Abstract
Sepsis accounts for an estimated 30 million cases and 6 million deaths globally each year. According to a multidisciplinary task force convened by the Society of Critical Care Medicine and European Society of Intensive Care Medicine, sepsis is defined as life-threatening organ dysfunction due to a dysregulated host response to infection. Sepsis is a medical emergency, so much so that the World Health Organization made it a global health priority.
Since patients with cardiovascular diseases have unique risk factors for sepsis, prompt and accurate diagnosis is critical. In this regard, the sepsis-specific Sequential Organ Failure Assessment (SOFA) helps clinicians identify the organ dysfunction and predict outcomes. Sepsis management is grouped into specific interventions called bundles, and completion of each bundle element is time sensitive. The U.S. Centers for Medicaid and Medicare Services and some state-specific regulations have made compliance with these bundles reportable as a quality measure. The updated Surviving Sepsis Campaign Hour-1 bundle recommends that lactate measurement, blood cultures procurement, broad spectrum antibiotics administration, resuscitation with 30 mL/kg crystalloid, and vasopressor initiation for hypotension all be initiated within 1 hour of time zero, which is from the time of triage in the emergency department or from sepsis diagnosis. Septic shock is defined as hypotension with a mean arterial pressure less than 65 mm Hg, requiring vasopressors despite adequate fluid resuscitation and/or lactic acid levels above 2 mmol/L. Both fluid resuscitation and clinical re-evaluation with lactate measurement guide the fluid and vasopressor therapy. Specific guidelines exist for organ support that address mechanical ventilation, blood transfusions, vasopressor choices, and nutrition.
Keywords: sepsis, septic shock, SOFA, qSOFA, lactate, vasopressor, sepsis bundles
DEFINITION
In early 2016, a multidisciplinary task force convened by the Society of Critical Care Medicine and the European Society of Intensive Care Medicine published the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3), thereby redefining sepsis as a life-threatening organ dysfunction due to dysregulated host response to infection.1 As opposed to the prior Sepsis-2 definition, which defined sepsis as a spectrum ranging from a systemic inflammatory response (SIRS) to sepsis, severe sepsis, and septic shock, the new definition emphasized organ dysfunction rather than inflammation. The task force used the Sequential Organ Failure Assessment (SOFA) score for quantifying the severity of organ dysfunction, and a score of ≥ 2 with suspicion of infection was determined to be consistent with sepsis (Table 1). The most severe stage, septic shock, is defined as persisting hypotension that requires vasopressors to achieve a mean arterial pressure ≥ 65 mm Hg despite adequate fluid resuscitation and a lactic acid level greater than 2 mmol/L.
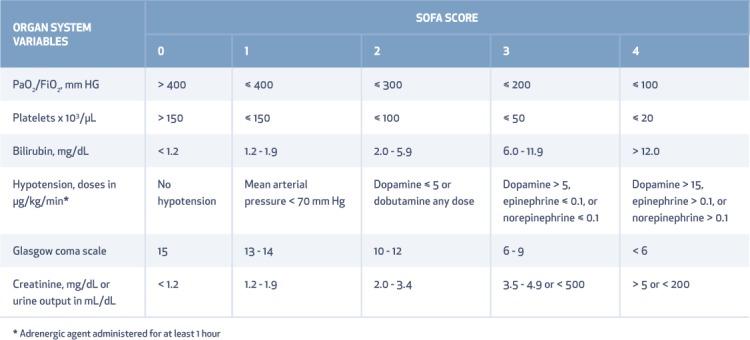
Table 1.
Sequential Organ Failure Assessment (SOFA) scoring system. Adapted from Vincent JL, et al. Intensive care med. 1996;22:707–10.1 PaO2: partial pressure of oxygen; FiO2: fraction of inspired oxygen
EPIDEMIOLOGY AND GLOBAL BURDEN
Globally, sepsis accounts for an estimated 30 million cases and 6 million deaths per year.2 Data from the National Hospital Ambulatory Medical Care Survey (NHAMCS) from 2009 to 2011 indicates that, there were 850,000 emergency department (ED) visits for sepsis in the United States, which accounts for 21% of all ED visits and a huge persistent burden of sepsis on healthcare.3 Septicemia is also the most expensive condition treated, accounting for $20.3 billion U.S. dollars in 2011 alone, per the U.S. Agency for Healthcare Research and Quality data.4 Across the continent, the 90-day mortality for sepsis is about 25%.5 Ensuring greater awareness of the need for early recognition and treatment among the public and clinicians is a crucial step for reducing the global sepsis burden. To that end, the World Health Organization has declared sepsis to be a global health priority and has proposed numerous resolutions that require coordinated efforts by politicians, policymakers, healthcare administrators, researchers, and clinicians working with people of all ages in all healthcare settings and in the community.
SEPSIS RISK FACTORS
There are several predisposing factors for sepsis: age > 60 years, male gender, underlying disease with reduced life expectancy (McCabe score > 0), immune deficiency, diabetes, chronic renal failure, malignancy, HIV, a prior history of sepsis, and chronic pulmonary disease.6 Lifestyle risk factors include smoking, alcohol consumption, intravenous (IV) drug use, poor hygiene, and lack of immunization. Risks for sepsis due to gram-negative bacteremia are renal disease, indwelling urinary catheter, hematologic malignancy, and neutropenia.7
SEPSIS RISK FACTORS IN UNIQUE CARDIAC COHORTS
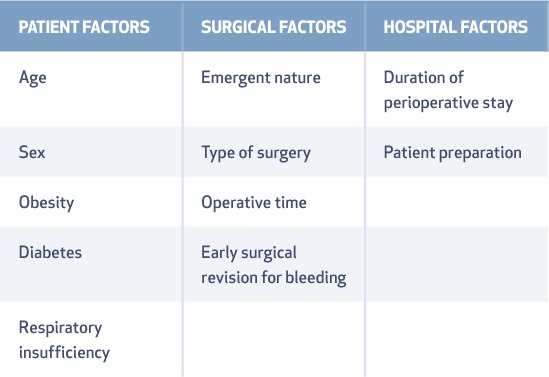
The risk of sepsis in cardiac patients revolves around the type of surgery, catheters, implanted devices, and the need for life-saving bridging devices such as extracorporeal membrane oxygenation (ECMO) and left ventricular assist devices (LVAD). Underlying cardiac disease can complicate early recognition of sepsis, hemodynamic monitoring, and resuscitation. Fever in cardiac surgical patients needs prompt evaluation since those who develop fever within 48 hours are less likely to have sepsis.8 Therefore, the time interval between the surgery and the onset of fever is key for predicting an infectious versus noninfectious cause. Surgical site infections are not common within the first 48 hours after surgery unless it is Clostridium or group A Streptococcus infection.8 Surgical site infections and mediastinitis are related to three important factors, as shown in Table 2.9
Table 2.
Risk factors for surgical site infections.
A study by Ramanathan et al. looking at the impact of sepsis on postsurgical patients found that the incidence of sepsis was highest in those undergoing cardiothoracic procedures (8.39%) compared to trauma/acute care surgery (7.5%) and plastic/reconstructive surgery (5.3%).10 In another study of 194 patients who underwent transcatheter aortic valve implantation (TAVI), 65 had postoperative fever, but only 17 of those (26.1%) had an infectious etiology such as pneumonia (52.9%) or urinary tract infection (41.2%).11 Van der Boon and colleagues determined that access site infection could contribute up to 12% of post-TAVI infections, with the most important determinants being access through the femoral artery, perioperative stroke, and obesity.12 Patients with unexplained fever and a prosthetic valve in place should be promptly evaluated for endocarditis. The overall incidence of blood stream infection is found to be 2.3 per 1,000 cardiac catheterizations and is commonly caused by Staphylococcus aureus, coagulase negative staphylococcus, and Escherichia coli.13 Bloodstream infection is a major complication in patients with a central venous catheter, and the risk increases with the duration of catheter use. The incidence of catheter-related bloodstream infection is 35 (antibiotic impregnated catheters) to 57 (nonimpregnated catheters) per 1,000 catheter days.14
The incidence of implantable cardiac device (ICD) infection is 1.9 per 1,000 device-years; it can be a pocket infection alone or associated with a blood stream infection or endocarditis. They are usually gram-positive infections (75%), with Staphylococcus aureus being the most common organism (41%). The predisposing factors for ICD infections include diabetes, heart failure, renal dysfunction, use of oral anticoagulants, use of pre-procedural temporary pacing, immunosuppression, failure to administer perioperative antibiotics, hematoma formation, operator experience, and device-related factors that can affect bacterial adherence.15
In patients undergoing ECMO, the SOFA score before ECMO initiation and the number of days of support were independently associated with a higher risk of blood stream infection (OR 1.23; 95% CI, 1.03–1.47).16 Ventricular assist device-related infections, such as culture-positive drive-line infection, pump pocket infection, bacteremia, or a combination of these, could be as high as 36% and may affect post-transplant survival (1-year survival 76% vs 81% in patients with and without infection, respectively).17
EARLY IDENTIFICATION OF SEPSIS AND SCREENING TOOLS
The crux of the matter lies in identifying time zero. Triage times are used as time zero in patients presenting from the emergency department or, if referred from another care location, from the earliest chart annotation consistent with all elements of sepsis (formerly severe sepsis) or septic shock ascertained through chart review; in both instances, a good screening tool is essential. Systemic inflammatory response syndrome or SIRS criteria based on the old definition of sepsis may be too sensitive and incorrectly identify patients. For example, a study by Vincent et al. showed a positive SIRS score in 87% of all ICU admissions, yet 14.3% of those with 2 or more SIRS criteria did not have infection.18 Furthermore, 12.1% of patients in one large study had SIRS-negative sepsis, accounting for 1 in 8 missed sepsis diagnoses.19 In the SHOCK trial, clinically significant signs of SIRS were seen in 18% of the patients with cardiogenic shock after acute myocardial infarction, but 26% of those were culture negative.20 Due to inaccuracies in the older diagnostic models, the new Sepsis-3 definition recommends using the SOFA score; however, it is not well known outside of the critical care world.1 SOFA aggregates scores from 0 to 4 for each of the objective variables specific to organ system functions, including respiratory, coagulation, liver, cardiovascular, renal, and central nervous systems (Table 1).21 An acute increase in the total score of 2 or more reflects an overall mortality risk of approximately 10% in patients suspected of infection.1 Calculating SOFA at the bedside or in noncritical care units and in patients who do not have full laboratory testing is challenging. Therefore, a quick SOFA (qSOFA) score is recommended for diagnosis of possible sepsis (Table 3). If 2 of 3 clinical variables are positive, it has a predictive validity similar to that of the full SOFA score when used outside the ICU setting.22
Table 3.
Variables measured in the quick Sepsis Related Organ Failure Assessment (qSOFA), a bedside prompt to help identify patients at risk of mortality due to suspected sepsis.

Although the SOFA score is a validated scoring system for prognostication, it needs further investigations to be used as a screening tool. Many institutions have developed screening tools specific to their patient population that range from paper checklists to electronic health record-based screening tools, such as modified early warning signs and Rothman Index.23,24 Some of the tools are listed in the Surviving Sepsis Campaign (SSC) website (www.survivingsepsis.org).
SURVIVING SEPSIS CAMPAIGN BUNDLE OF CARE
The guidelines of evidence-based care in the form of “sepsis bundles” have evolved since 2004 and are regularly updated with new evidence. The Surviving Sepsis Campaign guidelines on sepsis diagnosis and management were updated in 2016.
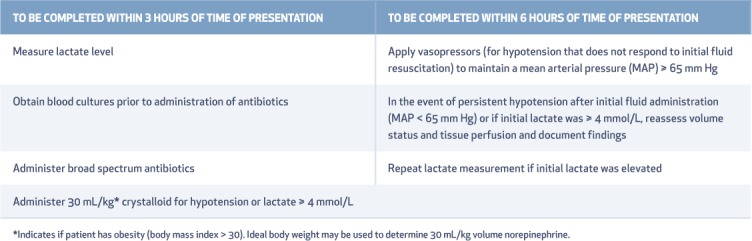
Grouped interventions, also called bundles, were recommended to be completed within a 3- and 6-hour period following sepsis diagnosis (Table 4).25 In May 2018, “hour-1 bundle” was adopted due to a strong association between compliance with bundles and improved survival in patients with sepsis and septic shock. In this new revision, both the 3- and 6-hour bundles have been combined into a single hour-1 bundle, not just to simplify the treatment regimen but also to improve outcomes. The hour-1 bundle elements are (1) measure lactate level, and re-measure if initial lactate is > 2 mmol/L; (2) obtain cultures prior to administration of antibiotics; (3) rapidly administer 30 mL/kg crystalloid for hypotension or lactate ≥ 4 mmol/L; and (4) apply vasopressors if patient is hypotensive during or after fluid resuscitation to maintain mean arterial pressure > 65 mm Hg.26
Table 4.
Sepsis treatment bundles identified in the Surviving Sepsis Campaign guidelines.55 From survivingsepsis.org. Reproduced with permission. Copyright © 2015 the Society of Critical Care Medicine and the European Society of Intensive Care Medicine.
ANTIMICROBIAL THERAPY
Prior to administering antimicrobial therapy, appropriate cultures should be procured to detect pathogens and test for antibiotic resistance in patients suspected of sepsis or septic shock, unless this creates a substantial delay in antimicrobial initiation. The SSC guidelines strongly recommend that broad spectrum antibiotics with one or more antimicrobials be given within the first hour following sepsis diagnosis to cover all likely pathogens because patients who receive appropriate antibiotic therapy within 1 hour in the ED have the greatest mortality benefit.27 Every additional hour without antibiotics is shown to increase the risk for death in septic shock patients by 7.6% during the first 6 hours.28 Combination therapy is preferred over monotherapy.
Risks for drug-resistant microorganisms—such as prior infections, recent antimicrobial therapy, hospitalizations at other institutions, recent indwelling lines or devices, and any previously documented cultures positive for drug-resistant organisms—should be assessed before choosing appropriate antibiotics. Samples that require invasive procedures (i.e., bronchoscopy, thoracentesis, or any open surgery) should be procured at the earliest time but should not delay immediate antimicrobial initiation. Pan culture is discouraged, and careful decisions should be made regarding which sites to culture to prevent inappropriate antimicrobial use. The SSC strongly recommends antibiotic stewardship in de-escalating antibiotics, tailored to the specific microorganism to prevent drug resistance. Procalcitonin measurement has been suggested to distinguish between infectious versus sterile inflammation or to guide antibiotic stewardship, but its use has not been confirmed as a strategy to reduce mortality, mechanical ventilation, clinical severity, reinfection, or duration of antimicrobial therapy in patients with septic conditions.29,30
SOURCE CONTROL
Delayed or inadequate source control or use of inappropriate antibiotics is associated with higher mortality. The time to source control plays an important role in outcomes, as there is a direct increase in mortality with each 6-hour delay in achieving source control.31 Similarly, Chu et al. studied cardiac patients with sepsis and septic shock associated with infective endocarditis and found that surgical treatment had a positive effect on in-hospital mortality and 1-year survival.32
FLUID RESUSCITATION
A total of 30 cc/kg should be given as a fluid challenge once the sepsis diagnosis is established. Clinical reevaluation and lactate measurement further guides the fluid and vasopressor therapy. A landmark trial in 2001 by Rivers et al. found a 16% improvement in mortality in patients who received early goal-directed resuscitation therapy.33 The researchers took a stepwise approach to protocolized septic shock resuscitation by monitoring central venous pressure, mean arterial pressures, and central venous oxygen saturation to guide the fluid dose, vasoactive agent initiation, and need for transfusion and inotropic agents. Results showed a 16% absolute risk reduction or number needed to treat of 6, which propelled this protocolized resuscitation (excluding blood transfusion intervention) to the forefront of sepsis therapy. Since then, three large trials—Protocolized Care for Early Septic Shock (ProCESS), Protocolised Management in Sepsis (ProMISe), and Australasian Resuscitation in Sepsis Evaluation (ARISE)—and a meta-analysis from the Protocolized Resuscitation in Sepsis Meta-Analysis (PRISM) have not shown any benefit from protocolized management compared to usual care.5,34–36 In all three trials, patients received early antibiotics and > 30 cc/kg of intravenous fluid prior to randomization, which may have contributed to the lack of observed mortality benefit. These studies did, however, establish the recommendation of the fixed volume of 30 mL/Kg in the early stages of resuscitation, although there is little evidence in the form of controlled data to support this. Even so, due to ethical considerations, it would not be feasible to replicate studies with a control arm of no fluids or anything less than 30 mL/kg fluid resuscitation. Of note, one must credit the Rivers study, which has definitely propagated early identification and goal-directed therapy and has influenced the standard of care. A majority of the patients will require more than the guideline-recommended 30 mL/kg fluids, and for these patients, functional hemodynamic measurements may still be necessary for ongoing reevaluation.
FLUIDS IN HEART FAILURE
In a large point-prevalence study that included patients with a history of congestive heart failure, compliance with the sepsis 3- and 6-hr bundles was independently associated with lower mortality.37 A large multicenter study the following year also showed that patients with a history of heart failure and/or kidney disease had a significant mortality benefit with bundle compliance, even patients with intermediate lactate values.38 Furthermore, in a study by Seymor et al., no adverse effects following bundle-based sepsis treatment were observed in patients with heart failure or on chronic hemodialysis.39 Generally, it is noted that patients with comorbid conditions of heart and renal failure experience delayed crystalloid administration, and the odds of death increase each hour until initiation of the fluid bolus.40 However, resuscitation using more than 5 L of crystalloids on the first day of septic shock treatment portends greater mortality.41
CHOICE OF FLUIDS
Crystalloids are considered the first-line therapy for resuscitation. Use of a buffered crystalloid compared with saline had not shown reduction in the risk of acute kidney injury, in-hospital mortality, receipt of renal replacement therapy, or persistent renal dysfunction.42 However, in a large recent pragmatic trial conducted by Semler and colleagues (SMART trial), balanced crystalloids showed 1.1% reduction in major adverse kidney event within 30 days (MAKE 30 - composite of death, new receipt of renal replacement therapy, or persistent renal dysfunction) when compared to normal saline in all critically ill adult patients.43 In their subgroup of patients with sepsis, in-hospital mortality was 25.2% with balanced crystalloids and 29.4% with saline, favoring balanced crystalloids in patients receiving large-volume resuscitation.43 Of all the balanced crystalloids available, lactated ringers is more cost efficient. Colloid options are starches or albumin, which increase oncotic pressure and expand intravascular volume without reducing extravasation into the extravascular tissue space. Multiple clinical trials and meta-analyses comparing hydroxyl-ethyl starch (HES) and normal saline have shown that patients who receive HES are more likely to require renal replacement therapy (although there is no difference in mortality).44–47 The 2004 Saline versus Albumin Fluid Evaluation (SAFE) trial comparing albumin and normal saline in critically ill patients showed some mortality benefit in the sepsis subgroup, although it did not meet statistical significance.48 However, the larger Albumin Italian Outcome Sepsis (ALBIOS) study showed no significant difference in organ dysfunction or mortality.49 Irrespective of the baseline albumin levels in septic patients, albumin is not recommended for sepsis resuscitation (unless substantial crystalloid therapy fails to correct fluid status) as it is not cost effective and has no mortality benefit.48,50,51
MAINTENANCE FLUIDS AND DE-RESUSCITATION
Fluids are very important in the treatment of sepsis and septic shock, but timing matters. Fluid resuscitation in septic shock can be described in four stages known as the ROSE concept: rescue, optimization, stabilization, and evacuation or de-resuscitation.52 In the ROSE concept, the first phase is to improve perfusion deficits, the second and third phases concentrate on maintaining perfusion, and the last phase is to remove excess fluids used during the first three phases to prevent edema. Fluid resuscitation with large volumes can cause volume overload, worsen already existing capillary leak, lead to tissue edema, increase extravascular lung water, increase intra-abdominal pressure leading to compartment syndrome, impede perfusion to encapsulated organs such as the liver and kidneys, and cause cerebral edema, thereby leading to poor cerebral perfusion pressure. Cautious use of diuretics and renal replacement therapy can help to mobilize excess fluid after the acute phase. A positive fluid balance has shown increased mortality in post hoc analyses of the Vasopressin and Septic Shock Trial and prolonged ventilator need in the Fluids and Catheters Treatment Trial.53,54 In a meta-analysis by Malbrain et al., restrictive fluid management was associated with improved mortality compared to a more liberal fluid management strategy (24.7% vs 33.2%).55
RED BLOOD CELL TRANSFUSIONS
The premise for restricted red blood cell transfusion in sepsis patients was that transfusion comes with significant risks, such as infection, pulmonary complications and transfusion-associated circulatory overload, transfusion-related immunomodulation and multiorgan failure, and increased mortality. However, large randomized clinical trials have not observed short- or long-term survival benefits in patients who were transfused at a hemoglobin threshold of 7 g/dL versus 9 g/dL.56,57 Cardiac surgery is a unique setting with significant cardiovascular comorbidities, so a slightly higher mortality rate was observed in the Transfusion Indication Threshold Reduction trial (TITRe 2) study comparing the restrictive-threshold group (4.2%) and the liberal-threshold group (2.6%).58 In septic shock or other critically ill patients who do not have acute coronary syndromes, blood transfusion should be withheld if the hemoglobin level is between 7 and 8 g/dL.
VASOPRESSORS AND INOTROPE THERAPY
The clinical criteria for identifying septic shock patients are hypotension requiring vasopressor therapy to maintain a mean arterial pressure (MAP) of 65 mm Hg or greater and a serum lactate level greater than 2 mmol/L (> 18 mg/dL) after adequate fluid resuscitation.59 The first-line vasopressor recommended in septic shock is norepinephrine, based on multiple randomized controlled studies and meta-analysis comparing dopamine and norepinephrine. Use of norepinephrine was found to be superior with regard to mortality and adverse cardiac events.60 Use of dobutamine can increase cardiac index but lowers MAP and increases oxygen extraction. Experts suggest there is a role for dobutamine in pump failure due to septic cardiomyopathy, as manifested by elevated cardiac filling pressures and low cardiac output. Epinephrine has potent inotropic and vasoconstrictive effects, but is less commonly used as a first-line agent in septic shock, which is typically associated with a hyperdynamic circulation. When epinephrine was compared to norepinephrine plus dobutamine, there were no significant mortality benefits although it was associated with greater cardiac index.60,61 Vasopressin is a useful drug in refractory shock, however the VASST study could not show any beneficial effects on mortality.53
CLINICAL SIGNIFICANCE OF LACTIC ACIDOSIS
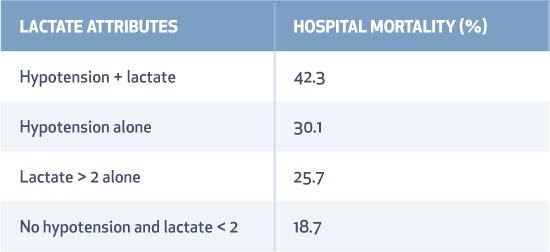
Lactate production in sepsis is multifactorial and incompletely understood. Increased production rather than delayed clearance possibly leads to high lactate levels in endotoxin-mediated sepsis. Traditionally, it was believed that lactate was produced from anaerobic metabolism, which led to multiple interventions to improve oxygen delivery, such as blood transfusion to a target of 10 mg/dL and use of inotropes to increase mixed venous oxygen saturation > 70%. But most patients with sepsis and elevated lactate have hyperdynamic circulation with adequate oxygen delivery. The source of lactate production is from the rapid rate of glycolysis and increased aerobic production that does not always take place in the muscle, so other tissues/cells are possible major contributors (Figure 1).62 Its greatest utility is as a guide to therapeutic response, an indicator of severity, and a prognostic tool for mortality (Table 5).59 Based on a large multicenter randomized controlled trial of two resuscitation protocols for early sepsis, a protocol targeting lactate clearance of at least 10% produces a similar short-term survival rate as a protocol using mixed venous oxygen saturation monitoring.63
Figure 1.
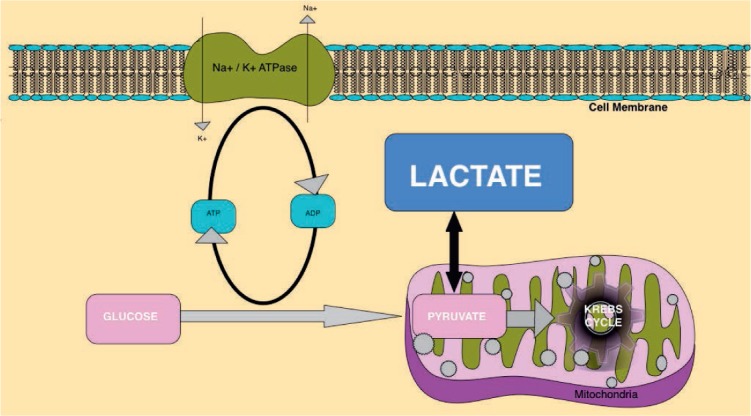
Lactate production from pyruvate, an intermediate step in glycolysis. Glycolysis is the metabolic process that serves as the foundation for both aerobic and anaerobic cellular respiration, where glucose is converted into pyruvate. Due to excess production of pyruvate or in anaerobic conditions, glucose is enzymatically catalyzed to lactate instead of entering the Krebs cycle in the mitochondria.
Table 5.
Lactate as a predictor of mortality.
HEMODYNAMIC MONITORING
Ideal hemodynamic monitoring should accurately report advanced parameters including intravascular volume status, fluid responsiveness, global blood flow, myocardial contractility, and cardiac afterload. Some of the available modalities are echocardiography, bioreactance, and calibrated/uncalibrated pulse contour analysis as well as functional testing such as the passive leg raise test and fluid challenge. Pulmonary artery catheter-guided therapy is associated with more complications than central venous catheter-guided therapy and thus is not recommended.60 While there are algorithms to guide fluid and vasopressor therapy using advanced hemodynamic monitoring,64 there are no studies demonstrating improved septic shock-related mortality. Instead of targeting distinct values of central venous pressure and central venous oxygen saturation, the SSC guidelines now recommend reassessing volume status and tissue perfusion by repeated focused examination and lactate clearance.
MYOCARDIAL DEPRESSION IN SEPSIS
Septic cardiomyopathy is a global phenomenon rather than just a reduction in left ventricular ejection fraction (LVEF). About 60% of septic shock patients experience LVEF reduction during the first 3 days of treatment, with an associated mortality rate of 15%.65 There is a complex interplay of vasoplegia, LV contractility, and LV afterload along with resuscitation efforts, such that LV performance varies throughout the course of sepsis. LVEF reflects the coupling between LV afterload and myocardial contractility, so a normal LVEF may still be observed when the arterial tone is severely depressed.66 Diastolic dysfunction is also known to be common and is a major predictor of mortality. In a study by Landesberg et al., patients with LVEF ≤ 50% only, e‘wave < 8 cm/s only (diastolic dysfunction), or with combined systolic and diastolic dysfunction had worse survival compared to those with normal cardiac function.67 Thirty percent of patients in early phases of septic shock have reduced right ventricular (RV) ejection fraction due to decreased preload (venous return) resulting from peripheral vasodilation.68 Myocardial depression and increased pulmonary vascular resistance due to adult respiratory distress syndrome (ARDS) may account for RV dysfunction as well. Elevated troponin-T levels can be difficult to interpret in such complex sepsis patients, and prevalence is estimated to be as high as 61%.69 The pathogenic mechanism is possibly due to demand ischemia, direct myocarditis, free radicals, elevated filling pressures, and ventricular wall stress. Left ventricular diastolic dysfunction and RV dilatation, a high Acute Physiology and Chronic Health Evaluation-II score (APACHE II), and low glomerular filtration rate correlate with high troponin-T concentrations and also predict higher mortality.70
ATRIAL FIBRILLATION ASSOCIATED WITH SEPSIS AND SEPTIC SHOCK
Sepsis and septic shock are potentially arrhythmogenic given the extreme stress from fluid shifts, catecholamine surges, autonomic dysfunction, proinflammatory cytokines, and cardiovascular compromise. The most common arrhythmia, atrial fibrillation, has an incidence ranging from 10% to 40% and is associated with increased mortality.71 The rates of atrial fibrillation are higher in patients maintained with a higher versus lower MAP (2.8% vs 6.7%), based on the Sepsis and Mean Arterial Pressure (SEPSISPAM) trial.72
RECOMMENDATIONS FOR ORGAN SUPPORT
Mechanical ventilation can improve cardiac performance by decreasing the stress of breathing and oxygen consumption on the respiratory muscles. For patients with sepsis-induced ARDS, SSC guidelines suggest using higher versus lower positive end expiratory pressure (PEEP), lower versus higher tidal volume settings on the mechanical ventilator, and prone positioning. Hypovolemia should be the first priority for hypotensive patients on mechanical ventilation. Even if there is dominant LV systolic failure, positive pressure ventilation is well tolerated due to an afterload reduction effect. However, diastolic dysfunction is associated with weaning failure from the ventilator, therefore it may be difficult to withdraw mechanical ventilation until LV function is stable.73
The SSC strongly recommends a protocolized approach to blood glucose management in ICU patients with sepsis, commencing insulin dosing when two consecutive blood glucose levels are > 180 mg/dL and maintaining an upper blood glucose level ≤ 180 mg/dL rather than ≤ 110 mg/dL. The Society of Critical Care Medicine and American Society for Parenteral and Enteral Nutrition guidelines recommend assessment of nutritional status using scoring systems such as the Nutrition Risk in the Critically Ill (NUTRIC) score and Nutritional Risk Screening (NRS 2002). Comorbid conditions should also be considered when assessing nutritional risk. Energy requirements may be calculated using indirect calorimetry or predicted equations. On average, a critically ill patient would need 25 to 30 kcal/kg and adequate protein of 1.2 to 2.0 gram/kg/day. Patients at high nutrition risk should be provided with > 80% of estimated requirements along with a high dose of protein and should be monitored for refeeding syndrome. High-protein hypocaloric foods should be used in obese patients to preserve lean body mass and minimize complications of over-feeding. Early oral or enteral feeding as tolerated is recommended rather than fasting or glucose-based intravenous therapies. Trophic feeding started during the initial acute phase of sepsis (≤ 500 kcal/day) should be advanced after 24 to 48 hours. Early parenteral nutrition is not known to improve outcomes.74
The SSC does not recommend using intravenous hydrocortisone to treat septic shock patients if adequate fluid resuscitation and vasopressor therapy can restore hemodynamic stability. If this is not achievable, intravenous hydrocortisone at a dose of 200 mg/day can be considered in refractory shock. Recently, the ADRENAL trial (ADjunctive coRticosteroid trEatment iN criticAlly ilL) compared continuous infusion of hydrocortisone in patients with septic shock who are on mechanical ventilation with placebo, and they did not report any mortality benefit in the steroid group.75 In the Activated Protein C and Corticosteroids for Human Septic Shock (APROCCHSS) trial of hydrocortisone plus fludrocortisone for adults with septic shock when compared to placebo showed lower 90-day all-cause mortality in the treatment arm; however, this study had a very long duration of patient accrual and withdrawal of the activated protein C arm.76 In early 2017, a combination therapy of hydrocortisone, vitamin C, and thiamine for severe sepsis and septic shock made waves in the news for lowering sepsis mortality by an overwhelming 32%. This was a single-center retrospective before-after study with a high baseline sepsis mortality and should be considered as hypothesis generating, with a further need for randomized trials to prove or disprove the success.77 Further considerations for steroid use can be reviewed in guidelines for critical illness-related corticosteroid insufficiency (CIRCI).78 In patients with acute kidney injury related to septic shock, the optimal timing for starting renal replacement therapy is still up for debate.79,80 Only about 59% of patients with sepsis-related renal failure recover renal function.81 Goals of care discussions should be incorporated into treatment and end-of-life care planning, using palliative care principles where appropriate.
SEPSIS GUIDELINES IMPLEMENTATION AND QUALITY INITIATIVES
The Surviving Sepsis Campaign strongly urges hospitals to develop an institution-specific SSC protocol and to establish a multidisciplinary working team with a solid commitment to enhanced sepsis screening, improved sepsis care bundle compliance, and outcomes. There are six LEADER steps for successful adoption of SSC guidelines in individual institutions: 1) Learn about sepsis quality improvement; 2) Establish baseline data to convince others that improvement is necessary; 3) Ask for buy-in from institutional leadership; 4) Develop an institution-specific SSC protocol; 5) Educate stakeholders; and 6) Remediate errors.
CONCLUSION
Sepsis in unique cardiovascular cohorts requires recommended sepsis bundle interventions, and compliance with these bundle elements improves mortality.
KEY POINTS
Sepsis has a new definition that is more focused on organ dysfunction rather than inflammation.
Sepsis management bundles guide clinicians through time-sensitive interventions.
Cardiovascular patients have complex attributes, and sepsis management, specifically fluid therapy, is often delayed.
Specific recommendations for organ support in sepsis should be followed.
Quality initiatives drive superior outcomes by establishing sepsis screening programs, institution-specific sepsis care bundle protocols, and multidisciplinary teams in charge of sepsis diagnosis and timely care.
Acknowledgments
The author would like to thank Janice L. Zimmerman, M.D., from Weill Cornell Medical College and Houston Methodist Hospital, for her help with this manuscript.
Footnotes
Conflict of Interest Disclosure: The author has completed and submitted the Methodist DeBakey Cardiovascular Journal Conflict of Interest Statement and none were reported.
REFERENCES
- 1. Singer M, Deutschman CS, Seymour CW, . et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016. February 23; 315 8: 801– 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fleischmann C, Scherag A, Adhikari NK, . et al .; International Forum of Acute Care Trialists Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am J Respir Crit Care Med. 2016. February 1; 193 3: 259– 72. [DOI] [PubMed] [Google Scholar]
- 3. Wang HE, Jones AR, Donnelly JP.. Revised National Estimates of Emergency Department Visits for Sepsis in the United States. Crit Care Med. 2017. September; 45 9: 1443– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Torio CM, Moore BJ. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013: Statistical Brief #204. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2006–2016. May. [PubMed] [Google Scholar]
- 5. Rowan KM, Angus DC, Bailey M, . et al .; PRISM Investigators Early, Goal-Directed Therapy for Septic Shock - A Patient-Level Meta-Analysis. N Engl J Med. 2017. June 8; 376 23: 2223– 34. [DOI] [PubMed] [Google Scholar]
- 6. Annane D, Aegerter P, Jars-Guincestre MC, Guidet B; CUB-Réa Network. . Current epidemiology of septic shock: the CUB-Réa Network. Am J Respir Crit Care Med. 2003. July 15; 168 2: 165– 72. [DOI] [PubMed] [Google Scholar]
- 7. Kang CI, Song JH, Chung DR, . et al .; Korean Network for Study of Infectious Diseases (KONSID) Risk factors and pathogenic significance of severe sepsis and septic shock in 2286 patients with gram-negative bacteremia. J Infect. 2011. January; 62 1: 26– 33. [DOI] [PubMed] [Google Scholar]
- 8. O'Grady NP, Barie PS, Bartlett JG, . et al .; American College of Critical Care Medicine; Infectious Diseases Society of America Guidelines for evaluation of new fever in critically ill adult patients: 2008 update from the American College of Critical Care Medicine and the Infectious Diseases Society of America. Crit Care Med. 2008. April; 36 4: 1330– 49. [DOI] [PubMed] [Google Scholar]
- 9. Lepelletier D, Bourigault C, Roussel JC, . et al. Epidemiology and prevention of surgical site infections after cardiac surgery. Med Mal Infect. 2013. October; 43 10: 403– 9. [DOI] [PubMed] [Google Scholar]
- 10. Ramanathan R, Leavell P, Mays C, Duane TM.. Impact of Sepsis on Surgical Outcomes. Surg Infect (Larchmt). 2015. August; 16 4: 405– 9. [DOI] [PubMed] [Google Scholar]
- 11. Orvin K, Kornowski R, Bishara J, . et al. The frequency and prognostic impact of fever following transcatheter aortic valve implantation. Cardiology. 2014; 127 3: 203– 10. [DOI] [PubMed] [Google Scholar]
- 12. van der Boon RM, Nuis RJ, Benitez LM, . et al. Frequency, determinants and prognostic implications of infectious complications after transcatheter aortic valve implantation. Am J Cardiol. 2013. July 1; 112 1: 104– 10. [DOI] [PubMed] [Google Scholar]
- 13. Dicks KV, Staheli R, Anderson DJ, . et al. “What the eyes don't see, the heart doesn't grieve over”: epidemiology and risk factors for bloodstream infections following cardiac catheterization. Infect Control Hosp Epidemiol. 2012. August; 33 8: 837– 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lai NM, Chaiyakunapruk N, Lai NA, O'Riordan E, Pau WS, Saint S.. Catheter impregnation, coating or bonding for reducing central venous catheter-related infections in adults. Cochrane Database Syst Rev. 2016. March 16; 3: CD007878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Baddour LM, Epstein AE, Erickson CC, . et al. ; American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; Council on Cardiovascular Disease in Young; Council on Cardiovascular Surgery and Anesthesia;et al. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010. January 26; 121 3: 458– 77. [DOI] [PubMed] [Google Scholar]
- 16. Aubron C, Cheng AC, Pilcher D, . et al. Infections acquired by adults who receive extracorporeal membrane oxygenation: risk factors and outcome. Infect Control Hosp Epidemiol. 2013. January; 34 1: 24– 30. [DOI] [PubMed] [Google Scholar]
- 17. Tong MZ, Smedira NG, Soltesz EG, . et al. Outcomes of Heart Transplant After Left Ventricular Assist Device Specific and Related Infection. Ann Thorac Surg. 2015. October; 100 4: 1292– 7. [DOI] [PubMed] [Google Scholar]
- 18. Vincent JL, Sakr Y, Sprung CL, . et al .; Sepsis Occurrence in Acutely Ill Patients Investigators Sepsis in European intensive care units: results of the SOAP study. Crit Care Med. 2006. February; 34 2: 344– 53. [DOI] [PubMed] [Google Scholar]
- 19. Kaukonen KM, Bailey M, Bellomo R.. Systemic Inflammatory Response Syndrome Criteria for Severe Sepsis. N Engl J Med. 2015. August 27; 373 9: 881. [DOI] [PubMed] [Google Scholar]
- 20. Kohsaka S, Menon V, Lowe AM, . et al. Systemic inflammatory response syndrome after acute myocardial infarction complicated by cardiogenic shock. Arch Intern Med. 2005. July 25; 165 14: 1643– 50. [DOI] [PubMed] [Google Scholar]
- 21. Vincent JL, Moreno R, Takala J, . et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med. 1996. July; 22 7: 707– 10. [DOI] [PubMed] [Google Scholar]
- 22. Seymour CW, Liu VX, Iwashyna TJ, . et al. Assessment of Clinical Criteria for Sepsis: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016. February 23; 315 8: 762– 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Roney JK, Whitley BE, Maples JC, Futrell LS, Stunkard KA, Long JD.. Modified early warning scoring (MEWS): evaluating the evidence for tool inclusion of sepsis screening criteria and impact on mortality and failure to rescue. J Clin Nurs. 2015. December; 24 23–24: 3343– 54. [DOI] [PubMed] [Google Scholar]
- 24. Rothman M, Levy M, Dellinger RP, . et al. Sepsis as 2 problems: Identifying sepsis at admission and predicting onset in the hospital using an electronic medical record-based acuity score. J Crit Care. 2017. April; 38: 237– 44. [DOI] [PubMed] [Google Scholar]
- 25. Rhodes A, Evans LE, Alhazzani W, . et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock: 2016. Intensive Care Med. 2017. March; 43 3: 304– 377. [DOI] [PubMed] [Google Scholar]
- 26. Levy MM, Evans LE, Rhodes A.. The Surviving Sepsis Campaign Bundle: 2018 update. Intensive Care Med. 2018. April 19 [epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 27. Sherwin R, Winters ME, Vilke GM, Wardi G.. Does Early and Appropriate Antibiotic Administration Improve Mortality in Emergency Department Patients with Severe Sepsis or Septic Shock? J Emerg Med. 2017. October; 53 4: 588– 95. [DOI] [PubMed] [Google Scholar]
- 28. Ferrer R, Martin-Loeches I, Phillips G, . et al. Empiric antibiotic treatment reduces mortality in severe sepsis and septic shock from the first hour: results from a guideline-based performance improvement program. Crit Care Med. 2014. August; 42 8: 1749– 55. [DOI] [PubMed] [Google Scholar]
- 29. Andriolo BN, Andriolo RB, Salomão R, Atallah ÁN.. Effectiveness and safety of procalcitonin evaluation for reducing mortality in adults with sepsis, severe sepsis or septic shock. Cochrane Database Syst Rev. 2017. January 18; 1: CD010959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Huang DT, Yealy DM, Filbin MR, . et al. , Procalcitonin-Guided Use of Antibiotics for Lower Respiratory Tract Infection. N Engl J Med. 2018. May 20 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rausei S, Pappalardo V, Ruspi L, . et al. Early Versus Delayed Source Control in Open Abdomen Management for Severe Intra-abdominal Infections: A Retrospective Analysis on 111 Cases. World J Surg. 2018. March; 42 3: 707– 12. [DOI] [PubMed] [Google Scholar]
- 32. Chu VH, Park LP, Athan E, . et al .; International Collaboration on Endocarditis (ICE) Investigators Association between surgical indications, operative risk, and clinical outcome in infective endocarditis: a prospective study from the International Collaboration on Endocarditis. Circulation. 2015. January 13; 131 2: 131– 40. [DOI] [PubMed] [Google Scholar]
- 33. Rivers E, Nguyen B, Havstad S, . et al .; Early Goal-Directed Therapy Collaborative Group Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001. November 8; 345 19: 1368– 77. [DOI] [PubMed] [Google Scholar]
- 34. Yealy DM, Kellum JA, Huang DT, . et al .; ProCESS Investigators A randomized trial of protocol-based care for early septic shock. N Engl J Med. 2014. May 1; 370 18: 1683– 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mouncey PR, Osborn TM, Power GS, . et al. Trial of early, goal-directed resuscitation for septic shock. N Engl J Med. 2015. April 2; 372 14: 1301– 11. [DOI] [PubMed] [Google Scholar]
- 36. Peake SL, Delaney A, Bailey M, . et al .; ARISE Investigators; ANZICS Clinical Trials Group Goal-directed resuscitation for patients with early septic shock. N Engl J Med. 2014. October 16; 371 16: 1496– 506. [DOI] [PubMed] [Google Scholar]
- 37. Rhodes A, Phillips G, Beale R, . et al. The Surviving Sepsis Campaign bundles and outcome: results from the International Multicentre Prevalence Study on Sepsis (the IMPreSS study). Intensive Care Med. 2015. September; 41 9: 1620– 8. [DOI] [PubMed] [Google Scholar]
- 38. Liu VX, Morehouse JW, Marelich GP, . et al. Multicenter Implementation of a Treatment Bundle for Patients with Sepsis and Intermediate Lactate Values. Am J Respir Crit Care Med. 2016. June 1; 193 11: 1264– 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Seymour CW, Gesten F, Prescott HC, . et al. Time to Treatment and Mortality during Mandated Emergency Care for Sepsis. N Engl J Med. 2017. June 8; 376 23: 2235– 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leisman DE, Goldman C, Doerfler ME, . et al. Patterns and Outcomes Associated With Timeliness of Initial Crystalloid Resuscitation in a Prospective Sepsis and Septic Shock Cohort. Crit Care Med. 2017. October; 45 10: 1596– 1606. [DOI] [PubMed] [Google Scholar]
- 41. Marik PE, Linde-Zwirble WT, Bittner EA, Sahatjian J, Hansell D.. Fluid administration in severe sepsis and septic shock, patterns and outcomes: an analysis of a large national database. Intensive Care Med. 2017. May; 43 5: 625– 32. [DOI] [PubMed] [Google Scholar]
- 42. Young P, Bailey M, Beasley R, . et al .; SPLIT Investigators; ANZICS CTG Effect of a Buffered Crystalloid Solution vs Saline on Acute Kidney Injury Among Patients in the Intensive Care Unit: The SPLIT Randomized Clinical Trial. JAMA. 2015. October 27; 314 16: 1701– 10. [DOI] [PubMed] [Google Scholar]
- 43. Semler MW, Self WH, Wanderer JP, . et al. Balanced Crystalloids versus Saline in Critically Ill Adults. N Engl J Med. 2018; 378 9: 829– 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Patel A, Pieper K, Myburgh JA, . et al. Reanalysis of the Crystalloid versus Hydroxyethyl Starch Trial (CHEST). N Engl J Med. 2017. July 20; 377 3: 298– 300. [DOI] [PubMed] [Google Scholar]
- 45. Bagshaw SM, Chawla LS. Hydroxyethyl starch for fluid resuscitation in critically ill patients. Can J Anaesth. 2013. July; 60 7: 709– 13. [DOI] [PubMed] [Google Scholar]
- 46. Crystalloid versus Hydroxyethyl Starch Trial (CHEST) Management Committee. . The Crystalloid versus Hydroxyethyl Starch Trial: protocol for a multi-centre randomised controlled trial of fluid resuscitation with 6% hydroxyethyl starch (130/0.4) compared to 0.9% sodium chloride (saline) in intensive care patients on mortality. Intensive Care Med. 2011. May; 37 5: 816– 23. [DOI] [PubMed] [Google Scholar]
- 47. Annane D, Siami S, Jaber S, . et al. Effects of fluid resuscitation with colloids vs crystalloids on mortality in critically ill patients presenting with hypovolemic shock: the CRISTAL randomized trial. JAMA. 2013. November 6; 310 17: 1809– 17. [DOI] [PubMed] [Google Scholar]
- 48. Finfer S, Bellomo R, McEvoy S, . et al .; SAFE Study Investigators Effect of baseline serum albumin concentration on outcome of resuscitation with albumin or saline in patients in intensive care units: analysis of data from the saline versus albumin fluid evaluation (SAFE) study. BMJ. 2006. November 18; 333 7577: 1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Caironi P, Tognoni G, Masson S, . et al .; ALBIOS Study Investigators Albumin replacement in patients with severe sepsis or septic shock. N Engl J Med. 2014. April 10; 370 15: 1412– 21. [DOI] [PubMed] [Google Scholar]
- 50. Jiang L, Jiang S, Zhang M, Zheng Z, Ma Y.. Albumin versus other fluids for fluid resuscitation in patients with sepsis: a meta-analysis. PLoS One. 2014. December 4; 9 12: e114666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Patel A, Laffan MA, Waheed U, Brett SJ.. Randomised trials of human albumin for adults with sepsis: systematic review and meta-analysis with trial sequential analysis of all-cause mortality. BMJ. 2014. July 22; 349: g4561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hoste EA, Maitland K, Brudney CS, . et al .; ADQI XII Investigators Group Four phases of intravenous fluid therapy: a conceptual model. Br J Anaesth. 2014. November; 113 5: 740– 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Russell JA, Walley KR, Singer J, . et al .; VASST Investigators Vasopressin versus norepinephrine infusion in patients with septic shock. N Engl J Med. 2008. February 28; 358 9: 877– 87. [DOI] [PubMed] [Google Scholar]
- 54. Wiedemann HP, Wheeler AP, Bernard GR, . et al .; National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006. June 15; 354 24: 2564– 75. [DOI] [PubMed] [Google Scholar]
- 55. Malbrain ML, Marik PE, Witters I, . et al. Fluid overload, de-resuscitation, and outcomes in critically ill or injured patients: a systematic review with suggestions for clinical practice. Anaesthesiol Intensive Ther. 2014. Nov-Dec; 46 5: 361– 80. [DOI] [PubMed] [Google Scholar]
- 56. Rygård SL, Holst LB, Wetterslev J, Johansson PI, Perner A; TRISS trial group; Scandinavian Critical Care Trials Group. . Higher vs lower haemoglobin threshold for transfusion in septic shock: subgroup analyses of the TRISS trial. Acta Anaesthesiol Scand. 2017. February; 61 2: 166– 75. [DOI] [PubMed] [Google Scholar]
- 57. Rygård SL, Holst LB, Wetterslev J, . et al .; TRISS Trial Group; Scandinavian Critical Care Trials Group Long-term outcomes in patients with septic shock transfused at a lower versus a higher haemoglobin threshold: the TRISS randomised, multicentre clinical trial. Intensive Care Med. 2016. November; 42 11: 1685– 94. [DOI] [PubMed] [Google Scholar]
- 58. Liberal or restrictive transfusion after cardiac surgery. N Engl J Med. 2015. June 4; 372 23: 2274. [DOI] [PubMed] [Google Scholar]
- 59. Shankar-Hari M, Phillips GS, Levy ML, . et al .; Sepsis Definitions Task Force Developing a New Definition and Assessing New Clinical Criteria for Septic Shock: For the Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016. February 23; 315 8: 775– 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhou F, Mao Z, Zeng X, . et al. Vasopressors in septic shock: a systematic review and network meta-analysis. Ther Clin Risk Manag. 2015. July 14; 11: 1047– 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Annane D, Vignon P, Renault A, . et al .; CATS Study Group Norepinephrine plus dobutamine versus epinephrine alone for management of septic shock: a randomised trial. Lancet. 2007. August 25; 370 9588: 676– 84. [DOI] [PubMed] [Google Scholar]
- 62. Michaeli B, Martinez A, Revelly JP, . et al. Effects of endotoxin on lactate metabolism in humans. Crit Care. 2012. July 27; 16 4: R139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA; Emergency Medicine Shock Research Network (EMShockNet) Investigators. . Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010. February 24; 303 8: 739– 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Saugel B, Huber W, Nierhaus A, Kluge S, Reuter DA, Wagner JY.. Advanced Hemodynamic Management in Patients with Septic Shock. Biomed Res Int. 2016; 2016: 8268569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Vieillard-Baron A, Caille V, Charron C, Belliard G, Page B, Jardin F.. Actual incidence of global left ventricular hypokinesia in adult septic shock. Crit Care Med. 2008. June; 36 6: 1701– 6. [DOI] [PubMed] [Google Scholar]
- 66. Antonucci E, Fiaccadori E, Donadello K, Taccone FS, Franchi F, Scolletta S.. Myocardial depression in sepsis: from pathogenesis to clinical manifestations and treatment. J Crit Care. 2014. August; 29 4: 500– 11. [DOI] [PubMed] [Google Scholar]
- 67. Landesberg G, Gilon D, Meroz Y, . et al. Diastolic dysfunction and mortality in severe sepsis and septic shock. Eur Heart J. 2012. April; 33 7: 895– 903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Vieillard Baron A, Schmitt JM, Beauchet A, . et al. Early preload adaptation in septic shock? A transesophageal echocardiographic study. Anesthesiology. 2001. March; 94 3: 400– 6. [DOI] [PubMed] [Google Scholar]
- 69. Zochios V, Valchanov K. Raised cardiac troponin in intensive care patients with sepsis, in the absence of angiographically documented coronary artery disease: A systematic review. J Intensive Care Soc. 2015. February; 16 1: 52– 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Landesberg G, Jaffe AS, Gilon D, . et al. Troponin elevation in severe sepsis and septic shock: the role of left ventricular diastolic dysfunction and right ventricular dilatation. Crit Care Med. 2014. April; 42 4: 790– 800. [DOI] [PubMed] [Google Scholar]
- 71. Klein Klouwenberg PM, Frencken JF, Kuipers S, . et al .; MARS Consortium Incidence, Predictors, and Outcomes of New-Onset Atrial Fibrillation in Critically Ill Patients with Sepsis. A Cohort Study. Am J Respir Crit Care Med. 2017. January 15; 195 2: 205– 11. [DOI] [PubMed] [Google Scholar]
- 72. Asfar P, Meziani F, Hamel JF, . et al .; SEPSISPAM Investigators High versus low blood-pressure target in patients with septic shock. N Engl J Med. 2014. April 24; 370 17: 1583– 93. [DOI] [PubMed] [Google Scholar]
- 73. Konomi I, Tasoulis A, Kaltsi I, . et al. Left ventricular diastolic dysfunction–an independent risk factor for weaning failure from mechanical ventilation. Anaesth Intensive Care. 2016. July; 44 4: 466– 73. [DOI] [PubMed] [Google Scholar]
- 74. Allingstrup MJ, Kondrup J, Wiis J, . et al. Early goal-directed nutrition versus standard of care in adult intensive care patients: the single-centre, randomised, outcome assessor-blinded EAT-ICU trial. Intensive Care Med. 2017. November; 43 11: 1637– 47. [DOI] [PubMed] [Google Scholar]
- 75. Venkatesh B, Finfer S, Cohen J, . et al. Adjunctive Glucocorticoid Therapy in Patients with Septic Shock. N Engl J Med. 2018. March 1; 378 9: 797– 808. [DOI] [PubMed] [Google Scholar]
- 76. Annane D, Renault A, Brun-Buisson C, . et al. Hydrocortisone plus Fludrocortisone for Adults with Septic Shock. N Engl J Med. 2018. March 1; 378 9: 809– 18. [DOI] [PubMed] [Google Scholar]
- 77. Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J.. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. Chest. 2017. June; 151 6: 1229– 38. [DOI] [PubMed] [Google Scholar]
- 78. Annane D, Pastores SM, Rochwerg B, . et al. Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive Care Med. 2017. December; 43 12: 1751– 63. [DOI] [PubMed] [Google Scholar]
- 79. Gaudry S, Chaïbi K, Bénichou N, Verney C, Hajage D, Dreyfuss D.. Renal replacement therapy for acute kidney injury in the intensive care unit. Nephrol Ther. 2017. April; 13 Suppl 1: S13– S21. [DOI] [PubMed] [Google Scholar]
- 80. Zarbock A, Kellum JA, Schmidt C, . et al. Effect of Early vs Delayed Initiation of Renal Replacement Therapy on Mortality in Critically Ill Patients With Acute Kidney Injury: The ELAIN Randomized Clinical Trial. JAMA. 2016. May 24–31; 315 20: 2190– 9. [DOI] [PubMed] [Google Scholar]
- 81. Kellum JA, Sileanu FE, Bihorac A, Hoste EA, Chawla LS.. Recovery after Acute Kidney Injury. Am J Respir Crit Care Med. 2017. March 15; 195 6: 784– 91. [DOI] [PMC free article] [PubMed] [Google Scholar]


