Abstract
A pair of diastereomeric (4S,5S)- and (4S,5R)-4-methoxycarbonyl-5-phenylselenomethyl-2-phenyl oxazolines, derived from L-vinylglycine, serve as precursors to protected, quaternary, L- and D-α-(2-tributylstannyl)vinyl amino acids, respectively, in three steps {(i) alkylative side chain installation, (ii) eliminative ring-opening and (iii) vinyl selenide to vinyl stannane interconversion}. The title compounds may be protodestannylated to the corresponding free, quaternary L- and D-vinyl amino acids. Alternatively, the 2-stannylvinyl α-branch (or the derivative 2-iodovinyl branch) may be exploited to access novel quaternary, L- and D-β,γ-unsaturated amino acids via a range of transition metal-mediated cross coupling reactions.
Keywords: self regeneration of stereocenters (SRS), β, γ-unsaturated amino acids, vinyl selenides, vinyl stannanes, chain extension
INTRODUCTION
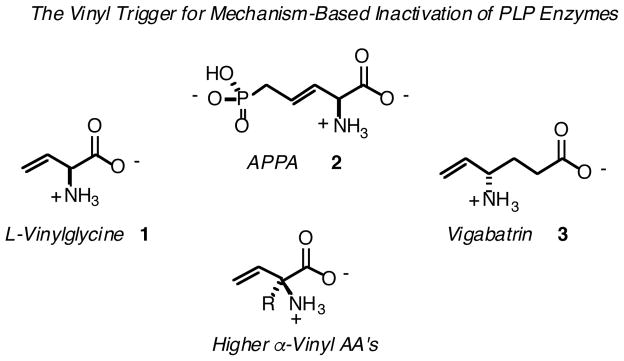
Nature produces a number of β,γ-unsaturated amino acids, generally bearing an α-hydrogen.1 The simplest member of this family, α-vinylglycine (1), has been isolated from mushroom sources.2 Such amino acids are potential mechanism-based inactivators (MBI’s) for enzymes that process amino acids by first removing the α-proton, including racemases, transaminases, and β- and γ-elimination and replacement enzymes. Indeed, vinylglycine is known to inactivate transaminases for L-aspartate, L-alanine, L-serine and D-alanine,3a–e as well as cysteine sulfinate decarboxylase.4 Incorporation of D-vinylglycine into an appropriate peptide leads to inhibition of peptidylglycine α-hydroxylating monooxygenase.5
Another member of this family, APPA (2-amino-5-phosphono-3-pentenoic acid (2); the β,γ-unsaturated phosphonate analogue of homoserine phosphate), inactivates the β-elimination enzyme, threonine synthase.6 E-APPA inhibits the γ-replacement enzyme, cystathionine γ-synthase.7 But perhaps the most well-studied example of an amino acid bearing unsaturation β- and γ- to the amino group that acts as a mechanism-based inhibitor is vigabatrin (3, γ-vinyl GABA). Vigabatrin inactivates GABA transaminase,8 thereby raising brain GABA levels. This drug has seen extensive clinical use as an anti-epileptic9 and, quite recently, has shown promise as a potential alternative to (methadone) replacement therapy in the treatment of substance addiction.10
If one wishes to apply a similar vinyl-triggering strategy to the mechanism-based inactivation of amino acid decarboxylase (AADC) enzymes, quaternary β,γ-unsaturated amino acids become attractive targets. That is, the usual AA side chain functional group is retained, to direct the inhibitor to the active site of interest, and an α-decarboxylation event, rather than an α-deprotonation event, is expected to conjugate the otherwise unreactive C-C double bond with the quinonoid intermediate along the PLP-enzyme reaction coordinate.11 Adding to the value of this family of higher12 α-vinyl AA’s, we have found that they conveniently serve as precursors to previously unexplored classes of potential AADC MBI’s, including α-oxiranyl,13 α-(2Echloro) vinyl,14 α-(1-chloro)vinyl14 and α-(2Z-fluoro)vinyl15 amino acids (Scheme 2).
Scheme 2.
The divergency of this approach is attractive from a tactical point of view, both in terms of overall synthetic efficiency and in terms of stereochemical control. That is, given the stability of these quaternary AA’s to racemization, control of absolute stereochemistry in the construction of the parent higher α-vinyl amino acids should translate into stereochemical control for all such derivative classes, as well. The ability to generate a variety of α-branched AA’s from parent α-vinyl or α-stannylvinyl AA’s also has implications for unnatural amino acid mutagenesis16 and for combinatorial chemistry. In the latter case, libraries of α-amino acids are often used and library diversity is a variable of great import.17 Indeed, it may be advantageous to broaden the number and type of quaternary AA’s included in such AA libraries, as α-branched AA’s, including those bearing unsaturation,18 tend to promote peptidyl secondary structure, particularly α-helical, and 310-helical motifs.19 At the same time, these unnatural AA’s often confer resistance to proteolysis upon their derivative peptides.20 In fact, of late, great success has been achieved with α-branched AA’s for the design of short peptides (i) that fold into helical structures in aqueous solution,19 (ii) that bind to receptors,20a,21 or (iii) that catalyze chemical reactions.22
ENANTIOSELECTIVE SYNTHESIS OF β,γ-UNSATURATED AA’S
For most of the applications enumerated in the Introduction, scalemic α-branched amino acids, of known handedness are preferable.23 This article will focus on the asymmetric synthesis of AA’s bearing a β,γ-unsaturation. At the outset, we wish to highlight several particularly elegant enantiocontrolled syntheses of β,γ-unsaturated AA’s for systems in which an α-proton is present (substituted alkenylglycines). From there, the discussion will move into a summary of available methods for the stereocontrolled construction of quaternary β,γ-unsaturated AA’s. Among these, the title α-(2-tributylstannyl)vinyl AA’s are especially attractive, as these are available in either D- or L-form, and lead either to unsubstituted α-vinyl AA’s (protodestannylation) or to more complex, chain-extended congeners, as desired.
I. Substituted Alkenylglycines
As noted before, the simplest β,γ-unsaturated vinyl AA is vinylglycine itself, for which a good number of asymmetric syntheses have been developed.24 Derivatives of vinylglycine in which the double bond bears one or more substituents, but in which the α-proton is retained, will be termed alkenylglycines here.
R. M. Williams and coworkers described a nice route to such compounds that passes through an interesting class of β,γ-alkynylglycines.25 The same 4,5-diphenyl-1,4-oxazin-2-one template that serves as the basis of Williams’ chiral glycine enolate equivalent, provides for a chiral electrophilic glycine equivalent here. Condensation of 4 with trialkylstannylalkynes leads to the protected α-alkynyl AA’s 5, presumably via the intermediacy of the corresponding α-iminium ion (Scheme 3).25a The alkyne is then reduced under dissolving metal conditions, with Na0-NH3(l) giving the higher chemical yields, but with Li0-NH3(l) giving better optical yields.
Scheme 3.
O’Donnell has developed the related electrophilic glycine equivalent, 7. This system is cleverly outfitted with the benzophenone imine protecting group on nitrogen, allowing for Pd-mediated allylic substitution at the α-carbon. Transmetallation can be effected with in situ-generated vinylaluminium reagents, thereby providing alkenylglycines directly.26a This cross-coupling chemistry was carried out in racemic fashion initially, but more recent developments suggest that enantioselective variants may be achievable. Thus, the O’Donnell group has successfully performed allylic substitution upon imine-protected glycine α-acetates, using a BINAP ligand as the source of chirality and potassium malonate as the nucleophile (Scheme 4).26b
Scheme 4.
Petasis has disclosed a nicely convergent, three component Mannich reaction which produces an allylic amine from the reaction of an alkenyl boronate with an amine and an aldehyde or electrophilic ketone. When glyoxylic acid is condensed with boronate 13 through the agency of L-phenylglycinol, protected D-α-cinnamylglycine is obtained in excellent ee. (Scheme 5).27
Scheme 5.
All of the approaches discussed heretofore involve disconnections at the Cα-R bond, wherein the unsaturated side chain is coupled with an electrophilic glycine equivalent. By contrast, Snapper and Hoveyda, target alkenylglycines by disconnecting at the Cα-CO2H bond. They have optimized the Ti(IV)-mediated 1,2- addition of cyanide to α,β-unsaturated iminium ions. Screening a library of chiral peptidic ligands for titanium, they identified the catalyst derived from tripeptide 16 as particularly effective (Scheme 6).28,29
Scheme 6.
II. Quaternary β,γ-Unsaturated AA’s
There are fewer methods available for the stereocontrolled synthesis of quaternary β,γ-unsaturated AA’s. These are more densely functionalized targets with the α-carbon directly bearing amino, carboxyl and alkenyl functional groups, in addition to the usual (and often functionalized) side chain R group itself. Among the earliest entries into this AA class is the work of Schöllkopf in which bis-lactim ether, 18, serves as a chiral alanine enolate equivalent.30 Here the chiral element is essentially an L-valine auxiliary built into 18. Condensation with acetone or acetophenone proceeds with high 1,4-stereoinduction. Subsequent dehydration and methanolysis produces methyl esters of substituted L-vinylalanine derivatives (20).
Seebach’s classic synthesis of higher vinyl AA’s served as part and parcel of a programmatic thrust to establish the principle of “self-regeneration of stereocenters” [SRS; previously referred to as SRC or selfr-egeneration (or reproduction) of chirality] in asymmetric synthesis. In this case, the α-stereocenter originally present in methionine is used to induce a second stereocenter at the N,X-acetal carbon in a cyclic oxazolidinone or imidazolidinone derivative (Scheme 8). Upon subsequent generation of the enolate, the α-stereocenter is temporarily removed, and then reinstalled via alkylative introduction of the side chain. The (methyl)thioethyl methionine side chain is used to install the requisite unsaturation, via oxidation and pyrolysis, either before or after the alkylation event. This chemistry has been used to access enantiomerically enriched α-vinyl AA’s bearing the alanine,31a butyrine,31a phenylalanine,31b and tyrosine31c side chains.
Scheme 8.
More recently, Hegedus and coworkers have combined their chromium carbene complex-mediated β-lactam synthesis with Ojima’s diastereoselective lactam enolate alkylation methodology, resulting in a novel entry into α-vinylalanine (Scheme 9).32 Chiral β-lactam, 28, may be regarded as a masked α-formylglycine equivalent. Alkylation of the potassium enolate thereof with methyl iodide proceeds with high diastereofacial selectivity. Unravelling of the formyl group, followed by methylenation and hydrolytic deprotection yields L- α-vinylalanine.
Scheme 9.
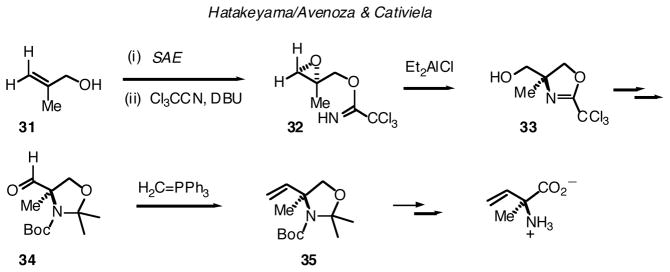
Very recently, Avenoza and Cativiela and their associates have reported an approach to α-vinylalanine that shares an “end game” methylenation with the Hegedus route.33 However, in this case, the requisite α-formylalanine equivalent is generated by entirely different chemistry. Namely, absolute stereochemistry is set 8 via a Sharpless-Katsuki asymmetric epoxidation (SAE)34 of allylic alcohol 31. Imidate formation and 5-exo cyclization is then performed under Et2AlCl-promotion according to the procedure of Hatakeyama.35 The α-amino group is thereby installed with inversion of configuration. Protecting group interchange and Swern oxidation set the stage for Wittig methylenation, leading to the targeted quaternary β,γ-unsaturated amino acid.36
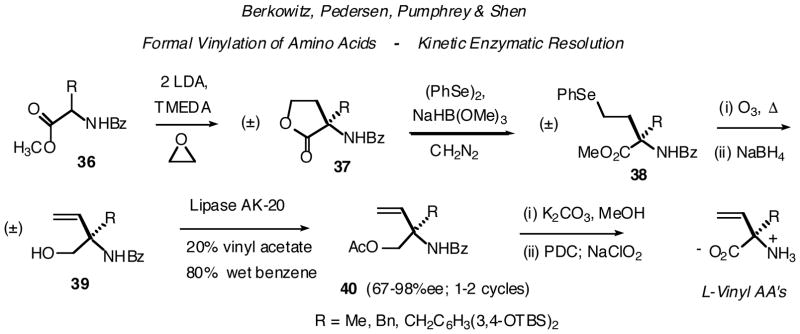
As part of a program directed at developing new AADC inhibitors, we have been engaged in a multipronged approach toward quaternary, α-vinyl amino acids, with three principal synthetic aims: (i) control of absolute stereochemistry; (ii) introduction of biologically relevant AA side chains and (iii) transformation of the parent α-vinyl AA’s into potential second generation inhibitor families (Scheme 2). Our initial approach was to formally α-vinylate an AA-derived dianion. These dianions are generated with two equivalents of base from N-benzoyl amino acid methyl esters. Ethylene oxide serves as an economical “vinyl cation equivalent” (Scheme 11).37 α-Alkylation predominates and yields are improved in the presence of TMEDA. Interestingly, Michelle Pedersen (Morris) found that, in addition to simple alkyl side chains (i.e. Ala, Phe, Val), with appropriate protection, heteroatom-functionalized side chains (DOPA, Lys, His, Orn, HomoSer) may be carried into these dianions, without deleterious effects. Given that these alkylations proceed smoothly with a modest electrophile such as ethylene oxide, we expect this dianion chemistry to be of utility for the generation of other quaternary AA analogues.
Scheme 11.
The resulting α-substituted homoserine lactones 37 undergo selective ring opening at the alkyl carbon-oxygen bond with a new, non-reducing phenylselenolate equivalent developed in the work.37b Oxidation and selenoxide pyrolysis then serves to install the α-vinyl group. At this stage, global deprotection (6 N HCl reflux) provides the racemic free, α-vinyl amino acids. Alternatively, James Pumphrey, an undergraduate co-worker, noted that if one first reduces the esters to the corresponding N-benzoyl α-vinyl amino alcohols, one can partially resolve these by enzymatic acylation. Chemical deacylation of the enzymatic product then permits a second round of resolution. In this way, enantiomerically enriched L-α-vinylalanine and L-α-vinylphenylalanine may be obtained.38
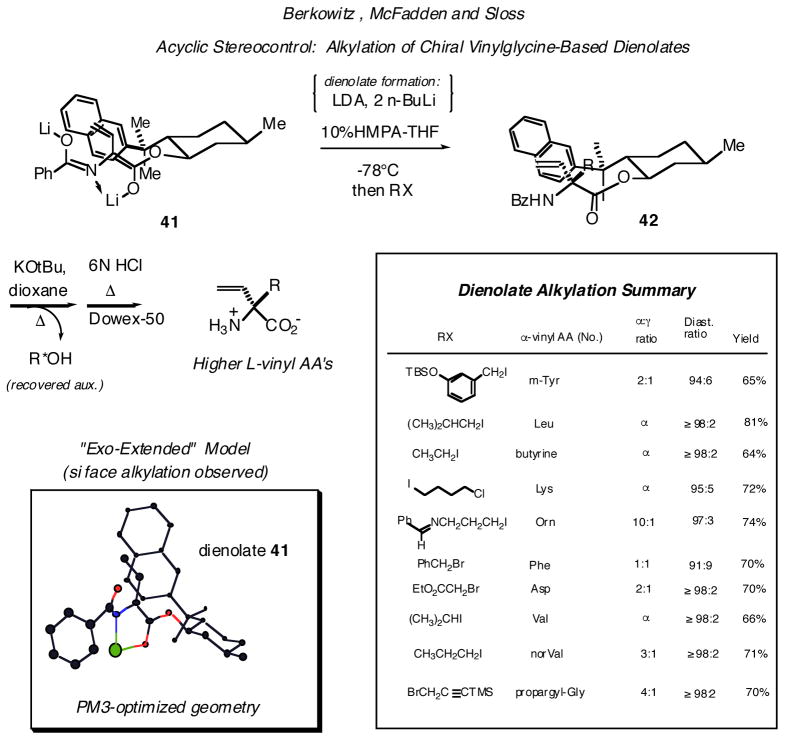
In light of the favorable reactivity of the aforementioned AA-derived dianions, it became attractive to investigate asymmetric versions of that chemistry. After all, it seemed likely that enolate geometry in these systems could be controlled via benzamidate nitrogen chelation to the enolate lithium. It would then remain only to control the facial selectivity of the alkylation reaction to achieve a high level of stereoselection. We envisioned doing so in an acyclic fashion, whereby the aryl π-system of an arylmenthyl type ester auxiliary would shield one face of the enolate. In fact, these goals were reduced to practice in the alkylation of the vinylglycine-derived dianionic dienolate 41, which is outfitted with d’Angelo’s 8-(β-naphthyl)menthyl auxiliary.39 Our working model for the reactive conformation of this dienolate is provided in Scheme 12. Through the diligent work of Jill McFadden, it was established that the alkylation of 41 with appropriate electrophiles provides a convergent route to a range of important L-α-vinyl AA’s, including the AADC inhibitors α-vinylornithine, α-vinyl-m-tyrosine and α-vinyllysine.40 If desired, the chiral auxiliary may be recovered and recycled via a modification of Gassman’s “anhydrous hydroxide” protocol for the cleavage of hindered esters.41
Scheme 12.
Parallel to this study, we had begun to explore a complementary approach to the targeted higher vinyl AA’s, also emanating from vinylglycine (Scheme 13).42 Here, rather than recruit chiral information from an external auxiliary, the chiral information inherent at Cα in L-vinylglycine would be parlayed divergently into enantiomeric directing centers at Cβ. An episelenonium ion-mediated 5-exo trig cyclization serves to mask the double bond and, in so doing, install the β-phenylselenomethyl directing group. Because the diastereomeric oxazolines 44t and 44c are separable by standard silica gel chromatography, yet yield mirror image enolates upon α-deprotonation, this may be regarded as a sort of divergent SRS approach. Oxazoline 44t serves as a synthon for higher L-α-vinyl AA’s, while 44c conveniently provides an entry into the D-series.
Scheme 13.
Treatment of the alkylation products 45 with KOtBu effectively unmasks the β,γ-unsaturation via eliminative oxazoline ring opening with release of an amidate leaving group. The resulting α-(2E-phenylseleno) vinyl AA’s (46) are of interest in their own right, both from a chemical and spectroscopic (77Se provides a potential NMR reporter group) point of view. However, they served here as the platform for the discovery of a new entry into vinyl stannanes. Namely, exposure of these vinyl selenides to trialkyltin radical leads to an efficient substitution reaction. A set of heretofore unexplored,43 α-(2E-trialkylstannyl)vinyl AA’s (47) results, and these may be obtained in high ee, and in either enantiomeric form, as desired. This simple substitution reaction had not been seen before and certainly merits further investigation. However, it is our purpose here to explore the chain extension chemistry of this novel class of quaternary, γ-stannyl-β,γ-unsaturated AA’s.44
If desired, protodestannylation may be carried out upon vinylstannanes 47, concommitant with global acidolytic deprotection (Scheme 13), to provide the parent α-vinyl AA’s. Alternatively, one can exploit the γ-stannyl group to position-specifically introduce a deuterium label by employing aqueous DCl (Scheme 14). In the case of L-α-(2E-tributylstannyl)vinylphenylalanine, this transformation proceeds cleanly and stereospecifically, with retention of configuration, as expected. Presumably, the same approach would provide a valuable means to introduce a tritium label into members of this AADC suicide substrate family for enzyme labeling studies.
Scheme 14.
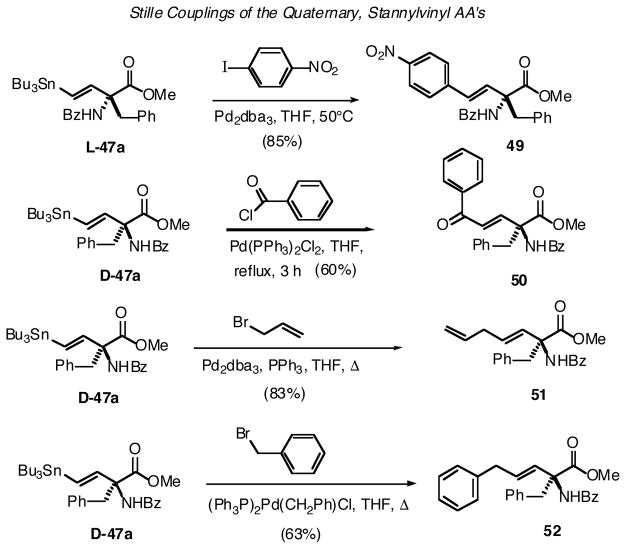
The same stannylvinyl AA, L-47a, was used to explore the possibility for extending the α-branch via Pd0-mediated couplings of the Stille variety.45 As is illustrated in Scheme 15, no significant complications were encountered with these quaternary AA scaffolds. Successful chain extensions were achieved with a range of electrophiles, from an aryl halide, to an acid chloride, to allylic and benzylic halides. In the latter case, the (Ph3P)2ClPdBn complex, originally reported by Stille for couplings with benzylic substrates,46 also proved successful here.
Scheme 15.
We were also pleased to find that the α-(2E-stannyl)vinyl branch in these densely functionalized AA’s may be conveniently transformed into an α-(2E-iodo)vinyl branch,47 in nearly quantitative yield, upon treatment with I2 (Scheme 16). This permitted us to explore transition metal catalyzed chain extension reactions of the opposite sense of polarity. That is, rather than enter the catalytic cycle “late” at the stage of the transmetallation, these new β,γ-unsaturated amino acids presumably enter the cycle “early” via oxidative addition to a PdL2 or [PdL2X]− species.48
Scheme 16.
Pleasingly, protected α-(2E-iodo)vinyl amino acids, 53, derived from L- and D-phenylalanine and D-serine could be successfully deployed in such Pd0-mediated C-C bond-forming reactions (Scheme 17). Employing vinyltributylstannane with L-53a, and Pd2dba3 as Pd0 source, one obtains 54, in which a 1,3- butadienyl branch now formally replaces the α-proton. Such quaternary amino acids hold potential as substrates for [4π+2π]-cycloadditions (vide infra). Alternatively, subjecting the antipode D-53a to a Negishi-type reaction49 with a 1-styrenylzinc reagent derived from Rieke Zn*50 leads to efficient cross-coupling. Given the variety of organozinc reagents available, and the functional group tolerance of such reagents, this opens up an important new manifold for chain extension. Finally, using the iodovinyl AA derived from D-serine (D-53c), good results were also obtained for an sp-sp2 coupling of the Sonagashira variety.51
Scheme 17.
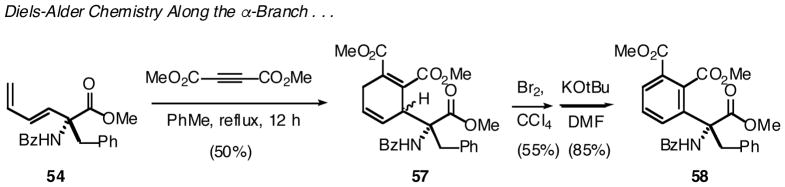
In a demonstration of proof of principle, the dienyl-AA 54 successfully participated in a Diels-Alder reaction with dimethyl acetylenedicarboxylate (DMAD) as dienophile (Scheme 18). There is particular significance to this result as this cycloaddition is expected to proceed via a sterically demanding transition state. Namely, two C-C bonds are formed along the α-branch, and one of these forms directly adjacent to the quaternary α-center. A bromine addition/HBr elimination sequence then allows one to aromatize the newly formed 6-membered ring. Overall, this chemistry provides a route into an interesting family of quaternary, arylglycine derivatives bearing the natural AA side chains.
Scheme 18.
Lastly, as a streamlined alternative to the Sonagashira route for the construction of γ,δ-sp2-sp bond, we have been able to apply of Shen’s recently reported variant52 of the Stille reaction to this system. In this reaction, 1,1-dibromoalkenes couple with alkenylstannanes, to give enynes, particularly in polar solvents and in the presence of electron rich ligands. Under these conditions, the quaternary, α-(2E-stannyl)vinyl AA, D-47a, couples with geminal dibromomethylene partner 59 to yield directly the functional equivalent of a Sonagashira product (60), without the need to synthesize the iodovinyl AA.
CONCLUSIONS
A divergent SRS-type approach to protected, D- or L- quaternary, α-(2-stannyl)vinyl AA’s has been developed. The enantiomeric purity of the starting L-vinylglycine is preserved through a sequence that involves:
installation of a directing β-stereocenter via an episelenonium ion mediated alkene-masking step,
chromatographic separation of the diastereomeric D- and L-vinyl AA synthons, 44c and 44t,
enolate generation with KHMDS and alkylative side chain introduction with nearly absolute 1,2-stereoinduction,
oxazoline opening/alkene unmasking under classical E2-type conditions to produce a family of α-(2Ephenylseleno) vinyl AA’s and
direct transformation of the α-(2E-phenylseleno)vinyl α-branch to an α-(2E-tributylstannyl)vinyl branch via treatment with tributyltin radical.
The α-(2E-stannyl)vinyl AA’s (or their iodovinyl derivatives), in turn, are shown to effectively participate in transition metal-mediated cross couplings of the Stille, Negishi, Sonagashira and Shen varieties. The AA products of these chain extension reactions bear a β,γ-unsaturated α-branch, in addition to the usual α-amino, α-carboxyl and side chain functional groups. Thus, the title stannylvinyl AA’s serve as convenient building blocks for a wide range of scalemic, sterically conjested amino acids, of either D- or L-handedness, that would otherwise be difficult to obtain.
EXPERIMENTAL
General
All reactions were conducted under an argon atmosphere using flame-dried glassware unless otherwise noted. Toluene, THF, and Et2O were distilled from sodium benzophenone ketyl. HMPA and DMF were distilled from Na, in vacuo. Methylene chloride was distilled from CaH2. NMR spectra were recorded on a Bruker-DRX-Avance-500 or a GE Omega-300 instrument. Chemical shifts are reported relative to residual CHCl3 (7.25 ppm, 1H; 77.0 ppm, 13C). Infrared spectra were obtained using an Nicolet Avatar 360 FTIR spectrometer. High resolution mass spectra were acquired at the Nebraska Center for Mass Spectrometry (University of Nebraska).
(4S,5S)-4-Methoxycarbonyl-5-(phenylseleno)methyl-2-phenyloxazoline (44t)/(4S,5R)-4-Methoxycarbonyl-5-(phenylseleno)methyl-2-phenyloxazoline (44c)
To a solution of phenylselenenyl chloride (1.92 g, 10.0 mmol) in THF (40 mL) at −78°C was added silver triflate (2.82 g, 10.9 mmol) in THF (30 mL). The resulting orange mixture was stirred at −78°C for 20 minutes and then cooled to −100 °C [EtOH-N2(l) slush bath]. A solution of L-4324c (2.00 g, 9.12 mmol) in THF (20 mL) at −100°C was then added via cannula and the reaction stirred for 2 h. EtOAc (60 mL) was added and the mixture was extracted with H2O (3 × 80 mL). The organic layer was dried (MgSO4), filtered, evaporated. Chromatography [PhCH3 or PhH/CH2Cl2/EtOAc (4:4:1)] provided trans-oxazoline (44t) (1.42 g, 42%) in a first fraction, and then cis-oxazoline (44c) (1.28 g, 37%). For 44t: [α]D24+50.3 (c 0.5, CHCl3); 1H NMR (500 MHz, CDCl3) δ 3.17 (dd, J = 7, 13 Hz, 1 H), 3.29 (dd, J = 7, 13 Hz, 1 H), 3.79 (s, 3 H), 4.71 (d, J = 7 Hz, 1 H), 5.12 (app dt, J = 5, 7 Hz, 1 H), 7.24 (m, 3 H), 7.36 (m, 2 H), 7.47 (m, 1 H), 7.56 (m, 2 H), 7.86 (d, J = 8 Hz, 2 H); 13C NMR (125 MHz, CDCl3) δ 31.6, 52.6, 73.7, 81.3, 126.9, 127.6, 128.2, 128.6, 128.7, 129.2, 131.8, 133.7, 165.3, 171.1; IR (ATR) 1743, 1643 cm−1. Anal. Calcd. for C18H17NO3Se: C, 57.76, H, 4.58; N, 3.74. Found: C, 57.57; H, 4.22; N, 3.93. For 44c: [α]D24 +16.2 (c 0.6, CHCl3); 1H NMR (500 MHz, CDCl3) δ 3.16 (dd, J = 5.5, 13 Hz, 1 H), 3.24 (dd, J = 8, 13 Hz, 1 H), 3.77 (s, 3 H), 5.02 (d, J = 10 Hz, 1 H), 5.09 (ddd, J = 5.5, 8, 10 Hz, 1 H), 7.24 (m, 3 H), 7.36 (m, 2 H), 7.47 (m, 1 H), 7.56 (m, 2 H), 7.88 (d, J = 8 Hz, 2 H); 13C NMR (125 MHz, CDCl3) δ 27.4, 52.4, 71.2 (CH), 81.0, 126.7, 127.6, 128.3, 128.6, 129.0, 129.2, 132.0, 133.5, 165.9, 170.1; IR (ATR) 1740, 1643 cm−1. Anal. Calcd. for C18 H17NO3Se: C, 57.76; H, 4.58; N, 3.74. Found: C, 57.88; H, 4.42; N, 3.98.
General Procedure A
(4R,5S)-4-Benzyl-4-methoxycarbonyl-5-(phenylseleno)methyl-2-phenyloxazoline (L-45a)
To a solution of KHMDS (7.0 mL, 0.5 M in toluene) and HMPA (3.0 mL) in THF (3.0 mL) at −78 °C was added 44t (98% ee, Chiralcel OD) (1.20 g, 3.20 mmol) in THF (10 mL) at −78 °C via cannula. The resulting deep orange/red solution was stirred for 20 min at −78 °C followed by addition of benzyl bromide (0.45 mL, d = 1.44, 3.80 mmol). After stirring for 3 h, the reaction mixture was poured into ether (15 mL) and NH4Cl (aqueous, 15 mL). Following further extraction with EtOAc (3 × 15 mL), the combined organics were dried (MgSO4), filtered, evaporated, and chromatographed (10% EtOAc/hexanes) to give L-45a (1.34 g, 90%, 98% ee, Chiralcel OD) as a white solid: mp 79–81 °C; [α]D24 +48.5 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3) δ 3.21–3.24 (m, 3 H), 3.49 (d, J = 13.7 Hz, 1 H), 3.84 (s, 3 H), 4.89 (t, J = 6.6 Hz, 1 H), 7.23–7.34 (m, 5 H), 7.36–7.38 (m, 3 H), 7.44 (t, J = 7.7 Hz, 2 H), 7.55 (t, J = 7.5 Hz, 1 H), 7.62–7.64 (m, 2 H), 7.88 (d, J = 7.3 Hz, 2 H); 13C NMR (125 MHz, CDCl3) δ 28.3, 44.3, 52.6, 81.1, 84.8, 126.8, 127.0, 127.4, 128.0, 128.2, 128.5, 129.2, 129.3, 130.9, 131.7, 133.6, 134.9, 164.2, 171.7. Anal. Calcd. For C25H23NO3Se: C, 64.66; H, 4.99; N, 3.02. Found: C, 64.98; H, 4.58; N, 3.03.
(4S,5R)-4-Benzyl-4-methoxycarbonyl-5-(phenylseleno)methyl-2-phenyloxazoline (D-45a)
From 44c (>99% ee, Chiralcel OD) (1.00 g, 2.67 mmol) and benzyl bromide (0.40 mL, d = 1.44, 3.20 mmol) following General Procedure A, was obtained D-45a (0.99 g, 80%, >99% ee, Chiralcel OD): [α]D24 −51.1 (c 1.0, CHCl3); 1H NMR (500 MHz, CDCl3) δ 3.21–3.25 (m, 3 H), 3.49 (d, J = 13.7 Hz, 1 H), 3.84 (s, 3 H), 4.89 (t, J = 6.6 Hz, 1 H), 7.23–7.34 (m, 5 H), 7.36–7.38 (m, 3 H), 7.44 (t, J = 7.7 Hz, 2 H), 7.55 (t, J = 7.5 Hz, 1 H), 7.62–7.64 (m, 2 H), 7.88 (d, J = 7.3 Hz, 2 H); 13C NMR (125 MHz, CDCl3) δ 28.4, 44.3, 52.6, 81.1, 84.8, 126.9, 127.5, 128.0, 128.2, 128.6, 129.2, 129.4, 130.9, 131.7, 133.4, 134.9, 142.0, 164.3, 171.7; IR (ATR) 3012, 1747, 1659 cm−1.
(4S,5R)-4-(Benzyloxy)methyl-4-methoxycarbonyl-5-(phenylseleno)methyl-2-phenyloxazoline (D-45c)
From 44c (>99% ee, Chiralcel OD) (1.00 g, 2.67 mmol) and benzyloxymethyl bromide (0.64 g, 3.20 mmol), following General Procedure A, was obtained D-45c (1.20 g, 90%, >99% ee, Chiralcel OD) as an oil, after flash chromatography (25% EtOAc/hexanes): [α]D24 −36.5 (c 1.0, CHCl3); 1H NMR (300 MHz, CDCl3) δ 3.17 (dd, J = 4.5, 8.5 Hz, 1 H), 3.26 (dd, J = 4, 8.5 Hz, 1 H), 3.77 (s, 3 H), 3.85 (d, J = 9.9 Hz, 1 H), 3.89 (d, J = 9.9 Hz, 1 H), 4.58 (s, 2 H), 5.00 (dd, J = 4.5, 8.8 Hz, 1 H), 7.23–7.28 (m, 7 H), 7.37–7.40 (m, 3 H), 7.48 (t, J = 7.3, 1 H), 7.56–7.57 (m, 2 H), 7.89 (d, J = 7.4 Hz, 2 H); 13C NMR (75 MHz, CDCl3) δ 28.2, 52.6, 73.2, 73.7, 80.9, 83.5, 126.9, 127.3, 127.6, 127.7, 128.2, 128.3, 128.6, 128.7, 129.1, 129.2, 129.5, 131.7, 133.1, 137.8, 165.2, 170.3; IR (ATR) 1734, 1652, 1450 cm−1; MS (FAB, 3-NBA), 518 (100, M+Na); HRMS (FAB, 3-NBA) m/z calcd for C26 H25NO4Se (M+Na) 518.0846, obsd 518.0839 (Δ = +1.4).
General Procedure B
Methyl N-Benzoyl-L-α-(2E-phenylseleno)vinylphenylalaninate (L-46a)
To a solution of L-45a (79 mg, 0.17 mmol) at 0 °C in DMF (2.3 mL) was added KOtBu (38 mg, 0.34 mmol). After warming to rt and stirring for 2 h, the resulting solution was poured into EtOAc (15 mL) and 1 N HCl (15 mL). Following a second extraction with EtOAc (10 mL), the combined organics were dried (MgSO4), filtered, and evaporated. The crude product was taken up in ether (10 mL) and treated with diazomethane. Evaporation of the solvent and flash chromatography (25% EtOAc/hexanes) yielded L-46a (60 mg, 76%): [α]D24 +21.4 (c 0.1, CDCl3); 1H NMR (500 MHz, CDCl3) δ 3.40 (d, J = 13 Hz, 1 H), 3.82 (s, 3 H), 3.91 (d, J = 13 Hz, 1 H), 6.29 (d, J = 16 Hz, 1 H), 6.70 (d, J = 16 Hz, 1 H), 6.97 (s, 1 H), 7.05 m, 2 H), 7.22 (m, 3 H), 7.27 (m, 3 H), 7.39 (t, J = 7.5 Hz, 2 H), 7.47 (m, 3 H), 7.68 (d, J = 7.5 Hz, 2 H); 13C NMR (125 MHz, CDCl3) δ 40.3, 53.1, 66.5, 121.5, 126.9, 127.1, 127.3, 128.3, 128.4, 128.6, 129.3, 129.9, 131.6, 132.3, 133.3, 134.7, 135.4, 166.3, 171.7; IR (ATR) 1736, 1650 cm−1. Anal. Calcd for C25H23NO3Se: C, 64.66; H, 4.99; N, 3.02. Found: C, 64.86; H, 5.30; N, 2.92.
Methyl N-Benzoyl-D-α-(2E-phenylseleno)vinylphenylalaninate (D-46a)
From D-45a (0.51 g, 1.1 mmol) in DMF (6 mL), folowing General Procedure B, was obtained D-46a (357 mg, 70%) as an oil after diazomethane esterification and flash chromatography (30% EtOAc/hexanes): [α]D24 −20.4 (c 0.3, CHCl3); 1H NMR (500 MHz, CDCl3) δ 3.40 (d, J = 13 Hz, 1 H), 3.82 (s, 3 H), 3.91 (d, J = 13 Hz, 1 H), 6.29 (d, J = 16 Hz, 1 H), 6.70 (d, J = 16 Hz, 1 H), 6.97 (s, 1 H), 7.05 m, 2 H), 7.22 (m, 3 H), 7.27 (m, 3 H), 7.39 (t, J = 7.5 Hz, 2 H), 7.47 (m, 3 H), 7.68 (d, J = 7.5 Hz, 2 H); 13C NMR (125 MHz, CDCl3) δ 40.3, 53.1, 66.5, 121.5, 126.9, 127.1, 127.3, 128.3, 128.4, 128.6, 129.3, 129.9, 131.6, 132.3, 133.3, 134.7, 135.4, 166.3, 171.7; IR (ATR) 1736, 1677 cm−1; MS (FAB, 3-NBA) 466 (M+H, 100); HRMS (FAB, 3-NBA) m/z calcd for C25H23NO3Se (M+H) 466.0921, obsd 466.0907 (Δ = +3.1 ppm).
Methyl N-Benzoyl-O-benzyl-D-α-(2E-phenylseleno)vinylserinate (D-46c)
From D-45c (1.2 g, 2.42 mmol) in THF (30 mL), following General Procedure B, was obtained D-46c (900 mg, 75%) after diazomethane esterification and flash chromatography (25% EtOAc/hexanes): [α]D24 +20.4 (c 2.0, CHCl3); 1H NMR (300 MHz, CDCl3) δ 3.82 (s, 3 H), 3.99 (d, J = 9.3 Hz, 1 H), 4.30 (d, J = 9.3 Hz, 1 H), 4.48 (d, J = 12.3 Hz, 1 H), 4.55 (d, J = 12.3 Hz, 1 H), 6.23 (d, J = 15.6 Hz, 1 H), 6.79 (d, J = 15.6 Hz, 1 H), 7.23–7.32 (m, 9 H), 7.46–7.48 (m, 4 H), 4.52 (m, 1 H), 7.84 (d, J = 7.5 Hz; 2 H); 13C NMR (75 MHz, CDCl3) δ 53.2, 65.9, 70.7, 73.4, 122.6, 127.0, 127.2, 127.6, 127.8, 128.3, 128.5, 129.3, 129.8, 130.0, 131.7, 132.2, 134.2, 137.6, 166.3, 170.7; IR (ATR) 3018, 1747, 1665 cm−1; MS (FAB, 3-NBA) 502 (100, M+Li); HRMS (FAB, 3-NBA) m/z calcd for C26 H25NO4Se (M+Li) 502.1109, obsd 502.1100 (Δ = +1.7 ppm).
General Procedure C
Methyl N-Benzoyl-D-α-(2E-tri-n-butylstannyl)vinylphenylalanine (D-47a)
To a deoxygenated solution of D-46a (0.52 g, 1.12 mmol) in toluene (15 mL) was added AIBN (42 mg, 0.26 mmol) and tributyltin hydride (0.58 mL, 2.24 mmol). The solution was then stirred at 85 °C for 1.5 h. After cooling the reaction mixture to rt, EtOAc (50 mL) and H2O (50 mL) were added. After further extraction with EtOAc (3 × 50 mL), the combined organics were dried (MgSO4), filtered, evaporated, and chromatographed (0–10% EtOAc/hexanes) to give D-47a (0.53 g, 80%): [α]D24 −15.7 (c 1.0, CHCl3); 1H NMR (300 MHz, CDCl3) δ 0 84–0.95 (m, 15 H), 1.25–1.34 (m, 6 H), 1.45–1.56 (m, 6 H), 3.55 (d, J = 13 Hz, 1 H), 3.80 (s, 3 H), 3.82 (d, J = 13 Hz, 1 H), 6.16 (d, J = 19.2 Hz, 1 H), 6.23 (d, J = 19.2 Hz, 1 H), 6.82 (s, 1 H), 7.07–7.11 (m, 2 H), 7.18–7.20 (m, 3 H), 7.42 (t, J = 7.3 Hz, 2 H), 7.48 (t, J = 7.3 Hz, 1 H), 7.71 (d, J = 7.3 Hz, 2 H); 13C NMR (75 MHz, CDCl3) δ 9.6, 13.7, 27.2, 28.9, 33.7, 52.8, 67.4, 126.8, 126.9, 128.1, 128.5, 130.1, 130.3, 131.4, 135.0, 136.3, 143.9, 166.3, 172.3; IR (ATR) 1737, 1483 cm−1; HREI, calcd for C31 H45NO3Sn (M-t-butyl) 542.1717, obsd 542.1709 (Δ = +1.5).
Methyl N-Benzoyl-L-α-(2E-tri-n-butylstannyl)vinylphenylalanine (L-47a)
From L-46a (45 mg, 0,10 mmol) in toluene (1.3 mL), following General Procedure C, was obtained L-47a (48 mg, 84%), after flash chromatography (20% EtOAc/hexanes): [α]D24 +14.3 (c 1.8, CHCl3); 1H NMR (300 MHz, CDCl3) δ 0 84–0.98 (m, 15 H), 1.25–1.33 (m, 6 H), 1.45–1.56 (m, 6 H), 3.55 (d, J = 13 Hz, 1 H), 3.79 (s, 3 H), 3.80 (d, J = 13 Hz, 1 H), 6.16 (d, J = 19.2 Hz, 1 H), 6.23 (d, J = 19.2 Hz, 1 H), 6.80 (s, 1 H), 7.07–7.17 (m, 2 H), 7.18–7.20 (m, 3 H), 7.42 (t, J = 7.3 Hz, 2 H), 7.48 (t, J = 7.3 Hz, 1 H), 7.71 (d, J = 7.3 Hz, 2 H); 13C NMR (75 MHz, CDCl3) δ 9.6, 13.7, 27.2, 28.9, 33.7, 52.8, 67.4, 126.8, 126.9, 128.1, 128.5, 130.1, 130.3, 131.4, 135.0, 136.3, 143.9, 166.3, 172.3; IR (ATR) 1738, 1482 cm−1. Anal. Calcd for C31H45NO3Sn: C, 62.22; H, 7.58; N, 2.34. Found: C, 62.40; H, 7.32; N, 2.28.
Methyl N-Benzoyl-O-benzyl-D-α-(2E-tri-n-butylstannyl)vinylserinate (D-47c)
From D-46c (800 mg, 1.6 mmol), following General Procedure C, was obtained D-47c (748 mg, 74%) after flash chromatography (20% EtOAc/hexanes): [α]D24 +24.4 (c 0.8, CHCl3); 1H NMR (300 MHz, CDCl3) δ 0.84–0.93 (m, 15 H), 1.24–1.32 (m, 6 H), 1.45–1.50 (m, 6 H), 3.78 (s, 3 H), 4.06 (d, J = 9.3 Hz, 1 H), 4.33 (d, J = 9.3 Hz, 1 H), 4.49 (d, J = 12 Hz, 1 H), 4.56 (d, J = 12 Hz, 1 H), 6.09 (d, J = 19.3 Hz, 1 H), 6.36 (d, J = 19.3 Hz, 1 H), 7.18 (s, 1 H), 7.24–7.32 (m, 5 H), 7.47 (t, J = 7.5 Hz, 2 H), 7.52 (t, J = 7.5 Hz, 1 H), 7.48 (d, J = 7.5 Hz, 2 H); 13C NMR (75 MHz, CDCl3) δ 9.6, 13.6, 27.1, 28.9, 52.9, 67.2, 70.9, 73.3, 17.1, 127.5, 127.6, 128.3, 128.5, 131.4, 131.6, 134.8, 138.0, 141.0, 166.1, 171.4; IR (ATR) 1735, 1443 cm−1; MS (FAB, 3-NBA), 636 (100, M+Li); HRMS (FAB, 3-NBA) m/z calcd for C32 H47NO4Sn (M+Li) 636.2687, obsd 636.2698 (Δ = −1.8 ppm).
(2′E-Deuterio)-L-α-vinylphenylalanine (L-48a)
A solution of L-47a (46 mg, 0.08 mmol) in 6 N DCl (1.0 mL) was stirred at light reflux for 4.5 h. Then the solution was cooled, taken up in H2O (5 mL) and extracted with CH2Cl2 (2 × 5.5 mL). The residue was applied to a Dowex 50 × 8 ion-exchange column. After the column was washed with H2O, elution with 1.3 M NH4OH afforded 2′-deuterio L-48a (11.3 mg, 75%): [α]D24 −6.6 (c 0.3, D2O); 1H NMR (300 MHz, D2O) δ 3.10 (d, J = 14.3 Hz, 1 H), 3.43 (d, J = 14.3 Hz, 1 H), 5.30 (d, J = 17.9 Hz, 1 H), 6.19 (d, J = 17.7 Hz, 1 H), 7.28 (m, 2 H), 7.38 (m, 3 H); 13C NMR (75 MHz, D2O) δ 42.1, 66.9, 117.4, 128.8, 129.7, 130.9, 134.4, 135.8, 174.4; MS (FAB, 3-NBA) 193 (69, M+1), 219 (100); HRMS (FAB, 3-NBA) m/z calcd for C11 H12NO2D (M+1) 193.1087, obsd 193.1085 (Δ = +1.1 ppm).
Methyl N-Benzoyl-α-1-[(4′-nitro)-E-styrenyl]-L-phenylalaninate (49)
A solution of Pd2(dba)3 (14 mg, 0.02 mmol) and 1-iodo-4-nitrobenzene (21.0 mg, 0.09 mmol) in THF (1.0 mL) was stirred for 10 min at rt. Then L-47a (50.0 mg, 0.09 mmol) in THF (1.0 mL) was added via cannula. The reaction was stirred at 50 °C for 2 h, then cooled to rt. Ethyl acetate (4 mL) was added and the mixture was filtered through Celite. The solvent was evaporated off and the crude product was chromatographed (10–30% EtOAc/hexanes) to give 49 (30.6 mg, 85%): [α]D24 +71.8 (c 0.6, CHCl3); 1H NMR (500 MHz, CDCl3) δ 3.49 (d, J = 13 Hz, 1 H), 3.88 (s, 3 H), 4.02 (d, J = 13 Hz, 1 H), 6.64 (d, J = 16 Hz, 1 H), 6.78 (d, J = 16 Hz, 1 H), 7.09 (m, 2 H), 7.17 (s, 1 H), 7.24 (m, 3 H), 7.45 (app t, J = 7.6 Hz, 2 H), 7.54 (m, 3 H), 7.76 (d, J = 7.2 Hz, 2 H), 8.18 (d, J = 8.8 Hz, 2 H); 13C NMR (125 MHz, CDCl3) δ 40.8, 53.3, 65.6, 123.9, 126.9, 127.3, 127.4, 128.5, 128.7, 128.9, 129.9, 131.9, 132.9, 135.1, 142.7, 143.3, 147.2, 166.3, 171.8; IR (ATR) 3020, 1740, 1653, 1515, 1483, 1342 cm−1; MS (FAB, 3-NBA) 431 (100, M+1); HRMS (FAB, 3-NBA) m/z calcd for C25H22N2O5 (M+1) 431.1607, obsd 431.1613 (Δ = −1.4 ppm).
Methyl N-Benzoyl-α-(2E-benzoyl)vinyl-D-phenylalaninate (50)
To a solution of D-47a (20 mg, 0.05 mmol) and Pd(PPh3)2Cl2 (7 mg, 0.01 mmol) in THF (0.5 mL) was added benzoyl chloride (6 μL, 0.05 mmol). The reaction mixture was refluxed for 3 h, cooled to rt, poured into EtOAc (0.5 mL) and extracted with H2O (2 × 0.5 mL). The organic layer was dried (MgSO4), filtered, evaporated and chromatographed (30% EtOAc/hexanes) to give 50 (8 mg, 60%) as an oil. [α]D24 −26.3 (c 0.4, CHCl3); 1H NMR (300 MHz, CDCl3) δ 3.48 (d, J = 13.4 Hz, 1 H), 3.84 (s, 3 H), 3.94 (d, J = 13.4 Hz, 1 H), 7.00 (d, J = 16.0 Hz, 1 H), 7.10–7.13 (m, 3 H), 7.22 (d, J = 16.0 Hz, 1 H), 7.25–7.28 (m, 3 H), 7.44–7.50 (m, 4 H), 7.56 (app t, J = 7.6 Hz, 2 H), 7.75 (d, J = 7.2 Hz, 2 H), 7.90 (d, J = 7.2 Hz, 2 H); 13C NMR (75 MHz, CDCl3) δ 40.4, 53.5, 65.6, 126.5, 127.0, 127.5, 128.5, 128.6, 128.7, 128.8, 129.8, 131.9, 133.0, 134.8, 144.7, 166.4, 171.2; IR (ATR) 2993, 1734, 1659, 1634, 1508 cm−1; MS (FAB, 3-NBA) 414 (100, M+1); HRMS (FAB, 3-NBA) m/z calcd for C26H23NO4 (M+1) 414.1705, obsd 414. 1718 (Δ = −3.0 ppm).
Methyl N-Benzoyl-α-1-(E-1,4-pentadienyl)-D-phenylalaninate (51)
To a solution of (PPh3)2Pd(PhCH2)Cl (1.1 mg, 1.5 μmol) in THF (0.4 mL) was added allyl bromide (4 μL, 0.05 mmol) and D-47a (30 mg, 0.05 mmol). The reaction mixture was stirred at reflux for 3 h. After cooling to rt, EtOAc (5 mL) was added and the mixture was filtered through Celite, evaporated and chromatographed (10% EtOAc/hexanes) to give 51 (15 mg, 84%)as an oil: [α]D24 −33.2 (c 1.1, CHCl3); 1H NMR (300 MHz, CDCl3) δ 2.86 (t, J = 6.4 Hz, 2 H), 3.44 (d, J = 13.5 Hz, 1 H), 3.84 (s, 3 H), 3.89 (d, J = 13.5. Hz, 1 H), 5.04 (br d, J = 10.5 Hz, 1 H), 5.06 (br d, J = 17 Hz, 1 H), 5.72 (dt, J = 6, 16 Hz, 1 H), 5.85 (d, J = 16 Hz, 1 H), 5.80–5.90 (m, 1 H), 6.96 (s, 1 H), 7.08–7.11 (m, 2 H), 7.19–7.27 (m, 3 H), 7.43 (app t, J = 7.4 Hz, 2 H), 7.51 (app t, J = 7.4 Hz, 1 H), 7.72 (d, J = 7.0 Hz, 2 H); 13C NMR (75 MHz, CDCl3) δ 36.1, 40.2, 52.9, 65.3, 115.9, 126.9, 127.0, 128.2, 128.6, 129.4, 129.9, 130.0, 134.9, 135.8, 135.9, 166.3, 172.6; IR (ATR) 3031, 1747, 1671, 1514, 1483 cm−1; MS (FAB, 3-NBA) 350 (100, M+1); HRMS (FAB, 3-NBA) m/z calcd for C22H23NO3 (M+1) 350.1756, obsd 350.1759 (Δ = +0.8 ppm).
Methyl N-Benzoyl-α-1-[E-(3-phenyl)propenyl]-D-phenylalaninate (52)
To a solution of (PPh3)2Pd(PhCH2)Cl (6 mg, 8 μmol) and benzyl bromide (10.5 mg, 0.06 mmol) in THF (1 mL) was added D-47a (30 mg, 0.05 mmol). The solution was refluxed for 3 h, cooled to rt, and H2O (5 mL) was added. The mixture was extracted with EtOAc (2 × 5 mL). The organics were combined, dried (MgSO4), filtered, evaporated and chromatographed (0–10% EtOAc/hexanes) to give 52 (13 mg, 63%) as an oil: [α]D24 −47.8 (c 0.7, CHCl3); 1H NMR (300 MHz, CDCl3) δ 3.46 (d, J = 13.4 Hz, 1 H), 3.47 (d, J = 6.2 Hz, 2 H), 3.84 (s, 3 H), 3.96 (d, J = 13.4 Hz, 1 H), 5.85 (dt, J = 6, 16 Hz, 1 H), 5.94 (d, J = 16 Hz, 1 H), 6.96 (s, 1 H), 7.07–7.10 (m, 2 H), 7.17–7.24 (m, 6 H), 7.27–7.33 (m, 2 H), 7.42 (app t, J = 7.2 Hz, 2 H), 7.51 (app t, J = 7.2 Hz, 1 H), 7.72 (d, J = 7.0 Hz, 2 H); 13C NMR (75 MHz, CDCl3) δ 38.4, 40.3, 65.1, 126.2, 126.8, 126.9, 128.2, 128.4, 128.6, 130.0, 130.1, 130.7, 131.5, 134.8, 135.9, 139.6, 166.3, 172.6; IR (ATR) 3018, 1741, 1671, 1527, 1482 cm−1; MS (FAB, 3-NBA) 400 (100, M+1); HRMS (FAB, 3-NBA) m/z calcd for C26H25NO3 (M+1) 400.1894, obsd 400.1895 (Δ = −0.1 ppm).
General Procedure D
Methyl N-Benzoyl-α-(2E-iodo)vinyl-L-phenylalaninate (L-53a)
To a solution of L-47a (150 mg, 0.26 mmol) in CCl4 (4.4 mL) at 4 °C was added iodine (167 mg, 0.66 mmol). The mixture was stirred at 4 °C for 30 min. Sodium bisulfite (10% aqueous) was added and the solution was extracted with ether (2 × 5 mL). The organics were combined, dried (MgSO4), filtered, evaporated and chromatographed (10% EtOAc/hexanes) to give L-53a (100 mg, 92%): [α]D24 +52.7 (c 0.7, CHCl3); 1H NMR (300 MHz, CDCl3).δ 3.35 (d, J = 13.6 Hz, 1 H), 3.86 (s, 3 H), 3.92 (d, J = 13.6 Hz, 1 H), 6.46 (d, J = 14.8 Hz, 1 H), 6.92 (d, J = 14.8 Hz, 1 H), 7.02 (s, 1 H), 7.07 (m, 2 H), 7.24 (m, 3 H), 7.44 (app t, J = 7.4 Hz, 2 H), 7.52 (app t, J = 7.3 Hz, 1 H), 7.77 (d, J = 7.1 Hz, 2 H); 13C NMR (75 MHz, CDCl3) δ 41.2, 54.3, 68.9, 80.2, 127.9, 128.4, 129.5, 129.7, 130.9, 132.8, 135.3, 135.8, 143.5, 167.3, 172.0; IR (ATR) 2949, 1735, 1653 cm−1; MS (FAB, 3-NBA) 436 (100, M+1); HRMS (FAB, 3-NBA) m/z calcd for C19 H18NO3I (M+1) 436.0410, obsd 436.0413 (Δ = −0.8 ppm).
Methyl N-Benzyl-α-(2E-iodo)vinyl-D-phenylalaninate (D-53a)
From D-47c (240 mg, 0.41 mmol), following General Procedure D, was obtained D-53a (160 mg, 90%) after flash chromatography (10% EtOAc/hexanes): [α]D24 −50.0 (c 0.5, CHCl3); 1H NMR (300 MHz, CDCl3).δ 3.35 (d, J = 13.6 Hz, 1 H), 3.86 (s, 3 H), 3.92 (d, J = 13.6 Hz, 1 H), 6.46 (d, J = 14.8 Hz, 1 H), 6.92 (d, J = 14.8 Hz, 1 H), 7.02 (s, 1 H), 7.07 (m, 2 H), 7.24 (m, 3 H), 7.44 (app t, J = 7.4 Hz, 2 H), 7.52 (app t, J = 7.3 Hz, 1 H), 7.77 (d, J = 7.1 Hz, 2 H); 13C NMR (75 MHz, CDCl3) δ 40.1, 53.3, 67.9, 79.3, 126.9, 127.4, 128.5, 128.7, 129.9, 131.8, 134.3, 134.8, 142.4, 167.3, 171.0; IR (ATR) 3031, 1753, 1671 cm−1; MS (FAB, 3-NBA) 436 (100, M+1); HRMS (FAB, 3-NBA) m/z calcd for C19 H18NO3I (M+1) 436.0410, obsd 436.0396 (Δ = +3.2 ppm).
Methyl N-Benzoyl-O-benzyl-α-(2E-iodo)vinyl-D-serinate (D-53c)
From D-47a (738 mg, 1.17 mmol), following General Procedure D, was obtained D-53c (540 mg, 98%) after flash chromatography (10% EtOAc/hexanes): [α]D24 +24.7 (c 0.6, CDCl3); 1H NMR (300 MHz, CDCl3) δ 3.82 (s, 3 H), 3.91 (d, J = 9.3 Hz, 1 H), 4.22 (d, J = 9.3 Hz, 1 H), 4.50 (d, J = 12.2 Hz, 1 H), 4.58 (d, J = 12.2 Hz, 1 H), 6.52 (d, J = 14.8, 1 H), 6.81 (d, J = 14.8 Hz, 1 H), 7.24–7.33 (m, 6 H), 7.46 (app t, J = 7.3 Hz, 2 H), 7.55 (app t, J = 7.3 Hz, 1 H), 7.82 (d, J = 7.4 Hz, 2 H); 13C NMR (75 MHz, CDCl3) δ 53.4, 67.3, 70.4, 73.4, 80.3, 127.1, 127.7, 127.9, 127.5, 128.6, 131.9, 133.9, 137.3, 139.8, 166.3, 170.0; IR (ATR) 3018, 1747, 1671, 1514 cm−1; MS (FAB, 3-NBA) 472 (100, M+Li); HRMS (FAB, 3-NBA) m/z calcd for C20H20NO4I (M+Li) 472.0597, obsd 472.0602 (Δ = −0.9 ppm).
Methyl N-Benzoyl-α-1-(E-1,3-butadienyl)-L-phenylalaninate (54)
To a solution of Pd2(dba)3 (1.9 mg, 2.00 μmol) and tri-(2-furyl)phosphine (4.3 mg, 0.02 mmol) in THF (2 mL) was added L-53a (50 mg, 0.12 mmol). The mixture was stirred for 10 min at rt. Then tributyl(vinyl)tin (38.1 mg, 0.12 mmol) was added and the reaction mixture was stirred at 50 °C for 2 h, filtered through Celite and the solvent was evaporated. The crude product was chromatographed (10% EtOAc/hexanes) to yield 54 (24.9 mg, 65%): [α]D24 +52.3 (c 0.5, CHCl3); 1H NMR (500 MHz, CDCl3) δ 3.42 (d, J = 13 Hz, 1 H), 3.82 (s, 3 H), 3.92 (d, J = 13 Hz, 1 H), 5.15 (d, J = 10 Hz, 1 H), 5.21 (d, J = 16.6 Hz, 1 H), 6.04 (d, J = 15.5 Hz, 1 H), 6.24 (dd, J = 15.4 Hz, 1 H), 6.38 (dt, J = 15.7, 10 Hz, 1 H), 7.00 (s, 1 H), 7.07 (m, 2 H), 7.20 (m, 3 H), 7.40 (app t, J = 7.6 Hz, 2 H), 7.50 (app t, J = 7.4 Hz, 1 H), 7.71 (d, J = 7.3 Hz, 2 H); 13C NMR (125 MHz, CDCl3) δ 40.3, 53.1, 65.3, 118.8, 126.9, 127.1, 128.3, 128.6, 129.9, 131.6, 131.8, 134.7, 135.7, 137.8, 142.0, 166.3, 172.3; IR (ATR) 3029, 1735, 1653 cm−1; MS (FAB, 3-NBA) 336 (100, M+1); HRMS (FAB, 3-NBA) m/z calcd for C21 H21NO3 (M+1) 336.1600, obsd 336.1598 (Δ = +0.4 ppm).
Methyl N-Benzoyl-α-1-[(3-phenyl)-E-1,3-butadienyl]-D-phenylalaninate (55)
To a solution of D-53a (30 mg, 0.07 mmol) and Pd2(dba)3 (11 mg, 14 μmol) in THF (0.6 mL)was added 1-phenylvinylzinc bromide [0.6 mL of a 0.5 M solution in THF (Rieke Metals, Inc.; Lincoln, Nebraska); 0.3 mmol]. The solution was then stirred at 40 °C for 8 h. The reaction mixture was cooled and poured into NH4Cl (5 mL) and extracted with EtOAc (2 × 5 mL). The combined organic layers were dried (MgSO4), filtered, evaporated and chromatographed (10% EtOAc/hexanes) to give 55 (22 mg, 79%) as an oil: [α]D24 −12.4 (c 1.1, CHCl3); 1H NMR (300 MHz, CDCl3) δ 3.46 (d, J = 13.6 Hz, 1 H), 3.84 (s, 3 H), 3.90 (d, J = 13.6 Hz, 1 H), 5.28 (d, J = 16.9 Hz, 2 H), 6.00 (d, J = 16 Hz, 1 H), 6.52 (d, J = 16 Hz, 1 H), 6.98 (s, 1 H), 7.07–7.09 (m, 2 H), 7.21–7.24 (m, 4 H), 7.33–7.39 (m, 4 H), 7.45 (app t, J = 7.3 Hz, 2 H), 7.53 (app t, J = 7.3 Hz, 1 H), 7.75 (d, J = 7.3 Hz, 2 H); 13C NMR (75 MHz, CDCl3) δ 40.3, 53.0, 65.3, 117.9,126.9, 127.0, 127.6, 128.2, 128.3, 128.6, 130.0, 131.3, 131.6, 132.4, 134.8, 135.8, 139.7, 146.7, 166.3, 172.3; IR (ATR) 3043, 1741, 1671, 1508 cm−1; MS (FAB,3-NBA) 434 (M+Na, 74); HRMS (FAB, 3-NBA) m/z calcd for C27 H25NO3 (M+Na) 434.1711, obsd 434.1711 (Δ = +0.7).
Methyl N-Benzoyl-O-benzyl-α-1-(E-oct-1-en-3-ynyl)-D-serinate (56)
To a solution of D-53a (20 mg, 0.04 mmol) CuI (400 μg, 2 μmol ) and (PPh3)2PdCl2 (300 μg, 0.43 μmol) in diethylamine (0.5 mL) was added 1-hexyne (5 μL, d = 0.715, 0.04 mmol). The reaction mixture was stirred at rt for 2 h. The diethylamine was evaporated and H2O (5 mL) was added to the residue. The mixture was extracted with EtOAc (2 × 5 mL). The combined organics were filtered through Celite, dried (MgSO4), evaporated and chromatographed (10% EtOAc/hexanes) to yield 56 (15.7 mg, 87%) as an oil: [α]D24 +23.8 (c 0.8, CHCl3); 1H NMR (300 MHz, CDCl3) δ 0.90 (t, J = 7.2 Hz, 3 H), 1.36–1.51 (m, 4 H), 2.28 (t, J = 7.0 Hz, 2 H), 3.80 (s, 3 H), 3.93 (d, J = 9.3 Hz, 1 H), 4.27 (d, J = 9.3 Hz, 1 H), 4.48 (d, J = 12.2 Hz, 1 H), 4.58 (d, J = 12.2 Hz, 1 H), 5.76 (d, J = 16.2 Hz, 1 H), 6.24 (d, J = 16.2 Hz, 1 H), 7.22–7.31 (m, 6 H), 7.45 (app t, J = 7.2 Hz, 2 H), 7.51 (app t, J = 7.2 Hz, 1 H), 7.82 (d, J = 7.2 Hz, 2 H); 13C NMR (75 MHz, CDCl3) δ 13.5, 19.1, 21.9, 30.6, 53.3, 65.0, 70.8, 73.4, 93.3, 113.1, 127.1, 127.6, 127.8, 128.4, 128.6, 131.7, 134.2, 135.8, 137.6, 166.2, 170.9; IR (ATR) 3043, 1741, 1678, 1508, 1489 cm−1; MS (FAB, 3-NBA) 420 (100, M+1); HRMS (FAB, 3-NBA) m/z calcd for C26H29NO4 (M+1) 420.2157, obsd 420. 2156 (Δ = +0.1 ppm).
Diels-Alder Adduct(s) 57
To a solution of 54 (100 mg, 0.30 mmol) in toluene (7 mL) was added dimethyl acetylenedicarboxylate (0.1 mL, 0.90 mmol). The reaction mixture was stirred at reflux for 12 h. Then the toluene was evaporated and the crude product was purified by chromatography (30% EtOAc/hexanes) to give 57 (71 mg, 50%): MS (FAB, 3-NBA) 478 (M+1, 45), 105 (100); HRMS (FAB, 3-NBA) m/z calcd for C27H27NO7 (M+1) 478.1848, obsd 478.1847 (Δ = +0.3).
Methyl N-Benzoyl-α-benzyl-L-2′,3′bis(carbomethoxy)phenylglycinate (58)
To a solution of 57 (27 mg, 0.06 mmol) in carbon tetrachloride (0.6 mL) was added bromine (47.9 mg, 0.3 mmol). The reaction mixture was stirred for 1 h at rt. Sodium thiosulfate (1 N) was added and the solution was extracted with EtOAc (3 mL), dried (MgSO4), filtered, evaporated and chromatographed (30% EtOAc/hexanes) to give the intermediate dibromide(s) (17.3 mg, 55%). To this product in DMF (0.3 mL) was added KOtBu (3.5 mg, 0.03 mmol). The reaction mixture was stirred at rt for 2 h. Then, 0.5N HCl (0.5 mL) was added and the solution was extracted with EtOAc (2 × 1 mL). The organics were combined, dried (MgSO4), filtered, evaporated and chromatographed (30% EtOAc/hexanes) to give 58 (12.7 mg, 86%): [α]D24 +24.4 (c 0.4, CHCl3); 1H NMR (300 MHz, CD2Cl2) δ 3.35 (s, 3 H), 3.73 (d, J = 12.6 Hz, 1 H), 3.76 (s, 3 H), 3.81 (s, 3 H), 4.33 (d, J = 12.6 Hz, 1 H), 6.99–7.02 (m, 2 H), 7.19–7.28 (m, 4 H), 7.41, (app t, J = 7.4 Hz, 2 H), 7.51 (app t, J = 7.4 Hz, 1 H), 7.59 (app d, J = 7 Hz, 2 H), 7.66 (t, J = 8.0 Hz, 1 H), 7.96 (d, J = 8.0 Hz, 1 H), 8.14 (d, J = 8.0 Hz, 1 H); 13C NMR (75 MHz, CDCl3) δ 40.0, 52.2, 53.3, 64.8, 126.9, 127.4, 128.2, 128.4, 129.3, 129.6, 131.6, 131.8, 132.9, 134.2, 134.8, 138.0, 166.9, 167.2, 169.1, 171.2; IR (ATR) 3018, 1747, 1665, 1514, 1482 cm−1; MS (FAB, 3-NBA) 476 (M+1, 24), 154 (100); HRMS (FAB, 3-NBA) m/z calcd for C27 H25NO7 (M+1) 476.1709, obsd 476.1708 (Δ = +0.2).
Methyl N-Benzoyl-α-1-[[(4′-acetoxy)-4-phenyl]-E-but-1-en-3-ynyl]-D-phenylalaninate (60)
A solution of D-47a (30 mg, 0.05 mmol), 1,1-dibromo-2[4-acetoxyphenyl]ethylene (15.5 mg, 0.05 mmol), diisopropylethylamine (9.4 mg, 0.07 mmol), Pd2(dba)3 (1.1 mg, 1.3 μmol) and (p-MeO-C6H4)3P (3.0 mg, 7.3 μmol) in DMF (0.5 mL) was stirred at 80 °C for 4 h. Then the reaction mixture was diluted with EtOAc (5 mL) and washed with H2O (10 mL). The organic layer was dried (MgSO4), filtered, evaporated and chromatographed (30% EtOAc/hexanes) to give 60 (13 mg. 55%): [α]D24 −67.7 (c 0.2, CHCl3); 1H NMR (300 MHz, CDCl3) δ 2.28 (s, 3 H), 2.39 (d, J = 13.4 Hz, 1 H), 3.84 (s, 3 H), 3.91 (d, J = 13.4 Hz, 1 H), 5.95 (d, J = 16.1 Hz, 1 H), 6.57 (d, J = 16.1 Hz, 1 H), 7.05–7.11 (m, 5 H), 7.24–7.27 (m, 3 H), 7.45 (d, J = 1.4 Hz, 2 H), 7.47 (d, J = 1.7 Hz, 2 H), 7.55 (app t, J = 7.0 Hz, 1 H), 7.72 (d, J = 7.0 Hz, 2 H); 13C NMR (75 MHz, CDCl3) δ 21.1, 40.3, 65.7, 111.6, 121.7, 127.0, 127.3, 128.5, 128.7, 129.9, 130.0, 131.8, 132.3, 132.7, 135.1, 140.4, 150.5, 166.4, 171.6, 172.5; IR (ATR) 3031, 1747, 1665, 1595 cm−1; MS (FAB, 3-NBA) 468 (M+H, 100); HRMS (FAB, 3-NBA) m/z calcd for C25H25NO5 (M+H) 468.1811, obsd 468.1791 (Δ = +4.1 ppm).
Scheme 1.
Scheme 7.
Scheme 10.
Scheme 19.
Acknowledgments
Financial support from the National Institutes of Health (CA 62034) is gratefully acknowledged. D.B.B. is an Alfred P. Sloan Research Fellow (1997–2001). We thank R. K. Shoemaker for assistance with 2D NMR experiments and R. Cerny (Nebraska Center for Mass Spectrometry) for mass spectral support.
REFERENCES AND NOTES
- 1.Williams RM. Synthesis of Optically Active Amino Acids. Pergamon Press; Oxford: 1989. [Google Scholar]
- 2.Dardenne G, Casimir J, Marlier M, Larsen PO. Phytochemistry. 1974;13:1897–1900. [Google Scholar]
- 3.(a) Cho C, Ishii R, Hyeon S, Suzuki A. Agric Biol Chem. 1987;51:2597–2598. [Google Scholar]; (b) Cornell NW, Zuurendonk PF, Kerich MJ, Straight CB. Biochem J. 1984;220:707–716. doi: 10.1042/bj2200707. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Soper TS, Manning JM, Marcotte PA, Walsh CT. J Biol Chem. 1977;252:1571–1575. [PubMed] [Google Scholar]; (d) Rando RR, Relyea N, Cheng L. J Biol Chem. 1976;251:3306–3312. [PubMed] [Google Scholar]; (e) Rando RR. Biochemistry. 1974;13:3859–3863. doi: 10.1021/bi00716a006. [DOI] [PubMed] [Google Scholar]
- 4.Griffith OW. J Biol Chem. 1983;258:1591–1598. [PubMed] [Google Scholar]
- 5.Zabriskie TM, Cheng H, Vederas JC. J Am Chem Soc. 1992;114:2270–2272. [Google Scholar]
- 6.Laber B, Gerbling K-P, Harde C, Neff K-H, Nordhoff E, Pohlenz H-D. Biochemistry. 1994;33:3413–3423. doi: 10.1021/bi00177a035. [DOI] [PubMed] [Google Scholar]
- 7.Clausen T, Wahl MC, Messerschmidt A, Huber R, Fuhrmann JC, Laber B, Streber W, Steegborn C. Biol Chem. 1999;380:1237–1242. doi: 10.1515/BC.1999.157. (APPA is said to be a reversible inhibitor in this active site) [DOI] [PubMed] [Google Scholar]
- 8.For structural mechanistic studies on the mode of GABA transaminase inactivation with a vinyl trigger see: Storici P, Capitani G, De Biase D, Moser M, John RA, Jansonius J, Schirmer T. Biochem. 1999;38:8628–8634. doi: 10.1021/bi990478j.Nanavati SM, Silverman RB. J Am Chem Soc. 1991;113:9341–9349.
- 9.Jung MJ, Palfreyman MG. Vigabatrin. In: Levy RH, Mattson RH, editors. Antiepileptic Drugs. 4. Raven Press; New York: 1995. [Google Scholar]
- 10.Brennan M. Chem Eng News. 1999 Aug 23;:8. [Google Scholar]
- 11.For references to the inactivation of AADC’s with α-vinyl AA’s see: Berkowitz DB, Jahng W-J, Pedersen ML. Bioorg Med Chem Lett. 1996;6:2151–2156. doi: 10.1016/0960-894X(96)00366-6.
- 12.We use the term higher α-vinyl amino acids to designate those members of this family bearing a side chain “higher” than the glycine side chain (i.e. R≠H).
- 13.Berkowitz DB, Pedersen ML. J Org Chem. 1995;60:5368–5369. doi: 10.1021/jo00122a002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berkowitz DB, Pedersen ML, Jahng W-J. Tetrahedron Lett. 1996;37:4309–4312. doi: 10.1016/0040-4039(96)00832-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jahng WJ, Salud-Bea R, Berkowitz DB. ORGN-351. 220th National ACS Meeting; August 20–24, 2000. [Google Scholar]
- 16.Mendel D, Ellman J, Schultz PG. J Am Chem Soc. 1993;115:4359–4360. [Google Scholar]
- 17.For studies relating to the incorporation of quaternary AA’s into amino acid or peptide libraries, see: Fornicola RS, Oblinger E, Montgomery J. J Org Chem. 1998;63:3528–3529.Griffith DL, O’Donnell MJ, Pottorf RS, Scott WL, Porco JA., Jr Tetrahedron Lett. 1997;38:8821–8824.O’Donnell MJ, Zhou C, Scott WL. J Am Chem Soc. 1996;118:6070–6071.
- 18.Use of an α-allylated AA for peptide design: Peggion C, Flammengo R, Mossel E, Broxterman QB, Kaptein B, Kamphuis J, Formaggio F, Crisma M, Toniolo C. Tetrahedron. 2000;56:3589–3601.
- 19.Theoretical study: Aleman C. J Phys Chem B. 1997;101:5046–5050.Helical peptide development: Formaggio F, Crisma M, Rossi P, Scrimin P, Kaptein B, Broxterman QB, Kamphuis J, Toniolo C. Chem Eur J. 2000;6:4498–4504. doi: 10.1002/1521-3765(20001215)6:24<4498::aid-chem4498>3.0.co;2-4.Schafmiester CE, Po J, Verdine GL. J Am Chem Soc. 2000;122:5891–5892.Mammi S, Rainaldi M, Bellanda M, Schievano E, Peggion E, Broxterman QB, Formaggio Fernando C, Marco, Toniolo C. J Am Chem Soc. 2000;122:11735–11736.Yokum TS, Gauthier TJ, Hammer RP, McLaughlin ML. J Am Chem Soc. 1997;119:1167–1168.Jaun B, Tanaka M, Seiler P, Kühnle FNM, Braun C, Seebach D. Liebigs Ann/Recueil. 1997:1697–1710.
- 20.(a) Medzihradszky-Schweiger H, Medzihradszky K, Nadasi H, Suli-Vargha H. In: Proc 25th Eur Pept Symp. Bajusz S, Hudecz F, editors. Akademiai Kiado; Budapest: 1999. pp. 606–607. [Google Scholar]; (b) Sall DJ, Shuman RT, Smith GF, Wiley MR. 5,484,772. US Patent. 1996 Jul 16;; (c) Frauer A, Mehlführer M, Thirring K, Berner H. J Org Chem. 1994;59:4215–4222. [Google Scholar]; (d) Veber DF, Freidinger RM. Trends Neurosci. 1985;8:392–396. [Google Scholar]; (e) Khosla A, Stachowiak K, Smeby RR, Bumpus FM, Piriou F, Lintner K, Fermandjian S. Proc Natl Acad Sci USA. 1981;78:757–760. doi: 10.1073/pnas.78.2.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.α-Branched AA’s in receptor ligands: Rovero P, Pellegrini M, Di Fenza A, Meini S, Quartara L, Maggi CA, Formaggio F, Toniolo C, Mierke DF. J Med Chem. 2001;44:274–278. doi: 10.1021/jm000319u.Yee NK. Org Lett. 2000;2:2781–2783. doi: 10.1021/ol000147v.
- 22.Branched AA’s in peptidyl catalysts: Rossi α-P, Felluga F, Tecilla P, Formaggio F, Crisma M, Toniolo C, Scrimin P. J Am Chem Soc. 1999;121:6948–6949.
- 23.Reviews: Cativiela C, Diaz-de-Villegas MD. Tetrahedron: Asymmetry. 1998;9:3517–3599.Davis FA, Zhou P, Chen B-C. Chem Soc Reviews. 1998;27:13–18.Wirth T. Angew Chem Int Ed Engl. 1997;36:225–227.Goodman M, Zhang J. Chemtracts. 1997;10:629–645.Seebach D, Sting AR, Hoffman M. Angew Chem Int Ed Engl. 1996;35:2708–2748.Ojima I. Acc Chem Res. 1995;28:383–389.Duthaler RO. Tetrahedron. 1994;50:1539–1650.
- 24.(a) Taylor RJKJCS. Chem Commun. 1999:217–227. [Google Scholar]; (b) Larksarp C, Alper H. J Am Chem Soc. 1997;119:3709–3715. [Google Scholar]; (b) Trost BM, Bunt RC. Angew Chem Int Ed Engl. 1996;35:99–102. [Google Scholar]; (c) Berkowitz DB, Smith MK. Synthesis. 1996:39–41. doi: 10.1055/s-1996-4177. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Griesbeck AG, Hirt J. Liebigs Ann. 1995:1957–1961. [Google Scholar]; (e) Carrasco M, Jones RJ, Kamel S, Rapoport H, Truong T. Org Syn. 1991;70:29–34. [Google Scholar]; (f) Pellicciari R, Natalini B, Marinozzi M. Synthetic Commun. 1988;18:1715–1721. [Google Scholar]; (g) Barton DHR, Crich D, Herve Y, Potier P, Thierry J. Tetrahedron. 1985;41:4347–4357. [Google Scholar]; (h) Hanessian S, Sahoo SP. Tetrahedron Lett. 1984;25:1425–1428. [Google Scholar]; (i) Schöllkopf U, Nozulak J, Groth U. Tetrahedron. 1984;40:1409–1417. [Google Scholar]
- 25.(a) Williams RM. Aldrichimica Acta. 1992:11–25. [Google Scholar]; (b) Zhai D, Zhai W, Williams RM. J Am Chem Soc. 1988;110:2501–2505. [Google Scholar]; (c) Williams RM, Zhai W. Tetrahedron. 1988;44:5425–5430. [Google Scholar]
- 26.(a) O’Donnell MJ, Min Li, Bennett WD, Grote T. Tetrahedron Lett. 1994;35:9383–9386. [Google Scholar]; (b) O’Donnell MJ, Chen N, Zhou C, Murray A, Kubiak C, Yang F, Stanley GG. J Org Chem. 1997;62:3962–3975. [Google Scholar]
- 27.(a) Petasis NA, Zavialov IA. J Am Chem Soc. 1998;120:11798–11799. [Google Scholar]; (b) Petasis NA, Zavialov IA. J Am Chem Soc. 1997;119:445–446. [Google Scholar]
- 28.Porter JR, Wirschun WG, Kuntz KW, Snapper ML, Hoveyda AH. J Am Chem Soc. 2000;122:2657–2658. [Google Scholar]
- 29.For other examples of stereocontrolled syntheses of alkenylglycines, see: Woiwode TF, Wandless TJ. J Org Chem. 1999;64:7670–7674.Griesback AG. Liebigs Ann. 1996:1951–1958.Mulzer J, Funk G. Synthesis. 1995:101–112.Clayden J, Collington EW, Warren S. Tetrahedron Lett. 1993;34:1327–1330.Mhemandoust M, Petit Y, Larcheveque M. Tetrahedron Lett. 1992;33:4313–3416.Beaulieu PL, Duceppe JS, Johnson C. J Org Chem. 1991;56:4196–4204.Duthaler RO. Angew Chem Int Ed Engl. 1991;30:705–707.Sasaki NA, Hashimoto C, Pauly R. Tetrahedron Lett. 1989;30:1943–1946.Baldwin JE, Moloney MG, North M. Tetrahedron. 1989;45:6319–6330.
- 30.Groth U, Schöllkopf U, Chiang Y-C. Synthesis. 1982:864–866. [Google Scholar]
- 31.Weber T, Aeschimann R, Maetzke T, Seebach D. Helv Chim Acta. 1986;69:1365–1377.Seebach D, Bürger HM, Schickli CP. Liebigs Ann Chim. 1991:669–684.For a recent example, that melds Seebach’s SRS approach and formal α-vinylation, see: Ma D, Zhu W. J Org Chem. 2001;66:348–350. doi: 10.1021/jo0013711.
- 32.(a) Colson PJ, Hegedus LS. J Org Chem. 1993;58:5918–5924. [Google Scholar]; (b) Ojima I, Komata T, Qiu X. J Am Chem Soc. 1990;112:770–774. [Google Scholar]
- 33.(a) Avenoza A, Cativiela C, Corzana F, Peregrina JM, Zurbano MM. J Org Chem. 1999;64:8220–8225. doi: 10.1021/jo990957o. [DOI] [PubMed] [Google Scholar]; (b) Avenoza A, Cativiela C, Peregrina JM, Sucunza D, Zurbano MM. Tet: Asymmetry. 1999;10:4653–4661. [Google Scholar]
- 34.Sharpless KB. Asymmetric Synthesis. Vol. 5. Academic Press; New York: 1985. pp. 247–308. [Google Scholar]
- 35.Hatakeyama S, Matsumoto H, Fukuyama H, Mukugi Y, Irie H. J Org Chem. 1997;62:2275–2279. doi: 10.1021/jo9618278. [DOI] [PubMed] [Google Scholar]
- 36.For another approach to a quaternary, α-vinyl AA involving the Beckmann rearrangement, see: Westermann B, Gedrath I. Synlett. 1996:665–666.
- 37.Pedersen ML, Berkowitz DB. J Org Chem. 1993;58:6966–6975.Pedersen ML, Berkowitz DB. Tetrahedron Lett. 1992;33:7315–7318.For important early work on phenylselenolate-mediated lactone cleavage, see: Liotta D, Sunay U, Santiesteban H, Markiewicz W, Yoneda F, Kuroda K. J Org Chem. 1981;46:2605–2610.Scarborough RM, Jr, Toder BH, Smith AB., III J Am Chem Soc. 1980;102:3904–3913.
- 38.Berkowitz DB, Pumphrey JA, Shen Q. Tetrahedron Lett. 1994;35:8743–8747. [Google Scholar]
- 39.Dumas F, Mezrhab B, d’Angelo J. J Org Chem. 1996;61:2293–2304. and refs therein. [Google Scholar]
- 40.Berkowitz DB, McFadden JM, Sloss MK. J Org Chem. 2000;65:2907–2918. doi: 10.1021/jo9918091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gassman PG, Schenk WN. J Org Chem. 1977;42:918–920. [Google Scholar]
- 42.Berkowitz DB, McFadden JM, Chisowa E, Semerad CL. J Am Chem Soc. 2000;122:11031–11032. doi: 10.1021/ja0055110. Though we are aware of no examples of the direct conversion of vinyl selenides to vinyl stannanes prior to this work, there had been reports of the conversion of vinyl sulfones or vinyl sulfides to vinyl stannanes (see this reference for such citations) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.To our knowledge there had only been one isolated report of a quaternary stannylvinyl AA prior to this work: Sewald N, Gaa K, Burger K. Heteroatom Chem. 1993;4:253–258.
- 44.For examples of the synthesis and use of glycine derivatives bearing alkenylstannane functionality, see: Kazmaier U, Schauss D, Pohlman M, Raddatz S. Synthesis. 2000:914–916.Reginato G, Mordini A, Valacchi M, Grandini E. J Org Chem. 1999;42:9211–9216.Reginato G, Mordini A, Caracciolo M. J Org Chem. 1997;62:6187–6192.Crisp GT, Gebauer MG. Tetrahedron Lett. 1995;36:3389–3392.Crisp GT, Glink PT. Tetrahedron. 1994;50:3213–3234.
- 45.Sheffy FK, Godschalx JP, Stille JK. J Am Chem Soc. 1984;106:4833–4840. [Google Scholar]
- 46.Milstein D, Stille JK. J Am Chem Soc. 1979;101:4992–4998. [Google Scholar]
- 47.For examples of the synthesis and use of glycine derivatives bearing iodoalkenyl functionality, see: Crisp GT, Glink PT. Tetrahedron. 1994;50:2623–2640.
- 48.Amatore C, Jutand A. Acc Chem Res. 2000;33:314–321. doi: 10.1021/ar980063a. [DOI] [PubMed] [Google Scholar]
- 49.(a) Negishi E-I. In: Organozinc Reagents. Knochel P, Jones P, editors. Oxford University Press; Oxford, UK: 1999. pp. 213–243. [Google Scholar]; (b) Negishi E-I, Takahashi T, Baba S. Org Synth. 1987;66:60–66. and refs therein. [Google Scholar]
- 50.Rieke RD. Aldrichimica Acta. 2000;33:52–60. [Google Scholar]
- 51.Sonagashira K, Tohda Y, Hagihara N. Tetrahedron Lett. 1975;16:4467–4470. [Google Scholar]
- 52.Shen W, Wang L. J Org Chem. 1999;64:8873–8879. doi: 10.1021/jo991116k. [DOI] [PubMed] [Google Scholar]