Abstract
Parkinson’s disease (PD) is one of the most common neurodegenerative movement disorder characterized by preferential loss of dopaminergic neurons of the substantia nigra pars compacta and the presence of Lewy bodies containing α-synuclein. Although the cause of PD remains elusive, remarkable advances have been made in understanding the possible causative mechanisms of PD pathogenesis. An explosion of discoveries during the past two decades has led to the identification of several autosomal dominant and recessive genes that cause familial forms of PD. The investigations of these familial PD gene products have shed considerable insights into the molecular pathogenesis of the more common sporadic PD. A growing body of evidence suggests that the etiology of PD is multifactorial and involves a complex interplay between genetic and environmental factors. Substantial evidence from human tissues, genetic and toxin-induced animal and cellular models indicates that mitochondrial dysfunction plays a central role in the pathophysiology of PD. Deficits in mitochondrial functions due to bioenergetics defects, alterations in the mitochondrial DNA, generation of reactive oxygen species, aberrant calcium homeostasis, and anomalies in mitochondrial dynamics and quality control are implicated in the underlying mechanisms of neuronal cell death in PD. In this review, we discuss how familial PD-linked genes and environmental factors interface the pathways regulating mitochondrial functions and thereby potentially converge both familial and sporadic PD at the level of mitochondrial integrity. We also provide an overview of the status of therapeutic strategies targeting mitochondrial dysfunction in PD. Unraveling potential pathways that influence mitochondrial homeostasis in PD may hold the key to therapeutic intervention for this debilitating neurodegenerative movement disorder.
Keywords: Parkinson’s disease, mitochondrial DNA, reactive oxygen species, permeability transition pore, mitochondrial dynamics, oxidative phosphorylation
1. Introduction
Parkinson’s disease (PD) is the second most prevalent neurodegenerative disease after Alzheimer’s disease affecting more than 4 million people worldwide (Dorsey et al., 2007). Clinically, PD is diagnosed by the presence of motor symptoms that includes rest tremor, rigidity, bradykinesia, and postural instability. The pathological hallmark of PD includes the progressive loss of dopaminergic (DA) neurons in the substantia nigra pars compacta (SNpc), and the presence of cytoplasmic and neuritic inclusions named as Lewy bodies (LBs) and Lewy neurites (LNs) that are composed of α-synuclein (Lang and Lozano, 1998a, Lang and Lozano, 1998b). Though the clinical hallmarks and pathological features of PD have been extensively studied, the exact molecular basis underlying DA neuron degeneration remains incompletely understood. Clinically PD cases are mostly sporadic and not classified traditionally as a genetic disease. However, rare familial forms have been identified that account for about 15% of all the PD cases. Despite these differences, both sporadic and familial forms of PD share common clinical, pathological and biochemical features and thus, potential insights into the function and dysfunction of PD-associated gene products have helped elucidate common pathways in PD pathogenesis. Accumulating evidence indicates that both sporadic and familial PD directly or indirectly coalesce on mitochondrial homeostasis thereby providing a link between mitochondrial dysfunction and PD pathogenesis (Banerjee et al., 2009). In the following sections, we discuss current perspectives of mitochondria in sporadic and familial PD and review therapeutic strategies targeting mitochondrial dysfunction in PD and the status of their clinical development.
2. Mitochondrial dysfunction in sporadic Parkinson’s disease and the role of environmental toxins
Mitochondria are membrane-bound organelles found in every eukaryotic cell and are especially abundant in tissues with high-energy demands, such as brain and muscle. The major function of mitochondria is energy metabolism, predominantly oxidative phosphorylation (OXPHOS). The OXPHOS system comprises of 5 major multi-subunit complexes: complex I (NADH dehydrogenase-ubiquinone oxidoreductase), complex II (succinate dehydrogenase-ubiquinone oxidoreductase), complex III (ubiquinone-cytochrome c oxidoreductase), complex IV (cytochrome c oxidase), and complex V (ATP synthase). Although the mitochondrial OXPHOS system generates adenosine triphosphate (ATP), crucial for many essential cellular processes, mitochondria are the main cellular source of reactive oxygen species (ROS) and involved in calcium homeostasis and in the regulation and initiation of cell destructive pathways, which could underlie selective DA neurodegeneration in PD (Banerjee et al., 2009). Complex I is a major entry point of the respiratory chain and its deficiencies can be translated into a dramatic loss of bioenergetics functions leading to mitochondrial instability. Complex I also produce most of the ROS generated in intact mitochondria. There are numerous complex cellular mechanisms in place to help minimize the harmful effects of ROS, and yet intracellular ROS production is implicated in aging and many neurodegenerative pathologies (Grimm and Eckert, 2017). A link between complex I dysfunction and PD was established when several groups reported reduced complex I activity in the SN of the human brain, the major site of neuronal loss in PD (Schapira et al., 1990a, Schapira et al., 1990b, Janetzky et al., 1994). Since then complex I defects has been reported in a variety of other tissues including frontal cortex, platelets, and skeletal muscle of patients with sporadic PD (Parker et al., 2008, Keeney et al., 2006, Parker et al., 1989, Krige et al., 1992, Benecke et al., 1993, Benecke et al., 1993, Bindoff et al., 1991, Blin et al., 1994, Cardellach et al., 1993). However, it is important to note that many laboratories could not confirm complex I defect in PD (Banerjee et al., 2009). Such inconsistency in the experimental data may likely be explained by significant methodological differences and individual variability of PD patients. The most compelling evidence of mitochondrial dysfunction as a causal source of PD was obtained in the 1980s following accidental exposure of drug abusers to an illicit drug contaminated with 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), an inhibitor of mitochondrial complex I, that resulted in irreversible parkinsonian syndromes almost indistinguishable from PD (Langston et al., 1983). The revelation of MPTP mechanisms has resulted in a gold mine of information regarding the involvement of mitochondria in PD. MPTP is metabolized to its toxic form MPP+ (1-methyl-4-phenylpyridinium ion) by mitochondrial monoamine oxidase B and undergoes selective uptake to DA neurons through the dopamine transporter (DAT) and is rapidly concentrated in the mitochondria by an energy-dependent process (Heikkila et al., 1984, Gainetdinov et al., 1997, Ramsay et al., 1987). Mitochondrial accumulation of MPP+ specifically inhibits the oxidation of NAD (nicotinamide adenine dinucleotide) -linked substrates (Nicklas et al., 1985) by blocking the electron transfer through the complex I of the electron transport chain. Several studies reproduced the parkinsonian features induced by MPTP in both primate and murine models (Blesa et al., 2012). A considerable body of evidence epidemiologically links exposure to environmental toxicants like rotenone and paraquat (also known to inhibit mitochondrial respiration) to PD (Blesa et al., 2012, Tanner et al., 2011). The herbicide paraquat is a free radical generator that inhibits mitochondrial respiratory chain activity and causes DA neuron loss, accompanied by α-synuclein aggregation (Day et al., 1999, Manning-Bog et al., 2002). Similarly, chronic administration of the classic complex I inhibitor rotenone in rodents has been reported to produce multisystem degeneration including loss of nigrostriatal DA neurons and LB-like inclusions (Betarbet et al., 2000, Hoglinger et al., 2003, Lapointe et al., 2004).
The general perception is that complex I inhibition causes bioenergetics failure leading to ATP depletion and subsequent cell death. Supporting this view, MPP+ treatment causes a significant depletion of ATP in whole brain and synaptosomal preparations (Scotcher et al., 1990, Cosi and Marien, 1998) (Figure 1). It appears that complex I activity should be reduced more than 50% to cause a significant ATP depletion in non-synaptic brain mitochondria (Davey and Clark, 1996). However ATP is only mildly reduced (~20%) in MPTP treated mouse midbrain and striata (Cosi and Marien, 1998). Given that complex I activity is only reduced by 25–30% in PD patients a direct role of ATP depletion in PD-related DA neurodegeneration is ruled out (Parker et al., 1989, Schapira et al., 1990a). Despite the discrepancies in complex I activity and ATP levels there is substantial evidence indicating that augmenting the function of complex I with pesticide resistant single subunit complex I from yeast is sufficient to counteract the effects of complex I inhibitors such as MPP+, rotenone, pyridaben, and annonacin (Marella et al., 2008, Richardson et al., 2007, Seo et al., 2002, Escobar-Khondiker et al., 2007, Sherer et al., 2007). Further support for a role of complex I dysfunction in PD-related DA neurodegeneration was established by the feeding of the mitochondrial electron transport chain directly at complex II by means of the ketone body D-β-hydroxybutyrate. Administration of D-β-hydroxybutyrate was shown to bypass complex I blockade, enhance oxidative phosphorylation, and attenuate DA neurodegeneration in MPTP treated mice (Tieu et al., 2003). Alternatively, administration of inorganic nitrite in phylogenetically distinct animal models of PD improved bioenergetics and rendered protective effects via mitochondrial complex I reversible S-nitrosation and activation of the antioxidant Nrf2 pathway (Milanese et al., 2018).
Fig 1.
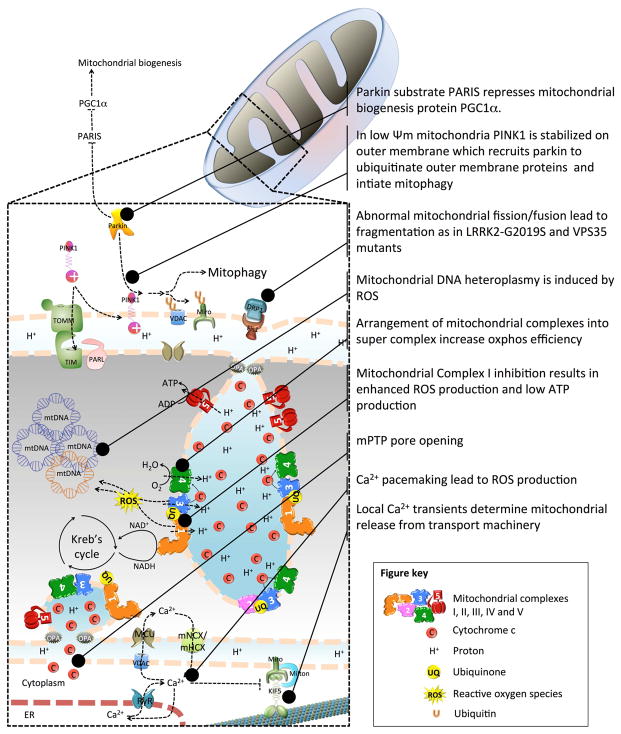
Mitochondrial pathways to Parkinson’s disease.
The OXPHOS components complex I, III, and IV form higher organized structures in the mitochondrial inner membrane called the super complexes. According to the current opinion assembly into super complexes improves enzymatic activities of individual complexes, and facilitates electron transfer through the OXPHOS chain, reducing ROS generation which in turn increases stability of individual complexes and results in efficient ATP synthesis (Enriquez, 2016, Maranzana et al., 2013). In a rodent PD model of 6-OHDA toxicity, neuronal degeneration was accompanied by decreased levels and activities of complex I in almost all forms of super complexes which was accompanied by decrease of complex IV activity in super complexes from striatum (Kuter et al., 2016). These observations were consistent with disassembly and loss of complex I and IV from the super complexes in mitochondria purified from fibroblasts derived from PD patients. The free complex III was only modestly affected, whereas its free versus super complex assembled forms decreased in PD compared to controls (Lopez-Fabuel et al., 2017). These findings suggest that besides complex I dysfunction and damage, the mitochondrial respiratory chain undergoes profound structural remodeling which is likely responsible for the energetic inefficiency and mitochondrial ROS over production observed in PD. Analysis of brain neurons and astrocytes in rodents revealed that the mitochondrial complex I in the neurons are predominantly assembled into super complexes, whereas in astrocytes the abundance of free complex I is higher. The presence of free complex I in astrocytes correlates with the several fold higher ROS production by astrocytes compared to neurons. Incidentally, complex I subunit NADH ubiquinone oxidoreductase core subunit S1 (NDUFS1) was more abundant in neurons than the astrocytes, whereas NDUFS1 knockdown in neurons decreased the association of complex I into super complexes, leading to impaired oxygen consumption and increased mitochondrial ROS. Conversely, overexpression of NDUFS1 in astrocytes promoted complex I incorporation into super complexes, decreasing ROS (Lopez-Fabuel et al., 2016). These findings suggest that complex I assembly into super complexes regulates ROS production and may contribute to bioenergetic differences between neurons and astrocytes. Collectively, these studies strongly support the view that complex I impairments may be central to the pathogenesis of DA neuronal death in sporadic PD. In the current scenario, it is unclear whether deficits in complex I is the triggering factor of all sporadic cases of PD, but systemic complex I deficiency might be a predominant feature of PD pathogenesis.
3. Genetic perspective of mitochondrial dysfunction in Parkinson’s disease
PD was long believed to be a prototypical, non-genetic disorder. However, in the last twenty years, the identification of distinct genetic loci responsible for rare monogenic Mendelian forms and the discovery of numerous genetic risk factors have refined and revolutionized this viewpoint. About 15% of patients with PD have a family history and in about 5–10% of subject’s PD is caused by monogenic mutations. To date, at least 23 loci and 19 disease-causing genes for parkinsonism have been found, but many more genetic risk loci and variants for sporadic PD phenotype have been identified in various genome-wide association studies (Deng et al., 2017, Domingo and Klein, 2018). The emerging view from the existing knowledge is that the etiology of PD is multifactorial and presumably involves a complex interplay between a myriad of gene networks, and the environment. Many of the causative or risk factor genes for PD directly or indirectly associate with mitochondrial biology and function (Bose and Beal, 2016) and have provided novel insights into the molecular pathogenesis of PD, which are discussed in this section (Table 1).
Table 1.
Familial PD genes and genetic risk factors and their potential role in mitochondrial biology
| Genes | Mitochondrial phenotype | References |
|---|---|---|
|
| ||
| α-synuclein (Park1/4) | Reduced Complex I activity, OCR | Subramaniam et al., 2014 |
| Abnormal mitochondrial morphology, Ca2+ homeostasis | Luth et al., 2014 | |
| Abnormal ER-mitochondria transport | Guardia-Laguarta et al., 2015 | |
|
| ||
| Parkin (Park2) | Reduced mitochondrial respiration, oxidative damage | Palacino et al., 2004 |
| Mitochondrial functional integrity | Chan et al., 2011 | |
| Reduced mitochondrial biogenesis/function | Shin et al., 2011 | |
| Abnormal mitochondria and high mitochondrial ROS | Chung et al., 2016 | |
|
| ||
| PINK1 (Park6) | Reduced ETC enzyme function | Papa et al., 2009 |
| Reduced ATP production, Ca2+ homeostasis | Heeman et al., 2011 | |
| Reduced mitochondrial function, fission | Park et al., 2006 | |
| Abnormal mitochondria and high mitochondrial ROS | Chung et al., 2016 | |
| Abnormal mitochondrial Ca2+ handling | Gandhi et al., 2009 | |
|
| ||
| DJ1 (Park7) | Abnormal mitochondrial morphology | Irrcher et al., 2010 |
| Uncoupled mitochondria, glycolytic shift | Shi et al., 2015 | |
| Mutants induce mitochondrial fragmentation | Wang et al., 2012a | |
|
| ||
| LRRK2 (Park8) | Reduced ATP production and membrane potential | Mortiboys et al., 2010 |
| Abnormal mitochondrial fission/fusion | Stafa et al., 2014 | |
| Delayed Miro degradation and mitophagy | Hsieh et al., 2016 | |
|
| ||
| ATP13A2 (Park9) | Mutant cause low mitochondrial oxygen consumption rate, ATP synthesis | Grunewald et al., 2012 |
|
| ||
| HTRA2 (Park13) | Mitochondrial morphological abnormalities | Martins et al., 2004 |
| Low respiration, sensitivity to apoptosis | Casadei et al., 2016 | |
|
| ||
| FBXO7 (Park15) | Impaired UPS, reduced mitophagy leading to accumulation of dysfunctional mitochondria | Zhou et al., 2016 |
| Mitochondrial accumulation of aggregates | Zhou et al., 2015 | |
|
| ||
| VPS35 Park17 | Mitochondrial fragmentation, reduced oxygen consumption | Wang et al., 2016 |
|
| ||
| CHCHD2 (Park22) | Decreased Complex I activity, respiration, increased ROS Transcription factor for Complex IV subunit COX4I2 | Aras et al., 2015, Aras et al., 2013 |
| Regulate apoptosis | Liu et al., 2015 | |
|
| ||
| PLA2G6 | Decreased mitochondrial membrane potential and function | Kinghorn et al., 2015 |
|
| ||
| GBA | Reduced macro autophagy leading to accumulation of dysfunctional mitochondria | Gegg and Schapira, 2016 |
3.1 α-synuclein (Park1, Park4)
Point mutations in the α-synuclein gene and multiplications of the wild-type gene are known to be associated with autosomal dominant PD (Schneider and Alcalay, 2017). α-synuclein protein is predominantly cytosolic, but a fraction has been identified in the mitochondria (Li et al., 2007), where it appears to interact directly with mitochondrial membranes (Nakamura et al., 2008). Given the affinity of α-synuclein for high-curvature detergent-resistant lipid-enriched membranes (Jensen et al., 2011, Middleton and Rhoades, 2010), perhaps it is not surprising that α-synuclein was found to have a role in the mitochondrial membrane. Upon binding to lipid membranes and vesicles, α-synuclein adopts an amphipathic α-helical structure, which can favor fibril formation, an important step in the initial process of oligomerization (Lee et al., 2002, Tsigelny et al., 2012). Mitochondrial localization of α-synuclein is also attributed to the presence of a cryptic mitochondrial targeting sequence (MTS) at its N-terminus of the α-synuclein gene (Devi et al., 2008). MTS is a short N-terminal peptide sequence that directs the transport of nuclear-encoded mitochondrial proteins into the mitochondria. Recent studies demonstrate that α-synuclein can disrupt mitochondrial protein import mechanisms in PD. The MTS is recognized by the translocase of the outer membrane (TOM) receptors, which is located on the outer membrane of the mitochondria. These matrix-targeted proteins are then translocated through the TOM complex to the translocase inner membrane (TIM) and finally into the matrix. In models of PD and postmortem PD brain tissues oligomeric, DA-modified, and Ser129E phosphomimetic α-synuclein can interact with members of the TOM complex (such as TOM40 and TOM20), and inhibit mitochondrial protein import resulting in excessive ROS production and mitochondrial impairment (Bender et al., 2013, Di Maio et al., 2016). Whether blockade of mitochondrial protein import is sufficient to drive nigrostriatal DA neurodegeneration remains to be determined, but it appears that accumulation of α-synuclein certainly impacts pathways involved in mitochondrial homeostasis. α-synuclein is known to inhibit mitochondrial complex I in a dose-dependent manner that reflects the regional brain expression of α-synuclein (Liu et al., 2009, Devi et al., 2008). Interestingly, Thy1-α-synuclein transgenic mice overexpressing the human wild-type α-synuclein showed defects in mitochondrial respiration in the nigrostriatal pathway but not in the cortex, suggest region-specific mitochondrial impairment due to α-synuclein overexpression (Subramaniam et al., 2014). In isolated mitochondria, the prefibrillar oligomeric form of α-synuclein cause complex I dysfunction (Luth et al., 2014). It is postulated that this inhibition of complex I is through direct association of α-synuclein with complex I (Devi et al., 2008). Transgenic mice overexpressing mutant human A53T α-synuclein in DA neurons display α-synuclein-induced inhibition of complex I function (Chinta et al., 2010). Interestingly, in mice overexpressing either human wild-type or mutant human A53T α-synuclein, it was concluded that α-synuclein has a regulatory role in the function of complex I (Loeb et al., 2010). However, contrary to the studies showing detrimental effects of α-synuclein on complex I, in isolated rat brain mitochondria, exposure to wild-type and mutant forms of α-synuclein leads to loss of mitochondrial transmembrane potential without affecting the activity of respiratory complexes (Banerjee et al., 2010). It is difficult to explain such confounding results between different transgenic mouse lines. Functional studies have shown that α-synuclein binding to mitochondria independent of complex I can lead to cytochrome c release, increased calcium, and generation of ROS levels culminating in cell death (Banerjee et al., 2015).
α-synuclein is also known to play an indirect role in modulating complex I activity through interaction with the anionic phospholipid cardiolipin, a protein necessary for complex I function (Fry and Green, 1981, Zhang et al., 2002). Cardiolipin appears to be required for the formation of the mitochondrial supercomplex that involves complex I, III, and IV. Therefore, physical interaction of cardiolipin with α-synuclein can disrupt electron transfer (Mileykovskaya and Dowhan, 2014). Monomeric and oligomeric forms of α-synuclein display a preference for cardiolipin-enriched inner mitochondrial membrane over the outer mitochondrial membrane (Camilleri et al., 2013, Zigoneanu et al., 2012). In response to mitochondrial damage, cardiolipin is externalized on mitochondria where it acts as a “self-destructive” signal and will interact with the autophagy protein LC3-II to mediate mitochondrial degradation machinery called mitophagy in neuronal cells (Chu et al., 2013). Interestingly, α-synuclein oligomers that bind to cardiolipin can form a complex and act as a substrate for cytochrome c peroxidase, which induce mitochondrial damage through permeabilization (Bayir et al., 2009). Collectively, these studies support a role for α-synuclein in regulating mitochondrial complex I activity, though there is some controversy regarding specific mechanisms resulting in complex I dysfunction. It is not yet clear as to which forms of α-synuclein, namely the oligomeric, monomeric, or the post-translationally modified α-synuclein versions are important in causing most significant damage to the mitochondria.
During conditions of oxidative stress, α-synuclein is known to localize in the nucleus where it binds to the promoter of peroxisome proliferator-activated receptor gamma-coactivator-1 alpha (PGC-1α) gene, a transcriptional coactivator known to play a major role in energy metabolism, mitochondrial physiology, and oxidative stress. Binding of α-synuclein to the PGC-1α promoter results in downregulation of PGC1-α target genes that are involved in the regulation of mitochondrial biogenesis and oxidative stress (Siddiqui et al., 2012). This is consistent with the presence of abnormal mitochondria, impaired mitochondrial respiration, fragmented endoplasmic reticulum, and increased susceptibility of nigral DA neurons to degeneration in the PGC1-α null mice following overexpression of human α-synuclein (Ciron et al., 2015). Moreover, α-synuclein-induced oxidative stress and neurodegeneration in PGC1-α null mice were rescued by expression of PGC1-α (Ciron et al., 2015). Other exogenous factors that link oxidative stress and mitochondrial dysfunction via α-synuclein are also suggested. For instance, in vivo studies using rats demonstrate the formation of α-synuclein aggregates following systemic exposure to mitochondrial toxins such as rotenone (Sherer et al., 2007) or dichlorvos (Binukumar et al., 2010). Similarly, α-synuclein transgenic mice exhibit increased proteinase-K-resistant α-synuclein inclusions following paraquat intoxication (Fernagut et al., 2007) and demonstrate increased sensitivity to neurodegeneration to MPTP (Nieto et al., 2006, Song et al., 2004, Lee et al., 2017a). Conversely, mice lacking α-synuclein are resistant to MPTP and other mitochondrial toxins such as malonate and 3-nitropropionic acid (Thomas et al., 2011, Drolet et al., 2004, Klivenyi et al., 2006, Dauer et al., 2002). Paradoxically, while acute MPTP administration to mice does not cause α-synuclein positive aggregates (Meredith and Rademacher, 2011), chronic infusion of MPTP via osmotic minipumps has been shown to produce ubiquitin and α-synuclein positive inclusions in the SN (Fornai et al., 2005, Thomas et al., 2007, Thomas et al., 2012).
There is growing body of evidence that mitochondrial dynamics which includes mitochondrial fission, fusion, transport and autophagic clearance of damaged mitochondria is essential for cellular health. These mechanisms are vital to neurons, as their alteration causes neurodegeneration. The dynamics of the mitochondrial network is intimately linked to the maintenance of mitochondrial activities. It is associated with cycles of fusion and fission events, regulated by fusion factors such as optic atrophy 1 (OPA1), mitofusin (Mfn1 and Mfn2), and by the fission GTPase Drp1. Mitochondrial membrane fusion allows redistribution of mitochondrial content and thus protect against the accumulation of damaged components, such as mitochondrial DNA (mtDNA). It constitutes a pro-survival response under stress conditions, ensuring optimal ATP production and regulating the autophagic removal of mitochondria (Tondera et al., 2009, Gomes et al., 2011, Shutt and McBride, 2013). Severe mitochondrial dysfunction inhibits mitochondrial fusion, resulting in mitochondrial fragmentation due to unopposed fission and subsequent mitophagy.
α-synuclein is known to affect mitochondrial dynamics by disrupting mitochondrial fission/fusion process. In Caenorhabditis elegans overexpression of α-synuclein leads to disruption in mitochondrial fusion resulting in mitochondrial fragmentation (Kamp et al., 2010), which is rescued by overexpression of PD proteins such as PINK1, Parkin and DJ-1. Interestingly, reintroduction of known mitochondrial fusion proteins such as Mfn1, Mfn2, and Opa1 failed to rescue these defects (Kamp et al., 2010). Studies from in vitro and in vivo model systems show that both wild-type and mutant α-synuclein overexpression induce mitochondrial fragmentation independent of Mfn2 or the fission protein Drp1 (Guardia-Laguarta et al., 2014, Kamp et al., 2010 Nakamura et al., 2011). In addition, mutant A53T α-synuclein is also reported to cause age-dependent abnormalities in mitochondrial morphology in vivo and impairments in mitochondrial transport in vitro (Xie and Chung, 2012). Indeed α-synuclein oligomers has been shown to interfere with axonal transport by disrupting the association of kinesin-1 motors with microtubules (Prots et al., 2013). It has been proposed that mechanisms that regulate axonal transport velocity and directionality are regulated by the number of active motors that interact with microtubules (Leidel et al., 2012, Fu and Holzbaur, 2014, Fu and Holzbaur, 2014). α-synuclein oligomers are known to decrease the velocity of microtubule gliding across kinesin-coated surfaces (Prots et al., 2013). These data link the intrinsic aggregation properties of α-synuclein to a mechanism for mitochondrial transport defects due to impairments in kinesin function. Incidentally, the considerable accumulation of proteins, vesicles and mitochondria within axonal swellings that are positive for α-synuclein staining in PD suggest that motility defects contribute to disease pathogenesis (Spillantini et al., 1998, Galvin et al., 1999). Thus, one of the plausible mechanisms by which α-synuclein might regulate mitochondrial homeostasis is by modifying mitochondrial mobility. In this regard, several studies have indicated that fusion-fission dynamics are linked to mitochondrial axonal transport (reviewed by Abeliovich and Gitler, 2016). It is therefore possible that defects in mitochondrial function in PD occur because of abnormal mitochondrial distribution and transport, which in turn could be induced by excessive levels of α-synuclein or due to its abnormal aggregation. Moreover, mitophagy is intricately linked to transport processes owing to the need for recovery of mitochondria for clearance. Thus, even if α-synuclein does not directly regulate mitochondrial clearance via autophagy, the role of α-synuclein fusion-fission and transport processes could indirectly regulate the rate of mitophagy (Pozo Devoto and Falzone, 2017). It will be of great interest to determine the role of α-synuclein in mitochondrial turnover. The existing viewpoint suggests that increase in α-synuclein levels or its aggregation does affect mitochondrial dynamics. Taken together, these studies suggest a direct reciprocal relationship between α-synuclein aggregation and mitochondrial dysfunction that can create a vicious cycle leading to neurodegeneration.
3.2 LRRK2 (Park8)
Autosomal dominant mutations in Leucine-rich repeat kinase-2 (LRRK2) are associated with both familial and late-onset PD. LRRK2 patients have clinical symptoms and pathology typical of sporadic PD (Healy et al., 2008, Martin et al., 2014a). LRRK2 is a complex and large protein constituted by multiple domains executing several functions, including GTP hydrolysis, kinase activity, and protein binding. Although the cellular function of LRRK2 is largely unknown there is increasing evidence that LRRK2 posits a role in autophagic regulation, microtubule dynamics, and mitochondrial function. LRRK2 resides mainly in the cytoplasm and is partially localized to the mitochondria. It is associated with membranes, such as those present in mitochondria, ER and synaptic vesicles (Vitte et al., 2010, West et al., 2005). Expression of mutant LRRK2 and overexpression of wild-type LRRK2 induce a variety of negative effects on mitochondria including increased fragmentation of mitochondria that produce more ROS and less ATP, resulting in increased vulnerability of cells to stressors. Skin biopsies from patients with the G2019S mutation in LRRK2 have reduced mitochondrial membrane potential, abnormal mitochondrial morphology, and decreased total intracellular ATP levels (Mortiboys et al., 2010).
It is likely that these mitochondrial deficits are due to dysregulated fission and fusion events as LRRK2 interacts with several key regulators of mitochondrial fission/fusion, thus indicating it has multiple regulatory roles (Ryan et al., 2015) (Figure 1). In neurons, it was shown that LRRK2 associates with the Dynamin-related protein 1 (Drp1 also referred as DLP1), a known mitochondrial fission factor (Wang et al., 2012b, Niu et al., 2012). Expression of LRRK2 G2019S and R1441C mutants in neurons induced mitochondrial fragmentation and increased their interaction rate with Drp1 which also displayed higher phosphorylation levels, resulting, among others, in an enhanced level of ROS. All these defects could be rescued by silencing of Drp1, suggesting a LRRK2-Drp1 pathway regulating mitochondrial fission events and their clearance (Wang et al., 2012b, Niu et al., 2012). It has been established that in murine primary neurons and human neuroblastoma, endogenous LRRK2 directly interacts with the fission regulator Drp1 at the mitochondrial membrane, by phosphorylating Drp1 leading to Drp1 activity and mitochondrial fission (Wang et al., 2012b, Niu et al., 2012). This LRRK2-Drp1-dependent mitochondrial fragmentation is enhanced by overexpressing wild-type LRRK2 or by expressing the PD-associated G2019S mutant protein and rescued by inhibiting Drp1 or increasing mitochondrial fusion (Wang et al., 2012b, Su and Qi, 2013). Furthermore, kinase-dead or GTP-binding-deficient LRRK2 displays greatly decreased Drp1 interaction (Wang et al. (2012b). Phosphorylation of Ser616 of Drp1 has been shown to promote fission and increased Ser616 Drp1 phosphorylation has been observed in sporadic PD patients (Chang and Blackstone, 2010). However, other data suggest that the G2019S mutant primarily phosphorylates Drp1 at Thr595 resulting in aberrant mitochondrial fragmentation (Su and Qi, 2013). LRRK2 also interacts with the mitochondrial fusion regulators Mfn1, Mfn2 and OPA1 modulating their activities. PD patients carrying the G2019S mutation demonstrate decreased levels of mature OPA1 (Stafa et al., 2014). Furthermore, fibroblasts and neuroblastoma cells expressing LRRK2 G2019S show a very high increase in basal oxygen consumption and a marked decrease in mitochondrial membrane potential, possibly due to mitochondrial proton leak caused by increased mitochondrial uncoupling protein 2 (UCP2) and UCP4 expression, restored to normal level with inhibition of LRRK2 (Papkovskaia et al., 2012). Additionally, UCP2 upregulation has been observed in induced pluripotent stem cell (iPSC) derived neurons from PD patients carrying the G2019S mutation (Grunewald et al., 2014). Together, these data demonstrate that the aberrant LRRK2 activity results in decreased mitochondrial fusion and increased fission and suggest that LRRK2 function may be an important factor in mitochondrial fission/fusion events in PD.
Another aspect of mitochondrial dynamics involves mitochondrial trafficking. Mitochondrial movements are tightly controlled to maintain energy homeostasis. Before initiation of mitophagy by which depolarized mitochondria are degraded through autophagosomes and lysosomes the process of mitochondrial motility ceases (Ashrafi et al., 2014, Liu et al., 2012, Wang et al., 2011). This arrest in mitochondrial motility is required to sequester damaged mitochondria, preventing them from moving and from reintroducing damage to other healthy mitochondria. Miro is an outer mitochondrial membrane protein that anchors the microtubule motors kinesin and dynein to mitochondria (Glater et al., 2006, Koutsopoulos et al., 2010, Wang and Schwarz, 2009). The depolarization-triggered mitochondrial arrest is achieved by removal of Miro from the damaged mitochondrial surface and its subsequent degradation by the proteasomes (Wang et al., 2011). A recent study discovered that LRRK2 promotes Miro removal from damaged mitochondria by forming a complex with Miro. PD associated LRRK2 G2019S mutant disrupt the LRRK2 Miro complex thus slowing Miro removal and mitochondrial arrest and delaying subsequent mitophagy, whereas partial depletion of Miro levels in LRRK2 G2019S human neurons and Drosophila PD models rescued neurodegeneration. Similar to LRRK2, other PD associated proteins PINK1 and Parkin have been shown to act in concert to target Miro for degradation (Ashrafi et al., 2014, Liu et al., 2012, Wang et al., 2011) suggesting that PINK1/Parkin pathways and LRRK2 function in parallel and converge on Miro that may be a common denominator for PD athogenesis.
3.3 PINK1 (Park6) and Parkin (Park2)
Two PD-associated proteins, the mitochondrial kinase PTEN-induced putative Kinase 1 (PINK1) and the E3-ubiquitin ligase Parkin, are central to mitochondrial quality control and provides the strongest evidence of mitochondrial dysfunction in PD pathogenesis (Pickrell and Youle, 2015). Mutations in PINK1 and Parkin are linked to early-onset familial PD (Pickrell and Youle, 2015). PINK1 localizes in the mitochondrial outer membrane and its kinase activity is critical for functions regulating mitochondrial homeostasis. Indeed, most mutations are found in the serine/threonine kinase domain of PINK1 protein, which suggest that loss of kinase activity plays a crucial part in the pathogenesis of PINK1-associated PD. Parkin on the other hand is located mainly in the cytosol and translocated to the mitochondria during stress or upon membrane depolarization in a PINK1-dependent manner to ubiquitinate several mitochondrial substrates mediating mitochondrial fragmentation, degradation and mitophagy (Matsuda et al., 2010). In healthy mitochondria with normal membrane potential, PINK1 is imported into the mitochondria and become degraded by proteolysis through the rhomboid protease presenilin-associated rhomboid-like protein (PARL) (Jin et al., 2010). However, in damaged mitochondria, the depolarization of the membrane potential as when treated with carbonyl cyanide m-chlorophenylhydrazone (CCCP-a mitochondrial uncoupler) allows the stabilization of PINK1 in the outer mitochondrial membrane. Activation and recruitment of Parkin onto damaged mitochondria involves PINK1-mediated phosphorylation of both Parkin and ubiquitin (Iguchi et al., 2013, Koyano et al., 2014). Through a stepwise process, Parkin is converted from an auto-inhibited enzyme into an active phosho-ubiquitin dependent E3 ligase. Upon activation, Parkin ubiquitinates itself in concert with many different substrates including mitochondrial proteins such as Mfn1 and Mfn2 (Gegg et al., 2010, Poole et al., 2010, Tanaka et al., 2010). The ubiquitin conjugates attached to these substrates can in turn be phosphorylated by PINK1, which triggers further cycles of Parkin recruitment and activation. This feed-forward amplification loop regulates both Parkin activity and mitophagy (Figure 1).
Depolarization of mitochondria induces PINK1/Parkin to associate with Miro, a mitochondrial outer membrane protein that recruit’s kinesin to the mitochondrial surface (Wang et al., 2011). PINK1 phosphorylates Miro to induce a Parkin and proteasomal-dependent degradation of Miro, thereby releasing kinesin from mitochondria, stalling the mitochondria, which is considered as an initial step prior to mitophagy (Wang et al., 2011). An additional mechanism for Parkin translocation to mitochondria involves the phosphorylation of Mfn2 by PINK1 which acts as a receptor for Parkin (Chen and Dorn, 2013). It is suggested that dysregulated PINK1/Parkin signaling leads to impaired mitophagy resulting in the development of PD.
Although PINK1/Parkin dependent mitophagy is highly reproducible in vitro, it is difficult to syncretize the contribution of PINK1/Parkin signaling to mitochondrial homeostasis in vivo as both PINK1 and Parkin null mice do not recapitulate the overt neuropathology or motor dysfunction that manifests in PD. Majority of the studies used cultured cells which poorly relate to the complex human nigrostriatal DA neurons that selectively degenerate in PD adding to the disconnect. Moreover, high levels of PINK1/Parkin are usually required to detect a robust induction of mitophagy and it is difficult to relate the cytotoxic induction stimuli (for e.g. CCCP/FCCP) to an analogous physiological correlate that evokes a similar level of mitochondrial clearance in vivo. It is also suggested that the high concentrations of the uncoupling agents (such as CCCP/FCCP) used to depolarize mitochondria in these studies also depolarize other cellular organelles such as lysosome raising the possibility that the observed effects may be caused by non-specific acidification of the cytosol (Berezhnov et al., 2016). However, two studies from the Dawson group reported that postnatal conditional Parkin null mice results in loss of nigral DA neurons, suggesting a compensatory mechanism that occurs during development in germline knockouts (Shin et al., 2011, Stevens et al., 2015). Although the nature of this compensatory mechanism is currently unclear, Parkin-independent mechanisms of mitophagy have been described and it is possible that one or more of these mechanisms may compensate for Parkin loss of function (Allen et al., 2013, Kageyama et al., 2014). Interestingly and unlike the PINK1 null mice the PINK1 knockout rat model exhibits locomotor deficits and age-dependent loss of nigral DA neurons (Dave et al., 2014, Villeneuve et al., 2016). In contrast, Parkin knockout rats do not exhibit significant PD-related pathology (Dave et al., 2014). However, a recent study failed to confirm the previous reports of loss of nigral DA neurons in the PINK1 knockout rats therefore questioning the validity of this model (Orr et al., 2017). Taken together these findings clearly indicate lack of robustness of nigrostrial pathway in PINK1 and Parkin null rodents in contrast to human, as functional loss of PINK1 or Parkin invariably results in nigral DA neurodegeneration in the latter.
Despite the lack of nigral DA neurodegeneration observed upon germline deletion of Parkin, two recent studies highlighted the importance of endogenous Parkin in mediating mitochondrial homeostasis in vivo in rodent models. In the first study Parkin null mice was crossed with a mouse strain that expresses a proofreading-deficient version of the mtDNA polymerase γ, associated with an age-dependent accumulation of mtDNA mutations, known as the “Mutator mouse” (Pickrell et al., 2015). While, individually, Parkin-null mice and Mutator mice did not show a neurodegenerative phenotype, there was a nearly 40 % loss in DA neurons in the SN of aged Parkin-deficient Mutator double mutant mice compared to wild-type mice suggesting the sensitivity to this double hit is selective for nigral DA neurons. Surprisingly, there was no increase in the mtDNA mutation frequency in the double mutants, suggesting that the influence of Parkin deletion in the mutator background was not a consequence of defect in the selective elimination of mutation-bearing mitochondria consistent with the biological role of Parkin in mitochondrial quality control. The second study explored the role of Parkin in mitochondrial quality control by crossing Parkin null mice with the PD-mito-Pstl male mouse, where the mtDNA undergoes double-strand breaks in DA neurons Pinto et al., 2017. The lack of Parkin promoted earlier onset of nigral DA neurodegeneration and motor defects in the PD-mito-Pstl mice, but it did not worsen the pathology. The lack of Parkin affected mitochondrial morphology in DA axons and increase in mutant and wild-type mtDNA levels without affecting mitophagy suggests that Parkin affects mtDNA levels in a mitophagy independent manner. These studies support the idea that Parkin protects DA neurons against mitochondrial dysfunction, but it fails to demonstrate that mitophagy as the underlying mechanism. Sterky and colleagues tested the hypothesis that absence of Parkin in vivo would prevent the clearance of defective mitochondria and thereby worsen the pathology in the MitoPark mice with conditional knockout of mitochondrial transcription factor A (TFAM) in DA neurons (Sterky et al., 2011). TFAM gene is known to regulate transcription of mtDNA and copy number and its deficiency in MitoPark mice results in progressive loss of DA neurons accompanied by low levels of mtDNA and lower respiratory chain activities (Ekstrand et al., 2007). Sterky and colleagues observed that the dysfunctional mitochondria in the MitoPark mice did not recruit Parkin in vivo, and neither affected the clearance of defective mitochondria nor the neurodegeneration phenotypes in the MitoPark Parkin double knockout mice (Sterky et al., 2011). These findings also failed to support a model in which Parkin-induced mitophagy protects against accumulation of mitochondria with severe respiratory chain dysfunction in vivo.
PINK1 and Parkin are also known to modulate mitochondrial homeostasis by regulating mitochondrial biogenesis. Parkin was shown to activate PGC-1α, a coregulator of mitochondrial biogenesis by promoting ubiquitin-dependent proteasomal degradation of the zinc finger transcriptional repressor called Parkin interacting substrate (PARIS) (Shin et al., 2011) (Figure 1). Loss of Parkin function results in the accumulation of PARIS, which in turn suppresses PGC-1α-dependent transcription. This was validated by analysis of postmortem SNpc tissue of PD patients, in which DA neurons displayed a reduction in PGC-1α levels. Moreover, viral vector-mediated overexpression of PARIS in the mouse SN led to DA neurodegeneration, which was prevented by Parkin or PGC-1α overexpression. Conversely, defective mitochondrial biogenesis and DA neuron loss following conditional Parkin deletion in adult mice was abrogated by PARIS gene silencing (Stevens et al., 2015). Recent studies indicate that PARIS is phosphorylated by PINK1 to regulate PARIS ubiquitination and clearance by Parkin where it controls PGC-1α levels indicating that PARIS provides a link between Parkin and PINK1 in the regulation of mitochondrial biogenesis (Lee et al., 2017b). Incidentally, some of the mitochondrial protective mechanisms are controlled by PINK1 independently of Parkin. This is highlighted by a specific role of PINK1 in the regulation of complex I activity. PINK1-deficiency in Drosophila and mouse models led to mitochondrial complex I defects (Morais et al., 2009), associated with loss of Ser250 phosphorylation of the complex I subunit NADH ubiquinone oxidoreductase subunit A10 (NDUFA10) (Morais et al., 2014). Incidentally, deficits in complex I activity and mitochondria-related phenotypes in PINK1 mutant flies, and cells derived from PINK1 null mice and patients with PINK1 mutations were rescued by Ser250 phosphorylation of NDUFA10. Another independent study showed that transgenic overexpression of NDUFA10 or its co-chaperone sicily rescued phenotypes in in PINK1 mutant flies, however, this study failed to confirm the phosphorylation of NDUFA10 in these phenotypes (Pogson et al., 2014). However, overexpression of NDUFA10 or sicily failed to restore phenotypes in Parkin null mutant flies, suggesting that PINK1 acts independently of Parkin in the maintenance of complex I activity. Interestingly, strategies bypassing or restoring complex I activity, through expression of the yeast Ndi1p NADH oxidoreductase or overexpression of NDUFA10 (Vilain et al., 2012, Morais et al., 2014, Pogson et al., 2014), attenuate phenotypes in PINK1 but not in Parkin mutant flies. Collectively, these studies provide strong evidence for the role of PINK1 and Parkin in mitochondrial quality control independent of its role in mitophagy and that future research should determine whether stimulating PINK1/Parkin mediated mitochondrial quality control represents a viable therapeutic target not just for PD but also for other mitochondrial disorders.
3.4 DJ1 (Park7)
Several other familial PD genes also influence either directly or indirectly the integrity of mitochondria in PD pathogenesis. Missense or deletion mutations of DJ-1 are associated with autosomal recessive PD (Bonifati et al., 2003). DJ-1 is a multifunctional protein involved in multiple cellular functions, including oxidative stress chaperone activity, transcription, and protecting mitochondria (Kinumi et al., 2004, Taira et al., 2004, Ren et al., 2011, Clements et al., 2006, Shendelman et al., 2004, Fan et al., 2008, Ishikawa et al., 2010, Ren et al., 2011, Hao et al., 2010). DJ-1 is mainly cytosolic and lacks a MTS but it translocate to mitochondria during oxidative stress-induced cell death (Canet-Aviles et al., 2004). Interestingly, PD causing mutants of DJ-1 such as L166P and M26I are localized on mitochondria and sensitize cells to oxidative stress (Ren et al., 2011). DJ-1 binds to complex I subunits NDUFA4 and ND1. Loss of DJ-1 decreases complex I activity consistent with mitochondrial defects observed in DJ-1 deficient cells (Hayashi et al., 2009). Incidentally, in fly models, DJ-1 mutant flies show compromised mitochondrial function with age whereas upregulation of DJ-1 can ameliorate toxic phenotypes of PINK1 but not Parkin mutant flies suggesting that DJ-1 acts downstream or parallel to the PINK1/Parkin pathway (Hao et al., 2010).
3.5 HTRA2 (Park13)
In humans, point mutations in HTRA2 are a susceptibility factor for PD (Table 1). The HTRA2 gene is a mitochondrial serine protease originally identified as a mammalian homolog to bacterial heat shock endoprotease (Faccio et al., 2000). In response to apoptosis, HTRA2 is released from mitochondria to the cytosol to cleave XIAP inducing apoptosis (Martins et al., 2002). Loss of function mutations in HTRA2 gene resulting in defective protease activity of HTRA2 is associated with autosomal recessive PD (Strauss et al., 2005). Loss of HTRA2 in mice leads to motor abnormalities, lethal neurodegenerative disorder, and accumulation of unfolded proteins in the mitochondria (Martins et al., 2004, Jones et al., 2003, Moisoi et al., 2009. HTRA2 knockout cells show a decrease in mitochondrial membrane potential, reduced mitochondrial density, and cause damage and mitochondrial DNA (mtDNA) mutations. In a fly model of HTRA2 loss of function, PD phenotypes were suppressed by the prosurvival Bcl-2 protein (M’Angale and Staveley, 2017). Additionally, PINK1 interacts with HTRA2 and facilitates HTRA2 phosphorylation, which contributes to increased resistance to mitochondrial stress (Plun-Favreau et al., 2007). Moreover, HTRA2 phosphorylation is decreased in brains from PD patients with PINK1 mutations, suggesting that PINK1 acts upstream of HTRA2 in mitochondrial stress signaling in PD (Plun-Favreau et al., 2007).
3.6 PLA2G6
Mutations in PLA2G6 cause PLA2G6-associated neurodegeneration (PLAN), including infantile neuroaxonal dystrophy (Khateeb et al., 2006) and adult-onset dystonia with parkinsonism (Paisan-Ruiz et al., 2009, Sina et al., 2009). PLA2G6 gene encodes for calcium-independent phospholipase A2b which hydrolyzes the sn-2 acyl chain of glycerophospholipids to release free fatty acids from phospholipids (Ma and Turk, 2001). It is distributed in the cytosol and membrane associated compartments, and considerably in the mitochondria (Seleznev et al., 2006). PLA2G6 null mice develop neuroaxonal dystrophy accompanied by increase phospho-Ser129 α-synuclein immunoreactivity in neuronal granules labeled with mitochondrial outer membrane protein TOM20 with degenerated mitochondria (Sumi-Akamaru et al., 2016). Similarly, loss of PLA2G6 orthologue in flies leads to age-dependent locomotor deficits and neurodegeneration with decreases in mitochondrial membrane potential and ATP production (Kinghorn et al., 2015). Overexpression of PLA2G6 protects cells from staurosporine-induced apoptosis by stabilizing mitochondrial membrane potential and reducing ROS levels (Seleznev et al., 2006). Moreover, PLA2G6 accumulates in LB from idiopathic and PLA2G6 patients (Miki et al., 2017) suggesting a role of PLA2G6 in mitochondrial dysfunction and LB formation (Sumi-Akamaru et al., 2015, Sumi-Akamaru et al., 2016).
3.7 CHCHD2 (Park22)
Missense mutation in CHCHD2 gene is associated with late-onset autosomal dominant PD and is a risk factor for sporadic PD (Funayama et al., 2015, Shi et al., 2016). CHCHD2 encodes coiled-coil-helix-coiled-coil-helix domain-containing protein 2, a protein originally identified as a transcription factor that binds to oxygen responsive element of COX4I2, a gene encoding cytochrome c oxidase (COX) (Aras et al., 2013). CHCHD2 contains an MTS and is localized to mitochondrial intermembrane space bound to COX and regulates its activity (Aras et al., 2015). CHCHD2 protein translocate from mitochondria to the nucleus in response to low oxygen tension and functions as a transcription factor to transactivate COX4I2 gene. Decrease of CHCHD2 protein level results in a decrease of COX activity and mitochondrial membrane potential, increase in ROS production and mitochondrial fragmentation, and disrupt mitochondrial cristae structure and destabilizes cytochrome c (Aras et al., 2015, Meng et al., 2017). Moreover, CHCHD2 protects against apoptosis via interaction with Bcl-xl to inhibit the oligomerization and mitochondrial accumulation of pro-apoptotic protein Bax (Liu et al., 2015). Taken together, CHCHD2 has a prominent role in the regulation of mitochondrial structure, synthesis of respiratory chain components and functions and modulation of apoptosis.
3.8 FBXO7 (Park15)
Mutations in F-box only 7 (FBXO7) results in early onset autosomal recessive PD (Shojaee et al., 2008). FBXO7 is a member of the Skp1-Cullin-F-box-type E3 ubiquitin ligase and plays a role in the ubiquitin proteasome system (Ho et al., 2008). FBXO7 is known to participate in mitochondrial maintenance through direct interaction with PINK1 and Parkin and modulates Parkin-mediated mitophagy and rescue phenotypes in the fly model of Parkin loss of function, whereas, PD-linked FBXO7 mutants can recruit Parkin to damaged mitochondria and facilitate its aggregation (Burchell et al., 2013, Zhou et al., 2016, Zhou et al., 2015). Incidentally, FBXO7 immunoreactivity has been reported in α-synuclein positive inclusions of PD and multiple system atrophy (MSA) brains (Zhao et al., 2013).
3.9 Vps35 (Park17)
Mutations in the vacuolar protein sorting 35 (Vps35) gene, which encode a core component of the retromer complex, is associated with late-onset autosomal dominant PD (Zimprich et al., 2011). The retromer complex is a critical coordinator of endosomal dynamics with a functional role in multiple cellular processes through sorting cargoes from endosomes to the trans-Golgi network or the plasma membrane. Retromer has been suggested to be associated with mitochondrial function by mediating vesicle transport between mitochondria and peroxisomes. The components of the mammalian retromer Vps35 and Vps26 functionally interact with the small ubiquitin-like modifier E3 ligase known as mitochondria-anchored protein ligase (MAPL) that is enriched in mitochondria-derived vesicles (MDVs) (Braschi et al., 2010). Silencing of Vps35 and Vps26 leads to defects in the formation of MAPL-positive MDVs and thus causes the reduction in the delivery of MAPL to peroxisomes (Braschi et al., 2010). A role of the retromer deficiency/mutation to impairments in mitochondrial dynamics and neurodegeneration is also suggested (Wang et al., 2016). During mitochondrial fission, DLP1 is recruited to mitochondrial outer-membrane to exert fission (Yoon et al., 1998). Retromer has been shown to regulate the removal of DLP1 complexes from mitochondria via a Vps35-DLP1 interaction, and is known to transport these complexes through MDV-dependent mechanisms to lysosomes for degradation (Wang et al., 2016). The Vps35 D620N mutant displays an enhanced interaction with DLP1 and promotes the turnover of mitochondrial DLP1 complexes, resulting in mitochondrial fragmentation and neuronal loss (Wang et al., 2016). A role of the retromer in the regulation of mitochondrial fusion is also suggested (Tang et al., 2015). Retromer promotes the degradation of mitochondrial E3 ubiquitin ligase (MUL1) and known to suppress MUL1-mediated Mfn2 degradation (Tang et al., 2015). In Vps35-deficient DA neurons, MUL1 levels are elevated leading to reduction in Mfn2 subsequently resulting in mitochondrial fragmentation and neurodegeneration. Notably, these phenotypes were restored by the overexpression of Vps35 but not Vps35 D620N mutant (Tang et al., 2015). Consistent with a role in mitochondria-dependent cell death, overexpression of Vps35 also protects DA neurons against MPP+-induced toxicity which is partially compromised by the presence of the PD-linked Vps35 D620N mutation (Bi et al., 2013). Together, these findings suggest a role of retromer in mitochondrial biology beyond its function in endosomal trafficking.
3.10 ATP13A2 (Park9)
Mutations in ATP13A2, which encodes a lysosomal P-type ATPase cause a rare autosomal recessive parkinsonian syndrome (Ramirez et al., 2006, Di Fonzo et al., 2007). Fibroblasts derived from PD patients with ATP13A2 mutation displays fragmented mitochondria with low oxygen consumption rate and ATP synthesis (Grunewald et al., 2012). However, mitochondrial phenotypes in patient-derived cells with the ATP13A2 mutation are circumstantial and might involve other factors that modify mitochondrial function such as α-synuclein, manganese, H2O2 or Zn2+ (Gitler et al., 2009, Del Fiacco et al., 1987, Daniel et al., 2015). Incidentally, ATP13A2 is known to regulate mitochondrial bioenergetics indirectly through macroautophagy (Gusdon et al., 2012).
3.11 GBA
The lysosomal hydrolase glucocerebrosidase (GCase) is encoded by the GBA gene. Homozygous GBA mutations cause Gaucher disease (GD), a lysosomal storage disorder. Although not known to cause monogenic form of PD, homozygous and heterozygous GBA mutations are numerically the greatest genetic risk factor for PD (Sidransky et al., 2009, Gegg and Schapira, 2016). The loss of GCase activity results in impairment of the autophagy-lysosomal pathway, which is required for the degradation of macromolecules and damaged organelles. The genetic and pharmacological inhibition of GCase activity via GCase shRNA or conduritol β-epoxide (CβE) respectively, results in significant decrease in mitochondrial membrane potential, inhibition of mitochondrial electron transport chain and mitochondrial fragmentation (Cleeter et al., 2013). Mitochondrial dysfunction is also observed in dermal fibroblasts from GD patients and in mouse models of GD. Fibroblasts from GD patients exhibited impaired oxygen consumption and significantly reduced levels of the respiratory chain electron carrier coenzyme Q10 and subsequent reduction in ATP levels and increased production of ROS (de la Mata et al., 2015). Both astrocytes and neurons derived from the GD null mice showed loss of mitochondrial membrane potential, reduced oxygen consumption and fragmented mitochondria (Osellame et al., 2013). Consistent with mitochondrial dysfunction in GD, GBA mutations or its deficiency in mice increased vulnerability of DA neurons to MPTP (Yun et al., 2018). At this juncture, a direct role of GCase in mitochondrial function is unknown, the loss of mitochondrial function in GD patients and preclinical models could be explained by impairment of the autophagy-lysosomal pathway, combined with changes in cellular lipid/sterol metabolism, neuroinflammation and perturbed calcium homeostasis (Gegg and Schapira, 2016).
To summarize the genetic factors, many familial PD gene products, which reside within the mitochondria or elsewhere in the cell play a crucial role in the maintenance of mitochondrial homeostasis. Deleterious mutations affecting the vital functions of these proteins therefore could dysregulate mitochondrial functions by interaction through innumerable signaling pathways. Based on the evidences a wide range of mitochondrial abnormalities has been reported in familial PD, including lowered ATP production, deficits in mitochondrial respiratory chain complex function, increased ROS production, abnormal morphology, disturbed mitochondrial transport, impaired mitophagy, and deficits in mitochondrial quality control. Future work should focus on resolving the impact of genes, environment, or a complex interaction of both in PD and should clarify which aspects of mitochondria become dysfunctional and when in the context of the entire pathological process leading to neurodegeneration to identify targets for therapeutic interventions.
4. Mitochondrial genome instability in Parkinson’s disease
Mitochondria maintain a circular double stranded mtDNA that is 16,569 base pair long in humans. Unlike the nuclear genome, which is present in two copies in each cell, mitochondrion maintains multiple copies of the mitochondrial genome with identical sequence in a normal cell (homoplasmy). This extra-nuclear DNA in the cell gives partial autonomy to mitochondria that codes for 37 genes including two rRNAs, 22 tRNAs and 13 polypeptides that assemble with other nuclear encoded proteins to constitute key enzymes involved in OXPHOS. In the absence of rigorous proof reading mechanism that exists in nuclear DNA, mtDNA accumulate errors resulting in nonidentical copies within the individual mitochondrion or between the mitochondria of the same cell (heteroplasmy). There is a minimal threshold level of mutant mtDNA required to manifest a disease and mitochondrial dysfunction. This threshold for disease is lower in tissues that are highly dependent on oxidative metabolism. Mutations of mtDNA may be inherited or somatic. Somatic mutations of mtDNA are known to develop with aging and are thought to represent cumulative damage due to excess exposure to free radicals (Cooper et al., 1992). The proximity to the respiratory chain is suggested to favor mtDNA damage by ROS production linked to complex I defects (Richter et al., 1988) (Figure 1). Consistent with this view, transgenic mice overexpressing mitochondrial targeted antioxidant enzyme catalase exhibit reduced accumulation of mtDNA mutations during aging (Vermulst et al., 2008).
In general, primary mutations of mtDNA, as opposed to mutations secondary to a nuclear housekeeping gene has rarely shown to manifest PD. Regardless, support for mtDNA encoded defects in PD patients were reported employing the use of cybrid cells. Cybrids are generated by replacing mtDNA of a normal cell with the one derived from a patient or control cell allowing exclusion of nuclear genome effects from that of the mtDNA. PD cybrids have depolarized mitochondria, reduced complex I activity, increased ROS production and lower ATP production (Swerdlow et al., 1996, Esteves et al., 2008). The cybrid studies suggest that complex I defects in PD are inherited either from the mitochondrial genome or from alteration in the somatic mtDNA. In PD, the maternal inheritance pattern of mtDNA mutation is rare (Swerdlow et al., 1998, Wooten et al., 1997, Thyagarajan et al., 2000), although several lines of studies provide genetic evidence that mtDNA abnormality may contribute to PD pathogenesis. A point mutation in mitochondrial 12SrRNA was found in a pedigree with Parkinsonism, with deafness and neuropathy (Thyagarajan et al., 2000), and there is occurrence of Parkinsonism with Leber’s optic atrophy with mitochondrial mutation G11778A (Simon et al., 1999). However, these are cases of atypical PD. Moreover, analysis of mtDNA found no homoplasmic mtDNA point mutations in coding regions in PD subjects in either DNA isolated from white blood cells or from the SN (Simon et al., 2000, Vives-Bauza et al., 2002). Whereas low levels of heteroplasmic mutations resulting in non-identical copies of mtDNA in the ND5 subunit of complex I were reported to be associated with PD, (Parker and Parks, 2005) the level of heteroplasmy was much less than seen in diseases caused by mtDNA mutations. Although, mitochondrial heteroplasmy has been highly anticipated, a clinical confirmation largely eluded, perhaps due to the shortcomings of technology to reach high enough resolution to sequence individual DNA molecule of individual mitochondria in large enough numbers. In contrast, higher burden of mtDNA mutations in SN were reported in individuals with early onset PD and incidental Lewy body disease (Lin et al., 2012).
MtDNA synthesis, replication and repair mechanisms are controlled by mtDNA polymerase gamma 1 (POLG1). POLG1 is a nuclear encoded protein and imported into the mitochondria to localize in the inner mitochondrial membrane. Mutations in POLG1 results in a wide range of complex syndromes including autosomal dominant or recessive progressive ophthalmoplegia and parkinsonism (Luoma et al., 2007, Mancuso et al., 2004, Hudson et al., 2007). PD associated with POLG1 mutations are L-DOPA responsive, characterized by loss of SNpc DA neurons lacking Lewy bodies, and by the presence of multiple mtDNA deletions (Hudson et al., 2007). However, POLG mutant mice (D257A) with reduced ability to proofread accumulate significant amount of mtDNA deletions without neuronal degeneration and mitochondrial dysfunction (Perier et al., 2013). Mutations/polymorphisms in mitochondrial transcription factor gene TFAM, that regulates transcription of mtDNA and copy number has been suspected for its association in PD (Gatt et al., 2013). In contrast to the POLG mutant mice the conditional knockout of TFAM in DA neurons of MitoPark mice showed progressive loss of DA neurons accompanied by low levels of mtDNA and lower respiratory chain activities (Ekstrand et al., 2007). These studies suggest that the presence of increased levels of mtDNA deletions below a certain threshold is not responsible for neurodegeneration in PD.
In European populations, the mtDNA haplotype J and K, associated with mild uncoupling of mitochondria allowing adaptation to colder climates, would lead to reduced mitochondrial ROS production. Haplotype J, determined by a single nucleotide polymorphism (SNP) at 10398G and haplotype K in the control region of mtDNA, reduced the incidence of PD by 50% in these European patients (Ghezzi et al., 2005). However, with increasing age the super-haplogroup HV increases the risk for development of the disease (Hudson et al., 2013). These studies highlight the fact that tiny changes in mitochondrial genome may determine the risk of developing PD. A role of mtDNA deletions in human SN neurons was found by the presence of clonally expanded mtDNA deletions that was associated with a decrease in cytochrome oxidase activity in the SN (Bender et al., 2006, Kraytsberg et al., 2006). The Bender et al., study used long-range PCR to amplify mtDNA from cytochrome oxidase deficient and control neurons, and the Kraytsberg et al., study developed a novel single-molecule PCR technique to quantify the total cellular burden of mtDNA deletions in cytochrome oxidase-deficient neurons harboring mitochondrial respiratory chain defect and control neurons. Both studies found that mtDNA deletions were present at higher levels in aged controls compared to younger individuals and that these deletions were clonally expanded. In addition, Bender et al., study identified that mtDNA deletions were higher still in individuals with PD compared to aged controls (52.3% and 43.3% respectively), although the difference was of borderline significance (P= 0.06). These findings of surprisingly high levels of clonally expanded mtDNA deletions in SN neurons from elderly subjects, and a correlation of mutations with cytochrome oxidase deficiency support the hypothesis that the age-related accumulation of somatic mtDNA mutations may contribute to the aging of human brain and to the pathogenesis of PD. Consistent with these observations Grunewald and colleagues recently showed that mitochondrial complex I and II are most consistently affected in single neurons that also displayed reduced mtDNA copy number (Grunewald et al., 2016). Also, in idiopathic PD cases increased mtDNA mutations were also reported in iPSC-derived neurons from patients carrying LRRK2 mutations (Sanders et al., 2014). A complete spectrum of mtDNA changes, including deletions, copy number variations, and point mutations in single DA neurons from SN and neurons from other brain regions of individuals with PD and healthy controls was recently studied (Dolle et al., 2016). Dölle and colleagues in this elegant study showed that SN DA neurons from PD patients accumulate higher levels of somatic mtDNA deletions, but not point mutations. Moreover, mtDNA copy number increases with age in healthy individuals, thus maintaining the pool of wild-type mtDNA population in spite of accumulating deletions. Conversely, mtDNA copy number does not increase in individuals with PD, resulting in depletion of the wild-type mtDNA population. Together, these observations suggest that there is a threshold beyond which mtDNA deletions accumulate in DA neurons of SN that results in mitochondrial defects and increase the risk of PD.
In summary, it is unclear if defects in mitochondrial genome are a direct cause of the PD pathogenesis or is a secondary consequence of ongoing mitochondrial dysfunction or neurodegeneration. Based on the studies thus far there is no clear evidence that mtDNA mutations are the primary culprit for PD. It appears that mtDNA mutations occur in the context of increasing cellular stress and decreasing fidelity of cellular defense systems against stress. However, clonal expansions of mtDNA mutations can functionally impair the respiratory capacity and thus lead to degeneration of vulnerable neurons. Despite these issues, the role of mtDNA should not be completely ignored and further investigations with more sophisticated tools are warranted to better understand its role in PD pathogenesis.
5. Mitochondrial permeability transition and the role of calcium homeostasis in Parkinson’s disease
Calcium (Ca2+) plays a significant role in the function of all mammalian cells and is especially important in the nervous system. Extracellular Ca2+ affects neuronal excitability and the release of neurotransmitters, whereas intracellular Ca2+ is known to function as a second messenger in cell signaling and execute a wide range of functions in the mitochondria and ER (Brini et al., 2014). Ca2+ mishandling is associated with pathogenesis of several neurodegenerative diseases, including PD (Surmeier et al., 2010). During normal synaptic activity, intracellular concentration of Ca2+ increases transiently with no adverse effects on neurons. Unlike most neurons in the brain, DA neurons in the SNpc are autonomously active through their pacemaking activity, generating action potentials in the absence of synaptic input (Chan et al., 2007). This pacemaking activity is driven by voltage-dependent L-type Ca2+ channels, leading to sustained elevations in cytosolic Ca2+ levels in the SNpc DA neurons. The large Ca2+ buffering burden created by pacemaking activity in these neurons ultimately compromises mitochondrial function, resulting in mitochondrial oxidative stress and oscillations in mitochondrial potential, the latter being associated with compromised ATP production (Guzman et al., 2010) (Figure 1). Supporting a pathogenic role for increased Ca2+ load linked to pacemaking activity, the L-type Ca2+ channel antagonist-isradipine can attenuate rotenone-induced dendritic loss in brain slices from the adult midbrain and attenuate MPTP-induced DA neurodegeneration in mice (Chan et al., 2007). These observations suggest that sustained mitochondrial Ca2+overload in adult SNpc DA neurons may render them selectively vulnerable to PD. In agreement with this, neighboring ventral tegmental area (VTA) DA neurons, which do not rely on L-type Ca2+ channels for pacemaking, are relatively preserved in PD (Chan et al., 2010, Khaliq and Bean, 2010). In addition to differences in pacemaking another proposed mechanism for differential vulnerability between SN vs VTA DA neurons in PD is because SN DA neurons have a very high basal rate of mitochondrial OXPHOS, a smaller reserve capacity, higher density of axonal mitochondria, elevated levels of oxidative stress, and a considerably more complex axonal arborization compared to VTA neurons (Pacelli et al., 2015). Additionally, in neurons Ca2+ plays a significant role in the release of neurotransmitters. A recent study identified that when Ca2+ levels in the neurons increase, such as upon neuronal signaling, the α-synuclein binds to synaptic vesicles at multiple points causing the vesicles to come together (Lautenschlager et al., 2018). This may indicate a normal role of α-synuclein to help with chemical transmission across neurons. However, there is a fine balance of Ca2+ and α-synuclein maintained in neurons, and in conditions of excessive Ca2+ α-synuclein undergoes aggregation resulting in neuronal degeneration (Lautenschlager et al., 2018). To maintain Ca2+ homeostasis, Ca2+ entering neurons is rapidly sequestered in intracellular organelles, such as the mitochondria and the endoplasmic reticulum (ER), or pumped back across the plasma membrane concentration gradient. Although, the most significant intracellular storage site of Ca2+, is the ER, the ability to accumulate, retain, and release Ca2+ is a fundamental property of mitochondria. Accumulation of Ca2+ within the mitochondrial matrix depends on both Ca2+ uptake into the mitochondria through an electrogenic uniporter, as well as extrusion of Ca2+ from the mitochondria through Na+/Ca2+ and H+/Ca2+ antiporters (Szabadkai et al., 2006, De Stefani et al., 2011). Ca2+ handling by mitochondria is involved in energy production for neuronal electrical activity, in buffering and shaping cytosolic Ca2+ rises and in determining cell fate by triggering or preventing apoptosis (Contreras et al., 2010).
The mitochondrial permeability transition is a phenomenon characterized by opening of a non-specific channel, known as the permeability transition pore (PTP), located in the inner membranes of the mitochondria (Figure 1). The PTP is opened by Ca2+ accumulation in the mitochondria and many other factors, and is traditionally linked to mitochondrial dysfunction because its occurrence leads to mitochondrial depolarization, cessation of ATP synthesis, Ca2+ release, pyridine nucleotide depletion, inhibition of respiration, matrix swelling, outer mitochondrial membrane (OMM) rupture and eventually release of pro-apoptotic proteins such as cytochrome c, endonuclease G and apoptosis inducing factor (AIF) (Bernardi et al., 2015). An important point to consider is that these detrimental effects on energy conservation and cell viability are only seen for long-lasting openings of the PTP (Petronilli et al., 2001) while short-term openings, which have been documented both in isolated mitochondria and in situ (Bernardi et al., 2015) may be involved in physiological regulation of Ca2+ and ROS homeostasis (Zorov et al., 2014), and provide mitochondria with a fast mechanism for Ca2+ release (Barsukova et al., 2011). Despite considerable experimental efforts, the molecular identity of the protein(s) that form the PTP channel remains a mystery. Although, there are multiple proteins implicated in pore formation and its regulation (Bernardi and Di Lisa, 2015, Giorgio et al., 2017), the major proteins proposed to comprise the PTP are-a) the voltage-dependent anion channel (VDAC) present in the outer membrane, b) the adenine nucleotide translocator (ANT) located in the inner membrane, c) cyclophilin D (CypD) found in the matrix, d) the F1F0 ATP synthase found in the inner mitochondrial membrane (IMM), and other molecules. According to the prevailing point of view, the formation of the pore is initiated by the CypD translocation from the mitochondrial matrix to the IMM to bind with ANT. CypD binding to ANT results in the formation of an ANT channel in IMM. The ANT formed channel together with the channel formed by VDAC in OMM constitutes a tunnel-like structure crossing the mitochondrial membranes, thus connecting mitochondrial matrix with the cytosol (Banerjee et al., 2009). The molecular nature of PTP was further refined with the identification of c-subunit ring of the F1F0 ATP synthase which forms a voltage-sensitive channel of the PTP following the dissociation of the F1 ATP synthase from CypD (Giorgio et al., 2013, Bonora et al., 2013, Alavian et al., 2014).
In the context of PD-related cell death, one of the earliest observations implicating Ca2+-induced mitochondrial dysfunction was that MPP+, 6-hydroxydopamine or dopamine strongly stimulated Ca2+ release from mitochondria and hydrolysis of intra-mitochondrial pyridine nucleotides (Frei and Richter, 1986) This was followed by the finding that MPP+ caused mitochondrial swelling and the release of cytochrome c (Cassarino et al., 1999), Ca2+ efflux and membrane depolarization (Packer et al., 1996), all of which were inhibited by the CypD inhibitor cyclosporine A, thereby implying a classical PTP. Consistent with these in vitro findings we showed that genetic ablation of PTP modulator CypD, which significantly increases the resistance of mitochondria to Ca2+-induced PTP opening, also strongly protects mice against acute MPTP-neurotoxicity (Thomas et al., 2012). Studies using cybrid cells containing mtDNA from PD patients also exhibit lower mitochondrial Ca2+ sequestration compared to controls when stimulated for Ca2+ entry into the mitochondria (Sheehan et al., 1997). Other studies that support a role for alterations in Ca2+ homeostasis in PD are related to dysfunctions in PD related proteins. For eg., both monomeric and oligomeric α-synuclein has been shown to favor entry of Ca2+ from extracellular space, whereas increase in intracellular Ca2+ is known to enhance α-synuclein aggregation, thus establishing a reciprocal relationship between α-synuclein and Ca2+ (Follett et al., 2013, Schmidt et al., 2012, Tsigelny et al., 2012). It is unclear whether this effect is due to increased plasma membrane permeability to Ca2+ or due to modulation of the Ca2+ channels. The finding that α-synuclein is present in the mitochondria-associated ER membrane fractions also known as (MAM) (Guardia-Laguarta et al., 2015) supports its role in stabilization/formation of the ER-mitochondria interactions and mitochondrial uptake of Ca2+. It is important to note that there is a significant interplay between mitochondria and ER about Ca2+. Both the ER and mitochondria are linked biochemically and physically and facilitate efficient Ca2+ transmission from ER to mitochondria (Csordas et al., 2006, de Brito and Scorrano, 2008). Interestingly, α-synuclein has been shown to interact with ANT, one of the components of PTP (Zhu et al., 2011), and genetic ablation of CypD, the PTP modulator, delayed disease onset, and extended lifespan in mutant human A53T α-synuclein transgenic mice (Martin et al., 2014b). These observations are consistent with increased mitochondrial swelling, PTP pore opening and Ca2+-induced cytochrome c release in the presence of soluble pre-fibrillar α-synuclein oligomers (Luth et al., 2014). The PD protein Parkin is known to exert a protective role by sustaining ATP production via stabilization of ER-mitochondria Ca2+ transfer, thus providing a possible mechanism through which Parkin mutations or deficiency might result in neuronal dysfunction (Cali et al., 2013, Gautier et al., 2016). Moreover, Parkin deficiency has been shown to be associated with increased basal Ca2+ levels (Sandebring et al., 2009). Similarly, the loss of PD protein PINK1 resulted impaired Ca2+ efflux and an overload of Ca2+ in the mitochondria (Gandhi et al., 2009). This resulted in lower mitochondrial respiration, an increased ROS production and a lower threshold for the opening of the PTP. In confirmation of this point of view, co-expression of mutant PINK1 and α-synuclein-induced mitochondrial alterations were partially rescued by the PTP inhibitor cyclosporine A (Marongiu et al., 2009). Moreover, loss of PINK1 has been shown to sensitize cells to PTP opening (Gandhi et al., 2009, Gautier et al., 2012) and reduced PKA signaling leading to impaired phosphorylation and activity of the mitochondrial Na+/Ca2+ exchanger (NCLX) which is likely responsible for increased mitochondrial Ca2+ accumulation (Kostic et al., 2015). An association of PINK1 in Ca2+ homeostasis and the development of PD is also established through the mitochondrial calcium uniporter (MCU), located in the inner mitochondrial membrane. Genetic and pharmacologic inactivation of the MCU prevented dopaminergic neuronal loss in PINK1Y431 mutant zebra fish (Soman et al., 2017). The PD related protein DJ-1 has been shown to act at the ER-mitochondria contact sites (Ottolini et al., 2013) and is known to mediate direct transfer of Ca2+ from ER to mitochondrial matrix. Interestingly, DJ-1 overexpression in a cellular model shows protective response by delaying the opening of the PTP and suggests that DJ-1 plays a crucial role in the control of Ca2+ buffering and oxidative stress via uncoupling proteins (UCP4 and UCP5) (Dongworth et al., 2014, Guzman et al., 2010). Another PD-related protein LRRK2 is also linked to PTP function. LRRK2 has been shown to interact with three components of the PTP including ANT, VDAC, and mitochondrial creatine kinase. Expression of both wild-type and mutant LRRK2 retain the mitochondrial creatine kinase at the outer mitochondrial membrane and block its mitochondrial import to promote the interaction between VDAC1 and ANT, thus sensitizing the cell to PTP opening and subsequent cell death (Cui et al., 2011).
In summary, there is convincing evidence for the involvement of derangements in Ca2+ signaling and mitochondrial Ca2+ mishandling in PD pathogenesis, and that nigral DA neurons act as a major site of neuronal Ca2+ impairments in the CNS due to their intrinsic susceptibility (Chan et al., 2009). Given the complexity and insufficient knowledge of the composition of PTP and its regulation, future research should address some important questions, such as the protein composition of the PTP channel, how they regulate PTP opening, and what PD relevant factors/mechanisms influence PTP function? Answering these questions may help establish whether PTP plays a role in the etiology of PD and develop therapeutic agents targeting PTP.
6. Therapeutic approaches targeting mitochondrial dysfunction in Parkinson’s disease
Despite intensive research, the etiology of PD remains poorly understood, leaving with no effective neuroprotective therapies. Current therapies are palliative at best by providing effective control of symptoms, particularly in the early stages of the disease. The current symptomatic therapies cannot completely ameliorate later-stage symptoms, nor can they address the ongoing degeneration that underlies PD pathology. For this reason, a great deal of research has focused on finding the cause of neurodegeneration in PD and on exploring protective, restorative, and replacement therapies. Substantial evidence as detailed in this review suggests a major role of mitochondrial dysfunction in the pathogenesis of both sporadic and familial PD. Given the importance of mitochondrial dysfunction in PD pathogenesis, therapeutics targeting mitochondria have been extensively studied to treat PD. Here, we briefly summarize therapeutic agents that reduced PD pathology in preclinical models of PD and those that eventually made it to human clinical trials (Table 2). Most of the agents tested for neuroprotective effects in clinical trials in PD have used compounds that would slow down neurodegeneration and disease progression by blocking mitochondrial dysfunction, reducing oxidative stress or both.
Table 2.
Current clinical trials targeting mitochondrial dysfunction in PD
| Drug | Mechanism of action | Clinical outcome | Reference |
|---|---|---|---|
| Rasagiline | MAO-B inhibitor Antioxidant |
Modest UPDRS gain, but inconsistent dose dependency between 1mg and 2mg |
NCT00256204* Smith et al., 2015 |
| Creatine | Antioxidant Mitochondrial biogenesis |
No improvement |
NCT00449865* Writing Group for the et al., 2015 |
| Ubiquinone (Coenzyme Q10) | Antioxidant Mitochondrial electron carrier |
Trial completed No improvement |
NCT00004731* Parkinson Study Group et al., 2014a |
| Mitoquinone (MitoQ) | Antioxidant Mitochondrial biogenesis |
Trial completed No improvement |
NCT00329056* Liu and Wang, 2014 |
| Isradipine | Calcium channel blocker | Safety study completed Phase III trial underway |
NCT00909545* Swart and Hurley, 2016 |
| Inosine | Antioxidant | Generally safe and tolerated; Slight dose dependent attenuation of clinical decline |
NCT00833690* Parkinson Study Group et al., 2014b |
| N-acetylcysteine | Antioxidant Glutathione precursor |
Moderately improved UPDRS. | Monti et al., 2016 |
| Pioglitazone | Anti-inflammatory PPAR-g agonist |
No improvement |
NCT01280123* Investigators, 2015 |
| Exenatide | Anti-apoptotic Anti-inflammatory Mitochondrial activity |
Trial completed | NCT01971242* |
| Resveratrol | Mitochondrial biogenesis | Safety study |
NCT01504854* Smoliga et al., 2011 |
| EPI-589 (R)-troloxamide quinone | Augments GSH levels | Recruitment stage | NCT02462603* |
| Ursodeoxycholic acid | Beneficial effect on mitochondrial dysfunction | Safety study |
NCT02967250* Mortiboys et al., 2013 |
ClinicalTrials.gov Identifier
The Deprenyl And Tocopherol Antioxidative Therapy Of Parkinsonism (DATATOP) trial was the largest and the first clinical trial to investigate the neuroprotective potential of selegiline, a monoamine oxidase-B inhibitor, along with tocopherol (vitamin E), in patients with early PD with putative rescue mechanisms on mitochondrial function and oxidative stress (Parkinson Study, 1993). The primary outcome was the onset of disability prompting the clinical decision to begin levodopa treatment in PD patients. Although the use of selegiline had a beneficial effect and significantly delayed the time of onset of levodopa treatment during the first 12 months, patients treated with tocopherol showed no benefits nor did the tocopherol added any benefit to selegiline. This study suggests that selegiline may delay disease progression, with mild symptomatic effects known to improve motor symptoms in PD rather than a neuroprotective effect. Rasagiline is a newer monoamine oxidase-B inhibitor that is more potent than selegiline and has metabolites with potential antioxidant properties. It has been studied using a delayed-start clinical trial design intended to reduce the confounding effect of symptomatic efficacy. The outcomes of this study suggest that early treatment confers a long-lasting improvement, but the overall duration of this study was relatively short, and the group sizes were small (Parkinson Study, 2002). However, study in a larger sample and over a longer time course failed to demonstrate disease-modifying effects of the drug at 2 mg dose which was observed with 1 mg. Moreover, small change in Unified Parkinson Disease Rating Scale (UPDRS) scores made the results difficult to interpret (Olanow et al., 2009).
Coenzyme Q10 (CoQ10) is a lipid-soluble endogenous compound that serves as a cofactor for the electron transport chain by accepting electrons from complex I, II and III (Beal, 2003). It serves as a potent free radical scavenger in the inner mitochondrial membranes and microsomal lipid membranes by reducing α-tocopheroxyl radical and by regenerating α-tocopherol (Beal, 2003). CoQ10 is also an obligatory cofactor for mitochondrial uncoupling proteins as they function by regulating ATP production and reducing free radical generation and reduce DA neurodegeneration in mouse PD models (Beal, 2002). A large multicenter QE3 study evaluated the potential neuroprotective effect of two different doses of CoQ10 (1200 and 2400 mg daily) in drug naive participants with PD (Parkinson Study Group et al., 2014a). All participants of this study also received 1200 IU/day of vitamin E. The patients were observed for 16 months or until symptomatic treatments were required. CoQ10 was safe and well tolerated but did not show any clinical benefits (Parkinson Study Group et al., 2014a). Another mitochondrial targeted antioxidant Mitoquinone (MitoQ-a derivative of mitochondrial quinolone) consists of the lipophilic cation triphenylphosphonium (TPP) covalently attached to the ubiquinone moiety of CoQ10 (Jin et al., 2014). The TPP cation enables MitoQ to cross membranes and to accumulate within the mitochondria (Snow et al., 2010). MitoQ is known to convert hydrogen peroxide to water and oxygen, to reduce toxic insults from free radicals in the mitochondria and enhance mitochondrial bioenergetics. It has been shown to be effective in numerous animal models of mitochondrial dysfunction including PD (Orsucci et al., 2011). A multicenter study in New Zealand and Australia that tested two doses of MitoQ compared to placebo in a double-blind study showed no difference in UPDRS score at 12-month follow-up. Failure of this study was attributed to small sample size and lack of adequate brain penetrance of MitoQ (Snow et al., 2010).
Creatine is a guanidine compound and serves as a crucial energy reservoir for ATP and is a component of the creatine-phosphate system. Most of the creatine is found in the skeletal muscle and is taken up by various organs by specific creatine transporters, and serves as a substrate for mitochondrial and cytosolic creatine kinase (Snow and Murphy, 2001). It is suggested that the creatine enables mitochondrial creatine kinase to remain functionally in the octameric state to inhibit the mitochondrial PTP and block apoptosis (Dolder et al., 2003). Free radicals enable the octameric form of mitochondrial creatine kinase to convert to a dimeric state, which causes opening of the PTP to mediate cell death (O’Gorman et al., 1997). However, the neuroprotective function of creatine/phosphocreatine through creatine kinase-mitochondrial PTP system has been challenged, and instead it enhances cytosolic high energy phosphates that maintain ATP levels (Klivenyi et al., 2004, Brustovetsky et al., 2001). Nevertheless, creatine has been shown in multiple independent studies to block neuronal death and increase lifespan in experimental animal models of neurodegenerative disorders (Beal, 2011). The NIH Exploratory Trials in Parkinson Long-term study-1 (NET-PD LS-1) that tested creatine monohydrate 5 g twice a day and patients followed up for a minimum of 5 years. This study failed to show any difference in UPDRS scores between the placebo and creatine groups and did not improve the outcome in patients with early or treated PD (Writing Group for the et al., 2015).
Pioglitazone is an FDA approved drug for the treatment of type 2 diabetes. It is a peroxisome proliferator-activated receptor γ (PPAR-γ) agonist known to reduce insulin resistance (Smith, 2001). It’s cofactor PGC-1α is a transcriptional coactivator and is a master regulator of mitochondrial biogenesis. Activation of PGC-1α is shown to be neuroprotective in many different models of neurodegenerative diseases (Agarwal et al., 2017). There is also strong evidence of impaired PGC-1α regulated transcriptional network in PD (Zheng et al., 2010). A multicenter double-blind, randomized, placebo-controlled, futility trial compared 15 or 45 mg per day pioglitazone or placebo at baseline and 44 weeks. The results were negative and suggested that pioglitazone is unlikely to modify progression in early PD (Investigators, 2015). In addition to PGC-1α, activation of sirtuins and AMPA kinase pathways are also implicated in mitochondrial biogenesis and serve as promising targets for PD therapeutics by preventing mitochondrial dysfunction (Valero, 2014). Another FDA-approved drug licensed for type-2 diabetes is the glucagon-like peptide (GLP-1) agonist Exenatide. The precise mechanism of this drug in PD remains unclear but preclinical studies in the MPTP mouse model of PD shows protective effects GLP-1 agonists by its stimulating effect on mitochondrial biogenesis (Li et al., 2009, Chen et al., 2015, Fan et al., 2010, Kang et al., 2015). A single-center, randomized, double-blind, placebo-controlled trial in 62 patients with moderate PD received subcutaneous injections of 2 mg or placebo once weekly for 48 weeks, followed by a 12-week washout period. At 60 weeks, the UPDRS improved by 1.0 point in the exenatide arm but worsened by 2.1 points in the placebo group (Athauda et al., 2017). Exenatide had positive effects on practically defined off-medication motor scores in PD, which were sustained beyond the period of exposure. Whether exenatide affects the underlying disease pathophysiology or simply induces long-lasting symptomatic effects is uncertain. Nevertheless, exenatide represents a major new avenue for investigation in PD, and effects on everyday symptoms should be examined in longer-term trials.
Isradipine, a L-type Ca2+ channel antagonist, which is FDA-approved for the treatment of high blood pressure. Preclinical studies with isradipine has been shown to protect against DA neuron degeneration in the MPTP mouse model of PD by preventing sustained elevations in cytosolic Ca2+ levels resulting in mitochondrial oxidative stress (Guzman et al., 2010, Ilijic et al., 2011, Wang et al., 2017). It has been used in a phase II trial to asses to establish dosage, safety and efficacy. The Safety, Tolerability, and Efficacy Assessment of Dynacirc controlled release for PD controlled release in PD (STEADY-PD) was undertaken (Parkinson Study, 2013, Biglan et al., 2017) and found that isradipine 10 mg daily to be safe and well tolerated in early PD with a dosage that achieves serum concentrations found to be neuroprotective in animal models of PD. The much-anticipated phase III efficacy trial is currently underway and provides a unique opportunity to evaluate the potential impact of this novel therapy to slow progression of PD disability.
Ursodeoxycholic acid (UDCA) is FDA approved for the use in treating primary biliary cirrhosis. Preclinical studies show that UDCA renders protective effect in both toxin and genetic models of PD by blocking mitochondrial perturbations (Abdelkader et al., 2016, Mortiboys et al., 2015). A phase I study is currently underway to determine brain bioenergetics profile and ATPase activity using MR-spectroscopy following repeated oral dosing of UDCA. Additional studies will characterize oral UDCA pharmacokinetics in plasma and develop a pharmacokinetic/pharmacodynamic model to characterize the relationship between peripheral measurements of UDCA and peripheral measures and brain bioenergetic measurements (Table 2). Another compound EPI-589, also known as (R)-troloxamide quinone developed by Edison Pharma for inherited mitochondrial disorders is claimed to have significant effects in modifying cellular glutathione (GSH) levels. Currently a phase II study is underway in idiopathic PD to determine its safety and the ability to alter the biochemical signature of PD as assessed by peripheral blood and brain biomarkers along with clinical measures (Table 2). The GSH precursor N-acetylcysteine currently approved to treat acetaminophen overdose and mucous build-up in cystic fibrosis also showed benefits in preclinical models of PD (Rahimmi et al., 2015, Martinez Banaclocha, 2000, Berman et al., 2011, Clark et al., 2010). Oral administration of N-acetylcysteine is known to boost blood and brain levels of GSH in PD patients (Holmay et al., 2013). A preliminary clinical study significantly increased dopamine transporter binding in the caudate and putamen with mean increase ranging from 4.4% to 7.8% in the PD group treated with N-acetylcysteine, and no measurable changes in the control group. UPDRS scores were also significantly improved in the N-acetylcysteine group with a mean improvement of 12.9% (Monti et al., 2016). These results demonstrate a positive clinical effects N-acetylcysteine in PD through a direct effect on DA neurons. A large-scale clinical trial to test the therapeutic efficacy of N-acetylcysteine in PD to better elucidate the mechanism of action is warranted.
A promising strategy for the treatment of neurodegenerative diseases involves drug-induced activation of a coordinated antioxidant genetic program to maintain redox equilibrium by the expression of pro-survival proteins and cytoprotective enzymes (Gazaryan and Thomas, 2016). A key transcription factor orchestrating this process is Nrf2. The ability to selectively induce a battery of cytoprotective and antioxidative enzymes that are directly under the genetic control of a single transcription factor such as Nrf2 may have significant advantages over conventional strategies. One can potentially induce multiple antioxidant pathways and associated neuroprotective responses, which would not be dependent on the antioxidant effects of a single molecule or combination of molecules that are frequently consumed while scavenging toxic-free radical species. Others and we have demonstrated that compounds such as synthetic triterpenoids and fumaric acid esters can activate this pathway and offer neuroprotection in animal models of PD (Kaidery et al., 2013, Ahuja et al., 2016, Lastres-Becker et al., 2016). Tecfidera is an oral formulation of dimethylfumarate used to treat multiple sclerosis. We showed that dimethylfumarate and its metabolite monomethylfumarate activate Nrf2 to upregulate genes involved in antioxidant, anti-inflammatory, and mitochondrial biogenesis to protect against MPTP-neurotoxicity via distinct S-alkylating properties and GSH depletion (Ahuja et al., 2016). Our data suggest that activating Nrf2 using MMF rather than DMF is a promising approach to block oxidative stress, neuroinflammation, and mitochondrial dysfunction for therapeutic intervention in PD while minimizing side effects (Ahuja et al., 2016, Gazaryan and Thomas, 2016). Another endogenous chemical Uric acid has been shown to render neuroprotective effects in cell culture and animal models of PD by activating the Nrf2 pathway (Zhang et al., 2014, Jhang et al., 2015). Interestingly, epidemiological studies have shown a decreased incidence of PD in subjects with high serum urate levels (Davis et al., 1996, Weisskopf et al., 2007) and in subjects with gout (Alonso et al., 2007). In patients with early PD, higher plasma urate levels correlate with slower disease progression and subjects on diets that promote high urate levels have a reduced risk of developing PD (Gao et al., 2008). Such a urate-rich diet could serve as a neuroprotective therapy in PD. However, the potential benefits of a urate-rich diet must be weighed against the risk of developing gout and cardiovascular disease. A large-scale clinical trial of the effectiveness of elevating urate in patients with PD is currently underway (Crotty et al., 2017). The Safety of Urate Elevation in Parkinson’s Disease (SURE-PD) study was conducted to test the precursor of urate, inosine and was found to be safe and effective in increasing serum urate levels, and plasma antioxidant capacity of PD patients and thus may prove to be a disease-modifying therapy for PD (Parkinson Study Group et al., 2014b, Bhattacharyya et al., 2016).
In summary, the lack of efficacy observed with many of the drugs described above except for exenatide in human PD may raise concerns regarding the utility of targeting mitochondria for therapeutic approaches. However, failure of a drug in a clinical trial only questions the usefulness of the specific test compound. Moreover, some of the studies appear to be underpowered and for many of the drugs data on blood-brain barrier penetrance in PD patients are not consistently available. The lack of valid and sensitive biomarkers for the diagnosis and monitoring of the treatment response in PD that would replace clinical endpoints or symptomatic markers to assess disease progression is also a limitation, as these are currently determined by neurological exams. Moreover, much of the disability associated with PD is not considered in the UPDRS scale, such as those related to autonomic dysfunction. Another reason for the failure of potential neuroprotective therapies could also be explained by the limitations of the current preclinical models. Currently, there is no one animal model for PD that mimics the full pathology and clinical symptomology of the illness. Most of the drugs tested thus far in clinical trials showed efficacy in toxin models which show degeneration of nigrostriatal DA neurons, but the time course and pathological features of these are different from human disease (Beal, 2010). The identification of familial PD-linked genes led to the development of genetic-based models as alternatives to toxin models and some of them recapitulate many of the cardinal features of human PD and will undoubtedly support drug development over the coming years (Visanji et al., 2016, Creed and Goldberg, 2018). The predictive power of animal models of PD will be confirmed only if there is success in demonstrating neuroprotection in humans. Lastly, our knowledge about the underlying molecular pathways involved in the pathogenesis of PD is still limited despite intensive research efforts invested in this field. A comprehensive discussion addressing many of these crucial issues combined with treatment modalities that restore mitochondrial function shall provide new ideas and avenues for future development of neuroprotective therapies with a goal to slow or arrest the progression of this disabling disorder.
7. Concluding remarks
Although the etio-pathogenic mechanisms for PD are not fully understood, ample empirical evidence suggests a significant role of mitochondrial dysfunction in disease development. Deficits in mitochondrial functions due to bioenergetics defects, generation of ROS, aberrant calcium homeostasis, and anomalies in mitochondrial dynamics and quality control are implicated as the underlying mechanisms of both sporadic and familial PD. Based on the existing knowledge of mitochondrial alterations in PD it could be speculated that strategies aimed at correcting the above mentioned biochemical abnormalities might be useful to halt or slowdown the progression of PD. In this regard, many of the candidate drugs that showed efficacy in experimental PD models have already made it to clinical trials. However, except for exenatide many other drugs failed to show any beneficial effects in human PD to date. These failures should not cast doubts on targeting mitochondrial rescue as a therapeutic strategy. Instead there should be better integration and meaningful translation from bench to the bedside through better understanding of disease mechanisms, discovery of reliable and sensitive biomarkers, rectifying therapeutic design of clinical trials and by selecting candidate drugs for clinical trials that show efficacy in multiple and mechanistically diverse preclinical models of PD. Such collective efforts should enable the PD community to ultimately find better therapies targeting mitochondrial dysfunction for this debilitating neurodegenerative movement disorder.
Acknowledgments
The authors acknowledge the financial support provided by National Institutes of Health grant NS101967 and grant from the Parkinson Support Group.
Footnotes
Conflicts of Interest: The authors declare no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abdelkader NF, Safar MM, Salem HA. Ursodeoxycholic Acid Ameliorates Apoptotic Cascade in the Rotenone Model of Parkinson’s Disease: Modulation of Mitochondrial Perturbations. Mol Neurobiol. 2016;53:810–817. doi: 10.1007/s12035-014-9043-8. [DOI] [PubMed] [Google Scholar]
- Abeliovich A, Gitler AD. Defects in trafficking bridge Parkinson’s disease pathology and genetics. Nature. 2016;539:207–216. doi: 10.1038/nature20414. [DOI] [PubMed] [Google Scholar]
- Agarwal S, Yadav A, Chaturvedi RK. Peroxisome proliferator-activated receptors (PPARs) as therapeutic target in neurodegenerative disorders. Biochem Biophys Res Commun. 2017;483:1166–1177. doi: 10.1016/j.bbrc.2016.08.043. [DOI] [PubMed] [Google Scholar]
- Ahuja M, Ammal Kaidery N, Yang L, Calingasan N, Smirnova N, Gaisin A, Gaisina IN, Gazaryan I, Hushpulian DM, Kaddour-Djebbar I, Bollag WB, Morgan JC, Ratan RR, Starkov AA, Beal MF, Thomas B. Distinct Nrf2 Signaling Mechanisms of Fumaric Acid Esters and Their Role in Neuroprotection against 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Experimental Parkinson’s-Like Disease. J Neurosci. 2016;36:6332–6351. doi: 10.1523/JNEUROSCI.0426-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alavian KN, Beutner G, Lazrove E, Sacchetti S, Park HA, Licznerski P, Li H, Nabili P, Hockensmith K, Graham M, Porter GA, Jr, Jonas EA. An uncoupling channel within the c-subunit ring of the F1FO ATP synthase is the mitochondrial permeability transition pore. Proc Natl Acad Sci U S A. 2014;111:10580–10585. doi: 10.1073/pnas.1401591111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen GF, Toth R, James J, Ganley IG. Loss of iron triggers PINK1/Parkin-independent mitophagy. EMBO Rep. 2013;14:1127–1135. doi: 10.1038/embor.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso A, Rodriguez LA, Logroscino G, Hernan MA. Gout and risk of Parkinson disease: a prospective study. Neurology. 2007;69:1696–1700. doi: 10.1212/01.wnl.0000279518.10072.df. [DOI] [PubMed] [Google Scholar]
- Aras S, Bai M, Lee I, Springett R, Huttemann M, Grossman LI. MNRR1 (formerly CHCHD2) is a bi-organellar regulator of mitochondrial metabolism. Mitochondrion. 2015;20:43–51. doi: 10.1016/j.mito.2014.10.003. [DOI] [PubMed] [Google Scholar]
- Aras S, Pak O, Sommer N, Finley R, Jr, Huttemann M, Weissmann N, Grossman LI. Oxygen-dependent expression of cytochrome c oxidase subunit 4-2 gene expression is mediated by transcription factors RBPJ, CXXC5 and CHCHD2. Nucleic Acids Res. 2013;41:2255–2266. doi: 10.1093/nar/gks1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashrafi G, Schlehe JS, LaVoie MJ, Schwarz TL. Mitophagy of damaged mitochondria occurs locally in distal neuronal axons and requires PINK1 and Parkin. J Cell Biol. 2014;206:655–670. doi: 10.1083/jcb.201401070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, Hibbert S, Budnik N, Zampedri L, Dickson J, Li Y, Aviles-Olmos I, Warner TT, Limousin P, Lees AJ, Greig NH, Tebbs S, Foltynie T. Exenatide once weekly versus placebo in Parkinson’s disease: a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1664–1675. doi: 10.1016/S0140-6736(17)31585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee K, Sinha M, Pham Cle L, Jana S, Chanda D, Cappai R, Chakrabarti S. Alpha-synuclein induced membrane depolarization and loss of phosphorylation capacity of isolated rat brain mitochondria: implications in Parkinson’s disease. FEBS Lett. 2010;584:1571–1576. doi: 10.1016/j.febslet.2010.03.012. [DOI] [PubMed] [Google Scholar]
- Banerjee R, Ammal Kaidery N, Thomas B. Oxidative Stress in Parkinson’s Disease: Role in Neurodegeneration and Targets for Therapeutics. In: Maria Hepel SA, editor. Oxidative Stress: Diagnostics, Prevention, and Therapy. Vol. 2. ACS Publications; 2015. pp. 147–176. [Google Scholar]
- Banerjee R, Starkov AA, Beal MF, Thomas B. Mitochondrial dysfunction in the limelight of Parkinson’s disease pathogenesis. Biochim Biophys Acta. 2009;1792:651–663. doi: 10.1016/j.bbadis.2008.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsukova A, Komarov A, Hajnoczky G, Bernardi P, Bourdette D, Forte M. Activation of the mitochondrial permeability transition pore modulates Ca2+ responses to physiological stimuli in adult neurons. Eur J Neurosci. 2011;33:831–842. doi: 10.1111/j.1460-9568.2010.07576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayir H, Kapralov AA, Jiang J, Huang Z, Tyurina YY, Tyurin VA, Zhao Q, Belikova NA, Vlasova II, Maeda A, Zhu J, Na HM, Mastroberardino PG, Sparvero LJ, Amoscato AA, Chu CT, Greenamyre JT, Kagan VE. Peroxidase mechanism of lipid-dependent cross-linking of synuclein with cytochrome C: protection against apoptosis versus delayed oxidative stress in Parkinson disease. J Biol Chem. 2009;284:15951–15969. doi: 10.1074/jbc.M900418200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF. Coenzyme Q10 as a possible treatment for neurodegenerative diseases. Free Radic Res. 2002;36:455–460. doi: 10.1080/10715760290021315. [DOI] [PubMed] [Google Scholar]
- Beal MF. Bioenergetic approaches for neuroprotection in Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S39–47. doi: 10.1002/ana.10479. discussion S47–38. [DOI] [PubMed] [Google Scholar]
- Beal MF. Parkinson’s disease: a model dilemma. Nature. 2010;466:S8–10. doi: 10.1038/466S8a. [DOI] [PubMed] [Google Scholar]
- Beal MF. Neuroprotective effects of creatine. Amino Acids. 2011;40:1305–1313. doi: 10.1007/s00726-011-0851-0. [DOI] [PubMed] [Google Scholar]
- Bender A, Desplats P, Spencer B, Rockenstein E, Adame A, Elstner M, Laub C, Mueller S, Koob AO, Mante M, Pham E, Klopstock T, Masliah E. TOM40 mediates mitochondrial dysfunction induced by alpha-synuclein accumulation in Parkinson’s disease. PLoS One. 2013;8:e62277. doi: 10.1371/journal.pone.0062277. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Bender A, Krishnan KJ, Morris CM, Taylor GA, Reeve AK, Perry RH, Jaros E, Hersheson JS, Betts J, Klopstock T, Taylor RW, Turnbull DM. High levels of mitochondrial DNA deletions in substantia nigra neurons in aging and Parkinson disease. Nat Genet. 2006;38:515–517. doi: 10.1038/ng1769. [DOI] [PubMed] [Google Scholar]
- Benecke R, Strumper P, Weiss H. Electron transfer complexes I and IV of platelets are abnormal in Parkinson’s disease but normal in Parkinson-plus syndromes. Brain. 1993;116(Pt 6):1451–1463. doi: 10.1093/brain/116.6.1451. [DOI] [PubMed] [Google Scholar]
- Berezhnov AV, Soutar MP, Fedotova EI, Frolova MS, Plun-Favreau H, Zinchenko VP, Abramov AY. Intracellular pH Modulates Autophagy and Mitophagy. J Biol Chem. 2016;291:8701–8708. doi: 10.1074/jbc.M115.691774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman AE, Chan WY, Brennan AM, Reyes RC, Adler BL, Suh SW, Kauppinen TM, Edling Y, Swanson RA. N-acetylcysteine prevents loss of dopaminergic neurons in the EAAC1−/− mouse. Ann Neurol. 2011;69:509–520. doi: 10.1002/ana.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P, Di Lisa F. The mitochondrial permeability transition pore: molecular nature and role as a target in cardioprotection. J Mol Cell Cardiol. 2015;78:100–106. doi: 10.1016/j.yjmcc.2014.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernardi P, Rasola A, Forte M, Lippe G. The Mitochondrial Permeability Transition Pore: Channel Formation by F-ATP Synthase, Integration in Signal Transduction, and Role in Pathophysiology. Physiol Rev. 2015;95:1111–1155. doi: 10.1152/physrev.00001.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S, Bakshi R, Logan R, Ascherio A, Macklin EA, Schwarzschild MA. Oral Inosine Persistently Elevates Plasma antioxidant capacity in Parkinson’s disease. Mov Disord. 2016;31:417–421. doi: 10.1002/mds.26483. [DOI] [PubMed] [Google Scholar]
- Bi F, Li F, Huang C, Zhou H. Pathogenic mutation in VPS35 impairs its protection against MPP(+) cytotoxicity. Int J Biol Sci. 2013;9:149–155. doi: 10.7150/ijbs.5617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biglan KM, Oakes D, Lang AE, Hauser RA, Hodgeman K, Greco B, Lowell J, Rockhill R, Shoulson I, Venuto C, Young D, Simuni T Parkinson Study Group SPDIIII. A novel design of a Phase III trial of isradipine in early Parkinson disease (STEADY-PD III) Ann Clin Transl Neurol. 2017;4:360–368. doi: 10.1002/acn3.412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bindoff LA, Birch-Machin MA, Cartlidge NE, Parker WD, Jr, Turnbull DM. Respiratory chain abnormalities in skeletal muscle from patients with Parkinson’s disease. J Neurol Sci. 1991;104:203–208. doi: 10.1016/0022-510x(91)90311-t. [DOI] [PubMed] [Google Scholar]
- Binukumar BK, Bal A, Kandimalla RJ, Gill KD. Nigrostriatal neuronal death following chronic dichlorvos exposure: crosstalk between mitochondrial impairments, alpha synuclein aggregation, oxidative damage and behavioral changes. Mol Brain. 2010;3:35. doi: 10.1186/1756-6606-3-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blesa J, Phani S, Jackson-Lewis V, Przedborski S. Classic and new animal models of Parkinson’s disease. J Biomed Biotechnol. 2012;2012:845618. doi: 10.1155/2012/845618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blin O, Desnuelle C, Rascol O, Borg M, Peyro Saint Paul H, Azulay JP, Bille F, Figarella D, Coulom F, Pellissier JF, et al. Mitochondrial respiratory failure in skeletal muscle from patients with Parkinson’s disease and multiple system atrophy. J Neurol Sci. 1994;125:95–101. doi: 10.1016/0022-510x(94)90248-8. [DOI] [PubMed] [Google Scholar]
- Bonifati V, Rizzu P, van Baren MJ, Schaap O, Breedveld GJ, Krieger E, Dekker MC, Squitieri F, Ibanez P, Joosse M, van Dongen JW, Vanacore N, van Swieten JC, Brice A, Meco G, van Duijn CM, Oostra BA, Heutink P. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science. 2003;299:256–259. doi: 10.1126/science.1077209. [DOI] [PubMed] [Google Scholar]
- Bonora M, Bononi A, De Marchi E, Giorgi C, Lebiedzinska M, Marchi S, Patergnani S, Rimessi A, Suski JM, Wojtala A, Wieckowski MR, Kroemer G, Galluzzi L, Pinton P. Role of the c subunit of the FO ATP synthase in mitochondrial permeability transition. Cell Cycle. 2013;12:674–683. doi: 10.4161/cc.23599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose A, Beal MF. Mitochondrial dysfunction in Parkinson’s disease. J Neurochem. 2016;139(Suppl 1):216–231. doi: 10.1111/jnc.13731. [DOI] [PubMed] [Google Scholar]
- Braschi E, Goyon V, Zunino R, Mohanty A, Xu L, McBride HM. Vps35 mediates vesicle transport between the mitochondria and peroxisomes. Curr Biol. 2010;20:1310–1315. doi: 10.1016/j.cub.2010.05.066. [DOI] [PubMed] [Google Scholar]
- Brini M, Cali T, Ottolini D, Carafoli E. Neuronal calcium signaling: function and dysfunction. Cell Mol Life Sci. 2014;71:2787–2814. doi: 10.1007/s00018-013-1550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brustovetsky N, Brustovetsky T, Dubinsky JM. On the mechanisms of neuroprotection by creatine and phosphocreatine. J Neurochem. 2001;76:425–434. doi: 10.1046/j.1471-4159.2001.00052.x. [DOI] [PubMed] [Google Scholar]
- Burchell VS, Nelson DE, Sanchez-Martinez A, Delgado-Camprubi M, Ivatt RM, Pogson JH, Randle SJ, Wray S, Lewis PA, Houlden H, Abramov AY, Hardy J, Wood NW, Whitworth AJ, Laman H, Plun-Favreau H. The Parkinson’s disease-linked proteins Fbxo7 and Parkin interact to mediate mitophagy. Nat Neurosci. 2013;16:1257–1265. doi: 10.1038/nn.3489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cali T, Ottolini D, Negro A, Brini M. Enhanced parkin levels favor ER-mitochondria crosstalk and guarantee Ca(2+) transfer to sustain cell bioenergetics. Biochim Biophys Acta. 2013;1832:495–508. doi: 10.1016/j.bbadis.2013.01.004. [DOI] [PubMed] [Google Scholar]
- Camilleri A, Zarb C, Caruana M, Ostermeier U, Ghio S, Hogen T, Schmidt F, Giese A, Vassallo N. Mitochondrial membrane permeabilisation by amyloid aggregates and protection by polyphenols. Biochim Biophys Acta. 2013;1828:2532–2543. doi: 10.1016/j.bbamem.2013.06.026. [DOI] [PubMed] [Google Scholar]
- Canet-Aviles RM, Wilson MA, Miller DW, Ahmad R, McLendon C, Bandyopadhyay S, Baptista MJ, Ringe D, Petsko GA, Cookson MR. The Parkinson’s disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc Natl Acad Sci U S A. 2004;101:9103–9108. doi: 10.1073/pnas.0402959101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardellach F, Marti MJ, Fernandez-Sola J, Marin C, Hoek JB, Tolosa E, Urbano-Marquez A. Mitochondrial respiratory chain activity in skeletal muscle from patients with Parkinson’s disease. Neurology. 1993;43:2258–2262. doi: 10.1212/wnl.43.11.2258. [DOI] [PubMed] [Google Scholar]
- Casadei N, Sood P, Ulrich T, Fallier-Becker P, Kieper N, Helling S, May C, Glaab E, Chen J, Nuber S, Wolburg H, Marcus K, Rapaport D, Ott T, Riess O, Kruger R, Fitzgerald JC. Mitochondrial defects and neurodegeneration in mice overexpressing wild-type or G399S mutant HtrA2. Hum Mol Genet. 2016;25:459–471. doi: 10.1093/hmg/ddv485. [DOI] [PubMed] [Google Scholar]
- Cassarino DS, Parks JK, Parker WD, Jr, Bennett JP., Jr The parkinsonian neurotoxin MPP+ opens the mitochondrial permeability transition pore and releases cytochrome c in isolated mitochondria via an oxidative mechanism. Biochim Biophys Acta. 1999;1453:49–62. doi: 10.1016/s0925-4439(98)00083-0. [DOI] [PubMed] [Google Scholar]
- Chan CS, Gertler TS, Surmeier DJ. Calcium homeostasis, selective vulnerability and Parkinson’s disease. Trends Neurosci. 2009;32:249–256. doi: 10.1016/j.tins.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Gertler TS, Surmeier DJ. A molecular basis for the increased vulnerability of substantia nigra dopamine neurons in aging and Parkinson’s disease. Mov Disord. 2010;25(Suppl 1):S63–70. doi: 10.1002/mds.22801. [DOI] [PubMed] [Google Scholar]
- Chan CS, Guzman JN, Ilijic E, Mercer JN, Rick C, Tkatch T, Meredith GE, Surmeier DJ. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature. 2007;447:1081–1086. doi: 10.1038/nature05865. [DOI] [PubMed] [Google Scholar]
- Chan NC, Salazar AM, Pham AH, Sweredoski MJ, Kolawa NJ, Graham RL, Hess S, Chan DC. Broad activation of the ubiquitin-proteasome system by Parkin is critical for mitophagy. Hum Mol Genet. 2011;20:1726–1737. doi: 10.1093/hmg/ddr048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CR, Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann N Y Acad Sci. 2010;1201:34–39. doi: 10.1111/j.1749-6632.2010.05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Dorn GW., 2nd PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang Y, Li L, Holscher C. Neuroprotective effects of geniposide in the MPTP mouse model of Parkinson’s disease. Eur J Pharmacol. 2015;768:21–27. doi: 10.1016/j.ejphar.2015.09.029. [DOI] [PubMed] [Google Scholar]
- Chinta SJ, Mallajosyula JK, Rane A, Andersen JK. Mitochondrial alpha-synuclein accumulation impairs complex I function in dopaminergic neurons and results in increased mitophagy in vivo. Neurosci Lett. 2010;486:235–239. doi: 10.1016/j.neulet.2010.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu CT, Ji J, Dagda RK, Jiang JF, Tyurina YY, Kapralov AA, Tyurin VA, Yanamala N, Shrivastava IH, Mohammadyani D, Wang KZQ, Zhu J, Klein-Seetharaman J, Balasubramanian K, Amoscato AA, Borisenko G, Huang Z, Gusdon AM, Cheikhi A, Steer EK, Wang R, Baty C, Watkins S, Bahar I, Bayir H, Kagan VE. Cardiolipin externalization to the outer mitochondrial membrane acts as an elimination signal for mitophagy in neuronal cells. Nat Cell Biol. 2013;15:1197–1205. doi: 10.1038/ncb2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung SY, Kishinevsky S, Mazzulli JR, Graziotto J, Mrejeru A, Mosharov EV, Puspita L, Valiulahi P, Sulzer D, Milner TA, Taldone T, Krainc D, Studer L, Shim JW. Parkin and PINK1 Patient iPSC-Derived Midbrain Dopamine Neurons Exhibit Mitochondrial Dysfunction and alpha-Synuclein Accumulation. Stem Cell Reports. 2016;7:664–677. doi: 10.1016/j.stemcr.2016.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciron C, Zheng L, Bobela W, Knott GW, Leone TC, Kelly DP, Schneider BL. PGC-1alpha activity in nigral dopamine neurons determines vulnerability to alpha-synuclein. Acta Neuropathol Commun. 2015;3:16. doi: 10.1186/s40478-015-0200-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark J, Clore EL, Zheng K, Adame A, Masliah E, Simon DK. Oral N-acetyl-cysteine attenuates loss of dopaminergic terminals in alpha-synuclein overexpressing mice. PLoS One. 2010;5:e12333. doi: 10.1371/journal.pone.0012333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleeter MW, Chau KY, Gluck C, Mehta A, Hughes DA, Duchen M, Wood NW, Hardy J, Mark Cooper J, Schapira AH. Glucocerebrosidase inhibition causes mitochondrial dysfunction and free radical damage. Neurochem Int. 2013;62:1–7. doi: 10.1016/j.neuint.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements CM, McNally RS, Conti BJ, Mak TW, Ting JP. DJ-1, a cancer- and Parkinson’s disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc Natl Acad Sci U S A. 2006;103:15091–15096. doi: 10.1073/pnas.0607260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras L, Drago I, Zampese E, Pozzan T. Mitochondria: the calcium connection. Biochim Biophys Acta. 2010;1797:607–618. doi: 10.1016/j.bbabio.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Cooper JM, Mann VM, Schapira AH. Analyses of mitochondrial respiratory chain function and mitochondrial DNA deletion in human skeletal muscle: effect of ageing. J Neurol Sci. 1992;113:91–98. doi: 10.1016/0022-510x(92)90270-u. [DOI] [PubMed] [Google Scholar]
- Cosi C, Marien M. Decreases in mouse brain NAD+ and ATP induced by 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine (MPTP): prevention by the poly(ADP-ribose) polymerase inhibitor, benzamide. Brain Res. 1998;809:58–67. doi: 10.1016/s0006-8993(98)00829-4. [DOI] [PubMed] [Google Scholar]
- Creed RB, Goldberg MS. New developments in genetic rat models of Parkinson’s disease. Mov Disord. 2018 doi: 10.1002/mds.27296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty GF, Ascherio A, Schwarzschild MA. Targeting urate to reduce oxidative stress in Parkinson disease. Exp Neurol. 2017;298:210–224. doi: 10.1016/j.expneurol.2017.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, Balla T, Mannella CA, Hajnoczky G. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui J, Yu M, Niu J, Yue Z, Xu Z. Expression of leucine-rich repeat kinase 2 (LRRK2) inhibits the processing of uMtCK to induce cell death in a cell culture model system. Biosci Rep. 2011;31:429–437. doi: 10.1042/BSR20100127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel G, Musso A, Tsika E, Fiser A, Glauser L, Pletnikova O, Schneider BL, Moore DJ. alpha-Synuclein-induced dopaminergic neurodegeneration in a rat model of Parkinson’s disease occurs independent of ATP13A2 (PARK9) Neurobiol Dis. 2015;73:229–243. doi: 10.1016/j.nbd.2014.10.007. [DOI] [PubMed] [Google Scholar]
- Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, Staal R, Tieu K, Schmitz Y, Yuan CA, Rocha M, Jackson-Lewis V, Hersch S, Sulzer D, Przedborski S, Burke R, Hen R. Resistance of alpha-synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci U S A. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dave KD, De Silva S, Sheth NP, Ramboz S, Beck MJ, Quang C, Switzer RC, 3rd, Ahmad SO, Sunkin SM, Walker D, Cui X, Fisher DA, McCoy AM, Gamber K, Ding X, Goldberg MS, Benkovic SA, Haupt M, Baptista MA, Fiske BK, Sherer TB, Frasier MA. Phenotypic characterization of recessive gene knockout rat models of Parkinson’s disease. Neurobiol Dis. 2014;70:190–203. doi: 10.1016/j.nbd.2014.06.009. [DOI] [PubMed] [Google Scholar]
- Davey GP, Clark JB. Threshold effects and control of oxidative phosphorylation in nonsynaptic rat brain mitochondria. J Neurochem. 1996;66:1617–1624. doi: 10.1046/j.1471-4159.1996.66041617.x. [DOI] [PubMed] [Google Scholar]
- Davis JW, Grandinetti A, Waslien CI, Ross GW, White LR, Morens DM. Observations on serum uric acid levels and the risk of idiopathic Parkinson’s disease. Am J Epidemiol. 1996;144:480–484. doi: 10.1093/oxfordjournals.aje.a008954. [DOI] [PubMed] [Google Scholar]
- Day BJ, Patel M, Calavetta L, Chang LY, Stamler JS. A mechanism of paraquat toxicity involving nitric oxide synthase. Proc Natl Acad Sci U S A. 1999;96:12760–12765. doi: 10.1073/pnas.96.22.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- de la Mata M, Cotan D, Oropesa-Avila M, Garrido-Maraver J, Cordero MD, Villanueva Paz M, Delgado Pavon A, Alcocer-Gomez E, de Lavera I, Ybot-Gonzalez P, Paula Zaderenko A, Ortiz Mellet C, Garcia Fernandez JM, Sanchez-Alcazar JA. Pharmacological Chaperones and Coenzyme Q10 Treatment Improves Mutant beta-Glucocerebrosidase Activity and Mitochondrial Function in Neuronopathic Forms of Gaucher Disease. Sci Rep. 2015;5:10903. doi: 10.1038/srep10903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Fiacco M, Levanti MC, Dessi ML, Zucca G. The human hippocampal formation and parahippocampal gyrus: localization of substance P-like immunoreactivity in newborn and adult post-mortem tissue. Neuroscience. 1987;21:141–150. doi: 10.1016/0306-4522(87)90328-9. [DOI] [PubMed] [Google Scholar]
- Deng H, Wang P, Jankovic J. The genetics of Parkinson disease. Ageing Res Rev. 2017;42:72–85. doi: 10.1016/j.arr.2017.12.007. [DOI] [PubMed] [Google Scholar]
- Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK. Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain. J Biol Chem. 2008;283:9089–9100. doi: 10.1074/jbc.M710012200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Fonzo A, Chien HF, Socal M, Giraudo S, Tassorelli C, Iliceto G, Fabbrini G, Marconi R, Fincati E, Abbruzzese G, Marini P, Squitieri F, Horstink MW, Montagna P, Libera AD, Stocchi F, Goldwurm S, Ferreira JJ, Meco G, Martignoni E, Lopiano L, Jardim LB, Oostra BA, Barbosa ER, Bonifati V Italian Parkinson Genetics N. ATP13A2 missense mutations in juvenile parkinsonism and young onset Parkinson disease. Neurology. 2007;68:1557–1562. doi: 10.1212/01.wnl.0000260963.08711.08. [DOI] [PubMed] [Google Scholar]
- Di Maio R, Barrett PJ, Hoffman EK, Barrett CW, Zharikov A, Borah A, Hu X, McCoy J, Chu CT, Burton EA, Hastings TG, Greenamyre JT. alpha-Synuclein binds to TOM20 and inhibits mitochondrial protein import in Parkinson’s disease. Sci Transl Med. 2016;8:342ra378. doi: 10.1126/scitranslmed.aaf3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolder M, Walzel B, Speer O, Schlattner U, Wallimann T. Inhibition of the mitochondrial permeability transition by creatine kinase substrates. Requirement for microcompartmentation. J Biol Chem. 2003;278:17760–17766. doi: 10.1074/jbc.M208705200. [DOI] [PubMed] [Google Scholar]
- Dolle C, Flones I, Nido GS, Miletic H, Osuagwu N, Kristoffersen S, Lilleng PK, Larsen JP, Tysnes OB, Haugarvoll K, Bindoff LA, Tzoulis C. Defective mitochondrial DNA homeostasis in the substantia nigra in Parkinson disease. Nat Commun. 2016;7:13548. doi: 10.1038/ncomms13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingo A, Klein C. Genetics of Parkinson disease. Handb Clin Neurol. 2018;147:211–227. doi: 10.1016/B978-0-444-63233-3.00014-2. [DOI] [PubMed] [Google Scholar]
- Dongworth RK, Mukherjee UA, Hall AR, Astin R, Ong SB, Yao Z, Dyson A, Szabadkai G, Davidson SM, Yellon DM, Hausenloy DJ. DJ-1 protects against cell death following acute cardiac ischemia-reperfusion injury. Cell Death Dis. 2014;5:e1082. doi: 10.1038/cddis.2014.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsey ER, Constantinescu R, Thompson JP, Biglan KM, Holloway RG, Kieburtz K, Marshall FJ, Ravina BM, Schifitto G, Siderowf A, Tanner CM. Projected number of people with Parkinson disease in the most populous nations, 2005 through 2030. Neurology. 2007;68:384–386. doi: 10.1212/01.wnl.0000247740.47667.03. [DOI] [PubMed] [Google Scholar]
- Drolet RE, Behrouz B, Lookingland KJ, Goudreau JL. Mice lacking alpha-synuclein have an attenuated loss of striatal dopamine following prolonged chronic MPTP administration. Neurotoxicology. 2004;25:761–769. doi: 10.1016/j.neuro.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Ekstrand MI, Terzioglu M, Galter D, Zhu S, Hofstetter C, Lindqvist E, Thams S, Bergstrand A, Hansson FS, Trifunovic A, Hoffer B, Cullheim S, Mohammed AH, Olson L, Larsson NG. Progressive parkinsonism in mice with respiratory-chain-deficient dopamine neurons. Proc Natl Acad Sci U S A. 2007;104:1325–1330. doi: 10.1073/pnas.0605208103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriquez JA. Supramolecular Organization of Respiratory Complexes. Annu Rev Physiol. 2016;78:533–561. doi: 10.1146/annurev-physiol-021115-105031. [DOI] [PubMed] [Google Scholar]
- Escobar-Khondiker M, Hollerhage M, Muriel MP, Champy P, Bach A, Depienne C, Respondek G, Yamada ES, Lannuzel A, Yagi T, Hirsch EC, Oertel WH, Jacob R, Michel PP, Ruberg M, Hoglinger GU. Annonacin, a natural mitochondrial complex I inhibitor, causes tau pathology in cultured neurons. J Neurosci. 2007;27:7827–7837. doi: 10.1523/JNEUROSCI.1644-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteves AR, Domingues AF, Ferreira IL, Januario C, Swerdlow RH, Oliveira CR, Cardoso SM. Mitochondrial function in Parkinson’s disease cybrids containing an nt2 neuron-like nuclear background. Mitochondrion. 2008;8:219–228. doi: 10.1016/j.mito.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Faccio L, Fusco C, Chen A, Martinotti S, Bonventre JV, Zervos AS. Characterization of a novel human serine protease that has extensive homology to bacterial heat shock endoprotease HtrA and is regulated by kidney ischemia. J Biol Chem. 2000;275:2581–2588. doi: 10.1074/jbc.275.4.2581. [DOI] [PubMed] [Google Scholar]
- Fan J, Ren H, Jia N, Fei E, Zhou T, Jiang P, Wu M, Wang G. DJ-1 decreases Bax expression through repressing p53 transcriptional activity. J Biol Chem. 2008;283:4022–4030. doi: 10.1074/jbc.M707176200. [DOI] [PubMed] [Google Scholar]
- Fan R, Li X, Gu X, Chan JC, Xu G. Exendin-4 protects pancreatic beta cells from human islet amyloid polypeptide-induced cell damage: potential involvement of AKT and mitochondria biogenesis. Diabetes Obes Metab. 2010;12:815–824. doi: 10.1111/j.1463-1326.2010.01238.x. [DOI] [PubMed] [Google Scholar]
- Fernagut PO, Hutson CB, Fleming SM, Tetreaut NA, Salcedo J, Masliah E, Chesselet MF. Behavioral and histopathological consequences of paraquat intoxication in mice: effects of alpha-synuclein over-expression. Synapse. 2007;61:991–1001. doi: 10.1002/syn.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett J, Darlow B, Wong MB, Goodwin J, Pountney DL. Potassium depolarization and raised calcium induces alpha-synuclein aggregates. Neurotox Res. 2013;23:378–392. doi: 10.1007/s12640-012-9366-z. [DOI] [PubMed] [Google Scholar]
- Fornai F, Schluter OM, Lenzi P, Gesi M, Ruffoli R, Ferrucci M, Lazzeri G, Busceti CL, Pontarelli F, Battaglia G, Pellegrini A, Nicoletti F, Ruggieri S, Paparelli A, Sudhof TC. Parkinson-like syndrome induced by continuous MPTP infusion: convergent roles of the ubiquitin-proteasome system and alpha-synuclein. Proc Natl Acad Sci U S A. 2005;102:3413–3418. doi: 10.1073/pnas.0409713102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frei B, Richter C. N-methyl-4-phenylpyridine (MMP+) together with 6-hydroxydopamine or dopamine stimulates Ca2+ release from mitochondria. FEBS Lett. 1986;198:99–102. doi: 10.1016/0014-5793(86)81192-9. [DOI] [PubMed] [Google Scholar]
- Fry M, Green DE. Cardiolipin requirement for electron transfer in complex I and III of the mitochondrial respiratory chain. J Biol Chem. 1981;256:1874–1880. [PubMed] [Google Scholar]
- Fu MM, Holzbaur EL. Integrated regulation of motor-driven organelle transport by scaffolding proteins. Trends Cell Biol. 2014;24:564–574. doi: 10.1016/j.tcb.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama M, Ohe K, Amo T, Furuya N, Yamaguchi J, Saiki S, Li Y, Ogaki K, Ando M, Yoshino H, Tomiyama H, Nishioka K, Hasegawa K, Saiki H, Satake W, Mogushi K, Sasaki R, Kokubo Y, Kuzuhara S, Toda T, Mizuno Y, Uchiyama Y, Ohno K, Hattori N. CHCHD2 mutations in autosomal dominant late-onset Parkinson’s disease: a genome-wide linkage and sequencing study. Lancet Neurol. 2015;14:274–282. doi: 10.1016/S1474-4422(14)70266-2. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Fumagalli F, Jones SR, Caron MG. Dopamine transporter is required for in vivo MPTP neurotoxicity: evidence from mice lacking the transporter. J Neurochem. 1997;69:1322–1325. doi: 10.1046/j.1471-4159.1997.69031322.x. [DOI] [PubMed] [Google Scholar]
- Galvin JE, Uryu K, Lee VM, Trojanowski JQ. Axon pathology in Parkinson’s disease and Lewy body dementia hippocampus contains alpha-, beta-, and gamma-synuclein. Proc Natl Acad Sci U S A. 1999;96:13450–13455. doi: 10.1073/pnas.96.23.13450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, Downward J, Latchman DS, Tabrizi SJ, Wood NW, Duchen MR, Abramov AY. PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death. Mol Cell. 2009;33:627–638. doi: 10.1016/j.molcel.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao X, Chen H, Choi HK, Curhan G, Schwarzschild MA, Ascherio A. Diet, urate, and Parkinson’s disease risk in men. Am J Epidemiol. 2008;167:831–838. doi: 10.1093/aje/kwm385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatt AP, Jones EL, Francis PT, Ballard C, Bateman JM. Association of a polymorphism in mitochondrial transcription factor A (TFAM) with Parkinson’s disease dementia but not dementia with Lewy bodies. Neurosci Lett. 2013;557(Pt B):177–180. doi: 10.1016/j.neulet.2013.10.045. [DOI] [PubMed] [Google Scholar]
- Gautier CA, Erpapazoglou Z, Mouton-Liger F, Muriel MP, Cormier F, Bigou S, Duffaure S, Girard M, Foret B, Iannielli A, Broccoli V, Dalle C, Bohl D, Michel PP, Corvol JC, Brice A, Corti O. The endoplasmic reticulum-mitochondria interface is perturbed in PARK2 knockout mice and patients with PARK2 mutations. Hum Mol Genet. 2016;25:2972–2984. doi: 10.1093/hmg/ddw148. [DOI] [PubMed] [Google Scholar]
- Gautier CA, Giaime E, Caballero E, Nunez L, Song Z, Chan D, Villalobos C, Shen J. Regulation of mitochondrial permeability transition pore by PINK1. Mol Neurodegener. 2012;7:22. doi: 10.1186/1750-1326-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazaryan IG, Thomas B. The status of Nrf2-based therapeutics: current perspectives and future prospects. Neural Regen Res. 2016;11:1708–1711. doi: 10.4103/1673-5374.194706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet. 2010;19:4861–4870. doi: 10.1093/hmg/ddq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gegg ME, Schapira AH. Mitochondrial dysfunction associated with glucocerebrosidase deficiency. Neurobiol Dis. 2016;90:43–50. doi: 10.1016/j.nbd.2015.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghezzi D, Marelli C, Achilli A, Goldwurm S, Pezzoli G, Barone P, Pellecchia MT, Stanzione P, Brusa L, Bentivoglio AR, Bonuccelli U, Petrozzi L, Abbruzzese G, Marchese R, Cortelli P, Grimaldi D, Martinelli P, Ferrarese C, Garavaglia B, Sangiorgi S, Carelli V, Torroni A, Albanese A, Zeviani M. Mitochondrial DNA haplogroup K is associated with a lower risk of Parkinson’s disease in Italians. Eur J Hum Genet. 2005;13:748–752. doi: 10.1038/sj.ejhg.5201425. [DOI] [PubMed] [Google Scholar]
- Giorgio V, Burchell V, Schiavone M, Bassot C, Minervini G, Petronilli V, Argenton F, Forte M, Tosatto S, Lippe G, Bernardi P. Ca(2+) binding to F-ATP synthase beta subunit triggers the mitochondrial permeability transition. EMBO Rep. 2017;18:1065–1076. doi: 10.15252/embr.201643354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giorgio V, von Stockum S, Antoniel M, Fabbro A, Fogolari F, Forte M, Glick GD, Petronilli V, Zoratti M, Szabo I, Lippe G, Bernardi P. Dimers of mitochondrial ATP synthase form the permeability transition pore. Proc Natl Acad Sci U S A. 2013;110:5887–5892. doi: 10.1073/pnas.1217823110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitler AD, Chesi A, Geddie ML, Strathearn KE, Hamamichi S, Hill KJ, Caldwell KA, Caldwell GA, Cooper AA, Rochet JC, Lindquist S. Alpha-synuclein is part of a diverse and highly conserved interaction network that includes PARK9 and manganese toxicity. Nat Genet. 2009;41:308–315. doi: 10.1038/ng.300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glater EE, Megeath LJ, Stowers RS, Schwarz TL. Axonal transport of mitochondria requires milton to recruit kinesin heavy chain and is light chain independent. J Cell Biol. 2006;173:545–557. doi: 10.1083/jcb.200601067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes LC, Di Benedetto G, Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol. 2011;13:589–598. doi: 10.1038/ncb2220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm A, Eckert A. Brain aging and neurodegeneration: from a mitochondrial point of view. J Neurochem. 2017;143:418–431. doi: 10.1111/jnc.14037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald A, Arns B, Meier B, Brockmann K, Tadic V, Klein C. Does uncoupling protein 2 expression qualify as marker of disease status in LRRK2-associated Parkinson’s disease? Antioxid Redox Signal. 2014;20:1955–1960. doi: 10.1089/ars.2013.5737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunewald A, Arns B, Seibler P, Rakovic A, Munchau A, Ramirez A, Sue CM, Klein C. ATP13A2 mutations impair mitochondrial function in fibroblasts from patients with Kufor-Rakeb syndrome. Neurobiol Aging. 2012;33:1843e1841–1847. doi: 10.1016/j.neurobiolaging.2011.12.035. [DOI] [PubMed] [Google Scholar]
- Grunewald A, Rygiel KA, Hepplewhite PD, Morris CM, Picard M, Turnbull DM. Mitochondrial DNA Depletion in Respiratory Chain-Deficient Parkinson Disease Neurons. Ann Neurol. 2016;79:366–378. doi: 10.1002/ana.24571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia-Laguarta C, Area-Gomez E, Rub C, Liu Y, Magrane J, Becker D, Voos W, Schon EA, Przedborski S. alpha-Synuclein is localized to mitochondria-associated ER membranes. J Neurosci. 2014;34:249–259. doi: 10.1523/JNEUROSCI.2507-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guardia-Laguarta C, Area-Gomez E, Schon EA, Przedborski S. A new role for alpha-synuclein in Parkinson’s disease: Alteration of ER-mitochondrial communication. Mov Disord. 2015;30:1026–1033. doi: 10.1002/mds.26239. [DOI] [PubMed] [Google Scholar]
- Gusdon AM, Zhu J, Van Houten B, Chu CT. ATP13A2 regulates mitochondrial bioenergetics through macroautophagy. Neurobiol Dis. 2012;45:962–972. doi: 10.1016/j.nbd.2011.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT, Surmeier DJ. Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1. Nature. 2010;468:696–700. doi: 10.1038/nature09536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao LY, Giasson BI, Bonini NM. DJ-1 is critical for mitochondrial function and rescues PINK1 loss of function. Proc Natl Acad Sci U S A. 2010;107:9747–9752. doi: 10.1073/pnas.0911175107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Ishimori C, Takahashi-Niki K, Taira T, Kim YC, Maita H, Maita C, Ariga H, Iguchi-Ariga SM. DJ-1 binds to mitochondrial complex I and maintains its activity. Biochem Biophys Res Commun. 2009;390:667–672. doi: 10.1016/j.bbrc.2009.10.025. [DOI] [PubMed] [Google Scholar]
- Healy DG, Falchi M, O’Sullivan SS, Bonifati V, Durr A, Bressman S, Brice A, Aasly J, Zabetian CP, Goldwurm S, Ferreira JJ, Tolosa E, Kay DM, Klein C, Williams DR, Marras C, Lang AE, Wszolek ZK, Berciano J, Schapira AH, Lynch T, Bhatia KP, Gasser T, Lees AJ, Wood NW, International LC. Phenotype, genotype, and worldwide genetic penetrance of LRRK2-associated Parkinson’s disease: a case-control study. Lancet Neurol. 2008;7:583–590. doi: 10.1016/S1474-4422(08)70117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heeman B, Van den Haute C, Aelvoet SA, Valsecchi F, Rodenburg RJ, Reumers V, Debyser Z, Callewaert G, Koopman WJ, Willems PH, Baekelandt V. Depletion of PINK1 affects mitochondrial metabolism, calcium homeostasis and energy maintenance. J Cell Sci. 2011;124:1115–1125. doi: 10.1242/jcs.078303. [DOI] [PubMed] [Google Scholar]
- Heikkila RE, Manzino L, Cabbat FS, Duvoisin RC. Protection against the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine by monoamine oxidase inhibitors. Nature. 1984;311:467–469. doi: 10.1038/311467a0. [DOI] [PubMed] [Google Scholar]
- Ho MS, Ou C, Chan YR, Chien CT, Pi H. The utility F-box for protein destruction. Cell Mol Life Sci. 2008;65:1977–2000. doi: 10.1007/s00018-008-7592-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoglinger GU, Feger J, Prigent A, Michel PP, Parain K, Champy P, Ruberg M, Oertel WH, Hirsch EC. Chronic systemic complex I inhibition induces a hypokinetic multisystem degeneration in rats. J Neurochem. 2003;84:491–502. doi: 10.1046/j.1471-4159.2003.01533.x. [DOI] [PubMed] [Google Scholar]
- Holmay MJ, Terpstra M, Coles LD, Mishra U, Ahlskog M, Oz G, Cloyd JC, Tuite PJ. N-Acetylcysteine boosts brain and blood glutathione in Gaucher and Parkinson diseases. Clin Neuropharmacol. 2013;36:103–106. doi: 10.1097/WNF.0b013e31829ae713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CH, Shaltouki A, Gonzalez AE, Bettencourt da Cruz A, Burbulla LF, St Lawrence E, Schule B, Krainc D, Palmer TD, Wang X. Functional Impairment in Miro Degradation and Mitophagy Is a Shared Feature in Familial and Sporadic Parkinson’s Disease. Cell Stem Cell. 2016;19:709–724. doi: 10.1016/j.stem.2016.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson G, Nalls M, Evans JR, Breen DP, Winder-Rhodes S, Morrison KE, Morris HR, Williams-Gray CH, Barker RA, Singleton AB, Hardy J, Wood NE, Burn DJ, Chinnery PF. Two-stage association study and meta-analysis of mitochondrial DNA variants in Parkinson disease. Neurology. 2013;80:2042–2048. doi: 10.1212/WNL.0b013e318294b434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson G, Schaefer AM, Taylor RW, Tiangyou W, Gibson A, Venables G, Griffiths P, Burn DJ, Turnbull DM, Chinnery PF. Mutation of the linker region of the polymerase gamma-1 (POLG1) gene associated with progressive external ophthalmoplegia and Parkinsonism. Arch Neurol. 2007;64:553–557. doi: 10.1001/archneur.64.4.553. [DOI] [PubMed] [Google Scholar]
- Iguchi M, Kujuro Y, Okatsu K, Koyano F, Kosako H, Kimura M, Suzuki N, Uchiyama S, Tanaka K, Matsuda N. Parkin-catalyzed ubiquitin-ester transfer is triggered by PINK1-dependent phosphorylation. J Biol Chem. 2013;288:22019–22032. doi: 10.1074/jbc.M113.467530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilijic E, Guzman JN, Surmeier DJ. The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson’s disease. Neurobiol Dis. 2011;43:364–371. doi: 10.1016/j.nbd.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Investigators N.E.T.i.P.D.F.-Z. Pioglitazone in early Parkinson’s disease: a phase 2, multicentre, double-blind, randomised trial. Lancet Neurol. 2015;14:795–803. doi: 10.1016/S1474-4422(15)00144-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irrcher I, Aleyasin H, Seifert EL, Hewitt SJ, Chhabra S, Phillips M, Lutz AK, Rousseaux MW, Bevilacqua L, Jahani-Asl A, Callaghan S, MacLaurin JG, Winklhofer KF, Rizzu P, Rippstein P, Kim RH, Chen CX, Fon EA, Slack RS, Harper ME, McBride HM, Mak TW, Park DS. Loss of the Parkinson’s disease-linked gene DJ-1 perturbs mitochondrial dynamics. Hum Mol Genet. 2010;19:3734–3746. doi: 10.1093/hmg/ddq288. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Taira T, Takahashi-Niki K, Niki T, Ariga H, Iguchi-Ariga SM. Human DJ-1-specific transcriptional activation of tyrosine hydroxylase gene. J Biol Chem. 2010;285:39718–39731. doi: 10.1074/jbc.M110.137034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janetzky B, Hauck S, Youdim MB, Riederer P, Jellinger K, Pantucek F, Zochling R, Boissl KW, Reichmann H. Unaltered aconitase activity, but decreased complex I activity in substantia nigra pars compacta of patients with Parkinson’s disease. Neurosci Lett. 1994;169:126–128. doi: 10.1016/0304-3940(94)90372-7. [DOI] [PubMed] [Google Scholar]
- Jensen MB, Bhatia VK, Jao CC, Rasmussen JE, Pedersen SL, Jensen KJ, Langen R, Stamou D. Membrane curvature sensing by amphipathic helices: a single liposome study using alpha-synuclein and annexin B12. J Biol Chem. 2011;286:42603–42614. doi: 10.1074/jbc.M111.271130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jhang JJ, Cheng YT, Ho CY, Yen GC. Monosodium urate crystals trigger Nrf2- and heme oxygenase-1-dependent inflammation in THP-1 cells. Cell Mol Immunol. 2015;12:424–434. doi: 10.1038/cmi.2014.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Kanthasamy A, Ghosh A, Anantharam V, Kalyanaraman B, Kanthasamy AG. Mitochondria-targeted antioxidants for treatment of Parkinson’s disease: preclinical and clinical outcomes. Biochim Biophys Acta. 2014;1842:1282–1294. doi: 10.1016/j.bbadis.2013.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol. 2010;191:933–942. doi: 10.1083/jcb.201008084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JM, Datta P, Srinivasula SM, Ji W, Gupta S, Zhang Z, Davies E, Hajnoczky G, Saunders TL, Van Keuren ML, Fernandes-Alnemri T, Meisler MH, Alnemri ES. Loss of Omi mitochondrial protease activity causes the neuromuscular disorder of mnd2 mutant mice. Nature. 2003;425:721–727. doi: 10.1038/nature02052. [DOI] [PubMed] [Google Scholar]
- Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA, Chen W, Hoke A, Dawson VL, Dawson TM, Gabrielson K, Kass DA, Iijima M, Sesaki H. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J. 2014;33:2798–2813. doi: 10.15252/embj.201488658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaidery NA, Banerjee R, Yang L, Smirnova NA, Hushpulian DM, Liby KT, Williams CR, Yamamoto M, Kensler TW, Ratan RR, Sporn MB, Beal MF, Gazaryan IG, Thomas B. Targeting Nrf2-mediated gene transcription by extremely potent synthetic triterpenoids attenuate dopaminergic neurotoxicity in the MPTP mouse model of Parkinson’s disease. Antioxid Redox Signal. 2013;18:139–157. doi: 10.1089/ars.2011.4491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamp F, Exner N, Lutz AK, Wender N, Hegermann J, Brunner B, Nuscher B, Bartels T, Giese A, Beyer K, Eimer S, Winklhofer KF, Haass C. Inhibition of mitochondrial fusion by alpha-synuclein is rescued by PINK1, Parkin and DJ-1. EMBO J. 2010;29:3571–3589. doi: 10.1038/emboj.2010.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang MY, Oh TJ, Cho YM. Glucagon-Like Peptide-1 Increases Mitochondrial Biogenesis and Function in INS-1 Rat Insulinoma Cells. Endocrinol Metab (Seoul) 2015;30:216–220. doi: 10.3803/EnM.2015.30.2.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeney PM, Xie J, Capaldi RA, Bennett JP., Jr Parkinson’s disease brain mitochondrial complex I has oxidatively damaged subunits and is functionally impaired and misassembled. J Neurosci. 2006;26:5256–5264. doi: 10.1523/JNEUROSCI.0984-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khaliq ZM, Bean BP. Pacemaking in dopaminergic ventral tegmental area neurons: depolarizing drive from background and voltage-dependent sodium conductances. J Neurosci. 2010;30:7401–7413. doi: 10.1523/JNEUROSCI.0143-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khateeb S, Flusser H, Ofir R, Shelef I, Narkis G, Vardi G, Shorer Z, Levy R, Galil A, Elbedour K, Birk OS. PLA2G6 mutation underlies infantile neuroaxonal dystrophy. Am J Hum Genet. 2006;79:942–948. doi: 10.1086/508572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinghorn KJ, Castillo-Quan JI, Bartolome F, Angelova PR, Li L, Pope S, Cocheme HM, Khan S, Asghari S, Bhatia KP, Hardy J, Abramov AY, Partridge L. Loss of PLA2G6 leads to elevated mitochondrial lipid peroxidation and mitochondrial dysfunction. Brain. 2015;138:1801–1816. doi: 10.1093/brain/awv132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinumi T, Kimata J, Taira T, Ariga H, Niki E. Cysteine-106 of DJ-1 is the most sensitive cysteine residue to hydrogen peroxide-mediated oxidation in vivo in human umbilical vein endothelial cells. Biochem Biophys Res Commun. 2004;317:722–728. doi: 10.1016/j.bbrc.2004.03.110. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Calingasan NY, Starkov A, Stavrovskaya IG, Kristal BS, Yang L, Wieringa B, Beal MF. Neuroprotective mechanisms of creatine occur in the absence of mitochondrial creatine kinase. Neurobiol Dis. 2004;15:610–617. doi: 10.1016/j.nbd.2003.12.014. [DOI] [PubMed] [Google Scholar]
- Klivenyi P, Siwek D, Gardian G, Yang L, Starkov A, Cleren C, Ferrante RJ, Kowall NW, Abeliovich A, Beal MF. Mice lacking alpha-synuclein are resistant to mitochondrial toxins. Neurobiol Dis. 2006;21:541–548. doi: 10.1016/j.nbd.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Kostic M, Ludtmann MH, Bading H, Hershfinkel M, Steer E, Chu CT, Abramov AY, Sekler I. PKA Phosphorylation of NCLX Reverses Mitochondrial Calcium Overload and Depolarization, Promoting Survival of PINK1-Deficient Dopaminergic Neurons. Cell Rep. 2015;13:376–386. doi: 10.1016/j.celrep.2015.08.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koutsopoulos OS, Laine D, Osellame L, Chudakov DM, Parton RG, Frazier AE, Ryan MT. Human Miltons associate with mitochondria and induce microtubule-dependent remodeling of mitochondrial networks. Biochim Biophys Acta. 2010;1803:564–574. doi: 10.1016/j.bbamcr.2010.03.006. [DOI] [PubMed] [Google Scholar]
- Koyano F, Okatsu K, Kosako H, Tamura Y, Go E, Kimura M, Kimura Y, Tsuchiya H, Yoshihara H, Hirokawa T, Endo T, Fon EA, Trempe JF, Saeki Y, Tanaka K, Matsuda N. Ubiquitin is phosphorylated by PINK1 to activate parkin. Nature. 2014;510:162–166. doi: 10.1038/nature13392. [DOI] [PubMed] [Google Scholar]
- Kraytsberg Y, Kudryavtseva E, McKee AC, Geula C, Kowall NW, Khrapko K. Mitochondrial DNA deletions are abundant and cause functional impairment in aged human substantia nigra neurons. Nat Genet. 2006;38:518–520. doi: 10.1038/ng1778. [DOI] [PubMed] [Google Scholar]
- Krige D, Carroll MT, Cooper JM, Marsden CD, Schapira AH. Platelet mitochondrial function in Parkinson’s disease. The Royal Kings and Queens Parkinson Disease Research Group. Ann Neurol. 1992;32:782–788. doi: 10.1002/ana.410320612. [DOI] [PubMed] [Google Scholar]
- Kuter K, Kratochwil M, Berghauzen-Maciejewska K, Glowacka U, Sugawa MD, Ossowska K, Dencher NA. Adaptation within mitochondrial oxidative phosphorylation supercomplexes and membrane viscosity during degeneration of dopaminergic neurons in an animal model of early Parkinson’s disease. Biochim Biophys Acta. 2016;1862:741–753. doi: 10.1016/j.bbadis.2016.01.022. [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson’s disease. First of two parts. N Engl J Med. 1998a;339:1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson’s disease. Second of two parts. N Engl J Med. 1998b;339:1130–1143. doi: 10.1056/NEJM199810153391607. [DOI] [PubMed] [Google Scholar]
- Langston JW, Ballard P, Tetrud JW, Irwin I. Chronic Parkinsonism in humans due to a product of meperidine-analog synthesis. Science. 1983;219:979–980. doi: 10.1126/science.6823561. [DOI] [PubMed] [Google Scholar]
- Lapointe N, St-Hilaire M, Martinoli MG, Blanchet J, Gould P, Rouillard C, Cicchetti F. Rotenone induces non-specific central nervous system and systemic toxicity. FASEB J. 2004;18:717–719. doi: 10.1096/fj.03-0677fje. [DOI] [PubMed] [Google Scholar]
- Lastres-Becker I, Garcia-Yague AJ, Scannevin RH, Casarejos MJ, Kugler S, Rabano A, Cuadrado A. Repurposing the NRF2 Activator Dimethyl Fumarate as Therapy Against Synucleinopathy in Parkinson’s Disease. Antioxid Redox Signal. 2016;25:61–77. doi: 10.1089/ars.2015.6549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager J, Stephens AD, Fusco G, Strohl F, Curry N, Zacharopoulou M, Michel CH, Laine R, Nespovitaya N, Fantham M, Pinotsi D, Zago W, Fraser P, Tandon A, St George-Hyslop P, Rees E, Phillips JJ, De Simone A, Kaminski CF, Schierle GSK. C-terminal calcium binding of alpha-synuclein modulates synaptic vesicle interaction. Nat Commun. 2018;9:712. doi: 10.1038/s41467-018-03111-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Choi C, Lee SJ. Membrane-bound alpha-synuclein has a high aggregation propensity and the ability to seed the aggregation of the cytosolic form. J Biol Chem. 2002;277:671–678. doi: 10.1074/jbc.M107045200. [DOI] [PubMed] [Google Scholar]
- Lee S, Oh ST, Jeong HJ, Pak SC, Park HJ, Kim J, Cho HS, Jeon S. MPTP-induced vulnerability of dopamine neurons in A53T alpha-synuclein overexpressed mice with the potential involvement of DJ-1 downregulation. Korean J Physiol Pharmacol. 2017a;21:625–632. doi: 10.4196/kjpp.2017.21.6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Stevens DA, Kang SU, Jiang H, Lee YI, Ko HS, Scarffe LA, Umanah GE, Kang H, Ham S, Kam TI, Allen K, Brahmachari S, Kim JW, Neifert S, Yun SP, Fiesel FC, Springer W, Dawson VL, Shin JH, Dawson TM. PINK1 Primes Parkin-Mediated Ubiquitination of PARIS in Dopaminergic Neuronal Survival. Cell Rep. 2017b;18:918–932. doi: 10.1016/j.celrep.2016.12.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leidel C, Longoria RA, Gutierrez FM, Shubeita GT. Measuring molecular motor forces in vivo: implications for tug-of-war models of bidirectional transport. Biophys J. 2012;103:492–500. doi: 10.1016/j.bpj.2012.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li WW, Yang R, Guo JC, Ren HM, Zha XL, Cheng JS, Cai DF. Localization of alpha-synuclein to mitochondria within midbrain of mice. Neuroreport. 2007;18:1543–1546. doi: 10.1097/WNR.0b013e3282f03db4. [DOI] [PubMed] [Google Scholar]
- Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, Powers K, Shen H, Egan JM, Sambamurti K, Brossi A, Lahiri DK, Mattson MP, Hoffer BJ, Wang Y, Greig NH. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106:1285–1290. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MT, Cantuti-Castelvetri I, Zheng K, Jackson KE, Tan YB, Arzberger T, Lees AJ, Betensky RA, Beal MF, Simon DK. Somatic mitochondrial DNA mutations in early Parkinson and incidental Lewy body disease. Ann Neurol. 2012;71:850–854. doi: 10.1002/ana.23568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G, Zhang C, Yin J, Li X, Cheng F, Li Y, Yang H, Ueda K, Chan P, Yu S. alpha-Synuclein is differentially expressed in mitochondria from different rat brain regions and dose-dependently down-regulates complex I activity. Neurosci Lett. 2009;454:187–192. doi: 10.1016/j.neulet.2009.02.056. [DOI] [PubMed] [Google Scholar]
- Liu J, Wang LN. Mitochondrial enhancement for neurodegenerative movement disorders: a systematic review of trials involving creatine, coenzyme Q10, idebenone and mitoquinone. CNS Drugs. 2014;28:63–68. doi: 10.1007/s40263-013-0124-4. [DOI] [PubMed] [Google Scholar]
- Liu S, Sawada T, Lee S, Yu W, Silverio G, Alapatt P, Millan I, Shen A, Saxton W, Kanao T, Takahashi R, Hattori N, Imai Y, Lu B. Parkinson’s disease-associated kinase PINK1 regulates Miro protein level and axonal transport of mitochondria. PLoS Genet. 2012;8:e1002537. doi: 10.1371/journal.pgen.1002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Clegg HV, Leslie PL, Di J, Tollini LA, He Y, Kim TH, Jin A, Graves LM, Zheng J, Zhang Y. CHCHD2 inhibits apoptosis by interacting with Bcl-x L to regulate Bax activation. Cell Death Differ. 2015;22:1035–1046. doi: 10.1038/cdd.2014.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb V, Yakunin E, Saada A, Sharon R. The transgenic overexpression of alpha-synuclein and not its related pathology associates with complex I inhibition. J Biol Chem. 2010;285:7334–7343. doi: 10.1074/jbc.M109.061051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Fabuel I, Le Douce J, Logan A, James AM, Bonvento G, Murphy MP, Almeida A, Bolanos JP. Complex I assembly into supercomplexes determines differential mitochondrial ROS production in neurons and astrocytes. Proc Natl Acad Sci U S A. 2016;113:13063–13068. doi: 10.1073/pnas.1613701113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Fabuel I, Martin-Martin L, Resch-Beusher M, Azkona G, Sanchez-Pernaute R, Bolanos JP. Mitochondrial respiratory chain disorganization in Parkinson’s disease-relevant PINK1 and DJ1 mutants. Neurochem Int. 2017;109:101–105. doi: 10.1016/j.neuint.2017.03.023. [DOI] [PubMed] [Google Scholar]
- Luoma PT, Eerola J, Ahola S, Hakonen AH, Hellstrom O, Kivisto KT, Tienari PJ, Suomalainen A. Mitochondrial DNA polymerase gamma variants in idiopathic sporadic Parkinson disease. Neurology. 2007;69:1152–1159. doi: 10.1212/01.wnl.0000276955.23735.eb. [DOI] [PubMed] [Google Scholar]
- Luth ES, Stavrovskaya IG, Bartels T, Kristal BS, Selkoe DJ. Soluble, prefibrillar alpha-synuclein oligomers promote complex I-dependent, Ca2+-induced mitochondrial dysfunction. J Biol Chem. 2014;289:21490–21507. doi: 10.1074/jbc.M113.545749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- M’Angale PG, Staveley BE. The HtrA2 Drosophila model of Parkinson’s disease is suppressed by the pro-survival Bcl-2 Buffy. Genome. 2017;60:1–7. doi: 10.1139/gen-2016-0069. [DOI] [PubMed] [Google Scholar]
- Ma Z, Turk J. The molecular biology of the group VIA Ca2+-independent phospholipase A2. Prog Nucleic Acid Res Mol Biol. 2001;67:1–33. doi: 10.1016/s0079-6603(01)67023-5. [DOI] [PubMed] [Google Scholar]
- Mancuso M, Filosto M, Bellan M, Liguori R, Montagna P, Baruzzi A, DiMauro S, Carelli V. POLG mutations causing ophthalmoplegia, sensorimotor polyneuropathy, ataxia, and deafness. Neurology. 2004;62:316–318. doi: 10.1212/wnl.62.2.316. [DOI] [PubMed] [Google Scholar]
- Manning-Bog AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA. The herbicide paraquat causes up-regulation and aggregation of alpha-synuclein in mice: paraquat and alpha-synuclein. J Biol Chem. 2002;277:1641–1644. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- Maranzana E, Barbero G, Falasca AI, Lenaz G, Genova ML. Mitochondrial respiratory supercomplex association limits production of reactive oxygen species from complex I. Antioxid Redox Signal. 2013;19:1469–1480. doi: 10.1089/ars.2012.4845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marella M, Seo BB, Nakamaru-Ogiso E, Greenamyre JT, Matsuno-Yagi A, Yagi T. Protection by the NDI1 gene against neurodegeneration in a rotenone rat model of Parkinson’s disease. PLoS One. 2008;3:e1433. doi: 10.1371/journal.pone.0001433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marongiu R, Spencer B, Crews L, Adame A, Patrick C, Trejo M, Dallapiccola B, Valente EM, Masliah E. Mutant Pink1 induces mitochondrial dysfunction in a neuronal cell model of Parkinson’s disease by disturbing calcium flux. J Neurochem. 2009;108:1561–1574. doi: 10.1111/j.1471-4159.2009.05932.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin I, Kim JW, Dawson VL, Dawson TM. LRRK2 pathobiology in Parkinson’s disease. J Neurochem. 2014a;131:554–565. doi: 10.1111/jnc.12949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Semenkow S, Hanaford A, Wong M. Mitochondrial permeability transition pore regulates Parkinson’s disease development in mutant alpha-synuclein transgenic mice. Neurobiol Aging. 2014b;35:1132–1152. doi: 10.1016/j.neurobiolaging.2013.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez Banaclocha M. N-acetylcysteine elicited increase in complex I activity in synaptic mitochondria from aged mice: implications for treatment of Parkinson’s disease. Brain Res. 2000;859:173–175. doi: 10.1016/s0006-8993(00)02005-9. [DOI] [PubMed] [Google Scholar]
- Martins LM, Iaccarino I, Tenev T, Gschmeissner S, Totty NF, Lemoine NR, Savopoulos J, Gray CW, Creasy CL, Dingwall C, Downward J. The serine protease Omi/HtrA2 regulates apoptosis by binding XIAP through a reaper-like motif. J Biol Chem. 2002;277:439–444. doi: 10.1074/jbc.M109784200. [DOI] [PubMed] [Google Scholar]
- Martins LM, Morrison A, Klupsch K, Fedele V, Moisoi N, Teismann P, Abuin A, Grau E, Geppert M, Livi GP, Creasy CL, Martin A, Hargreaves I, Heales SJ, Okada H, Brandner S, Schulz JB, Mak T, Downward J. Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice. Mol Cell Biol. 2004;24:9848–9862. doi: 10.1128/MCB.24.22.9848-9862.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, Kimura M, Komatsu M, Hattori N, Tanaka K. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng H, Yamashita C, Shiba-Fukushima K, Inoshita T, Funayama M, Sato S, Hatta T, Natsume T, Umitsu M, Takagi J, Imai Y, Hattori N. Loss of Parkinson’s disease-associated protein CHCHD2 affects mitochondrial crista structure and destabilizes cytochrome c. Nat Commun. 2017;8:15500. doi: 10.1038/ncomms15500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Rademacher DJ. MPTP mouse models of Parkinson’s disease: an update. J Parkinsons Dis. 2011;1:19–33. doi: 10.3233/JPD-2011-11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton ER, Rhoades E. Effects of curvature and composition on alpha-synuclein binding to lipid vesicles. Biophys J. 2010;99:2279–2288. doi: 10.1016/j.bpj.2010.07.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki Y, Tanji K, Mori F, Kakita A, Takahashi H, Wakabayashi K. PLA2G6 accumulates in Lewy bodies in PARK14 and idiopathic Parkinson’s disease. Neurosci Lett. 2017;645:40–45. doi: 10.1016/j.neulet.2017.02.027. [DOI] [PubMed] [Google Scholar]
- Milanese C, Tapias V, Gabriels S, Cerri S, Levandis G, Blandini F, Tresini M, Shiva S, Greenamyre JT, Gladwin MT, Mastroberardino PG. Mitochondrial Complex I Reversible S-Nitrosation Improves Bioenergetics and Is Protective in Parkinson’s Disease. Antioxid Redox Signal. 2018;28:44–61. doi: 10.1089/ars.2017.6992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileykovskaya E, Dowhan W. Cardiolipin-dependent formation of mitochondrial respiratory supercomplexes. Chem Phys Lipids. 2014;179:42–48. doi: 10.1016/j.chemphyslip.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moisoi N, Klupsch K, Fedele V, East P, Sharma S, Renton A, Plun-Favreau H, Edwards RE, Teismann P, Esposti MD, Morrison AD, Wood NW, Downward J, Martins LM. Mitochondrial dysfunction triggered by loss of HtrA2 results in the activation of a brain-specific transcriptional stress response. Cell Death Differ. 2009;16:449–464. doi: 10.1038/cdd.2008.166. [DOI] [PubMed] [Google Scholar]
- Monti DA, Zabrecky G, Kremens D, Liang TW, Wintering NA, Cai J, Wei X, Bazzan AJ, Zhong L, Bowen B, Intenzo CM, Iacovitti L, Newberg AB. N-Acetyl Cysteine May Support Dopamine Neurons in Parkinson’s Disease: Preliminary Clinical and Cell Line Data. PLoS One. 2016;11:e0157602. doi: 10.1371/journal.pone.0157602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morais VA, Haddad D, Craessaerts K, De Bock PJ, Swerts J, Vilain S, Aerts L, Overbergh L, Grunewald A, Seibler P, Klein C, Gevaert K, Verstreken P, De Strooper B. PINK1 loss-of-function mutations affect mitochondrial complex I activity via NdufA10 ubiquinone uncoupling. Science. 2014;344:203–207. doi: 10.1126/science.1249161. [DOI] [PubMed] [Google Scholar]
- Morais VA, Verstreken P, Roethig A, Smet J, Snellinx A, Vanbrabant M, Haddad D, Frezza C, Mandemakers W, Vogt-Weisenhorn D, Van Coster R, Wurst W, Scorrano L, De Strooper B. Parkinson’s disease mutations in PINK1 result in decreased Complex I activity and deficient synaptic function. EMBO Mol Med. 2009;1:99–111. doi: 10.1002/emmm.200900006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortiboys H, Aasly J, Bandmann O. Ursocholanic acid rescues mitochondrial function in common forms of familial Parkinson’s disease. Brain. 2013;136:3038–3050. doi: 10.1093/brain/awt224. [DOI] [PubMed] [Google Scholar]
- Mortiboys H, Furmston R, Bronstad G, Aasly J, Elliott C, Bandmann O. UDCA exerts beneficial effect on mitochondrial dysfunction in LRRK2(G2019S) carriers and in vivo. Neurology. 2015;85:846–852. doi: 10.1212/WNL.0000000000001905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortiboys H, Johansen KK, Aasly JO, Bandmann O. Mitochondrial impairment in patients with Parkinson disease with the G2019S mutation in LRRK2. Neurology. 2010;75:2017–2020. doi: 10.1212/WNL.0b013e3181ff9685. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Nemani VM, Azarbal F, Skibinski G, Levy JM, Egami K, Munishkina L, Zhang J, Gardner B, Wakabayashi J, Sesaki H, Cheng Y, Finkbeiner S, Nussbaum RL, Masliah E, Edwards RH. Direct membrane association drives mitochondrial fission by the Parkinson disease-associated protein alpha-synuclein. J Biol Chem. 2011;286:20710–20726. doi: 10.1074/jbc.M110.213538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura K, Nemani VM, Wallender EK, Kaehlcke K, Ott M, Edwards RH. Optical reporters for the conformation of alpha-synuclein reveal a specific interaction with mitochondria. J Neurosci. 2008;28:12305–12317. doi: 10.1523/JNEUROSCI.3088-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas WJ, Vyas I, Heikkila RE. Inhibition of NADH-linked oxidation in brain mitochondria by 1-methyl-4-phenyl-pyridine, a metabolite of the neurotoxin, 1-methyl-4-phenyl-1,2,5,6-tetrahydropyridine. Life Sci. 1985;36:2503–2508. doi: 10.1016/0024-3205(85)90146-8. [DOI] [PubMed] [Google Scholar]
- Nieto M, Gil-Bea FJ, Dalfo E, Cuadrado M, Cabodevilla F, Sanchez B, Catena S, Sesma T, Ribe E, Ferrer I, Ramirez MJ, Gomez-Isla T. Increased sensitivity to MPTP in human alpha-synuclein A30P transgenic mice. Neurobiol Aging. 2006;27:848–856. doi: 10.1016/j.neurobiolaging.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Niu J, Yu M, Wang C, Xu Z. Leucine-rich repeat kinase 2 disturbs mitochondrial dynamics via Dynamin-like protein. J Neurochem. 2012;122:650–658. doi: 10.1111/j.1471-4159.2012.07809.x. [DOI] [PubMed] [Google Scholar]
- O’Gorman E, Beutner G, Dolder M, Koretsky AP, Brdiczka D, Wallimann T. The role of creatine kinase in inhibition of mitochondrial permeability transition. FEBS Lett. 1997;414:253–257. doi: 10.1016/s0014-5793(97)01045-4. [DOI] [PubMed] [Google Scholar]
- Olanow CW, Rascol O, Hauser R, Feigin PD, Jankovic J, Lang A, Langston W, Melamed E, Poewe W, Stocchi F, Tolosa E Investigators AS. A double-blind, delayed-start trial of rasagiline in Parkinson’s disease. N Engl J Med. 2009;361:1268–1278. doi: 10.1056/NEJMoa0809335. [DOI] [PubMed] [Google Scholar]
- Orr AL, Rutaganira FU, de Roulet D, Huang EJ, Hertz NT, Shokat KM, Nakamura K. Long-term oral kinetin does not protect against alpha-synuclein-induced neurodegeneration in rodent models of Parkinson’s disease. Neurochem Int. 2017;109:106–116. doi: 10.1016/j.neuint.2017.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsucci D, Mancuso M, Ienco EC, LoGerfo A, Siciliano G. Targeting mitochondrial dysfunction and neurodegeneration by means of coenzyme Q10 and its analogues. Curr Med Chem. 2011;18:4053–4064. doi: 10.2174/092986711796957257. [DOI] [PubMed] [Google Scholar]
- Osellame LD, Rahim AA, Hargreaves IP, Gegg ME, Richard-Londt A, Brandner S, Waddington SN, Schapira AH, Duchen MR. Mitochondria and quality control defects in a mouse model of Gaucher disease--links to Parkinson’s disease. Cell Metab. 2013;17:941–953. doi: 10.1016/j.cmet.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottolini D, Cali T, Negro A, Brini M. The Parkinson disease-related protein DJ-1 counteracts mitochondrial impairment induced by the tumour suppressor protein p53 by enhancing endoplasmic reticulum-mitochondria tethering. Hum Mol Genet. 2013;22:2152–2168. doi: 10.1093/hmg/ddt068. [DOI] [PubMed] [Google Scholar]
- Pacelli C, Giguere N, Bourque MJ, Levesque M, Slack RS, Trudeau LE. Elevated Mitochondrial Bioenergetics and Axonal Arborization Size Are Key Contributors to the Vulnerability of Dopamine Neurons. Curr Biol. 2015;25:2349–2360. doi: 10.1016/j.cub.2015.07.050. [DOI] [PubMed] [Google Scholar]
- Packer MA, Miesel R, Murphy MP. Exposure to the parkinsonian neurotoxin 1-methyl-4-phenylpyridinium (MPP+) and nitric oxide simultaneously causes cyclosporin A-sensitive mitochondrial calcium efflux and depolarisation. Biochem Pharmacol. 1996;51:267–273. doi: 10.1016/0006-2952(95)02165-5. [DOI] [PubMed] [Google Scholar]
- Paisan-Ruiz C, Bhatia KP, Li A, Hernandez D, Davis M, Wood NW, Hardy J, Houlden H, Singleton A, Schneider SA. Characterization of PLA2G6 as a locus for dystonia-parkinsonism. Ann Neurol. 2009;65:19–23. doi: 10.1002/ana.21415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacino JJ, Sagi D, Goldberg MS, Krauss S, Motz C, Wacker M, Klose J, Shen J. Mitochondrial dysfunction and oxidative damage in parkin-deficient mice. J Biol Chem. 2004;279:18614–18622. doi: 10.1074/jbc.M401135200. [DOI] [PubMed] [Google Scholar]
- Papa S, Sardanelli AM, Capitanio N, Piccoli C. Mitochondrial respiratory dysfunction and mutations in mitochondrial DNA in PINK1 familial parkinsonism. J Bioenerg Biomembr. 2009;41:509–516. doi: 10.1007/s10863-009-9252-4. [DOI] [PubMed] [Google Scholar]
- Papkovskaia TD, Chau KY, Inesta-Vaquera F, Papkovsky DB, Healy DG, Nishio K, Staddon J, Duchen MR, Hardy J, Schapira AH, Cooper JM. G2019S leucine-rich repeat kinase 2 causes uncoupling protein-mediated mitochondrial depolarization. Hum Mol Genet. 2012;21:4201–4213. doi: 10.1093/hmg/dds244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Lee SB, Lee S, Kim Y, Song S, Kim S, Bae E, Kim J, Shong M, Kim JM, Chung J. Mitochondrial dysfunction in Drosophila PINK1 mutants is complemented by parkin. Nature. 2006;441:1157–1161. doi: 10.1038/nature04788. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr, Boyson SJ, Parks JK. Abnormalities of the electron transport chain in idiopathic Parkinson’s disease. Ann Neurol. 1989;26:719–723. doi: 10.1002/ana.410260606. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr, Parks JK. Mitochondrial ND5 mutations in idiopathic Parkinson’s disease. Biochem Biophys Res Commun. 2005;326:667–669. doi: 10.1016/j.bbrc.2004.11.093. [DOI] [PubMed] [Google Scholar]
- Parker WD, Jr, Parks JK, Swerdlow RH. Complex I deficiency in Parkinson’s disease frontal cortex. Brain Res. 2008;1189:215–218. doi: 10.1016/j.brainres.2007.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson Study G. Effects of tocopherol and deprenyl on the progression of disability in early Parkinson’s disease. N Engl J Med. 1993;328:176–183. doi: 10.1056/NEJM199301213280305. [DOI] [PubMed] [Google Scholar]
- Parkinson Study G. A controlled trial of rasagiline in early Parkinson disease: the TEMPO Study. Arch Neurol. 2002;59:1937–1943. doi: 10.1001/archneur.59.12.1937. [DOI] [PubMed] [Google Scholar]
- Parkinson Study G. Phase II safety, tolerability, and dose selection study of isradipine as a potential disease-modifying intervention in early Parkinson’s disease (STEADY-PD) Mov Disord. 2013;28:1823–1831. doi: 10.1002/mds.25639. [DOI] [PubMed] [Google Scholar]
- Parkinson Study Group, QEI. Beal MF, Oakes D, Shoulson I, Henchcliffe C, Galpern WR, Haas R, Juncos JL, Nutt JG, Voss TS, Ravina B, Shults CM, Helles K, Snively V, Lew MF, Griebner B, Watts A, Gao S, Pourcher E, Bond L, Kompoliti K, Agarwal P, Sia C, Jog M, Cole L, Sultana M, Kurlan R, Richard I, Deeley C, Waters CH, Figueroa A, Arkun A, Brodsky M, Ondo WG, Hunter CB, Jimenez-Shahed J, Palao A, Miyasaki JM, So J, Tetrud J, Reys L, Smith K, Singer C, Blenke A, Russell DS, Cotto C, Friedman JH, Lannon M, Zhang L, Drasby E, Kumar R, Subramanian T, Ford DS, Grimes DA, Cote D, Conway J, Siderowf AD, Evatt ML, Sommerfeld B, Lieberman AN, Okun MS, Rodriguez RL, Merritt S, Swartz CL, Martin WR, King P, Stover N, Guthrie S, Watts RL, Ahmed A, Fernandez HH, Winters A, Mari Z, Dawson TM, Dunlop B, Feigin AS, Shannon B, Nirenberg MJ, Ogg M, Ellias SA, Thomas CA, Frei K, Bodis-Wollner I, Glazman S, Mayer T, Hauser RA, Pahwa R, Langhammer A, Ranawaya R, Derwent L, Sethi KD, Farrow B, Prakash R, Litvan I, Robinson A, Sahay A, Gartner M, Hinson VK, Markind S, Pelikan M, Perlmutter JS, Hartlein J, Molho E, Evans S, Adler CH, Duffy A, Lind M, Elmer L, Davis K, Spears J, Wilson S, Leehey MA, Hermanowicz N, Niswonger S, Shill HA, Obradov S, Rajput A, Cowper M, Lessig S, Song D, Fontaine D, Zadikoff C, Williams K, Blindauer KA, Bergholte J, Propsom CS, Stacy MA, Field J, Mihaila D, Chilton M, Uc EY, Sieren J, Simon DK, Kraics L, Silver A, Boyd JT, Hamill RW, Ingvoldstad C, Young J, Thomas K, Kostyk SK, Wojcieszek J, Pfeiffer RF, Panisset M, Beland M, Reich SG, Cines M, Zappala N, Rivest J, Zweig R, Lumina LP, Hilliard CL, Grill S, Kellermann M, Tuite P, Rolandelli S, Kang UJ, Young J, Rao J, Cook MM, Severt L, Boyar K. A randomized clinical trial of high-dosage coenzyme Q10 in early Parkinson disease: no evidence of benefit. JAMA Neurol. 2014a;71:543–552. doi: 10.1001/jamaneurol.2014.131. [DOI] [PubMed] [Google Scholar]
- Parkinson Study Group SPDI. Schwarzschild MA, Ascherio A, Beal MF, Cudkowicz ME, Curhan GC, Hare JM, Hooper DC, Kieburtz KD, Macklin EA, Oakes D, Rudolph A, Shoulson I, Tennis MK, Espay AJ, Gartner M, Hung A, Bwala G, Lenehan R, Encarnacion E, Ainslie M, Castillo R, Togasaki D, Barles G, Friedman JH, Niles L, Carter JH, Murray M, Goetz CG, Jaglin J, Ahmed A, Russell DS, Cotto C, Goudreau JL, Russell D, Parashos SA, Ede P, Saint-Hilaire MH, Thomas CA, James R, Stacy MA, Johnson J, Gauger L, Antonelle de Marcaida J, Thurlow S, Isaacson SH, Carvajal L, Rao J, Cook M, Hope-Porche C, McClurg L, Grasso DL, Logan R, Orme C, Ross T, Brocht AF, Constantinescu R, Sharma S, Venuto C, Weber J, Eaton K. Inosine to increase serum and cerebrospinal fluid urate in Parkinson disease: a randomized clinical trial. JAMA Neurol. 2014b;71:141–150. doi: 10.1001/jamaneurol.2013.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perier C, Bender A, Garcia-Arumi E, Melia MJ, Bove J, Laub C, Klopstock T, Elstner M, Mounsey RB, Teismann P, Prolla T, Andreu AL, Vila M. Accumulation of mitochondrial DNA deletions within dopaminergic neurons triggers neuroprotective mechanisms. Brain. 2013;136:2369–2378. doi: 10.1093/brain/awt196. [DOI] [PubMed] [Google Scholar]
- Petronilli V, Penzo D, Scorrano L, Bernardi P, Di Lisa F. The mitochondrial permeability transition, release of cytochrome c and cell death. Correlation with the duration of pore openings in situ. J Biol Chem. 2001;276:12030–12034. doi: 10.1074/jbc.M010604200. [DOI] [PubMed] [Google Scholar]
- Pickrell AM, Huang CH, Kennedy SR, Ordureau A, Sideris DP, Hoekstra JG, Harper JW, Youle RJ. Endogenous Parkin Preserves Dopaminergic Substantia Nigral Neurons following Mitochondrial DNA Mutagenic Stress. Neuron. 2015;87:371–381. doi: 10.1016/j.neuron.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickrell AM, Youle RJ. The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease. Neuron. 2015;85:257–273. doi: 10.1016/j.neuron.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto M, Nissanka N, Moraes CT. Lack of Parkin anticipates the phenotype and affects mitochondrial morphology and mtDNA levels in a mouse model of Parkinson’s Disease. J Neurosci. 2017 doi: 10.1523/JNEUROSCI.1384-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plun-Favreau H, Klupsch K, Moisoi N, Gandhi S, Kjaer S, Frith D, Harvey K, Deas E, Harvey RJ, McDonald N, Wood NW, Martins LM, Downward J. The mitochondrial protease HtrA2 is regulated by Parkinson’s disease-associated kinase PINK1. Nat Cell Biol. 2007;9:1243–1252. doi: 10.1038/ncb1644. [DOI] [PubMed] [Google Scholar]
- Pogson JH, Ivatt RM, Sanchez-Martinez A, Tufi R, Wilson E, Mortiboys H, Whitworth AJ. The complex I subunit NDUFA10 selectively rescues Drosophila pink1 mutants through a mechanism independent of mitophagy. PLoS Genet. 2014;10:e1004815. doi: 10.1371/journal.pgen.1004815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole AC, Thomas RE, Yu S, Vincow ES, Pallanck L. The mitochondrial fusion-promoting factor mitofusin is a substrate of the PINK1/parkin pathway. PLoS One. 2010;5:e10054. doi: 10.1371/journal.pone.0010054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozo Devoto VM, Falzone TL. Mitochondrial dynamics in Parkinson’s disease: a role for alpha-synuclein? Dis Model Mech. 2017;10:1075–1087. doi: 10.1242/dmm.026294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prots I, Veber V, Brey S, Campioni S, Buder K, Riek R, Bohm KJ, Winner B. alpha-Synuclein oligomers impair neuronal microtubule-kinesin interplay. J Biol Chem. 2013;288:21742–21754. doi: 10.1074/jbc.M113.451815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimmi A, Khosrobakhsh F, Izadpanah E, Moloudi MR, Hassanzadeh K. N-acetylcysteine prevents rotenone-induced Parkinson’s disease in rat: An investigation into the interaction of parkin and Drp1 proteins. Brain Res Bull. 2015;113:34–40. doi: 10.1016/j.brainresbull.2015.02.007. [DOI] [PubMed] [Google Scholar]
- Ramirez A, Heimbach A, Grundemann J, Stiller B, Hampshire D, Cid LP, Goebel I, Mubaidin AF, Wriekat AL, Roeper J, Al-Din A, Hillmer AM, Karsak M, Liss B, Woods CG, Behrens MI, Kubisch C. Hereditary parkinsonism with dementia is caused by mutations in ATP13A2, encoding a lysosomal type 5 P-type ATPase. Nat Genet. 2006;38:1184–1191. doi: 10.1038/ng1884. [DOI] [PubMed] [Google Scholar]
- Ramsay RR, Kowal AT, Johnson MK, Salach JI, Singer TP. The inhibition site of MPP+, the neurotoxic bioactivation product of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine is near the Q-binding site of NADH dehydrogenase. Arch Biochem Biophys. 1987;259:645–649. doi: 10.1016/0003-9861(87)90531-5. [DOI] [PubMed] [Google Scholar]
- Ren H, Fu K, Wang D, Mu C, Wang G. Oxidized DJ-1 interacts with the mitochondrial protein BCL-XL. J Biol Chem. 2011;286:35308–35317. doi: 10.1074/jbc.M110.207134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson JR, Caudle WM, Guillot TS, Watson JL, Nakamaru-Ogiso E, Seo BB, Sherer TB, Greenamyre JT, Yagi T, Matsuno-Yagi A, Miller GW. Obligatory role for complex I inhibition in the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) Toxicol Sci. 2007;95:196–204. doi: 10.1093/toxsci/kfl133. [DOI] [PubMed] [Google Scholar]
- Richter C, Park JW, Ames BN. Normal oxidative damage to mitochondrial and nuclear DNA is extensive. Proc Natl Acad Sci U S A. 1988;85:6465–6467. doi: 10.1073/pnas.85.17.6465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan BJ, Hoek S, Fon EA, Wade-Martins R. Mitochondrial dysfunction and mitophagy in Parkinson’s: from familial to sporadic disease. Trends Biochem Sci. 2015;40:200–210. doi: 10.1016/j.tibs.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Sandebring A, Dehvari N, Perez-Manso M, Thomas KJ, Karpilovski E, Cookson MR, Cowburn RF, Cedazo-Minguez A. Parkin deficiency disrupts calcium homeostasis by modulating phospholipase C signalling. FEBS J. 2009;276:5041–5052. doi: 10.1111/j.1742-4658.2009.07201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders LH, Laganiere J, Cooper O, Mak SK, Vu BJ, Huang YA, Paschon DE, Vangipuram M, Sundararajan R, Urnov FD, Langston JW, Gregory PD, Zhang HS, Greenamyre JT, Isacson O, Schule B. LRRK2 mutations cause mitochondrial DNA damage in iPSC-derived neural cells from Parkinson’s disease patients: reversal by gene correction. Neurobiol Dis. 2014;62:381–386. doi: 10.1016/j.nbd.2013.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD. Mitochondrial complex I deficiency in Parkinson’s disease. J Neurochem. 1990a;54:823–827. doi: 10.1111/j.1471-4159.1990.tb02325.x. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Mann VM, Cooper JM, Dexter D, Daniel SE, Jenner P, Clark JB, Marsden CD. Anatomic and disease specificity of NADH CoQ1 reductase (complex I) deficiency in Parkinson’s disease. J Neurochem. 1990b;55:2142–2145. doi: 10.1111/j.1471-4159.1990.tb05809.x. [DOI] [PubMed] [Google Scholar]
- Schmidt F, Levin J, Kamp F, Kretzschmar H, Giese A, Botzel K. Single-channel electrophysiology reveals a distinct and uniform pore complex formed by alpha-synuclein oligomers in lipid membranes. PLoS One. 2012;7:e42545. doi: 10.1371/journal.pone.0042545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider SA, Alcalay RN. Neuropathology of genetic synucleinopathies with parkinsonism: Review of the literature. Mov Disord. 2017;32:1504–1523. doi: 10.1002/mds.27193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotcher KP, Irwin I, DeLanney LE, Langston JW, Di Monte D. Effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 1-methyl-4-phenylpyridinium ion on ATP levels of mouse brain synaptosomes. J Neurochem. 1990;54:1295–1301. doi: 10.1111/j.1471-4159.1990.tb01962.x. [DOI] [PubMed] [Google Scholar]
- Seleznev K, Zhao C, Zhang XH, Song K, Ma ZA. Calcium-independent phospholipase A2 localizes in and protects mitochondria during apoptotic induction by staurosporine. J Biol Chem. 2006;281:22275–22288. doi: 10.1074/jbc.M604330200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo BB, Nakamaru-Ogiso E, Flotte TR, Yagi T, Matsuno-Yagi A. A single-subunit NADH-quinone oxidoreductase renders resistance to mammalian nerve cells against complex I inhibition. Mol Ther. 2002;6:336–341. doi: 10.1006/mthe.2002.0674. [DOI] [PubMed] [Google Scholar]
- Sheehan JP, Swerdlow RH, Parker WD, Miller SW, Davis RE, Tuttle JB. Altered calcium homeostasis in cells transformed by mitochondria from individuals with Parkinson’s disease. J Neurochem. 1997;68:1221–1233. doi: 10.1046/j.1471-4159.1997.68031221.x. [DOI] [PubMed] [Google Scholar]
- Shendelman S, Jonason A, Martinat C, Leete T, Abeliovich A. DJ-1 is a redox-dependent molecular chaperone that inhibits alpha-synuclein aggregate formation. PLoS Biol. 2004;2:e362. doi: 10.1371/journal.pbio.0020362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherer TB, Richardson JR, Testa CM, Seo BB, Panov AV, Yagi T, Matsuno-Yagi A, Miller GW, Greenamyre JT. Mechanism of toxicity of pesticides acting at complex I: relevance to environmental etiologies of Parkinson’s disease. J Neurochem. 2007;100:1469–1479. doi: 10.1111/j.1471-4159.2006.04333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi CH, Mao CY, Zhang SY, Yang J, Song B, Wu P, Zuo CT, Liu YT, Ji Y, Yang ZH, Wu J, Zhuang ZP, Xu YM. CHCHD2 gene mutations in familial and sporadic Parkinson’s disease. Neurobiol Aging. 2016;38:217e219–217e213. doi: 10.1016/j.neurobiolaging.2015.10.040. [DOI] [PubMed] [Google Scholar]
- Shi SY, Lu SY, Sivasubramaniyam T, Revelo XS, Cai EP, Luk CT, Schroer SA, Patel P, Kim RH, Bombardier E, Quadrilatero J, Tupling AR, Mak TW, Winer DA, Woo M. DJ-1 links muscle ROS production with metabolic reprogramming and systemic energy homeostasis in mice. Nat Commun. 2015;6:7415. doi: 10.1038/ncomms8415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JH, Ko HS, Kang H, Lee Y, Lee YI, Pletinkova O, Troconso JC, Dawson VL, Dawson TM. PARIS (ZNF746) repression of PGC-1alpha contributes to neurodegeneration in Parkinson’s disease. Cell. 2011;144:689–702. doi: 10.1016/j.cell.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shojaee S, Sina F, Banihosseini SS, Kazemi MH, Kalhor R, Shahidi GA, Fakhrai-Rad H, Ronaghi M, Elahi E. Genome-wide linkage analysis of a Parkinsonian-pyramidal syndrome pedigree by 500 K SNP arrays. Am J Hum Genet. 2008;82:1375–1384. doi: 10.1016/j.ajhg.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutt TE, McBride HM. Staying cool in difficult times: mitochondrial dynamics, quality control and the stress response. Biochim Biophys Acta. 2013;1833:417–424. doi: 10.1016/j.bbamcr.2012.05.024. [DOI] [PubMed] [Google Scholar]
- Siddiqui A, Chinta SJ, Mallajosyula JK, Rajagopolan S, Hanson I, Rane A, Melov S, Andersen JK. Selective binding of nuclear alpha-synuclein to the PGC1alpha promoter under conditions of oxidative stress may contribute to losses in mitochondrial function: implications for Parkinson’s disease. Free Radic Biol Med. 2012;53:993–1003. doi: 10.1016/j.freeradbiomed.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen CM, Clark LN, Condroyer C, De Marco EV, Durr A, Eblan MJ, Fahn S, Farrer MJ, Fung HC, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen GJ, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan EK, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu YR, Zabetian CP, Zhao Y, Ziegler SG. Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med. 2009;361:1651–1661. doi: 10.1056/NEJMoa0901281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon DK, Mayeux R, Marder K, Kowall NW, Beal MF, Johns DR. Mitochondrial DNA mutations in complex I and tRNA genes in Parkinson’s disease. Neurology. 2000;54:703–709. doi: 10.1212/wnl.54.3.703. [DOI] [PubMed] [Google Scholar]
- Simon DK, Pulst SM, Sutton JP, Browne SE, Beal MF, Johns DR. Familial multisystem degeneration with parkinsonism associated with the 11778 mitochondrial DNA mutation. Neurology. 1999;53:1787–1793. doi: 10.1212/wnl.53.8.1787. [DOI] [PubMed] [Google Scholar]
- Sina F, Shojaee S, Elahi E, Paisan-Ruiz C. R632W mutation in PLA2G6 segregates with dystonia-parkinsonism in a consanguineous Iranian family. Eur J Neurol. 2009;16:101–104. doi: 10.1111/j.1468-1331.2008.02356.x. [DOI] [PubMed] [Google Scholar]
- Smith KM, Eyal E, Weintraub D Investigators A. Combined rasagiline and antidepressant use in Parkinson disease in the ADAGIO study: effects on nonmotor symptoms and tolerability. JAMA Neurol. 2015;72:88–95. doi: 10.1001/jamaneurol.2014.2472. [DOI] [PubMed] [Google Scholar]
- Smith U. Pioglitazone: mechanism of action. Int J Clin Pract Suppl. 2001:13–18. [PubMed] [Google Scholar]
- Smoliga JM, Baur JA, Hausenblas HA. Resveratrol and health--a comprehensive review of human clinical trials. Mol Nutr Food Res. 2011;55:1129–1141. doi: 10.1002/mnfr.201100143. [DOI] [PubMed] [Google Scholar]
- Snow BJ, Rolfe FL, Lockhart MM, Frampton CM, O’Sullivan JD, Fung V, Smith RA, Murphy MP, Taylor KM Protect Study G. A double-blind, placebo-controlled study to assess the mitochondria-targeted antioxidant MitoQ as a disease-modifying therapy in Parkinson’s disease. Mov Disord. 2010;25:1670–1674. doi: 10.1002/mds.23148. [DOI] [PubMed] [Google Scholar]
- Snow RJ, Murphy RM. Creatine and the creatine transporter: a review. Mol Cell Biochem. 2001;224:169–181. doi: 10.1023/a:1011908606819. [DOI] [PubMed] [Google Scholar]
- Soman S, Keatinge M, Moein M, Da Costa M, Mortiboys H, Skupin A, Sugunan S, Bazala M, Kuznicki J, Bandmann O. Inhibition of the mitochondrial calcium uniporter rescues dopaminergic neurons in pink1(−/−) zebrafish. Eur J Neurosci. 2017;45:528–535. doi: 10.1111/ejn.13473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DD, Shults CW, Sisk A, Rockenstein E, Masliah E. Enhanced substantia nigra mitochondrial pathology in human alpha-synuclein transgenic mice after treatment with MPTP. Exp Neurol. 2004;186:158–172. doi: 10.1016/S0014-4886(03)00342-X. [DOI] [PubMed] [Google Scholar]
- Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. alpha-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc Natl Acad Sci U S A. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafa K, Tsika E, Moser R, Musso A, Glauser L, Jones A, Biskup S, Xiong Y, Bandopadhyay R, Dawson VL, Dawson TM, Moore DJ. Functional interaction of Parkinson’s disease-associated LRRK2 with members of the dynamin GTPase superfamily. Hum Mol Genet. 2014;23:2055–2077. doi: 10.1093/hmg/ddt600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterky FH, Lee S, Wibom R, Olson L, Larsson NG. Impaired mitochondrial transport and Parkin-independent degeneration of respiratory chain-deficient dopamine neurons in vivo. Proc Natl Acad Sci U S A. 2011;108:12937–12942. doi: 10.1073/pnas.1103295108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens DA, Lee Y, Kang HC, Lee BD, Lee YI, Bower A, Jiang H, Kang SU, Andrabi SA, Dawson VL, Shin JH, Dawson TM. Parkin loss leads to PARIS-dependent declines in mitochondrial mass and respiration. Proc Natl Acad Sci U S A. 2015;112:11696–11701. doi: 10.1073/pnas.1500624112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss KM, Martins LM, Plun-Favreau H, Marx FP, Kautzmann S, Berg D, Gasser T, Wszolek Z, Muller T, Bornemann A, Wolburg H, Downward J, Riess O, Schulz JB, Kruger R. Loss of function mutations in the gene encoding Omi/HtrA2 in Parkinson’s disease. Hum Mol Genet. 2005;14:2099–2111. doi: 10.1093/hmg/ddi215. [DOI] [PubMed] [Google Scholar]
- Su YC, Qi X. Inhibition of excessive mitochondrial fission reduced aberrant autophagy and neuronal damage caused by LRRK2 G2019S mutation. Hum Mol Genet. 2013;22:4545–4561. doi: 10.1093/hmg/ddt301. [DOI] [PubMed] [Google Scholar]
- Subramaniam SR, Vergnes L, Franich NR, Reue K, Chesselet MF. Region specific mitochondrial impairment in mice with widespread overexpression of alpha-synuclein. Neurobiol Dis. 2014;70:204–213. doi: 10.1016/j.nbd.2014.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumi-Akamaru H, Beck G, Kato S, Mochizuki H. Neuroaxonal dystrophy in PLA2G6 knockout mice. Neuropathology. 2015;35:289–302. doi: 10.1111/neup.12202. [DOI] [PubMed] [Google Scholar]
- Sumi-Akamaru H, Beck G, Shinzawa K, Kato S, Riku Y, Yoshida M, Fujimura H, Tsujimoto Y, Sakoda S, Mochizuki H. High expression of alpha-synuclein in damaged mitochondria with PLA2G6 dysfunction. Acta Neuropathol Commun. 2016;4:27. doi: 10.1186/s40478-016-0298-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surmeier DJ, Guzman JN, Sanchez-Padilla J. Calcium, cellular aging, and selective neuronal vulnerability in Parkinson’s disease. Cell Calcium. 2010;47:175–182. doi: 10.1016/j.ceca.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart T, Hurley MJ. Calcium Channel Antagonists as Disease-Modifying Therapy for Parkinson’s Disease: Therapeutic Rationale and Current Status. CNS Drugs. 2016;30:1127–1135. doi: 10.1007/s40263-016-0393-9. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Davis JN, 2nd, Cassarino DS, Trimmer PA, Currie LJ, Dougherty J, Bridges WS, Bennett JP, Jr, Wooten GF, Parker WD. Matrilineal inheritance of complex I dysfunction in a multigenerational Parkinson’s disease family. Ann Neurol. 1998;44:873–881. doi: 10.1002/ana.410440605. [DOI] [PubMed] [Google Scholar]
- Swerdlow RH, Parks JK, Miller SW, Tuttle JB, Trimmer PA, Sheehan JP, Bennett JP, Jr, Davis RE, Parker WD., Jr Origin and functional consequences of the complex I defect in Parkinson’s disease. Ann Neurol. 1996;40:663–671. doi: 10.1002/ana.410400417. [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Simoni AM, Bianchi K, De Stefani D, Leo S, Wieckowski MR, Rizzuto R. Mitochondrial dynamics and Ca2+ signaling. Biochim Biophys Acta. 2006;1763:442–449. doi: 10.1016/j.bbamcr.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Taira T, Saito Y, Niki T, Iguchi-Ariga SM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO Rep. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A, Cleland MM, Xu S, Narendra DP, Suen DF, Karbowski M, Youle RJ. Proteasome and p97 mediate mitophagy and degradation of mitofusins induced by Parkin. J Cell Biol. 2010;191:1367–1380. doi: 10.1083/jcb.201007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang FL, Liu W, Hu JX, Erion JR, Ye J, Mei L, Xiong WC. VPS35 Deficiency or Mutation Causes Dopaminergic Neuronal Loss by Impairing Mitochondrial Fusion and Function. Cell Rep. 2015;12:1631–1643. doi: 10.1016/j.celrep.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner CM, Kamel F, Ross GW, Hoppin JA, Goldman SM, Korell M, Marras C, Bhudhikanok GS, Kasten M, Chade AR, Comyns K, Richards MB, Meng C, Priestley B, Fernandez HH, Cambi F, Umbach DM, Blair A, Sandler DP, Langston JW. Rotenone, paraquat, and Parkinson’s disease. Environ Health Perspect. 2011;119:866–872. doi: 10.1289/ehp.1002839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B, Banerjee R, Starkova NN, Zhang SF, Calingasan NY, Yang L, Wille E, Lorenzo BJ, Ho DJ, Beal MF, Starkov A. Mitochondrial permeability transition pore component cyclophilin D distinguishes nigrostriatal dopaminergic death paradigms in the MPTP mouse model of Parkinson’s disease. Antioxid Redox Signal. 2012;16:855–868. doi: 10.1089/ars.2010.3849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B, Mandir AS, West N, Liu Y, Andrabi SA, Stirling W, Dawson VL, Dawson TM, Lee MK. Resistance to MPTP-neurotoxicity in alpha-synuclein knockout mice is complemented by human alpha-synuclein and associated with increased beta-synuclein and Akt activation. PLoS One. 2011;6:e16706. doi: 10.1371/journal.pone.0016706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B, von Coelln R, Mandir AS, Trinkaus DB, Farah MH, Leong Lim K, Calingasan NY, Flint Beal M, Dawson VL, Dawson TM. MPTP and DSP-4 susceptibility of substantia nigra and locus coeruleus catecholaminergic neurons in mice is independent of parkin activity. Neurobiol Dis. 2007;26:312–322. doi: 10.1016/j.nbd.2006.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyagarajan D, Bressman S, Bruno C, Przedborski S, Shanske S, Lynch T, Fahn S, DiMauro S. A novel mitochondrial 12SrRNA point mutation in parkinsonism, deafness, and neuropathy. Ann Neurol. 2000;48:730–736. [PubMed] [Google Scholar]
- Tieu K, Perier C, Caspersen C, Teismann P, Wu DC, Yan SD, Naini A, Vila M, Jackson-Lewis V, Ramasamy R, Przedborski S. D-beta-hydroxybutyrate rescues mitochondrial respiration and mitigates features of Parkinson disease. J Clin Invest. 2003;112:892–901. doi: 10.1172/JCI18797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tondera D, Grandemange S, Jourdain A, Karbowski M, Mattenberger Y, Herzig S, Da Cruz S, Clerc P, Raschke I, Merkwirth C, Ehses S, Krause F, Chan DC, Alexander C, Bauer C, Youle R, Langer T, Martinou JC. SLP-2 is required for stress-induced mitochondrial hyperfusion. EMBO J. 2009;28:1589–1600. doi: 10.1038/emboj.2009.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsigelny IF, Sharikov Y, Wrasidlo W, Gonzalez T, Desplats PA, Crews L, Spencer B, Masliah E. Role of alpha-synuclein penetration into the membrane in the mechanisms of oligomer pore formation. FEBS J. 2012;279:1000–1013. doi: 10.1111/j.1742-4658.2012.08489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero T. Mitochondrial biogenesis: pharmacological approaches. Curr Pharm Des. 2014;20:5507–5509. doi: 10.2174/138161282035140911142118. [DOI] [PubMed] [Google Scholar]
- Vermulst M, Wanagat J, Kujoth GC, Bielas JH, Rabinovitch PS, Prolla TA, Loeb LA. DNA deletions and clonal mutations drive premature aging in mitochondrial mutator mice. Nat Genet. 2008;40:392–394. doi: 10.1038/ng.95. [DOI] [PubMed] [Google Scholar]
- Vilain S, Esposito G, Haddad D, Schaap O, Dobreva MP, Vos M, Van Meensel S, Morais VA, De Strooper B, Verstreken P. The yeast complex I equivalent NADH dehydrogenase rescues pink1 mutants. PLoS Genet. 2012;8:e1002456. doi: 10.1371/journal.pgen.1002456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villeneuve LM, Purnell PR, Boska MD, Fox HS. Early Expression of Parkinson’s Disease-Related Mitochondrial Abnormalities in PINK1 Knockout Rats. Mol Neurobiol. 2016;53:171–186. doi: 10.1007/s12035-014-8927-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visanji NP, Brotchie JM, Kalia LV, Koprich JB, Tandon A, Watts JC, Lang AE. alpha-Synuclein-Based Animal Models of Parkinson’s Disease: Challenges and Opportunities in a New Era. Trends Neurosci. 2016;39:750–762. doi: 10.1016/j.tins.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Vitte J, Traver S, Maues De Paula A, Lesage S, Rovelli G, Corti O, Duyckaerts C, Brice A. Leucine-rich repeat kinase 2 is associated with the endoplasmic reticulum in dopaminergic neurons and accumulates in the core of Lewy bodies in Parkinson disease. J Neuropathol Exp Neurol. 2010;69:959–972. doi: 10.1097/NEN.0b013e3181efc01c. [DOI] [PubMed] [Google Scholar]
- Vives-Bauza C, Andreu AL, Manfredi G, Beal MF, Janetzky B, Gruenewald TH, Lin MT. Sequence analysis of the entire mitochondrial genome in Parkinson’s disease. Biochem Biophys Res Commun. 2002;290:1593–1601. doi: 10.1006/bbrc.2002.6388. [DOI] [PubMed] [Google Scholar]
- Wang QM, Xu YY, Liu S, Ma ZG. Isradipine attenuates MPTP-induced dopamine neuron degeneration by inhibiting up-regulation of L-type calcium channels and iron accumulation in the substantia nigra of mice. Oncotarget. 2017;8:47284–47295. doi: 10.18632/oncotarget.17618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wang X, Fujioka H, Hoppel C, Whone AL, Caldwell MA, Cullen PJ, Liu J, Zhu X. Parkinson’s disease-associated mutant VPS35 causes mitochondrial dysfunction by recycling DLP1 complexes. Nat Med. 2016;22:54–63. doi: 10.1038/nm.3983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Petrie TG, Liu Y, Liu J, Fujioka H, Zhu X. Parkinson’s disease-associated DJ-1 mutations impair mitochondrial dynamics and cause mitochondrial dysfunction. J Neurochem. 2012a;121:830–839. doi: 10.1111/j.1471-4159.2012.07734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Schwarz TL. The mechanism of Ca2+-dependent regulation of kinesin-mediated mitochondrial motility. Cell. 2009;136:163–174. doi: 10.1016/j.cell.2008.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Winter D, Ashrafi G, Schlehe J, Wong YL, Selkoe D, Rice S, Steen J, LaVoie MJ, Schwarz TL. PINK1 and Parkin target Miro for phosphorylation and degradation to arrest mitochondrial motility. Cell. 2011;147:893–906. doi: 10.1016/j.cell.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Yan MH, Fujioka H, Liu J, Wilson-Delfosse A, Chen SG, Perry G, Casadesus G, Zhu X. LRRK2 regulates mitochondrial dynamics and function through direct interaction with DLP1. Hum Mol Genet. 2012b;21:1931–1944. doi: 10.1093/hmg/dds003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisskopf MG, O’Reilly E, Chen H, Schwarzschild MA, Ascherio A. Plasma urate and risk of Parkinson’s disease. Am J Epidemiol. 2007;166:561–567. doi: 10.1093/aje/kwm127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West AB, Moore DJ, Biskup S, Bugayenko A, Smith WW, Ross CA, Dawson VL, Dawson TM. Parkinson’s disease-associated mutations in leucine-rich repeat kinase 2 augment kinase activity. Proc Natl Acad Sci U S A. 2005;102:16842–16847. doi: 10.1073/pnas.0507360102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wooten GF, Currie LJ, Bennett JP, Harrison MB, Trugman JM, Parker WD., Jr Maternal inheritance in Parkinson’s disease. Ann Neurol. 1997;41:265–268. doi: 10.1002/ana.410410218. [DOI] [PubMed] [Google Scholar]
- Writing Group for the N.E.T.i.P.D.I. Kieburtz K, Tilley BC, Elm JJ, Babcock D, Hauser R, Ross GW, Augustine AH, Augustine EU, Aminoff MJ, Bodis-Wollner IG, Boyd J, Cambi F, Chou K, Christine CW, Cines M, Dahodwala N, Derwent L, Dewey RB, Jr, Hawthorne K, Houghton DJ, Kamp C, Leehey M, Lew MF, Liang GS, Luo ST, Mari Z, Morgan JC, Parashos S, Perez A, Petrovitch H, Rajan S, Reichwein S, Roth JT, Schneider JS, Shannon KM, Simon DK, Simuni T, Singer C, Sudarsky L, Tanner CM, Umeh CC, Williams K, Wills AM. Effect of creatine monohydrate on clinical progression in patients with Parkinson disease: a randomized clinical trial. JAMA. 2015;313:584–593. doi: 10.1001/jama.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Chung KK. Alpha-synuclein impairs normal dynamics of mitochondria in cell and animal models of Parkinson’s disease. J Neurochem. 2012;122:404–414. doi: 10.1111/j.1471-4159.2012.07769.x. [DOI] [PubMed] [Google Scholar]
- Yoon Y, Pitts KR, Dahan S, McNiven MA. A novel dynamin-like protein associates with cytoplasmic vesicles and tubules of the endoplasmic reticulum in mammalian cells. J Cell Biol. 1998;140:779–793. doi: 10.1083/jcb.140.4.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SP, Kim D, Kim S, Kim S, Karuppagounder SS, Kwon SH, Lee S, Kam TI, Lee S, Ham S, Park JH, Dawson VL, Dawson TM, Lee Y, Ko HS. alpha-Synuclein accumulation and GBA deficiency due to L444P GBA mutation contributes to MPTP-induced parkinsonism. Mol Neurodegener. 2018;13:1. doi: 10.1186/s13024-017-0233-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Mileykovskaya E, Dowhan W. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J Biol Chem. 2002;277:43553–43556. doi: 10.1074/jbc.C200551200. [DOI] [PubMed] [Google Scholar]
- Zhang N, Shu HY, Huang T, Zhang QL, Li D, Zhang GQ, Peng XY, Liu CF, Luo WF, Hu LF. Nrf2 signaling contributes to the neuroprotective effects of urate against 6-OHDA toxicity. PLoS One. 2014;9:e100286. doi: 10.1371/journal.pone.0100286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Severijnen LA, van der Weiden M, Zheng PP, Oostra BA, Hukema RK, Willemsen R, Kros JM, Bonifati V. FBXO7 immunoreactivity in alpha-synuclein-containing inclusions in Parkinson disease and multiple system atrophy. J Neuropathol Exp Neurol. 2013;72:482–488. doi: 10.1097/NEN.0b013e318293c586. [DOI] [PubMed] [Google Scholar]
- Zheng B, Liao Z, Locascio JJ, Lesniak KA, Roderick SS, Watt ML, Eklund AC, Zhang-James Y, Kim PD, Hauser MA, Grunblatt E, Moran LB, Mandel SA, Riederer P, Miller RM, Federoff HJ, Wullner U, Papapetropoulos S, Youdim MB, Cantuti-Castelvetri I, Young AB, Vance JM, Davis RL, Hedreen JC, Adler CH, Beach TG, Graeber MB, Middleton FA, Rochet JC, Scherzer CR, Global PDGEC. PGC-1alpha, a potential therapeutic target for early intervention in Parkinson’s disease. Sci Transl Med. 2010;2:52ra73. doi: 10.1126/scitranslmed.3001059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZD, Sathiyamoorthy S, Angeles DC, Tan EK. Linking F-box protein 7 and parkin to neuronal degeneration in Parkinson’s disease (PD) Mol Brain. 2016;9:41. doi: 10.1186/s13041-016-0218-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou ZD, Xie SP, Sathiyamoorthy S, Saw WT, Sing TY, Ng SH, Chua HP, Tang AM, Shaffra F, Li Z, Wang H, Ho PG, Lai MK, Angeles DC, Lim TM, Tan EK. F-box protein 7 mutations promote protein aggregation in mitochondria and inhibit mitophagy. Hum Mol Genet. 2015;24:6314–6330. doi: 10.1093/hmg/ddv340. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Duan C, Lu L, Gao H, Zhao C, Yu S, Ueda K, Chan P, Yang H. alpha-Synuclein overexpression impairs mitochondrial function by associating with adenylate translocator. Int J Biochem Cell Biol. 2011;43:732–741. doi: 10.1016/j.biocel.2011.01.014. [DOI] [PubMed] [Google Scholar]
- Zigoneanu IG, Yang YJ, Krois AS, Haque E, Pielak GJ. Interaction of alpha-synuclein with vesicles that mimic mitochondrial membranes. Biochim Biophys Acta. 2012;1818:512–519. doi: 10.1016/j.bbamem.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimprich A, Benet-Pages A, Struhal W, Graf E, Eck SH, Offman MN, Haubenberger D, Spielberger S, Schulte EC, Lichtner P, Rossle SC, Klopp N, Wolf E, Seppi K, Pirker W, Presslauer S, Mollenhauer B, Katzenschlager R, Foki T, Hotzy C, Reinthaler E, Harutyunyan A, Kralovics R, Peters A, Zimprich F, Brucke T, Poewe W, Auff E, Trenkwalder C, Rost B, Ransmayr G, Winkelmann J, Meitinger T, Strom TM. A mutation in VPS35, encoding a subunit of the retromer complex, causes late-onset Parkinson disease. Am J Hum Genet. 2011;89:168–175. doi: 10.1016/j.ajhg.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorov DB, Juhaszova M, Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]