This work was supported by NIDILRR (90DP0043-02-01 [DNH]), NIH (P50GM060338, R01GM056687, T32 GM008256 [DNH]; R01HD049471 [OES]; and R01GM112936 [CCF]), Shriners Hospitals for Children (84080, 80100, 71008, and 71000 [DNH]), the Department of Surgery at UTMB (2014-667 [LES]), and the Remembering the 15 Research Education Endowment Fund. This study was also conducted with the support of UTMB’s Institute for Translational Sciences, supported in part by a Clinical and Translational Science Award (UL1TR000071) from the National Center for Advancing Translational Sciences (NIH).
The authors would like to thank the medical and research staff of Shriners Hospitals for Children—Galveston for their valuable assistance as well as the Data Safety Monitoring Board. We would also like to thank Drs Sally Abston, Manu Desai, Steven Wolf, Arthur Sanford, Marc Jeschke, James Gallagher, Jong Lee, Carlos Jimenez, Ludwik Branski, and William Norbury for their clinical contributions and their work in maintaining the protocols; Clark Andersen for his assistance in statistical analysis; and Deborah Benjamin, Jamie Heffernan, Jason Scheaffer, Daralyn Johnson, Pamela Stevens, Lisa Molina, and Sharon Liang for their assistance in retrieving the data. We would also like to thank the respiratory therapy team of Shriners Hospitals for Children—Galveston, especially Dr Ronald Mlcak, Ms Vicki Walker, and Stacey Brewster, for their assistance in diagnosing inhalation injury.
Footnotes
Disclosure Information: Nothing to disclose.
Disclosures outside the scope of this work: Dr Herndon receives royalties from Elsevier.
Presented at the Southern Surgical Association 129th Annual Meeting, Hot Springs, VA, December 2017.
Disclaimer: None of the funding sources had any role in the design of the study, in the writing of the manuscript, or in the collection, analysis, and/or interpretation of the data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tredget EE, Yu YM. The metabolic effects of thermal injury. World J Surg. 1992;16:68–79. doi: 10.1007/BF02067117. [DOI] [PubMed] [Google Scholar]
- 2.Porter C, Herndon DN, Sidossis LS, et al. The impact of severe burns on skeletal muscle mitochondrial function. Burns. 2013;39:1039–1047. doi: 10.1016/j.burns.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simsek T, Simsek HU, Canturk NZ. Response to trauma and metabolic changes: Posttraumatic metabolism. Ulus Cerrahi Derg. 2014;30:153–159. doi: 10.5152/UCD.2014.2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hart DW, Wolf SE, Mlcak R, et al. Persistence of muscle catabolism after severe burn. Surgery. 2000;128:312–319. doi: 10.1067/msy.2000.108059. [DOI] [PubMed] [Google Scholar]
- 5.Newsome TW, Mason AD, Jr, Pruitt BA., Jr Weight loss following thermal injury. Ann Surg. 1973;178:215–217. doi: 10.1097/00000658-197308000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuo R, Herndon DN, Kobayashi M, et al. Cd4-cd8-tr alpha/beta+suppressor t cells demonstrated in mice 1 day after thermal injury. J Trauma. 1997;42:635–640. doi: 10.1097/00005373-199704000-00009. [DOI] [PubMed] [Google Scholar]
- 7.Low JF, Herndon DN, Barrow RE. Effect of growth hormone on growth delay in burned children: A 3-year follow-up study. Lancet. 1999;354:1789. doi: 10.1016/s0140-6736(99)02741-5. [DOI] [PubMed] [Google Scholar]
- 8.Weidenfeld S. Ueber den verbrennungstod. I. Abhangigkeit des verbrennungstodes von der grosse der verbrannten hautflache. Arch f Dermatol u Syph. 1902;61:33. [Google Scholar]
- 9.Hajek S, Stefan GJ, Kral Z, et al. Analysis of 147 fatal thermic injuries. Acta Chir Plast. 1963;5:193–204. [PubMed] [Google Scholar]
- 10.Finney DJ. Probit analysis; a statistical treatment of the sigmoid response curve. Cambridge Eng: University Press; 1947. [Google Scholar]
- 11.Finney DJ. Probit analysis: A statistical treatment of the sigmoid response curve. 2. Cambridge Eng: University Press; 1952. [Google Scholar]
- 12.Finney DJ. Probit analysis. 3. Cambridge Eng: University Press; 1971. [Google Scholar]
- 13.Bull JP, Squire JR. A study of mortality in a burns unit: Standards for the evaluation of alternative methods of treatment. Ann Surg. 1949;130:160–173. doi: 10.1097/00000658-194908000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bull JP, Fisher AJ. A study of mortality in a burns unit: A revised estimate. Ann Surg. 1954;139:269–274. doi: 10.1097/00000658-195403000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bull JP. Revised analysis of mortality due to burns. Lancet. 1971;2:1133. doi: 10.1016/s0140-6736(71)91286-4. [DOI] [PubMed] [Google Scholar]
- 16.Barnes BA. Mortality of burns at the massachusetts general hospital, 1939–1954. Ann Surg. 1957;145:210–222. doi: 10.1097/00000658-195702000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz MS, Soroff HS, Reiss E, Artz CP. USASRU Research Report MEDEW-RS-12–56. 1956. An evaluation of the mortality and the relative severity of second and third-degree injuries in burns. [Google Scholar]
- 18.Pruitt BA, Jr, Tumbusch WT, Mason AD, Jr, et al. Mortality in 1,100 consecutive burns treated at a burns unit. Ann Surg. 1964;159:396. doi: 10.1097/00000658-196403000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Curreri PW, Luterman A, Braun DW, Jr, et al. Burn injury. Analysis of survival and hospitalization time for 937 patients. Ann Surg. 1980;192:472–478. doi: 10.1097/00000658-198010000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shirani KZ, Pruitt BA, Jr, Mason AD., Jr The influence of inhalation injury and pneumonia on burn mortality. Ann Surg. 1987;205:82–87. doi: 10.1097/00000658-198701000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Osler T, Glance LG, Hosmer DW. Simplified estimates of the probability of death after burn injuries: Extending and updating the baux score. J Trauma. 2010;68:690–697. doi: 10.1097/TA.0b013e3181c453b3. [DOI] [PubMed] [Google Scholar]
- 22.Mlcak R, Buffalo M. Pre-hospital management, transport, and emergency care. In: Herndon DN, editor. Total burn care. Vol. 3. Philadelphia: Saunders; 2007. pp. 81–92. [Google Scholar]
- 23.Herndon DN, Barrow RE, Rutan RL, et al. A comparison of conservative versus early excision. Therapies in severely burned patients. Ann Surg. 1989;209:547–552. doi: 10.1097/00000658-198905000-00006. discussion 552–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finnerty CC, Ali A, McLean J, et al. Impact of stress-induced diabetes on outcomes in severely burned children. J Am Coll Surg. 2014;218:783–795. doi: 10.1016/j.jamcollsurg.2014.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hastie TJT. Generalized additive models. Chapman & Hall/CRC; 1990. [Google Scholar]
- 26.Efron BTRJ. Improvements on cross-validation: The 632+ bootstrap method. J Am Stat Assoc. 1997;92:548–560. [Google Scholar]
- 27.Osler T, Glance LG, Hosmer DW. Simplified estimates of the probability of death after burn injuries: Extending and updating the baux score. J Trauma Acute Care Surg. 2010;68:690–697. doi: 10.1097/TA.0b013e3181c453b3. [DOI] [PubMed] [Google Scholar]
- 28.Herndon DN, Gore D, Cole M, et al. Determinants of mortality in pediatric patients with greater than 70% full-thickness total body surface area thermal injury treated by early total excision and grafting. J Trauma. 1987;27:208–212. doi: 10.1097/00005373-198702000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Spies M, Herndon DN, Rosenblatt JI, et al. Prediction of mortality from catastrophic burns in children. Lancet. 2003;361:989–994. doi: 10.1016/S0140-6736(03)12824-3. [DOI] [PubMed] [Google Scholar]
- 30.Pruitt BA, Jr, Tumbusch WT, Mason AD, Jr, et al. Mortality in 1,100 consecutive burns treated at a burns unit. Ann Surg. 1964;159:396. doi: 10.1097/00000658-196403000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herndon DN, Parks DH. Comparison of serial debridement and autografting and early massive excision with cadaver skin overlay in the treatment of large burns in children. J Trauma. 1986;26:149–152. doi: 10.1097/00005373-198602000-00009. [DOI] [PubMed] [Google Scholar]
- 32.Warden GD. Burn shock resuscitation. World J Surg. 1992;16:16–23. doi: 10.1007/BF02067109. [DOI] [PubMed] [Google Scholar]
- 33.Barton RG, Saffle JR, Morris SE, et al. Resuscitation of thermally injured patients with oxygen transport criteria as goals of therapy. J Burn Care Rehabil. 1997;18:1–9. doi: 10.1097/00004630-199701000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Greenhalgh DG. Burn resuscitation. J Burn Care Res. 2007;28:555–565. doi: 10.1097/bcr.0b013e318093df01. [DOI] [PubMed] [Google Scholar]
- 35.Herndon DN, Voigt CD, Capek KD, et al. Reversal of growth arrest with the combined administration of oxandrolone and propranolol in severely burned children. Ann Surg. 2016;264:421–428. doi: 10.1097/SLA.0000000000001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Porter C, Tompkins RG, Finnerty CC, et al. The metabolic stress response to burn trauma: Current understanding and therapies. Lancet. 2016;388:1417–1426. doi: 10.1016/S0140-6736(16)31469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herndon DN, Tompkins RG. Support of the metabolic response to burn injury. Lancet. 2004;363:1895–1902. doi: 10.1016/S0140-6736(04)16360-5. [DOI] [PubMed] [Google Scholar]
- 38.Herndon DN, Barrow RE, Rutan TC, et al. Effect of propranolol administration on hemodynamic and metabolic responses of burned pediatric patients. Ann Surg. 1988;208:484–492. doi: 10.1097/00000658-198810000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herndon DN, Rodriguez NA, Diaz EC, et al. Long-term propranolol use in severely burned pediatric patients: A randomized controlled study. Ann Surg. 2012;256:402–411. doi: 10.1097/SLA.0b013e318265427e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jeschke MG, Finnerty CC, Suman OE, et al. The effect of oxandrolone on the endocrinologic, inflammatory, and hypermetabolic responses during the acute phase postburn. Ann Surg. 2007;246:351–360. doi: 10.1097/SLA.0b013e318146980e. discussion 360–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pereira CT, Herndon DN. The pharmacologic modulation of the hypermetabolic response to burns. Advances in Surg. 2005;39:245–261. doi: 10.1016/j.yasu.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Herndon DN, Hart DW, Wolf SE, et al. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–1229. doi: 10.1056/NEJMoa010342. [DOI] [PubMed] [Google Scholar]
- 43.Gore DC, Chinkes D, Heggers J, et al. Association of hyperglycemia with increased mortality after severe burn injury. J Trauma. 2001;51:540–544. doi: 10.1097/00005373-200109000-00021. [DOI] [PubMed] [Google Scholar]
- 44.Kraft R, Herndon DN, Al-Mousawi AM, et al. Burn size and survival probability in paediatric patients in modern burn care: a prospective observational cohort study. Lancet. 2012;379:1013–1021. doi: 10.1016/S0140-6736(11)61345-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jeschke MG, Herndon DN. Burns in children: Standard and new treatments. Lancet. 2014;383:1168–1178. doi: 10.1016/S0140-6736(13)61093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klein MB, Goverman J, Hayden DL, et al. Benchmarking outcomes in the critically injured burn patient. Ann Surg. 2014;259:833–841. doi: 10.1097/SLA.0000000000000438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mlcak R, Desai MH, Robinson E, et al. Lung function following thermal injury in children--an 8-year follow up. Burns. 1998;24:213–216. doi: 10.1016/s0305-4179(98)00012-6. [DOI] [PubMed] [Google Scholar]
- 48.Palmieri TL, Warner P, Mlcak RP, et al. Inhalation injury in children: a 10 year experience at Shriners Hospitals for Children. J Burn Care Res. 2009;30:206–208. doi: 10.1097/BCR.0b013e3181923ea4. [DOI] [PubMed] [Google Scholar]
- 49.Traber DL, Enkhabaatar P. Thermal lung injury and acute smoke inhalation. In: Fischman DA, editor. Fischman’s pulmonary diseases and disorders. Vol. 1. New York: McGraw-Hill Medical Publishing Company; 2008. pp. 1053–1064. [Google Scholar]
- 50.Pereira C, Murphy K, Herndon D. Outcome measures in burn care. Is mortality dead? Burns. 2004;30:761–771. doi: 10.1016/j.burns.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 51.Goverman J, Mathews K, Holavanahalli RK, et al. The National Institute on Disability, Independent Living, and Rehabilitation Research Burn Model System: twenty years of contributions to clinical service and research. J Burn Care Res. 2017;38:e240–e253. doi: 10.1097/BCR.0000000000000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Goverman J, Mathews K, Nadler D, et al. Satisfaction with life after burn: a Burn Model System National Database Study. Burns. 2016;42:1067–1073. doi: 10.1016/j.burns.2016.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kazis LE, Lee AF, Rose M, et al. Recovery curves for pediatric burn survivors: advances in patient-oriented outcomes. JAMA Pediatr. 2016;170:534–542. doi: 10.1001/jamapediatrics.2015.4722. [DOI] [PubMed] [Google Scholar]
- 54.Murphy ME, Holzer CE, 3rd, Richardson LM, et al. Quality of life of young adult survivors of pediatric burns using world health organization disability assessment scale ii and burn specific health scale-brief: A comparison. J Burn Care Res. 2015;36:521–533. doi: 10.1097/BCR.0000000000000156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rosenberg M, Mehta N, Rosenberg L, et al. Immediate and long-term psychological problems for survivors of severe pediatric electrical injury. Burns. 2015;41:1823–1830. doi: 10.1016/j.burns.2015.06.006. [DOI] [PubMed] [Google Scholar]
- 56.Rosenberg M, Ramirez M, Epperson K, et al. Comparison of long-term quality of life of pediatric burn survivors with and without inhalation injury. Burns. 2015;41:721–726. doi: 10.1016/j.burns.2015.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hussain A, Choukairi F, Dunn K. Predicting survival in thermal injury: A systematic review of methodology of composite prediction models. Burns. 2013;39:835–850. doi: 10.1016/j.burns.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 58.Altman DG, Vergouwe Y, Royston P, et al. Prognosis and prognostic research: validating a prognostic model. BMJ. 2009;338:b605. doi: 10.1136/bmj.b605. [DOI] [PubMed] [Google Scholar]