Introduction
Great achievements in acute stroke care have been made, to a large extent, because of increased stroke awareness. Earlier arrival of patients at dedicated stroke centers leads to a better chance of successful treatment. Nevertheless, to date, the therapeutic options for acute stroke are still limited to IV tissue plasminogen activator, mechanical thrombolysis, or delivery of fibrinolytics. While those therapies have had a significant impact on stroke outcome, there is still a remarkable lack of adjunct therapeutic options, such as neuroprotection and neurorestoration. Cell therapies represent a new investigational approach for the treatment of stroke. Pre-clinical reports are abundant and controlled clinical trials have begun to be performed around the globe. Many critical questions remain to be answered and a consortium of scientists and clinicians are joining forces to discuss open issues and provide potential recommendations based on the best available knowledge1.
One of the many critical questions is about the ideal route of delivery in terms of efficacy, safety, timing of delivery, and methods by which to monitor the process. Published clinical studies have mainly used intracerebral transplantation and intravenous injection. Smaller case series have reported intra-arterial (IA) cell delivery. Selection of the cell delivery route should be based on the primary therapeutic mechanisms. Systemic effects would favor IV route. If recovery depends on cell-cell interactions then intraparenchymal or IA injection may be most beneficial. There seems to be a good rationale for intravascular delivery. It is less invasive than intracerebral transplantation, it is repeatable, it would allow for a systemic biological effect, and could lead to a widespread distribution in the affected brain regions2. This potentially would compare favorably to the focal delivery achieved with stereotactic transplantation. Even in cases of permanent arterial occlusion, which is rare,a significant number of cells can home in to the ischemic brain through collateral circulation. With an increasing number of IA catheter interventions for stroke performed, it would also appear that IA cell injection would be ideally suited in the stroke setting, as well as being quite feasible. Pre-clinical data suggests that IA cell injection leads to a greater number of cells targeting the ischemia. The main reason for this is that cells bypass filtering organs, such as the lung, the spleen, and the liver3. Pre-clinical studies have also demonstrated that targeted delivery to the ischemic brain has well-defined molecular mechanisms, attracting cells from the intravascular to the intraparenchymal space4. Cell-sorting or cell engineering to improve the targeted delivery needs to be further investigated. In addition, the success of targeted delivery also appears to be strongly dependent on the cell type used. Mononuclear cells of different origins, for instance, have shown very limited to no tropism to the ischemic brain tissue. It has also been postulated that cell size determines, in part, the safety profile of an IA delivery, whereas larger cell types might lead to microembolic obstruction of capillaries and strokes5-7. The mechanistic theories about transendothelial migration and the safety concerns have led to an additional important consideration, which is the monitoring of cell delivery. In vivo cerebral blood flow measurements8 and advanced magnetic resonance imaging9 techniques to follow cell delivery in real-time are being developed and should ideally be implemented in future clinical trials.
In this review, we will focus on the current knowledge related to the safety of IA cell delivery in stroke and will present novel methods that would allow monitoring of the cell delivery process. We will also put these pre-clinical concepts into a clinical perspective.
Safety of IA injection after stroke
Uninterrupted cerebral blood flow is critical for preserving the structure and function of the nervous tissue. Preserving blood circulation is of even greater importance in the aftermath of stroke, as homeostasis is fragile and any disturbance of nutrient/oxygen supply triggered by IA intervention may exacerbate the secondary damage. Cerebral capillaries are about 5-10 micrometers in diameter and circulating cellular elements, including erythrocytes (7μm) or leukocytes (6-18μm), pass seamlessly through them. The adhesion of leukocytes and diapedesis occurs primarily at the site of postcapillary venules10, so the trophic function of capillaries is maintained. While some degree of temporary capillary blockage may be tolerated, if a critical threshold is exceeded, this inevitably leads to local hypoxia/ischemia and microembolic lesions. The density of cerebral capillaries varies in different brain structures, with the cortex having about five times the density of the corpus callosum11, and, in this context, white matter might be more vulnerable to capillary occlusion. During IA stem cell delivery, relatively large numbers of cells are infused, with the anticipation that they will be captured by the cerebral vasculature; however, the cell load or local pressure disturbances may compromise the safety of this procedure.
To date, over 50 IA cell delivery studies in stroke have been published and a number of these studies have reported procedure-related complications. Important lessons on safety were learned and several factors have been identified as critical for the safety of IA cell delivery. The most important variables that were identified are cell type and size, cell dose, infusion speed, and preservation of arterial blood flow in the feeding vessel during infusion. Other important factors to consider are timing after stroke onset and the anatomical considerations of the target (Figure 1). Comprehensive overview of the experimental conditions is included in Supplementary Table I. Details pertaining safety are included in Supplementary Table II.
Figure 1.
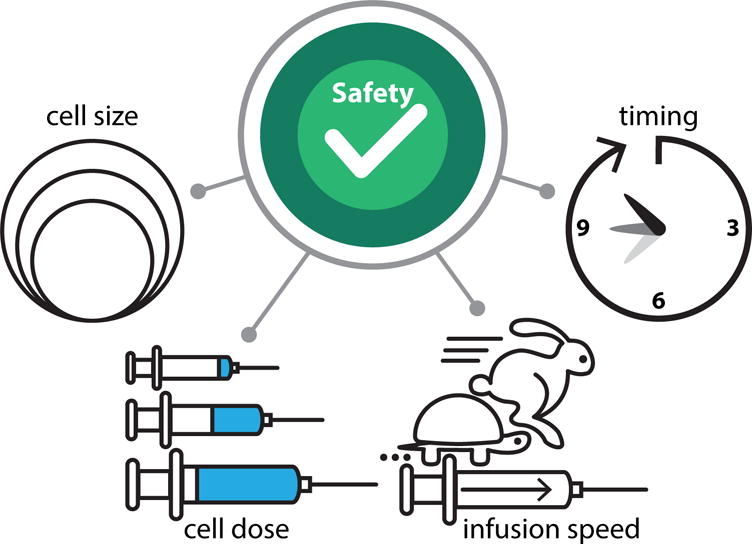
Safety and success of IA cell delivery has been shown to depend in part on cell type and size, cell dose, infusion speed and timing of cell transplantation after stroke onset.
Cell type and size
Stem cell diameters range from 7μm for bone marrow mononuclear cells, 13-15μm for neural stem cells (NSCs), and over 25μm for mesenchymal stem cells (MSCs). Mononuclear cells are a diverse population including lymphocytes, monocytes, hematopoietic progenitor cells and a small fraction of mesenchymal stem cells. MSCs are frequently selected based on adhesion on to plastic and characterized by large size and expression of specific markers including CD44, CD90, CD106 or Stro-1. With large size cells, such as MSCs, there is an obvious risk that the cells, rather than rolling and adhering to the postcapillary venule walls, clog up the entire capillary lumen, eliminating its function as a nutrient supplier and gas exchanger.
Of the reviewed studies, 19 report on the transplantation of bone marrow or cord blood mononuclear cells and none have reported any complications. Seven studies used neural stem cells and two of these reported on a minor increase in mortality or compromised cerebral perfusion8, 12. MSCs are associated with the highest risk of adverse effects, as, of 29 studies, 11 reported various adverse effects, including reduced cerebral blood flow, increased mortality, and neurological impairment. Notably, microembolic lesions frequently occur in the white matter6, 8, 13, indicating its vulnerability to capillary occlusion. Detailed references to individual studies are included in Supplementary Table I.
The cell dose
While IA injection has the potential for efficient cell targeting to the brain, there must be a balance between maximizing engraftment and maintaining sufficient perfusion, thus ensuring safety. A wide range of cell doses has been studied in several species, including the mouse, the rat, dogs, and humans (Supplementary Table II). In an attempt to normalize the dose across tested species, we divided the reported dose of injected cells over the average weight of the brain in grams (mouse=0.4g; rat=2g; dog=72g; human=1350g). In mouse studies, cell doses ranged between 0.1-12.5×105/g of brain tissue and complications were reported only for the highest doses, with 7.5×105/g of MSCs leading to micro-occlusions as detected by multi-photon microscopy7. At a dose of 12.5×105/g of C17.2 neural stem cells, increased mortality has been observed12.
The majority of preclinical studies have been performed in rat models, and injected cell doses ranged between 0.05-150×105/g . Notably, injection of bone marrow mononuclear cells, even at extremely high doses of 100×105/g14 or even 150×105/g15 were not associated with any adverse effects. This is reassuring and indicates the excellent safety of mononuclear cell injection, but, it also raises the question of the efficacy of endothelial capture. Six studies have reported the use of neural stem cells and only one listed microembolic complications with mouse neural stem cells at a dose of 5×105/g , but that complication was eliminated when cells were infused with preserved blood flow in the carotid artery8. The highest frequency of complications was associated with the use of MSCs, which has been reported in 12 studies. Microembolic lesions have been reported with cell doses as low as 1.2×105/g13, and were observed with high reproducibility across different studies when the dose exceeded 5×105/g . Notably, there is one research group that showed, in several studies, that a cell dose of 10×105/g resulted in significant functional benefit without any reported complications16, 17. Three studies report on the use of a dog model, with cell doses ranging from 0.14-0.69×105/g , and microembolic complications at doses of 0.4×105/g of MSCs18 and 0.69×105/g of injected adipose-derived pericyte progenitors19. There were 12 papers on clinical studies. All used bone marrow mononuclear cells at relatively low doses ranging between 0.002-3.3×105/g , and none reported adverse effects related to cell injection. Overall, mononuclear cells are safe at any dose, with the upper limit for neural stem cells below 7.5×105/g , and, for MSCs, the safety threshold seems to be at 1×105/g in rodents and below 0.4×105/g in dogs.
The infusion speed
IA cell injection in the clinical setting is part of a neurointerventional procedure, with the advancement of an endovascular microcatheter under X-ray guidance into the cerebral arteries. The correct placement of the microcatheter is confirmed by an arteriogram, which requires a bolus injection of a contrast agent, and frequently exceeds 5ml/sec, an extremely high speed beyond a physiological perfusion rate of ~3.4ml/sec for the basilar artery (BA) or ~2.3 ml for the middle cerebral artery20. An injection at a rate exceeding physiological perfusion may lead to increased pressure downstream from the catheter tip. Indeed, microcatheter contrast injections have been reported as a potential contributor to intracranial hemorrhage risk in the context of IA thrombolysis21. In a rat model, the infusion of phosphate-buffered saline (PBS) into the internal carotid artery at a very high velocity of 3 ml/min resulted in focal T2 hyperintensities on MRI consistent with vascular injury. After the infusion rate was reduced to 0.2ml/min, no injury was observed6. Microinfarcts were observed in another study with an injection velocity at 0.3ml/min22. Similar lesions were also observed with the injection of PBS at a rate of 0.16ml/min13. T2 abnormalities were similar to those observed in patients who undergo routine cerebral angiography23, 24.
The timing
The acute period of the initial hours and days after stroke is when the risk of complications is particularly high and complications from the IA injection of stem cells are frequently reported25-27. The risk elevation deserves a closer look, as the acute and subacute phases are also an opportune period for the initiation of cell-based treatment. Neurons and glia that are subject to secondary damage could be a target of cell therapy and any delay of the intervention could reduce the impact of such therapy. The vulnerability during the acute stroke phase coincides temporarily with endothelial injury, blood brain barrier breakdown28, and with the massive wave of leukocyte infiltration that peaks around 48-72hrs after stroke29. An arterial/endothelial system modified at that time to maximize the shuttling of leukocytes into the stroke lesion may offer a unique opportunity for effective IA delivery of stem cells. Ironically, complications observed following the IA injection of stem cells in acute stroke may be due to highly successful homing and excessive cell engraftment, which leads to microocclusions and local hypoperfusion26. This challenge may be addressed by introducing techniques to monitor cell infusion, and that will be discussed in a subsequent section.
The anatomical site
As mentioned above, the vulnerability to complications seems to be region-specific, with the white matter more susceptible6, 13. The microstrokes can be clinically silent or result in neurological deficits depending on their anatomical location. It is likely that the complications of IA injection in the brain stem could potentially lead to more severe consequences and these studies would particularly benefit from precise control and monitoring of cell infusion.
Monitoring and guiding IA delivery with imaging
The benefits of IA delivery are undisputed, but, with a few decades of experience in this area, there is a growing consensus about the need to monitor therapy non-invasively. The need is driven, to a large extent, by uncertainty about the destination of injected cells, as their biodistribution is affected by multiple factors, including the size of the ischemic lesion, the time after ischemia, hemodynamics, infusion velocity, cell type and size, etc.). Without detailed knowledge about cell biodistribution, as well as the precision and efficiency of targeting to a stroke lesion, it is difficult or impossible to fully evaluate and optimize the therapeutic procedure (Figure 2). Histopathology is still the gold standard for the assessment of preclinical studies, but, besides being labor-intensive and plagued with the unreliability of the quantification, terminal studies lack dynamic information about the entirety of the journey of the injected cells from the catheter to the arteries through the capillaries to their final destination. Another important motivation for incorporating imaging into therapy protocols is safety. As discussed above, the complications of IA injection have been reported in a relatively large number of studies. Excessive cell engraftment appears to be the primary source of complications, and, with high patient-to-patient variability in cell engraftment26, monitoring cell homing in real-time during the infusion procedure is very appealing. Finally, real-time imaging of IA cell injection may help improve the precision of cell injection and ensure their placement at the desired destination.
Figure 2.
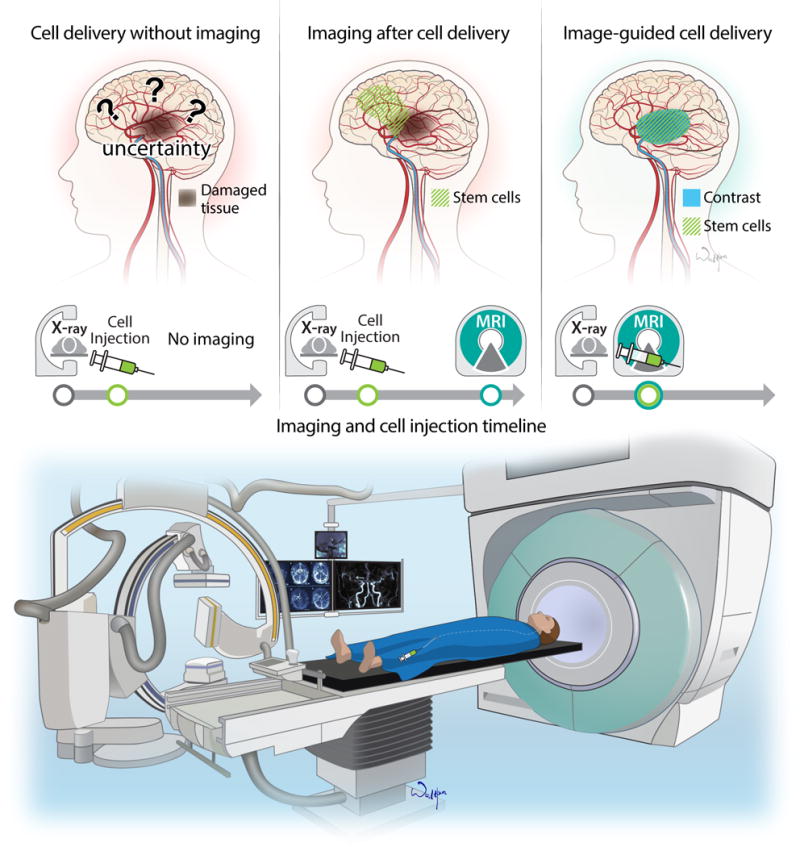
Clinical Perspective on therapeutic intra-arterial cell delivery after stroke. Imaging of cell delivery should become an integral part of cell transplantation studies. Modern imaging has the potential to demonstrate the anatomical biodistribution of cells after injection. Recent advances in MR imaging enables real-time monitoring of cellular delivery and anatomical distribution. Hybrid OR suite may offer the necessary infrastructure for state of the art IA cell transplantation.
Imaging after completed cell injection
Several imaging modalities have been used for the longitudinal assessment of cells after IA transplantation in a stroke setting, including MRI, Single-photon emission computed tomography (SPECT), and bioluminescent imaging (BLI). Each modality has its own strengths and limitations. The advantages of using MRI are the clinical applicability, the excellent anatomical detail, and the cell detection sensitivity at the single-cell level30, but a key limitation of nanoparticle-based cell labels is a dilution of the contrast, with cell division and transfer to phagocytes after cell death, limiting the reliability of long-term tracking31. MRI has been successfully applied to visualize IA-injected iron oxide nanoparticle (SPIO)-labeled MSCs in a rat stroke model immediately after injection and it was instrumental in demonstrating the high animal-to-animal variability of cell engraftment in the brain. MRI also showed that the highest engraftment correlated with reduced cerebral blood flow and led to increased mortality26. The propensity of MSCs to induce microocclusions was also observed in several other studies where MRI cell-tracking was instrumental in developing safe transplantation protocols6, 13, 22, 32, 33. Similarly to MSCs, biodistribution of neural stem cells (C17.2) injected IA in a mouse model of hypoxia/ischemia was visualized on MRI scans shortly after injection3. Several studies attempted to longitudinally assess IA-injected MSCs and showed gradual clearance of SPIO-labeled, cell-derived hypointensities from the brain, with some signal detectable at two weeks34 or even four weeks after injection18.
SPECT is a nuclear medicine technique that, compared to MRI, has much lower spatial resolution, but its sensitivity and specificity are high. SPECT also enables whole-body imaging and provides reliable data on global biodistribution of transplanted cells. An important disadvantage is the short imaging window related to the half-life of radioisotopes, allowing cell tracking from 24-48h. SPECT has been used to show the biodistribution of 111indium oxine-labeled neural stem cells in an MCAO rat model and IA, but not intravenous injection, resulted in cerebral engraftment. Detection sensitivity has been estimated at approximately 1000 cells and labeling was not detrimental to cell function35. In another study, SPECT was used to track human MSCs, and, while a large proportion of the cells were trapped in the brain immediately after injection, the majority disappeared after 24 hours, redistributing to filtering organs36. The same group has shown the differential clearance of rat vs. human MSCs within the first six hours after IA injection, with a faster washout of human cells37. Because SPECT is a clinically applicable technique, it has been used by several groups to track IA-injected cells in patients, thus providing unique data about the early biodistribution of cells. A case report of a study performed in Brazil in a patient who was IA-transplanted with autologous bone marrow mononuclear cells, nine days after stroke, showed accumulation of the cells in the ipsilateral hemisphere, as well as in the liver and spleen38. A subsequent report from this same group included six patients, and, while cell accumulation was detected in the ipsilateral hemisphere in all patients two hours after injection, at 24h, it was detectable in only two patients.
Currently, the most sensitive techniques for cellular imaging rely on labeling with contrast agents or radioactive tracers, and, while these clinically applicable techniques are reliable for cell tracking over a period of days (SPECT) or even the initial few weeks (MRI) after transplantation, long-term tracking with these techniques is not feasible due to either the decay of the tracer (in the case of SPECT), or low specificity (in the case of MRI)39. For reliable long-term tracking, the imaging tag must be replenished following cell division and rapidly lost after the death of labeled cells. These requirements are perfectly addressed by reporter genes. Reporter genes have been developed for several imaging modalities, including PET40 and MRI31, 41, but the most widely used systems are based on BLI. Although the spatial resolution of BLI is low, it lacks tomographic capabilities, and its use is limited to small rodents, it is an excellent tool for the longitudinal assessment of cell survival and biodistribution. Indeed, BLI has shown that IA-injected neural cells engraft in the hypoxia/ischemia-injured brain and engraftment efficiency for IA injection was 12 times higher compared to intravenous delivery. Cells were detectable in the brain for two weeks8. Neural stem cells injected intraparenchymally were detectable on BLI for several months39; thus, signal loss after IA injection may indicate overall long-term low engraftment. In response to the need to improve endothelial capture and diapedesis of IA-injected cells, there have been efforts to either select cells with a high expression of adhesion molecules42, or to engineer cells to induce the expression of such molecules13, 43, 44.
Monitoring the interventional procedure of IA cell injection in real-time
As discussed above, imaging provides unique information about the localization and even viability of IA-injected cells. This is helpful in improving transplantation protocols, but, from a clinical perspective, the practicality of this approach may be of limited value. At the time when the procedure of cell delivery is completed, imaging can show, the placement of the cells, but, should cells be misinjected or their biodistribution be suboptimal, with excessive or insufficient engraftment, it is too late to correct and potentially avoid complications. With recent progress in interventional MRI, and the development of fast imaging protocols along with the use of high sensitivity contrast agents, it is now possible to address this challenge. Indeed, it has been shown that interventional MRI can be used to track stem cells but also, more importantly, to predict the biodistribution of IA-injected cells prior to their administration44. After placement of an IA catheter under X-ray guidance, animals are transferred to the MRI scanner, and, prior to cell injection, MRI contrast agent is infused via IA catheter. That enables visualization of perfusion territory and the tuning of that territory. Once the perfusion territory was optimized, cells could be injected into a predetermined territory of the brain. Notably, MR imaging at high temporal resolution (2-3s) enables visualization of the cells as they are captured within the cerebral vasculature (Video 1). Real-time imaging can be used in combination with adjusting infusion speed, catheter position or dosing to assure desired and optimal cell biodistribution. In a related study, a similar approach was used for MRI-guided opening of the blood-brain barrier45. These studies are good examples of how non-invasive imaging can be used to improve the precision and safety of stem cell injection in a stroke setting.
Overall, the use of non-invasive imaging both to guide the procedure of cell infusion and assess cell status over time should be incorporated into pre-clinical and clinical protocols to improve the reproducibility of results and improve safety, which would, hopefully, translate into more effective therapies.
Cell sorting, preconditioning, and engineering to improve targeted cell delivery
Enhancing the capacity of stem cells to transmigrate from the vascular compartment into the ischemic brain has been one of the strategies used to improve therapeutic success. Studies have demonstrated a direct correlation between the number of cells that survive in the brain after IA transplantation and positive functional outcomes42. Several approaches have been described to enhance the potency of cellular targeting. They are all based on the fact that specific molecular mechanisms, such as adhesion and chemoattraction, are responsible for stem cell diapedesis. Using fluorescence-activated cell sorting to select for cell populations with a strong expression of adhesion molecules42 or chemokine receptors46 has been shown to significantly increase the number of cells homing to the brain. Another strategy has been to pre-treat stem cells with factors that enhance chemokine receptor expression. Interaction of SDF-1 with CXCR4 was shown to play an important role in facilitating homing to the ischemic brain47. It was shown that preconditioning of stem cells with BDNF12 or tetramethylpyrazine48 resulted in a dramatic increase in CXCR4 expression, and significantly improved migration in response to SDF-1. Other preconditioning strategies, such as hypoxia and exposure to inflammatory cytokines, have also been investigated49. Engineering cells to overexpress cell adhesion molecules and chemokine receptors50,13, 43, 51, or the use of cell surface modifications52 have been attempted to improve targeted cell delivery (Figure 3).
Figure 3.
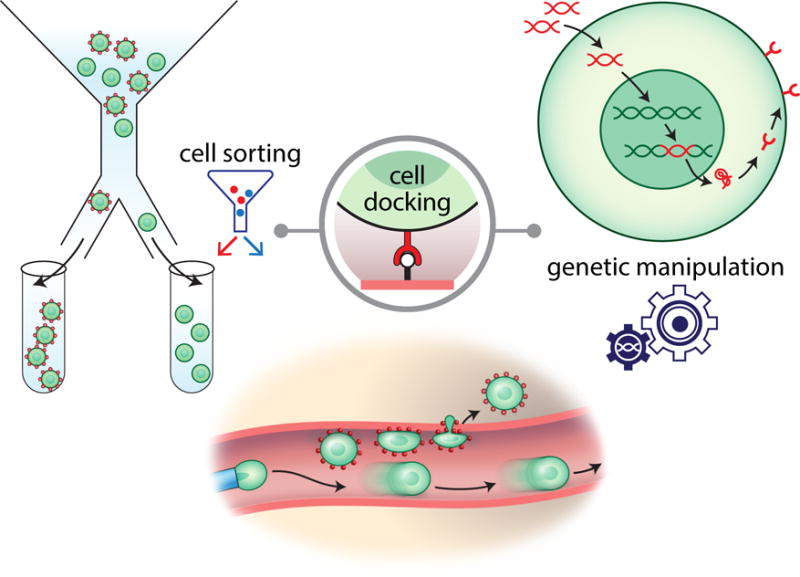
Improving the efficiency of cell delivery. Different strategies to improve transendothelial cell homing to the brain have been developed including fluorescence activated cell sorting (FACS) to select cells with high expression of surface adhesion molecules and genetic cell modification of enhance adhesion molecule and chemokine receptor expression.
Clinical perspective
Very recently, several clinical trials have shown the efficacy of mechanical clot removal (thrombectomy) through an IA catheter for emergent large-vessel occlusion (ELVO)53. Most importantly, IA procedures have a much longer therapeutic window as compared to intravenous treatments. The current standard therapeutic window is 6 hours. Novel clinical studies have shown (DAWN trial)54, or are still underway (DEFUSE3 trial) that the treatment window can be expanded to 24 hours among patients with large vessel anterior circulation occlusion who have a favorable imaging profile on computed tomography perfusion or magnetic resonance imaging. This will allow treatment of most patients with ELVO. However, despite the high rates of technical success with up to 90% successful revascularization, only 1/3 of patients will have a disability-free survival. Importantly, thrombectomy requires the placement of an IA catheter within the cerebral arteries, providing a coincident opportunity to deliver adjuvant therapies precisely and at an optimal dose to the infarcted territory55. The success of IA stroke therapies has led to a tremendous effort to expand the infrastructure to deliver state of the art treatments to stroke patients. In this context, adding IA cell delivery as an adjunct therapy to thrombectomy is a very appealing option. Interestingly, the newly developed interventional infrastructure could be used to treat patients with stroke who do not qualify for thrombectomy due to the absence of ELVO or missing the window of opportunity. The beneficial outcomes of thrombectomies encourage the performance of additional IA cell infusions as separate procedures at later time points. Therefore, logistically, there are favorable circumstances in which to initiate clinical trials to investigate the effectiveness of IA cell delivery.
There should however be a strong rationale behind the initiation of clinical trials with an IA route of cell therapy in stroke. These attempts should also be performed in a meaningful way to obtain a wide breadth of information, which could serve as a source for technical improvements. As mentioned above, there could be a particular role for real-time MR imaging to visualize the process of cell infusion. It is important to emphasize that any advantage of the IA route of cell delivery is only gained if infused cells are capable of homing at the first pass. For example, it is intriguing that infusion of bone marrow mononuclear cells at extremely high doses does not lead to complications which raises the question about the ability of those cells to home to the infarcted brain (Supplementary Table I). It is therefore evident that novel imaging methods are needed to monitor the cell delivery and then to document their biodistribution. SPIO nanoparticles are preferably used for MRI cell tracking; however, the SPION formulation (Feridex/Endorem) used in early clinical studies56, 57 has been withdrawn from the market and there is still no good replacement. Clinical-grade ferumoxytol is available and ferumoxytol-heparin-protamine complexes have been used to label adipose-derived stem cells injected into rats58; however, such complexes would still need a separate FDA approval to be used in a clinical setting. In terms of imaging equipment, X-ray fluoroscopic/MRI dual suites are available and are equipped with table transfer system, which ideally fit the current needs. While these facilities are currently not widespread, ongoing progress in interventional neuroradiology are strong drivers for further infrastructure development. In this scenario, after placement of a catheter with or without thrombectomy, a patient could be seamlessly moved from the X-ray fluoroscopic site to the MRI gantry to receive a cell infusion (Figure 3).
Summarizing, the IA route of cell delivery for the treatment of stroke is very appealing in the era of endovascular thrombectomy. The advancements in imaging methods, particularly real-time MRI, make it possible to visualize the transit of infused cells along the cerebral vasculature and the magnitude of endothelial capture, which is critically important for safety, as well as to better understand the biodistribution of transplanted cells. Engineering or sorting of cells prior to transplantation might be necessary to allow for improved cellular homing. These tools may improve efficacy of IA cell transplantation that should be applied in concert with standard treatment algorithms (Figure 4). Finally, further studies to elucidate the mechanism of action that leads to stem cell induced neuroprotection and neuroregeneration after IA cell delivery will be required.
Figure 4.
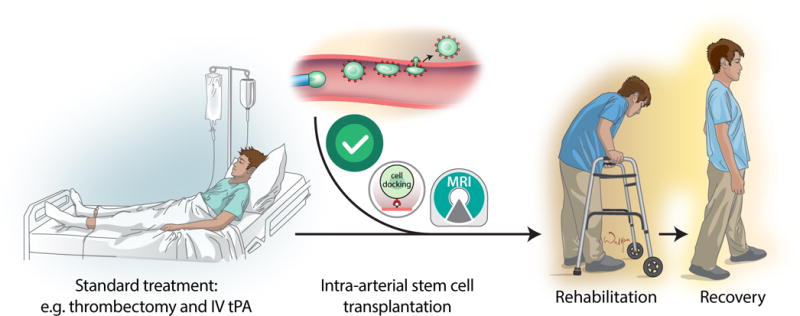
The role of IA stem cell-based therapy in the context of standard treatment algorithm
Supplementary Material
Acknowledgments
We thank Mary McAllister for editorial assistance.
Funding Sources
NIH R01NS091100 (PW) and R01NS091100 (MJ). SNF310030-146632 and SNF31003A-163305 (RG)
Footnotes
Disclosures
Authors declare no conflict of interest.
References
- 1.Savitz SI, Cramer SC, Wechsler L, Consortium S Stem cells as an emerging paradigm in stroke 3: Enhancing the development of clinical trials. Stroke. 2014;45:634–639. doi: 10.1161/STROKEAHA.113.003379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Misra V, Ritchie MM, Stone LL, Low WC, Janardhan V. Stem cell therapy in ischemic stroke: Role of iv and intra-arterial therapy. Neurology. 2012;79:S207–212. doi: 10.1212/WNL.0b013e31826959d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pendharkar AV, Chua JY, Andres RH, Wang N, Gaeta X, Wang H, et al. Biodistribution of neural stem cells after intravascular therapy for hypoxic-ischemia. Stroke. 2010;41:2064–2070. doi: 10.1161/STROKEAHA.109.575993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Auriat AM, Rosenblum S, Smith TN, Guzman R. Intravascular stem cell transplantation for stroke. Transl Stroke Res. 2011;2:250–265. doi: 10.1007/s12975-011-0093-1. [DOI] [PubMed] [Google Scholar]
- 5.Fukuda Y, Horie N, Satoh K, Yamaguchi S, Morofuji Y, Hiu T, et al. Intra-arterial transplantation of low-dose stem cells provides functional recovery without adverse effects after stroke. Cell Mol Neurobiol. 2015;35:399–406. doi: 10.1007/s10571-014-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Janowski M, Lyczek A, Engels C, Xu J, Lukomska B, Bulte JW, et al. Cell size and velocity of injection are major determinants of the safety of intracarotid stem cell transplantation. J Cereb Blood Flow Metab. 2013;33:921–927. doi: 10.1038/jcbfm.2013.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ge J, Guo L, Wang S, Zhang Y, Cai T, Zhao RC, et al. The size of mesenchymal stem cells is a significant cause of vascular obstructions and stroke. Stem Cell Rev. 2014;10:295–303. doi: 10.1007/s12015-013-9492-x. [DOI] [PubMed] [Google Scholar]
- 8.Chua JY, Pendharkar AV, Wang N, Choi R, Andres RH, Gaeta X, et al. Intra-arterial injection of neural stem cells using a microneedle technique does not cause microembolic strokes. J Cereb Blood Flow Metab. 2011;31:1263–1271. doi: 10.1038/jcbfm.2010.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walczak P, Wojtkiewicz J, Nowakowski A, Habich A, Holak P, Xu J, et al. Real-time mri for precise and predictable intra-arterial stem cell delivery to the central nervous system. J Cereb Blood Flow Metab. 2017;37:2346–2358. doi: 10.1177/0271678X16665853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller WA. Getting leukocytes to the site of inflammation. Vet Pathol. 2013;50:7–22. doi: 10.1177/0300985812469883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cavaglia M, Dombrowski SM, Drazba J, Vasanji A, Bokesch PM, Janigro D. Regional variation in brain capillary density and vascular response to ischemia. Brain Res. 2001;910:81–93. doi: 10.1016/s0006-8993(01)02637-3. [DOI] [PubMed] [Google Scholar]
- 12.Rosenblum S, Smith TN, Wang N, Chua JY, Westbroek E, Wang K, et al. Bdnf pretreatment of human embryonic-derived neural stem cells improves cell survival and functional recovery after transplantation in hypoxic-ischemic stroke. Cell Transplant. 2015;24:2449–2461. doi: 10.3727/096368914X679354. [DOI] [PubMed] [Google Scholar]
- 13.Cui LL, Kerkela E, Bakreen A, Nitzsche F, Andrzejewska A, Nowakowski A, et al. The cerebral embolism evoked by intra-arterial delivery of allogeneic bone marrow mesenchymal stem cells in rats is related to cell dose and infusion velocity. Stem Cell Res Ther. 2015;6:11. doi: 10.1186/scrt544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brenneman M, Sharma S, Harting M, Strong R, Cox CS, Jr, Aronowski J, et al. Autologous bone marrow mononuclear cells enhance recovery after acute ischemic stroke in young and middle-aged rats. J Cereb Blood Flow Metab. 2010;30:140–149. doi: 10.1038/jcbfm.2009.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vasconcelos-dos-Santos A, Rosado-de-Castro PH, Lopes de Souza SA, da Costa Silva J, Ramos AB, Rodriguez de Freitas G, et al. Intravenous and intra-arterial administration of bone marrow mononuclear cells after focal cerebral ischemia: Is there a difference in biodistribution and efficacy? Stem Cell Res. 2012;9:1–8. doi: 10.1016/j.scr.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 16.Li Y, Chen J, Wang L, Lu M, Chopp M. Treatment of stroke in rat with intracarotid administration of marrow stromal cells. Neurology. 2001;56:1666–1672. doi: 10.1212/wnl.56.12.1666. [DOI] [PubMed] [Google Scholar]
- 17.Zhang C, Li Y, Chen J, Gao Q, Zacharek A, Kapke A, et al. Bone marrow stromal cells upregulate expression of bone morphogenetic proteins 2 and 4, gap junction protein connexin-43 and synaptophysin after stroke in rats. Neuroscience. 2006;141:687–695. doi: 10.1016/j.neuroscience.2006.04.054. [DOI] [PubMed] [Google Scholar]
- 18.Lu SS, Liu S, Zu QQ, Xu XQ, Yu J, Wang JW, et al. In vivo mr imaging of intraarterially delivered magnetically labeled mesenchymal stem cells in a canine stroke model. PloS one. 2013;8:e54963. doi: 10.1371/journal.pone.0054963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Youn SW, Jung KH, Chu K, Lee JY, Lee ST, Bahn JJ, et al. Feasibility and safety of intra-arterial pericyte progenitor cell delivery following mannitol-induced transient blood-brain barrier opening in a canine model. Cell Transplant. 2015;24:1469–1479. doi: 10.3727/096368914X682413. [DOI] [PubMed] [Google Scholar]
- 20.Zarrinkoob L, Ambarki K, Wahlin A, Birgander R, Eklund A, Malm J. Blood flow distribution in cerebral arteries. J Cereb Blood Flow Metab. 2015;35:648–654. doi: 10.1038/jcbfm.2014.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khatri P, Broderick JP, Khoury JC, Carrozzella JA, Tomsick TA, Ims I, et al. Microcatheter contrast injections during intra-arterial thrombolysis may increase intracranial hemorrhage risk. Stroke. 2008;39:3283–3287. doi: 10.1161/STROKEAHA.108.522904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Namestnikova D, Gubskiy I, Gabashvili A, Sukhinich K, Melnikov P, Vishnevskiy D, et al. Mri evaluation of frequent complications after intra-arterial transplantation of mesenchymal stem cells in rats. Journal of Physics: Conference Series. 2017:886. [Google Scholar]
- 23.Brockmann C, Hoefer T, Diepers M, Neumaier-Probst E, Noelte I, Brockmann MA, et al. Abciximab does not prevent ischemic lesions related to cerebral angiography: A randomized placebo-controlled trial. Cerebrovasc Dis. 2011;31:353–357. doi: 10.1159/000323219. [DOI] [PubMed] [Google Scholar]
- 24.Bendszus M, Koltzenburg M, Burger R, Warmuth-Metz M, Hofmann E, Solymosi L. Silent embolism in diagnostic cerebral angiography and neurointerventional procedures: A prospective study. Lancet. 1999;354:1594–1597. doi: 10.1016/S0140-6736(99)07083-X. [DOI] [PubMed] [Google Scholar]
- 25.Mitkari B, Kerkela E, Nystedt J, Korhonen M, Jolkkonen J. Unexpected complication in a rat stroke model: Exacerbation of secondary pathology in the thalamus by subacute intraarterial administration of human bone marrow-derived mesenchymal stem cells. J Cereb Blood Flow Metab. 2015;35:363–366. doi: 10.1038/jcbfm.2014.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, et al. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39:1569–1574. doi: 10.1161/STROKEAHA.107.502047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yavagal DR, Lin B, Raval AP, Garza PS, Dong C, Zhao W, et al. Efficacy and dose-dependent safety of intra-arterial delivery of mesenchymal stem cells in a rodent stroke model. PloS one. 2014;9:e93735. doi: 10.1371/journal.pone.0093735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sandoval KE, Witt KA. Blood-brain barrier tight junction permeability and ischemic stroke. Neurobiol Dis. 2008;32:200–219. doi: 10.1016/j.nbd.2008.08.005. [DOI] [PubMed] [Google Scholar]
- 29.Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- 30.Shapiro EM, Sharer K, Skrtic S, Koretsky AP. In vivo detection of single cells by mri. Magn Reson Med. 2006;55:242–249. doi: 10.1002/mrm.20718. [DOI] [PubMed] [Google Scholar]
- 31.Gilad AA, McMahon MT, Walczak P, Winnard PT, Jr, Raman V, van Laarhoven HW, et al. Artificial reporter gene providing mri contrast based on proton exchange. Nature biotechnology. 2007;25:217–219. doi: 10.1038/nbt1277. [DOI] [PubMed] [Google Scholar]
- 32.Argibay B, Trekker J, Himmelreich U, Beiras A, Topete A, Taboada P, et al. Intraarterial route increases the risk of cerebral lesions after mesenchymal cell administration in animal model of ischemia. Scientific reports. 2017;7:40758. doi: 10.1038/srep40758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grudzenski S, Baier S, Ebert A, Pullens P, Lemke A, Bieback K, et al. The effect of adipose tissue-derived stem cells in a middle cerebral artery occlusion stroke model depends on their engraftment rate. Stem Cell Res Ther. 2017;8:96. doi: 10.1186/s13287-017-0545-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gutierrez-Fernandez M, Rodriguez-Frutos B, Alvarez-Grech J, Vallejo-Cremades MT, Exposito-Alcaide M, Merino J, et al. Functional recovery after hematic administration of allogenic mesenchymal stem cells in acute ischemic stroke in rats. Neuroscience. 2011;175:394–405. doi: 10.1016/j.neuroscience.2010.11.054. [DOI] [PubMed] [Google Scholar]
- 35.Lappalainen RS, Narkilahti S, Huhtala T, Liimatainen T, Suuronen T, Narvanen A, et al. The spect imaging shows the accumulation of neural progenitor cells into internal organs after systemic administration in middle cerebral artery occlusion rats. Neuroscience letters. 2008;440:246–250. doi: 10.1016/j.neulet.2008.05.090. [DOI] [PubMed] [Google Scholar]
- 36.Mitkari B, Kerkela E, Nystedt J, Korhonen M, Mikkonen V, Huhtala T, et al. Intra-arterial infusion of human bone marrow-derived mesenchymal stem cells results in transient localization in the brain after cerebral ischemia in rats. Exp Neurol. 2013;239:158–162. doi: 10.1016/j.expneurol.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 37.Khabbal J, Kerkela E, Mitkari B, Raki M, Nystedt J, Mikkonen V, et al. Differential clearance of rat and human bone marrow-derived mesenchymal stem cells from the brain after intra-arterial infusion in rats. Cell Transplant. 2015;24:819–828. doi: 10.3727/096368914X679336. [DOI] [PubMed] [Google Scholar]
- 38.Correa PL, Mesquita CT, Felix RM, Azevedo JC, Barbirato GB, Falcao CH, et al. Assessment of intra-arterial injected autologous bone marrow mononuclear cell distribution by radioactive labeling in acute ischemic stroke. Clin Nucl Med. 2007;32:839–841. doi: 10.1097/RLU.0b013e318156b980. [DOI] [PubMed] [Google Scholar]
- 39.Berman SC, Galpoththawela C, Gilad AA, Bulte JW, Walczak P. Long-term mr cell tracking of neural stem cells grafted in immunocompetent versus immunodeficient mice reveals distinct differences in contrast between live and dead cells. Magn Reson Med. 2011;65:564–574. doi: 10.1002/mrm.22613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roelants V, Labar D, de Meester C, Havaux X, Tabilio A, Gambhir SS, et al. Comparison between adenoviral and retroviral vectors for the transduction of the thymidine kinase pet reporter gene in rat mesenchymal stem cells. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2008;49:1836–1844. doi: 10.2967/jnumed.108.052175. [DOI] [PubMed] [Google Scholar]
- 41.Devor M, Gilad A, Arbilly M, Nissenbaum J, Yakir B, Raber P, et al. Sex-specific variability and a ‘cage effect’ independently mask a neuropathic pain quantitative trait locus detected in a whole genome scan. The European journal of neuroscience. 2007;26:681–688. doi: 10.1111/j.1460-9568.2007.05704.x. [DOI] [PubMed] [Google Scholar]
- 42.Guzman R, De Los Angeles A, Cheshier S, Choi R, Hoang S, Liauw J, et al. Intracarotid injection of fluorescence activated cell-sorted cd49d-positive neural stem cells improves targeted cell delivery and behavior after stroke in a mouse stroke model. Stroke. 2008;39:1300–1306. doi: 10.1161/STROKEAHA.107.500470. [DOI] [PubMed] [Google Scholar]
- 43.Gorelik M, Orukari I, Wang J, Galpoththawela S, Kim H, Levy M, et al. Use of mr cell tracking to evaluate targeting of glial precursor cells to inflammatory tissue by exploiting the very late antigen-4 docking receptor. Radiology. 2012;265:175–185. doi: 10.1148/radiol.12112212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nowakowski A, Andrzejewska A, Boltze J, Nitzsche F, Cui LL, Jolkkonen J, et al. Translation, but not transfection limits clinically relevant, exogenous mrna based induction of alpha-4 integrin expression on human mesenchymal stem cells. Scientific reports. 2017;7:1103. doi: 10.1038/s41598-017-01304-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ngen EJ, Bar-Shir A, Jablonska A, Liu G, Song X, Ansari R, et al. Imaging the DNA alkylator melphalan by cest mri: An advanced approach to theranostics. Mol Pharm. 2016;13:3043–3053. doi: 10.1021/acs.molpharmaceut.6b00130. [DOI] [PubMed] [Google Scholar]
- 46.Andres RH, Choi R, Pendharkar AV, Gaeta X, Wang N, Nathan JK, et al. The ccr2/ccl2 interaction mediates the transendothelial recruitment of intravascularly delivered neural stem cells to the ischemic brain. Stroke. 2011;42:2923–2931. doi: 10.1161/STROKEAHA.110.606368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hill WD, Hess DC, Martin-Studdard A, Carothers JJ, Zheng J, Hale D, et al. Sdf-1 (cxcl12) is upregulated in the ischemic penumbra following stroke: Association with bone marrow cell homing to injury. J Neuropathol Exp Neurol. 2004;63:84–96. doi: 10.1093/jnen/63.1.84. [DOI] [PubMed] [Google Scholar]
- 48.Li L, Chu L, Fang Y, Yang Y, Qu T, Zhang J, et al. Preconditioning of bone marrow-derived mesenchymal stromal cells by tetramethylpyrazine enhances cell migration and improves functional recovery after focal cerebral ischemia in rats. Stem Cell Res Ther. 2017;8:112. doi: 10.1186/s13287-017-0565-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cai H, Zhang Z, Yang GY. Preconditioned stem cells: A promising strategy for cell-based ischemic stroke therapy. Curr Drug Targets. 2014;15:771–779. doi: 10.2174/1389450115666140623120010. [DOI] [PubMed] [Google Scholar]
- 50.Yu X, Chen D, Zhang Y, Wu X, Huang Z, Zhou H, et al. Overexpression of cxcr4 in mesenchymal stem cells promotes migration, neuroprotection and angiogenesis in a rat model of stroke. J Neurol Sci. 2012;316:141–149. doi: 10.1016/j.jns.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 51.Cui LL, Nitzsche F, Pryazhnikov E, Tibeykina M, Tolppanen L, Rytkonen J, et al. Integrin alpha4 overexpression on rat mesenchymal stem cells enhances transmigration and reduces cerebral embolism after intracarotid injection. Stroke. 2017 doi: 10.1161/STROKEAHA.117.017809. [DOI] [PubMed] [Google Scholar]
- 52.Jeong JH, Schmidt JJ, Kohman RE, Zill AT, DeVolder RJ, Smith CE, et al. Leukocyte-mimicking stem cell delivery via in situ coating of cells with a bioactive hyperbranched polyglycerol. J Am Chem Soc. 2013;135:8770–8773. doi: 10.1021/ja400636d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campbell BC, Mitchell PJ, Investigators E-I Endovascular therapy for ischemic stroke. N Engl J Med. 2015;372:2365–2366. doi: 10.1056/NEJMc1504715. [DOI] [PubMed] [Google Scholar]
- 54.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2017 doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 55.Fraser JF, Maniskas M, Trout A, Lukins D, Parker L, Stafford WL, et al. Intra-arterial verapamil post-thrombectomy is feasible, safe, and neuroprotective in stroke. J Cereb Blood Flow Metab. 2017 doi: 10.1177/0271678X17705259. 271678X17705259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Janowski M, Walczak P, Kropiwnicki T, Jurkiewicz E, Domanska-Janik K, Bulte JW, et al. Long-term mri cell tracking after intraventricular delivery in a patient with global cerebral ischemia and prospects for magnetic navigation of stem cells within the csf. PloS one. 2014;9:e97631. doi: 10.1371/journal.pone.0097631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jozwiak S, Habich A, Kotulska K, Sarnowska A, Kropiwnicki T, Janowski M, et al. Intracerebroventricular transplantation of cord blood-derived neural progenitors in a child with severe global brain ischemic injury. Cell medicine. 2010;1:71–80. doi: 10.3727/215517910X536618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bryant LH, Jr, Kim SJ, Hobson M, Milo B, Kovacs ZI, Jikaria N, et al. Physicochemical characterization of ferumoxytol, heparin and protamine nanocomplexes for improved magnetic labeling of stem cells. Nanomedicine. 2017;13:503–513. doi: 10.1016/j.nano.2016.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


