Abstract
Trauma can affect any individual at any location and at any time over a lifespan. The disruption of macrobarriers and microbarriers induces instant activation of innate immunity. The subsequent complex response, designed to limit further damage and induce healing, also represents a major driver of complications and fatal outcome after injury. This Review aims to provide basic concepts about the posttraumatic response and is focused on the interactive events of innate immunity at frequent sites of injury: the endothelium at large, and sites within the lungs, inside and outside the brain and at the gut barrier.
An initial traumatic insult disrupts macrobarriers such as the skin, as well as microbarriers such as cell membranes, which causes the release of multiple danger molecules. This disruption constitutes the beginning of a rapidly activated innate immune response1 that aims to end the dangerous situation for the recipient of the traumatic insult but can also result in severe complications and death2–5. The innate immune response after severe tissue trauma or life-threatening multiple injury (polytrauma) results in a multi-faceted systemic disease with a complex and heterogeneous, although mainly compartmentalized, response6–8. To cover the organ systems most frequently affected by severe trauma—the head, chest and abdomen (in descending prevalence)9,10—this Review addresses mainly those systems, plus the endothelium as a ‘meta-organ’, and their interrelated changes in innate immunity after trauma.
Protective and harmful innate immune responses to trauma
Trauma elicits a series of rapid innate immune responses (Fig. 1), in an attempt to clear damaged tissues, that is followed by the activation of repair mechanisms, with the ultimate goal of restoring cells and tissues to their pre-injury state11,12. Severe injury can be associated with the presence of ‘non-self’ pathogen-associated molecular patterns (PAMPs) from infectious agents (bacteria, viruses and fungi), along with the release of large amounts of ‘self’ damage-associated molecular patterns (DAMPs) such as ATP, HMGB-1, matricryptins, cold-inducible RNA-binding protein, histones and mitochondrial DNA13–17.
Fig. 1. Protective and harmful innate immune responses to trauma.
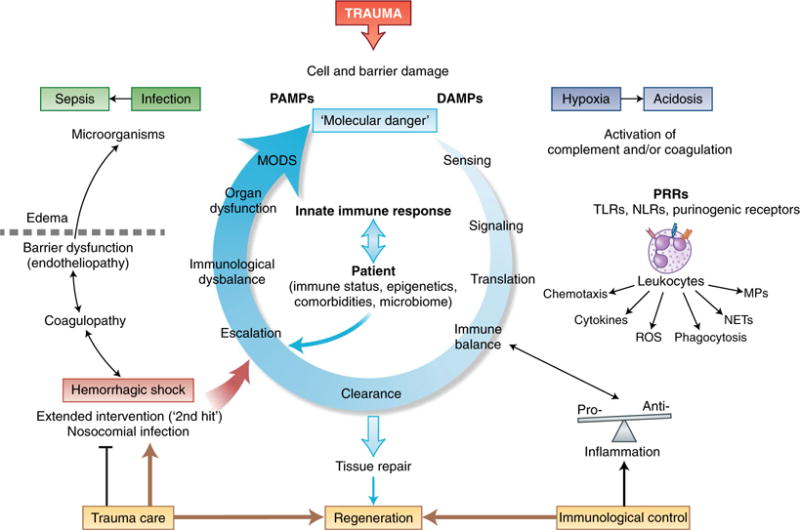
Trauma leads to the damage of external and Internal barriers and thus exposes the Immune system to DAMPs and PAMPs. Molecular danger signals and the destruction of local barriers are sensed by the complement and the coagulation systems and induce intracellular signaling in leukocytes via PRRs, which leads to translation into an instantaneous cellular immune response. Ideally, a balanced pro-inflammatory and anti-inflammatory reaction leads to rapid clearance of debris and the induction of effective tissue repair and regeneration; adverse events can be caused by individual factors of the patient or aggravated tissue damage after hemorrhage, nosocomial infection or extended surgical intervention. Escalation of the innate immune response in the form of coagulopathy and excessive inflammation leads to barrier disturbance, edema formation and compromised innate defense against invading microorganisms. Such changes can aggravate hypoxic conditions, the accumulation of metabolites and bacterial invasion, all of which can ‘feed in’ more DAMPs and PAMPs and thus generate a vicious cycle of the innate immune response. This eventually results in organ dysfunction and systemic infection, which emphasizes the importance of damage-adjusted trauma-care principles as well as control of the balance of the immune system, particularly in the acute phase after injury. MPs, microparticles.
The molecular danger signals noted above can be sensed by inflammatory fluid-phase pathways that contain proteins or lipids and participate in the so-called ‘first line of defense’. In particular, the serine protease system, composed of the kinin, coagulation and complement cascades, can detect DAMPs and PAMPs, become rapidly activated after trauma18,19 and be further bolstered in acidic (for example, hypoxic) microenvironments20. Either directly or via such activated systems, DAMPs and PAMPs can transmit their signals to leukocytes through pattern-recognition receptors (PRRs) such as TLRs, NLRs, RAGE, purinergic receptors or complement receptors11,12,21.
After severe trauma, a ‘genetic storm’ and functional reprioritizing of leukocytes have been described22 that seem in other studies to be unique to each injury pattern23. Overall, the cellular translation results in largely balanced pro-inflammatory and anti-inflammatory protective effects mediated by targeted chemotaxis, cytokine release (with the systemic appearance of, for example, IL-6, IL-8, IL-1Ra and IL-10), the generation of reactive oxygen species (ROS), phagocytosis, the formation of neutrophil extracellular traps (NETs) and the killing of bacteria6,24–28. The release of microvesicles from leukocytes can also enhance leukocyte adhesion and systemic inflammation and promote activation of the clotting system as a strategy for containing hemorrhage29,30. Of note, the systemic inflammatory response comprises not only multiple immune system-activating features but also considerably suppressive features that evolve within minutes or hours after trauma25,26,28. This balanced systemic inflammatory response is designed to clear the molecular danger and to induce tissue-repair mechanisms for healing—for example, by reprogramming macrophages from the pro-inflammatory M1 phenotype to the anti-inflammatory M2 phenotype31. Even extravasated neutrophils can help resolve inflammation and initiate regenerative processes12,25,32.
However, if the severity of the initial injury is substantial or is associated with hemorrhagic shock and/or extended surgical intervention, the innate immune response can become imbalanced6,14 with consequences that include dysregulated cascade systems (for example, acute trauma-induced complementopathy and coagulopathy)18,33 and reprogrammed and rapidly suppressed immunological function (for example, decreased expression of HLA-DR in macrophages)6,11,14,25,26,28. The inflammatory response can alter its kinetics34 and lose compartmentalization and diversity and thus become overwhelming. This ‘cytokine storm’ can lead to alterations in Na+-K+ ATPases and thereby result in electrophysiological membrane dysfunction35.
Factors that disturb an effective innate immune response include nosocomial infections, immunocompromising comorbidities and unfavorable epigenetic or microbiome perturbations. In addition, programmed cell death as a defense strategy against cellular damage and infection36 can be disturbed in immune cells. Neutrophils, for example, exhibit a prolonged lifetime soon after injury, and this prolongation can increase their autoaggressive potential37. On the other hand, lymphocytes and crypt intestinal epithelial cells can be driven to apoptosis after severe trauma38. Furthermore, an inability to normalize posttraumatic lymphopenia in a timely fashion is associated with a poor outcome, irrespective of the dynamics of the leukocytosis39. Excessive immune, coagulatory and ROS responses can lead to substantial endotheliopathy and dysfunction of cellular barriers40 that facilitate the transit and generation of more PAMPs and DAMPs, which amplifies a vicious cycle of tissue injury and damaging immunological processes6,11,14. In the acute situation, infection, sepsis and early multiple-organ-dysfunction syndrome (MODS) are clinical manifestations of those harmful immune responses. In the long term, beyond 14 days after trauma, patients receiving modern management in the intensive care unit might survive the consequences of trauma and not develop late MODS. However, such patients often show signs of persistent inflammation-immunosuppressive catabolism syndrome (PICS), which, in addition to being associated with ongoing protein catabolism, is associated with innate and adaptive immunosuppressive features41. These consist mainly of reduced generation of cytokines, loss of monocyte-macrophage function, a persistently increased number of myeloid-derived suppressor cells (MDSCs) and a reduction in the number and function of effector T cells. The PICS-based immunological-metabolic suppression correlates with poor wound healing, infections, a long decline and a high mortality rate41.
It is therefore vital in such circumstances that an immunological balance be re-established as early as possible. Clinically, various controversial trauma-care concepts have been developed to limit additional tissue damage caused by surgery42,43 that are intended to inhibit the further generation of DAMPs and PAMPs and an escalation of a damaging immune response. Damage-control orthopedic surgery (DCO)44 represents one example of the clinical realization of an immunological rationale. DCO has been directly connected to a diminished inflammatory response during the initial stage after polytrauma44. Early total care, in contrast, seems reasonable in hemodynamically stable patients but sets the more severely injured patients at increased risk of developing acute lung injury45. In the context of posttraumatic complications, however, more than one decade after the initial studies, DCO does not appear to be superior to early total care46, a result most probably due to improved minimally invasive techniques that minimize iatrogenic tissue damage and thereby restrict the immune response and allow novel strategies such as prompt, individualized, safe management47. Overall, it is important to realize that trauma care can be supportive or detrimental to the immune response. The development of control principles of the early immune response might beneficially support surgical and intensive-care strategies.
Interaction of innate immunity with the endothelium after trauma
The innate (fluid-phase and cellular) immune system and the autonomic nervous system (ANS) interact closely with the endothelium, the body’s largest, although functionally heterogeneous, ‘organ’48. Any injury instantly elicits a global stress reaction involving the ANS49,50 that modulates not only innate immune responses but also organ function and hemodynamics (Fig. 2). Activation of the sympathoadrenal system during hemorrhagic shock induces profound vasoconstriction that is counteracted by the release of vasodilatory nitric oxide from stressed and damaged endothelial cells, which leads to microcirculatory disturbances. The coagulation and complement systems and interacting platelets are rapidly activated after trauma18,19,51, which results in a stemming of hemorrhage and protection against invading bacteria. Of note, injured patients prospectively followed for the development of either a ‘sterile’ systemic inflammatory response or sepsis exhibited differential expression of plasma proteins in each sequela. Nearly 25% of those plasma proteins have been mapped to either the coagulation cascade or complement cascade and appeared well in advance of the clinical manifestation of complications52.
Fig. 2. Activation of innate immune responses and endothelial dysfunction after trauma.
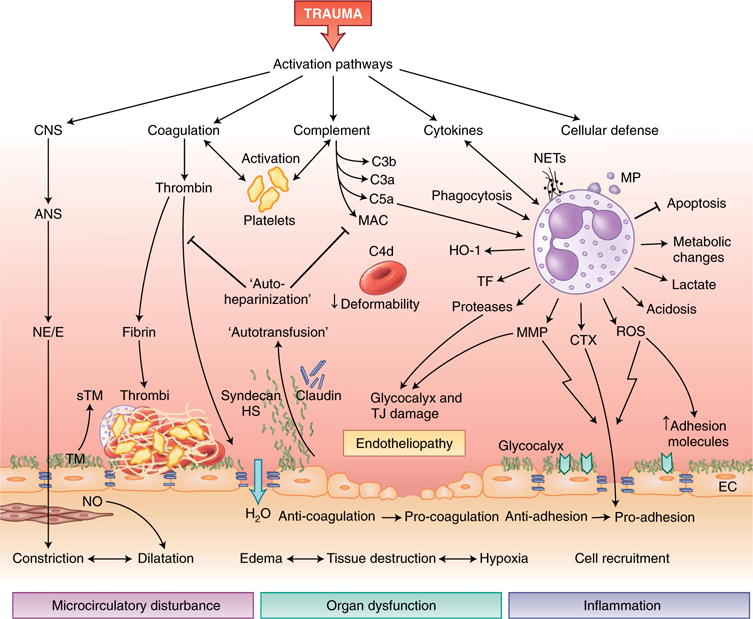
After trauma, various pathways of innate immunity can induce posttraumatic endotheliopathy and further tissue damage. Activation of the ANS and its systemic release of norepinephrine and/or epinephrine (NE/E) leads to instant vasoconstriction (centralization), activates the endothelium and induces the release of thrombomodulin (TM), which thereby diminishes the anticoagulant features of the endothelium. The dilation of subendothelial smooth muscle cells through stimulation with nitric oxide (NO) potentiates microcirculatory disturbances and hypoxia. Cleavage of thrombin during activation of the clotting cascade leads to the formation of a microthrombus on the endothelial surface and loosening of intercellular barriers, with efflux of water (H2O) into the interstitial tissues. Activated platelets, in concert with products of the activation of coagulation and/or complement and leukocytes, form the thromboinflammatory response. The activation of complement on red blood cells compromises their deformability. The activation of innate leukocytes, particularly neutrophil granulocytes, by complement and proinflammatory cytokines creates an overall pro-inflammatory microenvironment with released NETs and MPs, reduced apoptosis, and metabolic changes that lead to local generation of lactate. The generation of ROS and matrix metalloproteinase (MMP) increases endothelial expression of adhesion molecules and widens cell-cell junctions, which facilitate the migration of leukocytes into inflamed tissue. Proteases secreted from leukocytes can damage the glycocalyx layer and TJs, which leads to the intravascular release of glycosaminoglycans that exhibit colloidal-osmotic and heparin-like effects. CNS, central nervous system; sTM, soluble thrombomodulin; HS, heparan sulfate; HO-1, heme oxygenase-1; CTX, chemotaxis; EC, endothelial cell.
In patients who have suffered trauma, complement-activation products are deposited not only on bacteria, debris and platelets but also on the surface of erythrocytes; this suppresses cellular deformability and consequently oxygen delivery53. Tissue factor (TF) released by damaged tissues and also expressed on leukocytes, for which inflammatory mediators such as TNF and C5a act as a ‘pro-coagulatory switch’54, triggers the generation of thrombin. Thrombin, in turn, activates fibrinogen to form thrombi with platelets and also activates endothelial cells, which leads to the endothelial release of cytokines, cell contraction, enhancement of permeability and expression of adhesion molecules48. The consequences are vascular inflammation, (micro)perfusion disturbances and hypoxia, all of which aggravate the thromboinflammatory response51,55. Trauma-generated DAMPs (for example, mitochondrial DNA) and ROS also induce the endothelial expression of adhesion molecules that facilitate leukocyte adhesion56, which promotes extravasation into injured tissue that is controlled by vascular endothelial cadherin57.
Neutrophils interacting at the endothelial barrier are beneficially involved in the release of cytokines and chemokines, the engulfment and digestion of tissue debris as well as pathogens, and the formation of NETs58. Furthermore, these cells activate the heme-degradation pathway (with enhanced expression of haptoglobin and hemoxygenase-1) in order to clear the free heme as a DAMP that originates from the hemoglobin of damaged erythrocytes after polytrauma with septic complications59. Overall, after severe tissue trauma and hemorrhagic shock, the endothelium switches spatially and temporally from an anti-adhesive phenotype to a pro-adhesive phenotype and from an anti-coagulatory response to a pro-coagulatory response60,61.
Ischemic and inflammatory conditions often result in the disruption of both tight junctions (TJs) (for example, claudins) and glycocalyx components (for example, glucosaminoglycans (GAGs)), as well as their being shed into the intravascular space, as detected very early in clinical and experimental polytrauma62–65. In addition, the abundance of the bioactive lipid S1P, a preserver of endothelial integrity, is diminished in the plasma of patients who have suffered polytrauma; this unleashes metalloproteinase activity that contributes to damage to protective GAGs66. The resultant increasingly leaky endothelial barrier defines trauma-induced endotheliopathy50,61,64, which promotes the development of edema that hinders oxygen transport and aggravates hypoxic conditions67. The shed GAGs, such as syndecan-1 and heparan sulfate, can temporarily stabilize hemodynamics after trauma (‘autotransfusion’)61, exhibit heparinlike anti-coagulatory effects (‘autoheparinization’)64,68 and inhibit the antibacterial activity of plasma and thereby promote infection69.
Early after polytrauma, the interaction of innate immunity and the endothelium can become hyperactivated, especially in the presence of an additional hemorrhagic shock65. The defensive features of neutrophils can then result in the excessive release of proteases, massive generation of ROS, shedding of pro-inflammatory microparticles29,30, antiapoptotic features37, a prothrombotic surface29 and immuno-metabolic changes that generate a lactate-acidic microenvironment, as has been shown both in vitro and in vivo after exposure to C5a70. The energy metabolism of leukocytes can shift from oxidative phosphorylation toward aerobic glycolysis (the Warburg effect), as has been found during septic complications after tissue trauma and infection70,71. This shift also forms the metabolic basis of ‘trained innate immunity’ for monocytes and macrophages70–72 for attaining an enhanced and prolonged functional state after a second contact with a DAMP or PAMP, which represents a memory process mediated by epigenetic reprogramming dependent on the hypoxia-inducible transcription factor HIF-1α72. The immuno-metabolic response can also lead to the accumulation of metabolites that modulate epigenetic enzymes, which in turn can reprogram the immune response—for example, by histone modification, as seen in sepsis73. Overall, these dramatic immunological changes can lead to further dysfunction of the endothelium and tissue damage to associated organs.
Innate immune responses in the lungs following trauma
Acute lung injury (ALI), whether caused directly by a blunt or penetrating traumatic impact to the chest and lungs or as a common consequence of remote organ injury and/or hemorrhagic shock, is a leading cause of adult respiratory distress syndrome (ARDS), morbidity and death in patients who have suffered trauma74. Direct pulmonary trauma or contusion disrupts lung tissue and vessels, followed by immediate bleeding into the bronchoalveolar space and pulmonary interstitium, which immediately results in compromised oxygenation and a decrease in the ratio of the partial pressure of arterial oxygen to the fraction of inspired oxygen (paO2/FiO2). This disruption leads to rapid activation of the coagulation system and thrombus formation, which compromises both perfusion and gas exchange (Fig. 3). The complement system is synchronously activated, which generates C5a, whose pulmonary concentrations in patients who have suffered trauma are correlated with the volume of lung contusion75. Blockade of thrombin generation in experimental traumatic ALI inhibits the pulmonary formation of C5a and accumulation of neutrophils in the alveoli75. In both primary lung injury and secondary lung injury, complement-induced upregulation of leukocyte and endothelial adhesion molecules (for example, ICAM-1), together with a degraded pulmonary endothelial glycocalyx, results in the recruitment of leukocytes to the damaged area, a hallmark of ALI and ARDS76,77. After being recruited, neutrophils deploy their arsenal for the clearance of DAMPs and PAMPs: they release inflammatory mediators and proteases, generate ROS, remove phagocyte debris and form NETs78. However, neutrophils undergo early apoptosis and accumulate after pulmonary contusion; after their death, they are removed by alveolar macrophages77 and the inflammation is resolved. Interestingly, neutrophils can also switch to an anti-inflammatory phenotype together with alveolar macrophages79.
Fig. 3. Innate immune responses in the lungs following trauma.
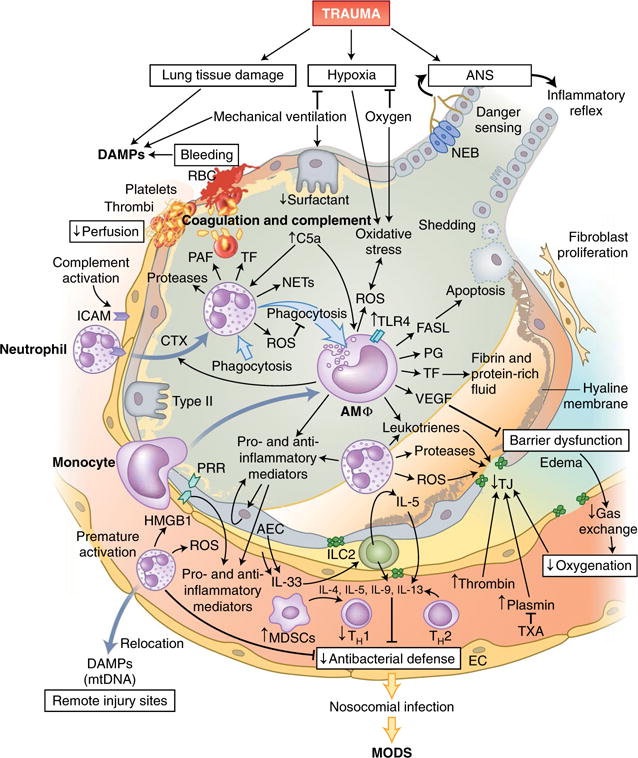
Tissue damage in the lungs and requisite mechanic ventilation lead to the release of large amounts of endogenous DAMPs, while bleeding and clot formation hinder the perfusion of alveolar capillaries. Leukocytes, primarily neutrophils and subsequently monocytes, which differentiate into macrophages on-site, invade the alveolar space via the activated endothelium and secrete inflammatory mediators and pro-coagulatory factors. Cellular debris and apoptotic neutrophils are removed by phagocytosis, which can induce a shift in macrophages from a pro-inflammatory phenotype to an anti-inflammatory phenotype. Alveolar macrophages (AMΦ) can also modulate the apoptosis of alveolar epithelial cells by secretion of the ligand for the death receptor Fas (FASL). Barrier degradation by neutrophil-derived proteases and ROS induces disruption of the air-blood-barrier, formation of edema in the extra-alveolar space, intra-alveolar accumulation of protein-rich fluids and the formation of hyaline membranes and thereby impairs gas exchange and blood oxygenation. Proteases of the coagulation cascade amplify the breakdown of TJs, which can be partially prevented by therapeutic application of the antifibrinolytic tranexamic acid (TXA). Furthermore, the local inflammatory response can be augmented by the recognition of DAMPs, including HMGB-1, by PRRs on alveolar endothelial and epithelial cells, which in turn release more DAMPs and mediators into alveoli and blood vessels. However, prematurely activated neutrophils are strongly attracted by other DAMPs, including mitochondrial DNA (mtDNA), and can relocate to remote injury sites and thus impair the local pulmonary antibacterial defense. IL-33 secreted by activated epithelial and endothelial lung cells activates group 2 ILCs (ILC2), which, via IL-5 production and a feed-forward loop, induce the secretion of more IL-5; this enhances a type 2 cytokine profile (IL-4, IL-5, IL-9 and IL-13) and further diminishes antibacterial potential. RBC, red blood cells; PAF, platelet-activating factor; Type II, type II alveolar epithelial cells; AEC, alveolar epithelial cells; NEB, neuroepithelial bodies; PG, prostaglandin; VEGF, vascular endothelial growth factor.
Overall, alveolar macrophages function as conductors of the orchestrated immune response after trauma77,80. They sense DAMPs and PAMPs and extracellular matrix fragments such as hyaluronan81 via PRRs and react to pulmonary and extra-pulmonary trauma with initially accentuated pro-inflammatory and pro-coagulatory responses. After lung contusion, alveolar macrophages upregulate their expression of the innate immunoreceptor TLR4 to bolster defenses against bacteria, generate ROS (particularly in an ischemic microenvironment), produce TF that contributes to fibrin formation, and release inflammatory mediators such as IL-1β, IL-6 and leukotrienes, which further amplifies the inflammation and damages the air-blood barrier. Furthermore, alveolar macrophages can induce the apoptosis of neutrophils and alveolar epithelial type II cells77,79,80.
In concert with neutrophils, alveolar macrophages can, independent of the initial injurious or infectious trigger(s), also contribute to the development of dysfunction of the air-blood barrier by releasing ROS, proteases and edemagenic mediators such as TF, thrombin, platelet-activating factor and IL-1β, all of which compromise alveolar and endothelial TJ biology. The results of this release are leakage, the development of edema, diminished gas exchange and hypoxemia, which in turn contribute to epithelial and endothelial barrier damage80.
Alveolar macrophages are highly ‘immuno-plastic’ regarding their immunological characteristics; depending on the posttraumatic microenvironment and the progress made in debris clearance, they can actively switch to an anti-inflammatory phenotype79. In this context, the generation of lipid mediators (such as lipoxins) induced by prostaglandins (for example, PGE2) promotes the phagocytosis of apoptotic neutrophils, inhibits the release of inflammatory mediators and induces tissue remodeling80. However, neutrophils inhibit the anti-inflammatory mechanisms of alveolar macrophages in a TLR-dependent fashion and thereby maintain lung inflammation after hemorrhagic shock82. The phagocytosis of apoptotic cells also results in alveolar macrophage-produced vascular endothelial growth factor, which helps repair damaged air-blood barriers80. In addressing the extra-pulmonary effects of lipopolysaccharide-induced ALI, alveolar macrophages communicate with respiratory epithelial cells not only via mediator signaling but also through direct cell-cell coupling (via the gap-junction channel connexin 43) to synchronize the anti-inflammatory responses83.
The respiratory epithelium, as an interface with the environment, also contributes substantially to the innate immune response after traumatic ALI. The airway epithelium secretes multiple antimicrobial peptides and mucus to enable mucociliary clearance of debris and bacteria. Whereas mucus production is massively enhanced during ventilator-associated ALI84, the generation of the antimicrobial peptides is compromised after severe tissue trauma. Physiologically, alveolar type II cells produce surfactant lipids to prevent alveolar collapse. These surfactants also incorporate several host-defense molecules (for example, members of the collectin family and tubular myelin) that improve both the opsonization and the clearance of PAMPs85. However, after lung contusion and during extended periods of ventilation, the quantity and quality of the surfactant, and thus its innate immunological defense properties, are considerably impaired86,87.
The alveolar epithelium expresses several PRRs (for example, TLR2 and TLR4, which are predominantly apical and basolateral, respectively), with corresponding downstream signaling. After exposure to trauma-induced DAMPs (such as HMGB-1, hyaluronan, the heat-shock protein HSP70 and surfactant protein-A), alveolar epithelial cells release additional cell-type-specific DAMPs and inflammatory mediators into the alveoli and circulation85,88. In this context, studies have described vital transfer of mitochondria via connexin 43-containing gap-junction channels from bone marrow-derived stromal cells to alveolar epithelial cells, which provides bioenergetic enhancement and protection against lipopolysaccharide-induced ALI89. Early after experimental trauma to the thorax, MDSCs undergo population expansion, which promotes the release of cytokines characteristic of the TH1 subset of helper T cells and inhibits T cell proliferation, thereby restricting the initial inflammatory response90. Damaged lung epithelial and endothelial cells secrete the alarmin IL-3391, which, after blunt trauma, appears systemically when IL-33 concentrations are associated with a type 2 cytokine profile (IL-4, IL-5, IL-9 and IL-13), infectious complications and organ dysfunction92. In a reverse-translation study, IL-33 has been found to activate group 2 innate lymphoid cells (ILCs) in the lungs early after extra-pulmonary trauma, with a subsequent IL-5 response. That activation, in turn, induces further release of IL-5 and type 2 cytokines from accumulated neutrophils via a feed-forward loop; these cytokines can induce repair mechanisms but also inhibit antibacterial defense, which results in nosocomial infection and impaired organ function92.
After fracture and in abdominal trauma models, mitochondrial DAMPs that mimic bacterial PAMPs (according to the endosymbiotic theory) are released and can suppress the remote neutrophil-driven pulmonary immune response: after lung contusion, neutrophils relocate from the contused lungs to sites of extra-pulmonary damage and cause a decrease in pulmonary antibacterial defense and an increased risk of pneumonia93,94. Input from the periphery also modulates the innate pulmonary immune response. In rats, exosomes in mesentery lymph after extra-pulmonary trauma and/or hemorrhagic shock activate alveolar macrophages via TLR4, which mounts a full ALI response95. Modulating the mesenteric lymph after traumatic shock in vivo with a pharmacologic vagal agonist results in a reduction in the activity of phosphorylated transcription factor STAT3 in circulating monocytes and attenuates the development of secondary ALI, as well as the systemic inflammatory response96.
Sensory input about trauma-caused alterations to the metabolic microenvironment and potential danger molecules is also provided by vagal nodose sensory nerve terminals within the alveolar epithelium and pulmonary neuroepithelial bodies, which form autonomous circuits and control the recruitment of macrophages via neuropeptides97. However, little is known about the consequences of trauma-induced disruption of these sensors. In the clinical setting of trauma-induced ALI, mechanical ventilation can enhance the innate immune response98, supplying oxygen can result in enhanced production of ROS, hyperoxia even produces an angiopoietin 2-dependent increase in macrophages and lung tissue injury99, and transfusion of erythrocytes can result in transfusion-related ALI, which has been attributed to antibodies to human neutrophil alloantigen 3a100. Of note, the lungs, with their numerous immune cells, not only represent the main target of the innate immune response after lung contusion and extra-pulmonary trauma but also can influence the responses of distant organs.
To complete the picture of the immunological consequences of chest trauma, the heart as the ‘central’ vital thorax organ is an underestimated target. After experimental polytrauma with trauma to the thorax (including primary heart trauma), a local cardiac innate immune response comes into play not only by generating inflammatory mediators (for example, IL-1β and IL-6) but also by featuring subcellular changes (for example, disturbances in connexin 43) that represent a mechanistic basis of possible functional alterations after severe trauma101. There is also evidence that remote trauma-inducing secondary cardiac injury involves the activation of innate immunity via the TLR system, production of cytokines, infiltration of leukocytes and generation of ROS, all of which can result in structure-function defects and a poor outcome102.
Cerebral and extracerebral challenges to the innate immune system
Traumatic brain injury (TBI) represents another major cause of mortality worldwide, and the outcome worsens considerably in the presence of combined injuries. The initial insult often consists of meningeal and neuronal contusion, axonal shearing and damage to blood vessels in the meninx and the brain as the ‘primary TBI’. That acutely leads to glia limitans damage, meningeal cell death, neuronal damage and the activation of glial cells such as microglia and astrocytes, which release further DAMPs and produce an inflammatory response in brain103,104 (Fig. 4). When exposed to DAMPs, phagocytic microglia are rapidly activated to clear debris (for example, a hematoma), seal defective barriers and produce neurotrophic factors105–107. However, microglia are also critical for an extensive and often sustainable (up to years) generation of cytokines (for example, IL-1β and IL-6) and ROS, which in turn recruit neutrophils and blood monocytes-macrophages to the injured area103,105,108. Furthermore, complement109 or lysophosphatidylcholine activates inflammasomes in microglia or astrocytes soon after TBI and during neuro-inflammation110. After exposure to IL-1β, astrocytes rapidly generate immune signals in the form of extracellular vesicles dispatched to the periphery, which results in the further recruitment of neutrophils and systemic release of cytokines111. The resulting inflammatory response is associated with additional generation of ROS and release of excitatory neurotransmitters, further cellular recruitment of non-classical monocytes and neutrophils112 and microcirculatory disturbances, which causes the progressive, often rapid transition to ‘secondary TBI’103. Immigrated neutrophils help to clear debris such as myelin fragments but also aggravate inflammation and even neuronal loss. Monocytes recruited mainly via a path dependent on the chemokine receptor CCR2 can polarize and contribute to tissue repair and the removal of debris, as well as hematoma clearance113, but can also contribute substantially to the progressive inflammation. Further pathophysiological consequences include breakdown of the blood-brain barrier (BBB) and cellular swelling. HMGB-1 from necrotic neurons, for example, prompts microglia to generate IL-6, which induces expression of the water channel aquaporin 4 in astrocytes, resulting in cytotoxic swelling114. The multifactor-driven development of edema, increased intracranial pressure and reduced cerebral perfusion pressure and blood flow constitute a vicious cycle that amplifies the hypoxic conditions that disrupt the energy supply (ATP) in the brain. These intracerebral changes often lead to additional damage to white and gray matter105 and a sustained reduction in synaptic plasticity.
Fig. 4. Cerebral and extracerebral challenges to the innate immune system.
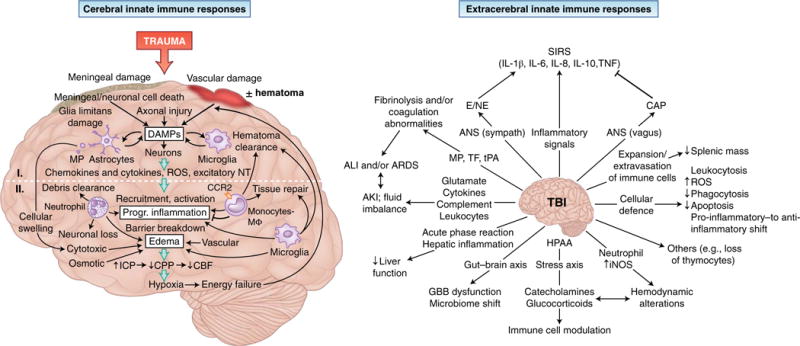
The innate immune response in the brain (left) is induced by meningeal damage, neuronal loss and axonal injury that results in substantial local release of DAMPs. The picture of primary brain injury comprises DAMP-induced activation of microglia cells and astrocytes, which generate chemokines and cytokines and ROS, as well as excitatory neurotransmitters (NT), and release, in an enhancing loop, additional DAMPs. These mechanisms can induce secondary brain injury via the recruitment and activation of leukocytes, which contribute not only to the clearance of damaged tissue but also to the progression of inflammation and the breakdown of barriers between cerebral compartments. Edema formation resulting from cytotoxic cell swelling, osmotic imbalances and impaired BBB function increases intracranial pressure (ICP) and decreases cerebral perfusion pressure (CPP); this leads to reduced cerebral blood flow (CBF) and hypoxia, which in turn cause further neuronal loss. On the extracerebral level (right), TBI alters a plethora of physiological processes. Inflammatory stimuli, together with ANS activation, can lead to systemic inflammation with deleterious effects. The release of brain-derived pro-coagulatory and fibrinolytic molecules (TF and tissue plasminogen activator (tPA)) and MPs with resulting coagulatory effects, as well as excessive secretion of mediators and activation of leukocytes, can induce remote organ injury in the lungs and kidneys. Hepatic function is altered by the launching of an acute-phase reaction, and the gut barrier can be affected by direct communication via the gut-brain axis. Furthermore, modulation of immune-cell function and hemodynamics after TBI can occur via the release of catecholamines and glucocorticoid by the hypothalamic-pituitary-adrenal axis (HPAA) and the generation of inducible nitric-oxide synthase (iNOS) by neutrophils. Splenic atrophy can result from the activation and population expansion of lymphocytes and their extravasation into the circulation. Finally, the systemic cellular defense mechanisms are modulated by TBI by an increased number of circulating leukocytes with enhanced production of ROS and prolonged survival, but with reduced phagocytic activity, and by a functional shift from a pro-inflammatory phenotype to an anti-inflammatory phenotype in circulating monocytes. Progr., progressive; SIRS, systemic inflammatory response syndrome; AKI, acute kidney injury; Sympath, sympathetic ; CAP, cholinergic anti-inflammatory pathway; GBB, gut-blood barrier; I, primary injury; II, secondary injury.
In the acute phase after TBI, the intense communication between the brain and the extra-cerebral immune system is often altered, compromised or lost. Although the brain itself depends on peripheral priming of immune cells to elicit an effective response115, DAMPs and pro-inflammatory mediators released from the injured tissue exit via a disrupted BBB or the glymphatic system116 and induce a systemic inflammatory response103. Furthermore, brain injury induces the breakdown of cerebral compartments with different immunological responses117. The peripheral inflammatory reaction can include both pro-inflammatory responses and anti-inflammatory responses and induce either further neurological damage or protection106,118,119. This systemic response is mainly controlled by the ANS via inflammatory reflex loops49.
Depending on the injury pattern, a traumatic impact can also affect parts of the ‘immunological homunculus’ within the central nervous system, an afferent and efferent system hardwired to monitor and modulate DAMPs120. The parasympathetic ANS (vagal nerve) stimulates splenic T lymphocytes and thereby inhibits the pro-inflammatory responses of macrophages121. This cholinergic anti-inflammatory pathway seems to be critically involved in the control of systemic inflammation and BBB breakdown119. Activation of the sympathetic ANS (the ‘sympathetic storm’) after TBI or after an additional hemorrhagic shock can result in massive peripheral release of epinephrine and/or norepinephrine, which causes enhanced, but also reprogrammed and suppressed, immune responses122–124. After TBI, plasma norepinephrine is associated with a poor outcome, and patients exhibit signs of endotheliopathy and coagulopathy125. Pro-coagulatory molecules and microparticles loaded with TF and phosphatidylserine derived from microglia and astrocytes can activate platelets and induce a hypercoagulatory state or even a consumptive coagulopathy126,127. Similarly, pro-inflammatory molecules in microglia-derived microparticles can add to the inflammatory response128.
In isolated TBI without major bleeding, brain-derived TF-induced consumptive coagulopathy129 and the release of plasminogen activators result in local and systemic fibrinolysis, intracerebral hemorrhage and the development of acute traumatic coagulopathy130. In addition, after TBI, the complement system has been shown to be locally and systemically activated in the acute phase and in a prolonged manner131.
Both complement anaphylatoxins and clotting factors account for the induction of ALI and ARDS as serious complications after TBI. Experimental TBI results in breakdown of the air-blood barrier, alveolar hemorrhage, an early peak of TF+ microparticles in the lung alveoli and deposition of fibrinogen in the alveolar space. Along the same lines, TBI-induced ALI can be ameliorated by inhibition of thrombin, which suggests that the ‘coagulo-immune response’ is the main driver of ALI after brain injury132. High concentrations of glutamate derived from neurons and glia after TBI cause a switch from anti-inflammatory signaling to pro-inflammatory signaling in peripheral leukocytes in response to ATP and adenosine, which results in ‘neurogenic ALI’133. Furthermore, TBI increases the recruitment of neutrophils to the bronchoalveolar space, but it can also suppress the pulmonary immune response via the cholinergic anti-inflammatory pathway, which puts the lungs at an enhanced risk of infection134.
Activation of the pathway noted above can also affect neurological-immunological interactions between the brain and kidneys135. Clinically, acute kidney injury manifests in nearly 10% of all patients with TBI, which results in remarkable mortality rates of more than 40% (ref. 136). Whereas non-immunological and immunological kidney-brain interactions are well described137, brain injury-induced immunological changes in the kidneys are less well established. Closed head injury can result in substantial activation of complement (C3d and C9) in the kidneys and in pro-apoptotic events that can be ameliorated by intravenous infusion of neuronal stem cells138. TBI can induce electrolyte-fluid imbalances and decreased glomerular perfusion via catecholamine-induced renal vasoconstriction, which in turn can intensify cerebral edema139. Various DAMPs released by damaged brain tissue can also be sensed by the proximal tubular epithelium interacting with immune cells140. However, understanding of the immunological brain-kidney axis135 and its interactions with non-immunological factors, such as electrolyte, osmotic and volume imbalances, is only beginning; nevertheless, stress-induced anti-inflammatory reflexes might modulate acute kidney injury after TBI as well as after ischemia141.
Similarly, the immune response in the liver is greatly affected by brain injury. TBI-derived cytokines trigger an early remote hepatic acute-phase response that produces ‘CC’ and ‘CXC’ chemokines and recruits leukocytes to the liver and damaged brain areas142. Furthermore, alterations to the homeostasis of hepatic and brain bile acid add to the systemic inflammation and neurological dysfunction143. After injury to the central nervous system, liver Kupffer cells mainly control the influx of neutrophils into remote sites of injury but contribute only partially to the full hepatic inflammatory response144. TBI also seems to downregulate the hepatic metabolization of multiple inflammatory mediators that results in an enhanced and prolonged systemic cytokine response145.
The well-established gut-brain axis is especially affected by TBI and contributes substantially to dysautonomia, gut-barrier dysfunction and alterations to the composition of local immune cells and the microbiome146. TBI induces, via unclear mechanisms, structural changes to the intestinal villi and epithelium, with the opening and loss of TJs and subsequent complications146. TNF released by local macrophages after stimulation by brain-derived DAMPs impairs TJ function and increases gut permeability, even in a TBI model in flies147.
After TBI, neutrophils can paradoxically exhibit both increased function and suppressed function. Microglia in the injured brain as well as neutrophils in extra-cerebral areas generate inducible nitricoxide synthase early after TBI, which contributes to hemodynamic instability through vasodilation148. In contrast, vasoconstriction is induced by catecholamines from the TBI-activated HPAA, which results in a stress reaction that modulates extra-cerebral immune responses (for example, activation of neutrophils and immunological-metabolic shifts) and is also associated with a poor outcome149.
Whereas early recruitment of peripheral neutrophils to the damaged brain is well established103,104, TBI-generated peripheral effects on leukocytes are less well understood. Even low concentrations of brain-derived cytokines in blood are capable of inducing leukocytosis and suppressing key functions of peripheral lymphocytes103,150, actions attributed mainly to enhanced intracranial pressure, vagal tone and release of catecholamines and glucocorticoids103,151. Presumably through increased C5a131, peripheral neutrophils react to TBI with enhanced numbers, release of ROS, decreased phagocytotic capacity and a considerably delayed apoptosis, all of which enhance an aggressive immune reaction that probably induces bystander injury151. Circulating monocytes are also increased but, on balance, indicate a transition toward an anti-inflammatory response. In contrast, the number of cytotoxic CD56dim natural killer cells seems to decrease in patients early after TBI151. ILCs have been detected within the meningeal space and lungs. Notably, experimental spinal injury changes the gene-expression profile of these cells152.
An increase in circulating splenic lymphocytes (due to mobilization of immune cells) occurs that is associated with a compromised BBB and the development of splenic atrophy early after experimental TBI153, and beyond 30 days after TBI, a decrease in F4/80+ macrophages in the red pulpa and of Ly6Clo tissue macrophages has been observed154. TBI is also associated with a loss of thymus mass as a result of a reduction in lineage marker-negative populations and mature T cells that is associated overall with immunosuppressive features154.
In conclusion, the highly dynamic extracerebral response to TBI is associated with functional compromise of innate immune and organ systems that can promote a sepsis-like picture. Under such conditions, treatment is highly challenging. In addition to surgical decompression and control of hemorrhage, the development of an effective control of the immune system to improve the outcome of patients with TBI is imperative.
Posttraumatic immune response and breakdown of protective cell barriers in the gut
Disturbed cellular barriers between different macro-and microenvironments are especially problematic for the gut-blood barrier that is formed by mucus, intestinal epithelial cells (IECs) and a large arsenal of immune cells, all of which separate a vast amount of generally protective commensal bacteria, as well as pathogenic microorganisms, from a sterile surrounding area155–157. After severe trauma, the stress response results in the centralization of blood flow via splanchnic vasoconstriction, which leads to hypoxia and ischemia in the intestinal tissue, especially in the highly vulnerable villi158 (Fig. 5). Consequently, IECs respond by releasing DAMPs and cytokines, which attract inflammatory leukocytes and induce remote organ injury (ALI)159. In hemorrhagic shock, locally and systemically activated complement can also contribute to the recruitment and activation of leukocytes, together with the deposition of complement and opsonization of damaged cells160.
Fig. 5. Trauma-induced breakdown of protective cell barriers in the gut.
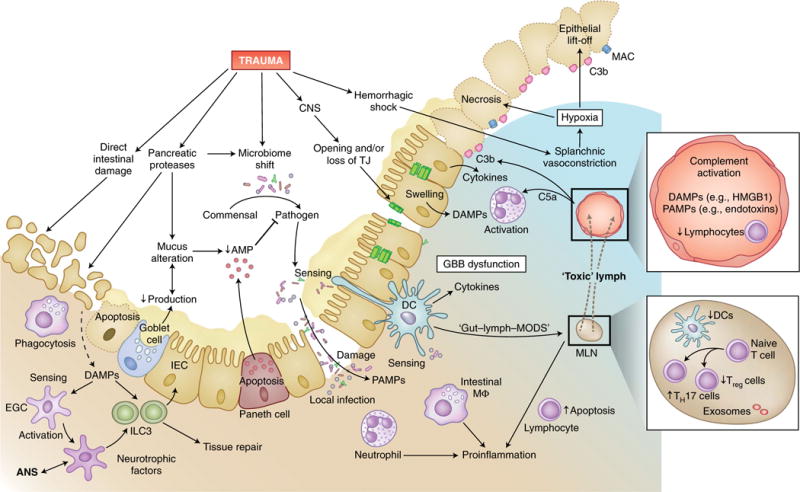
In addition to the direct tissue damage inflicted by abdominal injury, trauma can also indirectly induce dysfunction of the gut-blood barrier, with its central function of separating commensal and pathogenic intestinal bacteria from the predominantly sterile surrounding tissue and circulation. In intestinal tissue and blood vessels, trauma can induce lymphopenia, strong complement activation and large amounts of DAMPs and PAMPs, which in turn activate neutrophil granulocytes and recruit them to the intestine. Proteases increasingly secreted intraluminally from the pancreas in response to traumatic injury can alter the composition of mucus as well as its production in goblet cells and induce autodigestion of intestinal epithelial cells (IEC). Reduced secretion of host-defense peptides from Paneth cells leads to impaired detection and clearance of pathogenic bacteria, which can result in dysbiosis. Deteriorated barrier function and increased necrosis of intestinal epithelial cells, also due to the deposition of complement on damaged cells and hypoxia, as well as the loss of TJs (for example, after TBI), allow the transition of pathogens into the submucosal tissue. Local infection is sensed by neutrophils and resident intestinal macrophages, which induce a pro-inflammatory response and further damage the intestinal barrier. Intestinal DAMPs and PAMPs are screened by dendritic cells (DCs), which have spines that reach into the luminal space, as well as by enteric glial cells (EGC) that interact with neuronal cells of the ANS and group 3 ILCs (ILC3). Stimulated group 3 ILCs, in turn, promote intestinal tissue repair. Bacteria and their products can reach the lymph system either by direct translocation or, according to the ‘lymph-gut hypothesis’, by being transferred to mesenteric lymph nodes (MLN) by dendritic cells and intestinal macrophages, which results in the flow of ‘toxic’ lymph fluid into the venous system and presumably causes MODS. Furthermore, in the mesenteric lymph nodes, trauma can reduce the number of dendritic cells and thereby tip the TH17 cell/Treg cell balance toward a pro-inflammatory response. lympho, lymphocyte; AMP, antimicrobial peptide.
In response to the inflammatory milieu, IECs can undergo a reduction in TJ connections, changes in intracellular pH, cellular swelling and rapid apoptosis and necrosis38,158. Microscopically, these changes are often reflected by a ‘lift-off’ phenomenon in which the epithelial layer of the intestinal villi is released, together with the formation of profuse submucosal edema and destruction of the regenerative platform. Goblet cells produce mucus as an integral part of innate immunity. In response to trauma and/or hemorrhagic shock, the mucosal barrier is diminished and damaged by oxidative stress, with autodigestion of mucus and IECs by intraluminal pancreatic proteases161,162. Trauma also results in the apoptosis of Paneth cells, which results in a decrease in host-defense peptides (for example, antimicrobial peptides)158,163. These posttraumatic conditions evolve into a ‘dysbiosis’, often with a commensal-pathogen shift in the gut microbiome158.
There are two controversial paradigms used to explain whether and how bacteria and PAMPs that originate in the gut enter the circulation and induce a systemic immune response. Some groups have found no evidence of endotoxemia early after multiple trauma, either in the portal vein or systemically164, whereas others have detected endotoxemia systemically165,166. These differences are still unresolved and probably reflect differences in technical approaches164–166. In principle, either bacterial pathogens and related PAMPs can directly penetrate the damaged intestinal tissue to induce local inflammation and cause further damage by activating leukocytes, or they can be translocated to the intestinal lymph nodes via dendritic cells and intestinal macrophages. Furthermore, the damaged tissue and pathogen exposure can result in a ‘toxic’ lymph fluid that flows into the venous system, which leads to deformability of red blood cells, tissue injury, sepsis and MODS, as postulated by the gut-lymph-MODS hypothesis167–170. Thus, the balance of adaptive TH17 cells and regulatory T cells (Treg cells) in immunity and tolerance tilts toward a pro-inflammatory immune response171,172. In addition, the neuroenteric axis173 seems to diminish the abundance of dendritic cells in the mesenteric lymph nodes and thereby tips the TH17 cell/Treg cell balance after trauma and or hemorrhagic shock. The effect of the trauma-induced ‘toxic lymph’167 and shift in the ratio of TH17 cells to Treg cells can be counterbalanced by stimulation of the vagal nerve172. In particular, CD63+HSP70+ exosomes within the mesenteric lymph nodes represent a major trigger for the monocyte-macrophage pro-inflammatory response174.
Clinically, trauma-induced gut-driven changes in lymphatic biological activity can induce remote (immunological) organ injuries, such as apoptosis of cells in the spleen and thymus, or ALI167,169,175. It is remarkable that in fatal severe trauma, the intertwined adaptive immune response after trauma mainly shows enhanced apoptosis of lymphocytes in the intestine as well as systemic lymphocytopenia, which exposes the patient to an enhanced risk of infection38. The intestinal posttraumatic and ischemic immune response can also involve group 3 ILCs as well as enteric glial cells, which screen the intestinal environment for DAMPs and PAMPs and communicate via neurotrophic factors that in turn control the intestinal immunological milieu176. After either direct intestinal injury (ischemia-reperfusion) or remote injury (for example, TBI), enteric glial cells exhibit functional changes and their activation seems to be needed to shape the vagal anti-inflammatory response177,178. Overall, the innate immune response of the injured gut contributes substantially to the injury and dysfunction of remote organs, and vice versa.
Implications for therapeutic modulation of innate immunity after trauma
Most patients who have suffered trauma and survive the first 24-48 hours after severe injury have a relatively good prognosis179,180. Notably, deaths after that early phase are caused to a lesser extent directly by the trauma itself and instead are caused by infectious and septic complications180,181, which develop in particular during conditions of prolonged immunosuppression or, later, in the context of PICS41; therefore, not only preventing damaging immunological reactions but also reinforcing innate immunity might improve the outcome by overcoming the period of severe immunosuppression. In the context of trauma, various interventions to inhibit or augment the immune response, such as antibody to L-selectin, hydrocortisone, prostaglandin E1, aprotinin, immunoglobulins, interferon-γ, immunonutrition, probiotics, etc., have been applied, analyzed in detail and reviewed elsewhere1,182. By the standards of randomized clinical trials, however, most of the proposed approaches have failed to significantly improve mortality1. Pioneering immunomodulation has employed damage targeting; for example, by the detection of C3b-iC3b (deposited in injured tissue) using the complement modulator CD59-2a-CRIg (a dimer in which each monomer consists of CD59 (an inhibitor of the complement complex MAC) fused to CRIg (a complement receptor of the immunoglobulin superfamily) via IgG2a hinge region)109 or by the sensing of proteoglycan complexes in injured brain areas with the small peptide CAQK183. Promising innate immunomodulatory therapies have been applied early after trauma or in a delayed fashion27,104,109,113,160,162,183–196 (Table 1). Other innovative strategies could include glycocalyx compounds to seal broken barriers, exosomes to alter different functions (for example, coagulation), as well as bioelectronical devices to modulate the inflammatory ‘immune reflexes’121, or they might derive from in silico modeling of the complex trauma response. Furthermore, ex vivo-primed (innate) immune cells used to temporally and spatially aid in the clearance of debris and microbial infections, as well as to promote wound healing197, might represent a future therapeutic avenue.
Table 1.
Examples of therapeutic modulation of promising innate immune targets
| Immunological target | Specific therapy | Trauma | Species | Postulated mechanisms | Effect or clinical outcome (treatment vs control) | Ref. |
|---|---|---|---|---|---|---|
| DAMPs | Anti-HMGB1 | (Traumatic)-hemorrhagic shock; pseudo-fracture and soft-tissue injury; and TBI | C57BL/6 mice; rats (TBI) | Inhibition of HMGB1 as a major driver of multiple pro-inflammatory events, and its interaction with TLRs; modulation of HMGB1-induced population expansion of MDSCs; neutralization of HMGB1 as important DAMP in the brain after TBI | Lowering of IL-6 and IL-10; amelioration of ileal mucosal hyperpermeability; improved 24-hour survival (P < 0.04); amelioration of MDSCs in the spleen; less trauma-induced suppression of T cells with amelioration of the level of TH1 cytokines; protection of the BBB (P < 0.01); improvement of motor function 24 h after injury (P < 0.05) | 184–186 |
| DAMPs | Inhibition of the puringergic receptor P2X7 | TBI | C57BL/6J, CD-1 and C57BL/6 mice | Neutralizing of the ATP effects on microglia and astrocytes | Elimination of neutrophil recruitment; decrease in IL-1β and GFAP; less cerebral edema (P < 0.01); improved neurological outcome (P < 0.01) | 104,187 |
| DAMPs | ahscFv (scFv antibody to histone) | Soft tissue trauma | C57BL/6 mice | Inhibition of histone-induced immune cell activation, endothelial damage, and coagulation activation | Less endothelial damage; normalized activation of coagulation; attenuated cytokine response; amelioration of acute lung injury; less histone-related mortality | 188 |
| Coagulation and/or complement | C1 inhibitor | (Traumatic)-hemorrhagic shock and polytrauma with femur or pelvic fracture | Yorkshire swine and humans | rhC1-INH inactivates C1r, C1s, MASP2, factor XII, plasma kallikrein, factor XI, plasmin and tPA; suppression of the systemic inflammatory response (less IL-6) | In swine: less TNF; less deposition of C3, C5 and C5b-9 (MAC) in the small intestine and lungs; improvement of metabolic acidosis; less renal, intestinal, and lung tissue damage (P < 0.05); for humans, study terminated (limited feasibility) | 160,189 |
| Complement | Inhibition of C3 deposition by mTT30 (chimeric CR2-fH) | TBI | C57BL/6 mice | Targeted inhibition of complement at the site of injury and thus less neuroinflammation | Less deposition of C3 in brain; suppressed activation of microglia; less neuronal cell death (P < 0.05) | 190 |
| Complement | C6 antisense oligonucleotide or C5 blocker (OMCI) | TBI | C57BL/6 mice | Inhibition of MAC formation by C6 antisense oligonucleotide, or inhibition of C5 by OMCI | Inhibition of C6: less cerebral MAC (up to 96%); inhibition of C6 synthesis (>80%); inhibition of C5: decreased MAC formation; improved neurological outcome by delayed application (P < 0.05) | 191 |
| Complement | CD59-2a-CRIg | TBI | Mice | Targeted inhibition of MAC formation at injured sites with deposition of C3b and iC3b | Homing of dimers to sites of injury; less deposition of C9; inhibition of MAC-induced inflammasome activation in microglia; less accumulation of microglia; less axonal damage; enhanced neurological recovery (50% reduction; P < 0.001) | 109 |
| Neutrophils | BM-derived neutrophils (i.t.) | Abdominal injury (mtDNA, i.p.) and posttraumatic lung bacteria | CD1 and C57BL/6 mice | Debris inhibit neutrophil function and bacterial clearance; thus, application of neutrophils improves killing of bacteria | Clearance of various bacterial inoculi by exogenous BM neutrophils after traumatic simulation (P < 0.015); BM neutrophils had no untoward clinical effects | 27 |
| Macrophages | CCX872 (CCR2-selective antagonist) |
TBI | C57BL/6 mice | Inhibition of CCR2-dependent recruitment of macrophages to the injured brain and subsequent inflammation | Less cerebral accumulation of macrophages; less inflammation; sparing of TBI-induced cognitive dysfunction (P < 0.01) | 113 |
| Macrophages and microglia | EVT901 (antagonist of the neurotrophin receptor p75) |
TBI | C57BL/6 mice | Less microglial activation; effect on trafficking of peripheral immune cells to the injured brain | Attenuated infiltration of peripheral inflammatory monocytes-macrophages into the lesion site; fewer peripheral pro-inflammatory monocytes; improvement in motor function (P = 0.04) | 192 |
| Macrophages and microglia | Atorvastatin (lipidlowering statin) | TBI | C57BL/6 mice | Less recruitment of peripheral leukocytes; anti-inflammation; modulating macrophage polarization; increased NET formation | Less recruitment of peripheral leukocyte subsets to the injury site; decreased pro inflammatory cytokine/anti-inflammatory cytokine ratio; M1-to-M2 shift of microglia-macrophages; less neuronal apoptosis; improved behavioral deficits 72 h after TBI (P < 0.01) | 193 |
| Proteases | ANGD or tranexamic acid (enterally and i.p.) | Hemorrhagic shock | Rats | Blockade of pancreatic serine proteases as a proposed driver of innate immunity-mediated inflammation and MODS | Less protease activity in the intestinal wall; less tissue damage in the intestine, lungs and heart; improved survival (P ≤ 0.01) | 162 |
| ROS | GSH(br)(transcranially) | TBI | C57BL/6 mice | GSH scavenges ROS; fine tuning of the macrophage response to infection | 50% less death of meningeal cells after pretreatment; inhibition of neutrophil influx; 50% less death of parenchymal cells after delayed (12 h) treatment (P < 0.05) | 104 |
| Peptides | CAQK (short peptide) | TBI | Mice and humans | Targeting of a proteoglycan complex upregulated in the injured brain; delivery of drugs | Specific binding to injured brain tissue of CAQK nanoparticles; delivery of siRNA to injured site; binding to injured human brain (P < 0.01) | 183 |
| Peptides | Pep19-4LF (synthetic antimicrobial peptide) |
(Traumatic)-hemorrhagic shock | Rats | Host-defense and antimicrobial; neutralization of PAMPs; inhibition of DC migration and cytokine response | Hemodynamic stabilization; inhibition of TNF release by hemorrhagic shock-stimulated monocytes; inhibition of NF-κB and pro-inflammation; organ protection (kidneys, liver, lungs) (P < 0.05) | 194 |
| Others | Valproate | Polytrauma, hemorrhagic shock and TBI | Yorkshire swine | Inhibition of histone deacetylation; induction of neurogenic transcriptional program | Reduction in the size of early brain lesion; faster neurological recovery (P = 0.02) | 195 |
| Others | Artesunate (a prodrug that is rapidly converted to its active form, DHA) | Hemorrhagic shock-induced multiple injury | Rats | Anti-malarial activity; antiviral; anti-inflammation: decrease in the pro-inflammatory/anti-inflammatory ratio of macrophages; suppression of T cell activation | Decrease in pro-inflammatory cytokines IL-6 and TNF; activation of the kinase Akt-endothelial nitric oxide synthase survival pathway; improved MOF (P < 0.05) | 196 |
GFAP, glial fibrillary acidic protein; rhC1-INH, recombinant human C1 inhibitor; MASP2, mannan-binding lectin serine protease 2; CR2-fH, complement receptor 2-factor H; OMCI: ornithodoros moubata complement inhibitor; BM, bone marrow; i.t., intratracheally; i.p., intraperitoneally; CCR2, chemokine receptor; ANGD, 6-aminidino 2-naphthyl p-guanidinobenzoate di-methansulfate; GSH, glutathione; siRNA, small interfering RNA; NF-κB, transcription factor; DHA, dihydro-artemisinin; MOF, multi-organ failure.
Future perspectives
This Review has integrated major ‘cross-talking’ aspects into a clinical context, to elaborate how controlling the balance of the innate immune system can improve outcomes for patients who have suffered trauma. Although this strategy was proposed decades ago, it remains critical that the immune response be reliably monitored temporally and spatially before any therapeutic immunomodulation can be performed effectively. Therefore, in the future, real-time, profound functional monitoring of the innate immune and organ responses will be needed to precisely detect functional defects at the bedside. Because innate and adaptive immune responses can differ considerably depending on age, comorbidities and other preexisting conditions, efforts to delineate the underlying posttraumatic mechanisms need to be intensified in the context of different disturbance variables. Furthermore, in the era of precision medicine, big data-driven discovery in complex trauma situations such as TBI or polytrauma might be feasible through the use of bioinformatics tools such as topological data analysis. Such strategies might improve the phenotyping of injury patterns, precision diagnosing and treatment planning198. Here we focused on physical trauma but have neglected complex interactions with the psychological dimension; for example, the innate immune response after psychological trauma reveals in part similarities to the reactions described after physical trauma. The future might thus yield insights into multiple common and interactive immune responses, including immuno-metabolic and neuro-immunological switches and checkpoints after physical and psychological trauma. Overall, therapeutic immunomodulation after severe trauma may not only aim to stabilize fluid-phase and cellular innate immunity along with immunological barriers but also use ex vivo reprogrammed cells to induce regenerative processes and thereby promote healing and, finally, improve the quality of life following trauma.
Acknowledgments
We thank R. Halbgebauer and D. McClellan for editorial assistance, and S. Denk for graphical support. Supported by the German Research Foundation (DFG CRC1149 and DFG EI866/5-1), the US National Institutes of Health (AI068730, AI030040) and the European Community’s Seventh Framework Programme (under grant agreement number 602699 (DIREKT)).
Footnotes
Author contributions
All authors researched the data for the article, contributed to discussions of the content, wrote the text and reviewed or edited the article before submission.
Competing interests
M.H.-L. and P.A.W. hold a patent on compositions and methods for the diagnosis and treatment of sepsis (US 7455837). J.D.L. is the founder of Amyndas Pharmaceuticals, which is developing complement inhibitors (including third-generation compstatin analogs such as AMY-101), and is the inventor of patents or patent applications that describe the use of complement inhibitors for therapeutic purposes, some of which are developed by Amyndas Pharmaceuticals. J.D.L. is also the inventor of the compstatin technology licensed to Apellis Pharmaceuticals (4(1MeW)7W/POT-4/APL-1 and PEGylated derivatives such as APL-2).
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lord JM, et al. The systemic immune response to trauma: an overview of pathophysiology and treatment. Lancet. 2014;384:1455–1465. doi: 10.1016/S0140-6736(14)60687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sauaia A, Moore FA, Moore EE. Postinjury inflammation and organ dysfunction. Crit Care Clin. 2017;33:167–191. doi: 10.1016/j.ccc.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mira JC, et al. The epidemiology of chronic critical illness after severe traumatic injury at two level-one trauma centers. Crit Care Med. 2017;45:1989–1996. doi: 10.1097/CCM.0000000000002697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gabbe BJ, et al. Long-term health status and trajectories of seriously injured patients: a population-based longitudinal study. PLoS Med. 2017;14:e1002322. doi: 10.1371/journal.pmed.1002322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callcut RA, et al. Discovering the truth about life after discharge: Long-term trauma-related mortality. J Trauma Acute Care Surg. 2016;80:210–217. doi: 10.1097/TA.0000000000000930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keel M, Trentz O. Pathophysiology of polytrauma. Injury. 2005;36:691–709. doi: 10.1016/j.injury.2004.12.037. [DOI] [PubMed] [Google Scholar]
- 7.Adib-Conquy M, Cavaillon JM. Compensatory anti-inflammatory response syndrome. Thromb Haemost. 2009;101:36–47. [PubMed] [Google Scholar]
- 8.Cabrera CP, et al. Signatures of inflammation and impending multiple organ dysfunction in the hyperacute phase of trauma: A prospective cohort study. PLoS Med. 2017;14:e1002352. doi: 10.1371/journal.pmed.1002352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dijkink S, et al. Polytrauma patients in the Netherlands and the USA: A bi-institutional comparison of processes and outcomes of care. Injury. 2018;49:104–109. doi: 10.1016/j.injury.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Minei JP, et al. The changing pattern and implications of multiple organ failure after blunt injury with hemorrhagic shock. Crit Care Med. 2012;40:1129–1135. doi: 10.1097/CCM.0b013e3182376e9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Billiar TR, Vodovotz Y. Time for trauma immunology. PLoS Med. 2017;14:e1002342. doi: 10.1371/journal.pmed.1002342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Netea MG, et al. A guiding map for inflammation. Nat Immunol. 2017;18:826–831. doi: 10.1038/ni.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhao H, Kilgas S, Alam A, Eguchi S, Ma D. The role of extracellular adenosine triphosphate in ischemic organ injury. Crit Care Med. 2016;44:1000–1012. doi: 10.1097/CCM.0000000000001603. [DOI] [PubMed] [Google Scholar]
- 14.Gebhard F, Huber-Lang M. Polytrauma-pathophysiology and management principles. Langenbecks Arch Surg. 2008;393:825–831. doi: 10.1007/s00423-008-0334-2. [DOI] [PubMed] [Google Scholar]
- 15.Qiang X, et al. Cold-inducible RNA-binding protein (CIRP) triggers inflammatory responses in hemorrhagic shock and sepsis. Nat Med. 2013;19:1489–1495. doi: 10.1038/nm.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, et al. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature. 2010;464:104–107. doi: 10.1038/nature08780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis GE, Bayless KJ, Davis MJ, Meininger GA. Regulation of tissue injury responses by the exposure of matricryptic sites within extracellular matrix molecules. Am J Pathol. 2000;156:1489–1498. doi: 10.1016/S0002-9440(10)65020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burk AM, et al. Early complementopathy after multiple injuries in humans. Shock. 2012;37:348–354. doi: 10.1097/SHK.0b013e3182471795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ganter MT, et al. Role of the alternative pathway in the early complement activation following major trauma. Shock. 2007;28:29–34. doi: 10.1097/shk.0b013e3180342439. [DOI] [PubMed] [Google Scholar]
- 20.Kenawy HI, Boral I, Bevington A. Complement-coagulation cross-talk: a potential mediator of the physiological activation of complement by low pH. Front Immunol. 2015;6:215. doi: 10.3389/fimmu.2015.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cekic C, Linden J. Purinergic regulation of the immune system. Nat Rev Immunol. 2016;16:177–192. doi: 10.1038/nri.2016.4. [DOI] [PubMed] [Google Scholar]
- 22.Xiao W, et al. A genomic storm in critically injured humans. J Exp Med. 2011;208:2581–2590. doi: 10.1084/jem.20111354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lederer JA, et al. Comparison of longitudinal leukocyte gene expression after burn injury or trauma-hemorrhage in mice. Physiol Genomics. 2008;32:299–310. doi: 10.1152/physiolgenomics.00086.2007. [DOI] [PubMed] [Google Scholar]
- 24.Seshadri A, et al. Phenotyping the immune response to trauma: a multiparametric systems immunology approach. Crit Care Med. 2017;45:1523–1530. doi: 10.1097/CCM.0000000000002577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Munford RS, Pugin J. Normal responses to injury prevent systemic inflammation and can be immunosuppressive. Am J Respir Crit Care Med. 2001;163:316–321. doi: 10.1164/ajrccm.163.2.2007102. [DOI] [PubMed] [Google Scholar]
- 26.Hazeldine J, et al. Prehospital immune responses and development of multiple organ dysfunction syndrome following traumatic injury: A prospective cohort study. PLoS Med. 2017;14:e1002338. doi: 10.1371/journal.pmed.1002338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Itagaki K, et al. Mitochondrial DNA released by trauma induces neutrophil extracellular traps. PLoS One. 2015;10:e0120549. doi: 10.1371/journal.pone.0120549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Timmermans K, et al. Plasma levels of danger-associated molecular patterns are associated with immune suppression in trauma patients. Intensive Care Med. 2016;42:551–561. doi: 10.1007/s00134-015-4205-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mitchell TA, et al. Traumatic hemothorax blood contains elevated levels of microparticles that are prothrombotic but inhibit platelet aggregation. Shock. 2017;47:680–687. doi: 10.1097/SHK.0000000000000819. [DOI] [PubMed] [Google Scholar]
- 30.Matijevic N, et al. Microvesicle phenotypes are associated with transfusion requirements and mortality in subjects with severe injuries. J Extracell Vesicles. 2015;4:29338. doi: 10.3402/jev.v4.29338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nemeth K, et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E2-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat Med. 2009;15:42–49. doi: 10.1038/nm.1905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones HR, Robb CT, Perretti M, Rossi AG. The role of neutrophils in inflammation resolution. Semin Immunol. 2016;28:137–145. doi: 10.1016/j.smim.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 33.Frith D, et al. Definition and drivers of acute traumatic coagulopathy: clinical and experimental investigations. J Thromb Haemost. 2010;8:1919–1925. doi: 10.1111/j.1538-7836.2010.03945.x. [DOI] [PubMed] [Google Scholar]
- 34.Bastian OW, et al. Impaired bone healing in multitrauma patients is associated with altered leukocyte kinetics after major trauma. J Inflamm Res. 2016;9:69–78. doi: 10.2147/JIR.S101064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen W, et al. Cytokine cascades induced by mechanical trauma injury alter voltage-gated sodium channel activity in intact cortical neurons. J Neuroinflammation. 2017;14:73. doi: 10.1186/s12974-017-0847-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jorgensen I, Rayamajhi M, Miao EA. Programmed cell death as a defence against infection. Nat Rev Immunol. 2017;17:151–164. doi: 10.1038/nri.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paunel-Görgülü A, Kirichevska T, Lögters T, Windolf J, Flohé S. Molecular mechanisms underlying delayed apoptosis in neutrophils from multiple trauma patients with and without sepsis. Mol Med. 2012;18:325–335. doi: 10.2119/molmed.2011.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hotchkiss RS, et al. Rapid onset of intestinal epithelial and lymphocyte apoptotic cell death in patients with trauma and shock. Crit Care Med. 2000;28:3207–3217. doi: 10.1097/00003246-200009000-00016. [DOI] [PubMed] [Google Scholar]
- 39.Heffernan DS, et al. Failure to normalize lymphopenia following trauma is associated with increased mortality, independent of the leukocytosis pattern. Crit Care. 2012;16:R12. doi: 10.1186/cc11157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kottke MA, Walters TJ. Where’s the leak in vascular barriers? a review. Shock. 2016;46:20–36. doi: 10.1097/SHK.0000000000000666. [DOI] [PubMed] [Google Scholar]
- 41.Gentile LF, et al. Persistent inflammation and immunosuppression: a common syndrome and new horizon for surgical intensive care. J Trauma Acute Care Surg. 2012;72:1491–1501. doi: 10.1097/TA.0b013e318256e000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hietbrink F, Koenderman L, van Wessem KJ, Leenen LP. The impact of intramedullary nailing of tibia fractures on the innate immune system. Shock. 2015;44:209–214. doi: 10.1097/SHK.0000000000000405. [DOI] [PubMed] [Google Scholar]
- 43.Kanakaris NK, Anthony C, Papasotiriou A, Giannoudis PV. Inflammatory response after nailing. Injury. 2017;48:S10–S14. doi: 10.1016/j.injury.2017.04.017. [DOI] [PubMed] [Google Scholar]
- 44.Pape HC, et al. Impact of intramedullary instrumentation versus damage control for femoral fractures on immunoinflammatory parameters: prospective randomized analysis by the EPOFF Study Group. J Trauma. 2003;55:7–13. doi: 10.1097/01.TA.0000075787.69695.4E. [DOI] [PubMed] [Google Scholar]
- 45.Pape HC, et al. Impact of the method of initial stabilization for femoral shaft fractures in patients with multiple injuries at risk for complications (borderline patients) Ann Surg. 2007;246:491–499. doi: 10.1097/SLA.0b013e3181485750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rixen D, et al. Randomized, controlled, two-arm, interventional, multicenter study on risk-adapted damage control orthopedic surgery of femur shaft fractures in multiple-trauma patients. Trials. 2016;17:47. doi: 10.1186/s13063-016-1162-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Giannoudis PV, Giannoudis VP, Horwitz DS. Time to think outside the box: ‘prompt-individualised-safe management’ (PR.I.S.M.) should prevail in patients with multiple injuries. Injury. 2017;48:1279–1282. doi: 10.1016/j.injury.2017.05.026. [DOI] [PubMed] [Google Scholar]
- 48.Aird WC. The role of the endothelium in severe sepsis and multiple organ dysfunction syndrome. Blood. 2003;101:3765–3777. doi: 10.1182/blood-2002-06-1887. [DOI] [PubMed] [Google Scholar]
- 49.Tracey KJ. Reflex control of immunity. Nat Rev Immunol. 2009;9:418–428. doi: 10.1038/nri2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johansson PI, et al. Traumatic endotheliopathy: a prospective observational study of 424 severely injured patients. Ann Surg. 2017;265:597–603. doi: 10.1097/SLA.0000000000001751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ekdahl KN, et al. Dangerous liaisons: complement, coagulation, and kallikrein/kinin cross-talk act as a linchpin in the events leading to thromboinflammation. Immunol Rev. 2016;274:245–269. doi: 10.1111/imr.12471. [DOI] [PubMed] [Google Scholar]
- 52.Lissauer ME, et al. Coagulation and complement protein differences between septic and uninfected systemic inflammatory response syndrome patients. J Trauma. 2007;62:1082–1092. doi: 10.1097/TA.0b013e31804d23e1. [DOI] [PubMed] [Google Scholar]
- 53.Muroya T, et al. C4d deposits on the surface of RBCs in trauma patients and interferes with their function. Crit Care Med. 2014;42:e364–e372. doi: 10.1097/CCM.0000000000000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kambas K, et al. C5a and TNF-α up-regulate the expression of tissue factor in intra-alveolar neutrophils of patients with the acute respiratory distress syndrome. J Immunol. 2008;180:7368–7375. doi: 10.4049/jimmunol.180.11.7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kral JB, Schrottmaier WC, Salzmann M, SpringerAmpamp. Assinger A. Platelet interaction with innate immune cells. Transfus Med Hemother. 2016;43:78–88. doi: 10.1159/000444807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun S, et al. Mitochondrial DAMPs increase endothelial permeability through neutrophil dependent and independent pathways. PLoS One. 2013;8:e59989. doi: 10.1371/journal.pone.0059989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vestweber D. How leukocytes cross the vascular endothelium. Nat Rev Immunol. 2015;15:692–704. doi: 10.1038/nri3908. [DOI] [PubMed] [Google Scholar]
- 58.Jorch SK, Kubes P. An emerging role for neutrophil extracellular traps in noninfectious disease. Nat Med. 2017;23:279–287. doi: 10.1038/nm.4294. [DOI] [PubMed] [Google Scholar]
- 59.Rittirsch D, et al. An integrated clinico-transcriptomic approach identifies a central role of the heme degradation pathway for septic complications after trauma. Ann Surg. 2016;264:1125–1134. doi: 10.1097/SLA.0000000000001553. [DOI] [PubMed] [Google Scholar]
- 60.Deitch EA, et al. Trauma-hemorrhagic shock induces a CD36-dependent RBC endothelial-adhesive phenotype. Crit Care Med. 2014;42:e200–e210. doi: 10.1097/CCM.0000000000000119. [DOI] [PubMed] [Google Scholar]
- 61.Maegele M, Schöchl H, Cohen MJ. An update on the coagulopathy of trauma. Shock. 2014;41:21–25. doi: 10.1097/SHK.0000000000000088. [DOI] [PubMed] [Google Scholar]
- 62.Naumann DN, et al. Endotheliopathy of trauma is an on-scene phenomenon, and is associated with multiple organ dysfunction syndrome: a prospective observational study. Shock. 2017 doi: 10.1097/SHK.0000000000000999. [DOI] [PubMed] [Google Scholar]
- 63.Denk S, et al. Early detection of junctional adhesion molecule-1 (JAM-1) in the circulation after experimental and clinical polytrauma. Mediators Inflamm. 2015;2015:463950. doi: 10.1155/2015/463950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann Surg. 2011;254:194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- 65.Denk S, et al. Role of hemorrhagic shock in experimental polytrauma. Shock. 2018;49:154–163. doi: 10.1097/SHK.0000000000000925. [DOI] [PubMed] [Google Scholar]
- 66.Halbgebauer R, et al. Hemorrhagic shock drives glycocalyx, barrier and organ dysfunction early after polytrauma. J Crit Care. 2017;44:229–237. doi: 10.1016/j.jcrc.2017.11.025. [DOI] [PubMed] [Google Scholar]
- 67.White NJ, Ward KR, Pati S, Strandenes G, Cap AP. Hemorrhagic blood failure: oxygen debt, coagulopathy, and endothelial damage. J Trauma Acute Care Surg. 2017;82:S41–S49. doi: 10.1097/TA.0000000000001436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ostrowski SR, Johansson PI. Endothelial glycocalyx degradation induces endogenous heparinization in patients with severe injury and early traumatic coagulopathy. J Trauma Acute Care Surg. 2012;73:60–66. doi: 10.1097/TA.0b013e31825b5c10. [DOI] [PubMed] [Google Scholar]
- 69.Nelson A, Berkestedt I, Schmidtchen A, Ljunggren L, Bodelsson M. Increased levels of glycosaminoglycans during septic shock: relation to mortality and the antibacterial actions of plasma. Shock. 2008;30:623–627. doi: 10.1097/SHK.0b013e3181777da3. [DOI] [PubMed] [Google Scholar]
- 70.Denk S, et al. Complement C5a functions as a master switch for the ph balance in neutrophils exerting fundamental immunometabolic effects. J Immunol. 2017;198:4846–4854. doi: 10.4049/jimmunol.1700393. [DOI] [PubMed] [Google Scholar]
- 71.Cheng SC, et al. Broad defects in the energy metabolism of leukocytes underlie immunoparalysis in sepsis. Nat Immunol. 2016;17:406–413. doi: 10.1038/ni.3398. [DOI] [PubMed] [Google Scholar]
- 72.Cheng SC, et al. mTOR- and HIF-1α-mediated aerobic glycolysis as metabolic basis for trained immunity. Science. 2014;345:1250684. doi: 10.1126/science.1250684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van der Poll T, van de Veerdonk FL, Scicluna BP, Netea MG. The immunopathology of sepsis and potential therapeutic targets. Nat Rev Immunol. 2017;17:407–420. doi: 10.1038/nri.2017.36. [DOI] [PubMed] [Google Scholar]
- 74.Pfeifer R, Heussen N, Michalewicz E, Hilgers RD, Pape HC. Incidence of adult respiratory distress syndrome in trauma patients: a systematic review and meta-analysis over a period of three decades. J Trauma Acute Care Surg. 2017;83:496–506. doi: 10.1097/TA.0000000000001571. [DOI] [PubMed] [Google Scholar]
- 75.Hoth JJ, Wells JD, Jones SE, Yoza BK, McCall CE. Complement mediates a primed inflammatory response after traumatic lung injury. J Trauma Acute Care Surg. 2014;76:601–608. doi: 10.1097/TA.0000000000000129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmidt EP, et al. The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat Med. 2012;18:1217–1223. doi: 10.1038/nm.2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Niesler U, Palmer A, Radermacher P, Huber-Lang MS. Role of alveolar macrophages in the inflammatory response after trauma. Shock. 2014;42:3–10. doi: 10.1097/SHK.0000000000000167. [DOI] [PubMed] [Google Scholar]
- 78.Grommes J, Soehnlein O. Contribution of neutrophils to acute lung injury. Mol Med. 2011;17:293–307. doi: 10.2119/molmed.2010.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robb CT, Regan KH, Dorward DA, Rossi AG. Key mechanisms governing resolution of lung inflammation. Semin Immunopathol. 2016;38:425–448. doi: 10.1007/s00281-016-0560-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Herold S, Mayer K, Lohmeyer J. Acute lung injury: how macrophages orchestrate resolution of inflammation and tissue repair. Front Immunol. 2011;2:65. doi: 10.3389/fimmu.2011.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jiang D, et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–1179. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 82.Wen Z, et al. Neutrophils counteract autophagy-mediated anti-inflammatory mechanisms in alveolar macrophage: role in posthemorrhagic shock acute lung inflammation. J Immunol. 2014;193:4623–4633. doi: 10.4049/jimmunol.1400899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Westphalen K, et al. Sessile alveolar macrophages communicate with alveolar epithelium to modulate immunity. Nature. 2014;506:503–506. doi: 10.1038/nature12902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Koeppen M, et al. Detrimental role of the airway mucin Muc5ac during ventilator-induced lung injury. Mucosal Immunol. 2013;6:762–775. doi: 10.1038/mi.2012.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Whitsett JA, Alenghat T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol. 2015;16:27–35. doi: 10.1038/ni.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Raghavendran K, et al. Lung contusion: inflammatory mechanisms and interaction with other injuries. Shock. 2009;32:122–130. doi: 10.1097/SHK.0b013e31819c385c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Aufmkolk M, et al. Local effect of lung contusion on lung surfactant composition in multiple trauma patients. Crit Care Med. 1999;27:1441–1446. doi: 10.1097/00003246-199908000-00005. [DOI] [PubMed] [Google Scholar]
- 88.Hoth JJ, Wells JD, Yoza BK, McCall CE. Innate immune response to pulmonary contusion: identification of cell type-specific inflammatory responses. Shock. 2012;37:385–391. doi: 10.1097/SHK.0b013e3182478478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Islam MN, et al. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nat Med. 2012;18:759–765. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hüsecken Y, et al. MDSCs are induced after experimental blunt chest trauma and subsequently alter antigen-specific T cell responses. Sci Rep. 2017;7:12808. doi: 10.1038/s41598-017-13019-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liew FY, Girard JP, Turnquist HR. Interleukin-33 in health and disease. Nat Rev Immunol. 2016;16:676–689. doi: 10.1038/nri.2016.95. [DOI] [PubMed] [Google Scholar]
- 92.Xu J, et al. IL33-mediated ILC2 activation and neutrophil IL5 production in the lung response after severe trauma: A reverse translation study from a human cohort to a mouse trauma model. PLoS Med. 2017;14:e1002365. doi: 10.1371/journal.pmed.1002365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao C, et al. Mitochondrial damage-associated molecular patterns released by abdominal trauma suppress pulmonary immune responses. J Trauma Acute Care Surg. 2014;76:1222–1227. doi: 10.1097/TA.0000000000000220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li H, et al. Mitochondrial damage-associated molecular patterns from fractures suppress pulmonary immune responses via formyl peptide receptors 1 and 2. J Trauma Acute Care Surg. 2015;78:272–279. doi: 10.1097/TA.0000000000000509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kojima M, et al. Exosomes in postshock mesenteric lymph are key mediators of acute lung injury triggering the macrophage activation via Toll-like receptor 4. FASEB J. 2018;32:97–110. doi: 10.1096/fj.201700488R. [DOI] [PubMed] [Google Scholar]
- 96.Langness S, Costantini TW, Morishita K, Eliceiri BP, Coimbra R. Modulating the biologic activity of mesenteric lymph after traumatic shock decreases systemic inflammation and end organ injury. PLoS One. 2016;11:e0168322. doi: 10.1371/journal.pone.0168322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Branchfield K, et al. Pulmonary neuroendocrine cells function as airway sensors to control lung immune response. Science. 2016;351:707–710. doi: 10.1126/science.aad7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.van Wessem KJ, Hennus MP, van Wagenberg L, Koenderman L, Leenen LP. Mechanical ventilation increases the inflammatory response induced by lung contusion. J Surg Res. 2013;183:377–384. doi: 10.1016/j.jss.2012.12.042. [DOI] [PubMed] [Google Scholar]
- 99.Bhandari V, et al. Hyperoxia causes angiopoietin 2-mediated acute lung injury and necrotic cell death. Nat Med. 2006;12:1286–1293. doi: 10.1038/nm1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Greinacher A, et al. Characterization of the human neutrophil alloantigen-3a. Nat Med. 2010;16:45–48. doi: 10.1038/nm.2070. [DOI] [PubMed] [Google Scholar]
- 101.Kalbitz M, et al. Cardiac Depression in pigs after multiple trauma — characterization of posttraumatic structural and functional alterations. Sci Rep. 2017;7:17861. doi: 10.1038/s41598-017-18088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wilson NM, Wall J, Naganathar V, Brohi K, De’Ath HD. Mechanisms involved in secondary cardiac dysfunction in animal models of trauma and hemorrhagic shock. Shock. 2017;48:401–410. doi: 10.1097/SHK.0000000000000882. [DOI] [PubMed] [Google Scholar]
- 103.McKee CA, Lukens JR. Emerging roles for the immune system in traumatic brain injury. Front Immunol. 2016;7:556. doi: 10.3389/fimmu.2016.00556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roth TL, et al. Transcranial amelioration of inflammation and cell death after brain injury. Nature. 2014;505:223–228. doi: 10.1038/nature12808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Braun M, et al. White matter damage after traumatic brain injury: A role for damage associated molecular patterns. Biochim Biophys Acta 1863. 2017;10(Pt B):2614–2626. doi: 10.1016/j.bbadis.2017.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Russo MV, McGavern DB. Inflammatory neuroprotection following traumatic brain injury. Science. 2016;353:783–785. doi: 10.1126/science.aaf6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lan X, Han X, Li Q, Yang QW, Wang J. Modulators of microglial activation and polarization after intracerebral haemorrhage. Nat Rev Neurol. 2017;13:420–433. doi: 10.1038/nrneurol.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nizamutdinov D, Shapiro LA. Overview of traumatic brain injury: an immunological context. Brain Sci. 2017 doi: 10.3390/brainsci7010011. http://dx.doi.org/10.3390/brainsci7010011. [DOI] [PMC free article] [PubMed]
- 109.Ruseva MM, Ramaglia V, Morgan BP, Harris CL. An anticomplement agent that homes to the damaged brain and promotes recovery after traumatic brain injury in mice. Proc Natl Acad Sci USA. 2015;112:14319–14324. doi: 10.1073/pnas.1513698112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Freeman L, et al. NLR members NLRC4 and NLRP3 mediate sterile inflammasome activation in microglia and astrocytes. J Exp Med. 2017;214:1351–1370. doi: 10.1084/jem.20150237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bird L. Neuroimmunology: immune signals packaged in the brain. Nat Rev Immunol. 2017;17:278–279. doi: 10.1038/nri.2017.43. [DOI] [PubMed] [Google Scholar]
- 112.Makinde HM, Cuda CM, Just TB, Perlman HR, Schwulst SJ. Nonclassical monocytes mediate secondary injury, neurocognitive outcome, and neutrophil infiltration after traumatic brain injury. J Immunol. 2017;199:3583–3591. doi: 10.4049/jimmunol.1700896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Morganti JM, et al. CCR2 antagonism alters brain macrophage polarization and ameliorates cognitive dysfunction induced by traumatic brain injury. J Neurosci. 2015;35:748–760. doi: 10.1523/JNEUROSCI.2405-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Laird MD, et al. High mobility group box protein-1 promotes cerebral edema after traumatic brain injury via activation of toll-like receptor 4. Glia. 2014;62:26–38. doi: 10.1002/glia.22581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ransohoff RM, Engelhardt B. The anatomical and cellular basis of immune surveillance in the central nervous system. Nat Rev Immunol. 2012;12:623–635. doi: 10.1038/nri3265. [DOI] [PubMed] [Google Scholar]
- 116.Sullan MJ, Asken BM, Jaffee MS, DeKosky ST, Bauer RM. Glymphatic system disruption as a mediator of brain trauma and chronic traumatic encephalopathy. Neurosci Biobehav Rev. 2018;84:316–324. doi: 10.1016/j.neubiorev.2017.08.016. [DOI] [PubMed] [Google Scholar]
- 117.Engelhardt B, Vajkoczy P, Weller RO. The movers and shapers in immune privilege of the CNS. Nat Immunol. 2017;18:123–131. doi: 10.1038/ni.3666. [DOI] [PubMed] [Google Scholar]
- 118.Utagawa A, Truettner JS, Dietrich WD, Bramlett HM. Systemic inflammation exacerbates behavioral and histopathological consequences of isolated traumatic brain injury in rats. Exp Neurol. 2008;211:283–291. doi: 10.1016/j.expneurol.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Dash PK, et al. Activation of α7 cholinergic nicotinic receptors reduce blood-brain barrier permeability following experimental traumatic brain injury. J Neurosci. 2016;36:2809–2818. doi: 10.1523/JNEUROSCI.3197-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Diamond B, Tracey KJ. Mapping the immunological homunculus. Proc Natl Acad Sci USA. 2011;108:3461–3462. doi: 10.1073/pnas.1100329108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pavlov VA, Tracey KJ. Neural regulation of immunity: molecular mechanisms and clinical translation. Nat Neurosci. 2017;20:156–166. doi: 10.1038/nn.4477. [DOI] [PubMed] [Google Scholar]
- 122.Woiciechowsky C, et al. Sympathetic activation triggers systemic interleukin-10 release in immunodepression induced by brain injury. Nat Med. 1998;4:808–813. doi: 10.1038/nm0798-808. [DOI] [PubMed] [Google Scholar]
- 123.Di Battista AP, et al. Inflammatory cytokine and chemokine profiles are associated with patient outcome and the hyperadrenergic state following acute brain injury. J Neuroinflammation. 2016;13:40. doi: 10.1186/s12974-016-0500-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shein SL, et al. Hemorrhagic shock shifts the serum cytokine profile from pro- to anti-inflammatory after experimental traumatic brain injury in mice. J Neurotrauma. 2014;31:1386–1395. doi: 10.1089/neu.2013.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Di Battista AP, et al. Sympathoadrenal activation is associated with acute traumatic coagulopathy and endotheliopathy in isolated brain injury. Shock. 2016;46:96–103. doi: 10.1097/SHK.0000000000000642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tian Y, et al. Brain-derived microparticles induce systemic coagulation in a murine model of traumatic brain injury. Blood. 2015;125:2151–2159. doi: 10.1182/blood-2014-09-598805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Maegele M, et al. Coagulopathy and haemorrhagic progression in traumatic brain injury: advances in mechanisms, diagnosis, and management. Lancet Neurol. 2017;16:630–647. doi: 10.1016/S1474-4422(17)30197-7. [DOI] [PubMed] [Google Scholar]
- 128.Kumar A, et al. Microglial-derived microparticles mediate neuroinflammation after traumatic brain injury. J Neuroinflammation. 2017;14:47. doi: 10.1186/s12974-017-0819-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chang R, Cardenas JC, Wade CE, Holcomb JB. Advances in the understanding of trauma-induced coagulopathy. Blood. 2016;128:1043–1049. doi: 10.1182/blood-2016-01-636423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hijazi N, et al. Endogenous plasminogen activators mediate progressive intracerebral hemorrhage after traumatic brain injury in mice. Blood. 2015;125:2558–2567. doi: 10.1182/blood-2014-08-588442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Leinhase I, et al. Inhibition of the alternative complement activation pathway in traumatic brain injury by a monoclonal anti-factor B antibody: a randomized placebo-controlled study in mice. J Neuroinflammation. 2007;4:13. doi: 10.1186/1742-2094-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yasui H, Donahue DL, Walsh M, Castellino FJ, Ploplis VA. Early coagulation events induce acute lung injury in a rat model of blunt traumatic brain injury. Am J Physiol Lung Cell Mol Physiol. 2016;311:L74–L86. doi: 10.1152/ajplung.00429.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Dai SS, et al. Plasma glutamate-modulated interaction of A2AR and mGluR5 on BMDCs aggravates traumatic brain injury-induced acute lung injury. J Exp Med. 2013;210:839–851. doi: 10.1084/jem.20122196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hu PJ, Pittet JF, Kerby JD, Bosarge PL, Wagener BM. Acute brain trauma, lung injury, and pneumonia: more than just altered mental status and decreased airway protection. Am J Physiol Lung Cell Mol Physiol. 2017;313:L1–L15. doi: 10.1152/ajplung.00485.2016. [DOI] [PubMed] [Google Scholar]
- 135.Okusa MD, Rosin DL, Tracey KJ. Targeting neural reflex circuits in immunity to treat kidney disease. Nat Rev Nephrol. 2017;13:669–680. doi: 10.1038/nrneph.2017.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Moore EM, et al. The incidence of acute kidney injury in patients with traumatic brain injury. Ren Fail. 2010;32:1060–1065. doi: 10.3109/0886022X.2010.510234. [DOI] [PubMed] [Google Scholar]
- 137.Lu R, Kiernan MC, Murray A, Rosner MH, Ronco C. Kidney-brain crosstalk in the acute and chronic setting. Nat Rev Nephrol. 2015;11:707–719. doi: 10.1038/nrneph.2015.131. [DOI] [PubMed] [Google Scholar]
- 138.Gao M, et al. Systemic Administration of induced neural stem cells regulates complement activation in mouse closed head injury models. Sci Rep. 2017;7:45989. doi: 10.1038/srep45989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Nongnuch A, Panorchan K, Davenport A. Brain-kidney crosstalk. Crit Care. 2014;18:225. doi: 10.1186/cc13907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Molitoris BA. Therapeutic translation in acute kidney injury: the epithelial/endothelial axis. J Clin Invest. 2014;124:2355–2363. doi: 10.1172/JCI72269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Abe C, et al. C1 neurons mediate a stress-induced anti-inflammatory reflex in mice. Nat Neurosci. 2017;20:700–707. doi: 10.1038/nn.4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Campbell SJ, et al. Central nervous system injury triggers hepatic CC and CXC chemokine expression that is associated with leukocyte mobilization and recruitment to both the central nervous system and the liver. Am J Pathol. 2005;166:1487–1497. doi: 10.1016/S0002-9440(10)62365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nizamutdinov D, et al. Hepatic alterations are accompanied by changes to bile acid transporter-expressing neurons in the hypothalamus after traumatic brain injury. Sci Rep. 2017;7:40112. doi: 10.1038/srep40112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Campbell SJ, et al. Liver Kupffer cells control the magnitude of the inflammatory response in the injured brain and spinal cord. Neuropharmacology. 2008;55:780–787. doi: 10.1016/j.neuropharm.2008.06.074. [DOI] [PubMed] [Google Scholar]
- 145.Ma J, et al. Impacts of blast-induced traumatic brain injury on expressions of hepatic cytochrome P450 1A2, 2B1, 2D1, and 3A2 in rats. Cell Mol Neurobiol. 2017;37:111–120. doi: 10.1007/s10571-016-0351-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sundman MH, Chen NK, Subbian V, Chou YH. The bidirectional gut-brain-microbiota axis as a potential nexus between traumatic brain injury, inflammation, and disease. Brain Behav Immun. 2017;66:31–44. doi: 10.1016/j.bbi.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 147.Katzenberger RJ, Ganetzky B, Wassarman DA. The gut reaction to traumatic brain injury. Fly (Austin) 2015;9:68–74. doi: 10.1080/19336934.2015.1085623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kozlov AV, Bahrami S, Redl H, Szabo C. Alterations in nitric oxide homeostasis during traumatic brain injury. Biochim Biophys Acta. 2017;1863:2627–2632. doi: 10.1016/j.bbadis.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 149.Rizoli SB, et al. Catecholamines as outcome markers in isolated traumatic brain injury: the COMA-TBI study. Crit Care. 2017;21:37. doi: 10.1186/s13054-017-1620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6:775–786. doi: 10.1038/nrn1765. [DOI] [PubMed] [Google Scholar]
- 151.Hazeldine J, Lord JM, Belli A. Traumatic brain injury and peripheral immune suppression: primer and prospectus. Front Neurol. 2015;6:235. doi: 10.3389/fneur.2015.00235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gadani SP, Smirnov I, Smith AT, Overall CC, Kipnis J. Characterization of meningeal type 2 innate lymphocytes and their response to CNS injury. J Exp Med. 2017;214:285–296. doi: 10.1084/jem.20161982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Walker PA, et al. Intravenous multipotent adult progenitor cell therapy for traumatic brain injury: preserving the blood brain barrier via an interaction with splenocytes. Exp Neurol. 2010;225:341–352. doi: 10.1016/j.expneurol.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Schwulst SJ, Trahanas DM, Saber R, Perlman H. Traumatic brain injury-induced alterations in peripheral immunity. J Trauma Acute Care Surg. 2013;75:780–788. doi: 10.1097/TA.0b013e318299616a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Johansson ME, Hansson GC. Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol. 2016;16:639–649. doi: 10.1038/nri.2016.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Perez-Lopez A, Behnsen J, Nuccio SP, Raffatellu M. Mucosal immunity to pathogenic intestinal bacteria. Nat Rev Immunol. 2016;16:135–148. doi: 10.1038/nri.2015.17. [DOI] [PubMed] [Google Scholar]
- 157.Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–144. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 158.Patel JJ, Rosenthal MD, Miller KR, Martindale RG. The gut in trauma. Curr Opin Crit Care. 2016;22:339–346. doi: 10.1097/MCC.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 159.Sodhi CP, et al. Intestinal epithelial TLR-4 Activation is required for the development of acute lung injury after trauma/hemorrhagic shock via the release of HMGB1 from the Gut. J Immunol. 2015;194:4931–4939. doi: 10.4049/jimmunol.1402490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Dalle Lucca JJ, et al. Effects of C1 inhibitor on tissue damage in a porcine model of controlled hemorrhage. Shock. 2012;38:82–91. doi: 10.1097/SHK.0b013e31825a3522. [DOI] [PubMed] [Google Scholar]
- 161.Fishman JE, et al. Intraluminal nonbacterial intestinal components control gut and lung injury after trauma hemorrhagic shock. Ann Surg. 2014;260:1112–1120. doi: 10.1097/SLA.0000000000000631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.DeLano FA, Hoyt DB, Schmid-Schönbein GW. Pancreatic digestive enzyme blockade in the intestine increases survival after experimental shock. Sci Transl Med. 2013;5:169ra11. doi: 10.1126/scitranslmed.3005046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Grootjans J, et al. Level of activation of the unfolded protein response correlates with Paneth cell apoptosis in human small intestine exposed to ischemia/reperfusion. Gastroenterology. 2011;140:529–539. doi: 10.1053/j.gastro.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 164.Moore FA, et al. Gut bacterial translocation via the portal vein: a clinical perspective with major torso trauma. J Trauma. 1991;31:629–636. doi: 10.1097/00005373-199105000-00006. [DOI] [PubMed] [Google Scholar]
- 165.Buttenschoen K, et al. Plasma concentrations of endotoxin and antiendotoxin antibodies in patients with multiple injuries: a prospective clinical study. Eur J Surg. 1996;162:853–860. [PubMed] [Google Scholar]
- 166.Charbonney E, et al. Endotoxemia following multiple trauma: risk factors and prognostic implications. Crit Care Med. 2016;44:335–341. doi: 10.1097/CCM.0000000000001404. [DOI] [PubMed] [Google Scholar]
- 167.Levy G, et al. Parasympathetic stimulation via the vagus nerve prevents systemic organ dysfunction by abrogating gut injury and lymph toxicity in trauma and hemorrhagic shock. Shock. 2013;39:39–44. doi: 10.1097/SHK.0b013e31827b450d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Deitch EA. Gut-origin sepsis: evolution of a concept. Surgeon. 2012;10:350–356. doi: 10.1016/j.surge.2012.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lee MA, Yatani A, Sambol JT, Deitch EA. Role of gut-lymph factors in the induction of burn-induced and trauma-shock-induced acute heart failure. Int J Clin Exp Med. 2008;1:171–180. [PMC free article] [PubMed] [Google Scholar]
- 170.Fang JF, et al. Proteomic analysis of post-hemorrhagic shock mesenteric lymph. Shock. 2010;34:291–298. doi: 10.1097/SHK.0b013e3181ceef5e. [DOI] [PubMed] [Google Scholar]
- 171.Dai H, Sun T, Liu Z, Zhang J, Zhou M. The imbalance between regulatory and IL-17-secreting CD4+ T cells in multiple-trauma rat. Injury. 2013;44:1521–1527. doi: 10.1016/j.injury.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 172.Morishita K, Coimbra R, Langness S, Eliceiri BP, Costantini TW. Neuroenteric axis modulates the balance of regulatory T cells and T-helper 17 cells in the mesenteric lymph node following trauma/hemorrhagic shock. Am J Physiol Gastrointest Liver Physiol. 2015;309:G202–G208. doi: 10.1152/ajpgi.00097.2015. [DOI] [PubMed] [Google Scholar]
- 173.Matteoli G, Boeckxstaens GE. The vagal innervation of the gut and immune homeostasis. Gut. 2013;62:1214–1222. doi: 10.1136/gutjnl-2012-302550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kojima M, et al. Exosomes, not protein or lipids, in mesenteric lymph activate inflammation: unlocking the mystery of post-shock multiple organ failure. J Trauma Acute Care Surg. 2017;82:42–50. doi: 10.1097/TA.0000000000001296. [DOI] [PubMed] [Google Scholar]
- 175.Tiesi G, et al. Early trauma-hemorrhage-induced splenic and thymic apoptosis is gut-mediated and toll-like receptor 4-dependent. Shock. 2013;39:507–513. doi: 10.1097/SHK.0b013e318293d020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ibiza S, et al. Glial-cell-derived neuroregulators control type 3 innate lymphoid cells and gut defence. Nature. 2016;535:440–443. doi: 10.1038/nature18644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Langness S, Kojima M, Coimbra R, Eliceiri BP, Costantini TW. Enteric glia cells are critical to limiting the intestinal inflammatory response after injury. Am J Physiol Gastrointest Liver Physiol. 2017;312:G274–G282. doi: 10.1152/ajpgi.00371.2016. [DOI] [PubMed] [Google Scholar]
- 178.Ma EL, et al. Bidirectional brain-gut interactions and chronic pathological changes after traumatic brain injury in mice. Brain Behav Immun. 2017;66:56–69. doi: 10.1016/j.bbi.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Oyeniyi BT, et al. Trends in 1029 trauma deaths at a level 1 trauma center: Impact of a bleeding control bundle of care. Injury. 2017;48:5–12. doi: 10.1016/j.injury.2016.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Lefering R, et al. Epidemiology of in-hospital trauma deaths. Eur J Trauma Emerg Surg. 2012;38:3–9. doi: 10.1007/s00068-011-0168-4. [DOI] [PubMed] [Google Scholar]
- 181.Prin M, Li G. Complications and in-hospital mortality in trauma patients treated in intensive care units in the United States, 2013. Inj Epidemiol. 2016;3:18. doi: 10.1186/s40621-016-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Spruijt NE, Visser T, Leenen LP. A systematic review of randomized controlled trials exploring the effect of immunomodulative interventions on infection, organ failure, and mortality in trauma patients. Crit Care. 2010;14:R150. doi: 10.1186/cc9218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Mann AP, et al. A peptide for targeted, systemic delivery of imaging and therapeutic compounds into acute brain injuries. Nat Commun. 2016;7:11980. doi: 10.1038/ncomms11980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Yang R, et al. Anti-HMGB1 neutralizing antibody ameliorates gut barrier dysfunction and improves survival after hemorrhagic shock. Mol Med. 2006;12:105–114. doi: 10.2119/2006-00010.Yang. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ruan X, et al. Anti-HMGB1 monoclonal antibody ameliorates immunosuppression after peripheral tissue trauma: attenuated T-lymphocyte response and increased splenic CD11b+Gr-1+ myeloid-derived suppressor cells require HMGB1. Mediators Inflamm. 2015;2015:458626. doi: 10.1155/2015/458626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Okuma Y, et al. Anti-high mobility group box-1 antibody therapy for traumatic brain injury. Ann Neurol. 2012;72:373–384. doi: 10.1002/ana.23602. [DOI] [PubMed] [Google Scholar]
- 187.Kimbler DE, Shields J, Yanasak N, Vender JR, Dhandapani KM. Activation of P2X7 promotes cerebral edema and neurological injury after traumatic brain injury in mice. PLoS One. 2012;7:e41229. doi: 10.1371/journal.pone.0041229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Abrams ST, et al. Circulating histones are mediators of trauma-associated lung injury. Am J Respir Crit Care Med. 2013;187:160–169. doi: 10.1164/rccm.201206-1037OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Heeres M, et al. The effect of C1-esterase inhibitor on systemic inflammation in trauma patients with a femur fracture – The CAESAR study: study protocol for a randomized controlled trial. Trials. 2011;12:223. doi: 10.1186/1745-6215-12-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rich MC, et al. Site-targeted complement inhibition by a complement receptor 2-conjugated inhibitor (mTT30) ameliorates post-injury neuropathology in mouse brains. Neurosci Lett. 2016;617:188–194. doi: 10.1016/j.neulet.2016.02.025. [DOI] [PubMed] [Google Scholar]
- 191.Fluiter K, Opperhuizen AL, Morgan BP, Baas F, Ramaglia V. Inhibition of the membrane attack complex of the complement system reduces secondary neuroaxonal loss and promotes neurologic recovery after traumatic brain injury in mice. J Immunol. 2014;192:2339–2348. doi: 10.4049/jimmunol.1302793. [DOI] [PubMed] [Google Scholar]
- 192.Lee S, et al. A novel antagonist of p75NTR reduces peripheral expansion and CNS trafficking of pro-inflammatory monocytes and spares function after traumatic brain injury. J Neuroinflammation. 2016;13:88. doi: 10.1186/s12974-016-0544-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Xu X, et al. Anti-inflammatory and immunomodulatory mechanisms of atorvastatin in a murine model of traumatic brain injury. J Neuroinflammation. 2017;14:167. doi: 10.1186/s12974-017-0934-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Yamada N, et al. Novel synthetic, host-defense peptide protects against organ injury/dysfunction in a rat model of severe hemorrhagic shock. Ann Surg. 2017 doi: 10.1097/SLA.0000000000002186. http://doi.org/10.1097/SLA.0000000000002186. [DOI] [PubMed]
- 195.Nikolian VC, et al. Valproic acid decreases brain lesion size and improves neurologic recovery in swine subjected to traumatic brain injury, hemorrhagic shock, and polytrauma. J Trauma Acute Care Surg. 2017;83:1066–1073. doi: 10.1097/TA.0000000000001612. [DOI] [PubMed] [Google Scholar]
- 196.Sordi R, et al. Artesunate protects against the organ injury and dysfunction induced by severe hemorrhage and resuscitation. Ann Surg. 2017;265:408–417. doi: 10.1097/SLA.0000000000001664. [DOI] [PubMed] [Google Scholar]
- 197.Laplante P, et al. MFG-E8 reprogramming of macrophages promotes wound healing by increased bFGF production and fibroblast functions. J Invest Dermatol. 2017;137:2005–2013. doi: 10.1016/j.jid.2017.04.030. [DOI] [PubMed] [Google Scholar]
- 198.Nielson JL, et al. Topological data analysis for discovery in preclinical spinal cord injury and traumatic brain injury. Nat Commun. 2015;6:8581. doi: 10.1038/ncomms9581. [DOI] [PMC free article] [PubMed] [Google Scholar]


