Abstract
Hepatocellular carcinoma (HCC) is one of the most common primary hepatic malignancies and one of the fastest-growing causes of cancer-related mortality in the United States. The molecular basis of HCC carcinogenesis has not been clearly identified. Among the molecular signaling pathways implicated in the pathogenesis of HCC, the Wnt/β-catenin signaling pathway is one of the most frequently activated. A great effort is under way to clearly understand the role of this pathway in the pathogenesis of HCC and its role in the transition from chronic liver diseases, including viral hepatitis, to hepatocellular adenomas (HCAs) and HCCs and its targetability in novel therapies. In this article, we review the role of the β-catenin pathway in hepatocarcinogenesis and progression from chronic inflammation to HCC, the novel potential treatments targeting the pathway and its prognostic role in HCC patients, as well as the imaging features of HCC and their association with aberrant activation of the pathway.
Keywords: hepatocellular carcinoma, Wnt/β-catenin, gadoxetic acid-enhanced magnetic resonance imaging, molecular therapy
Introduction
Hepatocellular carcinoma (HCC) is the most common primary hepatic malignancy, constituting around 85–90% of primary liver cancers. The incidence of HCC is on the rise, and HCC is now the fastest-growing cause of cancer-related mortality in the United States.1–3 HCC is also the third leading cause of cancer-related mortality in the world,4,5 causing approximately 750,000 deaths worldwide in 2012.3 It is the sixth most common malignancy.4,6 The number of cases reported annually reaches more than half a million worldwide.7,8
HCC frequently develops in the setting of underlying chronic liver disease. Viral hepatitis (infection with either hepatitis C virus [HCV] or hepatitis B virus [HBV]) is thought to be the most common etiology of HCC.9 However, other factors may contribute to the development of HCC. The interaction of aflatoxin B1 (a contaminant found in food) with HBV infection is believed to increase the prevalence of HCC.10 Cirrhosis due to non-alcoholic steatohepatitis is also associated with the development of HCC.7 In addition to underlying liver disease, lifestyle factors such as tobacco use, alcohol use, and obesity may also contribute to the development of HCC.6,7,11
It is known that genetic mutations and abnormal activation of signal transduction pathways are involved in the development of HCC.1,6,7,11–13 However, it has been difficult to elucidate the specific molecular pathophysiology that leads to HCC tumor development.3,12 Unfortunately, this lack of understanding has limited the development of targeted molecular therapies to treat HCC,3 and the prognosis of HCC has remained poor.12,14,15 Signaling pathways involved in cell proliferation, apoptosis, metabolism, splicing, and the cell cycle are believed to play a vital role in the formation of HCC. The signaling pathways known to be activated in HCC tissue include the Wnt/β-catenin pathway (up to 50% of HCC), the phosphatidylinositol-3-kinase and protein kinase B (PI3K/Akt) pathway (40–60% of HCC), the Myc pathway (30–60%), the Hedgehog pathway (50–60%), and the MET pathway (30–40%).2,7,12,15–17 The Wnt/β-catenin pathway regulates multiple cellular processes that are involved in the initiation, growth, survival, migration, differentiation, and apoptosis of HCC.2,6,8,13,18 The Wnt/β-catenin pathway has shown significant promise as a potential target for novel molecular therapies. Also, β-catenin mutation has been shown to affect the prognosis of HCC, which warrants more understanding of the role of this pathway in developing HCC.
Materials and methods
In this article, we review the role of the β-catenin pathway in hepatocarcinogenesis and progression from chronic liver disease to HCC, the novel potential treatments targeting the pathway and its prognostic role in HCC patients, as well as the imaging features of HCC on magnetic resonance imaging (MRI) and computed tomography (CT) associated with aberrant activation of the pathway.
CTNNB1 and β-catenin
Previous attempts to understand the pathogenesis of HCC have identified the β-catenin gene (CTNNB1) as one of the primary oncogenes involved in HCC development.1,6,10 CTNNB1 encodes β-catenin, a protein that serves multiple important cellular functions. β-catenin is found in the adherens junctions and plays a key role in intercellular adhesion and communication.1,9,13,19–23 In particular, deletions or missense mutations in exon 3 of this gene are thought to be the most common genetic abnormality leading to the development of HCC.1,12,16,18,23–26 Previous studies have shown a correlation between CTNNB1 missense mutations and increased nuclear and cytoplasmic expression of β-catenin, suggesting that these mutations cause aberrant activation of the Wnt pathway and play a role in the development of HCC.1,7,18,22,26 Table 1 shows a summary of the clinical studies on β-catenin and HCC with their clinical significance.
Table 1.
Summary of different studies concerning β-catenin in HCC and their clinical significance
| Reference | Patient number | Cell lines | Test | Protein expression | Genetic mutation | Clinical significance |
|---|---|---|---|---|---|---|
| de La Coste et al, 199810 | 31 | Human HCC | Western blot, RT-PCR, Immunofluorescence staining | β-Catenin | CTNNB1 activating mutation (26%) | Dysregulation of Wnt β-catenin pathway is implicated in the development of cancer |
| Huang et al, 199933 | 22 | Human HCC with HCV infection | SSCP, direct DNA sequencing, immunohistochemistry | β-Catenin | APC and CTNNB1 mutations (41%) | β-catenin mutations contributes to HCC development in HCV patients |
| Kondo et al, 199916 | 38 | Human HCC | Immunohistochemistry, PCR-SSCP | β-Catenin | CTNNB1 mutation (24%) | Mutations of Exon3 leads to accumulation of β-catenin which is associated with the malignant progression of HCC |
| Nhieu et al, 199921 | 32 | Human HCC | Immunohistochemistry, PCR | β-Catenin | CTNNB1 somatic mutations in exon 3 (34%) | Nuclear expression of β-catenin correlated significantly with increased Ki-67 and associated with poor prognosis. |
| Hsu et al, 200019 | 434 | Human HCC | Immunohistochemistry, PCR, Southern blot for HBV | β-Catenin | GSK-3β phosphorylation site mutations (13.1%) | β-catenin mutations were more frequent with older patients and associated with Grade I HCC. HCC with mutant nuclear β-catenin expression had better 5-year survival than wild type. |
| Mao et al, 200120 | 372 | Human HCC | Immunohistochemistry, PCR | β-Catenin | CTNNB1 mutations (14.1%) | Nuclear β-catenin expression correlated strongly with gene mutation and is associated with good prognosis |
| Inagawa et al, 200237 | 51 | Human HCC grades I, II, III | Immunohistochemistry | Nuclear β-Catenin | NA | β-catenin promotes tumor progression by stimulating cell proliferation and reducing cell adhesion, associated with poor prognosis |
| Austinat et al, 200836 | 40 | Human HCC | Immunohistochemistry TOP-flash reporter PCR | β-catenin, glutamine synthase | CTNNB1 exon 3 mutation (25%) | Diagnostic value of GS expression for detection of β-catenin mutations is doubtful |
| Yuan et al, 201139 | 210 | Human HCC | Immunohistochemistry, direct DNA sequencing | CK19 | p53 (47.2%) and β-catenin (14.5%) mutations | CK19 expression with mutated p53 is associated with more aggressive HCC. β-catenin mutation is associated with low-grade HCC and better 5-year survival. |
| Lee, 20137 | 89 | Human HCC transfected with WT or mutant β-catenin | TOPFlash reporter activity | Nuclear and cytoplasmic β-catenin | β-catenin-T cell factor transactivation | CTNNB1 mutation and fibrosis are independent risk factors for the development of HCC. N/C β-catenin was associated with reduced fibrosis while C/N β-catenin with increased inflammation and proliferation. |
| Ueno et al, 201444 | 34 | Human HCC | qRT-PCR, immunohistochemistry | β-Catenin, OATP1B3 | Wnt/β-catenin target genes | OATP1B3 had strong correlation with tumor enhancement in hepatobiliary phase of EOB-MRI and with Wnt/β-catenin signaling |
| Lu et al, 201418 | 115 | Human HCC | Direct exon 3 sequencing of CTNNB1 | CTNNB1 somatic mutations (17.5% advanced 20% early stage) | Patients over 60 years more likely to have CTNNB1 mutations. No association with survival. | |
| Li et al, 2014 1 | 126 | Human HCC, paracarcinoma tissue and cirrhotic liver | RT-PCR, immunohistochemistry | Wnt-5a and β-catenin | Wnt-5a mRNA (73.1%) | Increased Wnt-5a mRNA expression (73.1%) and abnormal localization of β-catenin protein (72.9 %) in HCC samples |
| Friemel et al, 201511 | 23 | Human HCC | Immunohistochemistry, PCR | β-Catenin | TP53 and β-catenin mutations | Heterogeneous intratumor mutational status in 20% of HCC which may contribute to difficult classification and treatment failure. |
| Kitao, 201527 | 138 | Human HCC | Immunohistochemistry | β-Catenin, glutamine synthase and OATP1B3 | NA | HCCs with β-catenin mutations (19.5%) showed higher differentiation and higher enhancement on gadoxetic acid MRI especially during hepatobiliary phase and high ADCs at DWI MRI |
| Kim et al, 201630 | 245 | Human HCC with HBV infection | Genotyping by Golden gate genotyping assay kit | NA | SNPs in the AXIN1, CTNNB1, and WNT2 genes | Genetic polymorphisms in CTNNB1 and AXIN1 genes could be associated with HCC development and overall survival in HBV patients. |
| Rebouissou et al, 201640 | 220 HCA/373 HCC/17 borderline lesions | Human HCA/HCC | qRT-PCR, immunohistochemistry | β-Catenin | CTNNB1 weak, moderate, highly active mutations (25% of HCA, 39% of HCC, and 65% of borderline lesions) | High β-catenin by specific CTNNB1 mutations and S45 allele duplication is associated with malignant transformation of HCAs |
| Ziv et al, 201741 | 17 | Human HCC treated with embolization | 341-gene panel next generation sequence assay | NA | CTNNB1, MEN1 and NCOR1 gene mutations | Upregulation of the Wnt/β-catenin signaling pathway may be associated with sensitivity to embolization |
Abbreviations: HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HBV, hepatitis B virus; NA, not analyzed; MRI, magnetic resonance imaging; SSCP, single-strand conformation polymorphism; HCA, hepatocellular adenoma; qRT-PCR, quantitative real-time polymerase chain reaction; EOB-MRI, gadolinium-enhanced MRI; ADC, apparent diffusion coefficient; SNPs, single nuclear polymorphisms; DWI, diffusion weighted image.
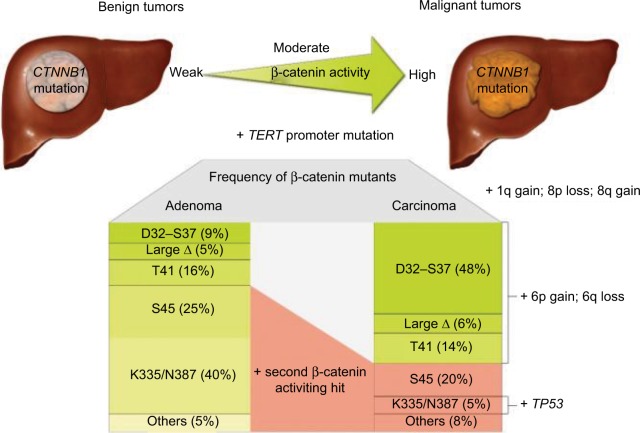
HCC can develop in healthy livers without the presence of fibrosis; in fact, most HCC tumors that contain β-catenin mutation develop from normal liver tissue. Some HCC tumors develop from the malignant transformation of hepatocellular adenomas (HCAs) (Figure 1). These HCC tumors typically have mutations in the β-catenin gene. Notably, pre-malignant liver lesions, such as cirrhotic nodules, have never been found to have mutations in the β-catenin gene.2,3,27
Figure 1.
Carcinogenesis in HCC with β-catenin mutation with different β-catenin mutants identified, leading to different levels of β-catenin activation depending on the state of tumor progression. Reproduced with permission from Rebouissou S, Franconi A, Calderaro J, et al. Genotype-phenotype correlation of CTNNB1 mutations reveals different β-catenin activity associated with liver tumor progression. Hepatology. 2016;64(6):2047–2061. Copyright John Wiley and Sons.40
Abbreviation: HCC, hepatocellular carcinoma.
Immunohistochemical staining reveals that β-catenin is localized primarily in the hepatocyte membrane in normal liver tissue, as shown by Li et al using clear linear brown staining.1 However, in cirrhosis and HCC, β-catenin shows significantly decreased expression in the membrane and increased expression in the cytoplasm and nucleus. This abnormal β-catenin protein expression was significantly greater in HCC tissue (72.94% [62/85]) compared with para-carcinoma tissues and hepatic cirrhosis tissues (22.35% [19/85] and 26.67% [4/15], respectively; P < 0.001).1,7,21
CTNBB1 and Axin1 mutation
CTNNB1 mutation leading to activation of the Wnt pathway and the resulting accumulation of β-catenin have been associated with well-to-moderately differentiated tumors with small size and good overall prognosis. Figure 2 demonstrates the immunohistochemical staining of β-catenin protein in moderately differentiated HCC with β-catenin mutation. However, the nuclear expression of β-catenin due to its mutation in HCC has been associated with an increased incidence of microvascular and macrovascular invasion (78% in HCC with β-catenin mutation vs. 38% in HCC without β-catenin mutation), doubled tumor size (compared with tumors without β-catenin mutation), and multiplicity of nodules.28
Figure 2.
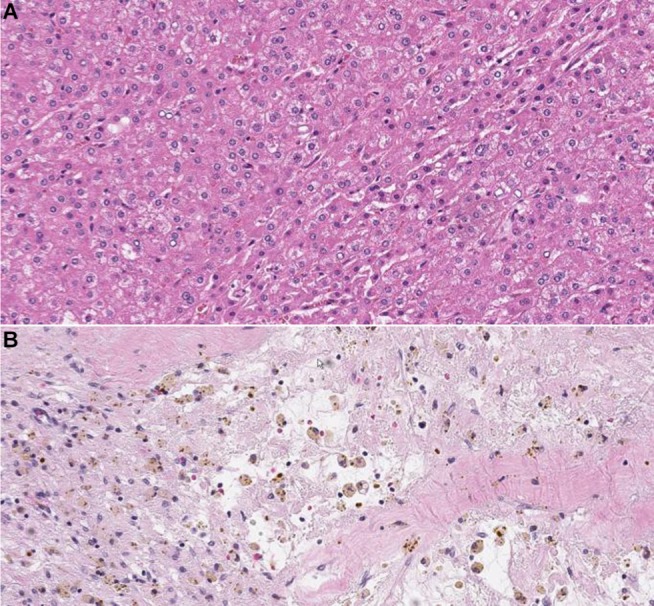
Hematoxylin-eosin staining showing moderately-differentiated HCC (A). At immunohistochemical staining (B), the tumor shows expression of β-catenin protein in the nucleus and/or cytoplasm. Magnification ×200.
Abbreviation: HCC, hepatocellular carcinoma.
There is strong evidence that in human HCC, gain-of-function β-catenin mutations are greatly different from inactivating mutations of Axin1. Axin mutations (and, rarely, β-catenin mutations) are found mainly in chromosomally unstable HCCs that are associated with HBV infection, while β-catenin mutations are found mainly in non-HBV, well-differentiated, chromosomally stable HCCs.8,29
WNT signaling
WNT signaling in normal tissue and HCC development
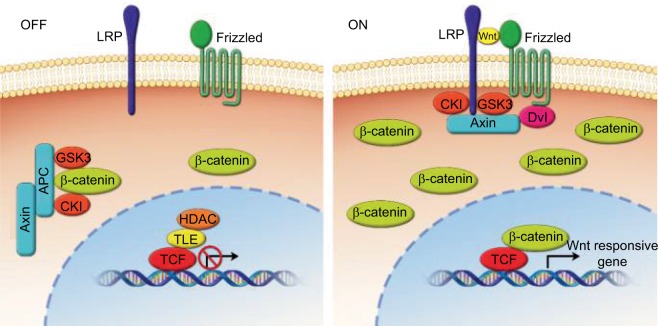
The WNT signaling pathway serves many vital functions in embryonic patterning, cell proliferation, differentiation, and angiogenesis. It is heavily involved in the development of embryonic tissues before birth and is also believed to play a role in the maintenance of stem cells in adult tissues.1,12,24 The Wnt pathway is categorized into a canonical pathway (β-catenin dependent) and a non-canonical pathway (β-catenin independent).9,12,14 Both categories of Wnt signaling begin when extracellular Wnt ligands bind to receptors on the cell membrane. In the canonical pathway, binding of Wnt ligands to the transmembrane receptor’s Wnt/FZD-related protein and to the low-density lipoprotein receptor results in the dissolution of the proteasome complex (APC protein, axin, and glycogen synthase kinase 3 [GSK-3]), and this complex is usually responsible for the destruction of β-catenin. Destruction of this complex results in the accumulation of β-catenin in the cytoplasm and its subsequent relocation into the nucleus, where β-catenin forms a binding complex with the transcription factor, i.e., lymphoid enhancer factor/T cell factor (LEF/TCF) proteins. This complex then regulates the transcription of target genes that are involved in cell proliferation, migration, cell cycle regulation, and metastasis (Figure 3).1,3,7,9,14,15,19–21,23,24,27,30,31
Figure 3.
Overview of Wnt/β-catenin signaling.
Notes: In the off-state, or absence of Wnt, cytoplasmic β-catenin forms a complex with APC, axin, CKI, and GSK-3 and then is targeted for proteosomal degradation while Wnt target genes are repressed by TCF/TLE and HDAC. In the on-state, or presence of Wnt, a receptor complex forms between Frizzled and lipoprotein receptor-related protein families, which leads to accumulation of β-catenin in the cytoplasm and nucleus, where it serves as a coactivator for T cell-factor proteins to activate Wnt-responsive genes.24
Abbreviations: CKI, cyclin-dependent kinase inhibitor; GSK-3, glycogen synthase kinase 3; TCF/TLE, T cell-factor proteins/transducin like enhancer of split; HDAC, histone deacetylases; LRP, low-density-lipoprotein receptor-related protein; APC, adenomatous polyposis coli.
Wnt/β-catenin signaling plays an important role in hepatocyte development and function.1,6,32 In normal hepatocytes, levels of β-catenin are low owing to the presence of the abovementioned β-catenin destruction complex (APC, axin, and GSK-3). However, there are multiple ways in which the Wnt/β-catenin pathway can become aberrantly activated and cause the development and progression of HCC. Mutations that involve the β-catenin gene and the AXIN1/2 gene result in sustained aberrant activation of the Wnt/β-catenin pathway and thus dysregulate multiple cellular functions, including proliferation, apoptosis, and cell motility.1,3,7,19,31–33 Mutations in CTNNB1 can result in the production of β-catenin proteins that are resistant to degradation by the proteasome.3,7,14,20,31 In addition, loss-of-function mutations in APC and axin, which are members of the destruction complex, lead to the accumulation of β-catenin and allow for oncogenic transcription.9,14 β-catenin mutation is reportedly present in approximately 20–40% of all cases of HCC.3,9,27 AXIN1 mutation is seen in 3–16% of all HCC cases, and AXIN2 mutation is seen in 3% of all HCC cases.3,19,33,34 However, it is important to note that up to 40–60% of HCC tumors do not have mutations in CTNNB1, AXIN1, or AXIN2.3
Mutations that affect CTNNB1 and correlate with the amount of nuclear β-catenin are considered functionally important with regard to the development of HCC.3,35 However, a previous study using glutamine synthase as an immunohistochemical marker of β-catenin activity suggested that mutations in Axin1 may also exert their oncogenic effects through non-Wnt pathway mechanisms.29,35,36 In addition, other studies have demonstrated that factors affecting E-cadherin production can also lead to the accumulation of β-catenin in the cytosol and subsequent translocation of β-catenin to the nucleus. Some pathogens are able to stimulate β-catenin signaling by disrupting intercellular junctions and thus mobilizing β-catenin from complexes with cadherins.1,9,13,19–21,23,37
Hypoxia and activation of Wnt/β-catenin signaling
In addition to genetic mutations in Wnt signaling components, aberrant Wnt/β-catenin signaling in HCC tissue may be stimulated by hypoxia. Experiments have demonstrated crosstalk between Wnt/β-catenin and hypoxia signaling pathways.38 B-cell lymphoma 9 (BCL9) is an important co-activator of β-catenin–mediated transcription. Hypoxia-inducible factors, including HIF-1α, regulate the expression of BCL9 and thus can increase transcriptional activity of β-catenin. This increased transcriptional activity of β-catenin can occur in the absence of other mutations affecting Wnt components. Evidence suggests that hypoxia-inducible factors play a role in HCC development, likely through crosstalk between hypoxia signaling pathways and the Wnt/β-catenin signaling pathway. Increased expression of BCL9 in HCC tumors has been shown to be a poor prognostic indicator.14
Wnt/β-catenin pathway in the progression from chronic HCV/HBV to HCC
HCV is one of the most common causes of HCC worldwide. The mechanism by which HCV affects the development of HCC is not well understood, but it is believed that proteins that make up the HCV virus, including the HCV core protein and nonstructural protein 5A, independently stimulate the Wnt/β-catenin signaling pathway.9,37 It has also been shown that HCV-related HCC tumors have a significantly increased frequency of CTNNB1 mutations compared with HBV-related and non-viral HCC tumors. Notably, HCV is an RNA virus that exerts its effects on cells without going through a DNA intermediate phase. Thus, HCV’s effect on the hepatocyte genome is likely through an indirect mechanism.9,12 As chronic hepatitis infection progresses to cirrhosis, genetic mutations accumulate gradually.
In patients with HCC associated with HBV infection, aberrant WNT/β-catenin signaling appears to be more frequently stimulated by Axin1-inactivating mutations rather than by CTNNB1 mutations.29,30,34 However, CTNNB1 mutations are also present in some HBV-associated HCC. Single-nucleotide polymorphisms in AXIN1 and CTNNB1 in HBV-associated HCC were correlated with overall patient survival.30
Prognostic role of b-catenin mutation
β-catenin is involved in the development and progression of HCC, but its role in prognostication is less clear.6 There have been conflicting reports as to whether CTNNB1 mutations typically occur late or early in the development of HCC.3,23,24 Mutation in the β-catenin gene has been associated with less aggressive tumors, which are less likely to invade the vasculature and which contain more highly differentiated cells. β-catenin mutation is also more frequently seen in earlier stages of HCC (I and II) than in more advanced stages (III and IV).6,18,20,39 Patients with HCC tumors containing only β-catenin mutation also have lower serum α-fetoprotein levels. Higher α-fetoprotein levels are seen in tumors that contain mutations in other genes, such as p53 and cytokeratin 19 (CK19), and are associated with decreased patient survival.20,25,27 Also, patients over the age of 60 years are more likely to have CTNNB1 mutation than younger patients are.18–20 According to one study, HCC tumors that contain mutations in CTNNB1 are associated with older age, male sex, low serum α-fetoprotein levels, well-differentiated cells, large tumors, high alcohol intake, microscopic vascular invasion, tumor capsule invasion, and intra-tumor cholestasis.40 Some of the aforementioned disease characteristics are associated with good prognosis, like old age, low serum α-fetoprotein levels and well-differentiated cells, while others are associated with poorer prognosis, like microscopic vascular invasion and tumor capsule invasion.
β-catenin mutation is associated with increased nuclear and cytoplasmic β-catenin expression,2,3,19–21,31 and while nuclear β-catenin expression is associated with a relatively good prognosis, cytoplasmic and membranous β-catenin expression is associated with a poorer prognosis.19,20,31 Of note, it appears that prognosis is affected by whether the β-catenin that accumulates in the nucleus is mutated. Tumors with an accumulation of mutated β-catenin in the nucleus are associated with a better 5-year survival than tumors with increased levels of wild-type β-catenin in the nucleus.19,20 However, in contrast, a study by Inagawa et al showed that nuclear β-catenin expression in poorly differentiated HCCs is associated with increased tumor progression, recurrence, and poor prognosis.37
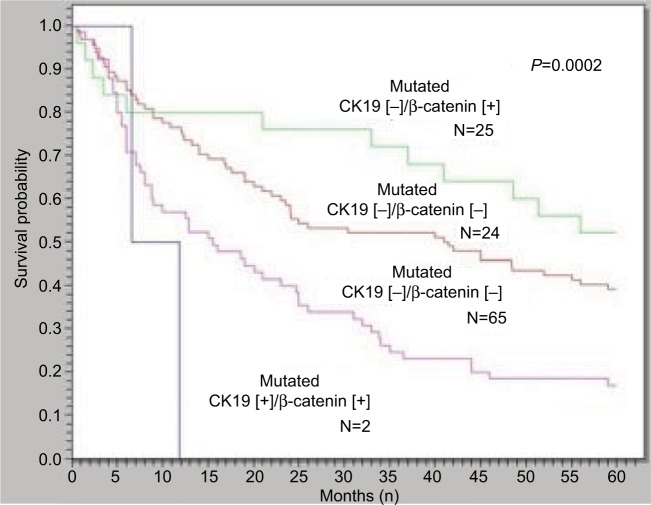
Previous studies have shown that 2.8–23.8% of patients with early-stage HCC have mutations in exon 3 of CTNNB1, which encodes the portion of the β-catenin protein that is involved in phosphorylation and degradation by the proteasome. In early HCC, these exon 3 mutations are associated with better prognosis in patients undergoing curative surgical resection of HCC tumors.10,19 According to one study showing the relation between β-catenin mutation and cytokeratin 19 (CK19) expression and their influence on HCC recurrence and prognosis, the frequency of vascular invasion and early tumor recurrence in HCCs with CK19 expression but not β-catenin mutation was higher than in HCCs without either β-catenin mutation or CK19 expression. HCCs with β-catenin mutation but not CK19 expression had the lowest frequencies of vascular invasion and early tumor recurrence.39 Additionally, the overall 5-year survival of HCCs with β-catenin mutation alone was significantly better than the HCCs without β-catenin mutation with CK19 expression (Figure 4).39
Figure 4.
HCCs with β-catenin mutation but not CK19 expression had the best 5-year survival rate, while HCCs with CK19 expression but not β-catenin mutation had the worst 5-year survival rate (P = 0.0002).
Notes: (+) = presence of CK19 expression or β-catenin mutation. (−) = absence of CK19 expression or β-catenin mutation. Reprinted by permission from Journal of Gastrointestinal Surgery, Springer Nature, Role of p53 and β-catenin mutations in conjunction with CK19 expression on early tumor recurrence and prognosis of hepatocellular carcinoma, Yuan RH, Jeng YM, Hu RH, et al, copyright 2010.39
Abbreviations: HCC, hepatocellular carcinoma; CK19, cytokeratin 19.
In one recent study, it was observed that the mutation rate of CTNNB1 in patients with advanced HCC was not higher than that in early-stage HCC tumors, suggesting that CTNNB1 mutation did not significantly affect prognosis.18
Wnt/b-catenin in predicting response to transarterial chemoembolization
An important goal of ongoing research is to predict the response of HCC tumors to embolization therapies. It has been demonstrated that there is no statistically significant difference in the overall survival of HCC patients treated with embolization and chemoembolization therapies, suggesting that the efficacy of embolization therapy is primarily mediated by hypoxia. However, hypoxia results in a wide variety of signaling interactions that ultimately may lead to cell death or even promote cell survival. HCC tumors with mutations in CTNNB1 and MEN1 (a promoter of Wnt signaling) have been shown to have better response to embolization therapy than those with wild-type CTNNB1 and MEN1. Conversely, tumors with mutations in NCOR1 (a negative regulator of β-catenin transcriptional target genes) do not respond as well to embolization therapy (Figure 5).14,41
Figure 5.
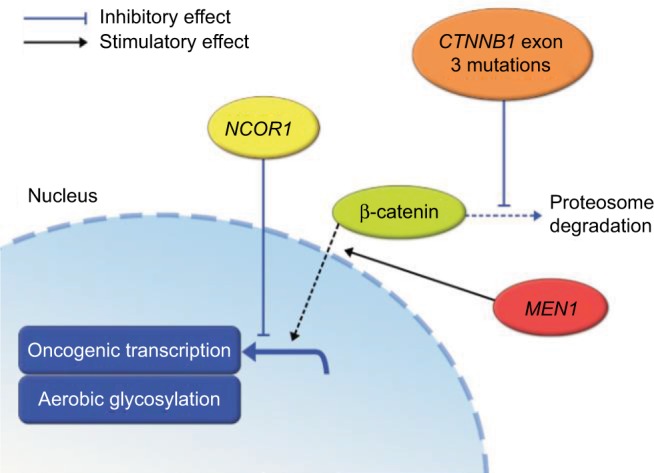
Relationship between β-catenin, MEN1, and NCOR1 mutations and the Wnt signaling pathway summarized.41
Imaging features of HCC associated with aberrant activation of Wnt/b-catenin
As discussed previously, the presence of β-catenin mutation in HCC tumors may provide important information regarding prognosis and treatment response. As a result, there have been attempts to determine whether non-invasive imaging methods can identify tumors that have β-catenin mutation and those that do not. Contrast-enhanced CT is one of the most common imaging modalities used for the detection and monitoring of HCC in patients. Overall, the sensitivity of CT for HCC has been reported to be approximately 68%, but triphasic multidetector CT may have a sensitivity of up to 100% for lesions greater than 2 cm and 96% for lesions between 1 and 2 cm in size.2 A study by Kitao et al demonstrated that the attenuation and enhancement during the arterial phase of dynamic CT images could not differentiate between tumors with β-catenin mutation and those without.27 The quantitative CT imaging features of HCC with β-catenin mutation were investigated at our institution to determine whether they could provide quantitative biomarkers to differentiate HCC with proven β-catenin mutation from those without mutation; this preliminary study demonstrated the feasibility of quantitative imaging feature extraction from contrast-enhanced CT imaging to differentiate HCC with proven β-catenin gene mutation and HCC without such mutation.42 However, future work is needed to verify those findings.
MRI is also an extremely common imaging modality used for the evaluation of HCC. MRI sensitivities for detecting HCC have been reported to be around 81%.2 On T1- and T2-weighted images, there appears to be no significant difference between HCC with and without β-catenin mutation. However, on diffusion-weighted imaging, HCC tumors with β-catenin mutation were shown to have lower contrast-to-noise ratios and higher apparent diffusion coefficients.27 A higher apparent diffusion coefficient on diffusion-weighted imaging correlates with more tumors that are well differentiated.
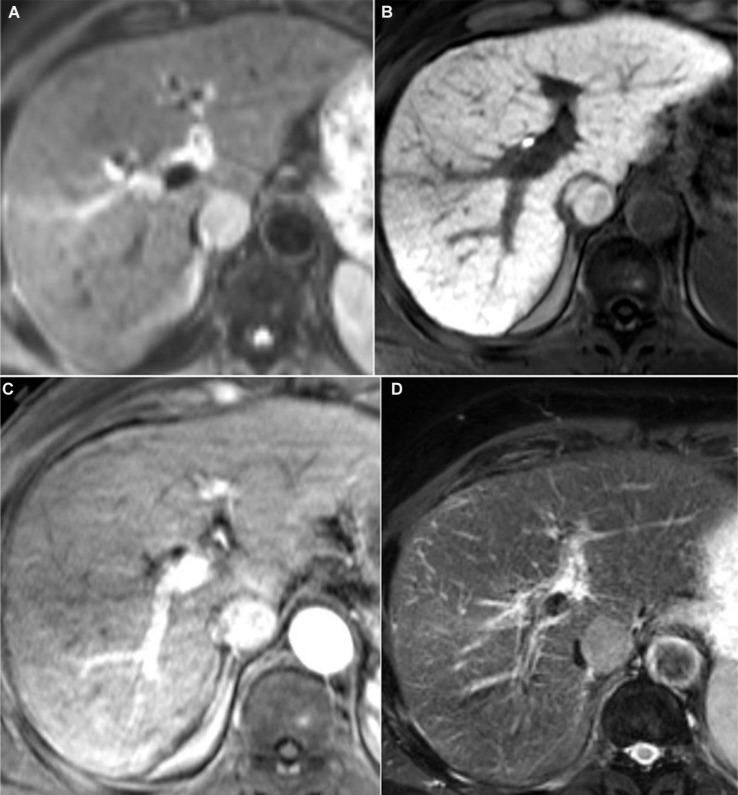
HCC tumors with β-catenin mutation were also shown to have higher contrast-to-noise ratios and higher enhancement ratios during the hepatobiliary phase (HBP) of gadoxetic acid-enhanced MRI (Figure 6).27,43
Figure 6.
Axial images in a 72-year-old man with HCC.
Notes: HCC with β-catenin mutation, hepatitis C, and cirrhosis showing increased tumor intensity on diffusion-weighted imaging (A) and retention of contrast on 20-minute hepatobiliary phase (B). The lesion is seen extending from the caudate lobe and compressing the inferior vena cava with adequate contrast uptake on arterial phase (C), the lesion is also seen to be slightly hyperintense on T2-weighted images (D).
Abbreviation: HCC, hepatocellular carcinoma.
Gadoxetic acid (Gd-EOB-DTPA) is a contrast agent that is specific to hepatocytes and is useful for the identification of focal hepatic lesions. It can also be used for HBP imaging. Most HCC tumors appear hypointense compared with surrounding liver tissue on HBP images. This hypointensity is believed to be primarily due to a decrease in the expression of the organic anion transporting polypeptide 1B3 (OATP1B3), which is a transporter that takes up Gd-EOB-DTPA into hepatocytes. Expression of this transporter is usually decreased during HCC carcinogenesis. However, up to 10–20% of HCC tumors are either hyperintense or isointense on HBP images. HCC tumors that are hypointense on HBP (Figure 7) have lower expression of OATP1B3, while HCC tumors that are hyperintense on HBP (Figure 6) have high expression of OATP1B3. Interestingly, increased expression of OATP1B3 is strongly associated with increased Wnt/β-catenin signaling (Figure 8).43,44 The presence of tumor enhancement on HBP images can accurately predict Wnt/β-catenin–activated HCC with a sensitivity of 78.9% and specificity of 81.7%.27,44 Furthermore, HCC tumors with increased expression of OATP1B3, in addition to hyperintensity on HBP imaging and low serum α-fetoprotein levels, have been associated with a better prognosis.27,43,45
Figure 7.
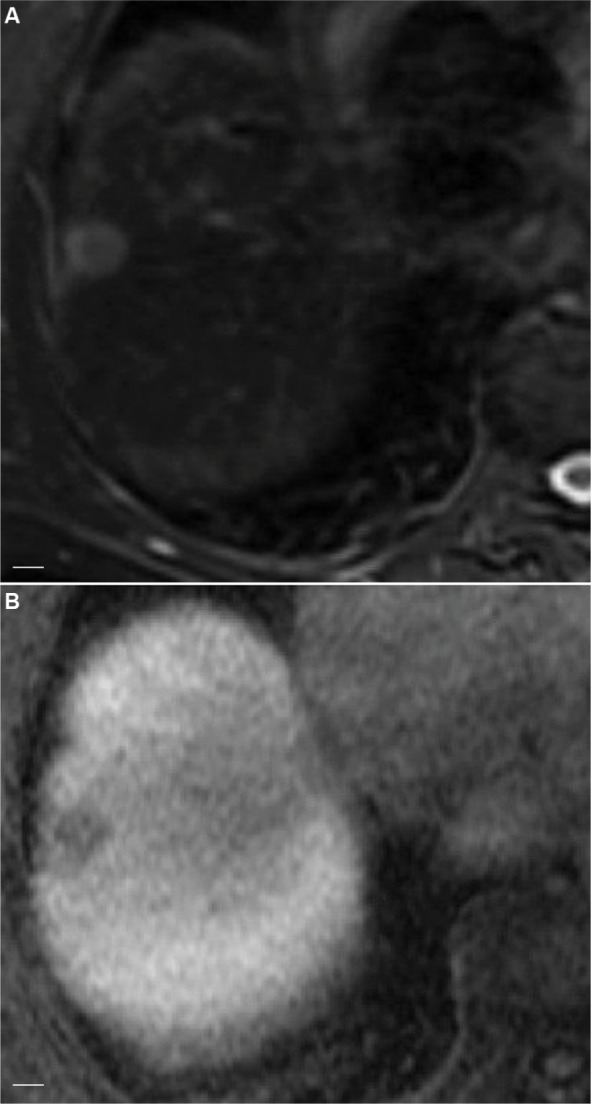
Axial image in HCV-related hepatitis showing HCC without β-catenin mutation.
Notes: The images show hyperintensity on T2-weighted images (A) and definite hypointensity on 20-minute hepatobiliary phase compared with background liver (B). Scale bar=1 cm.
Abbreviations: HCV, hepatitis C virus; HCC, hepatocellular carcinoma.
Figure 8.
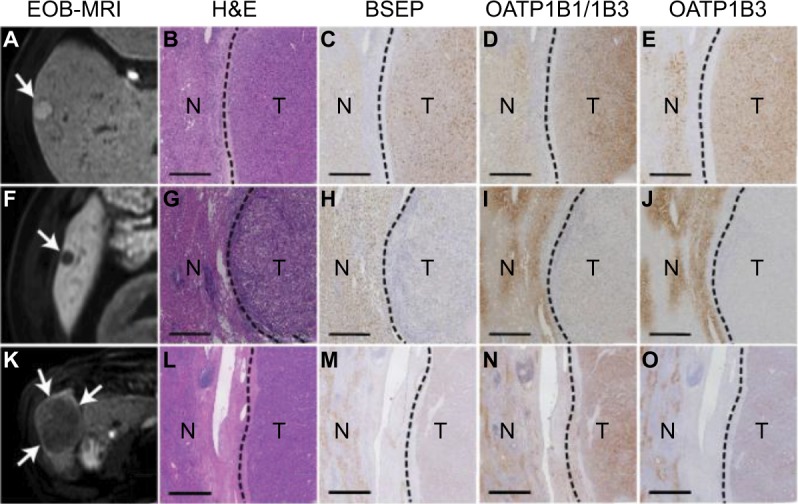
Relationship between expression of different genes and the tumor enhancement patterns in hepatobiliary phase images of EOB-MRI.44
Notes: (A) hyperintense nodule in hepatobiliary-phase (arrow) showed strong expression of (C) BSEP, (D) OATP1B1/1B3, and (E) OATP1B3. (F) markedly hypointense nodule (arrow) showed very low expression of (H) BSEP, (I) OATP1B1/1B3, and (J) OATP1B3. (K) slightly hypointense nodule (arrows) showed strong expression of (N) OATP1B1/1B3 but weak expression of (M) BSEP and (O) OATP1B3. (B, G, and L) are H&E staining of correspondent HCC nodules. Scale bars=0.5 mm. Reprinted from Journal of Hepatology, 61(5), Ueno A, Masugi Y, Yamazaki K, et al, OATP1B3 expression is strongly associated with Wnt/β-catenin signalling and represents the transporter of gadoxetic acid in hepatocellular carcinoma, 80–1087, Copyright (2014), with permission from Elsevier.44
Abbreviation: EOB-MRI, gadoxetic acid-enhanced magnetic resonance imaging; H&E, hematoxylin and eosin staining; N, non-tumoural liver; T, tumor; HCC, hepatocellular carcinoma.
Therapies targeting the Wnt/β-catenin pathway and their anti-tumor activity
There is ongoing research focused on developing specific therapies that target molecular mechanisms involved in the pathogenesis and progression of HCC. Currently, sorafenib (a VEGF-targeted therapy) is the standard therapy that provides a small prolongation of overall survival for patients with advanced HCC. Other agents (regorafenib and nivolumab) were recently approved by the US Food and Drug Administration (USFDA) for the treatment of advanced HCC in patients who have been previously treated with sorafenib.46,47 However, there is evidence that sorafenib and other VEGF- targeted therapies may actually increase tumor cell invasion and metastasis.12
Aberrant Wnt/β-catenin signaling has been shown to be common in HCC tumors and to have significant clinical impact on tumor behavior, prognosis, and response to treatment. As a result, Wnt/β-catenin may be a promising target for future HCC therapies.48
Combretastatin A-1 phosphate (CA1P) is a microtubule polymerization inhibitor that is currently being studied as a promising treatment for HCC. CA1P has been shown to have significant antitumor activity in HCC tumors both in vitro and in vivo. CA1P is believed to exert its antitumor effects through inactivation of AKT, which in turn activates GSK-3B and inhibits the Wnt/β-catenin pathway.48
FH535 is another small-molecule targeted therapy that is currently being studied in the treatment of HCC and hepatoblastoma. FH535 inhibits both peroxisome proliferator-activated receptor and β-catenin/LEF/TCF. This agent inhibits the proliferation of both HCC and hepatoblastoma cell lines. When combined with sorafenib, FH535 causes synergistic inhibition of HCC and hepatoblastoma cells. However, additional studies are still needed to determine the efficacy and side effects of FH535 in HCC patients.12,15
Currently, there are two clinical phase I/II trials studying the use of agents (such as PRI-724 and OMP-18R5) that specifically target the β-catenin signaling pathway to treat solid tumors and myeloid malignancies.12
Risk stratification of hepatic adenomas expressing beta catenin biomarkers
HCAs transforming into HCCs is a rare occurrence with a reported frequency of 4.2%.49 However, β-catenin mutation and the consequent Wnt signaling pathway activation play a big role in the proliferation of hepatic cells and the progression to malignancy.49 Zucman-Rossi et al analyzed the genotype–phenotype correlation of hepatic adenomas and their relationship with HCC and found out that among the β-catenin-activated adenomas (10–15% of all adenomas), around 46% progressed to HCC which was a rare occurrence in HCAs with other types of mutations.50 Glutamine synthase expression was associated with β-catenin activating mutations.40,50 Rebouissou et al found that in benign tumors, there are three levels of β-catenin activation depending on specific mutations: (1) weak activity by S45, K335, and N387 mutations; (2) moderate activity by T41 mutations; and (3) high activity through exon 3 deletions. High activity mutations were associated with malignancy and strong GS expression. Weak mutations were more frequent in HCA except for S45 mutation which was identified in 20% of mutated HCAs and HCCs.40 Mutations in CTNNB1 exon 7 or 8 were associated with mild activations of Wnt/β-catenin signaling and without increased risk of malignant transformation unlike CTNNB1 exon 3 mutations.51 HCAs with high/moderate or S45 mutations should be followed up for potential HCC transformation and may necessitate more aggressive treatment options.40
Conclusion
Mutations of the CTNNB1 gene are thought to be the most common genetic abnormality leading to the development of HCC. Multiple studies suggest that the presence of β-catenin mutation in HCC tumors is associated with a more favorable prognosis and provides useful information about treatment response and tumor behavior. However, the accumulation of β-catenin in the nucleus and cytoplasm may also be associated with increased vascular invasion, cell proliferation, and more poorly differentiated tumors. It has also been shown that HCV-related HCC tumors have a significantly increased frequency of CTNNB1 mutations compared with HBV-related and non-viral HCC tumors. While most HCC tumors appear hypointense compared with surrounding liver tissue on HBP images, the presence of tumor enhancement on HBP images can accurately predict Wnt/β-catenin-activated HCC.
More research is needed to determine the effect of β-catenin mutation on HCC tumor pathogenesis, prognosis, and behavior. Nowadays, small molecular therapy targeting the Wnt/β-catenin pathway is being investigated for treatment of HCC. Improving our understanding of the clinical and pathologic significance of the molecular mechanisms that lead to Wnt/β-catenin signaling activation in HCC tumors will guide the development of new targeted therapies for HCC.
Although HCA progression into HCC is a rare event, the β-catenin-activated subtype of HCAs was the most frequent to turn malignant, warranting more aggressive follow-up and treatment options such as complete resection.
Acknowledgments
The University of Texas is supported in part by the National Institutes of Health through Cancer Center Support Grant P30CA016672. The authors would also like to thank the department of scientific publication at The University of Texas, MD Anderson Cancer Center for their contribution.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Li P, Cao Y, Li Y, Zhou L, Liu X, Geng M. Expression of Wnt-5a and B-catenin in primary hepatocellular carcinoma. Int J Clin Exp Pathol. 2014;7(6):3190–3195. [PMC free article] [PubMed] [Google Scholar]
- 2.Shanbhogue AK, Prasad SR, Takahashi N, Vikram R, Sahani DV. Recent advances in cytogenetics and molecular biology of adult hepatocel-lular tumors: implications for imaging and management. Radiology. 2011;258(3):673–693. doi: 10.1148/radiol.10100376. [DOI] [PubMed] [Google Scholar]
- 3.Waisberg J, Saba GT. Wnt−/−β-catenin pathway signaling in human hepatocellular carcinoma. World J Hepatol. 2015;7(26):2631–2635. doi: 10.4254/wjh.v7.i26.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dahmani R, Just P-A, Perret C. The Wnt/β-catenin pathway as a therapeutic target in human hepatocellular carcinoma. Clin Res Hepatol Gastroenterol. 2011;35(11):709–713. doi: 10.1016/j.clinre.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 5.Oishi N, Wang XW. Novel therapeutic strategies for targeting liver cancer stem cells. Int J Biol Sci. 2011;7(5):517–535. doi: 10.7150/ijbs.7.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Z, Sheng Y, Gao X, et al. β-catenin mutation is correlated with a favorable prognosis in patients with hepatocellular carcinoma. Mol Clin Oncol. 2015;3(4):936–940. doi: 10.3892/mco.2015.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee JM, Yang J, Newell P, et al. β-Catenin signaling in hepatocellular cancer: implications in inflammation, fibrosis, and proliferation. Cancer Lett. 2014;343(1):90–97. doi: 10.1016/j.canlet.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu LJ, Xie SX, Chen YT, Xue JL, Zhang CJ, Zhu F. Aberrant regulation of WNT signaling in hepatocellular carcinoma. World J Gastroenterol. 2016;22(33):7486–7499. doi: 10.3748/wjg.v22.i33.7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, Pan Q, Fuhler GM, Smits R, Peppelenbosch MP. Action and function of Wnt/β-catenin signaling in the progression from chronic hepatitis C to hepatocellular carcinoma. J Gastroenterol. 2017;52(4):419–431. doi: 10.1007/s00535-016-1299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de La Coste A, Romagnolo B, Billuart P, et al. Somatic mutations of the beta-catenin gene are frequent in mouse and human hepatocellular carcinomas. Proc Natl Acad Sci U S A. 1998;95(15):8847–8851. doi: 10.1073/pnas.95.15.8847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friemel J, Rechsteiner M, Frick L, et al. Intratumor heterogeneity in hepatocellular carcinoma. Clin Cancer Res. 2015;21(8):1951–1961. doi: 10.1158/1078-0432.CCR-14-0122. [DOI] [PubMed] [Google Scholar]
- 12.Vilchez V, Turcios L, Marti F, Gedaly R. Targeting Wnt/B-catenin pathway in hepatocellular carcinoma treatment. World J Gastroenterol. 2016;22(2):823–832. doi: 10.3748/wjg.v22.i2.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Zhang J, Feng W, et al. PIN1 gene overexpression and ?-catenin gene mutation/expression in hepatocellular carcinoma and their significance. J Huazhong Univ Sci Technol. 2007;27(1):54–57. doi: 10.1007/s11596-007-0116-z. [DOI] [PubMed] [Google Scholar]
- 14.Xu W, Zhou W, Cheng M, et al. Hypoxia activates Wnt/β-catenin signaling by regulating the expression of BCL9 in human hepatocellular carcinoma. Sci Rep. 2017;7:40446. doi: 10.1038/srep40446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gedaly R, Galuppo R, Daily MF, et al. Targeting the Wnt/β-catenin signaling pathway in liver cancer stem cells and hepatocellular carcinoma cell lines with FH535. PLoS One. 2014;9(6):e99272. doi: 10.1371/journal.pone.0099272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kondo Y, Kanai Y, Sakamoto M, et al. β-catenin accumulation and mutation of exon 3 of the β-catenin gene in hepatocellular carcinoma. Jpn J Cancer Res. 1999;90(12):1301–1309. doi: 10.1111/j.1349-7006.1999.tb00712.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14(15):1837–1851. [PubMed] [Google Scholar]
- 18.Lu LC, Shao YY, Lee YH, et al. β-Catenin (CTNNB1) mutations are not associated with prognosis in advanced hepatocellular carcinoma. Oncology. 2014;87(3):159–166. doi: 10.1159/000362821. [DOI] [PubMed] [Google Scholar]
- 19.Hsu HC, Jeng YM, Mao TL, Chu JS, Lai PL, Peng SY. Beta-catenin mutations are associated with a subset of low-stage hepatocellular carcinoma negative for hepatitis B virus and with favorable prognosis. Am J Pathol. 2000;157(3):763–770. doi: 10.1016/s0002-9440(10)64590-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mao TL, Chu JS, Jeng YM, Lai PL, Hsu HC. Expression of mutant nuclear beta-catenin correlates with non-invasive hepatocellular carcinoma, absence of portal vein spread, and good prognosis. J Pathol. 2001;193(1):95–101. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH720>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 21.Nhieu JT, Renard CA, Wei Y, Cherqui D, Zafrani ES, Buendia MA. Nuclear accumulation of mutated β-catenin in hepatocellular carcinoma is associated with increased cell proliferation. Am J Pathol. 1999;155(3):703–710. doi: 10.1016/s0002-9440(10)65168-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Polakis P. The oncogenic activation of beta catenin. Curr Opin Genet Dev. 1999;9(1):15–21. doi: 10.1016/s0959-437x(99)80003-3. [DOI] [PubMed] [Google Scholar]
- 23.Suzuki T, Yano H, Nakashima Y, Nakashima O, Kojiro M. Beta-catenin expression in hepatocellular carcinoma: a possible participation of beta-catenin in the dedifferentiation process. J Gastroenterol Hepatol. 2002;17(9):994–1000. doi: 10.1046/j.1440-1746.2002.02774.x. [DOI] [PubMed] [Google Scholar]
- 24.MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Peng SY, Chen WJ, Lai PL, Jeng YM, Sheu JC, Hsu HC. High α-fetoprotein level correlates with high stage, early recurrence and poor prognosis of hepatocellular carcinoma: significance of hepatitis virus infection, age, p53 and β-catenin mutations. Int J Cancer. 2004;112(1):44–50. doi: 10.1002/ijc.20279. [DOI] [PubMed] [Google Scholar]
- 26.Terris B, Pineau P, Bregeaud L, et al. Close correlation between β-catenin gene alterations and nuclear accumulation of the protein in human hepatocellular carcinomas. Oncogene. 1999;18(47):6583–6588. doi: 10.1038/sj.onc.1203051. [DOI] [PubMed] [Google Scholar]
- 27.Kitao A, Matsui O, Yoneda N, et al. Hepatocellular carcinoma with β-catenin mutation: imaging and pathologic characteristics. Radiology. 2015;275(3):708–717. doi: 10.1148/radiol.14141315. [DOI] [PubMed] [Google Scholar]
- 28.Tien LT, Ito M, Nakao M, et al. Expression of beta-catenin in hepatocellular carcinoma. World J Gastroenterol. 2005;11(16):2398–2401. doi: 10.3748/wjg.v11.i16.2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zucman-Rossi J, Benhamouche S, Godard C, et al. Differential effects of inactivated Axin1 and activated β-catenin mutations in human hepatocellular carcinomas. Oncogene. 2007;26(5):774–780. doi: 10.1038/sj.onc.1209824. [DOI] [PubMed] [Google Scholar]
- 30.Kim SS, Cho HJ, Lee H-Y, et al. Genetic polymorphisms in the Wnt/B-catenin pathway genes as predictors of tumor development and survival in patients with hepatitis B virus-associated hepatocellular carcinoma. Clin Biochem. 2016;49(10–11):792–801. doi: 10.1016/j.clinbiochem.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 31.Wong CM, Fan ST, Ng IOL. β-catenin mutation and overexpression in hepatocellular carcinoma: clinicopathologic and prognostic significance. Cancer. 2001;92(1):136–145. doi: 10.1002/1097-0142(20010701)92:1<136::aid-cncr1301>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 32.Wang W, Xu L, Liu P, et al. Blocking Wnt secretion reduces growth of hepatocellular carcinoma cell lines mostly independent of β-catenin signaling. Neoplasia. 2016;18(12):711–723. doi: 10.1016/j.neo.2016.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang H, Fujii H, Sankila A, et al. Beta-catenin mutations are frequent in human hepatocellular carcinomas associated with hepatitis C virus infection. Am J Pathol. 1999;155(6):1795–1801. doi: 10.1016/s0002-9440(10)65496-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Levrero M, Zucman-Rossi J. Mechanisms of HBV-induced hepatocellular carcinoma. J Hepatol. 2016;64(1 Suppl):S84–S101. doi: 10.1016/j.jhep.2016.02.021. [DOI] [PubMed] [Google Scholar]
- 35.Zucman-Rossi J, Villanueva A, Nault JC, Llovet JM. Genetic landscape and biomarkers of hepatocellular carcinoma. Gastroenterology. 2015;149(5):1226–1239. doi: 10.1053/j.gastro.2015.05.061. [DOI] [PubMed] [Google Scholar]
- 36.Austinat M, Dunsch R, Wittekind C, Tannapfel A, Gebhardt R, Gaunitz F. Correlation between β-catenin mutations and expression of Wnt-signaling target genes in hepatocellular carcinoma. Mol Cancer. 2008;7(1):21. doi: 10.1186/1476-4598-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Inagawa S, Itabashi M, Adachi S, et al. Expression and prognostic roles of beta-catenin in hepatocellular carcinoma: correlation with tumor progression and postoperative survival. Clin Cancer Res. 2002;8(2):450–456. [PubMed] [Google Scholar]
- 38.Bogaerts E, Heindryckx F, Vandewynckel YP, Van Grunsven LA, Van Vlierberghe H. The roles of transforming growth factor-β, Wnt, Notch and hypoxia on liver progenitor cells in primary liver tumours (review) Int J Oncol. 2014;44(4):1015–1022. doi: 10.3892/ijo.2014.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yuan RH, Jeng YM, Hu RH, et al. Role of p53 and β-catenin mutations in conjunction with CK19 expression on early tumor recurrence and prognosis of hepatocellular carcinoma. J Gastrointest Surg. 2011;15(2):321–329. doi: 10.1007/s11605-010-1373-x. [DOI] [PubMed] [Google Scholar]
- 40.Rebouissou S, Franconi A, Calderaro J, et al. Genotype-phenotype correlation of CTNNB1 mutations reveals different β-catenin activity associated with liver tumor progression. Hepatology. 2016;64(6):2047–2061. doi: 10.1002/hep.28638. [DOI] [PubMed] [Google Scholar]
- 41.Ziv E, Yarmohammadi H, Boas FE, et al. Gene signature associated with upregulation of the Wnt/beta-catenin signaling pathway predicts tumor response to transarterial embolization. J Vasc Interv Radiol. 2017;28(3):349.e1–355.e1. doi: 10.1016/j.jvir.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khalaf AM, Fuentes DT, Ahmed K, et al. Quantitative CT imaging features for hepatocellular carcinoma (HCC) with b-catenin (CTNNB1) gene mutation. J Clin Oncol. 2017;35(4 Suppl):253–253. [Google Scholar]
- 43.Joo I, Lee JM. Recent advances in the imaging diagnosis of hepatocellular carcinoma: value of gadoxetic acid-enhanced MRI. Liver Cancer. 2015;5(1):67–87. doi: 10.1159/000367750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ueno A, Masugi Y, Yamazaki K, et al. OATP1B3 expression is strongly associated with Wnt/B-catenin signalling and represents the transporter of gadoxetic acid in hepatocellular carcinoma. J Hepatol. 2014;61(5):1080–1087. doi: 10.1016/j.jhep.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 45.Vilgrain V, Van Beers BE, Pastor CM. Insights into the diagnosis of hepatocellular carcinomas with hepatobiliary MRI. J Hepatol. 2016;64(3):708–716. doi: 10.1016/j.jhep.2015.11.016. [DOI] [PubMed] [Google Scholar]
- 46.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492–2502. doi: 10.1016/S0140-6736(17)31046-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim K, Jha R, Prins PA, et al. Regorafenib in advanced hepatocellular carcinoma (HCC): considerations for treatment. Cancer Chemother Pharmacol. 2017;80(5):945–954. doi: 10.1007/s00280-017-3431-5. [DOI] [PubMed] [Google Scholar]
- 48.Mao J, Wang D, Wang Z, et al. Combretastatin A-1 phosphate, a microtubule inhibitor, acts on both hepatocellular carcinoma cells and tumor-associated macrophages by inhibiting the Wnt/β-catenin pathway. Cancer Lett. 2016;380(1):134–143. doi: 10.1016/j.canlet.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 49.Stoot JHMB, Coelen RJS, De Jong MC, Dejong CHC. Malignant transformation of hepatocellular adenomas into hepatocellular carcinomas: a systematic review including more than 1600 adenoma cases. HPB (Oxford) 2010;12(8):509–522. doi: 10.1111/j.1477-2574.2010.00222.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zucman-Rossi J, Jeannot E, Van Nhieu JT, et al. Genotype-phenotype correlation in hepatocellular adenoma: new classification and relationship with HCC. Hepatology. 2006;43(3):515–524. doi: 10.1002/hep.21068. [DOI] [PubMed] [Google Scholar]
- 51.Nault JC, Couchy G, Balabaud C, et al. Molecular classification of hepatocellular adenoma associates with risk factors, bleeding, and malignant transformation. Gastroenterology. 2017;152(4):880–894. doi: 10.1053/j.gastro.2016.11.042. [DOI] [PubMed] [Google Scholar]