Abstract
The autacoid, adenosine, is present in the normoxic kidney and generated in the cytosol as well as at extracellular sites. The rate of adenosine formation is enhanced when the rate of ATP hydrolysis prevails over the rate of ATP synthesis during increased tubular transport work or during oxygen deficiency. Extracellular adenosine acts on adenosine receptor subtypes (A1, A2A, A2B, and A3) in the cell membranes to affect vascular and tubular functions. Adenosine lowers glomerular filtration rate by constricting afferent arterioles, especially in superficial nephrons, and thus lowers the salt load and transport work of the kidney consistent with the concept of metabolic control of organ function. In contrast, it leads to vasodilation in the deep cortex and the semihypoxic medulla, and exerts differential effects on NaCl transport along the tubular and collecting duct system. These vascular and tubular effects point to a prominent role of adenosine and its receptors in the intrarenal metabolic regulation of kidney function, and, together with its role in inflammatory processes, form the basis for potential therapeutic approaches in radiocontrast media-induced acute renal failure, ischemia reperfusion injury, and in patients with cardiorenal failure.
Keywords: Adenosine receptors electrolyte, Kidney, Tubuloglomerular feedback, Renin, Fluid and transport, Metabolic control, Acute renal failure, Acute kidney injury, Radiocontrast media, Ischemia reperfusion injury, Heart failure
1 Introduction
Adenosine is a tissue hormone that is locally generated in many organs and that binds to cell surface receptors to mediate various aspects of organ function. Many of these effects revolve around a role of adenosine in metabolic control of organ function, including local matching of blood flow with energy consumption. According to this concept, the interstitial concentration of adenosine rises when cells are in negative energy balance. Adenosine locally activates vasodilatory adenosine A2 receptor (A2AR) and adjusts blood flow to meet demand. The role of adenosine in the kidney is analogous but, as a consequence of the specific renal structural organization and function, more complicated than its role in other organs. We will first describe the differential effects of adenosine on the renal cortical and medullary vascular structures, and its role in tubuloglomerular feedback (TGF), the regulation of renin secretion and in transport processes in the tubular and collecting duct system. These issues are subsequently discussed with regard to a potential role of adenosine receptors as new potential targets in the treatment of patients with radiocontrast media-induced acute renal failure, ischemia-reperfusion injury, and in patients with acute decompensated heart failure or cardiorenal failure. Please see recent reviews on the expression of adenosine receptors in the kidney and the role of adenosine in kidney function in general (Vallon et al. 2006), and in acute renal failure (Osswald and Vallon 2008) and fluid retention in particular (Welch 2002; Modlinger and Welch 2003; Vallon et al. 2008).
2 Vascular Effects of Adenosine in Kidney Cortex and Medulla
In contrast to other organs, blood flow into the cortex of the kidney generates, via the formation of an ultrafiltrate, the metabolic burden for tubular electrolyte transport and thus the demand for energy. Hence, to recover from negative energy balance in the kidney, a mechanism is required that lowers glomerular filtration rate (GFR) or the ratio between glomerular filtration rate and cortical renal blood flow. In comparison, blood flow in the renal medulla is nutritive. It derives from the postglomerular circulation of deep nephrons, and due to the way the kidney concentrates the urine, blood flow and O2 supply are low in this area, although active NaCl reabsorption in the medullary thick ascending limb is essential for function. With regard to metabolic control, this requires a vasodilator to prevent hypoxic injury in the renal medulla. As outlined in the following, adenosine is a vasodilator in the renal medulla but induces cortical vasoconstriction and lowers GFR.
2.1 Activation of A1AR Lowers Glomerular Filtration Rate
Healthy volunteers responded to an intravenous infusion or direct application of adenosine into the renal artery with a reduction in GFR of 15–25% while blood pressure and renal blood flow were unchanged (Edlund and Sollevi 1993; Edlund et al. 1994; Balakrishnan et al. 1996). Adenosine infusion into the renal artery of rats or dogs reduced single-nephron GFR (SNGFR) in superficial nephrons to a larger extent than whole-kidney GFR, indicating that deep-cortical vasodilation (see below) counteracts superficial vasoconstriction (Osswald et al. 1978a, b; Haas and Osswald 1981). Adenosine lowers SNGFR in superficial nephrons due to afferent arteriolar vasoconstriction (Osswald et al. 1978b; Haas and Osswald 1981) (Fig. 1). Direct videometric assessment of pre- and postglomerular arteries using the “split-hydronephrotic” rat kidney technique revealed adenosine-induced constriction of afferent arterioles via high-affinity A1AR and dilation via activation of both high-affinity A2AAR and low-affinity A2BAR (Tang et al. 1999). Whereas activation of A1AR led to the constriction of mainly afferent arterioles near the glomerulus, A2AR activation lead to the dilation of mainly postglomerular arteries (Holz and Steinhausen 1987; Dietrich and Steinhausen 1993; Gabriels et al. 2000). A1AR-mediated afferent arteriolar constriction involves a pertussis toxin-sensitive Gi protein and subsequent activation of phospholipase C, presumably through βγ subunits released from Gαi (Hansen et al. 2003b). A2AAR-mediated renal vasodilation may involve activation of ATP-regulated potassium channels (Tang et al. 1999) and endothelial nitric oxide synthase (Hansen et al. 2005).
Fig. 1.
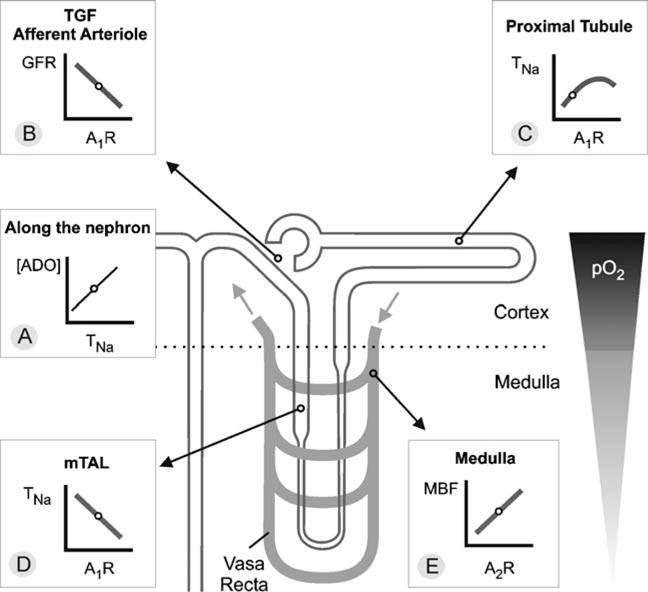
a–e Control of renal hemodynamics and transport by adenosine (ADO). The line plots illustrate the relationships between the given parameters. Small circles on these lines indicate ambient physiological conditions. In general, the medulla is at greater risk for hypoxic damage than the cortex due to a lower partial oxygen pressure (pO2). a In every nephron segment, an increase in reabsorption or transport of sodium (TNa) increases extracellular ADO. b ADO via A1AR mediates tubuloglomerular feedback (TGF) and constricts the afferent arteriole to lower GFR. c In the proximal tubule, ADO via A1AR stimulates TNa and thus lowers the Na+ load to segments residing in the semihypoxic medulla. d In contrast, ADO via A1AR inhibits TNa in the medulla, including medullary thick ascending limb (mTAL). e In addition, ADO via A2AR enhances medullary blood flow (MBF), which increases O2 delivery and further limits O2-consuming transport in the medulla (adapted from Vallon et al. 2006)
Oral application of the A1AR antagonist (+)-(R)-[(E)-3-(2-phenylpyrazolo [1,5-a]pyridin-3-yl)acryloyl]-2-piperidine ethanol (FK-453) to healthy male subjects increased GFR by ∼20% without significantly altering effective renal plasma flow or mean arterial blood pressure (Balakrishnan et al. 1993), providing evidence that endogenous adenosine elicits a tonic suppression of GFR through the activation of A1AR. Consistent with a prominent role of adenosine in the regulation of afferent arteriolar tone, autoregulation of renal blood flow and glomerular filtration rate (i.e., their constancy in spite of changes in renal perfusion pressure) is dependent upon the activation of A1AR (Hashimoto et al. 2006).
2.2 Factors Modulating Adenosine-Induced Cortical Vasoconstriction
Suppression of the renin–angiotensin system by dietary salt or pharmacological means reduces or blocks the renal vasoconstrictive action of adenosine (Osswald et al. 1975, 1982; Spielman and Osswald 1979; Arend et al. 1985; Macias-Nunez et al. 1985; Dietrich et al. 1991; Dietrich and Steinhausen 1993). In contrast, activation of the renin–angiotensin system potentiates adenosine-induced vasoconstriction and lowering of GFR (Osswald et al. 1975, 1978a, 1982). Further studies identified a mutual dependency and cooperation of adenosine and angiotensin II in producing afferent arteriolar constriction (Weihprecht et al. 1994; Traynor et al. 1998; Hansen et al. 2003a). Adenosine enhances angiotensin II-induced constriction of afferent arterioles by receptor-dependent and -independent pathways. The latter involves adenosine uptake and intracellular effects that increase the calcium sensitivity by phosphorylating the myosin light chain (Lai et al. 2006; Patzak et al. 2007). Moreover, inhibiting the synthesis of vasodilators like nitric oxide (NO) (Barrett and Droppleman 1993; Pflueger et al. 1999b) or prostaglandins (Spielman and Osswald 1978; Pflueger et al. 1999a) increases the sensitivity of the kidney to adenosine-induced vasoconstriction. The outlined interactions can be of clinical relevance.
2.3 Activation of A2AR Induces Medullary Vasodilation
Intrarenal adenosine infusion in rats initially induces vasoconstriction in all cortical zones; this is followed by persistent superficial cortical vasoconstriction but deep cortical vasodilation (Macias-Nunez et al. 1983; Miyamoto et al. 1988). While A1AR-mediated afferent arteriolar constriction dominates in superficial nephrons, deep cortical glomeruli, which supply the blood flow to the renal medulla, can respond to adenosine with A2AR-mediated vasodilation (Inscho et al. 1991; Weihprecht et al. 1992; Inscho 1996; Yaoita et al. 1999; Nishiyama et al. 2001). In accordance, renal interstitial infusion in rats of the A2AR agonist 2-[p-(2-carboxyethyl)phenethylamino]-5′-N-ethylcarboxamido adenosine (CGS-21680) increased medullary blood flow (Agmon et al. 1993), whereas intramedullary infusion of the selective A2AR antagonist 3,7-dimethyl-1-propargylxanthine (DMPX) (but not the A1AR antagonist 1,3-dipropyl-8-cyclopentylxanthine (DPCPX)) decreased medullary blood flow (Zou et al. 1999). This indicates that endogenous adenosine dilates medullary vessels and sustains medullary blood flow via activation of A2AR (Fig. 1).
2.4 Adenosine is a Mediator of Tubuloglomerular Feedback via Activation of A1AR
The mammalian kidney has a rather high GFR (∼180 l per day in humans). About 99% of the filtered fluid and NaCl are subsequently reabsorbed along the tubular and collecting duct system, such that urinary excretion closely matches intake. As a result, GFR is a significant determinant of renal transport work, and GFR and re-absorption have to be closely coordinated to avoid renal loss or retention of fluid and NaCl. The tubuloglomerular feedback (TGF) is a mechanism that helps to coordinate GFR with the tubular transport activity or capacity. In this mechanism, specialized tubular cells, the macula densa, sense the tubular NaCl load at the end of the thick ascending limb (TAL; where about 85% of the filtered NaCl has been reabsorbed), and induce a change in afferent arteriolar tone such that an inverse relationship is established between the tubular NaCl load and SNGFR of the same nephron. This way, the TGF stabilizes the NaCl load to further distal segments, where the fine regulation of NaCl and fluid balance takes place under systemic neurohumoral control.
The TGF response, in other words an inverse change in SNGFR or glomerular capillary pressure in response to changes in the NaCl concentration at the macula densa, is inhibited by unselective adenosine receptor blockers like theophylline or 1,3-dipropyl-8-sulfophenylxanthine (DPSPX) (Schnermann et al. 1977; Osswald et al. 1980; Franco et al. 1989), as well as by selective A1AR antagonists like DPCPX, 8-(noradamantan-3-yl)-1,3-dipropylxanthine (KW-3902, rolofylline), CVT-124 (the S-enantiomer of the highly selective racemic A1AR antagonist 1,3-dipropyl-8-[2-(5,6-epoxynorbornyl)] xanthine), or 6-oxo-3-(2-phenylpyrazolo [1,5-a]pyridin-3-yl)-1(6H)-pyridazinebutanoic acid (FK838) (Franco et al. 1989; Schnermann et al. 1990; Kawabata et al. 1998; Wilcox et al. 1999; Thomson et al. 2000; Ren et al. 2002a). Mice with gene knockout for A1AR lack the TGF response (Brown et al. 2001; Sun et al. 2001; Vallon et al. 2004), and have an impaired ability to stabilize the Na+ delivery to the distal tubule (Vallon et al. 2004). Most importantly, an intact TGF response requires local concentrations of adenosine to fluctuate depending on the NaCl concentration in the tubular fluid at the macula densa, indicating that adenosine serves as a mediator of TGF (Thomson et al. 2000).
In 1980, Osswald and colleagues proposed that adenosine may be a mediator of TGF. Figure 2 illustrates a current model. Changes in luminal concentrations of Na+, K+, and Cl− alter NaCl uptake by macula densa cells via the furosemide-sensitive Na–K–2Cl cotransporter in the luminal membrane. This triggers basolateral ATP release (Bell et al. 2003; Komlosi et al. 2004) as well as transport-dependent hydrolysis by basolateral Na+–K+-ATPase (Lorenz et al. 2006) of ATP to AMP. Plasma membrane-bound ectonucleoside triphosphate diphosphohydrolase 1 (CD39) converts ATP and ADP to AMP (Oppermann et al. 2008) and ecto-5′-nucleotidase (CD73) converts extracellular AMP to adenosine (Thomson et al. 2000; Castrop et al. 2004; Ren et al. 2004; Huang et al. 2006). Part of the extracellular adenosine involved in the TGF response is generated independent of ecto-5′-nucleotidase and may reflect direct adenosine release from macula densa cells (Huang et al. 2006). Extracellular adenosine binds to A1AR at the surface of extraglomerular mesangial cells (Olivera et al. 1989; Weaver and Reppert 1992; Toya et al. 1993; Smith et al. 2001) and increases cytosolic Ca2+ concentrations (Olivera et al. 1989). Gap junctions between extraglomerular mesangial cells and smooth muscle cells of glomerular arterioles can transmit intracellular Ca2+ transients to these target structures, inducing afferent arteriolar constriction (Iijima et al. 1991; Ren et al. 2002b). Potential candidates for the formation of gap junctions in the juxtaglomerular apparatus include connexins 37, 40, and 43 (Wagner et al. 2007; Takenaka et al. 2008a, b).
Fig. 2.
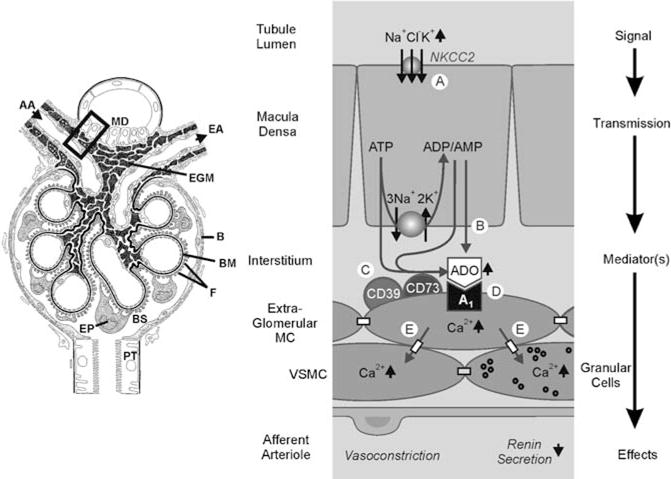
a–e Adenosine is a mediator of the tubuloglomerular feedback: a proposed mechanism. Left panel: schematic drawing illustrating the macula densa (MD) segment at the vascular pole with the afferent arteriole (AA) entering and the efferent arteriole (EA) leaving the glomerulus; extraglomerular mesangium (EGM); glomerular basement membrane (BM); epithelial podocytes (EP) with foot processes (F); Bowman’s capsule (B) and space (BS), respectively; proximal tubule (PT). (Adapted from Kriz, Nonnenmacher and Kaissling). Right panel: schematic enlargement of area in rectangle. An increase in concentration-dependent uptake of Na+, K+ and Cl− via the furosemide-sensitive Na+ − K+ − 2Cl− cotransporter (NKCC2) a leads to transport-related, intra- and/or extracellular generation of adenosine (ADO) b, c. Extracellular ADO activates A1AR, triggering an increase in cytosolic Ca2+ in extraglomerular mesangium cells (MC) d. The intensive coupling between extraglomerular MC, granular renin-containing cells, and vascular smooth muscle cells (VSMC) of the afferent arteriole by gap junctions allows propagation of the increased Ca2+ signal e, resulting in afferent arteriolar vasoconstriction and inhibition of renin release (adapted from Vallon et al. 2006)
3 Activation of A1AR Inhibits Renin Secretion
Tagawa and Vander reported in 1970 that adenosine infusion into the renal artery of salt-depleted dogs inhibited the renal secretion of renin into the venous blood (Tagawa and Vander 1970). This was confirmed in various species including humans (Edlund et al. 1994). Most notably, a single application of the A1AR antagonist FK-453 increased plasma renin concentrations in humans (Balakrishnan et al. 1993), indicating a tonic inhibition of renin secretion by A1AR activation. In accordance, knockout mice for A1AR have increased renal mRNA expression and content of renin (Schweda et al. 2003) as well as greater plasma renin activity (Brown et al. 2001; Rieg et al. 2007) compared with wild-type mice.
Jackson and coworkers proposed an extracellular cyclic adenosine monophosphate (cAMP)-adenosine pathway in the control of renin release: the increase in intracellular cAMP in renin-secreting cells causes efflux of cAMP, the latter being converted to adenosine in the extracellular space. The generated adenosine, by acting on A1AR on the renin-secreting cells, then acts as a negative-feedback control on renin release (Jackson and Raghvendra 2004). In addition, high NaCl concentrations in the tubular lumen enhance adenosine generation in a macula densa-dependent way, and the adenosine generated inhibits renin release via activation of A1AR (Itoh et al. 1985; Weihprecht et al. 1990; Lorenz et al. 1993; Kim et al. 2006) (Fig. 2). In contrast to A1AR stimulation, activation of A2AR can increase renin secretion (Churchill and Churchill 1985; Churchill and Bidani 1987). The latter may have contributed to the observation that the unselective adenosine receptor antagonist caffeine reduced plasma renin concentration in mice lacking A1AR (Rieg et al. 2007).
4 Differential Effects of Adenosine on Fluid and Electrolyte Transport
In addition to its effects on renal blood flow, GFR, and renin release, adenosine induces direct effects on fluid and electrolyte transport along the tubular and collecting duct system.
4.1 Activation of A1AR Increases Reabsorption in the Proximal Tubule
Endogenously formed adenosine can stimulate proximal tubular reabsorption of fluid, Na+, HCO3−, and phosphate by activation of A1AR (Takeda et al. 1993; Cai et al. 1994, 1995; Tang and Zhou 2003). Importantly, systemic application of selective A1AR antagonists (such as CVT-124, DPCPX, KW-3902, or FK-453) elicits diuresis and natriuresis predominantly by inhibiting reabsorption in the proximal tubule in rats and humans (Mizumoto and Karasawa 1993; Balakrishnan et al. 1993; van-Buren et al. 1993; Knight et al. 1993b; Wilcox et al. 1999; Miracle et al. 2007), indicating a tonic stimulation of proximal tubular reabsorption via A1AR activation (Fig. 1). As a consequence, selective A1AR antagonists are being developed as eukaliuretic natriuretics in Na+-retaining states such as heart failure (see below). A1AR-mediated increases in proximal tubular reabsorption may involve increases of intracellular Ca2+ (Di Sole et al. 2003), reductions of intracellular cAMP levels (Kost Jr et al. 2000), and activation of the Na+–H+ exchanger (NHE3) (Di Sole et al. 2003).
Similar to selective A1AR blockade, systemic application or consumption of the unselective adenosine receptor antagonist theophylline or caffeine induces natriuretic and diuretic responses. These responses to theophylline and caffeine are absent in mice lacking A1AR, strongly suggesting that A1AR blockade mediates the natriuresis and diuresis in response to these compounds (Rieg et al. 2005).
4.2 Activation of A1AR Inhibits Reabsorption in Medullary Thick Ascending Limb
In contrast to the proximal tubule, adenosine via activation of A1AR inhibits NaCl reabsorption in medullary TAL (Torikai 1987; Burnatowska-Hledin and Spielman 1991; Beach and Good 1992). Medullary TAL is a site of adenosine release, and adenosine release in this segment is transport dependent (Beach et al. 1991; Baudouin-Legros et al. 1995) and enhances significantly during hypoxic conditions (Beach et al. 1991). Studies using pharmacological inhibition (Zou et al. 1999) or gene knockout (Vallon et al. 2004) are consistent with a tonic inhibition of Na+ re-absorption in medullary TAL by A1AR activation (Fig. 1). This is relevant since the renal medulla has a low partial oxygen pressure (Brezis and Rosen 1995), and the described inhibitory effects of adenosine on transport work together with its A2AR-mediated renal medullary vasodilation (see above) to maintain metabolic balance in the renal medulla.
4.3 Effects of Adenosine on Transport in Distal Convolution and Cortical Collecting Duct
In general, natriuretics that act proximal to the aldosterone-sensitive distal nephron stimulate K+ secretion in the latter segment and thus increase renal K+ excretion. The natriuretic but eukaliuretic effect of A1AR inhibitors suggests an additional site of action in the aldosterone-sensitive distal nephron, but the exact site of action and the involved mechanisms are unclear.
A1AR activation can stimulate Mg2+ and Ca2+ uptake in the cortical collecting duct in vitro (Hoenderop et al. 1998, 1999; Kang et al. 2001), but the clinical relevance (e.g., during pharmacological inhibition of A1AR) is not known.
4.4 Activation of A1AR Counteracts Vasopressin Effects in Inner Medullary Collecting Duct
Extracellular adenosine feedback can inhibit vasopressin-induced cAMP-mediated stimulation of Na+ and fluid reabsorption in the inner medullary collecting duct (IMCD) (Yagil 1990; Yagil et al. 1994; Rieg et al. 2008) and decrease vasopressin-stimulated electrogenic Cl− secretion through the activation of A1AR (Moyer et al. 1995). Vasopressin-induced adenosine may derive from the extracellular cAMP–adenosine pathway (Jackson et al. 2003) or follow the cellular release and breakdown of ATP (Vallon 2008). Studies on water transport in knockout mice indicate efficient compensation by other pathways in the absence of A1AR, including upregulation of ATP-sensitive P2Y2 receptors (Rieg et al. 2008).
5 Adenosine and Metabolic Control of Kidney Function
The above outlined functions of adenosine can be integrated into the concept of metabolic control of renal function (Fig. 1). Adenosine-induced vasoconstriction via A1AR activation is predominant in the outer cortex by increasing the resistance of afferent arterioles, which lowers GFR and thus renal transport work. Under physiological conditions, adenosine-induced afferent arteriolar constriction primarily derives from tonic activation of the TGF, for which adenosine acts as a mediator. Adenosine via A1AR tonically stimulates NaCl reabsorption in the cortical proximal tubule, which is a tubular segment with a relatively high basal oxygen supply, thereby limiting the NaCl load to downstream medullary segments. In the deep cortex and medulla, adenosine induces vasodilation via A2AR activation, which is associated with an increase of medullary blood flow and thus increased medullary oxygenation. Moreover, adenosine inhibits NaCl reabsorption in medullary TAL and IMCD (i.e., nephron segments with relatively low oxygen delivery). In addition, the A2AR-mediated rise in medullary blood flow lowers medullary transport activity by washing out the high osmolality in the medullary interstitium (Zou et al. 1999). In accordance, interstitial infusion of adenosine in rat kidney decreased partial pressure of O2 in the cortex but increased it in the medulla, consistent with an important regulatory and protective role of adenosine in renal medullary O2 balance (Dinour and Brezis 1991).
6 Adenosine and Acute Renal Failure
The renal effects of adenosine fit into the concepts of acute renal failure (ARF) in as much as adenosine is an intrarenal metabolite that accumulates in the kidney during renal ischemia and that can lower GFR. In addition, ischemia or nephrotoxins can inhibit renal transport activity, with the resulting increase in the NaCl concentration at the macula densa further lowering GFR (Fig. 3). Moreover, experimental models of ARF can be associated with increased expression of A1AR in glomeruli, which may contribute to depressed GFR (Smith et al. 2000). Thus, inhibition of adenosine vasoconstrictor actions in the kidney could be beneficial in conditions of ARF. On the other hand, the ARF-associated reduction in GFR and thus in tubular NaCl load may, to some extent, protect the tubular system—especially the medulla—from hypoxic injury, and the body from excess NaCl loss. Moreover, adenosine can induce direct cytoprotective effects in renal cells. Therefore, inhibition of adenosine receptors in ARF could be a two-sided sword. In the following we discuss the role of adenosine in ARF induced by radiocontrast media and ischemia-reperfusion, respectively.
Fig. 3.
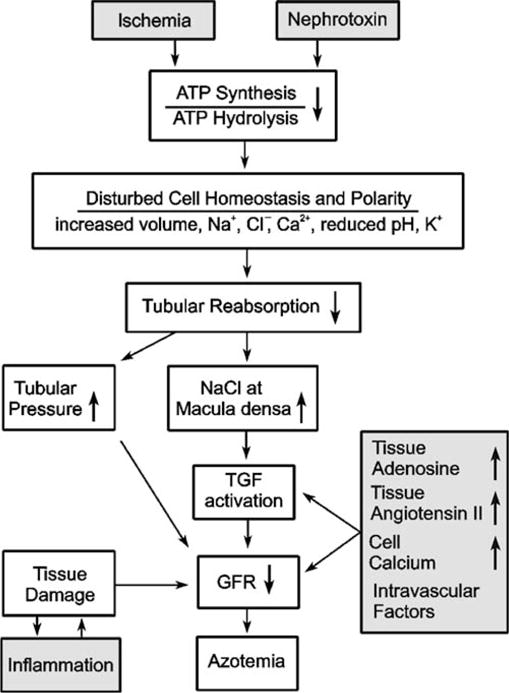
Schematic illustration of intrarenal mechanisms in acute renal failure. See text for further explanation (adapted from Osswald and Vallon 2008)
6.1 Radiocontrast Media-Induced Acute Renal Failure: Theophylline and A1AR Antagonists Induce Protective Effects
Application of radiocontrast media to humans can lead to an impairment of renal function, including a fall in GFR. Concomitant volume and NaCl depletion increases the severity and can result in ARF. Unselective or A1AR-selective antagonists can prevent renal impairment induced by radiocontrast media, as shown in dogs (Arend et al. 1987), rats (Erley et al. 1997), mice (Lee et al. 2006), and, most importantly, in humans (Erley et al. 1994; Katholi et al. 1995; Kolonko et al. 1998; Kapoor et al. 2002; Huber et al. 2002, 2003). In accordance, mice lacking A1AR preserved kidney function better, and had lesser renal cortical vacuolization and enhanced survival 24 h after radiocontrast media treatment compared with wild-type mice (Lee et al. 2006). In comparison, dipyridamole, which increases extracellular adenosine concentrations, augmented the severity of renal impairment in response to radio-contrast media in dogs (Arend et al. 1987) and humans (Katholi et al. 1995). Two studies indicated that the unselective adenosine receptor antagonist theophylline is as effective as saline hydration at preventing ARF in response to contrast media, but the benefits of the two maneuvers are not additive (Abizaid et al. 1999; Erley et al. 1999). Thus, use of theophylline can be beneficial in patients where sufficient hydration may be impossible or in patients with a concomitant decrease in renal blood flow (e.g., congestive heart failure or chronic renal insufficiency (Erley et al. 1999; Huber et al. 2002)). A recent meta-analysis of clinical trials concluded that theophylline may reduce the incidence of radiocontrast media-induced nephropathy, and recommended a large, well-designed trial to more adequately assess the role of theophylline in this condition (Bagshaw and Ghali 2005). Notably, unselective or A1AR-selective antagonists can also prevent renal impairment in response to other nephrotoxic substances (Table 1).
Table 1.
Adenosine receptor antagonists improve renal function in various models of nephrotoxic acute renal failure (ARF)
| Models of ARF | Species | Adenosine antagonist | References |
|---|---|---|---|
| Glycerol | Rat | Theophylline | Bidani and Churchill (1983); Bidani et al. (1987) |
| Injection | 8-Phenyl-theophylline |
Bowmer et al. (1986); Yates et al. (1987) Kellett et al. (1989); Panjehshahin et al. (1992) |
|
| DPCPX | |||
| FK-453 | Ishikawa et al. (1993) | ||
| KW-3902 | Suzuki et al. (1992) | ||
| Uranyl nitrate | Rat | Theophylline | Osswald et al. (1979) |
| Cisplatin | Rat | Theophylline | Heidemann et al. (1989) |
| Human | DPCPX | Knight et al. (1991) | |
| KW-3902 | Nagashima et al. (1995) | ||
| Theophylline | Benoehr et al. (2005) | ||
| Contrast media | Dog | Theophylline | Arend et al. (1987) |
| Human | Theophylline | Erley et al. (1994); Katholi et al. (1995); Kolonko et al. (1998); Kapoor et al. (2002); Huber et al. (2002, 2003) | |
| Rat | DPCPX, KW3902 | Erley et al. (1997); Yao et al. (2001) | |
| Endotoxin | Rat | DPCPX | Knight et al. (1993a) |
| Amphotericin B | Rat | Theophylline | Heidemann et al. (1983) |
| Dog | Theophylline | Gerkens et al. (1983) | |
| Gentamicin | Rat | KW-3902 | Yao et al. (1994) |
8-Cyclopentyl-1,3-dipropylxanthine (DPCPX), (+)-(R)-[(E)-3-(2-phenylpyrazolo[1,5-a]pyridin-3-yl)acryloyl]-2-piperidine ethanol (FK-453) and 8-(noradamantan-3-yl)-1,3 dipropylxanthine (KW-3902) are A1AR-selective antagonists. Adapted from Vallon et al. (2006)
6.2 Ischemia-Reperfusion Injury
Ischemia-reperfusion injury plays a major role in delayed graft function and long-term changes after kidney transplantation. It has become evident that the cellular and molecular mechanisms that operate during ischemia and reperfusion resemble an acute inflammatory response (Gueler et al. 2004). To what extent the acute cellular alterations persist and affect organ function later on remains unclear.
In the kidney, extracellular adenosine derives to a large extent from the extracellular breakdown of ATP and ADP to AMP and adenosine via ectonucleoside triphosphate diphosphohydrolases (ENTPDases) and CD73 (Grenz et al. 2007a, b). Using knockout mouse models for these ectoenzymes, Grenz et al. showed that CD39-dependent nucleotide phosphohydrolysis as well as CD73-dependent adenosine formation serve to protect against renal ischemia-reperfusion injury and to increase the ischemia tolerance of the kidney. In addition, the authors presented evidence that treatment with apyrase or soluble 5′-nucleotidase to increase extracellular adenosine concentrations could serve as potential novel pharmacological approaches to renal diseases precipitated by limited oxygen availability (Grenz et al. 2007a, b).
6.2.1 Theophylline Induces Protective Effects
Different animal studies assessed the effect of a single application of the unselective adenosine receptor antagonist theophylline in ischemia-reperfusion injury. Animals were pretreated with theophylline or it was given at day 5 after the renal ischemic/hypoxemic event. Pretreatment with a single dose of theophylline in rats attenuated the reduction in renal blood flow and GFR observed during the initiation phase of postischemic ARF as determined 1 h after releasing a 30 or 45 min occlusion of the renal artery (Lin et al. 1986). Similar results were obtained with theophylline in the rabbit (Gouyon and Guignard 1988). In rats subjected to 60 min occlusion of the left renal artery, theophylline given i.v. 20 min before the release of the renal artery clamp in doses which antagonize the renal actions of adenosine in vivo improved the recovery of renal function after ischemic injury by increasing urinary flow rate, GFR (measured by inulin clearance), and histology, as assessed by morphometric quantification of tubular damage, tubular obstruction and pathologic alteration of glomeruli at 3 h after initiating reperfusion (Osswald et al. 1979; Helmlinger 1979). In contrast, pretreating rats prior to renal artery occlusion for 30 min with dipyridamole, which increases extracellular adenosine concentrations, intensified the fall in renal blood flow and GFR determined about 1 h after releasing the clamp, and this impairment was blocked by theophylline (Lin et al. 1987).
Notably, single-dose pretreatment of rats with theophylline during a 30 min renal artery occlusion lead to increased renal blood flow and GFR during the maintenance phase of ARF after five days, indicating that the effects of theophylline in the acute phase affected the outcome in the maintenance phase. Similarly, a single dose of theophylline, given early after birth in asphyxiated full-term infants, has beneficial effects in reducing the renal involvement and fall in GFR as determined over the first five days (Bakr 2005). Finally, acute theophylline treatment given at five days after ischemia acutely increases renal blood flow and GFR in previously untreated rats, indicating that adenosine contributes to the suppression of renal blood flow and GFR in the maintenance phase of ischemia-reperfusion injury (Lin et al. 1988). These data provide strong evidence that pretreatment with theophylline can exert beneficial effects in the initiation and maintenance phase of ischemia-reperfusion injury.
6.2.2 Adenosine Induces Protective Effects via A1AR and A2AR
Similar to theophylline, systemic intravenous infusion of adenosine (1.75 mg kg−1 min−1 ×10 min, intravenously) 2 min before a 45 min ischemic insult protected renal function against ischemia and reperfusion injury, as indicated by lower blood urea nitrogen and creatinine and improved renal morphology after 24 h of reperfusion. The effects of adenosine were proposed to be mediated by A1AR (Lee and Emala 2000), involve Gi/o proteins and protein kinase C activation (Lee and Emala 2001a), and include a reduction in inflammation, necrosis, and apoptosis (Lee et al. 2004a). Direct cytoprotective effects of endogenous A1AR activation in renal proximal tubules involve modulation of heat-shock protein (HSP)27 due to A1AR-mediated enhancement of p38 and AP2 mitogen-activated protein kinase activities (Lee et al. 2007). In comparison, mice lacking A1AR exhibited significantly higher plasma creatinines and worsened renal histology compared with wild-type mice at 24 h after renal ischemia for 30 min (Lee et al. 2004b). Similarly, wild-type mice pretreated with an A1AR antagonist or agonist demonstrated worsened or improved renal function, respectively, after ischemia-reperfusion that was associated with increased or reduced markers of renal inflammation, respectively (Lee et al. 2004b) (Fig. 3). More recent work indicated that A1AR activation produces not only acute but also delayed renal protection; i.e., pretreatment with a selective A1AR agonist 24 h before renal ischemia was also protective against renal ischemia-reperfusion injury. Furthermore, the study showed that acute protection from A1AR activation is dependent on protein kinase C and Akt activation, whereas the delayed protection is dependent on Akt activation and induction of HSP27 (Joo et al. 2007).
Continuous application in the reperfusion period of 4-(3-(6-amino-9-(5-ethylcarbamoyl-3,4-dihydroxytetrahydrofuran-2-yl)-9H-purin-2-yl)prop-2-ynyl) cyclohexanecarboxylic acid methyl ester (DWH-146e), a selective A2AAR agonist, protected kidneys from ischemia-reperfusion injury, as evidenced by a lower rise in serum creatinine and blood urea nitrogen following 24 and 48 h of reperfusion. Histological examination revealed widespread tubular epithelial necrosis and vascular congestion in the outer medulla of vehicle-treated rats. These lesions were significantly reduced in DWH-146e-treated animals (Okusa et al. 1999). Similarly, systemic adenosine given after 45 min of renal ischemia but before reperfusion protected renal function, as indicated by lower rises in creatinine and less histologically evident renal tubular damage. Pharmacological maneuvers indicated that these effects of adenosine were mediated by A2AAR activation (Lee and Emala 2001b). Whereas A2AAR activation could improve medullary hypoxia, other studies suggested that protection from renal ischemia-reperfusion injury by A2AR agonists or endogenous adenosine requires activation of A2AR expressed on bone marrow-derived cells (Day et al. 2003). Activation of A2AAR on macrophages was also shown to inhibit inflammation in a rat model of glomerulonephritis (Garcia et al. 2008). Moreover, activation of A2BAR in the renal vasculature contributes to the increased ischemia tolerance produced by the procedure of renal ischemic preconditioning (Grenz et al. 2008).
Finally, A3AR stimulation in rats deteriorated renal ischemia-reperfusion injury, whereas inhibition of A3AR protected renal function as efficiently as preconditioning (Lee and Emala 2000). In accordance, mice lacking A3AR presented significant renal protection, functionally and morphologically, from ischemic or myoglobinuric renal failure (Lee et al. 2003). The mechanisms of these A3AR-mediated effects are not understood at present.
In summary, beneficial effects on GFR and renal morphology beyond 3–24 h of reperfusion after ischemia can be induced by (1) pretreatment with the unselective adenosine receptor antagonist theophylline, (2) pretreatment or treatment immediately before reperfusion with adenosine, (3) pretreatment with A1AR agonists, (4) treatment immediately before or during reperfusion with A2AAR agonists, (5) treatment with A2BAR agonists, and (6) deficiency of A3AR. In comparison, the outcome is worsened by (1) pretreatment with A1AR antagonists or deficiency of A1AR, (2) pretreatment with A2BAR antagonists or deficiency of A2BAR, and (3) pretreatment with A3AR agonists. The findings appear contradictory because theophylline can inhibit both A1AR and A2AAR, and possibly acts as an agonist at A3AR (Ezeamuzie 2001). Further studies are necessary to resolve this issue, which may relate to the nature of adenosine being a double-edged sword in ARF, and the situation being further complicated by the role of adenosine in inflammatory responses.
7 A1AR Antagonists in the Treatment of Cardiorenal Failure
Concomitant renal dysfunction is one of the strongest risk factors for mortality in ambulatory heart failure patients (Dries et al. 2000; Hillege et al. 2000; Mahon et al. 2002). In patients hospitalized for decompensated heart failure, worsening of renal function further predicts an adverse outcome (Forman et al. 2004). Intravenous loop diuretics are the mainstay of therapy for patients with both systemic volume overload and acute pulmonary edema decompensated heart failure. Treatment, however, may be complicated by diuretic resistance and/or worsening of renal function, indicating the need for alternative approaches.
Volume overload heart failure in dogs increases myocardial adenosine release (Newman et al. 1984), and circulating levels of adenosine can be increased in patients with chronic heart failure (∼200 vs 60 nM) (Funaya et al. 1997) (Fig. 4). Whether this increases circulating adenosine to an extent that affects afferent arteriolar tone and thus GFR is unclear. Nonetheless, the renal vasculature in heart failure patients can be sensitized to the GFR-lowering effects of adenosine by the associated activation of the renin–angiotensin system and/or impairment of the local formation of NO (endothelial dysfunction) or prostaglandins (see above and Fig. 4). In addition, impaired renal perfusion and hypoxia enhance adenosine formation within the kidney (Nishiyama et al. 1999). As a consequence, the normally homeostatic adenosine system may become maladaptive and overshoots with regard to the downregulation of GFR in patients with heart failure. Fluid retention is further potentiated by stimulation of NaCl and fluid reabsorption in the proximal tubule, a mechanism also mediated by A1AR activation (see above and Fig. 4). Based on this concept, pharmacological blockade of A1AR could improve kidney function and fluid retention in heart failure. Since adenosine (through the activation of A1AR) mediates TGF, the expected TGF-induced reduction in GFR in response to inhibition of proximal reabsorption by A1AR antagonists should be blunted. In accordance, a study in rats showed that A1AR antagonism with KW-3902 prevented the GFR-lowering effect of the proximal diuretic benzolamide, a carbonic anhydrase inhibitor (Miracle et al. 2007).
Fig. 4.
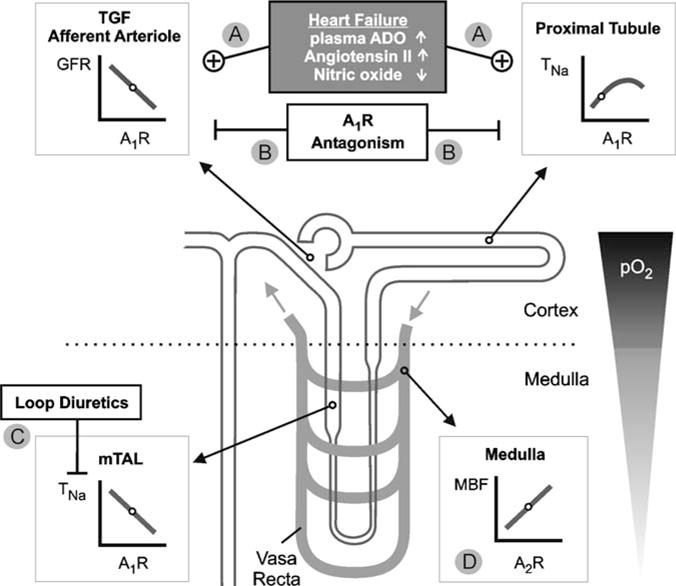
a–d Basis for a therapeutic effect of A1AR antagonism in heart failure. The basic effects of adenosine on renal functions are outlined in the legend to Fig. 1. a Heart failure can be associated with increased plasma concentrations of adenosine (ADO) and angiotensin II, and endothelial dysfunction can impair nitric oxide (NO) formation, all of which can enhance the A1AR-mediated lowering of GFR and may, in addition, stimulate proximal reabsorption. b A1AR antagonism induces natriuresis and diuresis by inhibiting proximal reabsorption and preserving or increasing GFR. c A1AR antagonism can enhance sodium transport (TNa) in semihypoxic medullary thick ascending limb (mTAL). This is prevented by coadministration of loop diuretics, and diuresis and natriuresis are potentiated. d A2AR-mediated medullary vasodilation is preserved (adapted from Vallon 2008)
7.1 Animal Studies
Lucas et al. used a pig model of systolic dysfunction and induction of chronic heart failure by pacer-induced tachycardia. They observed that acute application of the selective A1AR antagonist 1,3-dipropyl-8-[2-(5,6-epoxynorbornyl)xanthine (BG9719) (CVT-124) increased creatinine clearance and urinary flow rate and sodium excretion. This was associated with lower pulmonary capillary wedge pressure and pulmonary vascular resistance in the absence of significant changes in mean arterial blood pressure, heart rate or cardiac output compared with vehicle control (Lucas Jr et al. 2002). Similar effects were described by Jackson et al. in aged, lean SHHF/Mcc-fa(cp) rats, a rodent model of hypertensive dilated cardiomyopathy in response to the same compound (Jackson et al. 2001). The rats were pretreated for 72 h before experiments with the loop diuretic furosemide to mimic the clinical setting of chronic diuretic therapy, and were given 1% NaCl as drinking water to reduce dehydration/sodium depletion. Acute application of BG9719 increased GFR and urinary fluid and sodium excretion. In comparison, acute application of furosemide decreased renal blood flow and GFR and increased fractional potassium excretion. Neither drug altered afterload or left ventricular systolic function (+dP/dt (max)); however, furosemide, but not BG9719, decreased preload and attenuated diastolic function (decreased −dP/dt (max), increased tau). Thus, in the setting of left ventricular dysfunction, chronic salt loading and prior loop diuretic treatment, selective A1AR antagonists are effective diuretic/natriuretic agents that do not induce potassium loss and have a favorable renal hemodynamic/cardiac performance profile (Jackson et al. 2001).
7.2 Human Studies
Gottlieb et al. compared the acute effects of furosemide and BG9719 on renal function in 12 patients categorized as New York Heart Association (NYHA) functional classes II, III or IV (Gottlieb et al. 2000). Both BG9719 and furosemide increased sodium excretion compared with placebo. However, only furosemide lowered GFR. Subsequently, Gottlieb et al. compared BG9719 and furosemide in 63 patients categorized as NYHA functional classes II, III or IV, which despite receiving standard therapy, including furosemide (at least 80 mg daily) and angiotensin-converting enzyme inhibitors, remained edematous (Gottlieb et al. 2002). Patients received 7 h infusions of placebo or BG9719 to yield serum concentrations of 0.1, 0.75, or 2.5 μg ml−1. BG9719 tripled urine output without lowering GFR or inducing kaliuresis. In comparison, furosemide increased urine output eightfold and increased potassium excretion while reducing GFR. Notably, when BG9719 was given with furosemide, GFR remained unaltered compared with placebo and sodium excretion increased further. These results indicate that A1AR antagonism can preserve renal function while simultaneously promoting natriuresis during acute treatment of heart failure (Gottlieb et al. 2002).
Similar results were more recently reported in studies using the A1AR antagonist KW-3902 in patients with congestive heart failure and impaired renal function (Dittrich et al. 2007; Givertz et al. 2007). Dittrich et al. assessed baseline GFR and renal plasma flow 3 h before and over 8 h following the intravenous administration of furosemide along with KW-3902 (30 mg) or placebo. After a washout period of 3–8 days (median six days), the crossover portion of the study was performed. KW-3902 increased GFR by 32% and renal plasma flow by 48% compared with placebo. Notably, subjects who initially received KW-3902 had a statistically significant 10 ml min−1 increase in GFR when they returned for the crossover phase compared with the previous baseline. Thus, the increase in GFR persisted for several days longer than predicted by pharmacokinetics. These findings suggest that KW-3902 reset the complex network that determines kidney function in these patients, and provided first evidence for potential longer-term benefits of using A1AR antagonists (Dittrich et al. 2007). Greenberg et al. assessed the effects of the selective A1AR antagonist 1,3-dipropyl-8-[1-(4-propionate)-bicyclo-[2,2,2]octyl)]xanthine (BG9928) given orally for ten days to 50 patients with heart failure and left ventricular systolic dysfunction who were receiving standard therapy (Greenberg et al. 2007). BG9928 (3–225 mg per day) increased sodium excretion without causing kaliuresis or reducing GFR. Notably, these effects were maintained over the ten-day period. BG9928 at doses of 15, 75, or 225 mg also reduced body weight at the end of the study compared with placebo (Greenberg et al. 2007).
In summary, the abovedescribed acute and short-term studies employing A1AR antagonists in patients with heart failure yielded promising results. Since A1AR blockade may increase transport in the semihypoxic medullary TAL, combining A1AR antagonists with furosemide may potentiate natriuresis while helping to prevent transport-induced medullary hypoxia (Fig. 4). Whereas the presented animal and human studies were acute or short-term treatments, it remains to be determined whether longer-term application of A1AR antagonism has beneficial effects. These studies should also reveal whether a clinically relevant effect of A1AR blockade on renin release occurs. Consideration should also be given to the evidence that A1AR activation is potentially important for protection in response to ischemia of the kidney (see above) and the heart (Cohen and Downey 2008). Apart from these issues, A1AR blockade is unique in inducing natriuresis without potassium loss and lowering renal vascular resistance independent of all other organs. With regard to preserving renal function, this is an advantage over all vasodilator heart failure therapies that have been tried so far.
Acknowledgments
The work from our laboratories was supported by the Deutsche Forschungs-gemeinschaft (DFG VA 118/2-1, DFG OS 42/1–42/7), the Department of Veterans Affairs, the National Institutes of Health (DK56248, DK28602, GM66232, P30DK079337), and the American Heart Association (GiA 655232Y).
Abbreviations
- AA
Afferent arteriole
- ADO
Adenosine
- ARF
Acute renal failure
- AXAR
Adenosine receptor subtype x
- B
Bowman’s capsule
- BG9719
1,3-Dipropyl-8-[2-(5,6-epoxynorbornyl) xanthine
- BG9928
1,3-Dipropyl-8-[1-(4-propionate)-bicyclo-[2,2,2]octyl)]xanthine
- BM
Basement membrane
- BS
Bowman’s space
- cAMP
Cyclic adenosine monophosphate
- CD39
Ecto-nucleoside triphosphate diphosphohydrolase-1
- CD73
Ecto-5′-nucleotidase
- CGS21680
2-[p-(2-Carboxyethyl)phenethylamino]-5′-N-ethylcarboxamido adenosine
- CVT-124
S-Enantiomer of 1,3-dipropyl-8-[2-(5,6-epoxynorbornyl)] xanthine
- DMPX
3,7-Dimethyl-1-propargylxanthine
- DPCPX
1,3-Dipropyl-8-cyclopentylxanthine
- DPSPX
1,3-Dipropyl-8-sulfophenylxanthine
- DWH 146e
4-(3-(6-Amino-9-(5-ethylcarbamoyl-3,4-dihydroxytetrahydrofuran-2-yl)-9H-purin-2-yl)prop-2-ynyl)cyclohexanecarboxylic acid methyl ester
- EA
Efferent arteriole
- EGM
Extraglomerular mesangium
- ENTPDase
Ectonucleoside triphosphate diphosphohydrolase
- FK-453
(+)-(R)-[(E)-3-(2-Phenylpyrazolo[1,5-a]pyridin-3-yl)acryloyl]-2-piperidine ethanol
- FK-838
6-Oxo-3-(2-phenylpyrazolo[1,5-a]pyridin-3-yl)-1(6H)-pyridazinebutanoic ‘acid
- GFR
Glomerular filtration rate
- HSP27
Heat-shock protein 27
- IMCD
Inner medullary collecting duct
- KW-3902
8-(Noradamantan-3-yl)-1,3 dipropylxanthine
- MBF
Medullary blood flow
- MC
Mesangium cells
- mTAL
Medullary thick ascending limb
- NHE
Na + − H+ exchanger
- NKCC2
Na + − K+ −2Cl− cotransporter
- NO
Nitric oxide
- NYHA
New York Heart Association
- pO2
Partial oxygen pressure
- PT
Proximal tubule
- SNGFR
Single nephron glomerular filtration rate
- TAL
Thick ascending limb
- TGF
Tubuloglomerular feedback
- TNa
Transport of sodium
- VSMC
Vascular smooth muscle cells
References
- Abizaid AS, Clark CE, Mintz GS, Dosa S, Popma JJ, Pichard AD, Satler LF, Harvey M, Kent KM, Leon MB. Effects of dopamine and aminophylline on contrast-induced acute renal failure after coronary angioplasty in patients with preexisting renal insufficiency. Am J Cardiol. 1999;83:260–263. A5. doi: 10.1016/s0002-9149(98)00833-9. [DOI] [PubMed] [Google Scholar]
- Agmon Y, Dinour D, Brezis M. Disparate effects of adenosine A1- and A2-receptor agonists on intrarenal blood flow. Am J Physiol. 1993;265:F802–F806. doi: 10.1152/ajprenal.1993.265.6.F802. [DOI] [PubMed] [Google Scholar]
- Arend LJ, Thompson CI, Spielman WS. Dipyridamole decreases glomerular filtration in the sodium-depleted dog. Evidence for mediation by intrarenal adenosine. Circ Res. 1985;56:242–251. doi: 10.1161/01.res.56.2.242. [DOI] [PubMed] [Google Scholar]
- Arend LJ, Bakris GL, Burnett JC, Jr, Megerian C, Spielman WS. Role for intrarenal adenosine in the renal hemodynamic response to contrast media. J Lab Clin Med. 1987;110:406–411. [PubMed] [Google Scholar]
- Bagshaw SM, Ghali WA. Theophylline for prevention of contrast-induced nephropathy: a systematic review and meta-analysis. Arch Intern Med. 2005;165:1087–1093. doi: 10.1001/archinte.165.10.1087. [DOI] [PubMed] [Google Scholar]
- Bakr AF. Prophylactic theophylline to prevent renal dysfunction in newborns exposed to perinatal asphyxia: a study in a developing country. Pediatr Nephrol. 2005;20:1249–1252. doi: 10.1007/s00467-005-1980-z. [DOI] [PubMed] [Google Scholar]
- Balakrishnan VS, Coles GA, Williams JD. A potential role for endogenous adenosine in control of human glomerular and tubular function. Am J Physiol. 1993;265:F504–F510. doi: 10.1152/ajprenal.1993.265.4.F504. [DOI] [PubMed] [Google Scholar]
- Balakrishnan VS, Coles GA, Williams JD. Effects of intravenous adenosine on renal function in healthy human subjects. Am J Physiol. 1996;271:F374–F381. doi: 10.1152/ajprenal.1996.271.2.F374. [DOI] [PubMed] [Google Scholar]
- Barrett RJ, Droppleman DA. Interactions of adenosine A1 receptor-mediated renal vasoconstriction with endogenous nitric oxide and ANG II. Am J Physiol. 1993;265:F651–F659. doi: 10.1152/ajprenal.1993.265.5.F651. [DOI] [PubMed] [Google Scholar]
- Baudouin-Legros M, Badou A, Paulais M, Hammet M, Teulon J. Hypertonic NaCl enhances adenosine release and hormonal cAMP production in mouse thick ascending limb. Am J Physiol. 1995;269:F103–F109. doi: 10.1152/ajprenal.1995.269.1.F103. [DOI] [PubMed] [Google Scholar]
- Beach RE, Good DW. Effects of adenosine on ion transport in rat medullary thick ascending limb. Am J Physiol. 1992;263:F482–F487. doi: 10.1152/ajprenal.1992.263.3.F482. [DOI] [PubMed] [Google Scholar]
- Beach RE, Watts BA, Good DW, Benedict CR, DuBose TD., Jr Effects of graded oxygen tension on adenosine release by renal medullary and thick ascending limb suspensions. Kidney Int. 1991;39:836–842. doi: 10.1038/ki.1991.105. [DOI] [PubMed] [Google Scholar]
- Bell PD, Lapointe JY, Sabirov R, Hayashi S, Peti-Peterdi J, Manabe K, Kovacs G, Okada Y. Macula densa cell signaling involves ATP release through a maxi anion channel. Proc Natl Acad Sci USA. 2003;100:4322–4327. doi: 10.1073/pnas.0736323100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benoehr P, Krueth P, Bokemeyer C, Grenz A, Osswald H, Hartmann JT. Nephroprotection by theophylline in patients with cisplatin chemotherapy: a randomized, single-blinded, placebo-controlled trial. J Am Soc Nephrol. 2005;16:452–458. doi: 10.1681/ASN.2004030225. [DOI] [PubMed] [Google Scholar]
- Bidani AK, Churchill PC. Aminophylline ameliorates glycerol-induced acute renal failure in rats. Can J Physiol Pharmacol. 1983;61:567–571. doi: 10.1139/y83-087. [DOI] [PubMed] [Google Scholar]
- Bidani AK, Churchill PC, Packer W. Theophylline-induced protection in myoglobinuric acute renal failure: further characterization. Can J Physiol Pharmacol. 1987;65:42–45. doi: 10.1139/y87-008. [DOI] [PubMed] [Google Scholar]
- Bowmer CJ, Collis MG, Yates MS. Effect of the adenosine antagonist 8-phenyltheophylline on glycerol-induced acute renal failure in the rat. Br J Pharmacol. 1986;88:205–212. doi: 10.1111/j.1476-5381.1986.tb09488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezis M, Rosen S. Hypoxia of the renal medulla: its implications for disease. N Engl J Med. 1995;332:647–655. doi: 10.1056/NEJM199503093321006. [DOI] [PubMed] [Google Scholar]
- Brown R, Ollerstam A, Johansson B, Skott O, Gebre-Medhin S, Fredholm B, Persson AE. Abolished tubuloglomerular feedback and increased plasma renin in adenosine A1 receptor-deficient mice. Am J Physiol Regul Integr Comp Physiol. 2001;281:R1362–R1367. doi: 10.1152/ajpregu.2001.281.5.R1362. [DOI] [PubMed] [Google Scholar]
- Burnatowska-Hledin MA, Spielman WS. Effects of adenosine on cAMP production and cytosolic Ca2+ in cultured rabbit medullary thick limb cells. Am J Physiol. 1991;260:C143–C150. doi: 10.1152/ajpcell.1991.260.1.C143. [DOI] [PubMed] [Google Scholar]
- Cai H, Batuman V, Puschett DB, Puschett JB. Effect of KW-3902, a novel adenosine A1 receptor antagonist, on sodium-dependent phosphate and glucose transport by the rat renal proximal tubular cell. Life Sci. 1994;55:839–845. doi: 10.1016/0024-3205(94)00567-2. [DOI] [PubMed] [Google Scholar]
- Cai H, Puschett DB, Guan S, Batuman V, Puschett JB. Phosphate transport inhibition by KW-3902, an adenosine A1 receptor antagonist, is mediated by cyclic adenosine monophosphate. Am J Kidney Dis. 1995;26:825–830. doi: 10.1016/0272-6386(95)90451-4. [DOI] [PubMed] [Google Scholar]
- Castrop H, Huang Y, Hashimoto S, Mizel D, Hansen P, Theilig F, Bachmann S, Deng C, Briggs J, Schnermann J. Impairment of tubuloglomerular feedback regulation of GFR in ecto-5′-nucleotidase/CD73-deficient mice. J Clin Invest. 2004;114:634–642. doi: 10.1172/JCI21851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill PC, Bidani A. Renal effects of selective adenosine receptor agonists in anesthetized rats. Am J Physiol. 1987;252:F299–F303. doi: 10.1152/ajprenal.1987.252.2.F299. [DOI] [PubMed] [Google Scholar]
- Churchill PC, Churchill MC. A1 and A2 adenosine receptor activation inhibits and stimulates renin secretion of rat renal cortical slices. J Pharmacol Exp Ther. 1985;232:589–594. [PubMed] [Google Scholar]
- Cohen MV, Downey JM. Adenosine: trigger and mediator of cardioprotection. Basic Res Cardiol. 2008;103:203–215. doi: 10.1007/s00395-007-0687-7. [DOI] [PubMed] [Google Scholar]
- Day YJ, Huang L, McDuffie MJ, Rosin DL, Ye H, Chen JF, Schwarzschild MA, Fink JS, Linden J, Okusa MD. Renal protection from ischemia mediated by A2A adenosine receptors on bone marrow-derived cells. J Clin Invest. 2003;112:883–891. doi: 10.1172/JCI15483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MS, Steinhausen M. Differential reactivity of cortical and juxtaglomerullary glomeruli to adenosine-1 and adenosine-2 receptor stimulation and angiotensin converting-enzyme inhibition. Microvasc Res. 1993;45:122–133. doi: 10.1006/mvre.1993.1012. [DOI] [PubMed] [Google Scholar]
- Dietrich MS, Endlich K, Parekh N, Steinhausen M. Interaction between adenosine and angiotensin II in renal microcirculation. Microvasc Res. 1991;41:275–288. doi: 10.1016/0026-2862(91)90028-a. [DOI] [PubMed] [Google Scholar]
- Dinour D, Brezis M. Effects of adenosine on intrarenal oxygenation. Am J Physiol. 1991;261:F787–F791. doi: 10.1152/ajprenal.1991.261.5.F787. [DOI] [PubMed] [Google Scholar]
- Di Sole F, Cerull R, Petzke S, Casavola V, Burckhardt G, Helmle-Kolb C. Bimodal acute effects of A1 adenosine receptor activation on Na+/H+ exchanger 3 in opossum kidney cells. J Am Soc Nephrol. 2003;14:1720–1730. doi: 10.1097/01.asn.0000072743.97583.db. [DOI] [PubMed] [Google Scholar]
- Dittrich HC, Gupta DK, Hack TC, Dowling T, Callahan J, Thomson S. The effect of KW-3902, an adenosine A1 receptor antagonist, on renal function and renal plasma flow in ambulatory patients with heart failure and renal impairment. J Card Fail. 2007;13:609–617. doi: 10.1016/j.cardfail.2007.08.006. [DOI] [PubMed] [Google Scholar]
- Dries DL, Exner DV, Domanski MJ, Greenberg B, Stevenson LW. The prognostic implications of renal insufficiency in asymptomatic and symptomatic patients with left ventricular systolic dysfunction. J Am Coll Cardiol. 2000;35:681–689. doi: 10.1016/s0735-1097(99)00608-7. [DOI] [PubMed] [Google Scholar]
- Edlund A, Sollevi A. Renal effects of i.v. adenosine infusion in humans. Clin Physiol. 1993;13:361–371. doi: 10.1111/j.1475-097x.1993.tb00336.x. [DOI] [PubMed] [Google Scholar]
- Edlund A, Ohlsen H, Sollevi A. Renal effects of local infusion of adenosine in man. Clin Sci Colch. 1994;87:143–149. doi: 10.1042/cs0870143. [DOI] [PubMed] [Google Scholar]
- Erley CM, Duda SH, Schlepckow S, Koehler J, Huppert PE, Strohmaier WL, Bohle A, Risler T, Osswald H. Adenosine antagonist theophylline prevents the reduction of glomerular filtration rate after contrast media application. Kidney Int. 1994;45:1425–1431. doi: 10.1038/ki.1994.186. [DOI] [PubMed] [Google Scholar]
- Erley CM, Heyne N, Burgert K, Langanke J, Risler T, Osswald H. Prevention of radiocontrast-induced nephropathy by adenosine antagonists in rats with chronic nitric oxide deficiency. J Am Soc Nephrol. 1997;8:1125–1132. doi: 10.1681/ASN.V871125. [DOI] [PubMed] [Google Scholar]
- Erley CM, Duda SH, Rehfuss D, Scholtes B, Bock J, Muller C, Osswald H, Risler T. Prevention of radiocontrast-media-induced nephropathy in patients with pre-existing renal insufficiency by hydration in combination with the adenosine antagonist theophylline. Nephrol Dial Transplant. 1999;14:1146–1149. doi: 10.1093/ndt/14.5.1146. [DOI] [PubMed] [Google Scholar]
- Ezeamuzie CI. Involvement of A(3) receptors in the potentiation by adenosine of the inhibitory effect of theophylline on human eosinophil degranulation: possible novel mechanism of the antiinflammatory action of theophylline. Biochem Pharmacol. 2001;61:1551–1559. doi: 10.1016/s0006-2952(01)00613-x. [DOI] [PubMed] [Google Scholar]
- Forman DE, Butler J, Wang Y, Abraham WT, O’Connor CM, Gottlieb SS, Loh E, Massie BM, Rich MW, Stevenson LW, Young JB, Krumholz HM. Incidence, predictors at admission, and impact of worsening renal function among patients hospitalized with heart failure. J Am Coll Cardiol. 2004;43:61–67. doi: 10.1016/j.jacc.2003.07.031. [DOI] [PubMed] [Google Scholar]
- Franco M, Bell PD, Navar LG. Effect of adenosine A1 analogue on tubuloglomerular feedback mechanism. Am J Physiol. 1989;257:F231–F236. doi: 10.1152/ajprenal.1989.257.2.F231. [DOI] [PubMed] [Google Scholar]
- Funaya H, Kitakaze M, Node K, Minamino T, Komamura K, Hori M. Plasma adenosine levels increase in patients with chronic heart failure. Circulation. 1997;95:1363–1365. doi: 10.1161/01.cir.95.6.1363. [DOI] [PubMed] [Google Scholar]
- Gabriels G, Endlich K, Rahn KH, Schlatter E, Steinhausen M. In vivo effects of diadenosine polyphosphates on rat renal microcirculation. Kidney Int. 2000;57:2476–2484. doi: 10.1046/j.1523-1755.2000.00106.x. [DOI] [PubMed] [Google Scholar]
- Garcia GE, Truong LD, Li P, Zhang P, Du J, Chen JF, Feng L. Adenosine A2A receptor activation and macrophage-mediated experimental glomerulonephritis. FASEB J. 2008;22:445–454. doi: 10.1096/fj.07-8430com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerkens JF, Heidemann HT, Jackson EK, Branch RA. Effect of aminophylline on amphotericin B nephrotoxicity in the dog. J Pharmacol Exp Ther. 1983;224:609–613. [PubMed] [Google Scholar]
- Givertz MM, Massie BM, Fields TK, Pearson LL, Dittrich HC. The effects of KW-3902, an adenosine A1-receptor antagonist on diuresis and renal function in patients with acute decompensated heart failure and renal impairment or diuretic resistance. J Am Coll Cardiol. 2007;50:1551–1560. doi: 10.1016/j.jacc.2007.07.019. [DOI] [PubMed] [Google Scholar]
- Gottlieb SS, Skettino SL, Wolff A, Beckman E, Fisher ML, Freudenberger R, Gladwell T, Marshall J, Cines M, Bennett D, Liittschwager EB. Effects of BG9719 (CVT-124), an A1-adenosine receptor antagonist, and furosemide on glomerular filtration rate and natriuresis in patients with congestive heart failure. J Am Coll Cardiol. 2000;35:56–59. doi: 10.1016/s0735-1097(99)00532-x. [DOI] [PubMed] [Google Scholar]
- Gottlieb SS, Brater DC, Thomas I, Havranek E, Bourge R, Goldman S, Dyer F, Gomez M, Bennett D, Ticho B, Beckman E, Abraham WT. BG9719 (CVT-124), an A1 adenosine receptor antagonist, protects against the decline in renal function observed with diuretic therapy. Circulation. 2002;105:1348–1353. doi: 10.1161/hc1102.105264. [DOI] [PubMed] [Google Scholar]
- Gouyon JB, Guignard JP. Theophylline prevents the hypoxemia-induced renal hemodynamic changes in rabbits. Kidney Int. 1988;33:1078–1083. doi: 10.1038/ki.1988.114. [DOI] [PubMed] [Google Scholar]
- Greenberg B, Thomas I, Banish D, Goldman S, Havranek E, Massie BM, Zhu Y, Ticho B, Abraham WT. Effects of multiple oral doses of an A1 adenosine antagonist, BG9928, in patients with heart failure: results of a placebo-controlled, dose-escalation study. J Am Coll Cardiol. 2007;50:600–606. doi: 10.1016/j.jacc.2007.03.059. [DOI] [PubMed] [Google Scholar]
- Grenz A, Zhang H, Eckle T, Mittelbronn M, Wehrmann M, Kohle C, Kloor D, Thompson LF, Osswald H, Eltzschig HK. Protective role of ecto-5′-nucleotidase (CD73) in renal ischemia. J Am Soc Nephrol. 2007a;18:833–845. doi: 10.1681/ASN.2006101141. [DOI] [PubMed] [Google Scholar]
- Grenz A, Zhang H, Hermes M, Eckle T, Klingel K, Huang DY, Muller CE, Robson SC, Osswald H, Eltzschig HK. Contribution of E-NTPDase1 (CD39) to renal protection from ischemia-reperfusion injury. FASEB J. 2007b;21:2863–2873. doi: 10.1096/fj.06-7947com. [DOI] [PubMed] [Google Scholar]
- Grenz A, Osswald H, Eckle T, Yang D, Zhang H, Tran ZV, Klingel K, Ravid K, Eltzschig HK. The reno-vascular A2B adenosine receptor protects the kidney from ischemia. PLoS Med. 2008;5:e137. doi: 10.1371/journal.pmed.0050137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gueler F, Gwinner W, Schwarz A, Haller H. Long-term effects of acute ischemia and reperfusion injury. Kidney Int. 2004;66:523–527. doi: 10.1111/j.1523-1755.2004.761_11.x. [DOI] [PubMed] [Google Scholar]
- Haas JA, Osswald H. Adenosine induced fall in glomerular capillary pressure. Effect of ureteral obstruction and aortic constriction in the Munich–Wistar rat kidney. Naunyn–Schmiedeberg’s Arch Pharmacol. 1981;317:86–89. doi: 10.1007/BF00506263. [DOI] [PubMed] [Google Scholar]
- Hansen PB, Hashimoto S, Briggs J, Schnermann J. Attenuated renovascular constrictor responses to angiotensin II in adenosine 1 receptor knockout mice. Am J Physiol Regul Integr Comp Physiol. 2003a;285:R44–R49. doi: 10.1152/ajpregu.00739.2002. [DOI] [PubMed] [Google Scholar]
- Hansen PB, Castrop H, Briggs J, Schnermann J. Adenosine induces vasoconstriction through Gi-dependent activation of phospholipase C in isolated perfused afferent arterioles of mice. J Am Soc Nephrol. 2003b;14:2457–2465. doi: 10.1097/01.asn.0000086474.80845.25. [DOI] [PubMed] [Google Scholar]
- Hansen PB, Hashimoto S, Oppermann M, Huang Y, Briggs JP, Schnermann J. Vasoconstrictor and vasodilator effects of adenosine in the mouse kidney due to preferential activation of A1 or A2 adenosine receptors. J Pharmacol Exp Ther. 2005;315:1150–1157. doi: 10.1124/jpet.105.091017. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Huang Y, Briggs J, Schnermann J. Reduced autoregulatory effectiveness in adenosine 1 receptor-deficient mice. Am J Physiol Renal Physiol. 2006;290:F888–F891. doi: 10.1152/ajprenal.00381.2005. [DOI] [PubMed] [Google Scholar]
- Heidemann HT, Gerkens JF, Jackson EK, Branch RA. Effect of aminophylline on renal vaso-constriction produced by amphotericin B in the rat. Naunyn–Schmiedeberg’s Arch Pharmacol. 1983;324:148–152. doi: 10.1007/BF00497021. [DOI] [PubMed] [Google Scholar]
- Heidemann HT, Muller S, Mertins L, Stepan G, Hoffmann K, Ohnhaus EE. Effect of aminophylline on cisplatin nephrotoxicity in the rat. Br J Pharmacol. 1989;97:313–318. doi: 10.1111/j.1476-5381.1989.tb11956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmlinger J. Das experimentell erzeugte akute ischämische Nierenversagen bei der Ratte. University of Aachen; Germany: 1979. Dissertation. [Google Scholar]
- Hillege HL, Girbes AR, de Kam PJ, Boomsma F, de Zeeuw D, Charlesworth A, Hampton JR, van Veldhuisen DJ. Renal function, neurohormonal activation, and survival in patients with chronic heart failure. Circulation. 2000;102:203–210. doi: 10.1161/01.cir.102.2.203. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Hartog A, Willems PH, Bindels RJ. Adenosine-stimulated Ca2+ reabsorption is mediated by apical A1 receptors in rabbit cortical collecting system. Am J Physiol. 1998;274:F736–F743. doi: 10.1152/ajprenal.1998.274.4.F736. [DOI] [PubMed] [Google Scholar]
- Hoenderop JGJ, De Pont JJHH, Bindels RJM, Willems PHGM. Hormone-stimulated Ca2+ reabsorption in rabbit kidney cortical collecting system is cAMP-independent and involves a phorbol ester-insensitive PKC isotype. Kidney Int. 1999;55:225–233. doi: 10.1046/j.1523-1755.1999.00228.x. [DOI] [PubMed] [Google Scholar]
- Holz FG, Steinhausen M. Renovascular effects of adenosine receptor agonists. Renal Physiol. 1987;10:272–282. doi: 10.1159/000173135. [DOI] [PubMed] [Google Scholar]
- Huang DY, Vallon V, Zimmermann H, Koszalka P, Schrader J, Osswald H. Ecto-5′-nucleotidase (cd73)-dependent and -independent generation of adenosine participates in the mediation of tubuloglomerular feedback in vivo. Am J Physiol Renal Physiol. 2006;291:F282–F288. doi: 10.1152/ajprenal.00113.2005. [DOI] [PubMed] [Google Scholar]
- Huber W, Ilgmann K, Page M, Hennig M, Schweigart U, Jeschke B, Lutilsky L, Weiss W, Salmhofer H, Classen M. Effect of theophylline on contrast material-nephropathy in patients with chronic renal insufficiency: controlled, randomized, double-blinded study. Radiology. 2002;223:772–779. doi: 10.1148/radiol.2233010609. [DOI] [PubMed] [Google Scholar]
- Huber W, Schipek C, Ilgmann K, Page M, Hennig M, Wacker A, Schweigart U, Lutilsky L, Valina C, Seyfarth M, Schomig A, Classen M. Effectiveness of theophylline prophylaxis of renal impairment after coronary angiography in patients with chronic renal insufficiency. Am J Cardiol. 2003;91:1157–1162. doi: 10.1016/s0002-9149(03)00259-5. [DOI] [PubMed] [Google Scholar]
- Iijima K, Moore LC, Goligorsky MS. Syncytial organization of cultured rat mesangial cells. Am J Physiol. 1991;260:F848–F855. doi: 10.1152/ajprenal.1991.260.6.F848. [DOI] [PubMed] [Google Scholar]
- Inscho EW. Purinoceptor-mediated regulation of the renal microvasculature. J Auton Pharmacol. 1996;16:385–388. doi: 10.1111/j.1474-8673.1996.tb00059.x. [DOI] [PubMed] [Google Scholar]
- Inscho EW, Carmines PK, Navar LG. Juxtamedullary afferent arteriolar responses to P1 and P2 purinergic stimulation. Hypertension. 1991;17:1033–1037. doi: 10.1161/01.hyp.17.6.1033. [DOI] [PubMed] [Google Scholar]
- Ishikawa I, Shikura N, Takada K. Amelioration of glycerol-induced acute renal failure in rats by an adenosine A1 receptor antagonist (FR-113453) Renal Fail. 1993;15:1–5. doi: 10.3109/08860229309065565. [DOI] [PubMed] [Google Scholar]
- Itoh S, Carretero OA, Murray RD. Possible role of adenosine in the macula densa mechanism of renin release in rabbits. J Clin Invest. 1985;76:1412–1417. doi: 10.1172/JCI112118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson EK, Raghvendra DK. The extracellular cyclic AMP-adenosine pathway in renal physiology. Annu Rev Physiol. 2004;66:571–599. doi: 10.1146/annurev.physiol.66.032102.111604. [DOI] [PubMed] [Google Scholar]
- Jackson EK, Kost CK, Jr, Herzer WA, Smits GJ, Tofovic SP. A(1) receptor blockade induces natriuresis with a favorable renal hemodynamic profile in SHHF/Mcc-fa(cp) rats chronically treated with salt and furosemide. J Pharmacol Exp Ther. 2001;299:978–987. [PubMed] [Google Scholar]
- Jackson EK, Mi Z, Zhu C, Dubey RK. Adenosine biosynthesis in the collecting duct. J Pharmacol Exp Ther. 2003;307:888–896. doi: 10.1124/jpet.103.057166. [DOI] [PubMed] [Google Scholar]
- Joo JD, Kim M, Horst P, Kim J, D’Agati VD, Emala CW, Sr, Lee HT. Acute and delayed renal protection against renal ischemia and reperfusion injury with A1 adenosine receptors. Am J Physiol Renal Physiol. 2007;293:F1847–F1857. doi: 10.1152/ajprenal.00336.2007. [DOI] [PubMed] [Google Scholar]
- Kang HS, Kerstan D, Dai LJ, Ritchie G, Quamme GA. Adenosine modulates Mg(2+) uptake in distal convoluted tubule cells via A(1) and A(2) purinoceptors. Am J Physiol Renal Physiol. 2001;281:F1141–F1147. doi: 10.1152/ajprenal.2001.281.6.F1141. [DOI] [PubMed] [Google Scholar]
- Kapoor A, Kumar S, Gulati S, Gambhir S, Sethi RS, Sinha N. The role of theophylline in contrast-induced nephropathy: a case–control study. Nephrol Dial Transplant. 2002;17:1936–1941. doi: 10.1093/ndt/17.11.1936. [DOI] [PubMed] [Google Scholar]
- Katholi RE, Taylor GJ, McCann WP, Woods WT, Jr, Womack KA, McCoy CD, Katholi CR, Moses HW, Mishkel GJ, Lucore CL, et al. Nephrotoxicity from contrast media: attenuation with theophylline. Radiology. 1995;195:17–22. doi: 10.1148/radiology.195.1.7892462. [DOI] [PubMed] [Google Scholar]
- Kawabata M, Ogawa T, Takabatake T. Control of rat glomerular microcirculation by juxtaglomerular adenosine A1 receptors. Kidney Int. 1998;67(Suppl):S228–S230. doi: 10.1046/j.1523-1755.1998.06757.x. [DOI] [PubMed] [Google Scholar]
- Kellett R, Bowmer CJ, Collis MG, Yates MS. Amelioration of glycerol-induced acute renal failure in the rat with 8-cyclopentyl-1,3-dipropylxanthine. Br J Pharmacol. 1989;98:1066–1074. doi: 10.1111/j.1476-5381.1989.tb14639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SM, Mizel D, Huang YG, Briggs JP, Schnermann J. Adenosine as a mediator of macula densa-dependent inhibition of renin secretion. Am J Physiol Renal Physiol. 2006;290:F1016–F1023. doi: 10.1152/ajprenal.00367.2005. [DOI] [PubMed] [Google Scholar]
- Knight RJ, Collis MG, Yates MS, Bowmer CJ. Amelioration of cisplatin-induced acute renal failure with 8-cyclopentyl-1,3-dipropylxanthine. Br J Pharmacol. 1991;104:1062–1068. doi: 10.1111/j.1476-5381.1991.tb12550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RJ, Bowmer CJ, Yates MS. Effect of the selective A1 adenosine antagonist 8-cyclopentyl-1,3-dipropylxanthine on acute renal dysfunction induced by Escherichia coli endotoxin in rats. J Pharm Pharmacol. 1993a;45:979–984. doi: 10.1111/j.2042-7158.1993.tb05640.x. [DOI] [PubMed] [Google Scholar]
- Knight RJ, Bowmer CJ, Yates MS. The diuretic action of 8-cyclopentyl-1,3-dipropylxanthine, a selective A1 adenosine receptor antagonist. Br J Pharmacol. 1993b;109(1):271–277. doi: 10.1111/j.1476-5381.1993.tb13564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolonko A, Wiecek A, Kokot F. The nonselective adenosine antagonist theophylline does prevent renal dysfunction induced by radiographic contrast agents. J Nephrol. 1998;11:151–156. [PubMed] [Google Scholar]
- Komlosi P, Peti-Peterdi J, Fuson AL, Fintha A, Rosivall L, Bell PD. Macula densa basolateral ATP release is regulated by luminal [NaCl] and dietary salt intake. Am J Physiol Renal Physiol. 2004;286:F1054–F1058. doi: 10.1152/ajprenal.00336.2003. [DOI] [PubMed] [Google Scholar]
- Kost CK, Jr, Herzer WA, Rominski BR, Mi Z, Jackson EK. Diuretic response to adenosine A(1) receptor blockade in normotensive and spontaneously hypertensive rats: role of pertussis toxin-sensitive G-proteins. J Pharmacol Exp Ther. 2000;292:752–760. [PubMed] [Google Scholar]
- Lai EY, Martinka P, Fahling M, Mrowka R, Steege A, Gericke A, Sendeski M, Persson PB, Persson AE, Patzak A. Adenosine restores angiotensin II-induced contractions by receptor-independent enhancement of calcium sensitivity in renal arterioles. Circ Res. 2006;99:1117–1124. doi: 10.1161/01.RES.0000249530.85542.d4. [DOI] [PubMed] [Google Scholar]
- Lee HT, Emala CW. Protective effects of renal ischemic preconditioning and adenosine pretreatment: role of A(1) and A(3) receptors. Am J Physiol. 2000;278:F380–F387. doi: 10.1152/ajprenal.2000.278.3.F380. [DOI] [PubMed] [Google Scholar]
- Lee HT, Emala CW. Protein kinase C and G(i/o) proteins are involved in adenosine- and ischemic preconditioning-mediated renal protection. J Am Soc Nephrol. 2001a;12:233–240. doi: 10.1681/ASN.V122233. [DOI] [PubMed] [Google Scholar]
- Lee HT, Emala CW. Systemic adenosine given after ischemia protects renal function via A(2a) adenosine receptor activation. Am J Kidney Dis. 2001b;38:610–618. doi: 10.1053/ajkd.2001.26888. [DOI] [PubMed] [Google Scholar]
- Lee HT, Ota-Setlik A, Xu H, D’Agati VD, Jacobson MA, Emala CW. A3 adenosine receptor knockout mice are protected against ischemia- and myoglobinuria-induced renal failure. Am J Physiol Renal Physiol. 2003;284:F267–F273. doi: 10.1152/ajprenal.00271.2002. [DOI] [PubMed] [Google Scholar]
- Lee HT, Gallos G, Nasr SH, Emala CW. A1 adenosine receptor activation inhibits inflammation, necrosis, and apoptosis after renal ischemia-reperfusion injury in mice. J Am Soc Nephrol. 2004a;15:102–111. doi: 10.1097/01.asn.0000102474.68613.ae. [DOI] [PubMed] [Google Scholar]
- Lee HT, Xu H, Nasr SH, Schnermann J, Emala CW. A1 adenosine receptor knockout mice exhibit increased renal injury following ischemia and reperfusion. Am J Physiol Renal Physiol. 2004b;286:F298–F306. doi: 10.1152/ajprenal.00185.2003. [DOI] [PubMed] [Google Scholar]
- Lee HT, Jan M, Bae SC, Joo JD, Goubaeva FR, Yang J, Kim M. A1 adenosine receptor knock-out mice are protected against acute radiocontrast nephropathy in vivo. Am J Physiol Renal Physiol. 2006;290:F1367–F1375. doi: 10.1152/ajprenal.00347.2005. [DOI] [PubMed] [Google Scholar]
- Lee HT, Kim M, Jan M, Penn RB, Emala CW. Renal tubule necrosis and apoptosis modulation by A1 adenosine receptor expression. Kidney Int. 2007;71:1249–1261. doi: 10.1038/sj.ki.5002227. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Churchill PC, Bidani AK. Effect of theophylline on the initiation phase of postischemic acute renal failure in rats. J Lab Clin Med. 1986;108:150–154. [PubMed] [Google Scholar]
- Lin JJ, Churchill PC, Bidani AK. The effect of dipyridamole on the initiation phase of postischemic acute renal failure in rats. Can J Physiol Pharmacol. 1987;65:1491–1495. doi: 10.1139/y87-233. [DOI] [PubMed] [Google Scholar]
- Lin JJ, Churchill PC, Bidani AK. Theophylline in rats during maintenance phase of postischemic acute renal failure. Kidney Int. 1988;33:24–28. doi: 10.1038/ki.1988.4. [DOI] [PubMed] [Google Scholar]
- Lorenz JN, Weihprecht H, He XR, Skott O, Briggs JP, Schnermann J. Effects of adenosine and angiotensin on macula densa-stimulated renin secretion. Am J Physiol. 1993;265:F187–F194. doi: 10.1152/ajprenal.1993.265.2.F187. [DOI] [PubMed] [Google Scholar]
- Lorenz JN, Dostanic-Larson I, Shull GE, Lingrel JB. Ouabain inhibits tubuloglomerular feedback in mutant mice with ouabain-sensitive alpha1 Na, K-ATPase. J Am Soc Nephrol. 2006;17:2457–2463. doi: 10.1681/ASN.2006040379. [DOI] [PubMed] [Google Scholar]
- Lucas DG, Jr, Hendrick JW, Sample JA, Mukherjee R, Escobar GP, Smits GJ, Crawford FA, Jr, Spinale FG. Cardiorenal effects of adenosine subtype 1 (A1) receptor inhibition in an experimental model of heart failure. J Am Coll Surg. 2002;194:603–609. doi: 10.1016/s1072-7515(02)01136-5. [DOI] [PubMed] [Google Scholar]
- Macias-Nunez JF, Fiksen-Olsen MJ, Romero JC, Knox FG. Intrarenal blood flow distribution during adenosine-mediated vasoconstriction. Am J Physiol. 1983;244:H138–H141. doi: 10.1152/ajpheart.1983.244.1.H138. [DOI] [PubMed] [Google Scholar]
- Macias-Nunez JF, Garcia Iglesias C, Santos JC, Sanz E, Lopez-Novoa JM. Influence of plasma renin content, intrarenal angiotensin II, captopril, and calcium channel blockers on the vasoconstriction and renin release promoted by adenosine in the kidney. J Lab Clin Med. 1985;106:562–567. [PubMed] [Google Scholar]
- Mahon NG, Blackstone EH, Francis GS, Starling RC, III, Young JB, Lauer MS. The prognostic value of estimated creatinine clearance alongside functional capacity in ambulatory patients with chronic congestive heart failure. J Am Coll Cardiol. 2002;40:1106–1113. doi: 10.1016/s0735-1097(02)02125-3. [DOI] [PubMed] [Google Scholar]
- Miracle CM, Rieg T, Blantz RC, Vallon V, Thomson SC. Combined effects of carbonic anhydrase inhibitor and adenosine A1 receptor antagonist on hemodynamic and tubular function in the kidney. Kidney Blood Press Res. 2007;30:388–399. doi: 10.1159/000108625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto M, Yagil Y, Larson T, Robertson C, Jamison RL. Effects of intrarenal adenosine on renal function and medullary blood flow in the rat. Am J Physiol. 1988;255:F1230–F1234. doi: 10.1152/ajprenal.1988.255.6.F1230. [DOI] [PubMed] [Google Scholar]
- Mizumoto H, Karasawa A. Renal tubular site of action of KW-3902, a novel adenosine A-1-receptor antagonist, in anesthetized rats. Jpn J Pharmacol. 1993;31(3):251–253. doi: 10.1254/jjp.61.251. [DOI] [PubMed] [Google Scholar]
- Modlinger PS, Welch WJ. Adenosine A1 receptor antagonists and the kidney. Curr Opin Nephrol Hypertens. 2003;12:497–502. doi: 10.1097/00041552-200309000-00003. [DOI] [PubMed] [Google Scholar]
- Moyer BD, McCoy DE, Lee B, Kizer N, Stanton BA. Adenosine inhibits arginine vasopressin-stimulated chloride secretion in a mouse IMCD cell line (mIMCD-K2) Am J Physiol. 1995;269:F884–F891. doi: 10.1152/ajprenal.1995.269.6.F884. [DOI] [PubMed] [Google Scholar]
- Nagashima K, Kusaka H, Karasawa A. Protective effects of KW-3902, an adenosine A1-receptor antagonist, against cisplatin-induced acute renal failure in rats. Jpn J Pharmacol. 1995;67:349–357. doi: 10.1254/jjp.67.349. [DOI] [PubMed] [Google Scholar]
- Newman WH, Grossman SJ, Frankis MB, Webb JG. Increased myocardial adenosine release in heart failure. J Mol Cell Cardiol. 1984;16:577–580. doi: 10.1016/s0022-2828(84)80645-8. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Miyatake A, Aki Y, Fukui T, Rahman M, Kimura S, Abe Y. Adenosine A(1) receptor antagonist KW-3902 prevents hypoxia-induced renal vasoconstriction. J Pharmacol Exp Ther. 1999;291:988–993. [PubMed] [Google Scholar]
- Nishiyama A, Inscho EW, Navar LG. Interactions of adenosine A1 and A2a receptors on renal microvascular reactivity. Am J Physiol Renal Physiol. 2001;280:F406–F414. doi: 10.1152/ajprenal.2001.280.3.F406. [DOI] [PubMed] [Google Scholar]
- Okusa MD, Linden J, Macdonald T, Huang L. Selective A2A adenosine receptor activation reduces ischemia-reperfusion injury in rat kidney. Am J Physiol. 1999;277:F404–F412. doi: 10.1152/ajprenal.1999.277.3.F404. [DOI] [PubMed] [Google Scholar]
- Olivera A, Lamas S, Rodriguez-Puyol D, Lopez-Novoa JM. Adenosine induces mesangial cell contraction by an A1-type receptor. Kidney Int. 1989;35:1300–1305. doi: 10.1038/ki.1989.126. [DOI] [PubMed] [Google Scholar]
- Oppermann M, Friedman DJ, Faulhaber-Walter R, Mizel D, Castrop H, Enjyoji K, Robson SC, Schnermann JB. Tubuloglomerular feedback and renin secretion in NTPDase1/CD39-deficient mice. Am J Physiol Renal Physiol. 2008;294:F965–F970. doi: 10.1152/ajprenal.00603.2007. [DOI] [PubMed] [Google Scholar]
- Osswald H, Vallon V. Adenosine and tubuloglomerular feedback in the pathophysiology of acute renal failure. In: Ronco C, Bellomo R, Kellum JA, editors. Critical care nephrology. 2nd 2008. [Google Scholar]
- Osswald H, Schmitz HJ, Heidenreich O. Adenosine response of the rat kidney after saline loading, sodium restriction and hemorrhagia. Pflügers Arch. 1975;357:323–333. doi: 10.1007/BF00585986. [DOI] [PubMed] [Google Scholar]
- Osswald H, Schmitz HJ, Kemper R. Renal action of adenosine: effect on renin secretion in the rat. Naunyn–Schmiedeberg’s Arch Pharmacol. 1978a;303:95–99. doi: 10.1007/BF00496190. [DOI] [PubMed] [Google Scholar]
- Osswald H, Spielman WS, Knox FG. Mechanism of adenosine-mediated decreases in glomerular filtration rate in dogs. Circ Res. 1978b;43:465–469. doi: 10.1161/01.res.43.3.465. [DOI] [PubMed] [Google Scholar]
- Osswald H, Helmlinger J, Jendralski A, Abrar B. Improvement of renal function by theophylline in acute renal failure of the rat. Naunyn–Schmiedebergs Arch Pharmacol. 1979;307(Suppl):R 47. [Google Scholar]
- Osswald H, Nabakowski G, Hermes H. Adenosine as a possible mediator of metabolic control of glomerular filtration rate. Int J Biochem. 1980;12:263–267. doi: 10.1016/0020-711x(80)90082-8. [DOI] [PubMed] [Google Scholar]
- Osswald H, Hermes H, Nabakowski G. Role of adenosine in signal transmission of tubuloglomerular feedback. Kidney Int. 1982;22(Suppl 12):S136–S142. [PubMed] [Google Scholar]
- Panjehshahin MR, Munsey TS, Collis MG, Bowmer CJ, Yates MS. Further characterization of the protective effect of 8-cyclopentyl-1,3-dipropylxanthine on glycerol-induced acute renal failure in the rat. J Pharm Pharmacol. 1992;44:109–113. doi: 10.1111/j.2042-7158.1992.tb03572.x. [DOI] [PubMed] [Google Scholar]
- Patzak A, Lai EY, Fahling M, Sendeski M, Martinka P, Persson PB, Persson AE. Adenosine enhances long term the contractile response to angiotensin II in afferent arterioles. Am J Physiol Regul Integr Comp Physiol. 2007;293:R2232–R2242. doi: 10.1152/ajpregu.00357.2007. [DOI] [PubMed] [Google Scholar]
- Pflueger AC, Gross JM, Knox FG. Adenosine-induced renal vasoconstriction in diabetes mellitus rats: role of prostaglandins. Am J Physiol. 1999a;277:R1410–R1417. doi: 10.1152/ajpregu.1999.277.5.R1410. [DOI] [PubMed] [Google Scholar]
- Pflueger AC, Osswald H, Knox FG. Adenosine-induced renal vasoconstriction in diabetes mellitus rats: role of nitric oxide. Am J Physiol. 1999b;276:F340–F346. doi: 10.1152/ajprenal.1999.276.3.F340. [DOI] [PubMed] [Google Scholar]
- Ren Y, Arima S, Carretero OA, Ito S. Possible role of adenosine in macula densa control of glomerular hemodynamics. Kidney Int. 2002a;61:169–176. doi: 10.1046/j.1523-1755.2002.00093.x. [DOI] [PubMed] [Google Scholar]
- Ren Y, Carretero OA, Garvin JL. Role of mesangial cells and gap junctions in tubuloglomerular feedback. Kidney Int. 2002b;62:525–531. doi: 10.1046/j.1523-1755.2002.00454.x. [DOI] [PubMed] [Google Scholar]
- Ren Y, Garvin JL, Liu R, Carretero OA. Role of macula densa adenosine triphosphate (ATP) in tubuloglomerular feedback. Kidney Int. 2004;66:1479–1485. doi: 10.1111/j.1523-1755.2004.00911.x. [DOI] [PubMed] [Google Scholar]
- Rieg T, Steigele H, Schnermann J, Richter K, Osswald H, Vallon V. Requirement of intact adenosine A1 receptors for the diuretic and natriuretic action of the methylxanthines theophylline and caffeine. J Pharmacol Exp Ther. 2005;313:403–409. doi: 10.1124/jpet.104.080432. [DOI] [PubMed] [Google Scholar]
- Rieg T, Schnermann J, Vallon V. Adenosine A1 receptors determine effects of caffeine on total fluid intake but not caffeine appetite. Eur J Pharmacol. 2007;555:174–177. doi: 10.1016/j.ejphar.2006.10.039. [DOI] [PubMed] [Google Scholar]
- Rieg T, Pothula K, Schroth J, Satriano J, Osswald H, Schnermann J, Insel PA, Bundey RA, Vallon V. Vasopressin regulation of inner medullary collecting ducts and compensatory changes in mice lacking adenosine A1 receptors. Am J Physiol Renal Physiol. 2008;294:F638–F644. doi: 10.1152/ajprenal.00344.2007. [DOI] [PubMed] [Google Scholar]
- Schnermann J, Osswald H, Hermle M. Inhibitory effect of methylxanthines on feedback control of glomerular filtration rate in the rat kidney. Pflügers Arch. 1977;369:39–48. doi: 10.1007/BF00580808. [DOI] [PubMed] [Google Scholar]
- Schnermann J, Weihprecht H, Briggs JP. Inhibition of tubuloglomerular feedback during adenosine1 receptor blockade. Am J Physiol. 1990;258:F553–F561. doi: 10.1152/ajprenal.1990.258.3.F553. [DOI] [PubMed] [Google Scholar]
- Schweda F, Wagner C, Kramer BK, Schnermann J, Kurtz A. Preserved macula densa-dependent renin secretion in A1 adenosine receptor knockout mice. Am J Physiol Renal Physiol. 2003;284:F770–F777. doi: 10.1152/ajprenal.00280.2002. [DOI] [PubMed] [Google Scholar]
- Smith JA, Whitaker EM, Bowmer CJ, Yates MS. Differential expression of renal adenosine A(1) receptors induced by acute renal failure. Biochem Pharmacol. 2000;59:727–732. doi: 10.1016/s0006-2952(99)00369-x. [DOI] [PubMed] [Google Scholar]
- Smith JA, Sivaprasadarao A, Munsey TS, Bowmer CJ, Yates MS. Immunolocalisation of adenosine A(1) receptors in the rat kidney. Biochem Pharmacol. 2001;61:237–244. doi: 10.1016/s0006-2952(00)00532-3. [DOI] [PubMed] [Google Scholar]
- Spielman WS, Osswald H. Characterization of the postocclusive response of renal blood flow in the cat. Am J Physiol. 1978;235:F286–F290. doi: 10.1152/ajprenal.1978.235.4.F286. [DOI] [PubMed] [Google Scholar]
- Spielman WS, Osswald H. Blockade of postocclusive renal vasoconstriction by an angiotensin II antagonist: evidence for an angiotensin–adenosine interaction. Am J Physiol. 1979;237:F463–F467. doi: 10.1152/ajprenal.1979.237.6.F463. [DOI] [PubMed] [Google Scholar]
- Sun D, Samuelson LC, Yang T, Huang Y, Paliege A, Saunders T, Briggs J, Schnermann J. Mediation of tubuloglomerular feedback by adenosine: evidence from mice lacking adenosine 1 receptors. Proc Natl Acad Sci USA. 2001;98:9983–9988. doi: 10.1073/pnas.171317998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki F, Shimada J, Mizumoto H, Karasawa A, Kubo K, Nonaka H, Ishii A, Kawakita T. Adenosine A1 antagonists. 2. Structure–activity relationships on diuretic activities and protective effects against acute renal failure. J Med Chem. 1992;35:3066–3075. doi: 10.1021/jm00094a022. [DOI] [PubMed] [Google Scholar]
- Tagawa H, Vander AJ. Effects of adenosine compounds on renal function and renin secretion in dogs. Circ Res. 1970;26:327–338. doi: 10.1161/01.res.26.3.327. [DOI] [PubMed] [Google Scholar]
- Takeda M, Yoshitomi K, Imai M. Regulation of Na(+)–3HCO3–cotransport in rabbit proximal convoluted tubule via adenosine A1 receptor. Am J Physiol. 1993;265:F511–F519. doi: 10.1152/ajprenal.1993.265.4.F511. [DOI] [PubMed] [Google Scholar]
- Takenaka T, Inoue T, Kanno Y, Okada H, Hill CE, Suzuki H. Connexins 37 and 40 transduce purinergic signals mediating renal autoregulation. Am J Physiol Regul Integr Comp Physiol. 2008a;294:R1–R11. doi: 10.1152/ajpregu.00269.2007. [DOI] [PubMed] [Google Scholar]
- Takenaka T, Inoue T, Kanno Y, Okada H, Meaney KR, Hill CE, Suzuki H. Expression and role of connexins in the rat renal vasculature. Kidney Int. 2008b;73:415–422. doi: 10.1038/sj.ki.5002673. [DOI] [PubMed] [Google Scholar]
- Tang L, Parker M, Fei Q, Loutzenhiser R. Afferent arteriolar adenosine A2a receptors are coupled to KATP in in vitro perfused hydronephrotic rat kidney. Am J Physiol. 1999;277:F926–F933. doi: 10.1152/ajprenal.1999.277.6.F926. [DOI] [PubMed] [Google Scholar]
- Tang Y, Zhou L. Characterization of adenosine A1 receptors in human proximal tubule epithelial (HK-2) cells. Receptor Channel. 2003;9:67–75. [PubMed] [Google Scholar]
- Thomson S, Bao D, Deng A, Vallon V. Adenosine formed by 5′-nucleotidase mediates tubuloglomerular feedback. J Clin Invest. 2000;106:289–298. doi: 10.1172/JCI8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torikai S. Effect of phenylisopropyladenosine on vasopressin-dependent cyclic AMP generation in defined nephron segments from rat. Renal Physiol. 1987;10:33–39. doi: 10.1159/000173111. [DOI] [PubMed] [Google Scholar]
- Toya Y, Umemura S, Iwamoto T, Hirawa N, Kihara M, Takagi N, Ishii M. Identification and characterization of adenosine A1 receptor–cAMP system in human glomeruli. Kidney Int. 1993;43:928–932. doi: 10.1038/ki.1993.130. [DOI] [PubMed] [Google Scholar]
- Traynor T, Yang T, Huang YG, Arend L, Oliverio MI, Coffman T, Briggs JP, Schnermann J. Inhibition of adenosine-1 receptor-mediated preglomerular vasoconstriction in AT1A receptor-deficient mice. Am J Physiol. 1998;275:F922–F927. doi: 10.1152/ajprenal.1998.275.6.F922. [DOI] [PubMed] [Google Scholar]
- Vallon V. P2 receptors in the regulation of renal transport mechanisms. Am J Physiol Renal Physiol. 2008;294:F10–F27. doi: 10.1152/ajprenal.00432.2007. [DOI] [PubMed] [Google Scholar]
- Vallon V, Richter K, Huang DY, Rieg T, Schnermann J. Functional consequences at the single-nephron level of the lack of adenosine A1 receptors and tubuloglomerular feedback in mice. Pflugers Arch. 2004;448:214–221. doi: 10.1007/s00424-004-1239-8. [DOI] [PubMed] [Google Scholar]
- Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev. 2006;86:901–940. doi: 10.1152/physrev.00031.2005. [DOI] [PubMed] [Google Scholar]
- Vallon V, Miracle C, Thomson S. Adenosine and kidney function: potential implications in patients with heart failure. Eur J Heart Fail. 2008;10:176–187. doi: 10.1016/j.ejheart.2008.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van-Buren M, Bijlsma JA, Boer P, van-Rijn HJ, Koomans HA. Natriuretic and hypotensive effect of adenosine-1 blockade in essential hypertension. Hypertension. 1993;22:728–734. doi: 10.1161/01.hyp.22.5.728. [DOI] [PubMed] [Google Scholar]
- Wagner C, de Wit C, Kurtz L, Grunberger C, Kurtz A, Schweda F. Connexin40 is essential for the pressure control of renin synthesis and secretion. Circ Res. 2007;100:556–563. doi: 10.1161/01.RES.0000258856.19922.45. [DOI] [PubMed] [Google Scholar]
- Weaver DR, Reppert SM. Adenosine receptor gene expression in rat kidney. Am J Physiol. 1992;263:F991–F995. doi: 10.1152/ajprenal.1992.263.6.F991. [DOI] [PubMed] [Google Scholar]
- Weihprecht H, Lorenz JN, Schnermann J, Skott O, Briggs JP. Effect of adenosine1-receptor blockade on renin release from rabbit isolated perfused juxtaglomerular apparatus. J Clin Invest. 1990;85:1622–1628. doi: 10.1172/JCI114613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weihprecht H, Lorenz JN, Briggs JP, Schnermann J. Vasomotor effects of purinergic agonists in isolated rabbit afferent arterioles. Am J Physiol. 1992;263:F1026–F1033. doi: 10.1152/ajprenal.1992.263.6.F1026. [DOI] [PubMed] [Google Scholar]
- Weihprecht H, Lorenz JN, Briggs JP, Schnermann J. Synergistic effects of angiotensin and adenosine in the renal microvasculature. Am J Physiol. 1994;266:F227–F239. doi: 10.1152/ajprenal.1994.266.2.F227. [DOI] [PubMed] [Google Scholar]
- Welch WJ. Adenosine A1 receptor antagonists in the kidney: effects in fluid-retaining disorders. Curr Opin Pharmacol. 2002;2:165–170. doi: 10.1016/s1471-4892(02)00134-0. [DOI] [PubMed] [Google Scholar]
- Wilcox CS, Welch WJ, Schreiner GF, Belardinelli L. Natriuretic and diuretic actions of a highly selective adenosine A1 receptor antagonist. J Am Soc Nephrol. 1999;10:714–720. doi: 10.1681/ASN.V104714. [DOI] [PubMed] [Google Scholar]
- Yagil Y. Interaction of adenosine with vasopressin in the inner medullary collecting duct. Am J Physiol. 1990;259:F679–F687. doi: 10.1152/ajprenal.1990.259.4.F679. [DOI] [PubMed] [Google Scholar]
- Yagil C, Katni G, Yagil Y. The effects of adenosine on transepithelial resistance and sodium uptake in the inner medullary collecting duct. Pflügers Arch. 1994;427:225–232. doi: 10.1007/BF00374528. [DOI] [PubMed] [Google Scholar]
- Yao K, Kusaka H, Sato K, Karasawa A. Protective effects of KW-3902, a novel adenosine A1-receptor antagonist, against gentamicin-induced acute renal failure in rats. Jpn J Pharmacol. 1994;65:167–170. doi: 10.1254/jjp.65.167. [DOI] [PubMed] [Google Scholar]
- Yao K, Heyne N, Erley CM, Risler T, Osswald H. The selective adenosine A1 receptor antagonist KW-3902 prevents radiocontrast media-induced nephropathy in rats with chronic nitric oxide deficiency. Eur J Pharmacol. 2001;414:99–104. doi: 10.1016/s0014-2999(01)00764-6. [DOI] [PubMed] [Google Scholar]
- Yaoita H, Ito O, Arima S, Endo Y, Takeuchi K, Omata K, Ito S. Effect of adenosine on isolated afferent arterioles. Nippon Jinzo Gakkai Shi. 1999;41:697–703. [PubMed] [Google Scholar]
- Yates MS, Bowmer CJ, Kellett R, Collis MG. Effect of 8-phenyltheophylline, enprofylline and hydrochlorothiazide on glycerol-induced acute renal failure in the rat. J Pharm Pharmacol. 1987;39:803–808. doi: 10.1111/j.2042-7158.1987.tb05122.x. [DOI] [PubMed] [Google Scholar]
- Zou AP, Nithipatikom K, Li PL, Cowley AW., Jr Role of renal medullary adenosine in the control of blood flow and sodium excretion. Am J Physiol. 1999;45:R790–R798. doi: 10.1152/ajpregu.1999.276.3.R790. [DOI] [PubMed] [Google Scholar]


