SUMMARY
Background
Enterovirus D68 (EV-D68) is implicated in a widespread 2014 outbreak of severe respiratory illness across the United States, and has also been sporadically reported in patients with acute flaccid myelitis (AFM). The association between EV-D68 infection and AFM remains unclear.
Methods
Here we report metagenomic and molecular epidemiological analyses of 25 AFM cases in California and Colorado from 2012−2014.
Findings
EV-D68 was detected in respiratory secretions from 7 of 11 (64%) patients comprising two temporally and geographically linked AFM clusters at the height of the 2014 outbreak, and from 12 of 25 (48%) investigated AFM cases overall. Phylogenetic analysis revealed that all AFM-associated EV-D68 sequences grouped into a single novel clade B1 strain that originally emerged in 2010. Out of six observed coding polymorphisms in the clade B1 EV-D68 polyprotein, 5 of 6 polymorphisms were shared between neuropathogenic poliovirus and/or EV-D70. One child with AFM and a sibling with only upper respiratory illness were both infected by identical EV-D68 strains, suggesting a potential role for host-specific factors in differential responses to EV-D68 infection. Notably, EV-D68 viremia was identified in a child experiencing acute neurologic progression of his paralytic illness. Deep metagenomic sequencing of CSF from 14 AFM cases failed to reveal evidence of an alternative infectious etiology to EV-D68.
Interpretation
Taken together, these findings strengthen the putative association between EV-D68 and AFM, as well as the contention that AFM is a rare yet severe clinical manifestation of EV-D68 infection in susceptible hosts.
INTRODUCTION
Enteroviruses cause a broad spectrum of clinical illnesses including acute respiratory infection, febrile rash, hand-foot-and-mouth disease (HFMD), meningitis, encephalitis, and rarely, acute flaccid paralysis (AFP). Enterovirus D68 (EV-D68), first identified in 19621, is known to cause respiratory illness, but has also been sporadically detected in patients with AFP2–7. In 2014, the United States experienced a nationwide outbreak of EV-D68 in association with severe respiratory illness8, with more than 1,150 confirmed cases. This EV-D68 outbreak coincided with an apparent increased incidence in the number of reported AFP cases, including a temporally associated cluster in Colorado2, 7. To more specifically describe this syndrome, the Centers for Disease Control and Prevention (CDC) and California Department of Public Health (CDPH) have proposed the term acute flaccid myelitis (AFM) to include the subset of AFP cases with myelitis primarily involving the gray matter9. However, it remains unclear whether EV-D68 is an incidental finding in these patients or a newly emerging cause of AFM.
Metagenomic next-generation sequencing (NGS) is a promising approach for pathogen detection and discovery in diseases that remain challenging to diagnose, such as encephalitis10–12, and for outbreak investigation13, 14. Here we combine pathogen discovery by metagenomic NGS, viral genome recovery, and EV-D68 phylogenetic analysis to investigate cases of AFM in California and Colorado from 2012–2014, including two clusters in August to October 2014 occurring during the height of the 2014 EV-D68 respiratory outbreak in the United States. The genomics-centered approach presented here provides the most in-depth analysis of the putative association between EV-D68 infection and AFM to date.
METHODS
Study design and patients
For this study, AFM cases were defined as patients who (1) manifested acute flaccid weakness, (2) demonstrated radiologic (MRI) and/or neurophysiologic (EMG) evidence of acute spinal motor neuron injury, and (3) did not meet criteria for Guillain-Barré syndrome, West Nile virus infection, stroke, transverse myelitis, myasthenia gravis, botulism, or other known causes of AFP9. The clinical features of these patients have been summarized previously2, 5, 7. Local IRB approval was obtained from Children's Hospital Colorado (CHCO), Children's Hospital Los Angeles (CHLA), and University of California, San Francisco (UCSF) for collection and analysis of samples. De-identified clinical data and samples were also provided by the CDPH.
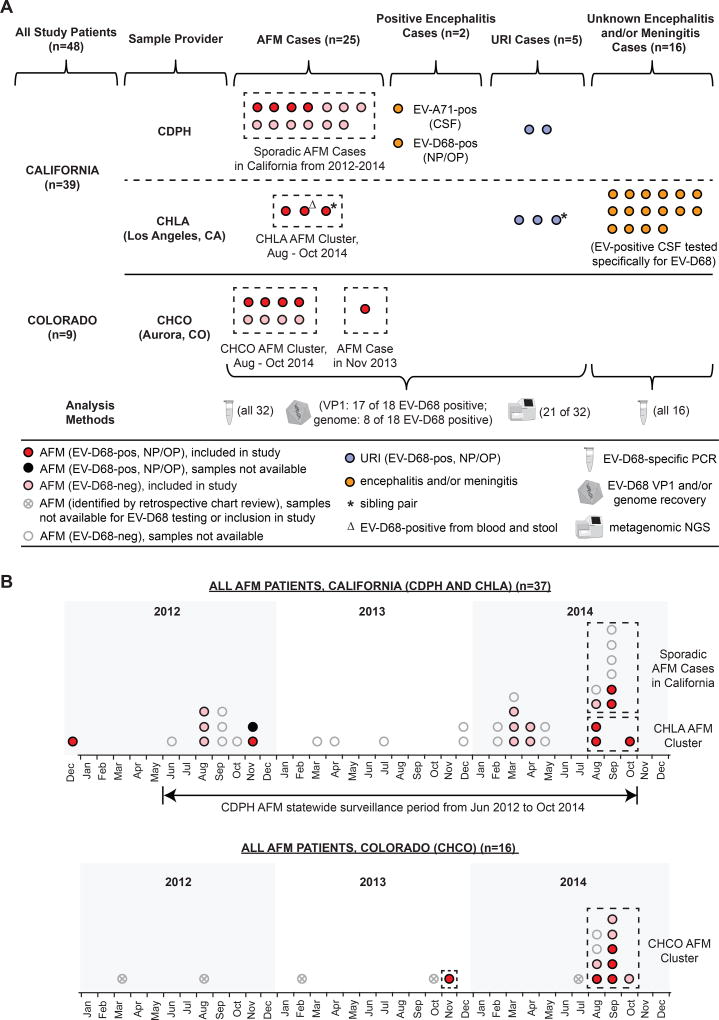
In total, our study included 48 patients, 25 patients with AFM, 2 patients with EV-associated encephalitis, 5 patients with EV-D68-associated upper respiratory infection (URI), and 16 consecutive pediatric patients with aseptic meningitis / encephalitis who tested positive at CHLA for EV in August to October 2014 and were subsequently screened for EV-D68 (Figure 1A). Among the 25 AFM patients, 16 patients originated from California and 9 patients from Colorado (Figure 1B). Three of the patients from California were part of a geographically and temporally linked cluster at Children's Hospital Los Angeles (CHLA) from Aug to Oct 2014. From Colorado, we analyzed samples collected from 8 of 10 AFM patients corresponding to a previously reported cluster in Aug to Oct 2014 at CHCO2, 7 (as consent was not available for the remaining 2 patients), and one additional patient diagnosed in late 2013. Thus 11 of 25 AFM cases analyzed in this study comprised part of a cluster, while the remaining 14 cases were sporadic.
Figure 1. Study patients in California and Colorado from 2012–2014.
(A) Flow chart of patients whose samples were analyzed in this study, including the sample provider, number of cases, and methods used for analysis. (B) Timeline of patients investigated by the CDPH and CHLA (California) and CHCO (Colorado) meeting the case definition of AFM. In California, cases of AFM were reported to CDPH beginning in Jun 2012 when surveillance and testing were initiated. As of Oct 2014, 37 AFM patients had been tested at the CDPH, 1 before (in Dec 2012) and 36 during the surveillance period (upper panel). At the CHCO in Aurora, Colorado, there were 16 hospitalized pediatric patients meeting the case definition of AFM from Jan 2012 to Oct 2014 (lower panel). Circles denote EV-D68-positive AFM patients (red), EV-D68-negative AFM patients (pink), EV-D68-positive URI patients (blue), non-AFM patients with encephalitis and/or meningitis (orange), or AFM patients not included in the study due to lack of sample availability or consent (translucent; either grey, marked with "x", or clear). The dashed boxes outline temporally linked clusters of AFM cases seen at CHLA and CHCO in Aug to Oct 2014, or additional sporadic AFM cases. Abbreviations: AFM, acute flaccid myelitis; URI, upper respiratory infection; CDPH, California Department of Public Health; CHLA, Children's Hospital Los Angeles; CHCO, Children's Hospital Colorado; NGS, next-generation sequencing.
Nasopharyngeal/oropharyngeal (NP/OP) swab or wash, serum, whole blood, rectal swab, stool, and cerebrospinal fluid (CSF) samples from patients were analyzed by PCR and/or metagenomic NGS (Table S1 and Supplementary Appendix). P-values comparing EV-D68-positive and EV-D68-negative AFM patients were calculated using the two-tailed Fisher's exact test for categorical variables, two-sample unpaired t-test for means, and Mann-Whitney U test for medians.
PCR and sequencing analyses
Clinical samples that had already undergone routine laboratory testing at CDPH, CHCO, and CHLA were screened for EV-D68 using a pan-rhinovirus (RV)/enterovirus (EV) RT-PCR targeting the 5' untranslated region (5'-UTR)15, followed by qPCR to further boost sensitivity (Supplementary Methods and Table S2). Samples positive for EV-D68 were further screened using a newly designed EV-D68 specific heminested RT-PCR assay targeting the VP1 gene (Tables 2 and S2), and a SYBR Green qRT-PCR assay16 to obtain viral copy numbers by standard curve analysis (Figure S1).
Table 2.
Microbiological testing of patients with AFM and EV-D68-positive patients with encephalitis or URI.
| Patient | Clinical Laboratory PCR TestingΔ |
EV PCR Screening | EV-D68 Sequencing |
Metagenomic NGS | |||
|---|---|---|---|---|---|---|---|
| CSF | NP/OP (Ct) | CSF | NP/OP | NP/OP | CSF | NP/OP (# reads) | |
| AFM, EV-D68 POS | |||||||
| US/CA/12-5641 | NEG | POS EV-D68 | − | POS EV-D68 | G (93.8%) | − | EV-D68 (1,702) |
| Anello (7)# | |||||||
| US/CA/12-5837*a | NEG | POS EV¥ (31.6) | − | POS EV-D68 | VP1 | − | EV-D68 (2,790) |
| HPV-136# (10) | |||||||
| US/CA/14-6067*b | NEG | POS EV¥ (35.6) | NEG | POS EV-D68 | VP1 | NONE | − |
| US/CA/14-6070*c | NEG | POS EV¶ (36.5) | NEG | POS EV-D68 | VP1 | aPV-10# | − |
| US/CA/14-6089 | NEG | POS EV-D68 | − | POS EV-D68 | VP1 | − | − |
| US/CA/14-6092 | NEG | POS EV¥ (26.2) | − | POS EV-D68 | G (95.9%) | − | − |
| US/CA/14-6100d | NEG | POS EV¶ (27.5) | NEG | POS EV-D68 | G (96.1%) | − | − |
| US/CO/13-60 | NEG | POS EV-D68 | NEG | POS EV-D68 | G (100%) | NONE | − |
| US/CO/14-93** | NEG | POS EV-D68 | NEG | POS EV-D68 | G (99.1%) | NONE | − |
| US/CO/14-94** | NEG | POS EV-D68 | NEG | POS EV-D68 | G (84.5%) | NONE | − |
| US/CO/14-98** | NEG | NEG | − | POS EV-D68 | unable to recover | − | − |
| US/CO/14-86** | NEG | POS EV-D68 | NEG | POS EV-D68 | VP1 | Anello# | − |
| AFM, EV-D68 NEG | |||||||
| US/CA/12-5806 | NEG | NEG | NEG | NEG | − | NONE | HAdV-C (1) |
| US/CA/12-5807 | NEG | NEG | NEG | NEG | − | NONE | HHV6A (5) |
| gPV-1 # (10) | |||||||
| US/CA/14-5809 | NEG | NEG | NEG | NEG | − | NONE | HSV-1 (5) |
| US/CA/14-5999 | NEG | NEG | NEG | NEG | − | NONE | HMPV (521) |
| US/CA/14-6000 | NEG | NEG | NEG | NEG | − | NONE | bPV # (2) |
| US/CA/14-6007e | NEG | NEG | NEG | POS HRV-A | − | Anello# | |
| US/CA/14-6010 | NEG | − | NEG | POS HRV-A | − | NONE | HRV-A65 (157k) |
| US/CA/14-6013 | NEG | NEG | NEG | NEG | − | BPV§ | NONE |
| US/CA/14-6072d | NEG | POS EV | − | POS EV-B (not subtyped) | − | − | − |
| US/CO/14-84** | NEG | POS HRV-B¥ | NEG | POS HRV-B | − | − | − |
| US/CO/14-88** | NEG | − | NEG | NEG | − | − | aPV-2 (60)# |
| US/CO/14-95** | NEG | POS AdV, HRV-A24 | NEG | NEG | − | − | HRV-A24 (18mil) |
| HBoV (8) | |||||||
| US/CO/14-101** | NEG | NEG | NEG | NEG | − | − | − |
| ENC, EV-D68 POS | |||||||
| US/CA/11-1767 | NEG | NEG | − | POS EV-D68 | G (100%) | − | EV-D68 (3mil) |
| URI, EV-D68 POS | |||||||
| US/CA/09-871 | − | NEG | − | POS EV-D68 | VP1 | − | EV-D68 (3,027) |
| US/CA/10-786 | − | NEG | − | POS EV-D68 | G (100%) | − | EV-D68 (46mil) |
| BDV (257)§ | |||||||
| US/CA/14-R1 | − | POS EV¶ (28.6) | − | POS EV-D68 | VP1 | − | − |
| US/CA/14-R2 | NEG | POS EV¶ (36.3) | − | POS EV-D68 | VP1 | − | − |
| US/CA/14-6103SIBd | NEG | POS EV¶ (32.1) | − | POS EV-D68 | G (100%) | − | − |
Abbreviations: ENC, encephalitis; URI, respiratory tract infection; NP/OP, nasopharyngeal/oropharyngeal swab testing; EV, enterovirus; POS, positive test; NEG, negative test; −, not applicable or sample not tested due to lack of availability; G, genome; NONE, no reads from credible pathogens found in NGS data; HRV, human rhinovirus; HAdV-C, human adenovirus C; HHV-6A, human herpesvirus 6A; HMPV, human metapneumovirus; HPV, human papillomavirus, gPV-1, gammapapillomavirus 1; bPV, betapapillomavirus; aPV, alphapapillomavirus; HBoV, human bocavirus; BPV, bovine parvovirus; BDV, bovine diarrheal virus; Anello, Anelloviridae
routine laboratory testing includes viral/bacterial/mycobacterial/fungal culture, respiratory virus PCR, EV PCR, herpes simplex virus 1/2 (HSV-1/2) PCR, Epstein-Barr virus (EBV) PCR, cytomegalovirus (CMV) PCR, varicella-zoster virus (VZV) PCR. For Colorado samples, anti- neuromyelitis optica (anti-NMO) Ab testing is also done for cases with bulbar involvement. For California samples, additional specific parechovirus PCR, human herpesvirus 6 (HHV-6) PCR, anti-NMDA (N-methyl-D-aspartate) receptor Ab, and Mycoplasma pneumoniae IgM/IgG Ab testing is performed.
part of 3-person AFM cluster in Los Angeles, California from August to October 2014
part of 8-person AFM cluster in Aurora, Colorado from August to October 2014
serum sample from the patient positive for Mycoplasma IgM (not shown in table)
stool sample from the patient positive for coxsackievirus A16 (CV-A16) (Ct 30.5) (not shown in table)
stool sample from the patient positive for EV-D68 (Ct 36.6) (not shown in table)
stool sample from the patient positive for EV-B (5'-UTR sequencing of PCR amplicon insufficient for subtyping) (not shown in table)
serum sample from this patient positive for West Nile Virus (WNV) IgG 10.72. WNV IgM 0.28 was negative (not shown in table)
quantitative pan-RV/EV RT-PCR assay with confirmatory Sanger sequencing16
quantitative pan-EV RT-PCR FOCUS Simplexa assay
considered to be part of normal microbial flora of the blood or skin
common viral laboratory contaminant of fetal bovine serum
Metagenomic NGS
Metagenomic NGS for pathogen detection is shotgun ("random") sequencing of the genomic nucleic acid (RNA and DNA) of a clinical sample, followed by microbial identification by comparison with comprehensive reference databases such as NIH GenBank17. Pathogen identification from metagenomic NGS data was performed using the SURPI computational pipeline (Supplementary Methods)18.
VP1 and Genome Sequencing of EV-D68
Full VP1 sequences were obtained using an EV-D68 specific heminested RT-PCR assay targeting the VP1 region (Table S2), followed by Sanger sequencing. Consensus genomes were recovered by mapping reads and de novo assembled contigs (contiguous sequences) obtained by metagenomic NGS (± viral probe enrichment) or Sanger sequencing to the reference EV-D68 Fermon strain (AF081348). Phylogenetic and molecular clock analyses were performed by alignment of recovered EV-D68 VP1 or complete genome sequences with all corresponding available sequences in GenBank. Full details are given in the Supplementary Methods.
ROLE OF THE FUNDING SOURCE
The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
RESULTS
Clinical characteristics of EV-D68-positive and EV-D68-negative patients with AFM
All 25 cases of AFP included in this study met the case definition for diagnosis of AFM, and presented with acute flaccid weakness, anterior horn cell gray matter involvement on MRI or EMG, and no identified etiology (Table 1). AFM cases were predominantly children, with a median age of seven years (range 0.3 to 73 years) and 60% male. Notably, 20 of 25 cases (80%) reported an upper respiratory illness (URI) prodrome, on average 5.6 days prior to appearance of AFM symptoms and 8.0 and 11.6 days prior to CSF or NP/OP collection, respectively. AFM cases with or without EV-D68 detected in NP/OP samples were largely comparable, with exceptions of fewer days between URI onset and NP/OP collection (7.8 vs. 15.1 days, p=0.01) and decreased likelihood of corticosteroid treatment after onset of AFM (33% vs. 78%, p=0.047) in EV-D68-positive patients. Clinical outcomes at 30-day follow-up were poor, with persistent neurologic deficits for all cases and 17 of 22 (77%) patients with no or minimal improvement.
Table 1.
Clinical characteristics of EV-D68-positive and EV-D68-negative patients with AFM.
| All AFM Patients (n=25) |
EV-D68-positive (n=12) |
EV-D68-negative (n=13) |
P-value | |
|---|---|---|---|---|
| Part of an AFM cluster in California (n=3) or Colorado (n=8) |
11 of 25 (44%) | 7 of 11 (64%) | 4 of 11 (36%) | |
| Age | 7·0±6·0 (0·3–73) | 4·5±3·0 (0·5–18) | 13·0±6·0 (0·3–73) | 0·07 |
| Male sex | 15 (60%) | 7 (58%) | 8 (62%) | 1·00 |
| Clinical presentation | ||||
| Fever | 21 (84%) | 11 (92%) | 10 (77%) | 0·22 |
| URI prodrome (fever, rhinorrhea, cough) | 20 (80%) | 11 (92%) | 9 (69%) | 0·10 |
| URI onset (# days prior to AFM onset) | 5·6±3·2 (0–13) | 5·1±1·8 (2–8) | 6·1±4·2 (0–13) | 0·47 |
| URI onset (# days prior to CSF collection) | 8·0±3·7 (0–17) | 7·5±2·1 (3–11) | 8·4±4·8 (0–17) | 0·59 |
| URI onset (# days prior to NP/OP collection) | 11·6±7·2 (3–33) | 7·8±2·4 (3–11) | 15·1±8·4 (6–33) | 0·01 |
| AFM onset (# days prior to CSF collection) | 2·8±3·5 (0–16) | 2·5±2·1 (0–6) | 3·2±4·4 (0–16) | 0·63 |
| Weakness / paralysis in 1 or more extremities | 25 (100%) | 12 (100%) | 13 (100%) | 1·00 |
| Ventilatory and/or feeding support required | 7 (28%) | 4 (33%) | 3 (23%) | 0·67 |
| Bowel and/or bladder dysfunction | 7 (28%) | 1 (8%) | 6 (46%) | 0·07 |
| Grey matter spinal cord injury on MRI / EMG | 25 (100%) | 12 (100%) | 13 (100%) | 1·00 |
| Final clinical diagnosis of AFM | 25 (100%) | 12 (100%) | 13 (100%) | 1·00 |
| Cerebrospinal fluid profile | ||||
| Leukocyte count (#×1000/ mm3) | 91±104 (0–396) | 63±43 (3–155) | 100±141 (0–396) | 0·39 |
| Pleocytosis (leukocyte count > 10,000 / mm3) | 18 (72%) | 11 (92%) | 7 (54%) | 0·07 |
| Neutrophilic predominance | 4 (17%) | 2 (18%) | 2 (17%) | 1·00 |
| Protein (normal 20-45 mg/dl) | 58±51 (16–234) | 45±25 (17–92) | 70±64 (16–234) | 0·24 |
| Glucose (normal 50-80 mg/dl) | 58±20 (28–124) | 57±7 (47–68) | 58±27 (28–124) | 0·88 |
| Treatment (after onset of AFM) | ||||
| Experimental antivirals (pocapavir) | 4 (16%) | 3 (25%) | 1 (8%) | 0·32 |
| Systemic corticosteroids | 14 (56%) | 4 (33%) | 10 (77%) | 0·047 |
| Intravenous immunoglobulin (IVIg) | 19 (76%) | 11 (92%) | 8 (62%) | 0·16 |
| Clinical follow-up in AFM at 30 days* | ||||
| No or minimal improvement in flaccid paralysis | 17 of 22 (77%) | 7 of 10 (70%) | 10 of 12 (83%) | 0·62 |
| Partial recovery with residual deficits | 5 of 22 (23%) | 3 of 10 (30%) | 2 of 12 (17%) | 0·62 |
Abbreviations: URI, upper respiratory infection; LP, lumbar puncture. For quantitative variables, the mean ± standard deviation and range are given, with the exception of age, for which the median is substituted for the mean.
30-day follow-up data unavailable for 3 of 25 (12%) patients.
EV-D68 detection in clinical samples from patients with AFM
In NP/OP samples from AFM patients, 12 of 25 total (48%) were positive for EV-D68 by screening and confirmatory sequencing using two independent RT-PCR assays. Among the patients comprising two clusters in California and Colorado (Figure 1), 7 of 11 (64%) tested EV-D68-positive. None of the 25 AFM cases in the study tested positive from the CSF using clinical assays for EV (Table 2), nor by EV-D68 5'-UTR heminested RT-PCR15. However, EV-D68 was detected in whole blood and stool as well as NP/OP samples from a 6-year old child (US/CA/14-6070) in the CHLA AFM cluster. This was the only case where EV-D68 was detected in whole blood or stool, and viral titers were much lower than in NP/OP samples (Figure S1). Notably, the child's blood was collected more than a week after URI onset during hospitalization for progressive paralysis.
EV-D68 testing of clinical samples from patients with aseptic meningitis or encephalitis
To search more broadly for potential neuroinvasive infections from EV-D68, we also analyzed EV-positive CSF from 16 consecutive California pediatric patients from CHLA presenting with aseptic meningitis or encephalitis in August 2014 (Figure 1A). These patients were part of the same pediatric population from which the 3 AFP cases in the CHLA outbreak were derived. None of the CSF samples were positive for EV-D68 by VP1 heminested RT-PCR screening followed by confirmatory sequencing.
VP1 phylogenetic analysis of EV-D68 reveals a clade associated with AFM
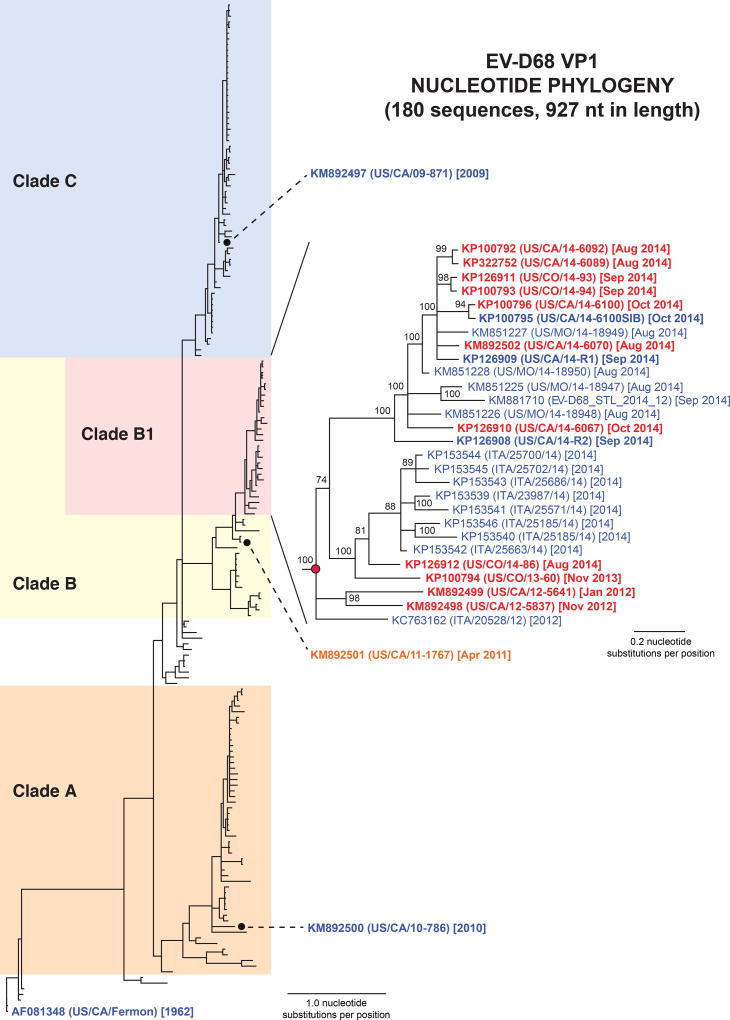
To further characterize EV-D68 strains associated with AFM patients or "non-AFM" patients (defined as URI or encephalitis without AFM), we recovered 17 new full-length EV-D68 VP1 sequences and 8 full-length genomes. Due to low concentrations of EV-D68 in several NP/OP samples (Figure S1), genome sequence recovery required a combination of metagenomic NGS, probe-based EV-D68 target enrichment, and PCR with Sanger sequencing to bridge remaining gaps (Supplementary Methods). Alignment and phylogenetic analysis of the new VP1 sequences in comparison to all published VP1 sequences from 1962–2014 confirmed that the original 1962 Fermon strain had diverged into 3 distinct clades (Figure 2), as previously reported19. All 11 (100%) of the AFM-associated EV-D68 viruses belonged to an evolutionarily recent cluster from 2012–2014 (clade B1), which also included 16 VP1 sequences from non-AFM 2014 respiratory outbreak isolates8, 20, 21. A 2011 EV-D68 isolate from a patient with non-AFM encephalitis (US/CA/11-1767) was positioned just outside of clade B1, while two strains from patients with non-AFM URI (US/CA/09-871 and US/CA/10-786) were phylogenetically distant. The two initial EV-D68-positive AFM cases, reported to the CDPH in Dec 2011 and Nov 20125, were positioned near the root of clade B1, consistent with the emergence of this clade approximately 4.5 years ago by molecular clock analysis (Figures S2 and S3).
Figure 2. Phylogeny of EV-D68 by VP1 gene sequence.
All 180 complete EV-D68 VP1 sequences available in GenBank as of Dec 2014, including the 17 new EV-D68 VP1 gene sequences in this study (boldface), were aligned using MUSCLE29, and phylogenetic trees were constructed using the MrBayes algorithm30. EV-D68 strains from AFM patients are grouped together in a novel clade (clade B1) and include sequences from patients with severe respiratory illness from the 2014 outbreak. AFM cases are marked in red text, encephalitis in orange text, and respiratory illness only in blue text. Abbreviations: nt, nucleotide.
Whole-genome analysis shows coding polymorphisms associated with clade B1
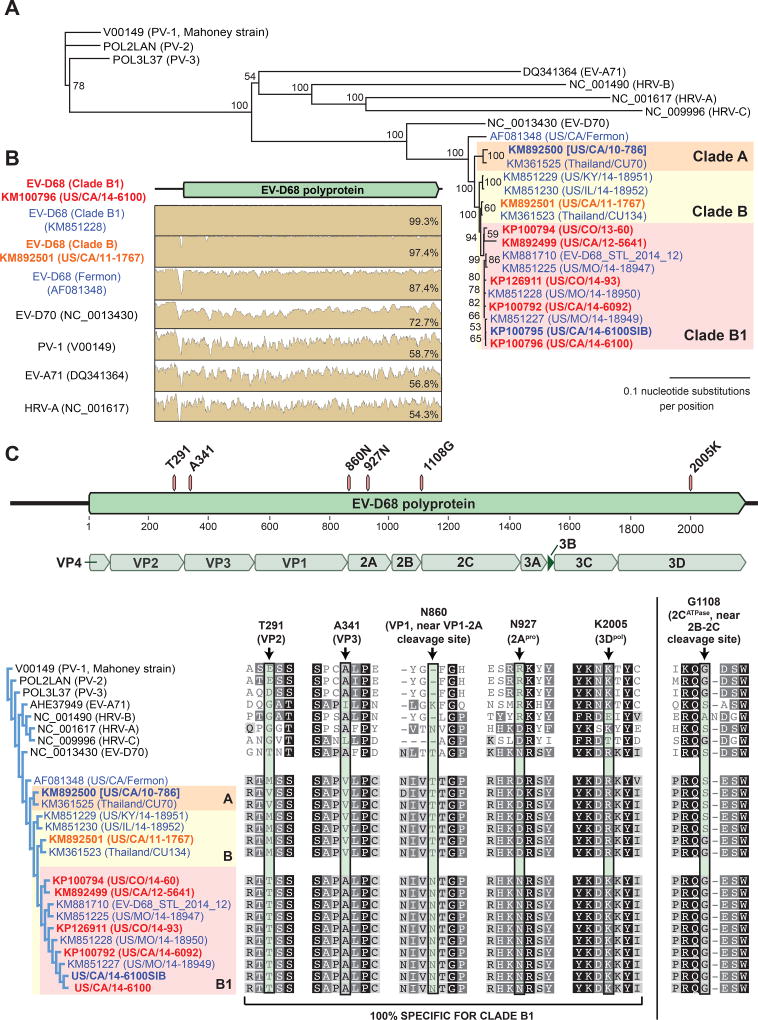
Phylogenetic analysis of all 17 EV-D68 genomes available as of Dec 2014 (9 genomes from GenBank and 8 newly sequenced EV-D68 genomes) recapitulated the same phylogenetic relationships observed in the VP1 tree, and all AFM-associated EV-D68 genomes belonged to clade B1 (Figure 3A). Clade B1 genomes were 87% identical to the ancestral 1962 Fermon strain, 87–97% identical to other EV-D68 strains outside of clade B1, and 97–100% identical to each other by pairwise alignment, with no evidence of recombination (Figure 3B).
Figure 3. Coding polymorphisms associated with EV-D68 clade B1.
(A) Eight EV-D68 genomes were recovered from clinical NP/OP samples harboring EV-D68 at levels sufficient for genome recovery (boldface). Translated polyproteins were aligned using MUSCLE29 and phylogenetically clustered using MrBayes30 with the 9 other complete EV-D68 genomes in GenBank as of Dec 2014 and the genomes of other representative enteroviruses. AFM cases are marked in red text, encephalitis in orange text, and respiratory illness only in blue text. The scale bar shows the number of amino acid substitutions per site. (B) Pairwise identity plots of a clade B1 EV-D68 genome (US/CA/14-6100) versus other enteroviruses. (C) Coding polymorphisms associated with the EV-D68 clade B1 polyprotein compared to non-clade B1 EV-D68 and other representative enteroviruses. Abbreviations: PV, poliovirus; HRV, human rhinovirus; EV, enterovirus.
The 5’UTR internal ribosome entry site (IRES) of enteroviruses has been associated with neurovirulence22. The sole nucleotide difference in the 5'-UTR of the clade B1 strain is U339 as compared to C339 in other EV-D68 strains. Among the three 5'-UTR nucleotides associated with neurovirulence in enterovirus 71 (U448, A/U700, and G272)22, only G272 was present in EV-D68, and this nucleotide was common to all sequenced EV-D68 genomes regardless of clade. However, analysis of the EV-D68 polyprotein revealed 5 coding single nucleotide polymorphisms (SNPs) specific to clade B1 (Figure 3C). An additional coding polymorphism 1108G (Figure 3C, far right), also seen in strains positioned just outside of clade B1, was situated at the 2B-2C proteolytic junction. Interestingly, five of the six coding SNPs matched with poliovirus or EV-D70 or both neuropathogenic viruses.
Sequencing of a clinically discordant sibling pair reveals identical EV-D68 viruses
One patient with AFM in the current study (US/CA/14-6100) developed AFM following a URI prodrome, while her sibling (US/CA/14-6100SIB) only exhibited URI symptoms (Figure 1A and Table 2). NP/OP samples from the two children, both collected 9 days after onset of URI symptoms, were positive for EV-D68. Full genome sequencing of EV-D68 strains from the two siblings revealed no nucleotide differences. Despite being infected with a clade B1 EV-D68 strain (Table 2), neither the unaffected sibling nor two additional patients with URI developed AFM within 60 days of follow-up.
Deep metagenomic sequencing of clinical samples is negative for alternative potential infectious etiologies of AFM
To search for other pathogens that may be associated with AFM, we performed metagenomic NGS of 15 CSF samples, including 14 from AFM patients (six EV-D68-positive and eight EV-D68-negative) and one from a non-AFM EV-A71 encephalitis case as a positive control (Table S1). Only 14 of 25 AFM CSF samples were analyzed given limited sample availability and to maximize the depth of sequencing achieved. Between 13 and 117 million sequence reads were generated per CSF sample (Table S3). No sequence reads corresponding to putative neuropathogenic viruses, bacteria, fungi or parasites were recovered from CSF samples collected from AFM patients (Supplementary Appendix and Tables S4 to S7). In contrast, the positive control CSF sample revealed 4,600 reads aligning to EV-A71 out of a total number of ~39 million sequenced reads, with recovery of 92% of the genome (Figure S4-A; Table S4).
To aid in the recovery of EV-D68 genome sequences and detect potential co-infections from other viruses, we also performed metagenomic NGS on 13 NP/OP samples (2 EV-D68-positive AFM, 9 EV-D68-negative AFM, 1 EV-D68-positive non-AFM encephalitis, and 1 EV-D68-positive URI), with 32 to 402 million reads generated per sample. Metagenomic NGS of NP/OP samples from the 2 EV-D68-positive AFM cases, US/CA/12-5641 and US/CA/12-5837, revealed 1,702 of ~381 million and 2,790 of ~402 million reads corresponding to EV-D68 (Figure S4-B; Table S4), consistent with the moderately low calculated EV-D68 titers of 53,575 and 11,141 copies / mL, respectively (Table S1). A virus other than EV-D68 was identified from 4 of 9 EV-D68-negative AFM cases by metagenomic sequencing, including human adenovirus C (1 read), human metapneumovirus (554 reads), a co-infection with human rhinovirus A24 (HRV-A24) (~18 million reads) and human bocavirus (8 reads), and HRV-A65 (~157,000 reads) (Table 2, Table S1, and Figure S4-C). By RT-PCR testing for enteroviruses15 and confirmatory Sanger sequencing of all 13 EV-D68-negative AFM cases (Table 2), 3 additional viruses, HRV-A, HRV-B, and EV-B, were detected. In total, NP/OP swabs from 7 of 13 (54%) EV-D68-negative AFM cases harbored a virus different from EV-D68.
DISCUSSION
Here we report PCR screening, EV-D68 genome sequencing, and metagenomic analysis of clinical samples from 25 AFM cases, predominantly in children, from 2012−2014. Our analysis detected EV-D68 in respiratory samples from 5 of 14 (36%) sporadic AFM cases and 7 of 11 (64%) AFM cases comprising two temporally linked clusters from August to October 2014, coincident with the widespread 2014 respiratory outbreak across the United States. The two clusters were also geographically linked to patients within the regions covered by CHCO and CHLA hospitals in Aurora, Colorado and Los Angeles, California, respectively. By phylogenetic analysis, AFM-associated EV-D68 strains grouped into a distinct novel clade B1 emerging 4.5 years ago, along with the majority of 2014 respiratory outbreak strains sequenced to date (Figure 2). Notably, the two initial EV-D68-positive AFM cases, reported from California in 20125, were situated near the root of the clade. Coding polymorphisms associated with the clade B1 EV-D68 polyprotein were found to share intriguing homology with poliovirus and EV-D70. Importantly, EV-D68 viremia was also detected in a child experiencing acute neurological progression.
Our data strengthen the putative association between EV-D68 respiratory infection and AFM, and suggest that detection of EV-D68 in respiratory secretions from AFM patients is unlikely to be incidental. The timing of the EV-D68-associated outbreak of severe respiratory illness across the United States in 20148, 20, 21 coincided with an apparent increased incidence of AFM cases (Figure 1)2, 5, 7. More than 80% of AFM patients reported fever and/or a viral URI prodrome, consistent with antecedent EV-D68 respiratory infection (Table 1). EV-D68 was also the most common virus detected in NP/OP samples from AFM patients, and none of the neurotropic enteroviruses classically associated with AFM (EV-70, EV-71, and poliovirus) were detected. Other respiratory viruses found in NP/OP samples from AFM patients, including four strains of rhinovirus, EV-B, bocavirus, and metapneumovirus, as well as coxsackievirus A16 and EV-B in the stool of two EV-D68-positive AFM patients, were detected only as single individual cases (Table 2). Finally, deep metagenomic sequencing of CSF samples from 14 AFM patients (13 – 117 million reads per sample) failed to reveal evidence of an alternative infectious etiology. Taken together, these findings indicate that EV-D68 at present can be considered the most likely etiologic candidate for AFM.
The anterior horn gray matter involvement observed in patients with AFM is consistent with spinal motor neuron injury from direct viral invasion of tissue, which is characteristic of poliovirus and enterovirus A71 infections23, 24. We speculate that the coding polymorphisms observed in the clade B1 polyprotein (Figure 3C) may have conferred on EV-D68 an increased propensity for neurovirulence. The N860 polymorphism in the VP1 protein as well as the G1108 polymorphism at the proteolytic 2B/2C junction (Figure 3C) are particularly intriguing as the VP1 capsid change is specific to clade B1 EV-D68 and protease profiling of enterovirus 3C proteins has demonstrated enhanced cleavage of Q/G compared to Q/S peptides25. However, as the functional consequences of these polymorphisms are completely unknown, further investigation will be needed to determine their clinical significance, if any, with regard to AFM.
Infections by clade B1 viruses are not restricted to AFM patients, but have been seen in patients with non-AFM respiratory illness (Figure 2)8, 20, 21. In addition, we report a sibling pair infected with identical clade B1 strains, one with a URI prodrome progressing to AFM and the other with a self-limited URI. These two findings suggest that the potential clinical manifestations and severity of EV-D68 infection are broad, and that host-specific (perhaps immunological) or environmental factors may play a role in differential responses to infection.
Delayed collection of clinical samples relative to URI onset, generally >7 days, is likely to have reduced overall titers and yield of EV-D68 in clinical samples. EV-D68 titers in the respiratory tract of several AFM patients were low, in one case requiring two rounds of RT-PCR for detection (Table S1). Notably, AFM patients testing EV-D68-negative had NP/OP samples collected on average ~7 days later relative to URI onset than EV-D68-positive AFM patients (p=0.01, Table 1). The comparable clinical findings between EV-D68-positive and EV-D68-negative patients also suggest that the identification of EV-D68 in only a subset of NP/OP samples may be due to insufficient detection sensitivity from delayed sample collection.
The failure to detect EV-D68 in CSF altgoether is not surprising given that reported rates of CSF detection for known neurotropic enteroviruses such as polioviruses and EV-A71 are as low as 0–5%23, 26, 27. Brain or spinal cord tissue from affected cases, which can assist in the diagnosis of viral encephalitis for which parallel CSF testing is negative10, was not available for testing. Yet another possibility accounting for the failure to detect EV-D68 in CSF is that the pathogenesis of AFM is related to an aberrant immune response to recent EV-D68 infection and not due to direct neuroinvasion by the virus.
Two previous cases from the literature suggest that EV-D68 is likely neurotropic. EV-D68 was previously detected from CSF in a young adult with AFP in 20054, and from CSF and brain on autopsy in a 5 year-old boy with fulminant encephalitis in 20083. The recent emergence of rare cases of AFM in 2014 occurring concomitantly with a widespread EV-D68 respiratory outbreak in the United States2, 5, 7 and globally6 is reminiscent of the large-scale outbreaks of acute hemorrhagic conjunctivitis accompanied by AFP in ~1:10,000 patients from EV-D70 during the 1970s28, and more recently, sporadic outbreaks of EV-A71-associated AFP throughout the Asia-Pacific region from the 1990s to the present23. Importantly, here we detected EV-D68 in whole blood as well as NP/OP and stool samples from a 6-year old child in California with AFM, more than a week after URI onset and during the progressive period of his paralytic illness. Prolonged viremia with EV-D68 may directly facilitate the development of neuroinvasive disease, akin to what is observed in poliomyelitis24. Given that all of the AFM patients in the current study continue to have residual limb weakness or other neurological deficits to date, further investigation of the potential neuropathogenesis of EV-D68, especially clade B1 strains, is urgently needed.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
A nationwide outbreak of enterovirus D68 (EV-D68) associated with severe cases of respiratory illness occurred in 2014. In addition to respiratory symptoms, a small number of patients infected with EV-D68, nearly all children, presented with acute flaccid myelitis (AFM). However, there is scant evidence to date addressing whether EV-D68 is an incidental finding in these patients or a newly emerging cause of AFM. In addition, a number of recent papers have reported the sequences of EV-D68 from single AFM or multiple respiratory cases, but EV-D68 genomic and metagenomic analyses from clusters of patients presenting with AFM have not been described.
Added value of this study
We report that a novel B1 strain emerging 4.5 years ago is associated with all of the investigated EV-D68 AFM cases from California and Colorado from 2012 to 2014, and with the majority of 2014 respiratory outbreak viruses sequenced to date. Seven of 11 (64%) AFM patients from two distinct clusters during the 2014 outbreak were infected with EV-D68. We also identified a sibling pair with discordant symptoms (one child with AFM and the other with only upper respiratory illness), yet infected with identical EV-D68 viruses. Notably, EV-D68 was detected for the first time in blood from a child with AFM during the progressive phase of his paralytic illness. No other candidate infectious etiology was found in the cerebrospinal fluid of patients with AFM by extensive metagenomic deep sequencing.
Implications of all the available evidence
Despite the inability to detect EV-D68 in CSF, the collective evidence provided in this study implicates EV-D68 infection as a potential trigger of AFM, including its detection in temporally and geographically linked AFM clusters, association with a recent novel EV-D68 B1 strain, its capacity for systemic infection (viremia), and the failure to identify an alternative infectious etiology by metagenomic deep sequencing. The finding of one child with AFM and her sibling with only upper respiratory illness shows that the clinical manifestations of EV-D68 infection can be highly variable and suggests a potential role for host-specific factors, such as differential immunological responses, in the pathogenesis of EV-D68. Although no single EV-D68 polymorphism was specific to AFM patients, the identified sequence homologies at several amino acid sites with neuropathogenic enteroviruses merits further investigation.
Acknowledgments
We thank the laboratory staff at Children's Hospital Los Angeles, Children's Hospital Colorado, the Viral and Rickettsial Disease Laboratory of the California Department of Public Health, and the US CDC for performing diagnostic testing. We also thank the clinicians who referred cases to the California Encephalitis Project.
Funding
This study was partially supported by grants from the National Institutes of Health (NIH) R01-HL105704 (to CYC) and UL1-TR001082 (to KM and SD), a University of California Discovery Award (to CYC), an Abbott Viral Discovery Award (to CYC), and the Centers for Disease Control and Prevention (CDC) Emerging Infections Program U50/CCU915546-09 (to CG).
Footnotes
CONTRIBUTORS
ALG, SNN, KM, SRD, GA, and CG and CYC conceived of and designed the study. ALG, SNN, GY, SS, CA, and SY performed the experiments. KM, AC, SM, DW, DX, JPW, SRD, CG, and GA contributed clinical samples. KM, AC, KVH, JPW, SRD, CG, GA, and CYC analyzed the clinical and epidemiological data. AG, SN, GY, SS, and CYC analyzed the sequencing data. SNN, SF, DS, and CYC developed and contributed software analysis tools. ALG, SNN, and CYC wrote the paper. All authors read the final manuscript and approved submission.
DECLARATIONS OF INTEREST
This study was funded in part by an Abbott Viral Discovery Award. CYC is the director of the UCSF-Abbott Viral Diagnostics and Discovery Center (VDDC).
References
- 1.Oberste MS, Maher K, Schnurr D, et al. Enterovirus 68 is associated with respiratory illness and shares biological features with both the enteroviruses and the rhinoviruses. The Journal of general virology. 2004;85(Pt 9):2577–84. doi: 10.1099/vir.0.79925-0. [DOI] [PubMed] [Google Scholar]
- 2.Pastula DM, Aliabadi N, Haynes AK, et al. Acute neurologic illness of unknown etiology in children - colorado, august-september 2014. MMWR Morbidity and mortality weekly report. 2014;63(40):901–2. [PMC free article] [PubMed] [Google Scholar]
- 3.Kreuter JD, Barnes A, McCarthy JE, et al. A fatal central nervous system enterovirus 68 infection. Archives of pathology & laboratory medicine. 2011;135(6):793–6. doi: 10.5858/2010-0174-CR.1. [DOI] [PubMed] [Google Scholar]
- 4.Khetsuriani N, Lamonte-Fowlkes A, Oberst S, Pallansch MA Centers for Disease C, Prevention. Enterovirus surveillance--United States, 1970-2005. Morbidity and mortality weekly report Surveillance summaries. 2006;55(8):1–20. [PubMed] [Google Scholar]
- 5.Ayscue P, Haren KV, Sheriff H, et al. Acute flaccid paralysis with anterior myelitis - california, june 2012-june 2014. MMWR Morbidity and mortality weekly report. 2014;63(40):903–6. [PMC free article] [PubMed] [Google Scholar]
- 6.Lang M, Mirand A, Savy N, et al. Acute flaccid paralysis following enterovirus D68 associated pneumonia, France, 2014. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2014;19(44) doi: 10.2807/1560-7917.es2014.19.44.20952. [DOI] [PubMed] [Google Scholar]
- 7.Messacar K, Schreiner TL, Maloney JA, et al. A cluster of acute flaccid paralysis and cranial nerve dysfunction temporally associated with an outbreak of enterovirus D68 in children in Colorado, USA. Lancet. 2015 doi: 10.1016/S0140-6736(14)62457-0. [DOI] [PubMed] [Google Scholar]
- 8.Midgley CM, Jackson MA, Selvarangan R, et al. Severe respiratory illness associated with enterovirus D68 - Missouri and Illinois, 2014. MMWR Morbidity and mortality weekly report. 2014;63(36):798–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Division of Viral Diseases NCfI, Respiratory Diseases CDC, Division of Vector-Borne Diseases DoH-CP, et al. Notes from the field: acute flaccid myelitis among persons aged </=21 years - United States, august 1-november 13, 2014. MMWR Morbidity and mortality weekly report. 2015;63(53):1243–4. [PMC free article] [PubMed] [Google Scholar]
- 10.Naccache SN, Peggs KS, Mattes FM, et al. Diagnosis of neuroinvasive astrovirus infection in an immunocompromised adult with encephalitis by unbiased next-generation sequencing. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015 doi: 10.1093/cid/ciu912. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilson MR, Naccache SN, Samayoa E, et al. Actionable diagnosis of neuroleptospirosis by next-generation sequencing. The New England journal of medicine. 2014;370(25):2408–17. doi: 10.1056/NEJMoa1401268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaser C, Bloch KC. Encephalitis: why we need to keep pushing the envelope. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009;49(12):1848–50. doi: 10.1086/648420. [DOI] [PubMed] [Google Scholar]
- 13.Grard G, Fair JN, Lee D, et al. A novel rhabdovirus associated with acute hemorrhagic fever in central Africa. PLoS pathogens. 2012;8(9):e1002924. doi: 10.1371/journal.ppat.1002924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.P AL, Chen EC, Sittler T, et al. A metagenomic analysis of pandemic influenza A (2009 H1N1) infection in patients from North America. PloS one. 2010;5(10):e13381. doi: 10.1371/journal.pone.0013381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imamura T, Suzuki A, Lupisan S, et al. Detection of enterovirus 68 in serum from pediatric patients with pneumonia and their clinical outcomes. Influenza and other respiratory viruses. 2014;8(1):21–4. doi: 10.1111/irv.12206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kares S, Lonnrot M, Vuorinen P, Oikarinen S, Taurianen S, Hyoty H. Real-time PCR for rapid diagnosis of entero- and rhinovirus infections using LightCycler. Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology. 2004;29(2):99–104. doi: 10.1016/s1386-6532(03)00093-3. [DOI] [PubMed] [Google Scholar]
- 17.Chiu CY. Viral pathogen discovery. Current opinion in microbiology. 2013;16(4):468–78. doi: 10.1016/j.mib.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naccache SN, Federman S, Veeraraghavan N, et al. A cloud-compatible bioinformatics pipeline for ultrarapid pathogen identification from next-generation sequencing of clinical samples. Genome research. 2014;24(7):1180–92. doi: 10.1101/gr.171934.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tokarz R, Firth C, Madhi SA, et al. Worldwide emergence of multiple clades of enterovirus 68. The Journal of general virology. 2012;93(Pt 9):1952–8. doi: 10.1099/vir.0.043935-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown BA, Nix WA, Sheth M, Frace M, Oberste MS. Seven Strains of Enterovirus D68 Detected in the United States during the 2014 Severe Respiratory Disease Outbreak. Genome announcements. 2014;2(6) doi: 10.1128/genomeA.01201-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wylie KM, Wylie TN, Orvedahl A, et al. Genome sequence of enterovirus D68 from St. Louis, Missouri USA. Emer Infect Dis. 2015;21(1) doi: 10.3201/eid2101.141605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R, Zou Q, Chen L, Zhang H, Wang Y. Molecular analysis of virulent determinants of enterovirus 71. PloS one. 2011;6(10):e26237. doi: 10.1371/journal.pone.0026237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ooi MH, Wong SC, Lewthwaite P, Cardosa MJ, Solomon T. Clinical features, diagnosis, and management of enterovirus 71. The Lancet Neurology. 2010;9(11):1097–105. doi: 10.1016/S1474-4422(10)70209-X. [DOI] [PubMed] [Google Scholar]
- 24.Racaniello VR. One hundred years of poliovirus pathogenesis. Virology. 2006;344(1):9–16. doi: 10.1016/j.virol.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 25.O'Donoghue AJ, Eroy-Reveles AA, Knudsen GM, et al. Global identification of peptidase specificity by multiplex substrate profiling. Nature methods. 2012;9(11):1095–100. doi: 10.1038/nmeth.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perez-Velez CM, Anderson MS, Robinson CC, et al. Outbreak of neurologic enterovirus type 71 disease: a diagnostic challenge. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2007;45(8):950–7. doi: 10.1086/521895. [DOI] [PubMed] [Google Scholar]
- 27.Grard G, Drexler JF, Lekana-Douki S, et al. Type 1 wild poliovirus and putative enterovirus 109 in an outbreak of acute flaccid paralysis in Congo, October-November 2010. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2010;15(47) doi: 10.2807/ese.15.47.19723-en. [DOI] [PubMed] [Google Scholar]
- 28.Neurovirulence of enterovirus 70. Lancet. 1982;1(8268):373–4. [PubMed] [Google Scholar]
- 29.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic acids research. 2004;32(5):1792–7. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17(8):754–5. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.