This case-control study investigates the prevalence and type of learning disability in a cohort of patients with posterior cortical atrophy compared with patients with logopenic variant primary progressive aphasia and amnestic Alzheimer disease.
Key Points
Question
What is the prevalence and category type of learning disability in patients with posterior cortical atrophy?
Findings
In this case-control study of 279 patients, learning disabilities were observed at higher rates among patients with posterior cortical atrophy and logopenic variant primary progressive aphasia than in the general population and a cohort with amnestic Alzheimer disease. Investigations into the category type of learning disability revealed that the patients with posterior cortical atrophy had a significantly higher amount of nonlanguage mathematical and visuospatial learning disabilities compared with the general population and the control group.
Meaning
Domain-specific learning differences may be associated with domain-specific neurodegenerative diseases, raising the possibility that neurodevelopment is associated with targeting and selective vulnerability to neurodegenerative disease.
Abstract
Importance
Increased prevalence of language-based learning disabilities (LDs) has been previously reported in patients with primary progressive aphasia (PPA). This study hypothesized that patients with focal neurodegenerative syndromes outside the language network, such as posterior cortical atrophy (PCA), would have a higher rate of nonlanguage LDs, congruent with their mainly visuospatial presentation.
Objective
To investigate the prevalence and type of LD (language and/or mathematical and visuospatial) in a large cohort of patients with PCA compared with patients with logopenic variant PPA (lvPPA) and amnestic Alzheimer disease (AD).
Design, Setting, and Participants
This case-control study reviewed 279 medical records from a university-based clinic and research center for patients with neurodegenerative diseases for LD history, including patients with PCA (n = 95), patients with lvPPA (n = 84), and a matched cohort with amnestic AD (n = 100). No records were excluded. The study compared cognitive and neuroimaging features of patients with PCA with and without LDs. A review of the records of patients presenting from March 1, 1999, to August 31, 2014, revealed 95 PCA cases and 84 lvPPA cases. Then 100 patients with amnestic AD from this same period were chosen for comparison, matching against the groups for age, sex, and disease severity. Data analysis was performed from September 8, 2013, to November 6, 2017.
Main Outcomes and Measures
Prevalence of total LD history and prevalence of language and mathematical or visuospatial LD history across all cohorts.
Results
A total of 179 atypical AD cases (95 with PCA and 84 with lvPPA) and 100 disease control cases (amnestic AD) were included in the study. The groups were not statistically different for mean (SD) age at first visit (PCA, 61.9 [7.0] years; lvPPA, 65.1 [8.7] years; amnestic AD, 64.0 [12.6] years; P = .08), mean (SD) age at first symptom (PCA, 57.5 [7.0] years; lvPPA, 61.1 [9.0] years; amnestic AD, 59.6 [13.7] years; P = .06), or sex (PCA, 66.3% female; lvPPA, 56.0% female; amnestic AD, 57.0% female; P = .30) but differed on non–right-hand preference (PCA, 18.3%; lvPPA, 20.2%; amnestic AD, 7.7%; P = .04), race/ethnicity (PCA, 88.3% white; lvPPA, 99.0% white; amnestic AD, 80.0% white; P < .001), and mean (SD) educational level (PCA, 15.7 [3.2] years; lvPPA, 16.2 [3.3] years; amnestic AD, 14.8 [3.5] years; P = .02). A total of 18 of the 95 patients with PCA (18.9%) reported a history of LD, which is greater than the 3 of 100 patients (3.0%) in the amnestic AD cohort (P < .001) and the 10.0% expected rate in the general population (P = .007). In the PCA cohort, 13 of 95 patients (13.7%) had a nonlanguage mathematical and/or visuospatial LD; this rate was greater than that in the amnestic AD (1 of 100 [1.0%]; P < .001) and lvPPA (2 of 84 [2.4%]; P = .006) cohorts and greater than the 6.0% expected general population rate of mathematical LD (P = .003). Compared with the patients with PCA without LDs, the group with LDs had greater preservation of global cognition and a more right-lateralized pattern of atrophy.
Conclusions and Relevance
Nonlanguage mathematical and visuospatial LDs were associated with focal, visuospatial predominant neurodegenerative clinical syndromes. This finding supports the hypothesis that neurodevelopmental differences in specific brain networks are associated with phenotypic manifestation of later-life neurodegenerative disease.
Introduction
The risk factors that predispose individuals to phenotypically atypical neurodegenerative disease remain largely unknown. Increasing evidence suggests that neurodevelopmental differences account, in part, for susceptibility of the language network to neurodegenerative disease later in life.1,2 Previously, we found more frequent language-based learning disabilities (LDs) in patients with logopenic variant primary progressive aphasia (lvPPA) than in healthy control individuals and those with other forms of primary progressive aphasia (PPA).2 Logopenic variant PPA, a disorder of the phonologic system, with degeneration of left posterior temporal and inferior parietal regions,3 has remarkable clinical and neuroanatomical parallels with dyslexia.4 These parallels between dyslexia and lvPPA suggest that the presence of language-based LDs might alter the neuroanatomical reserve of the phonologic language network, affecting the manifestation and clinical course of neurodegenerative disease in susceptible individuals.2 On the basis of these network-specific associations between language-based LDs and language domain neurodegenerative disease, we hypothesized that this association might also be true for another focal cortical neurologic disorder, posterior cortical atrophy (PCA), a disorder usually associated with Alzheimer disease (AD) pathologic findings that lacks any established susceptibility factors.
Posterior cortical atrophy is a neurodegenerative disorder that presents with selective visuospatial and visuoperceptual deficits and bilateral (most often right-sided greater than left-sided) occipito-temporo-parietal atrophy.5 A core symptom of PCA, simultagnosia,6 is a deficit in visual integration, often manifesting as impaired awareness of more than 1 visual stimuli at a time.7 Simultagnosia is associated with bilateral parieto-occipital damage with varying degrees of lateralization. In a series of stroke cases, simultagnosia was associated with right greater than left superior parietal damage,8 whereas in a series of patients with PCA and simultagnosia, the opposite was observed.9 These same biparieto-occipital localizations subserve mathematical abilities, with perhaps specializations between the left and right sides,10,11 the former with greater relevance to complex arithmetic, symbol mapping, and verbal-based counting12 and the latter with processing quantity,13 number intuition, and subitization (the rapid and accurate nonverbal recognition of 1-4 objects).12,14 Possibly as a consequence of visual integration disability and its anatomical overlap, mathematical abilities are severely impaired in patients with PCA.15,16
Selective deficits in arithmetic abilities, when out of proportion to generalized cognition, are the hallmark of dyscalculia—the most common mathematical LD in the general population.17 At the core of dyscalculia are speculated deficits in abstract numerical intuition or nonsymbolic number sense, also known as numerosity.17,18,19,20 Similar to dyslexia, developmental dyscalculia is highly heritable,21 and genetic models of developmental dyscalculia demonstrate functional and structural alterations of the right intraparietal sulcus.22 Dyscalculia can be temporarily induced in healthy adults without dyscalculia through transcranial magnetic stimulation of the right intraparietal sulcal region.23
The development of dyslexia and dyscalculia is thought to involve neuronal migration within neocortical structures.22,24 Because of the left hemisphere correlation between dyslexia and lvPPA and overlapping right hemisphere localizations between mathematical and visuospatial abilities and PCA disease patterns, we hypothesized that (1) LDs would appear at higher rates in cortically predominant AD syndromes, such as lvPPA and PCA, than in typical hippocampal- and entorhinal-based AD and (2) networks associated with right hemisphere developmental disabilities, especially mathematical and visuospatial abilities, would manifest more often in PCA than in lvPPA or amnestic AD. To examine these hypotheses, we reviewed the medical records of patients with PCA, patients with lvPPA, and matched patients with amnestic AD3,25 for a history of LD and then categorized these LDs into language and nonlanguage presentations.
Methods
Participants
We identified 95 patients diagnosed with PCA at first visit in the University of California, San Francisco (UCSF) Memory and Aging Center (MAC) database and 84 patients with lvPPA (including 48 previously described2) from March 1, 1999, to August 31, 2014. We selected a group of 100 patients with amnestic AD who were matched to the PCA and lvPPA groups by age (as measured at first visit, within 1.5 SDs of the combined mean of the lvPPA and PCA cohorts), sex, and disease severity (as measured by the Mini-Mental State Examination [MMSE]). Demographics, sex, hand preference, race/ethnicity, and educational level were obtained by open-ended questioning or patient self-disclosure on intake questionnaires. Race/ethnicity was included in this study to investigate bias. Data analysis was performed from September 8, 2013, to November 6, 2017. All patients provided written informed consent to share their clinical data for research purposes. All data were deidentified. The study was approved by the UCSF Human Research Committee.
Neurodegenerative disease diagnoses were established with current diagnostic criteria for PCA,6,26 lvPPA,3 and AD.27 Amyloid imaging data were available in 33 of 95 patients with PCA (34.7%), 35 of 84 patients with lvPPA (41.7%), and 4 of 100 patients with amnestic AD (4.0%). Autopsies were performed on 12 of 95 patients with PCA (12.6%), 10 of 84 patients with lvPPA (11.9%), and 18 of 100 patients with amnestic AD (18.0%) (4 PCA, 4 lvPPA, and 2 amnestic AD cases had overlapping amyloid imaging and autopsy results) (Table 1).
Table 1. Population Demographics of the PCA Cohort.
| Diagnostic Group | Amnestic AD (n = 100) | lvPPA (n = 84) | Total PCA (n = 95) | P Value | PCA With LDs (n = 18) | PCA Without LDs (n = 77) | P Value |
|---|---|---|---|---|---|---|---|
| Age at first visit, mean (SD), y | 64.0 (12.6) | 65.1 (8.7) | 61.9 (7.0) | .08 | 60.2 (7.4) | 62.2 (7.0) | .30 |
| Age at first symptom, mean (SD), y | 59.6 (13.7) | 61.1 (9.0) | 57.5 (7.0) | .06 | 55.9 (7.4) | 57.9 (7.0) | .30 |
| Non–right-handed hand preference, No./total No. (%) | 7/91 (7.7) | 17/84 (20.2) | 17/93 (18.3) | .04 | 4/18 (22.2) | 13/75 (17.3) | .70 |
| Female, No./total No. (%) | 57/100 (57.0) | 47/84 (56.0) | 63/95 (66.3) | .30 | 11/18 (61.1) | 52/77 (67.5) | .60 |
| White race, No./total No. (%) | 71/89 (80.0) | 77/78 (99.0) | 83/94 (88.3) | <.001 | 16/18 (88.9) | 67/76 (88.2) | >.99 |
| Educational level, mean (SD), y [n] | 14.8 (3.5) [90] | 16.2 (3.3) [79] | 15.7 (3.2) [95] | .02 | 16.7 (3.4) [18] | 15.5 (3.1) [77] | .20 |
| APOE4 allelic frequency, No./total No. (%) | 21/64 (32.8) | 21/98 (21.4) | 24/96 (25.0) | .30 | 5/24 (20.8) | 19/72 (26.4) | .60 |
| Positive amyloid biomarker imaging results, No./total No. (%) | 4/4 (100) | 34/35 (97.1) | 33/33 (100) | .60 | 13/13 (100) | 20/20 (100) | >.99 |
| Positive autopsy results for AD pathologic features, No./total No. (%)a | 18/18 (100)b | 10/10 (100)b | 12/12 (100)b,c | >.99 | 0 | 12/12 (100)b,c | >.99 |
| CDR total, mean (SD) [n] | 1.0 (0.6) [59] | 0.6 (0.3) [57] | 1 (0.6) [66] | <.001 | 0.8 (0.3) [14] | 1.0 (0.6) [52] | .20 |
| CDR sum of boxes, mean (SD) [n] | 5.8 (3.7) [59] | 3.0 (1.6) [57] | 5.3 (3.3) [66] | <.001 | 4.4 (1.6) [14] | 5.6 (3.6) [52] | .20 |
| MMSE score at first visit, mean (SD) [n] | 21.2 (5.8) [76] | 19.6 (6.7) [75] | 19.5 (7.5) [95] | .20 | 22.7 (5.4) [18] | 18.8 (7.5) [77] | .04 |
| CVLT 10-min free recall score, mean (SD) [n] | 1.2 (1.7) [74] | 2.4 (2.5) [67] | 2.7 (2.5) [75] | <.001 | 3.4 (2.6) [11] | 2.5 (2.5) [64] | .30 |
| Boston Naming test score, mean (SD) [n] | 11.2 (3.0) [74] | 9.0 (4.5) [71] | 11.3 (3.6) [72] | <.001 | 13 (2.4) [11] | 11 (3.7) [61] | .20 |
| Semantic fluency score, mean (SD) [n] | 9.4 (4.8) [78] | 7.2 (5.2) [73] | 10.3 (5.2) [82] | <.001 | 13.2 (4.0) [14] | 9.6 (5.2) [68] | .02 |
| Digits backward score, mean (SD) [n] | 3.5 (1.2) [74] | 2.9 (1.1) [72] | 2.9 (1.2) [84] | .005 | 3.6 (1.1) [14] | 2.8 (1.2) [70] | .03 |
| Benson copy test score, mean (SD) [n] | 12.1 (4.5) [72] | 13.3 (3.9) [73] | 4.7 (4.8) [77] | <.001 | 6.5 (5.1) [15] | 4.2 (4.7) [62] | .10 |
| Benson recall test score, mean (SD) [n] | 2.7 (3.2) [73] | 5.3 (3.6) [72] | 2.6 (3.4) [79] | <.001 | 2.9 (2.8) [15] | 2.5 (3.5) [64] | .70 |
| Calculations, mean (SD) [n] | 3.7 (5.0) [75] | 2.9 (1.2) [72] | 2.2 (1.3) [68] | .01 | 2.2 (0.9) [13] | 2.2 (1.4) [55] | .90 |
| VOSP, mean (SD) [n] | 6.9 (2.5) [34] | 7.2 (2.6) [54] | 4.6 (3.0) [48] | <.001 | 5.0 (2.4) [13] | 4.4 (3.1) [35] | .60 |
Abbreviations: AD, Alzheimer disease; APOE4, apolipoprotein E ε4; CDR, Clinical Dementia Rating; CVLT, California Verbal Learning Test; LDs, learning disability; lvPPA, logopenic variant primary progressive aphasia; PCA, posterior cortical atrophy; PPA, primary progressive aphasia; VOSP, Visual Object and Space Perception.
Amyloid and tau pathologic findings that met Braak, Consortium to Establish a Registry for Alzheimer Disease, Thal, and/or National Institute on Aging Reagan criteria as the primary or coprimary diagnosis.
Overlapping autopsy and amyloid biomarker imaging results.
One patient had copathologic Pick disease and AD.
Identification and Classification of LD
As a part of the standard clinical evaluation (detailed and thorough 1-hour history and physical examination), all patients at the UCSF MAC are asked not only about educational attainment but also about scholastic strengths and weaknesses. History of LD was obtained by medical record review, searching for evidence of developmental cognitive impairments in language (speaking or reading, including diagnoses of dyslexia and/or stuttering, and a history of delay in speaking or reading) and for other developmental impairments, including those in nonlanguage domains (diagnosis of dyscalculia, self-report of difficulties in mathematical and/or visuospatial functioning during childhood).
Statistical Analysis of Demographic, LD, and Neuropsychological Data
The χ2 and Fisher exact tests were used for prevalence comparisons of the amnestic AD, lvPPA, and PCA groups in total LD and nonlanguage LD presentations. The χ2 and Fisher exact tests were used for observed total LD prevalence across the various disease cohorts vs a 10.0% expected general population rate,17,28 as well as analyses of observed nonlanguage LD prevalence vs a 6.0% expected general population rate for nonlanguage LD.17,28,29,30 Demographic and neuropsychological group comparisons were examined using Mann-Whitney U nonparametric statistics when comparing continuous variables, and the χ2 and Fisher exact tests were performed for categorical variables.
Neuroimaging Analysis Comparing Patients With PCA With and Without LDs
Participants
Patients with PCA were included in this analysis if (1) their magnetic resonance images were acquired within 1 year of the clinical visit, (2) their image was obtained on a 3-T magnetic resonance scanner, (3) their MMSE score was above 15, and (4) their Clinical Dementia Rating was less than 2. A group of 20 healthy control individuals who also had 3-T magnetic resonance images and were matched for age, sex, handedness, and sample size were included. After comparisons between all PCA and controls, the PCA cohort was split into those with and without LD histories and compared with the same group of controls.
Image Acquisition
All patients underwent structural magnetic resonance imaging to obtain sequences previously described on a 3-T Siemens TrioTlm Snygo scanner.31 Healthy imaging controls were recruited from the UCSF MAC healthy aging cohort.
Voxel-Based Morphometry
Image analysis was performed using SPM12 software (http://www.fil.ion.ucl.ac.uk/spm) developed in the Wellcome Department of Imaging Neuroscience, Institute of Neurology, University College London, running in MATLAB R2014b (Mathworks). Voxel-based morphometry was performed following standard procedures, as previously described.2 Analysis of variance testing across the different groups was performed in SPM12. Contrasts of interest included the comparisons of the total PCA group, the PCA without LDs group, and the PCA with LDs group with the same group of matched healthy controls. All statistical analyses were performed controlling for age, sex, handedness, and total intracranial volume as covariates. Additional comparisons between the degree of atrophy on the left and right sides within the PCA with LDs and the PCA without LDs groups were evaluated using z scores. Statistical thresholds were set at 2-sided P < .05 corrected for familywise error.
Results
A total of 179 patients (95 with PCA and 84 with lvPPA) and 100 controls with disease (amnestic AD) were included in the study. The groups were not statistically different for mean (SD) age at first visit (PCA, 61.9 [7.0] years; lvPPA, 65.1 [8.7] years; amnestic AD, 64.0 [12.6] years; P = .08), mean (SD) age at first symptom (PCA, 57.5 [7.0] years; lvPPA, 61.1 [9.0] years; amnestic AD, 59.6 [13.7] years; P = .06), or sex (PCA, 66.3% female; lvPPA, 56.0% female; amnestic AD, 57.0% female; P = .30) but differed on non–right-hand preference (PCA, 18.3%; lvPPA, 20.2%; amnestic AD, 7.7%; P = .04), race/ethnicity (PCA, 88.3% white; lvPPA, 99.0% white; amnestic AD, 80.0% white; P < .001), and mean (SD) educational level (PCA, 15.7 [3.2] years; lvPPA, 16.2 [3.3] years; amnestic AD, 14.8 [3.5] years; P = .02).
Prevalence of LDs Across Cohorts
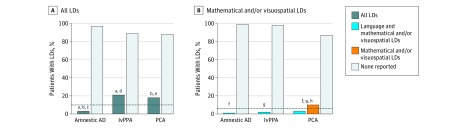
Among the 3 groups, we identified 42 individuals who had LDs. In the studied groups, 3 of 100 individuals with amnestic AD (3.0%), 21 of 84 with lvPPA (25.0%), and 18 of 95 with PCA (18.9%) had a history of LD. Comparison of prevalences of LD showed group-level differences (3/100 vs 21/84 vs 18/95, P < .001). Posthoc pairwise comparisons showed differences between the amnestic AD and PCA cohorts (3/100 vs 18/95, P < .001) and the amnestic AD and lvPPA cohorts (3/100 vs 21/84, P < .001) and no differences between the lvPPA and PCA cohorts (21/84 vs 18/95, P = .30). Compared with a 10.0% estimated general population rate of LDs,17,28 individuals with PCA and lvPPA had a significantly elevated prevalence of LDs (P = .007 for the PCA group and P < .001 for the lvPPA group), whereas the amnestic AD cohort had a statistically smaller frequency of LDs (P = .02) (Figure 1A).
Figure 1. Prevalence of Learning Disabilities (LDs) in Patients With Amnestic Alzheimer Disease (AD), Logopenic Variant Primary Progressive Aphasia (lvPPA), and Posterior Cortical Atrophy (PCA).
Data shown are for 100 patients with amnestic AD, 84 with lvPPA, and 95 with PCA. A, Comparisons across all cohorts revealed statistically significant differences in the prevalence of all LDs (amnestic AD vs lvPPA vs PCA, P < .001). The dashed line indicates the 10.0% estimated rate of all LDs in the general population. B, Comparisons across all cohorts revealed statistically significant differences in the prevalence of mathematical and/or visuospatial LDs (amnestic AD vs lvPPA vs PCA, P < .001). The dashed line indicates the 6.0% estimated rate of mathematical and/or visuospatial LDs in the general population.
aP < .001, post hoc pairwise comparisons of all LDs between amnestic AD and lvPPA.
bP < .001, post hoc pairwise comparisons of all LDs between amnestic AD and PCA.
cP = .02, observed vs expected rates of all LDs.
dP < .001, observed vs expected rates of all LDs.
eP = .007, observed vs expected rates of mathematical and/or visuospatial LDs.
fP < .001, post hoc pairwise comparisons of mathematical and/or visuospatial LDs between amnestic AD and PCA.
gP = .006, post hoc pairwise comparisons of mathematical and/or visuospatial LDs between lvPPA and PCA.
hP = .003, observed vs expected rates of mathematical and/or visuospatial LDs.
LD Type Across Diagnostic Cohorts
Subdividing LD type into language and nonlanguage yielded 32 language and 19 nonlanguage LD cases, including 9 overlapping language and nonlanguage LD cases. In the amnestic AD cohort, 2 individuals had sole language LD and 1 had overlapping language and mathematical LDs. In the lvPPA cohort, all individuals with LDs had language-based LDs, with 2 overlapping mathematical LD cases. In the PCA cohort, 5 had exclusively language-based LDs, 10 had exclusively nonlanguage mathematical and/or visuospatial LDs, and 3 had LDs in both domains.
Across the diagnostic cohorts, the prevalence of nonlanguage LDs revealed group differences (1/100 vs 2/84 vs 13/95, P < .001). Posthoc pairwise comparisons revealed differences between the amnestic AD and PCA cohorts (1/100 vs 13/95, P < .001) and the lvPPA and PCA cohorts (2/84 vs 13/95, P = .006) and no differences between the amnestic AD and lvPPA cohorts (1/100 vs 2/84, P = .50).
Because 13 of 95 members of the PCA cohort (13.7%) had a mathematical LD, assuming a 6.0% general population rate of mathematical LDs,17,28,29,30 the PCA cohort had an elevated prevalence of mathematical LDs compared with the general population (13 observed vs 6 expected, P = .003). The rates in the amnestic AD and lvPPA cohorts did not differ statistically from the general population rate (Figure 1B).
Demographic and Neuropsychological Data in Patients With PCA With and Without LDs
No differences were found between the patients with PCA with and without LDs in age at first visit, age at first symptoms, hand preference, sex, race/ethnicity, educational level, apolipoprotein E ε4 (APOE4) allelic frequency, total Clinical Dementia Rating, or Clinical Dementia Rating sum of boxes. Cognitive measures of global function (MMSE, semantic fluency, and digits backward) were spared in the patients with PCA with LDs compared with the patients with PCA without LDs, indicating a more focal visuospatial cognitive syndrome with relatively spared language functions in the PCA with LDs group (Table 1). In the PCA with LDs group, comparisons between the 5 patients with PCA with sole language LDs and the 10 with sole mathematical and/or visuospatial LDs revealed no differences across demographic and clinical measures.
Neuroimaging Study
For this imaging substudy, 19 of 95 individuals with PCA met the inclusion criteria, 10 of whom had a history of LDs. Of these 10 individuals with PCA with LDs, 6 had sole mathematical and/or visuospatial LDs, 3 had language and mathematical and/or visuospatial LDs, and 1 had a sole language LD. We used voxel-based morphometry (VBM) to compare the total PCA cohort (n = 19; age, 59.2 years; 10 women; 15 right-handed) and the healthy controls (n = 20; age, 63.2 years; 11 women; 16 right-handed). We also compared separately the PCA with LDs subgroup (n = 10, age, 56.9 years; 6 women; 6 right-handed) and the PCA without LDs subgroup (n = 9; age, 61.7 years; 4 women; 9 right-handed) with the same cohort of healthy controls. A post hoc analysis of demographic, functional, and cognitive scores between these cohorts used for VBM showed that the only difference was that the PCA with LDs group performed better on digits backward (Table 2).
Table 2. Demographics of the Voxel-Based Morphometry PCA Cohort.
| Diagnostic Group | Total PCA (n = 19) | PCA With LDs (n = 10)a | PCA Without LDs (n = 9) | P Value |
|---|---|---|---|---|
| Age at first visit, mean (SD), y | 59.2 (6.4) | 56.9 (6.4) | 61.7 (5.7) | .10 |
| Age at first symptom, mean (SD), y | 55.0 (6.7) | 52.6 (6.6) | 57.7 (6.0) | .10 |
| Non–right-handed hand preference, No./total No. (%) | 3/19 (15.8) | 3/10 (30.0) | 0 | .20 |
| Female, No./total No. (%) | 10/19 (52.6) | 6/10 (60.0) | 4/9 (44.4) | .70 |
| White race, No./total No. (%) | 18/19 (94.7) | 9/10 (90.0) | 9/9 (100) | >.99 |
| Educational level, mean (SD), y | 15.6 (2.1) | 16.3 (2.5) | 15 (1.5) | .20 |
| APOE4 allelic frequency, No./total No. (%) | 9/38 (23.7) | 4/20 (20.0) | 5/18 (27.8) | .70 |
| Positive amyloid biomarker imaging results, No./total No. (%) | 17/17 (100) | 10/10 (100) | 7/7 (100) | >.99 |
| Positive autopsy results for AD pathologic findings, No./total No. (%) | 1/1 (100) | 0 | 1/1 (100) | >.99 |
| CDR total, mean (SD) [n] | 0.8 (0.3) [18] | 0.9 (0.2) [9] | 0.7 (0.3) [9] | .06 |
| CDR sum of boxes, mean (SD) [n] | 3.9 (1.9) [18] | 4.7 (1.5) [9] | 3.1 (1.9) [9] | .08 |
| MMSE score at first visit, mean (SD) | 22.7 (3.8) | 23.1 (3.6) | 22.3 (4.2) | .70 |
| CVLT 10-min free recall score, mean (SD) [n] | 3.3 (2.8) [15] | 3.6 (2.6) [7] | 3.1 (3.1) [8] | .80 |
| Boston naming test score, mean (SD) [n] | 13.1 (1.7) [14] | 13.0 (2.2) [6] | 13.1 (1.6) [8] | .90 |
| Semantic fluency score, mean (SD) [n] | 11.7 (3.7) [17] | 12.9 (4.3) [9] | 10.4 (2.4) [8] | .20 |
| Digits backward score, mean (SD) [n] | 3.1 (1.0) [17] | 3.6 (1.1) [9] | 2.6 (0.5) [8] | .05 |
| Benson copy test score, mean (SD) [n] | 5.3 (4.3) [17] | 5.7 (4.3) [9] | 5.0 (4.5) [8] | .80 |
| Benson recall test score, mean (SD) [n] | 3.1 (2.6) [17] | 2.8 (2.2) [9] | 3.3 (3.1) [8] | .80 |
| Calculations, mean (SD) [n] | 2.1 (1.4) [16] | 2.1 (1.1) [8] | 2.1 (1.7) [8] | >.99 |
| VOSP, mean (SD) [n] | 4.4 (2.9) [15] | 5.0 (2.7) [8] | 3.7 (3.3) [7] | .40 |
Abbreviations: APOE4, apolipoprotein E ε4; CDR, Clinical Dementia Rating; CVLT, California Verbal Learning Test; LD, learning disability; MMSE, Mini-Mental State Examination; PCA, posterior cortical atrophy; VOSP, Visual Object and Space Perception.
Between the LD and non-LD PCA groups, there were no differences except for performance on digits backward.
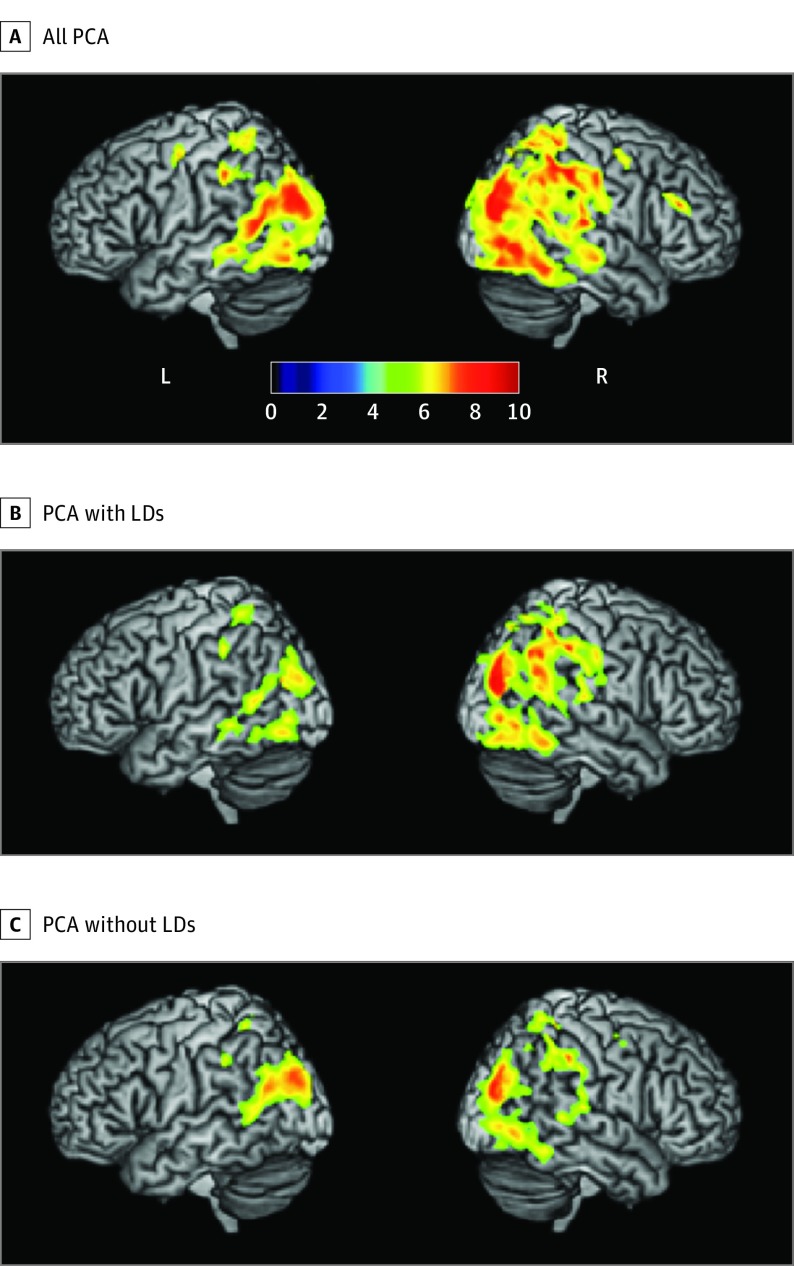
The VBM analyses found the expected pattern of bilateral, right-sided greater than left-sided occipito-temporo-parietal atrophy in the total PCA cohort. Splitting the PCA cohorts into groups with and without LDs produced atrophy maps where the amount of atrophy appears right lateralized in the PCA with LDs group and bilateral in distribution in the PCA without LDs group (Figure 2 and Table 3). To demonstrate the extent of atrophy asymmetry in the PCA with LDs cohort compared with the PCA without LDs cohort, we repeated the VBM analyses masking for left and right hemispheres and summed the cluster size for each side separately. Using these measures, we calculated an atrophy index of asymmetry (left − right/left + right) for each group, obtaining a value of −0.46 for the PCA with LDs cohort and −0.22 for PCA without LDs cohort.
Figure 2. Voxel-Based Morphometry (VBM) for All Patients With Posterior Cortical Atrophy (PCA), Patients With PCA and Learning Disabilities (LDs), and Patients With PCA Without LDs.
The atrophy patterns in all 19 patients with PCA along with the 10 patients with PCA and LDs and the 9 patients with PCA without LDs each vs 20 healthy controls are shown. A, The entire cohort had right-sided greater than left-sided atrophy focused on the middle and superior occipital gyri with a rightward extension into the intraparietal sulcus. B, The PCA and LDs cohort had a right-lateralized pattern of atrophy with especially prominent intraparietal sulcal involvement. C, In contrast, the PCA without LDs cohort had a more bilateral pattern of atrophy focused around the middle and superior occipital gyri. The colored bar indicates the t value in the VBM analysis.
Table 3. Voxel-Based Morphometry Clusters for All PCA, PCA With LDs, and PCA Without LDs.
| Region | Cluster Size | t Value | z Score | x | y | z |
|---|---|---|---|---|---|---|
| All PCA | ||||||
| Right middle occipital | 52 491 | 13.10 | 7.64 | 28 | −80 | 26 |
| Right superior occipital | 10.95 | 7.01 | 26 | −68 | 26 | |
| Right supramarginal | 10.63 | 6.90 | 48 | −36 | 44 | |
| Right middle cingulum | 10.32 | 6.80 | 14 | −38 | 42 | |
| Bilateral cuneus | 10.03 | 6.70 | 0 | −75 | 36 | |
| Right inferior temporal | 9.95 | 6.67 | 50 | −69 | −4 | |
| Right inferior occipital | 9.68 | 6.57 | 42 | −78 | 0 | |
| Right angular | 9.56 | 6.52 | 46 | −51 | 38 | |
| Right postcentral | 9.45 | 6.48 | 18 | −42 | 60 | |
| Right inferior parietal | 9.44 | 6.48 | 44 | −48 | 54 | |
| Left middle occipital | 9.43 | 6.48 | −30 | −84 | 26 | |
| Right middle frontal | 222 | 9.06 | 6.33 | 36 | 33 | 22 |
| Right precentral | 467 | 8.03 | 5.90 | 33 | −3 | 50 |
| Left precentral | 596 | 7.13 | 5.48 | −30 | −9 | 52 |
| Left hippocampus | 162 | 7.02 | 5.42 | −14 | −36 | 3 |
| Right hippocampus | 195 | 6.88 | 5.35 | 16 | −34 | 3 |
| Right inferior frontal | 186 | 6.67 | 5.24 | 39 | 10 | 32 |
| Left superior temporal | 201 | 6.00 | 4.87 | −52 | −21 | 14 |
| PCA With LDs | ||||||
| Right middle occipital | 14 490 | 11.54 | 7.20 | 30 | −80 | 18 |
| Right superior occipital | 8.95 | 6.29 | 26 | −68 | 26 | |
| Right inferior parietal | 8.71 | 6.19 | 44 | −48 | 54 | |
| Right angular | 8.30 | 6.02 | 57 | −51 | 26 | |
| Right superior parietal | 8.22 | 5.99 | 15 | −70 | 54 | |
| Right cingulum | 8.22 | 5.98 | 14 | −38 | 44 | |
| Right precuneus | 8.22 | 5.98 | 20 | −66 | 42 | |
| Right inferior temporal | 3506 | 8.50 | 6.10 | 54 | −51 | −15 |
| Right inferior occipital | 8.34 | 6.04 | 42 | −78 | 0 | |
| Right fusiform | 7.63 | 5.72 | 36 | −64 | −16 | |
| Left middle occipital | 2220 | 7.99 | 5.88 | −27 | −76 | 22 |
| Left inferior temporal | 7.15 | 5.49 | −54 | −58 | 14 | |
| Left superior occipital | 5.87 | 4.80 | −16 | −66 | 40 | |
| Left middle temporal | 5.79 | 4.75 | −48 | −63 | 24 | |
| Left inferior occipital | 1001 | 7.00 | 5.41 | −40 | −75 | −10 |
| Left inferior temporal | 6.42 | 5.11 | −50 | −56 | −8 | |
| Right superior temporal | 345 | 6.93 | 5.38 | 54 | −26 | 14 |
| Left middle temporal | 230 | 6.58 | 5.20 | −60 | −40 | −3 |
| Right middle temporal | 187 | 6.52 | 5.16 | 62 | −20 | −8 |
| PCA Without LDs | ||||||
| Right superior occipital | 17 368 | 9.92 | 6.66 | 28 | −81 | 26 |
| Right middle occipital | 9.65 | 6.56 | 30 | −81 | 20 | |
| Right middle cingulum | 8.61 | 6.15 | 12 | −33 | 36 | |
| Left precuneus | 8.35 | 6.04 | −2 | −75 | 36 | |
| Left middle occipital | 8.30 | 6.02 | −39 | −78 | 30 | |
| Right inferior parietal | 8.22 | 5.98 | 50 | −36 | 45 | |
| Right postcentral | 144 | 7.48 | 5.65 | 58 | −18 | 44 |
| Right precentral | 279 | 7.26 | 5.54 | 34 | −3 | 48 |
| Left superior parietal | 270 | 7.23 | 5.53 | −20 | −46 | 60 |
| Left inferior parietal | 555 | 6.88 | 5.35 | −44 | −38 | 40 |
| Bilateral cuneus | 258 | 6.36 | 5.07 | 4 | −86 | 21 |
| Left middle occipital | 107 | 6.17 | 4.97 | −50 | −74 | 0 |
Abbreviations: LD, learning disability; PCA, posterior cortical atrophy.
Discussion
Neurodevelopmental differences have been hypothesized as susceptibility factors toward the development of late-life focal neurodegenerative syndromes.32 We observe an increased prevalence of LDs among patients with PCA and lvPPA compared with patients with amnestic AD and reported general population rates. Subdividing LDs into language and nonlanguage mathematical and visuospatial types revealed that language-based LDs were more common in patients with lvPPA and nonlanguage mathematical and visuospatial LDs were more common in patients with PCA. The amount of mathematical and visuospatial LDs in patients with PCA was greater than in either AD cohort and greater than reported general population estimates. These findings support an association between domain-specific LDs and domain-specific neurodegenerative diseases, perhaps best understood within a network-specific view of neurodevelopmental and neurodegenerative syndromes.
Multiple studies1,2 have found that patients with PPA are more likely to have a language-based LD than the general population. Previously, we found a specific association between dyslexia, the most common language-based LD, and lvPPA, speculating that developmental weakness of phonologic processing within the left temporoparietal junction might be further associated with preferential neurodegeneration of this network later in life.2 The anatomical localizations of mathematical and visuospatial abilities are less clearly defined than their language counterparts because these abilities are complex and sustained by multiple brain networks. Increasing evidence supports the role of a network centered on the right intraparietal sulcus in humans and other species, especially within numerosity processing.20,33,34 Alterations in these intraparietal sulcal networks are associated with developmental dyscalculia,17,22,23 and in the context of our findings, we speculate that the presence of mathematical and/or visuospatial LDs might make right-sided parietal structures more susceptible to late-life neurodegeneration resulting in focal progressive disease of visuospatial and/or mathematical abilities.
In patients with PCA, 72.2% of all LDs were in nonlanguage domains, whereas the remaining 27.8% presented with language-only LDs. Comparisons between the PCA cohorts with and without LDs revealed greater global preservation of cognition along with greater rightward asymmetry of brain atrophy directly overlying the intraparietal sulcus in the PCA cohort with LDs. Because the PCA groups with and without LDs did not differ in disease duration or assessment of functional impairment, it is unlikely that the greater impairment of nonvisual abilities in the PCA without LDs cohort reflects a difference in disease severity. The greatest selectivity of domain association in this study was within lvPPA, in which all 21 patients with LDs had language-based LDs (including 2 with a comorbid mathematical LD), raising the possibility that this domain association is largely driven by the strength of association within the lvPPA cohort; nevertheless, other investigations lend support to our claims of a selective association between mathematical and visuospatial differences in PCA. A genome-wide association study35 of PCA identified 3 novel putative gene targets, 2 of which are involved in neurodevelopment, including 1 that plays a notable role in the maturation of the visual system. Furthermore, a series that investigated LDs in AD and frontotemporal dementia examined 85 patients with typical amnestic AD and 17 with atypical AD presentations, including 3 with lvPPA and 3 with PCA, and found increased prevalence of LDs in the atypical AD group. All 3 patients with lvPPA had language-only LDs, and 2 of the 3 patients with PCA reported dyscalculia.36 Thus, we believe that there is evidence and precedence to support a domain association between early-life learning differences and later-life neurodegenerative disease risk in lvPPA and PCA.
Although there is no perfect correlation between clinical syndrome and pathologic findings (lvPPA-like syndromes with underlying frontotemporal lobar degeneration pathologic features37,38 and corticobasal degeneration from dementia with Lewy body pathologic features in PCA6,39 have been described), the study cohort had a great degree of pathologic homogeneity. Almost half (40%) the total study cohort underwent amyloid imaging and/or autopsy evaluation, and of this group, only 1 patient with lvPPA had results inconsistent with AD as the primary cause of disease. Nonetheless, these developmental susceptibility findings need not be limited to AD pathologic features. Two previous studies40,41 found that dyslexia-relevant genetics may influence the atrophy patterns in frontotemporal dementia, including PPA, affecting the susceptibility of the language network to disease. The reason specific brain networks are susceptible to certain neurodegenerative diseases in individuals is likely a multifactorial process with contributions from genetics, neurodevelopment, and environmental factors.
Limitations
We did not observe increases in LDs in the amnestic AD cohort compared with the general population, possibly because language and mathematical and visuospatial difficulties, such as dyslexia and dyscalculia, are thought to develop through dysfunction of neocortical neuronal migration,22,24 whereas typical amnestic presentations of AD localize to allocortical structures. Regardless, neurodevelopmental susceptibility is relevant to this population because multiple studies42,43 have found lower gray matter volumes in AD-relevant anatomies in APOE4-positive infants compared with controls. Thus, we may not be adequately assessing the exact developmental domain to capture premorbid differences in typical AD. Alternatively, perhaps there is an ascertainment bias. The amnestic AD group had the largest number of non-white patients, and some researchers have suggested that nonwhite populations may have higher rates of undiagnosed LDs.44 Furthermore, discussions of early-life differences might be seen as less relevant to this cohort or, conversely, more relevant to atypical AD cohorts, leading participants and their study partners to have a recall bias when discussing premorbid strengths and weaknesses. These concerns highlight the significant limitations inherent to retrospective history review; nevertheless, medical record–based studies such as this one can be critical in laying the groundwork for future larger-scale investigations.
Conclusions
We observed an overlap of cognitive and neuroanatomical targets between early development and degeneration in language and nonlanguage domains. Our findings suggest that specific forms of LDs are associated with phenotypically atypical presentations of neurodegenerative disorders later in life and fit within a larger framework of research into the influence of neurodevelopment on vulnerability and susceptibility of neural systems to neurodegenerative disease.2,32,42,45,46 We hope that this study reaffirms the value of close phenotyping in neurodegenerative diseases and enables longitudinal explorations of individuals with learning differences to better establish the influence of the aging process on potentially at-risk populations. Whether the association between neurodevelopment and neurodegeneration is linked to a common underlying biological process, such as decreased synaptic plasticity or deficient neuronal pruning,47 or reflects decreased reserve in a network-specific manner remains an open question. Regardless, appreciation of developmental history may not only assist in disease prevention and earlier detection but also aid in prognosticating phenotypic presentation, prediction of clinical trajectory, and perhaps response to neurorehabilitation, underscoring the importance of capturing premorbid, neurodevelopmental differences in all adults being evaluated for cognitive impairment.
References
- 1.Rogalski E, Johnson N, Weintraub S, Mesulam M. Increased frequency of learning disability in patients with primary progressive aphasia and their first-degree relatives. Arch Neurol. 2008;65(2):244-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miller ZA, Mandelli ML, Rankin KP, et al. Handedness and language learning disability differentially distribute in progressive aphasia variants. Brain. 2013;136(pt 11):3461-3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76(11):1006-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shaywitz SE. Dyslexia. N Engl J Med. 1998;338(5):307-312. [DOI] [PubMed] [Google Scholar]
- 5.Crutch SJ, Lehmann M, Schott JM, Rabinovici GD, Rossor MN, Fox NC. Posterior cortical atrophy. Lancet Neurol. 2012;11(2):170-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang-Wai DF, Graff-Radford NR, Boeve BF, et al. Clinical, genetic, and neuropathologic characteristics of posterior cortical atrophy. Neurology. 2004;63(7):1168-1174. [DOI] [PubMed] [Google Scholar]
- 7.Chechlacz M, Humphreys GW. The enigma of Bálint’s syndrome: neural substrates and cognitive deficits. Front Hum Neurosci. 2014;8:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chechlacz M, Rotshtein P, Hansen PC, Riddoch JM, Deb S, Humphreys GW. The neural underpinings of simultanagnosia: disconnecting the visuospatial attention network. J Cogn Neurosci. 2012;24(3):718-735. [DOI] [PubMed] [Google Scholar]
- 9.Neitzel J, Ortner M, Haupt M, et al. Neuro-cognitive mechanisms of simultanagnosia in patients with posterior cortical atrophy. Brain. 2016;139(pt 12):3267-3280. [DOI] [PubMed] [Google Scholar]
- 10.Cappelletti M, Lee HL, Freeman ED, Price CJ. The role of right and left parietal lobes in the conceptual processing of numbers. J Cogn Neurosci. 2010;22(2):331-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piazza M, Pinel P, Le Bihan D, Dehaene S. A magnitude code common to numerosities and number symbols in human intraparietal cortex. Neuron. 2007;53(2):293-305. [DOI] [PubMed] [Google Scholar]
- 12.Demeyere N, Rotshtein P, Humphreys GW. The neuroanatomy of visual enumeration: differentiating necessary neural correlates for subitizing versus counting in a neuropsychological voxel-based morphometry study. J Cogn Neurosci. 2012;24(4):948-964. [DOI] [PubMed] [Google Scholar]
- 13.Ashkenazi S, Black JM, Abrams DA, Hoeft F, Menon V. Neurobiological underpinnings of math and reading learning disabilities. J Learn Disabil. 2013;46(6):549-569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mazza V. Simultanagnosia and object individuation. Cogn Neuropsychol. 2017;34(7-8):430-439. [DOI] [PubMed] [Google Scholar]
- 15.Delazer M, Karner E, Zamarian L, Donnemiller E, Benke T. Number processing in posterior cortical atrophy: a neuropsycholgical case study. Neuropsychologia. 2006;44(1):36-51. [DOI] [PubMed] [Google Scholar]
- 16.Spotorno N, McMillan CT, Powers JP, Clark R, Grossman M. Counting or chunking? mathematical and heuristic abilities in patients with corticobasal syndrome and posterior cortical atrophy. Neuropsychologia. 2014;64:176-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilson AJ, Andrewes SG, Struthers H, et al. Dyscalculia and dyslexia in adults: cognitive bases of comorbidity. Learn Individ Differ. 2015;37:118-132. [Google Scholar]
- 18.Thompson RF, Mayers KS, Robertson RT, Patterson CJ. Number coding in association cortex of the cat. Science. 1970;168(3928):271-273. [DOI] [PubMed] [Google Scholar]
- 19.Piazza M, Izard V. How humans count: numerosity and the parietal cortex. Neuroscientist. 2009;15(3):261-273. [DOI] [PubMed] [Google Scholar]
- 20.Dehaene S. The Number Sense: How the Mind Creates Mathematics. Oxford, England: Oxford University Press; 2011. [Google Scholar]
- 21.Shalev RS, Manor O, Kerem B, et al. Developmental dyscalculia is a familial learning disability. J Learn Disabil. 2001;34(1):59-65. [DOI] [PubMed] [Google Scholar]
- 22.Molko N, Cachia A, Rivière D, et al. Functional and structural alterations of the intraparietal sulcus in a developmental dyscalculia of genetic origin. Neuron. 2003;40(4):847-858. [DOI] [PubMed] [Google Scholar]
- 23.Cohen Kadosh R, Cohen Kadosh K, Schuhmann T, et al. Virtual dyscalculia induced by parietal-lobe TMS impairs automatic magnitude processing. Curr Biol. 2007;17(8):689-693. [DOI] [PubMed] [Google Scholar]
- 24.Galaburda AM, LoTurco J, Ramus F, Fitch RH, Rosen GD. From genes to behavior in developmental dyslexia. Nat Neurosci. 2006;9(10):1213-1217. [DOI] [PubMed] [Google Scholar]
- 25.Migliaccio R, Agosta F, Rascovsky K, et al. Clinical syndromes associated with posterior atrophy: early age at onset AD spectrum. Neurology. 2009;73(19):1571-1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mendez MF, Ghajarania M, Perryman KM. Posterior cortical atrophy: clinical characteristics and differences compared to Alzheimer’s disease. Dement Geriatr Cogn Disord. 2002;14(1):33-40. [DOI] [PubMed] [Google Scholar]
- 27.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging–Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 2011;7(3):263-269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lagae L. Learning disabilities: definitions, epidemiology, diagnosis, and intervention strategies. Pediatr Clin North Am. 2008;55(6):1259-1268, vii. [DOI] [PubMed] [Google Scholar]
- 29.Shalev RS, Auerbach J, Manor O, Gross-Tsur V. Developmental dyscalculia: prevalence and prognosis. Eur Child Adolesc Psychiatry. 2000;9(suppl 2):II58-II64. [DOI] [PubMed] [Google Scholar]
- 30.Reigosa-Crespo V, Valdés-Sosa M, Butterworth B, et al. Basic numerical capacities and prevalence of developmental dyscalculia: the Havana Survey. Dev Psychol. 2012;48(1):123-135. [DOI] [PubMed] [Google Scholar]
- 31.Bettcher BM, Wilheim R, Rigby T, et al. C-reactive protein is related to memory and medial temporal brain volume in older adults. Brain Behav Immun. 2012;26(1):103-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alberca R, Montes E, Russell E, Gil-Néciga E, Mesulam M. Left hemicranial hypoplasia in 2 patients with primary progressive aphasia. Arch Neurol. 2004;61(2):265-268. [DOI] [PubMed] [Google Scholar]
- 33.Harvey BM, Klein BP, Petridou N, Dumoulin SO. Topographic representation of numerosity in the human parietal cortex. Science. 2013;341(6150):1123-1126. [DOI] [PubMed] [Google Scholar]
- 34.Rugani R, Vallortigara G, Priftis K, Regolin L. Animal cognition. Number-space mapping in the newborn chick resembles humans’ mental number line. Science. 2015;347(6221):534-536. [DOI] [PubMed] [Google Scholar]
- 35.Schott JM, Crutch SJ, Carrasquillo MM, et al. Genetic risk factors for the posterior cortical atrophy variant of Alzheimer’s disease. Alzheimers Dement. 2016;12(8):862-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seifan A, Assuras S, Huey ED, Mez J, Tsapanou A, Caccappolo E. Childhood learning disabilities and atypical dementia: a retrospective chart review. PLoS One. 2015;10(6):e0129919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rohrer JD, Ridgway GR, Crutch SJ, et al. Progressive logopenic/phonological aphasia: erosion of the language network. Neuroimage. 2010;49(1):984-993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mesulam M, Wicklund A, Johnson N, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63(6):709-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Renner JA, Burns JM, Hou CE, McKeel DW Jr, Storandt M, Morris JC. Progressive posterior cortical dysfunction: a clinicopathologic series. Neurology. 2004;63(7):1175-1180. [DOI] [PubMed] [Google Scholar]
- 40.Paternicó D, Premi E, Alberici A, et al. Dyslexia susceptibility genes influence brain atrophy in frontotemporal dementia. Neurol Genet. 2015;1(3):e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paternicó D, Manes M, Premi E, et al. Frontotemporal dementia and language networks: cortical thickness reduction is driven by dyslexia susceptibility genes. Sci Rep. 2016;6:30848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dean DC III, Jerskey BA, Chen K, et al. Brain differences in infants at differential genetic risk for late-onset Alzheimer disease: a cross-sectional imaging study. JAMA Neurol. 2014;71(1):11-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chang L, Douet V, Bloss C, et al. ; Pediatric Imaging, Neurocognition, and Genetics (PING) Study Consortium . Gray matter maturation and cognition in children with different APOE ε genotypes. Neurology. 2016;87(6):585-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Morgan PL, Farkas G, Hillemeier MM, et al. Minorities are disproportionately underrepresented in special education: Longitudinal evidence across five disability conditions. Educ Res. 2015;44(5):278-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geschwind N, Galaburda AM. Cerebral lateralization. Biological mechanisms, associations, and pathology, III: a hypothesis and a program for research. Arch Neurol. 1985;42(7):634-654. [DOI] [PubMed] [Google Scholar]
- 46.Miller ZA, Hinkley LB, Herman A, et al. Anomalous functional language lateralization in semantic variant PPA. Neurology. 2015;84(2):204-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stephan AH, Barres BA, Stevens B. The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci. 2012;35:369-389. [DOI] [PubMed] [Google Scholar]