Abstract
Low attentional control (AC) and high anxiety are closely linked. Researchers often presume that high anxiety reduces AC; however, the reverse causal possibility – that low AC increases anxiety – is equally plausible. We addressed this question in people with elevated trait anxiety by evaluating the temporal precedence of the AC-anxiety association. We tested whether autonomic arousal (electrodermal activity) and subjective anxiety elicited by an anxiety induction were associated more strongly with AC measured either pre-induction (N = 40) or post-induction (N = 38). Low AC was indexed by distractibility during a visual search task requiring attentional inhibition of emotionally neutral distractors. Higher distractibility predicted higher autonomic activation but not higher increases in self-reported anxiety. Critically, this AC-anxiety association occurred for pre-induction but not post-induction AC. The results suggest that low AC may heighten subsequent anxious arousal. By implication, treatment interventions should specifically enhance AC to alleviate anxiety.
Keywords: Anxiety, Attentional control
1. Introduction
People who suffer from anxiety show signs of low attentional control (AC; Ansari & Derakshan, 2011; Bishop, 2009; Pacheco-Unguetti, Acosta, Callejas, & Lupiáñez, 2010; Qi, Ding, & Li, 2013). This impaired ability to control attention in a goal-directed way manifests as difficulty resisting interference from irrelevant information. The association between anxiety and attentional control is evident for transient anxious moods (i.e., state anxiety) (Richey, Keough, & Schmidt, 2012; Spada, Georgiou, & Wells, 2010) and for enduring anxious dispositions (i.e., trait anxiety) (Derryberry & Reed, 2002; Healy & Kulig, 2006; Judah, Grant, Mills, & Lechner, 2013; Moriya & Tanno, 2008).
A prominent view regarding the AC-anxiety association is that anxiety causes AC deficits, as codified in Attentional Control Theory (ACT; Eysenck, Derakshan, Santos, & Calvo, 2007; Eysenck & Derakshan, 2011). Evidence for the idea that high anxiety causes low AC emerges from studies in which anxiety was manipulated to determine its causal effects. For example, state anxiety induced by a verbal arithmetic task decreased self-reported AC (Putman, Verkuil, Arias-Garcia, Pantazi, & van Schie, 2013). Evidence suggests that a similar state anxiety induction creates a behavioral AC deficit (i.e., distractibility measured by response slowing associated with distractor stimuli) more in high- than low-trait-anxious people (Keogh & French, 1997).
However, despite the evidence above, the inferences to be drawn about the AC-anxiety association remain unclear for three reasons. First, experimental manipulations of anxiety do not always impair AC (Hoskin, Hunter, & Woodruff, 2014; Hu, Bauer, Padmala, & Pessoa, 2012; Robinson, Krimsky, and Grillon, 2013). Nevertheless, a substantial body of research shows that anxiety instilled via threat of shock does indeed impair AC. For example, Choi, Padmala, and Pessoa (2012) showed that threat of shock increased interference on a response-conflict task and that higher state anxiety was correlated with greater interference. Cornwell, Mueller, Kaplan, Grillon, and Ernst (2012) showed that threat of shock slowed response inhibition involving visual attention in an antisaccade task. Thus, although findings are mixed about the effects of manipulated anxiety on AC, the threat-of-shock literature in particular does nevertheless show some clear causal evidence that anxiety impairs AC.
Second, in a substantial subset of studies that show an AC-anxiety association, anxiety was not experimentally manipulated. This is often the case in studies examining AC in people with high versus low trait anxiety (e.g., Moser, Becker, & Moran, 2012) or social anxiety (Wieser, Pauli, & Muhlberger, 2009). Any AC-anxiety associations observed in such studies that did not manipulate anxiety were not necessarily caused by anxiety. For example, Derakshan, Ansari, Hansard, Shoker, and Eysenck (2009) clearly demonstrated that high trait anxiety is associated with a deficit in inhibiting visual attention, as measured in an antisaccade task involving neutral stimuli. It is unclear whether (a) trait anxiety dampens the ability to inhibit attention (i.e., anxiety is the cause, AC is the effect), (b) a chronic inability to inhibit attention creates frequent anxious moods that manifest as trait anxiety (i.e., AC is the cause, anxiety is the effect), (c) both, or (d) neither (i.e., there is no causal effect and rather an unmeasured, third variable explains the association).
Third, some theories (e.g., Lonigan, Vasey, Phillips, & Hazen, 2004; Nigg, 2006; Sportel, Nauta, de Hullu, de Jong, & Hartman, 2011) and recent empirical works are consistent with the idea that low AC causes high anxiety. By way of empirical example, lower preexisting AC predicts higher post-traumatic stress symptoms after a traumatic event, even after controlling for baseline levels of these symptoms (Bardeen, Fergus, & Orcutt, 2015). In addition, treatments that aim to improve AC have been shown to reduce negative affect (Callinan, Johnson, & Wells, 2014; Siegle, Ghinassi, & Thase, 2007). By extrapolation, low AC may contribute to negative affect, in which case shoring up AC in and of itself may hold therapeutic promise for treating anxiety. Indeed, Sari, Koster, Pourtois, and Derakshan (2015) found that a three-week working memory intervention led to gains in AC that were associated with reductions in anxiety. Nevertheless, the majority of anxiety interventions do not target low AC for treatment (for a review, see Deacon & Abramowitz, 2004).
Overall, although there is compelling evidence to suggest that low AC and high anxiety are related, it is still not known whether low AC causes high anxiety, high anxiety causes low AC, or both. Our lack of understanding about the causal relations underlying the anxiety-AC association represents a barrier to developing interventions that maximize symptom improvement. Although an experimental manipulation of AC would be the ideal way to determine whether an AC deficit causes anxiety (or whether trained AC protects against anxiety), experimental manipulations of AC are difficult to achieve (e.g., Calkins et al., 2011).
Fortunately, one does not need to manipulate AC to get closer to making causal inferences about the association between AC and anxiety. Instead, one can examine temporal precedence with respect to the relationship between these two constructs. Temporal precedence reflects the idea that whenever changes in one variable cause changes in another, the cause must precede the effect (Shadish, Cook, & Campbell, 2002). According to Kraemer, Stice, Kazdin, Offord, and Kupfer (2001), evaluating temporal precedence is a critical tool for understanding risk factors in psychopathology, of which problems of anxiety represent one common and pernicious example.
In the present study, we harnessed temporal precedence in the context of naturally-occurring fluctuations in both AC and anxiety. We reasoned that, if AC deficits indeed create a subsequent rise in anxiety, then low AC should predict high anxiety more strongly when AC is measured before than after an anxiety-eliciting event. In contrast, if anxiety results in a subsequent drop in AC, then the association should be stronger when AC is measured after than before the anxiety-eliciting event. Whether the anxious mood state impairs AC and/or low AC facilitates the rise of the anxious mood state, the nature of the association between the two constructs should be detectable – and indeed likely clarified – when investigated on a short time scale in a well controlled experimental design with a sizable participant sample. Based on this logic, we measured AC deficits behaviorally using a reaction time index of distraction cost derived from the Irrelevant Singleton Task (Moser et al., 2012), henceforth referred to as the distraction task. Previous research validates the use of this task for measuring AC that covaries with anxiety as expected. Specifically, higher distractibility scores in this distraction task have been associated with higher levels of trait anxiety among a sample of young adult women (Moser et al., 2012) and with post-traumatic stress symptoms in a sample of war veterans with trauma exposure (Esterman et al., 2013). Furthermore, the task measures AC objectively by way of reaction times, and it assesses AC in a non-threatening context so that pure AC can be isolated from the separate construct of attentional bias to threat, which is also associated with anxiety (Bar-Haim, Lamy, Pergamin, Bakermans-Kranenburg, & van IJzendoorn, 2007). These advantages make this task ideally suited to investigate the temporal precedence question in the present study.
Additionally, we measured anxiety objectively as the average level of electrodermal activity during an anxiety induction and subjectively based on ratings collected before and after the induction. The anxiety induction consisted of a modified version of the Trier Social Stress Test (TSST; Kirschbaum, Pirke, & Hellhammer, 1993) involving a pair of socially evaluative tasks in which participants delivered a speech and performed math problems aloud while being videotaped. The TSST was specifically chosen for its ability to increase activity of the autonomic nervous system (Hellhammer & Schubert, 2012) and specifically EDA (Hendrawan, Yamakawa, Kimura, Murakami, & Ohira, 2012).
To test temporal precedence, we manipulated the order of the distraction and anxiety tasks and assessed whether the AC-anxiety association was present when the distraction task preceded or followed the anxious induction, and, if so, whether preexisting AC (pre-induction) or subsequent AC (post-induction) was a better predictor of elicited anxiety. As such, half of the participants completed the AC measure directly before the anxiety induction, and the other half of participants completed the AC measure directly after the anxiety induction.
We tested three hypotheses. First, we hypothesized that higher distraction cost (i.e., lower AC) would be associated with higher trait anxiety, replicating prior literature (Moser et al., 2012). Second, we hypothesized that higher distraction cost would be associated with higher state anxiety as reflected in higher EDA during the induction and higher increases in self-reported anxiety from pre- to post-induction, also replicating prior literature regarding state anxiety. Third, and most importantly, we hypothesized that these effects could depend on temporal precedence. If the AC-anxiety association is mainly explained by distractibility creating subsequent anxiety, the anxiety measures should covary more with pre- than post-induction distraction cost. Conversely, if the association is mainly explained by anxiety leading to subsequent distractibility, the opposite result should occur. Finally, if the association is equally explained by both temporal orders, the anxiety measures should covary comparably with pre- and post-induction distraction cost. This research question of the moderating effect of temporal precedence depended on our ability to detect an AC-anxiety relationship. Because the relationship between low AC and state anxiety is most evident in people with elevated trait anxiety (e.g., Pacheco-Unguetti et al., 2010), we recruited people with moderate to high dispositional anxiety to test these hypotheses.
2. Method
2.1. Participants
All potential participants completed a confidential online survey to determine eligibility for the study. The survey was administered via Qualtrics for paid participants and via Sona Systems Ltd., for participants receiving course credit. Eligible participants had moderate to high trait anxiety as indicated by the State-Trait Anxiety Inventory, Form Y2 (STAI-Y2; Spielberger, 1983), with total scores greater than the median score (42) of a separate undergraduate sample at Tufts University (unpublished). Eligible participants were aged 18–65 years old and not colorblind.
Seventy-eight young adults (39 females and 39 males; M = 26.10 years old; SD = 11.84) from Tufts University and the nearby community participated for course credit or monetary compensation. Forty participants were randomly assigned to the distraction-first order, and 38 participants were randomly assigned to the anxiety elicitation-first order. We determined the sample size by recruiting an additional 10 participants above the suggested sample size of 68 calculated by the software program G*Power 3.1 (Faul, Erdfelder, Lang, & Buchner, 2007) for a two-tailed linear regression analysis with a medium a priori effect size of 0.15, power (1-beta) of .80, two predictors, and alpha = .05. Participants were 64.10% Caucasian, 20.51% Asian or Asian American, 7.69% Black or African American, 1.28% American Indian or Alaska Native; 3.85% declined to provide this information. Of the total sample, 5.13% endorsed being of Hispanic origin and 3.85% declined to provide this information. Information about income and socioeconomic status was not collected. All study procedures were approved by the Institutional Review Board at Tufts University, and all participants provided written informed consent prior to participating in the study.
2.1. Materials
2.2.1. Distraction task
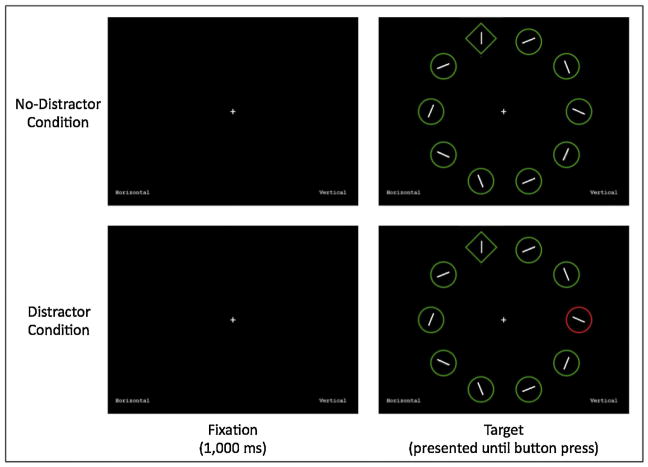
The distraction task involved the presentation of an array of ten shapes on the screen. Participants were instructed to search for the uniquely shaped object (e.g., the one circle amid nine diamonds) and then press one of two keys as quickly and accurately as possible to indicate whether the line inside that target shape was either horizontal or vertical. Half the trials included a colored distractor item that was never located in the same location as the target item (e.g., one red shape amid nine green shapes), and half the trials included no distractor (e.g., ten green shapes). Consistent with Moser et al. (2012), a distraction cost dependent variable was designed to reflect inefficient attentional focusing required to inhibit the processing of distractors. Therefore, we computed this measure as the difference between reaction times on distraction and non-distraction trials (distraction RT minus non-distraction RT). See Fig. 1 for a schematic representation of the task.
Fig. 1.
Trial design of the distraction task. Time advances during the trial from left to right. Two sample trials are shown for the no-distractor condition (top) and the distractor condition (bottom). The words “Horizontal” and “Vertical” appear on the bottom left and right sides of the screen, respectively, to remind participants that the left key, ‘f’, corresponds to horizontal targets and the right key, ‘g’, corresponds to vertical targets. The correct answer for both sample trials above is the ‘g’ key because the unique shape (the diamond among circles in both cases) contains a vertical line.
The distraction task consisted of a block of 20 practice trials followed by a block of 80 test trials. For the practice trials, the levels of the factor of Distraction (distraction trials, non-distraction trials) each consisted of 10 trials with a random order of presentation. For the test trials the levels of the factor of Distraction (distraction trials, non-distraction trials) each consisted of 40 trials with a random order of presentation. A trial began with a fixation cross, presented for 1000 ms. The array of ten shapes, including the target shape, was then presented until the participant made a button-press response (i.e., the “f” key for horizontal or the “g” key for vertical). Participants heard a brief, low-pitched sound immediately after making incorrect responses throughout the task in order to encourage accurate responding and to alert participants if their hands were mistakenly positioned over the wrong keys.
2.2.1.1. Anxious mood induction
During the anxiety-inducing TSST, participants were granted exactly three minutes to prepare a speech. During this brief period, they were instructed to take notes on a piece of paper that would be subsequently taken away from them before the speech began. The TSST proper then followed in two separate parts. First, participants delivered a five-minute speech about one of two pre-determined pairs of topics, which were randomly assigned for each participant. The first pair of topics was “your leadership skills and your ability to work as part of a team of people.” The second pair of topics was “your organization skills and your ability to meet deadlines under pressure.” The experimenters ensured that participants continued speaking up until the end of the 300-s task with no gaps in participant speaking longer than approximately 10 s. Then, participants completed mental arithmetic aloud for five minutes without the aid of pen and paper using one of two instructions, which were randomly assigned to each participant. One instruction was to count out loud backwards from 2104 in increments of 13, and the other instruction was to count out loud backwards from 2223 in increments of 17. The experimenter promptly told participants every time they made an error and instructed them to begin again from the original number. To increase anxiety stemming from social evaluative threat, the experimenter deceptively told participants that “the math is quite easy, and most people do not have a problem with it.”
The TSST was modified slightly from the standard version (Kirschbaum et al., 1993) to accommodate limited space in the experimental booth. Instead of a panel of judges being physically present in the same room as participants, there was a single judge (the experimenter) who communicated with participants via an intercom system. To maintain similarity with the traditional TSST, the experimenter instructed the participants deceptively before the TSST as follows: “Two of the research affiliates are trained in verbal and nonverbal communication. They will review the video recording together and will take notes regarding the manner and content of your speech, including notes about body language and the persuasiveness of your argument.” Given that the absence of a panel of in-person judges could potentially reduce the level of induced anxiety, we added an additional element to boost anxiety. Specifically, on the computer monitor 60 cm in front of them, participants saw a continuous full-screen video feed of their faces as they performed the speech and math tasks.
2.2.2. Questionnaires
2.2.2.1. Trait anxiety
The STAI-Y2 (Spielberger, 1983) is a 20-item measure of trait anxiety (e.g., I have disturbing thoughts; I get in a state of tension or turmoil as I think over my recent concerns and interests). This scale had excellent internal consistency reliability (Cronbach’s α = .91) for the present sample.
2.2.2.2. State anxiety
The State-Trait Inventory of Cognitive and Somatic Anxiety (STICSA; Grös, Antony, Simms, & McCabe, 2007) is a 21-item scale that indexes the severity of worrisome thoughts (cognitive state anxiety subscale; e.g., I picture some future misfortune; I think that the worst will happen) and anxious bodily symptoms (somatic state anxiety subscale; e.g., My muscles are tense; I feel trembly and shaky). The cognitive (Cronbach’s α = .87 at pre-induction and Cronbach’s α = .91 at post-induction) and somatic (Cronbach’s α = .91 at pre-induction and Cronbach’s α = .91 at post-induction) subscales each had high internal consistency reliability for the present sample.
2.2.2.3. Attentional control
The ACS (Derryberry & Rothbart, 1988) is a 20-item measure of attentional control. The focusing scale includes items assessing the ability to ignore irrelevant information (e.g., When concentrating, I can focus my attention so that I become unaware of what’s going on in the room around me; When trying to focus my attention on something, I have difficulty blocking out distracting thoughts). The shifting scale includes items assessing the ability to switch the focus of attention (e.g., When a distracting thought comes to mind, it is easy for me to shift my attention away from it; It is easy for me to alternate between two different tasks). Based on a total score across the two scales, the level of self-reported AC in the present sample (M = 48.52, SD = 8.74) was similar to the mean of a large sample of young adults (M = 50.17, SD = 7.49; Ólafsson et al., 2011). The focusing (Cronbach’s α = .81) and shifting (Cronbach’s α = .74) subscales each had high internal consistency reliability for the present sample.
2.2.2.4. Additional measures
In addition to the above measures, we administered a set of in-house mood rating triplets to assess a wide range of positive and negative feeling states. For the sake of brevity, we report only results for the STICSA measure of state anxiety below. We also administered a set of surveys to collect demographic information: age, sex, race, ethnicity, level of education, marital status, number of children, and number of people in household. Information about perceived stress, life satisfaction, and various symptoms related to mood, anxiety, and depression were also collected but will not be discussed further in the present manuscript.
2.2. Procedure
Interested potential participants were screened for eligibility via email. Eligible participants were scheduled for a laboratory session. After the informed consent process at the beginning of the lab session, two sets of electrodes were attached. Two EDA electrodes were attached to the index and middle fingertips of the nondominant hand, and a ground electrode was attached on the back of the neck. Two elecromyography electrodes were attached to the corrugator muscle, just above the left eyebrow. Note that results pertaining to the corrugator measure will not be reported for reasons elaborated in the next section.
Participants completed the distraction task to assess the AC deficit. They completed both the arithmetic and speaking components of the TSST to elicit state anxiety. Mood ratings (i.e., mood triplets and STICSA) were administered immediately before and immediately after the TSST. Critically, as our manipulation of time of AC measurement, approximately half of the participants completed the AC measure directly before the TSST (pre-induction time of measurement), and the other half of participants completed the AC measure directly after the TSST (post-induction time of measurement). A between-subjects manipulation was desirable because it prevented confounding our measure of AC with practice and sensitization effects due to prior exposure to the distraction task in the same session.
Following these tasks, participants completed additional tasks that will not be discussed further in this manuscript. These additional tasks were an Attention Bias Modification Treatment and a repetition of the distraction task and the TSST. Note that all participants completed the first instances of the distraction task and the TSST, which are the ones considered herein, before these additional tasks. The session ended with trait questionnaires administered on Qualtrics, a short humorous clip to improve participants’ mood, removal of electrodes, and debriefing. The total duration of the study session was approximately 120 min per participant. For-pay participants were compensated with 30 dollars, and for-credit participants received experimental credit in partial fulfillment of a psychology course requirement.
2.2.1. Physiological data collection, reduction, and analysis
As part of this study, we recorded electrodermal activity throughout the session, specifically average electrodermal activity (EDA). In addition to self-reported anxiety, EDA served as the primary index of anxious responding for two reasons. First, previous work examining AC and anxiety has often used self-reported measures of one or both constructs, which may lead to erroneously inflated estimates of variance (Derryberry & Reed, 2002; Healy, 2010; Healy & Kulig, 2006; Moriya & Tanno, 2008). Second, self-reported emotion collected before and after anxiety inductions is not always correlated with physiological anxiety during the inductions (Hellhammer & Schubert, 2012); subjective reports of anxiety are thus an unreliable proxy for the bodily manifestations of anxiety driven by the autonomic nervous system. We recorded EDA and corrugator muscle activity continuously during the tasks, but we report the results of EDA only since it is an appropriate measure of autonomic arousal (Dawson, Schell, & Filion, 2007) and since EDA may be less likely than corrugator activity to be affected by participants’ socially motivated attempts to show positive emotion on their faces during the videotaped TSST, especially during the mock job interview.
The following procedures for electrodermal activity were very similar to those reported by Urry (2010). Data were recorded with a sampling rate of 1000 Hz using Biopac MP150 hardware and Acq-Knowledge 3.8.2 software (Biopac, Goleta, CA, USA). Two disposable Ag/AgCl electrodes pregelled with 0.5% chloride isotonic gel (1 cm circular contact area) were attached to the distal phalanges of the index and middle fingers on the nondominant hand. EDA level was recorded with DC coupling and constant voltage electrode excitation at 31.25 Hz (sensitivity .7 nS). Offline, EDA was smoothed with a 1 Hz low-pass filter, decimated to 10 Hz, and linearly detrended on a trial-by-trial basis.
Average EDA was calculated across the duration of the distraction task beginning with the first fixation cross until the end of the last trial. The same measures were computed separately for the speech and math portions of the TSST. The average EDA across the TSST speech and TSST math tasks was computed for each participant as the key measure of anxiety-related autonomic arousal.
3. Results
3.1. Data retention
For analyses involving distraction cost (N = 76), one participant was excluded for poor performance indicative of non-compliance or failure to comprehend instructions (i.e., fewer than 2/3 of trials answered correctly for the distraction or no-distraction conditions; this accuracy rate was well below typical accuracy rates, as reported in the next section below). Participants with extreme outliers for key variables were excluded from the analyses. Extremely high and low values were defined as values more than three times the interquartile range above the 75th percentile and below the 25th percentile, respectively. One additional participant was excluded for having an extremely high RT for the no-distractor condition. No additional participants were excluded for having extreme values for the distractor condition or the distraction cost metric.
For analyses involving self-reported state anxiety measures (STICSA total, cognitive, and somatic scales; N = 73), one participant did not complete the STICSA measures before the TSST, and three participants did not complete the STICSA measures after the TSST. One participant opted not to complete the TSST speech preparation and speech tasks, and therefore all data related to the TSST were excluded for this participant.
For analyses involving EDA for the TSST (N = 76), one participant had no usable physiological data for any of the TSST tasks due to technical issues, and the aforementioned participant who did not complete the entire TSST was excluded. No further participants were excluded for having extreme values for EDA on the distraction task, TSST speech, or TSST math.
These exclusions result in N = 76 for hypothesis 1, N = 71 for testing hypotheses 2 and 3 with self-reported somatic anxiety as the dependent variable, and N = 74 for testing hypotheses 2 and 3 with EDA as the dependent variable.
3.2. Manipulation check for distraction cost
We conducted paired t-tests to determine whether the visual distractors increased reaction time and reduced accuracy in the distraction task. Indeed, reaction time was higher for the distractor condition (M = 1742.70 ms, SD = 537.36) than the no-distractor condition (M = 1496.27 ms, SD = 444.00), t(75) = 10.94, p < .001, Mdistraction-cost (SD) = 246.43 ms (196.47), 95% CI [201.53, 291.32], d = .445. Accuracy was marginally lower for the distractor condition (M = 96.18% correct, SD = 4.92%) than the no-distractor condition (M = 97.27% correct, SD = 3.61%), t(75) = −1.98, p = .052, Mdifference (SD) = −1.09% correct (4.78%), 95% CI [−2.18%, 0.01%], d = .248. Overall accuracy was thus very high. Overall accuracy was not correlated with distraction cost, r(74) = .10, p = .410, suggesting that the amount of error feedback encountered was unrelated to the measure of AC.
3.3. Manipulation check of TSST anxiety induction
We conducted paired t-tests to determine whether self-reported somatic and cognitive anxiety (measured by the STICSA) was induced by the TSST. Somatic anxiety increased from pre-TSST (M = 15.51, SD = 4.23) to post-TSST (M = 18.78, SD = 5.35), t(72) = 6.54, p < .001, Mchange (SD) = 3.27 (4.27), 95% CI [2.28, 4.27], d = 0.678, but self-reported cognitive anxiety did not increase significantly from pre-TSST (M = 18.88, SD = 6.77) to post-TSST (M = 19.33, SD = 6.22), t(72) = .82, p = .413, Mchange (SD) = .45 (4.65), 95% CI [−.64, 1.53], d = .069.
Similarly, we conducted paired t-tests to determine whether the EDA measure successfully indexed state anxiety induced by the TSST. The index of EDA during the distraction task, which is ostensibly not a stressor, was used as a control comparison in these tests. As expected, EDA was robustly elevated during the TSST speech task (M = 10.96 μS, SD = 5.54) relative to the distraction task (M = 8.66 μS, SD = 4.85), t(75) = 7.87, p < .001, Mchange (SD) = 2.31 (2.56), 95% CI [1.72, 2.89], d = .429, and during the TSST math task (M = 10.89 μS, SD = 5.45) relative to the distraction task (M = 8.66 μS, SD = 4.85), t(75) = 8.13, p < .001, Mchange (SD) = 2.24 (2.40), 95% CI [1.69, 2.79], d = .432.
In summary, the TSST was successful in creating a state of anxiety. It instilled subjectively experienced bodily symptoms of anxiety, and it resulted in heightened activation of the sympathetic branch of the autonomic nervous system.
3.4. Testing for differences in AC and anxiety associated with time of AC measurement
3.4.1. Preliminary analyses
Table 1 presents the correlations among the behavioral measure of AC deficit (distraction cost), the self-reported measure of AC (ACS total score), trait anxiety (STAI total score), the self-reported measures of state anxiety (STICSA somatic and cognitive scores), average EDA during the distraction task, average EDA during the TSST speech task, and average EDA during the TSST math task. There were no group differences in trait anxiety, self-reported AC, average EDA during the distraction task, average EDA during the TSST speech task, average EDA during the TSST math task, or distraction cost, all ps ≥ .192. Additionally, we used Levene’s test for equality of variances to verify that there were no group differences in the variability of distraction cost when measured before the TSST (SD = 171.51 ms) and when measured after the TSST (SD = 224.67 ms), F(1,73) = .883, p = .350, indicating that both the mean and variance of the critical measure of behavioral AC did not vary by time of measurement (pre-induction, post-induction).
Table 1.
Correlations of distraction cost, self-reported AC, trait anxiety, and state anxiety measures.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Distraction Cost | – | |||||||||
| 2. ACS Total Score | −.216† | – | ||||||||
| 3. STAI Total Score | .210† | −.582** | – | |||||||
| 4. STICSA Somatic Score Before TSST | .028 | −.402** | .297* | – | ||||||
| 5. STICSA Cognitive Score Before TSST | .203† | −.518** | .603** | .559** | – | |||||
| 6. STICSA Somatic Score After TSST | .107 | −.353** | .308** | .624** | .477** | – | ||||
| 7. STICSA Cognitive Score After TSST | .180 | −.390** | .603** | .322** | .754** | .458** | – | |||
| 8. Average EDA during Distraction Task | .139 | −.343** | .086 | .308** | .134 | .328** | .061 | – | ||
| 9. Average EDA during TSST Speech Task | .247* | −.321** | .085 | .346** | .116 | .295* | .062 | .887** | – | |
| 10. Average EDA during TSST Math Task | .261* | −.295* | .066 | .313** | .104 | .276* | .067 | .898** | .981** | – |
Note. Correlation coefficients (r) are indicated. The variables listed next to the ascending numbers in the rows are represented by the numbers alone in the columns. Only participants with usable data for all measures in the table are included so that all correlations represented above pertain to the same participants. AC = attentional control. ACS = Attentional Control Scale. STAI = State-Trait Anxiety Inventory. STICSA = State-Trait Inventory of Cognitive and Somatic Anxiety. TSST = Trier Social Stress Test. EDA = electrodermal activity.
Of interest and as expected, higher distraction cost showed a marginally significant association with lower self-reported ACS total scores, r(70) = −.216, p = .070. Though not significant, the direction of the association suggests that the behavioral measure of low AC may correspond somewhat to people’s self-reported perceptions of their own AC.
3.4.2. Hypothesis testing
To test hypothesis 1 that higher distraction cost would be associated with higher trait anxiety, we performed bivariate correlations. To test hypotheses 2 and 3 that higher distraction cost would be associated with higher state anxiety and that temporal precedence could moderate anxiety-AC associations, we computed separate linear regression models using PROCESS in SPSS (Hayes, 2013) for the measures of subjective and physiological state anxiety, as described below.
3.4.3. Is higher distraction cost associated with higher trait anxiety?
Contrary to hypothesis 1, higher distraction cost was not significantly correlated with higher self-reported trait anxiety, r(74) = .191, p = .098.1 Note, though, that the association represented a trend in the direction reported by Moser et al. (2012).
3.4.4. Is higher distraction cost associated with higher subjective anxiety and is this association moderated by temporal precedence?
The model for self-reported anxiety was constructed such that distraction cost predicted STICSA somatic scores. We entered the time of AC measurement (pre-induction, post-induction) as the moderator of this relationship to test whether the association between AC and anxiety depends on whether the measurement of AC precedes or follows the measurement of subjective anxiety. The somatic STICSA score at the start of the TSST was entered as a covariate on the dependent measure in order to control for individual differences in subjective anxiety that were unrelated to the anxiety induction. Since heightened error monitoring is linked with anxiety—albeit for trait rather than state anxiety (Olvet & Hajcak, 2008), the proportion of accurate trials during the distraction task was entered as an additional covariate on the dependent measure in order to control for the amount of rarely encountered error feedback as a potential correlate of anxiety. We focused here on STICSA somatic scores because the TSST specifically increased somatic but not cognitive subjective anxiety, as described above.
The overall model was significant, R2 = .41, F(5, 65) = 24.36, p < .001. Accuracy during the distraction task did not predict self-reported somatic anxiety, b = 16.028, p = .248, 95% CI [−11.424, 43.479]. Unexpectedly and contrary to hypothesis 2, higher distraction cost did not predict higher self-reported somatic anxiety, b = .002, p = .371, 95% CI [−.003, .007]. Time of AC measurement was not a significant predictor, b = −.484, p = .664, 95% CI [−2.695, 1.728]. Furthermore, there was no interaction effect of Distraction Cost × Time of AC Measurement, b < .001, p = .998, 95% CI [−.009, .009]. Regarding hypothesis 3, higher distraction cost did not predict higher self-reported somatic anxiety differently among participants for whom AC was measured pre-induction (p = .491) or post-induction (p = .559). Indeed, distraction cost did not predict self-reported anxiety at all in this experimental context.
3.4.5. Is higher distraction cost associated with higher autonomic arousal and is this association moderated by temporal precedence?
The model for the physiological measure of anxiety was constructed such that distraction cost predicted average EDA across the speech and math tasks of the TSST. As above, we entered the time of AC measurement (pre-induction, post-induction) as the moderator of this relationship. The average EDA during the distraction task was entered as a covariate on the dependent measure in order to control for individual differences in EDA that were unrelated to the anxiety induction. Also as above, the proportion of accurate trials during the distraction task was entered as an additional covariate on the dependent measure.
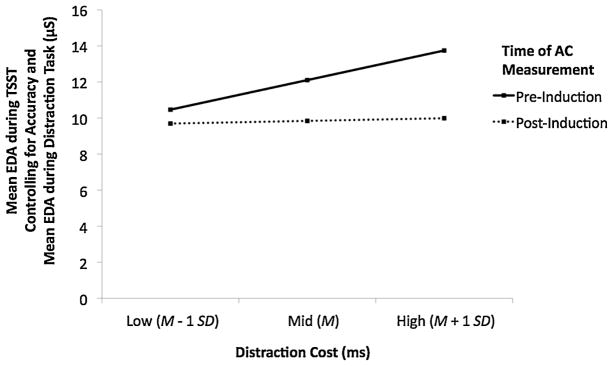
The overall model was significant, R2 = .88, F(5, 68) = 98.48, p < .001. Accuracy during the distraction task did not predict EDA during the TSST, b = −5.618, p = .293, 95% CI [−16.186, 4.951]. Supporting hypothesis 2, higher distraction cost predicted higher EDA during the TSST, b = .005, p = .002, 95% CI [.002, .007]. In addition, time-of-AC-measurement was a significant predictor, b = 2.264, p < .001, 95% CI [1.233, 3.296], such that pre-induction time of AC measurement was associated with higher EDA during the TSST than post-induction time of AC measurement. Critically, however, these two main effects were qualified by an interaction effect of Distraction Cost × Time of AC Measurement, b = .008, p = .010, 95% CI [.002, .013]. Supporting hypothesis 3, this interaction suggested that higher distraction cost predicted higher EDA during the TSST tasks among participants for whom AC was measured pre-induction, b = .008, p = .003, 95% CI [.003, .014], but not among participants for whom AC was measured post-induction, b = .001, p = .257, 95% CI [−.001, .002] (see Fig. 2). This result supports the notion that low AC may be a precursor to higher anxious arousal in this experimental context.
Fig. 2.
Distraction cost and time of AC measurement (pre-induction, post-induction) as predictors of average electrodermal activity (EDA) during the Trier Social Stress Test (TSST) tasks. Low, medium, and high distraction cost on the x-axis represent values that are one standard deviation below the mean, the mean, and one standard deviation above the mean, respectively. The estimates plotted on the y-axis are adjusted using the average EDA during the distraction task and accuracy rate during the distraction task as covariates in order to control for individual differences in autonomic arousal that are unrelated to state anxiety elicited by anxiety induction and autonomic arousal associated with the amount of error feedback encountered, respectively. Overall, higher distraction costs predicted a subsequently higher EDA response to the induction. Critically, this main effect was qualified by an interaction of Distraction Cost × Time of AC Measurement such that higher distraction costs only predicted higher EDA during the induction for the group with the pre-induction time of AC measurement (solid line) but not for the group with the post-induction time of AC measurement (dotted line).
4. Discussion
Previous research reveals a robust association between AC and anxiety, but it remains unknown whether AC deficits actually lead to subsequent anxiety and should thus be a major target for clinical interventions. In the present study, we used a controlled elicitation of state anxiety to evaluate the temporal precedence underlying the AC-anxiety association to gain insight into what explains it. The present findings supported the idea that preexisting variation in AC contributes to autonomic arousal experienced during a subsequent episode of anxiety. Specifically, higher distractibility predicted greater levels of autonomic arousal during an anxiety induction only when distractibility was measured prior to the induction. In contrast, no relationship existed between post-induction distractibility and autonomic arousal during the induction. Although we cannot definitively determine causality here, this pattern of results suggests that the relationship between low AC and anxiety may be better explained by distractibility preceding autonomic arousal than by autonomic arousal preceding distractibility. However, because AC scores varied naturally and were not experimentally controlled, we cannot rule out the possibility that a third variable accounts for the temporally specific association between AC and anxiety.
Why should low AC create high anxiety? First, AC may be a resource for some forms of emotion regulation; low AC may, thus, hamper emotion regulation processes that rely on this resource (Opitz, Gross, & Urry, 2012). AC recruits prefrontal brain regions implicated in reappraising the meaning of situations to regulate negative emotion (Ochsner & Gross, 2005). Therefore, improved emotion regulation success may explain why lower AC is associated with higher negative emotion in response to negative stimuli (Compton, 2000) and distressing situations (Gyurak et al., 2012). Second, perceived uncontrollability has been shown to lead to anxiety (Mineka & Kelly, 1989). Since low AC entails a lack of control over one’s own cognitive activity, which one may be able to perceive, it may instill beliefs about the uncontrollability of the present situation, thereby giving rise to anxiety.
Interestingly, there was no association between AC and changes in subjective state anxiety for either time of AC measurement; rather, the results were specific to autonomic arousal. One possibility regarding this discrepancy between physiology and subjective experience is that low AC confers a risk of elevated autonomic arousal when potentially anxiety-eliciting situations arise, and this risk may manifest in peripheral physiology even before heightened anxiety is consciously experienced. Thus, consistent with findings that emotional stimuli can elicit autonomic arousal even in the absence of conscious awareness (Silvert, Delplanque, Bouwalerh, Verpoort, & Sequeira, 2004), autonomic arousal may be a more sensitive indicator than self-reported anxiety of a newly burgeoning anxious episode. A second possibility is that the link between AC and the subjective conscious awareness of anxiety only becomes evident if the anxious episode entails an increase in worrisome thoughts (i.e., cognitive anxiety), which was not the case in the present study. A third possibility is that the measure of self-reported anxiety, unlike the measure of autonomic activity, failed to capture the time of maximal anxiety, which was during the TSST rather than at its end. Future research using subjective ratings that are easy and quick to complete repeatedly during the anxiety induction (e.g., the Subjective Units of Distress Scale; Wolpe, 1982) could help to address this last point.
The results have clear theoretical implications. If preexisting AC deficits indeed contribute to anxious arousal as proposed by some researchers (Lonigan et al., 2004; Nigg, 2006; Sportel et al., 2011), then the AC-anxiety relationship cannot be exclusively explained by anxiety’s deleterious effect on cognition. Indeed, in the present study there was actually no evidence consistent with the idea that anxiety led to a subsequent AC impairment. This consideration has implications for a broadening of the scope of ACT with respect to its strong claim that the AC deficits associated with anxiety are caused by anxiety’s impairment of the inhibition function and its lack of acknowledgement that the opposite sequence may also occur (see Eysenck & Derakshan, 2011). Specifically, ACT may need to broaden its scope to account for the possibility that AC deficits promote anxiety in addition to being one of anxiety’s unfortunate consequences.
Critically, this exploration of temporal precedence has clinical implications. If AC is a resource that can eventually be manipulated reliably (Calkins & Otto, 2013; Papageorgiou & Wells, 2000; Siegle et al., 2007), then the present findings suggest that chronic anxiety may be lowered by a successful AC-boosting intervention. Indeed, recent evidence suggests, for example, that three weeks of daily working memory training improved AC and that AC gains were associated with decreases in trait anxiety (Sari et al., 2015). Mindfulness meditation training may represent another promising avenue for increasing AC and thus decreasing anxiety given early evidence that it facilitates executive attention (e.g., Tang et al., 2007). Nevertheless, the majority of anxiety interventions do not target low AC for treatment (for a review, see Deacon & Abramowitz, 2004); the present results point to the importance of doing so.
The design and methodology of the present study afforded us several advantages, two of which bear underscoring here. First, the behavioral and physiological measures of AC and anxiety, respectively, do not share a common methodology and are each resistant to demand characteristics. Second, the time of AC measurement was a between-subjects factor. Thus, the measure of AC was unaffected by confounding practice and sensitization effects that would likely have falsely lowered the estimates of post-induction AC in a within-subjects design.
Despite these advantages, we acknowledge three noteworthy limitations of this design. First, we did not manipulate AC experimentally, and we therefore cannot assert with certainty that low AC caused subsequently higher levels of stress-related autonomic activity. However, that interpretation is consistent with preliminary empirical support showing a link between AC training and negative affect. Engaging AC processes extensively via cognitive control training can directly reduce the experience of negative affect (Calkins & Otto, 2013; Callinan et al., 2014; Siegle et al., 2007; but see Calkins et al., 2011). Research in the area of AC training represents an exciting avenue for future research and holds promise as an effective clinical intervention. Second, we did not assess individual differences in average EDA at rest in the absence of an active cognitive task. Instead, we used the average EDA during the distraction task to control for individual differences in autonomic activity that were unrelated to the anxiety induction. However, there were no differences in EDA during the distraction task between the groups with pre- and post-induction time of AC measurement, suggesting that this limitation cannot explain the observed pattern of results. Third, as mentioned above, the self-reported measure of anxiety occurred before and after but not during the anxiety induction when subjective anxiety was likely at its peak.
It should be noted that the association between low AC and anxiety is likely bidirectional. This possibility mirrors recent research suggesting that the association between attentional bias to threat and anxiety also is bidirectional (Van Bockstaele et al., 2014). The causal accounts proposed by proponents of ACT and theorists attempting to account for the development of anxiety may both be accurate. Though not supported by the present data, it is possible that positive feedback loops occur in which impaired cognition and escalating anxiety go hand in hand due to a bidirectional facilitation effect (e.g., low AC contributes to anxiety, which in turn further dampens AC). This cycle may help to explain the maintenance of anxiety.
It is unclear why we did not observe signs of bidirectional facilitation in this study in that AC and anxiety were not associated when AC was measured after the induction. One possibility is that the anxiety-inducing effect of the TSST did not persist long enough to exert an effect on distraction cost for participants who completed the distraction task after the TSST. This possibility is consistent with the lack of difference in autonomic arousal during the distraction task between the groups of participants for whom this task occurred before or after the TSST. In contrast to previous research that has found an effect of induced anxiety on subsequent cognitive performance (e.g., Choi et al., 2012; Cornwell et al., 2012), the present study did not involve continuous or even temporally proximal threat (e.g., within seconds) in the test of cognitive performance. A second possibility was that participants for whom AC was measured post-induction may have benefited from the prefrontal engagement required to perform the TSST beforehand. In other words, the AC-reducing effects of anxiety may have been obscured by the AC-enhancing effects of organizing and implementing a persuasive speech and/or using working memory intensively to perform mental arithmetic. This possibility could be tested by replacing the TSST with a state anxiety induction (e.g., film clip) that does not recruit intensive top–down control and working memory resources.
Although there was a marginally significant hint of such an association, we did not find significant support for the first hypothesis that higher distractibility should be associated with higher trait anxiety, contrary to previous research (Moser et al., 2012). This may be explained in at least two ways. First, it may be that the recruitment of participants with elevated trait anxiety reduced our ability to detect the correlation due to the truncated range of trait anxiety at the higher end of the distribution. Second, it is possible that the true effect is small and thus requires a larger sample to detect, although Moser et al. (2012) actually had a smaller sample than in the present study. An area for future research to explore is whether distractibility more strongly predicts state anxiety within minutes of AC measurement than it predicts trait anxiety more generally.
In light of our findings and the strengths and limitations of this research, we see three compelling additional directions for future research. First, in the present study we only examined the AC-anxiety relationship with respect to a single anxiety elicitation. Future research should extend this work by measuring distractibility and autonomic activation at multiple time points within a single group of participants. This approach will allow us to verify whether intact AC protects against the subsequent rise of anxious arousal in response to anxiety-eliciting events by testing whether the temporal precedence of AC deficits before anxious episodes is a reliable phenomenon.
Second, further experimental studies are warranted to investigate the causal explanations for the effect. The causal effect of induced anxiety on distractibility can be tested with a modified version of the TSST having a control condition designed not to elicit anxiety. Additionally, it is important to test whether the effect of temporal precedence replicates across a variety of anxiety-inducing contexts, including ones that are not explicitly social in nature (e.g., test anxiety). Future studies should explore the underlying mechanisms that could account for the possible adverse effect of AC deficits on anxiety such as impaired emotion regulation ability due to low AC (Ochsner & Gross, 2005) and perceived lack of control due to low AC (Rapee, Craske, Brown, & Barlow, 1996).
Finally, future research should address the possibility that momentarily bolstering AC on short time scales prior to expected anxiety inductions (e.g., job interviews) may help to curb the rise of anxious arousal. We have no evidence to suggest that the distraction task boosts AC, which underscores the need for future studies to try to do so. The present study suggests that the inability to concentrate and focus attention may spur anxiety. If future research bears out this claim in the ways outlined above, then efforts to develop clinical interventions should be refined to target this specific AC deficit of attentional focusing in order to manage and treat anxiety as effectively as possible.
In sum, in a sample of people who face moderate-to-high trait anxiety, we found that a behavioral measure of lower attentional focusing, an aspect of AC, predicted a greater response of the autonomic nervous system to a subsequent anxiety induction. There was no such association when attentional focusing was measured directly after the induction. Overall, these results support the notion that low AC may be a precursor to state anxiety among people who suffer from anxiety frequently. Since anxiety disorders continue to rank among the most prevalent mental health disturbances, collectively afflicting about 3 in 10 people in the United States (Kessler et al., 2007), understanding what instigates and maintains anxiety remains a critical research goal for affective and clinical psychological science.
Acknowledgments
We thank C. Casey, A. Chan, M. Chu, K. Cochran, E. Friedman, M. Hefyan, M. Hennessy, L. Katz, J. Merrin, J. Mow, R. O’Donnelly, E. Orlando, V. Powell, A. Rogers, and A. Yacoubian for assistance with data collection and processing. Finally, we thank S. Cavanagh, L. Vujovic, and V. Floerke for valuable feedback, and F. Wilhelm and P. Peyk for making ANSLAB, a suite of open source Matlab routines used to process physiological data, available as freeware in the Society for Psychophysiological Research Software Repository (http://www.sprweb.org/repository).
Footnotes
The association between distraction cost and trait anxiety reported in Table 1, r(70) = .210, p < .078, differs from the test of hypothesis 1, r(74) = .191, p < .098, due to a different number of included participants; the table represents only participants who completed all measures represented therein.
Authorship
J.L.B. and H.L.U. designed the study with important contributions from P.C.O. Data collection was conducted by J.L.B. and the research assistants noted in the acknowledgements section. J.L.B. performed the data analysis with guidance and feedback from H.L.U. and P.C.O. J.L.B. drafted the paper. P.C.O. and H.L.U. provided critical revisions. All authors approved the final version of the paper for submission.
References
- Ansari TL, Derakshan N. The neural correlates of impaired inhibitory control in anxiety. Neuropsychologia. 2011;49:1146–1153. doi: 10.1016/j.neuropsychologia.2011.01.019. http://dx.doi.org/10.1016/j.neuropsychologia.2011.01.019. [DOI] [PubMed] [Google Scholar]
- Bardeen JR, Fergus TA, Orcutt HK. Attentional control as a prospective predictor of posttraumatic stress symptomatology. Personality and Individual Differences. 2015:124–128. http://dx.doi.org/10.1016/j.paid.2014.09.010.
- Bar-Haim Y, Lamy D, Pergamin L, Bakermans-Kranenburg MJ, van IJzendoorn MH. Threat-related attentional bias in anxious and nonanxious individuals: a meta-analytic study. Psychological Bulletin. 2007;133:1–24. doi: 10.1037/0033-2909.133.1.1. [DOI] [PubMed] [Google Scholar]
- Bishop SJ. Trait anxiety and impoverished prefrontal control of attention. Nature Neuroscience. 2009;12:91–98. doi: 10.1038/nn.2242. [DOI] [PubMed] [Google Scholar]
- Calkins AW, Deveney CM, Weitzman ML, Hearon BA, Siegle GJ, Otto MW. The effects of prior cognitive control task exposure on responses to emotional tasks in healthy participants. Behavioural and Cognitive Psychotherapy. 2011;39:205–220. doi: 10.1017/S1352465810000652. [DOI] [PubMed] [Google Scholar]
- Calkins AW, Otto MW. Testing the boundaries of computerized cognitive control training on symptoms of obsessive compulsive disorder. Cognitive Therapy and Research. 2013;37:587–594. http://dx.doi.org/10.1007/s10608-012-9496-x. [Google Scholar]
- Callinan S, Johnson D, Wells A. A randomised controlled study of the effects of the attention training technique on traumatic stress symptoms, emotional attention set shifting and flexibility. Cognitive Therapy and Research. 2014:1–10. http://dx.doi.org/10.1007/s10608-014-9634-8.
- Choi JM, Padmala S, Pessoa L. Impact of state anxiety on the interaction between threat monitoring and cognition. Neuroimage. 2012;59(2):1912–1923. doi: 10.1016/j.neuroimage.2011.08.102. http://dx.doi.org/10.1016/j.neuroimage.2011.08.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton RJ. Ability to disengage attention predicts negative affect. Cognition & Emotion. 2000;14:401–415. http://dx.doi.org/10.1080/02699930037889. [Google Scholar]
- Cornwell BR, Mueller SC, Kaplan R, Grillon C, Ernst M. Anxiety, a benefit and detriment to cognition: behavioral and magnetoencephalographic evidence from a mixed-saccade task. Brain and Cognition. 2012;78(3):257–267. doi: 10.1016/j.bandc.2012.01.002. http://dx.doi.org/10.1016/j.bandc.2012.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Filion DL. 7 The Electrodermal System. Handbook of Psychophysiology. 2007:159–181. [Google Scholar]
- Deacon BJ, Abramowitz JS. Cognitive and behavioral treatments for anxiety disorders: a review of meta-analytic findings. Journal of Clinical Psychology. 2004;60(4):429–441. doi: 10.1002/jclp.10255. http://dx.doi.org/10.1002/jclp.10255. [DOI] [PubMed] [Google Scholar]
- Derakshan N, Ansari TL, Hansard M, Shoker L, Eysenck MW. Anxiety, inhibition, efficiency, and effectiveness: an investigation using the antisaccade task. Experimental Psychology. 2009;56(1):48–55. doi: 10.1027/1618-3169.56.1.48. http://dx.doi.org/10.1027/1618-3169.56.1.48. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111:225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Rothbart MK. Arousal, affect, and attention as components of temperament. Journal of Personality and Social Psychology. 1988;55:958–966. doi: 10.1037//0022-3514.55.6.958. [DOI] [PubMed] [Google Scholar]
- Esterman M, DeGutis J, Mercado R, Rosenblatt A, Vasterling JJ, Milberg W, et al. Stress-related psychological symptoms are associated with increased attentional capture by visually salient distractors. Journal of the International Neuropsychological Society. 2013;19:835–840. doi: 10.1017/S135561771300057X. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N, Santos R, Calvo MG. Anxiety and cognitive performance: attentional control theory. Emotion. 2007;7:336–353. doi: 10.1037/1528-3542.7.2.336. http://dx.doi.org/10.1037/1528-3542.7.2.336. [DOI] [PubMed] [Google Scholar]
- Eysenck MW, Derakshan N. New perspectives in attentional control theory. Personality and Individual Differences. 2011;50:955–960. http://dx.doi.org/10.1016/j.paid.2010.08.019. [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G* Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behavior Research Methods. 2007;39(2):175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Grös DF, Antony MM, Simms LJ, McCabe RE. Psychometric properties of the State-Trait Inventory for Cognitive and Somatic Anxiety (STICSA): comparison to the State-Trait Anxiety Inventory (STAI) Psychological Assessment. 2007;19(4):369. doi: 10.1037/1040-3590.19.4.369. http://dx.doi.org/10.1037/1040-3590.19.4.369. [DOI] [PubMed] [Google Scholar]
- Gyurak A, Hooker CI, Miyakawa A, Verosky S, Luerssen A, Ayduk ON. Individual differences in neural responses to social rejection: The joint effect of self-esteem and attentional control. Social Cognitive and Affective Neuroscience. 2012;7:322–331. doi: 10.1093/scan/nsr014. http://dx.doi.org/10.1093/scan/nsr014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes AF. Introduction to mediation, moderation, and conditional process analysis: a regression-based approach. Guilford Press; 2013. [Google Scholar]
- Healy B. The effect of attentional control and heart-period variability on negative affect and trait anxiety. The Journal of General Psychology: Experimental, Psychological, and Comparative Psychology. 2010;137:140–150. doi: 10.1080/00221301003645079. [DOI] [PubMed] [Google Scholar]
- Healy B, Kulig J. Temperament, anxiety and attentional control. Psychological Reports. 2006;98:23–29. doi: 10.2466/pr0.98.1.23-29. [DOI] [PubMed] [Google Scholar]
- Hellhammer J, Schubert M. The physiological response to trier social stress test relates to subjective measures of stress during but not before or after the test. Psychoneuroendocrinology. 2012;37:119–124. doi: 10.1016/j.psyneuen.2011.05.012. http://dx.doi.org/10.1016/j.psyneuen.2011.05.012. [DOI] [PubMed] [Google Scholar]
- Hendrawan D, Yamakawa K, Kimura M, Murakami H, Ohira H. Executive functioning performance predicts subjective and physiological acute stress reactivity: preliminary results. International Journal of Psychophysiology. 2012;84:277–283. doi: 10.1016/j.ijpsycho.2012.03.006. http://dx.doi.org/10.1016/j.ijpsycho.2012.03.006. [DOI] [PubMed] [Google Scholar]
- Hoskin R, Hunter MD, Woodruff PW. Neither state or trait anxiety alter the response to distracting emotionally neutral sounds. Experimental Psychology. 2014:1–8. doi: 10.1027/1618-3169/a000268. [DOI] [PubMed] [Google Scholar]
- Hu K, Bauer A, Padmala S, Pessoa L. Threat of bodily harm has opposing effects on cognition. Emotion. 2012;12(1):28–32. doi: 10.1037/a0024345. http://dx.doi.org/10.1037/a0024345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Judah MR, Grant DM, Mills AC, Lechner WV. Factor structure and validation of the attentional control scale. Cognition & Emotion. 2013:1–19. doi: 10.1080/02699931.2013.835254. http://dx.doi.org/10.1080/02699931.2013.835254 (ahead-of-print) [DOI] [PubMed]
- Keogh E, French CC. The effects of mood manipulation and trait anxiety on susceptibility to distraction. Personality and Individual Differences. 1997;22:141–149. [Google Scholar]
- Kessler RC, Angermeyer M, Anthony JC, de Graaf R, Demyttenaere K, Gasquet I, et al. Lifetime prevalence and age-of-onset distributions of mental disorders in the World Health Organization’s World Mental Health Survey Initiative. World Psychiatry. 2007;6:168. [PMC free article] [PubMed] [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The ‘Trier Social Stress Test’ —a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Stice E, Kazdin A, Offord D, Kupfer D. How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. American Journal of Psychiatry. 2001;158:848–856. doi: 10.1176/appi.ajp.158.6.848. http://dx.doi.org/10.1176/appi.ajp.158.6.848. [DOI] [PubMed] [Google Scholar]
- Lonigan CJ, Vasey MW, Phillips BM, Hazen RA. Temperament, anxiety, and the processing of threat-relevant stimuli. Journal of Clinical Child and Adolescent Psychology. 2004;33:8–20. doi: 10.1207/S15374424JCCP3301_2. [DOI] [PubMed] [Google Scholar]
- Mineka S, Kelly KA. The relationship between anxiety, lack of control, and loss of control. In: Steptoe A, Appels A, editors. Stress, control, and personal health. Chichester, UK: John Wiley & Sons; 1989. pp. 163–191. [Google Scholar]
- Moriya J, Tanno Y. Relationships between negative emotionality and attentional control in effortful control. Personality and Individual Differences. 2008;44(6):1348–1355. http://dx.doi.org/10.1016/j.paid.2007.12.003. [Google Scholar]
- Moser JS, Becker MW, Moran TP. Enhanced attentional capture in trait anxiety. Emotion. 2012;12:213–216. doi: 10.1037/a0026156. http://dx.doi.org/10.1037/a0026156. [DOI] [PubMed] [Google Scholar]
- Nigg JT. Temperament and developmental psychopathology. Journal of Child Psychology and Psychiatry. 2006;47:395–422. doi: 10.1111/j.1469-7610.2006.01612.x. [DOI] [PubMed] [Google Scholar]
- Ólafsson RP, Smári J, Guđmundsdóttir F, Olafsdóttir G, Harđardóttir HL, Einarsson SM. Self reported attentional control with the Attentional Control Scale: factor structure and relationship with symptoms of anxiety and depression. Journal of Anxiety Disorders. 2011;25(6):777–782. doi: 10.1016/j.janxdis.2011.03.013. http://dx.doi.org/10.1016/j.janxdis.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. http://dx.doi.org/10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Olvet DM, Hajcak G. The error-related negativity (ERN) and psychopathology: toward an endophenotype. Clinical Psychology Review. 2008;28:1343–1354. doi: 10.1016/j.cpr.2008.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz PC, Gross JJ, Urry HL. Selection, optimization, and compensation in the domain of emotion regulation: Applications to adolescence, older age, and major depressive disorder. Social and Personality Psychology Compass. 2012;6(2):142–155. http://dx.doi.org/10.1111/j.1751-9004.2011.00413.x. [Google Scholar]
- Pacheco-Unguetti AP, Acosta A, Callejas A, Lupiáñez J. Attention and anxiety: different attentional functioning under state and trait anxiety. Psychological Science. 2010;21:298–304. doi: 10.1177/0956797609359624. http://dx.doi.org/10.1177/0956797609359624. [DOI] [PubMed] [Google Scholar]
- Papageorgiou C, Wells A. Treatment of recurrent major depression with attention training. Cognitive and Behavioral Practice. 2000;7(4):407–413. [Google Scholar]
- Putman P, Verkuil B, Arias-Garcia E, Pantazi I, van Schie C. EEG theta/beta ratio as a potential biomarker for attentional control and resilience against deleterious effects of stress on attention. Cognitive, Affective, & Behavioral Neuroscience. 2013:1–10. doi: 10.3758/s13415-013-0238-7. [DOI] [PubMed] [Google Scholar]
- Rapee RM, Craske MG, Brown TA, Barlow DH. Measurement of perceived control over anxiety-related events. Behavior Therapy. 1996;27:279–293. [Google Scholar]
- Richey JA, Keough ME, Schmidt NB. Attentional control moderates fearful responding to a 35% CO2 challenge. Behavior Therapy. 2012;43:285–299. doi: 10.1016/j.beth.2011.06.004. http://dx.doi.org/10.1016/j.beth.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Robinson OJ, Krimsky M, Grillon C. The impact of induced anxiety on response inhibition. Frontiers in Human Neuroscience. 2013;7:1–5. doi: 10.3389/fnhum.2013.00069. http://dx.doi.org/10.3389/fnhum.2013.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi S, Ding C, Li H. Neural correlates of inefficient filtering of emotionally neutral distractors from working memory in trait anxiety. Cognitive, Affective, & Behavioral Neuroscience. 2013:1–13. doi: 10.3758/s13415-013-0203-5. http://dx.doi.org/10.3758/s13415-013-0203-5. [DOI] [PubMed]
- Shadish WR, Cook TD, Campbell DT. Experimental and Quasi-experimental Designs for Generalized Causal Inference. Boston: Houghton Mifflin; 2002. [Google Scholar]
- Sari BA, Koster EHW, Pourtois G, Derakshan N. Training working memory to improve attentional control in anxiety: a proof-of-principle study using behavioral and electrophysiological measures. Biological Psychology. 2015 doi: 10.1016/j.biopsycho.2015.09.008. http://dx.doi.org/10.1016/j.biopsycho.2015.09.008. [DOI] [PubMed]
- Siegle GJ, Ghinassi F, Thase ME. Neurobehavioral therapies in the 21st century: summary of an emerging field and an extended example of cognitive control training for depression. Cognitive Therapy and Research. 2007;31:235–262. http://dx.doi.org/10.1007/s10608-006-9118-6. [Google Scholar]
- Silvert L, Delplanque S, Bouwalerh H, Verpoort C, Sequeira H. Autonomic responding to aversive words without conscious valence discrimination. International Journal of Psychophysiology. 2004;53:135–145. doi: 10.1016/j.ijpsycho.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Spada MM, Georgiou GA, Wells A. The relationship among metacognitions, attentional control, and state anxiety. Cognitive Behaviour Therapy. 2010;39:64–71. doi: 10.1080/16506070902991791. http://dx.doi.org/10.1080/16506070902991791. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anxiety Inventory for Adults manual. Menlo Park, CA: Mind Garden, Inc; 1983. [Google Scholar]
- Sportel BE, Nauta MH, de Hullu E, de Jong PJ, Hartman CA. Behavioral inhibition and attentional control in adolescents: robust relationships with anxiety and depression. Journal of Child and Family Studies. 2011;20:149–156. doi: 10.1007/s10826-010-9435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YY, Ma Y, Wang J, Fan Y, Feng S, Lu Q, et al. Short-term meditation training improves attention and self-regulation. Proceedings of the National Academy of Sciences. 2007;104(43):17152–17156. doi: 10.1073/pnas.0707678104. http://dx.doi.org/10.1073/pnas.0707678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry HL. Seeing, thinking, and feeling: emotion-regulating effects of gaze-directed cognitive reappraisal. Emotion. 2010;10:125. doi: 10.1037/a0017434. http://dx.doi.org/10.1037/a0017434. [DOI] [PubMed] [Google Scholar]
- Van Bockstaele B, Verschuere B, Tibboel H, De Houwer J, Crombez G, Koster EH. A review of current evidence for the causal impact of attentional bias on fear and anxiety. Psychological Bulletin. 2014;140(3):682. doi: 10.1037/a0034834. http://dx.doi.org/10.1037/a0034834. [DOI] [PubMed] [Google Scholar]
- Wieser MJ, Pauli P, Mühlberger A. Probing the attentional control theory in social anxiety: an emotional saccade task. Cognitive, Affective, & Behavioral Neuroscience. 2009;9(3):314–322. doi: 10.3758/CABN.9.3.314. http://dx.doi.org/10.3758/cabn.9.3.314. [DOI] [PubMed] [Google Scholar]
- Wolpe J. The Practice of Behavioral Therapy. 3. New York: Pergamon Press; 1982. [Google Scholar]